- 1University of Minnesota Cancer Center and the Department of Pediatrics, Division of Blood & Marrow Transplant & Cellular Therapy, Minneapolis, MN, United States
- 2Division of Hematology, Mayo Clinic, Rochester, MN, United States
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a curative therapy for many types of cancer. Genetic disparities between donor and host can result in immune-mediated attack of host tissues, known as graft versus host disease (GVHD), a major cause of morbidity and mortality following HSCT. Regulatory CD4+ T cells (Tregs) are a rare cell type crucial for immune system homeostasis, limiting the activation and differentiation of effector T cells (Teff) that are self-reactive or stimulated by foreign antigen exposure. Adoptive cell therapy (ACT) with Treg has demonstrated, first in murine models and now in patients, that prophylactic Treg infusion can also suppress GVHD. While clinical trials have demonstrated Treg reduce severe GVHD occurrence, several impediments remain, including Treg variability and practical need for individualized Treg production for each patient. Additionally, there are challenges in the use of in vitro expansion techniques and in achieving in vivo Treg persistence in context of both immune suppressive drugs and in lymphoreplete patients being treated for GVHD. This review will focus on 3 main translational approaches taken to improve the efficacy of tTreg ACT in GVHD prophylaxis and development of treatment options, following HSCT: genetic modification, manipulating TCR and cytokine signaling, and Treg production protocols. In vitro expansion for Treg ACT presents a multitude of approaches for gene modification to improve efficacy, including: antigen specificity, tissue targeting, deletion of negative regulators/exhaustion markers, resistance to immunosuppressive drugs common in GVHD treatment. Such expansion is particularly important in patients without significant lymphopenia that can drive Treg expansion, enabling a favorable Treg:Teff ratio in vivo. Several potential therapeutics have also been identified that enhance tTreg stability or persistence/expansion following ACT that target specific pathways, including: DNA/histone methylation status, TCR/co-stimulation signaling, and IL-2/STAT5 signaling. Finally, this review will discuss improvements in Treg production related to tissue source, Treg subsets, therapeutic approaches to increase Treg suppression and stability during tTreg expansion, and potential for storing large numbers of Treg from a single production run to be used as an off-the-shelf infusion product capable of treating multiple recipients.
Introduction
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a curative therapy for many types of cancer (1). Genetic disparities between donor and host can result in graft-versus-host disease (GVHD), a major cause of morbidity and mortality following allo-HSCT (2). Regulatory CD4+25++FoxP3+ T cells (Tregs) are present at low frequency and are crucial for immune system homeostasis by limiting the activation and differentiation of effector T cells (Teff) that are self-reactive or stimulated by foreign antigen (Figure 1) exposure (3). Adoptive cell therapy (ACT) with Tregs has demonstrated, first in murine models and now in patients, that prophylactic Treg infusion can also suppress GVHD (4–6). While clinical trials have demonstrated Tregs reduce severe GVHD occurrence, several impediments remain, including the practical need for individualized Treg production for each patient (5, 7). Additional challenges exist in achieving in vivo Treg persistence, especially in the context of immune suppressive drugs given to patients for GVHD prevention or treatment (8).
This review will focus on translational approaches taken to improve the efficacy of Treg ACT such as manipulating T cell receptor (TCR) and cytokine signaling, in vitro expansion and genome modifications to improve antigen specificity, GVHD target tissue migration, and therapeutics to enhance Treg stability or persistence/expansion following ACT (9). Lastly, this review will discuss improvements in Treg production related to tissue source, Treg subsets, suppressor potency and stability, and potential for use as an off-the-shelf product capable of treating multiple recipients.
CD4 Treg background
CD4+ Tregs can be divided into three main classes based upon site of development. Thymic Tregs (tTregs) are CD4+25++FoxP3+ cells formed in the thymus. Peripheral Tregs (pTregs)and induced Tregs (iTregs) acquire Foxp3 and suppressor function in vivo or in vitro, respectively. Type 1 regulatory T (Tr1) cells also can arise in vivo in the periphery or induced in vitro; Tr1 cells do not express FoxP3, require the transcription factors Tbet and B lymphocyte-induced maturation protein-1 (Blimp-1) (10), and secrete IL-10 as the primary mechanism for their suppressive function (11).
Regulatory CD4+ T cells (Tregs) are a rare cell type crucial for immune system homeostasis, limiting the activation and differentiation of effector T cells (Teff) that are self-reactive or stimulated by foreign antigen exposure (3). Treg are characteristically defined by the constitutive expression of both Foxp3 and high expression of CD25, compared to conventional T-cells (Tcon) which typically express significantly lower levels of both CD25 and Foxp3 (12). However, human Tcon can also transiently express Foxp3 following TCR stimulation, thus human FoxP3+ T-cells consist of a heterogeneous population of both Treg and activated Tcon. CD127 expression has been shown to inversely correlate with the expression of Foxp3 in human T-cells (13). Therefore, human Treg are characterized as CD127lo (i.e. CD4+CD25+CD127loFoxp3+). CD4+25++ Treg also co-express high levels of several immunosuppressive functional markers, including CTLA-4, Lag3, TIGIT, Tim-3 and PD-1, which directly contribute to the critical suppressive function of this population (14–17), as well as CD39 and CD73 (18). Treg also constitutively express a number of co-stimulatory molecules, including 4-1BB, OX-40, TNFRII, TNFRSF25 (19). While expression is not restricted to Treg, Helios and neuropilin-1 expression have been shown to increase Treg stability in vivo (20–22).
Interestingly, several mechanisms used by Treg for suppression of Teff responses also help stabilize the Treg phenotype. For example, high expression of CD25 by tTreg and iTreg may preferentially facilitate IL-2 signaling to Treg and, via competition, diminish IL-2 signaling of Teffs (23). Similarly, multiple subsets of tTreg, iTreg and Tr1 cells produce the immunosuppressive cytokines TGFß and IL-10, which concomitantly promote Treg stability while limiting Teff activation and differentiation (11, 23, 24). Treg also secrete the immunosuppressive cytokine IL-35, which has recently been shown to induce infectious tolerance and/or T cell exhaustion (25, 26). Treg also use metabolic intermediates to suppress T cell activation, including extracellular production of adenosine through the concordant expression of CD39 and CD73 (27) and the direct transfer of the potent inhibitory second messenger cAMP to T cells (23). Treg expression of CTLA4 can induce DC expression of indoleamine 2,3- dioxygenase (IDO), which suppresses via depletion of tryptophan and commensurate production of kynurenines (28).
Treg can also directly induce T cell death by several pathways. Human Treg and Tr1 cells can directly lyse T cells via a perforin and granzyme A or B mechanism, respectively (29, 30). Alternatively, Treg can induce T cell apoptosis via a TRAIL-DR5 pathway or through expression of galectin-1 (31) or FasL (32).
CD4 Treg ACT clinical trials
Despite strong evidence of the in vivo efficacy of Tregs in murine and xenogeneic models, the initiation of clinical trials was slowed due to difficulties in obtaining sufficient numbers of Tregs without contaminating effector T cells (Teffs) that may subvert Treg potency and stability (33). Another consideration was that supra-physiological murine Treg numbers can cause generalized immunosuppression (34, 35). GVHD, a frequent and severe complicating factor in allo-HSCT (6), represented a unique Treg application venue as the GVHD risk period has a defined onset that begins with the infusion of a known number of donor T cells that can be controlled by certain T cell:Treg ratios. Furthermore, the goal of immunosuppression is to control donor anti-host reactions until the highest risk period has passed, facilitating the development of operational tolerance.
One of the biggest hurdles to the development of a successful GVHD therapy is maintaining the therapeutic GVL effect. There has been concern in the field that Treg ACT would result in global immunosuppression, interfere with an effective GVL response, and potentially induce an aggressive autoimmunity (36). Further concerns included the possibility that infused Treg would convert to Teffs, thereby worsening GVHD. However, murine and xenogeneic experiments showed that Treg did not exacerbate GVHD (32, 37–40). Indeed, over 20 reports on Treg ACT clinical trials found that Treg did not exacerbate GVHD. There is the potential loss of a GVL response. While preclinical studies do not support this as a substantial risk, clinical outcome parameters for cancer recurrence are not sufficiently mature to reach a definitive conclusion.
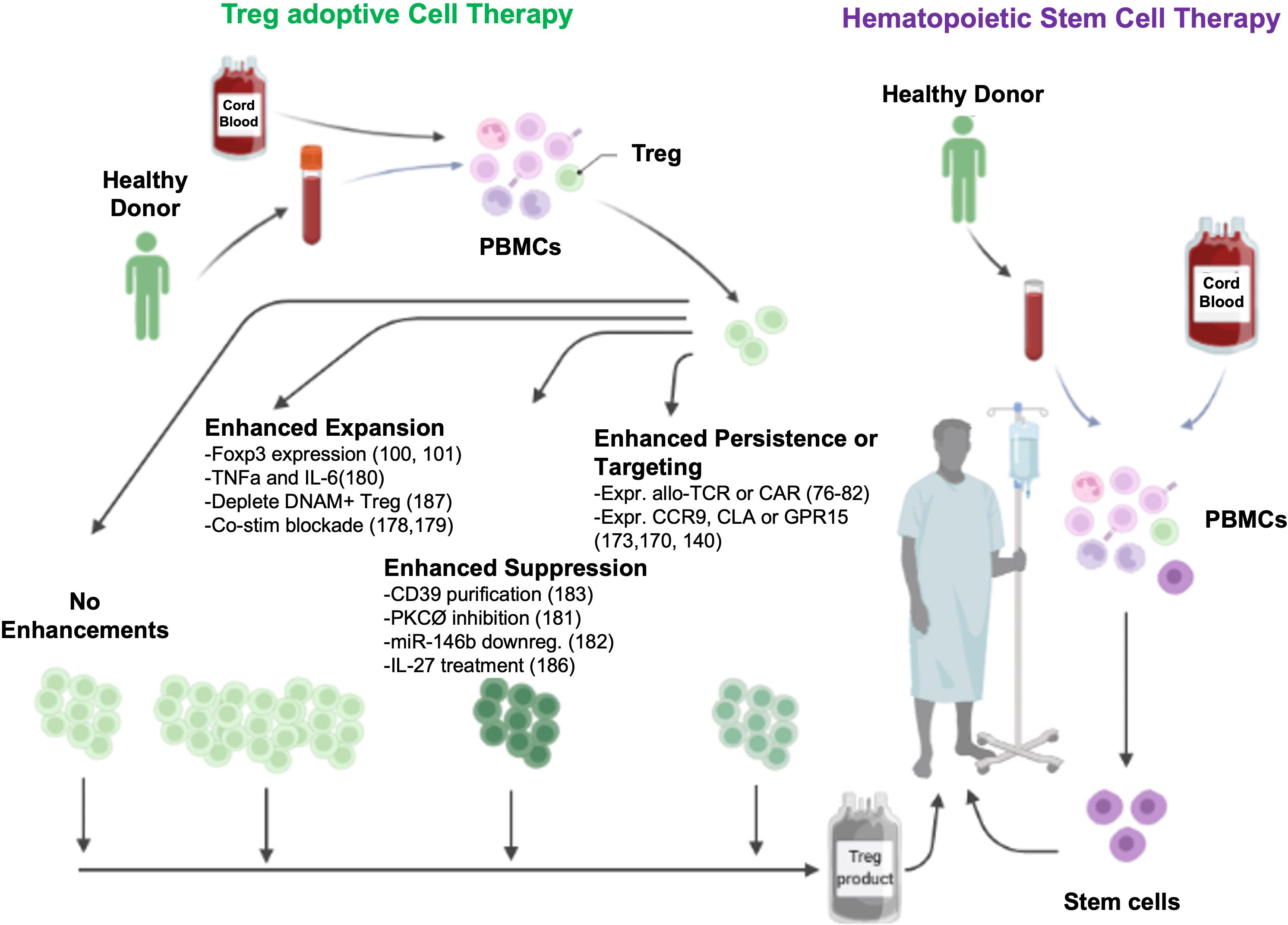
Several groups have now reported Treg ACT acute GVHD (aGVHD) prevention data with variations including whether Treg were in vitro expanded or freshly isolated and directly infused, type and source of Treg, and Treg dose (Table 1). In first-in-human Treg infusions, Treg were flow-sort purified from the initial allo-HSCT donors, expanded in vitro, and then infused into patients with acute or chronic GVHD. Transient improvement for aGVHD and significant reduction in symptoms and immune suppressive drugs were seen (46). In initial Treg ACT studies for GVHD prophylaxis, donor Tregs bead-purified from peripheral blood (PB), no toxicities were seen; however, a limited number of Tregs prevented dose escalation over 5x106/kg studies (41, 42). Efficacy was observed in patients receiving Tregs prior to Tcon infusions, allowing in vivo Treg expansion to occur in lymphopenic recipients, allowing for higher Treg : Tcon ratios (44). To achieve higher Treg cell doses, bead-purified Tregs were expanded in vitro, albeit with lower purity (Foxp3+CD127-) and suppressor function. Adding rapamycin that preferentially inhibits Tcon over Treg expansion (51–54) to bead purified Treg cultures increased purity and suppressor function, allowing assessment of the efficacy of donor Treg ACT on GVHD (NCT00725062). In other concurrent studies, Tregs were purified from umbilical cord blood (UCB); in vitro expansion was achieved with retention of high purity and suppressor function due to a relative lack of contaminating Tcons in UCB as compared to PB. The initial study showed modest reduction in aGVHD in recipients of third-party expanded UCB blood Tregs at a dose of 3x106/kg (4). In a follow-up study employing a second round of Treg expansion, doses of up to100x106/kg virtually eliminated aGVHD with a cumulative incidence of only 9% at 100 days (5).
