
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol., 08 July 2022
Sec. Autoimmune and Autoinflammatory Disorders
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.929155
This article is part of the Research TopicInsights in Autoimmune and Autoinflammatory Disorders: 2021View all 14 articles
Background: IgG and complement 3 (C3) are generally found to be deposited along the glomerular basement membrane (GBM) in human anti-GBM disease. The pathogenic role of complement activation in kidney damage of anti-GBM disease has been explored in recent years. Therefore, we investigated the relationship between serum C3 and outcomes among patients with anti-GBM disease in this study.
Methods: Ninety-four anti-GBM disease patients between January 2004 and December 2020 at the National Clinical Research Center of Kidney Diseases Jinling Hospital were retrospectively analyzed, and were divided into the low C3 group and the normal C3 group according to serum C3 levels at diagnosis. Fifty-six patients had undergone renal biopsy. We analyzed the clinical manifestations, laboratory tests, kidney pathology, treatment, and outcomes between the two groups. The primary endpoint was kidney failure. Cox regression and smooth curve fitting of generalized additive mixed model analysis were used to explore the correlation between serum C3 and kidney failure. The outcomes of the two groups were compared by the Kaplan–Meier curve.
Results: A total of 94 patients (aged 43.6 ± 16.2; male patients, 46%) with anti-GBM disease were enrolled. There were 26 patients with low C3 levels and 68 patients with normal C3 levels. Compared with the normal C3 group, patients in the low C3 group have a higher proportion of glomerular sclerosis progressing to kidney failure. Multivariate Cox regression analysis suggested that C3 is associated with kidney outcomes in patients with anti-GBM disease (HR = 0.782, 95% CI = 0.673–0.907, p = 0.001). Smooth curve fitting of generalized additive mixed model analysis indicated that the level of C3 had a linear relationship with the changing trend of kidney failure. The Kaplan–Meier curve showed that there was a statistical difference between the two groups in terms of kidney failure (p = 0.033).
Conclusion: The kidney outcomes of anti-GBM disease in the low C3 group were poorer than those in the normal C3 group. The influence of C3 on the kidney outcomes of patients with anti-GBM disease may be of clinical relevance.
Anti-glomerular basement membrane (GBM) disease is a rare but life-threatening autoimmune disorder that is characterized by rapidly progressive glomerulonephritis with or without pulmonary hemorrhage. Kidney biopsy of anti-GBM disease has shown that immunoglobulin G (IgG) linearly deposits along the GBM, which is usually accompanied by linear or granular deposition of complement 3 (C3) (1). This indicates that complement activation may participate in the pathogenesis of anti-GBM disease. The pathways of complement activation in anti-GBM disease are mainly studied by passive injection of heterologous antibodies against GBM. It has been found that the complement system is activated in human anti-GBM disease through classical and alternative pathways (2), which plays an aggressive role in the pathogenesis of kidney injury by proinflammatory effect or cell lysis effect (3, 4). Multiple studies also showed that low serum C3 levels at diagnosis are associated with poor kidney outcomes in ANCA-associated vasculitis (5) and IgA nephropathy (6). However, to the best of our knowledge, few studies have analyzed the effect of serum C3 levels as a prognostic parameter in anti-GBM disease.
Therefore, this study aimed to investigate whether patients with low serum C3 levels at diagnosis have different clinical and histopathological features or outcomes compared to patients with normal C3 levels in anti-GBM disease.
The patients diagnosed with anti-GBM disease in the National Clinical Research Center of Kidney Diseases Jinling Hospital from January 2004 to December 2020 were enrolled in this study. All patients had follow-up data for at least 3 months from time of presentation or until death, and those without serum C3 levels and missing follow-up records were excluded (Figure 1). The study complied with the Declaration of Helsinki and was approved by the independent ethics committee of Jinling Hospital (Approval 2022DZKY-033-01).
