- 1Lübeck Institute of Experimental Dermatology (LIED), University of Lübeck, Lübeck, Germany
- 2Department of Dermatology, University of Lübeck, Lübeck, Germany
Pemphigoid diseases (PD) are autoimmune skin blistering diseases characterized by autoantibodies directed against proteins of the cutaneous basement membrane zone (BMZ). One of the major antigens is type XVII collagen (BP180), a transmembrane glycoprotein, which is targeted in four PDs: bullous pemphigoid, mucous membrane pemphigoid, linear IgA dermatosis, and pemphigoid gestationis. To date, different epitopes on BP180 have been described to be recognized by PD disease patients’ autoantibodies. Different BP180 epitopes were associated with distinct clinical phenotypes while the underlying mechanisms are not yet fully understood. So far, the main effects of anti-BP180 reactivity are mediated by Fcγ-receptors on immune cells. More precisely, the autoantibody–antigen interaction leads to activation of complement at the BMZ and infiltration of immune cells into the upper dermis and, by the release of specific enzymes and reactive oxygen species, to the degradation of BP180 and other BMZ components, finally manifesting as blisters and erosions. On the other hand, inflammatory responses independent of Fcγ-receptors have also been reported, including the release of proinflammatory cytokines and internalization and depletion of BP180. Autoantibodies against BP180 can also be found in patients with neurological diseases. The assumption that the clinical expression of PD depends on epitope specificity in addition to target antigens, autoantibody isotypes, and antibody glycosylation is supported by the observation that epitopes of PD patients differ from those of PD patients. The aim of the present review is to describe the fine specificities of anti-BP180 autoantibodies in different PDs and highlight the associated clinical differences. Furthermore, the direct effects after binding of the autoantibodies to their target are summarized.
Introduction
The skin is the largest human organ. It serves as a barrier and protects the body from environmental influences, including heat, cold, dehydration, UV radiation, and pathogens, as well as many toxic and immunogenic substances. The integrity of the skin is established by cell–cell contacts among keratinocytes and the adhesion of the epidermis to the dermis at the dermo-epidermal junction (DEJ). Proteins of these structures target antigens in various autoimmune bullous disorders, i.e., pemphigus and pemphigoid diseases (PD) (1, 2).
In addition to providing structural integrity, the DEJ has multiple functions and regulates epithelial–mesenchymal interaction during skin homeostasis, growth, and wound healing; serves as a permeability barrier; and participates in signal transduction (3–8). The individual layers of the DEJ can electron microscopically be subdivided in the lamina lucida, directly adjacent to the plasma membrane of basal keratinocytes, the lamina densa, and the sublamina densa. The DEJ is mainly composed of widely conserved molecules, including collagen type IV, laminins, nidogen, and perlecan (6). Within the DEJ-specialized cell-substratum adhesion sites, the so-called hemidesmosomes mediate the interaction between the cytoskeleton and, via anchoring filaments and anchoring fibrils, dermal collagens (5, 9). Hemidesmosomes of the epidermis and surface-close epithelia are composed of an intracellular plaque where the intermediated filaments keratins K5 and K14 link with the plectin isoform 1a (P1a) and the bullous pemphigoid antigen 1 isoform e (BPAG1e), also termed BP230. The latter two hemidesmosomal molecules interact with the two transmembrane molecules α6β4 integrin and BP180 (also called type XVII collagen or BPAG2). As the fifth hemidesmosomal protein, the transmembrane tetraspanin CD151 associates with the extracellular loop of α6 integrin. In the extracellular space, α6β4 integrin and BP180 connect with anchoring filaments, mainly consisting of laminin 332, laminin 331, and uncein. Anchoring filaments interact with anchoring fibrils, mainly consisting of type VII collagen, that finally links with dermal collagen (9–11).
In PD, autoantibodies are directed against proteins of the DEJ, primarily BP180, BP230, laminin 332, the p200 antigen (laminin γ1), and collagen type VII (12–15).
The present review focuses on BP180, the main autoantigen of the DEJ, and highlights the fine autoantibody specificities against this autoantigen targeted in four PD, i.e. bullous pemphigoid (BP), mucous membrane pemphigoid (MMP), linear IgA dermatosis, and pemphigoid gestationis (16, 17). In addition, this review summarizes the described direct effects immediately after binding of anti-BP180 antibodies to their cellular target.
Epidemiology and clinical features of pemphigoid diseases
By far, the most common PD is BP, which predominantly affects patients with a mean age of between 75 and 80 years at the time of diagnosis (18–21). The incidence of BP has recently been prospectively estimated to be 19.6 patients/million/year in Northern Germany (22). About the same incidence has been reported in France, while incidences of 7.63 patients/million/year and 7.1 patients/million/year were described in UK and Sweden based on national health registries (23, 24). In other populations, like in Tehran and Israel, pemphigus is more frequent than BP (25). Annual incidences of the other BP180-related diseases MMP, pemphigoid gestationis, and linear IgA dermatosis were reported to be 1.3 and 2.0/million/year, 0.8–2.0, and 0.25–1.0, respectively (21, 26–29).
BP clinically presents with intense pruritus and tense blisters and erosions. In some patients, urticarial plaques can predominate, and in 20% of patients, no blisters are found (30–32). Pemphigoid gestationis is a mainly transient disease occurring during pregnancy with intense itch and urticarial erythema; vesicles may or may not arise (33). In linear IgA disease, autoantibodies are mainly of the IgA isotype and blistering tends to occur at the edge of lesions, presenting as the so-called crown of jewels sign (34). In contrast to other pemphigoid disorders, in MMP, mucosal surfaces are predominantly involved, manifesting as erosions and crusts, mainly affecting the mouth, conjunctivae, nose, and genital area (26, 35–37).
Anti-BP230 reactivity in pemphigoid diseases
In 50%–60% of BP patients, in addition to BP180-specific antibodies, reactivity against BP230 is found (38–45). BP patients with autoantibodies restricted to BP230 are rare (40). In pemphigoid gestations, linear IgA diseases, and MMP, anti-BP230 reactivity is less frequent and nearly always accompanied by anti-BP180 antibodies (14, 15, 46). While the main pathogenic effect is mediated by autoantibodies against BP180 (47, 48), the pathogenic effect of anti-BP230 IgG has also recently been described in vivo (49).
Epitopes on BP180
BP180 is a homotrimeric, transmembrane, hemidesmosomal glycoprotein with type II orientation, meaning that the amino-terminal end is located in the cytoplasm while the carboxy-terminus is located in the extracellular space (3, 46) (Figures 1A, B). The protein consists of a globular intracellular domain and a large extracellular domain (46, 50). Both domains contain multiple antigenic sites (51–56). The extracellular domain is composed of 15 collagenous domains interrupted by noncollagenous domains called NC1 to NC16 (3, 57, 58). The importance of BP180 for the structural integrity of the DEJ and the attachment of keratinocytes to the underlying dermis is highlighted by mutations in COL17 encoding for BP180 and resulting in variants of junctional epidermolysis bullosa characterized by blisters and erosions present at birth or in early childhood (59).
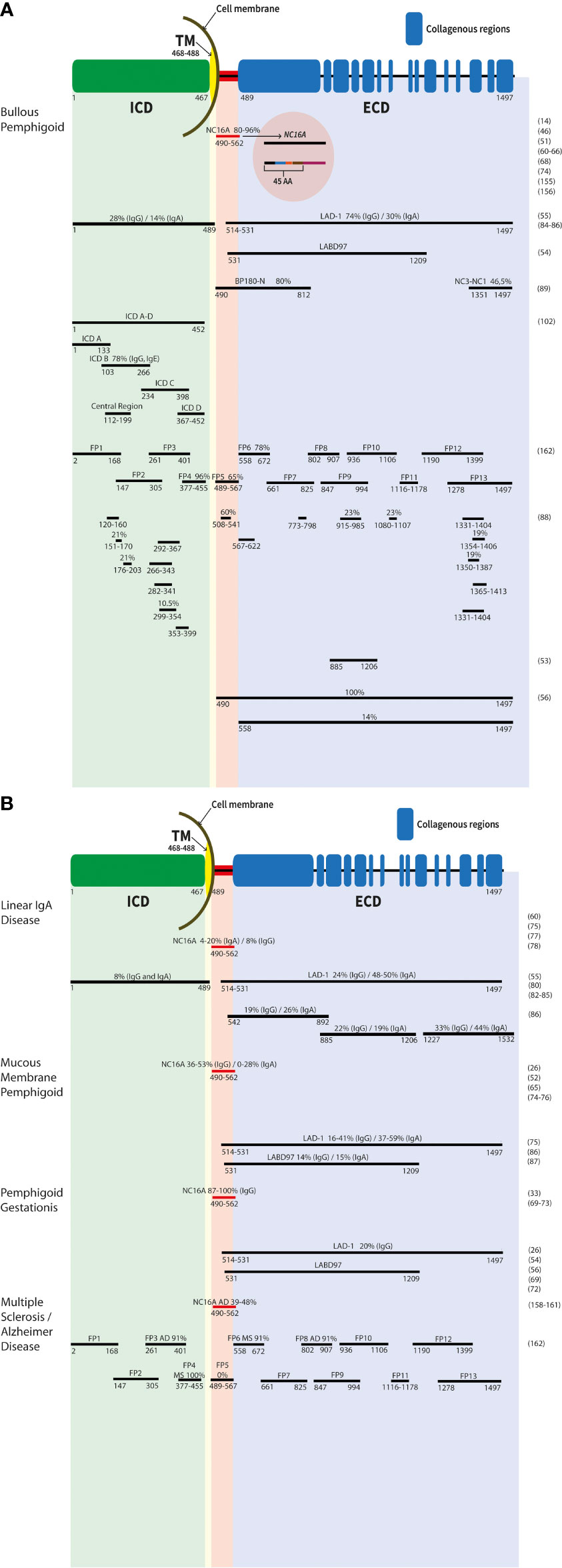
Figure 1 Schematic representation of the antigenic sites of the BP180 protein. The different epitopes of the molecule are shown. Numbers represent the first and last amino acid of each fragment/recombinat protein. The common names are listed above. The main targets are labelled with antibody reactivity (%) and IgG if not otherwise mentioned. Results in bullous pemphigoid (A), linear IgA disease, mucous membrane pemphigoid, pemphigoid gestationis, as well multiple sclerosis and Alzheimer’s disease (B) are depicetd. ECD, extracellular domain; ICD, intracellular domain; TM, transmembrane region.
