
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 08 August 2022
Sec. Molecular Innate Immunity
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.926236
This article is part of the Research Topic The key role of Mer receptor tyrosine kinase: where inflammation ends and fibrosis begins. View all 6 articles
 Rosaria Maria Pipitone1*
Rosaria Maria Pipitone1* Vincenza Calvaruso1
Vincenza Calvaruso1 Lorenza Di Marco2
Lorenza Di Marco2 Francesca Di Salvo3
Francesca Di Salvo3 Miriam Gaggianesi4
Miriam Gaggianesi4 Giulia Lupo1
Giulia Lupo1 Rossella Zito1
Rossella Zito1 Claudia La Mantia1
Claudia La Mantia1 Matteo Ramazzotti5
Matteo Ramazzotti5 Salvatore Petta1
Salvatore Petta1 Vito Di Marco1
Vito Di Marco1 Antonio Craxì1
Antonio Craxì1 Stefania Grimaudo1*
Stefania Grimaudo1*MerTK is a tyrosine kinase receptor that belongs to the TAM (Tyro3/Axl/Mer) receptor family. It is involved in different processes including cellular proliferation/survival, cellular adhesion/migration, and release of the inflammatory/anti-inflammatory cytokines. Although it is reported that MERTK polymorphisms affect the severity of viral and metabolic liver diseases, being able to influence fibrosis progression and hepatocellular carcinoma development, the mechanisms remain unknown. Methods: using a microarray approach, we evaluated the liver expression of genes involved in fibrogenesis and hepatocarcinogenesis in patient with chronic hepatitis C (CHC), stratified for MERTK genotype and MERTK expression. Results: we found that the rs 4374383 AA homozygosity is associated with lower MERTK expression in CHC patients and that, depending on MERTK genotype, Matrix Metallopeptidase 9 (MMP9), Matrix Metallopeptidase 7 (MMP7), Secreted Frizzled Related Protein 1 (SFRP1) and WNT gene family 11(WNT11) show differential expression in patients with CHC with or without neoplastic progression. Conclusions: our results confirm that MERTK represents a genetic biomarker for progression of liver disease and are suggestive of translational relevance for the study of downstream pathways involved in fibrogenesis and hepatocarcinogenesis.
Hepatitis C Virus (HCV) infection is a major etiology of hepatocellular carcinoma (HCC) worldwide. Even in the direct-acting antivirals era, HCV-related HCC occurrence remains significant (1). In this context, the role of genetic background in conditioning HCV-induced liver disease progression is crucial and host genetics should be considered in HCC surveillance to identify those patients who need more rigorous monitoring.
Mer tyrosine kinase (MerTK) is a major macrophage receptor involved in the clearance of apoptotic cells and it is expressed mainly in subsets of alternatively activated (M2) macrophages (2). Tumor-associated macrophages mostly display an M2-like phenotype; they are major sources of cytokines and participate in tumor progression, angiogenesis, and tissue remodeling (3, 4).
MerTK is involved mainly in efferocytosis, but it can also induce the fibrogenesis acting directly on the hepatic stellate cells (HSCs) (5, 6). Genome Wide Association Study (GWAS) reported that a Single Nucleotide Polymorphism (SNP) of MERTK (rs4374383 A>G) is associated with the risk of developing fibrosis in patients with Chronic Hepatitis C (CHC) (7). It seems that the polymorphic status of MERTK gene may play a key role not only in the control of apoptosis, in the epithelial-mesenchymal transition of HSCs and in the immune response, but also in the mechanisms involved in fibrosis progression and carcinogenesis (8).
Starting from this evidence, we investigated whether the MERTK variant A>G can influence the risk of progression of liver disease, in patients with chronic infection sustained by hepatitis viruses (C or B) or with nonalcoholic steatohepatitis (NASH).
Preliminary data showed that the AA MERTK genotype can significantly affect the risk of liver decompensation (LD) and HCC development in patients with HCV cirrhosis, treated with Peg-interferon alfa-2b and ribavirin (9).
