- 1National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V. I. Kulakov of Ministry of Healthcare of Russian Federation, Moscow, Russia
- 2Peoples’ Friendship University of Russia, Medical Institute, Moscow, Russia
- 3A. P. Avtsyn Research Institute of Human Morphology, Laboratory of Growth and Development, Moscow, Russia
The fatal outcomes of COVID-19 are related to the high reactivity of the innate wing of immunity. Estrogens could exert anti-inflammatory effects during SARS-CoV-2 infection at different stages: from increasing the antiviral resistance of individual cells to counteracting the pro-inflammatory cytokine production. A complex relationship between sex hormones and immune system implies that menopausal hormone therapy (MHT) has pleiotropic effects on immunity in peri- and postmenopausal patients. The definite immunological benefits of perimenopausal MHT confirm the important role of estrogens in regulation of immune functionalities. In this review, we attempt to explore how sex hormones and MHT affect immunological parameters of the organism at different level (in vitro, in vivo) and what mechanisms are involved in their protective response to the new coronavirus infection. The correlation of sex steroid levels with severity and lethality of the disease indicates the potential of using hormone therapy to modulate the immune response and increase the resilience to adverse outcomes. The overall success of MHT is based on decades of experience in clinical trials. According to the current standards, MHT should not be discontinued in COVID-19 with the exception of critical cases.
Introduction
The new coronavirus infection (COVID-19/SARS-Cov-2) has quickly reached pandemic proportions following the initial outbreak in Wuhan (1, 2). A wide range of measures are being taken around the world with occasional declarations of emergency and restrictions on daily life. Over 2020–2021, the pandemic with over 160 million recorded cases of coronavirus disease (COVID-19) caused almost 3.5 million deaths (3). The situation continues to pose a threat to the world economy and human well-being. Efficient therapies for SARS-Cov-2 acute coronavirus infection represent an urgent global challenge to laboratory and clinical research.
Severe complications and high mortality of COVID-19 result from the massive cytokine storm that triggers inflammatory infiltration of the lungs and ends in the acute respiratory distress syndrome. The cytokine storm may also promote acute cardiac failure, secondary infections, generalized sepsis, and multiple organ failure, all of them potentially lethal. Prevention of the cytokine storm in SARS-Cov-2-infected individuals is pivotal. Steroid hormones, e.g. estrogens, are renowned anti-inflammatory agents. Cytokines, hormones, and neurotransmitters coordinate and integrate the immune, nervous, and endocrine functionalities through interactions with specific receptors in target cells (4). The majority of immune cells are known to express estrogen receptors (5). An association between estradiol intake (as a component of menopausal hormone therapy, MHT, as well as a component of hormone replacement therapy, HRT) and reduced risks of lethal outcome in SARS-Cov-2 has been demonstrated in a number of studies (6–8). At the same time, immune status of women receiving MHT has not been studied in detail. In this review, we attempt to explore how MHT affects immunological parameters of the body and what mechanisms are involved in its protective response to the new coronavirus infection. The review is based on PubMed searches (https://pubmed.ncbi.nlm.nih.gov/) using the keywords: COVID-19, SARS-CoV-2, coronavirus, novel coronavirus infection, autoimmunity, sex hormones, estradiol, progesterone, testosterone, immune response, menopausal hormone therapy, hormone replacement therapy, oophorectomy, postmenopausal women, immune aging.
Sex-based differences in COVID-19 outcomes
The differences in hormonal status that distinguish men and women throughout their lives contribute to considerable immunological dimorphism reflected by skewed epidemiological profiles. The situation can be illustrated by reciprocal incidence proportions of autoimmune disorders and cancers: the former are more frequently diagnosed in women, and vice versa (9–11).
Female sex hormones, including estrogens and progesterone, act as regulators of innate and adaptive immunity; hence the higher flexibility of humoral and cell-mediated immune responses in women. A number of studies have come to conclusion that women show increased resistance to viral diseases and SARS-CoV-2 infections in particular (12).
Indeed, the incidence of severe cases and deaths of COVID-19 in women is lower (13, 14). In the International Severe Acute Respiratory and emerging Infections Consortium (ISARIC) World Health Organization (WHO) Clinical Characterization Protocol UK prospective observational study (CCP-UK) enrolling approximately 20,000 hospital patients with COVID-19 in early 2020, female lethality was lower by 20% (15).
Two previous outbreaks of zoonotic β-coronavirus encountered in this century showed similar epidemiological patterns. Among a total of 1,755 patients hospitalized during the 2002 outbreak of SARS-CoV in the Guangdong province of China, mortality rates constituted 13% for women and 22% for men (16). During the 2012 coronavirus epidemic in Saudi Arabia, the mortality rates were 23% and 52%, respectively (17).
As shown by the analysis of COVID-19 data from Italy, Spain, Germany, Switzerland, Belgium, and Norway, mortality rates in males exceed those in females for all age groups except under-20-year-olds (18). The established fact that adult men of all ages and women over 50 have the highest risks of severe complicated COVID-19 rekindles the point on the role of sex steroids in the clinical course of COVID-19 (19). Female immune system appears to produce a better coordinated and more flexible antiviral response with the overall impact on the morbidity, severity, and associated mortality. The trend may be explained by the modulatory effects of estrogens on leukocyte functionalities, both in circulating pools and resident populations of cells recruited from the bloodstream to peripheral tissues (12, 20–23). However, a number of reports emphasize the lack of difference in estradiol levels between deceased and surviving patients with COVID-19 (24, 25). Here we focus on the relationship between sex hormone levels and immune status of the body with a special regard to SARS-CoV-2 infection.
Sex hormones as chefs d’orchestre for the immune system
Estrogens define immunological parameters
Steroid hormones play important roles by tuning immune responses through modulatory effects on diverse cell populations representing both innate (neutrophils, macrophages/monocytes, natural killers, and dendritic cells) and acquired immunity (T and B cells) (4), including in immune-mediated diseases (26).
Sex hormones, which include estrogens, progesterone, and androgens, are produced in all humans; however their plasma levels, physiological duties, and target organs in men and women are different (27). In reproductive age women, estrogens and progesterone are produced cyclically by the ovaries, while small amounts of testosterone are produced by ovaries and adrenal glands. Estrogens are also produced locally by aromatization of androgens in adipose tissue, bones, and mammary glands. In men, estrogen blood levels are maintained through aromatization of testosterone in peripheral tissues, whereas Leydig and Sertoli cells of the testes are engaged in local synthesis (27).
Endogenous estrogens include estrone (E1), 17β-estradiol (E2), and estriol (E3) (28). Estradiol is the predominant and most biologically active estrogen. Estradiol is a physiological derivative of testosterone, whereas estrone is derived from androstenedione; the syntheses are catalyzed by aromatase. Estrone, which prevails in postmenopause, has weaker effects compared with estradiol.
Estrogen receptors are expressed in all immune cells and participate in transcriptional regulation (12, 20–23). Estrogen receptors fall into two types: intracellular (ERα and ERβ) and membrane-bound (G-protein coupled estrogen receptor, GPER). Accordingly, the routes of estrogen signaling involve genomic and non-genomic options. Genomic estrogen signaling involves interactions of genomic DNA with ligand-bound ER, either direct (classical) or mediated by other transcription factors, whereas non-genomic estrogen signaling acts by triggering cytoplasmic protein phosphorylation cascades (29). The classical genomic estrogen signaling (characteristic of steroid hormones in general) consists of the following steps (1): the hormone enters the cytoplasm and meets its nuclear receptor (2); the hormone-receptor complex is translocated to the nucleus (3); the complex binds specific recognition elements in promoter regions of effector genes (30). However, some effects of estrogens are too fast to be a consequence of gene expression (which takes time invariably). Instead, the fast effects of estrogens result from non-genomic signaling routes triggered by engagement of the membrane-bound GPERs (31–34). Such effects include the estrogen-mediated activatory phosphorylation of the endothelial nitric oxide synthase (eNOS) (35); similar non-genomic effects have been reported for other steroid hormones. Thus, immune cells have multiple routes of responding to the circulating estrogen levels, and exploit them in accordance with receptor profiles expressed by particular cells (36, 37).
The net effect of estrogens on immune functionalities is anti-inflammatory. Studies show that physiological estrogen levels in premenopausal women suppress the release of pro-inflammatory cytokines, notably interleukins IL-6 and IL-8, and tumor necrosis factor α (TNF-α) (38). By contrast, low physiological estrogen levels in postmenopausal women fail to counteract the release of pro-inflammatory cytokines. Quite indicatively, the elevated levels of IL-1, IL-6, and TNF-α, encountered in postmenopause, can be effectively mitigated by MHT (39, 40).
Cellular mechanisms of the anti-inflammatory action of estrogens are likely to involve diverse leukocyte populations of the body. Estrogens have been shown to regulate cell numbers and functional activities of neutrophils by affecting the release of many chemokines (e.g. monocyte chemoattractant protein MCP-1) and cytokines (TNF-α, IL-1β, and IL-6) (41). The suppressive effect of estrogens on the production of TNF-α, IL-1β, and IL-6 by neutrophils and macrophages has been independently confirmed in rats (42), mice (43), and humans (44).
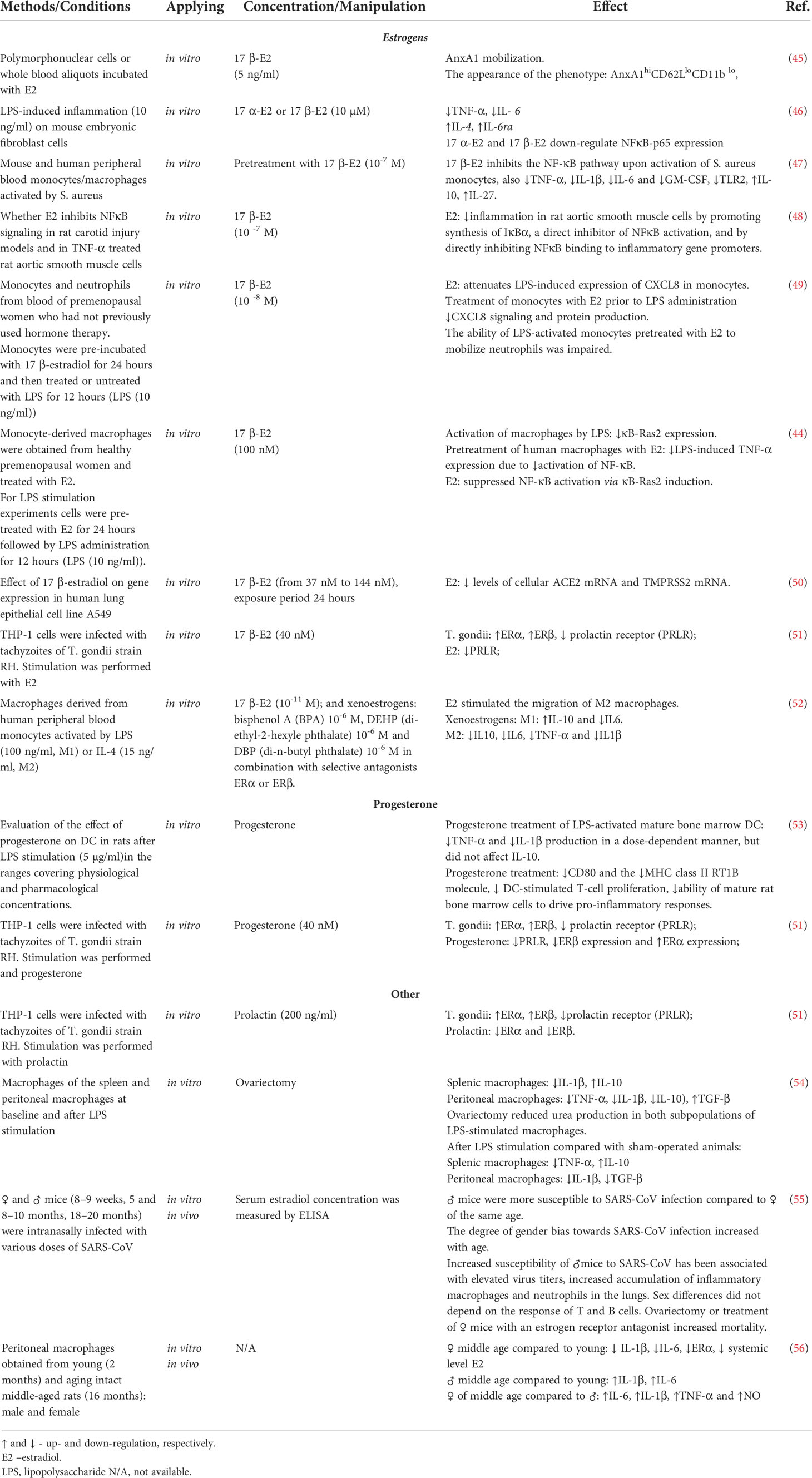
The protective effect of estrogens on polymorphonuclear leukocytes involves activation of the regulatory pathway controlled by the prominent anti-inflammatory protein annexin A1. The response to female steroids in neutrophils is accompanied by a rapid increase in annexin A1 levels. Depletion of this protein by immunoneutralization or genetic modification abolishes the mitigating effect of estrogen on neutrophil extravasation both in vitro and in vivo (45) (Table 1).
Monocyte-macrophage lineages are also highly responsive to estrogens. Many studies identify monocyte populations as the key anti-inflammatory effector of estrogens (57, 58). Estrogens inhibit expression of chemokine receptors CCR2 and CXCR3 in monocytes, thereby reducing their sensitivity to pro-inflammatory factors (59–61) (Table 2). Elevated blood levels of 17β-estradiol promote expression of anti-inflammatory markers in monocytes (66) while inhibiting the production of pro-inflammatory cytokines by these cells (67–69). More specifically, estrogens can modulate macrophage phenotypes (polarization status) in favor of anti-inflammatory profiles (70, 71). Toniolo et al. have demonstrated decreased estrogen levels observed in postmenopause negatively affects the macrophage capability of polarization towards anti-inflammatory phenotypes in response to microenvironmental stimuli (72).