In BP, the major antigenic site is the NC16A domain (40). The NC16A domain consists of 76 amino acids and is located immediately outside the transmembrane part (amino acids (aa) 490-562, Figure 1A) (46). Interestingly, all reactive sites recognized by autoantibodies are located within the N-terminal region of the NC16A domain and are recognized by IgG autoantibodies in 80%–96% of BP sera (14, 51, 60–65) (Figure 1A). For the detection of autoantibodies to BP180, highly sensitive and specific enzyme-linked immunosorbent assays using the NC16A domain as an antigenic target, have been developed recently (60, 61, 64). It has also been shown that disease activity in BP patients correlates with the serum levels of autoantibodies to BP180 NC16A in addition to IgG antibodies, also IgE and IgA autoantibodies are found in BP sera, mainly targeting the NC16A domain (46, 63, 66–68). The NC16A domain of BP180 is also the main target of autoantibodies in pemphigoid gestationis. In total, 87%–100% of cases exhibit IgG autoantibodies against this domain (33, 69–73). Reactivities for IgG and IgA with the NC16A domain were detected in 36%–53% and 0%–28% of MMP patients, respectively (26, 52, 65, 74–76). In LAD, 8% and 4%–20% of analyzed sera exhibit anti-BP180 NC16A IgG or IgA autoantibodies, respectively (75, 77, 78) (Figure 1B).
The BP180 ectodomain can be shed within the NC16A domain via a disintegrin and metalloprotease (ADAM) 9, 10, and 17 (79–81), resulting in a 120-kDa protein fragment named linear IgA bullous dermatosis autoantigen 1 (LAD-1) and a 97-kDa protein (97-kDa linear IgA bullous dermatosis autoantigen (LABD97)), both originally described as autoantigens in LAD (Figures 1A,B) (82–84). By this cleavage within the NC16A domain, different neoepitopes are induced, which might be important for the detection of autoantibodies in LAD, since it was shown that autoantibodies are preferentially bound to LAD-1 than to full-length BP180 (55, 80). By immunoblot analysis, sera from LAD patients showed IgA reactivity against keratinocyte-derived LAD-1 in 48%-50% and IgG reactivity in 24% of cases, whereas sera from BP patients had IgG against LAD-1 in 74% and IgA in 30% of cases (Figures 1A,B) (55, 85, 86). In addition, also patients with pemphigoid gestationis and MMP revealed serum reactivity with these two proteins (26, 54, 56, 72). In MMP, 16%–41% and 14% of analyzed sera contained IgG against LAD-1 and LABD97, respectively, and 37%–59% and 15% of sera exhibited IgA reactivity against LAD-1 and LABD97, respectively (Figure 1B) (75, 87).
Besides the soluble keratinocyte-derived proteins of the BP180 ectodomain, several recombinant fragments of the BP180 ectodomain have been generated by different investigators (Figures 1A,B) (53, 56, 88, 89). In total, 7.8%–47% of BP sera exhibit autoantibodies (IgG and IgA) that are directed against different recombinant proteins of the BP180 ectodomain, excluding the NC16A domain (46, 88–90). IgE against these epitopes has also been detected (91). Recently, in patients without frank blistering but erythematosus lesions, exclusive IgM autoantibodies against BP180 have been reported (92, 93). Boch et al. described three patients who had IgM reactivity against the DEJ by direct IF microscopy and serum IgM against the BP180 ectodomain BMZ. In one patient, additional IgM reactivity against the NC16A domain was present (93). Interestingly, in the last years, it has been reported, that patients with diabetes mellitus treated with dipeptidyl peptidase-4 inhibitors (DPP-4i or gliptins) develop a BP with autoantibodies against epitopes on the C-terminus of BP180 (94–96). In Japanese patients with DPP-4i-associated BP, a noninflammatory phenotype has been described and an association with HLA-DQB1_03:01 was suggested (94, 97).
In MMP, autoantibodies against C-terminal epitopes on BP180 outside NC16A were detected in 16%–53% (IgG) and 4%–37% (IgA) of patients, respectively (26, 46, 52, 74, 75, 98, 99). In pemphigoid gestationis, individual patients exhibit IgG and IgA extracellular epitopes outside NC16A (46, 72). In linear IgA disease, autoantibodies against C-terminal epitopes on BP180 outside NC16A were detected in 50%–100% (46, 55, 77, 80, 100, 101).
Antigenic regions targeted by pemphigoid patients’ autoantibodies can also be found on the intracellular portion of BP180 (88, 102). Overall, 28%–82% of BP sera (IgG reactivity) can recognize at least one epitope of the intracellular domain (56, 88, 102, 103), and also IgE reactivity against this site was described (102). For MMP, serum IgG and IgA against the intracellular part of BP180 were observed in about one-fifth of patients (75). In a small cohort of pemphigoid gestationis patients, about half of the patients showed IgG reactivity with a least one recombinant protein of the intracellular domain of BP180 (72) and in linear IgA disease, IgG and IgA reactivity was detected in 8% (for both isotypes) of analyzed sera (85).
It is currently assumed that intracellular epitopes are only recognized by autoantibodies after there is already reactivity to extracellular domains. Internalization via macropinocytosis of a BP180 NC16A IgG immune complex has recently been described (104). Together with the observation that anti-BP230 IgG alone is pathogenic in vivo (49, 105), it may be speculated that even autoantibodies against the intracellular part of BP180 per se are pathogenic. Of note, the autoantibody profile in individual PD patients is dynamic and may change over time, a phenomenon called epitope spreading. So far, little is known about the pathogenic relevance of autoantibodies against the intracellular and non-NC16A epitopes on the BP180 ectodomain. Alike, clinical phenotypes within a distinct PD depending on the targeted epitopes have not yet been sufficiently explored. Setterfield et al., however, have found concomitant serum IgG and IgA reactivity against the DEJ to be associated with a more severe phenotype in MMP (106).
Fcγ-receptor-independent effects of BP180-specific antibodies
Tissue destruction in BP is mediated by a variety of Fcγ-receptor (FcγR)-mediated effects. The antigen-autoantibody complexes lead to the activation of complement, followed by the infiltration of inflammatory cells such as mast cells, macrophages, and neutrophils to the upper dermis (107–110). These infiltrating cells release reactive oxygen species and specific proteases which finally degrade the DEJ, clinically leading to blister formation (109, 111). Most data about the essential role of complement activation in BP stems from observations in the neonatal mouse model of this disease (107, 108, 112). These data were supported by the high impact of complement activation in the mouse models of EBA and MMP (113–115).
Of note, antibodies against BP180 also promote blister formation in a FcγR- and complement-independent manner (116). The binding of anti-BP180 antibodies led to reduced adhesion of keratinocytes to the substrate/basement membrane using cultures of human keratinocytes and skin by macropinocytosis/internalization (104, 117–119). In detail, it was shown that BP-IgG induced a 45%–50% depletion of BP180 from normal human keratinocytes and DJM-1 cells (malignant trichilemmal cyst cells) after 2 h of incubation and about 75% in DJM-1 cells and 85% in normal human keratinocytes after 6 h. BP patient’s sera had the same depletion ability. A depletion of 80%–95% of BP180 from cells compared to normal control sera could be observed in seven of nine sera, the remaining two had a depletion activity of about 50%. The depletion of BP180 led to a loss of adhesion strength of about 40% in normal human keratinocytes and DJM-1 cells (119).
The internalization of BP180 upon binding of anti-BP180 IgE to cultured keratinocytes was also observed by confocal microscopy. After a time period of 15 min, the anti-BP180 IgE staining was detectable on the cell surface and 45 min later, the staining had moved from the periphery to the center, i.e., the cytoplasm of the cultured keratinocytes (117). The authors suggested that the possible blistering-inducing potential of anti-BP180 IgE is derived from its more potent cytokine-inducing potential compared to anti-BP180 IgG. This view was based on the observation that the relation of serum anti-BP180 IgE to total serum IgE was 30%–40% considerably higher compared to less than 1% of anti-BP180 IgG in relation to total serum IgG (117). These data are in line with the results Hiroyasu et al. observed for the internalization of BP IgG (118). They used BP-IgG to treat subconfluent/confluent normal human keratinocyte cultures that expressed green fluorescent protein-BP180 (GFP-BP180) and observed the beginning of an internalization, described as spot-like structures, from the surface into the cytoplasm after 20/105 min. By additionally labeling the BP-IgG with Fluor 647, it turned out that BP180 is internalized together with its antibody no matter if the antibodies are bound to the intracellular domain or extracellular domain (104). Internalization occurred via a non-inflammatory pathway and is FcγR-independent. This was tested using BP-IgG Fab fragments for cell stimulation, where an internalization of GFP-BP180 still occurred. By additionally treating the cells with EIPA, a Na+/H+ exchanger inhibitor, and cytochalasin D, an actin polymerization inhibitor, the internalization of GFP-BP180 failed. This indicates that internalization occurs via a macropinocytotic pathway (118). This process was suggested to be mediated by the protein kinase C pathway via phosphorylation of BP180 followed by the internalization and degradation of immune complexes, leading to a deficiency of BP180 in cell membranes and, consequently, loss of adhesion strength in keratinocytes (104). Furthermore, Kamaguchi and co-workers showed for MMP that the interaction between collagen type IV and BP180 is disrupted by MMP IgG directed against the BP180 C-terminus in keratinocytes of the oral mucosa, resulting in a reduction of the adhesion to the BMZ. However, the mechanism of lesion formation in the mucosa is still not fully understood. It has been shown that the expression level of BP180 is higher in the oral mucosa compared to the skin (120). This in turn is thought to compensate for the depletion of BP180 induced by anti-BP180 NC16A IgG.