Data of clinical, laboratory, and pathological variables were collected from medical records at diagnosis and during follow-up, which included the following: demographics, medical history and duration, clinical manifestations, serum C3 and C4, serum creatinine (SCr) at presentation, peak SCr, estimated glomerular filtration rate (eGFR), oliguria or anuria, hypertension, and kidney replacement therapy (KRT) at onset. Serum C3 and C4 levels were measured at the time of diagnosis, using a turbidimetric test. Our laboratory’s serum C3 reference range is 0.8–1.8 g/L, and serum C4 reference range is 0.1–0.4 g/L.
The main endpoint of the event was kidney failure, which was defined as the persistent need for KRT for more than 3 months. The need for KRT at onset was defined as the requirement for KRT during the first hospital stay. The eGFR was determined using the CKD-EPI equation (7). The extent of acute tubular–interstitial lesions includes tubular epithelial brush-border loss or interstitial edema and inflammatory cell infiltration area. Kidney tubular lesions used semi-quantitative scores: 0, not present; 1, present in 1%–25%; 2, present in 25%–50%; 3, present in >50%.
A kidney biopsy was performed at the time of diagnosis. Kidney specimens were evaluated by direct immunofluorescence and light and electron microscopy and were forwarded to two pathologists who examined the specimens separately, blinded to each other and the patients’ data.
For direct immunofluorescence, frozen sections were stained with a panel of fluorescein isothiocyanate-conjugated rabbit anti-human antibodies to IgG, IgM, IgA, C3, C1q, and fibrinogen. On light microscopy, the following indicators were evaluated by pathologists: the percentage of crescents (cellular, fibrocellular, and fibrous crescents), acute tubular–interstitial lesions, interstitial fibrosis, and tubular atrophy.
Values are expressed as mean ± standard (SD), or as median and interquartile range for continuous variables, and percentages for categorical variables. First, continuous variables were analyzed using the t-test. Meanwhile, the χ2 test and Mann–Whitney U test were used for categorical variables. Next, generalized additive model smooth curve fitting was used to address the relationship between serum C3 and kidney failure in anti-GBM disease, and univariate and multivariate Cox regression models were performed to examine whether C3 is associated with kidney failure in the disease. The Kaplan–Meier curve was used to analyze two groups’ kidney survival. In addition, an interaction test was conducted to evaluate whether patients’ characteristics influence the relationship between C3 and kidney failure. Data analysis was performed using R (http://www.r-project.org) and EmpowerStats (www.empowerstats.com.X&YSolutionsInc). A two-sided p < 0.05 was considered statistically significant.
A total of 94 patients diagnosed with anti-GBM disease were included in the study, 26 (27.7%) of whom had low C3 levels (below the normal range limit, <0.8 g/L). Patients were categorized according to the measurement of C3 into two groups, one group with low C3 and another group with normal C3. We found that percentage of glomerular sclerosis was higher in the low C3 group than the normal C3 group (p = 0.012), while there were no statistical differences in demographics, clinical, laboratory, and other pathological variables. The main clinical characteristics of the patients at presentation are summarized in Table 1. Among 94 patients, the average age at diagnosis was 43.6 ± 16.2, with 43 men and 51 women. The SCr at presentation was 661.9 ± 422.6 μmol/L, and the peak SCr was 896.1 ± 389.3 μmol/L. Mean serum C3 and C4 levels were 1.0 ± 0.3 and 0.2 ± 0.1 g/L, respectively. Kidney biopsies showed linear staining of IgG and linear or granular staining of C3 along GBM in the 36 (69%) patients. The average total crescent formation shown in the glomeruli was 68.5% (46.9%–88.8%) (Table 2). There were no statistical differences between the two groups of patients who received the standard induction therapy including glucocorticoids and cyclophosphamide combined with plasma purification technology. The median follow-up of the patients was 23.3 (7.1–74.0) months. At the last follow-up, 83 patients progressed into kidney failure, of which 26 were in the low C3 group and 57 (84%) were in the normal C3 group. There was no difference in patient survival between the low C3 group and the normal C3 group (p > 0.05).
