
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol. , 04 July 2022
Sec. Cancer Immunity and Immunotherapy
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.927233
This article is part of the Research Topic New Strategies to Boost Tumor Immunotherapy: from the Perspective of Neutrophil Metabolic Heterogeneity View all 8 articles
In cancer, neutrophils are an important part of the tumour microenvironment (TME). Previous studies have shown that circulating and infiltrating neutrophils are associated with malignant progression and immunosuppression in gliomas. However, recent studies have shown that neutrophils have an antitumour effect. In this review, we focus on the functional roles of neutrophils in the circulation and tumour sites in patients with glioma. The mechanisms of neutrophil recruitment, immunosuppression and the differentiation of neutrophils are discussed. Finally, the potential of neutrophils as clinical biomarkers and therapeutic targets is highlighted. This review can help us gain a deeper and systematic understanding of the role of neutrophils, and provide new insights for treatment in gliomas.
Gliomas are heterogeneous and primary malignant tumour in the brain. Glioblastoma (GBM) is the most lethal form of glioma, accounting for 70-75% of all diagnoses of diffuse glioma and having a median overall survival (OS) time of 14-17 months (1). The current standard of treatment includes maximal surgical resection and combined radiochemotherapy (2, 3). The significance of Stupp protocol has become the standard of care for the treatment of GBM. It consists of radiotherapy and concomitant chemotherapy with temozolomide, an alkylating agent (4). Over the years, many arts have been improved to aid the surgeon in the resection about the brain cancer. Improvements such as surgical microscopes, high-resolution imaging, fluorescence-guided surgery and neuronavigation are widely used in glioma treatment (5–7). Despite aggressive treatment strategies over the past few decades, the OS of glioma patients has not improved significantly due to the rapid proliferation, extensive invasion, and treatment resistance of gliomas (8). GBM tumours are highly resistant to treatment and the resistance can be explained by characteristics of TME (9). The GBM microenvironment contains many different non-cancerous cell types in addition to cancer cells, including endothelial cells, pericytes, fibroblasts and immune cells. These cells interact with one another and with tumour cells to perpetuate brain tumour growth (10). A state of immunosuppression characterizes GBM’s TME, thanks to the secretion of several cytokines by tumour cells, microglia, and tumour associated macrophages (TAMs) (11). In contrast to other immune cells, comparatively less is known about the contributions of neutrophils.
Neutrophils play various roles in different diseases. Neutrophils exert antimicrobial and inflammatory functions through phagocytosis, degranulation, release of neutrophil extracellular traps (NETs) and antigen presentation (12, 13). Neutrophils release decondensed DNA fibres and antimicrobial peptides, known as NETs (14). These web-like structures trap and kill different bacteria (14), fungi (15, 16), and parasites (17). At present, the importance and role of neutrophils in cancer have increased over the past decades (18). And neutrophils play an oncogenic role primarily by increasing DNA damage, angiogenesis and immunosuppression (19). The association between tumour initiation and progression, cancer-associated thrombosis and NETs has been reported (20–24).
Increasing evidence reveals that the numbers of circulating and tumour-infiltrating neutrophils are relevant to immunosuppression, poor survival and a poor prognosis in patients with cancer (25–27). However, the role of neutrophils in cancer is a controversial issue. The results of many studies have shown that tumour-associated neutrophils (TANs) are able to stimulate tumour cell migration and invasion (28–30). Conversely, findings from many other studies have suggested that TANs have various antitumour properties, such as direct cytotoxicity against tumour cells and inhibition of metastasis (31–33). Additionally, neutrophil classification in the TME, such as N1/N2 neutrophils and polymorphonuclear myeloid-derived suppressor cells (PMN-MDSCs), is also a controversial topic (34, 35). In gliomas, Using immuno-histochemical analysis of glioma sections, Fossati G et al. reported that neutrophil infiltration into tumours was significantly correlated with glioma grade (36). Subsequently, researchers found that increased neutrophil recruitment during antiangiogenic therapy promoted glioma progression and might promote treatment resistance (37). In addition, studies have found that the neutrophil-to-lymphocyte ratio (NLR) of patients with glioma is valuable for prognosis and diagnosis (38). We reviewed the recent association of neutrophils with gliomas and found that gliomas are characterized by an immunosuppressive TME. Pathologically activated neutrophils, called PMN-MDSCs, are a type of myeloid-derived suppressor cell (MDSC) and one of the major contributors to the immunosuppressive properties of gliomas (39, 40). As a consequence, neutrophils are now the subject of intense research in gliomas.
However, our understanding of the roles of neutrophils in gliomas is still limited to date. This article aims to review neutrophil research in cancer patients. The search was focused on the association of circulating neutrophils and tumour-infiltrating neutrophils with prognosis in glioma patients. A PubMed search using the keywords “neutrophils”, “gliomas”, “tumour microenvironment”, “myeloid-derived suppressor cells” and “neutrophil-to-lymphocyte ratio” was performed. Reference lists were then searched for additional articles. Available data were obtained from patients with glioma to elucidate the roles of neutrophils with various phenotypes in gliomas. In addition, we dissected the pathways that mediate the transport of neutrophils to the tumour site, described their role once they arrived in the tumour microenvironment, and integrated this with the current understanding of glioma progression. A vast body of evidence supports the importance of the neutrophils in the progression of gliomas, and the possibility of neutrophils in the treatment of glioma is further discussed in this paper in combination with recent studies. Therefore, elucidating the mechanisms by which glioma cells interact with neutrophils can uncover multiple potential therapeutic targets for clinical applications.
Neutrophils are derived from the bone marrow and give rise to multiple granulocytic immune cell subsets (41). In a steady state, normal adults produce more than 1×1011 neutrophils per day (42). Neutrophils have long been considered as cells playing a crucial role in the immune system. They participate in the inflammatory response in the body and are the first line of defense against pathogen invasion (12). Inflammation responds to infection and carries out wound healing and tissue regeneration. Inflammation plays an important role in protecting the body. However, chronic inflammation induces cancer by destroying tissues. For example, chronic hepatitis increases the risk of liver cancer (43). Neutrophils provide a link between inflammation and cancer.
In recent years, researchers found neutrophils within tumours in the majority of solid tumour samples (44). Several studies have revealed a correlation between the presence of neutrophils and a poor prognosis in patients with early-stage melanoma, head and neck cancer or hepatocellular carcinoma and demonstrated that the presence of neutrophils is independently associated with a poor prognosis (45–47). In an in-depth study of neutrophils, it was found that neutrophils are an important component of the TME (48). In the TME, neutrophils have varied functions and have been classified using different terms, including N1/N2 neutrophils, TANs, and PMN-MDSCs (49–51). In 2009, Fridlender et al. classified the types of antitumorigenic and protumorigenic TANs, named N1 and N2, respectively. They showed that transforming growth factor-β (TGF-β), an immunosuppressive cytokine overexpressed by tumour cells, polarized neutrophils into a protumorigenic phenotype (N2) and that neutrophil depletion caused a small decrease in tumour growth in mouse models. However, the presence of interferon β (IFN-β) or blockade of TGF-β with SM16, an oral inhibitor of TGF–β receptor kinase, led to the aggregation of neutrophils with an antitumorigenic phenotype (N1) (52, 53). In this case, TANs depletion led to increased tumour growth (35, 54). Despite the existence of functional differences, no definitive surface markers have been identified to distinguish N1 and N2 TANs (35). Although there is no obvious surface marker of N1/N2 at present, The classification of N1 and N2 used to refer to antitumour and protumour neutrophils is important for our understanding of the role of neutrophils in tumours. We hope that interested readers can conduct follow-up studies to distinguish the N1/N2 classification of neutrophils.
TANs have important roles in cancer initiation and progression, and high densities of neutrophils are correlated with more advanced-stage disease in patients with gastric cancer and are more likely to be detected in more aggressive pancreatic tumours (55, 56). Several studies involving patients with early-stage melanoma, head and neck cancer, and hepatocellular carcinoma have revealed a correlation between presence of TANs and a poor prognosis (45–47). However, other papers of mouse metastatic renal cell carcinoma models have highlighted the antitumour potential of neutrophils. The antitumour neutrophils recruited to the lung by tumour-secreted chemokines build an antimetastatic barrier (54, 57). Hepatocyte growth factor/MET proto-oncogene-dependent nitric oxide release by antitumour neutrophils promotes cytotoxicity, which abates mouse Murine Lewis lung carcinoma cells, melanoma cells and human non-small-cell lung carcinoma cells growth and metastasis (58). Interestingly, in the colorectal cancer (CRC), the prognostic relevance of TANs is controversial. Rao H.L et al. discovered that the presence of CD66b+ neutrophils detected in 229 CRC patients using tissue microarray and immunohistochemistry. And neutrophils were identified as an independent factor for a poor prognosis in patients with CRC (59). In contrast, data from early stages of colon cancers patients have suggested that infiltration of CD66b+ neutrophils in the tumour front is associated with a favourable prognosis in patients with colon cancers (60). The differences in the conclusions of these studies may differ from the selected study patients, which included only colon cancers and not rectal cancers in the second study. In addition, manual counting of neutrophils according to their morphology may influence the results. The role of neutrophils in lung cancer is also controversial. In a study involving patients with early-stage (stage I–III) non-small cell lung carcinoma (NSCLC), high CD66b+ neutrophil density had a significantly effect on increased relapse following surgical resection and had a trend toward decreased OS (61). The presence of CD66b+ TANs show diverging prognostic effect in NSCLC patients according to histological subgroups. CD66b+ TANs described as a positive prognostic factor in patients with squamous cell carcinoma but an adverse prognostic factor in those with adenocarcinoma (62). Since there is no consensus on methods for staining and identifying neutrophils in cancer tissues, the prognostic implications of neutrophil infiltration in these patients clearly require further investigation.
Apart from the TANs, when describing the role and importance of neutrophils in cancer, PMN-MDSCs cannot be ignored. In 2007, MDSCs were confirmed and defined with this canonical name. MDSCs are a heterogeneous population of immature myeloid cells with immunosuppressive functions. Granulocytic or polymorphonuclear MDSCs (G/PMN-MDSCs), early-stage MDSCs (eMDSCs) and monocytic MDSCs (M-MDSCs) are the main types of MDSCs that have been detected (34, 63). Based on the typical suppressive functional characteristics of MDSCs, it has been suggested that PMN-MDSCs are a population of neutrophils with immunosuppressive activity (29, 64, 65). MDSC production follows the same differentiation pathway as the production of neutrophils and monocytes, both of which are produced by granulocyte colony-stimulating factor (G-CSF) and macrophage colony-stimulating factor (M-CSF) stimulation (39, 66, 67). Accumulating evidence indicates that the ability to suppress T cells is an important characteristic of MDSCs. The potent immunosuppressive activity of MDSCs is the reason that the function of MDSCs is different from that of monocytes and neutrophils. In addition, mature neutrophils (CD14- CD15+ CD66b+ CD16+) express specific cell-surface proteins (68). In mice, SiglecFhigh CD11b+ Ly-6G+ Gr-1+ cells resemble neutrophils (69). MDSCs are generally characterized as expressing the myeloid lineage differentiation antigen Gr-1 (Ly6G and C); CD11b, M-MDSCs typically have the phenotype CD11b+ Ly6Chigh Ly6G−; and PMN-MDSCs are typically defined as CD11b+ Ly6Clow Ly6G+ (70). A more complex panel of markers is typically used to identify human MDSCs (CD11b, CD14, CD15, CD66b, HLA-DR and CD33), M-MDSCs (CD14+ CD15- HLA-DR−/low) and PMN-MDSCs (CD14- CD15+ CD66b+ CD16+ CD11b+ CD33+ HLA-DR-) (71–73) (The markers summarized in Table 1). These markers have also been shown to be expressed by neutrophils. Therefore, we have concluded that the term PMN-MDSCs actually describes a subset of neutrophils until more definitive evidence is found.
Circulating neutrophils are non-negligible component of the inflammation, which plays important roles in cancer development and progression (74). The NLR, a systemic cellular inflammation marker, is a noninvasive biomarker for patients with cancer. We calculated the NLR as follows: NLR = neutrophil count/lymphocyte count (75). The NLR is a low-cost method, as lymphocyte and neutrophil counts can be easily derived using the common complete blood count (76). The NLR has become a prognostic indicator for survival in many tumour types, including CRC, hepatocellular carcinoma, breast cancer and gliomas (38, 77–79).
Concerning gliomas, the NLR is a widely used parameter for diagnosis and OS prediction (80, 81). The approach has shown diagnostic value in differentiating isocitrate dehydrogenase-mutant (IDH-mt) GBM from IDH-wild-type (IDH-wt) GBM (82). Auezova et al. found lower NLR values in patients with IDH-mt GBM (83). A systematic review found that high NLR values were associated with lower overall survival and that patients with a high NLR value were associated with high-grade gliomas (38). In addition, a retrospective review reported that a lower NLR was associated with longer OS during focal radiotherapy and concomitant temozolomide treatment (84). However, NLR can potentially be affected by bacterial or viral infections or drug treatments (85). For example, bacterial infections and steroid usage can increase neutrophil counts, while viral infections may increase lymphocyte counts. The effects of acute disease conditions on NLR may overlap with chronic persistent inflammation. In addition, hypertension, diabetes mellitus, metabolic syndrome, left ventricular dysfunction and hypertrophy, acute coronary syndromes, cardiovascular diseases, abnormal thyroid function tests, renal or hepatic dysfunction, previous history of infection (<3 months), inflammatory diseases, and some medications (e.g. steroids) can potentially affect the measurement of NLR (76). Therefore, the measurement of NLR should consider the potential effects of other conditions or drug use.