The importance of the release of inflammatory cytokines like IL-6 and IL-8 in the pathogenesis of BP is supported by the finding of increased levels of both cytokines in the blister fluid of BP patients compared to suction blister controls (111, 121). Furthermore, a correlation between IL-6 and IL-8 serum levels and BP disease activity was shown (122). The release of IL-6 and IL-8 upon stimulation of cultured human keratinocytes with BP patient IgG indicates that FcγR-independent events might be relevant for disease development. Schmidt et al. observed a dose- and time-dependent release of the two cytokines. The elevated IL-6 and IL-8 levels in this approach were shown to be mediated by anti-BP180 IgG and were also observed at the mRNA level (123). Subsequently, the IL-8 release was shown to be significantly inhibited by dapsone but not doxycycline (124), two drugs regularly used in the treatment of BP (125–127). These data were corroborated by the observation that the treatment of cultured keratinocytes with IgE against BP180 also leads to the release of IL-6 and IL-8 (117). These cytokines may directly attract neutrophils without complement activation, leading to blister formation (128).
In addition, the injection of anti-BP180 IgG into humanized neonatal C3-deficient mice resulted in skin detachment by depletion and degradation of BP180 without complement deposits (107). This was somehow unexpected since, in C5-deficient mice, no dermal–epidermal separation occurred (107). Supporting the findings in C3-deficient animals, injection of F(ab′)2 fragments resulted in skin fragility without complement activation and neither skin detachment nor complement deposits in C5-deficient mice (107). Collectively, these data support the hypothesis that both FcγR- and complement-independent processes are involved in the pathophysiology of BP.
Anti-BP180 reactivity in neurological disease with and without concomitant bullous pemphigoid
BP is often associated with neurological diseases such as Alzheimer’s disease, Parkinson’s disease, and multiple sclerosis (129–133). Proteins associated with neurological diseases, like amyloidogenic proteins, were detected in human skin (134). In Alzheimer’s disease patients, skin physiology was altered and their risk of developing BP was 2.6 times higher than in individuals without the neurological disease (134–136). On the other hand, patients with nonmelanoma skin cancer had a lower risk of developing Alzheimer’s disease (137). One explanation for both observations is that the main antigen for BP, BP180, is expressed not only in the skin but also in the central nervous system (CNS) (138, 139). It has further been suggested that due to the neurodegenerative processes in neurological diseases the immune privilege of the CNS may be lost, i.e., that the blood–brain barrier no longer protects against harmful substances, pathogens, and toxins (140). This loss can then lead to the accessibility of the antigens in the brain and a subsequent autoimmune response against BP180 and BP230 (129, 133). More precisely, Seppänen et al. localized BP180 in the soma and proximal axons, but not in glial cells (139). BP180 was mostly found in the pyramidal cells of hippocampal regions, the ganglionic layer of the cerebral cortex, the hypoglossal nucleus (nucleus XII), oculomotor nucleus (nucleus III), nucleus basalis of Meynert, supraoptic nucleus, and subthalamic nuclei (139, 141). The observation that the neurological disease precedes BP by an average of 5.5 years supports the hypothesis that, indeed, the neurological disease triggers the autoimmune blistering disorder (130, 142). In fact, the relationship between BP and stroke, dementia, Parkinson’s disease, multiple sclerosis, and epilepsy has been well established in various populations (129–131, 133, 142–153). Since both neurological diseases such as stroke or dementia increase in the elderly and an age-related impairment of the blood-brain barrier has been described, this may also explain the striking occurrence of BP above the age of 70 years (140, 154–158).
To provide further experimental evidence for the generation of anti-BP180 IgG in patients with neurological diseases, Kokkonen and co-workers tested 115 Alzheimer’s disease patients and 40 neurologically healthy controls for anti-BP180 IgG. 21% of the patients with Alzheimer’s disease but only 7.5% of the controls showed IgG against BP180 NC16A by ELISA (159). All positive samples were further analyzed by immunoblotting against recombinant full-length BP180. In total, 18% of Alzheimer’s disease patients revealed IgG against NC16A and full-length BP180 in contrast to 3% in controls. Interestingly, Alzheimer’s disease patients with higher serum anti-BP180 IgG levels had more severe dementia (159). Comparable results were obtained by Wang et al., who found NC16A ELISA reactivity in 48% of 23 Alzheimer’s disease patients (160) (Figure 1B). In the sex- and age-matched healthy control group, only 8% of the tested 50 sera reacted with NC16A. Validation by immunoblot analyses with recombinant full-length BP180 or NC16A showed that nine of the 23 ELISA-positive Alzheimer’s disease patients (39%) and one of four (25%) controls reacted with the proteins (Figure 1B). In addition, 11 of the 23 ELISA-positive Alzheimer’s disease sera reacted with a 180-kDa protein from the human brain extract, but none of the controls did. The brain extract was obtained from the human hippocampus, which is a known BP180 expression locus (160). In contrast, Recke et al. found no significant increase in autoreactivity of autoantibodies against BP180 in patients with multiple sclerosis and Parkinson’s disease (161). The latter study is compatible with further work by Tuusa et al. that detected serum anti-BP180 NC16A IgG in only eight of 143 (5.6%) patients with multiple sclerosis and two of 140 (1.4%) neurologically healthy controls by ELISA, while none of these sera reacted with a glutathione-S-transferase NC16A fusion protein by immunoblotting (162). When different fusion proteins (FP1-FP13) covering BP180 (Figures 1A,B) were employed, BP sera preferentially reacted with the extracellular fragment FP5, corresponding to the NC16A domain, while there was no reactivity in sera of patients with multiple sclerosis, Alzheimer’s disease and healthy controls. Of note, patients with multiple sclerosis primarily reacted with the intracellular fragment FP4 (in 100%) and the extracellular fragments FP6 outside the NC16A domain (in 91%). Alzheimer’s disease sera predominantly recognized the intracellular fragment FP3 (in 91%) and the extracellular fragments FP8 outside the NC16A domain (in 91%; Figure 1) (162). Collectively, these data indicate that autoantibodies against different epitopes in BP180 are associated with different pathologies and as such, it can be expected that the pathogenic effect of anti-BP180 autoantibodies not only depends on their complement-activating potential, their glycosylation status, and isotype but also on the targeted epitope.
Outlook and conclusions
It has been well documented that different epitopes on BP180 are targeted in different PD as well as in patients with neurodegenerative disorders. The main pathogenic effect of anti-BP180 autoantibodies appears to be mediated by FcγR. Correspondingly, the pathogenicity of anti-BP180 autoantibodies was shown to depend on the autoantibody isotype, IgG subclass, and glycosylation status, leading to a varying extent of complement activation at the DEJ and attraction of inflammatory cells to the upper dermis. A more puzzling observation was that in different PDs, different regions of BP180 are predominantly targeted. In addition, most in vitro and all animal models have shown pathogenicity only against the NC16A domain and its murine homolog, not against intracellular or extracellular epitopes outside of this domain. In line, only serum levels of antibodies against the NC16A domain were shown to correlate with the disease activity of patients. As such, different epitopes on BP180 may convey different pathologies. This hypothesis, however, cannot be well explained by FcγR-dependent mechanisms alone.
Increasing evidence is provided for FcγR-independent mechanisms of anti-BP180 IgG in different in vitro and in vivo models. These mechanisms would allow us to more closely investigate the role of different epitopes in the pathophysiology of PD. The targeted epitope(s) may vary among the different disease stages and may also play a role in the transition of pemphigoid predisease, i.e., when autoantibodies against the DEJ are present but no skin or mucosal lesions have yet evolved. The in-depth characterization of epitope-dependent disease mechanisms in PD will not only allow a better understanding of autoantibody-triggered inflammation but may also provide novel therapeutic approaches that are urgently needed.
