
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Immunol. , 15 September 2022
Sec. Cancer Immunity and Immunotherapy
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.944013
 Qian Xing1,2
Qian Xing1,2 Zhongwei Zhang1,2*
Zhongwei Zhang1,2* Biao Zhu1,2*
Biao Zhu1,2* Qionghua Lin1,2
Qionghua Lin1,2 Lihua Shen1,2
Lihua Shen1,2 Fangfang Li1,2
Fangfang Li1,2 Zhili Xia1,2
Zhili Xia1,2 Zhiyong Zhao1,2
Zhiyong Zhao1,2Introduction: Immune therapy has ushered in a new era of tumor treatment, at the expense of immune-related adverse events, including rare but fatal adverse cardiovascular events, such as myocarditis. Steroids remain the cornerstone of therapy for immune-related myocarditis, with no clear consensus on additional immunosuppressive treatment for steroid-refractory cases yet.
Case report: Here, we report a patient with stage IV nasopharyngeal carcinoma who developed immune-related myocarditis in the fourth course of therapy with immune checkpoint inhibitors. The patient presented with precordial discomfort with elevation of cardiac enzymes and interleukin-6, atypical electrocardiographic abnormalities, and reduced left ventricular ejection fraction. Coronary computed tomography angiography excluded the possibility of acute coronary syndrome. The therapy with tofacitinib targeting the Janus kinase-signal transducer and activator of transcription signal pathway was successfully conducted, since there was no significant improvement in troponin under high-dose steroid and intravenous immunoglobulin treatment. The patient recovered without major adverse cardiac events during hospitalization.
Discussion: The safety and efficacy of tofacitinib in a patient with steroid-refractory immune-related myocarditis were investigated, hoping to provide a basis for prospective therapeutic strategies. Tofacitinib led to remarkable remissions in primary autoimmune disease by blocking the inflammatory cascade, indicating its potential therapeutic use in immune-related adverse events.
Immune checkpoint inhibitors (ICIs) potentiate T cell cytotoxicity against tumor cells through targeting programmed cell death 1 (PD-1), programmed cell death-ligand 1, or cytotoxic T-lymphocyte-associated protein 4, preventing the escape of tumor cells from the immune system. ICIs have revolutionized cancer treatment over the past decade, although at the expense of immune-related adverse events (irAEs), which are clinically detected in 70–90% of patients on ICIs (1). Meanwhile, high grades of irAEs are detected in 10–15% of patients, among which, cardiovascular adverse events could manifest as arrhythmias, pericardial disease, vasculitis, Takotsubo-like syndrome, dyslipidemia and, most frequently, myocarditis with a fatality rate over 50% (1–4). Here, we review the treatment of a patient with nasopharyngeal carcinoma, who presented with precordial discomfort with an elevation of cardiac enzymes who was hospitalized with immune-related myocarditis and treated with tofacitinib. We hope that this report provides a basis for prospective therapeutic strategies for a new option for treatment with tofacitinib, in the absence of clinical trials, and to improve the prognosis of immune-related myocarditis. Written informed consent was obtained from the patient in this report.
A 67-year-old male was admitted to hospital in January 2021 with a new onset mass in the right neck. Magnetic resonance imaging indicated a thickening and enhancement of the nasopharyngeal wall, as well as multiple cervical lymphadenopathies. A nasopharyngoscopy revealed a neoplasm on the top left wall of the nasopharynx. Pathological findings of the mass confirmed non-keratinizing squamous cell carcinoma. Multiple bone metastases were detected by radionuclide bone scans. As a result, he was diagnosed with nasopharyngeal carcinoma (cT4N3M1).
He then participated in a clinical trial for metastatic nasopharyngeal carcinoma at initial diagnosis investigating the efficacy and safety of a PD-1 inhibitor (toripalimab) combined with gemcitabine and cisplatin (toripalimab 240 mg on day 1 + gemcitabine 1 g/m2 on days 1 and 8 + cisplatin 50 mg on days 1–3). There were no adverse reactions in the first three courses of treatment. However, he complained of precordial discomfort on the sixth day of the fourth course, in the absence of any history or heart disease. Blood results showed increased high-sensitivity cardiac troponin (hs-cTn) up to 0.553 (normal 0.013–0.025) ng/ml, creatine kinase-MB (CK-MB) up to 176 (normal 0–2.88) ng/ml, serum myoglobin up to 405 (normal 28–72) ng/ml, pro-brain natriuretic peptide (proBNP) up to 205 (normal 0–14.8) pmol/L, and interleukin-6 (IL-6) up to 17.41 (normal 0–5.4) pg/ml. While the value of IL-10 and interferon (IFN)–γ were, respectively, 17.2 and 26 pg/ml, slightly higher than the normal range.