Univariate analysis showed that C3 is markedly correlated with the risk of kidney failure. In addition, hypertension, oliguria or anuria, anti-GBM levels, eGFR, SCr at presentation, peak SCr, KRT at onset, crescents, and fibrocellular crescents were found to be associated with the risk of kidney failure (p < 0.05) (Table 3). After adjustment for age, gender, hypertension, anti-GBM levels, eGFR, KRT at onset, and crescents, multivariate analysis still showed that C3 at diagnosis was an independent protective factor for kidney outcomes of anti-GBM disease. For every 0.1 g/L increase in C3, the risk of developing kidney failure decreases by 22% (HR = 0.782, 95% CI = 0.673–0.907, p = 0.001) (Table 3). In addition, oliguria/anuria and peak SCr were also associated with kidney failure in multivariate analysis (Table 3).
We analyzed the patient and kidney survival according to C3 levels, finding that patients in the low C3 group had significantly worse kidney survival than the normal C3 group (p = 0.029). We further compared kidney survival of patients with anti-GBM disease in two groups by the Kaplan–Meier curve, which showed that low C3 was associated with a significantly lower kidney survival (p = 0.033) (Figure 2).
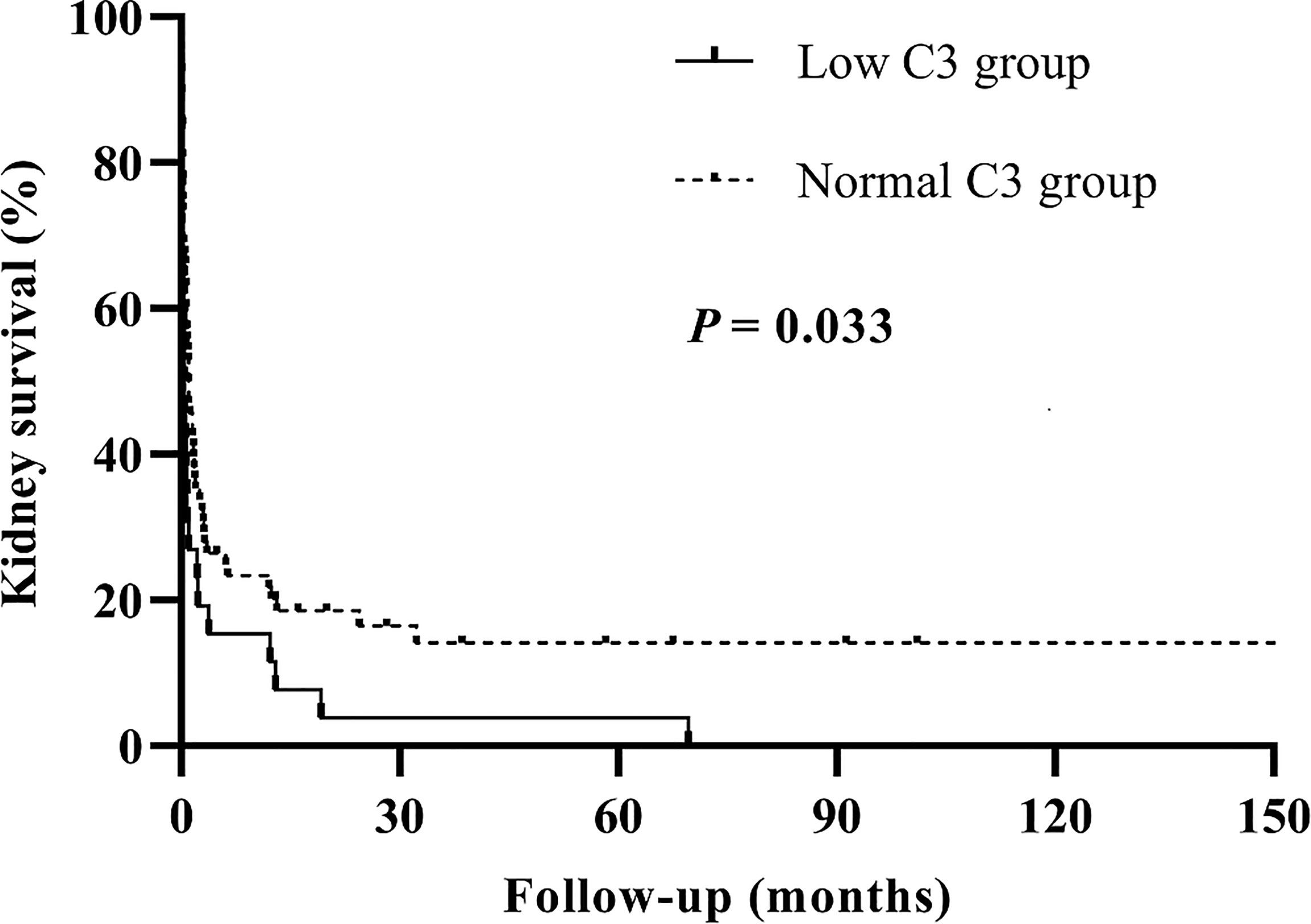
Figure 2 Kaplan–Meier survival analysis for kidney outcomes between the low C3 group and the normal C3 group.
