
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 06 October 2022
Sec. Nutritional Immunology
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.949629
This article is part of the Research Topic Nutritional Immunology and Fibrosis View all 5 articles
 Rui Tang1,2†
Rui Tang1,2† Xiaohong Lyu3†
Xiaohong Lyu3† Yi Liu4†
Yi Liu4† Mingzhi Zhu4
Mingzhi Zhu4 Xukai Yang4
Xukai Yang4 Zhoujie Wu4
Zhoujie Wu4 Bingnan Han5*
Bingnan Han5* Shandong Wu4*
Shandong Wu4* Jinlyu Sun1,2*
Jinlyu Sun1,2*Background: Cow’s milk protein allergy (CMPA) is a common allergy. Immunoglobulin E (IgE)-mediated cow’s milk allergy is associated with a high mortality risk and poor prognosis. The study aims to investigate whether there are different clinically CMPA phenotypes in China and to explore the association between CMPA phenotypes and specific IgE (sIgE) antibodies against different dairy products.
Methods: Serum sIgE against different animal milk and cow’s milk products and different milk components was measured by an allergen array. Four CMPA classifications were identified by the presence of serum sIgE: boiled milk-positive, yogurt-positive, buttermilk-positive, and raw milk-positive.
Results: Among the 234 participants included in the study, 9 were boiled milk sIgE-positive, 50 were yogurt sIgE-positive, 17 were buttermilk sIgE-positive, and 158 were only raw milk sIgE-positive. The boiled milk-positive group had the highest levels of raw milk sIgE and casein sIgE antibodies, followed sequentially by the yogurt-positive, buttermilk-positive, and raw milk-positive groups. The boiled milk group observed the highest levels of sIgE against raw milk, casein, α-lactalbumin, and β-lactoglobulin. These levels differed significantly from those in the other three groups. Allergic symptoms were distributed differently among the four study groups. The percentages of allergic patients with gastrointestinal tract symptoms in the above mentioned four groups ranged from high to low, and the percentages of patients with skin symptoms in the four groups ranged from low to high, respectively.
Conclusion: Based on dairy product sIgE antibody levels associated with different milk components and various clinical allergic symptom tendencies, we could distinguish four CMPA phenotypes.
Food allergies are a health problem of increasing concern worldwide. Food allergy is defined as a specific immune response caused by a portion of specific food, which can occur repeatedly and cause disorders involving the skin or gastrointestinal or respiratory tract. Milk, eggs, wheat, soybeans, peanuts, nuts, fish, and shellfish are considered to be the common causes of food allergy (1). Cow’s milk protein allergy (CMPA) is a specific immune response to various proteins in milk. CMPA can be attributed to immunoglobulin E (IgE)-mediated and non-IgE-mediated mechanisms (2–8). CMPA is the most common food protein allergy in infants and children (9). In previous studies, the prevalence of IgE-mediated CMPA was 2%-9% (9). A prospective study of 6768 children with suspected CMPA conducted jointly by seven hospitals in southern China showed that 182 children were diagnosed with CMPA, and the prevalence of CMPA was 2.69% (10).
A food allergy may endanger life safety and greatly affect quality of life. An accurate food allergy diagnosis is necessary to avoid severe allergic reactions and unnecessary dietary restrictions. However, accurate diagnosis can be difficult. According to practice guidelines (11), the initial diagnosis of CMPA should be based on the exclusion of milk protein from the diet. Then, a double-blind placebo-controlled oral food challenge (OFC), the gold standard for the diagnosis of CMPA (3, 12), should be performed. However, the trial requires the cooperation of patients and parents, which is time-consuming and expensive. It is challenging to implement under real-world conditions in China. Even nondouble-blind OFC has the risk of an immediate or severe allergic reaction (13).
There are diagnostic methods for diagnosing CMPA other than OFC, including screening scoring systems, skin prick tests (SPTs) and serum milk-specific Immunoglobulin E (sIgE) detection. However, the cutoff point value of the milk-related symptom score has not been agreed upon in the academic community, and the sensitivity and specificity of this diagnostic tool are low (14). The area under the receiver operating characteristic (ROC) curve (AUC) was only 0.68 (14). An SPT is used to detect the presence of IgE tissue-binding antibodies, which can reflect IgE-mediated allergic diseases to a certain extent. However, this test is prone to false-negative results in infants because infants usually have a poor response to the SPT (15). Because of the convenience and ability to detect various allergens, serum sIgE detection (16–20) is widely used to assist in the diagnosis of CMPA in China.
CMPA patients need to strictly avoid milk in daily life to prevent the occurrence of an allergic reaction. However, this common management practice has coincided with the delayed resolution of the allergy. Strict milk avoidance does not improve long-term outcomes and significantly affects patients’ quality of life. Many CMPA patients believe from experience that they can tolerate some milk-containing products. Kim JS et al. reported that ingesting extensively heated milk products (baked milk) accelerates and increases the overall likelihood of milk allergy resolution in CMPA patients without significant adverse effects (21). Furthermore, Cansin Sackesen et al. found that CMPA patients had different reactivity levels to baked, fermented, and whole milk according to a new Luminex-based peptide assay (22). It seems that CMPA patients could be divided into different phenotypes according to reactivity to different milk products (23).
We designed this cross-sectional study to investigate whether there are different clinically CMPA phenotypes in North China and to explore the association between the CMPA phenotypes and sIgE antibodies against different dairy products. In addition, we investigated the clinical characteristics of the different CMPA phenotypes.
This study was a cross-sectional study. Participants with CMPA underwent evaluation at the department of allergy at Peking Union Medical College Hospital between May 2019 and July 2019. Clinical information was collected through either a questionnaire or chart review. Written informed consent was obtained. CMPA was documented when a patient reported a convincing history of acute reaction after food ingestion in the previous 12 months, considering the results of milk sIgE, SPT, or a previous positive OFC result. The study was approved by the Institutional Ethics Committee of Peking Union Medical College Hospital (JS-3427).