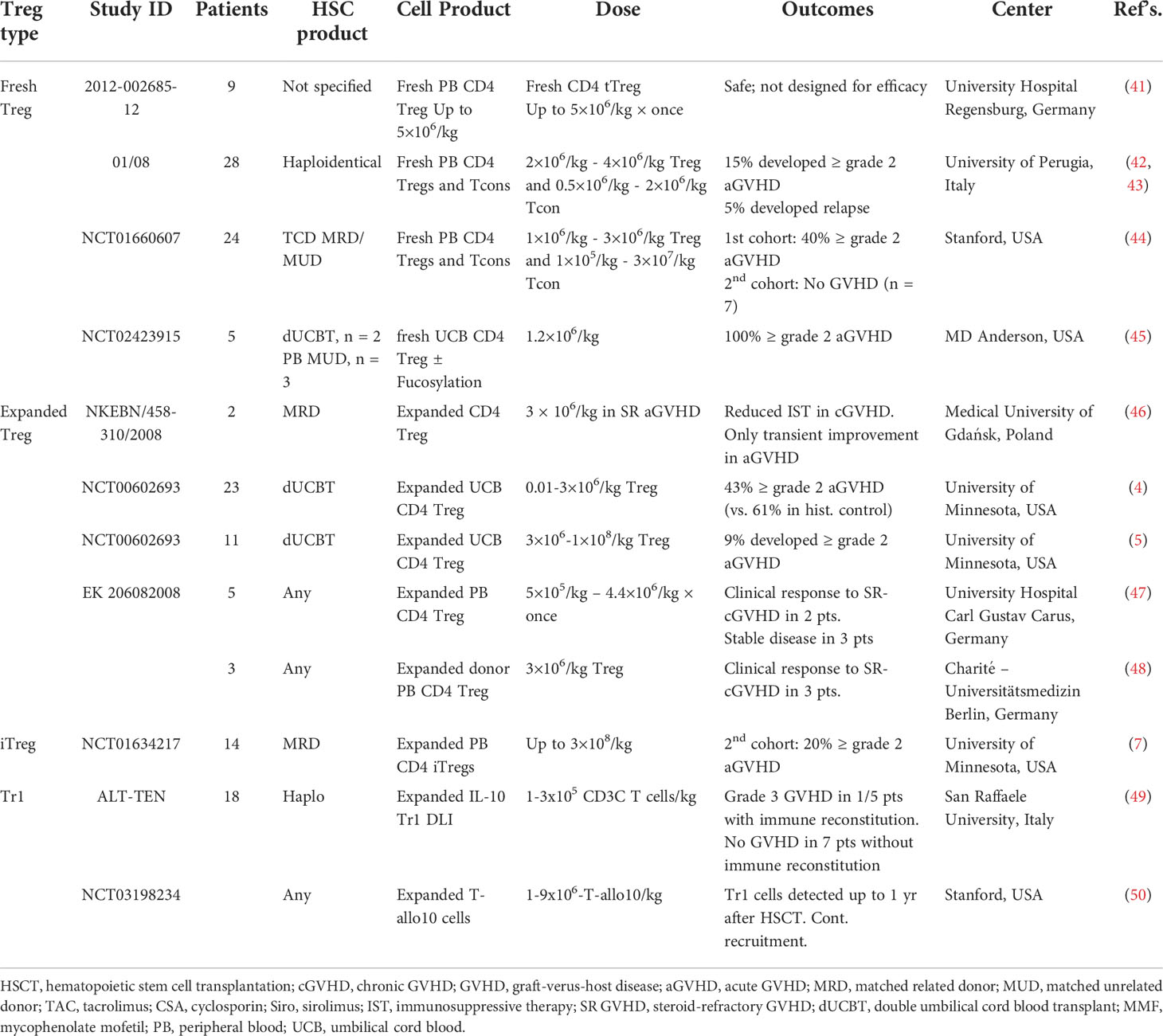
Table 1 Completed clinical trials with results involving adoptive Treg therapy in GVHD (search date March 30, 2022).
Protocols have been developed to induce regulatory function in PB CD4 non-Treg cells by expanding Tcons in the presence of anti-CD3 antibody, TGFβ and rapamycin (37). These iTregs were as suppressive in vitro and in vivo as pTregs. Because PB Tcons are far more abundant than Tregs, yields were as much as 50-fold higher than initial PB and UCB Treg clinical trials. Despite concerns for iTreg de-differentiation to Teffs (termed plasticity), iTregs given as GVHD prophylaxis were well-tolerated at doses of 300x106/kg with no clinical or laboratory evidence of iTreg plasticity (7).
Tr1s, initially shown to mediate tolerance following allo-HSCT in severe combined immune deficiency patients, have desirable properties such as antigen specificity and a direct graft-vs-leukemia (GVL) effect against some tumors (55). Tr1 ACT was then used in a proof-of-concept study treating patients receiving allo-HSCT for hematological malignancies (49). Their in vivo suppressive role is can best be demonstrated in situations in which Tregs are present at low to negligible levels such as aGVHD, wherein Tr1 become the main Treg subset; conversely, under these conditions, Tr1 deficiency can lead to GVHD progression (11). Roncarolo, Bachetta and colleagues are conducting a dose-escalation study (1-9x106/kg) with host allo-antigen driven Tr1 cells; preliminary analysis shows that therapy is well-tolerated, with long-term persistence of Tr1 cells (50, 56).
In addition to the varied types of Treg used for ACT, these products differed in their state of differentiation. Most Treg ACT trials have used cells purified from PB as a readily accessible Treg cell source. The majority (>80%) of PB Tregs (and Tcons) are antigen-experienced (i.e. CD45RO+) and have been shown to expand to a lesser extent than their naïve counterparts (57–59). In contrast, Tregs isolated from UCB are >90% naïve (33) as are tTregs isolated from pediatric thymi often removed to better expose the operating field in children born with congenital heart defects (60, 61).
Impact of different immunosuppressive drugs on Treg function
One significant consideration for the use of Treg ACT for either prophylaxis or treatment of GVHD are the wide range of immunosuppressants used in the transplant setting. Studies with murine and human T cells have shown that treatment with JAK inhibitors (Ruxolitinib, JAK1/2 or Pacritinib, JAK2) can increase the relative proportion of Treg following transplant (62, 63). Similarly, Treg expression of aldehyde dehydrogenase preferentially allows Treg compared to Teffector (Teff) survival in the presence of cyclophosphamide treatment during HSCT (64). Rapamycin, an mTOR inhibitor, allows preferential survival of Treg over Teff in vitro and in vivo (40, 52, 53), owing to Foxp3-mediated expression of Pim2, a kinase with substrate overlap with Akt and, by extension, mTOR (51). In contrast, cyclosporin A (CsA) inhibits Treg persistence and suppressor function in vitro and in an ACT model in vivo (65).
Genetic engineering to improve Treg specificity and suppressor function
In preclinical studies, antigen-specific Tregs have superior potency on a per cell basis as compared to polyclonal Tregs and as a result of antigen-specificity, decreased risk of global immunosuppression (66–68). Although alloantigen-reactive Tregs can be expanded via repetitive stimulation with host antigen-presenting cells (APCs), clinical translation has proven to be challenging due to the low frequency of such tTregs and pTregs present in PB (69, 70).
To confer antigen specificity, polyclonal Tregs can be transduced with a recombinant antigen-specific TCR or CAR directed to the desired antigen (71–73). TCR delivery has been tested in various preclinical models of autoimmune diseases and transplantation (74–77). In the context of GVHD, Semple et al. showed that iTregs generated from chicken ovalbumin (OVA)-reactive CD4 OT-II TCR transgenic T cells efficiently prevented aGVHD induced by polyclonal Teffs in allogeneic recipients that expressed OVA protein, but not in OVA(-) recipients (78). In a subsequent study, Li et al. generated iTregs reactive to minor histocompatibility antigens that are encoded on the Y-chromosome. Male histocompatibility (H-Y)-specific iTregs isolated from TCR transgenic mice were highly effective in controlling GVHD in an antigen-dependent manner while sparing the GVL effect against acute or pre-established leukemia (79). While these studies provide a rationale for further development of TCR-specific Treg therapies, translating TCR gene modifications into the clinic for use in GVHD prophylaxis and treatment is hampered by the necessity that the host target antigens need to be presented in the context of a specific HLA determinant, or of the direct allorecognition of the “foreign” host HLA-determinant itself. Furthermore, mispairing of the endogenous and engineered TCR chains can cause undesired reactivity and off-target effects (80). Various strategies have been explored to reduce this issue, including genome editing techniques to partially knockdown or knockout endogenous TCR expression, as well as using TCR chains that are structurally modified in the constant region, such that they pair with endogenous chains with lower efficiency (81–83).
While TCRs can recognize both intracellular and surface antigens, CAR recognition is limited to cell surface proteins. However, CARs have the advantage of being MHC independent and their function can further be regulated via co-stimulatory signal potentiation (84, 85). Furthermore, Tregs possess a unique feature of bystander suppression which enables targeting of third-party antigens present in the same tissue to induce endogenous tolerogenic cells through a process known as infectious tolerance (86–89). This modality is particularly advantageous in diseases with no defined causative antigen (Figure 1).
The first CAR Tregs developed with the specific aim of reducing alloimmunity were targeted against HLA-A2, a frequently mismatched antigen in allo-HSCT (90). Tregs expressing an HLA-A2 CAR were shown to inhibit xenogeneic GvHD more effectively than polyclonal Tregs on a per cell basis (90). In subsequent studies, HLA-A2 CAR Tregs were shown to migrate to HLA-A2 expressing skin and islet grafts, alleviating the alloimmune-mediated graft rejection in humanized mice (91, 92). These promising results have led to the authorization of the first CAR-Treg clinical trial in the UK and the Netherlands (STeadfast) to evaluate the safety and tolerability of an autologous HLA-A2-specific Treg therapy (TX200-TR101 product) for HLA-A2 mismatched kidney transplant recipients (EUCTR2019-001730-34-NL and NCT04817774). Results of the STeadfast trial,may further support the application of CAR Tregs in a clinical trial setting, further expanding the possibility of using CAR Tregs in other disease conditions. As such, the results of this study are highly anticipated.
Another antigen recently applied to CAR Tregs for preventing GVHD in preclinical studies is CD19 expressed on B cells (85, 93). Using a xenogeneic GVHD model, Imura et al. showed that GvHD-suppressing effect of human CD19-CAR Tregs was greater than that of polyclonal Tregs in immune deficient mice given peripheral blood mononuclear cells, probably because such Tregs could specifically expand in response to B cells (93). As such, CD19-CAR Tregs may also be a potential candidate for treating chronic GVHD and antibody-mediated autoimmune conditions due to their capacity to inhibit antibody production (93).Several studies have investigated the effects of incorporating different costimulatory motifs into CAR Tregs. Dawson et al, compared 10 costimulatory domains, including CD28, 4-1BB, ICOS, CTLA-4, PD-1, GITR, OX40 and TNFR2, in a xenogeneic GVHD model using the HLA-A2 CAR Treg platform (85). These data, as well as those of three other independent studies, confirmed that CAR Tregs encoding a CD28 signal have superior in vitro and in vivo suppressor function (85, 93–95). These studies highlight the fact that intracellular signaling domains most effective in CAR-T cells do not necessarily apply to CAR-Tregs. Understanding how different CAR designs affect Treg function merits further exploration (71).