Administration of exogenous estradiol (E2) to male or ovariectomized female mice significantly reduced the expression of pro-inflammatory cytokine IL-1β by peritoneal macrophages in vivo (73). Zhang et al. reported alleviation of inflammatory response in macrophage cell line RAW264.7 by estradiol; the mechanism involved a reduction in expression and secretion of IL-1β (74). In a study by Stanojević et al., stimulation with endotoxin (lipopolysaccharide, LPS) reduced secretion of IL-1β and TNF-α by peritoneal macrophages in ovariectomized mice (54). However, no decrease in IL-1β production after treatment with estradiol was observed in monocytic macrophages derived from women in postmenopause (52). Ćuruvija et al. showed that LPS-stimulated peritoneal macrophages of middle-aged female rats with significantly reduced levels of circulating estradiol secrete less IL-1β than matched cells of young females (56). Galván-Ramirez et al. demonstrated that immortalized monocytic cells THP-1 treated with estradiol prior to stimulation by exposure to Toxoplasma gondii have decreased rates of production of the pro-inflammatory cytokine IL-12 (51). Estrogens have been also shown to suppress production of major neutrophil chemoattractants CXCL1, CXCL2, and CXCL3 within inflammatory foci in rodent models of colon (75), lung and vascular injury (48). Estradiol has been also demonstrated to inhibit the release of a potent neutrophil chemoattractant, chemokine CXCL8, by human monocytes ex vivo (49). Myeloid lineages isolated from ERα knockout mice (Esr1-/-) showed poor performance in macrophage clearance tests and defective polarization of macrophages from classically activated (pro-inflammatory) to alternatively activated (anti-inflammatory) phenotypes (76).
A number of studies explore molecular mechanisms of the estrogen effects on cytokine production. The decrease in cytokine production under the action of estrogens apparently involves inhibition of NF-κB signaling pathway. Pre-treatment with estrogens has been shown to interfere with NF-κB signaling and ultimately inhibit the LPS-induced production of TNF-α by human macrophages in vitro (72). Santos et al. demonstrated that estrogens mitigate the LPS-induced inflammation through inhibition of the NF-κB–p65 axis in embryonic fibroblasts (46, 59, 77). Besides, estrogens are capable suppressors of the non-receptor Bruton’s tyrosine kinase (BTK) essential for monocyte functionalities and the interleukin-1 receptor-associated kinase IRAK2 at transcriptional level (78). In vitro exposure to estrogens mitigates the functional performance of immune cells challenged with LPS/interferon-γ: the production of TNF-α and IL-10 decreases, reflecting the interference of estrogens with NF-κB signaling via both genomic and non-genomic mechanisms (44). In particular, estrogens boost the production (48) and prevent the degradation of IκB-α — the chief endogenous NF-κB inhibitor (44).
Estrogens are also capable of influencing lymphocytes, albeit in this case the effects are obviously multidirectional. The available experimental findings advocate both pro- and anti-inflammatory effects. For instance, estrogens have been confirmed to modulate negative selection of the high-affinity autoreactive B cells and meddle with their functionalities, orchestrating a Тh 2 type response (22). Estrogens have been also shown to suppress thymopoiesis (65), promote T cell activation (79), and stimulate NF-κB signaling which controls numerous genes of immune response, cell cycle, and apoptosis (80). Estrogens also promote the synthesis of IL-1, IL-10, and interferon-γ by lymphocytes (81, 82), while supporting Th1 and Th17 differentiation (83), regulatory Т (Treg) cell maintenance, and the expression of immunosuppressive gene FoxP3 (84–87). According to other studies, estrogens reinforce the immunosuppressive functionalities of Treg cells (88, 89) and boost the expression of chemokine receptors CCR1-5 (90), as well as the levels of chemokines MCP1, MCP5, eotaxin, and SDF1β (81, 91). On the other hand, estrogens stimulate the production of anti-inflammatory cytokines (e.g. IL-4 and IL-10) by CD4+ T helper cells. Estrogens also reduce the production of IL-17 by pro-inflammatory helper cells Th17 and stimulate Treg cell proliferation thus facilitating immune tolerance (92). Exposure of normal killer cells (NK cells) to estradiol in vitro promotes secretion of interferon γ by these cells and reinforces their cytotoxicity (93). At the same time, estradiol has been shown to suppress the expression of surface activation markers and FAS ligand by NK cells while inhibiting secretion of granzyme B serine protease by these cells in murine model (64).
Apart from in vitro studies, the immunity-related effects of estrogens were tested in a number of pro-inflammatory disease models in vivo. In the majority of experimental models, the anti-inflammatory effects of estrogens predominantly involved innate immunity: the reduced production of pro-inflammatory cytokines (e.g. IL-6, IL-1β, and TNF-α) by monocytes and macrophages along with chemokine production inhibition alleviated monocytic infiltration of inflammatory foci.
In pre-clinical studies using influenza A virus infection models, estrogens exhibited potent immunomodulatory effects leading to a more balanced innate immune response in the lungs, associated with reduced local levels of pro-inflammatory cytokines and reduced chemokine reactions before the onset of clinical symptoms (94–96).
In a model of acute pneumonia induced by instillation of bacterial LPS, male and ovariectomized female mice showed increased infiltration of the lungs with polymorphonuclear cells producing high amounts of IL-6, IL-1β, and the inter-cellular adhesion molecule 1 (ICAM-1); these symptoms were reduced upon exogenous administration of estradiol (62).
A major influence of estrogens on immune response in humans involves their meddling with the neutrophil recruitment to acute inflammation foci. Several studies identify two basic routes of such effects (1): control of the neutrophil chemotaxis and (2) modulation of the interactions between neutrophils and endothelial cells (97).
Acute lung injury in ovariectomized mice was successfully treated with estrogen replacement therapy (62). Administration of estrogen receptor antagonists (or ovariectomy) increased the lethality of SARS-CoV infection among female mice (55). Estrogens have been also demonstrated to play an important role in protection of the lung tissue through spatial confinement of local inflammation. This capacity was confirmed by in vivo experiments with administration of LPS to male or ovariectomized female mice. Despite an initial boost in the levels of IL-1β in response to LPS, administration of estradiol reduced both the albumin levels and the degree of LPS-induced lung injury (16, 62).
Progesterone impacts immune responses
Progesterone is another important immunomodulatory and anti-inflammatory hormone. Its cognate receptors are expressed by multiple cell types of the immune system, including macrophages, dendritic cells, lymphocytes, mast cells, and eosinophils. Progesterone also binds and activates glucocorticoid and mineralocorticoid receptors, which results in suppressed production of pro-inflammatory IL-1β and IL-12 cytokines by macrophages and dendritic cells (98). According to a number of studies, progesterone inhibits T cell proliferation, promotes apoptosis, facilitates production of IL-4 while reducing production of interferon β and IL-17, and also inhibits Th1 and Th17 activities while supporting Treg cell differentiation (99, 100).
Progesterone has a broad spectrum of anti-inflammatory effects. After exposure to progesterone, macrophages and dendritic cells have inferior activation status and produce less IL-1β and TNF compared with untreated cells (53, 101). Progesterone is also known to facilitate proliferation of Treg cells and thus support immune tolerance (92). Exposure to progesterone promotes expression of FIZZ1 and YM1 (the alternatively activated anti-inflammatory macrophage markers) and inhibits expression of the inducible nitric oxide synthase (iNOS) with a concomitant decrease in NO production in bone marrow-derived murine macrophages. The Toll-like receptor (TLR) and NF-κB signaling pathways can be antagonized by progesterone-mediated effects. Cytokine storm is the climax of severe COVID-19 and its mechanisms clearly have a TLR-dependent component. Accordingly, the role of TLR signaling pathways in COVID-19 pathogenesis represents a continuous research focus (102, 103). Despite the predominant implication of TLR7/8, other receptors of this family appear to be involved as well. For instance, the TLR3/TLR4 double-knockout mice are more susceptible to SARS-CoV-2 infections (104) and many studies emphasize the significance of TLR4 in the cytokine storm development (105).
Exposure of human NK cells to progesterone mitigates their activation and inhibits production of interferon γ by these cells through caspase-dependent apoptosis. Progesterone also modulates Th cell-mediated responses by promoting a Th1-to-Th2 shift in Th phenotypes and facilitating production of anti-inflammatory cytokines (e.g. IL-4 and IL-10) by Th cells. In addition, progesterone inhibits production of pro-inflammatory cytokines(e.g. IL-1β and IL-12) by dendritic cells (98, 106).
Androgens impact immune responses
Testosterone exerts immunosuppressive action targeted at several constituents of the immune system, including effector cells of innate and adaptive immunities. Testosterone can inhibit production and release of pro-inflammatory cytokines (IL-1β, IL-6, TNF-α, interferon γ, and IL-12), while promoting production of anti-inflammatory cytokines (IL-10 and IL-4) (107). Androgens have been shown to suppress Th1 lineages and support Th2 differentiation, while inhibiting В lymphopoiesis and production of antibodies by B cells (108). A number of studies confirm the overall anti-inflammatory effect on androgens using experimental models of autoimmune and inflammatory diseases, progression of which can be slowed down by testosterone administration. Large prospective studies associated lower testosterone levels and increased estradiol-to-testosterone ratio in men with severe course of COVID-19 and high levels of pro-inflammatory cytokines (109). However, this association cannot be considered a direct evidence of the pro-inflammatory effect of estradiol in men, as it likely reflects a reduction in testosterone levels associated with visceral obesity — a risk factor on its own.
The main histocompatibility complex (MHC types I and II) engaged in the pathogenic antigen presentation plays a pivotal role in immune response. Testosterone is known to reduce the levels of MHC II expression on dendritic cells, while estrogens exert the opposite action by increasing the MHC II expression levels (110). The sex-based difference in immunity reactions may be related to Х-chromosome localization of certain immunoregulatory genes (9). At least, FoxP3 and CD40L genes are expressed at higher levels in women. The variable patterns of X inactivation in immune cells and the pleiotropic functional spectrum of many genes provide a favorable playground for sex hormones to finely orchestrate the immune system capacity of breaking tolerance to exogenous or endogenous agents (9, 111).
Sex hormones not only control the reproductive system, but also largely tune the immunity. The hormones regulate immune response in the whole diversity of its aspects and forms (innate and adaptive, humoral and cell-mediated), so that any flaws in the mechanisms of such regulation contribute to the development of immune-mediated diseases, including autoimmune conditions (98, 112–117). Although the exact molecular mechanisms of the immunological impact of sex hormones are not yet fully understood, studies show that sex hormones profoundly control development, homeostasis, gene expression, and signaling in T and B lymphocytes, monocytes, macrophages, dendritic cells, and granulocytes, deeply affecting their functionalities under normal and pathological conditions.
Non-immune effects of sex hormones
Among their diverse systemic effects, sex steroids are known to interfere with local immunityby stimulating local immunocompetent cells and modifying the properties of epithelial barrier. Although steroid receptors are expressed by many mammalian cell types, the action of sex steroids on mucous membranes of the genital tract is surely the main focus (118). Estradiol promotes secretion of leukocyte protease inhibitor and β-defensin-2 by human uterine epithelial cells thus enhancing their antimicrobial properties (119). With the onset of menopause, the barrier function of the endometrium declines, which is associated with changes in composition of subepithelial lymphocyte populations (118), thinning of the epithelium (120), and disruption of epithelial cell junctions, especially those involving cadherins (121). Vulnerability of other types of cell junctions in the uterine epithelium during postmenopause is questionable, although certain pathogens, such as HIV, have been reported to facilitate destruction of tight and adherens junctions (122). Noteworthy, estradiol inhibits secretion of IL-6, IL-8, and MIF by uterine epithelium in response to TLR3/4 stimulation (119), as well as the INFγ-induced gene expression, while progesterone has the opposite effect (123). The effect of sex steroids on other mucous membranes is less pronounced. Decreased 17β-estradiol and progesterone levels are accompanied by decreased salivary levels of IgA and higher incidence of upper respiratory tract infections (124). In addition, salivary levels of secretory IgA in women are known to be significantly higher than in men (125, 126); although the menstrual cycle-related dynamics are negligible. Estrogens promote IgA transport across the epithelia, thus contributing to the barrier function of mucosa, for instance, in the intestine (127).
The effects of sex steroids on mucous membranes of digestive tract are widespread. For instance, estradiol administration reduced the symptoms of eosinophilic esophagitis (128), a typical dysfunction of the epithelial barrier. At the same time, estrogens, in contrast to testosterone, can impede wound healing in the oral cavity (129).
A human herpesvirus 2 (HSV-2) vaccine administered intranasally against the background of E2 estradiol ensures more pronounced Th17 responses, longer persistence of CD4+ T cells, and higher numbers of memory Th-cells in the upper respiratory tract mucosa-associated lymphoid tissue (130). The diverse effects of sex hormones are determined by robust expression of their receptors in a variety of mammalian cell types.
Estrogens attenuate the pro-inflammatory cytokine storm
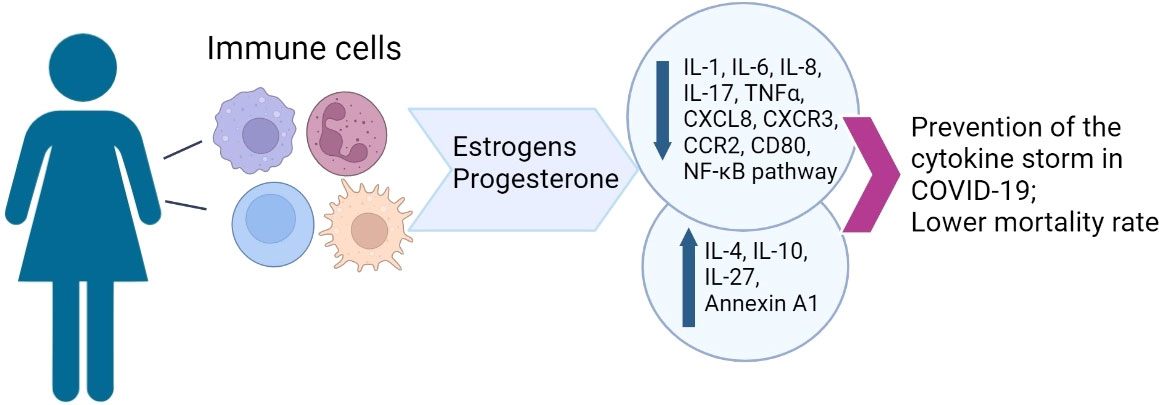
The fatal outcomes of COVID-19 are due to the intrinsically high reactivity of the innate wing of immunity. Under conditions of severe respiratory illness, the innate immune system overreacts by critical hypercytokinemia and massive migration of the activated immune cells to the lungs. The patients die not of the minor tissue damage caused by viral replication per se, but as a consequence of generalized devastating immune response with characteristic off-scale burst in systemic levels of pro-inflammatory cytokines leading to the acute distress-syndrome and multiple organ failure (92). These devastating consequences can be considerably mitigated by estrogens at different planes of SARS-CoV-2 infection: from increasing the antiviral resistance of individual cells to counteracting the pro-inflammatory cytokine production.
