
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Immunol. , 13 June 2022
Sec. Autoimmune and Autoinflammatory Disorders
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.928621
Background: Bullous pemphigoid (BP) is the most common autoimmune subepidermal bullous disease of the skin. First-line treatment of systemic corticosteroids may cause serious adverse events. Rituximab, omalizumab, and dupilumab should be explored as alternative treatment options to improve outcomes.
Objective: To systematically review the rituximab, omalizumab, and dupilumab treatment outcomes in bullous pemphigoid.
Methods: A PubMed, Embase, Web of Science, and Cochrane library search were conducted on March 10, 2022. A total of 75 studies were included using Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines.
Results: Use of rituximab (n=122), omalizumab (n=53) and dupilumab (n=36) were reported in 211 patients with BP. Rituximab led to complete remission in 70.5% (n=86/122) and partial remission in 23.8% (n=29/122) of patients within 5.7 months, with a recurrence rate of 20.5% (n=25/122). 9.0% (n=11/122) of patients died and infection (6.6%, n=8/122) was the most common adverse event. Omalizumab led to complete remission in 67.9% (n=36/53) and partial remission in 20.8% (n=11/53) of patients within 6.6 months, with a recurrence rate of 5.7% (n=3/53). 1.9% (n=1/53) of patients died and thrombocytopenia (1.9%, n=1/53) was observed as the most common adverse event. Dupilumab led to complete remission in 66.7% (n=24/36) and partial remission in 19.4% (n=7/36) of patients within 4.5 months of treatment without any reported adverse events, with a recurrence rate of 5.6% (n=2/36).
Conclusions: Rituximab, omalizumab, and dupilumab have similar clinical benefits for BP patients. However, rituximab resulted in higher recurrence rates, adverse events, and mortality rates.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/, identifier CRD42022316454.
Bullous pemphigoid (BP) is the most common autoimmune subepidermal bullous disease of the skin, which mainly affects older adults about 70 years of age (1). The cumulative incidence of BP is estimated as 8.2% per million people, whereas the incidence rate was 34.2% per million people per year (2). The mortality from BP annually in the United States, Europe, and Asia are 11%-23%,13%-41%, and 12-27%, respectively (3). The most common reason for death is opportunistic infections because of long-term iatrogenic immunosuppression (3). BP is characterized by stiff, generally clear blisters, and erythema which are frequently associated with urticarial plaques and almost all patients experience severe pruritus. Typical clinical signs, histology, features on direct or indirect immunofluorescence assays, and positive specific antibodies can all be used to diagnose BP.
The treatment of localized or mild BP is mainly based on topical corticosteroids and can be combined with antibiotics and nicotinamide. Systemic or topical corticosteroids combined with immunosuppressants such as methotrexate, azathioprine, mycophenolate mofetil, cyclophosphamide, or cyclosporine A are used to treat moderate and severe BP. Other treatment modalities include intravenous immunoglobulin, plasma exchange, and immunoadsorption. Long-term application of corticosteroids may cause serious side effects. New therapeutic pharmacologic biologic agents such as rituximab, omalizumab, and dupilumab can selectively inhibit autoantibody formation and inflammatory cascade and it may be a safer and more effective method to treat BP.
Rituximab is a human-mouse chimeric monoclonal antibody against B lymphocyte CD20, consisting of mouse Fab and human FC. Relative molecular mass is about 145000, which can specifically bind to the transmembrane antibody CD20 on the surface of B lymphocytes and clear peripheral B lymphocytes through antibody-dependent cell-mediated cytotoxicity and complement-mediated apoptosis turn, which affects the corresponding antibody production. Rituximab is the earliest and most common treatment for bullous dermatoses, which has been approved by the Food and Drug Administration (FDA) for treating Pemphigus vulgaris. The European Academy of Dermatology and Venereology (EADV) currently suggests it as a third-line treatment for BP (4).
Omalizumab is a recombinant humanized anti-immunoglobulin E monoclonal antibody. It blocks the binding of IgE to FcϵRI and FcϵRII on the surface of mast cells, basophils, and dendritic cells by specifically binding to the free IgE Cϵ3 region to reduce inflammatory cell activation and inflammatory cascade (5, 6). Studies have shown that IgE is involved in BP pathogenesis. IgE deposition was observed in the epidermal basement membrane zone in 41% of BP patients (7), and there is a higher Bullous Pemphigoid Disease Area Index (BPDAI) score in patients with BP with IgE deposits along the dermal-epidermal junction than in patients without linear IgE deposits (8). The level of Anti-BP180 IgE correlates with BP pathogenesis and BP activity. However, due to varying assays, the fraction of BP patients with combined anti-BP180 IgE autoantibodies remains unknown, with anti-BP180 IgE autoantibody-positive rates ranging from 22% to 100% (9).
Dupilumab is a humanized IgG4 monoclonal antibody that targets the interleukin (IL)-4 receptor alpha chain. Dupilumab can inhibit T helper (Th) 2 cell differentiation, the transformation of Treg cells into ex-Treg cells in the context of allergic inflammation, and IgE production by B cells, driven by T follicular helper-derived IL-4. It can also prevent IL-4-related vascular endothelium dysfunction and inhibit ILC2 induction via eosinophils and basophils (10). The pathogenesis of BP involves the Th1/Th2 inflammatory reaction and the expression of the cytokine IL-4 is increased in the skin, serum, and herpes fluid of BP patients (11).
Therefore, this comprehensive study aims to systematically assess and evaluate the available reports on rituximab, omalizumab, and dupilumab and their outcomes in BP patients.
The systematic review was performed following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (12) and registered with the PROSPERO international prospective registry (CRD42022316454). A comprehensive review was performed on articles published from inception to March 10, 2022, in the following databases: PubMed, Embase, Web of Science, and Cochrane library. The following search string combining Medical Subject Headings (MeSH) terms and related words: ((Pemphigoid, Bullous) OR (Bullous Pemphigoid) OR (Pemphigoid) OR (Pemphigoids)) AND ((Rituximab) OR (CD20 Antibody, Rituximab) OR (Rituximab CD20 Antibody) OR (Mabthera) OR (IDEC-C2B8 Antibody) OR (IDEC C2B8 Antibody) OR (IDEC-C2B8) OR (IDEC C2B8) OR (GP2013) OR (Rituxan)) were searched. Vocabulary and syntax were adapted to be appropriate for each database. Additionally, the reviewers manually searched the references of the articles independently to identify any additional articles that search engines may have otherwise missed. A similar strategy was used in the search for omalizumab and dupilumab publications.
Studies were included that documented patients diagnosed with BP and reported resolved outcomes for the treatment of BP with rituximab, omalizumab, and dupilumab. The diagnosis of BP needs to meet at least one of the followings:(1) Histopathology reveals subepidermal blistering with eosinophilic infiltration. (2) Direct immunofluorescence: basement membrane containing IgG, IgM, and C3 deposits. (3) Indirect immunofluorescence:the presence of anti-basement band antibody IgG in the patient’s serum. (4) Positivity for BP180 and/or BP230 in an enzyme-linked immunosorbent assay. Studies were excluded if they did not report any efficacy data and that documented patients diagnosed with drug-induced bullous pemphigoid. There will not be any language or geographic restrictions.
1. Resolution outcomes on biologic treatment:
1) Complete remission: The total resolution of BP lesions. The publications used the terms “complete remission”, “complete response”, “complete control”, and “symptom-free”.
