
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol. , 15 August 2022
Sec. Cytokines and Soluble Mediators in Immunity
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.946422
This article is part of the Research Topic Insights into the functional role of extracellular vesicles with specific cell types following infection and inflammation View all 8 articles
Extracellular vesicles (EVs) are membrane-bound particles released by cells in various (patho)physiological conditions. EVs can transfer effector molecules and elicit potent responses in recipient cells, making them attractive therapeutic agents and drug delivery platforms. In contrast to their tremendous potential, only a few EV-based therapies and drug delivery have been approved for clinical use, which is largely attributed to limited therapeutic loading technologies and efficiency. As EV cargo has major influence on their functionality, understanding and translating the biology underlying the packaging and transferring of biomolecule cargos (e.g. miRNAs, pathogen antigens, small molecule drugs) into EVs is key in harnessing their therapeutic potential. In this review, through recent insights into EVs’ content packaging, we discuss different mechanisms utilized by EVs during cargo packaging, and how one might therapeutically exploit this process. Apart from the well-characterized EVs like exosomes and microvesicles, we also cover the less-studied and other EV subtypes like apoptotic bodies, large oncosomes, bacterial outer membrane vesicles, and migrasomes to highlight therapeutically-diverse opportunities of EV armoury.
At any given moment cells constitutively release signals enabling them to communicate with other cells. Among these signals are small, heterogenous populations of membrane-bound vesicles known as extracellular vesicles (EVs). EVs are traditionally classed into 3 major categories, which are exosomes, microvesicles (MVs), and apoptotic bodies (ApoBDs) (Figure 1). However, the EV field has since expanded considerably to include the emergence other EV subtypes (1). They are released under varying conditions such as in the case of cell transformation, cell migration and other forms of programmed cell death like necroptosis (2–5). EVs can also be released by microbes such as in the case of bacterial outer-membrane vesicles (OMVs), fungal EVs and parasitic EVs (6–8).
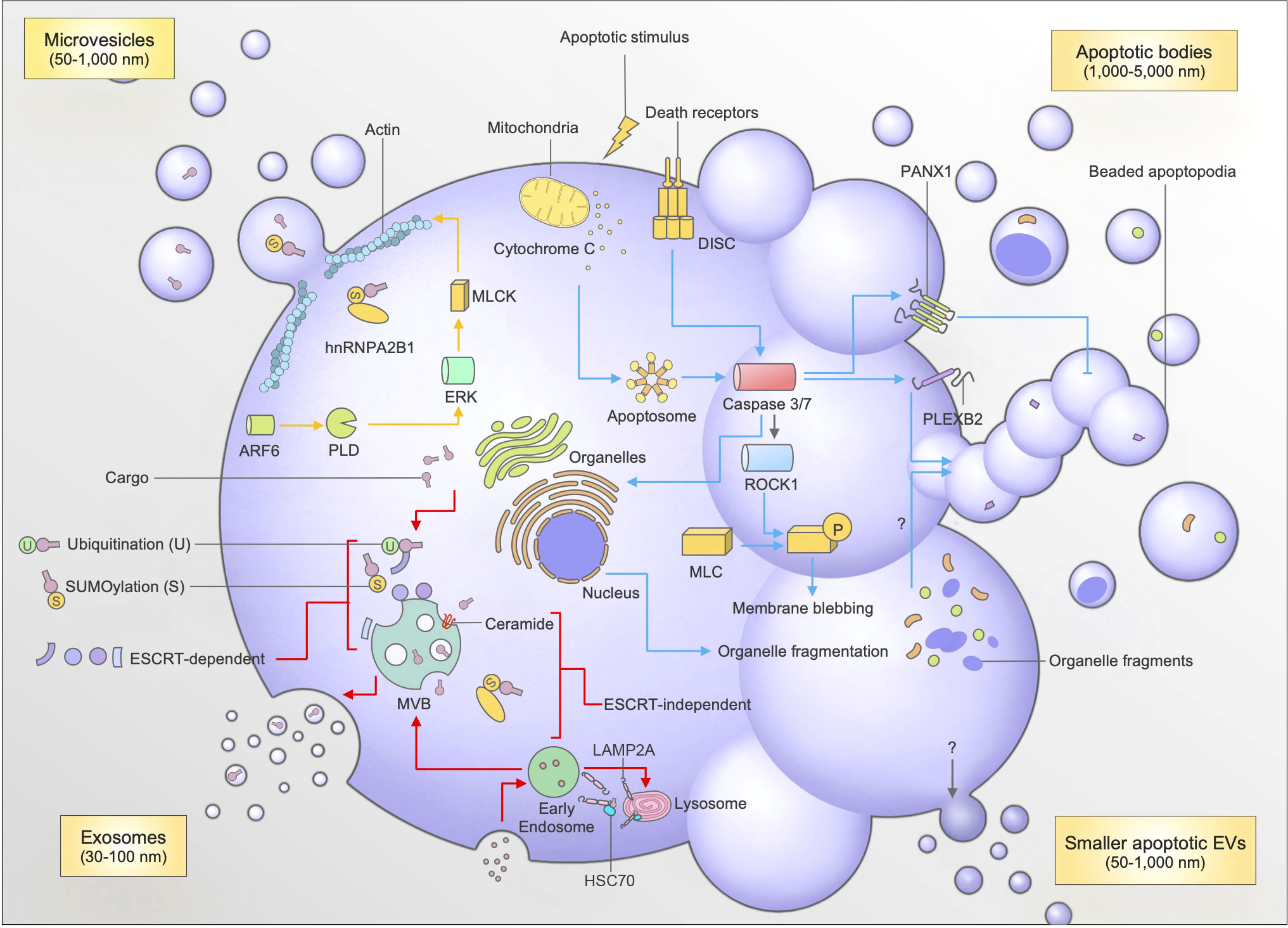
Figure 1 Mechanisms of biogenesis and cargo packaging for exosomes, microvesicles (MVs), apoptotic bodies (ApoBDs), and small apoptotic EVs. Exosome biogenesis (red arrows) involves the formation of intraluminal vesicles (ILVs) which contain cargo trafficked to the multivesicular body (MVB). The trafficking of cargo to the MVB involves post-translational modifications like sumoylation and ubiquitination by proteins like hnRNPA2B1, and interaction between ESCRT machinery (ESCRT-dependent), or sphingolipid ceramide, and LAMP2A-HSC70 complexes (ESCRT-independent). The fusion of the MVB with the plasma membrane causes the release of exosomes into the extracellular milleu. The formation of microvesicles (yellow arrows) occurs through plasma membrane budding, which requires actomyosin contractions facilitated by ARF6. Apoptotic bodies are generated following an apoptotic stimulus (blue arrows), which facilitates the induction of the extrinsic (death receptor mediated) or intrinsic (mitochondrial) pathway of apoptosis. The subsequent formation of the Apoptosome or DISC activates caspases 3 and 7. Caspases 3 and 7 cleave and activate PANX1 (negative regulator of apoptotic cell disassembly), ROCK1 (to facilitate membrane blebbing), and PLEXB2 (regulator of beaded apoptopodia formation). Caspases 3 and 7 are also able to cleave proteins to aid organellar fragmentation, which can then subsequently be packaged into ApoBDs through an unknown mechanism. Apoptotic EVs are also released during apoptosis, however the mechanisms are currently unknown.
Through transfer of functionally active biomolecules such as proteins and nucleic acids, EVs act as important mediators for intercellular communication in multiple physiological and pathological settings. Notably, EV amounts and cargo can be dysregulated during and contribute to the progression of infections, cancer, and neurodegeneration (9–12). As a consequence, EVs are considered attractive targets for novel therapeutic designs, which is why a number of EV-targeting approaches have been newly devised to control the formation and contents of endogenously formed EVs (13–15). Additionally, EVs display inherently clinically-desirable characteristics for therapeutic use such as (i) the ability to contain diverse biomolecular cargos, (ii) the ability of said cargo to elicit potent cellular responses, (iii) the ability to cross biological barriers, (iii) availability, (iv) bioengineerability and (v) scalability (10, 16–18). Furthermore, EVs have been shown to be safe for clinical use, for instance, loading EVs with a common chemotherapeutic drug, doxorubicin, was able to increase efficacy of the drug whilst simultaneously significantly reducing side effects in patients (11, 19). Other pre-clinical and clinical trials have also demonstrated the safety of mesenchymal stem cell (MSC) derived-EVs, citing low immunogenicity, although this is currently still an active area of investigation (see Janockova et al., 2021 for an in-depth review) (20, 21). Additionally, the malleability of EVs has provided opportunities for ingenuity, leading to favorable treatment outcomes for patients undergoing anticancer therapy, pathogen vaccination, immunotherapy and regenerative therapies (11, 12, 22–26). Innovative approaches such as formation of synthetic vesicles as cancer immunotherapies, as well as chimeric apoptotic vesicles have provided strong promise for such use (27, 28). Many groups have also investigated the use of EVs like exosomes, MVs, ApoBDs and large oncosomes (LOs) in minimally-invasive diagnostic strategies (26, 29–32). More recently, the approach of using EVs to help vaccinate and protect against SARS-CoV-2 infection is being investigated (33, 34). Nevertheless, the development of EV-based therapies and diagnostics is a fast-growing and promising research area.
The particular importance of EV contents in EV functions, disease progression and for therapeutic success emphasizes that understanding the biology underlying the trafficking and packaging of biomolecule cargos into EVs is key to fully unleash their therapeutic potential. In fact, EV researchers have acquired a substantial amount of knowledge to uncover the biology of cargo packaging over the past few years. Intriguingly, each EV subtype reportedly undergoes distinct cargo sorting mechanisms, implying subtype-specific functionality and therapeutic applications and collectively furthering EV diversity. Because recent EV-focused reviews have overlooked this significant feature of EV biology and had a tendency to focus on the traditional subtypes (9, 10, 12, 35), we herein capture recent advances in understanding the biogenesis of various EV subtypes, and in particular discuss the mechanisms behind cargo sorting into EVs. Furthermore, we aim to discuss the possible strategies available to utilize these EV packaging mechanisms for the purpose of developing a diverse repertoire of EV-based therapeutics.
Exosomes are perhaps the most well-characterized type of EV following their initial discovery in reticulocytes in 1987 (1). Ranging from 30-100 nm in diameter, exosomes originate as intraluminal vesicles (ILVs), which are formed through budding of the multivesicular body (MVB) (26). The fusion of the MVB with the plasma membrane causes the release of exosomes into the extracellular environment (Figure 1). During the formation of the MVB, many factors contribute to the biogenesis and subsequent packaging of cargo into exosomes. Additionally, the identification of certain exosomal markers has helped to identify the specific mechanisms behind biogenesis and packaging. For instance, the identification of tetraspanins CD63, Alix, TSG101 enrichment in exosomes has helped in elucidating an ‘endosomal sorting complexes required for transport (ESCRT)-dependent’ pathway of exosome biogenesis (26, 36).