On the other hand, the association between the presence of A allele of MERTK and the risk of HCC development was observed in patients with chronic Hepatitis B Virus (HBV) infection with active HBV replication or virological suppression. By evaluating the incidence of HCC according to the disease stage and the genotype, the probability of HCC development was higher in patients with liver compensation (LC) and AA/GA genotype compared to patients with LC and GG genotype. In addition, the incidence of HCC in Chronic Hepatitis B (CHB) patients without cirrhosis and with GA/AA or GG genotype was lower and similar, respectively, compared to those with cirrhosis (10).
These results confirm that the association between MERTK polymorphism and HCC is virus-independent, being observed both in HCV and HBV infections, and correlates with the inflammatory response and probably with the mechanisms of angiogenesis and tumorigenesis.
In this perspective the polymorphic status of MERTK gene represents a genetic condition able to influence the progression of liver disease: on one hand, AA/GA genotype of rs4374383 is protective against fibrosis progression according to Patin, on the other, the A allele confers a significant additional risk for HCC development. It has been reported that AA genotype, which is associated with lower intrahepatic expression of MERTK, is protective against severe fibrosis also in Non-Alcoholic Fatty Liver Disease (NAFLD) through a mechanism involving the modulation of HCS activation (11).
However, the rs4374383 (A>G at chr2:112013193) SNP of MERTK gene does not fall within regulatory regions and locates in the 8thintron. The aim of the present paper is to investigate the molecular mechanism involved in both protection from liver fibrosis and HCC development in CHC patients with AA genotype.
The rs4374383 is in string Linkage Disequilibrium (LD) with rs6726639 (A>C at chr2:111995520), localized at the beginning of the 4thintron on MERTK gene (r2 = 0.94, 17673 bp distance). The LD between the two variants is very strong in the 1000 genomes project (12, 13)
Actually, the rs4374383 and rs6726639 variants holds many other variants at very high LD (i.e. r2 ± 0.9). In Italy (Tuscany in Italy dataset), at least 78 other variants are shared. The GTEx database indicate that the expression of MERTK in liver is mainly due to isoforms 5 and 6. Accordingly, it makes no sense to investigate rs4374383 and rs6726639 separately because their LD is so high that their results will always be the same.
The GTEx portal contains per-tissue/organ differential quantitative RNA data (termed expression quantitative trait loci, eQTLs), i.e. genes that have differential expression in the presence of a variant. GTEx indicates that the two variants rs4374383 and rs6726639 (reasonably the entire LD region) in liver are associate with altered expression levels of several genes. It has been reported that the causal SNP in MERTK locus is represented by rs6726639, located within the binding sites of Transcriptional Repressor Factor (TF) which is able to bind to the A allele more strongly (12, 14). On the other hand, we suggested in our previous work that the down regulation of MERTK, observed in NAFLD patients with rs4374383 AA genotype, could be due to the association with rs6726639 AA genotype (11). In the same way, this genotype could influence the transcription of genes involved in fibrogenesis and in tumor suppression in CHC patients. Here, we evaluated the expression of MERTK and downstream signaling molecules in liver biopsies from CHC patients with AA (n=16) and GG (n=19) genotypes in the absence of HCC.
Secondly, in 12 selected liver samples genotyped for rs4374383 A>G and rs6726639 A>C SNPs of MERTK, we analyzed the expression of genes involved in fibrogenesis and HCC development. Depending on the polymorphic status of MERTK, we found a differential expression of Matrix Metallopeptidase 9 (MMP9), Matrix Metallopeptidase 7 (MMP7), Secreted Frizzled Related Protein 1 (SFRP1) and WNT gene family 11(WNT11) in the distinct groups of patients with CHC with or without HCC progression, thus confirming the role of MMPs and noncanonical WNT cascade in liver fibrogenesis and carcinogenesis.