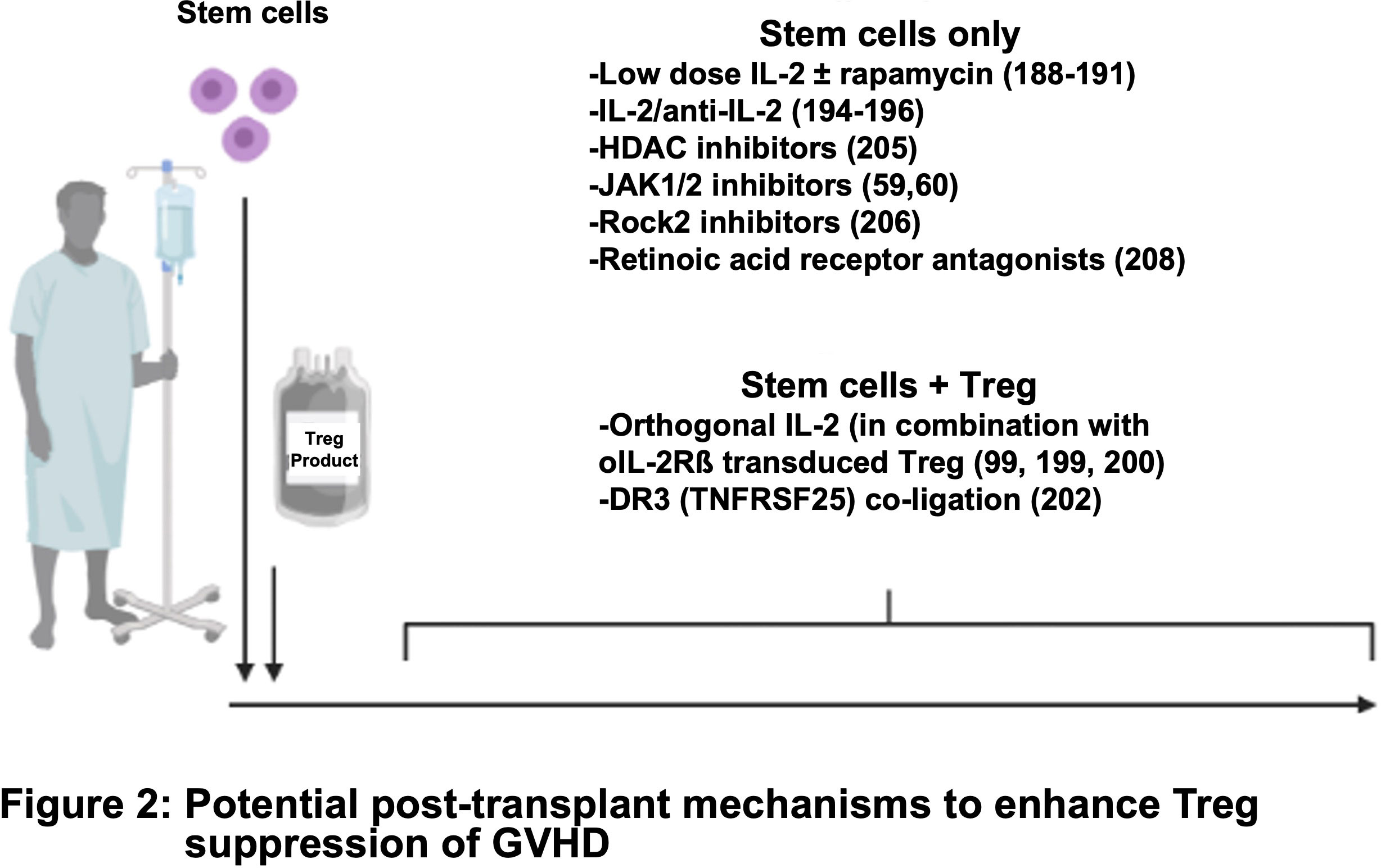
Recent advances in the field of cancer immunotherapy have inspired the adoption of innovative CAR designs. Rana et al. compared the functionality of a FVIII-specific second-generation CAR Treg with that of a TCR fusion construct (TruC) generated via linking of the FVIII scFV to CD3ϵ TCR chain (96). High-affinity second-generation CAR engagement led to strong TCR independent signaling and loss of Treg suppressor function along with limited in vivo persistence. In contrast, TruC Tregs delivered controlled antigen-specific, TCR-dependent signaling via engagement of the CAR along with the TCR complex to suppress FVIII-specific antibody response (96). Modular CARs, also known as universal CARs or switchable CARs, have also been applied to the field of CAR Tregs (97, 98). In this approach, the target antigen is not recognized directly by the CAR but rather by an adaptor encoding a tag such as biotin or fluorescein isothiocyanate (FITC) that is recognized by the CAR. A single CAR can thus be used to recognize a wide range of target antigens via a designated FITC- or biotin-conjugated antibody (97, 98). More recently, third generation CARs with two costimulatory motifs and fourth generation CARs which co-express constitutive or inducible factors such as cytokines or transcription factors have been developed (99–101). These have not been reported for Tregs to date; however, one can envision that a similar approach can be used to engineer a fourth generation CAR Treg with tailored cytokine support in order to modulate their function and stability more precisely (Figure 2) (102).
FoxP3 gene editing to generate Tregs
Because of the challenges associated with isolating a pure population of Tregs, genetic engineering has been used to enforce FoxP3 expression (103, 104). Although initial studies showed that ectopic expression of FoxP3 could induce a regulatory phenotype, subsequent studies have shown that FoxP3 expression alone is not sufficient to imprint a stable (resistant to plasticity) and fully functional Treg phenotype (105–107). The difference between tTregs, pTregs and FoxP3-converted T cells may lie in the FoxP3 expression level needed to stabilize the Treg phenotype (106). Allan et al. highlighted the importance of delivering the FoxP3 gene with a strong promoter to drive constitutive expression with limited fluctuation depending on the cell activation state (105). Similar findings were reported by Honaker et al, who used DNA editing techniques together with a homology directed repair to insert a strong promoter into the endogenous FOXP3 locus (108). More recently, Lam et al. published an optimized method for efficient and stable human Treg expansion with CRISPR-mediated FoxP3 gene knock-in (109). Collectively, these efforts highlight the importance of novel directed gene editing techniques in the design and development of next-generation Treg therapies.
Tissue targeting
It is well-established that Tregs found within different tissue niches can represent phenotypically and functionally distinct Treg subsets critical for local immune homeostasis and regulation of tissue-specific inflammatory disease, including GVHD (110–112). Treg heterogeneity is directly influenced by the immense diversity of cellular and non-cellular mediators in each specialized tissue microenvironment (110, 113, 114). As such, tissue niche-specific Treg subsets often have differential gene expression, including cytokine receptors that can provide a selective advantage within each tissue microenvironment (112, 115). Further, the mechanisms by which Treg migrate and infiltrate into these peripheral tissues have also been shown to play a critical role in immune regulation. Therefore, ex vivo Treg manipulation to facilitate homing to and survival within these tissue-specific niches may enhance the efficacy of Tregs in vivo in controlling those local environments.
Organ systems often take advantage of local tissue-specific stimuli to modulate local immune responses. In particular, tissue-specific Tregs are readily influenced by diverse environmental mediators within each distinct tissue microenvironment which may directly contribute to local immune homeostasis and the pathology of a wide-range of human disease, including GVHD (110–112, 116–119). For example, While.bone marrow (BM)-Tregs have several distinct characteristics and functional requirements that differ from other peripheral Treg populations, including differential upregulation of cytokine and chemokine receptors that may provide BM-Tregs with a unique selective advantage in that compartment (112). The BM niche is an extremely diverse and complex tissue (120–122). Previous work has suggested that the variable distribution and composition of different niches even within the BM itself can differentially impact important T-cell functions including proliferation, differentiation, migration and quiescence (112, 123). Similarly, unlike splenic Tregs, BM-Tregs proved to be minimally responsive to exogenous IL-2 given in vivo; instead, recombinant IL-9 significantly increased BM-Treg frequency while having no impact on the frequency of splenic Tregs (112). IL-9 is required for optimal maintenance of Treg suppressor function (124, 125). We observed both an upregulated expression of IL-9R in BM-Treg as well as an enhanced capacity to respond to IL-9 both in vitro and in vivo. Collectively, these data suggest that differential cytokine signaling within the BM niche may provide a distinct survival and functional advantage for BM-Tregs.
Similarly, within the gastrointestinal (GI) tract, differential expression and release of local simulants have been shown to both induce the production of pTregs within the gut and help to promote Treg localization and retention within the GI tract (126–130). The release of environmental factors, including TGF-β and retinoic acid (RA), drives local pTreg differentiation in the gut tissue (131–136) by contributing to gut immune homeostasis even under inflammatory conditions (137–139). Interestingly, T-cell in vitro exposure to RA and TGF-β is also associated with the induction of gut tropism and enhances the expression of several gut-associated T-cell homing receptors (126, 128).
Lymphocyte migration is well-established as a fundamental mechanism for the maintenance of normal immune function and is integral in controlling the pathology of inflammatory disease (140–142). Within the context of GVHD, T-cell and Treg homing can influence the initiation, severity, and prevention of GVHD (139, 143–150). Tissue-specific pathology within GVHD target organs, including the skin, liver, and GI tract is illustrative of the significance of T-cell and Treg homing mechanisms in GVHD pathology (139, 149, 151). In response to local inflammation and associated tissue damage, homing receptor ligands and chemoattractant receptors are upregulated by injured stromal cells (142, 152), providing directional cues for Teff and Treg migration to inflamed tissue. Because GI tract injury and inflammation are major drivers of disease severity (139, 145, 153–155), targeted the specific targeting of Tregs to the GI tract may be highly advantageous in mitigating disease severity and improving outcomes. Beilhack et al. (149), demonstrated that allogeneic donor T-cells first expanded within secondary lymphoid organs (SLO) then migrated to GVHD target organs. Similarly, this group later reported that Tregs were able to colocalize with allogeneic donor T-cells during GVHD, initially expanding within SLOs then migrating into inflamed tissues (148). Inflammation caused by irradiation and GVHD-associated pathology provided crucial stimuli for early Treg migration to these sites of donor T cell localization, reducing allogeneic T-cell proliferation and activation in vivo (148). Several studies have reported an integral role for GI homing of T-cells for both the initiation and prevention of GVHD (143, 145, 147, 156), although these findings can vary depending upon the intensity of conditioning and the pathogenic mechanisms responsible for GVHD (156). T-cell homing the GI tract is facilitated by distinct tissue-specific mechanisms that attract T-cells to the small or large intestines (126, 157–161). These pathways are primarily regulated by the expression of CCR9, α4β7 and GPR-15 (126, 127, 142, 157, 162–165). In particular, the expression of CCR9 and integrin α4β7 are integral to T-cell trafficking during GVHD. In a 2006 study Waldman et al. (145) demonstrated that alloreactive donor T-cells from α4β7-/- transgenic mice had a reduced capacity to cause GVHD, with a corresponding reduction in T-cell infiltration and tissue injury in both the gut and liver. Similarly, a retrospective case study of 59 allo-HSCT patients demonstrated that α4β7 expression was significantly upregulated in memory and naïve T-cell populations and CCR9 in CD8+ memory T-cells in patients who subsequently developed intestinal GVHD (147), studies that led to the testing of anti-α4β7 blocking antibody to prevent and treat aGVHD in the clinic (166–168).
Likewise, the expression of GI tract homing receptors has also been found to play a central role in Treg efficacy during allogeneic HSCT. Engelhardt et al. (143) recently reported that allo-HSCT patients with higher frequencies α4β7+ Treg post-transplant saw a significant increase in Treg infiltration within the GI tract, and correspondingly a reduced organ-specific risk and reduced GVHD severity. Interestingly, this study also reported a distinct negative correlation between the expression of cutaneous leukocyte antigen (CLA) in allogenic T-cell and the associated risk and severity of GVHD of the skin (143). During GVHD, skin involvement is often one of the first and most commonly manifestations of disease, with skin involvement occurring in >80% of aGVHD patients (169, 170). Like GI tract involvement, aGVHD of the skin can significantly impact allo-HSCT patient morbidity. CLA mediates T-cell homing to the skin by interacting its ligand, E-selectin, which is highly expressed on the microvasculature structure within the skin (171–173). This, in combination with the co-expression of several chemokine receptors, including CCR4, CCR6, CCR8, and CCR10, drives T-cell migration towards epithelial surfaces including the skin and GI tract (142, 146, 171, 174, 175). Varona et al. (146) also demonstrated a correlation between CCR6 expression in MHC class II–mismatched T-cells and the associated risk of GVHD in both the skin and GI tract with a significant reduction in the incidence and severity of GVHD in allogenic recipients of CCR6-deficient T-cells. Together, these studies support the notion that tissue-targeted Treg therapy may be a novel approach for GVHD therapies.
This then raises the question of how we can harness tissue-specific homing mechanisms for clinical translation? Recently, Hoeppli et al. (176) described an ex vivo human Treg product tailored to mimic gut-homing primed Tregs. Here, they utilized ex vivo RA stimulation to induced CCR9 expression in human PB CD4+Foxp3+ Tregs (176) and demonstrated that the ex vivo induction of CCR9 expression was sufficient to enhance Treg migration to the GI tract and reduce disease severity in a xenogeneic GVHD model (176). GPR-15 expression, an understudied chemoattractant homing receptor (127, 143, 176), has been shown to be highly dependent on environmental stimuli and regulated by TGF-β within the GI tract (127, 128) and an environmental chemical sensor, aryl hydrocarbon receptor (AHR) (177, 178). The ligand of GPR-15, GPR-15L, has been reported to be highly expressed in epithelial tissues exposed to the environment, including the skin and GI tract (179, 180). Together, these data suggest that GPR-15 is another promising target for a targeted Treg therapy. In addition to the ex vivo induction of tissue-targeted Treg products, genome modification of Tregs to achieve ectopic expression of T-cell homing receptors The generation of tailored tissue-targeted Tregs has the potential to increase the targeted efficacy of Tregs in vivo while reducing the risk of more global immunosuppression by providing a selective advantage for targeted Treg products.
