
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol., 06 October 2022
Sec. Microbial Immunology
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.947897
The aim of this study was to assess the association of vitamin B12 level and single nucleotide polymorphisms (SNPs) in vitamin B12 metabolic genes with pulmonary tuberculosis (PTB) in Chinese Han population. The plasma vitamin B12 expression level was detected using ELISA. Ten SNPs in six key genes (TCN1, TCN2, CUBN, MMACHC, FUT6, and MUT) of vitamin B12 metabolic pathway were included for genotyping by the SNPscan technique among 454 PTB patients and 467 controls. Our results found that vitamin B12 level was significantly reduced in PTB patients when compared with controls. There was no significant association between TCN1 rs526934, TCN2 rs1801198, CUBN rs7906242, rs10904861, rs1801222, MMACHC rs10789465, FUT6 rs3760776, rs3760775, MUT rs9473555, rs9381784 variants, and PTB susceptibility. TCN2 rs1801198 CC genotype, C allele was significantly associated with hypoproteinemia in PTB patients. In CUBN, rs7906242 GG genotype, G allele, rs10904861 TT genotype, and T allele were significantly related to the decreased frequency of sputum smear-positive, and rs10904861 variant affected the occurrence of drug resistance in PTB patients. In addition, the increased frequency of CUBN rs1801222 AA genotype was significantly associated with leukopenia. The decreased frequency of MUT rs9473555 CC genotype was found in the PTB patients with hypoproteinemia. However, vitamin B12 expression was not associated with the genotype distribution of above SNPs. In conclusion, vitamin B12 level was significantly decreased in PTB patients and genetic variants in vitamin B12 metabolic genes were not contributed to PTB susceptibility. Several SNPs in TCN2, CUBN, and MUT gene might associate with multiple clinical manifestations in PTB.
Tuberculosis (TB) is a common, serious infectious disease caused by Mycobacterium tuberculosis (MTB) and remains a major threat to public health in many countries (1). There are approximately 9.9 million new incident TB patients in 2021 around the world (2). The people infected with MTB finally develop the possible outcomes as follows: MTB clearance, primary TB, latent tuberculosis infection (LTBI), and active TB (3). Many studies indicated that the risk of developing TB was strongly associated with host-pathogen interactions, external environment, and genetic factor (4). Although genetic variations in multiple genes had been found to be closely associated with TB susceptibility, it was interesting to continue to explore the role of genetic variations in TB susceptibility, which could be helpful in developing appropriate approaches to TB prevention, diagnosis, and treatment (5, 6).
Malnutrition was a risk factor for the progression of active TB and an important predictor of recurrence among TB patients (7). Because of the important role of vitamins in host nutrition and immunity, and several vitamins were essential for the survival and virulence of most organisms, including mycobacteria (8, 9). Hence, vitamin deficiency had the ability to influence the host immunity to a variety of infectious diseases. In addition, altered vitamin statuses were associated with various viral infections such as human immunodeficiency virus, influenza, and bacterial infections including dental caries and TB (10, 11). Recent meta-analysis suggested that vitamins A, D, and E expression levels were significantly lower in the TB patients than that in the control group (12).
Vitamin B12 was an essential water-soluble micronutrient to host maintain health, and vitamin B12 deficiency was linked to the development of many diseases (13). Vitamin B12 also played important roles in the development of pulmonary tuberculosis (PTB), and detecting the serum vitamin B12 and vitamin A levels could be used as an effective measure for identifying active PTB and monitoring the efficacy of PTB treatment (14). Studies had shown that the role of genetic influence on vitamin B12 expression was considerable and genetic variations might alter vitamin B12 tissue status through a variety of mechanisms (15, 16). Many genes associated with vitamin B12 metabolic pathway, such as transcobalamin 1 (TCN1), TCN2, fucosyltransferase 6 (FUT6), and cubulin (CUBN), were involved in the host susceptibility to many diseases by affecting the vitamin B12 level (17–19). However, there were no studies to analyze the relationship of these genes and PTB susceptibility. Furthermore, another report showed that the clinical manifestation and progression of PTB patients might be impacted by the host genetic variation (20). Therefore, we conducted this study to explore the effects of vitamin B12 level and vitamin B12 metabolic pathway genes (TCN1, TCN2, CUBN, MMACHC, FUT6, and MUT) variation on PTB susceptibility, clinical manifestations in a Chinese Han population.
