- 1Institute of Allergology, Charité – Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Berlin, Germany
- 2Fraunhofer Institute for Translational Medicine and Pharmacology (ITMP), Allergology and Immunology, Berlin, Germany
- 3Institute of Clinical Physiology, Charité – Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Berlin, Germany
- 4Department of Dermatology and Venerology, Kepler University Hospital, Linz, Austria
Introduction: In mycosis fungoides (MF), the most common cutaneous T-cell lymphoma, itch is a frequent clinical symptom. Whether mast cells (MCs), eosinophils (Eos) or their mediators play a role in MF-associated itch or disease severity is controversially discussed. Here, we explored the role of MC and Eo numbers in the skin as well as blood levels of their mediators in disease severity and itch.
Methods: In 10 patients with MF and 10 matched control subjects we assessed disease severity, itch, and quality of life impairment using dedicated tools such as the mSWAT, ItchyQoL and DLQI. We analyzed skin biopsies and measured serum levels of tryptase, a mast cell mediator, as well as of the eosinophil products eosinophil cationic protein (ECP) and major basic protein (MBP).
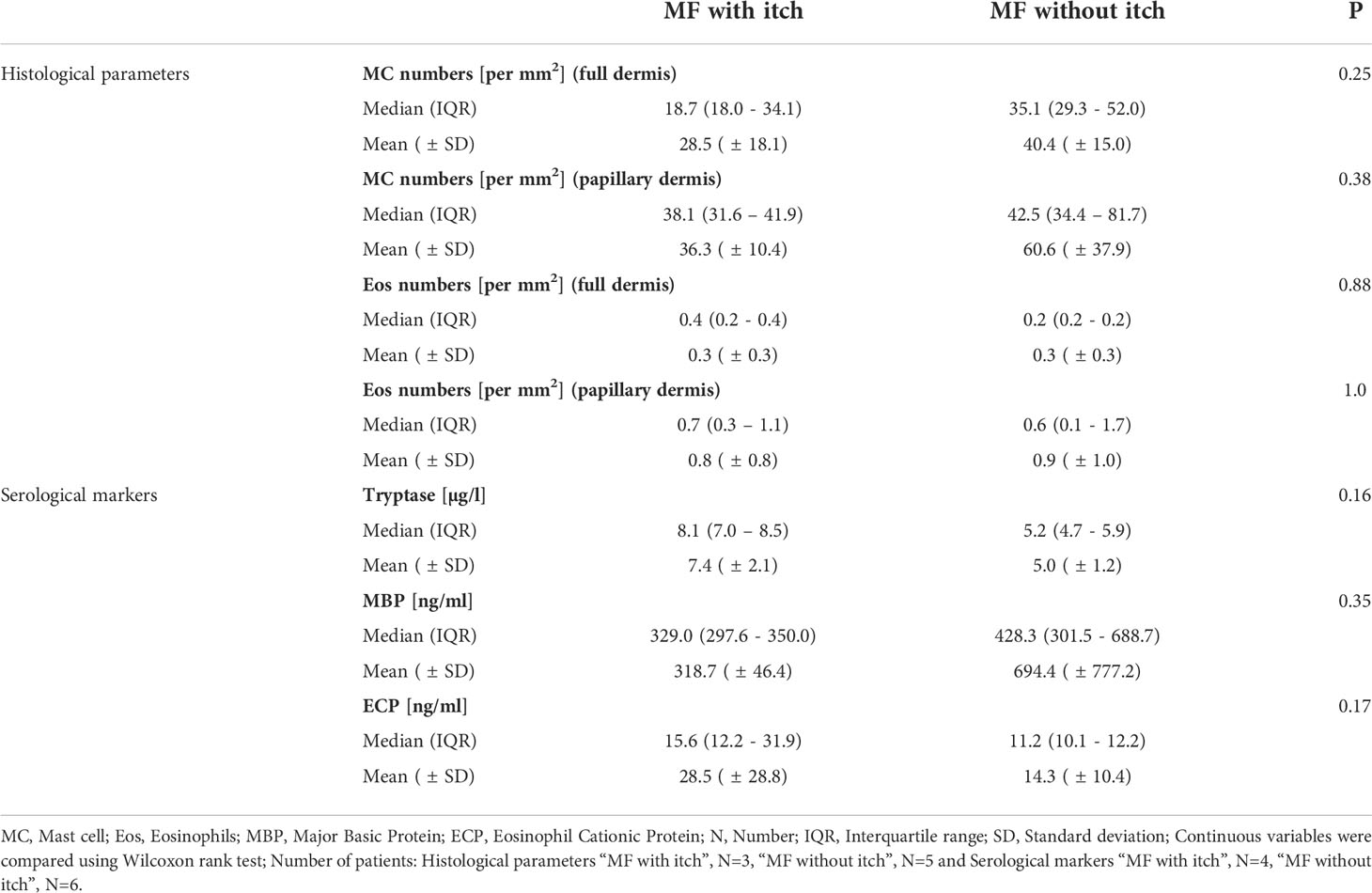
Results: The presence of chronic itch, in four of 10 patients, was associated with significantly higher disease severity (mSwat), larger body surface area affected, and stronger QoL impairment (Itchy-Qol, DLQI). Serum levels of tryptase, but not ECP and MBP, were linked with patient-reported disease severity, body surface area affected, and the presence of itch. Three of the four patients with chronic itch, but none of the six patients without, had tryptase levels above >6µg/l. Numbers of MCs in the papillary dermis were higher in MF skin lesions then in non-lesional skin of MF patients and skin of healthy controls.
Discussion: The MC-mediator tryptase, in MF, is linked to disease activity and impact, most prominently to itch. Our findings call for larger studies that explore the role of MCs, tryptase and other MC mediators as drivers of itch and their role in MF pathogenesis.
Introduction
Mycosis fungoides (MF) is the most common form of cutaneous T-cell lymphoma (CTCL). MF is caused by the uncontrolled growth of atypical T-cells, usually CD4+ T-lymphocytes, and associated inflammation in the epidermis. MF most commonly occurs between 50–70 years of age (1), but can affect people of any age. Neoplastic cells in MF typically express CLA and CCR4, exhibiting the phenotype of skin-homing CD4+ T cells (2). The disease can progress through several stages and is characterized by a skin rash, eczematous lesions and often pruritus (1, 3, 4). In the later stages, intradermal tumors can develop, which may spread into the peripheral blood, lymph nodes and other organs (4). In this stage of the disease, the prognosis is poor with a median survival of less than three years. Monitoring of the disease is crucial in the disease management of these patients. However, no readily assessable biomarkers for disease characterization and monitoring have been identified to date (5).