Enhancing ex vivo Treg expansion and stability
As discussed earlier, rapamycin improves both culture purity and suppressor function for clinical Treg ACT. A platform has been developed for solid organ transplant in which allo(donor)-specific Tregs from healthy donors or recipients post-transplantation are expanded in the presence of co-stimulatory blockade. Such Tregs maintain Foxp3 demethylation status which strongly correlates with stability (181, 182).
Expansion of sort-purified human Treg in the presence of TNFα and IL-6 increases expansion ~3-fold while maintaining Foxp3 expression, demethylation status, and in vitro and in vivo suppressive function (183). PKC-Ø is a negative regulator of Treg suppressive function, and acute treatment of expanded Treg with a non-competitive PKC-Ø inhibitor (AEB071) increased in vitro and in vivo suppressor function (184). Downregulation of miR-146b, which targets Traf6, increased Treg suppressive function in vitro and GVHD efficacy in vivo (185). Following in vitro expansion, purified CD39hi vs CD39lo Tregs were more suppressive in a xenogeneic GVHD model (186). Adoptive transfer of IL-33 stimulated Tregs were more effective than control Tregs at preventing murine aGVHD (187) an effect dependent on Treg expression of amphiregulin that can mediate tissue repair. In response to IL-33, engineered human ST2 (IL-33R)-expressing Tregs had increased expansion, maintained suppressor function, produced amphiregulin and had a heightened ability to induce anti-inflammatory M2 macrophages (188). IL-27, a member of the IL-12 family, has been shown to increase tTreg suppressive function and aGVHD efficacy in murine studies. Acute IL-27 stimulation increased the in vitro and in vivo suppressive function of human iTregs in a xenogeneic GVHD model (189). Lastly, CD155+ (DNAM+) Treg were less stable; depleting these cells at the beginning of culture increased Foxp3 expression, demethylation, and suppressive function in vitro (190).
In vivo strategies to enhance Treg efficacy
Tregs have high expression of CD25 (the high-affinity subunit of the IL-2 receptor) and IL-2 is required for stability and expansion. Clinical trials have shown that prophylactic administration of low doses of IL-2 can expand graft-associated Tregs after allo-HSCT and reduce the incidence of acute and chronic GVHD (191–193). Low dose IL-2/rapamycin enhanced the long-term persistence of adoptively transferred Tregs in non-human primates in a non-GVHD setting (194). PEGylation of IL-2 was found to increase half-life in vivo and expand Tregs in a xenogeneic GVHD model (195). In other studies, murine and human Treg containing IL-2 nanogel ‘backpacks’ that deliver IL-2 to Tregs in an autocrine fashion under certain conditions that trigger the TCR at sites of antigen encounter showed increased suppression of skin graft rejection in murine and xenogeneic models of disease (196). Infusion of IL-2/anti-IL-2 complexes increased both IL-2 half-life and Treg numbers, along with suppressing murine diabetes, colitis, and skin allograft rejection (197–199). One group also showed that IL-2/anti-IL-2 could reduce disease in a xenogeneic GVHD model, although efficacy in the context of Treg ACT was not assessed (199).
However, IL-2 also can stimulate CD8 T cells and NK cells that express the high affinity IL-2 receptor. Exogenous low-dose IL-2 and IL-2/anti-IL2 complexes decreased Treg efficacy when given at the time of donor T-cell infusion in either xenogeneic or allogeneic GVHD models, respectively, likely though expansion of contaminating cells (200, 201). To circumvent IL-2 augmentation of CD8 T-cells and NK cells, the Garcia group engineered orthogonal IL-2/IL-2Rβ pairs for murine and human systems. Following introduction of an ortho-IL-2Rß subunit and administration of ortho-IL2 protein into murine and human T cells, these neo-cytokines increased in vivo tumor killing in T cell, and CAR T cell, ACT (102, 202, 203). Infusion of ortho-IL-2 protein that has a markedly reduced capacity to bind to cells expressing wildtype IL-2Rβ, with Tregs transduced to express the ortho-IL-2Rß subunit was effective in ameliorating murine heart allograft rejection (204).
In vivo Treg expansion and suppression of GVHD were augmented by stimulation through TNFRSF25 (DR3) with either an agonistic antibody or a form of the natural ligand (TL1A-Ig) (205). These in vivo expanded Tregs also had increased efficacy following adoptive transfer (206). Activation of TNFRSF-member (TNFR2) expanded Tregs in vivo and ameliorated GVHD, without the need for exogenous IL-2 (207). Additionally, several pharmacologic agents favor Treg over Teff cell expansion post-HSCT, including: histone deacetylase inhibitors (vorinostat), hypomethylating agents (decitabine), JAK1/2 inhibitors (Ruxolitinib), ROCK1/2 inhibitors (Belumosudil) (62, 169, 208–210) and RA receptor agonists (211).
Concluding remarks
Treg ACT for GVHD prevention is now a reality, although barriers remain to common clinical practice. Pre-clinical advances are being made to enhance Treg efficacy, specificity, and tissue targeting. The clinical efficacy of adoptive Treg therapy for aGVHD is still being optimized. Comparable to the confluence in timing between Treg persistence and the relatively short-term immunosuppression needed in allo-HSCT and the fact that third party Treg ACT suppresses GVHD makes possible the production of banked (stored) Tregs that could be used to treat a multitude of patients. Importantly, Treg can also be highly expanded in vitro without obvious signs of exhaustion (212), enabling many of the culture or genetic manipulations discussed herein. Since Treg cryopreservation, an intricate part of banking, has proven very challenging (38, 61, 213), with varying recoveries, effects on Foxp3 expression, and in vitro suppressive functions, cryopreservation and thawing parameters that maintain a Treg phenotype and in vivo suppressive function after thawing is key to fully unlocking Treg ACT for GVHD and other indications such as graft rejection and autoimmune disease.
Author contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Funding
This work was supported by grants from the Children’s Cancer Research Fund and National Institutes of Health, National Heart, Lung and Blood Institute grant and R01 HL114512-01 (K.L.H.), and R01 HL11879 and HL155114, National Cancer Institute grants P01 CA142106 and P01 CA065493, National Institute of Allergy and Infectious Diseases grants P01 AI056299, and R37 AI344495 (B.R.B.).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ACT, Adoptive cell therapy; AHR, aryl hydrocarbon receptor; allo-HSCT, allogeneic hematopoietic stem cell transplantation; APCs, antigen-presenting cells; Blimp-1, B lymphocyte-induced maturation protein-1; BM-Treg, bone marrow Treg; CLA, cutaneous leukocyte antigen; Tcons, conventional T cells; Teff, effector T cells; FITC, fluorescein isothiocyanate; GI, gastrointestinal; GVL, graft-vs-leukemia; GVHD, graft versus host disease; iTregs, induced Treg; PB, peripheral blood; pTregs, peripheral Tregs; SLO, secondary lymphoid organs; Tregs, regulatory T cells; TCR, T cell receptor; TruC, TCR fusion construct; tTregs, Thymic Tregs; Tr1, type 1 regulatory T cells.
References
1. Li HW, Sykes M. Emerging concepts in haematopoietic cell transplantation. Nat Rev Immunol (2012) 12:403–16. doi: 10.1038/nri3226
2. Zeiser R, Blazar BR. Acute graft-versus-host disease - biologic process, prevention, and therapy. N Engl J Med (2017) 377:2167–79. doi: 10.1056/NEJMra1609337
3. Shevach EM, Thornton AM. tTregs, pTregs, and iTregs: similarities and differences. Immunol Rev (2014) 259:88–102. doi: 10.1111/imr.12160
4. Brunstein CG, Miller JS, Cao Q, McKenna DH, Hippen KL, Curtsinger J, et al. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood (2011) 117:1061–70. doi: 10.1182/blood-2010-07-293795
5. Brunstein CG, Miller JS, McKenna DH, Hippen KL, DeFor TE, Sumstad D, et al. Umbilical cord blood-derived T regulatory cells to prevent GVHD: kinetics, toxicity profile, and clinical effect. Blood (2016) 127:1044–51. doi: 10.1182/blood-2015-06-653667
6. Riley JL, June CH, Blazar BR. Human T regulatory cell therapy: take a billion or so and call me in the morning. Immunity (2009) 30:656–65. doi: 10.1016/j.immuni.2009.04.006
7. MacMillan ML, Hippen KL, McKenna DH, Kadidlo D, Sumstad D, DeFor TE, et al. First-in-human phase 1 trial of induced regulatory T cells for graft-versus-host disease prophylaxis in HLA-matched siblings. Blood Adv (2021) 5:1425–36. doi: 10.1182/bloodadvances.2020003219
8. MacDonald KN, Piret JM, Levings MK. Methods to manufacture regulatory T cells for cell therapy. Clin Exp Immunol (2019) 197:52–63. doi: 10.1111/cei.13297
9. Hefazi M, Bolivar-Wagers S, Blazar BR. Regulatory t cell therapy of graft-versus-host disease: advances and challenges. Int J Mol Sci (2021) 22(18):9676. doi: 10.3390/ijms22189676
10. Zhang P, Lee JS, Gartlan KH, Schuster IS, Comerford I, Varelias A, et al. Eomesodermin promotes the development of type 1 regulatory T (TR1) cells. Sci Immunol (2017) 2(10):eaah7152. doi: 10.1126/sciimmunol.aah7152
11. Roncarolo MG, Gregori S, Bacchetta R, Battaglia M, Gagliani N. The biology of t regulatory type 1 cells and their therapeutic application in immune-mediated diseases. Immunity (2018) 49:1004–19. doi: 10.1016/j.immuni.2018.12.001
12. Ohue Y, Nishikawa H. Regulatory T (Treg) cells in cancer: can treg cells be a new therapeutic target? Cancer Sci (2019) 110:2080–9. doi: 10.1111/cas.14069
13. Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, et al. Groth. 2006. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med (2006) 203(7):1701–11. doi: 10.1084/jem.20060772
14. Qureshi OS, Zheng Y, Nakamura K, Attridge K, Manzotti C, Schmidt EM, et al. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science (2011) 332:600–3. doi: 10.1126/science.1202947
15. Huang C-T, Workman CJ, Flies D, Pan X, Marson AL, Zhou G, et al. Role of LAG-3 in regulatory T cells. Immunity (2004) 21:503–13. doi: 10.1016/j.immuni.2004.08.010
16. Kamada T, Togashi Y, Tay C, Ha D, Sasaki A, Nakamura Y, et al. PD-1+ regulatory T cells amplified by PD-1 blockade promote hyperprogression of cancer. Proc Natl Acad Sci (2019) 116:9999–10008. doi: 10.1073/pnas.1822001116
17. Banerjee H, Nieves-Rosado H, Kulkarni A, Murter B, McGrath KV, Chandran UR, et al. Expression of Tim-3 drives phenotypic and functional changes in treg cells in secondary lymphoid organs and the tumor microenvironment. Cell Rep (2021) 36:109699. doi: 10.1016/j.celrep.2021.109699
18. Bopp T, Becker C, Klein M, Klein-Hessling S, Palmetshofer A, Serfling E, et al. Cyclic adenosine monophosphate is a key component of regulatory T cell-mediated suppression. J Exp Med (2007) 204:1303–10. doi: 10.1084/jem.20062129
19. Copsel S, Wolf D, Komanduri KV, Levy RB. The promise of CD4(+)FoxP3(+) regulatory T-cell manipulation in vivo: applications for allogeneic hematopoietic stem cell transplantation. Haematologica (2019) 104:1309–21. doi: 10.3324/haematol.2018.198838
20. Baine I, Basu S, Ames R, Sellers RS, Macian F. Helios Induces epigenetic silencing of IL2 gene expression in regulatory T cells. J Immunol (2013) 190:1008–16. doi: 10.4049/jimmunol.1200792
