
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol., 14 July 2022
Sec. Autoimmune and Autoinflammatory Disorders
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.935700
A correction has been applied to this article in:
Corrigendum: Urine soluble CD163 is a promising biomarker for the diagnosis and evaluation of lupus nephritis
Introduction: Urine-soluble CD163 (usCD163) is released from alternatively activated macrophages involved in the resolution of inflammation in glomeruli and plays an important role in glomerulonephritis. This study explored the role of usCD163 in patients with systemic lupus erythematosus (SLE).
Materials and Methods: usCD163 concentrations were measured cross-sectionally in 261 SLE patients in Taiwan. Clinical and laboratory data were collected, and SLE disease activity scores were calculated to assess the correlation with usCD163.
Results: SLE patients with high usCD163 levels tended to be younger, with a higher hospital admission rate, higher prednisolone dose, lower estimated glomerular filtration rate, higher urine protein creatinine ratio (UPCR), more pyuria and hematuria, higher levels of inflammatory markers, higher rates of anemia, neutropenia, and lymphopenia, lower complement 3 (C3) levels, higher anti-double-stranded DNA antibody (anti-dsDNA Ab) levels, and higher disease activity scores (p < 0.05). usCD163 levels were significantly higher in patients with active lupus nephritis (LN) than in those with extrarenal or inactive SLE and correlated with UPCR, disease activity, and anti-dsDNA Ab levels. SLE patients with high usCD163 levels tended to have a higher chronic kidney disease stage.
Discussion and conclusion: The usCD163 level correlates with the severity of LN and disease activity in renal SLE.
CD163 is a 130-kDa type I glycosylated transmembrane protein belonging to the cysteine-rich scavenger receptor superfamily type B that can bind the hemoglobin–haptoglobin complex during intravascular hemolysis, both physiologically and pathologically (1, 2). CD163-positive macrophages are alternatively activated M2 macrophages exhibiting an anti-inflammatory function and contribute to wound healing (3). CD163-positive macrophages are found in several human glomerular diseases, such as poststreptococcal glomerulonephritis, ANCA-associated vasculitis, diabetic nephropathy, and lupus nephritis (LN) (4, 5). Soluble CD163 is derived from the cleavage of the CD163 macrophage receptor by metalloproteinase and is released into inflammatory tissue, blood, urine, cerebrospinal fluid, and synovial fluid (6) during systemic inflammatory responses such as sepsis, liver disease, malaria, autoimmune disorders, and reactive hemophagocytic syndrome (7). Serum CD163 concentrations in peripheral blood reflect the disease severity in autoimmune diseases such as rheumatoid arthritis, systemic sclerosis, and idiopathic inflammatory myositis (6, 8). However, urine-soluble CD163, but not serum CD163, correlated with the severity of LN in some studies- (4, 9).
Serious complications of systemic lupus erythematosus (SLE) include hemolytic anemia, refractory severe thrombocytopenia, cardiac tamponade, massive bilateral pleural effusions, central nervous system lupus, macrophage activation syndrome, and proliferative LN (10, 11). More than half of SLE patients have renal disease, with the high risk of renal function deterioration and mortality, particularly patients who present with proliferative glomerular disease composed of the renal histology of International Society of Nephrology/Renal Pathology Society (ISN/RPS) class III/VI (12, 13). Approximately 10%–17% of LN patients will progress to end-stage renal disease, despite immunosuppressants (14). Markers of SLE activity, including serum complement 3 (C3), C4, and anti-double stranded DNA antibody (anti-dsDNA Ab), are conventional biomarkers for assessing disease severity (15) but lack sufficient sensitivity and specificity for histological classification of LN (16). Renal biopsy is the gold standard for histological assessment of LN but has shortcomings in terms of sampling error, invasiveness, and the complication of hemorrhage (17). An alternative non-invasive and reproducible biomarker, such as urine CD163, is needed for evaluating LN (18, 19). Specific urinary biomarkers that discriminate active LN could be instrumental in guiding its management (9, 20).
In this study, we evaluated usCD163 as a potential biomarker for LN and investigated its correlation with clinical characteristics, laboratory parameters, and disease activity, thereby providing a theoretical basis for novel therapeutics for LN.
This cross-sectional study was performed to evaluate usCD163 as a potential biomarker of LN disease activity in Taiwan. The study was approved by the Institutional Review Board of Chang Gung Memorial Hospital, and informed consent was obtained from all patients. Blood and urine samples were collected from 261 patients with SLE on admission to Chang Gung Memorial Hospital and its affiliated hospitals between January 2019 and September 2021. All patients met the 1997 American College of Rheumatology revised criteria (21) or 2012 Systemic Lupus International Collaborating Clinics (SLICC) classification criteria (22) for SLE. There was no severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection among our SLE cohort.
The Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K) covers seizures, psychosis, organic brain syndromes, visual disturbance, cranial nerve disorder, lupus headache, cerebrovascular accident, vasculitis, urinary casts, hematuria, proteinuria, pyuria, pleurisy, pericarditis, and fever (23). The renal SLEDAI (rSLEDAI) provides a sum score for the renal domains of SLEDAI, including hematuria (>5 red blood cells/high-power field), pyuria (>5 white blood cells/high-power field), proteinuria (>0.5 g/24 h), and urinary casts. SLE patients were classified into three groups: rSLEDAI score ≥4 (active LN), SLEDAI-2k score ≥5 (active extrarenal SLE), and rSLEDAI score = 0. The inactive (or low-disease activity) group included SLE patients with a SLEDAI-2k score ≤ 4, and the clinical SLEDAI (omitting anti-dsDNA and complement) included those with a SLEDAI-2k score ≤ 2.