Itch can be a bothersome symptom and affects more than half of the patients with MF. Itch, in MF, is one of the main drivers of quality of life impairment in these patients (6–8). The exact mechanisms of itch induction in MF lesions are not understood. The degranulation of skin mast cells (MC) and subsequent release of histamine could account for pruritus via histamine 1 or 4 receptors. Both receptors are found on sensory skin nerves (9). H1 antihistamines are used for the treatment of pruritus in MF, but this treatment has limited effect (10). In a more recent publication, a role of eosinophils for the itch component in MF was proposed (11), but eosinophils have not been described as common infiltrating cells in early disease stages (12).
Mast cells in the skin, the gastrointestinal tract, and the airways (13, 14) are commonly recognized for their role in IgE-dependent and independent immune responses. MC numbers have been reported to be increased in several hematological and solid cancers (15, 16). Depending on the type of malignancy, MCs have been suggested to have pro-tumorigenic functions, protective effects, or serve as bystanders (17). For example, MCs have been linked to prostate, skin and pancreatic cancer progression (18–20), but have been reported to have protective, anti-tumorigenic effects in breast and lung cancer (21, 22). As for MF, earlier reports have shown that MC numbers are increased in skin lesions and linked to tumor stage and level of invasiveness, suggesting that MCs have a pro-tumorigenic role in MF (10, 15, 16). In support of this notion, MC-deficient mice showed markedly decreased tumor growth in experimental cutaneous lymphoma (4).
The role and relevance of MCs in MF are ill characterized. Human skin MCs produce and release a comprehensive range of biologically active mediators (23). In addition, their activation can drive the recruitment of other proinflammatory cells including basophils, neutrophils and eosinophils (Eos). Changes in Eos numbers in the blood and skin have been linked to MF progression (12, 24, 25). The detection of MC and Eos activation in human skin is challenging, and serological markers have been established as surrogate markers for their activation. Tryptase is only produced by MCs and the most abundant secretory serine protease in MC granules. It is released constantly as well as upon MC activation and is used as a marker for MC numbers as well as MC activation (26). For Eos, blood levels of eosinophilic cationic protein (ECP) and major basic protein (MBP) are used as surrogate markers for cell numbers as well as activation.
Taken together, MC and Eos as well as their mediators appear to be linked to pathogenetic features and the course of MF. To address the gap of knowledge regarding their role on itch and disease severity, we explored the link of MCs, Eos and their activation products with MF disease severity, itch severity and quality of life impairment.
Materials and methods
Study participants
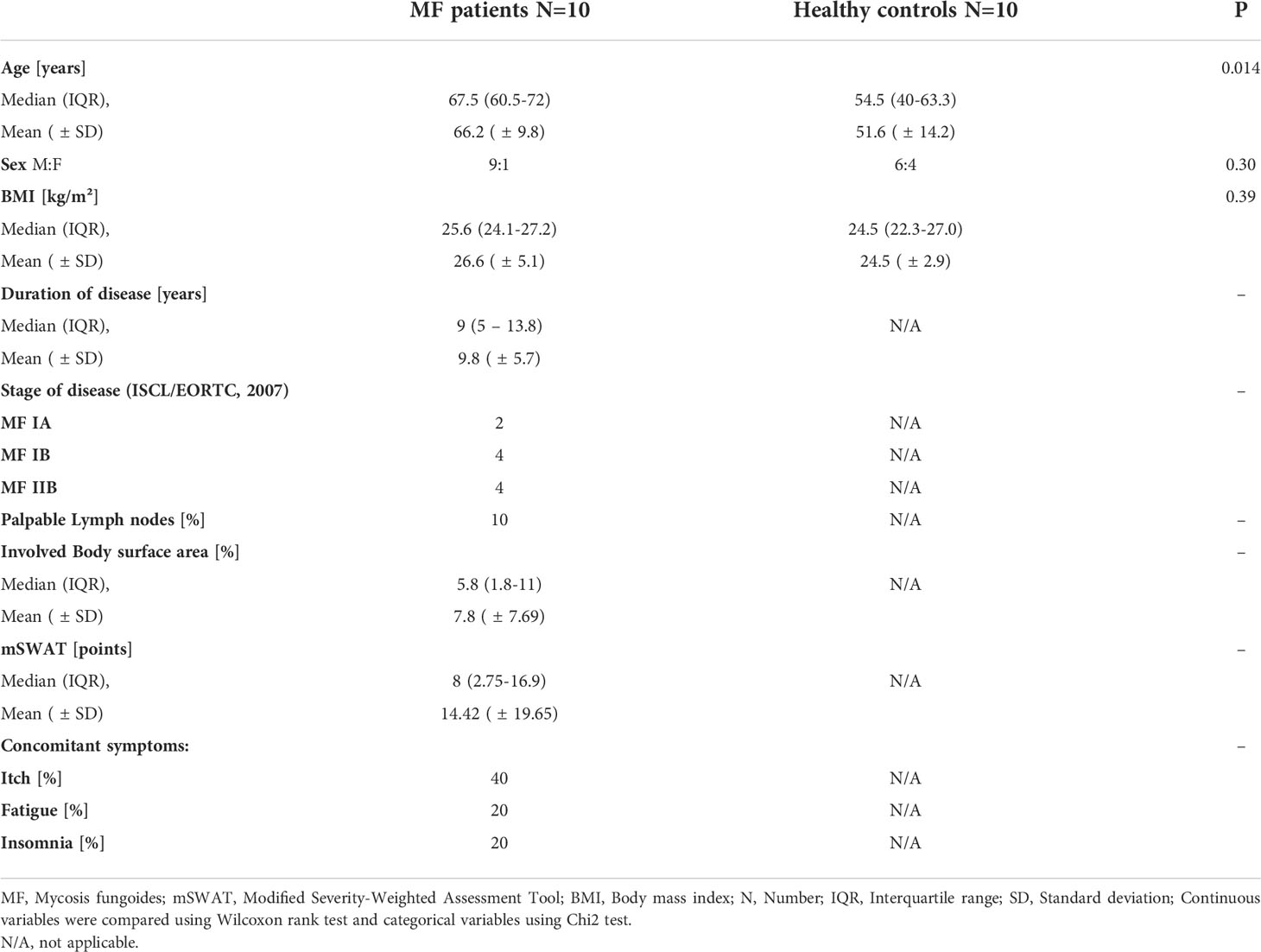
In this case-control study, patients with MF were recruited from the Department of Dermatology and Allergy, Charité - Universitätsmedizin Berlin between July 2017 and April 2018. We diagnosed CTCL according to the WHO-EORTC classification for cutaneous lymphomas (27). Patients were required to stop taking local or systemic steroids therapy for the previous two weeks before we performed the tests. We recorded a detailed medical history, took blood samples and biopsies from lesional and non-lesional skin. Supplementary Figure 1 shows a representative patient photo with characteristic skin lesions. We also recruited ten healthy subjects as a control group who underwent identical procedures (except lesional skin biopsy). Demographic and clinical characteristics of the MF patients and healthy controls are shown in Table 1. All 20 participants were skin type II or III according to the classification of Fitzpatrick (28).