An interesting aspect of these pathways in exosome biogenesis is that they can interact with other accessory proteins to assist with packaging of exosomal cargo. Recent work has uncovered the importance of post-translational modifications (PTM) to biomolecules in facilitating interactions with accessory molecules to chaperone biomolecules to the MVB for packaging into exosomes (37, 38). One of the primary signals associated with protein sorting into exosomes is ubiquitination (Figure 1). Ubiquitination requires the addition of one (mono-) or multiple (poly-) ubiquitin molecules onto lysine residues in protein cargo (37, 38). Following this process, ubiquitinated proteins can associate with ESCRT members ESCRT-0 and -1 by interacting with their specific proteins known as Hrs and TSG101, respectively (26, 39, 40). The clustering of ubiquitinated proteins to ESCRT-0 and subsequent recruitment to the endosomal membrane by other ESCRT members through interaction with membrane phosphoinositol PI3P ensures the sorting of specific protein cargo into exosomes during their biogenesis (26, 38–40). For instance, ubiquitination is important for transporting soluble Mycobacterium tuberculosis proteins into exosomes (39). Interestingly, this can have therapeutic implications as mice vaccinated with a combination of the BCG vaccine and macrophage-derived exosomes containing M. tuberculosis proteins were protected against tuberculosis challenge (41).
Another PTM like ubiquitination called sumoylation has also been shown to assist with cargo packaging during exosome biogenesis (Figure 1). Sumoylation involves the conjugation of a small ubiquitin-like modifier (SUMO) to a protein of interest, thereby targeting it for trafficking to MVBs during vesicle formation (37, 38). This process has been shown to be paramount in facilitating trafficking of miRNAs into exosomes, through the binding of sumoylated RNA binding protein hnRNPA2B1 to specific targeting motifs on miRNAs of interest (42). Sumoylation can also assist with trafficking α-synuclein particles to small EVs through interactions with ESCRT machinery TSG101, and accessory tetraspanin Alix (43). Additionally, the associations of proteins with tetraspanin-enriched microdomains (TEMs), which are responsible for the packaging of certain cargo like MHC II into dendritic cell (DC)-derived exosomes, has been shown to be regulated by palmitoylation, which is another form of PTM (37).
To add further complexities to this pathway, cargo-sorting into exosomes can occur independent of ESCRT machinery as well. In fact, the identification of alternative exosomal sorting pathways is a subject continually under investigation. Groups have discovered the role of sphingolipid ceramide (enriched on exosomal membranes) in trafficking proteolipid proteins in murine oligodendrogial cells (44). Other recent work continues to unveil additional mechanisms utilizing other proteins to traffic specific cargo such as toll-like receptor trafficking protein UNC93B1, a syndecan-syntenin-Alix pathway, and more recently a joint pathway primarily involving LAMP2A and HSC70 to chaperone proteins like HIF1A by targeting KFERQ-like motifs (44, 45). This no doubt provides vast potential for use in EV-based therapeutics by offering alternative targets for compound loading (37, 38, 45). It also should be noted that other EV subtypes display their own selective and unique mechanisms of cargo packaging, which are described further below (Table 1).
MVs are another major category of EVs released by cells. They are generated through the budding of the plasma membrane primarily from healthy cells, and typically range from 50-1,000 nm in diameter. The biogenesis of MVs, although not as well characterized as exosomes, relies on specific cytoskeletal rearrangements and phospholipid redistributions (36, 46, 47). For membrane budding to occur, ADP-ribosylation factor (ARF6) activates phospholipase D (PLD) (48). Next, ERK is recruited to the plasma membrane to initiate phosphorylation of myosin light chain kinase (MLCK) (48). This initiates an actomyosin contraction, which then triggers the ‘pinching’ and release of the MVs from the plasma membrane into the extracellular milieu (Figure 1) (48). Other external factors may also facilitate MV release, like calcium influx to induce phospholipid redistribution, indicated by PS exposure on the outer membrane leaflet (36, 46, 47). Additionally, in hypoxic conditions, ARF1 and small GTPases like RhoA and Rab22A are known to facilitate vesicle budding through actomyosin contractions through a Rho associated protein kinase (ROCK) mediated pathway (48, 49). Interestingly, although not well characterized, these pathways may bare similarities in the generation of other membrane-derived EVs including ApoBDs or large oncosomes (Table 1).
MVs have displayed multiple selective mechanisms for the assortment of cargo during biogenesis (50). ARF6, which is involved in MV biogenesis, is also a key mediator for cargo selection. For instance, ARF6 is responsible for the packaging of vesicle associated membrane protein (VAMP3), integrin β-1, and MHC I into tumor-cell derived MVs, as well as the simultaneous exclusion of transferrin receptors (46, 48, 50). Furthermore, recently an ARF6-exportin 5 dependent pathway was described for the packaging of miRNAs into tumor-derived MVs (51). Other packaging mechanisms also involve the use of SNARE proteins, particularly VAMP3, which when associated with CD9 facilitates its packaging into tumor-derived MVs (36, 52). Additionally, caveolin-1, which is a MV membrane protein marker, is known to interact with hnRNPA2B1 in noxious conditions to facilitate transfer of miR-17, 93 and 20a into MVs (53).
In contrast to other EVs, ApoBDs are released strictly by cells undergoing a form of programmed cell death called apoptosis. Initially thought to be generated via a stochastic process, recent work has revealed a highly coordinated mechanism of ApoBD formation via a process known as apoptotic cell disassembly (54, 55). This process details the morphological steps required for an apoptotic cell to fragment into ApoBDs, which could aid the efficient clearance of apoptotic materials (56). Previous studies have described a number of molecular regulators of ApoBD formation, namely ROCK1, Pannexin-1 (PANX1), and Plexin-B2 (PLEXB2) (Figure 1) (55, 57–59). ROCK1 was found to promote apoptotic cell removal by phagocytes through controlling membrane blebbing, which is a key morphological step in ApoBD formation for certain cell types (57, 60). Furthermore, targeting the key negative regulator of apoptotic cell disassembly, PANX1, induced generation of thin string-like membrane protrusions known as apoptopodia, which promoted ApoBD formation and subsequent uptake by phagocytes (55, 58). More recently, PLEXB2 was demonstrated to regulate the generation of monocyte-derived ApoBDs, which was also important in aiding apoptotic monocyte clearance in vitro and in vivo (59).
To further assist cell clearance, ApoBDs can contain organellar constituents that could aid recognition by immune surveillance mechanisms. For instance, the release of nuclear material like histones from membrane lysed ApoBDs could aid the recruitment of macrophages to apoptotic cells (Table 1) (56, 60). Similarly, the exposure of ER proteins, ERp57 and calreticulin, could promote the immunogenicity and clearance of apoptotic material (61). Furthermore, fragments of the nucleus, cis-Golgi, mitochondria, and lysosome have also been found in ApoBDs (58, 62, 63). Although the exact functional consequences of sorting organellar fragments into ApoBDs is currently unknown, evidence suggests that this process could assist with preferential clearance of apoptotic material (64). Notably, the removal of nuclear contents from apoptotic cells is especially important in preventing inflammation due to autoantigen production, as antibodies toward nuclear autoantigens appear in high titres in many conditions including the autoimmune disease systemic lupus erythematosus (SLE) (65, 66).
Besides packaging cargo into ApoBDs for clearance and degradation, ApoBDs have also been shown to transfer specific cargo to facilitate intercellular communication (Table 1). Like other EVs, ApoBDs are known to contain nucleic acids such as DNA and miRNA (17, 54). One of the initial studies on ApoBD cargo detailed the horizontal transfer of both genomic and EBV-DNA to recipient cells (67). A follow up study by the same group also demonstrated that oncogenes H-RASV12 and c-Myc could be transferred via ApoBDs to recipient p53-/- cells, thereby promoting a tumorigenic phenotype (68). Although these studies did not specifically isolate ApoBDs, it provided the basis for many studies into ApoBD packaging and function. More recently, in vivo studies showed the carriage of Wnt8a particles in ApoBDs could enhance stem cell proliferation in zebrafish (24). Although it was proposed that ApoBDs could only transfer molecules to benefit the recipient cell (68), it is still unclear whether ApoBD contents are packaged passively or selectively during apoptosis. For instance, exposure and enrichment of apoptotic membrane markers (like PS) and cell-type specific markers on ApoBDs is likely to be acquired passively as these molecules are readily present at the plasma membrane (63). Additionally, although there is debate in the field, there are currently no known specific ApoBD membrane markers, which would typically help with elucidating packaging mechanisms (as mentioned for exosomes and MVs above) (69). For the acquisition or exclusion of organelle-derived content, it is slightly more complex. In packaging nuclear material, it is known that the ‘tearing apart’ action facilitated by membrane blebbing is key in aiding the partition of nuclear fragments into blebs and subsequently ApoBDs (60, 62). This is further exemplified by the exclusion of nuclear material from ApoBDs generated by THP-1 monocytes, which do not readily undergo a dynamic blebbing process (17, 58). Furthermore, as the fragmentation of the Golgi due to caspase-dependent cleavage of structural proteins during apoptosis is well documented, it is unclear whether such Golgi fragments could be shuttled in ApoBDs selectively through a distinct mechanism, or passively packaged into ApoBDs as Golgi fragments are dispersed in the cytosol (70, 71). Notably, further studies into ApoBD content have unveiled less conventional biomolecules, like the transfer of Influenza A virions in monocyte-derived ApoBDs to promote viral dissemination via a ‘trojan-horse’ mechanism (72). Whilst these mechanisms are complex, and vastly contrasted to other EV subtypes, there is no doubt that ApoBD packaging mechanisms display intriguing potential for exploitation in therapeutics.
Besides ApoBDs, small EVs can also be released during apoptosis, namely small apoptotic-derived EVs (Figure 1) (73). Interestingly, Schiller et al. (2008) demonstrated that small apoptotic-derived EVs (~500 nm) could selectively carry histone proteins whilst excluding cytochrome C, prohibitin, HSP70 and lamin B (64). Likewise, small apoptotic EVs can carry a host of other effector molecules including Sjögren’s syndrome-associated autoantigen α-fodrin and 20S proteosome complexes (73–75). More recent studies into MSC-derived apoVs have shown they harbor the capability to contain multitudes of proteins and carry specific ligands like Fas-L to promote wound healing and attenuate sepsis (76, 77). However, it must be noted that whilst groups have isolated and analyzed these apoptotic-derived EVs, their characteristics such as markers, size, and method of isolation remains to be fully defined (69). Additionally, although there is evidence for selective packaging of cellular contents into apoptotic-derived EVs, their exact mechanisms of packaging and biogenesis have also not been fully elucidated. Similarly, small EVs released under other cell death conditions have also been described including pyroptosis and necroptosis (2, 78–80). Although they have only recently been described, further investigations into their contents and packaging mechanisms may provide broader treatment options and further expand treatment for inflammatory disease conditions that pyroptosis and necroptosis are associated with.
