
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Immunol. , 18 October 2022
Sec. Molecular Innate Immunity
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.932055
This article is part of the Research Topic Lactate Metabolism and Regulation of the Immune Response View all 5 articles
Recent findings about the new roles of lactate have changed our understanding of this end product of glycolysis or fermentation that was once considered only a waste product. It is now well accepted that lactate acts as a signaling molecule and fuel source for cancer cells in a glucose-restricted environment. Moreover, lactate and lactate dehydrogenase are markers of poor prognosis of many cancers and regulate many functions of immune cells. The presence of lactate in the tumor microenvironment (TME) leads to polarization of the immunosuppressive phenotypes of dendritic cells and impairs the cytotoxic abilities of T cells and NK cells, and as such lactate is a major obstacle to immune-cell effector functions and the efficacy of cell-based immunotherapies. Emerging evidence suggests that lactate in the TME might be a novel therapeutic target to enhance the immunotherapeutic potential of cell-based therapies. This review describes our current understanding of the role of lactate in tumor biology, including its detrimental effects on cell-based immunotherapy in cancer. We also highlight how the role of lactate in the TME must be considered when producing cell therapies designed for adoptive transfer and describe how targeted modulation of lactate in the TME might boost immune-cell functions and positively impact cellular immunotherapy, with a focus on NK cell.
Lactate is the end product of anaerobic glycolysis when oxygen levels are insufficient. In addition, our understanding of lactate function has shifted over the last decade; now, lactate is also seen as an important signaling mediator both at the cellular level and the systemic level as a modulator of cell behavior in health and disease (1–3).
During cancer, rapidly proliferating tumor cells produce lactate at high concentrations as a waste molecule of anaerobic and aerobic glycolysis. The extracellular concentrations of lactate released by tumor cells can reach up to 40mM, which is approximately 20 times higher than levels in healthy blood or tissue (4, 5). This metabolic state, in which cells rely on glycolysis rather than on oxidative phosphorylation in an oxygen-rich environment, is known as the Warburg effect. The rapid gain of ATP in a case of sufficient glucose supply is not the only benefit of this metabolic program (6, 7), as other intermediates arising from glycolysis serve as building blocks in other metabolic pathways linked to cell proliferation and protein synthesis (8, 9). The Warburg effect, originally described in tumor cells (10), has recently been reported in many immune cell types such as T cells (11), macrophages (12) or natural killer (NK) cells (13).
Lactate production is tightly linked with the activity of the cytoplasmic enzyme lactate dehydrogenase (LDHA), which mediates the reduction of pyruvate to lactate as well as the oxidation of NADH to NAD+ (14). Lactate is then exported outside of the cytosol by one of the monocarboxylate transporters (MCT) (15, 16).
Lactate not only serves as an end product of glycolysis, it is involved in NADPH production (Figure 1) in glucose-restricted environments, such as the one found in the tumor microenvironment (TME) (17). NADPH is crucial for maintaining redox balance (18) and reductive biosynthesis (19), which are key conditions for tumor growth. The cells activated through specific activation membrane receptors have increased energy uptake (20), which is linked with high lactate production. Lactic acid selectively disables activation of cytotoxic cells including NK cells and therefore impairs immune surveillance and possibly also the cytotoxic properties of therapeutic NK cells prepared for adoptive transfer (21). While many roles of lactate in solid tumors are broadly reported, the role of lactic acid in haemato-oncology disorders seems to be more nuanced. Nevertheless, increased levels of lactate and LDHA have been frequently reported as markers of poor prognosis in different types of leukemia (22, 23) such as acute lymphoblastic leukemia (ALL) (24, 25), acute myeloid leukemia (AML) (26–29), chronic lymphoblastic leukemia (CLL) (22) and chronic myeloid leukemia (CML) (30). Highly active tumor cells, as well as activated immune cells, utilize glucose and glutamine to produce ATP, and also catabolize lactate to produce NADPH.
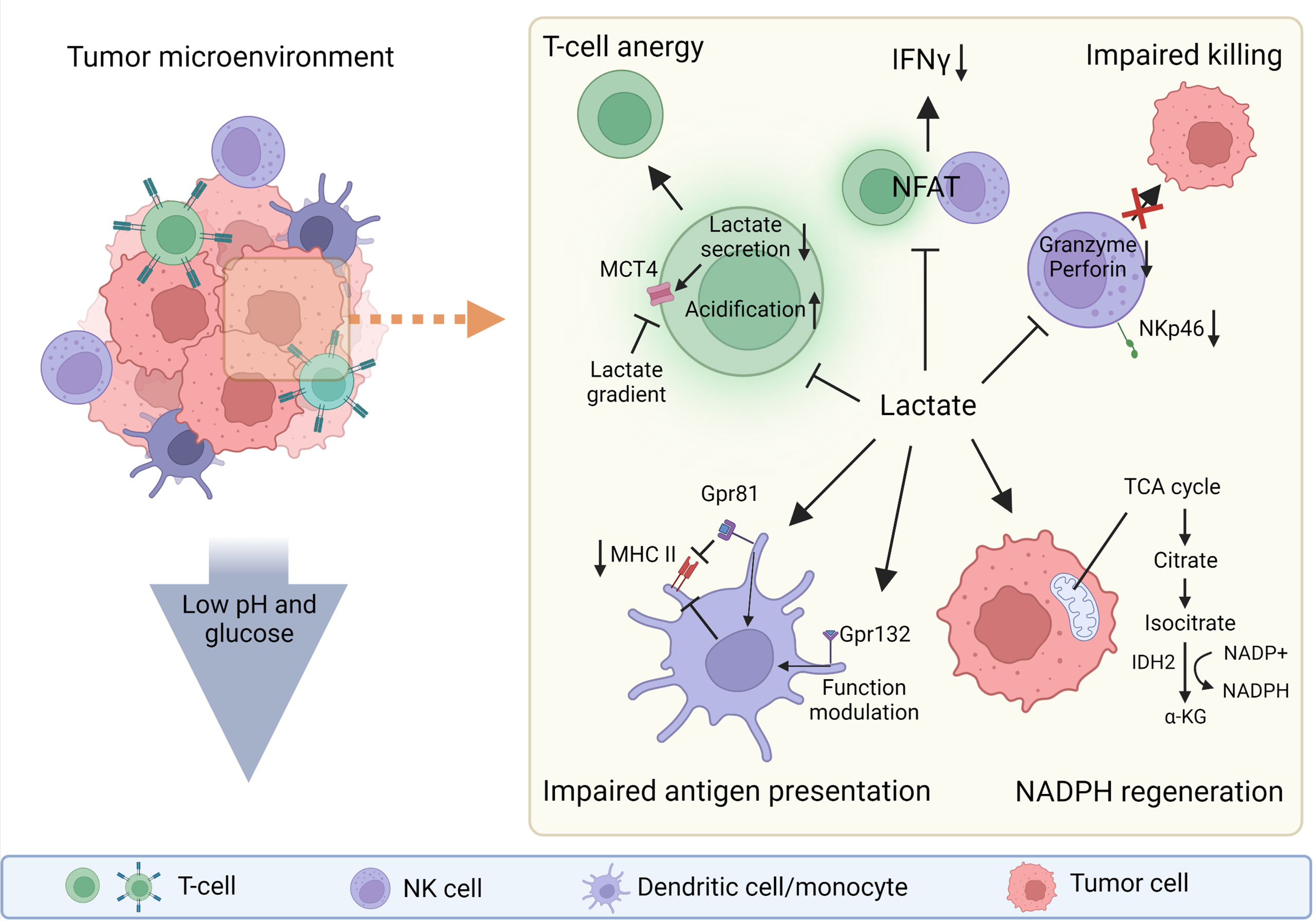
Figure 1 The roles of lactate in tumor microenvironment (TME). Lactate can be strongly immunosuppressive in the tumor microenvironment (TME) or can act as a molecule that helps tumor cells to regenerate NADPH and thus maintain redox balance and support their biosynthetic demands. The low pH and low glucose concentration in the TME also support the immunosuppressive function of the TME. Lactate directly or indirectly influences every cell in the TME. Gpr81, lactate receptor; MHC II, major histocompatibility complex class II. Created with BioRender.com.