The Ethics Committee of the Charité - Universitätsmedizin Berlin approved this study (EA4/124/10). The study is registered in the German Clinical Trials Registry (DRKS-ID: DRKS00004277). Presented data are from subcohorts of the trial “ROBERTIS”. Other subcohorts will be presented elsewhere.
Assessment of clinical parameters
We assessed patients’ global assessment of disease severity via a visual analogue scale (VAS) and a Likert scale (0–3). Physicians also assessed disease severity using a Likert scale (0–3). We evaluated the affected body surface area (BSA) using the patient’s palm as a rough reference point for 1%. We determined the skin tumor burden in patients with MF using the modified severity-weighted assessment tool (mSWAT) (29). The mSWAT score is calculated as the sum of affected BSA per body region multiplied by a lesion type-specific weighting factor, with higher values indicating more active disease (30).
Patient-reported outcome measures were applied: All patients assessed itch severity in the last 24 hours, the previous week, and the last month via a VAS, as well as itch-related quality of life (QoL) impairment with the help of the ItchyQol questionnaire (31, 32). Patients assessed their skin-related QoL impairment by using the Dermatological Life Quality Index (DLQI) (33).
Serological analyses
At a central laboratory (Labor Berlin GmbH, Berlin, Germany) the following blood parameters were assessed: differential blood count, total IgE serum levels, tryptase.
Eosinophil-related proteins were analyzed using the following ELISAs: Eosinophilic cationic protein (ECP; MyBioSource MBS700481, San Diego, California, USA) and major basic protein (MBP; MyBioSource MBS9308460). Determinations were performed according to the manufacturer’s instructions.
Histological analyses
For histological analyses, we took two skin-punch biopsies of 6 mm diameter in the study group (lesional and non-lesional) and one biopsy in the control group (only non-lesional). The non-lesional biopsy was taken approx. 6 cm away from the biopsy of the lesional skin. The proximity of both biopsies assures comparability of MC counts as MC numbers have been shown to vary in different parts of the body (34). After collection, tissue biopsies were immediately fixed in 5% buffered formalin overnight and subsequently embedded in paraffin wax as per routine protocol (Department of Pathology, Charité – Universitätsmedizin Berlin). The wax blocks were cut into 5‐μm sections and stained with Giemsa (Merck KG, Darmstadt, Germany) for histology. Supplementary Figure 2 shows exemplary stainings.
MCs and Eos were counted by two independent and blinded trained investigators in five or more horizontally adjacent high-power fields (× 400, 0.15 mm2) per skin layer, and mean cell numbers per horizontal layer were calculated. At least three and up to six skin layers were counted per skin sample.
Statistical Analyses
Datasets were small and hence tests for normal distribution (visually via QQ plot, computationally via Shapiro-Wilk test) missed normal distribution for most values. For descriptive statistics continuous variables were summarized using median and interquartile range as well as mean and standard deviation. Categorical variables are given as number and percentage. Continuous variables were compared using Wilcoxon rank test and categorical variables using Chi2 test. For sets of matched samples, the Wilcoxon signed rank test and for correlations the Spearman correlation was used. Any missing data was excluded for the respective statistical analysis. A p-value below 0.05 was considered statistically significant. All statistics were performed using RStudio Version 3.6.3 and stored in an encrypted Excel sheet on an internal Charité university hospital server. All analyses were exploratory in nature.
Results
Itch is more prevalent in patients with severe MF and drives quality of life impairment
In our analyzed patient cohort, four of 10 MF patients reported regular itching of their lesional skin. MF patients who presented with itch were more severely affected, with significantly higher disease severity scores in the mSwat (p=0.042) and a larger affected body surface area (BSA, p=0.014) compared to patients without itch (Table 2A).
In MF patients with itch, itch-related quality of life was significantly more impaired (Itchy-Qol), but also their overall quality of life impairment as assessed by general patient assessment (VAS, Likert) and DLQI (Table 2A), indicating a substantial higher disease burden in MF patients with concomitant itch.
MF is linked to higher numbers of skin mast cells, but not eosinophils
Mast cell (MC) counts in the papillary dermis of MF skin lesions and non-lesional skin were higher than in the skin of healthy controls (Figure 1), albeit not statistically significantly. Of note, two MF patients showed a 3-4 fold increase in MC numbers in lesional skin. No differences were seen in MC numbers in the lower parts of the dermis of lesional, non-lesional and healthy skin. Eosinophil numbers were very low in virtually all of skin samples, of MF patients and healthy controls (below 1 cell/HPF); only one MF patient displayed higher eosinophil numbers, i.e. cells/mm² (Supplementary Figure 2).
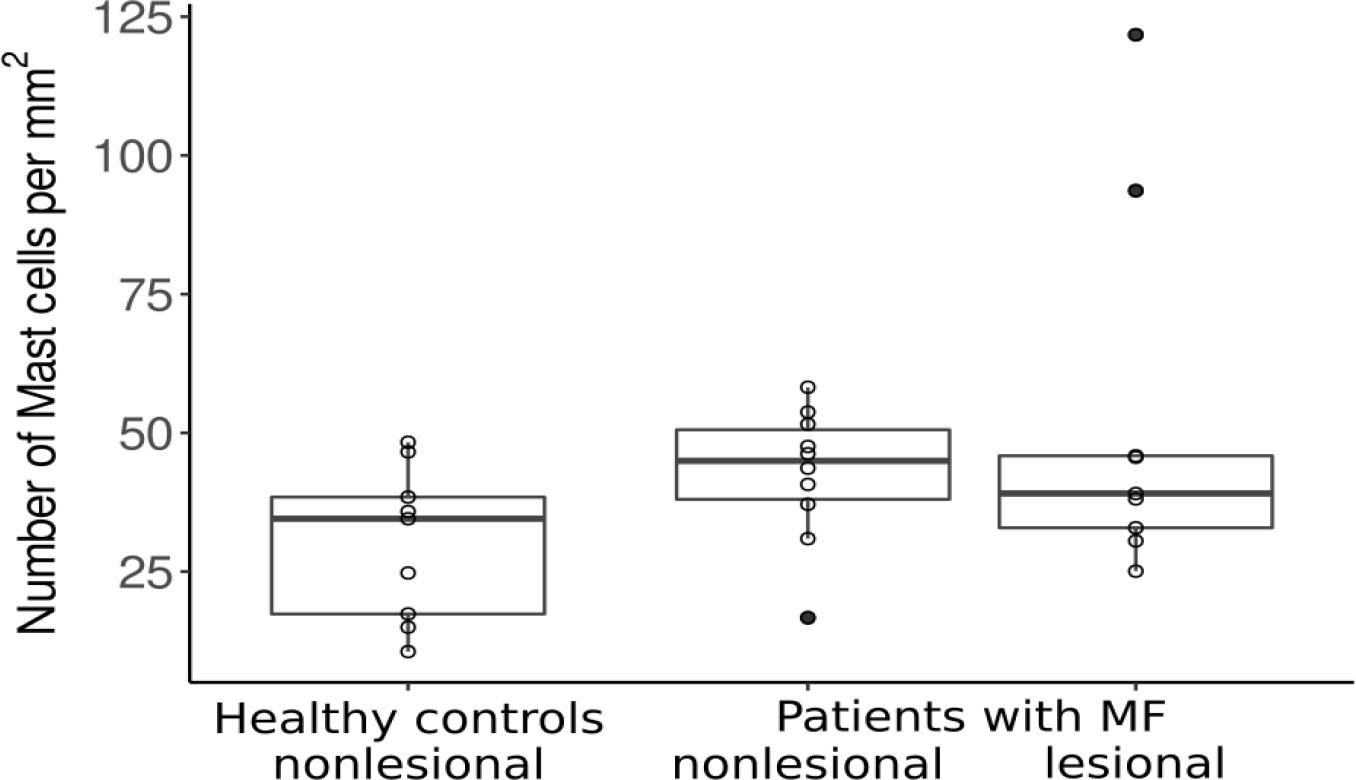
Figure 1 Mast cell counts in the papillary dermis of healthy patients and patients with MF. MF, Mycosis fungoides.