Smooth curve fitting of C3 and kidney failure was conducted after adjustments for age, gender, hypertension, anti-GBM levels, eGFR, KRT at onset, and crescents. The results showed that C3 level was linearly correlated with the changing trend of kidney failure risk (p = 0.001), which means that as C3 levels increase, the risk of kidney failure occurrence gradually decreases (Figure 3).
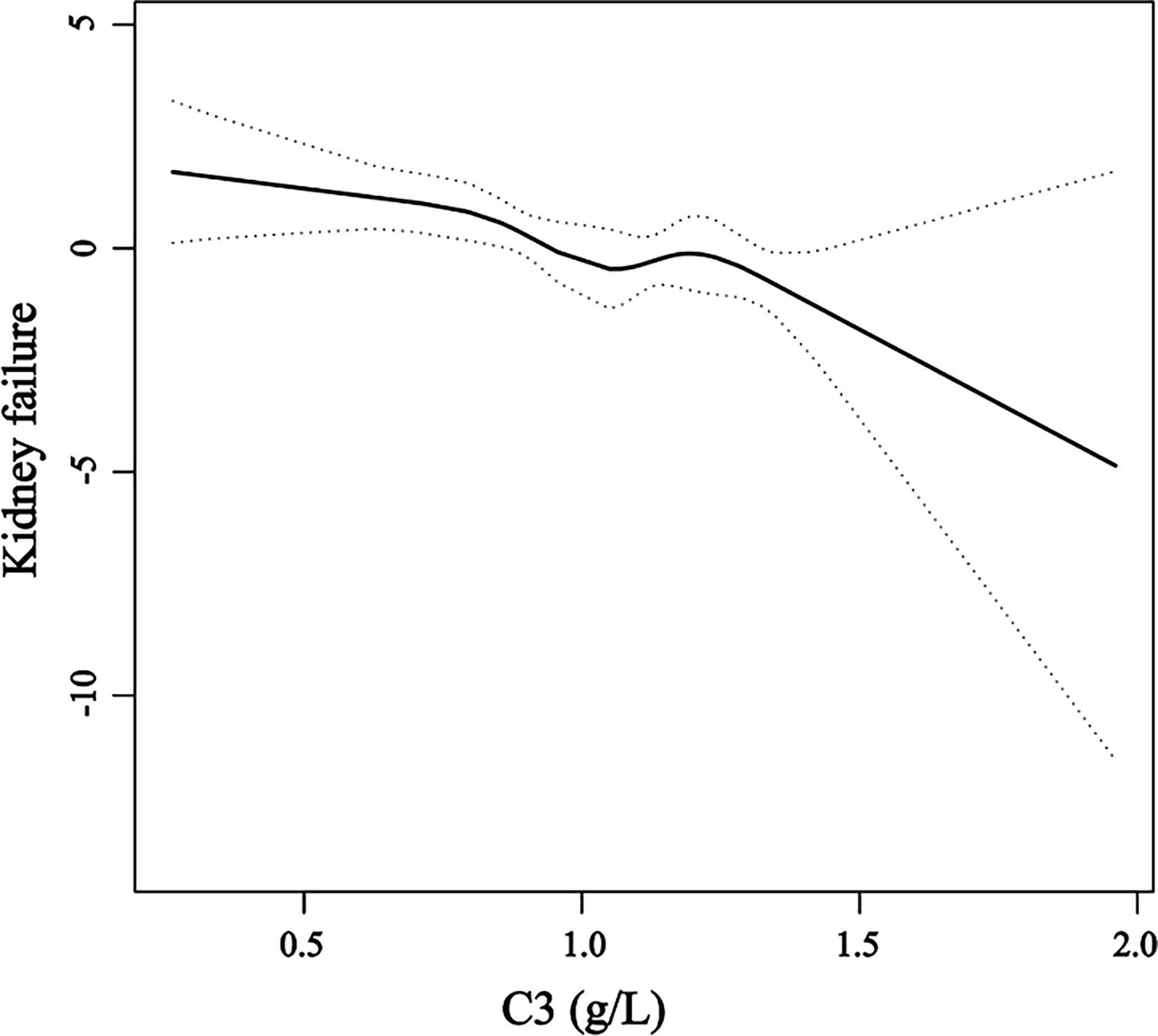
Figure 3 A smooth curve fitting of the relationship between serum C3 levels and the risk of kidney failure. The black straight line represents the smooth curve fit between the variables. The dotted line represents the 95% confidence interval of the fit.
In this study, the relationship between C3 and kidney failure was further stratified by age, hemoglobin, serum albumin, SCr at presentation, oliguria or anuria, and crescents. The results suggested that when the serum albumin <30 g/L, C3 had a protective effect on outcomes in patients with anti-GBM disease, and serum albumin could modify the relationship between C3 and kidney failure. In addition, the protective role in outcomes of C3 was observed only in patients without oliguria/anuria (HR = 0.029, 95% CI = 0.002–0.335, p = 0.005). Serum albumin levels and oliguria/anuria were considered the prominent interactive factors that affect the association between C3 and the risk of kidney failure by the interaction analysis, which remained robust under the grouping of other indicators (Table 4).