Over the years, investigation into exosomes, MVs and ApoBDs have demonstrated the possession of unique mechanisms required for the packaging of effector molecules. As the EV field expands to include newly characterized EV subtypes released under different conditions, it is of utmost importance to investigate their cargo and packaging mechanisms to further understand the intricacies of EV biology. This is essential, as biomolecule carriage and transfer is closely linked to function. Importantly, further understanding these mechanisms may provide insight into the causation, and treatment of patho(physiological) conditions. Here, we review other EV subtypes and recent advances that provide insight into packaging mechanisms and address their potential therapeutic use (Table 1).
Bacterial OMVs are a type of EV released by both gram-negative and gram-positive strains of bacteria. Initially observed during Vibrio cholerae growth, OMVs were thought to be cell debris or microscopic artefacts due to their small size of 20-250 nm (81). Since then, research has demonstrated that OMVs are extremely important to bacterial pathogenesis, as they are key in aiding the transferral of virulence factors, DNA, and contributing to biofilm formation (82, 83). With perhaps a more diverse mechanism of biogenesis among EV subtypes, OMV biogenesis can occur through a myriad of mechanisms, including changes in lipoprotein Lpp and peptidoglycan (PG) crosslinks within the membrane, accumulation of periplasmic cargo, and increase in membrane curvature (6) (Figure 2A). Notably, the mechanism of OMV biogenesis may vary depending on bacterial species. It is well known that OMVs, like other EVs, can contain many parental cell-derived constituents. This includes periplasmic and cytoplasmic proteins, lipopolysaccharide (LPS), PG, DNA, RNA and enzymes (81, 84). Interestingly, certain bacterial species have demonstrated selective packaging properties for the movement of cargo into OMVs (Table 1). Although, it must be noted that the exact machinery behind packaging has remained elusive. For instance, Porphyromonas gingivalis was able to specifically package a major group of virulence factors known as gingipans into OMVs through an unspecified LPS-mediated mechanism (82). Furthermore, it was found that LPS was also important for the exclusion of outer membrane proteins like RagA/B, further exemplifying the selectivity of this process (82). Other species of bacteria like Bacteroides fragilis and Bacteroides thetaiotaomicron could, through unspecified molecular machinery, select proteins for OMV packaging based on charge, as a majority of the cargo enriched in OMVs were acidic proteases and glycosidases (85). Notably, Helicobacter pylori also displayed undetermined selection mechanisms, as OMVs derived from H. pylori contained most T4SS components except VirD4 (6, 86). Interestingly, it has been suggested that exclusion of VirD4 from OMVs could benefit the parental bacterium (6, 86). Proteomic analysis demonstrated that OMVs from Neisseria meningitidis were highly enriched with 5 out of the 6 known autotransporter proteins (6, 87). Although OMV-based vaccines have been approved for use in the protection against meningococcal B, further investigations into the machinery behind bacterial OMV packaging may provide further opportunities for vaccine-based approaches. This is currently under investigation particularly in fight against COVID-19 infection (33).
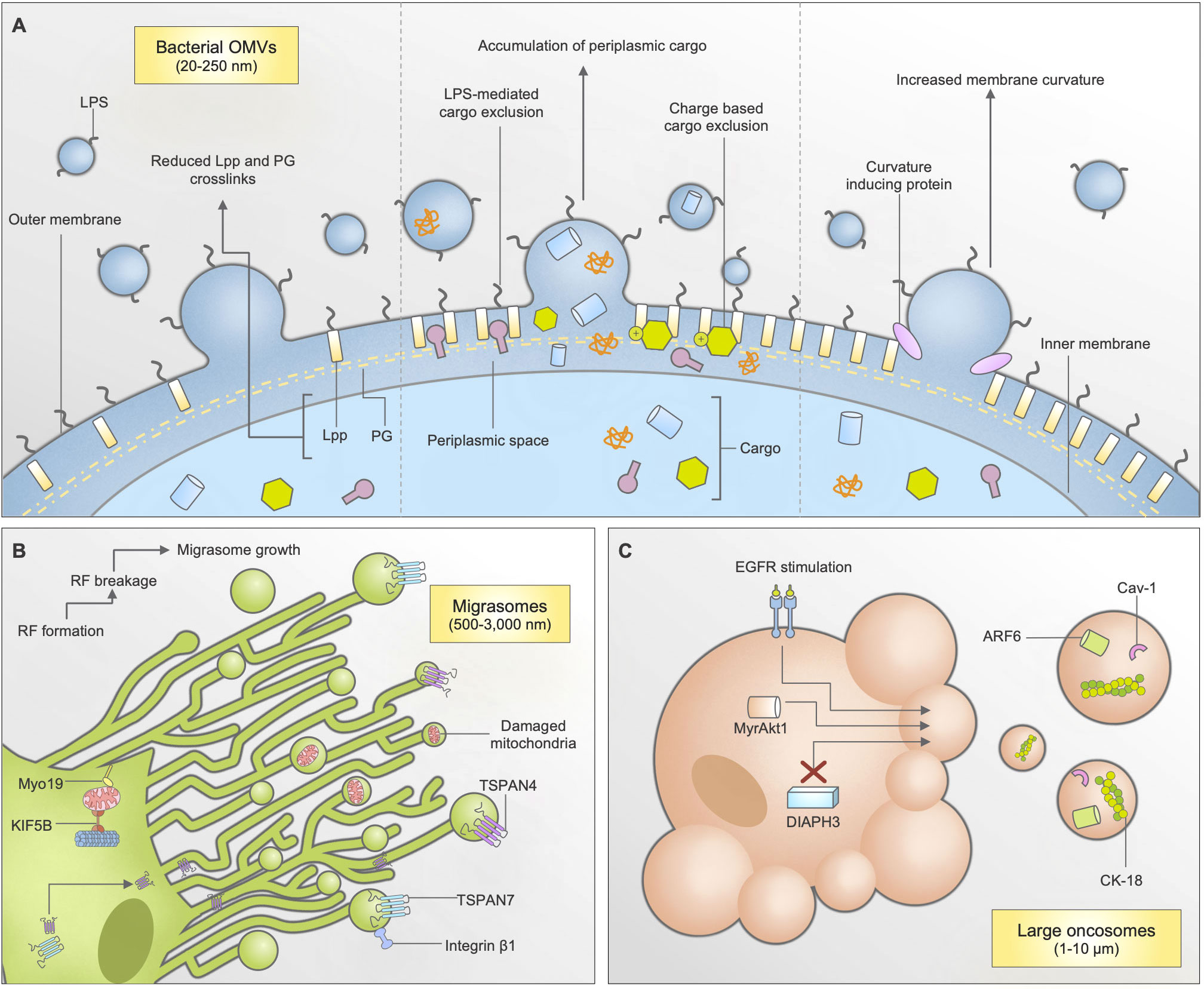
Figure 2 Biogenesis and cargo packaging mechanisms of bacterial outer membrane vesicles (OMVs), migrasomes, and large oncosomes (LOs). (A) Bacterial OMVs can be released through multiple mechanisms: through reduction in lipoprotein (lpp) and peptidoglycan (PG) crosslinks, accumulation of cargo within the periplasmic space, and increases in membrane curvature due to curvature inducing proteins. Cargo packaging mechanisms are largely unknown, however some bacterial species have indicated charge-based and lipopolysaccharide (LPS) mediated mechanisms. (B) Migrasomes are formed through a migration-dependent mechanisms, predominantly found in epithelial cells. As cells migrate, retraction fibres (RFs) form on the extracellular matrix. As the cell continues to move, the RF breaks and begins to form small, bulbous structures known as migrasomes. Tetraspanins TSPAN4 and TSPAN7 are known to be important for this process, and are recruited through the retraction fibres. When mitochondrial stress occurs, cells can also shuttle damaged mitochondria for disposal using mitocytosis, dependent on KIF5B and Myo19. (C) Large oncosomes are generated by prostate cancer cells during a non-apoptotic form of membrane blebbing. This occurs following EGFR stimulation, DIAPH3 silencing, or by activation of MyrAkt1.
Migrasomes are a newly discovered type of EV. Ranging from 500-3,000 nm in diameter, they are formed through a unique, migration-dependent mechanism whereby the breakage of retraction fibres left behind by migrating cells begin to form small, bulbous structures over time (Figure 2B) (4). The formation of migrasomes is dependent on ROCK1, tetraspanins TSPAN4, TSPAN7, integrins α5, β1, and cholesterol, in which TSPAN4, TSPAN7, and integrin-β1 are primarily used as membrane markers for migrasome isolation (88–91). Interestingly, migrasomes are formed by a broad range of cell types and have been observed in mice and zebrafish tissue (4, 88). Notably, their role in zebrafish gastrulation revealed that migrasomes, like other EVs, are enriched in many cellular signaling factors including chemokines, growth factors and morphogens (88). Intriguingly, fluorescence recovery after photobleaching (FRAP) studies have suggested that migrasomes are able to quickly recruit cellular contents as demonstrated by rapid movement of TSPAN4 into migrasomal membranes during formation, by using retraction fibres as a path (90). More recently, studies have revealed a new cargo-packaging method that neutrophils can utilize migrasomes as “disposal” systems, by specifically shuttling damaged mitochondria into them as part of a mitochondrial quality control process (92). In comparison to cargo recruitment mechanisms displayed by other EVs, these mechanisms are vastly unique and suggests a rapid and specific intracellular mechanism of recruitment, harness able for therapeutic usage. Additionally, the enrichment of enzyme proteins like N-sulfotransferase-1 (NDST1) and carboxypeptidase (CPQ), and simultaneous exclusion of organelle-derived proteins like Sec61a and GM130 suggests a selective process of packaging, although this has not been further investigated (93). The disposal of “faulty” cargo into migrasomes (whether it be proteins or organelle-specific) remains to be further investigated.
LOs are the largest subtype of EVs formed specifically by tumor cells, at 1-10 μm in diameter (94, 95). Their biogenesis is associated with a non-apoptotic form of plasma membrane blebbing that occurs during cell transition to an ‘amoeboid’ phenotype (Figure 2C) (95, 96). LO formation is enhanced through a variety of ways, including DIAPH3 silencing, epidermal growth factor (EGF) stimulation, and induced expression of oncoproteins like MyrAkt1 (95). LOs are distinct from exosomes in their size, and that they carry negligible levels of exosomal markers like CD81, TSG101 and CD9 (Table 1) (3). Furthermore, they are known to carry a substantial amount of DNA compared to smaller EVs, which can assist in understanding cancer-specific genomic alterations (94). Interestingly, they bare a resemblance to MVs through their enrichment of ARF6 and Cav-1 (97). Another protein marker important for LO isolation is cytokeratin-18, which can be used to visualize LOs in human tissues through immunohistological studies (3).