Angiotensin-converting enzyme 2 (ACE2) was identified as a unique cognate receptor for SARS-CoV-2, key for penetration of the virus into human cells. Patterns of ACE2 expression in human body (in terms of distribution and intensity) play pivotal role in the course of the infection accomplished through the binding of ACE2 to the SARS-CoV-2 spike glycoprotein (S-protein) (131–133). ACE2 protein is a membrane-bound aminopeptidase expressed in a variety of organs and tissues including heart, intestine, kidneys, lungs, lymph nodes, and ovaries (134). ACE2 is predominantly expressed by endothelial cells, as well as myocardium, intestinal mucosa, and type II pneumocytes — the pulmonary surfactant-producing spherical cells found in lung alveoli (135). ACE2 is also expressed by other cell types and structures of the respiratory tract, from nasopharyngeal mucosa to the transient secretory cells of the bronchi. The difference of ACE2 protein expression patterns observed in men and women may partially explain the sex-based differences in COVID-19 morbidity and mortality (136–139). The ACE2 receptor protein is abundantly expressed in hormone-producing organs and structures, notably in testes, the thyroid, and adipose tissue, and to a lesser extent also in adrenal glands, while the tendency for its increased expression identified in males and older individuals is consistent with the higher morbidity observed for these groups (131).
Estrogens and particularly their anti-SARS-CoV-2 immunomodulatory effects are currently a close focus. The acute respiratory distress syndrome (ARDS) is prevalent in severe COVID-19. Pathogenetic routes of this condition vary; one of them involves dysfunction of alveolar epithelial cells leading to the gas exchange disruption. The favorable outcome in ARDS is thought to depend on the effectiveness of alveolar fluid clearance (AFC) (140), which involves active transport of sodium ions (141). Sex steroids likely participate in regulation of this process, as women with ARDS show higher AFC rates and get more favorable prognosis than men (140). The same sex-based difference has been observed in preterm infants with respiratory distress syndrome (142). Several experimental studies show that administration of estrogen and progesterone supports the synthesis of pore-forming α-subunit of ENaC and Na,K-ATPase thus stimulating sodium transport and ultimately AFC, which may provide important link in the treatment of ARDS (143–145). Another study argues that the apparent beneficial effect of estradiol in LPS-induced acute lung injury may involve the PI3K/Akt/SGK1 signaling pathway activation (146). In addition, administration of 17β-estradiol has been shown to prevent the development of age-related changes in the lungs in female mice: it mitigates cell death, inhibits MMP2 expression, and restores interalveolar septa (147).
Estrogens have been shown to inhibit expression of the transmembrane serine protease 2 (TMPRSS2) in various cell lines (50). TMPRSS2 is required for the activation of spike protein in some coronaviruses, notably in SARS-CoV and SARS-CoV-2 (148). In human respiratory airways SARS-CoV-2 virus infects cells through interaction of the capsid S-protein with ACE2 (149). The inhibition of TMPRSS2 expression by estrogens can prevent the infection since TMPRSS2 and ACE2 are co-expressed, i.e. “interact” at transcriptional level (150). A recent study by Baristaite et al. shows that estradiol may alleviate the symptoms by regulating ACE2 and TMPRSS2, as their expression decreased upon 17β-estradiol treatment of A549 human lung epithelial cells in vitro.
The antiviral potential of estrogens is partially explained by its direct impact on transcription (mediated by nuclear receptors), as many genes known to participate in immune response and inflammation have estrogen-responsive elements in their promoter regions (151). Apart from that, estrogens counteract vasoconstriction by stimulating the nitric oxide synthesis and reducing the intracellular calcium levels in vascular smooth muscle cells (152). Estrogens are thought to mobilize the resting endothelial progenitors to proliferating pools, as well as to reduce the rates of apoptosis among endothelial cells (153). Besides, estrogens exert anti-inflammatory action on the endothelium by inhibiting leukocyte chemotaxis and formation of reactive oxygen species through activation of the renin-angiotensin-aldosterone system. Estrogen deficiency is accompanied by elevated levels of renin and increased expression of angiotensin receptor 1 with ensuing vasoconstriction and pro-inflammatory cytokine shift (154). These negative effects can be neutralized by exogenous estrogens at the non-genomic level.
Toll-like receptor 7 (TLR7) is expressed on dendritic cells (155). Berghöfer et al. demonstrated that, upon stimulation with TLR7 ligands, peripheral blood plasmacytoid dendritic cells of women produce more interferons type I than corresponding cells of men (156). The authors also observed the loss of TLR7-mediated responses in plasmacytoid dendritic cells during postmenopause and showed that it could be partially rescued with MHT. The TLR7 encoding gene is located on X chromosome (103), which suggests sex-based differences in the effectiveness of antivirals (111), including those against SARS-CoV-2, consistently with clinical observations (157). Female dendritic cells, monocytes and B lymphocytes tend to avoid inactivation of the TLR7 second copy (158), leading to increased TLR7 dosage and ultimately to higher levels of type I IFN (IFN-I) (158). The higher levels of IFN-I production by dendritic cells observed in women have been suggested to depend on estrogen levels (159–161). At the same time, dendritic cells of female mice transplanted into males continued to produce higher levels of IFN-I, which indicates a strong relationship of this phenomenon to the number of X chromosomes i.e. TLR7 copy number (161). A prominent role of estrogens in regulation of TLR-mediated responses has been confirmed by several studies (162, 163).The involvement of TLR7 pathway in systemic response to SARS-CoV-2, genomic sequences of which can activate the endosomal TLR7/8, has been supported by multiple evidence (164). The concomitant activation of TLR7 RNA sensor pathways, followed by activation of NFκB signaling, promotes secretion of IFN-I, IFN-γ, and IFN-λ3 within 48 h of active SARS-CoV-2 infection (165). The TLR7/8 agonist imiquimod has been shown to stimulate the production of TNF-α, IL-1, IL-2, IL-6, IL-8, IL-12, as well as IFN-α (166).
The relationship between TLR7 levels and sex-based differences in the clinical course of SARS-CoV-2 infection has been also demonstrated in studies featuring male patients with deleterious variants of TLR7 (Xp22.2) (167) leading to compromised TLR7 activation and severe COVID-19 (168).
But even accounting for both the unfavorable TLR7 variants in men (169) and the stimulating effect of estrogens, the enhanced TLR7 expression in women is difficult to explain. The discovery of X-chromosome inactivation avoidance makes an important point, as about 15–20% of active genes on X chromosome have been shown to escape inactivation of the extra copy (170). The degree of avoidance, as well as the tissue-specific signatures of non-inactivated sequences, require further investigation (171, 172). X-linked differences in antiviral immunity have been described for SARS-CoV-2, hepatitis C virus, and HIV.
As long as estrogens suppress the production of pro-inflammatory cytokines, they can have a decisive impact in the prevention of cytokine storm, which is the principal cause of death associated with severe COVID-19 pneumonia (173). In perspective, estrogen levels appear capable of modulating lung inflammation and damage, and potentially affect the outcomes of respiratory diseases such as SARS-CoV-2 pneumonia (174). Estrogens, and to a lesser extent also progesterone, modulate the release of cytokines, as well as proliferation, differentiation, and polarization in diverse immune cell lineages. The use of antiestrogens (tamoxyfen, toremifene) may interfere with differentiation and maturation of dendritic cells (175).
Some experts see MHT as a plausible part of therapeutic strategy aimed at restoring immunological tolerance and curbing the cytokine storm during coronavirus infection (176).
MHT
For the adequate clinical management of patients during the period of menopausal transition and early postmenopause, it is necessary to adhere to general criteria for the stages of female reproductive system aging STRAW (Stages of Reproductive Aging Workshop), developed in 2001 and revised in 2011, incorporating the results of large cohort studies conducted over the first decade of the new millenium (STRAW+10). The criteria were developed on the basis of studying the relationship between changes in hormonal parameters and the characteristics of menstrual cycle, which is extremely important for clinical practice when choosing therapy. Despite the universality of endocrine changes during reproductive aging, different stages of this process may differ individually in duration and be accompanied by various specific symptoms (vasomotor, psycho-emotional, vaginal, sexual, etc.) and systemic disorders like the loss of bone mass, unfavorable cardiovascular risk profile associated with the development of visceral obesity, dyslipidemia, endothelial dysfunction, impaired glucose tolerance, etc. (177–180).
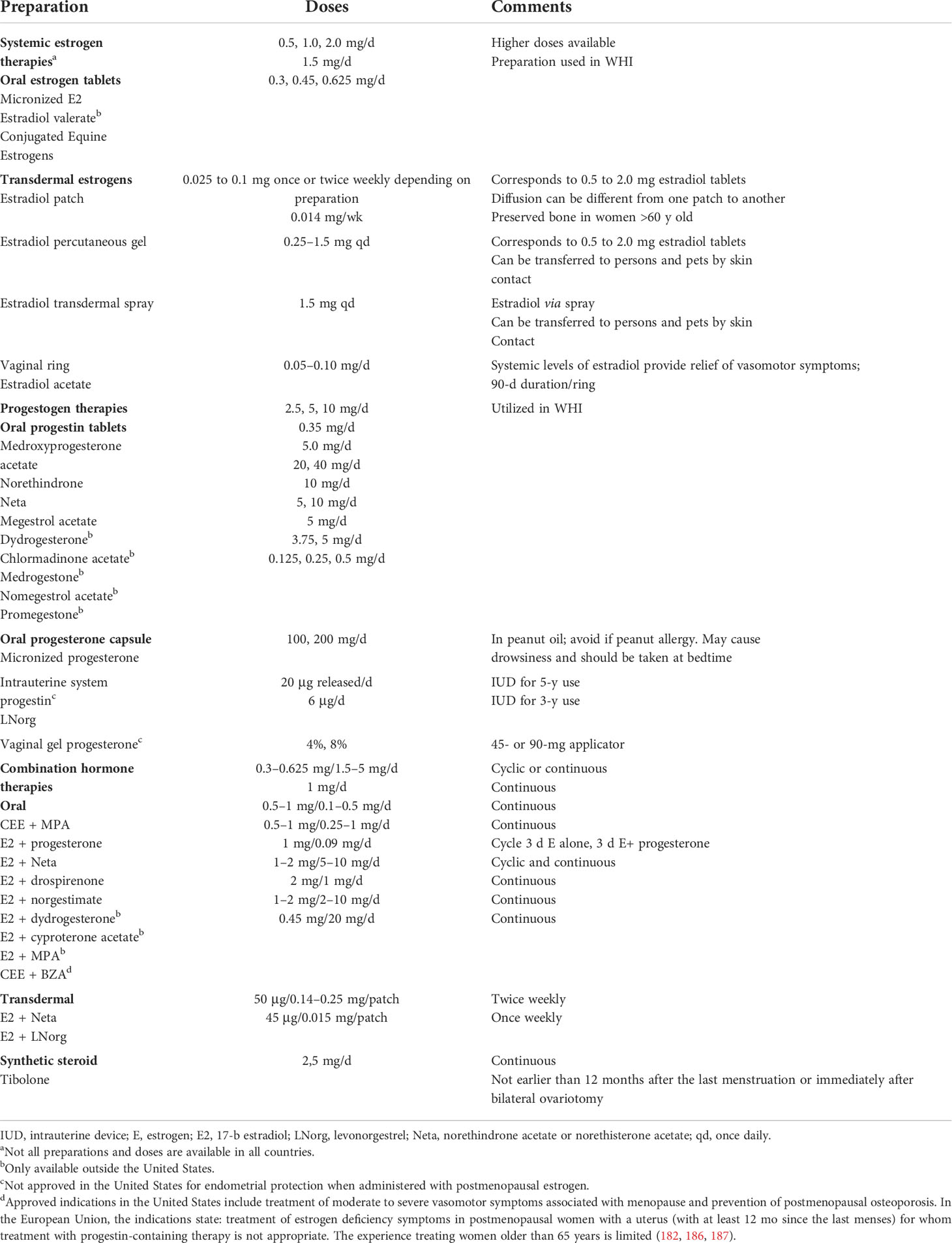
Currently, all leading international menopause societies recommend starting MHT in the peri- and postmenopausal period, at an age younger than 60 years and at menopause duration less than 10 years, when the benefit/risk ratio of MHT is most favorable in terms of relieving menopausal symptoms and preventing osteoporosis (181–185). The purpose of using MHT in peri- and postmenopausal women is to partially compensate for the sex hormone deficit. MHT is the most effective treatment for vasomotor symptoms and prevention of postmenopausal osteoporosis in women under the age of 60 years. A wide variety of MHT formulations are currently available worldwide, differing in components, doses, and forms (Table 3). The main components of MHT are estrogens and progestogens. Women after hysterectomy are prescribed MHT containing estrogens only. Micronized progesterone or synthetic progestogens are added to MHT for women with intact uterus to reduce the risk of endometrial hyperplasia and carcinoma conferred by estrogen monotherapy (188).
The combined estrogen-gestagenic therapy in a cyclic regime is prescribed to women with intact uterus in perimenopause, but not earlier than 6 months after the last menstruation, as a treatment for menopausal symptoms and prophylaxis of postmenopausal osteoporosis (181, 183, 185, 189). The monophasic combined low-dose and ultra-low-dose continuous estrogen-progestogen therapy is recommended for postmenopausal women with intact uterus (12 months after the last menstrual period) (181, 183, 185, 190). Estrogen delivery methods can be oral, transdermal (patches, gels, and sprays), subcutaneous (implants), and vaginal, whereas progestogens can be delivered orally, transdermally, or intrauterally. Tibolone is a synthetic steroid with estrogenic, progestogenic, and weak androgenic activities, indicated for the treatment of menopausal syndrome in postmenopausal women (186). An additional advantage of this drug is the absence of proliferative activity in relation to the endometrium and mammary glands, as well as a significant effect on the growth of myoma nodules. Tibolone has been associated with negligibly increased risks of breast cancer (191, 192) and decreased risks of venous thromboembolism (VTE) (181, 184, 186, 193) (Table 4).