MC and eosinophil numbers did not show significant correlations with any of the assessed clinical markers, like disease severity scores or quality of life scores (see Supplementary Table 1A). Of note, the two patients with highly increased MC numbers in lesional skin had lower total skin involvement (BSA of 1-2%) as compared to the other patients. The MF patient with the lowest MC count in lesional skin was among the patients with the highest BSA score (11%), resulting in a trend towards lower MC numbers as a marker for higher skin involvement in MF (but not disease stage).
When looking at the two subgroups of patients with and without itch, we saw comparable MC and eosinophil numbers in both groups (see Table 2B).
In MF, blood levels of tryptase, but not eosinophil mediators, are linked to disease activity, quality of life impairment, and itch
Overall, blood tryptase levels did not differ between MF patients and healthy controls. Also, MBP and ECP serum levels were comparable (see Supplementary Table 2).
Blood tryptase was to some extent positively correlated with patient-reported disease severity as assessed by VAS (rS=0.53, p=0.1; Figure 2). Patients with more severe disease based on patient or physician global assessment had the highest tryptase levels (Figure 2). Tryptase also correlated with affected body surface area (rS=0.63, p=0.05; Figure 2). Serum ECP and MBP levels did not correlate with any of the assessed disease severity markers (see Supplementary Table 1B).
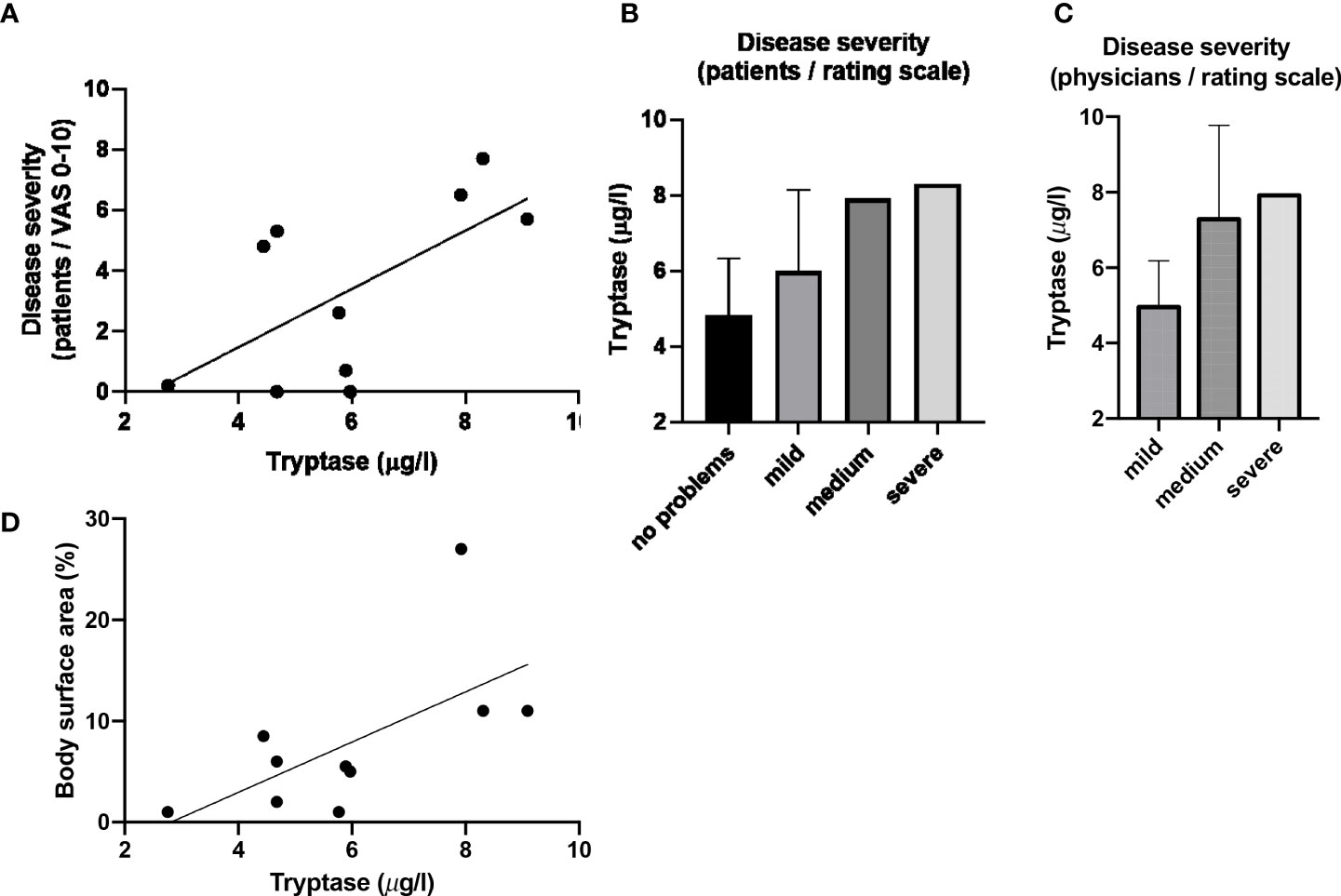
Figure 2 Blood tryptase counts correlate with disease severity and affected body surface area. (A) Serum Tryptase levels plottet against severity evaluated by patients using VAS (including depiction of linear correlation). (B) Tryptase versus disease severity evaluated by patients using rating scale (values given as mean and SD). (C) Tryptase versus disease severity evaluated by physicians using rating scale (values given as mean and SD). (D) Serum Tryptase levels plotted against affected body surface area (including depiction of linear correlation).