This is the first retrospective study to explore the relationship between serum C3 levels measured at diagnosis and the risk of kidney failure in patients with anti-GBM disease. We observed that patients with low C3 levels had more severe glomerular sclerosis and poorer kidney survival than normal C3 levels. Serum C3 may be a protective factor for kidney outcomes. Furthermore, we confirmed that C3 was associated with kidney outcomes by univariable and multivariable Cox regression. Meanwhile, there was a difference in the Kaplan–Meier curve between the low C3 and the normal C3 groups. Therefore, we thought that serum C3 at diagnosis has a protective effect on kidney prognosis in patients with anti-GBM disease. In addition, our work suggested that serum albumin and oliguria/anuria were considered the prominent interactive factors that affect the relationship between C3 and the risk of kidney failure by the interaction analysis.
The complement system plays an essential role in immune-mediated glomerulonephritis. C3 deposition of the glomerular capillary wall can be shown by immunofluorescence, which is consistent with the assumption that complement activation participated in the generation of kidney damage of human anti-GBM disease. Previous studies of humans and animals both have found that the complement system is activated through the classical and alternative pathways in anti-GBM disease (2, 8). Some researchers supported the idea that antibody-directed complement activation initiates a cascade involving cytokine production, upregulation of adhesion molecules and chemokine production, and recruitment and activation of platelets and neutrophils, which ultimately lead to this early glomerular injury (9–12). Ma et al. have found that the complement cascade played a pathogenic role in kidney injury, as shown by the possible proinflammatory effect of C5a and/or cell lysis effect of C5b-9 in 20 patients with kidney biopsy-confirmed anti-GBM disease (8).