SLEDAI, rSLEDAI, complete blood count, blood urea nitrogen, serum creatinine, urinalysis, urine protein creatinine ratio (UPCR), C3, C4, and anti-dsDNA data were collected. LN histology was determined based on the ISN/RPS classification, and histological scores (evaluated according to acute and chronic indices) were recorded. The Modification of Diet in Renal Disease formula was used to calculate the estimated glomerular filtration rate (eGFR, as follows:
in females). The results are expressed as mL/min/1.73 m2.
Clean-catch midstream urine samples were collected and refrigerated within 1 h of collection. The samples were then aliquoted and stored at -80°C. To detect usCD163, an enzyme-linked immunosorbent assay (ELISA) was performed using a human soluble CD163 ELISA Kit (Euroimmun, Lübeck, Germany) in accordance with the manufacturer’s instructions. The lower detection limit of the sCD163 ELISA is 0.15 ng/ml. A cutoff for usCD163 positivity of 1.27 ng/ml was used, or a normalized cutoff of 242.30 ng/mmol creatinine that was referenced from the instruction brochure from Euroimmun.
Count data are expressed as frequencies and rates, and comparisons of rates were done using the cube test. Normally distributed data are expressed as the mean and standard deviation. The t-test was used for group comparisons, and Pearson’s correlation analysis was also performed. Continuous variables were compared using Welch’s t-test. The coefficient of determination was taken to indicate the strength of the association between two continuous variables. The chi-squared and Fisher’s exact tests were used for comparing two groups, and analysis of variance was used for comparing multiple groups, with post-hoc pairwise comparisons. The non-parametric Spearman correlation test was also performed. All data are presented as box plots, where the upper whiskers represent the 75th percentile + 1.5 × the interquartile range (IQR), and the lower whiskers represent the 25th percentile - 1.5 × IQR. IBM SPSS Statistics for Windows software (version 25.0; IBM Corp., Armonk, NY, USA) was used for data processing, and p < 0.05 was considered to indicate a statistically significant difference.
The 261 SLE patients in Taiwan had an average age of 44.4 years, and women predominated (95.4%) (Table 1). Over half of the patients were taking prednisolone (79%, 9 mg/day) and/or hydroxychloroquine (69%, 266 mg/day). The average creatinine level was 0.8 mg/dl, the eGFR was 100 ml/min/1.73 m2, and the UPCR was 920 mg protein/g creatinine. The average usCD163 level was 2.8 ng/ml, and the urine usCD163/creatinine ratio was 842 ng/mmol. The average SLEDAI-2K score was 7.7 points in those with a high anti-ds DNA Ab level (170 IU/ml), low C3 level (81 mg/dl), orC4 (15 mg/dl) level.
Patients were divided into a high usCD163 group (n = 63, average usCD163 level = 10 ng/ml) and low usCD163 group (n = 198, average usCD163 level = 0.5 ng/ml) (Table 2). The former group of patients tended to be younger and had a higher hospital admission rate, mycophenolate sodium usage, and prednisolone dose (p < 0.05). They also had a lower eGFR, higher UPCR, higher rates of pyuria, hematuria, anemia, neutropenia, and lymphopenia, higher inflammatory marker levels, lower C3 levels, higher anti-dsDNA Ab levels, higher SLEDAI-2K scores, and higher urine usCD163/creatinine ratio (p < 0.05). Six (9.5%) inactive SLE patients and no extrarenal SLE patients had high usCD163 levels. The histological results of the initial kidney biopsy for 43 LN patients, according to the ISN/RPS class and activity and chronicity indices, are shown in Table 3. The glomerular sclerosis of the chronicity index correlated with both high usCD163 and high usCD163/creatinine in urine with clinical significance.
There were modest correlations with statistical significance of the usCD163 level with the UPCR, anti-dsDNA Ab level, and SLEDAI-2K and rSLEDAI scores in the SLE patients. Also, there were moderate correlations with statistical significance of the urine usCD163/creatinine ratio with the UPCR and SLEDAI-2K and rSLEDAI scores (Table 4). A usCD163 level of 0.443 ng/ml and a urine usCD163/creatinine ratio of 110.2 ng/mmol predicted high SLEDAI scores (more than six points) (Supplementary Figure 1, 2).

Table 4 Correlation between usCD163 and UPCR/anti-ds DNA Ab/SLEDAI/rSLEDAI in SLE patients; P < 0.0001.
The renal SLE patients with high usCD163 levels or usCD163/creatinine ratios had higher SLEDAI-2K scores compared with those with low usCD163 levels (Figures 1, 2). However, no difference in SLEDAI-2K score was seen between the inactive and extrarenal SLE patients. The clinical characteristics of the inactive, extrarenal, and renal SLE patients are shown in Supplementary Table S1.