He was then transferred to ICU. QT prolongation, horizontal ST segment depression (0.5–1 mm) of V5 and V6 leads, and T wave inversion in I, AVL, II, III, AVF, V5, and V6 leads were observed following a dynamic electrocardiogram. A bedside echocardiogram suggested the left ventricular ejection fraction (LVEF) decreased to 40%. A coronary computed tomography angiography showed that the proximal occlusion in left anterior descending, right coronary artery, and left circumflex branch was 70, 90, and 90%, respectively, with collateral formation and distal development, indicating the diagnosis of chronic and already compensatory coronary heart disease. Therefore, the new onset of chest symptoms and laboratory abnormalities were inferred as immune-related myocarditis and the patient was excluded from the clinical trial. He was quite depressed about it.
We immediately continued immunosuppressive therapy with methylprednisolone 4 mg/kg per day intravenously, as well as anti-platelet therapy with aspirin and anti-atherosclerosis therapy with atorvastatin. However, there was no improvement with his clinical performance; indeed, we noted an aggravation in the value of the myocardial zymogram. Consequently, intravenous immunoglobulin (IVIG, 0.4g/kg per day for 5 days) and a higher dosage of methylprednisolone (500 mg for 3 days, followed by a step-by-step dose reduction) were administered on the second day in the ICU, combined with trimetazidine and coenzyme Q10 to nourish cardiac muscle. There was a subsequent decline in the value of the myocardial zymogram as his symptoms improved.
However, this was short lived; the value of hs-cTn elevated on the sixth day since admission to ICU. Considering the aggravated laboratory parameters, he was treated with tofacitinib (5 mg twice daily for a week) for intensive immunosuppression. The level of hs-cTn, CK-MB, IL-6, IL-10, and IFN-γ gradually decreased to nearly normal a month after admission. Variation in lab tests and treatment strategies are demonstrated in Figure 1. Regular cardiac assessment by electrocardiogram and echocardiogram showed few changes over time. The patient felt much better recovering without a major adverse cardiac event (MACE), and his confidence to fight the tumor was restored. Unfortunately, the enlarging neck mass indicated tumor progression and he was transferred to the oncology department for further anti-tumor therapy.
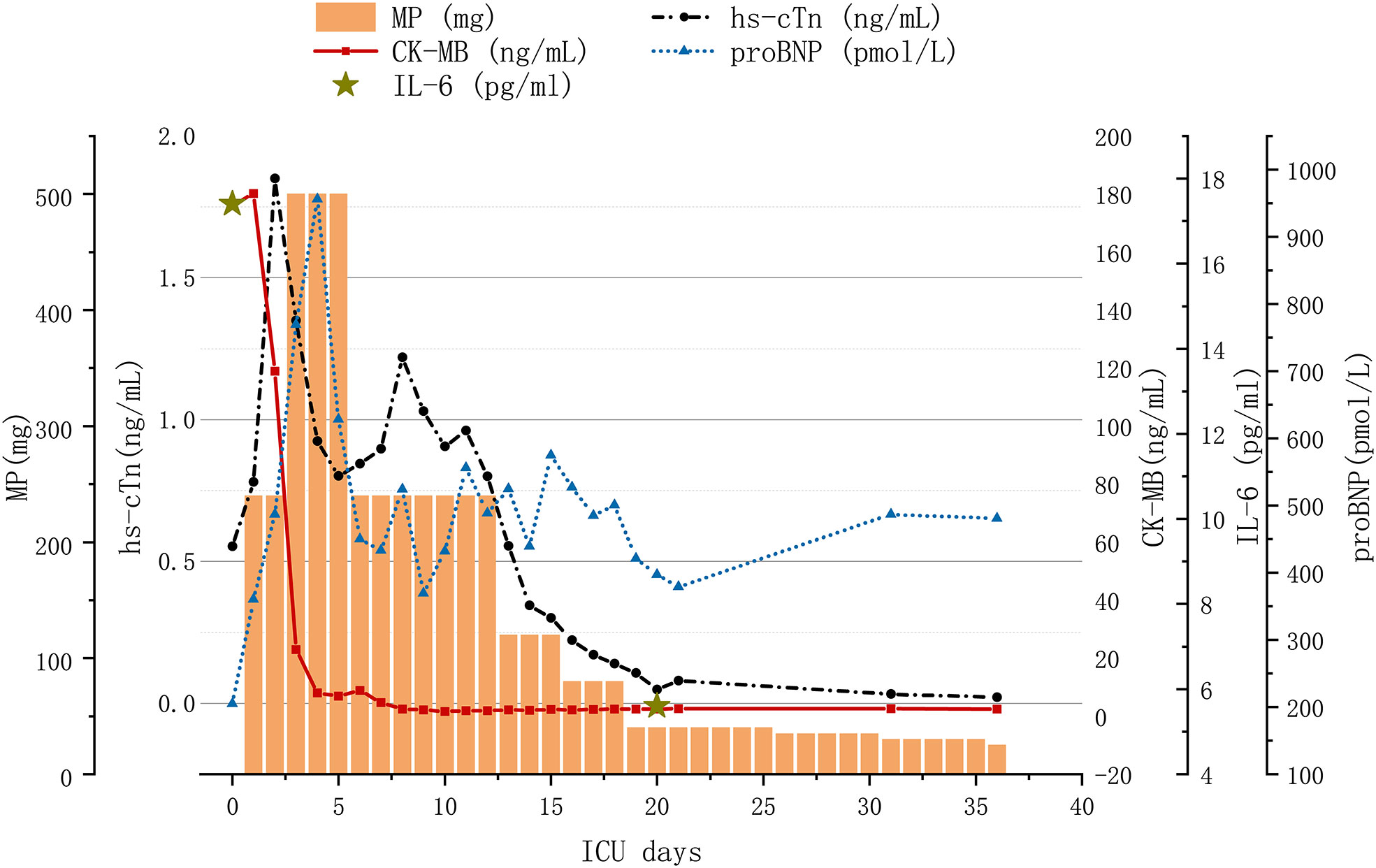
Figure 1 Variation in lab tests and treatment strategy with immune-related myocarditis. MP, methylprednisolone; hs-cTN, high-sensitivity cardiac troponin; CK-MB, creatine kinase MB Form; proBNP, pro-brain natriuretic peptide; IL-6, interleukin-6; IVIG, intravenous immunoglobulin.