Unlike other EVs, the majority of LO studies has been in the context of prostate cancer. Thus, this provides the basis that their packaging mechanisms may be cancer-cell specific. In particular, LO-like EVs isolated from prostate-cancer free patients showed they were completely absent of DNA in comparison to patients with prostate cancer, thus suggesting that DNA packaging into LOs is purely a cancer cell-specific mechanism (94). Beyond this, the mechanisms of LO packaging have not been further elucidated. Many studies into LO content have revealed that the containment of certain biomolecules are essential in promoting cancer phenotypes in recipient cells. For instance, the transfer of miR-1227 from LOs to recipient cancer-associated fibroblasts promoted cell migration (98). Additionally, further investigation revealed the enrichment of proteins important for metabolic processes like glucose and glutamine metabolism, which are exceedingly important in cancer progression (3). More recently, the transfer of phosphorylated-Akt1 by LOs into non-cancerous normal human prostate fibroblasts could induce reprogramming to establish tumor-supportive environments (29).
As described above, EVs are released under vastly different contexts. Thus, the contents of each EV whether it be released from a healthy cell, cancerous cell, or from bacteria can differ greatly. Interestingly, recent studies have unveiled that this variation in cargo harbors strong potential to be used therapeutically. Here we highlight how certain EVs could undergo cargo engineering for the purpose of expanding the repertoire for EV-based therapeutics (Table 2).
As EVs can carry constituents of cells, EVs can be used to promote immune responses. For instance, the use of EVs in vaccine development has proven to be an effective method of establishing immunoprotective effects. Notably, the use of exosomes in vaccine development has been under investigation for some time as seen through DC-derived exosomes being able to induce protection in mice against Toxoplasma gondii oral challenge (99). Additionally, exosomes were also able to induce a protective immune response by triggering an increase in Th1-specific responses due to the packaging of bacterial antigens (25, 99). For macrophage-derived exosomes, similar findings were also observed in studies against Mycobacterium tuberculosis challenge in mice (41). Along with the carriage of bacterial antigens, exosomes are able to carry MHC II molecules to assist in antigen presentation, which is a unique method to initiate immune response (39, 41, 100). Furthermore, EVs from dying cells have also displayed potential for vaccine development. For protection against tumor progression, tumor-baring rats vaccinated with monocyte-derived antigen presenting cells that had engulfed ApoBDs had increased survival by 80% (101). Furthermore, DCs that had engulfed ApoBDs derived from leukemic-B cells promoted T cell activation, proliferation and IFN-γ release (102). Although this has not been extensively investigated, this potential could be extended to use other dead cell-derived EVs including necroptotic EVs, where their lysis may aid in the sustained release of tumor-derived and pathogen-derived antigens to mount immune responses. As such, further investigations will need to be conducted to ascertain the true potential of dead cell-derived EVs like ApoBDs and necroptotic EVs in this context.
Notably, bacterial OMVs have exciting potential for use in vaccines against bacterial and viral infections. Along with the carriage of bacterial antigens, OMVs can also induce immunoprotective effects. This is largely due to the fact that they are non-replicative clones of their parental bacteria, yet highly immunogenic (81). OMVs derived from bacterial species like E. coli, H. pylori, and P. gingivalis were able to induce protection against pathogen challenge by promoting innate and adaptive immune responses as measured by increase IgG titres, and upregulation of pro-inflammatory mediators like NF-κB (81). Interestingly, this could be associated with the selective packaging of major virulence factors from pathogens, like RagA/B from P. gingivalis (82). As mention above, bacteria have displayed selective packaging mechanisms through the inclusion of virulence factors and simultaneous exclusion of membrane proteins in OMVs (82). This provides the potential for bacterial OMVs to be engineered to contain pathogen-derived cargo for vaccine development. Although this has yet to be investigated extensively, OMVs have been engineered to display pathogen-derived antigens on their surface. E.coli derived OMVs were engineered to display M2e (an Influenza A virus matrix protein) on the surface of OMVs, which induced effective immunoprotection against influenza A virus when used to vaccinate BALB/C mice (103). Of note, OMV-based vaccines have also displayed promise in providing protection against viruses like Influenza strain H1N1 and MERS-CoV, and SARS-CoV-2 (104). Importantly, OMV-based vaccine RMenB-OMV (also known as BEXSERO®) has been approved for protection against meningococcal B disease, and is currently under investigation for protection against STIs like Gonorrhoea and HIV (NCT04415424, NCT04597424) (105) (Table 2). A recent intranasal vaccine candidate based off Salmonella typhimurium OMVs has also been investigated. These OMVs are modified with SARS-CoV-2 spike receptor binding domains, and successfully created neutralizing antibody responses in vaccinated participants exposed to wild-type and delta variants, thus expanding the world’s vaccine repertoire to protect against COVID-19 variants of concern (33).
An interesting development of EV-based therapies is their use in regenerative medicines. For instance, the use of mesenchymal stem cell (MSC)-derived EVs has garnered recent interest as they have been used to treat many diseases. This includes cardiovascular, renal, lung and liver pathologies (16, 18, 106). Notably, MSC-derived EVs are also known to promote wound healing as shown in a rat skin burn model. Interestingly, these healing properties were attributed to the delivery of Wnt4 molecules by MSC-exosomes, thus activating Wnt/β-catenin signaling to induce skin cell proliferation and migration (107). MSC-derived ApoBDs were also able to rescue stem cells through Wnt pathway activation through the transferral of E3 ligase RNF146 and miR-328-3p (108). As an alternative mechanism, recently small EVs generated from apoptotic MSCs were found to promote bone and adipocyte formation following engulfment in MRL/lpr and Casp3-/- mice by upregulating pro-angiogenic genes THSN1 and VASH1 (109). Although this study did not investigate the contents of the small apoptotic EVs, it suggests that like other MSC-derived EVs, EVs of this origin could contain specific cargo to assist in the proliferation of many cell types.
Interestingly, ApoBDs derived from cells besides stem cells are also able to promote cell proliferation. Again through Wnt signaling, ApoBDs derived from zebrafish epithelial cells were able to promote stem cell proliferation through caspase-dependent packaging of Wnt8a molecules (24). Furthermore, ApoBDs from osteoclasts could also induce osteoclastogenesis and differentiation through RANK-L packaging (110, 111). These studies highlight the potential that ApoBDs could be specifically engineered or harnessed for use in the regeneration and proliferation in different pathologies. Other dead cell-derived EVs like necroptotic EVs could also be used for regenerative medicine, as recent evidence suggests continued synthesis of cytokines following lysis of necrotic cell ‘corpses’ could be harnessed to contain new cargo to promote proliferation (2, 112). Although promising, further studies will need to be conducted in assessing the feasibility and efficacy of these EV-based treatments.
As there are a myriad of ways to deliver therapeutics, EVs are currently being explored as promising drug delivery vehicles. There are a number of benefits in utilizing EVs as a drug delivery platform, including their size, similarity to parental cells, and ability to avoid immunosurveillance mechanisms (113). Notably, using EVs to deliver small molecule drugs may provide a way to enhance drug bioavailability by avoiding increased drug dosages, which can be detrimental to patients. Furthermore, there is a potential to deliver gene therapies via EVs as well. Exosomes and MVs in particular have been well-characterized in the delivery of many different molecules including nucleic acids and small molecule drugs (36). For instance, exosomes could be loaded with siRNA to induce significant knockdown of beta-secretase 1 in mice, providing gene-based therapies for Alzheimer’s disease (114). Furthermore, the packaging of small molecule drug paclitaxel (PTX) into exosomes and MVs was able to increase cytotoxicity against PC3 and LNCaP cells (115). Interestingly, macrophage-derived exosomes packaged with PTX was able to reduce the size of tumors in mice with pulmonary metastases in comparison to mice treated with the drug Taxol only (116). This was also reflected in bovine milk-derived exosomes also packaged with PTX and other drugs like withaferin A, as they displayed higher anti-cancer, anti-inflammatory effects, and increased bioavailability of the drugs in vivo (113). Furthermore, packaging of MVs with MTX or cisplatin were able to reverse drug-resistant properties in tumor cells from patients with end-stage lung cancer, as well as cholangiocarcinomas (117, 118). As such, the therapeutic effects of EV use in drug loading has progressed such that the loading of molecules like curcumin into exosomes and MVs is currently under investigation in clinical trials (119, 120) (Table 2).
Besides exosomes and MVs, other EV subtypes also exhibit drug delivery properties. For example, migrasomes are also able to transport cytosolic contents and organelles as indicated by the quick transfer of TSPAN4-GFP along retraction fibres during biogenesis, and movement of damaged mitochondria (4, 90, 92). The recent discovery of ROCK1 regulating migrasome formation also provides an avenue for their formation to be exploited therapeutically (91). Although these avenues have not been further investigated due to the early stages of migrasome research, it suggests another potential avenue for drug therapies to be transferred to recipient cells. Additionally, other dead cell-derived EVs have displayed promise through their ability to contain and transfer important bioactive molecules (60, 62, 63, 121, 122). Apoptotic tumor-derived EVs loaded with methotrexate (MTX), doxorubicin, cisplatin or PTX were able to inhibit tumor growth in vivo without adverse side-effects (123). Providing further demonstration for the use of dead-cell derived vesicles in therapeutics, promising work has shown apoptotic vesicles can be loaded with either anti-inflammatory molecules like curcumin, or various pro-drugs in order to provide benefit (12, 28, 122). In the case of the latter, the loading of apoptotic vesicles with disulphide-linked drugs, camptothecin and PR104A (anti-cancer agents) could effectively promote drug penetration in whole tumors promoting tumor destruction (122). Nevertheless, there are still many avenues to explore in determining the best use of EVs in drug delivery, including development of mass-production methods and quality control (113).
EVs are shed by virtually all cells under normal and pathological conditions. Importantly, because they carry protein and nucleic acids that reflect their parental cell origin, they are thought to provide the key in early detection of various diseases. For instance, numerous studies have indicated that analysis of miRNAs packaged into urinary EVs from prostate cancer patients can assist with early diagnosis (32, 124, 125). This has also extended to include cardiovascular diseases, pathogen-specific conditions, neurological diseases, and traumatic brain injuries (126–128). Additionally, because EVs are widely distributed in biological fluids, they are more readily attainable through liquid biopsies using blood, urine, saliva, sperm, or breast milk (36, 113). This has already provided vast advancements within the field, as the use of EV-based tests in conjunction with mainstay diagnostic techniques can increase test specificity. This was exemplified through combination of ExoDx® Prostate(IntelliScore) (a urine exosome gene expression assay, Bio-techne) and prostate serum antigen (PSA) testing to detect high-grade prostate cancer prior to tissue biopsies (32, 124). This assay has also been adapted to detect lung cancer markers, known as ExoDx® Lung(ALK) (129) (Table 2).