2) Partial remission: Improvement yet lack the complete resolution of BP lesions. The publications used the terms “partial remission,” “partial response”, “improved”, and “clinical improvement”.
3) No remission: no changes in BP lesions. The publications used the terms “no resolution” or “no response”.
4) Deterioration: exacerbation of BP lesions.
2. Time to remission:
The duration between biologic treatment starting and reports of resolution outcomes.
3. Recurrence:
Recurrence of BP lesions during biologic treatment or after biologic treatment stopped. The publications used the terms “new blister” or “recurrence of bullae”.
4. Adverse events.
Two reviewers (Cao and Xu) independently screened all the studies identified by the search strategy, screen titles, and abstracts, followed by the full text of potentially eligible studies. Any disagreement was resolved through discussion with a third reviewer (Zhang).
For each selected study, the following information was extracted into an electronic form: first author, publication year, study design, number of patients, sex, age, BP duration, follow-up period, detected antibodies (anti-BP180 IgG, anti-BP230 IgG, anti-BP180 IgE, anti-BP230 IgE, elevated total IgE), eosinophilia, previous treatment, concomitant treatment, resolution outcomes (complete remission, partial remission, no remission, deterioration), time to remission, recurrence, and adverse events.
The Oxford Centre for Evidence-Based Medicine 2009 Levels of Evidence was used to evaluate the quality of evidence.
Data were analyzed using descriptive statistics. Categorical variables are presented as numbers and percent and continuous variables as mean and range.
As shown in Figure 1, a total of 75 publications were included for analysis as 412 duplicated publications were excluded, 764 were excluded after reading titles and abstracts, and 46 were excluded after further reading from the 1,297 retrieved publications. The rituximab group had 36 publications (13–48), including 5 prospective studies, 9 retrospective studies, and 22 case series or reports. The omalizumab group had 28 publications (17, 49–75), including 1 prospective study, 1 retrospective study, and 26 case series or reports. While the dupilumab group had 11 publications (59, 73, 76–84), including 2 retrospective studies and 9 case series or reports.
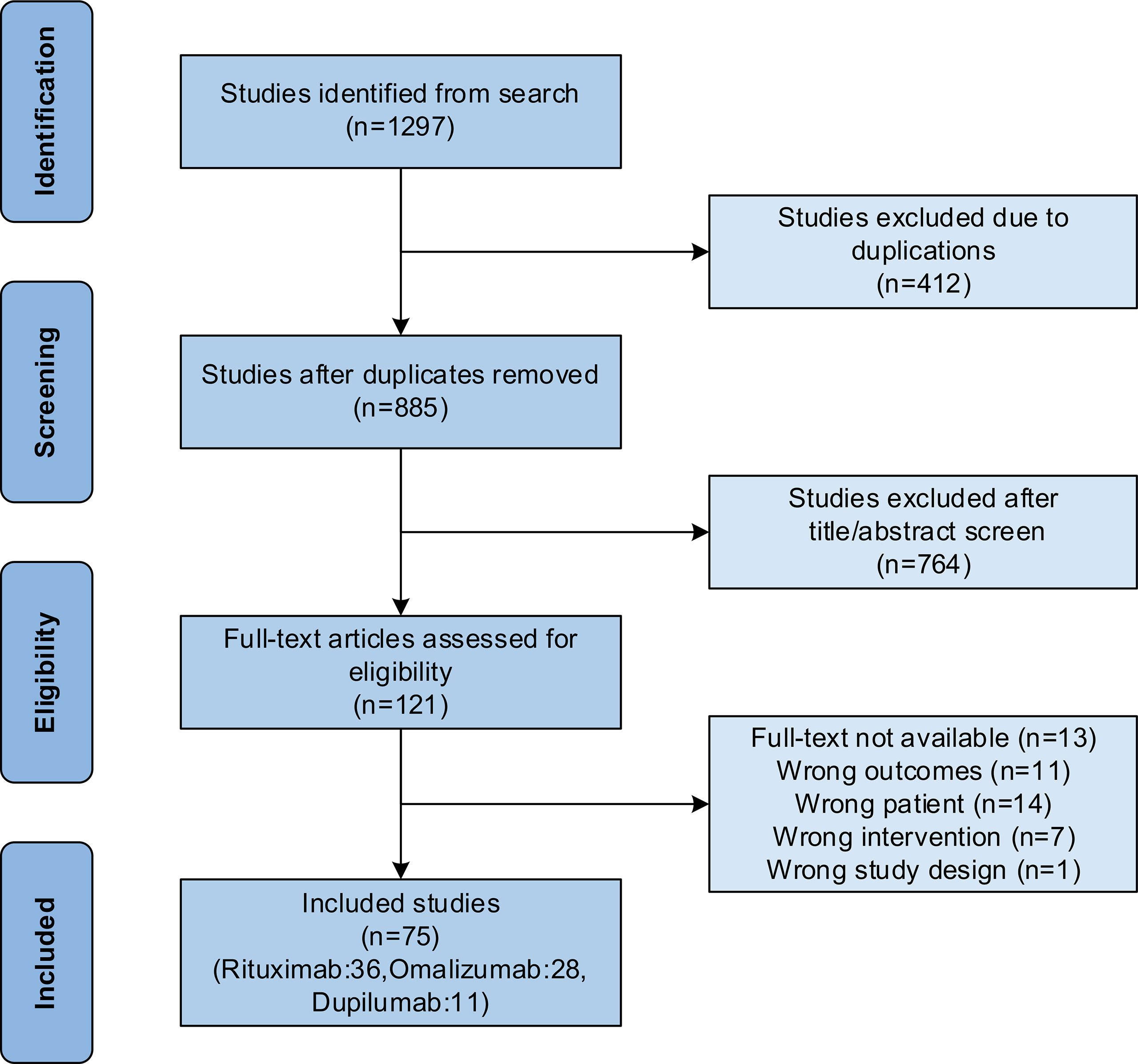
Figure 1 Flow chart of study selection following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
A total of 211 patients were included, of whom 122 received rituximab, 53 received omalizumab, and 36 received dupilumab (Table 1). Among these 211 patients (mean age were 68.0 years old, ranging from 0.3 to 95.0 years old), 43.6% (n=92/211) were women, 42.2% (n=89/211) were men, and 14.2% (n=30/211) had no gender information reported.
The average course of the disease was 25.4 months (ranging from 1.0 to 92.0 months). No IgG antibody information was reported for 32.8% (n=40/122) of patients in the rituximab groups, and 65.6% (n=80/122) presented anti-BP180 IgG positive and 48.4% (n=59/122) were anti-BP230 IgG positive.
Before rituximab treatment, 54.9% (n=67/122) received corticosteroids but failed to be treated; 10.7% (n=13/122), 18.0% (n=22/122), 11.5% (n=14/122), 4.1% (n=5/122), 4.9% (n=6/122), 0.8% (n=1/122) received immunosuppressants methotrexate, mycophenolate mofetil, azathioprine, cyclosporine, cyclophosphamide, and tacrolimus, respectively, but failed; 18.0% (n=22/122), 1.6% (n=2/122), 3.3% (n=4/122) received antibiotics dapsone, doxycycline and minocycline, respectively, but failed; 3.3% (n=4/122), 12.3% (n=15/122), 0.8% (n=1/122), 0.8% (n=1/122), 1.6% (n=2/122) received nicotinamide, intravenous immunoglobulin, immunoadsorption, plasma exchange, antihistamines, respectively, but failed; 0.8% (n=1/122) received omalizumab but failed, and 0.8% (n=1/122) did not receive any treatment.