Immune-related myocarditis often occurs in men (71%); studies show that its prevalence was only 1.14% with a mean onset age of 65 ± 13 years and a median onset time of 34 days after the initiation of ICIs (3). The ensuing myocarditis, 3 months later, exhibited significantly worse heart failure with left ventricular systolic dysfunction compared to the relatively early cases happening within the first three months (5). Consistent data suggest that patients receiving combination ICIs or with pre-existing cardiovascular disease had an increased incidence of myocarditis, whereas diabetes mellitus and obesity were unclear as risk factors (1). Patients with immune-related myocarditis could present from asymptomatic to acute respiratory or cardiac syndrome; 46% of the cases experienced MACE, including ventricular arrythmias, complete heart block, cardiogenic shock, cardiac arrest, and cardiovascular death, leading to high mortality (6, 7). In addition, clinicians should be alerted that complicated neuromuscular irAEs could occur with immune-related myocarditis, since there are increasing reports of the triple M overlap syndrome of myositis-myocarditis-myasthenia gravis (8–10). Consequently, a prompt and accurate diagnosis is critical for initiating treatment.
It is reported that nearly all cases with myocarditis demonstrated an elevated troponin (94%) and abnormal electrocardiogram (89%), whereas 66% had an increased proBNP and 51% presented with preserved LVEF on their echocardiograms (3). Higher troponin, CK, and CK-MB levels were associated with increased mortality, whereas the presence of prolonged QRS duration trended toward increased the risk of subsequent MACE (11, 12). Compared with LVEF, which was also measured by echocardiogram, a lower global longitudinal strain was strongly associated with the incidence of MACE in myocarditis patients (13, 14). Retrospective studies suggested that the application of T1 mapping with cardiac magnetic resonance can provide diagnostic value of immune-related myocarditis and predict the risk of MACE (15, 16). In addition, 68 Ga-FAPI PET/CT, reduced absolute lymphocyte count and increased neutrophil/lymphocyte ratio were also identified as new methods to detect immune-related myocarditis (17, 18). As the gold-standard test, endomyocardial biopsy indicated infiltration with T lymphocytes and macrophages among immune-related myocarditis cases, although rarely pursued for its invasive nature and potential complications (19, 20). In addition, the application of novel techniques, such as single-cell RNA sequencing and the gene knockout mice model, may allow better characterization of infiltrating immune cells (21, 22). On the basis of these tests, this patient was definitively diagnosed with myocarditis under the criteria by Bonaca (23).