The baseline neutrophil count is a current biomarker used to predict the efficacy of bevacizumab in the treatment of GBM (86). It has been found that an increased NLR has been associated with increased peritumoral infiltration of macrophages and upregulation of several cytokines, such as interleukin (IL)-6, IL-7, IL-8, IL-9, IL-12, IL-17, and IFNγ (87, 88). In the study of the immunosuppressive effect of GBM patient peripheral blood, it was found that peripheral cellular immunosuppression in GBM patients is correlated with increased neutrophil degranulation and elevated levels of serum arginase I (As shown on the right side of Figure 1) (98). Neutrophil degranulation is the process by which neutrophil cytoplasmic granules fuse with the cell membrane or phagosomal membrane, leading to the exocytosis of soluble granule proteins or exposure of membrane granule proteins to the cell surface (99). And arginase I is a factor known to be present within in granulocytes and has immunosuppressive activity (98). Arginase I expression suppress T cell function in patients with GBM and T cell function can be restored by targeting serum arginase I (96). Correlations of phenotypic characteristics between neutrophils in the blood and high-grade tumours have recently been reported. When compared to healthy controls, individuals with glioma expressed a few activation markers (CD11b, CD16, CD54, and CD63) and L-selectin (CD62L) at lower levels on neutrophils. Moreover, neutrophils showed higher expression of the surface receptor CD16 in the context of grade III gliomas in GBM (100). Activation of neutrophils expressing CD11b is an early predictor of tumour progression in GBM patients (97).
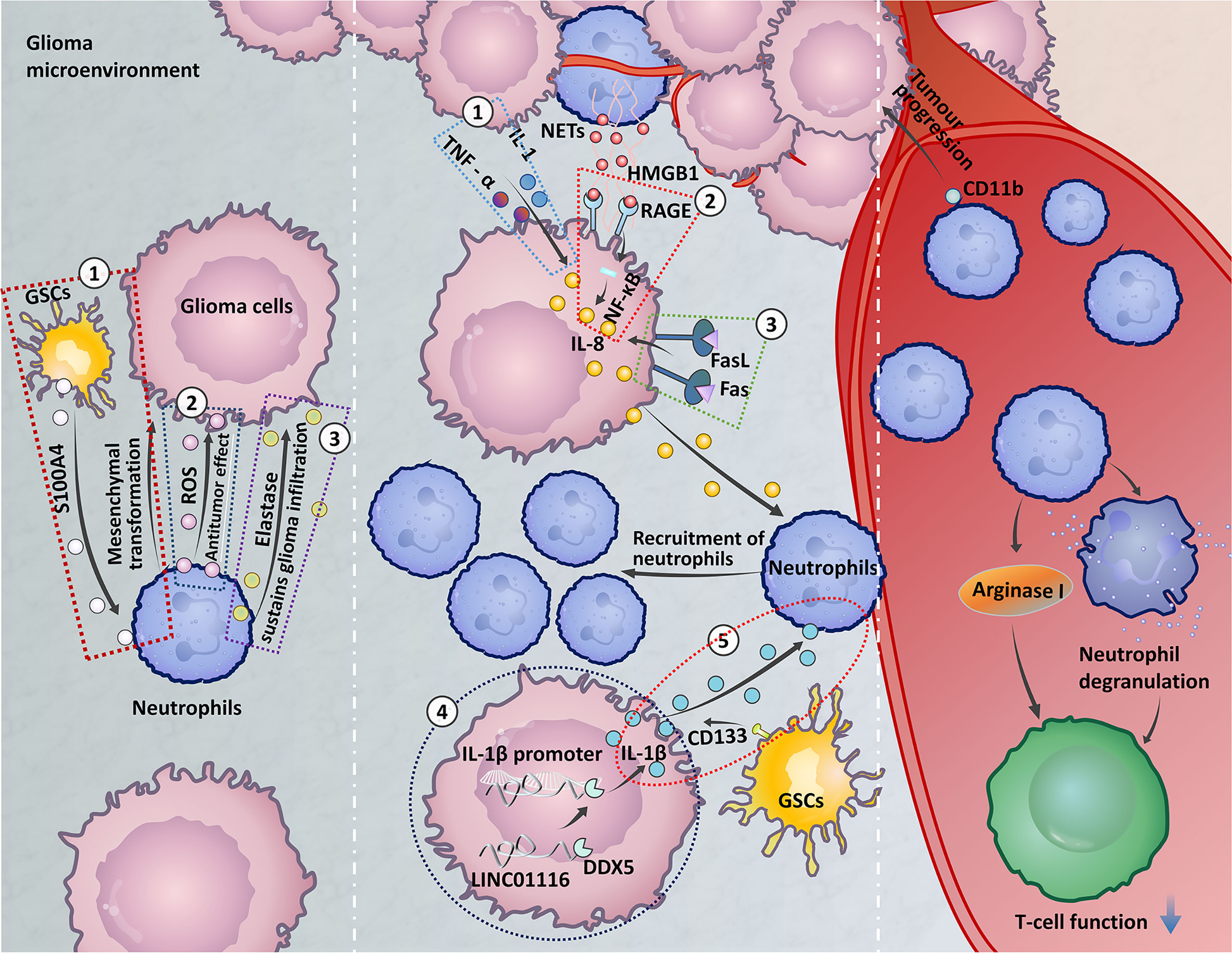
Figure 1 The role of circulating neutrophils and neutrophils at the tumour site. Left: ① Glioma stem cells (GSCs) express S100A4 to promote the mesenchymal transition of glioma cells (37). ② The release of reactive oxygen species (ROS) by neutrophils in the early stage of glioma development may be related to the antitumour neutrophil effect (89). ③ Neutrophils secrete elastase, which destroys brain tissue and aids glioma invasion (90). Middle: ① Astrocytoma and GBM cells express IL-1 and TNF and high levels of IL-8 under alpha stimulation, which recruit neutrophils (91). ② Neutrophils form high-mobility group box 1 (HMGB1) and bind to receptor for advanced glycation end products (RAGE) expressed in glioma tissues, activates the NF-κB signaling pathway to secrete IL-8, and promote neutrophil infiltration (92). ③ Expression of FasL on glioma cells activates Fas signaling in the TME to express IL-8, leading to neutrophil aggregation (93). ④ LINC01116 promotes the expression of IL-1β by recruiting the transcription regulator DDX5 to the IL-1β promoter, which promotes the recruitment of neutrophils (94). ⑤ The ectopic expression of CD133 induces an increase in IL-1β expression, which causes neutrophils to aggregate in the TME (95). Right: Neutrophil degranulation and elevated levels of serum arginase I induce immunosuppression in GBM patients (96). Neutrophil expression of CD11b is an early predictor of tumour progression (97).
Taken together, these findings illustrate that circulating neutrophils play important roles in the diagnosis, OS, immunosuppression, tumour growth promotion, and treatment resistance of patients with glioma. Data demonstrate the association between elevated peripheral blood NLR levels and increased TANs infiltration (101). However, the molecular mechanisms by which the NLR is associated with TANs remain unknown.
The normal brain has traditionally been recognized as an immune-privileged organ due to the presence of the endothelial blood-brain barrier (BBB) and the absence of a conventional lymphatic system (102). However, this viewpoint has recently been challenged, as even in the presence of an intact BBB, adaptive immune cells can traffic into the central nervous system (CNS) (103). Functional lymphatic vessels lining the dural sinuses were recently reported. These structures can carry immune cells from the cerebrospinal fluid and are connected to the cervical lymph nodes (104). Kipnis et al. described the cellular and molecular orchestration of the dural sinuses as a unique interface where the CNS and the immune system communicate with one another (105). Indeed, cells from the bone marrow, including neutrophils and monocytes, may directly from nearby bone marrow cavities in the skull and vertebrae (106–108). Moreover, in certain brain tumours, BBB dysfunction can be accompanied by increased leukocyte infiltration from the peripheral circulation (109). Nonetheless, the microenvironment of the glioma is generally immunosuppressive, with essentially no trafficking or patrolling by peripheral immune cells (110).
GBM cells produce cytokines, chemokines, growth factors and extra-cellular matrix modifying enzymes, extracellular vesicles and proteins to construct a favourable tumour microenvironment (111). Also, cells in TME interact with each other and with the neoplastic cells through different suppressor receptors like programmed cell death protein-1 (PD-1), T-lymphocyte-associated protein 4 (CTLA-4), CD70 and gangliosides that increase the tumour immune escaping (112, 113). The modulation of these cell populations in the brain TME could improve the efficacy of immunotherapy against brain malignancies.
In the case of glioma, the inflammation-enriched TME has many tumour-promoting effects (114). The glioma microenvironment exhibits a diverse immune cell landscape with substantial infiltration of resident microglia (115), circulating blood monocytes (macrophages) (116), dendritic cells (DCs), lymphoid cells, and neutrophils (117, 118). Microglia are tissue-resident macrophages that arise from embryonic yolk sac precursors (119). These cells regulate the innate immune response in the brain and play a major role in normal brain development (120–122). Few studies have investigated other populations of immune cells in the brain. Indeed, a recent discovery identified small populations of T cells and B cells that regulate microglial maturation and promote oligodendrocyte precursor cell proliferation, respectively (123, 124). TAMs consist of bone marrow-derived macrophages and tissue-resident microglia (125). In GBM, TAMs have a protumour role, and increased TAM accumulation is associated with increased tumour grade (126–129). There is increasing evidence that TAMs promote glioma growth and invasion (130). DCs are myeloid-derived cells that can stimulate T lymphocytes and natural killer (NK) cells to become potent antitumour effectors (131). Recent studies have reported the clinical effectiveness of DC-based vaccine therapy in malignant glioma (132). T cells, B cells, and NK cells migrate through the lymphatic system. Low levels of CD4+ T helper (Th) cells and CD8+ cytotoxic T lymphocytes (CTLs) within the T cell population have been shown to infiltrate gliomas (133). High levels of CD8+ CTLs are commonly regarded as having antitumoral activity, whereas high levels of CD4+ Th cells are related to favouring tumour development (134). NK cells are known to play a role in the apoptotic killing of both tumour cells and virus-infected cells (134). The role of B cells in glioma development is unclear. A comprehensive understanding of the complex glioma microenvironment will greatly expand the range of therapeutic strategies for this deadly disease.
Growing evidence has highlighted the role of neutrophils in promoting tumour progression in the brain TME. Neutrophil functions in the glioma microenvironment are described in more detail below. The modulation of neutrophils in the brain TME could improve the efficacy of therapy against brain malignancies.
Neutrophils are generated under steady-state conditions from haematopoietic stem and progenitor cells in the bone marrow. However, during infection or cancer, neutrophils are used up in large quantities, and the steady-state condition is converted to emergency granulopoiesis (135). In mouse models and patients with invasive cancer, the spleen also produces neutrophils during cancer progression (136). Growth factors (G-CSF and granulocyte-macrophage colony-stimulating factor (GM-CSF)) and inflammatory cytokines (IL-6, IL-1β, and IL-17) produced by tumour cells, tumour-associated stromal cells, and tumour-infiltrating leukocytes (including T cells) can modulate haematopoiesis (48). G-CSF is the principal cytokine regulating neutrophil generation and differentiation (137, 138). In addition to G-CSF, stem cell factor, IL-6, and GM-CSF induce an increase in neutrophils (139–141). The chemokine receptors CXCR1 and CXCR2 are expressed by neutrophils, and activation of these receptors is key to neutrophil recruitment. Tumour-infiltrating leukocytes, endothelial cells, and fibroblasts express the CXC chemokine ligands CXCL1, CXCL2, CXCL5, CXCL6, and CXCL8 (also known as IL-8) (142, 143). The chemokine receptor CXCR2 was originally found to be expressed on neutrophils (144). In mouse colon cancer models, the chemokines CXCL1, CXCL2, and CXCL5 are CXCR2 ligands that are observed to promote neutrophil recruitment (143, 145–147). CXCR2 is also the receptor for IL-8 and mediates neutrophil activation. The expression of CXCR2 proteins in gliomas has been significantly correlated with glioma recurrence (148).
The mechanism of TANs recruitment to gliomas remains limited (In the middle of the Figure 1). IL-8 is associated with the recruitment of neutrophils via the activation of multiple intracellular signalling pathways (149). Glioma cells produce a cytokine-induced neutrophil chemoattractant, IL-8, which attracts granulocytes to the tumour site (150). Astrocytoma and GBM cells express high levels of IL-8 under stimulation with IL-1 and TNF-α, and IL-8 has chemotactic effects on human neutrophils (91). Neutrophils exert their functions through the formation of NETs. In glioma, high-mobility group box 1 (HMGB1) derived from NETs binds to receptor for advanced glycation end products (RAGE) expressed in glioma tissue, activating the NF-κB signalling pathway to promote IL-8 secretion, which promotes neutrophil infiltration (92). In addition, IL-1β is involved in many diseases and tissue inflammation (151). LINC01116, a long noncoding RNA expressed in glioma tissue, can promote IL-1β expression by recruiting the transcriptional regulator DDX5 to the IL-1β promoter. Then, IL-1β expression in glioma cells promotes TANs recruitment (94). CD133 is a surface marker of glioma stem cells (GSCs). Increases in the expression of IL-1β induced by ectopic expression of CD133 recruit neutrophils to the TME and increase neutrophil migration (95). FasL expression on gliomas activates Fas signalling in the TME, and glioma cells express IL-8 in response to Fas activation, which leads to an accumulation of neutrophils (93). In addition, a recent study reported the upregulation of CXCL8, ITGA3, and CXCL17 by brain metastases. These chemokines are involved in neutrophil tissue infiltration. Increased expression of MET was found in neutrophils in brain metastases, and MET has been related to the recruitment of immunosuppressive neutrophils. The increased expression of the cell-surface receptor CD117 was correlated with neutrophil migration and activation (152). Whether these chemokines are involved in neutrophil infiltration in the glioma microenvironment needs to be further investigated.