Selected tissue liver samples, available for 39 of the 349 CHC patients previously recruited at the Gastrointestinal and Hepatic Unit of the University of Palermo and genotyped for rs4374383 A> G SNP and for rs6726639 A> C SNP of MERTK were used (Table 1). Among them, 35 were analyzed in Real Time PCR to quantify the expression of MERTK and downstream pathways genes (Tables 2, 3). Hepatic expression of genes involved in fibrogenesis or in tumor development/progression, listed in Table 4, was investigated in a subgroup of 9 patients chronically infected with HCV genotype-1b, monitored since 2013 in our hospital, and showing F3-F4 grade fibrosis (CHC). Among them, 2 developed HCC during the follow-up. The healthy subjects, used as control group, were constituted by two patients with histological diagnosis of NAFLD but without fibrosis and one patient without liver disease who underwent surgery for another pathology. The distribution of rs4374383 SNP and linked rs6726639 SNP genotypes was respectively: 7 AA/AA and 5 GG/CC (Table 5).
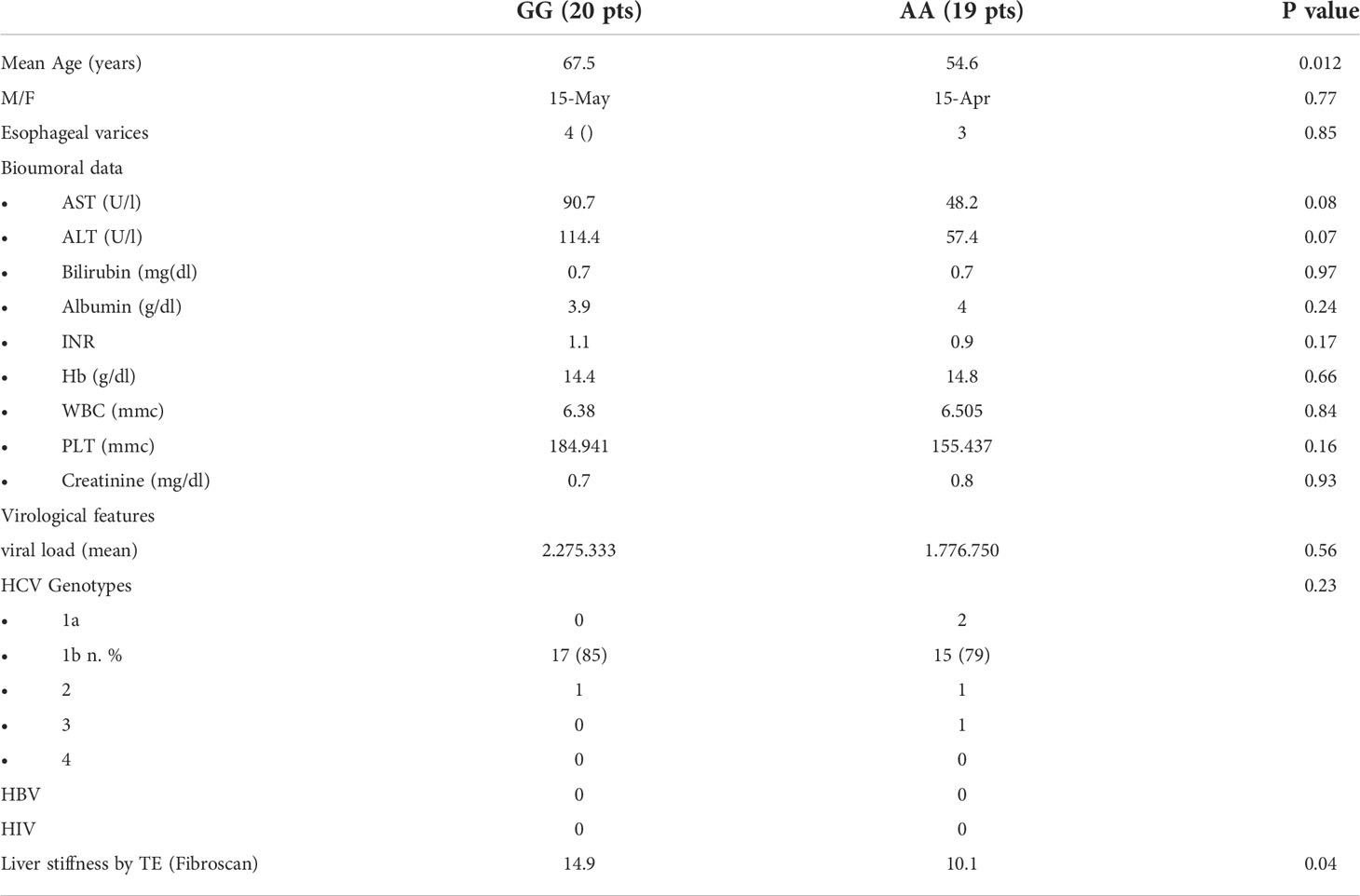
Table 1 Demographic, clinical, laboratory, and histological characteristics of CHC patients according to rs4374383 MERTK genotype.