The precise mechanisms of immune-related myocarditis still remain unclear. Suggested possibilities include the inhibition of the cardio-protective PD-1 and PD-L1 pathways by hyperactivated cytotoxic T cells in myocardiocytes, shared antigens between the tumor and the myocardium (24). Prompt initiation of steroids are recommended as first line therapy by the latest global guidelines, lacking consensus on the initial dosing or tapering protocols (25). Intensified immunosuppressive therapy (IIST), including abatacept, mycophenolate mofetil, IVIG, alemtuzumab, infliximab, and anti-thymocyte globulin, should be added as soon as evolution is unfavorable within 24 h on steroids especially when hemodynamic/electrical instability or neuromuscular adverse events occur (25–27). However, recommendations from current guidelines for IIST were adapted from the experience of primary autoimmune diseases, without taking into consideration the personalized treatments under specific immunohistopathological findings of affected organs (28).
Except for the IIST recommended by current guidelines, Janus kinase (JAK) inhibitors and IL-6 receptor inhibitors have attracted much attention with their performance on irAEs. As the first generation of pan-JAK inhibitors, tofacitinib primarily suppressed JAK1/JAK3, disturbed the JAK–signal transducer and activator of transcription (STAT) pathway downstream of inflammatory cytokine receptors, and blocked most inflammatory cytokines, including IL-6 and the subsequent inflammatory storm (Figure 2) (29–31). IL-6, a critical driver of inflammation, can also induce the differentiation of pathogenic TH17 from naive T cells and inhibit the regulatory T cells, in addition to the JAK-STAT pathway (32). Tofacitinib has been approved for rheumatoid arthritis, psoriatic arthritis, and ulcerative colitis and was recommended for immune-related colitis (33). The differentiation from CD8+ tissue-resident memory T cells to cytotoxic T-lymphocytes-induced cytokines, including IFN-γ, was considered to participate in the emergence of immune-related colitis (34). Tofacitinib has showed efficacy for immune-related colitis, probably through the inhibition of IFN-γ signaling (Figure 2) (33, 35). The patient in our case presented with elevation of IL-6, IL-10, IFN-γ, and deterioration in troponin under therapy with steroids and IVIG, was treated with tofacitinib to target JAK-STAT signal pathway and inhibit these cytokines. In addition, highly expressed phosphorylated proteins with the JAK-STAT pathway were found in the rat model of autoimmune myocarditis, suggesting that the JAK inhibitors may suppress the development of myocarditis (36).
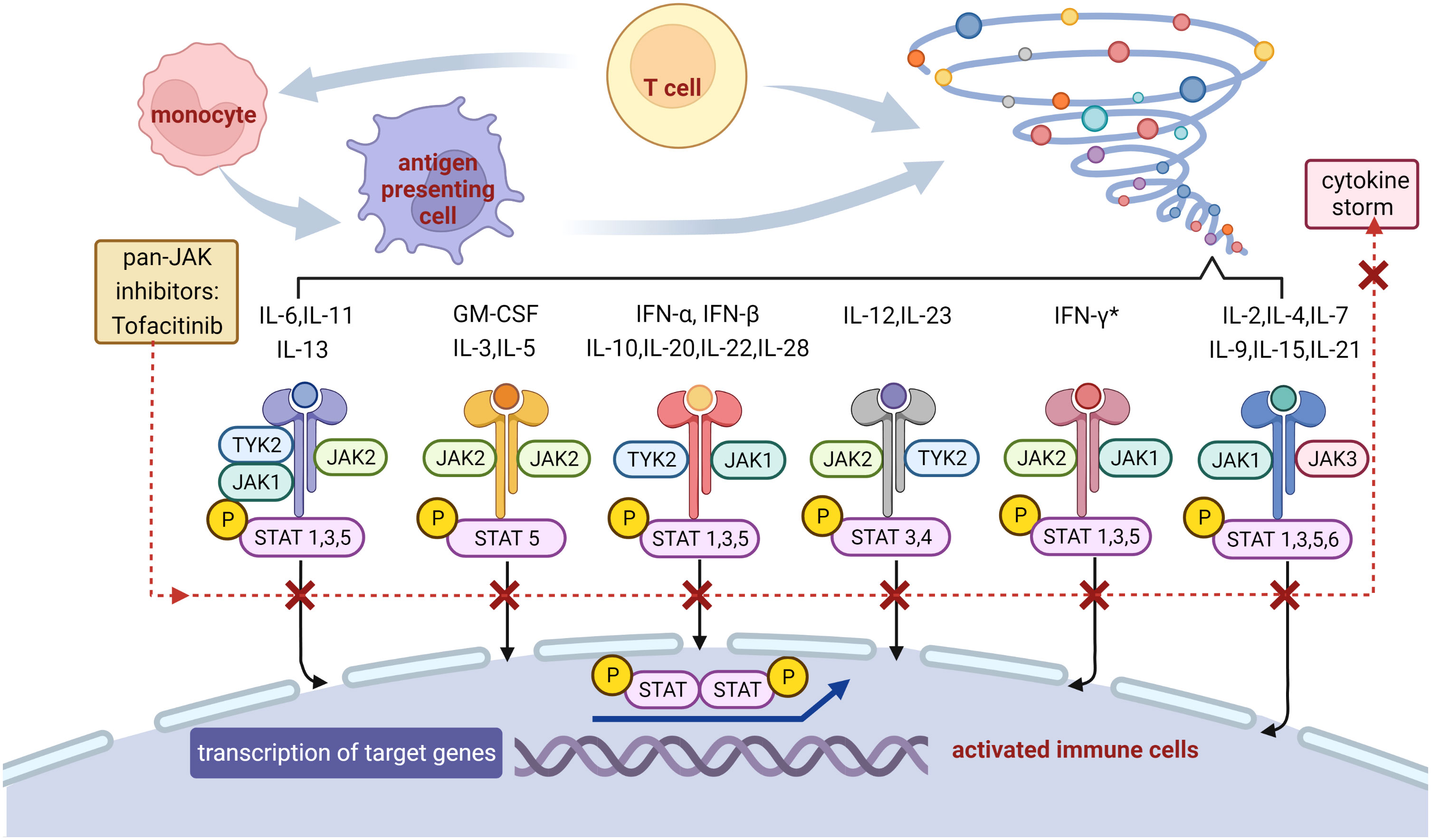