Given the growing efforts to employ the cellular responses of NKs and CD8+ T cells in immunotherapies against tumor and leukemic cells, the connection between lactate metabolism and lactate levels and the control of the cytotoxic responses of T and NK cells is an area of intense study. Here we overview current knowledge of the role of lactic acid, particularly that derived from the TME, on the function of immune cells, with a focus on NK cells. We also highlight the remaining research gaps that need further study to gain a deeper understanding of lactate metabolism and improve the efficacy of immunotherapies driven by NK cells.
Robust evidence of the role of lactate in shaping the function of many immune cells has recently emerged (31–34). The ability of lactate to modulate the function of immune cells in the TME is well-described; in the TME lactate serves as a pro-tumorogenic molecule by inhibiting the function of effector cell types such as effector T cells (CD4+ (33), CD8+ (28, 35, 36)) and NK cells (34, 37) and supports the development of suppressor cells such as T regulatory cells (Treg) (32, 38), myeloid-derived suppressor cells (MDSCs) (37, 39) and tolerogenic dendritic cells (tDCs) (32).
Among several described mechanisms of lactate-mediated immunosuppression in the TME is the metabolically driven effect of lactate on T cells. Lactate limits the glycolytic flux of T cells and thus shifts them toward tolerance through several mechanisms. These mechanisms involve lactate accumulation resulting in decreased glyceraldehyde 3-phosphate dehydrogenase (GAPDH) activity. Because the activity of glycolytic enzymes favors the production of pro-inflammatory cytokines, the decrease in GAPDH activity limits their synthesis. Secondly, the lactate-rich environment does not limit Treg function and expansion, thus supporting the induction of tolerance (32, 38). In addition, lactate is required for intra-tumoral Tregs to support tumor progression (40). Lactate also suppresses the proliferation and function of cytotoxic (CD8+) T lymphocytes (CTLs) by selectively inhibiting p38 and c-Jun N-terminal kinase activity, resulting in reduced IFN-γ production (41). Lactic acid also impairs the recruitment of CTLs to the TME by blocking their motility. Hass et al. observed that chemotaxis of CD4+ and CD8+ T cells is reduced by differently expressed lactate transporters (Slc16a1 on CD8+ T cells and Slc5a12 on CD4+ T cells). The authors also showed that the lactate and glycolytic pathways are key regulators of chemokine-induced T-cell migration (35). Lactate diminishes the cytotoxicity of CTLs by lowering intracellular levels of perforin and granzyme B and reducing lytic granule exocytosis (35, 36, 42). Moreover, activated T cells in the TME not only have to compete with tumor cells for glucose, but must also cope with intracellular acidification resulting from tumor cells via MCT-mediated lactate transmission (43). High levels of extracellular tumor-derived lactate in the TME prevent activated T cells from secreting lactate into extracellular space due to the concentration gradient of lactate across the membrane. This gradient causes endogenous lactate to accumulate, which hampers the antitumor activity of effector T cells (1). The similar principles should apply in CD8+, CART and/or NK cells. Nevertheless, this field and comparison still lack consistent data, so our discussion remains rather speculative. Neutralization of the acidic TME and proton-pump inhibitors can reverse the suppression of antitumor immunity and improve immunotherapy (44).
Lactate also suppresses inflammasome assembly, lipopolysaccharide (LPS)-stimulated cytokine secretion and migration of macrophages and monocytes (39). The effects of lactate and the overall acidity of the TME are dependent on the lactate concentration. On one hand, lactate promote the differentiation of monocytes to dendritic cells (DCs) with an immunosuppressive phenotype by stabilizing HIF1α. On the other hand, high levels of lactate in the TME prevent the differentiation of monocytes to DCs (45, 46). Lactic acid suppresses the inflammatory functions of macrophages (M1-like) and enhances regulatory (M2-like) polarization, and these effects are dependent on MCT transport and HIF activation (47). Interestingly subsets of macrophages (M2-like) are able to directly monitor the levels of lactate through G protein-coupled receptor 132 and modulate its functions according the lactate presence (48). Similar to the inhibitory effects of lactic acid reported in monocytes and macrophages, lactic acid reduces DCs maturation and suppresses LPS-induced cytokine production (39). Lactic acid limits cell presentation of tumor antigens by activating G protein-coupled receptor 81 (Gpr81; a receptor for lactate) and inhibits the expression of major histocompatibility complex II (MHC-II) (49) (Figure 1). Moreover, lactic acid suppresses immunoglobulin (Ig)E- and IL-33-dependant inflammatory cytokine and chemokine production (50). In neutrophils, lactate induces the formation of neutrophil extracellular traps (51).
Since the production of lactate acidifies the TME, several studies have investigated the effects of an acid environment on cellular function. Lowering the pH from 6.8 to 6.0 in NK cells decreases in levels of granzyme B and perforin mRNA; furthermore, lactate may interfere with secretory pathways in NK cells and thus modulating the activity of the cytolytic machinery (15, 37). Exposure of NK cells in vitro to 15mM lactate, which is comparable to in-vivo concentrations in tumors (4, 52), lowered the expression of the NKp46 activation receptor (37) (Figure 1). However, the levels of lactate may vary based on type of the tumour (5, 52). Another study showed that lactic acid produced by colorectal cancer cells causes apoptosis of isolated liver resident NK cells in vitro and lowers the amount of tumor-infiltrating NK cells in vivo (34). Brand and colleagues showed that the production of lactic acid by cancer cells limits CTL and NK cell activation (Figure 1) and impairs IFN-γ production; curiously this pattern was not present in CD4+ and IL-17+ CD4+ (Th17) T cells. Lactic acid-induced acidification also inhibits the transcription factor nuclear factor of activated T cells (NFAT), which results in decreased IFN-γ production (21) (Figure 1). The direct link between lactate levels and NFAT activity is an important finding, as NFAT plays a major role in orchestrating activities not only in T cells but also in other cell types including NK cells (53, 54). Interestingly lactate can influence cytosolic calcium (Ca2+) availability through pyruvate and α-ketoglutarate concentrations (55, 56). This could possibly influence NFAT function since it is regulated by Ca2+ abundance (54). The expression of LDHA negatively correlates with the survival of cancer patients, and reduces the numbers and activity of CD8+ T cells (21). Elevated lactate levels indirectly inhibit NK cell function by increasing the number of MDSCs (57). MDSCs are a heterogeneous population of immature myeloid cells that mediate the immunosuppressive environment in the TME. As well as suppressing NK cell activity, MDSCs prevent DCs maturation and inhibit T cell activation (37). The findings from the studies that have investigated influence of lactate on various immune cells need to be considered during further research leading to the use of adoptive transfer of NK cells to target solid tumors.