The current study enrolled 80 PTB patients and 84 normal controls to detect the vitamin B12 level, and a total of 454 PTB patients and 467 normal controls were included for analyzing the association between vitamin B12 metabolic genes variation and PTB susceptibility. All PTB patients were recruited from Anhui Chest Hospital and diagnosed by clinical experts according to the following criteria: suspicious clinical symptoms, chest radiography, sputum and/or bronchoalveolar lavage fluid MTB culture, microscopy for acid fast bacilli (AFB), and effect of anti-TB treatment. The PTB patients, which were accompanied by HIV positivity, hepatitis, malignancy, and immunodeficiency, were finally excluded from this study. This study selected the healthy individuals with no history of TB, HIV positivity, malignant tumor, or other infectious diseases from the health examine center in the same area as normal controls.
The study was approved by the Medical Ethics Committee of Anhui Medical University (approval number 20200250), and the informed consent of each subject was obtained. Then, we collected peripheral blood samples of all study subjects and the clinical data and laboratory indicators of PTB patients with the support of experienced clinicians.
In this study, the plasma was obtained by Ficoll-Hypaque density gradient centrifugation from 2 ml of peripheral blood among 80 PTB patients and 84 normal controls. Then, we adopted Enzyme-linked immunosorbent assay (ELISA) kits (MyBioSource Inc., San Diego, CA, USA) to detect plasma vitamin B12 expression level of these study participants. The result was expressed as picomole per liter.
Six vitamin B12 metabolic genes, including TCN1, TCN2, CUBN, MMACHC, FUT6, and MUT, were selected for analyses in this study. We first identified some specific single nucleotide polymorphisms (SNPs) associated with human disease susceptibility by searching the studies regarding the association of vitamin B12 metabolic genes polymorphisms with human disease. Then, the genotype data on these six genes of Han Chinese people Beijing were obtained from Ensembl Genome Browser 85 and CHBS_1000g, and then we selected the tag SNPs with the HaploView 4.0 software (Cambridge, MA). All the selected SNPs must satisfy the following conditions: minor allele frequency (MAF) ≥ 0.05 in CHB and r2 threshold > 0.8. Finally, we selected one SNP (rs526934) of TCN1, one SNP (rs1801198) of TCN2, three SNPs (rs7906242, rs10904861, and rs1801222) of CUBN, one SNP (rs10789465) of MMACHC, two SNPs (rs3760776 and rs3760775) of FUT6, and two SNPs (rs9473555 and rs9381784) of MUT for genotyping.
About 5 ml of peripheral blood samples were drawn from subjects and used to extract genomic DNA by Flexi Gene-DNA Kit (Qiagen, Valencia, CA). Genotyping for above selected SNP was performed using the SNPscan Kit, with the technical support of the Center for Genetic and Genomic Analysis (Genesky Biotechnologies Inc., Shanghai, China). The SNPscan assay was a rapid multiplex genetic screening system, and its basic principle was to recognize SNP alleles by using the high specificity of ligase binding reactions (21). The specific process of SNPscan genotyping was mentioned in previous study (22). The sample could be included in the final analysis, only when all SNPs were successfully genotyped in this sample.
Hardy–Weinberg equilibrium test was performed in normal controls using Chi-square test. Chi-square test was also used to compare the differences in genotype, allele frequencies differences of all SNPs between the PTB patients and normal controls. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated by logistic regression models. We analyzed the associations of each SNP with the risk for PTB under dominant, recessive model, and conducted haplotype analysis using SHEsis software (23). The vitamin B12 expression level was shown as M ± SD, and the differences in vitamin B12 expression level between two and three groups were analyzed by t-test and ANOVA, respectively. Statistical analyses were performed with SPSS 23.0, and a two-sided P-value of less than 0.05 was considered as the threshold of statistical significance. In SNP analysis, Bonferroni correction was adopted for multiple testing, and P-value of less than 0.005 (0.05/10) was considered as a significant level.
This investigated case control included 80 PTB patients (25 women and 55 men, mean age was 49.71 ± 19.08 years) and 84 normal controls (31 women and 53 men, mean age was 50.76 ± 9.69 years). As illustrated in Figure 1, the plasma vitamin B12 level was significantly decreased in PTB patients than normal controls (P <0.001). The association between vitamin B12 level and several clinical features of PTB patients was also analyzed. We found that vitamin B12 level was not associated with fever, drug resistant, DILI, pulmonary infection, and so forth in PTB patients (Table S1). In addition, no significant correlation was found between vitamin B12 level with ESR, TBIL, ALT, and AST in PTB (Figure 2).