Figure 2 Potential mechanism of the blockade on inflammatory storm with tofacitinib. JAKs phosphorylate tyrosine residues on their cytokine receptors, leading to recruitment and phosphorylation of STATs. Phosphorylated STATs dimerize and translocate to the nucleus, where they promote the transcription of genes encoding proteins with functions in proliferation and inflammation. Tofacitinib binds to JAKs thus prevents downstream gene transcription, resulting in reduction in cytokine storm and inflammatory cascade. * Tofacitinib has showed efficacy for immune-related colitis, probably through the inhibition of IFN-γ signaling. JAK, Janus kinase; STAT, signal transducer and activator of transcription; P, phosphorylation; TYK, tyrosine kinase; IL, interleukin; GM-CSF, granulocyte macrophage colony stimulating factor; IFN, interferon.
Tofacitinib has been reported to be beneficial to treat steroid and cyclosporin refractory myocarditis result from drug rash with eosinophilia and systemic symptoms by inhibition of cytokines with an anti-eosinophilic effect (37). Wang et al. proposed a new classification of steroid-resistant myocarditis based on troponin rebound during corticosteroid tapering, and tofacitinib was proven clinically beneficial for these patients (38, 39). Tocilizumab, a humanized monoclonal antibody for IL-6, was reported to behave possible synergistic anticancer effect with ICIs and efficacy against irAEs including myocarditis (40–42). However, similar to tofacitinib, only limited cases could support the efficacy for myocarditis, lacking evidence-based medical proof. More recently, a multicenter randomized placebo-controlled trial among patients hospitalized with COVID-19 pneumonia demonstrated that tofacitinib led to a lower risk of death and respiratory failure (43). The action of tofacitinib on multiple critical pathways of the inflammatory cascade may ameliorate inflammation-driven lung injury caused by COVID-19 (43). Considering similar immune dysfunction and potential cytokine storms in immune-related myocarditis, tofacitinib holds promise owing to its oral route of administration and its ability to disrupt both innate and adaptive branches of the immune system (28). Although loss-of-function mutations in JAK are worried to be associated with subsequent inefficacy of immune therapy, most of our patients indeed had to discontinue their immunotherapy permanently once diagnosed with immune-related myocarditis (32, 44). The long-term safety associated with tofacitinib in RA exhibited risks of MACE, which will not be underestimated if used in the setting of immune-related myocarditis (44).
In conclusion, tofacitinib can offer additional clinical benefit in patients with steroid refractory and provides a new option for the IIST strategy in immune-related myocarditis. In the future, further meta-analysis based on more cases and large-scale trials may be conducted to explore the drug safety, efficacy, and intensive mechanism of tofacitinib. With the variable manifestation and various immunosuppressive agents, it is essential to involve a multi-disciplinary team to optimize management of these complications in patients.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
(I) Conception and design: QX, BZ. (II) Administrative support: QL, LS. (III) Provision of study materials or patients: FL. (IV) Collection and assembly of data: QX, ZX, ZYZ. (V) Data analysis and interpretation: QX, ZWZ. (VI) Manuscript writing: QX. All authors contributed to the article and approved the submitted version.