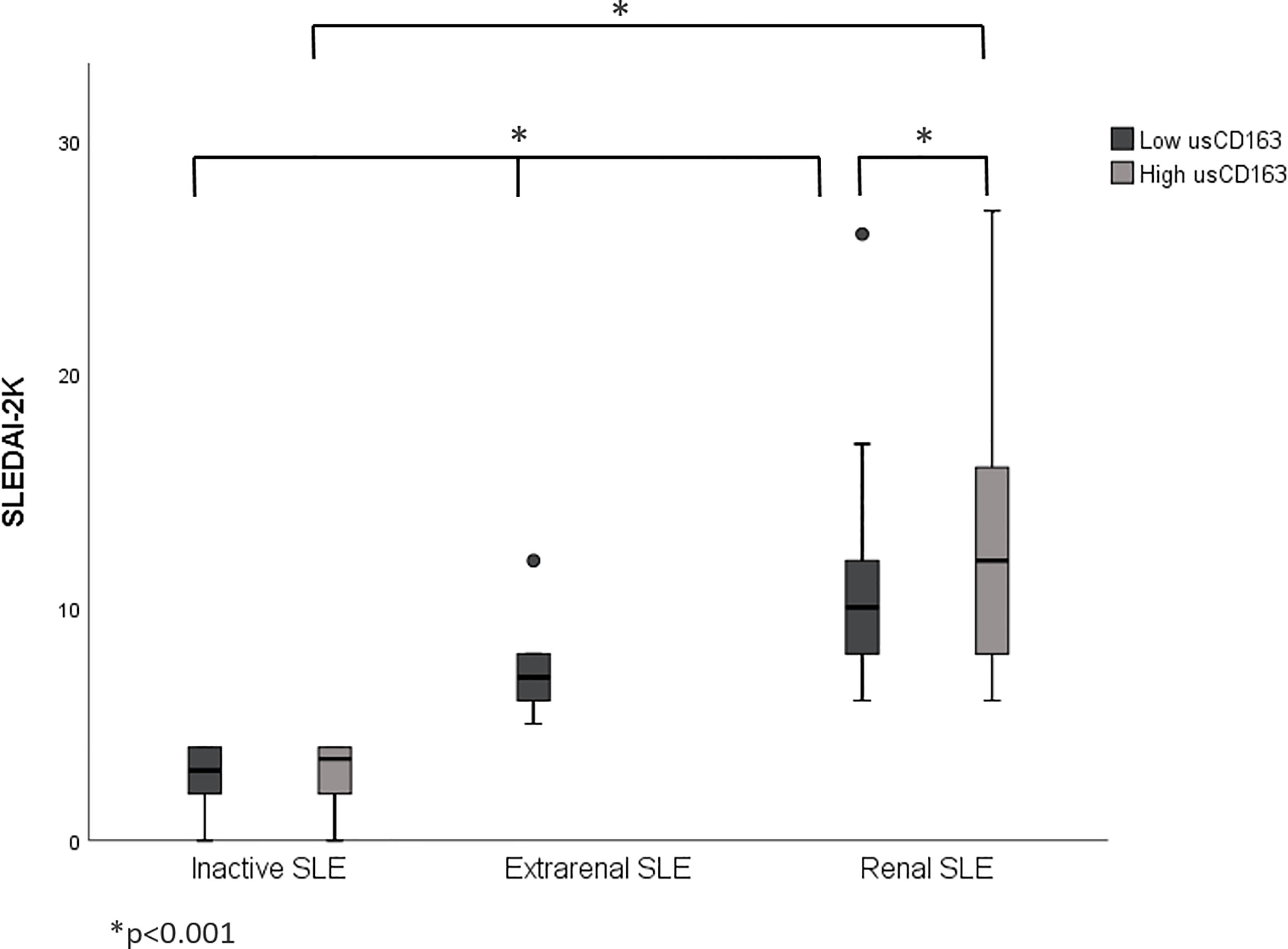
Figure 1 Correlation between usCD163 and SLEDAI-2K in SLE patients. The renal SLE patients with high usCD163 levels had higher SLEDAI-2K scores compared with those with low usCD163 levels. However, no difference in SLEDAI-2K score was seen between the inactive and extrarenal SLE patients. SLEDAI-2k, Systemic Lupus Erythematosus Disease Activity Index 2000.