Author contributions
BO, ES, and SG contributed to the writing of the manuscript. ES contributed to the revision of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
RTG 2633, Autoimmune Pre-Disease, University of Lübeck, Spokesperson: Ralf Ludwig.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Schmidt E, Kasperkiewicz M, Joly P. Pemphigus. Lancet (2019) 394(10201):882–94. doi: 10.1016/S0140-6736(19)31778-7
2. Schmidt E, Zillikens D. Pemphigoid diseases. Lancet (2013) 381(9863):320–32. doi: 10.1016/S0140-6736(12)61140-4
3. Goletz S, Zillikens D, Schmidt E. Structural proteins of the dermal-epidermal junction targeted by autoantibodies in pemphigoid diseases. Exp Dermatol (2017) 26(12):1154–62. doi: 10.1111/exd.13446
4. Borradori L, Sonnenberg A. Structure and function of hemidesmosomes: More than simple adhesion complexes. J Invest Dermatol (1999) 112(4):411–8. doi: 10.1046/j.1523-1747.1999.00546.x
5. Has C, Nystrom A, Saeidian AH, Bruckner-Tuderman L, Uitto J. Epidermolysis bullosa: Molecular pathology of connective tissue components in the cutaneous basement membrane zone. Matrix Biol (2018) 71(72):313–29. doi: 10.1016/j.matbio.2018.04.001
6. Pastor-Pareja JC. Atypical basement membranes and basement membrane diversity - what is normal anyway? J Cell Sci (2020) 133(8). doi: 10.1242/jcs.241794
7. Briggaman RA, Wheeler CE Jr. The epidermal-dermal junction. J Invest Dermatol (1975) 65(1):71–84. doi: 10.1111/1523-1747.ep12598050
8. Jayadev R, Sherwood DR. Basement membranes. Curr Biol (2017) 27(6):R207–R11. doi: 10.1016/j.cub.2017.02.006
9. Walko G, Castanon MJ, Wiche G. Molecular architecture and function of the hemidesmosome. Cell Tissue Res (2015) 360(2):363–78. doi: 10.1007/s00441-014-2061-z
10. Sakai LY, Keene DR, Morris NP, Burgeson RE. Type VII collagen is a major structural component of anchoring fibrils. J Cell Biol (1986) 103(4):1577–86. doi: 10.1083/jcb.103.4.1577
11. Owaribe K, Kartenbeck J, Stumpp S, Magin TM, Krieg T, Diaz LA, et al. The hemidesmosomal plaque. i. characterization of a major constituent protein as a differentiation marker for certain forms of epithelia. Differentiation (1990) 45(3):207–20. doi: 10.1111/j.1432-0436.1990.tb00475.x
12. Giudice GJ, Emery DJ, Zelickson BD, Anhalt GJ, Liu Z, Diaz LA. Bullous pemphigoid and herpes gestationis autoantibodies recognize a common non-collagenous site on the BP180 ectodomain. J Immunol (1993) 151(10):5742–50. doi: 10.1016/0923-1811(93)90940-Q
13. Giudice GJ, Emery DJ, Diaz LA. Cloning and primary structural analysis of the bullous pemphigoid autoantigen BP180. J Invest Dermatol (1992) 99(3):243–50. doi: 10.1111/1523-1747.ep12616580
14. Tampoia M, Giavarina D, Di Giorgio C, Bizzaro N. Diagnostic accuracy of enzyme-linked immunosorbent assays (ELISA) to detect anti-skin autoantibodies in autoimmune blistering skin diseases: A systematic review and meta-analysis. Autoimmun Rev (2012) 12(2):121–6. doi: 10.1016/j.autrev.2012.07.006
15. Schmidt E, Obe K, Brocker EB, Zillikens D. Serum levels of autoantibodies to BP180 correlate with disease activity in patients with bullous pemphigoid. Arch Dermatol (2000) 136(2):174–8. doi: 10.1001/archderm.136.2.174
16. van Beek N, Zillikens D, Schmidt E. Bullous autoimmune dermatoses. Dtsch Arztebl Int (2021) 118(24):413–20. doi: 10.3238/arztebl.m2021.0136
17. van Beek N, Zillikens D, Schmidt E. Diagnosis of autoimmune bullous diseases. J Dtsch Dermatol Ges (2018) 16(9):1077–91. doi: 10.1111/ddg.13637
18. Bernard P, Vaillant L, Labeille B, Bedane C, Arbeille B, Denoeux JP, et al. Incidence and distribution of subepidermal autoimmune bullous skin diseases in three French regions. bullous diseases French study group. Arch Dermatol (1995) 131(1):48–52. doi: 10.1001/archderm.1995.01690130050009
19. Marazza G, Pham HC, Scharer L, Pedrazzetti PP, Hunziker T, Trueb RM, et al. Incidence of bullous pemphigoid and pemphigus in Switzerland: A 2-year prospective study. Br J Dermatol (2009) 161(4):861–8. doi: 10.1111/j.1365-2133.2009.09300.x
20. Jung M, Kippes W, Messer G, Zillikens D, Rzany B. Increased risk of bullous pemphigoid in Male and very old patients: A population-based study on incidence. J Am Acad Dermatol (1999) 41(2 Pt 1):266–8. doi: 10.1016/S0190-9622(99)70061-7
21. Kridin K. Subepidermal autoimmune bullous diseases: Overview, epidemiology, and associations. Immunol Res (2018) 66(1):6–17. doi: 10.1007/s12026-017-8975-2
22. van Beek N, Weidinger A, Schneider SW, Kleinheinz A, Glaser R, Holtsche MM, et al. Incidence of pemphigoid diseases in northern Germany in 2016 - first data from the schleswig-Holstein registry of autoimmune bullous diseases. J Eur Acad Dermatol Venereol (2021) 35(5):1197–202. doi: 10.1111/jdv.17107
23. Persson MSM, Harman KE, Vinogradova Y, Langan SM, Hippisley-Cox J, Thomas KS, et al. Incidence, prevalence and mortality of bullous pemphigoid in England 1998-2017: A population-based cohort study. Br J Dermatol (2021) 184(1):68–77. doi: 10.1111/bjd.19022
24. Thorslund K, Seifert O, Nilzen K, Gronhagen C. Incidence of bullous pemphigoid in Sweden 2005-2012: A nationwide population-based cohort study of 3761 patients. Arch Dermatol Res (2017) 309(9):721–7. doi: 10.1007/s00403-017-1778-4
25. Kridin K, Schmidt E. Epidemiology of pemphigus. JID Innov (2021) 1(1):100004. doi: 10.1016/j.xjidi.2021.100004
26. Du G, Patzelt S, van Beek N, Schmidt E. Mucous membrane pemphigoid. Autoimmun Rev (2022) 21(4):103036. doi: 10.1016/j.autrev.2022.103036
27. Bertram F, Brocker EB, Zillikens D, Schmidt E. Prospective analysis of the incidence of autoimmune bullous disorders in lower franconia, Germany. J Dtsch Dermatol Ges (2009) 7(5):434–40. doi: 10.1111/j.1610-0387.2008.06976.x
28. Nanda A, Dvorak R, Al-Saeed K, Al-Sabah H, Alsaleh QA. Spectrum of autoimmune bullous diseases in Kuwait. Int J Dermatol (2004) 43(12):876–81. doi: 10.1111/j.1365-4632.2004.02292.x
29. Wong SN, Chua SH. Spectrum of subepidermal immunobullous disorders seen at the national skin centre, Singapore: A 2-year review. Br J Dermatol (2002) 147(3):476–80. doi: 10.1046/j.1365-2133.2002.04919.x
30. della Torre R, Combescure C, Cortes B, Marazza G, Beltraminelli H, Naldi L, et al. Clinical presentation and diagnostic delay in bullous pemphigoid: A prospective nationwide cohort. Br J Dermatol (2012) 167(5):1111–7. doi: 10.1111/j.1365-2133.2012.11108.x
31. Lamberts A, Meijer JM, Jonkman MF. Nonbullous pemphigoid: A systematic review. J Am Acad Dermatol (2018) 78(5):989–95.e2. doi: 10.1016/j.jaad.2017.10.035
32. Schmidt E, della Torre R, Borradori L. Clinical features and practical diagnosis of bullous pemphigoid. Dermatol Clin (2011) 29(3):427–38. viii-ix. doi: 10.1016/j.det.2011.03.010
33. Huilaja L, Makikallio K, Tasanen K. Gestational pemphigoid. Orphanet J Rare Dis (2014) 9:136. doi: 10.1186/s13023-014-0136-2
34. Koszory K, Sardy M. Linear IgA disease. In: Schmidt E, editor. Diseases of the oral mucosa, 1st edition. Berlin, Heidelberg: Springer (2022).
35. Lamberts A, Schmidt E, Horvath A. Mucous membrane pemphigoid. In: Schmidt E, editor. Diseases of the oral mucosa, 1st edition. Berlin, Heidelberg: Springer (2022).