Another marker of poor prognosis in oncology patients is serum levels of LDH, as shown in CLL (22) and AML (24). One study suggested that tumors with elevated lactate dehydrogenase A (LDHA) levels are more prone to immune evasion, and therefore tumor progression occurs due to limited anti-tumor mechanisms (21). In contrast, another study showed that LDHA is crucial for the anti-tumor and anti-viral functions of murine NK cells (58). However, it is difficult to obtain data that directly indicates the role of lactate on the different mechanisms of NK cell-mediated killing in the TME. Since the environment is very complex and lactate influences every cell present, NK cell inhibition can occur due to many mechanisms. These mechanisms include the direct inhibitory effects of lactate on NK cells (34) or indirect effects, whereby lactate alters the function of other immune cells, which then inhibit the function of NK cells,37,40.
Thus, overcoming the negative effects of lactate on cell-based immunotherapies remains elusive. A study that used LDH isolated from various cell lines showed that millimolar concentrations of exogenous pyruvate can inhibit LDH function in an MCT-1 dependent manner (59). But since these experiments have not been performed in the TME, it can only be speculated that a similar mechanism occurs in the TME. Hence, this finding needs to be validated in the TME before pyruvate could eventually be used in a clinical setting. In vivo experiments that injected a melanoma cells into mice showed that LDHA knockdown decreases PD-L1 expression, and thus makes tumor cells more susceptible to anti-PD-1 treatment. Mice with LDHA knockdown also have higher infiltration of NK and CD8+ T cells into tumors. These findings stimulate further research, opening a space for the eventual pharmacological targeting of LDH (60). Furthermore, strategies that remove lactate from the TME are emerging (15, 16, 61). In recent years the various efforts to lactate removal appeared. They aim mainly to lactate export by blocking MCT (15, 16, 62), however also systemic alkalization (61) approaches or lactate traps (63) have been tested. Blocking MCT4 (the main transporter for lactate secretion) in cancer cells restores the cytotoxicity of NK cells that was inhibited by the lactate environment (16). Another approach showed that alkalization of the tissue milieu by oral administration of sodium bicarbonate in a mouse model of λ-myc lymphoma restores IFN-γ production by NK cells. However, this approach did not restore the cytotoxic functions of NK cells (61). Overall, lactate is an omnipresent substance in the TME, and as such it directly influences the outcome of anti-tumor immunity (37, 64, 65).
The production and export of lactate by rapidly proliferating tumor cells not only (8, 66) influences the function of immune cells and supports the ability of the tumor to grow and escape immune recognition (as discussed above); the acidification of the TME also limits the efficacy of cell-based immunotherapies (15, 45, 62, 67). The cells used for adoptive transfer thus need to be in the best possible fitness and metabolic state to be able to overcome TME-mediated inhibition (13, 16, 58). The findings of lactate interference with NFAT signaling possibly also impair not only IFNγ expression but also key NK cell maintenance cytokines as IL-2 as it is NFAT dependent (68, 69).
NK cells, the key cells responsible for cancer immunosurveillance, eliminate cancerous cells by a multistep process. First, the cancer cell is recognized through a series of specific activatory and inhibitory receptors that screen for the density of human leukocyte antigen (HLA) molecules and other damage markers on the surface of controlled cells. The eventual cytotoxic elimination of the target cells is then initiated by several mechanisms including integrin- or antibody-mediated adhesion of NK cells to the target cell, followed by the formation of an immunologic synapse and release of lytic granules (70–72). As we and others reviewed earlier, this process is energetically demanding and therefore tightly orchestrated to maintain the energetic homeostasis of organism (20, 73).
Recent research has focused on developing new protocols for the use of adoptively transferred immune cells to treat various disorders including cancers (74–76) and autoimmune diseases (77, 78). Indeed immunotherapies that use different approaches and cell types are under investigation, including DC-based therapies, CAR T cells, NK cell-based therapies and CAR NK cells (75, 79–81). The efforts to treat various haemato-oncologic disorders using adoptively transferred immune cells are expanding; since 2020, there are 158 newly registered clinical studies (Figure 2).
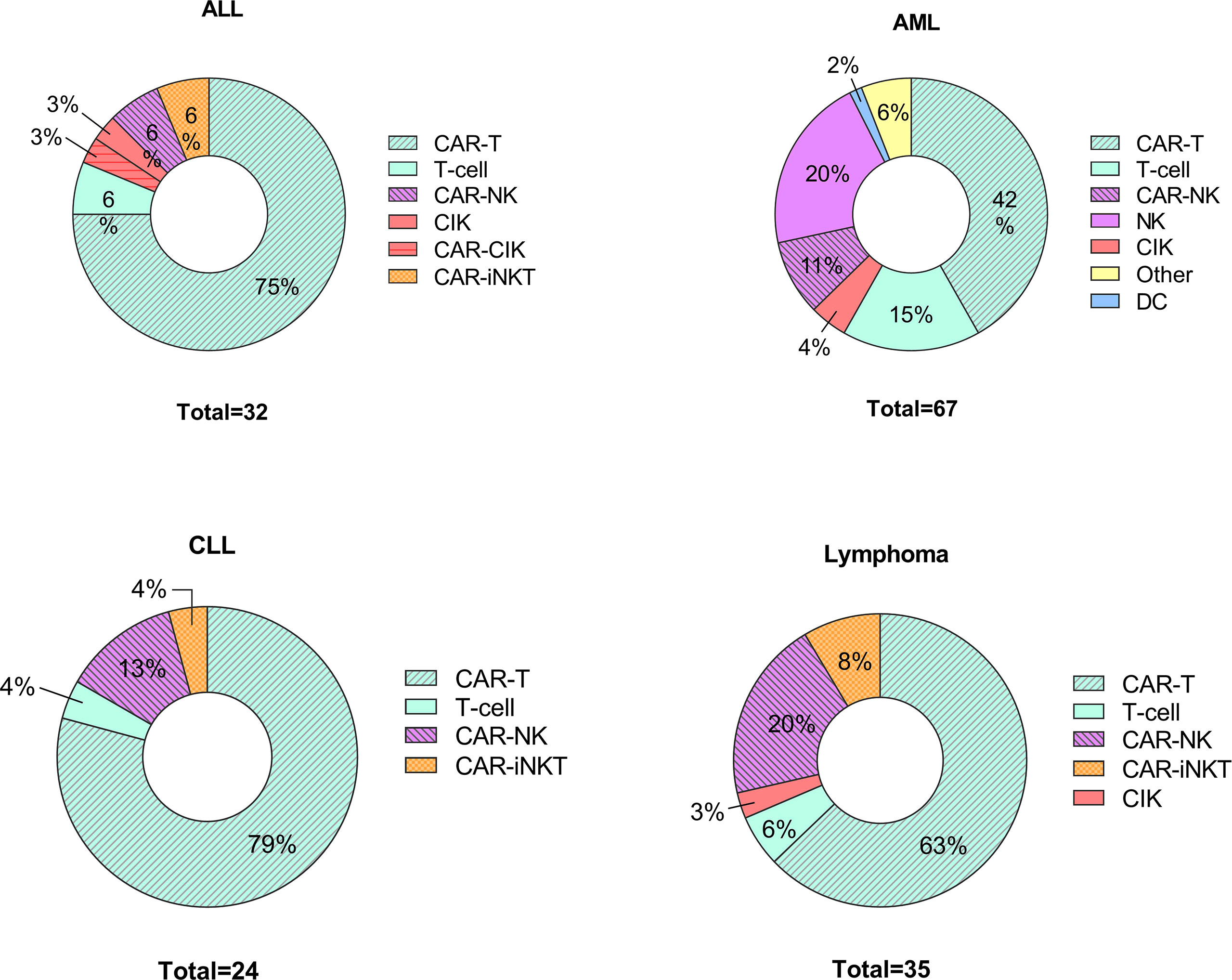
Figure 2 Cell-based therapies in hematological disorders. Graphical representations of clinical trials of adoptive cell transfer in hemato-oncological malignancies posted to clintrials.gov between 1st of January 2020 – 1st of March 2022. ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia including myelodysplastic syndrome; CLL, chronic lymphoblastic leukemia; CIK, cytokine induced killers; iNK, invariant NK cells.