21. Kim HJ, Barnitz RA, Kreslavsky T, Brown FD, Moffett H, Lemieux ME, et al. Stable inhibitory activity of regulatory T cells requires the transcription factor Helios. Science (2015) 350:334–9. doi: 10.1126/science.aad0616
22. Overacre-Delgoffe AE, Chikina M, Dadey RE, Yano H, Brunazzi EA, Shayan G, et al. Interferon-gamma drives treg fragility to promote anti-tumor immunity. Cell (2017) 169 1130–1141.e1111. doi: 10.1016/j.cell.2017.05.005
23. Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol (2008) 8:523–32. doi: 10.1038/nri2343
24. Chaudhry A, Samstein RM, Treuting P, Liang Y, Pils MC, Heinrich JM, et al. Interleukin-10 signaling in regulatory T cells is required for suppression of Th17 cell-mediated inflammation. Immunity (2011) 34:566–78. doi: 10.1016/j.immuni.2011.03.018
25. Sawant DV, Yano H, Chikina M, Zhang Q, Liao M, Liu C, et al. Adaptive plasticity of IL-10(+) and IL-35(+) treg cells cooperatively promotes tumor T cell exhaustion. Nat Immunol (2019) 20:724–35. doi: 10.1038/s41590-019-0346-9
26. Wei X, Zhang J, Gu Q, Huang M, Zhang W, Guo J, et al. Reciprocal expression of il-35 and il-10 defines two distinct effector treg subsets that are required for maintenance of immune tolerance. Cell Rep (2017) 21:1853–69. doi: 10.1016/j.celrep.2017.10.090
27. Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med (2007) 204:1257–65. doi: 10.1084/jem.20062512
28. Fallarino F, Grohmann U, Hwang KW, Orabona C, Vacca C, Bianchi R, et al. Modulation of tryptophan catabolism by regulatory T cells. Nat Immunol (2003) 4:1206–12. doi: 10.1038/ni1003
29. Cieniewicz B, Uyeda MJ, Chen PP, Sayitoglu EC, Liu JM, Andolfi G, et al. Engineered type 1 regulatory T cells designed for clinical use kill primary pediatric acute myeloid leukemia cells. Haematologica (2021) 106:2588–97. doi: 10.3324/haematol.2020.263129
30. Grossman WJ, Verbsky JW, Tollefsen BL, Kemper C, Atkinson JP, Ley TJ. Differential expression of granzymes a and b in human cytotoxic lymphocyte subsets and T regulatory cells. Blood (2004) 104:2840–8. doi: 10.1182/blood-2004-03-0859
31. Garin MI, Chu CC, Golshayan D, Cernuda-Morollon E, Wait R, Lechler RI. Galectin-1: a key effector of regulation mediated by CD4+CD25+ T cells. Blood (2007) 109:2058–65. doi: 10.1182/blood-2006-04-016451
32. Janssens W, Carlier V, Wu B, VanderElst L, Jacquemin MG, Saint-Remy JM. CD4+CD25+ T cells lyse antigen-presenting b cells by fas-fas ligand interaction in an epitope-specific manner. J Immunol (2003) 171:4604–12. doi: 10.4049/jimmunol.171.9.4604
33. Hippen KL, Riley JL, June CH, Blazar BR. Clinical perspectives for regulatory T cells in transplantation tolerance. Semin Immunol (2011) 23:462–8. doi: 10.1016/j.smim.2011.07.008
34. Belkaid Y. Regulatory T cells and infection: a dangerous necessity. Nat Rev Immunol (2007) 7:875–88. doi: 10.1038/nri2189
35. Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol (2006) 6:295–307. doi: 10.1038/nri1806
36. Braaten D. Trials race rashly ahead for regulatory immune cells. Nat Med (2007) 13:227–7. doi: 10.1038/nm0307-227
37. Hippen KL, Merkel SC, Schirm DK, Nelson C, Tennis NC, Riley JL, et al. Generation and large-scale expansion of human inducible regulatory T cells that suppress graft-versus-host disease. Am J Transplant (2011) 11:1148–57. doi: 10.1111/j.1600-6143.2011.03558.x
38. Hippen KL, Merkel SC, Schirm DK, Sieben CM, Sumstad D, Kadidlo DM, et al. Massive ex vivo expansion of human natural regulatory T cells (T(regs)) with minimal loss of in vivo functional activity. Sci Transl Med (2011) 3:83ra41. doi: 10.1126/scitranslmed.3001809
39. Taylor PA, Lees CJ, Blazar BR. The infusion of ex vivo activated and expanded CD4(+)CD25(+) immune regulatory cells inhibits graft-versus-host disease lethality. Blood (2002) 99:3493–9. doi: 10.1182/blood.V99.10.3493
40. Zeiser R, Leveson-Gower DB, Zambricki EA, Kambham N, Beilhack A, Loh J, et al. Differential impact of mammalian target of rapamycin inhibition on CD4+CD25+Foxp3+ regulatory T cells compared with conventional CD4+ T cells. Blood (2008) 111:453–62. doi: 10.1182/blood-2007-06-094482
41. Edinger M, Hoffmann P. Regulatory T cells in stem cell transplantation: strategies and first clinical experiences. Curr Opin Immunol (2011) 23:679–84. doi: 10.1016/j.coi.2011.06.006
42. Di Ianni M, Falzetti F, Carotti A, Terenzi A, Castellino F, Bonifacio E, et al. Tregs prevent GVHD and promote immune reconstitution in HLA-haploidentical transplantation. Blood (2011) 117:3921–8. doi: 10.1182/blood-2010-10-311894
43. Martelli MF, Di Ianni M, Ruggeri L, Falzetti F, Carotti A, Terenzi A, et al. HLA-haploidentical transplantation with regulatory and conventional T-cell adoptive immunotherapy prevents acute leukemia relapse. Blood (2014) 124:638–44. doi: 10.1182/blood-2014-03-564401
44. Meyer EH, Laport G, Xie BJ, MacDonald K, Heydari K, Sahaf B, et al. Transplantation of donor grafts with defined ratio of conventional and regulatory T cells in HLA-matched recipients. JCI Insight (2019) 4(10):e127244. doi: 10.1172/jci.insight.127244
45. Kellner JN, Delemarre EM, Yvon E, Nierkens S, Boelens JJ, McNiece I, et al. Third party, umbilical cord blood derived regulatory T-cells for prevention of graft versus host disease in allogeneic hematopoietic stem cell transplantation: feasibility, safety and immune reconstitution. Oncotarget (2018) 9:35611–22. doi: 10.18632/oncotarget.26242
46. Trzonkowski P, Bieniaszewska M, Juscinska J, Dobyszuk A, Krzystyniak A, Marek N, et al. First-in-man clinical results of the treatment of patients with graft versus host disease with human ex vivo expanded CD4+CD25+CD127- T regulatory cells. Clin Immunol (2009) 133:22–6. doi: 10.1016/j.clim.2009.06.001
47. Theil A, Tuve S, Oelschlagel U, Maiwald A, Dohler D, Ossmann D, et al. Adoptive transfer of allogeneic regulatory T cells into patients with chronic graft-versus-host disease. Cytotherapy (2015) 17:473–86. doi: 10.1016/j.jcyt.2014.11.005
48. Landwehr-Kenzel S, Muller-Jensen L, Kuehl JS, Abou-El-Enein M, Hoffmann H, Muench S, et al. Adoptive transfer of ex vivo expanded regulatory T cells improves immune cell engraftment and therapy-refractory chronic GvHD. Mol Ther (2022) 30:2298–314. doi: 10.1016/j.ymthe.2022.02.025
49. Bacchetta R, Lucarelli B, Sartirana C, Gregori S, Lupo Stanghellini MT, Miqueu P, et al. Immunological outcome in haploidentical-HSC transplanted patients treated with il-10-anergized donor T cells. Front Immunol (2014) 5:16. doi: 10.3389/fimmu.2014.00016
50. Chen PP, Cepika AM, Agarwal-Hashmi R, Saini G, Uyeda MJ, Louis DM, et al. Alloantigen-specific type 1 regulatory T cells suppress through CTLA-4 and PD-1 pathways and persist long-term in patients. Sci Transl Med (2021) 13:eabf5264. doi: 10.1126/scitranslmed.abf5264
51. Basu S, Golovina T, Mikheeva T, June CH, Riley JL. Cutting edge: Foxp3-mediated induction of pim 2 allows human T regulatory cells to preferentially expand in rapamycin. J Immunol (2008) 180:5794–8. doi: 10.4049/jimmunol.180.9.5794
52. Battaglia M, Stabilini A, Migliavacca B, Horejs-Hoeck J, Kaupper T, Roncarolo M-G. Rapamycin promotes expansion of functional CD4+CD25+FOXP3+ regulatory T cells of both healthy subjects and type 1 diabetic patients. J Immunol (2006) 177(12):8338–47. doi: 10.4049/jimmunol.177.12.8338
53. Battaglia M, Stabilini A, Roncarolo MG. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood (2005) 105:4743–8. doi: 10.1182/blood-2004-10-3932
54. Strauss L, Whiteside TL, Knights A, Bergmann C, Knuth A, Zippelius A. Selective survival of naturally occurring human CD4+CD25+Foxp3+ regulatory T cells cultured with rapamycin. J Immunol (2007) 178:320–9. doi: 10.4049/jimmunol.178.1.320
55. Locafaro G, Andolfi G, Russo F, Cesana L, Spinelli A, Camisa B, et al. IL-10-Engineered human CD4(+) Tr1 cells eliminate myeloid leukemia in an hla class i-dependent mechanism. Mol Ther (2017) 25:2254–69. doi: 10.1016/j.ymthe.2017.06.029
56. Sayitoglu EC, Freeborn RA, Roncarolo MG. The yin and yang of type 1 regulatory t cells: from discovery to clinical application. Front Immunol (2021) 12:693105. doi: 10.3389/fimmu.2021.693105
57. Bergstrom M, Muller M, Karlsson M, Scholz H, Vethe NT, Korsgren O. Comparing the effects of the mtor inhibitors azithromycin and rapamycin on in vitro expanded regulatory T cells. Cell Transplant (2019) 28:1603–13. doi: 10.1177/0963689719872488
58. Hoffmann P, Boeld TJ, Eder R, Huehn J, Floess S, Wieczorek G, et al. Loss of FOXP3 expression in natural human CD4+CD25+ regulatory T cells upon repetitive in vitro stimulation. Eur J Immunol (2009) 39:1088–97. doi: 10.1002/eji.200838904
59. Lam AJ, Uday P, Gillies JK, Levings MK. Helios Is a marker, not a driver, of human treg stability. Eur J Immunol (2022) 52:75–84. doi: 10.1002/eji.202149318
60. Dijke IE, Hoeppli RE, Ellis T, Pearcey J, Huang Q, McMurchy AN, et al. Discarded human thymus is a novel source of stable and long-lived therapeutic regulatory t cells. Am J Transplant (2016) 16:58–71. doi: 10.1111/ajt.13456
61. MacDonald KN, Ivison S, Hippen KL, Hoeppli RE, Hall M, Zheng G, et al. Cryopreservation timing is a critical process parameter in a thymic regulatory T-cell therapy manufacturing protocol. Cytotherapy (2019) 21:1216–33. doi: 10.1016/j.jcyt.2019.10.011
62. Betts BC, Bastian D, Iamsawat S, Nguyen H, Heinrichs JL, Wu Y, et al. Targeting JAK2 reduces GVHD and xenograft rejection through regulation of T cell differentiation. Proc Natl Acad Sci USA (2018) 115:1582–7. doi: 10.1073/pnas.1712452115
63. Spoerl S, Mathew NR, Bscheider M, Schmitt-Graeff A, Chen S, Mueller T, et al. Activity of therapeutic JAK 1/2 blockade in graft-versus-host disease. Blood (2014) 123:3832–42. doi: 10.1182/blood-2013-12-543736
64. Kanakry CG, Ganguly S, Zahurak M, Bolanos-Meade J, Thoburn C, Perkins B, et al. Aldehyde dehydrogenase expression drives human regulatory T cell resistance to posttransplantation cyclophosphamide. Sci Transl Med (2013) 5:211ra157. doi: 10.1126/scitranslmed.3006960
65. Zeiser R, Nguyen VH, Beilhack A, Buess M, Schulz S, Baker J, et al. Inhibition of CD4+CD25+ regulatory T-cell function by calcineurin-dependent interleukin-2 production. Blood (2006) 108:390–9. doi: 10.1182/blood-2006-01-0329
66. Sagoo P, Ali N, Garg G, Nestle FO, Lechler RI, Lombardi G. Human regulatory T cells with alloantigen specificity are more potent inhibitors of alloimmune skin graft damage than polyclonal regulatory T cells. Sci Trans Med (2011) 3:1–22. doi: 10.1126/scitranslmed.3002076
67. Tang Q, Henriksen KJ, Bi M, Finger EB, Szot G, Ye J, et al. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J Exp Med (2004) 199:1455–65. doi: 10.1084/jem.20040139
68. Veerapathran A, Pidala J, Beato F, Yu X-Z, Anasetti C. Ex vivo expansion of human tregs specific for alloantigens presented directly or indirectly. Blood (2011) 118:5671–80. doi: 10.1182/blood-2011-02-337097
69. Lee LM, Zhang H, Lee K, Liang H, Merleev A, Vincenti F, et al. A comparison of ex vivo expanded human regulatory t cells using allogeneic stimulated b cells or monocyte-derived dendritic cells. Front Immunol (2021) 12:679675–5. doi: 10.3389/fimmu.2021.679675