Table 3 Distribution of MERTK rs4374383 and rs6726639 SNPs in CHC patients selected for MERTK and downstream pathways panel.

Table 4 List of 88 genes target of Prime PCR card of epithelial-mesenchymal transition, stemness and metastagenesis panel.

Table 5 Distribution of MERTK rs4374383 and rs6726639 SNPs in patients selected for epithelial-mesenchymal transition, stemness and metastagenesis panel.
Patients with alcohol intake> 20g/per day, autoimmune hepatitis, hereditary hemochromatosis, α1-antitrypsin deficiency, and severe obesity (BMI> 40Kg/m2) were excluded.
The study was conducted in accordance with the principles of the Declaration of Helsinki and with local and national laws. The approval was obtained by the Comitato Etico Palermo 1 ID 2014 - AOUP “Paolo Giaccone” in Palermo.
DNA was purified using the QIAmp blood Mini Kit (Qiagen, Mainz,Germany) and DNA samples were quantified using spectrophotometric determination. Genotyping for MERTK (rs4374383 A>G SNP and rs6726639 A>C SNP) was carried out using the TaqMan SNP genotyping allelic discrimination method (Applied Biosystems, Foster City, CA, USA). Genotyping assays were commercially available. Genotypes were called using Sequence Detection Software (SDS v.2.3) (Applied Biosystems, USA). Genotyping was conducted in a blinded fashion relative to patient characteristics.
Total RNA was purified from liver biopsies by miRNeasy Micro Kit (Qiagen) and quantified using NanoDrop ™ 1000 Spectrophotometer (ThermoFisher Scientific). 1 µg of RNA retro-transcribed using the iScript ™ gDNA Clear cDNA Synthesis Kit according to manufacturer’s recommendations (Bio-Rad, CA, USA). Relative expression level of target genes was evaluated by real-time PCR using custom RT2 Profiler PCR Array (Qiagen) or Prime PCR Custom Panel as recommended by manufacturer (Bio-Rad, CA, USA). Quantitative Real Time PCR was performed using 96 wells plates (Qiagen, 24 target genes listed in Table 3) or 384 well-plate pre-designed Prime PCR card of epithelium-to-mesenchyme transition stemness and metastagenesis panel (Bio-Rad, CA, USA, 88 target genes listed in Table 4). All the plates contained primers for genomic DNA detection (gDNA), positive PCR control (PCR), RNA Quality Assay (RQ1 and RQ2), Reverse Transcription Control (RT) and 2 housekeeping genes: TATA-box binding protein (TBP), and hypoxanthine phospho-ribosyl-transferase 1 (HPRT1). Data were expressed as fold change using 2 − ΔΔCt method referred to NAFLD F0-F1 patient as control sample. Differences among experimental groups were analyzed by Student t test and used for comparison with PRIME PCR analysis software (Bio-Rad) or data analysis software is available at http://www.qiagen.com/it/shop/genes-and-pathways/data-analysis-center-overview-page/.