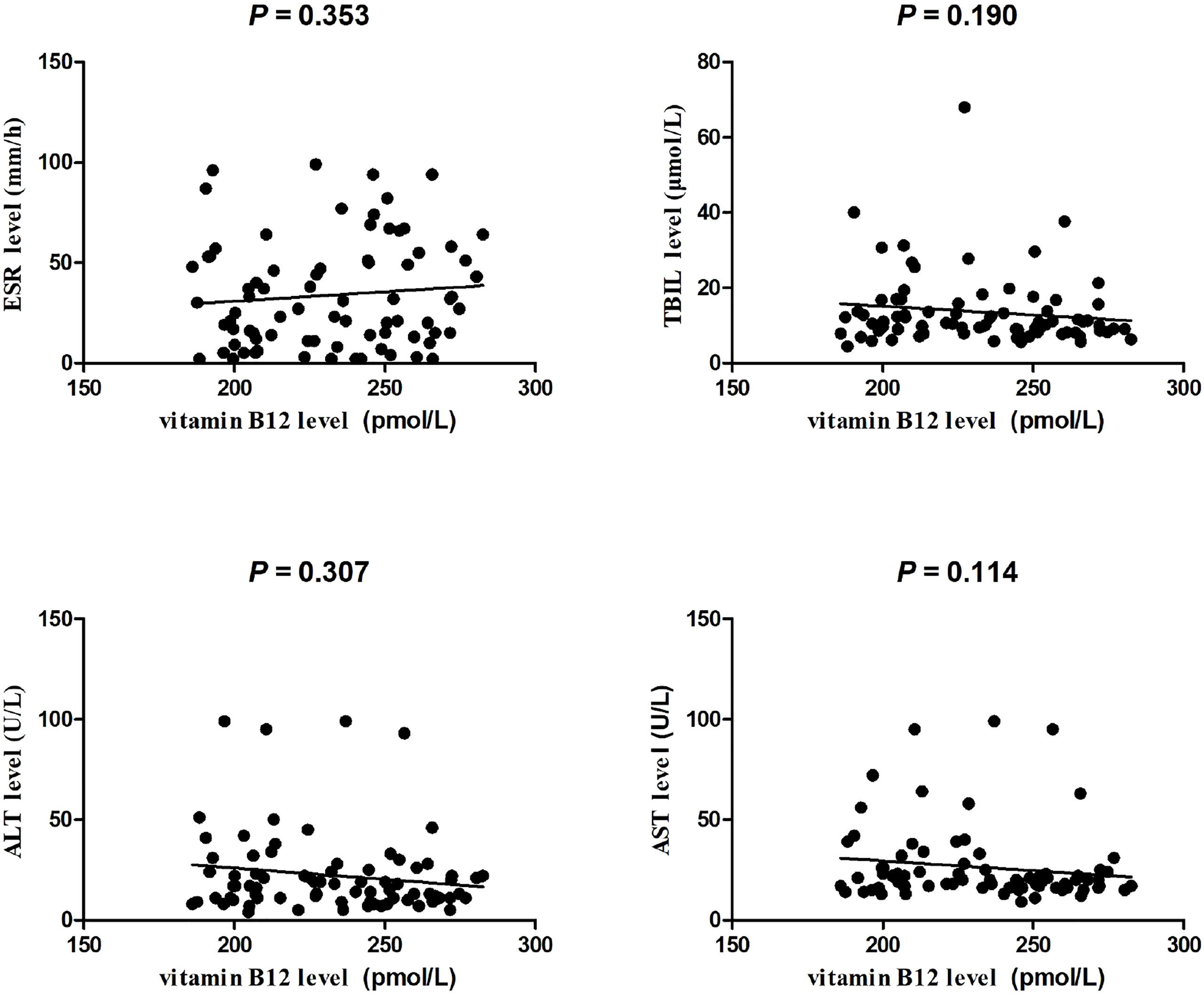
Figure 2 The correlation between ESR, TBIL, ALT, AST levels, and plasma vitamin B12 level in patients with PTB.
In this study, the average age of 454 PTB patients, including 194 women and 260 men, was 45.35 + 17.75 years, whereas the mean age of 467 normal controls, including 264 women and 203 men, was 43.37 + 13.87 years. We found that all SNP genotype distributions in normal controls were conformed to Hardy–Weinberg equilibrium, and the results of allele and genotype frequencies of these SNPs were shown in Table 1.