Detection of serum sIgE against different animal milks, different cow’s milk products and different milk components was performed with an allergen array (Hangzhou Zheda Dixun Biological Genetic Engineering Co., Ltd.). The lower and upper detection limits were 0.0 and 1000 kU/L, respectively. Serum sIgE values of 0.35 kU/L (4) or higher were considered indicative of positive results. The animal milks included raw milk, buffalo’s milk, goat’s milk, and mare’s milk. The cow’s milk products included boiled milk (representative of extensively heated milk), hydrolyzed milk powder, yogurt, cheddar cheese, buttermilk, defatted milk powder, cottage cheese, and cow whey. The cow’s milk components included casein, α-lactalbumin, β-lactoglobulin and bovine serum albumin (4).
The items of serological testing used in this study include allergenic component proteins of milk-casein (NA-BD8-1), alpha-lactalbumin (NA-BD4-1), beta-lactoglobulin (NA-BD5-1) and bovine serum albumin (NA-BD6-1) purified through HPLC or colum chromatography were purchased from Indoor biotechnologies. On the other hand, food allergen of raw milk, buffalo’s milk, goats’ milk, mare’s milk, boiled milk, hydrolyzed milk powder, yogurt, cheddar cheese, buttermilk, defatted milk powder, cottage cheese and cow whey are extract from natural food.
For protein extraction from natural foods, 10 mL of raw milk/buffalo’s milk/sheep’s milk/mare’s milk/boiled milk was centrifuged at 4000 rpm and 4°C for 15 min, add 50 mL of cold acetone to degrease, remove the acetone and air dry, add 100 mL of Coca’s solution (0.1M Na2CO3/NaHCO3, 0.1M NaCl, 2mM EDTA, 20mM DIECA) to the air-dried sample and stir overnight. After centrifugation at 10,000 rpm and 4°C for 30 min, the supernatant was taken for ultrafiltration. The ultra-filtered liquid was centrifuged at 12,000 rpm and 4°C for 30 min, and the supernatant was collected. Then the dialysis was carried out in PBS at 4°C under magnetic stirring for 10 h to obtain the final milk extract.
10 g of hydrolyzed milk powder/yogurt/cheddar cheese/buttermilk/defatted milk powder/cottage cheese/cow whey powder was dissolved in 100 mL purified water. 10 mL was taken for extraction. The extraction method was the same as raw milk.
Three hundred microlitre of undiluted serum is applied on chips wetted by washing buffer in the kit and incubated for 45 min, the secondary antibody is applied and incubated for 45 min after washing five times, then enzyme solution is applied and incubated for 20 min after washing five times. All steps are operated at room temperature. The DX-allergen Analysis Software (version 7.0) export testing results and interpretation of the results was based on international classification standards (24). The allergen specific IgE antibody contents are calculated according to a typical standard curve established with WHO IgE human serum (3rd International Standard) 11/234 (25).
The methodology is based on antigen-antibody specific reaction. The antigen proteins are immobilized on the matrix membrane. During the experiment, the antibody in the serum specifically binds to the corresponding antigen on the matrix membrane. After removing excess serum, a secondary antibody that recognizes human antibodies is added and then washed. An enzyme that can be linked to the secondary antibody is added, and this enzyme can undergo a chromogenic enzymatic reaction with the substrate. Determine the concentration of allergen-specific antibodies in serum based on the degree of colour development.
The comparative results of the sIgE test kit in this study and ImmunoCAP-250 system (Thermo Fisher Scientific) were a positive coincidence rate of 100.00% and a negative coincidence rate of 84.62%. The sIgE test kit in this study was also used in previous studies which have been published (26, 27). Similar sIgE test kit developed by another company was used in published studies also (28, 29).
According to guidelines recently, there is no symptom that is specific for CMA as its every manifestation can be caused by multiple conditions (30). The participants’ allergic symptoms were evaluated according to five systems. The allergic symptom of the upper respirational tract included allergic rhinitis. Allergic symptoms of the lower respiratory tract included cough and allergic asthma. Allergic symptoms of the skin included urticaria, allergic purpura, rash and blister. Allergic symptoms of the gastrointestinal tract included abdominal pain, diarrhea, and bloody stool caused by food hypersensitivity. Allergic symptoms of severe anaphylactic reactions were severe, life-threatening systemic allergic reactions.
All data were analyzed using SPSS statistical software, version 26.0 for Mac (SPSS Inc, Chicago, IL) and GraphPad Prism version 9.0.0 for Mac (GraphPad Software, San Diego, California USA). Milk sIgE levels were analyzed by ANOVA and t tests. P values of less than 0.05 were regarded as significant.
Two hundred and thirty-four CMPA participants were enrolled. The baseline characteristics of the participants are presented in Table 1. The average age of the CMPA participants was 5.8 ± 9.4 years old, ranging from 6 month to 61 years old. There were 151 males and 83 females. Almost all the participants completed all the milk-related sIgE detection examinations, except 49 participants with missing mare’s milk sIgE data and 185 participants with missing defatted milk powder, cottage cheese or cow whey sIgE data. More details regarding the numbers of milk-related product sIgE-positive participants are provided in Table 1.
A heatmap of the sIgE results of the participants is shown in Figure 1. The heatmap indicated that the positive results of sIgE against various dairy products were consistent with that of raw milk.