Based on the relative quantification method, the amount of target, normalized to the endogenous reference HPRT1 and relative with respect to a control sample (NAFLD f0-f1 patient), was computed. A list of gene expression, for which RQ is statistically significant when analyzed among the experimental groups has been identified. The mean and the standard deviation was identified for relative gene expressions inside experimental groups of subjects with CHC and respectively AA or GG genotype, with NAFLD and AA or GG genotype, and without liver disease and GG genotype for rs rs4374383 SNP of MERTK; explorative analyses were carried out to verify conditions on distributions and similar population variability. The student’s t-test for independent experiments was performed for testing differences in fold expression of genes between the experimental groups; the Bonferroni correction for multiple hypothesis testing was applied to t-test and P-value < 0.05 was statistically significant. Statistical analysis was performed using R Statistical Software version 3.6.3 (15).
We evaluated liver expression of MERTK and downstream signaling pathways in liver biopsies from CHC patients with AA (n = 16) and GG (n = 19) genotypes in the absence of HCC.
Interestingly, we found that, although AA homozygosity was associated with lower MERTK expression (1.85 folds), the phosphatidylinositol-3-kinase (PI3K) was expressed in the same way, while other molecules downstream were downregulated in AA (Figure 1).

Figure 1 Differential gene expression in CHC patients with GG MERTK genotype (N = 19) and AA genotype (N = 16). P-VALUE: MERTK 0.01; GRB2 0.10; STAT1 0.36; STAT6 0.2; FAS 0.07; AKTIP 0.03.
Gene expression analyses were performed in a subset of 12 patients, listed in Table 4, with different MERTK genotypes for whom frozen liver biopsies were available (9 with CHC, 2 with NAFLD and 1 without liver disease). In order to test for conditional independence of up/down regulated results and different groups with respect to each gene, the Mantel-Haenszel X-squared test (MH) was implemented on the correspondent frequency tables, after having stratified by genes (Cochran-Mantel-Haenszel M^2 = 106.62, df = 12, p-value <0.001). Using an RT Profiler PCR microarray approach, a list of 4 genes, MMP7, MMP9, SFRP1 and WNT11, among the 54 analyzed, showed evidence of differential expression between the experimental groups CHC (AA genotype), CHC (GG genotype), HCC (AA genotype). The magnitude of these differences could be reasonably estimated and tested even with small sample sizes assuming standard hypothesis. The T-test detected statistically significant differences in fold expression of genes MMP9, MMP7, SFRP1 and WNT11. Evidence for differential expression was evaluated using statistical significance with p-values <0.05.
MMP9 was more strongly up-regulated in HCC-AA (mean 10.3-fold increase) than in either CHC-AA (mean 4.41-fold increase) and CHC-GG groups (mean 4.02-fold increase). In both comparisons differences turn out to be significant with p-values respectively 0.045 and 0.04 (Figure 2A). The mean fold expression of HCC-AA group is 2.33 times higher than CHC-AA and 2.55 times higher than CHC-GG mean. MMP7 is up regulated in CHC-AA group, and its mean fold expression (6.63) is statistically different with p-value <0.01 and 2.7 times higher when compared with mean fold expression of CHC-GG group (2.43) (Figure 2B). For WNT11 the mean fold change difference is both statistically significant when CHC-GG group (mean= 7.42) is compared with CHC-AA (mean=0.11 and p value= 0.01) and with HCC-AA (mean=1.88 and p value=0.03). Mean expression in CHC-GG is 5.8 higher than in CHC-AA and 3.9 times higher than HCC-AA (Figure 2C). For SFRP1, the mean fold expression of CHC-GG and HCC-AA groups (1.29 and 1.94, with relative normalized expression in CHC-GG 2.5 times higher than HCC-AA) are statistically different (t-test=8.63, p value <0.01) (Figure 2D).