The TIMER2.0 database, R programming language, and so on have been used to analyse tyrosine protein tyrosine kinase binding protein, and CD96 expression has been correlated significantly with neutrophil infiltration (153, 154). Gene ontology (GO) enrichment analysis and gene set enrichment analysis (GSEA) showed that BLC7A was mainly enriched in neutrophil activation. Immunohistochemical (IHC) analysis revealed that low BCL7A expression was correlated with robust infiltration of neutrophils in gliomas (155). However, more studies are required to determine the underlying mechanisms.
Neutrophils have long been known to be responders in innate and adaptive immune responses that defend against infectious agents (156). Once neutrophils are recruited to the glioma microenvironment, they adopt new cellular and molecular identities.
IHC staining was used to detect neutrophil infiltration in human glioma tissues of different grades. The neutrophil infiltration level was positively correlated with glioma grade (36). In addition to the discovery of neutrophil cells infiltrating glioma tissue, in in vitro coculture models, neutrophils may be partially responsible for enhanced glioma proliferation (Summarized on the left side of Figure 1) (37). Subsequent studies investigating neutrophil function in depth described that neutrophils secrete elastase. Neutrophils elastase is a neutral protease and cytotoxic mediator that can damage brain tissue and aid in glioma invasion (90). Apart from invasion, neutrophils modulate tumour angiogenesis. S100A4 is a novel biomarker expressed in GSCs (157) that induces the tumorigenic activity of neutrophils. Neutrophils promote the mesenchymal transformation of gliomas via increased expression of S100A4 within the gliomas and increase vascularization, which induces resistance to anti-VEGF therapy (37). In mouse tumours, PMN-MDSCs and TANs express Ly6G (158, 159). Radiation-induced infiltrating Ly6G+ neutrophils secrete Nitric oxide (NO) that promotes the activity of the NOS-ID4 signalling axis, which converts GBM cells into GSCs, this conversion is negatively associated with survival and radiation therapy outcomes (160). It is important to note that telomerase reverse transcriptase mutation is accompanied by neutrophil infiltration and neutrophil chemokine expression in the IDH-wt glioma microenvironment, which may be partly responsible for the poor prognosis of IDH-wt gliomas (161). Furthermore, the reduced neutrophil infiltration in IDH-mt gliomas may contribute, in part, to the improved clinical outcomes observed in these patients (162).
Although previous studies have shown that neutrophils contribute to the malignant progression of gliomas, neutrophils can also limit glioma growth. It was recently reported that neutrophils are recruited during the early stages of glioma development and exert an antitumour function in tumour-bearing mice. Increased reactive oxygen species (ROS) release levels might be responsible for the role of antineoplastic neutrophils. Unfortunately, as the tumour progressed, neutrophils lost the ability to prevent tumour progression (89). The antitumorigenic property of neutrophils during early stages of glioma suggests that these cells may contribute to improved immunotherapeutic outcomes in patients with glioma.
Based on MDSC function, PMN-MDSCs should refer to neutrophil subsets with proven immunosuppressive activity. MDSCs in the tumour site play a major role in T cell suppression (The immunosuppressive function of MDSCs is summarized in Figure 2). The important factors implicated in the MDSC-mediated suppression of T cell function include the metabolism of L-arginine, increased production of ROS, and increased levels of peroxynitrite (ONOO−) (170). M-MDSCs and PMN-MDSCs regulate different aspects of immune suppression. M-MDSCs suppress the T cell response by utilizing NO, whereas PMN-MDSCs use ROS, peroxynitrite, and arginase to mediate immune suppression (170, 171). Because of the increased arginase activity of MDSCs, L-arginine is catabolized into urea and L-ornithine. The created L-arginine deficiency inhibits T cell proliferation (163, 164). MDSCs are induced to express inducible nitric oxide synthase-2 (iNOS2), which converts L-arginine into NO and L-citrulline (165). NO is thought to interfere with T cell JAK/STAT signalling proteins required for T cell activation, inhibit MHC class II gene transcription, and induce T cell apoptosis (166–168). ROS are another important factor that mediate the immunosuppressive activity of MDSCs, which has been demonstrated in in vitro studies (172–174). MDSCs produce high levels of peroxynitrite and ROS when in direct contact with T cells. The superoxide anion () interacts with NO to form peroxynitrite. An in vivo experimental model found that MDSCs produce ROS and peroxynitrite to induce modification of TCR and CD8 molecules, resulting in CD8+ T cells losing the ability to bind to pMHC complexes and inducing nonresponsiveness in tumour-specific CD8+ T cells in the peripheral blood (169). Comparison of MDSCs between the peripheral blood and TME shows that tumour MDSCs have more effective inhibitory activity. After migrating to a tumour, MDSCs are exposed to inflammation and hypoxia in the TME. This results in increases in arginase and iNOS, downregulation of ROS production, and upregulation of inhibitory PD-1 ligand (PD-L1) expression on the MDSC surface (171).
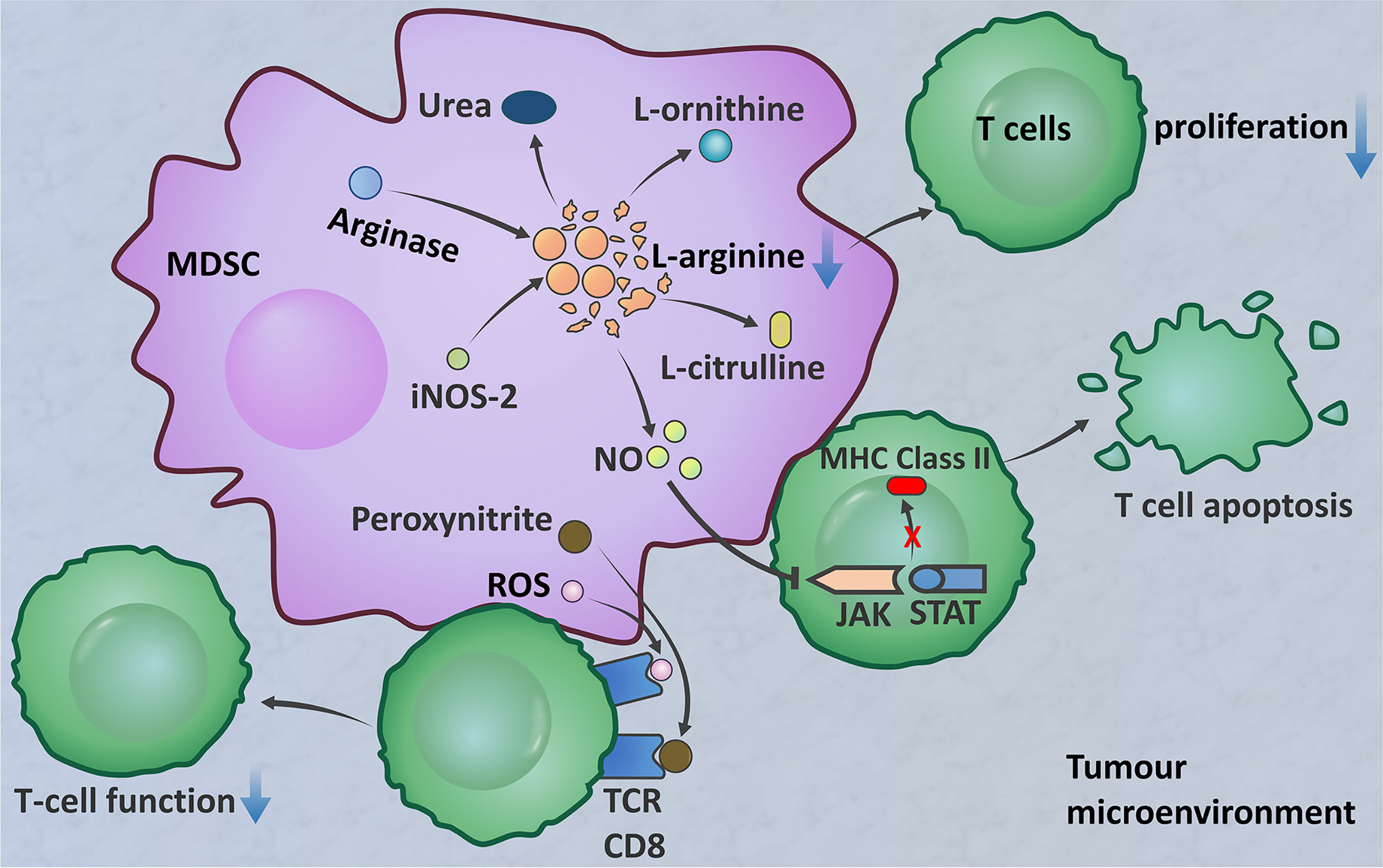
Figure 2 The immunosuppressive function of MDSCs. MDSCs produce arginase, which decomposes l-arginine into urea and l-ornithine (163, 164). MDSCs express iNOS2, which converts l-arginine into NO and l-citrulline (165). L-Arginine deficiency inhibits T cell proliferation. NO interferes with JAK/STAT signalling proteins, inhibits the transcription of MHC class II genes, and induces T cell apoptosis (166–168). MDSCs produce high levels of peroxynitrite and ROS when in direct contact with T cells to induce TCR and CD8 molecular modification, resulting in CD8+ T cells losing the ability to bind to the pMHC complex; this results in nonresponse of peripheral blood tumour-specific CD8+ T cells (169).
In gliomas, the abilities of MDSC subsets to express arginase I and produce ROS have been confirmed. Arginase I is expressed in tumour-derived MDSCs, predominantly M-MDSCs. Only a small portion of MDSCs in the blood of glioma patients express arginase. In contrast, both MDSC subsets can produce ROS (175). MDSCs were found to be increased in the peripheral blood of patients with GBM, and the largest population, comprising more than 60% of cells, was neutrophil MDSC subsets. MDSCs in the peripheral blood of patients with GBM were shown to suppress T cell IFN–γ production (176). Immunohistochemistry confirmed that CD15+ granulocytic MDSC (PMN-MDSC) subsets are dominant in glioma tissue (177). Blood-derived neutrophilic MDSCs inhibit T cell proliferation in vitro. There is a correlation between granulocytic MDSCs and effector memory CD4+ T cells in gliomas. Effector memory CD4+ T cells are dysfunctional and express high levels of PD-L1, an immunoinhibitory receptor that is involved in functional T cell exhaustion (175). The results of these studies have important clinical implications for immune-based interventions in GBM. Strategies to target MDSCs in peripheral blood and tumour tissue should be implemented into immunotherapeutic approaches.
The treatment of gliomas has been particularly challenging due to the high invasive growth and treatment resistance of these tumours (178, 179). In the context of glioma, neutrophils typically promote cancer cell proliferation, immunosuppression, and angiogenesis in support of tumour growth and metastasis (9, 76). Hence, significant attention has been drawn towards development of glioma immunotherapies targeting these neutrophils; either depleting them from tumour, blocking their infiltration, or using neutrophil-delivered drug system to exert immunostimulatory/tumoricidal properties (180–184).
Blocking VEGF to inhibit neovascularization has emerged as a primary strategy for glioma treatment (37, 185). Bevacizumab is a humanized monoclonal antibody against VEGF that improves progression-free survival in GBM patients (186). However, neutrophil infiltration into tumours is significantly correlated with acquired resistance to anti-VEGF therapy (37). Therefore, further research is needed to determine the exact mechanism by which neutrophils mediate anti-VEGF treatment resistance in GBM and to propose potential approaches for glioma treatment.
In addition, as mentioned above, in patients with glioma, increased neutrophil infiltration is associated with glioma progression and a poor prognosis. R. E. Kast et al. hypothesized that dapsone, an antibiotic, could target neutrophils by blocking IL-8-mediated neutrophil infiltration and subsequently limiting glioma cell migration (182). The results demonstrated in a modified rat T9 GBM model that glioma cells genetically engineered to secrete IL-6 invoke an effective, antitumour response in which the early stages may be mediated by neutrophils (181). These studies provided valuable information on neutrophils response to glioma in vitro and in vivo. In contrast to previous neutrophil depletion approaches, Yun Chang et al. established a new platform for producing neutrophils. They used chimeric antigen receptors (CARs) to enhance neutrophil antitumour cytotoxicity for targeted therapy of glioma (180). This strategy may complement current standard glioma treatments and boost their efficacy. Other strategies of cancer immunotherapy are to prevent the interaction between PD-1 on T cells and PD-L1 on tumour cells or host cells. Anti-neutrophil reagents have been observed to enhance the treatment efficacy of PD-1 inhibitors in most glioma mouse models (187) (Table 2). Future investigation is encouraged to target neutrophils in gliomas to alleviate their negative effects on PD-1 inhibitors.
Drug delivery directly into the CNS is a strong strategy because it circumvents the obstacle of the BBB (These methods are summarized in Figure 3) (183, 184). Neutrophils have the natural abilities to penetrate glioma sites and cross the BBB. Treatment with neutrophils carrying paclitaxel (PTX)-loaded liposomes produced superior suppressive effects on tumour recurrence in glioma mouse models (184). A neutrophil-derived exosome (NEs-Exos) drug delivery system for the treatment of glioma was recently reported. The anticancer drug doxorubicin (DOX) was loaded into this nanocarrier, which could efficiently cross the BBB into the brain and target inflamed brain tumours. NEs-Exos have been confirmed to efficiently suppress tumour growth and prolong survival time (183). These novel strategies hold positive clinical prospects for brain targeting if explored further in the right direction.