The choice of MHT should be personalized by accounting for risk factors, including cardiovascular diseases, VTE, breast cancer, and postmenopausal osteoporosis, and comorbidities. The main principle is selection of minimum effective dosage, determination of optimal dosage and form of MHT, and the choice of a regimen accounting for physical age, the stage of reproductive aging (STRAW+10), and the needs of the patient (184). In perimenopause, standard (2 mg) and low doses (1 mg) of estradiol as part of MHT are used, in postmenopause, low and ultra-low doses (0.5 mg) of estrogens are used. In postmenopausal women, the reference level of estradiol is ≤ 10 pg/mL and varies with age, presence of vasomotor symptoms and vulvovaginal atrophy, and body mass index (204). In a study evaluating serum estradiol levels in postmenopausal women using MHT, when using estradiol hemihydrate or estradiol valerate serum estradiol levels increased with increasing dose of the drug, however, the degree of increase was not directly proportional to the dose; in particular, for oral estradiol, increasing the dose from 1 to 2 mg resulted in an increase of serum estradiol levels to approximately 60% instead of a doubling. This finding suggests that “low doses” of estrogen may be adequate from the start of MHT (205). Data from the Women’s Health Initiative (WHI) randomized controlled trial and other studies support the safe use of MHT for at least 5 years in healthy women commencing treatment under the age of 60 while being less than 10 years in postmenopause. The question of continuing therapy is decided individually, taking into account the possible risks (184). The North American Menopause Society experts published a statement in 2015 on the possible continuation of the use of MHT at the lowest effective dose in women over 65 years of age for the treatment of persistent hot flashes, given that the patient has received detailed information about the possible risks and is under close medical supervision (206).
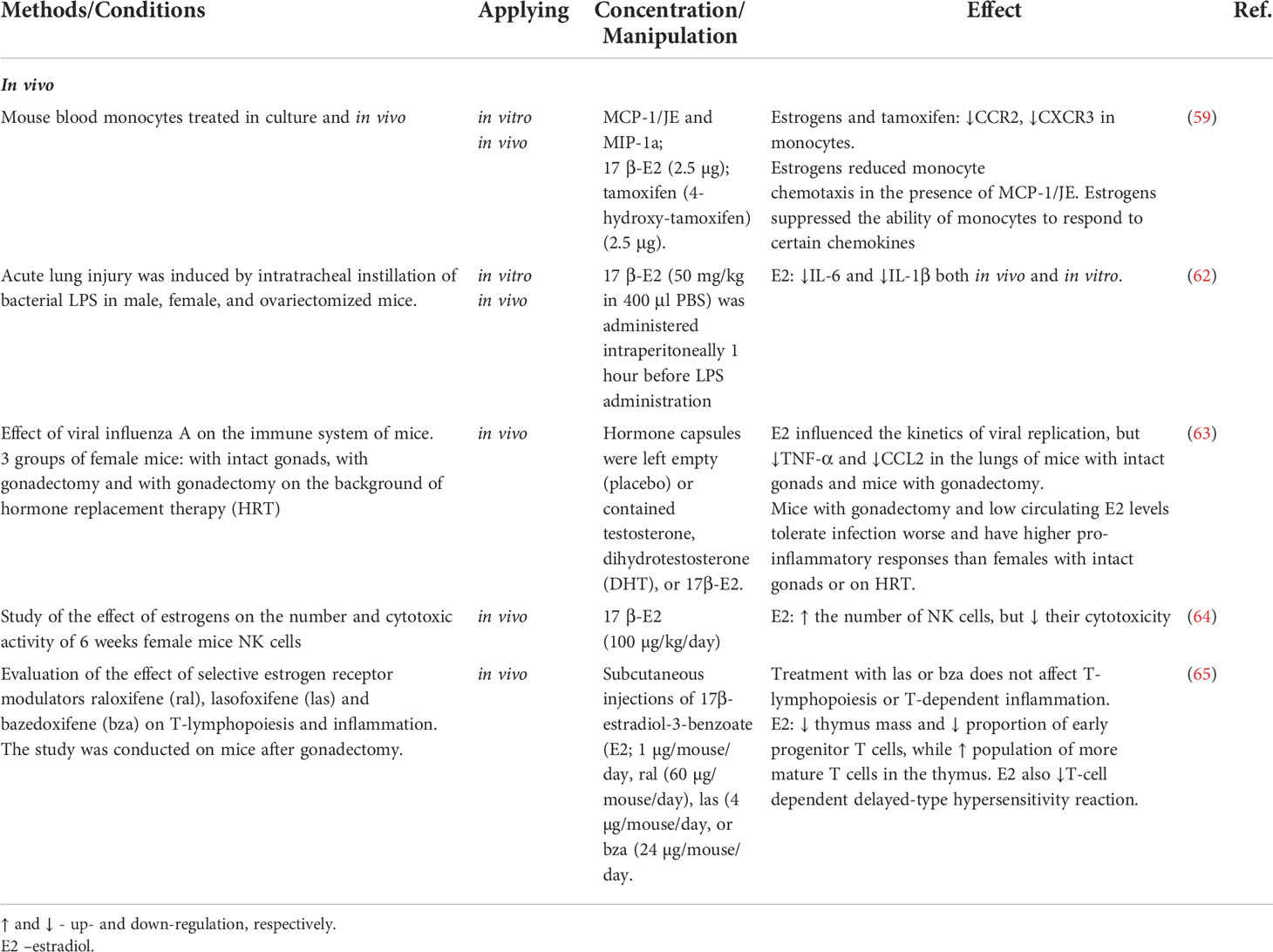
MHT reshapes immunological parameters
The gradual decline of ovarian function with age is a physiological process, but only in a small percentage of women the perimenopause proceeds unnoticed. Estimated 50–82% of women develop a symptom complex called “climacteric syndrome” with early vasomotor manifestations. The broader concept of climacteric syndrome involves psychoemotional and somatic components, as well as the increasing risk of cardiometabolic and cognitive dysfunctions in the long-range. Pathogenetic treatment of the climacteric syndrome aims to compensate the deficiency of sex hormones, first and foremost estrogens; the strategy allows slowing down the progression of the deficiency and thereby delay the onset of organic changes in target tissues and systems of the female body. Partial replenishment of the sex hormone deficiency slows down natural aging and eliminates long-term consequences.
The choice of MHT regimen depends on the stage of reproductive aging; the possibilities include combined estrogen-gestagen therapy in a cyclic mode using biphasic drugs in perimenopausal patients with intact uterus; monophasic combination therapy in postmenopausal patients with intact uterus, and estrogen monotherapy in hysterectomized patients. The modern principles of MHT prescription provide for an assessment of the risks of VTE individually for each patient. In postmenopausal women with low risk of VTE, estrogen in MHT could be administered both orally and transdermally at the lowest effective doses (low/ultra-low), according to existing recommendations (2016 IMS Recommendations on women’s midlife health and menopause hormone therapy) (184). For patients with increased risk of VTE and indications for MHT, transdermal forms of estrogens are prescribed as part of MHT, while in women with a history of VTE, MHT is contraindicated. During the pandemic in Russia, patients on MHT and infected with SARS-Cov-2 are prescribed anticoagulants to prevent VTE according to the guidelines ``Prevention, diagnosis and treatment of a new coronavirus infection (COVID-19)” 2021 and local clinical protocol for the Treatment of Patients with a New Coronavirus Infection (COVID-19). Summing up the available data, our personal opinion as the authors of this review is that MHT should not be cancelled in case of COVID-19 infection.
A complex relationship between sex hormones and immune system implies that MHT can exert pleiotropic effects on immunity in peri- and postmenopausal patients (207). The majority of studies focusing on such effects demonstrate a decrease in the production of pro-inflammatory cytokines (TNF-α, IL-1β, IL-6) by peripheral blood mononuclear cells of MHT recipients ex vivo or in vivo (208).
The influence of MHT on systemic inflammatory status may partially depend on estrogen administration routes. For instance, transdermal estrogens attenuated the response of the hypothalamic-pituitary-adrenal axis to low doses of endotoxin in vivo. The effect was accompanied by alleviation of the endotoxin-induced expression of pro-inflammatory cytokines IL-6 and TNF-α, as well as IL-1 receptor antagonist (IL-1ra). Oral administration of the same doses failed to reproduce this effect, probably due to the primary passage of the ingested hormones through the liver, where they triggered production of C-reactive protein and other pro-inflammatory molecules (209–211).
Clinical research data suggest that surgical menopause (upon ovariectomy) is accompanied by immunodeficiency with a decline in B cell counts and decreased serum levels of interferon γ. More generally, the rates of cellular and humoral immune response in women on MHT are higher than in matching controls (212). The definite immunological benefits of perimenopausal MHT confirm the important role of estrogens in regulation of immune functionalities (207). At the same time, it should be admitted that our understanding of MHT effects on immunological parameters is still fragmentary.
MHT and COVID-19
In 2020, at the first peak of the pandemic, the Italian Society of Contraception [Società Medica Italiana per la Contraccezione] issued the following opinion: MHT, as well as combined oral contraception regimens, should not be discontinued in patients with mild and moderate symptomatic COVID-19, whereas in severe cases the use of these medications is superfluous and should be replaced with anticoagulant therapy upon aggravation (213). Concomitantly, a board of experts representing specialized medical societies of Spain [Asociación Española para el Estudio de la Menopausia; Sociedad Española de Ginecología y Obstetricia; Sociedad Española de Trombosis y Hemostasia] recommended switching to parenteral routes of MHT administration, continue with parenteral MHT while adding anticoagulants, and fully replace MHT with anticoagulant therapy in, respectively, mild, moderate, and severe symptomatic COVID-19 (214). Large-scale self-monitoring data from the UK-based COVID-19 symptom tracking app showed lower incidence of hospitalization and reduced need for respiratory support in MHT recipients compared with matched controls (7, 215). A recent population study enrolling 151,193 MHT recipients and 152,637 matching non-recipients recorded significantly lower risks of adverse COVID-19 outcome for the former (adjusted odds ratio 0.22, 95% confidence interval 0.05–0.94) (7);. In a retrospective study, MHT reduced the risks of lethal COVID-19 outcomes in women over 50 by over 50% (odds ratio 0.33, 95% confidence interval 0.18–0.62; hazard ratio 0.29, 95% confidence interval 0.11–0.76) (8). Another retrospective study conducted in Sweden confirmed the reduced lethality of COVID-19 among postmenopausal MHT recipients (216). Given the design of the study, randomization was not considered. The weakness of the study is the lack of indication of the duration of endocrine/MHT intake. The level of sex hormones in postmenopausal patients was not determined, however, the distribution into groups suggested that in patients receiving endocrine therapy for breast cancer, the level of estrogen was reduced; in patients receiving MHT, estrogen levels were elevated; and in a control group of postmenopausal patients, without treatment, estrogen levels were consistent with postmenopausal values. A phase 2 clinical trial for possible alleviation of COVID-19 symptom severity through administration of transdermal estrogen patches was launched in the USA (NCT04359329).
Some experts argue that caution should be exercised regarding claims that the skewed sex ratio of COVID-19 morbidity and associated mortality is really determined by circulating steroid hormones. For instance, congenital genetic breakdowns in the immune system are arguably more impactful with regard to the critically aggravated course of the disease. The purposeful use of estrogens and progesterone in COVID-19 remains an intuitive concept that has not been supported by biochemical, physiological, or clinical evidence (217). Some authors believe that genetics and innate immune system disorders are more relevant to the sex-based difference in COVID-19 mortality than the circulating steroid hormone levels. Noteworthy, heritable defects in IFN-I immunity responsible for the deployment of life-threatening pneumonia in COVID-19 were detected in at least 2.6% of women and 12.5% of men (217). Bastard et al. hypothesized that congenital defects of cytokine system may contribute to the observed difference in disease severity between women and men. Apparently, germline mutations in IFN-I genes and neutralizing autoantibodies against corresponding proteins may underlie the diversity of respiratory complications (218). As reported by Zhang et al., at least 3.5% of patients with life-threatening COVID-19 pneumonia carry either autosomal recessive mutations in IRF7 or IFNAR1, autosomal dominant mutations in TLR3, TICAM1, TBK1 or IRF3, or de novo autosomal dominant mutations in UNC93B1, IRF7, IFNAR1 or IFNAR2 (219).
The plausible effects of MHT on the incidence and lethality of COVID-19 need further investigation. Although sex steroids appear to play an important role in modulating susceptibility to SARS-CoV-2, they cannot fully account for the skewed demographic patterns of COVID-19 mortality, indicating that other causes and mechanisms are yet to be understood (220).
Conclusion
High physiological concentrations of estrogens and progesterone synergistically reduce the production of pro-inflammatory cytokines by innate immune cells and also promote the anti-inflammatory response of T cells and immune tolerance, while stimulating the antibody production by B cells. In COVID-19, MHT may mitigate the clinical symptoms while increasing the antibody production (92). This knowledge is clinically relevant, as MHT is quite common, while dedicated development of new antivirals is hampered by the ongoing pandemic. Specific effects of MHT on hemostasis require careful assessment for the risks of its continued use in symptomatic COVID-19. The orchestrating role of estrogens in immune response and their protective effect on vascular endothelium should not be neglected. The correlation of sex steroid levels with severity and lethality of the disease indicates the potential of using hormone therapy to modulate the immune response and increase the resilience to adverse outcomes (9) (Figure 1). The overall success of MHT is based on decades of experience in clinical trials. According to the current standards, MHT should not be discontinued in COVID-19 with the exception of critical illness.
Author contributions
Writing—original draft preparation, МA, PV, and SY; writing—review and editing, МA, PV, SY, OY, TF, AE, and GS. All authors have read and agreed to the published version of the manuscript.
Funding
The research was performed within the framework of State Assignment No 121032500100-3. Part of the work concerning monocytes was supported by a grant for young Russian scientists MK-1573.2022.3. This work was supported by Russian Science Foundation [grant number 22-15-00241].