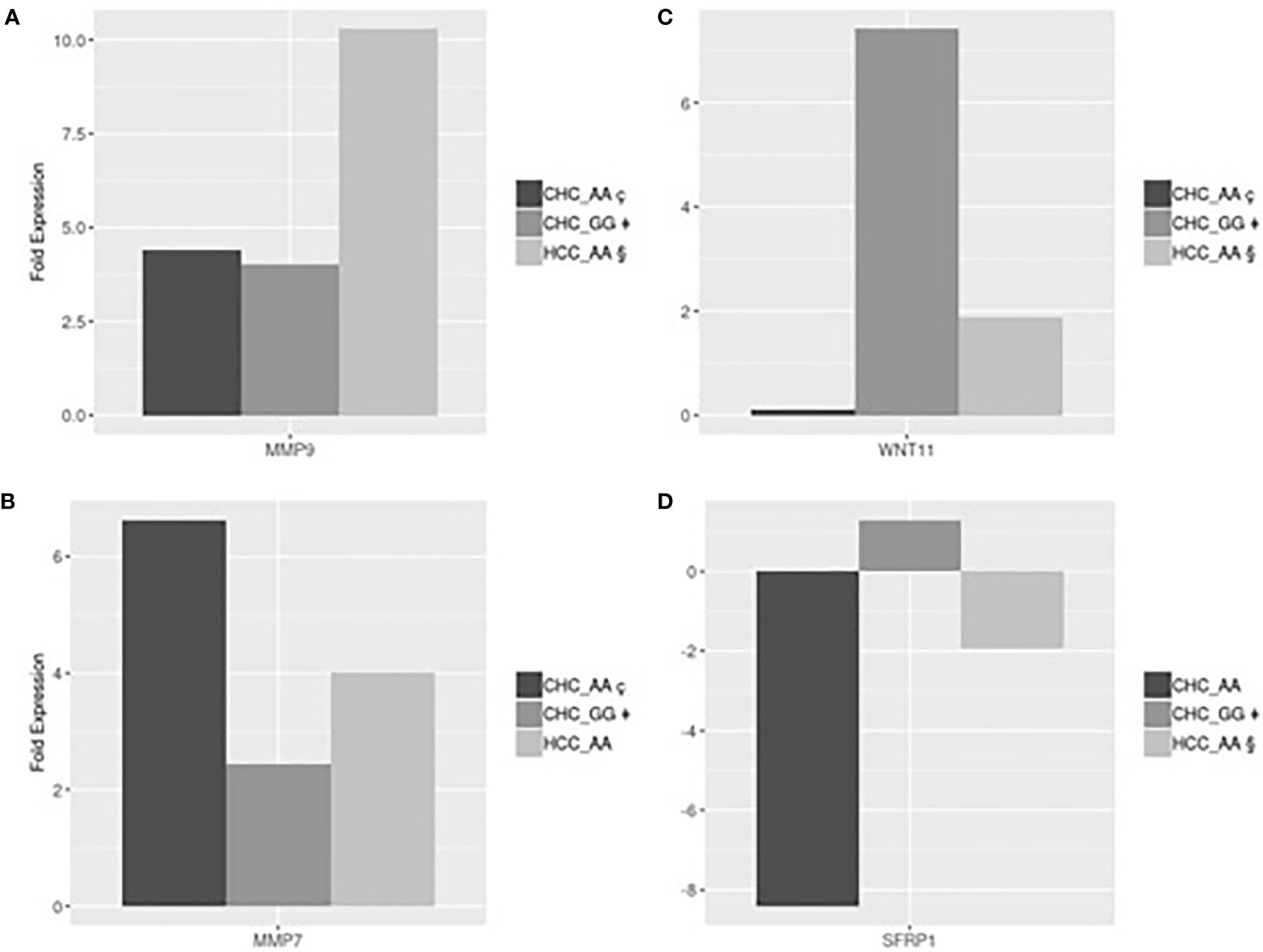
Figure 2 (A) HCC-AA vs CHC-AA and CHC-GG: statistically significant mean fold changes for MMP9 gene. PMMP9_§ç=0.045, PMMP9_§‡=0.04; (B) CHC-AA vs CHC-GG: statistically significant mean fold changes for MMP7 gene. PMMP7_ ç‡=0.01; (C) CHC-GG vs HCC-AA and vs CHC-AA: statistically significant mean fold changes for WNT11 gene. PWNT11_§‡=0.04; PWNT11_ç‡=0.03; (D) HCC-AA vs CHC-GG: statistically significant mean fold changes for SFRPI gene. PSFRPI §‡<0.01.