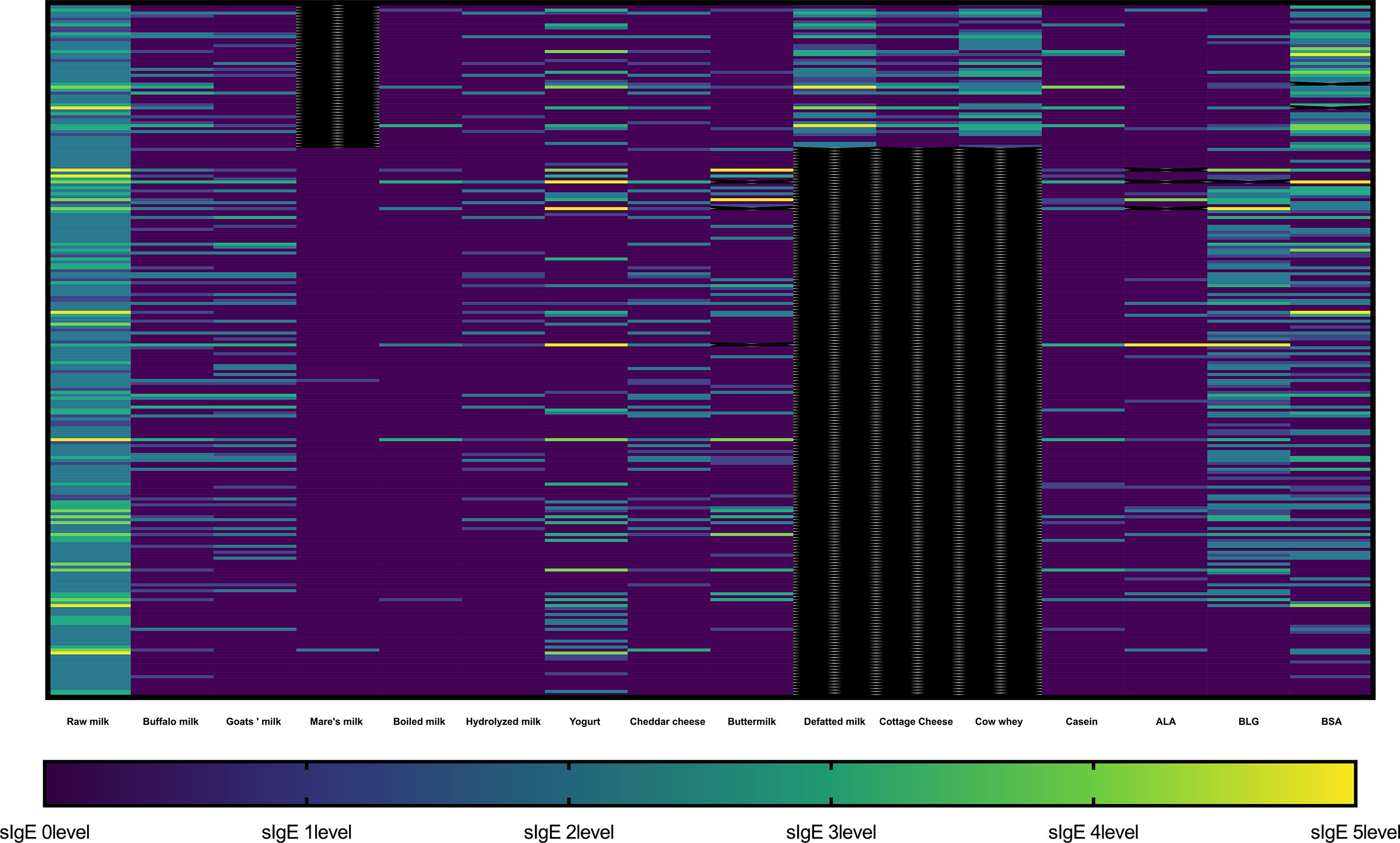
Figure 1 Heatmap representing sIgE levels against different milk-related product in each participant. The sIgE result was divided into 6 levels: level 0 (< 0.35 kU/L), level 1 (≥ 0.35 kU/L and < 0.7 kU/L), level 2 (≥ 0.7 kU/L and < 3.5 kU/L), level 3 (≥ 3.5 kU/L and < 17.5 kU/L), level 4 (≥ 17.5 kU/L and < 50 kU/L), level 5 (≥ 50 kU/L and < 100 kU/L), and level 6 (≥ 100 kU/L). The black color represents missing data. ALA, α-lactalbumin; BLG, β-lactoglobulin (BLG); BSA, bovine serum albumin (BSA).
The comparison of the raw milk sIgE levels among the raw milk sIgE-positive participants and the other dairy sIgE-positive participants is shown in Figure 2. The raw milk sIgE level in boiled milk sIgE-positive participants was highest, with a median of 30.13 and interquartile range (IQR) of 9.165 to 44.550, which was significantly higher than that in the raw milk sIgE-positive participants (median 1.685, IQR 0.898-4.183, P<0.0001). In addition, the raw milk sIgE levels in the yogurt- and buttermilk-positive participants were significantly higher than those in the raw milk-positive participants (P<0.0001 and P=0.0041). Therefore, reactivity to cow’s milk in participants in the boiled milk-positive group, yogurt-positive group, buttermilk-positive group, and raw milk-positive group were likely different. It is reasonable to explore the detailed milk component sIgE results and the clinical characteristics of these four groups.
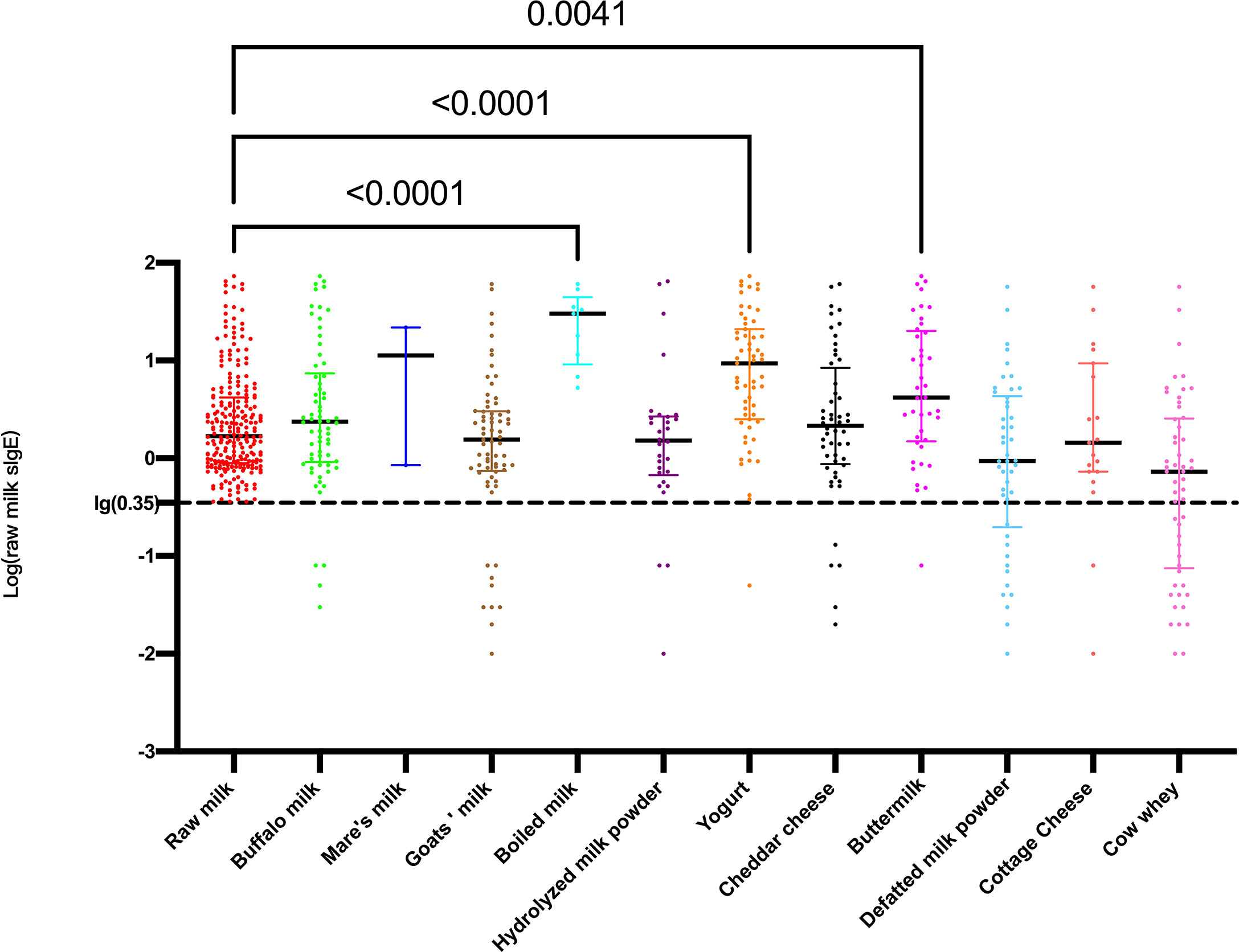
Figure 2 Levels of log-transformed sIgE against raw milk among different dairy product sIgE-positive participants.