After rituximab treatment, 70.5% (n=86/122) of patients had complete remission, 23.8% (n=29/122) had partial remission, 4.9% (n=6/122) showed no remission, and 0.8% (n=1/122) had deteriorated (Table 2). The average time to remission was 5.7 months (range, 1.0-13.0 months). The mean follow-up time after treatment was 21.9 months (range, 1.0-38.0 months). Also, 20.5% (n=25/122) patients recurred, 70.5% (n=86/122) did not recur, and 3.3% (n=4/122) did not report.
No adverse events occurred in 59.8% (n=73/122) of patients, information related to adverse events was not reported in 15.6% (n=19/122), and 9.0% (n=11/122) of patients died. The most common adverse event was infection (6.6%, n=8/122), followed by altered mental status (3.3%, n=4/122), anemia (1.6%, n=2/122), tachycardia (0.8%, n=1/122), compression fracture (0.8%, n=1/122), prostate cancer (0.8%, n=1/122), metastatic breast cancer (0.8%, n=1/122), mucoepidermoid carcinoma (0.8%, n=1/122) and dyspnea (0.8%, n=1/122).
Among all patients, the average course of disease was 9.6 months (ranging from 1.0 to 48.0 months). No IgG antibody information was reported for 49.1% (n=26/53) of patients in the omalizumab groups, and 47.2% (n=25/53) presented anti-BP180 IgG positive and 26.4% (n=14/53) were anti-BP230 IgG positive, while 7.5% (n=4/53) presented anti-BP180 IgE positive and 5.7% (n=3/53) were anti-BP230 IgE positive. Also, 64.2% (n=34/53) of patients showed an apparent increase in total IgE levels and eosinophils levels of 34.0% (n=18/53).
Before omalizumab treatment, 96.2% (n=51/53) received corticosteroids but failed; 9.4% (n=5/53), 13.2% (n=7/53), 17.0% (n=9/53), 1.9% (n=1/53), 3.8% (n=2/53) received immunosuppressants methotrexate, mycophenolate mofetil, azathioprine, cyclosporine, and cyclophosphamide, respectively, but failed; 13.2% (n=7/53), 20.8% (n=11/53), 3.8% (n=2/53), 5.7% (n=3/53), 1.9% (n=1/53) received antibiotics dapsone, doxycycline, minocycline, tetracycline, and azithromycin, respectively, but failed; 13.2% (n=7/53), 20.8% (n=11/53), 9.4% (n=5/53) received nicotinamide, intravenous immunoglobulin, and antihistamines, respectively, but failed; and 11.3% (n=6/53) received rituximab but failed.
After omalizumab treatment, 67.9% (n=36/53) of patients had complete remission, 20.8% (n=11/53) had partial remission, 11.3% (n=6/53) showed no remission, and no patients had deteriorated. The average time to remission was 6.6 months (range, 0.5-15.0 months). The mean follow-up time after treatment was 5.6 months (range, 2.0-10.0 months), 5.7% (n=3/53) patients recurred, 79.2% (n=42/53) did not recur, and 3.8% (n=2/53) did not report.
No adverse events occurred in 64.2% (n=34/53) of patients, information related to adverse events was not reported in 32.1% (n=17/53), and 1.9% (n=1/53) of patients died. The most common adverse event was thrombocytopenia (1.9%, n=1/53).
Among all patients, the average course of the disease was 19.2 months (ranging from 1.0 to 240.0 months). No IgG antibody information was reported for 91.7% (n=33/36) of patients in the dupiluma groups, 8.3% (n=3/36) presented anti-BP180 IgG positive, and 2.8% (n=1/36) were anti-BP230 IgG positive. Moreover, 16.7% (n=6/36) appeared to increase in total IgE level and eosinophils level of 11.1% (n=4/36) patients raised.
Before dupilumab treatment, 66.7% (n=24/36) received corticosteroids but failed; 22.2% (n=8/36), 13.9% (n=5/36), 5.6% (n=2/36), 2.8% (n=1/36), 2.8% (n=1/36) received immunosuppressants methotrexate, mycophenolate mofetil, azathioprine, cyclosporine, and cyclophosphamide, respectively, but failed; 2.8% (n=1/36), 16.7% (n=6/36) received antibiotics dapsone and doxycycline respectively but failed; 13.9% (n=5/36), 13.9% (n=5/36), 2.8% (n=1/36) received nicotinamide, intravenous immunoglobulin, and antihistamines, respectively, but failed; 5.6% (n=2/36), 13.9% (n=5/36) received rituximab and omalizumab, respectively, but failed.
After dupilumab treatment, 66.7% (n=24/36) of patients had complete remission, 19.4% (n=7/36) had partial remission, 13.9% (n=5/36) showed no remission, and no patients had deteriorated. The average time to remission was 4.5 months (range, 1.0-15.0 months). The mean follow-up time after treatment was 8.6 months (range, 5.0-12.0 months), 5.6% (n=2/36) patients recurred, 72.2% (n=26/36) did not recur, and 8.3% (n=3/36) did not report.
No adverse events occurred in 83.3% (n=30/36) of patients, information related to adverse events was not reported in 16.7% (n=6/36), and no patients died.
A total of 211 patients were included in this systematic review and the ratio of men to women was 0.97/1. There was no gender difference and the average age was 68.0, which was lower than the previously reported age of BP at the end of 70 years (1). This may be since the number of patients included in this systematic review was less but in comparison, it may be due to the tendency of BP patients to seek medical treatment and agree to receive biological agents decreasing with age. Whether there is a younger trend in the incidence of BP needs a broader epidemiological investigation.
Rituximab, omalizumab, and dupilumab have similar clinical benefits in treating BP. The complete remission ratio was 70.5%, 67.9% and 66.7%, respectively, and the partial remission ratio was 23.8%, 20.8% and 19.4%, respectively. One patient received complete remission by rituximab after the failure of omalizumab, five patients received complete remission by dupilumab after the failure of omalizumab, and one patient received partial remission by dupilumab after the failure of rituximab. This indicates the intricacies of the types of autoantibodies, their specific pathogenic actions, and the wide inherent inter-individual variations in BP (85). One patient received both omalizumab and dupilumab and achieved complete remission after 3 months of treatment and no recurrence was found after 10 months of follow-up. Whether treatment with two or more new biological agents simultaneously can produce more clinical benefits needs to be further explored.
Some individuals with refractory BP were resistant to treatment or had a high recurrence rate. In this group of patients, standard corticosteroids and immunosuppressants were ineffective. Some patients with refractory BP have benefited from the clinical use of rituximab, omalizumab, and dupilumab. Of the 211 patients included in the systematic review, a large proportion received biologics after failing treatment with corticosteroids, immunosuppressants, and antibiotics. It achieved complete or partial remission, and rituximab, omalizumab, and dupilumab could be the safe and effective alternative therapy for refractory BP. Only one patient, who had not received any treatment before receiving the biologic, achieved complete remission and no recurrence after rituximab treatment suggests that patients can benefit from the direct use of biologics in the early stage of the disease.
The exact mechanism by which rituximab, omalizumab, and dupilumab produce clinical remission in BP is currently unclear. Rituximab against the CD20 surface protein expressed on B-cell lymphocytes which produce the autoantibodies involved in BP pathogenesis. Once bound, the Fc portion of the antibody recruits immune effector cells for lysis of such antibody-producing B cells, leading to BP remission (86). Protected and non-pathogenic B cells were generated during repopulation when rituximab cleared pathogenic B cells that might contribute to long-term clinical remission (87).