The authors thank the patient and his family for allowing the publication of this work.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Zhang L, Reynolds KL, Lyon AR, Palaskas N, Neilan TG. The evolving immunotherapy landscape and the epidemiology, diagnosis, and management of cardiotoxicity: JACC: CardioOncology primer. JACC CardioOncol (2021) 3:35–47. doi: 10.1016/j.jaccao.2020.11.012
2. Wang DY, Salem JE, Cohen JV, Chandra S, Menzer C, Ye F, et al. Fatal toxic effects associated with immune checkpoint inhibitors: A systematic review and meta-analysis. JAMA Oncol (2018) 4:1721–8. doi: 10.1001/jamaoncol.2018.3923
3. Mahmood SS, Fradley MG, Cohen JV, Nohria A, Reynolds KL, Heinzerling LM, et al. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol (2018) 71:1755–64. doi: 10.1016/j.jacc.2018.02.037
4. Dolladille C, Akroun J, Morice PM, Dompmartin A, Ezine E, Sassier M, et al. Cardiovascular immunotoxicities associated with immune checkpoint inhibitors: A safety meta-analysis. Eur Heart J (2021) 42:4964–77. doi: 10.1093/eurheartj/ehab618
5. Dolladille C, Ederhy S, Allouche S, Dupas Q, Gervais R, Madelaine J, et al. Late cardiac adverse events in patients with cancer treated with immune checkpoint inhibitors. J Immunother Cancer (2020) 8:e000261. doi: 10.1136/jitc-2019-000261
6. Lehmann LH, Cautela J, Palaskas N, Baik AH, Meijers WC, Allenbach Y, et al. Clinical strategy for the diagnosis and treatment of immune checkpoint inhibitor-associated myocarditis: A narrative review. JAMA Cardiol (2021) 6:1329–37. doi: 10.1001/jamacardio.2021.2241
7. Noseda R, Ruinelli L, Gaag L, Ceschi A. Pre-existing cardiovascular conditions as clinical predictors of myocarditis reporting with immune checkpoint inhibitors: A VigiBase study. Cancers (Basel) (2020) 12(11):3480. doi: 10.3390/cancers12113480
8. Lipe DN, Galvis-Carvajal E, Rajha E, Wechsler AH, Gaeta S. Immune checkpoint inhibitor-associated myasthenia gravis, myositis, and myocarditis overlap syndrome. Am J Emerg Med (2021) 46:51–5. doi: 10.1016/j.ajem.2021.03.005
9. Luo YB, Tang W, Zeng Q, Duan W, Li S, Yang X, et al. Case report: The neuromusclar triad of immune checkpoint inhibitors: A case report of myositis, myocarditis, and myasthenia gravis overlap following toripalimab treatment. Front Cardiovasc Med (2021) 8:714460. doi: 10.3389/fcvm.2021.714460
10. Xing Q, Zhang ZW, Lin QH, Shen LH, Wang PM, Zhang S, et al. Myositis-myasthenia gravis overlap syndrome complicated with myasthenia crisis and myocarditis associated with anti-programmed cell death-1 (sintilimab) therapy for lung adenocarcinoma. Ann Transl Med (2020) 8:250. doi: 10.21037/atm.2020.01.79
11. Puzanov I, Subramanian P, Yatsynovich YV, Jacobs DM, Chilbert MR, Sharma UC, et al. Clinical characteristics, time course, treatment and outcomes of patients with immune checkpoint inhibitor-associated myocarditis. J Immunother Cancer (2021) 9(6):e002553. doi: 10.1136/jitc-2021-002553
12. Zlotoff DA, Hassan M, Zafar A, Alvi RM, Awadalla M, Mahmood SS, et al. Electrocardiographic features of immune checkpoint inhibitor associated myocarditis. J Immunother Cancer (2021) 9(3):e002007. doi: 10.1136/jitc-2020-002007
13. Matzen E, Bartels LE, Logstrup B, Horskaer S, Stilling C, Donskov F. Immune checkpoint inhibitor-induced myocarditis in cancer patients: A case report and review of reported cases. Cardiooncology (2021) 7:27. doi: 10.1186/s40959-021-00114-x
14. Awadalla M, Mahmood SS, Groarke JD, Hassan M, Nohria A, Rokicki A, et al. Global longitudinal strain and cardiac events in patients with immune checkpoint inhibitor-related myocarditis. J Am Coll Cardiol (2020) 75:467–78. doi: 10.1016/j.jacc.2019.11.049