There was no significant association between TCN1 rs526934, TCN2 rs1801198 variants, and PTB susceptibility (all P >0.005). Regarding CUBN gene, no significant difference in allele frequencies of rs7906242, rs10904861, and rs1801222 was observed between PTB patients and controls, and the same result was also observed in the genotype frequencies. We compared the differences in FUT6 rs3760775, rs3760776 variants genotype, and allele frequencies among PTB patients and controls, and none of these differences were statistically significant. Similarly, there were no significant differences in allele and genotype distributions of MMACHC rs10789465, MUT rs9473555, rs9381784 polymorphism between PTB patients and controls (all P > 0.005). Moreover, we did not detect significant associations between these SNPs and PTB susceptibility under dominant and recessive model.
We also analyzed whether vitamin B12 metabolic gene SNPs influenced the clinical manifestations, such as drug resistance, DILI, pulmonary infection, and hypoproteinemia, of PTB patients (Table 2). Our results found that PTB patients with TCN2 rs1801198 CC genotype, C allele were likely to suffer from hypoproteinemia (P = 0.013 and P = 0.004, respectively), whereas the patient with MUT rs9473555 CC genotype was less likely to suffer from hypoproteinemia (P = 0.034). The GG genotype and G allele frequencies of CUBN rs10904861 variant were significantly associated with the increased risk of drug resistance in PTB patients (P = 0.033 and P = 0.029, respectively). Regarding the sputum smear results, the results demonstrated that CUBN rs7906242 GG genotype, G allele, rs10904861 TT genotype, and T allele frequencies were significantly decreased in PTB patients with sputum smear-positive when compared with PTB patients with sputum smear-negative (P = 0.003, P = 0.001, P = 0.019, and P = 0.009, respectively). In addition, the increased frequency of rs1801222 AA genotype was significantly associated with leukopenia in PTB patients (P < 0.001). No significant association was found between FUT6, TCN1, and MUT gene variations and the clinical features of PTB.
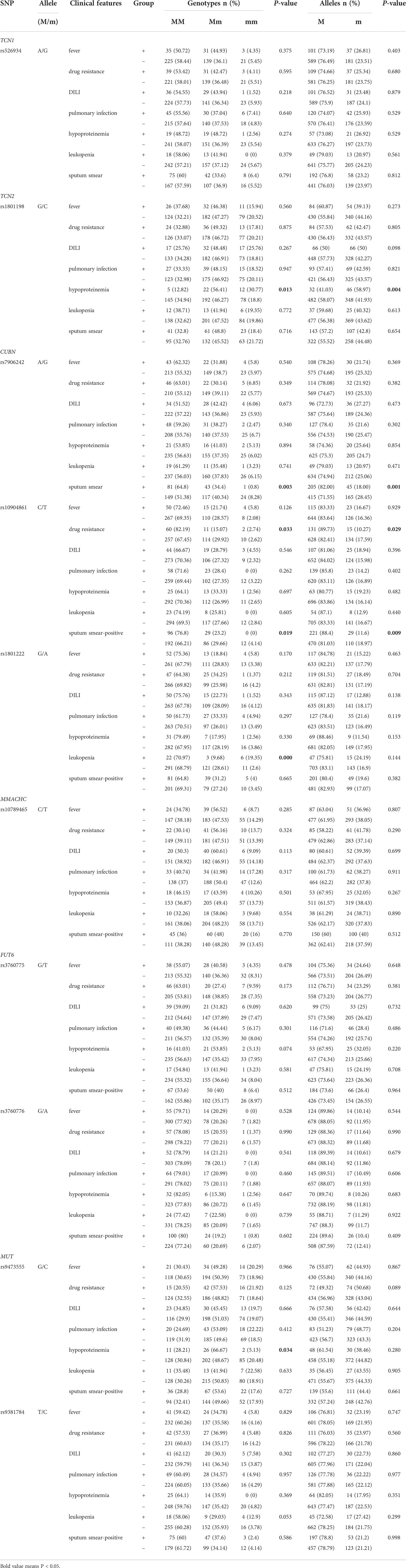
Table 2 Association of vitamin B12 metabolic genes polymorphisms with the clinical manifestations of PTB.
The main haplotypes with frequencies ≥ 3% in both PTB patients and controls of CUBN, FUT6, and MUT genes was detected using SHEsis software in this study. The frequency distributions of these haplotypes, including five haplotypes (ACG, ATG, GCA, and GCG) for CUBN, four main haplotypes (GA, GG, TA, and TG) for FUT6, three main haplotypes (CC, CG, and TG) for MUT, were summarized in Table 3. None of these haplotypes was significantly associated with the risk of PTB by comparing the differences in haplotype frequency between PTB patients and normal controls.