36. Rashid H, Lamberts A, Borradori L, Alberti-Violetti S, Barry RJ, Caproni M, et al. European Guidelines (S3) on diagnosis and management of mucous membrane pemphigoid, initiated by the European academy of dermatology and venereology - part I. J Eur Acad Dermatol Venereol (2021) 35(9):1750–1764. doi: 10.1111/jdv.17397
37. Schmidt E, Rashid H, Marzano AV, Lamberts A, Di Zenzo G, Diercks GFH, et al. European Guidelines (S3) on diagnosis and management of mucous membrane pemphigoid, initiated by the European academy of dermatology and venereology - part ii. J Eur Acad Dermatol Venereol (2021) 35(10):1926–1948. doi: 10.1111/jdv.17395
38. Ishiura N, Fujimoto M, Watanabe R, Nakashima H, Kuwano Y, Yazawa N, et al. Serum levels of IgE anti-BP180 and anti-BP230 autoantibodies in patients with bullous pemphigoid. J Dermatol Sci (2008) 49(2):153–61. doi: 10.1016/j.jdermsci.2007.08.008
39. Hamada T, Nagata Y, Tomita M, Salmhofer W, Hashimoto T. Bullous pemphigoid sera react specifically with various domains of BP230, most frequently with c-terminal domain, by immunoblot analyses using bacterial recombinant proteins covering the entire molecule. Exp Dermatol (2001) 10(4):256–63. doi: 10.1034/j.1600-0625.2001.100405.x
40. Blocker IM, Dahnrich C, Probst C, Komorowski L, Saschenbrecker S, Schlumberger W, et al. Epitope mapping of BP230 leading to a novel enzyme-linked immunosorbent assay for autoantibodies in bullous pemphigoid. Br J Dermatol (2012) 166(5):964–70. doi: 10.1111/j.1365-2133.2012.10820.x
41. Kromminga A, Sitaru C, Hagel C, Herzog S, Zillikens D. Development of an ELISA for the detection of autoantibodies to BP230. Clin Immunol (2004) 111(1):146–52. doi: 10.1016/j.clim.2003.12.007
42. Thoma-Uszynski S, Uter W, Schwietzke S, Hofmann SC, Hunziker T, Bernard P, et al. BP230- and BP180-specific auto-antibodies in bullous pemphigoid. J Invest Dermatol (2004) 122(6):1413–22. doi: 10.1111/j.0022-202X.2004.22603.x
43. Yoshida M, Hamada T, Amagai M, Hashimoto K, Uehara R, Yamaguchi K, et al. Enzyme-linked immunosorbent assay using bacterial recombinant proteins of human BP230 as a diagnostic tool for bullous pemphigoid. J Dermatol Sci (2006) 41(1):21–30. doi: 10.1016/j.jdermsci.2005.11.002
44. Tampoia M, Lattanzi V, Zucano A, Villalta D, Filotico R, Fontana A, et al. Evaluation of a new ELISA assay for detection of BP230 autoantibodies in bullous pemphigoid. Ann N Y Acad Sci (2009) 1173:15–20. doi: 10.1111/j.1749-6632.2009.04630.x
45. Charneux J, Lorin J, Vitry F, Antonicelli F, Reguiai Z, Barbe C, et al. Usefulness of BP230 and BP180-NC16a enzyme-linked immunosorbent assays in the initial diagnosis of bullous pemphigoid: A retrospective study of 138 patients. Arch Dermatol (2011) 147(3):286–91. doi: 10.1001/archdermatol.2011.23
46. Horvath B, Niedermeier A, Podstawa E, Muller R, Hunzelmann N, Karpati S, et al. IgA autoantibodies in the pemphigoids and linear IgA bullous dermatosis. Exp Dermatol (2010) 19(7):648–53. doi: 10.1111/j.1600-0625.2010.01080.x
47. Daneshpazhooh M, Ghiasi M, Lajevardi V, Nasiri N, Balighi K, Teimourpour A, et al. BPDAI and ABSIS correlate with serum anti-BP180 NC16A IgG but not with anti-BP230 IgG in patients with bullous pemphigoid. Arch Dermatol Res (2018) 310(3):255–9. doi: 10.1007/s00403-018-1817-9
48. Liu Y, Li L, Xia Y. BP180 is critical in the autoimmunity of bullous pemphigoid. Front Immunol (2017) 8:1752. doi: 10.3389/fimmu.2017.01752
49. Haeberle S, Wei X, Bieber K, Goletz S, Ludwig RJ, Schmidt E, et al. Regulatory T-cell deficiency leads to pathogenic bullous pemphigoid antigen 230 autoantibody and autoimmune bullous disease. J Allergy Clin Immunol (2018) 142(6):1831–42.e7. doi: 10.1016/j.jaci.2018.04.006
50. Hirako Y, Usukura J, Nishizawa Y, Owaribe K. Demonstration of the molecular shape of BP180, a 180-kDa bullous pemphigoid antigen and its potential for trimer formation. J Biol Chem (1996) 271(23):13739–45. doi: 10.1074/jbc.271.23.13739
51. Nakatani C, Muramatsu T, Shirai T. Immunoreactivity of bullous pemphigoid (BP) autoantibodies against the NC16A and c-terminal domains of the 180 kDa BP antigen (BP180): Immunoblot analysis and enzyme-linked immunosorbent assay using BP180 recombinant proteins. Br J Dermatol (1998) 139(3):365–70. doi: 10.1046/j.1365-2133.1998.02396.x
52. Murakami H, Nishioka S, Setterfield J, Bhogal BS, Black MM, Zillikens D, et al. Analysis of antigens targeted by circulating IgG and IgA autoantibodies in 50 patients with cicatricial pemphigoid. J Dermatol Sci (1998) 17(1):39–44. doi: 10.1016/S0923-1811(97)00067-4
53. Nie Z, Hashimoto T. IgA antibodies of cicatricial pemphigoid sera specifically react with c-terminus of BP180. J Invest Dermatol (1999) 112(2):254–5. doi: 10.1046/j.1523-1747.1999.00501.x
54. Egan CA, Reddy D, Nie Z, Taylor TB, Schmidt LA, Meyer LJ, et al. IgG anti-LABD97 antibodies in bullous pemphigoid patients' sera react with the mid-portion of the BPAg2 ectodomain. J Invest Dermatol (2001) 116(2):348–50. doi: 10.1046/j.1523-1747.2001.01246.x
55. Schumann H, Baetge J, Tasanen K, Wojnarowska F, Schacke H, Zillikens D, et al. The shed ectodomain of collagen XVII/BP180 is targeted by autoantibodies in different blistering skin diseases. Am J Pathol (2000) 156(2):685–95. doi: 10.1016/S0002-9440(10)64772-4
56. Perriard J, Jaunin F, Favre B, Budinger L, Hertl M, Saurat JH, et al. IgG autoantibodies from bullous pemphigoid (BP) patients bind antigenic sites on both the extracellular and the intracellular domains of the BP antigen 180. J Invest Dermatol (1999) 112(2):141–7. doi: 10.1046/j.1523-1747.1999.00497.x
57. Zillikens D, Giudice GJ. BP180/type XVII collagen: Its role in acquired and inherited disorders or the dermal-epidermal junction. Arch Dermatol Res (1999) 291(4):187–94. doi: 10.1007/s004030050392
58. Li K, Tamai K, Tan EM, Uitto J. Cloning of type XVII collagen. complementary and genomic DNA sequences of mouse 180-kilodalton bullous pemphigoid antigen (BPAG2) predict an interrupted collagenous domain, a transmembrane segment, and unusual features in the 5'-end of the gene and the 3'-untranslated region of the mRNA. J Biol Chem (1993) 268(12):8825–34. doi 10.1016/S0021-9258(18)52948-3
59. Bardhan A, Bruckner-Tuderman L, Chapple ILC, Fine JD, Harper N, Has C, et al. Epidermolysis bullosa. Nat Rev Dis Primers (2020) 6(1):78. doi: 10.1038/s41572-020-0210-0
60. Zillikens D, Mascaro JM, Rose PA, Liu Z, Ewing SM, Caux F, et al. A highly sensitive enzyme-linked immunosorbent assay for the detection of circulating anti-BP180 autoantibodies in patients with bullous pemphigoid. J Invest Dermatol (1997) 109(5):679–83. doi: 10.1111/1523-1747.ep12338088
61. Kobayashi M, Amagai M, Kuroda-Kinoshita K, Hashimoto T, Shirakata Y, Hashimoto K, et al. BP180 ELISA using bacterial recombinant NC16a protein as a diagnostic and monitoring tool for bullous pemphigoid. J Dermatol Sci (2002) 30(3):224–32. doi: 10.1016/S0923-1811(02)00109-3
62. Zillikens D, Rose PA, Balding SD, Liu Z, Olague-Marchan M, Diaz LA, et al. Tight clustering of extracellular BP180 epitopes recognized by bullous pemphigoid autoantibodies. J Invest Dermatol (1997) 109(4):573–9. doi: 10.1111/1523-1747.ep12337492
63. Dopp R, Schmidt E, Chimanovitch I, Leverkus M, Brocker EB, Zillikens D. IgG4 and IgE are the major immunoglobulins targeting the NC16A domain of BP180 in bullous pemphigoid: Serum levels of these immunoglobulins reflect disease activity. J Am Acad Dermatol (2000) 42(4):577–83. doi: 10.1067/mjd.2000.103986
64. Sitaru C, Dahnrich C, Probst C, Komorowski L, Blocker I, Schmidt E, et al. Enzyme-linked immunosorbent assay using multimers of the 16th non-collagenous domain of the BP180 antigen for sensitive and specific detection of pemphigoid autoantibodies. Exp Dermatol (2007) 16(9):770–7. doi: 10.1111/j.1600-0625.2007.00592.x
65. Kromminga A, Sitaru C, Meyer J, Arndt R, Schmidt E, Christophers E, et al. Cicatricial pemphigoid differs from bullous pemphigoid and pemphigoid gestationis regarding the fine specificity of autoantibodies to the BP180 NC16A domain. J Dermatol Sci (2002) 28(1):68–75. doi: 10.1016/S0923-1811(01)00144-X