Among the 234 participants included in the study (Figure 3), 9 were boiled milk sIgE-positive. Fifty were boiled milk sIgE-negative but yogurt sIgE-positive. Seventeen were boiled milk and yogurt sIgE-negative but buttermilk-sIgE positive. One hundred fifty-eight were boiled milk, yogurt, and buttermilk sIgE-negative but raw milk sIgE-positive. These four groups were the main study groups of this study.
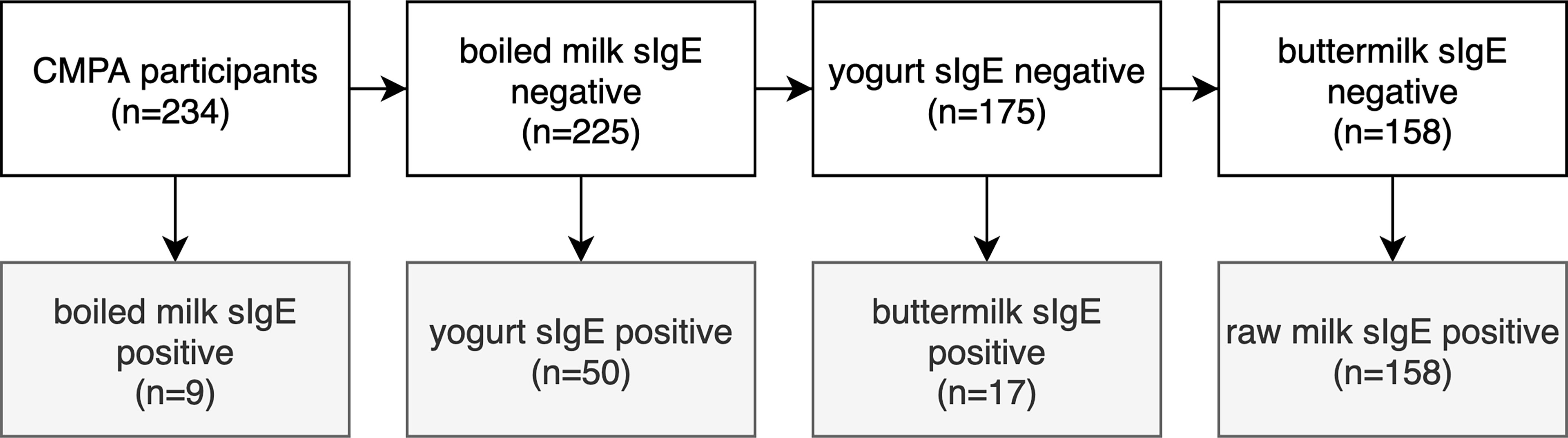
Figure 3 Flow diagram showing the distributions of participants according to the boiled milk, yogurt, butter milk and raw milk sIgE results.
The levels of antibodies against raw milk and casein were significantly different across the four clinical milk allergy groups (boiled milk, yogurt, buttermilk and raw milk) (Figure 4A, B). The highest levels were observed in the boiled milk group, and these levels differed significantly from those in the other three groups; this difference was more pronounced and significant for the levels of sIgE against raw milk (Figure 4A), casein (Figure 4B), α-lactalbumin (Figure 4C) and β-lactoglobulin (Figure 4D). In contrast, the raw milk group observed the lowest levels for raw milk, casein, α-lactalbumin, and β-lactoglobulin. The casein sIgE level in the yogurt and buttermilk groups were lower than those in the boiled milk group (P<0.0001, Figure 4B).

Figure 4 Levels of log-transformed sIgE among the four groups, namely, the boiled milk, yogurt, buttermilk, raw milk. (A); casein (B); α-lactalbumin (C); β-lactoglobulin (D); bovine serum albumin (E). The graph shows medians and quartiles.
As shown in Figure 5, allergic symptoms were distributed differently among the four study groups. The percentage of participants with upper respiratory tract allergic symptoms in the raw milk group was 20% (32/158), which was significantly lower than that in the yogurt group (34%, 17/50) (P=0.037). The percentage of participants with skin allergic reactions in the raw milk group was highest (59%, 94/158), which was significantly higher than that in the boiled milk group (22%, 2/9, P=0.032). The percentage of participants with gastrointestinal tract allergic symptom in the boiled milk group was highest (44%, 4/9), significantly higher than that in the raw milk group (P=0.017). In addition, there was also a higher percentage of patients with gastrointestinal tract symptoms in the yogurt group than in the raw milk group (P<0.001). There was a higher percentage of severe anaphylactic reactions in the boiled milk group than in the other groups, although this did not reach statistical significance.