As mentioned above, it is currently well known that analysis of miRNAs packaged in EVs can assist with classification of cancers and understanding their complexities (94). This was particularly important for unveiling therapeutic resistance in ovarian tumors, which was indicated by specific miRNA enrichment (130). Likewise, analysis of miRNA in semen-derived exosomes demonstrated the overexpression of miR-142-3p, miR-142-5p, and miR-223-3p in malignant and benign prostate tumors from patients, in comparison with healthy controls (124). Aside from miRNAs, other proteins like cancer-specific biomarkers have been located within MVs to assist with diagnosis of specific cancers. For instance, colon cancer biomarkers CEACAM1 and MUC13 could be found in MVs derived from colon cancer cells, which could assist in the diagnoses of colorectal cancers (131). Similarly, protein tyrosine kinase 2 (PTK2) (which indicates oncogenic transformation) could also be tracked in MVs derived from MDAMB231 cells, further suggesting that analysis of EV content is an attractive target for diagnostics in breast cancer (132). This is also the case for other pathological conditions such as Alzheimer’s disease. Notably, cerebrospinal fluid-derived MVs from Alzheimer’s patients displayed reduced concentration of tau and APP, which can indicate disease severity and associated cognitive decline (133).
LOs can also be useful in diagnostics as they can be detected in the bloodstream of mice and patients with prostate cancer (3). For instance, expression of Cav-1 in LOs is known to be an indicator of metastatic disease in prostate cancer patients (98). Additionally, the deletion of DIAPH3, which is important in LO biogenesis, may also be an indicator of metastasis as its deletion was detected in 64% of metastatic tumors (95). Furthermore, as LOs are known to carry oncoproteins like MyrAkt1 and metalloproteinases (which are important in maintaining tumor microenvironments), their detection could further assist with the diagnosis and monitoring of other cancers (97).
Additionally, as a variety of diseases can be associated with death of specific cell types, the analysis of dead-cell EVs could be used to monitor the level of cell death of a particular cell origin. For instance, cell-type specific ApoBDs can be isolated from biological tissues by using cell-type specific markers, which can potentially aid diagnosis of different conditions (69). Furthermore, the analysis of EV content may also assist in diagnosis, as analysis of apoptotic EVs revealed packaging of spliceosomes, which is important in identifying glioblastoma (63, 134). For other conditions like graft-vs-host-disease (GVHD), the presence of ApoBDs within the crypts of gastrointestinal tracts can aid in diagnosis. However, because further investigation is required to diagnose GVHD, analysis of ApoBD content could mitigate this step and create a quicker and easier process of diagnosis (30). Likewise, the detection and isolation of bacterial OMVs may also assist with early diagnosis and prevention of bacterial infection. The advent of a DNA-aptamer shown to be highly sensitive to bacterial OMVs may allow this (135). Although many advances have been made to improve on diagnostic techniques, the use of EV cargo analysis requires further investigation. Advancements into EV isolation techniques, characterisation, detection, and safety under different pathological conditions is needed to further advance diagnostic outcomes.
Over the years, many advances have been made in better understanding the mechanisms underpinning the importance of EV packaging pathways. The subtle, yet important differences within biomolecule carriage and transfer mechanisms among traditional EV subtypes vs newly discovered EVs have highlighted. Their potential to be utilized in providing therapeutic benefit for many conditions. In brief, it is known exosomes, MVs and ApoBDs can contain and package specific cargo through protein-specific pathways. Recently emerging research has shown unique packaging mechanisms harnessed by OMVs, LOs and migrasomes related to their biogenesis. They can import cellular material through new avenues, providing new protein targets, insights, and avenues for researchers to utilize and ultimately advance EV-based research. In this review, we have discussed how these characteristics could be exploited therapeutically, in vaccine development, regenerative medicines, drug delivery and lastly within diagnostic studies. Furthermore, we have highlighted how various groups have harnessed these bioengineering aspects to enhance characteristics of EVs to provide more suitable treatment options for different pathologies. This includes drug loading and expressing viral proteins for vaccine development, among others. It is no doubt that each EV subtype discussed in this review could be utilized in treatments, as there is no “one-size-fits-all” option. Overall, additional insight into the content and packaging mechanisms of less studied EV subtypes may hold the key to advancing modern-EV based therapeutics and creating a diverse library of options available for the treatment of various pathophysiological conditions.
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
This work was funded by the National Health and Medical Research Council [GNT1125033, GNT1140187].
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem (1987) 262(19):9412–20.
2. Zargarian S, Shlomovitz I, Erlich Z, Hourizadeh A, Ofir-Birin Y, Croker BA, et al. Phosphatidylserine externalization, “necroptotic bodies” release, and phagocytosis during necroptosis. PloS Biol (2017) 15(6):e2002711. doi: 10.1371/journal.pbio.2002711
3. Minciacchi VR, You S, Spinelli C, Morley S, Zandian M, Aspuria PJ, et al. Large Oncosomes contain distinct protein cargo and represent a separate functional class of tumor-derived extracellular vesicles. Oncotarget (2015) 6(13):11327–41. doi: 10.18632/oncotarget.3598
4. Ma L, Li Y, Peng J, Wu D, Zhao X, Cui Y, et al. Discovery of the migrasome, an organelle mediating release of cytoplasmic contents during cell migration. Cell Res (2015) 25(1):24–38. doi: 10.1038/cr.2014.135
5. Jiao H, Jiang D, Hu X, Du W, Ji L, Yang Y, et al. Mitocytosis, a migrasome-mediated mitochondrial quality-control process. Cell (2021) 184(11):2896–910. doi: 10.1016/j.cell.2021.04.027
6. Schwechheimer C, Kuehn MJ. Outer-membrane vesicles from gram-negative bacteria: Biogenesis and functions. Nat Rev Microbiol (2015) 13:605–19. doi: 10.1038/nrmicro3525
7. Dawson CS, Garcia-Ceron D, Rajapaksha H, Faou P, Bleackley MR, Anderson MA. Protein markers for candida albicans EVs include claudin-like Sur7 family proteins. J Extracell Vesicles (2020) 9(1):1750810. doi: 10.1080/20013078.2020.1750810
8. Garcia-Ceron D, Bleackley MR, Anderson MA. Fungal extracellular vesicles in pathophysiology. Subcellular Biochem (2021) 97:151–77. doi: 10.1007/978-3-030-67171-6_7
9. Caruso S, Poon IKH. Apoptotic cell-derived extracellular vesicles: More than just debris. Front Immunol (2018) 9:1486. doi: 10.3389/fimmu.2018.01486
10. Wiklander OPB, Brennan M, Lötvall J, Breakefield XO, Andaloussi SEL. Advances in therapeutic applications of extracellular vesicles. Sci Trans Med (2019) 11:eaav8521. doi: 10.1126/scitranslmed.aav8521
11. Villa F, Quarto R, Tasso R. Extracellular vesicles as natural, safe and efficient drug delivery systems. Pharmaceutics (2019) 11(11):557. doi: 10.3390/pharmaceutics11110557
12. Phan TK, Ozkocak DC, Poon IKH. Unleashing the therapeutic potential of apoptotic bodies. Biochem Soc Trans (2020) 48(5):2079–2088. doi: 10.1042/BST20200225
13. Nishida-Aoki N, Tominaga N, Takeshita F, Sonoda H, Yoshioka Y, Ochiya T. Disruption of circulating extracellular vesicles as a novel therapeutic strategy against cancer metastasis. Mol Ther (2017) 25(1):181–91. doi: 10.1016/j.ymthe.2016.10.009
14. Catalano M, O’Driscoll L. Inhibiting extracellular vesicles formation and release: A review of EV inhibitors. J Extracellular Vesicles (2020) 9(1):1703244. doi: 10.1080/20013078.2019.1703244
15. Richards KE, Zeleniak AE, Fishel ML, Wu J, Littlepage LE, Hill R. Cancer-associated fibroblast exosomes regulate survival and proliferation of pancreatic cancer cells. Oncogene (2017) 36(13):1770–8. doi: 10.1038/onc.2016.353
16. Baek G, Choi H, Kim Y, Lee HC, Choi C. Mesenchymal stem cell-derived extracellular vesicles as therapeutics and as a drug delivery platform. Stem Cells Trans Med (2019) 8:880–6. doi: 10.1002/sctm.18-0226
17. Phan TK, Fonseka P, Tixeira R, Pathan M, Ang C, Ozkocak DC, et al. Pannexin-1 channel regulates nuclear content packaging into apoptotic bodies and their size. Proteomics (2021) 21(13–14):2000097. doi: 10.1002/pmic.202000097
18. Mendt M, Rezvani K, Shpall E. Mesenchymal stem cell-derived exosomes for clinical use. Vol 54 Bone Marrow Transplant (2019) p:789–92. doi: 10.1038/s41409-019-0616-z
19. Martins-Marques T, Pinho MJ, Zuzarte M, Oliveira C, Pereira P, Sluijter JP, et al. Presence of Cx43 in extracellular vesicles reduces the cardiotoxicity of the anti-tumour therapeutic approach with doxorubicin. J Extracell vesicles (2016) 5(1):32538. doi: 10.3402/jev.v5.32538