One of the major benefits of the NK approach (compared with approaches that use other types of immune cells) is the partial prevention of graft versus host disease, as shown in preclinical studies (82, 83). The therapy setups include ex vivo cytokine-primed NKs (84–86), CAR NKs (79, 87, 88) and bi- or tri-specific killer engagers (BiKEs or TriKEs) (83, 89, 90). BiKEs and TriKEs are NK cells with two or three single-chain variable fragments, respectively, with various antigen specificity enabling precise cell-to-cell contact with tumor cell (91). A phase I/II clinical trial (NCT01904136) of an NK-based therapy showed promising results. The study aimed to decrease cancer relapse after a stem-cell transplant using ex vivo mb-IL21-expanded NK cells. The disease-free survival was 66% in patients who received multiple high doses of NK cells and 44% in controls, and the relapse rate was 9.5 times lower at 24-months follow-up (81). However, cells that are expanded in the optimal conditions of cell culture still have to face the strongly immunosuppressive niche of TME after adoptive transfer (92, 93). Since lactate is one of the most abundant metabolites in the TME (64, 94) its presence causes NFAT-regulated NK and CD8+ T cell suppression (21), impairment of NK cell cytolytic function (15, 34, 37) or inhibition of antigen presentation by DCs (49), which impairs the anti-tumor functions of these cell types (21, 45, 49, 95).
Intensive efforts have been invested into NK cell-based immunotherapy (96–98). The research field was boosted by the enormous success of several commercialized CAR T cell products. Improving the half-life of adoptively transferred cells and maintaining their cytotoxic capacity remain important research tasks. Efforts to improve these therapies drive research into a better molecular understanding of metabolic processes in T cells and NK cells, which closely control their cellular functions. Immunometabolism in the cytotoxic cells used for immunotherapy is essential to maintain their massive proliferation ex vivo, but the immunometabolism further changes after adoptive transfer as cytotoxic granules develop and the cells successfully adapt to their environment (13, 99, 100). NK cells are beneficial for both solid and hematological tumors (82, 101–103). Nersesian et al. examined 53 studies of various solid cancers and concluded that NK-cell infiltration into the tumor correlates with improved overall survival (102). Results from an experimental model of NK cell infiltration into tumor tissue support these clinical data. In these experiments, cancerous pancreatic cells were treated with an NK cell-recruiting protein-conjugated antibody (NRP-body), which after binding to the pancreatic tumor cell released a chemotactic molecule for NK cells. This boosted the ability of NK cells to infiltrate the tumor stroma and thus improved the outcome of immunotherapy (104). In hematological tumors, NK cell-based therapies benefit from a possible graft versus leukemia (GvL) effect by alloreactive NK cells (103, 105) and from the ability of NK cells to migrate into the bone marrow to eliminate leukemic cells (106). This latter feature of NK cells can be improved by incorporating chemokine receptors into the NK-based therapy that are specific for homing into the bone-marrow niche (107).
However, despite the initial success of NK cell-based therapies, the TME is one of the major obstacles to their success (92, 93). As well as lactate, transforming growth factor β (TGF-β) also suppresses immune cells in TME and other immune-suppressive molecules may be present (39, 108, 109). Nevertheless, studies show that NK cells expanded with IL-21-expressing feeder cells are not suppressed by TME in a model of ovarian cancer (13). Terren et al. showed that cytokines modulate the metabolism of NK cells and glycolysis is important for NK cell effector functions (86).
However, specific evidence about immunometabolism changes in adoptively transferred immunotherapeutic cells is scarce as it is difficult to obtain data. Therefore, the molecular mechanisms that orchestrate immunometabolism are highly underexplored. Adoptively transferred NK cells are dependent on the balance of activity of activator and inhibitory receptors and their cytotoxic machinery consisting of cytotoxic mediators including granzyme B and perforin. The success of adoptive transfer and further cytotoxicity are tightly dependent on NK cell status, fitness and donor variability (110). Therefore, the presence of lactate in the TME must be taken into account during the production of immunotherapeutic cells.
The functions of lactate, originally described and long-understood as an end product of metabolism, have recently been intensively studied and completely reconsidered. The plethora of roles of lactate now includes cancer biomarkers and target molecules for therapies. Together with LDHA, lactate is a marker of poor prognosis in haemato-oncological patients (23, 24, 62). As one of the main inhibitory molecules produced in the TME, lactate is a crucial obstacle to a patient´s immune response or the efficacy of cell-based immunotherapies in various cancers (92, 93). Lactate can trigger signaling in immune cells and thus limit their effector functions. These recent findings open the door to lactate targeting to boost the immune response to cancer. To date, lactate has been targeted almost exclusively by blocking its secretion from tumor cells, and this strategy shows promising results in some cancers (16, 62). However, with the advent of cell-based immunotherapies (NK cells, T cells), new questions need to be addressed. Contemporary research accepts the crucial role of metabolism in the function of immune effector cells (73), and so efforts are underway to try to modulate immunometabolism to produce superior therapeutic cells (73, 99). Nevertheless, this approach is still in its infancy and needs further research (20, 73, 111). In order to improve the outcome of NK cell-based therapies, the deeper understanding of NK cell cytotoxicity in patient´s body is needed. We also need to better understand the trajectories of therapeutic NK cells after adoptive transfer. This knowledge could be reached with the use of new models of bone marrow or tumor niche microenvironmnet. Furthermore, the precise map of NK cell metabolism might lead to possibility of precise metabolic-based modification during expansion and preparation of therapeutic cells in order to overcome inhibitory niche of TME. To conclude, even though new protocols to enhance the immunotherapeutic potential of cell-based therapies are emerging, lactate still plays an important role in thwarting of success of these approaches and so is a key obstacle to better cancer treatment.
MJ wrote and drafted the manuscript. TF, MH-K, and JF reviewed and edited the manuscript. LJ create the figures. All authors contributed to the article and approved the submitted version.
The authors were supported by the Ministry of Health of the Czech Republic grant nr. – NU22-08-00287 all rights reserved and DRO (Institute of Hematology and Blood Transfusion – IHBT, 00023736) and by the European Social Fund and European Regional Development Fund – Project MAGNET (No. CZ.02.1.01/0.0/0.0/15_003/0000492) and ENOCH (CZ.02.1.01/0.0/0.0/16_019/0000868).