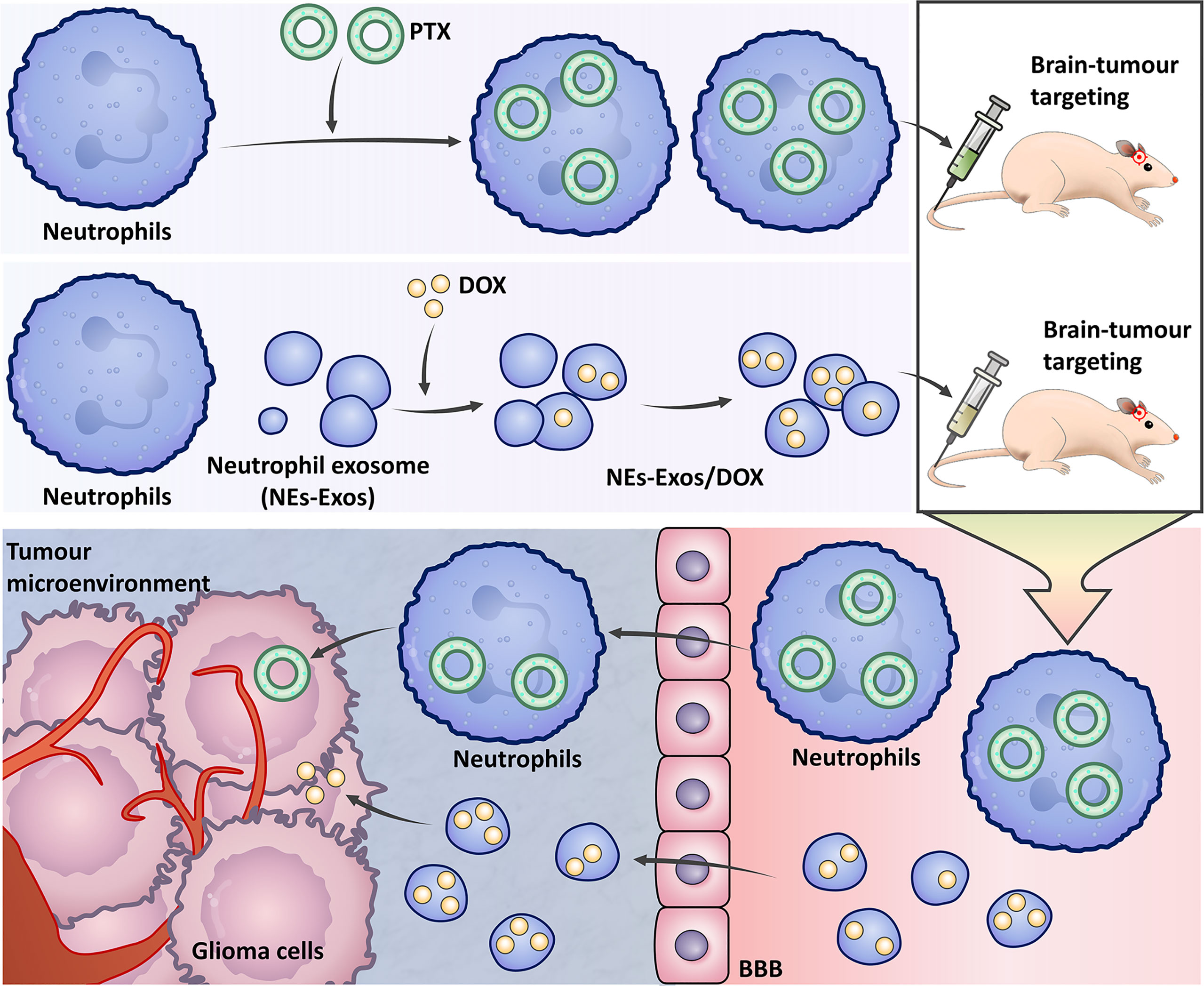
Figure 3 The strategy uses neutrophils to treat gliomas. Neutrophils carry paclitaxel (PTX) liposomes to treat gliomas. In the neutrophil-derived exosome (NEs-Exos) drug delivery system, the anticancer drug doxorubicin (DOX) is loaded into the nanocarrier for the treatment of gliomas.
The presence of NETs in tumours of CNS has rarely been reported. Recently, NETs were detected in grade IV glioma tissues by staining for MPO and CitH3. The levels of NETs in high-grade glioma tissues were significantly higher than those in low-grade glioma tissues. Furthermore, NETs participate in the proliferation and invasion of GBM cells by binding HMGB1 to RAGE to activate the NF-κB signaling pathway (92).
Injection of DNase I into experimental animals degraded extracellular DNA fibres and significantly inhibited the invasion and metastasis of pancreatic cancer cells (188). A study by Meurer et al. reported host DNase 1 promoted the killing of S. suis by neutrophils by cleaving DNA fibers in NETs (189). In addition to DNase, certain drugs or compounds have been shown to inhibit or destroy NETs and may play a therapeutic role in CNS diseases. Cl-amidine and BB-Cl-amidine are nonspecific PAD inhibitors that inhibit PAD4 and reduce the formation of NETs (190). HMGB1 plays an important role in ischaemic cerebral infarction and promotes the production of NETs. Studies have shown that the use of anti-HMGB1 antibodies can reduce the formation of NETs (191). The antidiabetic drug metformin has also been shown to reduce NETs concentrations in vitro (192). These drugs targeting NETs may arouse interest in treating gliomas. Futures potential therapeutic strategy for gliomas are needed to refine our knowledge on NETs.
The important role of neutrophils in tumour progression and their potential as therapeutic targets have been extensively studied in recent years (182, 193). To date, studies on neutrophils in cancer have investigated not only the ability of these cells to promote or prevent tumour progression but also the recruitment mechanism of neutrophils and their phenotypic classification (41). Each of these findings opens up new opportunities for therapeutic intervention in glioma patients.
The presence and significance of neutrophils in gliomas have long been overlooked. Clarifying the roles of neutrophils in the peripheral blood and TME of patients with glioma will help improve the potential of targeted glioma therapies and incorporate these cells into current treatment regimens. Circulating neutrophils are closely correlated with clinicopathological parameters such as tumour stage, tumour progression, and OS, so neutrophils can be used as biomarkers for diagnosis and prognosis (194–196). Most previous studies in patients with glioma have shown that neutrophils infiltration at the tumour site has negative effects on tumour progression, patient survival, and treatment response (197–199). Further study of the effects of neutrophils in the TME and analysis of their diversity has revealed new insights into TANs in gliomas, showing that neutrophils can directly exert important antineoplastic activity (89, 200). The goal of the previous hypothetical approach was to block neutrophils from infiltrating into the tumour site (201), and the discovery of the role of antitumour neutrophils provides a new way to improve the efficiency of current treatments (89). In conclusion, neutrophils perform different functional roles in the progression of glioma. Targeting neutrophils can block the growth of glioma cells and improve the immune response in the lesional area, and tumour progression can also be systematically inhibited using targeted metabolic drug delivery systems based on neutrophils (182–184). In addition, many drugs or compounds have been shown to inhibit the formation of NETs through different mechanisms (202, 203). We speculate that the use of these drugs or compounds is beneficial for the treatment of gliomas and hope to confirm this in future studies.
This is expected to be a new direction for the clinical treatment of glioma. However, the role of neutrophils in gliomas has not been sufficiently studied, and more studies are needed to elucidate the role and mechanism of neutrophils in gliomas. In addition, the clinical application prospects of neutrophils, whether for neutrophil recruitment or NETs, are expected to be confirmed in subsequent studies. Therefore, we hope that this paper can provide inspiration or useful information for follow-up study on neutrophils in glioma to promote progress in the diagnosis and treatment of glioma.
GW wrote the manuscript; JW retrieved literature; PW, YZ, and CN critically revised the manuscript. All authors have read and approved the final manuscript.
This research was supported by the Anhui Province Key Laboratory of Translational Cancer Research (Bengbu Medical College) (KFZZ202203).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Molinaro AM, Taylor JW, Wiencke JK, Wrensch MR. Genetic and Molecular Epidemiology of Adult Diffuse Glioma. Nature Reviews. Neurology (2019) 15(7):405–17. doi: 10.1038/s41582-019-0220-2
2. Gusyatiner O, Hegi ME. Glioma Epigenetics: From Subclassification to Novel Treatment Options. Semin Cancer Biol (2018) 51:50–8. doi: 10.1016/j.semcancer.2017.11.010
3. Sanai N, Berger MS. Surgical Oncology for Gliomas: The State of the Art. Nat Rev Clin Oncol (2018) 15(2):112–25. doi: 10.1038/nrclinonc.2017.171
4. Andrews DW, Judy KD, Scott CB, Garcia S, Harshyne LA, Kenyon L, et al. Phase Ib Clinical Trial of IGV-001 for Patients With Newly Diagnosed Glioblastoma. Clin Cancer Res (2021) 27(7):1912–22. doi: 10.1158/1078-0432.CCR-20-3805
5. Chen KT, Chai WY, Lin YJ, Lin CJ, Chen PY, Tsai HC, et al. Neuronavigation-Guided Focused Ultrasound for Transcranial Blood-Brain Barrier Opening and Immunostimulation in Brain Tumors. Sci Adv (2021) 7(6):eabd0772. doi: 10.1126/sciadv.abd0772
6. Ji M, Lewis S, Camelo-Piragua S, Ramkissoon SH, Snuderl M, Venneti S, et al. Orringer D.A. Detection of Human Brain Tumor Infiltration With Quantitative Stimulated Raman Scattering Microscopy. Sci Transl Med (2015) 7(309):309ra163. doi: 10.1126/scitranslmed.aab0195
7. Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ. Fluorescence-Guided Surgery With 5-Aminolevulinic Acid for Resection of Malignant Glioma: A Randomised Controlled Multicentre Phase III Trial. Lancet Oncol (2006) 7(5):392–401. doi: 10.1016/S1470-2045(06)70665-9
8. van Solinge TS, Nieland L, Chiocca EA, Broekman MLD. Advances in Local Therapy for Glioblastoma - Taking the Fight to the Tumour. Nat Rev Neurol (2022) 18(4):221–36. doi: 10.1038/s41582-022-00621-0
9. Khan S, Mittal S, McGee K, Alfaro-Munoz KD, Majd N, Balasubramaniyan V, et al. Role of Neutrophils and Myeloid-Derived Suppressor Cells in Glioma Progression and Treatment Resistance. Int J Mol Sci (2020) 21(6):1954. doi: 10.3390/ijms21061954
10. Markwell SM, Ross JL, Olson CL, Brat DJ. Necrotic Reshaping of the Glioma Microenvironment Drives Disease Progression. Acta Neuropathol (2022) 143(3):291–310. doi: 10.1007/s00401-021-02401-4
11. Locarno CV, Simonelli M, Carenza C, Capucetti A, Stanzani E, Lorenzi E, et al. Role of Myeloid Cells in the Immunosuppressive Microenvironment in Gliomas. Immunobiology (2020) 225(1):151853. doi: 10.1016/j.imbio.2019.10.002
12. Li Y, Wang W, Yang F, Xu Y, Feng C, Zhao Y. The Regulatory Roles of Neutrophils in Adaptive Immunity. Cell Commun Signal (2019) 17(1):147. doi: 10.1186/s12964-019-0471-y
13. Rosales C. Neutrophil: A Cell With Many Roles in Inflammation or Several Cell Types? Front Physiol (2018) 9:113. doi: 10.3389/fphys.2018.00113
14. Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil Extracellular Traps Kill Bacteria. Science (New York N Y) (2004) 303(5663):1532–5. doi: 10.1126/science.1092385
15. Muniz VS, Silva JC, Braga YAV, Melo RCN, Ueki S, Takeda M, et al. Eosinophils Release Extracellular DNA Traps in Response to Aspergillus Fumigatus. J Allergy Clin Immunol (2018) 141(2):571–585.e577. doi: 10.1016/j.jaci.2017.07.048
16. Silva JC, Rodrigues NC, Thompson-Souza GA, Muniz VS, Neves JS, Figueiredo RT. Mac-1 Triggers Neutrophil DNA Extracellular Trap Formation to Aspergillus Fumigatus Independently of PAD4 Histone Citrullination. J Leukoc Biol (2020) 107(1):69–83. doi: 10.1002/JLB.4A0119-009RR
17. Guimarães-Costa AB, Nascimento MT, Froment GS, Soares RP, Morgado FN, Conceição-Silva F, et al. Leishmania Amazonensis Promastigotes Induce and Are Killed by Neutrophil Extracellular Traps. Proc Natl Acad Sci U S A (2009) 106(16):6748–53. doi: 10.1073/pnas.0900226106
18. Borregaard N. Neutrophils, From Marrow to Microbes. Immunity (2010) 33(5):657–70. doi: 10.1016/j.immuni.2010.11.011
19. Xiong S, Dong L, Cheng L. Neutrophils in Cancer Carcinogenesis and Metastasis. J Hematol Oncol (2021) 14(1):173. doi: 10.1186/s13045-021-01187-y
20. Arelaki S, Arampatzioglou A, Kambas K, Papagoras C, Miltiades P, Angelidou I, et al. Gradient Infiltration of Neutrophil Extracellular Traps in Colon Cancer and Evidence for Their Involvement in Tumour Growth. PLoS One (2016) 11(5):e0154484. doi: 10.1371/journal.pone.0154484
21. Boone BA, Murthy P, Miller-Ocuin J, Doerfler WR, Ellis JT, Liang X, et al. Chloroquine Reduces Hypercoagulability in Pancreatic Cancer Through Inhibition of Neutrophil Extracellular Traps. BMC Cancer (2018) 18(1):678. doi: 10.1186/s12885-018-4584-2
22. Hisada Y, Grover SP, Maqsood A, Houston R, Ay C, Noubouossie DF, et al. Neutrophils and Neutrophil Extracellular Traps Enhance Venous Thrombosis in Mice Bearing Human Pancreatic Tumors. Haematologica (2020) 105(1):218–25. doi: 10.3324/haematol.2019.217083
23. Pieterse E, Rother N, Garsen M, Hofstra JM, Satchell SC, Hoffmann M, et al. Neutrophil Extracellular Traps Drive Endothelial-To-Mesenchymal Transition. Arterioscler Thromb Vasc Biol (2017) 37(7):1371–9. doi: 10.1161/ATVBAHA.117.309002
24. Tohme S, Yazdani HO, Al-Khafaji AB, Chidi AP, Loughran P, Mowen K, et al. Neutrophil Extracellular Traps Promote the Development and Progression of Liver Metastases After Surgical Stress. Cancer Res (2016) 76(6):1367–80. doi: 10.1158/0008-5472.CAN-15-1591
25. Burn GL, Foti A, Marsman G, Patel DF, Zychlinsky A. The Neutrophil. Immunity (2021) 54(7):1377–91. doi: 10.1016/j.immuni.2021.06.006
26. Güç E, Pollard JW. Redefining Macrophage and Neutrophil Biology in the Metastatic Cascade. Immunity (2021) 54(5):885–902. doi: 10.1016/j.immuni.2021.03.022