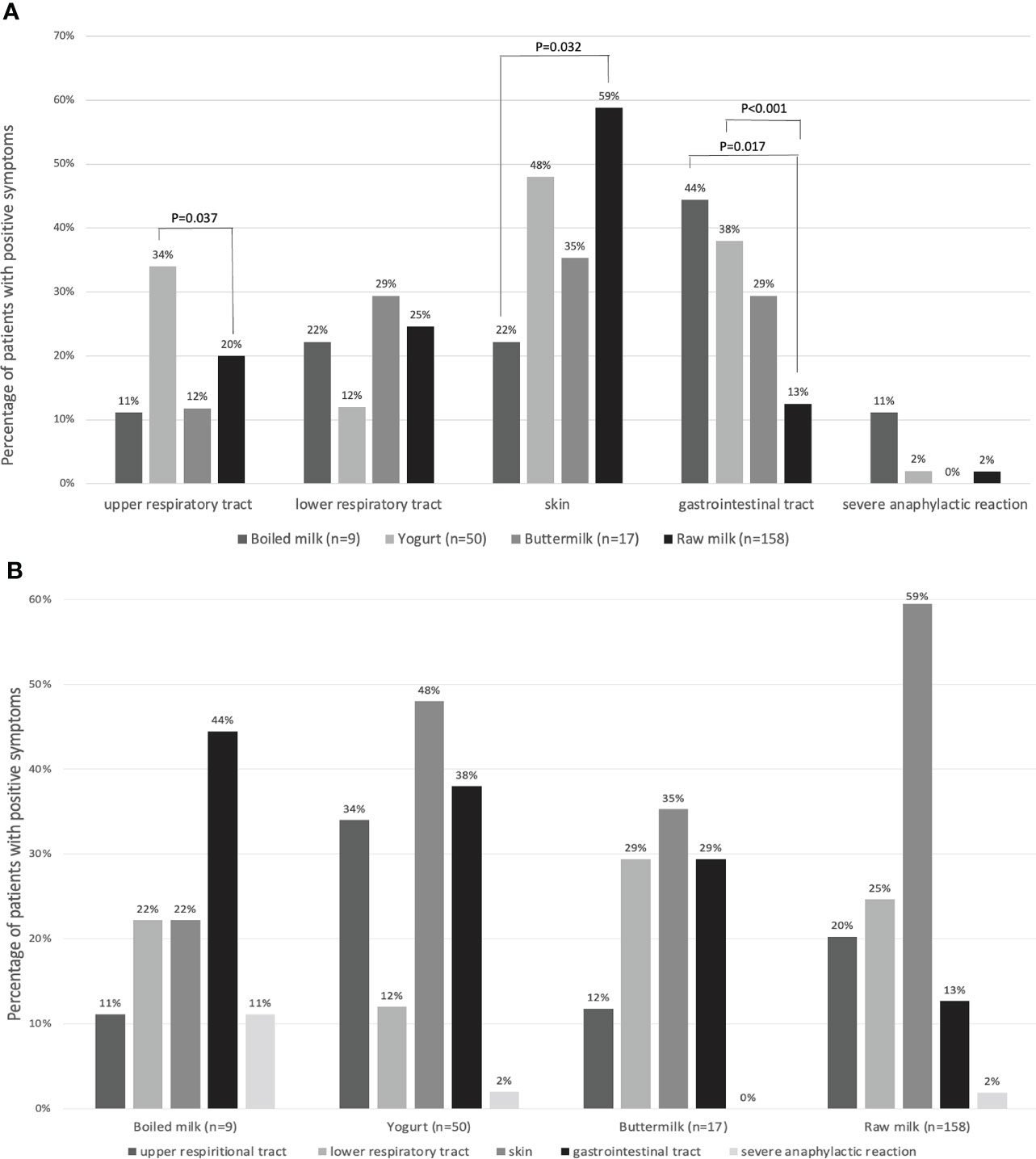
Figure 5 (A) The percentages of patients with allergic symptoms in four groups: boiled milk (n = 9), yogurt (n = 50), buttermilk (n = 17), and raw milk (n = 158), reported as groups by affected body systems. (B) The percentages of patients with allergic symptoms, reported as four groups.
In this cross-sectional study, we sought to differentiate distinct clinical phenotypes of CMPA according to traditional serum dairy product sIgE levels. The identified dairy product sIgE sequence (boiled milk, yogurt and buttermilk, raw milk) differentiated four classifications of participants:
● Patients who reacted to boiled milk;
● Patients who were negative for boiled milk sIgE and positive for yogurt sIgE;
● Patients who were negative for boiled milk sIgE, negative for yogurt sIgE, and positive for buttermilk sIgE;
● Patients who were negative for boiled milk sIgE, negative for yogurt sIgE, negative for buttermilk sIgE, and positive for raw milk sIgE.
Moreover, as previously reported, the raw milk sIgE levels and casein sIgE levels among the four groups were significantly different from high to low. Allergic symptoms were distributed differently among the four groups.
In this study, we proposed a classification of CMPA based on dairy product sIgE levels in North China for the first time. Boiled milk is an extensively heated milk product usually referred to as baked milk in other studies (22, 31). Extensively heated milk-tolerant subjects with negative extensively heated milk sIgE results have been shown to have lower levels of IgE against casein proteins, lower IgE-binding diversity to milk epitopes (32–37), and a better prognosis of CMPA resolution. A recent study reported the occurrence of intermediate-severity milk product allergy based on reactivity to fermented milk products such as yogurt and cheese. Therefore, the field gradually realizes the classification of clinical CMPA. However, there have been no studies on CMPA phenotypes in China. This classification based on sIgE results may not only help divide CMPA patients into allergic risk groups for management but also help increase quality of life, for example, by reducing stress levels and promoting social communication with others (38).