Several animal models have been employed to better understand the complement system’s role in the development of anti-GBM disease. Hammer et al. discovered that the primary stage of nephrotoxic serum nephritis produced by rabbit nephrotoxic serum appears to a great extent, but not wholly, upon the participation of serum complement (13). Sheerin et al., using C3-deficient mice, found that complement acts synergistically with heterologous antibodies, resulting in neutrophil infiltration and glomerular injury in experimental anti-GBM disease. Their studies also supported that classical and alternative pathways of the complement system are involved in the development of anti-GBM disease (14). Moreover, in the different phases of anti-GBM disease, the role of complement is contradictory. They thought that the effect of complement in the pathogenesis of glomerular disease might be dependent on the stage of the disease (15). More importantly, they also compared the contribution of systemic and local production of C3, concluding that circulating C3 is a critical factor in reducing the glomerular accumulation of immune complexes, while local synthesis of C3 did not have a major influence on this aspect of glomerular disease (16).
Our study found that lower C3 levels are associated with poorer kidney survival. There were no statistical differences between the two groups of patients who received the standard induction therapy including glucocorticoids and cyclophosphamide combined with a plasma purification technique. Even after adjusting for age, gender, hypertension, GBM, eGFR, KRT at onset, and crescents, C3 levels remained significantly associated with kidney survival. We further found that the C3 level was linearly related to the changing trend of kidney failure, and the risk of kidney failure gradually decreased as the C3 levels increased, which would suggest that serum C3 levels at diagnosis of anti-GBM disease could be an independent protective factor, but this needs further confirmation.
In addition, we found that serum albumin level was considered the prominent interactive factor that affects the association between C3 and the risk of kidney failure by the interaction analysis, which may be related to complement involvement in the pathogenesis of proteinuria (17). Considering that both albumin and C3 may be related to the nutritional status of patients and are potential systemic inflammatory response proteins, there may be other factors at play resulting in a concurrent decrease in both albumin and C3 (and maybe C4), which, in turn, results in worse outcomes that need to be further explored. As for C3, there was no protective role in patients with oliguria or anuria, which may be explained by oliguria or anuria being a stronger predictor of kidney survival in patients with anti-GBM disease compared with other variables, and almost all patients with initial oliguria or anuria end up entering kidney failure (18). Serum C4 levels and local synthesis of C3 have not been found to be associated with kidney outcomes of the disease in our study. We discovered that oliguria/anuria and peak SCr were risk factors for kidney failure in multivariable regression analysis, similar to previous reports.
Although C3 has been rarely studied in human anti-GBM disease, the crucial role of complement in pathogenesis has been explored in IgA nephropathy (6), C3 glomerulonephritis (19), membranous nephropathy (20), and other nephritis (21). Many recent studies demonstrated that C3 levels are correlated to patient and kidney survival in ANCA-associated vasculitis as well (22–24). The critical role of C3 at the intersection of all complement activation pathways and its synergistic effect in multiple immune and inflammatory networks have facilitated the development of C3-based therapies (25). Now, C3 intervention is emerging as a viable therapeutic strategy for rare inflammatory kidney diseases, such as C3 glomerulopathy (26).
Nevertheless, this study still has some limitations, including the small sample size and the single-center design. Moreover, some patients did not have a kidney biopsy at diagnosis, thus limiting the histological analysis. Despite these limitations, we demonstrated clearly that serum C3 plays a protective role in the progress of anti-GBM disease and that low serum C3 level at diagnosis is associated with poor kidney outcomes.
In conclusion, this study retrospectively compared the clinicopathological features and outcomes between the low C3 group and the normal C3 group, and found that patients with low serum C3 levels had a higher proportion of glomerular sclerosis progressing into kidney failure. Serum C3 is closely related to the kidney outcomes of patients with anti-GBM disease, and the pathogenesis of C3 in the disease needs to be further studied.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Independent ethics committee of Jinling Hospital. Written informed consent from the participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
JW and HZ contributed to the conception of the study. WL screened the data, and MZ analyzed and interpreted the data. FX completed the pathological analysis. MZ, JW, YJ, and CJ contributed to the follow-up. MZ finished the manuscript. JW and HZ supervised and edited the manuscript. All authors contributed to the work and approved the submitted version for publication.