We finally assessed the association between vitamin B12 metabolic genes (TCN1, TCN2, CUBN, MMACHC, FUT6, and MUT) variation and vitamin B12 level among 68 PTB patients. Our results showed that there was no significant difference in vitamin B12 level among their different genotypes (all P > 0.05) (Table S2).
Studies had suggested that vitamins might play important roles in the prevention of PTB, and genetic variation and abnormal expression of most vitamins were closely related to the susceptibility to PTB (24, 25). At present, there were many studies regarding the role of vitamins A, D, and E in the development of PTB, whereas few studies on vitamin B12. Our previous study found that some SNPs in vitamin D metabolic pathway genes were associated with susceptibility to PTB and might contribute to several clinical phenotypes of PTB patients (26). It is worth noting that MTB had the ability to regulate core metabolic functions according to B12 availability, that is, whether vitamin B12 was acquired by endogenous synthesis or through uptake from the host environment (9). Therefore, vitamin B12 played a pivotal role in the pathogenesis of PTB, whereas the mechanism still needed to be further explored. This study was the first to explore the role of vitamin B12 metabolic genes variation in the development of PTB. We finally selected 10 SNPs to examine the association between vitamin B12 metabolic genes (TCN1, TCN2, CUBN, MMACHC, FUT6, and MUT) and PTB susceptibility.
These genes were key genes in vitamin B12 metabolism, including four co-factors or regulators of vitamin B12 transport (FUT6, MMACHC, TCN1, and TCN2), one membrane transporters (CUBN), and one mitochondrial protein (MUT) (13). TCN1 gene was located on chromosome 11 and coded the vitamin B12 binding protein (27), which facilitated the entry of vitamin B12 into the cells through receptor mediated endocytosis. The TCN2 gene was located on chromosome 22 and had the capacity to make a vitamin B12 binding protein called transcobalamin II (TC) (28). FUT6 gene was located on chromosome 19, which encoded a Golgi stack membrane protein, and associated with vitamin B12 deficiency (29). The MMACHC gene was located on chromosome region 1p34.1, and encoded a chaperone protein MMACHC (cblC protein), which could bind to vitamin B12 in the cytoplasm (30). CUBN gene was located on chromosome 10 and expressed in intestinal and renal epithelial cells, which was involved in the uptake of the intrinsic factor-vitamin B12 complex (31). The MUT gene was located on chromosome 6, and provided instructions for the formation of methylmalonyl CoA mutase, which was a mitochondrial enzyme (13). Several SNPs in these genes had been shown to mediate disease progression by affecting vitamin B12 levels.
The high level of methylmalonic acid (MMA) has been proved to be a biomarker reflecting vitamin B12 deficiency with high sensitivity and specificity; therefore, Oh et al. detected the expression level of MMA in TB patients (24). They found that MMA level was significantly higher in PTB patients than that in controls, suggesting that vitamin B12 deficiency existed in PTB patients. Similarly, we found that the plasma vitamin B12 level was significantly decreased in PTB patients than normal controls. We believed that vitamin B12 was closely related to the pathogenesis of PTB, and vitamin B12 metabolic gene variation might affect susceptibility to PTB by affecting vitamin B12 expression level. The rs526934 variant in TCN1 gene was found to be associated with lower circulating vitamin B12 concentrations and an increased risk of developing gastric cancer (18). A significant association between TCN2 gene rs1801198 and serum vitamin B12 levels was observed in a male Irish population whereas not in Caucasian populations. Moreover, TCN2 rs1801198 and rs9606756 variations were significantly correlated with ulcerative colitis (32). Other studies also confirmed that CUBN gene rs1801222, MMACHC gene rs10789465, FUT6 gene rs3760776, rs3760775, MUT gene rs9473555, and rs9381784 polymorphisms were associated with vitamin B12 concentrations (13, 29, 33). In the present study, we assessed the potential association between TCN1 gene rs526934, TCN2 gene rs1801198, CUBN gene rs7906242, rs10904861, rs1801222, MMACHC gene rs10789465, FUT6 gene rs3760776, rs3760775, MUT gene rs9473555, rs9381784 polymorphisms, and PTB susceptibility and found that these SNPs were not contributed to PTB development. Association studies based on haplotypes of multiple markers could increase the efficiency of mapping and characterizing disease-causing genes (34); however, we still found no association between multiple haplotypes of CUBN, FUT6, MUT genes, and the risk of PTB. In addition, no statistically significant association between these SNPs and vitamin B12 expression level was observed in patients with PTB. The differences between our results and other studies might be due to the different race and disease characteristics. The association between vitamin B12 metabolic genes variation and vitamin B12 expression level were influenced by race, as well as disease characteristics. At present, studies on gene variation of vitamin B12 metabolic pathway and susceptibility to PTB were very limited, and different experimental methods and sample sizes might also affect the accuracy of the results. Therefore, reproducible studies were still necessary to investigate the precise role of vitamin B12-related genes variation in PTB.