Serum tryptase levels were numerically higher in MF patients with itch (Table 2B). Three of the four patients with chronic itch, but none of the six patients without, had tryptase levels above >6µg/l. ECP and MBP levels were not linked to itch (Table 2B; Figure 3).
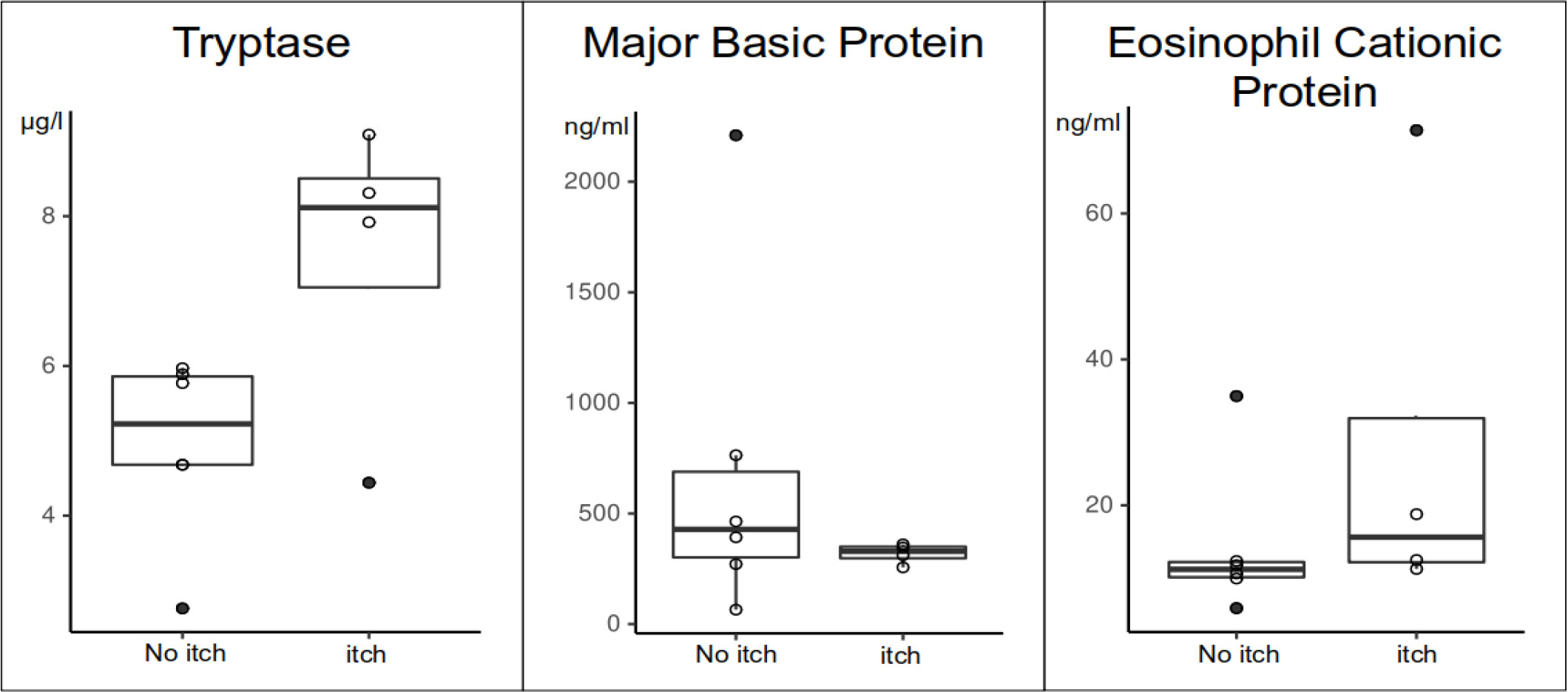
Figure 3 Differences of blood tryptase, MBP and ECP levels in MF patients with itch (itch) and without itch (no itch).
Discussion
Here, for the first time, we aimed to investigate the possible role of MCs, Eos and their mediators in MF by analyzing both skin and blood samples of patients.
Of our ten MF patients, four had chronic pruritus, which is in line with previous reports that itch in MF is frequent, with up to two thirds of patients affected in some studies (35–37). Our findings also confirm that pruritus, in patients with MF, is linked to poor quality of life. Previous MF studies had shown that itch can significantly affect many aspects of quality of life, including sleep (38) and mental wellbeing (39). In line with these reports, we also saw a significantly stronger quality of life impairment in MF patients with pruritus compared to patients without itch in our study. Interestingly, we found a strong and significant difference regarding higher disease severity assessed by BSA and mSWAT in MF patients with itch, irrespective of their disease stage (see Table 2). To our knowledge, this has not been reported before, as published reports have shown association with advanced disease stages, but not clinical severity (37, 40).
The underlying pathomechanisms of pruritus in MF and other cutaneous lymphomas remain unknown. MCs have been suspected to play an important role, since elevated numbers had been reported, especially in patients with more advanced stages and pruritus (4, 41). We also reinforce the evidence that MCs are increased in the skin of patients with MF, in our cohort particularly in lesional skin. Of note, the two patients with markedly elevated levels of MCs in the papillary dermis of lesional skin had low BSA involvement (up to 2%) and a lower mSWAT compared to the other patients. This would rather suggest a protective role of MCs in disease control and would be in contrast to prior publications that have shown that increased MC numbers were linked to tumor stage and advanced level of invasiveness, suggesting that MCs have a pro-tumorigenic role in MF (10, 15, 16). These conflicting data should encourage studies that further investigate the role of MCs in MF, since their presence in MF could not only be seen as a pro-tumorigenic, but also as a mechanism of the innate immune system attempting to control the tumor cells. However, since our cohort was very small, no clear conclusion can be drawn for the role on MCs in MF.
A recent study from Japan showed that the number of eosinophils, but not MCs, in the skin of patients with MF were increased in patients with intense itch (11). In our study, neither the number of eosinophils in the skin of MF patients, nor eosinophil-related mediators were elevated or correlated with disease severity, itch, or BSA. Further studies, ideally in larger and more diverse and multicenter patient populations are needed to better characterize the role of eosinophils and eosinophil-related markers in MF.