This work was supported by the National Key Research and Development Project of China (2021YFC2501302) and the Jiangsu Clinical Research Center Project (YXZXA2016003).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Fischer EG, Lager DJ. Anti-Glomerular Basement Membrane Glomerulonephritis: A Morphologic Study of 80 Cases. Am J Clin Pathol (2006) 125:445–50. doi: 10.1309/nptp-4ukv-7ju3-elmq
2. Otten MA, Groeneveld TW, Flierman R, Rastaldi MP, Trouw LA, Faber-Krol MC, et al. Both Complement and IgG Fc Receptors are Required for Development of Attenuated Antiglomerular Basement Membrane Nephritis in Mice. J Immunol (2009) 183:3980–8. doi: 10.4049/jimmunol.0901301
3. Ma R, Cui Z, Liao YH, Zhao MH. Complement Activation Contributes to the Injury and Outcome of Kidney in Human Anti-Glomerular Basement Membrane Disease. J Clin Immunol (2013) 33:172–8. doi: 10.1007/s10875-012-9772-2
4. Chen M, Daha MR, Kallenberg CG. The Complement System in Systemic Autoimmune Disease. J Autoimmun (2010) 34:J276–86. doi: 10.1016/j.jaut.2009.11.014
5. Xiao H, Dairaghi DJ, Powers JP, Ertl LS, Baumgart T, Wang Y, et al. C5a Receptor (CD88) Blockade Protects Against MPO-ANCA GN. J Am Soc Nephrol (2014) 25:225–31. doi: 10.1681/ASN.2013020143
6. Zhu L, Zhai YL, Wang FM, Hou P, Lv JC, Xu DM, et al. Variants in Complement Factor H and Complement Factor H-Related Protein Genes, CFHR3 and CFHR1, Affect Complement Activation in IgA Nephropathy. J Am Soc Nephrol (2015) 26:1195–204. doi: 10.1681/ASN.2014010096
7. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A New Equation to Estimate Glomerular Filtration Rate [Published Correction Appears in Ann Intern Med. 2011 Sep 20;155(6):408]. Ann Intern Med (2009) 150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006
8. Ma R, Cui Z, Hu SY, Jia XY, Yang R, Zheng X, et al. The Alternative Pathway of Complement Activation may be Involved in the Renal Damage of Human Anti-Glomerular Basement Membrane Disease. PloS One (2014) 9:e91250. doi: 10.1371/journal.pone.0091250
9. Mulligan MS, Johnson KJ, Todd RF 3rd, Issekutz TB, Miyasaka M, Tamatani T, et al. Requirements for Leukocyte Adhesion Molecules in Nephrotoxic Nephritis. J Clin Invest (1993) 91:577–87. doi: 10.1172/JCI116237
10. Wu X, Wittwer AJ, Carr LS, Crippes BA, DeLarco JE, Lefkowith JB. Cytokine-Induced Neutrophil Chemoattractant Mediates Neutrophil Influx in Immune Complex Glomerulonephritis in Rat. J Clin Invest (1994) 94:337–44. doi: 10.1172/JCI117326
11. Quigg RJ, Kozono Y, Berthiaume D, Lim A, Salant DJ, Weinfeld A, et al. Blockade of Antibody-Induced Glomerulonephritis With Crry-Ig, a Soluble Murine Complement Inhibitor. J Immunol (1998) 160:4553–60.