Acknowledgments
We acknowledge Natalia Usman for helpful discussions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Arentz M, Yim E, Klaff L, Lokhandwala S, Riedo FX, Chong M, et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington state. JAMA (2020) 323(16):1612–4. doi: 10.1001/jama.2020.4326
2. Gallo Marin B, Aghagoli G, Lavine K, Yang L, Siff EJ, Chiang SS, et al. Predictors of COVID-19 severity: A literature review. Rev Med Virol (2021) 31(1):1–10. doi: 10.1002/rmv.2146
3. Mølhave M, Agergaard J, Wejse C. Clinical management of COVID-19 patients – an update. Semin Nucl Med (2022) 52(1):4–10. doi: 10.1053/j.semnuclmed.2021.06.004
4. Bhatia A, Sekhon HK, Kaur G. Sex hormones and immune dimorphism. ScientificWorldJournal (2014) 2014:159150. doi: 10.1155/2014/159150
5. Cunningham M, Gilkeson G. Estrogen receptors in immunity and autoimmunity. Clin Rev Allergy Immunol (2011) 40(1):66–73. doi: 10.1007/s12016-010-8203-5
6. Dambha-Miller H, Hinton W, Wilcox CR, Joy M, Feher M, de Lusignan S. Mortality in COVID-19 among women on hormone replacement therapy: a retrospective cohort study. Fam Pract (2022), 1–7. doi: 10.1093/fampra/cmac041
7. Costeira R, Lee KA, Murray B, Christiansen C, Castillo-Fernandez J, Ni Lochlainn M, et al. Estrogen and COVID-19 symptoms: Associations in women from the COVID symptom study. PloS One (2021) 16(9):e0257051. doi: 10.1371/journal.pone.0257051
8. Seeland U, Coluzzi F, Simmaco M, Mura C, Bourne PE, Heiland M, et al. Evidence for treatment with estradiol for women with SARS-CoV-2 infection. BMC Med (2020) 18(1):369. doi: 10.1186/s12916-020-01851-z
9. Taneja V. Sex hormones determine immune response. Front Immunol (2018) 9:1931. doi: 10.3389/fimmu.2018.01931
10. Mangalam AK, Taneja V, David CS. HLA class II molecules influence susceptibility versus protection in inflammatory diseases by determining the cytokine profile. J Immunol (2013) 190(2):513–8. doi: 10.4049/jimmunol.1201891
11. Ngo ST, Steyn FJ, McCombe PA. Gender differences in autoimmune disease. Front Neuroendocrinol (2014) 35(3):347–69. doi: 10.1016/j.yfrne.2014.04.004
12. Ghare Naz MS, Banaei M, Dashti S, Tehrani FR. An overview of sex hormones in relation to SARS-CoV-2 infection. Future Virol (2021), 16. doi: 10.2217/fvl-2021-0058
13. Gebhard C, Regitz-Zagrosek V, Neuhauser HK, Morgan R, Klein SL. Impact of sex and gender on COVID-19 outcomes in Europe. Biol Sex Differ (2020) 11(1):29. doi: 10.1186/s13293-020-00304-9
14. Strope JD, Chau CH, Figg WD. Are sex discordant outcomes in COVID-19 related to sex hormones? Semin Oncol (2020) 47(5):335–40. doi: 10.1053/j.seminoncol.2020.06.002
15. Docherty AB, Harrison EM, Green CA, Hardwick HE, Pius R, Norman L, et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO clinical characterisation protocol: prospective observational cohort study. BMJ (2020) 369:m1985. doi: 10.1136/bmj.m1985
16. Karlberg J, Chong DSY, Lai WY. Re: Do men have a higher case fatality rate of severe acute respiratory syndrome than women do? Am J Epidemiol (2004) 159(3):229–31. doi: 10.1093/aje/kwh056
17. Alghamdi IG, Hussain II, Almalki SS, Alghamdi MS, Alghamdi MM, El-Sheemy MA. The pattern of middle East respiratory syndrome coronavirus in Saudi Arabia: a descriptive epidemiological analysis of data from the Saudi ministry of health. Int J Gen Med (2014) 7:417–23. doi: 10.2147/IJGM.S67061
18. Marina S, Piemonti L. Gender and age effects on the rates of infection and deaths in individuals with confirmed SARS-CoV-2 infection in six European countries. SSRN Electron J (2020), 16. doi: 10.2139/ssrn.3576790
19. Scully EP, Haverfield J, Ursin RL, Tannenbaum C, Klein SL. Considering how biological sex impacts immune responses and COVID-19 outcomes. Nat Rev Immunol (2020) 20(7):442–7. doi: 10.1038/s41577-020-0348-8
20. Bouman A, Heineman MJ, Faas MM. Sex hormones and the immune response in humans. Hum Reprod Update (2005) 11(4):411–23. doi: 10.1093/humupd/dmi008
21. Beagley KW, Gockel CM. Regulation of innate and adaptive immunity by the female sex hormones oestradiol and progesterone. FEMS Immunol Med Microbiol (2003) 38(1):13–22. doi: 10.1016/S0928-8244(03)00202-5
22. Grimaldi CM, Jeganathan V, Diamond B. Hormonal regulation of b cell development: 17 beta-estradiol impairs negative selection of high-affinity DNA-reactive b cells at more than one developmental checkpoint. J Immunol (2006) 176(5):2703–10. doi: 10.4049/jimmunol.176.5.2703
23. Salem ML. Estrogen, a double-edged sword: modulation of TH1- and TH2-mediated inflammations by differential regulation of TH1/TH2 cytokine production. Curr Drug Targets Inflammation Allergy (2004) 3(1):97–104. doi: 10.2174/1568010043483944
24. van Zeggeren IE, Boelen A, van de Beek D, Heijboer AC, Vlaar APJ, Brouwer MC. Sex steroid hormones are associated with mortality in COVID-19 patients: Level of sex hormones in severe COVID-19. Med (Baltimore) (2021) 100(34):e27072. doi: 10.1097/MD.0000000000027072
25. Beltrame A, Salguero P, Rossi E, Conesa A, Moro L, Bettini LR, et al. Association between sex hormone levels and clinical outcomes in patients with COVID-19 admitted to hospital: An observational, retrospective, cohort study. Front Immunol (2022) 13:834851. doi: 10.3389/fimmu.2022.834851
26. Ackerman LS. Sex hormones and the genesis of autoimmunity. Arch Dermatol (2006) 142(3):371–6. doi: 10.1001/archderm.142.3.371
27. Hammes SR, Levin ER. Impact of estrogens in males and androgens in females. J Clin Invest (2019) 129(5):1818–26. doi: 10.1172/JCI125755
28. Tee MK, Rogatsky I, Tzagarakis-Foster C, Cvoro A, An J, Christy RJ, et al. Estradiol and selective estrogen receptor modulators differentially regulate target genes with estrogen receptors alpha and beta. Mol Biol Cell (2004) 15(3):1262–72. doi: 10.1091/mbc.e03-06-0360
29. Safe S, Kim K. Non-classical genomic estrogen receptor (ER)/specificity protein and ER/activating protein-1 signaling pathways. J Mol Endocrinol (2008) 41(5):263–75. doi: 10.1677/JME-08-0103
30. Yaşar P, Ayaz G, User SD, Güpür G, Muyan M. Molecular mechanism of estrogen-estrogen receptor signaling. Reprod Med Biol (2017) 16(1):4–20. doi: 10.1002/rmb2.12006
31. Barton M. Position paper: The membrane estrogen receptor GPER–clues and questions. Steroids (2012) 77(10):935–42. doi: 10.1016/j.steroids.2012.04.001
32. Stelzig KE, Canepa-Escaro F, Schiliro M, Berdnikovs S, Prakash YS, Chiarella SE. Estrogen regulates the expression of SARS-CoV-2 receptor ACE2 in differentiated airway epithelial cells. Am J Physiol Lung Cell Mol Physiol (2020) 318(6):L1280–1. doi: 10.1152/ajplung.00153.2020
33. Kim KH, Young BD, Bender JR. Endothelial estrogen receptor isoforms and cardiovascular disease. Mol Cell Endocrinol (2014) 389(1–2):65–70. doi: 10.1016/j.mce.2014.02.001
34. Cheskis BJ, Greger JG, Nagpal S, Freedman LP. Signaling by estrogens. J Cell Physiol (2007) 213(3):610–7. doi: 10.1002/jcp.21253
35. Puglisi R, Mattia G, Carè A, Marano G, Malorni W, Matarrese P. Non-genomic effects of estrogen on cell homeostasis and remodeling with special focus on cardiac Ischemia/Reperfusion injury. Front Endocrinol (Lausanne) (2019) 10:733. doi: 10.3389/fendo.2019.00733
36. Boncler M, Watała C. Regulation of cell function by isoforms of c-reactive protein: a comparative analysis. Acta Biochim Pol (2009) 56(1):17–31. doi: 10.18388/abp.2009_2513
37. Al-kuraishy HM, Al-Gareeb AI, Faidah H, Al-Maiahy TJ, Cruz-Martins N, Batiha GES. The looming effects of estrogen in covid-19: A rocky rollout. Front Nutr (2021) 8:1–8. doi: 10.3389/fnut.2021.649128
38. Millas I, Duarte Barros M. Estrogen receptors and their roles in the immune and respiratory systems. Anat Rec. (2021) 304(6):1185–93. doi: 10.1002/ar.24612
39. Giefing-Kröll C, Berger P, Lepperdinger G, Grubeck-Loebenstein B. How sex and age affect immune responses, susceptibility to infections, and response to vaccination. Aging Cell (2015) 14(3):309–21. doi: 10.1111/acel.12326
40. Gaskins AJ, Wilchesky M, Mumford SL, Whitcomb BW, Browne RW, Wactawski-Wende J, et al. Endogenous reproductive hormones and c-reactive protein across the menstrual cycle: the BioCycle study. Am J Epidemiol (2012) 175(5):423–31. doi: 10.1093/aje/kwr343
41. Nadkarni S, McArthur S. Oestrogen and immunomodulation: new mechanisms that impact on peripheral and central immunity. Curr Opin Pharmacol (2013) 13(4):576–81. doi: 10.1016/j.coph.2013.05.007
42. Yu H-P, Hwang T-L, Hsieh P-W, Lau Y-T. Role of estrogen receptor-dependent upregulation of P38 MAPK/heme oxygenase 1 in resveratrol-mediated attenuation of intestinal injury after trauma-hemorrhage. Shock (2011) 35(5):517–23. doi: 10.1097/SHK.0b013e318209e931
43. Toyoda Y, Miyashita T, Endo S, Tsuneyama K, Fukami T, Nakajima M, et al. Estradiol and progesterone modulate halothane-induced liver injury in mice. Toxicol Lett (2011) 204(1):17–24. doi: 10.1016/j.toxlet.2011.03.031
44. Murphy AJ, Guyre PM, Pioli PA. Estradiol suppresses NF-kappa b activation through coordinated regulation of let-7a and miR-125b in primary human macrophages. J Immunol (2010) 184(9):5029–37. doi: 10.4049/jimmunol.0903463
45. Nadkarni S, Cooper D, Brancaleone V, Bena S, Perretti M. Activation of the annexin A1 pathway underlies the protective effects exerted by estrogen in polymorphonuclear leukocytes. Arterioscler Thromb Vasc Biol (2011) 31(11):2749–59. doi: 10.1161/ATVBAHA.111.235176
46. Santos RS, de Fatima LA, Frank AP, Carneiro EM, Clegg DJ. The effects of 17 alpha-estradiol to inhibit inflammation in vitro. Biol Sex Differ (2017) 8(1):30. doi: 10.1186/s13293-017-0151-9
47. Souza CLSe., Barbosa CD, Coelho HILN, Santos JúniorMN, Barbosa EN, Queiroz ÉC, et al. Effects of 17β-estradiol on Monocyte/Macrophage response to staphylococcus aureus: An in vitro study. Front Cell Infect Microbiol (2021) 11:1–12. doi: 10.3389/fcimb.2021.701391
48. Xing D, Oparil S, Yu H, Gong K, Feng W, Black J, et al. Estrogen modulates NFκB signaling by enhancing IκBα levels and blocking p65 binding at the promoters of inflammatory genes via estrogen receptor-β. PloS One (2012) 7(6):e36890. doi: 10.1371/journal.pone.0036890
49. Pioli PA, Jensen AL, Weaver LK, Amiel E, Shen Z, Shen L, et al. Estradiol attenuates lipopolysaccharide-induced CXC chemokine ligand 8 production by human peripheral blood monocytes. J Immunol (2007) 179(9):6284–90. doi: 10.4049/jimmunol.179.9.6284
50. Baristaite G, Gurwitz D. Estradiol reduces ACE2 and TMPRSS2 mRNA levels in A549 human lung epithelial cells. Drug Dev Res (2022) 83:961–66. doi: 10.1002/ddr.21923
51. Galván-Ramírez M de la L, Ramírez De Arellano A, Rodríguez-Pérez LR, Lopez-Pulido EI, Muñoz-Valle JF, Pereira-Suárez AL. Hormonal modulation of toxoplasma gondii infection: Regulation of hormonal receptors and cytokine production in THP-1 cells. Exp Parasitol (2019), 204:107721. doi: 10.1016/j.exppara.2019.107721
52. Teixeira D, Marques C, Pestana D, Faria A, Norberto S, Calhau C, et al. Effects of xenoestrogens in human M1 and M2 macrophage migration, cytokine release, and estrogen-related signaling pathways. Environ Toxicol (2016) 31(11):1496–509. doi: 10.1002/tox.22154
53. Butts CL, Shukair SA, Duncan KM, Bowers E, Horn C, Belyavskaya E, et al. Progesterone inhibits mature rat dendritic cells in a receptor-mediated fashion. Int Immunol (2007) 19(3):287–96. doi: 10.1093/intimm/dxl145
54. Stanojević S, Kovačević-Jovanović V, Dimitrijević M, Vujić V, Ćuruvija I, Blagojević V, et al. Unopposed estrogen Supplementation/Progesterone deficiency in post-reproductive age affects the secretory profile of resident macrophages in a tissue-specific manner in the rat. Am J Reprod Immunol (2015) 74(5):445–56. doi: 10.1111/aji.12424
55. Channappanavar R, Fett C, Mack M, Ten Eyck PP, Meyerholz DK, Perlman S. Sex-based differences in susceptibility to severe acute respiratory syndrome coronavirus infection. J Immunol (2017) 198(10):4046–53. doi: 10.4049/jimmunol.1601896
56. Ćuruvija I, Stanojević S, Arsenović-Ranin N, Blagojević V, Dimitrijević M, Vidić-Danković B, et al. Sex differences in macrophage functions in middle-aged rats: Relevance of estradiol level and macrophage estrogen receptor expression. Inflammation (2017) 40(3):1087–101. doi: 10.1007/s10753-017-0551-3
57. Kramer PR, Kramer SF, Guan G. 17 beta-estradiol regulates cytokine release through modulation of CD16 expression in monocytes and monocyte-derived macrophages. Arthritis Rheumatol (2004) 50(6):1967–75. doi: 10.1002/art.20309
58. Margaryan S, Hyusyan A, Martirosyan A, Sargsian S, Manukyan G. Differential modulation of innate immune response by epinephrine and estradiol. Horm Mol Biol Clin Investig (2017) 30(3). doi: 10.1515/hmbci-2016-0046
59. Janis K, Hoeltke J, Nazareth M, Fanti P, Poppenberg K, Aronica SM. Estrogen decreases expression of chemokine receptors, and suppresses chemokine bioactivity in murine monocytes. Am J Reprod Immunol (2004) 51(1):22–31. doi: 10.1046/j.8755-8920.2003.00117.x
60. Lang TJ. Estrogen as an immunomodulator. Clin Immunol (2004) 113(3):224–30. doi: 10.1016/j.clim.2004.05.011
61. Lee D-H, Kim S-C, Joo J-K, Kim H-G, Na Y-J, Kwak J-Y, et al. Effects of 17β-estradiol on the release of monocyte chemotactic protein-1 and MAPK activity in monocytes stimulated with peritoneal fluid from endometriosis patients. J Obstet Gynaecol Res (2012) 38(3):516–25. doi: 10.1111/j.1447-0756.2011.01734.x
62. Speyer CL, Rancilio NJ, McClintock SD, Crawford JD, Gao H, Sarma JV, et al. Regulatory effects of estrogen on acute lung inflammation in mice. Am J Physiol Cell Physiol (2005) 288(4):C881–90. doi: 10.1152/ajpcell.00467.2004