Several clinical trials have investigated heated milk tolerance in children with milk allergy (39, 40). Nowak-Wegrzyn et al. (31) conducted a study in children aged 2-17 years with CMPA. The children were challenged with extensively heated milk products. Seventy-five percent of the children with CMPA could tolerate the extensively heated milk product but not unheated milk. The 25% who reacted to the extensively heated milk product had significantly larger SPT wheals and higher milk-specific and casein-specific IgE levels than those in the other groups. In this study, as shown in Figure 4, in the boiled milk group, the levels of sIgE against raw milk, casein, α-lactalbumin, and β-lactoglobulin were significantly higher than those in the other three groups. In addition, the milk component sIgE levels in the yogurt- or buttermilk- positive patients were higher than those in the only raw milk sIgE-positive patients but lower than those in the boiled milk sIgE-positive patients. The mechanism might be related to the thermal processing of cow’s milk (41). Thermal processing can disrupt conformational IgE-binding epitopes but not usually continuous IgE-binding epitopes, which seems to play a role in milk allergy. In addition to altering IgE epitopes, thermal processing may change different biophysical and immunological properties of food proteins, such as their structure, function, solubility and digestibility and the T cell response.
This study is the first to report the allergic symptoms in CMPA patients among these four groups differentiated by sIgE levels. Wipa Jessadapakorn et al. reported that among patients with cow’s milk allergy, the casein sIgE level in the urticaria group tended to be higher than that in the atopic dermatitis (AD) group (42). Arianna Giannetti et al. reported that patients with AD showed higher rates of polysensitization to foods and higher levels of both total IgE and sIgE against milk, casein, wheat, peanuts, and cat dander at different ages than patients without AD (43). Although the classification of clinical CMPA was explored in a previous study, the levels of sIgE against different dairy products in allergic patients with different symptoms were evaluated. However, the distribution of clinical allergic symptoms was not reported according to the new classification. As seen in Figure 5, the percentages of allergic patients with gastrointestinal tract allergic symptoms among the following four groups were as follows, from high to low: boiled milk group, yogurt group, buttermilk group, and raw milk group. Interestingly, the percentages of patients with allergic skin reactions followed the opposite sequence. The boiled milk sIgE-positive patients had the lowest percentage of skin symptoms but the highest percentage of gastrointestinal tract symptoms. This order was the opposite in only raw milk sIgE-positive patients. We performed pairwise comparisons between each pair of groups, and more details are shown in Figure 5.
The method to detect the sIgE of different dairy product is an allergen array. The cutoff threshold was >=0.35 kU/L in this study. Although several studies might argue about the cutoff in sIgE test (44), which reported that the level of IgE positivity using 0.35 represented sensitization rather than actual allergy. However, the diagnosis of the patients with CMPA in this study was based on a combination of clinical presentation, sIgE, past medical history, SPT, or previous OFC results. According to previous guideline and studies, it was reasonable to adopt the traditional cutoff 0.35 (3, 4). In addition, in recent studies, there was also some other new method to identify milk allergens also. Fierro’s study measured the traces of milk and/or egg allergens in biscuits by two different liquid-chromatography-mass spectrometry methods (45).
There are some limitations to this study. All the diagnoses of CMPA were based on medical histories, clinical symptoms, sIgE levels and previous diagnoses instead of an OFC. It is difficult to carry out OFCs in China because of immature technology, patient resistance and the danger associated with the test. In addition, the sample was limited to the boiled milk sIgE-positive group, although the total sample was relatively large. All of the intergroup comparisons were performed by strict statistical tests to reduce the limitation of sample size. The low boiled milk sIgE positivity rate might suggest the rarity of this CMPA subtype. Another limitation of this study was that the lack of CMPA onset age or evolution years of these patients, which might imply clinical manifestations.
In conclusion, the new dairy product sIgE sequence (boiled milk, yogurt or buttermilk, raw milk) differentiated four groups of CMPA participants. Among the CMPA patients in the four groups, the levels of sIgE against raw milk and casein tended to range from high to low, according to the sequence above. The percentages of allergic patients with gastrointestinal tract symptoms in the four groups also ranged from high to low. However, the percentages of patients with skin symptoms in the four groups ranged from low to high. This study helps remind clinicians to pay attention to boiled milk, yogurt, and buttermilk sIgE-positive CMPA patients. It might be reasonable to adopt stratified measures for the management of patients who are positive for different dairy product, for example, stricter for boiled milk allergy patients.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by Institutional Ethics Committee of Peking Union Medical College Hospital. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
JS, BH, and SW designed and directed the project. YL, MZ, XY, ZW performed the experiments. XL analyzed data. RT and XL wrote the article. All authors contributed to the article and approved the submitted version.
Research Application of Egg and Milk Allergen Component Diagnostic Series Products (Hangzhou science external specialties 2021 No. 79).