IgE autoantibodies were involved in BP pathogenesis. The binding of Anti-BP180 IgE to FcϵRI on the surface of mast cells and eosinophils promotes inflammatory cell activation and inflammatory cascade response, thus leading to tissue damage and blister formation. In contrast, the binding of Anti-BP180 IgE to the extracellular region of BP180 might lead to internalization of BP180 and blister formation (74, 88, 89). Omalizumab leads to BP remission by directly blocking the process of IgE binding to cell surface FcϵRI. In addition, serum total IgE levels were elevated in 64.2% (n=34/53) of patients in the omalizumab group. Elevated serum total IgE levels correlate with the severity of BP, and omalizumab isolates free IgE, and prevents its binding to the IgE receptor FcϵRI, thus helping such BP patients to achieve clinical remission (89, 90).
Complex immune interactions associated with the Th2 axis, including the cytokines IL-4 and IL-13 and chemokines and eosinophils, are involved in the pathogenesis of BP. The IL-4 receptor alpha antagonist dupilumab inhibits B-cell proliferation, eosinophil chemotaxis, and Th2 chemokines expression by blocking IL-4 and IL-13 signaling and ultimately leads to BP remission (91).
The recurrence rate in the rituximab group was higher than that in omalizumab and dupilumab groups (20.5% vs. 5.7% vs. 5.6%). This may be due to the longer duration of BP in the rituximab group (25.4 vs. 9.6 vs. 19.2 months). One study found that the recurrence rate increased in patients with a longer duration of disease who received rituximab and the remission rate decreased (92). Another study found that patients with pemphigus who received rituximab after a long course of the disease and after conventional treatment failed, had a higher recurrence rate than those who received rituximab directly at the early stage of the disease (93). The later introduction of rituximab means more pathogenic B cell clones. The failure of rituximab treatment and the recurrence of patients after treatment are associated with the prolongation of the course of disease caused by conventional treatment (94).
Furthermore, longer follow-up time in the rituximab group was also associated with a higher recurrence rate (21.9 vs. 5.6 vs. 8.6 months). Relapse of BP generally occurs 12-18 months after treatment, which means that the rituximab group with a follow-up of 21.9 months was able to assess recurrence rates accurately. In contrast, the omalizumab and dupilumab groups may have had a lower recurrence rate than the actual value because of the short follow-up. Mechanistically, relapse is associated with a higher percentage of memory B cells, a decreased proportion of naïve and/or transitional B cells, or lower peak serum levels of B-cell activating factors (13). Besides, in vitro studies confirmed that rituximab could not clear the IgA-secreting plasma cells (95), which is one of the reasons for the high recurrence rate in the rituximab group.
The incidence of no adverse events in the dupilumab group was higher than in the omalizumab and rituximab groups. In contrast, the incidence of no adverse events in the omalizumab group was similar to that in the rituximab group. Noa Kremer et al. reported that adverse events in BP treated with omalizumab and rituximab were similar at 20% and 24%, respectively (96).
The main adverse event of rituximab treatment of BP is infection (6.6%, n=8/122), which is related to rituximab’s mechanism in clearing B lymphocytes. Rituximab should not be used in patients with active infection and impaired immune function. Infection caused by rituximab treatment of BP is generally mild. However, older patients and patients treated with large doses of corticosteroids or immunosuppressants should guard against the emergence of severe infection (97). The main adverse event of omalizumab in the treatment of BP is thrombocytopenia (1.9%, n=1/53). In a report on the treatment of asthma, the main adverse event related to omalizumab is anemia, with an incidence of 1-2/1000 (98). No adverse events have been reported in the treatment of BP with dupilumab. In treating atopic dermatitis, the most common side effect of dupilumab is injection site reaction, which mainly consists of transient erythema or edema (99) and the only specific side effect is conjunctivitis (100).
There was no death in the dupilumab group. One patient in the omalizumab group died of pneumonia. Two patients in the rituximab group died of acute respiratory failure. Two patients died of heart failure, two patients died of severe pneumonia, two patients died of systemic state changes, one patient died of gastrointestinal bleeding, and one patient died of bacterial septicemia with progressive renal failure. One patient with heart disease died 10 days after rituximab infusion. The patient mortality rates of 9.0% in the rituximab group, 1.9% in the omalizumab group, and 0.0% in the dupilumab group were all lower than the 11%-41% mortality rates of BP patients reported in other reports (3). The mean age of patients who died in the rituximab group was 59.3 years, including a 0.6-year-old child with BP, which led to this low figure. The mean age of patients who died after excluding this patient was 65.2 years, approximately equal to the mean age of patients in the rituximab group of 65.9 years. The age of patients who died in the omalizumab group was 78.0 years, higher than the mean age of patients in the omalizumab group of 68.2 years. The cause of death may be related to the patient’s advanced age and medical condition. However, the possibility that it was related to treatment with biologics cannot be ruled out, especially in the case of the one patient who died 10 days after rituximab treatment. However, the patient had a previous history of heart disease. Rituximab is currently the earliest and most widely used biological agent in treating BP. However, considering the incidence of adverse events and mortality, omalizumab or dupilumab may be more advantageous in treating BP.
A total of four children were included in the systematic review, of which three achieved complete remission after receiving rituximab, and one achieved partial remission after receiving rituximab. There were no adverse events but one patient died of respiratory failure three months after the last injection of rituximab. BP in children is very rare. The number of reported cases is less than 100 cases (101). The first-line treatment of BP in children is systemic corticosteroids but children are highly vulnerable to the side effects of systemic corticosteroids, notably infections and growth retardation (102). Rituximab is expected to replace corticosteroids as a more effective treatment for children with BP with fewer side effects, though more research data is needed. Some scholars believe that rituximab may lead to infection, drug fever, and other adverse reactions which should be used when a certain dose of corticosteroid is still unable to control the condition of the child. At present, there are no reports about the application of omalizumab and dupilumab in the treatment of BP in children. However, omalizumab has been widely used in the treatment of allergic asthma and urticaria in children and dupilumab has been widely used in the treatment of atopic dermatitis in children.
Limitations of this review include a small sample size and a lack of a control group. In addition, publication bias represents another limitation since studies with negative results are less likely to be published. Data reported in this systematic review are subject to publication bias of the underlying included studies consisting of case series and case reports (76.0%, n=57/75), retrospective (16.0%, n = 12/75), and small prospective studies (8.0%, n = 6/75). In addition, BP severity may affect rituximab, omalizumab, and dupilumab efficacy. However, the absence of this data and inconsistency of scoring methods in most of the included studies prevented further analysis.