In the process of PTB treatment, patients usually accompanied by pulmonary infection, hypoproteinemia, adverse drug reactions, and other adverse clinical manifestations, which were a major challenge to PTB treatment. According to one previous study, side effects had become the leading cause of unsuccessful response to treatment in PTB patients (35). Hence, it was of great significance to assess the roles of SNPs in the development of multiple clinical manifestations. Our previous studies showed that CYP27A1 gene rs17470271 T; rs933994 T allele frequencies were respectively significantly related to leukopenia, drug resistance, and lncRNA NEAT1 gene rs3825071 TT genotype; T allele frequencies were significantly increased in sputum smear-positive PTB patients (24, 36). In the present study, we revealed that TCN2 gene rs1801198, MUT gene rs9473555 variants was significantly associated with hypoproteinemia, and CUBN gene rs10904861 variant was significantly corrected with the occurrence of drug resistance in PTB patients. In addition, a significant association between CUBN gene rs7906242 GG genotype, G allele, rs10904861 TT genotype, T allele frequencies, and sputum smear-positive was found, and CUBN gene rs1801222 AA genotype could affect the susceptibility of the patients to leukopenia. These findings could further improve our understanding of the roles of vitamin B12 metabolism genes in the development of PTB and contribute to making more appropriate treatment options.
In conclusion, our study demonstrated that vitamin B12 level was significantly decreased in PTB patients, and vitamin B12 metabolic pathway genes (TCN1, TCN2, CUBN, MMACHC, FUT6, and MUT) variation might not contribute to PTB susceptibility. In addition, several SNPs in TCN2, CUBN, and MUT genes appeared to be more prone to the occurrence of multiple clinical manifestations, including drug resistance, hypoproteinemia, and leukopenia in PTB patients. Some limitations existed in this study should be noted. First, the sample size was relatively insufficient, which might affect the accuracy of our results. Second, the mechanism of vitamin B12 metabolic genes variation in PTB susceptibility needed to be further explored. Therefore, functional and reproducible studies with larger sample sizes were required to further understand the genetic mechanisms of vitamin B12 metabolic genes in PTB development.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
This study was reviewed and approved by the Ethics Committee of Anhui Medical University (20200250). The patients/participants provided their written informed consent to participate in this study.
H-ML, T-PZ and FT designed the study. H-ML conducted the experiment. RL performed the statistical analyses. L-JW and FT participated in sample collection. T-PZ drafted the manuscript. H-ML contributed to the manuscript revision. All authors contributed to the article and approved the submitted version.
This work was supported by grants from the National Natural Science Foundation of China (82003515), and Anhui Provincial Medical and Health Key Specialty Construction Project (No.[2021]273).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.947897/full#supplementary-material
1. Chitnis AS, Cheung R, Gish RG, Wong RJ. Epidemiology and prevention of tuberculosis and chronic hepatitis b virus infection in the united states. J Immigr Minor Health (2021) 23(6):1267–79. doi: 10.1007/s10903-021-01231-6
2. World Health Organization. Global tuberculosis report (2021). Available at: https://www.who.int/tb/publications/global_report/en/.
3. Zhang JX, Gong WP, Zhu DL, An HR, Yang YR, Liang Y, et al. Mannose-binding lectin 2 gene polymorphisms and their association with tuberculosis in a Chinese population. Infect Dis Poverty (2020) 9(1):46. doi: 10.1186/s40249-020-00664-9
4. Cooke GS, Hill AV. Genetics of susceptibility to human infectious disease. Nat Rev Genet (2001) 2(12):967–77. doi: 10.1038/35103577
5. Harishankar M, Selvaraj P, Bethunaickan R. Influence of genetic polymorphism towards pulmonary tuberculosis susceptibility. Front Med (Lausanne) (2018) 5:213. doi: 10.3389/fmed.2018.00213
6. Stein CM, Sausville L, Wejse C, Sobota RS, Zetola NM, Hill PC, et al. Genomics of human pulmonary tuberculosis: From genes to pathways. Curr Genet Med Rep (2017) 5:149–66. doi: 10.1007/s40142-017-0130-9
7. World Health Organization. WHO guidelines approved by the guidelines review committee. in: Guideline: nutritional care and support for patients with tuberculosis. Geneva: World Health Organization; (2013).