27. Hedrick CC, Malanchi I. Neutrophils in Cancer: Heterogeneous and Multifaceted. Nat Rev Immunol (2022) 22(3):173–87. doi: 10.1038/s41577-021-00571-6
28. Li MO, Wolf N, Raulet DH, Akkari L, Pittet MJ, Rodriguez PC, et al. Innate Immune Cells in the Tumor Microenvironment. Cancer Cell (2021) 39(6):725–9. doi: 10.1016/j.ccell.2021.05.016
29. Shaul ME, Fridlender ZG. Tumour-Associated Neutrophils in Patients With Cancer. Nat Rev Clin Oncol (2019) 16(10):601–20. doi: 10.1038/s41571-019-0222-4
30. Tian S, Chu Y, Hu J, Ding X, Liu Z, Fu D, et al. Tumour-Associated Neutrophils Secrete AGR2 to Promote Colorectal Cancer Metastasis via Its Receptor CD98hc-xCT. Gut (2022). doi: 10.1136/gutjnl-2021-325137
31. Bodac A, Meylan E. Neutrophil Metabolism in the Cancer Context. Semin Immunol (2021) 101583. doi: 10.1016/j.smim.2021.101583
32. Granot Z, Henke E, Comen EA, King TA, Norton L, Benezra R. Tumor Entrained Neutrophils Inhibit Seeding in the Premetastatic Lung. Cancer Cell (2011) 20(3):300–14. doi: 10.1016/j.ccr.2011.08.012
33. Rogers T, DeBerardinis RJ. Metabolic Plasticity of Neutrophils: Relevance to Pathogen Responses and Cancer. Trends Cancer (2021) 7(8):700–13. doi: 10.1016/j.trecan.2021.04.007
34. Gabrilovich DI, Bronte V, Chen SH, Colombo MP, Ochoa A, Ostrand-Rosenberg S, et al. The Terminology Issue for Myeloid-Derived Suppressor Cells. Cancer Res (2007) 67(1):425; author reply 426. doi: 10.1158/0008-5472.CAN-06-3037
35. Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, et al. Polarization of Tumor-Associated Neutrophil Phenotype by TGF-Beta: "N1" Versus "N2" TAN. Cancer Cell (2009) 16(3):183–94. doi: 10.1016/j.ccr.2009.06.017
36. Fossati G, Ricevuti G, Edwards SW, Walker C, Dalton A, Rossi ML. Neutrophil Infiltration Into Human Gliomas. Acta Neuropathol (1999) 98(4):349–54. doi: 10.1007/s004010051093
37. Liang J, Piao Y, Holmes L, Fuller GN, Henry V, Tiao N, et al. Neutrophils Promote the Malignant Glioma Phenotype Through S100A4. Clin Cancer Res (2014) 20(1):187–98. doi: 10.1158/1078-0432.CCR-13-1279
38. Gomes Dos Santos A, de Carvalho RF, de Morais A, Silva TM, Baylão VMR, Azevedo M, et al. Role of Neutrophil-Lymphocyte Ratio as a Predictive Factor of Glioma Tumor Grade: A Systematic Review. Crit Rev Oncol Hematol (2021) 163:103372. doi: 10.1016/j.critrevonc.2021.103372
39. Veglia F, Perego M, Gabrilovich D. Myeloid-Derived Suppressor Cells Coming of Age. Nat Immunol (2018) 19(2):108–19. doi: 10.1038/s41590-017-0022-x
40. Raychaudhuri B, Rayman P, Huang P, Grabowski M, Hambardzumyan D, Finke JH, et al. Myeloid Derived Suppressor Cell Infiltration of Murine and Human Gliomas Is Associated With Reduction of Tumor Infiltrating Lymphocytes. J Neurooncol (2015) 122(2):293–301. doi: 10.1007/s11060-015-1720-6
41. Coffelt SB, Wellenstein MD, de Visser KE. Neutrophils in Cancer: Neutral No More. Nat Rev Cancer (2016) 16(7):431–46. doi: 10.1038/nrc.2016.52
42. Dancey JT, Deubelbeiss KA, Harker LA, Finch CA. Neutrophil Kinetics in Man. J Clin Invest (1976) 58(3):705–15. doi: 10.1172/JCI108517
43. Greten FR, Grivennikov SI. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity (2019) 51(1):27–41. doi: 10.1016/j.immuni.2019.06.025
44. Gentles AJ, Newman AM, Liu CL, Bratman SV, Feng W, Kim D, et al. The Prognostic Landscape of Genes and Infiltrating Immune Cells Across Human Cancers. Nat Med (2015) 21(8):938–45. doi: 10.1038/nm.3909
45. Trellakis S, Bruderek K, Dumitru CA, Gholaman H, Gu X, Bankfalvi A, et al. Polymorphonuclear Granulocytes in Human Head and Neck Cancer: Enhanced Inflammatory Activity, Modulation by Cancer Cells and Expansion in Advanced Disease. Int J Cancer (2011) 129(9):2183–93. doi: 10.1002/ijc.25892
46. Li YW, Qiu SJ, Fan J, Zhou J, Gao Q, Xiao YS, et al. Intratumoral Neutrophils: A Poor Prognostic Factor for Hepatocellular Carcinoma Following Resection. J Hepatol (2011) 54(3):497–505. doi: 10.1016/j.jhep.2010.07.044
47. Jensen TO, Schmidt H, Møller HJ, Donskov F, Høyer M, Sjoegren P, et al. Intratumoral Neutrophils and Plasmacytoid Dendritic Cells Indicate Poor Prognosis and Are Associated With Pstat3 Expression in AJCC Stage I/II Melanoma. Cancer (2012) 118(9):2476–85. doi: 10.1002/cncr.26511
48. Jaillon S, Ponzetta A, Di Mitri D, Santoni A, Bonecchi R, Mantovani A. Neutrophil Diversity and Plasticity in Tumour Progression and Therapy. Nat Rev Cancer (2020) 20(9):485–503. doi: 10.1038/s41568-020-0281-y
49. Giese MA, Hind LE, Huttenlocher A. Neutrophil Plasticity in the Tumor Microenvironment. Blood (2019) 133(20):2159–67. doi: 10.1182/blood-2018-11-844548
50. Lu C, Rong D, Zhang B, Zheng W, Wang X, Chen Z, et al. Current Perspectives on the Immunosuppressive Tumor Microenvironment in Hepatocellular Carcinoma: Challenges and Opportunities. Mol Cancer (2019) 18(1):130. doi: 10.1186/s12943-019-1047-6
51. Veglia F, Sanseviero E, Gabrilovich DI. Myeloid-Derived Suppressor Cells in the Era of Increasing Myeloid Cell Diversity. Nat Rev Immunol (2021) 21(8):485–98. doi: 10.1038/s41577-020-00490-y
52. Jablonska J, Leschner S, Westphal K, Lienenklaus S, Weiss S. Neutrophils Responsive to Endogenous IFN-Beta Regulate Tumor Angiogenesis and Growth in a Mouse Tumor Model. J Clin Invest (2010) 120(4):1151–64. doi: 10.1172/JCI37223
53. Jablonska J, Wu CF, Andzinski L, Leschner S, Weiss S. CXCR2-Mediated Tumor-Associated Neutrophil Recruitment Is Regulated by IFN-β. Int J Cancer (2014) 134(6):1346–58. doi: 10.1002/ijc.28551
54. Andzinski L, Kasnitz N, Stahnke S, Wu CF, Gereke M, von Köckritz-Blickwede M, et al. Type I IFNs Induce Anti-Tumor Polarization of Tumor Associated Neutrophils in Mice and Human. Int J Cancer (2016) 138(8):1982–93. doi: 10.1002/ijc.29945
55. Reid MD, Basturk O, Thirabanjasak D, Hruban RH, Klimstra DS, Bagci P, et al. Tumor-Infiltrating Neutrophils in Pancreatic Neoplasia. Mod Pathol (2011) 24(12):1612–9. doi: 10.1038/modpathol.2011.113
56. Caruso RA, Bellocco R, Pagano M, Bertoli G, Rigoli L, Inferrera C. Prognostic Value of Intratumoral Neutrophils in Advanced Gastric Carcinoma in a High-Risk Area in Northern Italy. Mod Pathol (2002) 15(8):831–7. doi: 10.1097/01.MP.0000020391.98998.6B
57. López-Lago MA, Posner S, Thodima VJ, Molina AM, Motzer RJ, Chaganti RS. Neutrophil Chemokines Secreted by Tumor Cells Mount a Lung Antimetastatic Response During Renal Cell Carcinoma Progression. Oncogene (2013) 32(14):1752–60. doi: 10.1038/onc.2012.201
58. Finisguerra V, Di Conza G, Di Matteo M, Serneels J, Costa S, Thompson AA, et al. MET Is Required for the Recruitment of Anti-Tumoural Neutrophils. Nature (2015) 522(7556):349–53. doi: 10.1038/nature14407
59. Rao HL, Chen JW, Li M, Xiao YB, Fu J, Zeng YX, et al. Increased Intratumoral Neutrophil in Colorectal Carcinomas Correlates Closely With Malignant Phenotype and Predicts Patients' Adverse Prognosis. PLoS One (2012) 7(1):e30806. doi: 10.1371/journal.pone.0030806
60. Wikberg ML, Ling A, Li X, Öberg Å, Edin S, Palmqvist R. Neutrophil Infiltration Is a Favorable Prognostic Factor in Early Stages of Colon Cancer. Hum Pathol (2017) 68:193–202. doi: 10.1016/j.humpath.2017.08.028
61. Ilie M, Hofman V, Ortholan C, Bonnetaud C, Coëlle C, Mouroux J, et al. Predictive Clinical Outcome of the Intratumoral CD66b-Positive Neutrophil-to-CD8-Positive T-Cell Ratio in Patients With Resectable Nonsmall Cell Lung Cancer. Cancer (2012) 118(6):1726–37. doi: 10.1002/cncr.26456
62. Rakaee M, Busund LT, Paulsen EE, Richardsen E, Al-Saad S, Andersen S, et al. Prognostic Effect of Intratumoral Neutrophils Across Histological Subtypes of non-Small Cell Lung Cancer. Oncotarget (2016) 7(44):72184–96. doi: 10.18632/oncotarget.12360
63. Yang R, Cai Z, Zhang Y, W.H.t Y, Roby KF, Roden RB. CD80 in Immune Suppression by Mouse Ovarian Carcinoma-Associated Gr-1+CD11b+ Myeloid Cells. Cancer Res (2006) 66(13):6807–15. doi: 10.1158/0008-5472.CAN-05-3755
64. Sagiv JY, Michaeli J, Assi S, Mishalian I, Kisos H, Levy L, et al. Phenotypic Diversity and Plasticity in Circulating Neutrophil Subpopulations in Cancer. Cell Rep (2015) 10(4):562–73. doi: 10.1016/j.celrep.2014.12.039
65. Brandau S, Moses K, Lang S. The Kinship of Neutrophils and Granulocytic Myeloid-Derived Suppressor Cells in Cancer: Cousins, Siblings or Twins? Semin Cancer Biol (2013) 23(3):171–82. doi: 10.1016/j.semcancer.2013.02.007
66. Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated Regulation of Myeloid Cells by Tumours. Nat Rev Immunol (2012) 12(4):253–68. doi: 10.1038/nri3175
67. Barreda DR, Hanington PC, Belosevic M. Regulation of Myeloid Development and Function by Colony Stimulating Factors. Dev Comp Immunol (2004) 28(5):509–54. doi: 10.1016/j.dci.2003.09.010
68. Dumitru CA, Moses K, Trellakis S, Lang S, Brandau S. Neutrophils and Granulocytic Myeloid-Derived Suppressor Cells: Immunophenotyping, Cell Biology and Clinical Relevance in Human Oncology. Cancer Immunol Immunother (2012) 61(8):1155–67. doi: 10.1007/s00262-012-1294-5
69. Pfirschke C, Engblom C, Gungabeesoon J, Lin Y, Rickelt S, Zilionis R, et al. Tumor-Promoting Ly-6g(+) SiglecF(high) Cells Are Mature and Long-Lived Neutrophils. Cell Rep (2020) 32(12):108164. doi: 10.1016/j.celrep.2020.108164
70. Nagaraj S, Gabrilovich DI. Myeloid-Derived Suppressor Cells in Human Cancer. Cancer J (Sudbury Mass) (2010) 16(4):348–53. doi: 10.1097/PPO.0b013e3181eb3358
71. Gustafson MP, Lin Y, Maas ML, Van Keulen VP, Johnston PB, Peikert T, et al. A Method for Identification and Analysis of non-Overlapping Myeloid Immunophenotypes in Humans. PLoS One (2015) 10(3):e0121546. doi: 10.1371/journal.pone.0121546
72. Damuzzo V, Pinton L, Desantis G, Solito S, Marigo I, Bronte V, et al. Complexity and Challenges in Defining Myeloid-Derived Suppressor Cells. Cytometry B Clin Cytom (2015) 88(2):77–91. doi: 10.1002/cytob.21206
73. Abeles RD, McPhail MJ, Sowter D, Antoniades CG, Vergis N, Vijay GK, et al. CD14, CD16 and HLA-DR Reliably Identifies Human Monocytes and Their Subsets in the Context of Pathologically Reduced HLA-DR Expression by CD14(hi) /CD16(neg) Monocytes: Expansion of CD14(hi) /CD16(pos) and Contraction of CD14(lo) /CD16(pos) Monocytes in Acute Liver Failure. Cytometry A (2012) 81(10):823–34. doi: 10.1002/cyto.a.22104
74. Hanahan D, Weinberg RA. Hallmarks of Cancer: The Next Generation. Cell (2011) 144(5):646–74. doi: 10.1016/j.cell.2011.02.013
75. Templeton AJ, McNamara MG, Šeruga B, Vera-Badillo FE, Aneja P, Ocaña A, et al. Prognostic Role of Neutrophil-to-Lymphocyte Ratio in Solid Tumors: A Systematic Review and Meta-Analysis. J Natl Cancer Inst (2014) 106(6):dju124. doi: 10.1093/jnci/dju124