Previous reports suggest that MCs may be drivers of itch in MF (41), but that histamine is not the key player (3), suggesting that other inflammatory MC mediators such as cytokines, prostaglandin D2, or proteases including tryptase may importantly contribute to itch in MF (42). Tryptase levels in biological fluids have previously been used as an indicator of MC numbers and activation status (43) and increase with age (44) The recently published data from a large population-based cohort hints at lower reference ranges in adults in comparison with the currently applied upper reference limit (44). Tryptase, in our MF patients, was linked to higher disease severity (reported by both patients and physicians). More importantly, serum tryptase levels were higher in the slightly younger MF patients with itch, and three of the four patients with itch had tryptase levels above >6µg/l, which is higher than the average serum tryptase of healthy individuals (45). Indeed, MC tryptase could not only be a surrogate for MC activation, it has been shown to also directly elicit itching in mice through activation of the proteinase-activated receptor-2 (PAR-2), and that the PAR-2 antagonist FSLLRY, inhibits scratching induced by tryptase (46). We, therefore, propose that tryptase could be a significant pruritogen in MF, and further studies should be conducted to investigate this.
Taken together, our results indicate that itch is an important clinical marker in MF which can be easily assessed using simple questionnaires (7, 32) and could be even explored as a tool for disease severity monitoring. Furthermore, our report suggests that MC and their mediator tryptase play a role in itch in MF.
Due to the lack of efficacy of antihistamines (3), alternative treatments for itch in MF should be explored. Currently, patients with severe itch are treated off-label with substances like aprepitant, naloxone, naltrexone, butorphanol, mirtazapine, gabapentin or thalidomide (47), with limited success and often marked side effects. Newer directed therapies (48, 49) aimed at the inhibition or depletion of skin MCs may help treat pruritus, alongside direct lymphoma targeted therapies (47).
The main limitation of our study is the small patient number of only 10, but these patients were thoroughly investigated clinically, serologically, and histologically. Small numbers limit the meaningfulness of statistical tests, and the low patient numbers is likely to be the main reason for some of our comparisons to not show statistical significance. Also, patients were not followed over time to see whether clinical changes were reflected in changes of the assessed histological or serological markers. Also, healthy controls were somewhat younger, but comparable in the assessed cellular and serological marker. However, they should be better matched in regard to sex, age and localization of the biopsies in future studies which should also include patients with more severe and extensive disease.
Nevertheless, our data encourage further exploration of a possible role of MC and their mediators in driving itch and disease severity in MF.
In conclusion, we demonstrated that itch is an important, easily assessable marker in MF and that MCs and the MC mediator tryptase could play an important pathogenic and pruritogenic role. Of course, further analysis, ideally prospective studies in larger cohorts, need to be undertaken to analyze the pathophysiological roles of MCs and tryptase in MF. The investigation of their role as potential therapeutic targets or as markers for disease severity could improve MF patient management.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Charité. The patients/participants provided their written informed consent to participate in this study.
Author contributions
DT-M has collected patient data, was involved in statistical analysis and drafted the manuscript. KL and VP have collected patient data and performed laboratory analyses. KG performed statistical analysis and drafted the manuscript. MMe, MH, and MMa were involved in study planning and proof-reading of the manuscript. SA has planned the study, coordinated the study, collected patient data, performed statistical analysis and drafted the manuscript. All authors were involved in proof-reading of the manuscript and provided input. All authors contributed to the aricle and approved the submitted version.
Conflict of interest
DT-M has received research funds and was advisor for Celldex, Novartis, Sanofi and Moxie. MMe is or recently was a speaker and/or advisor for AbbVie, Amgen, ArgenX, AstraZeneca, Bayer, Celldex, Celgene, Escient, Galderma, Grünenthal, GSK, Menlo, Novartis, Pfizer, Pharvaris, Roche, Sanofi-Aventis, Third Harmonic Bio. MMa is or recently was a speaker and/or advisor for and/or has received research funding from Allakos, Aralez, Genentech, GSK, Menarini, Merckle Recordati, Moxie, Novartis, Sanofi, MSD, and Uriach. SA has conducted studies for received research funds/was advisor for Allakos, ALK, AstraZeneca, CSL Behring, LeoPharma, Moxie, Novartis, Sanofi, Takeda, Thermofisher. Published results are part of the study ROBERTIS, funded by AstraZeneca.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.930979/full#supplementary-material
Supplementary Figure 1 | Representative photos of a patient with mycosis fungoides. (A) An overview of a patient with MF and (B) A close-up of the arm, from which both skin biopsies of lesional and non-lesional skin were taken.
Supplementary Figure 2 | Mast cell numbers are increased in lesional skin of MF patients. Histological pictures of MC in Giemsa stainings are depicted of healthy control skin (A, B), lesional skin (C, D) and non-lesional skin (E, F) of a representative MF patient. Pictures (A, C, E) are shown as 20 fold magnification, pictures (B, D, F) in 40 fold magnification of marked area. Arrows point towards MCs.
Supplementary Figure 3 | Postulated mechanism of pruritus induction in MF patients (1).Higher number of mast cells in the papillary dermis (2). Increased levels of tryptase in the skin (3). Tryptase-induced, PAR-2-mediated pruritus
Supplementary Table 1 | Correlation of MC numbers in skin with clinical markers of MF. MF, Mycosis fungoides; mSWAT, Modified Severity-Weighted Assessment Tool; BSA, Body surface area; PGA, Patient Global Assessment; QoL, Quality of Life; DLQI, Dermatology Life Quality Index; N, Number; R, Spearman’s R; for correlations the Spearman correlation was used
Supplementary Table 1 | Correlation of serological markers with clinical markers of MF. MF, Mycosis fungoides; MBP, Major Basic Protein; ECP, Eosinophil Cationic Protein; mSWAT, Modified Severity-Weighted Assessment Tool; BSA, Body surface area; PGA, Patient Global Assessment; QoL, Quality of Life; DLQI, Dermatology Life Quality Index; N, Number; R, Spearman’s R; for correlations the Spearman correlation was used.
Supplementary Table 2 | Comparison of serological and histological markers of MF patients and healthy controls. MF, Mycosis fungoides; MC, Mast cell; Eos, Eosinophils; MBP, Major Basic Protein; ECP, Eosinophil Cationic Protein; N, Number; IQR, Interquartile range; SD, Standard deviation; Continuous variables were compared using Wilcoxon rank test.; Number of patients: Serological markers N=10 and Histological parameters N=9.
Abbreviations
CTCL, cutaneous T-cell lymphoma; ECP, eosinophil cationic protein; Eos, eosinophil granulocytes; MBP, major basic protein; MC, mast cell; MF, Mycosis fungoides.
References
1. Dermatologists B. Mycosis fungoides - patient information leaflet. (2019). Available at: https://www.bad.org.uk/pils/mycosis-fungoides/ (Accessed 30th July 2022).