63. Robinson DP, Lorenzo ME, Jian W, Klein SL. Elevated 17β-estradiol protects females from influenza a virus pathogenesis by suppressing inflammatory responses. PloS Pathog (2011) 7(7):e1002149. doi: 10.1371/journal.ppat.1002149
64. Hao S, Zhao J, Zhou J, Zhao S, Hu Y, Hou Y. Modulation of 17beta-estradiol on the number and cytotoxicity of NK cells in vivo related to MCM and activating receptors. Int Immunopharmacol (2007) 7(13):1765–75. doi: 10.1016/j.intimp.2007.09.017
65. Bernardi AI, Andersson A, Stubelius A, Grahnemo L, Carlsten H, Islander U. Selective estrogen receptor modulators in T cell development and T cell dependent inflammation. Immunobiology (2015) 220(10):1122–8. doi: 10.1016/j.imbio.2015.05.009
66. Habib P, Dreymueller D, Rösing B, Botung H, Slowik A, Zendedel A, et al. Estrogen serum concentration affects blood immune cell composition and polarization in human females under controlled ovarian stimulation. J Steroid Biochem Mol Biol (2018) 178:340–7. doi: 10.1016/j.jsbmb.2018.02.005
67. Miller AP, Feng W, Xing D, Weathington NM, Blalock JE, Chen Y-F, et al. Estrogen modulates inflammatory mediator expression and neutrophil chemotaxis in injured arteries. Circulation (2004) 110(12):1664–9. doi: 10.1161/01.CIR.0000142050.19488.C7
68. Hsieh Y-C, Frink M, Hsieh C-H, Choudhry MA, Schwacha MG, Bland KI, et al. Downregulation of migration inhibitory factor is critical for estrogen-mediated attenuation of lung tissue damage following trauma-hemorrhage. Am J Physiol Lung Cell Mol Physiol (2007) 292(5):L1227–32. doi: 10.1152/ajplung.00479.2006
69. Lambert KC, Curran EM, Judy BM, Lubahn DB, Estes DM. Estrogen receptor-alpha deficiency promotes increased TNF-alpha secretion and bacterial killing by murine macrophages in response to microbial stimuli in vitro. J Leukoc Biol (2004) 75(6):1166–72. doi: 10.1189/jlb.1103589
70. Villa A, Rizzi N, Vegeto E, Ciana P, Maggi A. Estrogen accelerates the resolution of inflammation in macrophagic cells. Sci Rep (2015) 5:15224. doi: 10.1038/srep15224
71. Pepe G, Braga D, Renzi TA, Villa A, Bolego C, D’Avila F, et al. Self-renewal and phenotypic conversion are the main physiological responses of macrophages to the endogenous estrogen surge. Sci Rep (2017) 7:44270. doi: 10.1038/srep44270
72. Toniolo A, Fadini GP, Tedesco S, Cappellari R, Vegeto E, Maggi A, et al. Alternative activation of human macrophages is rescued by estrogen treatment in vitro and impaired by menopausal status. J Clin Endocrinol Metab (2015) 100(1):E50–8. doi: 10.1210/jc.2014-2751
73. Souza CLSE, Barbosa CD, Coelho HILN, Santos Júnior MN, Barbosa EN, Queiroz ÉC, et al. Effects of 17β-estradiol on Monocyte/Macrophage response to staphylococcus aureus: An In vitro study. Front Cell Infect Microbiol (2021) 11:701391. doi: 10.3389/fcimb.2021.701391
74. Zhang X, Goncalves R, Mosser DM. The isolation and characterization of murine macrophages. Curr Protoc Immunol (2008) 14:14.1. doi: 10.1002/0471142735.im1401s83
75. Doucet D, Badami C, Palange D, Bonitz RP, Lu Q, Xu D-Z, et al. Estrogen receptor hormone agonists limit trauma hemorrhage shock-induced gut and lung injury in rats. PloS One (2010) 5(2):e9421. doi: 10.1371/journal.pone.0009421
76. Ribas V, Drew BG, Le JA, Soleymani T, Daraei P, Sitz D, et al. Myeloid-specific estrogen receptor alpha deficiency impairs metabolic homeostasis and accelerates atherosclerotic lesion development. Proc Natl Acad Sci U S A (2011) 108(39):16457–62. doi: 10.1073/pnas.1104533108
77. Kadel S, Kovats S. Sex hormones regulate innate immune cells and promote sex differences in respiratory virus infection. Front Immunol (2018) 9:1653. doi: 10.3389/fimmu.2018.01653
78. Cavaliere FM, Prezzo A, Bilotta C, Iacobini M, Quinti I. The lack of BTK does not impair monocytes and polymorphonuclear cells functions in X-linked agammaglobulinemia under treatment with intravenous immunoglobulin replacement. PloS One (2017) 12(4):e0175961. doi: 10.1371/journal.pone.0175961
79. Mohammad I, Starskaia I, Nagy T, Guo J, Yatkin E, Väänänen K, et al. Estrogen receptor α contributes to T cell-mediated autoimmune inflammation by promoting T cell activation and proliferation. Sci Signal (2018) 11(526). doi: 10.1126/scisignal.aap9415
80. Dai R, Phillips RA, Ahmed SA. Despite inhibition of nuclear localization of NF-kappa b p65, c-rel, and RelB, 17-beta estradiol up-regulates NF-kappa b signaling in mouse splenocytes: the potential role of bcl-3. J Immunol (2007) 179(3):1776–83. doi: 10.4049/jimmunol.179.3.1776
81. Lengi AJ, Phillips RA, Karpuzoglu E, Ahmed SA. Estrogen selectively regulates chemokines in murine splenocytes. J Leukoc Biol (2007) 81(4):1065–74. doi: 10.1189/jlb.0606391
82. Karpuzoglu E, Phillips RA, Dai R, Graniello C, Gogal RMJ, Ahmed SA. Signal transducer and activation of transcription (STAT) 4beta, a shorter isoform of interleukin-12-induced STAT4, is preferentially activated by estrogen. Endocrinology (2009) 150(3):1310–20. doi: 10.1210/en.2008-0832
83. Khan D, Dai R, Karpuzoglu E, Ahmed SA. Estrogen increases, whereas IL-27 and IFN-gamma decrease, splenocyte IL-17 production in WT mice. Eur J Immunol (2010) 40(9):2549–56. doi: 10.1002/eji.201040303
84. Nie J, Li YY, Zheng SG, Tsun A, Li B. FOXP3(+) treg cells and gender bias in autoimmune diseases. Front Immunol (2015) 6:493. doi: 10.3389/fimmu.2015.00493
85. Polanczyk MJ, Carson BD, Subramanian S, Afentoulis M, Vandenbark AA, Ziegler SF, et al. Cutting edge: estrogen drives expansion of the CD4+CD25+ regulatory T cell compartment. J Immunol (2004) 173(4):2227–30. doi: 10.4049/jimmunol.173.4.2227
86. Dinesh RK, Hahn BH, Singh RP. PD-1, gender, and autoimmunity. Autoimmun Rev (2010) 9(8):583–7. doi: 10.1016/j.autrev.2010.04.003
87. Prieto GA, Rosenstein Y. Oestradiol potentiates the suppressive function of human CD4 CD25 regulatory T cells by promoting their proliferation. Immunology (2006) 118(1):58–65. doi: 10.1111/j.1365-2567.2006.02339.x
88. Luo CY, Wang L, Sun C, Li DJ. Estrogen enhances the functions of CD4(+)CD25(+)Foxp3(+) regulatory T cells that suppress osteoclast differentiation and bone resorption in vitro. Cell Mol Immunol (2011) 8(1):50–8. doi: 10.1038/cmi.2010.54
89. Adurthi S, Kumar MM, Vinodkumar HS, Mukherjee G, Krishnamurthy H, Acharya KK, et al. Oestrogen receptor-α binds the FOXP3 promoter and modulates regulatory T-cell function in human cervical cancer. Sci Rep (2017) 7(1):17289. doi: 10.1038/s41598-017-17102-w
90. Mo R, Chen J, Grolleau-Julius A, Murphy HS, Richardson BC, Yung RL. Estrogen regulates CCR gene expression and function in T lymphocytes. J Immunol (2005) 174(10):6023–9. doi: 10.4049/jimmunol.174.10.6023
91. Moulton VR. Sex hormones in acquired immunity and autoimmune disease. Front Immunol (2018) 9:2279. doi: 10.3389/fimmu.2018.02279
92. Mauvais-Jarvis F, Klein SL, Levin ER. Estradiol, progesterone, immunomodulation, and COVID-19 outcomes. Endocrinology (2020) 161(9). doi: 10.1210/endocr/bqaa127
93. Nakaya M, Tachibana H, Yamada K. Effect of estrogens on the interferon-gamma producing cell population of mouse splenocytes. Biosci Biotechnol Biochem (2006) 70(1):47–53. doi: 10.1271/bbb.70.47
94. Robinson DP, Lorenzo ME, Jian W, Klein SL. Elevated17b-EstradiolProtectsFemalesfromInfluenzaAVirusPathogenesisbySuppressingInflammatoryResponses. PloS Pathog (2011) 7(7). doi: 10.1371/journal.ppat.1002149
95. Robinson DP, Hall OJ, Nilles TL, Jay H, Klein SL. Responses in the lungs 17 NL -estradiol protects females against influenza by recruiting. (2014) 88:4711–20. doi: 10.1128/JVI.02081-13
96. Vermillion MS, Ursin RL, Attreed SE, Klein SL. Estriol reduces pulmonary immune cell recruitment and inflammation to protect female mice from severe influenza. Endocrinology (2018) 159(9):3306–20. doi: 10.1210/en.2018-00486
97. MacNeil LG, Baker SK, Stevic I, Tarnopolsky MA. 17β-estradiol attenuates exercise-induced neutrophil infiltration in men. Am J Physiol Regul Integr Comp Physiol (2011) 300(6):R1443–51. doi: 10.1152/ajpregu.00689.2009
98. Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol (2016) 16(10):626–38. doi: 10.1038/nri.2016.90
99. Hughes GC. Progesterone and autoimmune disease. Autoimmun Rev (2012) 11(6–7):A502–14. doi: 10.1016/j.autrev.2011.12.003
100. Tan IJ, Peeva E, Zandman-Goddard G. Hormonal modulation of the immune system - a spotlight on the role of progestogens. Autoimmun Rev (2015) 14(6):536–42. doi: 10.1016/j.autrev.2015.02.004
101. Hardy DB, Janowski BA, Corey DR, Mendelson CR. Progesterone receptor plays a major antiinflammatory role in human myometrial cells by antagonism of nuclear factor-kappaB activation of cyclooxygenase 2 expression. Mol Endocrinol (2006) 20(11):2724–33. doi: 10.1210/me.2006-0112
102. Patra R, Chandra Das N, Mukherjee S. Targeting human TLRs to combat COVID-19: A solution? J Med virol (2021) 93:615–7. doi: 10.1002/jmv.26387
103. Khanmohammadi S, Rezaei N. Role of toll-like receptors in the pathogenesis of COVID-19. J Med Virol (2021) 93(5):2735–9. doi: 10.1002/jmv.26826
104. Totura AL, Whitmore A, Agnihothram S, Schäfer A, Katze MG, Heise MT, et al. Toll-like receptor 3 signaling via TRIF contributes to a protective innate immune response to severe acute respiratory syndrome coronavirus infection. MBio (2015) 6(3):e00638–15. doi: 10.1128/mBio.00638-15
105. Sohn KM, Lee SG, Kim HJ, Cheon S, Jeong H, Lee J, et al. COVID-19 patients upregulate toll-like receptor 4-mediated inflammatory signaling that mimics bacterial sepsis. J Korean Med Sci (2020) 35(38):e343. doi: 10.3346/jkms.2020.35.e343
106. Chen Q, Qi X, Zhang W, Zhang Y, Bi Y, Meng Q, et al. Catalpol inhibits macrophage polarization and prevents postmenopausal atherosclerosis through regulating estrogen receptor alpha. Front Pharmacol (2021) 12:1–16. doi: 10.3389/fphar.2021.655081
107. Gubbels Bupp MR, Jorgensen TN. Androgen-induced immunosuppression. Front Immunol (2018) 9:794. doi: 10.3389/fimmu.2018.00794
108. Pakpoor J, Goldacre R, Goldacre MJ. Associations between clinically diagnosed testicular hypofunction and systemic lupus erythematosus: a record linkage study. Clin Rheumatol (2018) 37(2):559–62. doi: 10.1007/s10067-017-3873-5
109. Stasi V, Rastrelli G. The role of sex hormones in the disparity of COVID-19 outcomes based on gender. J Sex Med (2021) 18(12):1950–4. doi: 10.1016/j.jsxm.2021.09.003
110. Kovats S, Carreras E. Regulation of dendritic cell differentiation and function by estrogen receptor ligands. Cell Immunol (2008) 252(1–2):81–90. doi: 10.1016/j.cellimm.2007.10.008
111. Conti P, Younes A. Coronavirus COV-19/SARS-CoV-2 affects women less than men: clinical response to viral infection. J Biol regulators homeostatic agents (2020) 34:339–43. doi: 10.23812/Editorial-Conti-3
112. Trombetta AC, Meroni M, Cutolo M. Steroids and autoimmunity. Front Horm Res (2017) 48:121–32. doi: 10.1159/000452911
113. Edwards M, Dai R, Ahmed SA. Our environment shapes us: The importance of environment and sex differences in regulation of autoantibody production. Front Immunol (2018) 9:478. doi: 10.3389/fimmu.2018.00478
114. Lahita RG. The immunoendocrinology of systemic lupus erythematosus. Clin Immunol (2016) 172:98–100. doi: 10.1016/j.clim.2016.08.014
115. Hughes GC, Choubey D. Modulation of autoimmune rheumatic diseases by oestrogen and progesterone. Nat Rev Rheumatol (2014) 10(12):740–51. doi: 10.1038/nrrheum.2014.144
116. Ortona E, Pierdominici M, Maselli A, Veroni C, Aloisi F, Shoenfeld Y. Sex-based differences in autoimmune diseases. Ann Ist Super Sanita (2016) 52(2):205–12. doi: 10.4415/ANN_16_02_12
117. Kovats S. Estrogen receptors regulate innate immune cells and signaling pathways. Cell Immunol (2015) 294(2):63–9. doi: 10.1016/j.cellimm.2015.01.018
118. Rodriguez-Garcia M, Patel MV, Shen Z, Wira CR. The impact of aging on innate and adaptive immunity in the human female genital tract. Aging Cell (2021) 20(5):e13361. doi: 10.1111/acel.13361
119. Fahey JV, Wright JA, Shen L, Smith JM, Ghosh M, Rossoll RM, et al. Estradiol selectively regulates innate immune function by polarized human uterine epithelial cells in culture. Mucosal Immunol (2008) 1(4):317–25. doi: 10.1038/mi.2008.20
120. Farage M, Maibach H. Lifetime changes in the vulva and vagina. Arch Gynecol Obstet (2006) 273(4):195–202. doi: 10.1007/s00404-005-0079-x
121. Thurman AR, Yousefieh N, Chandra N, Kimble T, Asin S, Rollenhagen C, et al. Comparison of mucosal markers of human immunodeficiency virus susceptibility in healthy premenopausal versus postmenopausal women. AIDS Res Hum Retroviruses (2017) 33(8):807–19. doi: 10.1089/aid.2016.0320
122. Nazli A, Chan O, Dobson-Belaire WN, Ouellet M, Tremblay MJ, Gray-Owen SD, et al. Exposure to HIV-1 directly impairs mucosal epithelial barrier integrity allowing microbial translocation. PloS Pathog (2010) 6(4):e1000852. doi: 10.1371/journal.ppat.1000852
123. Patel MV, Hopkins DC, Barr FD, Wira CR. Sex hormones and aging modulate interferon lambda 1 production and signaling by human uterine epithelial cells and fibroblasts. Front Immunol (2021) 12:718380. doi: 10.3389/fimmu.2021.718380
124. Shimizu K, Suzuki N, Nakamura M, Aizawa K, Imai T, Suzuki S, et al. Mucosal immune function comparison between amenorrheic and eumenorrheic distance runners. J strength Cond Res (2012) 26(5):1402–6. doi: 10.1519/JSC.0b013e31822e7a6c
125. Gillum T, Kuennen M, Miller T, Riley L. The effects of exercise, sex, and menstrual phase on salivary antimicrobial proteins. Exerc Immunol Rev (2014) 20:23–38.