70. Putnam AL, Safinia N, Medvec A, Laszkowska M, Wray M, Mintz MA, et al. Clinical grade manufacturing of human alloantigen-reactive regulatory T cells for use in transplantation. Am J Transplant (2013) 13:3010–20. doi: 10.1111/ajt.12433
71. Arjomandnejad M, Kopec AL, Keeler AM. CAR-T regulatory (CAR-treg) cells: engineering and applications. Biomedicines (2022) 10:1–21. doi: 10.3390/biomedicines10020287
72. Brusko TM, Koya RC, Zhu S, Lee MR, Putnam AL, McClymont SA, et al. Human antigen-specific regulatory T cells generated by T cell receptor gene transfer. PLoS One (2010) 5:e11726–6. doi: 10.1371/journal.pone.0011726
73. McGovern JL, Wright GP, Stauss HJ. Engineering specificity and function of therapeutic regulatory T cells. Front Immunol (2017) 8:1517–7. doi: 10.3389/fimmu.2017.01517
74. De Paula Pohl A, Schmidt A, Zhang A-H, Maldonado T, Königs C, Scott DW. Engineered regulatory T cells expressing myelin-specific chimeric antigen receptors suppress EAE progression. Cell Immunol (2020) 358:104222–2. doi: 10.1016/j.cellimm.2020.104222
75. Hull CM, Nickolay LE, Estorninho M, Richardson MW, Riley JL, Peakman M, et al. Generation of human islet-specific regulatory T cells by TCR gene transfer. J Autoimmun (2017) 79:63–73. doi: 10.1016/j.jaut.2017.01.001
76. Kim YC, Zhang A-H, Su Y, Rieder SA, Rossi RJ, Ettinger RA, et al. Engineered antigen-specific human regulatory T cells: immunosuppression of FVIII-specific T- and b-cell responses. Blood (2015) 125:1107–15. doi: 10.1182/blood-2014-04-566786
77. Tsang JYS, Tanriver Y, Jiang S, Xue SA, Ratnasothy K, Chen D, et al. Conferring indirect allospecificity on CD4+CD25+ tregs by TCR gene transfer favors transplantation tolerance in mice. J Clin Invest (2008) 118:3619–28. doi: 10.1172/JCI33185
78. Semple K, Yu Y, Wang D, Anasetti C, Yu X-Z. Efficient and selective prevention of GVHD by antigen-specific induced tregs via linked-suppression in mice. Biol Blood Marrow Transplant (2011) 17:309–18. doi: 10.1016/j.bbmt.2010.12.710
79. Li J, Heinrichs J, Haarberg K, Semple K, Veerapathran A, Liu C, et al. HY-specific induced regulatory T cells display high specificity and efficacy in the prevention of acute graft-versus-Host disease. J Immunol (2015) 195:717–25. doi: 10.4049/jimmunol.1401250
80. Bendle GM, Linnemann C, Hooijkaas AI, Bies L, de Witte MA, Jorritsma A, et al. Lethal graft-versus-host disease in mouse models of T cell receptor gene therapy. Nat Med (2010) 16:565–70. doi: 10.1038/nm.2128
81. Berdien B, Mock U, Atanackovic D, Fehse B. TALEN-mediated editing of endogenous T-cell receptors facilitates efficient reprogramming of T lymphocytes by lentiviral gene transfer. Gene Ther (2014) 21:539–48. doi: 10.1038/gt.2014.26
82. Ochi T, Fujiwara H, Okamoto S, An J, Nagai K, Shirakata T, et al. Novel adoptive T-cell immunotherapy using a WT1-specific TCR vector encoding silencers for endogenous TCRs shows marked antileukemia reactivity and safety. Blood (2011) 118:1495–503. doi: 10.1182/blood-2011-02-337089
83. Provasi E, Genovese P, Lombardo A, Magnani Z, Liu P-Q, Reik A, et al. Editing T cell specificity towards leukemia by zinc finger nucleases and lentiviral gene transfer. Nat Med (2012) 18:807–15. doi: 10.1038/nm.2700
84. Chmielewski M, Hombach AA, Abken H. Antigen-specific T-cell activation independently of the mhc: chimeric antigen receptor-redirected T cells. Front Immunol (2013) 4:371–1. doi: 10.3389/fimmu.2013.00371
85. Dawson NAJ, Rosado-Sánchez I, Novakovsky GE, Fung VCW, Huang Q, McIver E, et al. Functional effects of chimeric antigen receptor co-receptor signaling domains in human regulatory T cells. Sci Trans Med (2020) 12(557):eaaz3866. doi: 10.1126/scitranslmed.aaz3866
86. Andersson J, Tran DQ, Pesu M, Davidson TS, Ramsey H, O'Shea JJ, et al. CD4+ FoxP3+ regulatory T cells confer infectious tolerance in a TGF-beta-dependent manner. J Exp Med (2008) 205:1975–81. doi: 10.1084/jem.20080308
87. Elinav E, Waks T, Eshhar Z. Redirection of regulatory T cells with predetermined specificity for the treatment of experimental colitis in mice. Gastroenterology (2008) 134:2014–24. doi: 10.1053/j.gastro.2008.02.060
88. Karim M, Feng G, Wood KJ, Bushell AR. CD25+CD4+ regulatory T cells generated by exposure to a model protein antigen prevent allograft rejection: antigen-specific reactivation in vivo is critical for bystander regulation. Blood (2005) 105:4871–7. doi: 10.1182/blood-2004-10-3888
89. Yoon J, Schmidt A, Zhang A-H, Königs C, Kim YC, Scott DW. FVIII-specific human chimeric antigen receptor T-regulatory cells suppress T- and b-cell responses to FVIII. Blood (2017) 129:238–45. doi: 10.1182/blood-2016-07-727834
90. MacDonald KG, Hoeppli RE, Huang Q, Gillies J, Luciani DS, Orban PC, et al. Alloantigen-specific regulatory T cells generated with a chimeric antigen receptor. J Clin Invest (2016) 126:1413–24. doi: 10.1172/JCI82771
91. Boardman DA, Philippeos C, Fruhwirth GO, Ibrahim MAA, Hannen RF, Cooper D, et al. Expression of a chimeric antigen receptor specific for donor hla class i enhances the potency of human regulatory t cells in preventing human skin transplant rejection. Am J Transplant (2017) 17:931–43. doi: 10.1111/ajt.14185
92. Muller YD, Ferreira LMR, Ronin E, Ho P, Nguyen V, Faleo G, et al. Precision engineering of an anti-hla-a2 chimeric antigen receptor in regulatory t cells for transplant immune tolerance. Front Immunol (2021) 12:686439–9. doi: 10.3389/fimmu.2021.686439
93. Imura Y, Ando M, Kondo T, Ito M, Yoshimura A. CD19-targeted CAR regulatory T cells suppress b cell pathology without GvHD. JCI Insight (2020) 5(14):e136185. doi: 10.1172/jci.insight.136185
94. Boroughs AC, Larson RC, Choi BD, Bouffard AA, Riley LS, Schiferle E, et al. Chimeric antigen receptor costimulation domains modulate human regulatory T cell function. JCI Insight (2019) 5(8):e126194. doi: 10.1172/jci.insight.126194
95. Lamarthée B, Marchal A, Charbonnier S, Blein T, Leon J, Martin E, et al. Transient mTOR inhibition rescues 4-1BB CAR-tregs from tonic signal-induced dysfunction. Nat Commun (2021) 12:6446–6. doi: 10.1038/s41467-021-26844-1
96. Rana J, Perry DJ, Kumar SRP, Muñoz-Melero M, Saboungi R, Brusko TM, et al. CAR- and TRuC-redirected regulatory T cells differ in capacity to control adaptive immunity to FVIII. Mol Ther (2021) 29(9):2660–76. doi: 10.1016/j.ymthe.2021.04.034
97. Koristka S, Kegler A, Bergmann R, Arndt C, Feldmann A, Albert S, et al. Engrafting human regulatory T cells with a flexible modular chimeric antigen receptor technology. J Autoimmun (2018) 90:116–31. doi: 10.1016/j.jaut.2018.02.006
98. Pierini A, Iliopoulou BP, Peiris H, Pérez-Cruz M, Baker J, Hsu K, et al. T Cells expressing chimeric antigen receptor promote immune tolerance. JCI Insight (2017) 2:1–17. doi: 10.1172/jci.insight.92865
99. Chmielewski M, Hombach AA, Abken H. Of CARs and TRUCKs: Chimeric antigen receptor (CAR) T cells engineered with an inducible cytokine to modulate the tumor stroma. Immunol Rev (2014) 257:83–90. doi: 10.1111/imr.12125
100. Chmielewski M, Kopecky C, Hombach AA, Abken H. IL-12 release by engineered T cells expressing chimeric antigen receptors can effectively muster an antigen-independent macrophage response on tumor cells that have shut down tumor antigen expression. Cancer Res (2011) 71:5697–706. doi: 10.1158/0008-5472.CAN-11-0103
101. Fu RY, Chen AC, Lyle MJ, Chen C-Y, Liu CL, Miao CH. CD4+ T cells engineered with FVIII-CAR and murine Foxp3 suppress anti-factor VIII immune responses in hemophilia a mice. Cell Immunol (2020) 358:104216–6. doi: 10.1016/j.cellimm.2020.104216
102. Sockolosky JT, Trotta E, Parisi G, Picton L, Su LL, Le AC, et al. Selective targeting of engineered T cells using orthogonal IL-2 cytokine-receptor complexes. Science (2018) 359:1037–42. doi: 10.1126/science.aar3246
103. Aarts-Riemens T, Emmelot ME, Verdonck LF, Mutis T. Forced overexpression of either of the two common human Foxp3 isoforms can induce regulatory T cells from CD4(+)CD25(-) cells. Eur J Immunol (2008) 38:1381–90. doi: 10.1002/eji.200737590
104. Allan SE, Alstad AN, Merindol N, Crellin NK, Amendola M, Bacchetta R, et al. Generation of potent and stable human CD4+ T regulatory cells by activation-independent expression of FOXP3. Mol Ther (2008) 16:194–202. doi: 10.1038/sj.mt.6300341
105. Allan SE, Passerini L, Bacchetta R, Crellin N, Dai M, Orban PC, et al. The role of 2 FOXP3 isoforms in the generation of human CD4+ tregs. J Clin Invest (2005) 115:3276–84. doi: 10.1172/JCI24685
106. Fu W, Ergun A, Lu T, Hill JA, Haxhinasto S, Fassett MS, et al. A multiply redundant genetic switch 'locks in' the transcriptional signature of regulatory T cells. Nat Immunol (2012) 13:972–80. doi: 10.1038/ni.2420
107. Seng A, Krausz KL, Pei D, Koestler DC, Fischer RT, Yankee TM, et al. Coexpression of FOXP3 and a Helios isoform enhances the effectiveness of human engineered regulatory T cells. Blood Adv (2020) 4:1325–39. doi: 10.1182/bloodadvances.2019000965
108. Honaker Y, Hubbard N, Xiang Y, Fisher L, Hagin D, Sommer K, et al. Gene editing to induce FOXP3 expression in human CD4+ T cells leads to a stable regulatory phenotype and function. Sci Trans Med (2020) 12(546):eaay6422. doi: 10.1126/scitranslmed.aay6422
109. Lam AJ, Lin DTS, Gillies JK, Uday P, Pesenacker AM, Kobor MS, et al. Optimized CRISPR-mediated gene knockin reveals FOXP3-independent maintenance of human treg identity. Cell Rep (2021) 36:109494. doi: 10.1016/j.celrep.2021.109494
110. Zhou X, Tang J, Cao H, Fan H, Li B. Tissue resident regulatory T cells: novel therapeutic targets for human disease. Cell Mol Immunol (2015) 12:543–52. doi: 10.1038/cmi.2015.23
111. Burzyn D, Benoist C, Mathis D. Regulatory T cells in nonlymphoid tissues. Nat Immunol (2013) 14:1007–13. doi: 10.1038/ni.2683
112. Nicholls J, Cao B, Le Texier L, Xiong LY, Hunter CR, Llanes G, et al. Bone marrow regulatory t cells are a unique population, supported by niche-specific cytokines and plasmacytoid dendritic cells, and required for chronic graft-versus-host disease control. Front Cell Dev Biol (2021) 9. doi: 10.3389/fcell.2021.737880
113. Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol (2010) 10:490–500. doi: 10.1038/nri2785
114. Shevyrev D, Tereshchenko V. Treg heterogeneity, function, and homeostasis. Front Immunol (2020) 10:3100. doi: 10.3389/fimmu.2019.03100
115. Miragaia RJ, Gomes T, Chomka A, Jardine L, Riedel A, Hegazy AN, et al. Single-cell transcriptomics of regulatory T cells reveals trajectories of tissue adaptation. Immunity (2019) 50:493–504. doi: 10.1016/j.immuni.2019.01.001
116. Zaiss MM, Frey B, Hess A, Zwerina J, Luther J, Nimmerjahn F, et al. Regulatory T cells protect from local and systemic bone destruction in arthritis. J Immunol (2010) 184:7238–46. doi: 10.4049/jimmunol.0903841
117. Burzyn D, Kuswanto W, Kolodin D, Shadrach JL, Cerletti M, Jang Y, et al. A special population of regulatory T cells potentiates muscle repair. Cell (2013) 155:1282–95. doi: 10.1016/j.cell.2013.10.054
118. Chen X, Wu Y, Wang L. Fat-resident T regs: an emerging guard protecting from obesity-associated metabolic disorders. Obes Rev (2013) 14:568–78. doi: 10.1111/obr.12033
119. Richards DM, Delacher M, Goldfarb Y, Kägebein D, Hofer A-C, Abramson J, et al. Treg cell differentiation: from thymus to peripheral tissue. Prog Mol Biol Trans Sci (2015) 136:175–205. doi: 10.1016/bs.pmbts.2015.07.014