66. van Beek N, Schulze FS, Zillikens D, Schmidt E. IgE-mediated mechanisms in bullous pemphigoid and other autoimmune bullous diseases. Expert Rev Clin Immunol (2016) 12(3):267–77. doi: 10.1586/1744666X.2016.1123092
67. van Beek N, Luttmann N, Huebner F, Recke A, Karl I, Schulze FS, et al. Correlation of serum levels of IgE autoantibodies against BP180 with bullous pemphigoid disease activity. JAMA Dermatol (2017) 153(1):30–8. doi: 10.1001/jamadermatol.2016.3357
68. Hashimoto T, Ohzono A, Teye K, Numata S, Hiroyasu S, Tsuruta D, et al. Detection of IgE autoantibodies to BP180 and BP230 and their relationship to clinical features in bullous pemphigoid. Br J Dermatol (2017) 177(1):141–51. doi: 10.1111/bjd.15114
69. Tani N, Kimura Y, Koga H, Kawakami T, Ohata C, Ishii N, et al. Clinical and immunological profiles of 25 patients with pemphigoid gestationis. Br J Dermatol (2015) 172(1):120–9. doi: 10.1111/bjd.13374
70. Al Saif F, Jouen F, Hebert V, Chiavelli H, Darwish B, Duvert-Lehembre S, et al. Sensitivity and specificity of BP180 NC16A enzyme-linked immunosorbent assay for the diagnosis of pemphigoid gestationis. J Am Acad Dermatol (2017) 76(3):560–2. doi: 10.1016/j.jaad.2016.09.030
71. Sadik CD, Pas HH, Bohlmann MK, Mousavi S, Benoit S, Sardy M, et al. Value of BIOCHIP technology in the serological diagnosis of pemphigoid gestationis. Acta Derm Venereol (2017) 97(1):128–30. doi: 10.2340/00015555-2460
72. Di Zenzo G, Calabresi V, Grosso F, Caproni M, Ruffelli M, Zambruno G. The intracellular and extracellular domains of BP180 antigen comprise novel epitopes targeted by pemphigoid gestationis autoantibodies. J Invest Dermatol (2007) 127(4):864–73. doi: 10.1038/sj.jid.5700594
73. Sitaru C, Powell J, Messer G, Brocker EB, Wojnarowska F, Zillikens D. Immunoblotting and enzyme-linked immunosorbent assay for the diagnosis of pemphigoid gestationis. Obstet Gynecol (2004) 103(4):757–63. doi: 10.1097/01.AOG.0000115506.76104.ad
74. Hayakawa T, Furumura M, Fukano H, Li X, Ishii N, Hamada T, et al. Diagnosis of oral mucous membrane pemphigoid by means of combined serologic testing. Oral Surg Oral Med Oral Pathol Oral Radiol (2014) 117(4):483–96. doi: 10.1016/j.oooo.2013.12.402
75. Schmidt E, Skrobek C, Kromminga A, Hashimoto T, Messer G, Brocker EB, et al. Cicatricial pemphigoid: IgA and IgG autoantibodies target epitopes on both intra- and extracellular domains of bullous pemphigoid antigen 180. Br J Dermatol (2001) 145(5):778–83. doi: 10.1046/j.1365-2133.2001.04471.x
76. Ali S, Kelly C, Challacombe SJ, Donaldson AN, Dart JK, Gleeson M, et al. Salivary IgA and IgG antibodies to bullous pemphigoid 180 noncollagenous domain 16a as diagnostic biomarkers in mucous membrane pemphigoid. Br J Dermatol (2016) 174(5):1022–9. doi: 10.1111/bjd.14351
77. Zillikens D, Herzele K, Georgi M, Schmidt E, Chimanovitch I, Schumann H, et al. Autoantibodies in a subgroup of patients with linear IgA disease react with the NC16A domain of BP1801. J Invest Dermatol (1999) 113(6):947–53. doi: 10.1046/j.1523-1747.1999.00808.x
78. Ohata C, Ishii N, Koga H, Nakama T. A clinical and serological study of linear IgA bullous dermatosis without linear immunoglobulin deposition other than IgA at the basement membrane zone using direct immunofluorescence. Br J Dermatol (2017) 177(1):152–7. doi: 10.1111/bjd.15232
79. Nishie W, Lamer S, Schlosser A, Licarete E, Franzke CW, Hofmann SC, et al. Ectodomain shedding generates neoepitopes on collagen XVII, the major autoantigen for bullous pemphigoid. J Immunol (2010) 185(8):4938–47. doi: 10.4049/jimmunol.1001524
80. Yamauchi T, Matsushita S, Hashimoto T, Hirako Y. Major cleavage-dependent epitopes for linear IgA bullous dermatosis are formed at the boundary between the non-collagenous 16A and collagenous 15 domains of BP180. J Dermatol Sci (2014) 76(1):25–33. doi: 10.1016/j.jdermsci.2014.07.008
81. Franzke CW, Bruckner-Tuderman L, Blobel CP. Shedding of collagen XVII/BP180 in skin depends on both ADAM10 and ADAM9. J Biol Chem (2009) 284(35):23386–96. doi: 10.1074/jbc.M109.034090
82. Marinkovich MP, Taylor TB, Keene DR, Burgeson RE, Zone JJ. LAD-1, the linear IgA bullous dermatosis autoantigen, is a novel 120-kDa anchoring filament protein synthesized by epidermal cells. J Invest Dermatol (1996) 106(4):734–8. doi: 10.1111/1523-1747.ep12345782
83. Nishie W. Collagen XVII processing and blistering skin diseases. Acta Derm Venereol (2020) 100(5):adv00054. doi: 10.2340/00015555-3399
84. Zone JJ, Taylor TB, Meyer LJ, Petersen MJ. The 97 kDa linear IgA bullous disease antigen is identical to a portion of the extracellular domain of the 180 kDa bullous pemphigoid antigen, Bpag2. J Invest Dermatol (1998) 110(3):207–10. doi: 10.1046/j.1523-1747.1998.00129.x
85. Kromminga A, Scheckenbach C, Georgi M, Hagel C, Arndt R, Christophers E, et al. Patients with bullous pemphigoid and linear IgA disease show a dual IgA and IgG autoimmune response to BP180. J Autoimmun (2000) 15(3):293–300. doi: 10.1006/jaut.2000.0437
86. Georgi M, Scheckenbach C, Kromminga A, Partscht K, Messer G, Brocker EB, et al. Mapping of epitopes on the BP180 ectodomain targeted by IgA and IgG autoantibodies in patients with the lamina lucida-type of linear IgA disease. Arch Dermatol Res (2001) 293(3):109–14. doi: 10.1007/s004030000205
87. Oyama N, Setterfield JF, Powell AM, Sakuma-Oyama Y, Albert S, Bhogal BS, et al. Bullous pemphigoid antigen II (BP180) and its soluble extracellular domains are major autoantigens in mucous membrane pemphigoid: The pathogenic relevance to HLA class II alleles and disease severity. Br J Dermatol (2006) 154(1):90–8. doi: 10.1111/j.1365-2133.2005.06998.x
88. Di Zenzo G, Grosso F, Terracina M, Mariotti F, De Pita O, Owaribe K, et al. Characterization of the anti-BP180 autoantibody reactivity profile and epitope mapping in bullous pemphigoid patients. J Invest Dermatol (2004) 122(1):103–10. doi: 10.1046/j.0022-202X.2003.22126.x
89. Hofmann S, Thoma-Uszynski S, Hunziker T, Bernard P, Koebnick C, Stauber A, et al. Severity and phenotype of bullous pemphigoid relate to autoantibody profile against the NH2- and COOH-terminal regions of the BP180 ectodomain. J Invest Dermatol (2002) 119(5):1065–73. doi: 10.1046/j.1523-1747.2002.19529.x
90. Fairley JA, Bream M, Fullenkamp C, Syrbu S, Chen M, Messingham KN. Missing the target: Characterization of bullous pemphigoid patients who are negative using the BP180 enzyme-linked immunosorbant assay. J Am Acad Dermatol (2013) 68(3):395–403. doi: 10.1016/j.jaad.2012.09.012
91. Fairley JA, Fu CL, Giudice GJ. Mapping the binding sites of anti-BP180 immunoglobulin e autoantibodies in bullous pemphigoid. J Invest Dermatol (2005) 125(3):467–72. doi: 10.1111/j.0022-202X.2005.23853.x
92. Hirano Y, Iwata H, Tsujuwaki M, Mai S, Mai Y, Imafuku K, et al. Super-resolution imaging detects BP180 autoantigen in immunoglobulin m pemphigoid. J Dermatol (2022) 49(3):374–8. doi: 10.1111/1346-8138.16260
93. Boch K, Hammers CM, Goletz S, Kamaguchi M, Ludwig RJ, Schneider SW, et al. Immunoglobulin m pemphigoid. J Am Acad Dermatol (2021) 85(6):1486–92. doi: 10.1016/j.jaad.2021.01.017
94. Tasanen K, Varpuluoma O, Nishie W. Dipeptidyl peptidase-4 inhibitor-associated bullous pemphigoid. Front Immunol (2019) 10:1238. doi: 10.3389/fimmu.2019.01238
95. Kridin K, Bergman R. Association of bullous pemphigoid with dipeptidyl-peptidase 4 inhibitors in patients with diabetes: Estimating the risk of the new agents and characterizing the patients. JAMA Dermatol (2018) 154(10):1152–8. doi: 10.1001/jamadermatol.2018.2352
96. Patel PM, Jones VA, Kridin K, Amber KT. The role of dipeptidyl peptidase-4 in cutaneous disease. Exp Dermatol (2021) 30(3):304–18. doi: 10.1111/exd.14228
97. Ujiie H, Muramatsu K, Mushiroda T, Ozeki T, Miyoshi H, Iwata H, et al. HLA-DQB1*03:01 as a biomarker for genetic susceptibility to bullous pemphigoid induced by DPP-4 inhibitors. J Invest Dermatol (2018) 138(5):1201–4. doi: 10.1016/j.jid.2017.11.023