Authors would like to thank Dr. Jessica Tamanini from Insight Editing London for critically reviewing the manuscript before submission.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Brown TP, Ganapathy V. Lactate/GPR81 signaling and proton motive force in cancer: Role in angiogenesis, immune escape, nutrition, and warburg phenomenon. Pharmacol Ther (2020) 206:107451. doi: 10.1016/j.pharmthera.2019.107451
2. Barros LF. Metabolic signaling by lactate in the brain. Trends Neurosci (2013) 36(7):396–404. doi: 10.1016/j.tins.2013.04.002
3. Philp A, Macdonald AL, Watt PW. Lactate - a signal coordinating cell and systemic function. J Exp Biol (2005) 208(24):4561–75. doi: 10.1242/jeb.01961
4. Pérez-Tomás R, Pérez-Guillén I. Lactate in the tumor microenvironment: An essential molecule in cancer progression and treatment. Cancers (Basel) (2020) 12(11):1–29. doi: 10.3390/cancers12113244
5. Walenta S, Schroeder T, Mueller-Klieser W. Lactate in solid malignant tumors: Potential basis of a metabolic classification in clinical oncology. Curr Med Chem (2012) 11(16):2195–204. doi: 10.2174/0929867043364711
6. Liberti MV, Locasale JW. The warburg effect: How does it benefit cancer cells? Trends Biochem Sci (2016) 41(3):211–8. doi: 10.1016/j.tibs.2015.12.001
7. Shestov AA, Liu X, Ser Z, Cluntun AA, Hung YP, Huang L, et al. Quantitative determinants of aerobic glycolysis identify flux through the enzyme GAPDH as a limiting step. Elife 3:e03342. doi: 10.7554/eLife.03342
8. DeBerardinis RJ, Chandel NS. We need to talk about the warburg effect. Nat Metab (2020) 2(2):127–9. doi: 10.1038/s42255-020-0172-2
9. Vander Heiden MG, Cantley LC, Thompson CB. Understanding the warburg effect: The metabolic requirements of cell proliferation. Sci (80- ) (2009) 324(5930):1029–33. doi: 10.1007/978-3-540-29678-2_5605
10. Warburg O, Wind F, Negelein E. I . killing-off of tumor cells in vitro. J Gen Physiol (1927) 8(6):519–30.
11. Ma EH, Verway MJ, Johnson RM, Roy DG, Steadman M, Hayes S, et al. Metabolic profiling using stable isotope tracing reveals distinct patterns of glucose utilization by physiologically activated CD8+ T cells. Immunity (2019) 51(5):856–870.e5. doi: 10.1016/j.immuni.2019.09.003
12. Jing C, Castro-Dopico T, Richoz N, Tuong ZK, Ferdinand JR, Lok LSC, et al. Macrophage metabolic reprogramming presents a therapeutic target in lupus nephritis. Proc Natl Acad Sci U S A (2020) 117(26):15160–71. doi: 10.1073/pnas.2000943117
13. Poznanski SM, Singh K, Ritchie TM, Aguiar JA, Fan IY, Portillo AL, et al. Metabolic flexibility determines human NK cell functional fate in the tumor microenvironment. Cell Metab (2021) 33(6):1205–1220.e5. doi: 10.1016/j.cmet.2021.03.023
14. Urbańska K, Orzechowski A. Unappreciated role of LDHA and LDHB to control apoptosis and autophagy in tumor cells. Int J Mol Sci (2019) 20(9):1–15. doi: 10.3390/ijms20092085
15. Doherty JR, Yang C, Scott KEN, Cameron MD, Fallahi M, Li W, et al. Blocking lactate export by inhibiting the myc target MCT1 disables glycolysis and glutathione synthesis. Cancer Res (2014) 74(3):908–20. doi: 10.1158/0008-5472.CAN-13-2034
16. Long Y, Gao Z, Hu X, Xiang F, Wu Z, Zhang J, et al. Downregulation of MCT4 for lactate exchange promotes the cytotoxicity of NK cells in breast carcinoma. Cancer Med (2018) 7(9):4690–700. doi: 10.1002/cam4.1713
17. Ying M, You D, Zhu X, Cai L, Zeng S, Hu X. Lactate and glutamine support NADPH generation in cancer cells under glucose deprived conditions. Redox Biol (2021) 46:102065. doi: 10.1016/j.redox.2021.102065
18. Tao R, Zhao Y, Chu H, Wang A, Zhu J, Chen X, et al. Genetically encoded fluorescent sensors reveal dynamic regulation of NADPH metabolism. Nat Methods (2017) 14(7):720–8. doi: 10.1038/nmeth.4306
19. Fan J, Ye J, Kamphorst JJ, Shlomi T, Thompson CB, Rabinowitz JD. Quantitative flux analysis reveals folate-dependent NADPH production. Nature (2014) 510(7504):298–302. doi: 10.1038/nature13236
20. Hortová-Kohoutková M, Lázničková P, Frič J. How immune-cell fate and function are determined by metabolic pathway choice: The bioenergetics underlying the immune response. BioEssays (2021) 43(2):1–15. doi: 10.1002/bies.202000067
21. Brand A, Singer K, Koehl GE, Kolitzus M, Schoenhammer G, Thiel A, et al. LDHA-associated lactic acid production blunts tumor immunosurveillance by T and NK cells. Cell Metab (2016) 24(5):657–71. doi: 10.1016/j.cmet.2016.08.011
22. Autore F, Strati P, Innocenti I, Corrente F, Trentin L, Cortelezzi A, et al. Elevated lactate dehydrogenase has prognostic relevance in treatment-naïve patients affected by chronic lymphocytic leukemia with trisomy 12. Cancers (Basel) (2019) 11(7):1–12. doi: 10.3390/cancers11070896
23. Elbossaty WFM. Lactate dehydrogenase (LDH) as prognostic marker in acute leukemia quantitative method. J Blood Disord Transfus (2017) 08(01):1–7. doi: 10.4172/2155-9864.1000375
24. Hafiz G, Mannan MA. Serum lactate dehydrogenase level in childhood acute lymphoblastic leukemia. Bangladesh Med Res Counc Bull (2007) 33(3):88–91. doi: 10.3329/bmrcb.v33i3.1139
25. Yu H, Yin Y, Yi Y, Cheng Z, Kuang W, Li R, et al. Targeting lactate dehydrogenase a (LDHA) exerts antileukemic effects on T-cell acute lymphoblastic leukemia. Cancer Commun (2020) 40(10):501–17. doi: 10.1002/cac2.12080
26. Kalaycio M, Rybicki L, Pohlman B, Dean R, Sweetenham J, Andresen S, et al. Elevated lactate dehydrogenase is an adverse predictor of outcome in HLA-matched sibling bone marrow transplant for acute myelogenous leukemia. Bone Marrow Transplant (2007) 40(8):753–8. doi: 10.1038/sj.bmt.1705811
27. Brault C, Zerbib Y, Delette C, Marc J, Gruson B, Marolleau JP, et al. The warburg effect as a type b lactic acidosis in a patient with acute myeloid leukemia: A diagnostic challenge for clinicians. Front Oncol. (2018) 8:232. doi: 10.3389/fonc.2018.00232
28. Chen Y, Feng Z, Kuang X, Zhao P, Chen B, Fang Q. Increased lactate in AML blasts upregulates TOX expression. leading to exhaustion CD8 + cytolytic T Cells (2021) 11(11):5726–42.