Authors YL, MZ, XY, ZW, SW are employed by Hangzhou Zheda Dixun Biological Gene Engineering Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Sicherer SH, Sampson HA. Food allergy. J Allergy Clin Immunol (2010) 125(2 Suppl 2):S116–25. doi: 10.1016/j.jaci.2009.08.028
2. Johansson SG, Bieber T, Dahl R, Friedmann PS, Lanier BQ, Lockey RF, et al. Revised nomenclature for allergy for global use: Report of the nomenclature review committee of the world allergy organization, October 2003. J Allergy Clin Immunol (2004) 113(5):832–6. doi: 10.1016/j.jaci.2003.12.591
3. Fiocchi A, Bognanni A, Brożek J, Ebisawa M, Schünemann H. World allergy organization (WAO) diagnosis and rationale for action against cow's milk allergy (DRACMA) guidelines update - I - plan and definitions. World Allergy Organ J (2022) 15(1):100609. doi: 10.1016/j.waojou.2021.100609
4. Fiocchi A, Brozek J, Schünemann H, Bahna SL, von Berg A, Beyer K, et al. World allergy organization (WAO) diagnosis and rationale for action against cow's milk allergy (DRACMA) guidelines. Pediatr Allergy Immunol (2010) 21 Suppl 21:1–125. doi: 10.1111/j.1399-3038.2010.01068.x
5. Mennini M, Fiocchi AG, Cafarotti A, Montesano M, Mauro A, Villa MP, et al. Food protein-induced allergic proctocolitis in infants: Literature review and proposal of a management protocol. World Allergy Organ J (2020) 13(10):100471. doi: 10.1016/j.waojou.2020.100471
6. Pecora V, Valluzzi R, Dahdah L, Fierro V, Mennini M, Fiocchi A. Food protein-induced enterocolitis syndrome epidemiology, diagnosis, and treatment. Curr Opin Allergy Clin Immunol (2020) 20(3):316–22. doi: 10.1097/ACI.0000000000000643
7. Leonard SA, Pecora V, Fiocchi AG, Nowak-Wegrzyn A. Food protein-induced enterocolitis syndrome: A review of the new guidelines. World Allergy Organ J (2018) 11(1):4. doi: 10.1186/s40413-017-0182-z
8. Walsh J, Meyer R, Shah N, Quekett J, Fox AT. Differentiating milk allergy (IgE and non-IgE mediated) from lactose intolerance: Understanding the underlying mechanisms and presentations. Br J Gen Pract (2016) 66(649):e609–11. doi: 10.3399/bjgp16X686521
9. Rona RJ, Keil T, Summers C, Gislason D, Zuidmeer L, Sodergren E, et al. The prevalence of food allergy: A meta-analysis. J Allergy Clin Immunol (2007) 120(3):638–46. doi: 10.1016/j.jaci.2007.05.026
10. Yang M, Tan M, Wu J, Chen Z, Long X, Zeng Y, et al. Prevalence, characteristics, and outcome of cow's milk protein allergy in Chinese infants: A population-based survey. JPEN J Parenter Enteral Nutr (2019) 43(6):803–8. doi: 10.1002/jpen.1472
11. Koletzko S, Niggemann B, Arato A, Dias JA, Heuschkel R, Husby S, et al. Diagnostic approach and management of cow's-milk protein allergy in infants and children: ESPGHAN GI committee practical guidelines. J Pediatr Gastroenterol Nutr (2012) 55(2):221–9. doi: 10.1097/MPG.0b013e31825c9482
12. Plaza Martin AM, Martín Mateos MA, Giner Muñoz MT, Sierra Martínez JI. Challenge testing in children with allergy to cow's milk-proteins. Allergologia Immunopathol (2001) 29(2):50–4. doi: 10.1016/S0301-0546(01)79017-9
13. Kattan JD, Wang J. Allergen component testing for food allergy: Ready for prime time? Curr Allergy Asthma Rep (2013) 13(1):58–63. doi: 10.1007/s11882-012-0311-2
14. Prasad R, Venkata RSA, Ghokale P, Chakravarty P, Anwar F. Cow's milk-related symptom score as a predictive tool for cow's milk allergy in Indian children aged 0-24 months. Asia Pac Allergy (2018) 8(4):e36. doi: 10.5415/apallergy.2018.8.e36
15. Heinzerling LM, Burbach GJ, Edenharter G, Bachert C, Bindslev-Jensen C, Bonini S, et al. GA(2)LEN skin test study I: GA(2)LEN harmonization of skin prick testing: novel sensitization patterns for inhalant allergens in Europe. Allergy (2009) 64(10):1498–506. doi: 10.1111/j.1398-9995.2009.02093.x
16. García-Ara C, Boyano-Martínez T, Díaz-Pena JM, Martín-Muñoz F, Reche-Frutos M, Martín-Esteban M. Specific IgE levels in the diagnosis of immediate hypersensitivity to cows’ milk protein in the infant. J Allergy Clin Immunol (2001) 107(1):185–90. doi: 10.1067/mai.2001.111592
17. Sampson HA. Utility of food-specific IgE concentrations in predicting symptomatic food allergy. J Allergy Clin Immunol (2001) 107(5):891–6. doi: 10.1067/mai.2001.114708
18. Martorell A, García Ara MC, Plaza AM, Boné J, Nevot S, Echeverria L, et al. The predictive value of specific immunoglobulin e levels in serum for the outcome of the development of tolerance in cow's milk allergy. Allergol Immunopathol (Madr). (2008) 36(6):325–30. doi: 10.1016/S0301-0546(08)75864-6
19. El-Sebay HM, Badr EA, El-Ghobashi Y, Khalil MM, El-Mashad GM. The role of specific IgE antibodies in infants with cow milk protein allergy. Menoufia Med J (2016) 29(4):874. doi: 10.4103/1110-2098.202533
20. Rottem M, Shostak D, Foldi S. The predictive value of specific immunoglobulin e on the outcome of milk allergy. Israel Med Assoc J (2008) 10(12):862.
21. Kim JS, Nowak-Wegrzyn A, Sicherer SH, Noone S, Moshier EL, Sampson HA. Dietary baked milk accelerates the resolution of cow's milk allergy in children. J Allergy Clin Immunol (2011) 128(1):125–31 e2. doi: 10.1016/j.jaci.2011.04.036
22. Sackesen C, Suarez-Farinas M, Silva R, Lin J, Schmidt S, Getts R, et al. A new luminex-based peptide assay to identify reactivity to baked, fermented, and whole milk. Allergy (2019) 74(2):327–36. doi: 10.1111/all.13581
23. Cuomo B, Indirli GC, Bianchi A, Arasi S, Caimmi D, Dondi A, et al. Specific IgE and skin prick tests to diagnose allergy to fresh and baked cow's milk according to age: a systematic review. Ital J pediatr (2017) 43(1):93. doi: 10.1186/s13052-017-0410-8
24. Lambert C, Sarrat A, Bienvenu F, Brabant S, Nicaise-Roland P, Alyanakian MA, et al. The importance of EN ISO 15189 accreditation of allergen-specific IgE determination for reliable in vitro allergy diagnosis. Allergy (2015) 70(2):180–6. doi: 10.1111/all.12546