98. Balding SD, Prost C, Diaz LA, Bernard P, Bedane C, Aberdam D, et al. Cicatricial pemphigoid autoantibodies react with multiple sites on the BP180 extracellular domain. J Invest Dermatol (1996) 106(1):141–6. doi: 10.1111/1523-1747.ep12329728
99. Lee JB, Liu Y, Hashimoto T. Cicatricial pemphigoid sera specifically react with the most c-terminal portion of BP180. J Dermatol Sci (2003) 32(1):59–64. doi: 10.1016/S0923-1811(03)00035-5
100. Roh JY, Yee C, Lazarova Z, Hall RP, Yancey KB. The 120-kDa soluble ectodomain of type XVII collagen is recognized by autoantibodies in patients with pemphigoid and linear IgA dermatosis. Br J Dermatol (2000) 143(1):104–11. doi: 10.1046/j.1365-2133.2000.03598.x
101. Cozzani E, Di Zenzo G, Gasparini G, Salemme A, Agnoletti AF, Vassallo C, et al. Autoantibody profile of a cohort of 54 Italian patients with linear IgA bullous dermatosis: LAD-1 denoted as a major auto-antigen of the lamina lucida subtype. Acta Derm Venereol (2020) 100(4):adv00070. doi: 10.2340/00015555-3415
102. Dresow SK, Sitaru C, Recke A, Oostingh GJ, Zillikens D, Gibbs BF. IgE autoantibodies against the intracellular domain of BP180. Br J Dermatol (2009) 160(2):429–32. doi: 10.1111/j.1365-2133.2008.08858.x
103. Laffitte E, Skaria M, Jaunin F, Tamm K, Saurat JH, Favre B, et al. Autoantibodies to the extracellular and intracellular domain of bullous pemphigoid 180, the putative key autoantigen in bullous pemphigoid, belong predominantly to the IgG1 and IgG4 subclasses. Br J Dermatol (2001) 144(4):760–8. doi: 10.1046/j.1365-2133.2001.04130.x
104. Iwata H, Kamaguchi M, Ujiie H, Nishimura M, Izumi K, Natsuga K, et al. Macropinocytosis of type XVII collagen induced by bullous pemphigoid IgG is regulated via protein kinase c. Lab Invest (2016) 96(12):1301–10. doi: 10.1038/labinvest.2016.108
105. Makita E, Matsuzaki Y, Fukui T, Matsui A, Minakawa S, Nakano H, et al. Autoantibodies to BPAG1e trigger experimental bullous pemphigoid in mice. J Invest Dermatol (2021) 141(5):1167–76.e3. doi: 10.1016/j.jid.2020.08.031
106. Setterfield J, Shirlaw PJ, Kerr-Muir M, Neill S, Bhogal BS, Morgan P, et al. Mucous membrane pemphigoid: A dual circulating antibody response with IgG and IgA signifies a more severe and persistent disease. Br J Dermatol (1998) 138(4):602–10. doi: 10.1046/j.1365-2133.1998.02168.x
107. Liu Z, Giudice GJ, Swartz SJ, Fairley JA, Till GO, Troy JL, et al. The role of complement in experimental bullous pemphigoid. J Clin Invest (1995) 95(4):1539–44. doi: 10.1172/JCI117826
108. Liu Z, Giudice GJ, Zhou X, Swartz SJ, Troy JL, Fairley JA, et al. A major role for neutrophils in experimental bullous pemphigoid. J Clin Invest (1997) 100(5):1256–63. doi: 10.1172/JCI119639
109. Chen R, Fairley JA, Zhao ML, Giudice GJ, Zillikens D, Diaz LA, et al. Macrophages, but not T and b lymphocytes, are critical for subepidermal blister formation in experimental bullous pemphigoid: Macrophage-mediated neutrophil infiltration depends on mast cell activation. J Immunol (2002) 169(7):3987–92. doi: 10.4049/jimmunol.169.7.3987
110. Bieber K, Sun S, Ishii N, Kasperkiewicz M, Schmidt E, Hirose M, et al. Animal models for autoimmune bullous dermatoses. Exp Dermatol (2010) 19(1):2–11. doi: 10.1111/j.1600-0625.2009.00948.x
111. Schmidt E, Wehr B, Tabengwa EM, Reimer S, Brocker EB, Zillikens D. Elevated expression and release of tissue-type, but not urokinase-type, plasminogen activator after binding of autoantibodies to bullous pemphigoid antigen 180 in cultured human keratinocytes. Clin Exp Immunol (2004) 135(3):497–504. doi: 10.1111/j.1365-2249.2004.02401.x
112. Liu Z, Sui W, Zhao M, Li Z, Li N, Thresher R, et al. Subepidermal blistering induced by human autoantibodies to BP180 requires innate immune players in a humanized bullous pemphigoid mouse model. J Autoimmun (2008) 31(4):331–8. doi: 10.1016/j.jaut.2008.08.009
113. Sitaru C, Chiriac MT, Mihai S, Buning J, Gebert A, Ishiko A, et al. Induction of complement-fixing autoantibodies against type VII collagen results in subepidermal blistering in mice. J Immunol (2006) 177(5):3461–8. doi: 10.4049/jimmunol.177.5.3461
114. Karsten CM, Beckmann T, Holtsche MM, Tillmann J, Tofern S, Schulze FS, et al. Tissue destruction in bullous pemphigoid can be complement independent and may be mitigated by C5ar2. Front Immunol (2018) 9:488. doi: 10.3389/fimmu.2018.00488
115. Heppe EN, Tofern S, Schulze FS, Ishiko A, Shimizu A, Sina C, et al. Experimental laminin 332 mucous membrane pemphigoid critically involves C5aR1 and reflects clinical and immunopathological characteristics of the human disease. J Invest Dermatol (2017) 137(8):1709–18. doi: 10.1016/j.jid.2017.03.037
116. Iwata H, Ujiie H. Complement-independent blistering mechanisms in bullous pemphigoid. Exp Dermatol (2017) 26(12):1235–9. doi: 10.1111/exd.13367
117. Messingham KN, Srikantha R, DeGueme AM, Fairley JA. FcR-independent effects of IgE and IgG autoantibodies in bullous pemphigoid. J Immunol (2011) 187(1):553–60. doi: 10.4049/jimmunol.1001753
118. Hiroyasu S, Ozawa T, Kobayashi H, Ishii M, Aoyama Y, Kitajima Y, et al. Bullous pemphigoid IgG induces BP180 internalization via a macropinocytic pathway. Am J Pathol (2013) 182(3):828–40. doi: 10.1016/j.ajpath.2012.11.029
119. Iwata H, Kamio N, Aoyama Y, Yamamoto Y, Hirako Y, Owaribe K, et al. IgG from patients with bullous pemphigoid depletes cultured keratinocytes of the 180-kDa bullous pemphigoid antigen (Type XVII collagen) and weakens cell attachment. J Invest Dermatol (2009) 129(4):919–26. doi: 10.1038/jid.2008.305
120. Kamaguchi M, Iwata H, Nishie W, Toyonaga E, Ujiie H, Natsuga K, et al. The direct binding of collagen XVII and collagen IV is disrupted by pemphigoid autoantibodies. Lab Invest (2019) 99(1):48–57. doi: 10.1038/s41374-018-0113-9
121. Schmidt E, Bastian B, Dummer R, Tony HP, Brocker EB, Zillikens D. Detection of elevated levels of IL-4, IL-6, and IL-10 in blister fluid of bullous pemphigoid. Arch Dermatol Res (1996) 288(7):353–7. doi: 10.1007/BF02507102
122. Inaoki M, Takehara K. Increased serum levels of interleukin (IL)-5, IL-6 and IL-8 in bullous pemphigoid. J Dermatol Sci (1998) 16(2):152–7. doi: 10.1016/S0923-1811(97)00044-3
123. Schmidt E, Reimer S, Kruse N, Jainta S, Brocker EB, Marinkovich MP, et al. Autoantibodies to BP180 associated with bullous pemphigoid release interleukin-6 and interleukin-8 from cultured human keratinocytes. J Invest Dermatol (2000) 115(5):842–8. doi: 10.1046/j.1523-1747.2000.00141.x
124. Schmidt E, Reimer S, Kruse N, Brocker EB, Zillikens D. The IL-8 release from cultured human keratinocytes, mediated by antibodies to bullous pemphigoid autoantigen 180, is inhibited by dapsone. Clin Exp Immunol (2001) 124(1):157–62. doi: 10.1046/j.1365-2249.2001.01503.x
125. Schmidt E, Sticherling M, Sardy M, Eming R, Goebeler M, Hertl M, et al. S2k guidelines for the treatment of pemphigus Vulgaris/Foliaceus and bullous pemphigoid: 2019 update. J Dtsch Dermatol Ges (2020) 18(5):516–26. doi: 10.1111/ddg.14097
126. Sticherling M, Franke A, Aberer E, Glaser R, Hertl M, Pfeiffer C, et al. An open, multicentre, randomized clinical study in patients with bullous pemphigoid comparing methylprednisolone and azathioprine with methylprednisolone and dapsone. Br J Dermatol (2017) 177(5):1299–305. doi: 10.1111/bjd.15649
127. Williams HC, Wojnarowska F, Kirtschig G, Mason J, Godec TR, Schmidt E, et al. Doxycycline versus prednisolone as an initial treatment strategy for bullous pemphigoid: A pragmatic, non-inferiority, randomised controlled trial. Lancet (2017) 389(10079):1630–8. doi: 10.1016/S0140-6736(17)30560-3
128. Van den Bergh F, Eliason SL, Burmeister BT, Giudice GJ. Collagen XVII (BP180) modulates keratinocyte expression of the proinflammatory chemokine, IL-8. Exp Dermatol (2012) 21(8):605–11. doi: 10.1111/j.1600-0625.2012.01529.x
129. Bastuji-Garin S, Joly P, Lemordant P, Sparsa A, Bedane C, Delaporte E, et al. Risk factors for bullous pemphigoid in the elderly: A prospective case-control study. J Invest Dermatol (2011) 131(3):637–43. doi: 10.1038/jid.2010.301