76. Lin YJ, Wei KC, Chen PY, Lim M, Hwang TL. Roles of Neutrophils in Glioma and Brain Metastases. Front Immunol (2021) 12:701383. doi: 10.3389/fimmu.2021.701383
77. Li MX, Liu XM, Zhang XF, Zhang JF, Wang WL, Zhu Y, et al. Prognostic Role of Neutrophil-to-Lymphocyte Ratio in Colorectal Cancer: A Systematic Review and Meta-Analysis. Int J Cancer (2014) 134(10):2403–13. doi: 10.1002/ijc.28536
78. Corbeau I, Jacot W, Guiu S. Neutrophil to Lymphocyte Ratio as Prognostic and Predictive Factor in Breast Cancer Patients: A Systematic Review. Cancers (2020) 12(4):958. doi: 10.3390/cancers12040958
79. Arvanitakis K, Mitroulis I, Germanidis G. Tumor-Associated Neutrophils in Hepatocellular Carcinoma Pathogenesis, Prognosis, and Therapy. Cancers (2021) 13(12):2899. doi: 10.3390/cancers13122899
80. Zheng SH, Huang JL, Chen M, Wang BL, Ou QS, Huang SY. Diagnostic Value of Preoperative Inflammatory Markers in Patients With Glioma: A Multicenter Cohort Study. J Neurosurg (2018) 129(3):583–92. doi: 10.3171/2017.3.JNS161648
81. Wang PF, Meng Z, Song HW, Yao K, Duan ZJ, Yu CJ, et al. Preoperative Changes in Hematological Markers and Predictors of Glioma Grade and Survival. Front Pharmacol (2018) 9:886. doi: 10.3389/fphar.2018.00886
82. Sharma G, Jain SK, Sinha VD. Peripheral Inflammatory Blood Markers in Diagnosis of Glioma and IDH Status. J Neurosci Rural Pract (2021) 12(1):88–94. doi: 10.1055/s-0040-1721166
83. Auezova R, Ivanova N, Akshulakov S, Zhetpisbaev B, Kozhakhmetova A, Ryskeldiyev N, et al. Isocitrate Dehydrogenase 1 Mutation Is Associated With Reduced Levels of Inflammation in Glioma Patients. Cancer Manage Res (2019) 11:3227–36. doi: 10.2147/CMAR.S195754
84. Mason M, Maurice C, McNamara MG, Tieu MT, Lwin Z, Millar BA, et al. Neutrophil-Lymphocyte Ratio Dynamics During Concurrent Chemo-Radiotherapy for Glioblastoma Is an Independent Predictor for Overall Survival. J Neurooncol (2017) 132(3):463–71. doi: 10.1007/s11060-017-2395-y
85. Balta S, Demirkol S, Cakar M, Arslan Z, Unlu M, Celik T. Other Inflammatory Markers Should Not be Forgetten When Assessing the Neutrophil-to-Lymphocyte Ratio. Clin Appl Thromb Hemost (2013) 19(6):693–4. doi: 10.1177/1076029613486019
86. Bertaut A, Truntzer C, Madkouri R, Kaderbhai CG, Derangère V, Vincent J, et al. Blood Baseline Neutrophil Count Predicts Bevacizumab Efficacy in Glioblastoma. Oncotarget (2016) 7(43):70948–58. doi: 10.18632/oncotarget.10898
87. Motomura T, Shirabe K, Mano Y, Muto J, Toshima T, Umemoto Y, et al. Neutrophil-Lymphocyte Ratio Reflects Hepatocellular Carcinoma Recurrence After Liver Transplantation via Inflammatory Microenvironment. J Hepatol (2013) 58(1):58–64. doi: 10.1016/j.jhep.2012.08.017
88. Kantola T, Klintrup K, Väyrynen JP, Vornanen J, Bloigu R, Karhu T, et al. Stage-Dependent Alterations of the Serum Cytokine Pattern in Colorectal Carcinoma. Br J Cancer (2012) 107(10):1729–36. doi: 10.1038/bjc.2012.456
89. Magod P, Mastandrea I, Rousso-Noori L, Agemy L, Shapira G, Shomron N, et al. Exploring the Longitudinal Glioma Microenvironment Landscape Uncovers Reprogrammed Pro-Tumorigenic Neutrophils in the Bone Marrow. Cell Rep (2021) 36(5):109480. doi: 10.1016/j.celrep.2021.109480
90. Iwatsuki K, Kumara E, Yoshimine T, Nakagawa H, Sato M, Hayakawa T. Elastase Expression by Infiltrating Neutrophils in Gliomas. Neurol Res (2000) 22(5):465–8. doi: 10.1080/01616412.2000.11740701
91. Kasahara T, Mukaida N, Yamashita K, Yagisawa H, Akahoshi T, Matsushima K. IL-1 and TNF-Alpha Induction of IL-8 and Monocyte Chemotactic and Activating Factor (MCAF) mRNA Expression in a Human Astrocytoma Cell Line. Immunology (1991) 74(1):60–7.
92. Zha C, Meng X, Li L, Mi S, Qian D, Li Z, et al. Neutrophil Extracellular Traps Mediate the Crosstalk Between Glioma Progression and the Tumor Microenvironment via the HMGB1/RAGE/IL-8 Axis. Cancer Biol Med (2020) 17(1):154–68. doi: 10.20892/j.issn.2095-3941.2019.0353
93. Hor WS, Huang WL, Lin YS, Yang BC. Cross-Talk Between Tumor Cells and Neutrophils Through the Fas (APO-1, CD95)/FasL System: Human Glioma Cells Enhance Cell Viability and Stimulate Cytokine Production in Neutrophils. J Leukoc Biol (2003) 73(3):363–8. doi: 10.1189/jlb.0702375
94. Wang T, Cao L, Dong X, Wu F, De W, Huang L, et al. LINC01116 Promotes Tumor Proliferation and Neutrophil Recruitment via DDX5-Mediated Regulation of IL-1β in Glioma Cell. Cell Death Dis (2020) 11(5):302. doi: 10.1038/s41419-020-2506-0
95. Lee SY, Kim JK, Jeon HY, Ham SW, Kim H. CD133 Regulates IL-1β Signaling and Neutrophil Recruitment in Glioblastoma. Mol Cells (2017) 40(7):515–22. doi: 10.14348/molcells.2017.0089
96. Sippel TR, White J, Nag K, Tsvankin V, Klaassen M, Kleinschmidt-DeMasters BK, et al. Neutrophil Degranulation and Immunosuppression in Patients With GBM: Restoration of Cellular Immune Function by Targeting Arginase I. Clin Cancer Res (2011) 17(22):6992–7002. doi: 10.1158/1078-0432.CCR-11-1107
97. Rahbar A, Cederarv M, Wolmer-Solberg N, Tammik C, Stragliotto G, Peredo I, et al. Enhanced Neutrophil Activity Is Associated With Shorter Time to Tumor Progression in Glioblastoma Patients. Oncoimmunology (2016) 5(2):e1075693. doi: 10.1080/2162402X.2015.1075693
98. Munder M, Mollinedo F, Calafat J, Canchado J, Gil-Lamaignere C, Fuentes JM, et al. Arginase I Is Constitutively Expressed in Human Granulocytes and Participates in Fungicidal Activity. Blood (2005) 105(6):2549–56. doi: 10.1182/blood-2004-07-2521
99. Mollinedo F. Neutrophil Degranulation, Plasticity, and Cancer Metastasis. Trends Immunol (2019) 40(3):228–42. doi: 10.1016/j.it.2019.01.006
100. Raghavan JV, Ganesh RA, Sonpatki P, Naik D, John AE, Arunachalam P, et al. Immuno-Phenotyping of IDH-Mutant Grade 3 Astrocytoma and IDH-Wildtype Glioblastoma Reveals Specific Differences in Cells of Myeloid Origin. Oncoimmunology (2021) 10(1):1957215. doi: 10.1080/2162402X.2021.1957215
101. Han S, Liu Y, Li Q, Li Z, Hou H, Wu A. Pre-Treatment Neutrophil-to-Lymphocyte Ratio Is Associated With Neutrophil and T-Cell Infiltration and Predicts Clinical Outcome in Patients With Glioblastoma. BMC Cancer (2015) 15:617. doi: 10.1186/s12885-015-1629-7
102. Engelhardt B, Vajkoczy P, Weller RO. The Movers and Shapers in Immune Privilege of the CNS. Nat Immunol (2017) 18(2):123–31. doi: 10.1038/ni.3666
103. Aspelund A, Antila S, Proulx ST, Karlsen TV, Karaman S, Detmar M, et al. A Dural Lymphatic Vascular System That Drains Brain Interstitial Fluid and Macromolecules. J Exp Med (2015) 212(7):991–9. doi: 10.1084/jem.20142290
104. Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, et al. Structural and Functional Features of Central Nervous System Lymphatic Vessels. Nature (2015) 523(7560):337–41. doi: 10.1038/nature14432
105. Rustenhoven J, Drieu A, Mamuladze T, de Lima KA, Dykstra T, Wall M, et al. Functional Characterization of the Dural Sinuses as a Neuroimmune Interface. Cell (2021) 184(4):1000–1016.e1027. doi: 10.1016/j.cell.2020.12.040
106. Brioschi S, Wang WL, Peng V, Wang M, Shchukina I, Greenberg ZJ, et al. Heterogeneity of Meningeal B Cells Reveals a Lymphopoietic Niche at the CNS Borders. Science (New York N Y) (2021) 373(6553):eabf9277. doi: 10.1126/science.abf9277
107. Cugurra A, Mamuladze T, Rustenhoven J, Dykstra T, Beroshvili G, Greenberg ZJ, et al. Skull and Vertebral Bone Marrow Are Myeloid Cell Reservoirs for the Meninges and CNS Parenchyma. Science (New York N Y) (2021) 373(6553):eabf7844. doi: 10.1126/science.abf7844
108. Schafflick D, Wolbert J, Heming M, Thomas C, Hartlehnert M, Börsch AL, et al. Single-Cell Profiling of CNS Border Compartment Leukocytes Reveals That B Cells and Their Progenitors Reside in non-Diseased Meninges. Nat Neurosci (2021) 24(9):1225–34. doi: 10.1038/s41593-021-00880-y
109. Weiss N, Miller F, Cazaubon S, Couraud PO. The Blood-Brain Barrier in Brain Homeostasis and Neurological Diseases. Biochim Biophys Acta (2009) 1788(4):842–57. doi: 10.1016/j.bbamem.2008.10.022
110. Quail DF, Joyce JA. The Microenvironmental Landscape of Brain Tumors. Cancer Cell (2017) 31(3):326–41. doi: 10.1016/j.ccell.2017.02.009
111. Fanelli GN, Grassini D, Ortenzi V, Pasqualetti F, Montemurro N, Perrini P, et al. Decipher the Glioblastoma Microenvironment: The First Milestone for New Groundbreaking Therapeutic Strategies. Genes (Basel) (2021) 12(3):445. doi: 10.3390/genes12030445
112. Glass R, Synowitz M. CNS Macrophages and Peripheral Myeloid Cells in Brain Tumours. Acta Neuropathol (2014) 128(3):347–62. doi: 10.1007/s00401-014-1274-2
113. Sevenich L. Brain-Resident Microglia and Blood-Borne Macrophages Orchestrate Central Nervous System Inflammation in Neurodegenerative Disorders and Brain Cancer. Front Immunol (2018) 9:697. doi: 10.3389/fimmu.2018.00697
114. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-Related Inflammation. Nature (2008) 454(7203):436–44. doi: 10.1038/nature07205
115. Simmons GW, Pong WW, Emnett RJ, White CR, Gianino SM, Rodriguez FJ, et al. Neurofibromatosis-1 Heterozygosity Increases Microglia in a Spatially and Temporally Restricted Pattern Relevant to Mouse Optic Glioma Formation and Growth. J Neuropathol Exp Neurol (2011) 70(1):51–62. doi: 10.1097/NEN.0b013e3182032d37
116. Herisson F, Frodermann V, Courties G, Rohde D, Sun Y, Vandoorne K, et al. Direct Vascular Channels Connect Skull Bone Marrow and the Brain Surface Enabling Myeloid Cell Migration. Nat Neurosci (2018) 21(9):1209–17. doi: 10.1038/s41593-018-0213-2
117. Magaña-Maldonado R, Chávez-Cortez EG, Olascoaga-Arellano NK, López-Mejía M, Maldonado-Leal FM, Sotelo J, et al. Immunological Evasion in Glioblastoma. BioMed Res Int (2016) 2016:7487313. doi: 10.1155/2016/7487313
118. Gieryng A, Pszczolkowska D, Walentynowicz KA, Rajan WD, Kaminska B. Immune Microenvironment of Gliomas. Lab Invest (2017) 97(5):498–518. doi: 10.1038/labinvest.2017.19
119. Li Q, Barres BA. Microglia and Macrophages in Brain Homeostasis and Disease. Nat Rev Immunol (2018) 18(4):225–42. doi: 10.1038/nri.2017.125
120. Sierra A, Encinas JM, Deudero JJ, Chancey JH, Enikolopov G, Overstreet-Wadiche LS, et al. Microglia Shape Adult Hippocampal Neurogenesis Through Apoptosis-Coupled Phagocytosis. Cell Stem Cell (2010) 7(4):483–95. doi: 10.1016/j.stem.2010.08.014
121. Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, et al. Synaptic Pruning by Microglia Is Necessary for Normal Brain Development. Science (New York N Y) (2011) 333(6048):1456–8. doi: 10.1126/science.1202529