12. Feng L, Xia Y, Yoshimura T, Wilson CB. Modulation of Neutrophil Influx in Glomerulonephritis in the Rat With Anti-Macrophage Inflammatory Protein-2 (MIP-2) Antibody. J Clin Invest (1995) 95:1009–17. doi: 10.1172/JCI117745
13. Hammer Dk, Dixon FJ. Experimental Glomerulonephritis. II. Immunologic Events in the Pathogenesis of Nephrotoxic Serum Nephritis in the Rat. J Exp Med (1963) 117:1019–34. doi: 10.1084/jem.117.6.1019
14. Sheerin NS, Springall T, Carroll MC, Hartley B, Sacks SH. Protection Against Anti-Glomerular Basement Membrane (GBM)-Mediated Nephritis in C3- and C4-Deficient Mice. Clin Exp Immunol (1997) 110:403–9. doi: 10.1046/j.1365-2249.1997.4261438.x
15. Sheerin NS, Springall T, Abe K, Sacks SH. Protection and Injury: The Differing Roles of Complement in the Development of Glomerular Injury. Eur J Immunol (2001) 31:1255–60. doi: 10.1002/1521-4141(200104)31:4<1255::aid-immu1255>3.0.co;2-w
16. Sheerin NS, Abe K, Risley P, Sacks SH. Accumulation of Immune Complexes in Glomerular Disease is Independent of Locally Synthesized C3. J Am Soc Nephrol (2006) 17:686–96. doi: 10.1681/ASN.2004070515
17. Berger SP, Daha MR. Complement in Glomerular Injury. Semin Immunopathol (2007) 29:375–84. doi: 10.1007/s00281-007-0090-3
18. Alchi B, Griffiths M, Sivalingam M, Jayne D, Farrington K. Predictors of Renal and Patient Outcomes in Anti-GBM Disease: Clinicopathologic Analysis of a Two-Centre Cohort. Nephrol Dial Transplant (2015) 30:814–21. doi: 10.1093/ndt/gfu399
19. Smith RJH, Appel GB, Blom AM, Cook HT, D'Agati VD, Fakhouri F, et al. C3 Glomerulopathy - Understanding a Rare Complement-Driven Renal Disease. Nat Rev Nephrol (2019) 15:129–43. doi: 10.1038/s41581-018-0107-2
20. Zhang MF, Huang J, Zhang YM, Qu Z, Wang X, Wang F, et al. Complement Activation Products in the Circulation and Urine of Primary Membranous Nephropathy. BMC Nephrol (2019) 20:313. doi: 10.1186/s12882-019-1509-5
21. Naik A, Sharma S, Quigg RJ. Complement Regulation in Renal Disease Models. Semin Nephrol (2013) 33:575–85. doi: 10.1016/j.semnephrol.2013.08.008
22. Augusto JF, Langs V, Demiselle J, Lavigne C, Brilland B, Duveau A, et al. Low Serum Complement C3 Levels at Diagnosis of Renal ANCA-Associated Vasculitis Is Associated With Poor Prognosis. PloS One (2016) 11:e0158871. doi: 10.1371/journal.pone.0158871
23. Lionaki S, Marinaki S, Liapis G, Kalaitzakis E, Fragkioudaki S, Kalogeropoulos P, et al. Hypocomplementemia at Diagnosis of Pauci-Immune Glomerulonephritis Is Associated With Advanced Histopathological Activity Index and High Probability of Treatment Resistance. Kidney Int Rep (2021) 6:2425–35. doi: 10.1016/j.ekir.2021.05.043
24. Crnogorac M, Horvatic I, Kacinari P, Ljubanovic DG, Galesic K. Serum C3 Complement Levels in ANCA Associated Vasculitis at Diagnosis is a Predictor of Patient and Renal Outcome. J Nephrol (2018) 31:257–62. doi: 10.1007/s40620-017-0445-3
25. Mastellos DC, Reis ES, Yancopoulou D, Hajishengallis G, Ricklin D, Lambris JD. From Orphan Drugs to Adopted Therapies: Advancing C3-Targeted Intervention to the Clinical Stage. Immunobiology (2016) 221:1046–57. doi: 10.1016/j.imbio.2016.06.013
Keywords: anti-GBM disease, complement, C3, kidney failure, outcome
Citation: Zhu M, Wang J, Le W, Xu F, Jin Y, Jiao C and Zhang H (2022) Relationship Between Serum Complement C3 Levels and Outcomes Among Patients With Anti-GBM Disease. Front. Immunol. 13:929155. doi: 10.3389/fimmu.2022.929155
Received: 26 April 2022; Accepted: 15 June 2022;
Published: 08 July 2022.
Edited by:
Raphaela Goldbach-Mansky, National Institutes of Health (NIH), United StatesReviewed by:
Desmond Yat Hin Yap, University of Hong Kong, Hong Kong SAR, ChinaCopyright © 2022 Zhu, Wang, Le, Xu, Jin, Jiao and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haitao Zhang, aHR6aGFuZzE2M0AxNjMuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.