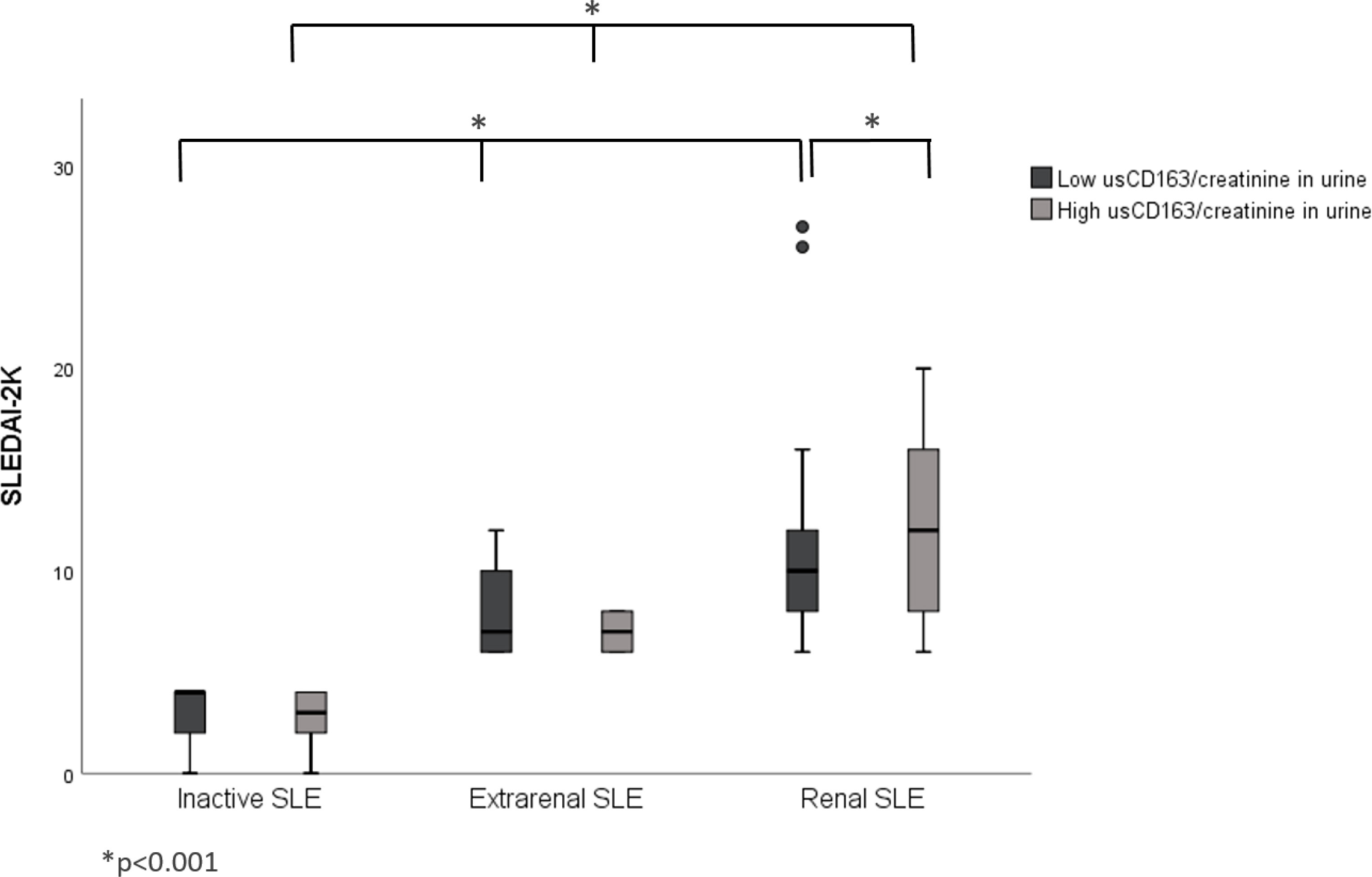
Figure 2 Correlation between usCD163/creatinine in urine and SLEDAI-2K in SLE patients. The renal SLE patients with high usCD163/creatinine ratios had higher SLEDAI-2K scores compared with those with low usCD163 levels. However, no difference in SLEDAI-2K score was seen between the inactive and extrarenal SLE patients. SLEDAI-2k, Systemic Lupus Erythematosus Disease Activity Index 2000.
The relative proportions of chronic kidney disease (CKD) stages I and II were higher in the low than high usCD163 group. The relative proportions of CKD stages II–V were higher in the high than low usCD163 group (Figure 3). SLE patients with high usCD163 levels tended to have a higher CKD stage.
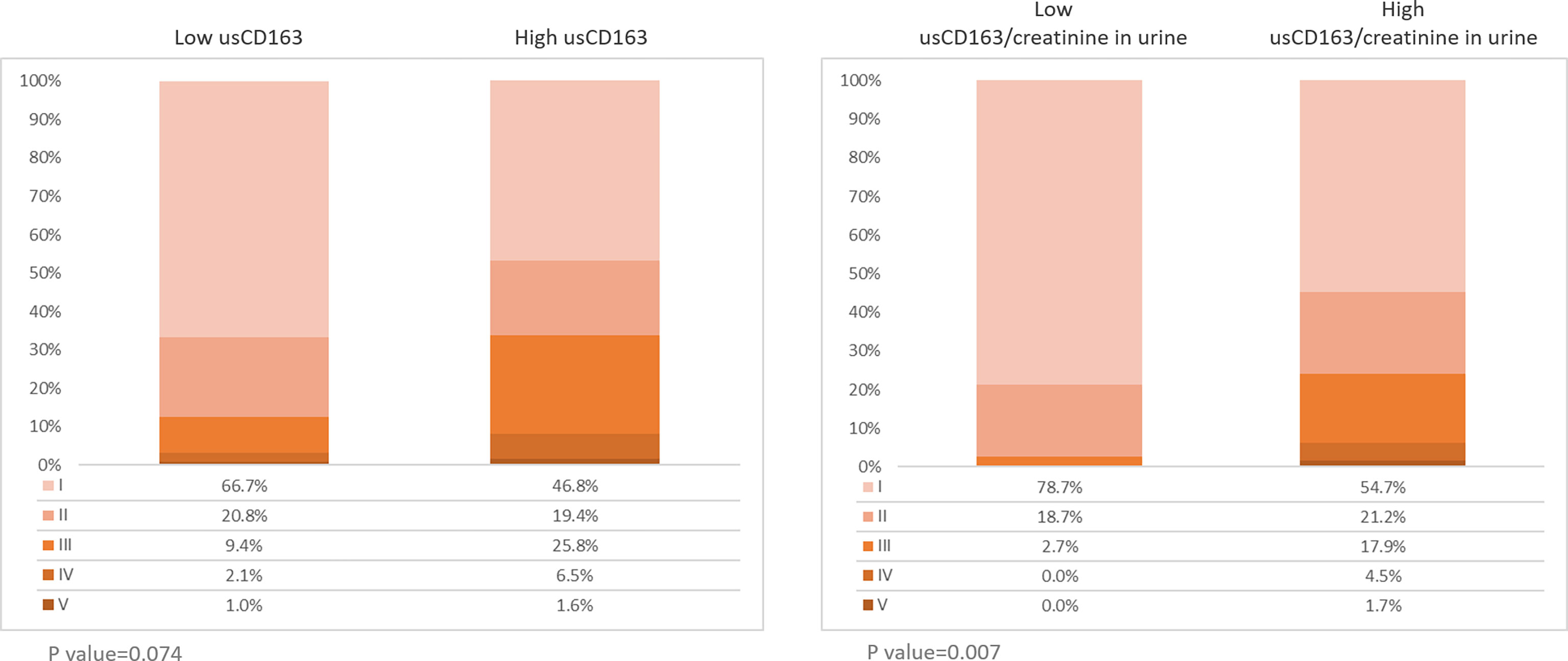
Figure 3 Stages of chronic kidney diseases in SLE patients. Two hundred sixty-one patients with SLE were divided into low or high usCD163 and usCD163/creatinine in urine groups. The stages of CKD were divided into stages I (EGFR≧90), II (EGFR 60-89), III (EGFR 30-59), IV (EGFR 15-29), and V (EGFR≦15).
Macrophages have been implicated in the pathogenesis of SLE, including classically activated inflammatory M1 macrophages and the alternately activated M2 macrophages M2a, M2b, and M2c, which demonstrate pro-fibrotic, immune-regulatory, remodeling, and anti-inflammatory effects, respectively. Initially, M1 macrophages were reported to be predominant in SLE, with M2a and M2c macrophage expressions being reduced (24). However, Gregor et al. detected more M2c than M2a macrophages, and few M1 macrophages, in all ISN/RPS classes of LN (25). Several reports suggest that M2 macrophages play an important role in driving or regulating interstitial inflammation, cellular crescent formation, and fibrinoid necrosis (9, 26, 27). These studies suggested that CD163+ M2c-like macrophages may be associated with the pathogenesis of SLE.
UsCD163 is a marker of the status of LN and could also serve as a biomarker of SLE disease activity. Mejia-Vilet et al. reported an association between usCD163 and proteinuria (9). Zhang et al. reported that the usCD163 level was correlated with the SLEDAI, rSLEDAI, and Physician Global Assessment scores in various ethnic groups (28). Our results showed that the usCD163 level and urine usCD163/creatinine ratio were correlated with the UPCR, anti-dsDNA Ab level, and SLEDAI and rSLEDAI scores. Furthermore, the usCD163 level of 0.443 ng/ml and the urine usCD163/creatinine ratio of 110.2 ng/mmol predicted high SLEDAI scores. Therefore, usCD163 might be considered as a marker to measure the disease activity of systemic lupus erythematosus or lupus nephritis in the future.