15. Zhang L, Awadalla M, Mahmood SS, Nohria A, Hassan M, Thuny F, et al. Cardiovascular magnetic resonance in immune checkpoint inhibitor-associated myocarditis. Eur Heart J (2020) 41:1733–43. doi: 10.1093/eurheartj/ehaa051
16. Thavendiranathan P, Zhang L, Zafar A, Drobni ZD, Mahmood SS, Cabral M, et al. Myocardial t1 and t2 mapping by magnetic resonance in patients with immune checkpoint inhibitor-associated myocarditis. J Am Coll Cardiol (2021) 77:1503–16. doi: 10.1016/j.jacc.2021.01.050
17. Finke D, Heckmann MB, Herpel E, Katus HA, Haberkorn U, Leuschner F, et al. Early detection of checkpoint inhibitor-associated myocarditis using (68)Ga-FAPI PET/CT. Front Cardiovasc Med (2021) 8:614997. doi: 10.3389/fcvm.2021.614997
18. Drobni ZD, Zafar A, Zubiri L, Zlotoff DA, Alvi RM, Lee C, et al. Decreased absolute lymphocyte count and increased Neutrophil/Lymphocyte ratio with immune checkpoint inhibitor-associated myocarditis. J Am Heart Assoc (2020) 9:e18306. doi: 10.1161/JAHA.120.018306
19. Johnson DB, Balko JM, Compton ML, Chalkias S, Gorham J, Xu Y, et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med (2016) 375:1749–55. doi: 10.1056/NEJMoa1609214
20. Ji C, Roy MD, Golas J, Vitsky A, Ram S, Kumpf SW, et al. Myocarditis in cynomolgus monkeys following treatment with immune checkpoint inhibitors. Clin Cancer Res (2019) 25:4735–48. doi: 10.1158/1078-0432.CCR-18-4083
21. Lozano AX, Chaudhuri AA, Nene A, Bacchiocchi A, Earland N, Vesely MD, et al. T Cell characteristics associated with toxicity to immune checkpoint blockade in patients with melanoma. Nat Med (2022) 28:353–62. doi: 10.1038/s41591-021-01623-z
22. Wei SC, Meijers WC, Axelrod ML, Anang N, Screever EM, Wescott EC, et al. A genetic mouse model recapitulates immune checkpoint inhibitor-associated myocarditis and supports a mechanism-based therapeutic intervention. Cancer Discovery (2021) 11:614–25. doi: 10.1158/2159-8290.CD-20-0856
23. Bonaca MP, Olenchock BA, Salem JE, Wiviott SD, Ederhy S, Cohen A, et al. Myocarditis in the setting of cancer therapeutics: Proposed case definitions for emerging clinical syndromes in cardio-oncology. Circulation (2019) 140:80–91. doi: 10.1161/CIRCULATIONAHA.118.034497
24. Khunger A, Battel L, Wadhawan A, More A, Kapoor A, Agrawal N. New insights into mechanisms of immune checkpoint inhibitor-induced cardiovascular toxicity. Curr Oncol Rep (2020) 22:65. doi: 10.1007/s11912-020-00925-8
25. Brahmer JR, Abu-Sbeih H, Ascierto PA, Brufsky J, Cappelli LC, Cortazar FB, et al. Society for immunotherapy of cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. J Immunother Cancer (2021) 9(6):e002435. doi: 10.1136/jitc-2021-002435
26. Schneider BJ, Naidoo J, Santomasso BD, Lacchetti C, Adkins S, Anadkat M, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO guideline update. J Clin Oncol (2021) 39:4073–126. doi: 10.1200/JCO.21.01440
27. Cautela J, Zeriouh S, Gaubert M, Bonello L, Laine M, Peyrol M, et al. Intensified immunosuppressive therapy in patients with immune checkpoint inhibitor-induced myocarditis. J Immunother Cancer (2020) 8(2):e001887. doi: 10.1136/jitc-2020-001887
28. Esfahani K, Elkrief A, Calabrese C, Lapointe R, Hudson M, Routy B, et al. Moving towards personalized treatments of immune-related adverse events. Nat Rev Clin Oncol (2020) 17:504–15. doi: 10.1038/s41571-020-0352-8
29. McLornan DP, Pope JE, Gotlib J, Harrison CN. Current and future status of JAK inhibitors. Lancet (2021) 398:803–16. doi: 10.1016/S0140-6736(21)00438-4