25. Thorpe SJ, Heath A, Fox B, Patel D, Egner W. The 3rd International Standard for serum IgE: international collaborative study to evaluate a candidate preparation. Clin Chem Lab Med. (2014) 52(9):1283–9. doi: 10.1515/cclm-2014-0243
26. Xu Q, Jiang Q, Yang L, Li W, Huang N, Yang Y, et al. IgE and IgG4 repertoire in asymptomatic HDM-sensitized and HDM-induced allergic rhinitis patients. Int Arch Allergy Immunol (2021) 182(12):1200–11. doi: 10.1159/000517824
27. Yang L, Yang Y, Xu Q, Zhang W, Jiang Q, Li W, et al. Specific IgE and IgG4 profiles of house dust mite components in allergen-specific immunotherapy. Front Immunol (2021) 12:786738. doi: 10.3389/fimmu.2021.786738
28. Celakovska J, Vankova R, Bukac J, Cermakova E, Andrys C, Krejsek J. Atopic dermatitis and sensitisation to molecular components of alternaria, cladosporium, penicillium, aspergillus, and malassezia-results of allergy explorer ALEX 2. J Fungi (Basel). (2021) 7(3): 183. doi: 10.3390/jof7030183
29. Hoang JA, Celik A, Lupinek C, Valenta R, Duan L, Dai R, et al. Modeling the conversion between specific IgE test platforms for nut allergens in children and adolescents. Allergy (2021) 76(3):831–41. doi: 10.1111/all.14529
30. Vandenplas Y, Brough HA, Fiocchi A, Miqdady M, Munasir Z, Salvatore S, et al. Current guidelines and future strategies for the management of cow's milk allergy. J Asthma Allergy (2021) 14:1243–56. doi: 10.2147/JAA.S276992
31. Nowak-Wegrzyn A, Bloom KA, Sicherer SH, Shreffler WG, Noone S, Wanich N, et al. Tolerance to extensively heated milk in children with cow's milk allergy. J Allergy Clin Immunol (2008) 122(2):342–7, 7 e1-2. doi: 10.1016/j.jaci.2008.05.043
32. Ford LS, Bloom KA, Nowak-Wegrzyn AH, Shreffler WG, Masilamani M, Sampson HA. Basophil reactivity, wheal size, and immunoglobulin levels distinguish degrees of cow's milk tolerance. J Allergy Clin Immunol (2013) 131(1):180–6 e1-3. doi: 10.1016/j.jaci.2012.06.003
33. Treudler R. [Allergic diseases in pregnancy. Overview of diagnosis and therapy]. Hautarzt (2010) 61(12):1027–33. doi: 10.1007/s00105-010-2008-6
34. Beyer K, Jarvinen KM, Bardina L, Mishoe M, Turjanmaa K, Niggemann B, et al. IgE-binding peptides coupled to a commercial matrix as a diagnostic instrument for persistent cow's milk allergy. J Allergy Clin Immunol (2005) 116(3):704–5. doi: 10.1016/j.jaci.2005.05.007
35. Han N, Jarvinen KM, Cocco RR, Busse PJ, Sampson HA, Beyer K. Identification of amino acids critical for IgE-binding to sequential epitopes of bovine kappa-casein and the similarity of these epitopes to the corresponding human kappa-casein sequence. Allergy (2008) 63(2):198–204. doi: 10.1111/j.1398-9995.2007.01539.x
36. Lin J, Bardina L, Shreffler WG, Andreae DA, Ge Y, Wang J, et al. Development of a novel peptide microarray for large-scale epitope mapping of food allergens. J Allergy Clin Immunol (2009) 124(2):315–22, 22 e1-3. doi: 10.1016/j.jaci.2009.05.024
37. García-Ara MC, Boyano-Martínez MT, Díaz-Pena JM, Martín-Muñoz MF, Martín-Esteban M. Cow's milk-specific immunoglobulin e levels as predictors of clinical reactivity in the follow-up of the cow's milk allergy infants. Clin Exp Allergy (2004) 34(6):866–70. doi: 10.1111/j.1365-2222.2004.01976.x
38. Indinnimeo L, Baldini L, De Vittori V, Zicari AM, De Castro G, Tancredi G, et al. Duration of a cow-milk exclusion diet worsens parents' perception of quality of life in children with food allergies. BMC Pediatr (2013) 13:203. doi: 10.1186/1471-2431-13-203
39. Bloom KA, Huang FR, Bencharitiwong R, Bardina L, Ross A, Sampson HA, et al. Effect of heat treatment on milk and egg proteins allergenicity. Pediatr Allergy Immunol (2014) 25(8):740–6. doi: 10.1111/pai.12283
40. Needs EC, Capellas M, Bland AP, Manoj P, MacDougal D, Paul G. Comparison of heat and pressure treatments of skim milk, fortified with whey protein concentrate, for set yogurt preparation: Effects on milk proteins and gel structure. J Dairy Res (2000) 67(3):329–48. doi: 10.1017/S0022029900004301
41. Huang F, Nowak-Wegrzyn A. Extensively heated milk and egg as oral immunotherapy. Curr Opin Allergy Clin Immunol (2012) 12(3):283–92. doi: 10.1097/ACI.0b013e3283535bc3
42. Jessadapakorn W, Sangsupawanich P, Wootipoom N, Suddeaugrai O, Yuenyongviwat A. Component-resolved diagnostics in Thai children with cow's milk and egg allergy. Asian Pacific J Allergy Immunol (2017) 35(4):179–85. doi: 10.12932/AP0820
43. Giannetti A, Cipriani F, Indio V, Gallucci M, Caffarelli C, Ricci G. Influence of atopic dermatitis on cow's milk allergy in children. Med (Kaunas). (2019) 55(8): 460. doi: 10.3390/medicina55080460
44. Siles RI, Hsieh FH. Allergy blood testing: A practical guide for clinicians. Cleveland Clinic J Med (2011) 78(9):585–92. doi: 10.3949/ccjm.78a.11023
Keywords: cow’s milk allergy, IgE, Fermented milk, boiled milk, raw milk
Citation: Tang R, Lyu X, Liu Y, Zhu M, Yang X, Wu Z, Han B, Wu S and Sun J (2022) Four clinical phenotypes of cow’s milk protein allergy based on dairy product specific IgE antibody types in North China. Front. Immunol. 13:949629. doi: 10.3389/fimmu.2022.949629
Received: 21 May 2022; Accepted: 31 August 2022;
Published: 06 October 2022.
Edited by:
Rachel Marion-Letellier, Université de Rouen, FranceReviewed by:
Scott P. Commins, University of North Carolina at Chapel Hill, United StatesCopyright © 2022 Tang, Lyu, Liu, Zhu, Yang, Wu, Han, Wu and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinlyu Sun, MTg2MTEzODIwNTBAMTYzLmNvbQ==; Shandong Wu, d3VzaGFuZG9uZ0B6ZGJpb2dlbmUuY29t; Bingnan Han, aGFuYmluZ25hbkB6c3R1LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.