8. Katona P, Katona-Apte J. The interaction between nutrition and infection. Clin Infect Dis (2008) 46:1582e8. doi: 10.1086/587658
9. Gopinath K, Moosa A, Mizrahi V, Warner DF. Vitamin B(12) metabolism in mycobacterium tuberculosis. Future Microbiol (2013) 8(11):1405–18. doi: 10.2217/fmb.13.113
10. Kearns MD, Alvarez JA, Seidel N, Tangpricha V. The impact of vitamin d on infectious disease: a systematic review of controlled trials. Am J Med Sci (2015) 349:245. doi: 10.1097/MAJ.0000000000000360
11. Surman SL, Penkert RR, Sealy RE, Jones BG, Marion TN, Vogel P, et al. Consequences of vitamin a deficiency: Immunoglobulin dysregulation, squamous cell metaplasia, infectious disease, and death. Int J Mol Sci (2020) 21(15):5570. doi: 10.3390/ijms21155570
12. Xu F, Ma B, Wang D, Lu J, Xiong K, Wang J. Associating the blood vitamin a, c, d and e status with tuberculosis: a systematic review and meta-analysis of observational studies. Food Funct (2022) 13(9):4825–38. doi: 10.1039/D1FO02827H
13. Surendran S, Adaikalakoteswari A, Saravanan P, Shatwaan IA, Lovegrove JA, Vimaleswaran KS. An update on vitamin B12-related gene polymorphisms and B12 status. Genes Nutr (2018) 13:2. doi: 10.1186/s12263-018-0591-9
14. Gebremicael G, Alemayehu M, Sileshi M, Geto Z, Gebreegziabxier A, Tefera H, et al. The serum concentration of vitamin B12 as a biomarker of therapeutic response in tuberculosis patients with and without human immunodeficiency virus (HIV) infection. Int J Gen Med (2019) 12:353–61. doi: 10.2147/IJGM.S218799
15. Quadros EV. Advances in the understanding of cobalamin assimilation and metabolism. Br J Haematol (2010) 148(2):195–204. doi: 10.1111/j.1365-2141.2009.07937.x
16. Nilsson SE, Read S, Berg S, Johansson B. Heritabilities for fifteen routine biochemical values: findings in 215 Swedish twin pairs 82 years of age or older. Scand J Clin Lab Invest (2009) 69(5):562–9. doi: 10.1080/00365510902814646
17. Nongmaithem SS N, Joglekar CV, Krishnaveni GV, Sahariah SA, Ahmad M, Ramachandran S, et al. GWAS identifies population-specific new regulatory variants in FUT6 associated with plasma B12 concentrations in indians. Hum Mol Genet (2017) 26(13):2589. doi: 10.1093/hmg/ddx156.
18. Zhao L, Wei Y, Song A, Li Y. Association study between genome-wide significant variants of vitamin B12 metabolism and gastric cancer in a han Chinese population. IUBMB Life (2016) 68(4):303–10. doi: 10.1002/iub.1485
19. Lahner E, Gentile G, Purchiaroni F, Mora B, Simmaco M, Annibale B. Single nucleotide polymorphisms related to vitamin B12 serum levels in autoimmune gastritis patients with or without pernicious anaemia. Dig Liver Dis (2015) 47(4):285–90. doi: 10.1016/j.dld.2015.01.147
20. Zhao Z, Zhang M, Ying J, Hu X, Zhang J, Zhou Y, et al. Significance of genetic polymorphisms in long non-coding RNA AC079767.4 in tuberculosis susceptibility and clinical phenotype in Western Chinese han population. . Sci Rep (2017) 7(1):965. doi: 10.1038/s41598-017-01163-y
21. Wang MG, Wang J, He JQ. Genetic association of TOLLIP gene polymorphisms and HIV infection: a case-control study. BMC Infect Dis (2021) 21(1):590. doi: 10.1186/s12879-021-06303-4
22. Peng W, Chen H, Zhao Z, Hu X, Zhou Y, Li Y, et al. TLR1 polymorphisms are significantly associated with the occurrence, presentation and drug-adverse reactions of tuberculosis in Western Chinese adults. Oncotarget. (2017) 9(2):1691–704. doi: 10.18632/oncotarget.23067
23. Li Z, Zhang Z, He Z, Tang W, Li T, Zeng Z, et al. A partition-ligation-combination-subdivision EM algorithm for haplotype inference with multiallelic markers: update of the SHEsis (http://analysis.bio-x.cn). Cell Res (2009) 19:519–23. doi: 10.1038/cr.2009.33