126. Van Anders SM. Gonadal steroids and salivary IgA in healthy young women and men. Am J Hum Biol Off J Hum Biol Counc (2010) 22(3):348–52. doi: 10.1002/ajhb.20997
127. Diebel ME, Diebel LN, Liberati DM. Gender dimorphism in the gut: mucosal protection by estrogen stimulation of IgA transcytosis. J Trauma (2011) 71(2):474–9. doi: 10.1097/TA.0b013e318228239d
128. Wheeler JC, Vanoni S, Zeng C, Waggoner L, Yang Y, Wu D, et al. 17β-estradiol protects the esophageal epithelium from IL-13-induced barrier dysfunction and remodeling. J Allergy Clin Immunol (2019) 143(6):2131–46. doi: 10.1016/j.jaci.2018.10.070
129. Engeland CG, Sabzehei B, Marucha PT. Sex hormones and mucosal wound healing. Brain Behav Immun (2009) 23(5):629–35. doi: 10.1016/j.bbi.2008.12.001
130. Bagri P, Ghasemi R, McGrath JJC, Thayaparan D, Yu E, Brooks AG, et al. Estradiol enhances antiviral CD4(+) tissue-resident memory T cell responses following mucosal herpes simplex virus 2 vaccination through an IL-17-Mediated pathway. J Virol (2020) 95(1):e01206–20. doi: 10.1128/JVI.01206-20
131. Li M-Y, Li L, Zhang Y, Wang X-S. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis poverty (2020) 9(1):45. doi: 10.1186/s40249-020-00662-x
132. Kirchdoerfer RN, Cottrell CA, Wang N, Pallesen J, Yassine HM, Turner HL, et al. Pre-fusion structure of a human coronavirus spike protein. Nature (2016) 531(7592):118–21. doi: 10.1038/nature17200
133. Ashour HM, Elkhatib WF, Rahman MM, Elshabrawy HA. Insights into the recent 2019 novel coronavirus (SARS-CoV-2) in light of past human coronavirus outbreaks. Pathog (Basel Switzerland) (2020) 9(3):186. doi: 10.3390/pathogens9030186
134. Pradhan A, Olsson P-E. Sex differences in severity and mortality from COVID-19: are males more vulnerable? Biol Sex Differ (2020) 11(1):53. doi: 10.1186/s13293-020-00330-7
135. Zhang H, Penninger JM, Li Y, Zhong N, Slutsky AS. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med (2020) 46(4):586–90. doi: 10.1007/s00134-020-05985-9
136. Majdic G. Could Sex/Gender differences in ACE2 expression in the lungs contribute to the Large gender disparity in the morbidity and mortality of patients infected with the SARS-CoV-2 virus? Front Cell Infect Microbiol (2020) 10:327. doi: 10.3389/fcimb.2020.00327
137. Lukassen S, Chua RL, Trefzer T, Kahn NC, Schneider MA, Muley T, et al. SARS-CoV- 2 receptor ACE 2 and TMPRSS 2 are primarily expressed in bronchial transient secretory cells. (2020), 1–15. doi: 10.15252/embj.20105114
138. Wu M, Ma L, Xue L, Zhu Q, Zhou S, Dai J, et al. Co-Expression of the SARS-CoV-2 entry molecules ACE2 and TMPRSS2 in human ovaries: Identification of cell types and trends with age. Genomics (2021) 113(6):3449–60. doi: 10.1016/j.ygeno.2021.08.012
139. Allegretti M, Cesta MC, Zippoli M, Beccari A, Talarico C, Mantelli F, et al. Repurposing the estrogen receptor modulator raloxifene to treat SARS-CoV-2 infection. Cell Death Differ (2022) 29(1):156–66. doi: 10.1038/s41418-021-00844-6
140. Ware LB, Matthay MA. Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med (2001) 163(6):1376–83. doi: 10.1164/ajrccm.163.6.2004035
141. Laube M, Küppers E, Thome UH. Modulation of sodium transport in alveolar epithelial cells by estradiol and progesterone. Pediatr Res (2011) 69(3):200–5. doi: 10.1203/PDR.0b013e3182070ec8
142. Hallman M, Haataja R. Genetic influences and neonatal lung disease. Semin Neonatol (2003) 8(1):19–27. doi: 10.1016/S1084-2756(02)00196-3
143. Haase M, Laube M, Thome UH. Sex-specific effects of sex steroids on alveolar epithelial na(+) transport. Am J Physiol Lung Cell Mol Physiol (2017) 312(3):L405–14. doi: 10.1152/ajplung.00275.2016
144. Sweezey N, Tchepichev S, Gagnon S, Fertuck K, O’Brodovich H. Female gender hormones regulate mRNA levels and function of the rat lung epithelial Na channel. Am J Physiol (1998) 274(2):C379–86. doi: 10.1152/ajpcell.1998.274.2.C379
145. Greenlee MM, Mitzelfelt JD, Yu L, Yue Q, Duke BJ, Harrell CS, et al. Estradiol activates epithelial sodium channels in rat alveolar cells through the G protein-coupled estrogen receptor. Am J Physiol Lung Cell Mol Physiol (2013) 305(11):L878–89. doi: 10.1152/ajplung.00008.2013
146. Qi D, He J, Wang D, Deng W, Zhao Y, Ye Y, et al. 17β-estradiol suppresses lipopolysaccharide-induced acute lung injury through PI3K/Akt/SGK1 mediated up-regulation of epithelial sodium channel (ENaC) in vivo and in vitro. Respir Res (2014) 15(1):159. doi: 10.1186/s12931-014-0159-1
147. Glassberg MK, Choi R, Manzoli V, Shahzeidi S, Rauschkolb P, Voswinckel R, et al. 17β-estradiol replacement reverses age-related lung disease in estrogen-deficient C57BL/6J mice. Endocrinology (2014) 155(2):441–8. doi: 10.1210/en.2013-1345
148. Strope JD, PharmD CHC, Figg WD. TMPRSS2: Potential biomarker for COVID-19 outcomes. J Clin Pharmacol (2020) 60(7):801–7. doi: 10.1002/jcph.1641
149. Rivellese F, Prediletto E. ACE2 at the centre of COVID-19 from paucisymptomatic infections to severe pneumonia. Autoimmun Rev (2020) 19:102536. doi: 10.1016/j.autrev.2020.102536
150. Song H, Seddighzadeh B, Cooperberg MR, Huang FW. Expression of ACE2, the SARS-CoV-2 receptor, and TMPRSS2 in prostate epithelial cells. bioRxiv (2020) 78(2):296–98. doi: 10.1101/2020.04.24.056259
151. Okpechi SC, Fong JT, Gill SS, Harman JC, Nguyen TH, Chukwurah QC, et al. Global sex disparity of COVID-19: A descriptive review of sex hormones and consideration for the potential therapeutic use of hormone replacement therapy in older adults. Aging Dis (2021) 12(2):671–83. doi: 10.14336/AD.2020.1211
152. Chakrabarti S, Davidge ST. G-Protein coupled receptor 30 (GPR30): a novel regulator of endothelial inflammation. PloS One (2012) 7(12):e52357. doi: 10.1371/journal.pone.0052357
153. Strehlow K, Werner N, Berweiler J, Link A, Dirnagl U, Priller J, et al. Estrogen increases bone marrow-derived endothelial progenitor cell production and diminishes neointima formation. Circulation (2003) 107(24):3059–65. doi: 10.1161/01.CIR.0000077911.81151.30
154. Hinojosa-Laborde C, Craig T, Zheng W, Ji H, Haywood JR, Sandberg K. Ovariectomy augments hypertension in aging female Dahl salt-sensitive rats. Hypertens (2004) 44(4):405–9. doi: 10.1161/01.HYP.0000142893.08655.96
155. Pisitkun P, Deane JA, Difilippantonio MJ, Tarasenko T, Satterthwaite AB, Bolland S. Autoreactive b cell responses to RNA-related antigens due to TLR7 gene duplication. Science (2006) 312(5780):1669–72. doi: 10.1126/science.1124978
156. Berghöfer B, Frommer T, Haley G, Fink L, Bein G, Hackstein H. TLR7 ligands induce higher IFN-alpha production in females. J Immunol (2006) 177(4):2088–96. doi: 10.4049/jimmunol.177.4.2088
157. de Groot NG, Bontrop RE. COVID-19 pandemic: is a gender-defined dosage effect responsible for the high mortality rate among males? Immunogenetics (2020) 72:275–7. doi: 10.1007/s00251-020-01165-7
158. Hagen SH, Henseling F, Hennesen J, Savel H, Delahaye S, Richert L, et al. Heterogeneous escape from X chromosome inactivation results in sex differences in type I IFN responses at the single human pDC level. Cell Rep (2020) 33(10):108485. doi: 10.1016/j.celrep.2020.108485
159. Meier A, Chang JJ, Chan ES, Pollard RB, Sidhu HK, Kulkarni S, et al. Sex differences in the toll-like receptor-mediated response of plasmacytoid dendritic cells to HIV-1. Nat Med (2009) 15(8):955–9. doi: 10.1038/nm.2004
160. Webb K, Peckham H, Radziszewska A, Menon M, Oliveri P, Simpson F, et al. Sex and pubertal differences in the type 1 interferon pathway associate with both X chromosome number and serum sex hormone concentration. Front Immunol (2018) 9:3167. doi: 10.3389/fimmu.2018.03167
161. Laffont S, Rouquié N, Azar P, Seillet C, Plumas J, Aspord C, et al. X-Chromosome complement and estrogen receptor signaling independently contribute to the enhanced TLR7-mediated IFN-α production of plasmacytoid dendritic cells from women. J Immunol (2014) 193(11):5444–52. doi: 10.4049/jimmunol.1303400
162. Griesbeck M, Ziegler S, Laffont S, Smith N, Chauveau L, Tomezsko P, et al. Sex differences in plasmacytoid dendritic cell levels of IRF5 drive higher IFN-α production in women. J Immunol (2015) 195(11):5327–36. doi: 10.4049/jimmunol.1501684
163. Mateus D, Sebastião AI, Carrascal MA, Carmo A, Matos AM, Cruz MT. Crosstalk between estrogen, dendritic cells, and SARS-CoV-2 infection. Rev Med Virol (2021) 32:e2290. doi: 10.1002/rmv.2290
164. Salvi V, Nguyen HO, Sozio F, Schioppa T, Gaudenzi C, Laffranchi M, et al. SARS-CoV-2-associated ssRNAs activate inflammation and immunity via TLR7/8. JCI Insight (2021) 6(18):e150542. doi: 10.1172/jci.insight.150542
165. Bortolotti D, Gentili V, Rizzo S, Schiuma G, Beltrami S, Strazzabosco G, et al. TLR3 and TLR7 RNA sensor activation during SARS-CoV-2 infection. Microorganisms (2021) 9(9):1820. doi: 10.3390/microorganisms9091820
166. Florindo HF, Kleiner R, Vaskovich-Koubi D, Acúrcio RC, Carreira B, Yeini E, et al. Immune-mediated approaches against COVID-19. Nat Nanotechnol (2020) 15(8):630–45. doi: 10.1038/s41565-020-0732-3
167. van der Made CI, Simons A, Schuurs-Hoeijmakers J, van den Heuvel G, Mantere T, Kersten S, et al. Presence of genetic variants among young men with severe COVID-19. JAMA (2020) 324(7):663–73. doi: 10.1001/jama.2020.13719
168. Asano T, Boisson B, Onodi F, Matuozzo D, Moncada-Velez M, Maglorius Renkilaraj MRL, et al. X-Linked recessive TLR7 deficiency in ~1% of men under 60 years old with life-threatening COVID-19. Sci Immunol (2021) 6(62). doi: 10.1126/sciimmunol.abl4348
169. Spiering AE, de Vries TJ. Why females do better: The X chromosomal TLR7 gene-dose effect in COVID-19. Front Immunol (2021) 12:756262. doi: 10.3389/fimmu.2021.756262
170. Carrel L, Cottle AA, Goglin KC, Willard HF. A first-generation X-inactivation profile of the human X chromosome. Proc Natl Acad Sci U S A (1999) 96(25):14440–4. doi: 10.1073/pnas.96.25.14440
171. Berletch JB, Yang F, Xu J, Carrel L, Disteche CM. Genes that escape from X inactivation. Hum Genet (2011) 130(2):237–45. doi: 10.1007/s00439-011-1011-z
172. Tukiainen T, Villani A-C, Yen A, Rivas MA, Marshall JL, Satija R, et al. Landscape of X chromosome inactivation across human tissues. Nature (2017) 550(7675):244–8. doi: 10.1038/nature24265
173. Shabbir S, Hafeez A, Rafiq MA, Khan MJ. Estrogen shields women from COVID-19 complications by reducing ER stress. Med Hypotheses (2020) 143:110148. doi: 10.1016/j.mehy.2020.110148
174. Pegiou S, Rentzeperi E, Koufakis T, Metallidis S, Kotsa K. The role of sexual dimorphism in susceptibility to SARS-CoV-2 infection, disease severity, and mortality: facts, controversies and future perspectives. Microbes Infect (2021) 23(9–10):104850. doi: 10.1016/j.micinf.2021.104850
175. Komi J, Lassila O. Nonsteroidal anti-estrogens inhibit the functional differentiation of human monocyte-derived dendritic cells. Blood (2000) 95(9):2875–82. doi: 10.1182/blood.V95.9.2875.009k12_2875_2882
176. Leng Z, Zhu R, Hou W, Feng Y, Yang Y, Han Q, et al. Transplantation of ACE2(-) mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Dis (2020) 11(2):216–28. doi: 10.14336/AD.2020.0228