122. Cunningham CL, Martínez-Cerdeño V, Noctor SC. Microglia Regulate the Number of Neural Precursor Cells in the Developing Cerebral Cortex. J Neurosci (2013) 33(10):4216–33. doi: 10.1523/JNEUROSCI.3441-12.2013
123. Tanabe S, Yamashita T. B-1a Lymphocytes Promote Oligodendrogenesis During Brain Development. Nat Neurosci (2018) 21(4):506–16. doi: 10.1038/s41593-018-0106-4
124. Pasciuto E, Burton OT, Roca CP, Lagou V, Rajan WD, Theys T, et al. Microglia Require CD4 T Cells to Complete the Fetal-To-Adult Transition. Cell (2020) 182(3):625–640.e624. doi: 10.1016/j.cell.2020.06.026
125. Bowman RL, Klemm F, Akkari L, Pyonteck SM, Sevenich L, Quail DF, et al. Macrophage Ontogeny Underlies Differences in Tumor-Specific Education in Brain Malignancies. Cell Rep (2016) 17(9):2445–59. doi: 10.1016/j.celrep.2016.10.052
126. Ye XZ, Xu SL, Xin YH, Yu SC, Ping YF, Chen L, et al. Tumor-Associated Microglia/Macrophages Enhance the Invasion of Glioma Stem-Like Cells via TGF-β1 Signaling Pathway. J Immunol (Baltimore Md 1950) (2012) 189(1):444–53. doi: 10.4049/jimmunol.1103248
127. Markovic DS, Glass R, Synowitz M, Rooijen N, Kettenmann H. Microglia Stimulate the Invasiveness of Glioma Cells by Increasing the Activity of Metalloprotease-2. J Neuropathol Exp Neurol (2005) 64(9):754–62. doi: 10.1097/01.jnen.0000178445.33972.a9
128. Lu-Emerson C, Snuderl M, Kirkpatrick ND, Goveia J, Davidson C, Huang Y, et al. Increase in Tumor-Associated Macrophages After Antiangiogenic Therapy Is Associated With Poor Survival Among Patients With Recurrent Glioblastoma. Neuro Oncol (2013) 15(8):1079–87. doi: 10.1093/neuonc/not082
129. Chen X, Zhang L, Zhang IY, Liang J, Wang H, Ouyang M, et al. RAGE Expression in Tumor-Associated Macrophages Promotes Angiogenesis in Glioma. Cancer Res (2014) 74(24):7285–97. doi: 10.1158/0008-5472.CAN-14-1240
130. Hambardzumyan D, Gutmann DH, Kettenmann H. The Role of Microglia and Macrophages in Glioma Maintenance and Progression. Nat Neurosci (2016) 19(1):20–7. doi: 10.1038/nn.4185
131. Kirkwood JM, Butterfield LH, Tarhini AA, Zarour H, Kalinski P, Ferrone S. Immunotherapy of Cancer in 2012. CA Cancer J Clin (2012) 62(5):309–35. doi: 10.3322/caac.20132
132. Anguille S, Smits EL, Lion E, van Tendeloo VF, Berneman ZN. Clinical Use of Dendritic Cells for Cancer Therapy. Lancet Oncol (2014) 15(7):e257–267. doi: 10.1016/S1470-2045(13)70585-0
133. Yu JS, Lee PK, Ehtesham M, Samoto K, Black KL, Wheeler CJ. Intratumoral T Cell Subset Ratios and Fas Ligand Expression on Brain Tumor Endothelium. J Neurooncol (2003) 64(1-2):55–61. doi: 10.1007/BF02700020
134. Hamilton A, Sibson NR. Role of the Systemic Immune System in Brain Metastasis. Mol Cell Neurosci (2013) 53:42–51. doi: 10.1016/j.mcn.2012.10.004
135. Manz MG, Boettcher S. Emergency Granulopoiesis. Nat Rev Immunol (2014) 14(5):302–14. doi: 10.1038/nri3660
136. Cortez-Retamozo V, Etzrodt M, Newton A, Rauch PJ, Chudnovskiy A, Berger C, et al. Origins of Tumor-Associated Macrophages and Neutrophils. Proc Natl Acad Sci U S A (2012) 109(7):2491–6. doi: 10.1073/pnas.1113744109
137. Richards MK, Liu F, Iwasaki H, Akashi K, Link DC. Pivotal Role of Granulocyte Colony-Stimulating Factor in the Development of Progenitors in the Common Myeloid Pathway. Blood (2003) 102(10):3562–8. doi: 10.1182/blood-2003-02-0593
138. Liu F, Wu HY, Wesselschmidt R, Kornaga T, Link DC. Impaired Production and Increased Apoptosis of Neutrophils in Granulocyte Colony-Stimulating Factor Receptor-Deficient Mice. Immunity (1996) 5(5):491–501. doi: 10.1016/S1074-7613(00)80504-X
139. Seymour JF, Lieschke GJ, Grail D, Quilici C, Hodgson G, Dunn AR. Mice Lacking Both Granulocyte Colony-Stimulating Factor (CSF) and Granulocyte-Macrophage CSF Have Impaired Reproductive Capacity, Perturbed Neonatal Granulopoiesis, Lung Disease, Amyloidosis, and Reduced Long-Term Survival. Blood (1997) 90(8):3037–49. doi: 10.1182/blood.V90.8.3037
140. Molineux G, Migdalska A, Szmitkowski M, Zsebo K, Dexter TM. The Effects on Hematopoiesis of Recombinant Stem Cell Factor (Ligand for C-Kit) Administered In Vivo to Mice Either Alone or in Combination With Granulocyte Colony-Stimulating Factor. Blood (1991) 78(4):961–6. doi: 10.1182/blood.V78.4.961.961
141. Liu F, Poursine-Laurent J, Wu HY, Link DC. Interleukin-6 and the Granulocyte Colony-Stimulating Factor Receptor Are Major Independent Regulators of Granulopoiesis In Vivo But Are Not Required for Lineage Commitment or Terminal Differentiation. Blood (1997) 90(7):2583–90. doi: 10.1182/blood.V90.7.2583
142. Mollica Poeta V, Massara M, Capucetti A, Bonecchi R. Chemokines and Chemokine Receptors: New Targets for Cancer Immunotherapy. Front Immunol (2019) 10:379. doi: 10.3389/fimmu.2019.00379
143. Jamieson T, Clarke M, Steele CW, Samuel MS, Neumann J, Jung A, et al. Inhibition of CXCR2 Profoundly Suppresses Inflammation-Driven and Spontaneous Tumorigenesis. J Clin Invest (2012) 122(9):3127–44. doi: 10.1172/JCI61067
144. Oppenheim JJ, Zachariae CO, Mukaida N, Matsushima K. Properties of the Novel Proinflammatory Supergene "Intercrine" Cytokine Family. Annu Rev Immunol (1991) 9:617–48. doi: 10.1146/annurev.iy.09.040191.003153
145. Shang K, Bai YP, Wang C, Wang Z, Gu HY, Du X, et al. Crucial Involvement of Tumor-Associated Neutrophils in the Regulation of Chronic Colitis-Associated Carcinogenesis in Mice. PLoS One (2012) 7(12):e51848. doi: 10.1371/journal.pone.0051848
146. Popivanova BK, Kitamura K, Wu Y, Kondo T, Kagaya T, Kaneko S, et al. Blocking TNF-Alpha in Mice Reduces Colorectal Carcinogenesis Associated With Chronic Colitis. J Clin Invest (2008) 118(2):560–70. doi: 10.1172/JCI32453
147. Antonio N, Bønnelykke-Behrndtz ML, Ward LC, Collin J, Christensen IJ, Steiniche T, et al. The Wound Inflammatory Response Exacerbates Growth of Pre-Neoplastic Cells and Progression to Cancer. EMBO J (2015) 34(17):2219–36. doi: 10.15252/embj.201490147
148. Yang L, Liu Z, Wu R, Yao Q, Gu Z, Liu M. Correlation of C-X-C Chemokine Receptor 2 Upregulation With Poor Prognosis and Recurrence in Human Glioma. Onco Targets Ther (2015) 8:3203–9. doi: 10.2147/OTT.S91626
149. Waugh DJ, Wilson C. The Interleukin-8 Pathway in Cancer. Clin Cancer Res (2008) 14(21):6735–41. doi: 10.1158/1078-0432.CCR-07-4843
150. Chen Y, Douglass T, Jeffes EW, Xu Q, Williams CC, Arpajirakul N, et al. Living T9 Glioma Cells Expressing Membrane Macrophage Colony-Stimulating Factor Produce Immediate Tumor Destruction by Polymorphonuclear Leukocytes and Macrophages via a "Paraptosis"-Induced Pathway That Promotes Systemic Immunity Against Intracranial T9 Gliomas. Blood (2002) 100(4):1373–80. doi: 10.1182/blood-2002-01-0174
151. Dinarello CA. Immunological and Inflammatory Functions of the Interleukin-1 Family. Annu Rev Immunol (2009) 27:519–50. doi: 10.1146/annurev.immunol.021908.132612
152. Klemm F, Maas RR, Bowman RL, Kornete M, Soukup K, Nassiri S, et al. Interrogation of the Microenvironmental Landscape in Brain Tumors Reveals Disease-Specific Alterations of Immune Cells. Cell (2020) 181(7):1643–1660.e1617. doi: 10.1016/j.cell.2020.05.007
153. Zhang Q, Zhong H, Fan Y, Liu Q, Song J, Yao S, et al. mmune and Clinical Features of CD96 Expression in Glioma by in Silico Analysis. Front Bioeng Biotechnol (2020) 8:592. doi: 10.3389/fbioe.2020.00592
154. Lu J, Peng Y, Huang R, Feng Z, Fan Y, Wang H, et al. Elevated TYROBP Expression Predicts Poor Prognosis and High Tumor Immune Infiltration in Patients With Low-Grade Glioma. BMC Cancer (2021) 21(1):723. doi: 10.1186/s12885-021-08456-6
155. Liu J, Gao L, Ji B, Geng R, Chen J, Tao X, et al. BCL7A as a Novel Prognostic Biomarker for Glioma Patients. J Trans Med (2021) 19(1):335. doi: 10.1186/s12967-021-03003-0
156. Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the Activation and Regulation of Innate and Adaptive Immunity. Nat Rev Immunol (2011) 11(8):519–31. doi: 10.1038/nri3024
157. Chow KH, Park HJ, George J, Yamamoto K, Gallup AD, Graber JH, et al. S100A4 Is a Biomarker and Regulator of Glioma Stem Cells That Is Critical for Mesenchymal Transition in Glioblastoma. Cancer Res (2017) 77(19):5360–73. doi: 10.1158/0008-5472.CAN-17-1294
158. Hawila E, Razon H, Wildbaum G, Blattner C, Sapir Y, Shaked Y, et al. CCR5 Directs the Mobilization of CD11b(+)Gr1(+)Ly6C(low) Polymorphonuclear Myeloid Cells From the Bone Marrow to the Blood to Support Tumor Development. Cell Rep (2017) 21(8):2212–22. doi: 10.1016/j.celrep.2017.10.104
159. Coffelt SB, Kersten K, Doornebal CW, Weiden J, Vrijland K, Hau CS, et al. IL-17-Producing γδ T Cells and Neutrophils Conspire to Promote Breast Cancer Metastasis. Nature (2015) 522(7556):345–8. doi: 10.1038/nature14282
160. Jeon HY, Ham SW, Kim JK, Jin X, Lee SY, Shin YJ, et al. Ly6G(+) Inflammatory Cells Enable the Conversion of Cancer Cells to Cancer Stem Cells in an Irradiated Glioblastoma Model. Cell Death Differ (2019) 26(10):2139–56. doi: 10.1038/s41418-019-0282-0
161. Gao M, Lin Y, Liu X, Zhao Z, Zhu Z, Zhang H, et al. TERT Mutation Is Accompanied by Neutrophil Infiltration and Contributes to Poor Survival in Isocitrate Dehydrogenase Wild-Type Glioma. Front Cell Dev Biol (2021) 9:654407. doi: 10.3389/fcell.2021.654407
162. Amankulor NM, Kim Y, Arora S, Kargl J, Szulzewsky F, Hanke M, et al. Mutant IDH1 Regulates the Tumor-Associated Immune System in Gliomas. Genes Dev (2017) 31(8):774–86. doi: 10.1101/gad.294991.116
163. Rodriguez PC, Zea AH, Culotta KS, Zabaleta J, Ochoa JB, Ochoa AC. Regulation of T Cell Receptor CD3zeta Chain Expression by L-Arginine. J Biol Chem (2002) 277(24):21123–9. doi: 10.1074/jbc.M110675200
164. Rodriguez PC, Quiceno DG, Ochoa AC. L-Arginine Availability Regulates T-Lymphocyte Cell-Cycle Progression. Blood (2007) 109(4):1568–73. doi: 10.1182/blood-2006-06-031856
165. Waldron TJ, Quatromoni JG, Karakasheva TA, Singhal S, Rustgi AK. Myeloid Derived Suppressor Cells: Targets for Therapy. Oncoimmunology (2013) 2(4):e24117. doi: 10.4161/onci.24117
166. Rivoltini L, Carrabba M, Huber V, Castelli C, Novellino L, Dalerba P, et al. Immunity to Cancer: Attack and Escape in T Lymphocyte-Tumor Cell Interaction. Immunol Rev (2002) 188:97–113. doi: 10.1034/j.1600-065X.2002.18809.x
167. Harari O, Liao JK. Inhibition of MHC II Gene Transcription by Nitric Oxide and Antioxidants. Curr Pharm Des (2004) 10(8):893–8. doi: 10.2174/1381612043452893
168. Bingisser RM, Tilbrook PA, Holt PG, Kees UR. Macrophage-Derived Nitric Oxide Regulates T Cell Activation via Reversible Disruption of the Jak3/STAT5 Signaling Pathway. J Immunol (Baltimore Md 1950) (1998) 160(12):5729–34.