2. Dummer R, Vermeer MH, Scarisbrick JJ, Kim YH, Stonesifer C, Tensen CP, et al. Cutaneous T cell lymphoma. Nat Rev Dis Primers (2021) 7(1):61. doi: 10.1038/s41572-021-00296-9
3. Meyer N, Paul C, Misery L. Pruritus in cutaneous T-cell lymphomas: frequent, often severe and difficult to treat. Acta Derm Venereol (2010) 90(1):12–7. doi: 10.2340/00015555-0789
4. Rabenhorst A, Schlaak M, Heukamp LC, Förster A, Theurich S, von Bergwelt-Baildon M, et al. Mast cells play a protumorigenic role in primary cutaneous lymphoma. Blood (2012) 120(10):2042–54. doi: 10.1182/blood-2012-03-415638
5. Dulmage B, Geskin L, Guitart J, Akilov OE. The biomarker landscape in mycosis fungoides and sézary syndrome. Exp Dermatol (2017) 26(8):668–76. doi: 10.1111/exd.13261
6. Holahan HM, Farah RS, Fitz S, Mott SL, Ferguson NN, McKillip J, et al. Health-related quality of life in patients with cutaneous T-cell lymphoma? Int J Dermatol (2018) 57(11):1314–9. doi: 10.1111/ijd.14132
7. Ottevanger R, van Beugen S, Evers AWM, Willemze R, Vermeer MH, Quint KD. Quality of life in patients with mycosis fungoides and sézary syndrome: a systematic review of the literature. J Eur Acad Dermatol Venereol (2021) 35(12):2377–87. doi: 10.1111/jdv.17570
8. Sampogna F, Frontani M, Baliva G, Lombardo GA, Alvetreti G, Di Pietro C, et al. Quality of life and psychological distress in patients with cutaneous lymphoma. Br J Dermatol (2009) 160(4):815–22. doi: 10.1111/j.1365-2133.2008.08992.x
9. Shim WS, Oh U. Histamine-induced itch and its relationship with pain. Mol Pain (2008) 4:29. doi: 10.1186/1744-8069-4-29
10. Misery L. Frontiers in neuroscience pruritus in cutaneous T-cell lymphomas. In: Carstens E, Akiyama T, editors. Itch: Mechanisms and treatment. Boca Raton (FL: CRC Press/Taylor & Francis © 2014 by Taylor & Francis Group, LLC (2014).
11. Shimizu K, Andoh T, Makino T, Yoshihisa Y, Mizawa M, Shimizu T. Mechanisms of itching in mycosis fungoides: grade of itching correlates with eosinophil infiltration and kallikrein 5 expression. Eur J Dermatol (2019) 29(3):268–73. doi: 10.1684/ejd.2019.3560
12. Dalton SR, Chandler WM, Abuzeid M, Hossler EW, Ferringer T, Elston DM, et al. Eosinophils in mycosis fungoides: an uncommon finding in the patch and plaque stages. Am J Dermatopathol (2012) 34(6):586–91. doi: 10.1097/DAD.0b013e31823d921b
13. Shelburne CP, Abraham SN. The mast cell in innate and adaptive immunity. Adv Exp Med Biol (2011) 716:162–85. doi: 10.1007/978-1-4419-9533-9_10
14. Tsai M, Grimbaldeston M, Galli SJ. Mast cells and immunoregulation/immunomodulation. Adv Exp Med Biol (2011) 716:186–211. doi: 10.1007/978-1-4419-9533-9_11
15. Melillo RM, Guarino V, Avilla E, Galdiero MR, Liotti F, Prevete N, et al. Mast cells have a protumorigenic role in human thyroid cancer. Oncogene (2010) 29(47):6203–15. doi: 10.1038/onc.2010.348
16. Ribatti D, Ennas MG, Vacca A, Ferreli F, Nico B, Orru S, et al. Tumor vascularity and tryptase-positive mast cells correlate with a poor prognosis in melanoma. Eur J Clin Invest (2003) 33(5):420–5. doi: 10.1046/j.1365-2362.2003.01152.x
17. Varricchi G, Galdiero MR, Loffredo S, Marone G, Iannone R, Marone G, et al. Are mast cells MASTers in cancer? Front Immunol (2017) 8. doi: 10.3389/fimmu.2017.00424
18. Li L, Dang Q, Xie H, Yang Z, He D, Liang L, et al. Infiltrating mast cells enhance prostate cancer invasion via altering LncRNA-HOTAIR/PRC2-androgen receptor (AR)-MMP9 signals and increased stem/progenitor cell population. Oncotarget (2015) 6(16):14179–90. doi: 10.18632/oncotarget.3651
19. Strouch MJ, Cheon EC, Salabat MR, Krantz SB, Gounaris E, Melstrom LG, et al. Crosstalk between mast cells and pancreatic cancer cells contributes to pancreatic tumor progression. Clin Cancer Res (2010) 16(8):2257–65. doi: 10.1158/1078-0432.CCR-09-1230
20. Weber A, Maurer M. Skin site mast cell numbers correlate with rates of nodular growth, but not incidence, of basal cell carcinoma. Dermatology (2005) 211(3):298–9. doi: 10.1159/000087030
21. Rajput AB, Turbin DA, Cheang MC, Voduc DK, Leung S, Gelmon KA, et al. Stromal mast cells in invasive breast cancer are a marker of favourable prognosis: a study of 4,444 cases. Breast Cancer Res Treat (2008) 107(2):249–57. doi: 10.1007/s10549-007-9546-3
22. Welsh TJ, Green RH, Richardson D, Waller DA, O'Byrne KJ, Bradding P. Macrophage and mast-cell invasion of tumor cell islets confers a marked survival advantage in non-small-cell lung cancer. J Clin Oncol (2005) 23(35):8959–67. doi: 10.1200/JCO.2005.01.4910
23. Krystel-Whittemore M, Dileepan KN, Wood JG. Mast cell: A multi-functional master cell. Front Immunol (2015) 6:620. doi: 10.3389/fimmu.2015.00620
24. Tancrède-Bohin E, Ionescu MA, de la Salmonière P, Dupuy A, Rivet J, Rybojad M, et al. Prognostic value of blood eosinophilia in primary cutaneous T-cell lymphomas. Arch Dermatol (2004) 140(9):1057–61. doi: 10.1001/archderm.140.9.1057
25. Terada T. Mycosis fungoides in plaque stage with pronounced eosinophilic infiltration, folliculotropism, and concomitant invasive squamous cell carcinoma. Int J Clin Exp Pathol (2013) 6(4):749–56.