This systematic review comprehensively summarized the reports of rituximab, omalizumab, and dupilumab for BP treatment to date. Our data suggest that rituximab, omalizumab, and dupilumab have similar clinical benefits in BP. However, rituximab resulted in higher recurrence rates, adverse events, and mortality rates. Future randomized clinical trials are required to conclude the safety and efficacy of biologics in patients with BP.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
PC and WX: conception and design of the work, data collection, data analysis and interpretation, drafting the article. LZ: conception and design of the work, data collection, data analysis and interpretation, article revision and approval of the publication. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Schmidt E, Zillikens D. Pemphigoid Diseases. Lancet (2013) 381(9863):320–32. doi: 10.1016/S0140-6736(12)61140-4
2. Persson MSM, Begum N, Grainge MJ, Harman KE, Grindlay D, Gran S. The Global Incidence of Bullous Pemphigoid: A Systematic Review and Meta-Analysis. Br J Dermatol (2022) 186(3):414–25. doi: 10.1111/bjd.20743
3. Phoon YW, Fook-Chong SM, Koh HY, Thirumoorthy T, Pang SM, Lee HY. Infectious Complications in Bullous Pemphigoid: An Analysis of Risk Factors. J Am Acad Dermatol (2015) 72(5):834–9. doi: 10.1016/j.jaad.2015.01.029
4. Feliciani C, Joly P, Jonkman MF, Zambruno G, Zillikens D, Ioannides D, et al. Management of Bullous Pemphigoid: The European Dermatology Forum Consensus in Collaboration With the European Academy of Dermatology and Venereology. Br J Dermatol (2015) 172(4):867–77. doi: 10.1111/bjd.13717
5. Holgate ST. New Strategies With Anti-IgE in Allergic Diseases. World Allergy Organ J (2014) 7(1):17. doi: 10.1186/1939-4551-7-17
6. Ledford DK. Omalizumab: Overview of Pharmacology and Efficacy in Asthma. Expert Opin Biol Ther (2009) 9(7):933–43. doi: 10.1517/14712590903036060
7. Yayli S, Pelivani N, Beltraminelli H, Wirthmuller U, Beleznay Z, Horn M, et al. Detection of Linear IgE Deposits in Bullous Pemphigoid and Mucous Membrane Pemphigoid: A Useful Clue for Diagnosis. Br J Dermatol (2011) 165(5):1133–7. doi: 10.1111/j.1365-2133.2011.10481.x
8. Kamata A, Kurihara Y, Funakoshi T, Takahashi H, Kuroda K, Hachiya T, et al. Basement Membrane Zone IgE Deposition Is Associated With Bullous Pemphigoid Disease Severity and Treatment Results. Br J Dermatol (2020) 182(5):1221–7. doi: 10.1111/bjd.18364
9. Van Beek N, Luttmann N, Huebner F, Recke A, Karl I, Schulze FS, et al. Correlation of Serum Levels of IgE Autoantibodies Against BP180 With Bullous Pemphigoid Disease Activit. JAMA Dermatol (2017) 153(1):30–8. doi: 10.1001/jamadermatol.2016.3357
10. Harb H, Chatila TA. Mechanisms of Dupilumab. Clin Exp Allergy (2020) 50(1):5–14. doi: 10.1111/cea.13491
11. Hendricks AJ, Yosipovitch G, Shi VY. Dupilumab Use in Dermatologic Conditions Beyond Atopic Dermatitis - A Systematic Review. J Dermatolog Treat (2021) 32(1):19–28. doi: 10.1080/09546634.2019.1689227
12. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PloS Med (2009) 6(7):e1000097. doi: 10.1371/journal.pmed.1000097
13. Ahmed AR, Shetty S, Kaveri S, Spigelman ZS. Treatment of Recalcitrant Bullous Pemphigoid (BP) With a Novel Protocol: A Retrospective Study With a 6-Year Follow-Up. J Am Acad Dermatol (2016) 74(4):700–8.e3. doi: 10.1016/j.jaad.2015.11.030
14. Aljasser MI, Sladden C, Crawford RI, Au S. Bullous Pemphigoid Associated With Acquired Hemophilia A: A Rare Association of Autoimmune Disease. J Cutaneous Med Surg (2014) 18(2):123–6. doi: 10.2310/7750.2013.13060
15. Batts AF, Jalalat SZ, Hunter-Ellul L, Wilkerson MG. Exacerbation of Bullous Pemphigoid After Hand, Foot, and Mouth Disease Treated With Rituximab. JAAD Case Rep (2016) 2(1):7–9. doi: 10.1016/j.jdcr.2015.11.006
16. Berkani N, Joly P, Golinski ML, Colliou N, Lim A, Larbi A, et al. B-Cell Depletion Induces a Shift in Self Antigen Specific B-Cell Repertoire and Cytokine Pattern in Patients With Bullous Pemphigoid. Sci Rep (2019) 9(1):3525. doi: 10.1038/s41598-019-40203-7
17. Bilgiç Temel A, Bassorgun CI, Akman-Karakaş A, Alpsoy E, Uzun S. Successful Treatment of a Bullous Pemphigoid Patient With Rituximab Who Was Refractory to Corticosteroid and Omalizumab Treatments. Case Rep Dermatol (2017) 9(1):38–44. doi: 10.1159/000452828
18. Chiaravalloti A. Treatment of Recalcitrant Bullous Pemphigoid With Rituximab. J Am Acad Dermatol (2014) 70(5):AB99. doi: 10.1016/j.jaad.2014.01.411
19. Cho YT, Chu CY, Wang LF. First-Line Combination Therapy With Rituximab and Corticosteroids Provides a High Complete Remission Rate in Moderate-to-Severe Bullous Pemphigoid. Br J Dermatol (2015) 173(1):302–4. doi: 10.1111/bjd.13633
20. Cruz MJ, Santos P, Morais P, Barreto F, Azevedo F. Refractory Bullous Pemphigoid With Fatal Outcome in a Young Patient. Int J Dermatol (2013) 52(5):601–2. doi: 10.1111/j.1365-4632.2011.05317.x
21. Eriksson H, Hansson J, Krynitz B, Masucci G, Girnita A, Lapins J, et al. Bullous Pemphigoid-Like Eruption Triggered by Targeted Therapy for Metastatic Melanoma. Acta Dermato-Venereologica (2020) 100(18):1–3. doi: 10.2340/00015555-3648
22. Fuertes I, Luelmo J, Leal L, Romaní J, Sánchez S, Mascaró JM Jr, et al. Refractory Childhood Pemphigoid Successfully Treated With Rituximab. Pediatr Dermatol (2013) 30(5):e96–7. doi: 10.1111/pde.12057
23. Hall RP 3rd, Streilein RD, Hannah DL, McNair PD, Fairley JA, Ronaghy A, et al. Association of Serum B-Cell Activating Factor Level and Proportion of Memory and Transitional B Cells With Clinical Response After Rituximab Treatment of Bullous Pemphigoid Patients. J Invest Dermatol (2013) 133(12):2786–8. doi: 10.1038/jid.2013.236
24. Hamann C, Castillo S, Mann J, Glass J, LeBlanc RE, Chapman MS. Immune-Related Adverse Events: A New Great Mimicker? A Case of Prurigo Nodularis-Like Bullous Pemphigoid Due to Anti-CTLA-1 and Anti-PD-1 Inhibitors Responding to Rituximab. J Am Acad Dermatol (2019) 81(4):AB222. doi: 10.1016/j.jaad.2019.06.816