30. Zhu KJ, Yang PD, Xu Q. Tofacitinib treatment of refractory cutaneous leukocytoclastic vasculitis: A case report. Front Immunol (2021) 12:695768. doi: 10.3389/fimmu.2021.695768
31. Hosseini A, Gharibi T, Marofi F, Javadian M, Babaloo Z, Baradaran B. Janus kinase inhibitors: A therapeutic strategy for cancer and autoimmune diseases. J Cell Physiol (2020) 235:5903–24. doi: 10.1002/jcp.29593
32. Liu X, Wu W, Fang L, Liu Y, Chen W. TNF-α inhibitors and other biologic agents for the treatment of immune checkpoint inhibitor-induced myocarditis. Front Immunol (2022) 13:922782. doi: 10.3389/fimmu.2022.922782
33. Esfahani K, Hudson M, Batist G. Tofacitinib for refractory immune-related colitis from PD-1 therapy. N Engl J Med (2020) 382:2374–5. doi: 10.1056/NEJMc2002527
34. Tang L, Wang J, Lin N, Zhou Y, He W, Liu J, et al. Immune checkpoint inhibitor-associated colitis: From mechanism to management. Front Immunol (2021) 12:800879. doi: 10.3389/fimmu.2021.800879
35. Bishu S, Melia J, Sharfman W, Lao CD, Fecher LA, Higgins PDR. Efficacy and outcome of tofacitinib in immune checkpoint inhibitor colitis. Gastroenterology (2021) 160(3):932–934.e3. doi: 10.1053/j.gastro.2020.10.029
36. Liu X, Zhang X, Ye L, Yuan H. Protective mechanisms of berberine against experimental autoimmune myocarditis in a rat model. BioMed Pharmacother (2016) 79:222–30. doi: 10.1016/j.biopha.2016.02.015
37. Chowdhury M, Azari BM, Desai NR, Ahmad T. A novel treatment for a rare cause of cardiogenic shock. JACC Case Rep (2020) 2:1461–5. doi: 10.1016/j.jaccas.2020.02.004
38. Wang C, Lin J, Wang Y, Hsi DH, Chen J, Liu T, et al. Case series of steroid-resistant immune checkpoint inhibitor associated myocarditis: A comparative analysis of corticosteroid and tofacitinib treatment. Front Pharmacol (2021) 12:770631. doi: 10.3389/fphar.2021.770631
39. Liu Y, Jiang L. Tofacitinib for treatment in immune-mediated myocarditis: The first reported cases. J Oncol Pharm Pract (2021) 27(3):739–46. doi: 10.1177/1078155220947141
40. Campochiaro C, Farina N, Tomelleri A, Ferrara R, Lazzari C, De Luca G, et al. Tocilizumab for the treatment of immune-related adverse events: a systematic literature review and a multicentre case series. Eur J Intern Med (2021) 93:87–94. doi: 10.1016/j.ejim.2021.07.016
41. Doms J, Prior JO, Peters S, Obeid M. Tocilizumab for refractory severe immune checkpoint inhibitor-associated myocarditis. Ann Oncol (2020) 31(9):1273–5. doi: 10.1016/j.annonc.2020.05.005
42. Wang H, Tian R, Gao P, Wang Q, Zhang L. Tocilizumab for fulminant programmed death 1 inhibitor-associated myocarditis. J Thorac Oncol (2020) 15(3):e31–2. doi: 10.1016/j.jtho.2019.09.080
43. Guimaraes PO, Quirk D, Furtado RH, Maia LN, Saraiva JF, Antunes MO, et al. Tofacitinib in patients hospitalized with covid-19 pneumonia. N Engl J Med (2021) 385:406–15. doi: 10.1056/NEJMoa2101643
Keywords: immune checkpoint inhibitors, myocarditis, steroid-refractory, immunosuppression, tofacitinib
Citation: Xing Q, Zhang Z, Zhu B, Lin Q, Shen L, Li F, Xia Z and Zhao Z (2022) Case Report: Treatment for steroid-refractory immune-related myocarditis with tofacitinib. Front. Immunol. 13:944013. doi: 10.3389/fimmu.2022.944013
Received: 14 May 2022; Accepted: 30 August 2022;
Published: 15 September 2022.
Edited by:
Arabella Young, The University of Utah, United StatesReviewed by:
Stephen Blake, South Australian Health and Medical Research Institute (SAHMRI), AustraliaCopyright © 2022 Xing, Zhang, Zhu, Lin, Shen, Li, Xia and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhongwei Zhang, d2Vpemhvbmd6aGFuZzEwMDJAMTYzLmNvbQ==; Biao Zhu, emh1Ymlhb3pzQDEyNi5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.