20. Janockova J, Slovinska L, Harvanova D, Spakova T, Rosocha J. New therapeutic approaches of mesenchymal stem cells-derived exosomes [Internet]. vol. 28, journal of biomedical science. BioMed Cent (2021), 28:1–26. doi: 10.1186/s12929-021-00736-4
21. Fang SB, Zhang HY, Wang C, He BX, Liu XQ, Meng XC, et al. Small extracellular vesicles derived from human mesenchymal stromal cells prevent group 2 innate lymphoid cell-dominant allergic airway inflammation through delivery of miR-146a-5p. J Extracell Vesicles (2020) 9(1):1723260. doi: 10.1080/20013078.2020.1723260
22. Choi SJ, Kim MH, Jeon J, Kim OY, Choi Y, Seo J, et al. Active immunization with extracellular vesicles derived from staphylococcus aureus effectively protects against staphylococcal lung infections, mainly via Th1 cell-mediated immunity. PloS One (2015) 10(9):e0136021. doi: 10.1371/journal.pone.0136021
23. Sicco CL, Reverberi D, Balbi C, Ulivi V, Principi E, Pascucci L, et al. Mesenchymal stem cell-derived extracellular vesicles as mediators of anti-inflammatory effects: Endorsement of macrophage polarization. Stem Cells Transl Med (2017) 6(3):1018–28. doi: 10.1002/sctm.16-0363%4010.1002/%28ISSN%292157-6580.SCTM_Best_Of_2018
24. Brock CK, Wallin ST, Ruiz OE, Samms KM, Mandal A, Sumner EA, et al. Stem cell proliferation is induced by apoptotic bodies from dying cells during epithelial tissue maintenance. Nat Commun (2019) 10(1):1–11. doi: 10.1038/s41467-019-09010-6
25. Smith VL, Cheng Y, Bryant BR, Schorey JS. Exosomes function in antigen presentation during an in vivo mycobacterium tuberculosis infection. Sci Rep (2017) 7(1):1–12. doi: 10.1038/srep43578
26. Huda MN, Nafiujjaman M, Deaguero IG, Okonkwo J, Hill ML, Kim T, et al. Potential use of exosomes as diagnostic biomarkers and in targeted drug delivery: Progress in clinical and preclinical applications. ACS Biomater Sci Eng (2021) 7(6):2106–49. doi: 10.1021/acsbiomaterials.1c00217
27. Park K-S, Svennerholm K, Crescitelli R, Lässer C, Gribonika I, Lötvall J. Synthetic bacterial vesicles combined with tumour extracellular vesicles as cancer immunotherapy. J Extracell Vesicles (2021) 10(9):e12120. doi: 10.1002/jev2.12120
28. Dou G, Tian R, Liu X, Yuan P, Ye Q, Liu J, et al. Chimeric apoptotic bodies functionalized with natural membrane and modular delivery system for inflammation modulation. Sci Adv (2020) 6(30):eaba2987. doi: 10.1126/sciadv.aba2987
29. Minciacchi VR, Spinelli C, Reis-Sobreiro M, Cavallini L, You S, Zandian M, et al. MYC mediates large oncosome-induced fibroblast reprogramming in prostate cancer. Cancer Res (2017) 77(9):2306–17. doi: 10.1158/0008-5472.CAN-16-2942
30. Karamchandani DM, Review RC. Apoptotic colopathy: A pragmatic approach to diagnosis. J Clin Pathol (2018) 71:1033–40. doi: 10.1136/jclinpath-2018-205388
31. Hong BS, Cho JH, Kim H, Choi EJ, Rho S, Kim J, et al. Colorectal cancer cell-derived microvesicles are enriched in cell cycle-related mRNAs that promote proliferation of endothelial cells. BMC Genomics (2009) 10(1):556. doi: 10.1186/1471-2164-10-556
32. Tutrone R, Donovan MJ, Torkler P, Tadigotla V, McLain T, Noerholm M, et al. Clinical utility of the exosome based ExoDx Prostate(IntelliScore) EPI test in men presenting for initial biopsy with a PSA 2–10 ng/mL. Prostate Cancer Prostatic Dis (2020) 23(4):607–14. doi: 10.1038/s41391-020-0237-z
33. Jiang L, Driedonks TAP, Jong WSP, Dhakal S, Bart van den Berg van Saparoea H, Sitaras I, et al. A bacterial extracellular vesicle-based intranasal vaccine against SARS-CoV-2 protects against disease and elicits neutralizing antibodies to wild-type and delta variants. J Extracell Vesicles (2022) 11(3):e12192. doi: 10.1002/jev2.12192
34. Rocha JLM, de Oliveira WCF, Noronha NC, dos Santos NCD, Covas DT, Picanço-Castro V, et al. Mesenchymal stromal cells in viral infections: Implications for COVID-19. Stem Cell Rev Rep (2021) 17(1):71–93. doi: 10.1007/s12015-020-10032-7
35. Xu X, Lai Y, Hua ZC. Apoptosis and apoptotic body: Disease message and therapeutic target potentials. Bioscience Rep (2019) 39(1):BSR20180992. doi: 10.1042/BSR20180992
36. Akers JC, Gonda D, Kim R, Carter BS, Chen CC. Iogenesis of extracellular vesicles (EV): Exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J Neuro-Oncol (2013) 113:1–11. doi: 10.1007/s11060-013-1084-8
37. Anand S, Samuel M, Kumar S, Mathivanan S. Ticket to a bubble ride: Cargo sorting into exosomes and extracellular vesicles. Biochim Biophys Acta - Proteins Proteomics (2019) 1867:140203. doi: 10.1016/j.bbapap.2019.02.005
38. Atukorala I, Mathivanan S. The role of post-translational modifications in targeting protein cargo to extracellular vesicles. Subcellular Biochem (2021), 97:45–60. doi: 10.1007/978-3-030-67171-6_3
39. Smith VL, Jackson L, Schorey JS. Ubiquitination as a mechanism to transport soluble mycobacterial and eukaryotic proteins to exosomes. J Immunol (2015) 195(6):2722–30. doi: 10.4049/jimmunol.1403186
40. Williams RL, Urbé S. The emerging shape of the ESCRT machinery. Nat Rev Mol Cell Biol (2007) 8:355–68. doi: 10.1038/nrm2162
41. Cheng Y, Schorey JS. Exosomes carrying mycobacterial antigens can protect mice against mycobacterium tuberculosis infection. Eur J Immunol (2013) 43(12):3279–90. doi: 10.1002/eji.201343727
42. Villarroya-Beltri C, Gutiérrez-Vázquez C, Sánchez-Cabo F, Pérez-Hernández D, Vázquez J, Martin-Cofreces N, et al. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat Commun (2013) 4(1):1–10. doi: 10.1038/ncomms3980
43. Kunadt M, Eckermann K, Stuendl A, Gong J, Russo B, Strauss K, et al. Extracellular vesicle sorting of α-synuclein is regulated by sumoylation. Acta Neuropathol (2015) 129(5):695–713. doi: 10.1007/s00401-015-1408-1
44. Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science (2008) 319(5867):1244–7. doi: 10.1126/science.1153124
45. Ferreira JV, Soares A da R, Ramalho J, Carvalho CM, Cardoso MH, Pintado P, et al. LAMP2A regulates the loading of proteins into exosomes. Sci Adv (2022) 8(12):1140. doi: 10.1126/sciadv.abm1140
46. Sedgwick AE, D’Souza-Schorey C. The biology of extracellular microvesicles. Traffic (2018) 19(5):319–27. doi: 10.1111/tra.12558
47. Agrahari V, Agrahari V, Burnouf PA, Chew CH, Burnouf T. Extracellular microvesicles as new industrial therapeutic frontiers. Trends Biotechnol (2019) 37:707–29. doi: 10.1016/j.tibtech.2018.11.012
48. Muralidharan-Chari V, Clancy J, Plou C, Romao M, Chavrier P, Raposo G, et al. ARF6-regulated shedding of tumor cell-derived plasma membrane microvesicles. Curr Biol (2009) 19(22):1875–85. doi: 10.1016/j.cub.2009.09.059
49. Wang T, Gilkes DM, Takano N, Xiang L, Luo W, Bishop CJ, et al. Hypoxia-inducible factors and RAB22A mediate formation of microvesicles that stimulate breast cancer invasion and metastasis. Proc Natl Acad Sci U.S.A. (2014) 111(31):E3234–42. doi: 10.1073/pnas.1410041111
50. Tricarico C, Clancy J, D’Souza-Schorey C. Biology and biogenesis of shed microvesicles. Small GTPases (2017) 8(4):220–32. doi: 10.1080/21541248.2016.1215283
51. Clancy JW, Zhang Y, Sheehan C, D’Souza-Schorey C. An ARF6–Exportin-5 axis delivers pre-miRNA cargo to tumour microvesicles. Nat Cell Biol (2019) 21(7):856–66. doi: 10.1038/s41556-019-0345-y
52. Clancy JW, Sedgwick A, Rosse C, Muralidharan-Chari V, Raposo G, Method M, et al. Regulated delivery of molecular cargo to invasive tumour-derived microvesicles. Nat Commun (2015) 6:6919. doi: 10.1038/ncomms7919
53. Lee H, Li C, Zhang Y, Zhang D, Otterbein LE, Jin Y. Caveolin-1 selectively regulates microRNA sorting into microvesicles after noxious stimuli. J Exp Med (2019) 216(9):2202–20. doi: 10.1084/jem.20182313
54. Atkin-Smith GK, Poon IKH. Disassembly of the dying: Mechanisms and functions. Trends Cell Biol (2017) 27:151–62. doi: 10.1016/j.tcb.2016.08.011
55. Poon IKH, Chiu YH, Armstrong AJ, Kinchen JM, Juncadella IJ, Bayliss DA, et al. Unexpected link between an antibiotic, pannexin channels and apoptosis. Nature (2014) 507(7492):329–34. doi: 10.1038/nature13147
56. Poon IKH, Lucas CD, Rossi AG, Ravichandran KS. Apoptotic cell clearance: Basic biology and therapeutic potential. Nat Rev Immunol (2014) 14:166–80. doi: 10.1038/nri3607
57. Tixeira R, Phan TK, Caruso S, Shi B, Atkin-Smith GK, Nedeva C, et al. ROCK1 but not LIMK1 or PAK2 is a key regulator of apoptotic membrane blebbing and cell disassembly. Cell Death Differ (2020) 27(1):102–16. doi: 10.1038/s41418-019-0342-5
58. Atkin-Smith GK, Tixeira R, Paone S, Mathivanan S, Collins C, Liem M, et al. A novel mechanism of generating extracellular vesicles during apoptosis via a beads-on-a-string membrane structure. Nat Commun (2015) 6(1):7439. doi: 10.1038/ncomms8439
59. Atkin-Smith GK, Miles MA, Tixeira R, Lay FT, Duan M, Hawkins CJ, et al. Plexin B2 is a regulator of monocyte apoptotic cell disassembly. Cell Rep (2019) 29(7):1821–1831.e3. doi: 10.1016/j.celrep.2019.10.014
60. Wickman GR, Julian L, Mardilovich K, Schumacher S, Munro J, Rath N, et al. Blebs produced by actin-myosin contraction during apoptosis release damage-associated molecular pattern proteins before secondary necrosis occurs. Cell Death Differ (2013) 20(10):1293–305. doi: 10.1038/cdd.2013.69