25. Kasperkiewicz M, Shimanovich I, Ludwig RJ, Rose C, Zillikens D, Schmidt E. Rituximab for Treatment-Refractory Pemphigus and Pemphigoid: A Case Series of 17 Patients. J Am Acad Dermatol (2011) 65(3):552–8. doi: 10.1016/j.jaad.2010.07.032
26. Kern M, Wong HK. Bullous Pemphigoid in a Father and Daughter: A Case Report. J Am Acad Dermatol (2019) 81(4):AB28. doi: 10.1016/j.jaad.2019.06.141
27. Kianfar N, Dasdar S, Mahmoudi H, Tavakolpour S, Balighi K, Daneshpazhooh M. Rituximab in Childhood and Juvenile Autoimmune Bullous Diseases as First-Line and Second-Line Treatment: A Case Series of 13 Patients. J Dermatol Treat (2020) 33(2):869–74. doi: 10.1080/09546634.2020.1788702
28. Kolesnik M, Becker E, Reinhold D, Ambach A, Heim MU, Gollnick H, et al. Treatment of Severe Autoimmune Blistering Skin Diseases With Combination of Protein A Immunoadsorption and Rituximab: A Protocol Without Initial High Dose or Pulse Steroid Medication. J Eur Acad Dermatol Venereol (2014) 28(6):771–80. doi: 10.1111/jdv.12175
29. Lourari S, Herve C, Doffoel-Hantz V, Meyer N, Bulai-Livideanu C, Viraben R, et al. Bullous and Mucous Membrane Pemphigoid Show a Mixed Response to Rituximab: Experience in Seven Patients. J Eur Acad Dermatol Venereol (2011) 25(10):1238–40. doi: 10.1111/j.1468-3083.2010.03889.x
30. Ma E, Kelly J, Ip W, Su J, Varigos G, Orchard D. Effective Treatment With Rituximab for Severe Infantile Bullous Pemphigoid. Australas J Dermatol (2010) 51:A34. doi: 10.1111/j.1440-0960.2010.00644.x
31. Maglie R, Antiga E, Quintarelli L, Verdelli A, Caproni M. Dramatic Exacerbation of Bullous Pemphigoid Following Rituximab and Successful Treatment With Omalizumab. Eur J Dermatol (2019) 29(2):213–5. doi: 10.1684/ejd.2019.3499
32. Nguyen T, Ahmed AR. Positive Clinical Outcome in a Patient With Recalcitrant Bullous Pemphigoid Treated With Rituximab and Intravenous Immunoglobulin. Clin Exp Dermatol (2017) 42(5):516–9. doi: 10.1111/ced.13092
33. Nieto-Benito LM, Bergón-Sendín M, Pulido-Pérez A, Suárez-Fernández RM. Clinical Outcome and Safety Profile of Rituximab for the Treatment of Dipeptidyl Peptidase 4 Inhibitors-Induced Bullous Pemphigoid. Exp Dermatol (2021) 31(4):641–2. doi: 10.1111/exd.14483
34. Parajuli S, Vidhan J, Pokhrel DB, Paudel U. Efficacy and Safety of Rituximab in Immunobullous Diseases: A Retrospective Study From a Tertiary Care Centre of Nepal. J Clin Diagn Res (2020) 14(9):WC01–4. doi: 10.7860/JCDR/2020/43460.13979
35. Polansky M, Eisenstadt R, DeGrazia T, Zhao X, Liu Y, Feldman R. Rituximab Therapy in Patients With Bullous Pemphigoid: A Retrospective Study of 20 Patients. J Am Acad Dermatol (2019) 81(1):179–86. doi: 10.1016/j.jaad.2019.03.049
36. Rashid H, Meijer JM, Bolling MC, Horváth B. Clinical Response to Rituximab and Improvement in Quality of Life in Patients With Bullous Pemphigoid and Mucous Membrane Pemphigoid British. J Dermatol (2021) 186(4):721–3. doi: 10.1111/bjd.20881
37. Reguiaï Z, Tchen T, Perceau G, Bernard P. Efficacy of Rituximab in a Case of Refractory Bullous Pemphigoid. Ann Dermatol Venereol (2009) 136(5):431–4. doi: 10.1016/j.annder.2008.10.038
38. Ridpath AV, Rzepka PV, Shearer SM, Scrape SR, Olencki TE, Kaffenberger BH. Novel Use of Combination Therapeutic Plasma Exchange and Rituximab in the Treatment of Nivolumab-Induced Bullous Pemphigoid. Int J Dermatol (2018) 57(11):1372–4. doi: 10.1111/ijd.13970
39. Saouli Z, Papadopoulos A, Kaiafa G, Girtovitis F, Kontoninas Z. A New Approach on Bullous Pemphigoid Therapy. Ann Oncol (2008) 19(4):825–6. doi: 10.1093/annonc/mdn046
40. Schmidt E, Seitz CS, Benoit S, Bröcker EB, Goebeler M. Rituximab in Autoimmune Bullous Diseases: Mixed Responses and Adverse Effects. Br J Dermatol (2007) 156(2):352–6. doi: 10.1111/j.1365-2133.2006.07646.x
41. Schulze J, Bader P, Henke U, Rose MA, Zielen S. Severe Bullous Pemphigoid in an Infant–Successful Treatment With Rituximab. Pediatr Dermatol (2008) 25(4):462–5. doi: 10.1111/j.1525-1470.2008.00751.x
42. Silva N, Costa A, Salvador F, Serradeiro E. Bullous Pemphigoid Successfully Treated With Rituximab. Acta Med Port (2017) 30(3):243–6. doi: 10.20344/amp.7812
43. Szabolcs P, Reese M, Yancey KB, Hall RP, Kurtzberg J. Combination Treatment of Bullous Pemphigoid With Anti-CD20 and Anti-CD25 Antibodies in a Patient With Chronic Graft-Versus-Host Disease. Bone Marrow Transplant (2002) 30(5):327–9. doi: 10.1038/sj.bmt.1703654
44. Tomsitz D, Stefaniak R, Worm M. Rituximab in Patients With Recalcitrant Autoimmune Blistering Diseases: Experience in a Cohort of 22 Patients. Br J Dermatol (2015) 172(3):829–31. doi: 10.1111/bjd.13307
45. Wang TS, Tsai TF. Remission of Bullous Pemphigoid After Rituximab Treatment in a Psoriasis Patient on Regular Low-Dose Methotrexate. Acta Derm Venereol (2014) 94(1):108–9. doi: 10.2340/00015555-1619
46. Wardlaw S, Raza A, Boumitri C. An Unexpected Cause of Dysphagia in a Patient Hospitalized for Nephrectomy. Am J Gastroenterol (2020) 115(SUPPL):S1006. doi: 10.14309/01.ajg.0000709780.43132.3e
47. Watson N, Carrozzo M, Hampton P. A Retrospective Cohort Study Reporting Rituximab Treatment for 33 Patients With Immunobullous Disease. J Oral Pathol Med (2021) 50(1):92–7. doi: 10.1111/jop.13123
48. Zhang X, Guo J, Guo X, Pan J. Successful Treatment of Acquired Haemophilia in a Patient With Bullous Pemphigoid With Single-Dosing Regimen of Rituximab. Haemophilia (2012) 18(5):e393–5. doi: 10.1111/j.1365-2516.2012.02917.x
49. De Brito M, Blake S, Murrell DF. 14th Annual Medical Dermatology Meeting British. J Dermatol (2019) 180(5):146–7. doi: 10.1111/bjd.17718
50. Balakirski G, Alkhateeb A, Merk HF, Leverkus M, Megahed M. Successful Treatment of Bullous Pemphigoid With Omalizumab as Corticosteroid-Sparing Agent: Report of Two Cases and Review of Literature. J Eur Acad Dermatol Venereology (2016) 30(10):1778–82. doi: 10.1111/jdv.13758
51. Balakirski G, Merk HF, Leverkus M, Megahed M. Omalizumab- Treatment of Therapy Resistant Bullous Pemphigoid. J Der Deutschen Dermatologischen Gesellschaft (2015) 13(12):1322–3.