29. Geva M, Shouval R, Fein JA, Yerushalmi R, Shimoni A, Nagler A, et al. The prognostic role of pretransplant serum lactate dehydrogenase levels in acute myeloid leukemia and lymphoma patients undergoing allogenic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant (2020) 26(3):S111–2. doi: 10.1016/j.bbmt.2019.12.620
30. Silvano A, Menegazzi G, Peppicelli S, Mancini C, Biagioni A, Tubita A, et al. Lactate maintainsBCR/Abl expression and signalinginChronic myeloid leukemia cells under nutrient restriction. Oncol Res Featur Preclin Clin Cancer Ther. (2022) 29(1):33–46 doi: 10.3727/096504022x16442289212164
31. Noe JT, Rendon BE, Geller AE, Conroy LR, Morrissey SM, Young LEA, et al. Lactate supports a metabolic-epigenetic link in macrophage polarization. Sci Adv (2021) 7(46):1–13. doi: 10.1126/sciadv.abi8602
32. Marin E, Bouchet-Delbos L, Renoult O, Louvet C, Nerriere-Daguin V, Managh AJ, et al. Human tolerogenic dendritic cells regulate immune responses through lactate synthesis. Cell Metab (2019) 30(6):1075–1090.e8. doi: 10.1016/j.cmet.2019.11.011
33. Comito G, Iscaro A, Bacci M, Morandi A, Ippolito L, Parri M, et al. Lactate modulates CD4 + T-cell polarization and induces an immunosuppressive environment, which sustains prostate carcinoma progression via TLR8/miR21 axis. Oncogene (2019) 38(19):3681–95. doi: 10.1038/s41388-019-0688-7
34. Harmon C, Robinson MW, Hand F, Almuaili D, Mentor K, Houlihan DD, et al. Lactate-mediated acidification of tumor microenvironment induces apoptosis of liver-resident NK cells in colorectal liver metastasis. Cancer Immunol Res (2019) 7(2):335–46. doi: 10.1158/2326-6066.CIR-18-0481
35. Haas R, Smith J, Rocher-Ros V, Nadkarni S, Montero-Melendez T, D'Acquisto F, et al. Lactate regulates metabolic and proinflammatory circuits in control of T cell migration and effector functions. PloS Biol (2015) 13(7):1–24. doi: 10.1371/journal.pbio.1002202
36. Feichtinger RG, Lang R. Targeting l-lactate metabolism to overcome resistance to immune therapy of melanoma and other tumor entities. J Oncol (2019) 2019:1–12. doi: 10.1155/2019/2084195
37. Husain Z, Huang Y, Seth P, Sukhatme VP. Tumor-derived lactate modifies antitumor immune response: Effect on myeloid-derived suppressor cells and NK cells. J Immunol (2013) 191(3):1486–95. doi: 10.4049/jimmunol.1202702
38. Angelin A, Gil-de-Gómez L, Dahiya S, Jiao J, Guo L, Levine MH, et al. Foxp3 reprograms T cell metabolism to function in low-glucose, high-lactate environments. Cell Metab (2017) 25(6):1282–1293.e7. doi: 10.1016/j.cmet.2016.12.018
39. Caslin HL, Abebayehu D, Pinette JA, Ryan JJ. Lactate is a metabolic mediator that shapes immune cell fate and function. Front Physiol (2021) 12:688485. doi: 10.3389/fphys.2021.688485
40. Watson MLJ, Vignali PDA, Mullett SJ, Overacre-Delgoffe AE, Peralta RM, Grebinoski S, et al. Metabolic support of tumour-infiltrating regulatory T cells by lactic acid. Nature (2021) 591(7851):645–51. doi: 10.1038/s41586-020-03045-2
41. Mendler AN, Hu B, Prinz PU, Kreutz M, Gottfried E, Noessner E. Tumor lactic acidosis suppresses CTL function by inhibition of p38 and JNK/c-jun activation. Int J Cancer (2012) 131(3):633–40. doi: 10.1002/ijc.26410
42. Wang ZH, Peng WB, Zhang P, Yang XP, Zhou Q. Lactate in the tumour microenvironment: From immune modulation to therapy. EBioMedicine (2021) 73:103627. doi: 10.1016/j.ebiom.2021.103627
43. Fischer K, Hoffmann P, Voelkl S, Meidenbauer N, Ammer J, Edinger M, et al. Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood (2007) 109(9):3812–9. doi: 10.1182/blood-2006-07-035972
44. Ippolito L, Morandi A, Giannoni E, Chiarugi P. Lactate: A metabolic driver in the tumour landscape. Trends Biochem Sci (2019) 44(2):153–66. doi: 10.1016/j.tibs.2018.10.011
45. Certo M, Tsai CH, Pucino V, Ho PC, Mauro C. Lactate modulation of immune responses in inflammatory versus tumour microenvironments. Nat Rev Immunol (2021) 21(3):151–61. doi: 10.1038/s41577-020-0406-2
46. Gottfried E, Kunz-Schughart LA, Ebner S, Mueller-Klieser W, Hoves S, Andreesen R, et al. Tumor-derived lactic acid modulates dendritic cell activation and antigen expression. Blood (2006) 107(5):2013–21. doi: 10.1182/blood-2005-05-1795
47. Zhang D, Tang Z, Huang H, Zhou G, Cui Ch, Weng Y, et al. Metabolic regulation of gene expression by histone lactylation. Nature (2019) 574(7779):575–80. doi: 10.1038/s41586-019-1678-1
48. Chen P, Zuo H, Xiong H, Kolar MJ, Chu Q, Saghatelian A, et al. Gpr132 sensing of lactate mediates tumor-macrophage interplay to promote breast cancer metastasis. Proc Natl Acad Sci U S A (2017) 114(3):580–5. doi: 10.1073/pnas.1614035114
49. Brown TP, Bhattacharjee P, Ramachandran S, Sivaprakasam S, Ristic B, Sikder MOF, et al. The lactate receptor GPR81 promotes breast cancer growth via a paracrine mechanism involving antigen-presenting cells in the tumor microenvironment. Oncogene (2020) 39(16):3292–304. doi: 10.1038/s41388-020-1216-5
50. Abebayehu D, Spence AJ, Caslin H, Taruselli M, Haque TT, Kiwanuka KN, et al. Lactic acid suppresses IgE-mediated mast cell function in vitro and in vivo. Cell Immunol (2019) 341:103918. doi: 10.1016/j.cellimm.2019.04.006
51. Awasthi D, Nagarkoti S, Sadaf S, Chandra T, Kumar S, Dikshit M. Glycolysis dependent lactate formation in neutrophils: A metabolic link between NOX-dependent and independent NETosis. Biochim Biophys Acta - Mol Basis Dis (2019) 1865(12):165542. doi: 10.1016/j.bbadis.2019.165542
52. Brizel DM, Schroeder T, Scher RL, Walenta S, Clough RW, Dewhirst MW, et al. Elevated tumor lactate concentrations predict for an increased risk of metastases in head-and-neck cancer. Int J Radiat Oncol Biol Phys (2001) 51(2):349–53. doi: 10.1016/S0360-3016(01)01630-3
53. Fric J, Zelante T, Wong AYW, Mertes A, Yu HB, Ricciardi-Castagnoli P. NFAT control of innate immunity. Blood (2012) 120(7):1380–9. doi: 10.1182/blood-2012-02-404475
54. Bendickova K, Tidu F, Fric J. Calcineurin– NFAT signalling in myeloid leucocytes: new prospects and pitfalls in immunosuppressive therapy. EMBO Mol Med (2017) 9(8):990–9. doi: 10.15252/emmm.201707698
55. Fendt S-M, Bell EL, Keibler MA, Olenchock BA, Mayers JR, et al. Reductive glutamine metabolism is a function of the αketoglutarate to citrate ratio in cells. Nat Commun (2013) 4:1–21. doi: 10.1038/ncomms3236