177. Gordon JL, Rubinow DR, Thurston RC, Paulson J, Schmidt PJ, Girdler SS. Cardiovascular, hemodynamic, neuroendocrine, and inflammatory markers in women with and without vasomotor symptoms. Menopause (2016) 23(11):1189–98. doi: 10.1097/GME.0000000000000689
178. Thurston RC, Chang Y, Barinas-Mitchell E, Jennings JR, von Känel R, Landsittel DP, et al. Physiologically assessed hot flashes and endothelial function among midlife women. Menopause (2018) 25(11):1354–61. doi: 10.1097/GME.0000000000001239
179. van Dijk GM, Maneva M, Colpani V, Dhana K, Muka T, Jaspers L, et al. The association between vasomotor symptoms and metabolic health in peri- and postmenopausal women: a systematic review. Maturitas (2015) 80(2):140–7. doi: 10.1016/j.maturitas.2014.11.016
180. Muka T, Oliver-Williams C, Colpani V, Kunutsor S, Chowdhury S, Chowdhury R, et al. Association of vasomotor and other menopausal symptoms with risk of cardiovascular disease: A systematic review and meta-analysis. PloS One (2016) 11(6):e0157417. doi: 10.1371/journal.pone.0157417
181. Адамян Л.В., Андреева Е.Н., Аполихина И.А., Артымук Н.В., Ашрафян Л.А. (2021) БВЕ. No Title. Клинические рекомендации РОАГ Менопауза и климактерическое состояние у женщины
182. Stuenkel CA, Davis SR, Gompel A, Lumsden MA, Murad MH, Pinkerton JV, et al. Treatment of symptoms of the menopause: An endocrine society clinical practice guideline. J Clin Endocrinol Metab (2015) 100(11):3975–4011. doi: 10.1210/jc.2015-2236
183. Armeni E, Lambrinoudaki I, Ceausu I, Depypere H, Mueck A, Pérez-López FR, et al. Maintaining postreproductive health: A care pathway from the European menopause and andropause society (EMAS). Maturitas (2016) 89:63–72. doi: 10.1016/j.maturitas.2016.04.013
184. Baber RJ, Panay N, Fenton A. 2016 IMS recommendations on women’s midlife health and menopause hormone therapy. Climacteric (2016) 19(2):109–50. doi: 10.3109/13697137.2015.1129166
185. Pinkerton JAV, Aguirre FS, Blake J, Cosman F, Hodis H, Hoffstetter S, et al. The 2017 hormone therapy position statement of the north American menopause society. Menopause (2017) 24(7):728–53. doi: 10.1097/GME.0000000000000921
186. Formoso G, Perrone E, Maltoni S, Balduzzi S, Wilkinson J, Basevi V, et al. Short-term and long-term effects of tibolone in postmenopausal women. Cochrane Database Syst Rev (2016) 10(10):CD008536. doi: 10.1002/14651858.CD008536.pub3
188. Hirschberg AL, Bitzer J, Cano A, Ceausu I, Chedraui P, Durmusoglu F, et al. Topical estrogens and non-hormonal preparations for postmenopausal vulvovaginal atrophy: An EMAS clinical guide. Maturitas (2021) 148:55–61. doi: 10.1016/j.maturitas.2021.04.005
189. Lumsden MA, Davies M, Sarri G. Diagnosis and management of menopause: The national institute of health and care excellence (NICE) guideline. JAMA Intern Med (2016) 176(8):1205–6. doi: 10.1001/jamainternmed.2016.2761
190. Sanchez-Rodriguez D, Bergmann P, Body JJ, Cavalier E, Gielen E, Goemaere S, et al. The Belgian bone club 2020 guidelines for the management of osteoporosis in postmenopausal women. Maturitas (2020), 139:69–89. doi: 10.1016/j.maturitas.2020.05.006
191. Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of breast cancer: nested case-control studies using the QResearch and CPRD databases. BMJ (2020) 371:m3873. doi: 10.1136/bmj.m3873
192. Lyytinen H, Pukkala E, Ylikorkala O. Breast cancer risk in postmenopausal women using estradiol-progestogen therapy. Obstet Gynecol (2009) 113(1):65–73. doi: 10.1097/AOG.0b013e31818e8cd6
193. Moro E, Degli Esposti E, Borghese G, Manzara F, Zanello M, Raimondo D, et al. The impact of hormonal replacement treatment in postmenopausal women with uterine fibroids: A state-of-the-Art review of the literature. Medicina (Kaunas) (2019) 55(9):549. doi: 10.3390/medicina55090549
194. Mehta J, Kling JM, Manson JAE. Risks, benefits, and treatment modalities of menopausal hormone therapy: Current concepts. Front Endocrinol (Lausanne) (2021) 12:1–14. doi: 10.3389/fendo.2021.564781
195. Chlebowski RT, Anderson GL, Aragaki AK, Manson JE, Stefanick ML, Pan K, et al. Association of menopausal hormone therapy with breast cancer incidence and mortality during long-term follow-up of the women’s health initiative randomized clinical trials. JAMA (2020) 324(4):369–80. doi: 10.1001/jama.2020.9482
196. Hamoda H, Panay N, Pedder H, Arya R, Savvas M. The British menopause society & women’s health concern 2020 recommendations on hormone replacement therapy in menopausal women. Post Reprod Heal (2020) 26(4):181–209. doi: 10.1177/2053369120957514
197. Okano H, Higuchi T, Kurabayashi T, Makita K, Mizunuma H, Mochizuki Y, et al. Japan Society of obstetrics and gynecology and Japan society for menopause and women’s health 2017 guidelines for hormone replacement therapy. J Obstet Gynaecol Res (2018) 44(8):1355–68. doi: 10.1111/jog.13684
198. Ortmann O, Beckermann MJ, Inwald EC, Strowitzki T, Windler E, Tempfer C. Peri- and postmenopause–diagnosis and interventions interdisciplinary S3 guideline of the association of the scientific medical societies in Germany (AWMF 015/062): short version. Arch Gynecol Obstet (2020) 302(3):763–77. doi: 10.1007/s00404-020-05682-4
199. Armeni E, Cano A, Rees M, Lambrinoudaki I. Menopausal hormone therapy and breast cancer risk: Individualization is the key to safety. Maturitas (2020) 141:85–6. doi: 10.1016/j.maturitas.2020.08.001
200. Boardman HMP, Hartley L, Eisinga A, Main C, Roqué i Figuls M, Bonfill Cosp X, et al. Hormone therapy for preventing cardiovascular disease in post-menopausal women. Cochrane Database Syst Rev (2015) 3):CD002229. doi: 10.1002/14651858.CD002229.pub4
201. Liu JH. What providers need to know about progestogens in hormone therapy. Menopause (2020) 28(3):325–6.
202. Miller VM, Taylor HS, Naftolin F, Manson JE, Gleason CE, Brinton EA, et al. Lessons from KEEPS: the kronos early estrogen prevention study. Climacteric (2021) 24(2):139–45. doi: 10.1080/13697137.2020.1804545
203. Hodis HN, Mack WJ, Henderson VW, Shoupe D, Budoff MJ, Hwang-Levine J, et al. Vascular effects of early versus late postmenopausal treatment with estradiol. N Engl J Med (2016) 374(13):1221–31. doi: 10.1056/NEJMoa1505241
204. Santen RJ, Pinkerton JV, Liu JH, Matsumoto AM, Lobo RA, Davis SR, et al. Workshop on normal reference ranges for estradiol in postmenopausal women, September 2019, Chicago, Illinois. Menopause (2020) 27(6):614–24. doi: 10.1097/GME.0000000000001556
205. Kim S-M, Kim SE, Lee D-Y, Choi D. Serum estradiol level according to dose and formulation of oral estrogens in postmenopausal women. Sci Rep (2021) 11(1):3585. doi: 10.1038/s41598-021-81201-y
206. The north American menopause society statement on continuing use of systemic hormone therapy after age 65. Menopause (2015) 22(7):693. doi: 10.1097/GME.0000000000000492
207. Kumru S, Godekmerdan A, Yilmaz B. Immune effects of surgical menopause and estrogen replacement therapy in peri-menopausal women. J Reprod Immunol (2004) 63(1):31–8. doi: 10.1016/j.jri.2004.02.001
208. Rachoń D, Myśliwska J, Suchecka-Rachoń K, Wieckiewicz J, Myśliwski A. Effects of oestrogen deprivation on interleukin-6 production by peripheral blood mononuclear cells of postmenopausal women. J Endocrinol (2002) 172(2):387–95. doi: 10.1677/joe.0.1720387
209. Puder JJ, Freda PU, Goland RS, Wardlaw SL. Estrogen modulates the hypothalamic-pituitary-adrenal and inflammatory cytokine responses to endotoxin in women. J Clin Endocrinol Metab (2001) 86(6):2403–8. doi: 10.1210/jc.86.6.2403
210. Lovre D, Peacock E, Katalenich B, Moreau C, Xu B, Tate C, et al. Conjugated estrogens and bazedoxifene improve β cell function in obese menopausal women. J Endocr Soc (2019) 3(8):1583–94. doi: 10.1210/js.2019-00074
211. Mauvais-Jarvis F, Manson JE, Stevenson JC, Fonseca VA. Menopausal hormone therapy and type 2 diabetes prevention: Evidence, mechanisms, and clinical implications. Endocr Rev (2017) 38(3):173–88. doi: 10.1210/er.2016-1146
212. Abdi F, Mobedi H, Mosaffa N, Dolatian M, Ramezani Tehrani F. Effects of hormone replacement therapy on immunological factors in the postmenopausal period. Climacteric (2016) 19(3):234–9. doi: 10.3109/13697137.2016.1164136
213. Fruzzetti F, Cagnacci A, Primiero F, De Leo V, Bastianelli C, Bruni V, et al. Contraception during coronavirus-covid 19 pandemia. recommendations of the board of the Italian society of contraception. Eur J Contracept Reprod Heal Care Off J Eur Soc Contracept (2020) 25(3):231–2. doi: 10.1080/13625187.2020.1766016
214. Ramírez I, de la Viuda E, Baquedano L, Coronado P, Llaneza P, Mendoza N, et al. Managing thromboembolic risk with menopausal hormone therapy and hormonal contraception in the COVID-19 pandemic: Recommendations from the Spanish menopause society, sociedad española de ginecología y obstetricia and sociedad española de trombosis y hemo. Maturitas (2020) 137:57–62. doi: 10.1016/j.maturitas.2020.04.019
215. Gargaglioni LH, Marques DA. Let’s talk about sex in the context of COVID-19. J Appl Physiol (2020) 128(6):1533–8. doi: 10.1152/japplphysiol.00335.2020
216. Sund M, Fonseca-Rodríguez O, Josefsson A, Welen K, Fors Connolly A-M. Association between pharmaceutical modulation of oestrogen in postmenopausal women in Sweden and death due to COVID-19: a cohort study. BMJ Open (2022) 12(2):e053032. doi: 10.1136/bmjopen-2021-053032
217. Traish AM. Sex steroids and COVID-19 mortality in women. Trends Endocrinol Metab (2021) 32(8):533–6. doi: 10.1016/j.tem.2021.04.006
218. Bastard P, Rosen LB, Zhang Q, Michailidis E, Hoffmann H-H, Zhang Y, et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science (2020) 370(6515):eabd4585.
219. Zhang Q, Bastard P, Liu Z, Le Pen J, Moncada-Velez M, Chen J, et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science (2020) 370(6515):eabd4570.
Keywords: sex hormones, steroids, immune system, menopausal hormone therapy, cytokines
Citation: Averyanova M, Vishnyakova P, Yureneva S, Yakushevskaya O, Fatkhudinov T, Elchaninov A and Sukhikh G (2022) Sex hormones and immune system: Menopausal hormone therapy in the context of COVID-19 pandemic. Front. Immunol. 13:928171. doi: 10.3389/fimmu.2022.928171
Received: 25 April 2022; Accepted: 11 July 2022;
Published: 02 August 2022.
Edited by:
Eva Reali, University of Milano-Bicocca, ItalyReviewed by:
Anna Lisa Giuliani, University of Ferrara, ItalyEleni Armeni, Aretaieion Hospital, Greece
Charles Wira, Dartmouth University, United States
Copyright © 2022 Averyanova, Vishnyakova, Yureneva, Yakushevskaya, Fatkhudinov, Elchaninov and Sukhikh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Polina Vishnyakova, dmlzaG55YWtvdmFwb2xpbmFAZ21haWwuY29t
 Marina Averyanova
Marina Averyanova Polina Vishnyakova1,2*
Polina Vishnyakova1,2* Svetlana Yureneva
Svetlana Yureneva Oksana Yakushevskaya
Oksana Yakushevskaya Timur Fatkhudinov
Timur Fatkhudinov Andrey Elchaninov
Andrey Elchaninov Gennady Sukhikh
Gennady Sukhikh