C3, C4, and anti-ds DNA Ab are traditional biomarkers of SLE disease activity; the SLEDAI-2K score is also based on these markers. We found that the usCD163 level and usCD163/creatinine ratio were also correlated with the SLEDAI-2K score and anti-ds DNA Ab level. Serum-soluble CD163 (29), monocyte chemoattractant protein-1 (MCP-1), neutrophil gelatinase-associated lipocalin (NGAL), TNF-like weak inducer of apoptosis (TWEAK), and vascular cell adhesion molecule-1 (VCAM) are new biomarkers of SLE disease activity (30).
Renal biopsy is the gold standard to diagnose LN. The identification of non-invasive biomarkers, such as usCD163, of active LN that are strongly correlated with renal biopsy results and underlying disease mechanisms has been a priority. Other non-invasive biomarkers of LN include anti-C1q antibody and urine MCP-1 (18, 31). The treatment goal should be an at least 25% and 50% reduction in the proteinuria level after 3 and 6 months, respectively, and a complete renal response (500–700 mg/day) at 12 months (32). However, persistent proteinuria (lasting for 6 months) makes it difficult to titrate immunosuppressants. Mejia-Vilet et al. found that the level of usCD163 after treatment could be monitored to predict the renal response and kidney histology score CD163 > 370 ng/mmol perfectly agreed (k = 1.0) with a histologic activity index > 1 in repeated biopsies after lupus nephritis flare-up (9).
The in vivo expression of CD163 on macrophages is influenced by several medications including glucocorticoids, mycophenolate mofetil (MMF), tacrolimus, rituximab, and cyclophosphamide (25, 33–36). However, the observed elevation in urine sCD163 in active and proliferative LN was not attributed to medications such as MMF and glucocorticoids in clinical studies (4, 28).
In our study, we found that mycophenolate sodium was more likely in patients with high usCD163. Actually, mycophenolate sodium and mycophenolate mofetil are all mycophenolate acids (MPAs) (37). Unlike oral mycophenolate mofetil, which releases MPA in the stomach, enteric-coated mycophenolate sodium releases MPA in the small intestine. Renal function, serum albumin levels, sex, ethnicity, food, concurrent antacids, metal-containing medications, and proton pump inhibitors were identified in some studies as having a significant influence on the pharmacokinetics of mycophenolate (38). The reason why patients with mycophenolate sodium tend to have high usCD163 is still unknown.