120. Babyn PS, Ranson M, McCarville ME. Normal bone marrow: signal characteristics and fatty conversion. Magnetic Resonance Imaging Clinics North America (1998) 6:473–95. doi: 10.1016/S1064-9689(21)00233-6
121. Romaniuk A, Lyndina Y, Sikora V, Lyndin M, Karpenko L, Gladchenko O, et al. Structural features of bone marrow. Interventional Med Appl Sci (2016) 8:121–6. doi: 10.1556/1646.8.2016.3.3
122. Travlos GS. Normal structure, function, and histology of the bone marrow. Toxicologic Pathol (2006) 34:548–65. doi: 10.1080/01926230600939856
123. Shafat MS, Gnaneswaran B, Bowles KM, Rushworth SA. The bone marrow microenvironment–home of the leukemic blasts. Blood Rev (2017) 31:277–86. doi: 10.1016/j.blre.2017.03.004
124. Rauber S, Luber M, Weber S, Maul L, Soare A, Wohlfahrt T, et al. Resolution of inflammation by interleukin-9-producing type 2 innate lymphoid cells. Nat Med (2017) 23:938. doi: 10.1038/nm.4373
125. Karagiannis F, Wilhelm C. More is less: IL-9 in the resolution of inflammation. Immunity (2017) 47:403–5. doi: 10.1016/j.immuni.2017.09.004
126. Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song S-Y. Retinoic acid imprints gut-homing specificity on T cells. Immunity (2004) 21:527–38. doi: 10.1016/j.immuni.2004.08.011
127. Kim SV, Xiang WV, Kwak C, Yang Y, Lin XW, Ota M, et al. GPR15-mediated homing controls immune homeostasis in the large intestine mucosa. Science (2013) 340:1456–9. doi: 10.1126/science.1237013
128. Konkel JE, Zhang D, Zanvit P, Chia C, Zangarle-Murray T, Jin W, et al. Transforming growth factor-β signaling in regulatory T cells controls T helper-17 cells and tissue-specific immune responses. Immunity (2017) 46:660–74. doi: 10.1016/j.immuni.2017.03.015
129. Bacchetta R, Gregori S, Serafini G, Sartirana C, Schulz U, Zino E, et al. Molecular and functional characterization of allogantigen-specific anergic T cells suitable for cell therapy. Haematologica (2010) 95:2134–43. doi: 10.3324/haematol.2010.025825
130. Alegre ML, Mannon RB, Mannon PJ. The microbiota, the immune system and the allograft. Am J Transplant (2014) 14:1236–48. doi: 10.1111/ajt.12760
131. Hu W, Pasare C. Location, location, location: Tissue-specific regulation of immune responses. J Leukoc Biol (2013) 94:409–21. doi: 10.1189/jlb.0413207
132. Denning TL, Y.-c. Wang, Patel SR, Williams IR, Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17–producing T cell responses. Nat Immunol (2007) 8:1086–94. doi: 10.1038/ni1511
133. Coombes JL, Siddiqui KR, Arancibia-Cárcamo CV, Hall J, Sun C-M, Belkaid Y, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β–and retinoic acid–dependent mechanism. J Exp Med (2007) 204:1757–64. doi: 10.1084/jem.20070590
134. Fantini MC, Becker C, Monteleone G, Pallone F, Galle PR, Neurath MF. Cutting edge: TGF-β induces a regulatory phenotype in CD4+ CD25– T cells through Foxp3 induction and down-regulation of Smad7. J Immunol (2004) 172:5149–53. doi: 10.4049/jimmunol.172.9.5149
135. Raverdeau M, Mills KH. Modulation of T cell and innate immune responses by retinoic acid. J Immunol (2014) 192:2953–8. doi: 10.4049/jimmunol.1303245
136. Liu Z-M, Wang K-P, Ma J, Guo Zheng S. The role of all-trans retinoic acid in the biology of Foxp3+ regulatory T cells. Cell Mol Immunol (2015) 12:553–7. doi: 10.1038/cmi.2014.133
137. Hooper LV, Gordon JI. Commensal host-bacterial relationships in the gut. Science (2001) 292:1115–8. doi: 10.1126/science.1058709
138. Farhadi A, Banan A, Fields J, Keshavarzian A. Intestinal barrier: an interface between health and disease. J Gastroenterol Hepatol (2003) 18:479–97. doi: 10.1046/j.1440-1746.2003.03032.x
139. Hill GR, Ferrara JL. The primacy of the gastrointestinal tract as a target organ of acute graft-versus-host disease: rationale for the use of cytokine shields in allogeneic bone marrow transplantation. Blood (2000) 95:2754–9. doi: 10.1182/blood.V95.9.2754.009k25_2754_2759
140. Picker LJ, Butcher EC. Physiological and molecular mechanisms of lymphocyte homing. Annu Rev Immunol (1992) 10:561–91. doi: 10.1146/annurev.iy.10.040192.003021
141. Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science (1996) 272:60–7. doi: 10.1126/science.272.5258.60
142. Islam SA, Luster AD. T Cell homing to epithelial barriers in allergic disease. Nat Med (2012) 18:705. doi: 10.1038/nm.2760
143. Engelhardt B, Jagasia M, Savani B, Bratcher N, Greer J, Jiang A, et al. Regulatory T cell expression of CLA or α 4 β 7 and skin or gut acute GVHD outcomes. Bone Marrow Transplant (2011) 46:436. doi: 10.1038/bmt.2010.127
144. Beilhack A, Schulz S, Baker J, Beilhack GF, Nishimura R, Baker EM, et al. Prevention of acute graft-versus-host disease by blocking T-cell entry to secondary lymphoid organs. Blood J Am Soc Hematol (2008) 111:2919–28. doi: 10.1182/blood-2007-09-112789
145. Waldman E, Lu SX, Hubbard VM, Kochman AA, Eng JM, Terwey TH, et al. Absence of β7 integrin results in less graft-versus-host disease because of decreased homing of alloreactive T cells to intestine. Blood (2006) 107:1703–11. doi: 10.1182/blood-2005-08-3445
146. Varona R, Cadenas V, Goímez L, Martiínez-A C, Maírquez G. CCR6 regulates CD4+ t-cell–mediated acute graft-versus-host disease responses. Blood (2005) 106:18–26. doi: 10.1182/blood-2004-08-2996
147. Chen Y-B, Kim HT, McDonough S, Odze RD, Yao X, Lazo-Kallanian S, et al. Up-regulation of α4β7 integrin on peripheral T cell subsets correlates with the development of acute intestinal graft-versus-host disease following allogeneic stem cell transplantation. Biol Blood Marrow Transplant (2009) 15:1066–76. doi: 10.1016/j.bbmt.2009.05.003
148. Nguyen VH, Zeiser R, Chang DS, Beilhack A, Contag CH, Negrin RS. In vivo dynamics of regulatory T-cell trafficking and survival predict effective strategies to control graft-versus-host disease following allogeneic transplantation. Blood (2007) 109:2649–56. doi: 10.1182/blood-2006-08-044529
149. Beilhack A, Schulz S, Baker J, Beilhack GF, Wieland CB, Herman EI, et al. In vivo analyses of early events in acute graft-versus-host disease reveal sequential infiltration of T-cell subsets. Blood (2005) 106:1113–22. doi: 10.1182/blood-2005-02-0509
150. Anderson BE, Taylor PA, McNiff JM, Jain D, Demetris AJ, Panoskaltsis-Mortari A, et al. Effects of donor T-cell trafficking and priming site on graft-versus-host disease induction by naive and memory phenotype CD4 T cells. Blood (2008) 111:5242–51. doi: 10.1182/blood-2007-09-107953
151. Ferrara JL, Deeg HJ. Graft-versus-host disease. New Engl J Med (1991) 324:667–74. doi: 10.1056/NEJM199103073241005
152. Luster AD, Alon R, von Andrian UH. Immune cell migration in inflammation: present and future therapeutic targets. Nat Immunol (2005) 6(12):1182–90. doi: 10.1038/ni1275
153. Staffas A, Burgos da Silva M, van den Brink MR. The intestinal microbiota in allogeneic hematopoietic cell transplant and graft-versus-host disease. Blood (2017) 129:927–33. doi: 10.1182/blood-2016-09-691394
154. Takatsuka H, Iwasaki T, Okamoto T, Kakishita E. Intestinal graft-versus-host disease. Drugs (2003) 63:1–15. doi: 10.2165/00003495-200363010-00001
155. Hill GR, Crawford JM, Cooke KR, Brinson YS, Pan L, Ferrara JL. Total body irradiation and acute graft-versus-host disease: the role of gastrointestinal damage and inflammatory cytokines. Blood J Am Soc Hematol (1997) 90:3204–13. doi: 10.1182/blood.V90.8.3204
156. Murai M, Yoneyama H, Ezaki T, Suematsu M, Terashima Y, Harada A, et al. Peyer's patch is the essential site in initiating murine acute and lethal graft-versus-host reaction. Nat Immunol (2003) 4:154–60. doi: 10.1038/ni879
157. Rivera-Nieves J, Olson T, Bamias G, Bruce A, Solga M, Knight RF, et al. L-selectin, α4β1, and α4β7 integrins participate in CD4+ T cell recruitment to chronically inflamed small intestine. J Immunol (2005) 174:2343–52. doi: 10.4049/jimmunol.174.4.2343
158. Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, et al. Treg induction by a rationally selected mixture of clostridia strains from the human microbiota. Nature (2013) 500:232–6. doi: 10.1038/nature12331
159. Agace WW. T-Cell recruitment to the intestinal mucosa. Trends Immunol (2008) 29:514–22. doi: 10.1016/j.it.2008.08.003
160. Mora JR, Iwata M, von Andrian UH. Vitamin effects on the immune system: vitamins a and d take centre stage. Nat Rev Immunol (2008) 8:685–98. doi: 10.1038/nri2378
161. Sigmundsdottir H, Butcher EC. Environmental cues, dendritic cells and the programming of tissue-selective lymphocyte trafficking. Nat Immunol (2008) 9:981–7. doi: 10.1038/ni.f.208
162. Hamann A, Andrew DP, Jablonski-Westrich D, Holzmann B, Butcher EC. Role of alpha 4-integrins in lymphocyte homing to mucosal tissues in vivo. J Immunol (1994) 152:3282–93.
163. Svensson M, Marsal J, Ericsson A, Carramolino L, Brodén T, Márquez G, et al. CCL25 mediates the localization of recently activated CD8αβ+ lymphocytes to the small-intestinal mucosa. J Clin Invest (2002) 110:1113–21. doi: 10.1172/JCI0215988
164. Kadowaki A, Saga R, Lin Y, Sato W, Yamamura T. Gut microbiota-dependent CCR9+ CD4+ T cells are altered in secondary progressive multiple sclerosis. Brain (2019) 142:916–31. doi: 10.1093/brain/awz012
165. Nguyen LP, Pan J, Dinh TT, Hadeiba H, O'Hara E, Ebtikar A, et al. Role and species-specific expression of colon T cell homing receptor GPR15 in colitis. Nat Immunol (2015) 16:207–13. doi: 10.1038/ni.3079
166. Chen YB, Shah NN, Renteria AS, Cutler C, Jansson J, Akbari M, et al. Vedolizumab for prevention of graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Blood Adv (2019) 3:4136–46. doi: 10.1182/bloodadvances.2019000893
167. Floisand Y, Lazarevic VL, Maertens J, Mattsson J, Shah NN, Zachee P, et al. Safety and effectiveness of vedolizumab in patients with steroid-refractory gastrointestinal acute graft-versus-host disease: a retrospective record review. Biol Blood Marrow Transplant (2019) 25:720–7. doi: 10.1016/j.bbmt.2018.11.013
168. Mehta RS, Saliba RM, Jan A, Shigle TL, Wang E, Nieto Y, et al. Vedolizumab for steroid refractory lower gastrointestinal tract graft-versus-host disease. Transplant Cell Ther (2021) 27:272 e271–72.e275. doi: 10.1016/j.jtct.2020.12.011
169. Choi SW, Braun T, Chang L, Ferrara JL, Pawarode A, Magenau JM, et al. Vorinostat plus tacrolimus and mycophenolate to prevent graft-versus-host disease after related-donor reduced-intensity conditioning allogeneic haemopoietic stem-cell transplantation: a phase 1/2 trial. Lancet Oncol (2014) 15:87–95. doi: 10.1016/S1470-2045(13)70512-6
170. Martin PJ, Schoch G, Fisher L, Byers V, Anasetti C, Appelbaum FR, et al. A retrospective analysis of therapy for acute graft-versus-host disease: initial treatment. Blood (1990) 76:1464–72. doi: 10.1182/blood.V76.8.1464.1464
171. Kupper TS, Fuhlbrigge RC. Immune surveillance in the skin: mechanisms and clinical consequences. Nat Rev Immunol (2004) 4:211. doi: 10.1038/nri1310
172. Babi LS, Moser R, Soler MP, Picker LJ, Blaser K, Hauser C. Migration of skin-homing T cells across cytokine-activated human endothelial cell layers involves interaction of the cutaneous lymphocyte-associated antigen (CLA), the very late antigen-4 (VLA-4), and the lymphocyte function-associated antigen-1 (LFA-1). J Immunol (1995) 154:1543–50.