24. Oh J, Choi R, Park HD, Lee H, Jeong BH, Park HY, et al. Evaluation of vitamin status in patients with pulmonary tuberculosis. J Infect (2017) 74(3):272–80. doi: 10.1016/j.jinf.2016.10.009
25. Ganmaa D, Uyanga B, Zhou X, Gantsetseg G, Delgerekh B, Enkhmaa D, et al. Vitamin d supplements for prevention of tuberculosis infection and disease. N Engl J Med (2020) 383(4):359–68. doi: 10.1056/NEJMoa1915176
26. Zhang TP, Chen SS, Zhang GY, Shi SJ, Wei L, Li HM. Association of vitamin d pathway genes polymorphisms with pulmonary tuberculosis susceptibility in a Chinese population. Genes Nutr (2021) 16(1):6. doi: 10.1186/s12263-021-00687-3
27. Johnston J, Yang-Feng T, Berliner N. Genomic structure and mapping of the chromosomal gene for transcobalamin I (TCN1): comparison to human intrinsic factor. Genomics. (1992) 12(3):459–64. doi: 10.1016/0888-7543(92)90435-U
28. Porck HJ, Frants RR, Lindemans J, Hooghwinkel GJ, Planta RJ. Variant-specific differences in human unsaturated transcobalamin II. Biochem Genet (1986) 24(1-2):103–14. doi: 10.1007/BF00502982
29. Lin X, Lu D, Gao Y, Tao S, Yang X, Feng J, et al. Genome-wide association study identifies novel loci associated with serum level of vitamin B12 in Chinese men. Hum Mol Genet (2012) 21(11):2610–7. doi: 10.1093/hmg/dds062
30. Lerner-Ellis JP, Tirone JC, Pawelek PD, Doré C, Atkinson JL, Watkins D, et al. Identification of the gene responsible for methylmalonic aciduria and homocystinuria, cblC type. Nat Genet (2006) 38(1):93–100. doi: 10.1038/ng1683
31. Drögemüller M, Jagannathan V, Howard J, Bruggmann R, Drögemüller C, Ruetten M, et al. A frameshift mutation in the cubilin gene (CUBN) in beagles with imerslund-gräsbeck syndrome (selective cobalamin malabsorption). Anim Genet (2014) 45(1):148–50. doi: 10.1111/age.12094
32. Zheng S, Wu H, Ye F, Xia X, Xia S, Lin X, et al. Genetic architecture of vitamin B12 and folate levels uncovered applying deeply sequenced large datasets. Plos Genet (2013) 9(6):e1003530. doi: 10.1371/journal.pgen.1003530
33. Lin X, Lu D, Gao Y, Tao S, Yang X, Feng J, et al. Genome-wide association study identifies novel loci associated with serum level of vitamin B12 in Chinese men. Hum Mol Gene (2012) 21:2610–7. doi: 10.1093/hmg/dds062
34. Manolio TA, Brooks LD, Collins FS. A HapMap harvest of insights into the genetics of common disease. J Clin Invest (2008) 118:1590–605. doi: 10.1172/JCI34772
35. Wang L, Zhang H, Ruan Y, Chin DP, Xia Y, Cheng S, et al. Tuberculosis prevalence in China, 1990-2010; a longitudinal analysis of national survey data. Lancet (2014) 383(9934):2057–2064. doi: 10.1016/S0140-6736(13)62639-2
Keywords: pulmonary tuberculosis, infectious disease, Mycobacterium tuberculosis, vitamin B12, single nucleotide polymorphisms
Citation: Zhang T-P, Li R, Wang L-J, Tang F and Li H-M (2022) Clinical relevance of vitamin B12 level and vitamin B12 metabolic gene variation in pulmonary tuberculosis. Front. Immunol. 13:947897. doi: 10.3389/fimmu.2022.947897
Received: 19 May 2022; Accepted: 09 September 2022;
Published: 06 October 2022.
Edited by:
Deepak Kaushal, Southwest National Primate Research Center (S​NPRC), United StatesReviewed by:
Yun Chen, Seventh Affiliated Hospital, Sun Yat-sen University, ChinaCopyright © 2022 Zhang, Li, Wang, Tang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong-Miao Li, eXVxaWNhaTE5OTBAcXEuY29t; Fei Tang, dGFuZ2ZlaTEyMDVAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.