52. Blake S, Murrell D. Omalizumab (Xolair) in Treatment Resistant Bullous Pemphigoid. Australas J Dermatol (2019) 60:20–. doi: 10.1111/ajd.13027
53. De A, Chowdhury B. Biologics Beyond Boundaries: Innovative Use of Biologics in Dermatology. Indian J Dermatol (2021) 66(3):314–7. doi: 10.4103/ijd.IJD_128_20
54. De D, Kaushik A, Handa S, Mahajan R, Schmidt E. Omalizumab: An Underutilized Treatment Option in Bullous Pemphigoid Patients With Co-Morbidities. J Eur Acad Dermatol Venereology (2021) 35(7):E469–72. doi: 10.1111/jdv.17229
55. Dufour C, Souillet AL, Chaneliere C, Jouen F, Bodemer C, Jullien D, et al. Successful Management of Severe Infant Bullous Pemphigoid With Omalizumab. Br J Dermatol (2012) 166(5):1140–2. doi: 10.1111/j.1365-2133.2011.10748.x
56. Ewy S, Pham H, Quan K, Su B, Tachdjian R. Successful Omalizumab Therapy for Bullous Pemphigoid Despite Transient Reaction. J Drugs Dermatol (2019) 18(9):947–9.
57. Fairley JA, Baum CL, Brandt DS, Messingham KA. Pathogenicity of IgE in Autoimmunity: Successful Treatment of Bullous Pemphigoid With Omalizumab. J Allergy Clin Immunol (2009) 123(3):704–5. doi: 10.1016/j.jaci.2008.11.035
58. Gönül M, Keseroglu HO, Ergin C, Özcan I, Erdem O. Bullous Pemphigoid Successfully Treated With Omalizumab. Indian J Dermatol Venereology Leprol (2016) 82(5):577–9. doi: 10.4103/0378-6323.183628
59. Jafari SMS, Feldmeyer L, Bossart S, Simon D, Schlapbach C, Borradori L. Case Report: Combination of Omalizumab and Dupilumab for Recalcitrant Bullous Pemphigoid. Front Immunol (2021) 11:611549. doi: 10.3389/fimmu.2020.611549
60. Jafari SMS, Gadaldi K, Feldmeyer L, Yawalkar N, Borradori L, Schlapbach C. Effects of Omalizumab on Fc Epsilon RI and IgE Expression in Lesional Skin of Bullous Pemphigoid. Front Immunol (2019) 10:1919. doi: 10.3389/fimmu.2019.01919
61. James T, Salman S, Stevenson B, Bundell C, Kelly G, Nolan D, et al. IgE Blockade in Autoimmunity: Omalizumab Induced Remission of Bullous Pemphigoid. Clin Immunol (2019) 198:54–6. doi: 10.1016/j.clim.2018.12.015
62. Lanoue D, Pham H, Valois J, Yang W. STEROID SPARING BENEFIT OF OMALIZUMAB IN A PATIENT WITH BULLOUS PEMPHIGOID AND METASTATIC SALIVARY CANCER. Ann Allergy Asthma Immunol (2020) 125(5):S115. doi: 10.1016/j.anai.2020.08.385
63. London VA, Kim GH, Fairley JA, Woodley DT. Successful Treatment of Bullous Pemphigoid With Omalizumab. Arch Dermatol (2012) 148(11):1241–3. doi: 10.1001/archdermatol.2012.1604
64. Lonowski S, Sachsman S, Patel N, Truong A, Holland V. Increasing Evidence for Omalizumab in the Treatment of Bullous Pemphigoid. JAAD Case Rep (2020) 6(3):228–33. doi: 10.1016/j.jdcr.2020.01.002
65. Maglie R, Antiga E, Quintarelli L, Verdelli A, Caproni M. Dramatic Exacerbation of Bullous Pemphigoid Following Rituximab and Successful Treatment With Omalizumab. Eur J Dermatol (2019) 29(2):213–5. doi: 10.1684/ejd.2019.3499
66. Mangin MA, Lienhart A, Gouraud A, Roux S, Hodique F, Jouen F, et al. Onset of Acquired Haemophilia A After Omalizumab Treatment in Severe Bullous Pemphigoid - A Report on Two Cases Successfully Treated With Mycophenolate Mofetil. Annales Dermatologie Et Venereologie (2021) 148(1):57–9. doi: 10.1016/j.annder.2020.09.577
67. Menzinger S, Kaya G, Schmidt E, Fontao L, Laffitte E. Biological and Clinical Response to Omalizumab in a Patient With Bullous Pemphigoid. Acta Dermato-Venereologica (2018) 98(2):284–6. doi: 10.2340/00015555-2845
68. Navarro-Trivino FJ, Llamas-Molina JM, Ayen-Rodriguez A, Cancela-Diez B, Ruiz-Villaverde R. Dramatic Improvement of Bullous Pemphigoid With Omalizumab in an Elderly Patient. Eur J Hosp Pharm (2021) 28(6):350–2. doi: 10.1136/ejhpharm-2020-002418
69. Pham H, Lanoue D, Valois J, Yang W. SUCCESSFUL TREATMENT AND COMPLETE STEROID SPARING USING OMALIZUMAB IN BULLOUS PEMPHIGOID MASQUERADING AS REFRACTORY URTICARIA. Ann Allergy Asthma Immunol (2020) 125(5):S115. doi: 10.1016/j.anai.2020.08.384
70. Salman S, James T, Stevenson B, Bundell C, Kelly G, Nolan D, et al. Highlighting The Role OF IgE Autoantibodies In Bullous Pemphigoid. Pathology (2019) 51:S49–50. doi: 10.1016/j.pathol.2018.12.114
71. Sarrazin M, Jouen F, Duvert-Lehembre S. Refractory Bullous Pemphigoid With IgE Anti-BP230 and IgG Anti-P200 Antibodies Successfully Treated With Omalizumab. Ann Dermatol Venereol (2021) 148(1):60–2. doi: 10.1016/j.annder.2020.08.053
72. Turkowski Y, Ahmed A, Konnikov N, Ahmed AR. IgE Bullous Pemphigoid and Nodular Regenerative Hyperplasia of the Liver in a Patient With Heterozygous Alpha1-Antitrypsin Deficiency. J Am Acad Dermatol (2018) 79(3):AB156. doi: 10.1016/j.jaad.2018.05.643
73. Velin M, Dugourd PM, Sanchez A, Bahadoran P, Montaudié H, Passeron T. Efficacy and Safety of Methotrexate, Omalizumab and Dupilumab for Bullous Pemphigoid in Patients Resistant or Contraindicated to Oral Steroids. A Monocentric Real-Life Study. J Eur Acad Dermatol Venereology (2022). doi: 10.1111/jdv.17999
74. Vico-Alonso C, Calleja-Algarra A, Aragon-Miguel R, Sanchez-Velazquez A, Velasco-Tamariz V, Ortiz-Romero PL, et al. Omalizumab as an Alternative Therapeutic Tool in the Treatment of Bullous Pemphigoid: A Case Report. Dermatologic Ther (2019) 32(2):e12829. doi: 10.1111/dth.12829
75. Yalcin AD, Genc GE, Celik B, Gumuslu S. Anti-IgE Monoclonal Antibody (Omalizumab) is Effective in Treating Bullous Pemphigoid and Effects on Soluble CD200. Allergy (2013) 68:20–. doi: 10.7754/clin.lab.2013.130642
76. Abdat R, Waldman RA, de Bedout V, Czernik A, McLeod M, King B, et al. Dupilumab as a Novel Therapy for Bullous Pemphigoid: A Multicenter Case Series. J Am Acad Dermatol (2020) 83(1):46–52. doi: 10.1016/j.jaad.2020.01.089
77. Kaye A, Gordon SC, Deverapalli SC, Her MJ, Rosmarin D. Dupilumab for the Treatment of Recalcitrant Bullous Pemphigoid. JAMA Dermatol (2018) 154(10):1225–6. doi: 10.1001/jamadermatol.2018.2526
78. Li W, Cai S, Man X. The Treatment of Refractory Atypical Bullous Pemphigoid With Generalized Eczema and Intense Pruritus With Dupilumab. Dermatologic Ther (2022) 35(2):e15243. doi: 10.1111/dth.15243
79. Liu X, Ma J, Qiu X, Hong D, Wang L, Shi Z. Dupilumab, an Emerging Therapeutic Choice for Recalcitrant Subepidermal Autoimmune Bullous Diseases: A Case Series of Three Patients. Eur J Dermatol (2021) 31(6):846–7. doi: 10.1684/ejd.2021.4190
80. Saleh M, Reedy M, Torok H, Weaver J. Successful Treatment of Bullous Pemphigoid With Dupilumab: A Case and Brief Review of the Literature. Dermatol Online J (2021) 27(4):13030/qt0dv3f9h6. doi: 10.5070/D3274053155
81. Seidman JS, Eichenfield DZ, Orme CM. Dupilumab for Bullous Pemphigoid With Intractable Pruritus. Dermatol Online J (2019) 25(11):13030/qt25q9w6r9. doi: 10.5070/D32511046147
82. Singh G, Patel T. Dupilumab for the Treatment of Recalcitrant Bullous Pemphigoid. J Dermatol Nurses' Assoc (2020), 12(2).