173. Butcher EC, Williams M, Youngman K, Rott L, Briskin M. Lymphocyte trafficking and regional immunity. Adv Immunol (1999) 1999:209–53. doi: 10.1016/S0065-2776(08)60022-X
174. Cahill R, Poskitt D, Frost D, Trnka Z. Two distinct pools of recirculating T lymphocytes: migratory characteristics of nodal and intestinal T lymphocytes. J Exp Med (1977) 145:420–8. doi: 10.1084/jem.145.2.420
175. Tubo NJ, McLachlan JB, Campbell JJ. Chemokine receptor requirements for epidermal T-cell trafficking. Am J Pathol (2011) 178:2496–503. doi: 10.1016/j.ajpath.2011.02.031
176. Hoeppli R, MacDonald K, Leclair P, Fung V, Mojibian M, Gillies J, et al. Tailoring the homing capacity of human tregs for directed migration to sites of Th1-inflammation or intestinal regions. Am J Transplant (2019) 19:62–76. doi: 10.1111/ajt.14936
177. Xiong L, Dean JW, Fu Z, Oliff KN, Bostick JW, Ye J, et al. Ahr-Foxp3-RORγt axis controls gut homing of CD4+ T cells by regulating GPR15. Sci Immunol (2020) 5(48):eaaz7277. doi: 10.1126/sciimmunol.aaz7277
178. Swaminathan G, Nguyen LP, Namkoong H, Pan J, Haileselassie Y, Patel A, et al. The aryl hydrocarbon receptor regulates expression of mucosal trafficking receptor GPR15. Mucosal Immunol (2021) 14(4):852–861. doi: 10.1038/s41385-021-00390-x
179. Suply T, Hannedouche S, Carte N, Li J, Grosshans B, Schaefer M, et al. A natural ligand for the orphan receptor GPR15 modulates lymphocyte recruitment to epithelia. Sci Signaling (2017) 10:eaal0180. doi: 10.1126/scisignal.aal0180
180. Ocón B, Pan J, Dinh TT, Chen W, Ballet R, Bscheider M, et al. A mucosal and cutaneous chemokine ligand for the lymphocyte chemoattractant receptor GPR15. Front Immunol (2017) 8 1111. doi: 10.3389/fimmu.2017.01111
181. Guinan EC, Cole GA, Wylie WH, Kelner RH, Janec KJ, Yuan H, et al. Ex vivo costimulatory blockade to generate regulatory t cells from patients awaiting kidney transplantation. Am J Transplant (2016) 16:2187–95. doi: 10.1111/ajt.13725
182. Shimozawa K, Contreras-Ruiz L, Sousa S, Zhang R, Bhatia U, Crisalli KC, et al. Ex vivo generation of regulatory T cells from liver transplant recipients using costimulation blockade. Am J Transplant (2022) 22:504–18. doi: 10.1111/ajt.16842
183. Skartsis N, Peng Y, Ferreira LMR, Nguyen V, Ronin E, Muller YD, et al. IL-6 and TNFalpha drive extensive proliferation of human tregs without compromising their lineage stability or function. Front Immunol (2021) 12:783282. doi: 10.3389/fimmu.2021.783282
184. McDonald-Hyman C, Muller JT, Loschi M, Thangavelu G, Saha A, Kumari S, et al. The vimentin intermediate filament network restrains regulatory T cell suppression of graft-versus-host disease. J Clin Invest (2018) 128:4604–21. doi: 10.1172/JCI95713
185. Lu Y, Hippen KL, Lemire AL, Gu J, Wang W, Ni X, et al. miR-146b antagomir-treated human tregs acquire increased GVHD inhibitory potency. Blood (2016) 128:1424–35. doi: 10.1182/blood-2016-05-714535
186. Gu J, Ni X, Pan X, Lu H, Lu Y, Zhao J, et al. Human CD39(hi) regulatory T cells present stronger stability and function under inflammatory conditions. Cell Mol Immunol (2017) 14:521–8. doi: 10.1038/cmi.2016.30
187. Yang J, Ramadan A, Reichenbach DK, Loschi M, Zhang J, Griesenauer B, et al. Rorc restrains the potency of ST2+ regulatory T cells in ameliorating intestinal graft-versus-host disease. JCI Insight (2019) 4(5):e122014. doi: 10.1172/jci.insight.122014
188. Lam AJ, MacDonald KN, Pesenacker AM, Juvet SC, Morishita KA, Bressler B, et al. Innate control of tissue-reparative human regulatory t cells. J Immunol (2019) 202:2195–209. doi: 10.4049/jimmunol.1801330
189. Le HT, Keslar K, Nguyen QT, Blazar BR, Hamilton BK, Min B. Interleukin-27 enforces regulatory t cell functions to prevent graft-versus-host disease. Front Immunol (2020) 11:181. doi: 10.3389/fimmu.2020.00181
190. Fuhrman CA, Yeh WI, Seay HR, Saikumar Lakshmi P, Chopra G, Zhang L, et al. Divergent phenotypes of human regulatory t cells expressing the receptors tigit and cd226. J Immunol (2015) 195:145–55. doi: 10.4049/jimmunol.1402381
191. Betts BC, Pidala J, Kim J, Mishra A, Nishihori T, Perez L, et al. IL-2 promotes early treg reconstitution after allogeneic hematopoietic cell transplantation. Haematologica (2017) 102:948–57. doi: 10.3324/haematol.2016.153072
192. Kennedy-Nasser AA, Ku S, Castillo-Caro P, Hazrat Y, Wu MF, Liu H, et al. Ultra low-dose IL-2 for GVHD prophylaxis after allogeneic hematopoietic stem cell transplantation mediates expansion of regulatory T cells without diminishing antiviral and antileukemic activity. Clin Cancer Res (2014) 20:2215–25. doi: 10.1158/1078-0432.CCR-13-3205
193. Klatzmann D, Abbas AK. The promise of low-dose interleukin-2 therapy for autoimmune and inflammatory diseases. Nat Rev Immunol (2015) 15:283–94. doi: 10.1038/nri3823
194. Furlan SN, Singh K, Lopez C, Tkachev V, Hunt DJ, Hibbard J, et al. IL-2 enhances ex vivo-expanded regulatory T-cell persistence after adoptive transfer. Blood Adv (2020) 4:1594–605. doi: 10.1182/bloodadvances.2019001248
195. Zhang B, Sun J, Wang Y, Ji D, Yuan Y, Li S, et al. Site-specific PEGylation of interleukin-2 enhances immunosuppression via the sustained activation of regulatory T cells. Nat BioMed Eng (2021) 5:1288–305. doi: 10.1038/s41551-021-00797-8
196. Wang H, Wang S, Cheng L, Jiang Y, Melo MAS, Weir MD, et al. Novel dental composite with capability to suppress cariogenic species and promote non-cariogenic species in oral biofilms. Mater Sci Eng C Mater Biol Appl (2019) 94:587–96. doi: 10.1016/j.msec.2018.10.004
197. Pilat N, Wiletel M, Weijler AM, Steiner R, Mahr B, Warren J, et al. Treg-mediated prolonged survival of skin allografts without immunosuppression. Proc Natl Acad Sci USA (2019) 116:13508–16. doi: 10.1073/pnas.1903165116
198. Spangler JB, Trotta E, Tomala J, Peck A, Young TA, Savvides CS, et al. Engineering a single-agent cytokine/antibody fusion that selectively expands regulatory t cells for autoimmune disease therapy. J Immunol (2018) 201:2094–106. doi: 10.4049/jimmunol.1800578
199. Trotta E, Bessette PH, Silveria SL, Ely LK, Jude KM, Le DT, et al. A human anti-IL-2 antibody that potentiates regulatory T cells by a structure-based mechanism. Nat Med (2018) 24:1005–14. doi: 10.1038/s41591-018-0070-2
200. Hippen KL, O'Connor RS, Lemire AM, Saha A, Hanse EA, Tennis NC, et al. In vitro induction of human regulatory t cells using conditions of low tryptophan plus kynurenines. Am J Transplant (2017) 17:3098–113. doi: 10.1111/ajt.14338
201. McDonald-Hyman C, Flynn R, Panoskaltsis-Mortari A, Peterson N, MacDonald KP, Hill GR, et al. Therapeutic regulatory T-cell adoptive transfer ameliorates established murine chronic GVHD in a CXCR5-dependent manner. Blood (2016) 128:1013–7. doi: 10.1182/blood-2016-05-715896
202. Aspuria PJ, Vivona S, Bauer M, Semana M, Ratti N, McCauley S, et al. An orthogonal IL-2 and IL-2Rbeta system drives persistence and activation of CAR T cells and clearance of bulky lymphoma. Sci Transl Med (2021) 13:eabg7565. doi: 10.1126/scitranslmed.abg7565
203. Zhang Q, Hresko ME, Picton LK, Su L, Hollander MJ, Nunez-Cruz S, et al. A human orthogonal IL-2 and IL-2Rbeta system enhances CAR T cell expansion and antitumor activity in a murine model of leukemia. Sci Transl Med (2021) 13:eabg6986. doi: 10.1126/scitranslmed.abg6986
204. Hirai T, Ramos TL, Lin PY, Simonetta F, Su LL, Picton LK, et al. Selective expansion of regulatory T cells using an orthogonal IL-2/IL-2 receptor system facilitates transplantation tolerance. J Clin Invest (2021) 131(8):e139991. doi: 10.1172/JCI139991
205. Mavers M, Simonetta F, Nishikii H, Ribado JV, Maas-Bauer K, Alvarez M, et al. Activation of the DR3-TL1A axis in donor mice leads to regulatory T cell expansion and activation with reduction in graft-Versus-Host disease. Front Immunol (2019) 10:1624. doi: 10.3389/fimmu.2019.01624
206. Wolf D, Barreras H, Bader CS, Copsel S, Lightbourn CO, Pfeiffer BJ, et al. Marked in vivo donor regulatory T cell expansion via interleukin-2 and TL1A-ig stimulation ameliorates graft-versus-host disease but preserves graft-versus-leukemia in recipients after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant (2017) 23:757–66. doi: 10.1016/j.bbmt.2017.02.013
207. Chopra M, Biehl M, Steinfatt T, Brandl A, Kums J, Amich J, et al. Exogenous TNFR2 activation protects from acute GvHD via host T reg cell expansion. J Exp Med (2016) 213:1881–900. doi: 10.1084/jem.20151563
208. Choi SW, Gatza E, Hou G, Sun Y, Whitfield J, Song Y, et al. Histone deacetylase inhibition regulates inflammation and enhances tregs after allogeneic hematopoietic cell transplantation in humans. Blood (2015) 125:815–9. doi: 10.1182/blood-2014-10-605238
209. Flynn R, Paz K, Du J, Reichenbach DK, Taylor PA, Panoskaltsis-Mortari A, et al. Targeted rho-associated kinase 2 inhibition suppresses murine and human chronic GVHD through a Stat3-dependent mechanism. Blood (2016) 127:2144–54. doi: 10.1182/blood-2015-10-678706
210. Tkachev V, Furlan SN, Watkins B, Hunt DJ, Zheng HB, Panoskaltsis-Mortari A, et al. Combined OX40L and mTOR blockade controls effector T cell activation while preserving treg reconstitution after transplant. Sci Transl Med (2017) 9(408):eaan3085. doi: 10.1126/scitranslmed.aan3085
211. Thangavelu G, Wang C, Loschi M, Saha A, Osborn MJ, Furlan SN, et al. Repurposing a novel anti-cancer RXR agonist to attenuate murine acute GVHD and maintain graft-versus-leukemia responses. Blood (2021) 137:1090–103. doi: 10.1182/blood.2020005628
212. Hippen KL, Furlan SN, Roychoudhuri R, Wang E, Zhang Y, Osborn MJ, et al. Multiply restimulated human thymic regulatory T cells express distinct signature regulatory T-cell transcription factors without evidence of exhaustion. Cytotherapy (2021) 23(8):704–714. doi: 10.1016/j.jcyt.2021.02.118
Keywords: tTreg, pTreg, iTreg, CAR, GVHD
Citation: Hippen KL, Hefazi M, Larson JH and Blazar BR (2022) Emerging translational strategies and challenges for enhancing regulatory T cell therapy for graft-versus-host disease. Front. Immunol. 13:926550. doi: 10.3389/fimmu.2022.926550
Received: 22 April 2022; Accepted: 27 June 2022;
Published: 28 July 2022.
Edited by:
José Antonio Pérez Simón, Sevilla University, SpainReviewed by:
Robert Zeiser, University of Freiburg, GermanyAntonio Pierini, University of Perugia, Italy
Copyright © 2022 Hippen, Hefazi, Larson and Blazar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Keli L. Hippen, aGlwcGUwMDJAdW1uLmVkdQ==; Bruce R. Blazar, YmxhemEwMDFAdW1uLmVkdQ==
 Keli L. Hippen
Keli L. Hippen Mehrdad Hefazi
Mehrdad Hefazi Jemma H. Larson
Jemma H. Larson Bruce R. Blazar
Bruce R. Blazar