56. Gellerich FN, Gizatullina Z, Trumbeckaite S, Nguyen HP, Pallas T, Arandarcikaite O, et al. The regulation of OXPHOS by extramitochondrial calcium. Biochim Biophys Acta - Bioenerg (2010) 1797(6-7):1018–27. doi: 10.1016/j.bbabio.2010.02.005
57. Husain Z, Seth P, Sukhatme VP. Tumor-derived lactate and myeloid-derived suppressor cells: Linking metabolism to cancer immunology. Oncoimmunology (2013) 2(11):9–12. doi: 10.4161/onci.26383
58. Sheppard S, Santosa EK, Lau CM, Violante S, Giovanelli P, Kim H, et al. Lactate dehydrogenase a-dependent aerobic glycolysis promotes natural killer cell anti-viral and anti-tumor function. Cell Rep (2021) 35(9):1–17. doi: 10.1016/j.celrep.2021.109210
59. Rao Y, Gammon ST, Sutton MN, Zacharias NM, Bhattacharya P, Piwnica-Worms D. Excess exogenous pyruvate inhibits lactate dehydrogenase activity in live cells in an MCT1-dependent manner. J Biol Chem (2021) 297(1):100775. doi: 10.1016/j.jbc.2021.100775
60. Daneshmandi S, Wegiel B. Blockade of lactate dehydrogenase-a ( LDH-a ) improves e ffi cacy of anti-programmed cell death-1. Cancers (Basel) (2019) 11(4):450. doi: 10.3390/cancers11040450
61. Pötzl J, Roser D, Bankel L, Hömberg N, Geishauser A, Brenner CD, et al. Reversal of tumor acidosis by systemic buffering reactivates NK cells to express IFN-γ and induces NK cell-dependent lymphoma control without other immunotherapies. Int J Cancer (2017) 140(9):2125–33. doi: 10.1002/ijc.30646
62. Saulle E, Spinello I, Quaranta MT, Pasquini L, Pelosi E, Iorio E, et al. Targeting lactate metabolism by inhibiting MCT1 or MCT4 impairs leukemic cell proliferation, induces two different related death-pathways and increases chemotherapeutic sensitivity of acute myeloid leukemia cells. Front Oncol (2021) 10:621458. doi: 10.3389/fonc.2020.621458
63. He R, Zang J, Zhao Y, Liu Y, Ruan S, Zheng X, et al. Nanofactory for metabolic and chemodynamic therapy: pro-tumor lactate trapping and anti-tumor ROS transition. J Nanobiotechnology (2021) 19(1):1–14. doi: 10.1186/s12951-021-01169-9
64. Elia I, Haigis MC. Metabolites and the tumour microenvironment: from cellular mechanisms to systemic metabolism. Nat Metab (2021) 3(1):21–32. doi: 10.1038/s42255-020-00317-z
65. Colegio OR, Chu NQ, Szabo AL, Chu T, Rhenberg AM, Jairam V, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature (2014) 513(7519):559–63. doi: 10.1038/nature13490
66. Rogatzki MJ, Ferguson BS, Goodwin ML, Gladden LB. Lactate is always the end product of glycolysis. Front Neurosci (2015) 9:22. doi: 10.3389/fnins.2015.00022
67. Wu H, Estrella V, Beatty M, Abrahams D, El-Kenawi A, Russell S, et al. T-Cells produce acidic niches in lymph nodes to suppress their own effector functions. Nat Commun (2020) 11(1):1–13. doi: 10.1038/s41467-020-17756-7
68. Bendickova K, Fric J. Roles of IL-2 in bridging adaptive and innate immunity, and as a tool for cellular immunotherapy. J Leukoc Biol (2020) 108(1):427–37. doi: 10.1002/JLB.5MIR0420-055R
69. Wang H, Grzywacz B, Sukovich D, McCullar V, Cao Q, Lee AB, et al. The unexpected effect of cyclosporin a on CD56+CD16- and CD56+CD16+ natural killer cell subpopulations. Blood (2007) 110(5):1530–9. doi: 10.1182/blood-2006-10-048173
70. Prager I, Watzl C. Mechanisms of natural killer cell-mediated cellular cytotoxicity. J Leukoc Biol (2019) 105(6):1319–29. doi: 10.1002/JLB.MR0718-269R
71. Orange JS. Formation and function of the lytic NK-cell immunological synapse. Nat Rev Immunol (2008) 8(9):713–25. doi: 10.1038/nri2381
72. Ochoa MC, Minute L, Rodriguez I, Garasa S, Perez-Ruiz E, Inogés S, et al. Antibody-dependent cell cytotoxicity: Immunotherapy strategies enhancing effector NK cells. Immunol Cell Biol (2017) 95(4):347–55. doi: 10.1038/icb.2017.6
73. O’Brien KL, Finlay DK. Immunometabolism and natural killer cell responses. Nat Rev Immunol (2019) 19(5):282–90. doi: 10.1038/s41577-019-0139-2
74. Zemanova M, Cernovska M, Havel L, Bartek T, Lukesova S, Jakesova J, et al. Autologous dendritic cell-based immunotherapy (DCVAC/LuCa) and carboplatin/paclitaxel in advanced non-small cell lung cancer: A randomized, open-label, phase I/II trial. Cancer Treat Res Commun (2021) 28:100427. doi: 10.1016/j.ctarc.2021.100427
75. Bloy N, Pol J, Aranda F, Eggermont A, Cremer I, Fridman WH, et al. Trial watch: Dendritic cell-based anticancer therapy. Oncoimmunology (2014) 3(11):e963424–1-e963424-16. doi: 10.4161/21624011.2014.963424
76. Sterner RC, Sterner RM. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J (2021) 11(4):1–11. doi: 10.1038/s41408-021-00459-7
77. Esensten JH, Muller YD, Bluestone JA, Tang Q. Regulatory T-cell therapy for autoimmune and autoinflammatory diseases: The next frontier. J Allergy Clin Immunol (2018) 142(6):1710–8. doi: 10.1016/j.jaci.2018.10.015
78. Rad F, Ghorbani M, Mohammadi Roushandeh A, Habibi Roudkenar M. Mesenchymal stem cell-based therapy for autoimmune diseases: emerging roles of extracellular vesicles. Mol Biol Rep (2019) 46(1):1533–49. doi: 10.1007/s11033-019-04588-y
79. Marofi F, Abdul-Rasheed OF, Rahman HS, Budi HS, Jalil AT, Yumashev AV, et al. CAR-NK cell in cancer immunotherapy; a promising frontier. Cancer Sci (2021) 112(9):3427–36. doi: 10.1111/cas.14993
80. Wang X, Rivière I. Clinical manufacturing of CAR T cells: Foundation of a promising therapy. Mol Ther - Oncolytics (2016) 3:16015. doi: 10.1038/mto.2016.15
81. Ciurea SO, Kongtim P, Soebbing D, Trikha P, Behbehani G, Rondon G, et al. Decrease post-transplant relapse using donor-derived expanded NK-cells. Leukemia (2021) 36(1):155–64. doi: 10.1038/s41375-021-01349-4
82. Ruggeri L, Vago L, Eikema DJ, de Wreede LC, Ciceri F, Diaz MA, et al. Natural killer cell alloreactivity in HLA-haploidentical hematopoietic transplantation: a study on behalf of the CTIWP of the EBMT. Bone Marrow Transplant (2021) 56(8):1900–7. doi: 10.1038/s41409-021-01259-0
83. Myers JA, Miller JS. Exploring the NK cell platform for cancer immunotherapy. Nat Rev Clin Oncol (2021) 18(2):85–100. doi: 10.1038/s41571-020-0426-7
84. Burns LJ, Weisdorf DJ, DeFor TE, Vesole DH, Repka TL, Blazar BR, et al. IL-2-based immunotherapy after authologous transplantation for lymphoma and breast cancer induces immune activation and cytokine release: A phase I/II trial. Bone Marrow Transplant (2003) 32(2):177–86. doi: 10.1038/sj.bmt.1704086
85. Mac Cheever MA. Twelve immunotherapy drugs that could cure cancers. Immunol Rev (2008) 222(1):357–68. doi: 10.1111/j.1600-065X.2008.00604.x