As a biomarker, usCD163 can be evaluated non-invasively and directly reflects macrophage-mediated glomerular inflammation; it can also distinguish between class III/IV and V LN in patients showing proteinuria relapse during their clinical course (4, 9, 28). The reason why usCD163 correlates with the proteinuria level and UPCR, but not with class V LN, needs further study.
The studies about the correlation of serum CD163 with systemic lupus erythematosus were conflicting. Serum CD163 was reported to be a biomarker that could be measured in lupus nephritis, accelerated atherosclerosis, and macrophage activation syndrome of SLE (39–41). On the other hand, Nobuhide et al. pointed out that serum CD163 levels did not show a difference between lupus nephritis, ANCA-associated vasculitis, and diabetic nephropathy (4). Moreover, there was a weak association between serum CD163 concentration and histological activity in lupus nephritis.
One of novel points in our study is that we compared both usCD163 and usCD163/creatinine in urine in systemic lupus erythematosus patients. Next, we analyzed the CKD stages in SLE patients with high and low usCD163 that was not discussed by other papers. Third, we found out that the patients with mycophenolate sodium but not mycophenolate mofetil might have a higher usCD163 level that was not mentioned by others.
The first limitation of our study was that patients with lupus nephritis did not receive a renal biopsy (53/96) for several reasons including low lupus nephritis disease activity, contraindication to renal biopsy, or lupus nephritis easily controlled by conventional disease-modifying antirheumatic drugs. Besides, the initial renal histopathology cannot represent the subsequent disease activity of lupus nephritis during the follow-up period. However, a previous study showed that usCD163 was associated with proliferative LN classes III and IV. Second, serial usCD163 measurements and long-term follow-up of renal were not performed, as this was a cross-sectional study. We will further analyze serum CD163, usCD163, anti-C1q antibody, and urine MCP-1 levels in LN in future studies. Lastly, we did not include a matched healthy control population in our study.
Most of our patients with a high usCD163 level or usCD163/creatinine ratio were renal SLE patients and had high SLEDAI and rSLEDAI scores. The usCD163 level and usCD163/creatinine ratio correlated with the UPCR, anti-ds DNA level, and SLEDAI and rSLEDAI scores. The strong correlation between renal SLE and disease activity indicates that further study of usCD163 is merited, as a promising biomarker of LN.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Institutional Review Board of Chang Gung Memorial Hospital. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Conceptualization, C-FK; methodology, Y-JH and C-HL; software, Y-JH and C-HL; validation, C-FK; formal analysis, YJH and C-HL; investigation, Y-JH; resources, H-YY and C-FK; data curation, Y-JH and C-HL; writing—original draft preparation, Y-JH; writing—review and editing, Y-JH and CFK; visualization, Y-JH; supervision, S-FL; project administration, C-FK and S-FL; funding acquisition, C-FK. All authors have read and agreed to the published version of the manuscript.
This study received funding from Key Development Project of Department of Science and Technology (2015C03Bd051) and Chang Gung Memorial Hospital Research Program (CMRPG3J0031).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors thank the Departments of Rheumatology, Allergy and Immunology and Big Data Analytics and Statistics of Chang Gung Memorial Hospital.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.935700/full#supplementary-material
1. Møller HJ. Soluble Cd163. Scand J Clin Lab Invest (2012) 72(1):1–13. doi: 10.3109/00365513.2011.626868
2. Etzerodt A, Moestrup SK. CD163 and Inflammation: Biological, Diagnostic, and Therapeutic Aspects. Antioxid Redox Signal (2013) 18(17):2352–63. doi: 10.1089/ars.2012.4834
3. Murray PJ. Macrophage Polarization. Annu Rev Physiol (2017) 79:541–66. doi: 10.1146/annurev-physiol-022516-034339
4. Endo N, Tsuboi N, Furuhashi K, Shi Y, Du Q, Abe T, et al. Urinary Soluble CD163 Level Reflects Glomerular Inflammation in Human Lupus Nephritis. Nephrol Dial Transplant (2016) 31(12):2023–33. doi: 10.1093/ndt/gfw214