61. Panaretakis T, Joza N, Modjtahedi N, Tesniere A, Vitale I, Durchschlag M, et al. The co-translocation of ERp57 and calreticulin determines the immunogenicity of cell death. Cell Death Differ (2008) 15(9):1499–509. doi: 10.1038/cdd.2008.67
62. Lane JD, Allan VJ, Woodman PG. Active relocation of chromatin and endoplasmic reticulum into blebs in late apoptotic cells. J Cell Sci (2005) 118(17):4059–71. doi: 10.1242/jcs.02529
63. Jiang L, Paone S, Caruso S, Atkin-Smith GK, Phan TK, Hulett MD, et al. Determining the contents and cell origins of apoptotic bodies by flow cytometry. Sci Rep (2017) 7(1):14444. doi: 10.1038/s41598-017-14305-z
64. Schiller M, Bekeredjian-Ding I, Heyder P, Blank N, Ho AD, Lorenz HM. Autoantigens are translocated into small apoptotic bodies during early stages of apoptosis. Cell Death Differ (2008) 15(1):183–91. doi: 10.1038/sj.cdd.4402239
65. Janko C, Schorn C, Grossmayer GE, Frey B, Herrmann M, Gaipl US, et al. Inflammatory clearance of apoptotic remnants in systemic lupus erythematosus (SLE). Autoimmun Rev (2008) 8:9–12. doi: 10.1016/j.autrev.2008.07.015
66. Zirngibl M, Fürnrohr BG, Janko C, Munoz LE, Voll RE, Gregory CD, et al. Loading of nuclear autoantigens prototypically recognized by systemic lupus erythematosus sera into late apoptotic vesicles requires intact microtubules and myosin light chain kinase activity. Clin Exp Immunol (2015) 179(1):39–49. doi: 10.1111/cei.12342
67. Holmgren L, Szeles A, Rajnavölgyi E, Folkman J, Klein G, Ernberg I, et al. Horizontal transfer of DNA by the uptake of apoptotic bodies. Blood (1999) 93(11):3956–63. doi: 10.1182/blood.V93.11.3956.411k05_3956_3963
68. Bergsmedh A, Szeles A, Henriksson M, Bratt A, Folkman MJ, Spetz AL, et al. Horizontal transfer of oncogenes by uptake of apoptotic bodies. Proc Natl Acad Sci U S A (2001) 98(11):6407–11. doi: 10.1073/pnas.101129998
69. Poon IKH, Parkes MAF, Jiang L, Atkin-Smith GK, Tixeira R, Gregory CD, et al. Moving beyond size and phosphatidylserine exposure: Evidence for a diversity of apoptotic cell-derived extracellular vesicles in vitro. J Extracell Vesicles (2019) 8(1):1608786. doi: 10.1080/20013078.2019.1608786
70. Walker A, Ward C, Sheldrake TA, Dransfield I, Rossi AG, Pryde JG, et al. Golgi fragmentation during fas-mediated apoptosis is associated with the rapid loss of GM130. Biochem Biophys Res Commun (2004) 316(1):6–11. doi: 10.1016/j.bbrc.2004.02.015
71. Mancini M, Machamer CE, Roy S, Nicholson DW, Thornberry NA, Casciola-Rosen LA, et al. Caspase-2 is localized at the golgi complex and cleaves golgin-160 during apoptosis. J Cell Biol (2000) 149(3):603–12. doi: 10.1083/jcb.149.3.603
72. Atkin-Smith GK, Duan M, Zanker DJ, Loh L, Nguyen THO, Koutsakos M, et al. Monocyte apoptotic bodies are vehicles for influenza a virus propagation. Commun Biol (2020) 3(1):1–14. doi: 10.1038/s42003-020-0955-8
73. Migneault F, Dieudé M, Turgeon J, Beillevaire D, Hardy MP, Brodeur A, et al. Apoptotic exosome-like vesicles regulate endothelial gene expression, inflammatory signaling, and function through the NF-κB signaling pathway. Sci Rep (2020) 10(1):12562. doi: 10.1038/s41598-020-69548-0
74. Ainola M, Porola P, Takakubo Y, Przybyla B, Kouri VP, Tolvanen TA, et al. Activation of plasmacytoid dendritic cells by apoptotic particles - mechanism for the loss of immunological tolerance in sjögren’s syndrome. Clin Exp Immunol (2018) 191(3):301–10. doi: 10.1111/cei.13077
75. Dieudé M, Bell C, Turgeon J, Beillevaire D, Pomerleau L, Yang B, et al. The 20S proteasome core, active within apoptotic exosome-like vesicles, induces autoantibody production and accelerates rejection. Sci Transl Med (2015) 7(318):318ra200. doi: 10.1126/scitranslmed.aac9816
76. Zheng C, Sui B, Zhang X, Hu J, Chen J, Liu J, et al. Apoptotic vesicles restore liver macrophage homeostasis to counteract type 2 diabetes. J Extracell Vesicles (2021) 10(7):e12109. doi: 10.1002/jev2.12109
77. Ou Q, Tan L, Shao Y, Lei F, Huang W, Yang N, et al. Electrostatic charge-mediated apoptotic vesicle biodistribution attenuates sepsis by switching neutrophil NETosis to apoptosis. Small (2022), 18:2200306. doi: 10.1002/smll.202200306
78. Baxter AA, Phan TK, Hanssen E, Liem M, Hulett MD, Mathivanan S, et al. Analysis of extracellular vesicles generated from monocytes under conditions of lytic cell death. Sci Rep (2019) 9(1):1–13. doi: 10.1038/s41598-019-44021-9
79. Sarkar A, Mitra S, Mehta S, Raices R, Wewers MD. Monocyte derived microvesicles deliver a cell death message via encapsulated caspase-1. PloS One (2009) 4(9):7140. doi: 10.1371/journal.pone.0007140
80. Zhang Y, Liu F, Yuan Y, Jin C, Chang C, Zhu Y, et al. Inflammasome-derived exosomes activate NF-κB signaling in macrophages. J Proteome Res (2017) 16(1):170–8. doi: 10.1021/acs.jproteome.6b00599
81. Pathirana RD, Kaparakis-Liaskos M. Bacterial membrane vesicles: Biogenesis, immune regulation and pathogenesis. Cell Microbiol (2016) 18:1518–24. doi: 10.1111/cmi.12658
82. Haurat MF, Aduse-Opoku J, Rangarajan M, Dorobantu L, Gray MR, Curtis MA, et al. Selective sorting of cargo proteins into bacterial membrane vesicles. J Biol Chem (2011) 286(2):1269–76. doi: 10.1074/jbc.M110.185744
83. Bitto NJ, Chapman R, Pidot S, Costin A, Lo C, Choi J, et al. Bacterial membrane vesicles transport their DNA cargo into host cells. Sci Rep (2017) 7(1):1–11. doi: 10.1038/s41598-017-07288-4
84. Kaparakis-Liaskos M, Ferrero RL. Immune modulation by bacterial outer membrane vesicles. Nat Rev Immunol (2015) 15:375–87. doi: 10.1038/nri3837
85. Elhenawy W, Debelyy MO, Feldman MF. Preferential packing of acidic glycosidases and proteases into bacteroides outer membrane vesicles. MBio (2014) 5(2):e00909–14. doi: 10.1128/mBio.00909-14
86. Olofsson A, Vallström A, Petzold K, Tegtmeyer N, Schleucher J, Carlsson S, et al. Biochemical and functional characterization of helicobacter pylori vesicles. Mol Microbiol (2010) 77(6):1539–55. doi: 10.1111/j.1365-2958.2010.07307.x
87. Lappann M, Otto A, Becher D, Vogel U. Comparative proteome analysis of spontaneous outer membrane vesicles and purified outer membranes of neisseria meningitidis. J Bacteriol (2013) 195(19):4425–35. doi: 10.1128/JB.00625-13
88. Jiang D, Jiang Z, Lu D, Wang X, Liang H, Zhang J, et al. Migrasomes provide regional cues for organ morphogenesis during zebrafish gastrulation. Nat Cell Biol (2019) 21(8):966–77. doi: 10.1038/s41556-019-0358-6
89. Wu D, Xu Y, DIng T, Zu Y, Yang C, Yu L. Pairing of integrins with ECM proteins determines migrasome formation. Cell Res (2017) 27:1397–400. doi: 10.1038/cr.2017.108
90. Huang Y, Zucker B, Zhang S, Elias S, Zhu Y, Chen H, et al. Migrasome formation is mediated by assembly of micron-scale tetraspanin macrodomains. Nat Cell Biol (2019) 21(8):991–1002. doi: 10.1038/s41556-019-0367-5
91. Lu P, Liu R, Lu D, Xu Y, Yang X, Jiang Z, et al. Chemical screening identifies ROCK1 as a regulator of migrasome formation. Cell Discovery (2020) 6(1):1–4. doi: 10.1038/s41421-020-0179-6
92. Jiao H, Jiang D, Hu X, Du W, Ji L, Yang Y, et al. Mitocytosis, a migrasome-mediated mitochondrial quality-control process. Cell (2021) 184(11):2896–2910.e13. doi: 10.1016/j.cell.2021.04.027
93. Zhao X, Lei Y, Zheng J, Peng J, Li Y, Yu L, et al. Identification of markers for migrasome detection. Cell Discovery (2019) 5(1):27. doi: 10.1038/s41421-019-0093-y
94. Vagner T, Spinelli C, Minciacchi VR, Balaj L, Zandian M, Conley A, et al. Large Extracellular vesicles carry most of the tumour DNA circulating in prostate cancer patient plasma. J Extracell Vesicles (2018) 7(1). doi: 10.1080/20013078.2018.1505403
95. Di Vizio D, Kim J, Hager MH, Morello M, Yang W, Lafargue CJ, et al. Oncosome formation in prostate cancer: Association with a region of frequent chromosomal deletion in metastatic disease. Cancer Res (2009) 69(13):5601–9. doi: 10.1158/0008-5472.CAN-08-3860
96. Hager MH, Morley S, Bielenberg DR, Gao S, Morello M, Holcomb IN, et al. DIAPH3 governs the cellular transition to the amoeboid tumour phenotype. EMBO Mol Med (2012) 4(8):743–60. doi: 10.1002/emmm.201200242
97. Di Vizio D, Morello M, Dudley AC, Schow PW, Adam RM, Morley S, et al. Large Oncosomes in human prostate cancer tissues and in the circulation of mice with metastatic disease. Am J Pathol (2012) 181(5):1573–84. doi: 10.1016/j.ajpath.2012.07.030
98. Morello M, Minciacchi VR, De Candia P, Yang J, Posadas E, Kim H, et al. Large Oncosomes mediate intercellular transfer of functional microRNA. Cell Cycle (2013) 12(22):3526–36. doi: 10.4161/cc.26539
99. Aline F, Bout D, Amigorena S, Roingeard P, Dimier-Poisson I. Toxoplasma gondii antigen-pulsed-dendritic cell-derived exosomes induce a protective immune response against t. gondii infection. Infect Immun (2004) 72(7):4127–37. doi: 10.1128/IAI.72.7.4127-4137.2004
100. Buschow SI, Nolte-’t Hoen ENM, van Niel G, Pols MS, ten Broeke T, Lauwen M, et al. MHC II in dendritic cells is targeted to lysosomes or T cell-induced exosomes via distinct multivesicular body pathways. Traffic (2009) 10(10):1528–42. doi: 10.1111/j.1600-0854.2009.00963.x
101. Henry F, Boisteau O, Bretaudeau L, Lieubeau B, Meflah K, Grégoire M. Antigen-presenting cells that phagocytose apoptotic tumor-derived cells are potent tumor vaccines. Cancer Res (1999) 59(14):3329–32.