130. Chen YJ, Wu CY, Lin MW, Chen TJ, Liao KK, Chen YC, et al. Comorbidity profiles among patients with bullous pemphigoid: A nationwide population-based study. Br J Dermatol (2011) 165(3):593–9. doi: 10.1111/j.1365-2133.2011.10386.x
131. Langan SM, Groves RW, West J. The relationship between neurological disease and bullous pemphigoid: A population-based case-control study. J Invest Dermatol (2011) 131(3):631–6. doi: 10.1038/jid.2010.357
132. Langer-Gould A, Albers KB, Van Den Eeden SK, Nelson LM. Autoimmune diseases prior to the diagnosis of multiple sclerosis: A population-based case-control study. Mult Scler (2010) 16(7):855–61. doi: 10.1177/1352458510369146
133. Stinco G, Codutti R, Scarbolo M, Valent F, Patrone P. A retrospective epidemiological study on the association of bullous pemphigoid and neurological diseases. Acta Derm Venereol (2005) 85(2):136–9. doi: 10.1080/00015550410024481
134. Akerman SC, Hossain S, Shobo A, Zhong Y, Jourdain R, Hancock MA, et al. Neurodegenerative disease-related proteins within the epidermal layer of the human skin. J Alzheimers Dis (2019) 69(2):463–78. doi: 10.3233/JAD-181191
135. Clos AL, Kayed R, Lasagna-Reeves CA. Association of skin with the pathogenesis and treatment of neurodegenerative amyloidosis. Front Neurol (2012) 3:5. doi: 10.3389/fneur.2012.00005
136. Forsti AK, Jokelainen J, Ansakorpi H, Seppanen A, Majamaa K, Timonen M, et al. Psychiatric and neurological disorders are associated with bullous pemphigoid - a nationwide Finnish care register study. Sci Rep (2016) 6:37125. doi: 10.1212/WNL.0b013e3182941990
137. White RS, Lipton RB, Hall CB, Steinerman JR. Nonmelanoma skin cancer is associated with reduced Alzheimer disease risk. Neurology (2013) 80(21):1966–72. doi: 10.1212/WNL.0b013e3182941990
138. Claudepierre T, Manglapus MK, Marengi N, Radner S, Champliaud MF, Tasanen K, et al. Collagen XVII and BPAG1 expression in the retina: Evidence for an anchoring complex in the central nervous system. J Comp Neurol (2005) 487(2):190–203. doi: 10.1002/cne.20549
139. Seppanen A, Suuronen T, Hofmann SC, Majamaa K, Alafuzoff I. Distribution of collagen XVII in the human brain. Brain Res (2007) 1158:50–6. doi: 10.1016/j.brainres.2007.04.073
140. Chen J, Li L, Chen J, Zeng Y, Xu H, Song Y, et al. Sera of elderly bullous pemphigoid patients with associated neurological diseases recognize bullous pemphigoid antigens in the human brain. Gerontology (2011) 57(3):211–6. doi: 10.1159/000315393
141. Seppanen A, Autio-Harmainen H, Alafuzoff I, Sarkioja T, Veijola J, Hurskainen T, et al. Collagen XVII is expressed in human CNS neurons. Matrix Biol (2006) 25(3):185–8. doi: 10.1016/j.matbio.2005.11.004
142. Taghipour K, Chi CC, Vincent A, Groves RW, Venning V, Wojnarowska F. The association of bullous pemphigoid with cerebrovascular disease and dementia: A case-control study. Arch Dermatol (2010) 146(11):1251–4. doi: 10.1001/archdermatol.2010.322
143. Forsti AK, Huilaja L, Schmidt E, Tasanen K. Neurological and psychiatric associations in bullous pemphigoid-more than skin deep? Exp Dermatol (2017) 26(12):1228–34. doi: 10.1111/exd.13401
144. Kridin K, Hubner F, Recke A, Linder R, Schmidt E. The burden of neurological comorbidities in six autoimmune bullous diseases: A population-based study. J Eur Acad Dermatol Venereol (2021) 35(10):2074–8. doi: 10.1111/jdv.17465
145. Foureur N, Descamps V, Lebrun-Vignes B, Picard-Dahan C, Grossin M, Belaich S, et al. Bullous pemphigoid in a leg affected with hemiparesia: A possible relation of neurological diseases with bullous pemphigoid? Eur J Dermatol (2001) 11(3):230–3.
146. Kwan Z, Lai YN, Ch'ng CC, Tan AH, Tan LL, Robinson S, et al. The association between bullous pemphigoid and neurological disorders in a selected Malaysian population. Med J Malaysia (2015) 70(2):81–5.
147. Casas-de-la-Asuncion E, Ruano-Ruiz J, Rodriguez-Martin AM, Velez Garcia-Nieto A, Moreno-Gimenez JC. Association between bullous pemphigoid and neurologic diseases: A case-control study. Actas Dermosifiliogr (2014) 105(9):860–5. doi: 10.1016/j.adengl.2014.09.010
148. Teixeira VB, Cabral R, Brites MM, Vieira R, Figueiredo A. Bullous pemphigoid and comorbidities: A case-control study in Portuguese patients. Bras Dermatol (2014) 89(2):274–8. doi: 10.1590/abd1806-4841.20142516
149. Brick KE, Weaver CH, Savica R, Lohse CM, Pittelkow MR, Boeve BF, et al. A population-based study of the association between bullous pemphigoid and neurologic disorders. J Am Acad Dermatol (2014) 71(6):1191–7. doi: 10.1016/j.jaad.2014.07.052
150. Jedlickova H, Hlubinka M, Pavlik T, Semradova V, Budinska E, Vlasin Z. Bullous pemphigoid and internal diseases - a case-control study. Eur J Dermatol (2010) 20(1):96–101. doi: 10.1684/ejd.2010.0805
151. Li J, Zuo YG, Zheng HY. Mortality of bullous pemphigoid in China. JAMA Dermatol (2013) 149(1):106–8. doi: 10.1001/archdermatol.2012.2994
152. Kibsgaard L, Rasmussen M, Lamberg A, Deleuran M, Olesen AB, Vestergaard C. Increased frequency of multiple sclerosis among patients with bullous pemphigoid: A population-based cohort study on comorbidities anchored around the diagnosis of bullous pemphigoid. Br J Dermatol (2017) 176(6):1486–91. doi: 10.1111/bjd.15405
153. Daneshpazhooh M, Khorassani J, Balighi K, Ghandi N, Mahmoudi H, Tohidinik H, et al. Neurological diseases and bullous pemphigoid: A case-control study in Iranian patients. Indian J Dermatol Venereol Leprol (2017) 83(2):195–9. doi: 10.4103/0378-6323.191132
154. Hofman A, Darwish Murad S, van Duijn CM, Franco OH, Goedegebure A, Ikram MA, et al. The Rotterdam study: 2014 objectives and design update. Eur J Epidemiol (2013) 28(11):889–926. doi: 10.1007/s10654-013-9866-z
155. Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: A systematic analysis for the global burden of disease study 2010. Lancet (2012) 380(9859):2197–223. doi: 10.1016/S0140-6736(12)61689-4
156. Bejot Y, Bailly H, Graber M, Garnier L, Laville A, Dubourget L, et al. Impact of the ageing population on the burden of stroke: The Dijon stroke registry. Neuroepidemiology (2019) 52(1-2):78–85. doi: 10.1159/000492820
157. Seppanen A. Collagen XVII: A shared antigen in neurodermatological interactions? Clin Dev Immunol (2013) 2013:240570. doi: 10.1155/2013/240570
158. Bejot Y, Yaffe K. Ageing population: A neurological challenge. Neuroepidemiology (2019) 52(1-2):76–7. doi: 10.1159/000495813
159. Kokkonen N, Herukka SK, Huilaja L, Kokki M, Koivisto AM, Hartikainen P, et al. Increased levels of the bullous pemphigoid BP180 autoantibody are associated with more severe dementia in alzheimer's disease. J Invest Dermatol (2017) 137(1):71–6. doi: 10.1016/j.jid.2016.09.010
160. Wang YN, Hammers CM, Mao X, Jin HZ, Yuan J, Li L. Analysis of the autoimmune response against BP180 in patients with alzheimer's disease. Ann Transl Med (2021) 9(2):107. doi: 10.21037/atm-20-5343
161. Recke A, Oei A, Hubner F, Fechner K, Graf J, Hagenah J, et al. Parkinson Disease and multiple sclerosis are not associated with autoantibodies against structural proteins of the dermal-epidermal junction. Br J Dermatol (2016) 175(2):407–9. doi: 10.1111/bjd.14538
Keywords: BP180, epitopes, pemphigoid, autoimmunity, bullous disease, autoantigen
Citation: Opelka B, Schmidt E and Goletz S (2022) Type XVII collagen: Relevance of distinct epitopes, complement-independent effects, and association with neurological disorders in pemphigoid disorders. Front. Immunol. 13:948108. doi: 10.3389/fimmu.2022.948108
Received: 19 May 2022; Accepted: 07 July 2022;
Published: 10 August 2022.
Edited by:
Kyle T. Amber, Rush University, United StatesReviewed by:
Takashi Hashimoto, Osaka City University, JapanJun Yamagami, Tokyo Women’s Medical University, Japan
Zhi Liu, University of North Carolina at Chapel Hill, United States
Giulia Gasparini, University of Genoa, Italy
Copyright © 2022 Opelka, Schmidt and Goletz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stephanie Goletz, stephanie.goletz@uksh.de
 Bianca Opelka
Bianca Opelka