5. Arai M, Mii A, Kashiwagi T, Shimizu A, Sakai Y. The Severity of Glomerular Endothelial Cell Injury is Associated With Infiltrating Macrophage Heterogeneity in Endocapillary Proliferative Glomerulonephritis. Sci Rep (2021) 11(1):13339. doi: 10.1038/s41598-021-92655-5
6. Zhou D, Wang Y, Chen LU, Zhang W, Luan J. Soluble CD163: A Novel Biomarker With Diagnostic and Therapeutic Implications in Autoimmune Diseases. J Rheumatol (2016) 43(4):830. doi: 10.3899/jrheum.151317
7. Van Gorp H, Delputte PL, Nauwynck HJ. Scavenger Receptor CD163, a Jack-Of-All-Trades and Potential Target for Cell-Directed Therapy. Mol Immunol (2010) 47(7-8):1650–60. doi: 10.1016/j.molimm.2010.02.008
8. Matsushita T, Takehara K. Soluble CD163 Is a Potential Biomarker in Systemic Sclerosis. Expert Rev Mol Diagn (2019) 19(3):197–9. doi: 10.1080/14737159.2019.1571911
9. Mejia-Vilet JM, Zhang XL, Cruz C, Cano-Verduzco ML, Shapiro JP, Nagaraja HN, et al. Urinary Soluble CD163: A Novel Noninvasive Biomarker of Activity for Lupus Nephritis. J Am Soc Nephrol (2020) 31(6):1335–47. doi: 10.1681/ASN.2019121285
10. Yang H, Liu H, Zhou Z, Zhao L, Fei Y, Chen H, et al. Management of Severe Refractory Systemic Lupus Erythematosus: Real-World Experience and Literature Review. Clin Rev Allergy Immunol (2021) 60(1):17–30. doi: 10.1007/s12016-020-08817-2
11. Ichinose K. [Unmet Needs in Systemic Lupus Erythematosus]. Nihon Rinsho Meneki Gakkai Kaishi (2017) 40(6):396–407. doi: 10.2177/jsci.40.396
12. Mohan C, Putterman C. Genetics and Pathogenesis of Systemic Lupus Erythematosus and Lupus Nephritis. Nat Rev Nephrol (2015) 11(6):329–41. doi: 10.1038/nrneph.2015.33
13. Bajema IM, Wilhelmus S, Alpers CE, Bruijn JA, Colvin RB, Cook HT, et al. Revision of the International Society of Nephrology/Renal Pathology Society Classification for Lupus Nephritis: Clarification of Definitions, and Modified National Institutes of Health Activity and Chronicity Indices. Kidney Int (2018) 93(4):789–96. doi: 10.1016/j.kint.2017.11.023
14. Tektonidou MG, Dasgupta A, Ward MM. Risk of End-Stage Renal Disease in Patients With Lupus Nephritis, 1971-2015: A Systematic Review and Bayesian Meta-Analysis. Arthritis Rheumatol (2016) 68(6):1432–41. doi: 10.1002/art.39594
15. Tofighi T, Morand EF, Touma Z. Systemic Lupus Erythematosus Outcome Measures for Systemic Lupus Erythematosus Clinical Trials. Rheum Dis Clin North Am (2021) 47(3):415–26. doi: 10.1016/j.rdc.2021.04.007
16. Arriens C, Wren JD, Munroe ME, Mohan C. Systemic Lupus Erythematosus Biomarkers: The Challenging Quest. Rheumatol (Oxford) (2017) 56(suppl_1):i32–45. doi: 10.1093/rheumatology/kew407
17. Hull KL, Adenwalla SF, Topham P, Graham-Brown MP. Indications and Considerations for Kidney Biopsy: An Overview of Clinical Considerations for the non-Specialist. Clin Med (Lond) (2021) 22:34–40. doi: 10.7861/clinmed.2021-0472
18. Rodríguez-Almaraz E, Gutiérrez-Solís E, Rabadán E, Rodríguez P, Carmona L, Morales E, et al. Something New About Prognostic Factors for Lupus Nephritis? A Systematic Review. Lupus (2021) 30:9612033211061475. doi: 10.1177/09612033211061475
19. Radin M, Miraglia P, Barinotti A, Fenoglio R, Roccatello D, Sciascia S, et al. Prognostic and Diagnostic Values of Novel Serum and Urine Biomarkers in Lupus Nephritis: A Systematic Review. Am J Nephrol (2021) 52(7):559–71. doi: 10.1159/000517852
20. Aragón CC, Tafúr RA, Suárez-Avellaneda A, Martínez T, de Las Salas A, Tobón GJ, et al. Urinary Biomarkers in Lupus Nephritis. J Transl Autoimmun (2020) 3:100042. doi: 10.1016/j.jtauto.2020.100042
21. Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, et al. The 1982 Revised Criteria for the Classification of Systemic Lupus Erythematosus. Arthritis Rheum (1982) 25(11):1271–7. doi: 10.1002/art.1780251101
22. Petri M, Orbai AM, Alarcón GS, Gordon C, Merrill JT, Fortin PR, et al. Derivation and Validation of the Systemic Lupus International Collaborating Clinics Classification Criteria for Systemic Lupus Erythematosus. Arthritis Rheum (2012) 64(8):2677–86. doi: 10.1002/art.34473
23. Gladman DD, Ibañez D, Urowitz MB. Systemic Lupus Erythematosus Disease Activity Index 2000. J Rheumatol (2002) 29(2):288–91.