86. Terrén I, Orrantia A, Mosteiro A, Vitallé J, Zenarruzabeitia O, Borrego F. Metabolic changes of interleukin-12/15/18-stimulated human NK cells. Sci Rep (2021) 11(1). doi: 10.1038/s41598-021-85960-6
87. Albinger N, Hartmann J, Ullrich E. Current status and perspective of CAR-T and CAR-NK cell therapy trials in Germany. Gene Ther (2021) 28(9):513–27. doi: 10.1038/s41434-021-00246-w
88. Daher M, Melo Garcia L, Li Y, Rezvani K. CAR-NK cells: the next wave of cellular therapy for cancer. Clin Transl Immunol (2021) 10(4):1–16. doi: 10.1002/cti2.1274
89. Vallera DA, Felices M, McElmurry R, McCullar V, Zhou X, Schmohl U, et al. IL15 trispecific killer engagers (TriKE) make natural killer cells specific to CD33+ targets while also inducing persistence, in vivo expansion, and enhanced function. Clin Cancer Res (2016) 22(14):3440–50. doi: 10.1158/1078-0432.CCR-15-2710
90. Reusing SB, Vallera DA, Manser AR, Vatrin T, Bhatia S, Felices M, et al. CD16xCD33 bispecific killer cell engager (BiKE) as potential immunotherapeutic in pediatric patients with AML and biphenotypic ALL. Cancer Immunol Immunother (2021) 70(12):3701–8. doi: 10.1007/s00262-021-03008-0
91. Demaria O, Gauthier L, Debroas G, Vivier E. Natural killer cell engagers in cancer immunotherapy: Next generation of immuno-oncology treatments. Eur J Immunol (2021) 51(8):1934–42. doi: 10.1002/eji.202048953
92. Kaymak I, Williams KS, Cantor JR, Jones RG. Immunometabolic interplay in the tumor microenvironment. Cancer Cell (2021) 39(1):28–37. doi: 10.1016/j.ccell.2020.09.004
93. Bader JE, Voss K, Rathmell JC. Targeting metabolism to improve the tumor microenvironment for cancer immunotherapy. Mol Cell (2020) 78(6):1019–33. doi: 10.1016/j.molcel.2020.05.034
94. Witney TH, James ML, Shen B, Chang E, Pohling C, Arksey N, et al. PET imaging of tumor glycolysis downstream of hexokinase through noninvasive measurement of pyruvate kinase M2. Sci Transl Med (2015) 7(310):1–9. doi: 10.1126/scitranslmed.aac6117
95. Deng H, Kan A, Lyu N, He M, Huang X, Qiao S, et al. Tumor-derived lactate inhibit the efficacy of lenvatinib through regulating PD-L1 expression on neutrophil in hepatocellular carcinoma. J Immunother Cancer (2021) 9(6):1–14. doi: 10.1136/jitc-2020-002305
96. Liu S, Galat V, Galat Y, Lee YKA, Wainwright D, Wu J. NK cell-based cancer immunotherapy: from basic biology to clinical development. J Hematol Oncol (2021) 14(1):1–24. doi: 10.1186/s13045-020-01014-w
97. Tarazona R, Lopez-Sejas N, Guerrero B, Hassouneh F, Valhondo I, Pera A, et al. Current progress in NK cell biology and NK cell-based cancer immunotherapy. Cancer Immunol Immunother (2020) 69(5):879–99. doi: 10.1007/s00262-020-02532-9
98. Becker PSA, Suck G, Nowakowska P, Ullrich E, Seifried E, Bader P, et al. Selection and expansion of natural killer cells for NK cell-based immunotherapy. Cancer Immunol Immunother (2016) 65(4):477–84. doi: 10.1007/s00262-016-1792-y
99. Zhang M, Jin X, Sun R, Xiong X, Wang J, Xie D, et al. Optimization of metabolism to improve efficacy during CAR-T cell manufacturing. J Transl Med (2021) 19(1):1–11. doi: 10.1186/s12967-021-03165-x
100. Terrén I, Orrantia A, Vitallé J, Zenarruzabeitia O, Borrego F. NK cell metabolism and tumor microenvironment. Front Immunol (2019) 10:2278. doi: 10.3389/fimmu.2019.02278
101. Habif G, Crinier A, André P, Vivier E, Narni-Mancinelli E. Targeting natural killer cells in solid tumors. Cell Mol Immunol (2019) 16(5):415–22. doi: 10.1038/s41423-019-0224-2
102. Nersesian S, Schwartz SL, Grantham SR, MacLean LK, Lee SN, Pugh-Toole M, et al. NK cell infiltration is associated with improved overall survival in solid cancers: A systematic review and meta-analysis. Transl Oncol (2021) 14(1):100930. doi: 10.1016/j.tranon.2020.100930
103. Locatelli F, Pende D, Falco M, Della Chiesa M, Moretta A, Moretta L. NK cells mediate a crucial graft-versus-Leukemia effect in haploidentical-HSCT to cure high-risk acute leukemia. Trends Immunol (2018) 39(7):577–90. doi: 10.1016/j.it.2018.04.009
104. Lee J, Kang TH, Yoo W, Choi H, Jo S, Kong K, et al. An antibody designed to improve adoptive NK-cell therapy inhibits pancreatic cancer progression in a murine model. Cancer Immunol Res (2019) 7(2):219–29. doi: 10.1158/2326-6066.CIR-18-0317
105. Guo H, Chang YJ, Hong Y, Xu LP, Wang Y, Zhang XH, et al. Dynamic immune profiling identifies the stronger graft-versus-leukemia (GVL) effects with haploidentical allografts compared to HLA-matched stem cell transplantation. Cell Mol Immunol (2021) 18(5):1172–85. doi: 10.1038/s41423-020-00597-1
106. Valent P, Sadovnik I, Eisenwort G, Bauer K, Herrmann H, Gleixner KV, et al. Immunotherapy-based targeting and elimination of leukemic stem cells in AML and CML. Int J Mol Sci (2019) 20(17). doi: 10.3390/ijms20174233
107. Ng YY, Du Z, Zhang X, Chng WJ, Wang S. CXCR4 and anti-BCMA CAR co-modified natural killer cells suppress multiple myeloma progression in a xenograft mouse model. Cancer Gene Ther (2022) 29(5):475–83. doi: 10.1038/s41417-021-00365-x
108. Slattery K, Woods E, Zaiatz-Bittencourt V, Marks S, Chew S, Conroy M, et al. TGFβ drives NK cell metabolic dysfunction in human metastatic breast cancer. J Immunother Cancer (2021) 9(2):1–13. doi: 10.1136/jitc-2020-002044
109. Batlle E, Massagué J. Transforming growth factor-β signaling in immunity and cancer. Immunity (2019) 50(4):924–40. doi: 10.1016/j.immuni.2019.03.024
110. Thomas S, Rouilly V, Patin E, Alanio C, Dubois A, Delval C, et al. The milieu intérieur study - an integrative approach for study of human immunological variance. Clin Immunol (2015) 157(2):277–93. doi: 10.1016/j.clim.2014.12.004
Keywords: lactate, immunotherapy, NK cell, T cell, cytotoxicity, immunosuppression,, immunometabolism
Citation: Jedlička M, Feglarová T, Janstová L, Hortová-Kohoutková M and Frič J (2022) Lactate from the tumor microenvironment - A key obstacle in NK cell-based immunotherapies. Front. Immunol. 13:932055. doi: 10.3389/fimmu.2022.932055
Received: 29 April 2022; Accepted: 30 September 2022;
Published: 18 October 2022.
Edited by:
Joao P.B. Viola, National Cancer Institute (INCA), BrazilReviewed by:
Gabriele Multhoff, Technical University of Munich, GermanyCopyright © 2022 Jedlička, Feglarová, Janstová, Hortová-Kohoutková and Frič. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jan Frič, amFuLmZyaWNAZm51c2EuY3o=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.