102. Kokhaei P, Rezvany MR, Virving L, Choudhury A, Rabbani H, Österborg A, et al. Dendritic cells loaded with apoptotic tumour cells induce a stronger T-cell response than dendritic cell-tumour hybrids in b-CLL. Leukemia (2003) 17(5):894–9. doi: 10.1038/sj.leu.2402913
103. Rappazzo CG, Watkins HC, Guarino CM, Chau A, Lopez JL, DeLisa MP, et al. Recombinant M2e outer membrane vesicle vaccines protect against lethal influenza a challenge in BALB/c mice. Vaccine (2016) 34(10):1252–8. doi: 10.1016/j.vaccine.2016.01.028
104. Shehata MM, Mostafa A, Teubner L, Mahmoud SH, Kandeil A, Elshesheny R, et al. Bacterial outer membrane vesicles (OMVs)-based dual vaccine for influenza a H1N1 virus and MERS-CoV. Vaccines (2019) 7(2):46. doi: 10.3390/vaccines7020046
105. Perrett KP, McVernon J, Richmond PC, Marshall H, Nissen M, August A, et al. Immune responses to a recombinant, four-component, meningococcal serogroup b vaccine (4CMenB) in adolescents: A phase III, randomized, multicentre, lot-to-lot consistency study. Vaccine (2015) 33(39):5217–24. doi: 10.1016/j.vaccine.2015.06.103
106. Rani S, Ryan AE, Griffin MD, Ritter T. Mesenchymal stem cell-derived extracellular vesicles: Toward cell-free therapeutic applications. Mol Ther (2015) 23:812–23. doi: 10.1038/mt.2015.44
107. Zhang B, Wang M, Gong A, Zhang X, Wu X, Zhu Y, et al. HucMSc-exosome mediated-Wnt4 signaling is required for cutaneous wound healing. Stem Cells (2015) 33(7):2158–68. doi: 10.1002/stem.1771
108. Liu D, Kou X, Chen C, Liu S, Liu Y, Yu W, et al. Circulating apoptotic bodies maintain mesenchymal stem cell homeostasis and ameliorate osteopenia via transferring multiple cellular factors. Cell Res (2018) 28(9):918–33. doi: 10.1038/s41422-018-0070-2
109. Liu H, Liu S, Qiu X, Yang X, Bao L, Pu F, et al. Donor MSCs release apoptotic bodies to improve myocardial infarction via autophagy regulation in recipient cells. Autophagy (2020) 16(12):2140–55. doi: 10.1080/15548627.2020.1717128
110. Kogianni G, Mann V, Noble BS. Apoptotic bodies convey activity capable of initiating osteoclastogenesis and localized bone destruction. J Bone Miner Res (2008) 23(6):915–27. doi: 10.1359/jbmr.080207
111. Ma Q, Liang M, Wu Y, Ding N, Duan L, Yu T, et al. Mature osteoclast- derived apoptotic bodies promote osteogenic differentiation via RANKL-mediated reverse signaling. J Biol Chem (2019) 294(29):11240–7. doi: 10.1074/jbc.RA119.007625
112. Orozco SL, Daniels BP, Yatim N, Messmer MN, Quarato G, Chen-Harris H, et al. RIPK3 activation leads to cytokine synthesis that continues after loss of cell membrane integrity. Cell Rep (2019) 28(9):2275–87. doi: 10.1016/j.celrep.2019.07.077
113. Munagala R, Aqil F, Jeyabalan J, Gupta RC. Bovine milk-derived exosomes for drug delivery. Cancer Lett (2016) 371(1):48–61. doi: 10.1016/j.canlet.2015.10.020
114. Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJA. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol (2011) 29(4):341–5. doi: 10.1038/nbt.1807
115. Saari H, Lázaro-Ibáñez E, Viitala T, Vuorimaa-Laukkanen E, Siljander P, Yliperttula M. Microvesicle- and exosome-mediated drug delivery enhances the cytotoxicity of paclitaxel in autologous prostate cancer cells. J Control Release (2015) 220:727–37. doi: 10.1016/j.jconrel.2015.09.031
116. Kim MS, Haney MJ, Zhao Y, Mahajan V, Deygen I, Klyachko NL, et al. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomed Nanotechnol Biol Med (2016) 12(3):655–64. doi: 10.1016/j.nano.2015.10.012
117. Ma J, Zhang Y, Tang K, Zhang H, Yin X, Li Y, et al. Reversing drug resistance of soft tumor-repopulating cells by tumor cell-derived chemotherapeutic microparticles. Cell Res (2016) 26(6):713–27. doi: 10.1038/cr.2016.53
118. Gao Y, Zhang H, Zhou N, Xu P, Wang J, Gao Y, et al. Methotrexate-loaded tumour-cell-derived microvesicles can relieve biliary obstruction in patients with extrahepatic cholangiocarcinoma. Nat BioMed Eng (2020), 4:1–11. doi: 10.1038/s41551-020-0583-0
119. Weir MA, Walsh M, Cuerden MS, Sontrop JM, Chambers LC, Garg AX. Micro-particle curcumin for the treatment of chronic kidney disease-1: Study protocol for a multicenter clinical trial. Can J Kidney Heal Dis (2018) 5:2054358118813088. doi: 10.1177/2054358118813088
120. Wu K, Xing F, Wu SY, Watabe K. Extracellular vesicles as emerging targets in cancer: Recent development from bench to bedside. Biochim Biophys Acta Rev Cancer (2017) 1868(2):538–63. doi: 10.1016/j.bbcan.2017.10.001
121. Zernecke A, Bidzhekov K, Noels H, Shagdarsuren E, Gan L, Denecke B, et al. Delivery of microRNA-126 by apoptotic bodies induces CXCL12-dependent vascular protection. Sci Signal (2009) 2(100):ra81. doi: 10.1126/scisignal.2000610
122. Zhao D, Tao W, Li S, Chen Y, Sun Y, He Z, et al. Apoptotic body–mediated intercellular delivery for enhanced drug penetration and whole tumor destruction. Sci Adv (2021) 7(16):eabg0880. doi: 10.1126/sciadv.abg0880
123. Tang K, Zhang Y, Zhang H, Xu P, Liu J, Ma J, et al. Delivery of chemotherapeutic drugs in tumour cell-derived microparticles. Nat Commun (2012) 3(1):1–11. doi: 10.1038/ncomms2282
124. Barceló M, Castells M, Bassas L, Vigués F, Larriba S. Semen miRNAs contained in exosomes as non-invasive biomarkers for prostate cancer diagnosis. Sci Rep (2019) 9(1):1–16. doi: 10.1038/s41598-019-50172-6
125. Nilsson J, Skog J, Nordstrand A, Baranov V, Mincheva-Nilsson L, Breakefield XO, et al. Prostate cancer-derived urine exosomes: A novel approach to biomarkers for prostate cancer. Br J Cancer (2009) 100(10):1603–7. doi: 10.1038/sj.bjc.6605058
126. Chen Y, Li G, Liu ML. Microvesicles as emerging biomarkers and therapeutic targets in cardiometabolic diseases. Genomics Proteomics Bioinf (2018) 16:50–62. doi: 10.1016/j.gpb.2017.03.006
127. Yu H, Sun T, An J, Wen L, Liu F, Bu Z, et al. Potential roles of exosomes in parkinson’s disease: From pathogenesis, diagnosis, and treatment to prognosis [Internet]. vol. 8, frontiers in cell and developmental biology. Front Media S.A (2020), 86. doi: 10.3389/fcell.2020.00086
128. Ko J, Hemphill M, Yang Z, Beard K, Sewell E, Shallcross J, et al. Multi-dimensional mapping of brain-derived extracellular vesicle MicroRNA biomarker for traumatic brain injury diagnostics. J Neurotrauma (2020) 37(22):2424–34. doi: 10.1089/neu.2018.6220
129. Jayaseelan VP. Emerging role of exosomes as promising diagnostic tool for cancer. Cancer Gene Ther (2020) 27:395–8. doi: 10.1038/s41417-019-0136-4
130. Taylor DD, Gercel-Taylor C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol Oncol (2008) 110(1):13–21. doi: 10.1016/j.ygyno.2008.04.033
131. Suwakulsiri W, Rai A, Xu R, Chen M, Greening DW, Simpson RJ. Proteomic profiling reveals key cancer progression modulators in shed microvesicles released from isogenic human primary and metastatic colorectal cancer cell lines. Biochim Biophys Acta - Proteins Proteomics (2019) 1867(12):140171. doi: 10.1016/j.bbapap.2018.11.008
132. Kreger BT, Dougherty AL, Greene KS, Cerione RA, Antonyak MA. Microvesicle cargo and function changes upon induction of cellular transformation. J Biol Chem (2016) 291(38):19774–85. doi: 10.1074/jbc.M116.725705
133. Spitzer P, Mulzer LM, Oberstein TJ, Munoz LE, Lewczuk P, Kornhuber J, et al. Microvesicles from cerebrospinal fluid of patients with alzheimer’s disease display reduced concentrations of tau and APP protein. Sci Rep (2019) 9(1):1–10. doi: 10.1038/s41598-019-43607-7
134. Pavlyukov MS, Yu H, Bastola S, Minata M, Shender VO, Lee Y, et al. Apoptotic cell-derived extracellular vesicles promote malignancy of glioblastoma Via intercellular transfer of splicing factors. Cancer Cell (2018) 34(1):119–135.e10. doi: 10.1016/j.ccell.2018.05.012
Keywords: extracellular vesicles, cargo packaging, EV therapies, drug delivery, EV biogenesis
Citation: Ozkocak DC, Phan TK and Poon IKH (2022) Translating extracellular vesicle packaging into therapeutic applications. Front. Immunol. 13:946422. doi: 10.3389/fimmu.2022.946422
Received: 17 May 2022; Accepted: 18 July 2022;
Published: 15 August 2022.
Edited by:
Bin Gong, University of Texas Medical Branch at Galveston, United StatesReviewed by:
Alin Rai, Baker Heart and Diabetes Institute, AustraliaCopyright © 2022 Ozkocak, Phan and Poon. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Thanh Kha Phan, dGhhbmgucGhhbkBsYXRyb2JlLmVkdS5hdQ==; Ivan K. H. Poon, aS5wb29uQGxhdHJvYmUuZWR1LmF1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.