83. Zhang YH, Xu QY, Chen LH, Chen JW, Zhang J, Zou Y, et al. Efficacy and Safety of Dupilumab in Moderate-To-Severe Bullous Pemphigoid. Front Immunol (2021) 12:738907. doi: 10.3389/fimmu.2021.738907
84. Zhang YH, Zhang J, Chen JW, Lin MT, Gong T, Cheng B, et al. Dupilumab Successfully Treated Refractory Bullous Pemphigoid With Early Clinical Manifestations Imitating Atopic Dermatitis: A Case Letter Australasian. J Dermatol (2021) 62(4):525–7. doi: 10.1111/ajd.13692
85. Bishnoi A, De D, Handa S, Mahajan R. Biologics in Autoimmune Bullous Diseases: Current Scenario. Indian J Dermatol Venereol Leprol (2021) 87(5):611–20. doi: 10.25259/ijdvl_886_19
86. Lytvyn Y, Rahat S, Mufti A, Witol A, Bagit A, Sachdeva M, et al. Biologic Treatment Outcomes in Mucous Membrane Pemphigoid: A Systematic Review. J Am Acad Dermatol (2021) S0190-9622(21)00010-4. doi: 10.1016/j.jaad.2020.12.056
87. Manjarrez-Orduno N, Quach TD, Sanz I. B Cells and Immunological Tolerance. J Invest Dermatol (2009) 129(2):278–88. doi: 10.1038/jid.2008.240
88. Messingham KA, Holahan HM, Fairley JA. Unraveling the Significance of IgE Autoantibodies in Organ-Specific Autoimmunity: Lessons Learned From Bullous Pemphigoid. Immunol Res (2014) 59(1-3):273–8. doi: 10.1007/s12026-014-8547-7
89. Seyed Jafari SM, Gadaldi K, Feldmeyer L, Yawalkar N, Borradori L, Schlapbach C. Effects of Omalizumab on FcepsilonRI and IgE Expression in Lesional Skin of Bullous Pemphigoid. Front Immunol (2019) 10:1919. doi: 10.3389/fimmu.2019.01919
90. Serrano-Candelas E, Martinez-Aranguren R, Valero A, Bartra J, Gastaminza G, Goikoetxea MJ, et al. Comparable Actions of Omalizumab on Mast Cells and Basophils. Clin Exp Allergy (2016) 46(1):92–102. doi: 10.1111/cea.12668
91. Wang SH, Zuo YG. Commentary: Efficacy and Safety of Dupilumab in Moderate-To-Severe Bullous Pemphigoid. Front Immunol (2021) 12:800609. doi: 10.3389/fimmu.2021.800609
92. Amber KT, Hertl M. An Assessment of Treatment History and its Association With Clinical Outcomes and Relapse in 155 Pemphigus Patients With Response to a Single Cycle of Rituximab. J Eur Acad Dermatol Venereol (2015) 29(4):777–82. doi: 10.1111/jdv.12678
93. Anandan V, Jameela WA, Sowmiya R, Kumar MMS, Lavanya P. Rituximab: A Magic Bullet for Pemphigus. J Clin Diagn Res (2017) 11(4):WC01–6. doi: 10.7860/JCDR/2017/21868.9717
94. Vinay K, Dogra S. Rituximab in Pemphigus: Road Covered and Challenges Ahead. Indian Dermatol Online J (2018) 9(6):367–72. doi: 10.4103/idoj.IDOJ_290_18
95. He Y, Shimoda M, Ono Y, Villalobos IB, Mitra A, Konia T, et al. Persistence of Autoreactive IgA-Secreting B Cells Despite Multiple Immunosuppressive Medications Including Rituximab. JAMA Dermatol (2015) 151(6):646–50. doi: 10.1001/jamadermatol.2015.59
96. Kremer N, Snast I, Cohen ES, Hodak E, Mimouni D, Lapidoth M, et al. Rituximab and Omalizumab for the Treatment of Bullous Pemphigoid: A Systematic Review of the Literature. Am J Clin Dermatol (2019) 20(2):209–16. doi: 10.1007/s40257-018-0401-6
99. Seegraber M, Srour J, Walter A, Knop M, Wollenberg A. Dupilumab for Treatment of Atopic Dermatitis. Expert Rev Clin Pharmacol (2018) 11(5):467–74. doi: 10.1080/17512433.2018.1449642
100. Wollenberg A, Ariens L, Thurau S, Van Luijk C, Seegraber M, De Bruin-Weller M. Conjunctivitis Occurring in Atopic Dermatitis Patients Treated With Dupilumab-Clinical Characteristics and Treatment. J Allergy Clin Immunol Pract (2018) 6(5):1778–80.e1. doi: 10.1016/j.jaip.2018.01.034
101. Yang B, Wu M, Yan X, Bao F, Yue Z, Pei Z, et al. Childhood Bullous Pemphigoid: A Report of Three Cases in China. Int J Dermatol (2016) 55(6):691–4. doi: 10.1111/ijd.12979
Keywords: rituximab, omalizumab, dupilumab, biologics, bullous pemphigoid
Citation: Cao P, Xu W and Zhang L (2022) Rituximab, Omalizumab, and Dupilumab Treatment Outcomes in Bullous Pemphigoid: A Systematic Review. Front. Immunol. 13:928621. doi: 10.3389/fimmu.2022.928621
Received: 26 April 2022; Accepted: 16 May 2022;
Published: 13 June 2022.
Edited by:
Takashi Hashimoto, Osaka City University, JapanReviewed by:
Cezary Kowalewski, Medical University of Warsaw, PolandCopyright © 2022 Cao, Xu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Litao Zhang, emhhbmdsaXRhb0BtZWRtYWlsLmNvbS5jbg==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.