24. Orme J, Mohan C. Macrophage Subpopulations in Systemic Lupus Erythematosus. Discovery Med (2012) 13(69):151–8.
25. Olmes G, Büttner-Herold M, Ferrazzi F, Distel L, Amann K, Daniel C, et al. CD163+ M2c-Like Macrophages Predominate in Renal Biopsies From Patients With Lupus Nephritis. Arthritis Res Ther (2016) 18:90. doi: 10.1186/s13075-016-0989-y
26. Tao J, Zhao J, Qi XM, Wu YG. Complement-Mediated M2/M1 Macrophage Polarization may be Involved in Crescent Formation in Lupus Nephritis. Int Immunopharmacol (2021) 101(Pt A):108278. doi: 10.1016/j.intimp.2021.108278
27. Gupta R, Yadav A, Aggarwal A. Urinary Soluble CD163 Is a Good Biomarker for Renal Disease Activity in Lupus Nephritis. Clin Rheumatol (2021) 40(3):941–8. doi: 10.1007/s10067-020-05343-6
28. Zhang T, Li H, Vanarsa K, Gidley G, Mok CC, Petri M, et al. Association of Urine Scd163 With Proliferative Lupus Nephritis, Fibrinoid Necrosis, Cellular Crescents and Intrarenal M2 Macrophages. Front Immunol (2020) 11. doi: 10.3389/fimmu.2020.00671
29. Lindblom J, Mohan C, Parodis I. Diagnostic, Predictive and Prognostic Biomarkers in Systemic Lupus Erythematosus: Current Insights. Curr Opin Rheumatol (2022) 34(2):139–49. doi: 10.1097/BOR.0000000000000862
30. Soliman S, Mohan C. Lupus Nephritis Biomarkers. Clin Immunol (2017) 185:10–20. doi: 10.1016/j.clim.2016.08.001
31. Dias R, Hasparyk UG, Lopes MP, de Barros JLVM, Silva ACSE. Novel Biomarkers for Lupus Nephritis in the "OMICS" Era. Curr Med Chem (2021) 28(29):6011–44. doi: 10.2174/0929867328666210212102438
32. Kostopoulou M, Fanouriakis A, Cheema K, Boletis J, Bertsias G, Jayne D, et al. Management of Lupus Nephritis: A Systematic Literature Review Informing the 2019 Update of the Joint EULAR and European Renal Association-European Dialysis and Transplant Association (EULAR/ERA-EDTA) Recommendations. RMD Open (2020) 6(2):713–23. doi: 10.1136/rmdopen-2020-001263
33. Tang Z, Niven-Fairchild T, Tadesse S, Norwitz ER, Buhimschi CS, Buhimschi IA, et al. Glucocorticoids Enhance CD163 Expression in Placental Hofbauer Cells. Endocrinology (2013) 154(1):471–82. doi: 10.1210/en.2012-1575
34. Kannegieter NM, Hesselink DA, Dieterich M, Kraaijeveld R, Rowshani AT, Leenen PJM, et al. The Effect of Tacrolimus and Mycophenolic Acid on CD14+ Monocyte Activation and Function. PloS One (2017) 12(1):e0170806. doi: 10.1371/journal.pone.0170806
35. Guo ZS, Parimi V, O'Malley ME, Thirunavukarasu P, Sathaiah M, Austin F, et al. The Combination of Immunosuppression and Carrier Cells Significantly Enhances the Efficacy of Oncolytic Poxvirus in the Pre-Immunized Host. Gene Ther (2010) 17(12):1465–75. doi: 10.1038/gt.2010.104
36. Lapuc I, Bolkun L, Eljaszewicz A, Rusak M, Luksza E, Singh P, et al. Circulating Classical CD14++CD16- Monocytes Predict Shorter Time to Initial Treatment in Chronic Lymphocytic Leukemia Patients: Differential Effects of Immune Chemotherapy on Monocyte-Related Membrane and Soluble Forms of CD163. Oncol Rep (2015) 34(3):1269–78. doi: 10.3892/or.2015.4088
37. Tett SE, Saint-Marcoux F, Staatz CE, Brunet M, Vinks AA, Miura M, et al. Mycophenolate, Clinical Pharmacokinetics, Formulations, and Methods for Assessing Drug Exposure. Transplant Rev (Orlando) (2011) 25(2):47–57. doi: 10.1016/j.trre.2010.06.001
38. Abd Rahman AN, Tett SE, Staatz CE. Clinical Pharmacokinetics and Pharmacodynamics of Mycophenolate in Patients With Autoimmune Disease. Clin Pharmacokinet (2013) 52(5):303–31. doi: 10.1007/s40262-013-0039-8
39. Nishino A, Katsumata Y, Kawasumi H, Hirahara S, Kawaguchi Y, Yamanaka H, et al. Usefulness of Soluble CD163 as a Biomarker for Macrophage Activation Syndrome Associated With Systemic Lupus Erythematosus. Lupus (2019) 28(8):986–94. doi: 10.1177/0961203319860201
40. David C, Divard G, Abbas R, Escoubet B, Chezel J, Chauveheid MP, et al. Soluble CD163 is a Biomarker for Accelerated Atherosclerosis in Systemic Lupus Erythematosus Patients at Apparent Low Risk for Cardiovascular Disease. Scand J Rheumatol (2020) 49(1):33–7. doi: 10.1080/03009742.2019.1614213
Keywords: Systemic lupus erythematosus, lupus nephritis, urine soluble CD163, urine biomarker, chronic kidney disease, macrophage, SLEDA
Citation: Huang Y-J, Lin C-H, Yang H-Y, Luo S-F and Kuo C-F (2022) Urine Soluble CD163 Is a Promising Biomarker for the Diagnosis and Evaluation of Lupus Nephritis. Front. Immunol. 13:935700. doi: 10.3389/fimmu.2022.935700
Received: 04 May 2022; Accepted: 16 June 2022;
Published: 14 July 2022.
Edited by:
Margaret Hibbs, Monash University, AustraliaReviewed by:
Katherine R. Martin, The University of Melbourne, AustraliaCopyright © 2022 Huang, Lin, Yang, Luo and Kuo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chang-Fu Kuo, emFuZGlzQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.