169. Nagaraj S, Gupta K, Pisarev V, Kinarsky L, Sherman S, Kang L, et al. Altered Recognition of Antigen Is a Mechanism of CD8+ T Cell Tolerance in Cancer. Nat Med (2007) 13(7):828–35. doi: 10.1038/nm1609
170. Gabrilovich DI, Nagaraj S. Myeloid-Derived Suppressor Cells as Regulators of the Immune System. Nat Rev Immunol (2009) 9(3):162–74. doi: 10.1038/nri2506
171. Kumar V, Patel S, Tcyganov E, Gabrilovich DI. The Nature of Myeloid-Derived Suppressor Cells in the Tumor Microenvironment. Trends Immunol (2016) 37(3):208–20. doi: 10.1016/j.it.2016.01.004
172. Szuster-Ciesielska A, Hryciuk-Umer E, Stepulak A, Kupisz K, Kandefer-Szerszeń M. Reactive Oxygen Species Production by Blood Neutrophils of Patients With Laryngeal Carcinoma and Antioxidative Enzyme Activity in Their Blood. Acta Oncol (Stockholm Sweden) (2004) 43(3):252–8. doi: 10.1080/02841860410029708
173. Schmielau J, Finn OJ. Activated Granulocytes and Granulocyte-Derived Hydrogen Peroxide Are the Underlying Mechanism of Suppression of T-Cell Function in Advanced Cancer Patients. Cancer Res (2001) 61(12):4756–60.
174. Kusmartsev S, Nefedova Y, Yoder D, Gabrilovich DI. Antigen-Specific Inhibition of CD8+ T Cell Response by Immature Myeloid Cells in Cancer Is Mediated by Reactive Oxygen Species. J Immunol (Baltimore Md 1950) (2004) 172(2):989–99. doi: 10.4049/jimmunol.172.2.989
175. Dubinski D, Wölfer J, Hasselblatt M, Schneider-Hohendorf T, Bogdahn U, Stummer W, et al. CD4+ T Effector Memory Cell Dysfunction Is Associated With the Accumulation of Granulocytic Myeloid-Derived Suppressor Cells in Glioblastoma Patients. Neuro Oncol (2016) 18(6):807–18. doi: 10.1093/neuonc/nov280
176. Raychaudhuri B, Rayman P, Ireland J, Ko J, Rini B, Borden EC, et al. Myeloid-Derived Suppressor Cell Accumulation and Function in Patients With Newly Diagnosed Glioblastoma. Neuro Oncol (2011) 13(6):591–9. doi: 10.1093/neuonc/nor042
177. Gielen PR, Schulte BM, Kers-Rebel ED, Verrijp K, Petersen-Baltussen HM, ter Laan M, et al. Increase in Both CD14-Positive and CD15-Positive Myeloid-Derived Suppressor Cell Subpopulations in the Blood of Patients With Glioma But Predominance of CD15-Positive Myeloid-Derived Suppressor Cells in Glioma Tissue. J Neuropathol Exp Neurol (2015) 74(5):390–400. doi: 10.1097/NEN.0000000000000183
178. Cordell EC, Alghamri MS, Castro MG, Gutmann DH. T Lymphocytes as Dynamic Regulators of Glioma Pathobiology. Neuro Oncol (2022). doi: 10.1093/neuonc/noac055
179. Gatto L, Di Nunno V, Franceschi E, Tosoni A, Bartolini S, Brandes AA. Pharmacotherapeutic Treatment of Glioblastoma: Where Are We to Date? Drugs (2022) 82(5):491–510. doi: 10.1007/s40265-022-01702-6
180. Chang Y, Syahirah R, Wang X, Jin G, Torregrosa-Allen SE, Elzey BD, et al. Engineering Chimeric Antigen Receptor Neutrophils From Human Pluripotent Stem Cells for Targeted Cancer Immunotherapy. BioRxiv (2022). 2022.2003.2002.482679. doi: 10.1101/2022.03.02.482679
181. Graf MR, Prins RM, Merchant RE. IL-6 Secretion by a Rat T9 Glioma Clone Induces a Neutrophil-Dependent Antitumor Response With Resultant Cellular, Antiglioma Immunity. J Immunol (Baltimore Md 1950) (2001) 166(1):121–9. doi: 10.4049/jimmunol.166.1.121
182. Karpel-Massler G, Kast RE, Siegelin MD, Dwucet A, Schneider E, Westhoff MA, et al. Anti-Glioma Activity of Dapsone and Its Enhancement by Synthetic Chemical Modification. Neurochem Res (2017) 42(12):3382–9. doi: 10.1007/s11064-017-2378-6
183. Wang J, Tang W, Yang M, Yin Y, Li H, Hu F, et al. Inflammatory Tumor Microenvironment Responsive Neutrophil Exosomes-Based Drug Delivery System for Targeted Glioma Therapy. Biomaterials (2021) 273:120784. doi: 10.1016/j.biomaterials.2021.120784
184. Xue J, Zhao Z, Zhang L, Xue L, Shen S, Wen Y, et al. Neutrophil-Mediated Anticancer Drug Delivery for Suppression of Postoperative Malignant Glioma Recurrence. Nat Nanotechnol (2017) 12(7):692–700. doi: 10.1038/nnano.2017.54
185. Batchelor TT, Reardon DA, de Groot JF, Wick W, Weller M. Antiangiogenic Therapy for Glioblastoma: Current Status and Future Prospects. Clin Cancer Res (2014) 20(22):5612–9. doi: 10.1158/1078-0432.CCR-14-0834
186. Chinot OL, Wick W, Mason W, Henriksson R, Saran F, Nishikawa R, et al. Bevacizumab Plus Radiotherapy-Temozolomide for Newly Diagnosed Glioblastoma. N Engl J Med (2014) 370(8):709–22. doi: 10.1056/NEJMoa1308345
187. Wang PF, Zhang YX, Su J, Yao K, Li SW, Huang GR, et al. Neutrophil Depletion Enhances the Therapeutic Effect of PD-1 Antibody on Glioma. Aging (2020) 12(15):15290–301. doi: 10.18632/aging.103428
188. Wen F, Shen A, Choi A, Gerner EW, Shi J. Extracellular DNA in Pancreatic Cancer Promotes Cell Invasion and Metastasis. Cancer Res (2013) 73(14):4256–66. doi: 10.1158/0008-5472.CAN-12-3287
189. Meurer M, Öhlmann S, Bonilla MC, Valentin-Weigand P, Beineke A, Hennig-Pauka I, et al. Role of Bacterial and Host DNases on Host-Pathogen Interaction During Streptococcus Suis Meningitis. Int J Mol Sci (2020) 21(15):5289. doi: 10.3390/ijms21155289
190. Knight JS, Subramanian V, O'Dell AA, Yalavarthi S, Zhao W, Smith CK, et al. Peptidylarginine Deiminase Inhibition Disrupts NET Formation and Protects Against Kidney, Skin and Vascular Disease in Lupus-Prone MRL/lpr Mice. Ann Rheum Dis (2015) 74(12):2199–206. doi: 10.1136/annrheumdis-2014-205365
191. Tadie JM, Bae HB, Jiang S, Park DW, Bell CP, Yang H, et al. HMGB1 Promotes Neutrophil Extracellular Trap Formation Through Interactions With Toll-Like Receptor 4. American Journal of Physiology. Lung Cell Mol Physiol (2013) 304(5):L342–349. doi: 10.1152/ajplung.00151.2012
192. Menegazzo L, Scattolini V, Cappellari R, Bonora BM, Albiero M, Bortolozzi M, et al. The Antidiabetic Drug Metformin Blunts NETosis In Vitro and Reduces Circulating NETosis Biomarkers In Vivo. Acta Diabetol (2018) 55(6):593–601. doi: 10.1007/s00592-018-1129-8
193. Lyne SB, Yamini B. An Alternative Pipeline for Glioblastoma Therapeutics: A Systematic Review of Drug Repurposing in Glioblastoma. Cancers (2021) 13(8):1953. doi: 10.3390/cancers13081953
194. Chim ST, Sanfilippo P, O'Brien TJ, Drummond KJ, Monif M. Pretreatment Neutrophil-to-Lymphocyte/Monocyte-to-Lymphocyte Ratio as Prognostic Biomarkers in Glioma Patients. J Neuroimmunol (2021) 361:577754. doi: 10.1016/j.jneuroim.2021.577754
195. Han S, Qu FW, Wang PF, Liu YX, Li SW, Yan CX. Development and Validation of a Nomogram Model Based on Hematological Indicators for Predicting the Prognosis of Diffused Gliomas. Front Surg (2022) 9:803237. doi: 10.3389/fsurg.2022.803237
196. Wang Z, Li J, Yuan Y, Li T, Zuo M, Liu Y. Prognostic Significance of Preoperative Systemic Inflammation Response Index in Newly Diagnosed Glioblastoma Patients Underwent Gross Total Resection: A Propensity Score Matching Analysis. World J Surg Oncol (2022) 20(1):137. doi: 10.1186/s12957-022-02588-0
197. Basheer AS, Abas F, Othman I, Naidu R. Role of Inflammatory Mediators, Macrophages, and Neutrophils in Glioma Maintenance and Progression: Mechanistic Understanding and Potential Therapeutic Applications. Cancers (2021) 13(16):4226. doi: 10.3390/cancers13164226
198. Qu S, Liu J, Wang H. EVA1B to Evaluate the Tumor Immune Microenvironment and Clinical Prognosis in Glioma. Front Immunol (2021) 12:648416. doi: 10.3389/fimmu.2021.648416
199. Yee PP, Wei Y, Kim SY, Lu T, Chih SY, Lawson C, et al. Neutrophil-Induced Ferroptosis Promotes Tumor Necrosis in Glioblastoma Progression. Nat Commun (2020) 11(1):5424. doi: 10.1038/s41467-020-19193-y
200. Massara M, Persico P, Bonavita O, Mollica Poeta V, Locati M, Simonelli M, et al. Neutrophils in Gliomas. Front Immunol (2017) 8:1349. doi: 10.3389/fimmu.2017.01349
201. Rennard SI, Dale DC, Donohue JF, Kanniess F, Magnussen H, Sutherland ER, et al. CXCR2 Antagonist MK-7123. A Phase 2 Proof-Of-Concept Trial for Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med (2015) 191(9):1001–11. doi: 10.1164/rccm.201405-0992OC
202. Lavoie SS, Dumas E, Vulesevic B, Neagoe PE, White M, Sirois MG. Synthesis of Human Neutrophil Extracellular Traps Contributes to Angiopoietin-Mediated In Vitro Proinflammatory and Proangiogenic Activities. J Immunol (Baltimore Md 1950) (2018) 200(11):3801–13. doi: 10.4049/jimmunol.1701203
Keywords: glioma, neutrophils, tumour microenvironment, immunosuppression, treatment
Citation: Wang G, Wang J, Niu C, Zhao Y and Wu P (2022) Neutrophils: New Critical Regulators of Glioma. Front. Immunol. 13:927233. doi: 10.3389/fimmu.2022.927233
Received: 24 April 2022; Accepted: 06 June 2022;
Published: 04 July 2022.
Edited by:
Stefano Ugel, University of Verona, ItalyReviewed by:
Elena Zenaro, University of Verona, ItalyCopyright © 2022 Wang, Wang, Niu, Zhao and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pengfei Wu, d3VwZW5nZmVpQHVzdGMuZWR1LmNu; Yan Zhao, emhhb3lhbjMwMDBAMTYzLmNvbQ==; Chaoshi Niu, bml1Y2hhb3NoaUB1c3RjLmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.