26. Payne V, Kam PC. Mast cell tryptase: a review of its physiology and clinical significance. Anaesthesia (2004) 59(7):695–703. doi: 10.1111/j.1365-2044.2004.03757.x
27. Willemze R, Cerroni L, Kempf W, Berti E, Facchetti F, Swerdlow SH, et al. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood (2019) 133(16):1703–14. doi: 10.1182/blood-2018-11-881268
28. Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol (1988) 124(6):869–71. doi: 10.1001/archderm.1988.01670060015008
29. Olsen EA, Kim YH, Kuzel TM, Pacheco TR, Foss FM, Parker S, et al. Phase IIb multicenter trial of vorinostat in patients with persistent, progressive, or treatment refractory cutaneous T-cell lymphoma. J Clin Oncol (2007) 25(21):3109–15. doi: 10.1200/JCO.2006.10.2434
30. Olsen EA, Whittaker S, Kim YH, Duvic M, Prince HM, Lessin SR, et al. Clinical end points and response criteria in mycosis fungoides and sézary syndrome: a consensus statement of the international society for cutaneous lymphomas, the united states cutaneous lymphoma consortium, and the cutaneous lymphoma task force of the European organisation for research and treatment of cancer. J Clin Oncol (2011) 29(18):2598–607. doi: 10.1200/JCO.2010.32.0630
31. Desai NS, Poindexter GB, Monthrope YM, Bendeck SE, Swerlick RA, Chen SC. A pilot quality-of-life instrument for pruritus. J Am Acad Dermatol (2008) 59(2):234–44. doi: 10.1016/j.jaad.2008.04.006
32. Krause K, Kessler B, Weller K, Veidt J, Chen SC, Martus P, et al. German Version of ItchyQoL: validation and initial clinical findings. Acta Derm Venereol (2013) 93(5):562–8. doi: 10.2340/00015555-1544
33. Finlay AY, Khan GK. Dermatology life quality index (DLQI)–a simple practical measure for routine clinical use. Clin Exp Dermatol (1994) 19(3):210–6. doi: 10.1111/j.1365-2230.1994.tb01167.x
34. Weber A, Knop J, Maurer M. Pattern analysis of human cutaneous mast cell populations by total body surface mapping. Br J Dermatol (2003) 148(2):224–8. doi: 10.1046/j.1365-2133.2003.05090.x
35. Bradford PT, Devesa SS, Anderson WF, Toro JR. Cutaneous lymphoma incidence patterns in the united states: a population-based study of 3884 cases. Blood (2009) 113(21):5064–73. doi: 10.1182/blood-2008-10-184168
36. Ku LS, Lo KK. Mycosis fungoides–a retrospective study of 40 cases in Hong Kong. Int J Dermatol (2005) 44(3):215–20. doi: 10.1111/j.1365-4632.2004.02362.x
37. Vij A, Duvic M. Prevalence and severity of pruritus in cutaneous T cell lymphoma. Int J Dermatol (2012) 51(8):930–4. doi: 10.1111/j.1365-4632.2011.05188.x
38. Hawro T, Hawro M, Zalewska-Janowska A, Weller K, Metz M, Maurer M. Pruritus and sleep disturbances in patients with psoriasis. Arch Dermatol Res (2020) 312(2):103–11. doi: 10.1007/s00403-019-01998-7
39. Molloy K, Jonak C, Woei AJF, Guenova E, Busschots AM, Bervoets A, et al. Characteristics associated with significantly worse quality of life in mycosis fungoides/Sézary syndrome from the prospective cutaneous lymphoma international prognostic index (PROCLIPI) study. Br J Dermatol (2020) 182(3):770–9. doi: 10.1111/bjd.18089
40. Green SB, Byar DP, Lamberg SI. Prognostic variables in mycosis fungoides. Cancer (1981) 47(11):2671–7. doi: 10.1002/1097-0142(19810601)47:11<2671::AID-CNCR2820471125>3.0.CO;2-X
41. Yamamoto T, Katayama I, Nishioka K. Role of mast cell and stem cell factor in hyperpigmented mycosis fungoides. Blood (1997) 90(3):1338–40. doi: 10.1182/blood.V90.3.1338
42. Garcovich S, Maurelli M, Gisondi P, Peris K, Yosipovitch G, Girolomoni G. Pruritus as a distinctive feature of type 2 inflammation. Vaccines (Basel) (2021) 9(3):303. doi: 10.3390/vaccines9030303
43. Schwartz LB. Clinical utility of tryptase levels in systemic mastocytosis and associated hematologic disorders. Leuk Res (2001) 25(7):553–62. doi: 10.1016/S0145-2126(01)00020-0
44. Slot MC, Claessen LHJ, Bons JAP, Menheere P, Nieuwhof CMG, de Boer D. Tryptase reference ranges are age-dependent in a large population-based cohort. Allergy (2022). doi: 10.1111/all.15369
45. Valent P, Sperr WR, Sotlar K, Reiter A, Akin C, Gotlib J, et al. The serum tryptase test: an emerging robust biomarker in clinical hematology. Expert Rev Hematol (2014) 7(5):683–90. doi: 10.1586/17474086.2014.955008
46. Ui H, Andoh T, Lee JB, Nojima H, Kuraishi Y. Potent pruritogenic action of tryptase mediated by PAR-2 receptor and its involvement in anti-pruritic effect of nafamostat mesilate in mice. Eur J Pharmacol (2006) 530(1-2):172–8. doi: 10.1016/j.ejphar.2005.11.021
47. Ahern K, Gilmore ES, Poligone B. Pruritus in cutaneous T-cell lymphoma: a review. J Am Acad Dermatol (2012) 67(4):760–8. doi: 10.1016/j.jaad.2011.12.021
48. Altrichter S, Staubach P, Pasha M, Singh B, Chang AT, Bernstein JA, et al. An open-label, proof-of-concept study of lirentelimab for antihistamine-resistant chronic spontaneous and inducible urticaria. J Allergy Clin Immunol (2021) 149(5):1683–90.e7. doi: 10.1016/j.jaci.2021.12.772
Keywords: mycosis fungoides, cutaneous T-cell lymphoma, mast cell, eosinophil, tryptase, itch (pruritus)
Citation: Terhorst-Molawi D, Lohse K, Ginter K, Puhl V, Metz M, Hu M, Maurer M and Altrichter S (2022) Mast cells and tryptase are linked to itch and disease severity in mycosis fungoides: Results of a pilot study. Front. Immunol. 13:930979. doi: 10.3389/fimmu.2022.930979
Received: 28 April 2022; Accepted: 20 July 2022;
Published: 10 August 2022.
Edited by:
Frans J. Van Overveld, University College Roosevelt, NetherlandsReviewed by:
Vadim V. Sumbayev, University of Kent, United KingdomManuel Pedro Pereira, University Hospital Münster, Germany
Miriam Margareta Düll, University Hospital Erlangen, Germany
Copyright © 2022 Terhorst-Molawi, Lohse, Ginter, Puhl, Metz, Hu, Maurer and Altrichter. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marcus Maurer, bWFyY3VzLm1hdXJlckBjaGFyaXRlLmRl
 Dorothea Terhorst-Molawi
Dorothea Terhorst-Molawi Katharina Lohse1,2
Katharina Lohse1,2 Katharina Ginter
Katharina Ginter Martin Metz
Martin Metz Man Hu
Man Hu Marcus Maurer
Marcus Maurer Sabine Altrichter
Sabine Altrichter