MerTK is expressed in human HSCs and acts directly on these cells by promoting their activation, supporting their survival, increasing their migration, and inducing the expression of profibrogenic genes, such as procollagen type I. We have previously showed that the AA genotypes of MERTK, associated with lower MerTK hepatic expression, is protective against severe fibrosis in NAFLD patients and is able to condition the progression of this chronic liver disease. By contrast, the GG genotype has been associated with fibrotic phenotype in NAFLD, showing a potential direct profibrotic action of MerTK (11).
MerTK, one of the three RTKs of TAM family, is expressed predominantly in M2 cells and acts inhibiting the inflammatory responses and controlling the tissue tolerance (16; 17). It plays a functional role in the clearance of apoptotic cells and this function is restricted to a subset of M2 macrophages, named M2c, that are characterized by secretion of IL-10 and up-regulation of MerTK (18). In fact, M2 differentiation is a key process that regulates inflammation and fibrosis (19). In chronic disease such as NAFLD, these macrophage populations, through MerTK play a crucial role to induce the HSCs trans differentiation in myofibroblasts promoting fibrogenesis. Gas6, together with the other ligands, binds to MerTK and induces its dimerization and autophosphorylation, so activating the downstream signaling (Linger et Al.,2008; 20). In addition, MerTK suppresses TLR signaling and down-regulates the production of pro-inflammatory cytokines through the phosphorylation of STAT1 which promotes the induction of SOCS-1 and SOCS-3 (21, 22). Normally, MerTK releases factors involved in tissue remodeling, immune responses suppression and tumor-promoting (23, 24).
Our evidence suggests that detection of the MERTK polymorphic status rs4374383, along with rs6726639, should warn in favor of more stringent monitoring to early detect disease progression and cancer development in patients with CHC and CHB and genotype AA/AG or progression to cirrhosis and its complications in patients with NAFLD and genotype GG/AG (9–11).
However, how MerTK can condition the progression of liver disease remains unknown. The aim of our work was to investigate the functional mechanism that links MerTK and progression of liver disease in CHC patients especially in neoplastic direction. We have focused our attention on the polymorphic status of MERTK rs6726639 because the A allele is able to differentially bind to transcription factors, thus conditioning MERTK expression and affecting downstream pathways (12). In this regard, in our CHC patients, we found that AA genotype is associated with lower expression of MERTK (1.8 folds) and with consequent lower expression of other downstream molecules compared to GG genotype (Figure 1).
In the same way, the AA genotype could negatively affect the transcription of genes involved in fibrogenesis and in tumor suppression. For this purpose, we have selected a subset of patients with CHC, for whom liver samples were available, with or without neoplastic progression in respect to the polymorphic MERTK status. The main finding of the present work was the differential expression of
MMP9, MMP7, SFRP1 and WNT11 in the different groups.
MMPs play a key role in several processes including epithelial-mesenchymal transition and are involved in fibroproliferative process and HCC development and progression during HCV infection (25, 26). Our results are in line with these suggestions showing, in Figure 2A, that MMP9 was upregulated in HCC-AA compared to both CHC-AA and CHC-GG groups, confirming the role of MMP9 in hepatocarcinogenesis. On the other hand, MMP7 was found upregulated in CHC-AA group compared with CHC-GG group (Figure 2B), confirming the role of AA genotype in conditioning the expression of genes involved in neoplastic progression.
Another pathway that was found differentially expressed was the WNT11/SRP1 pathway.
It has been demonstrated that WNT pathway is regulator of hepatic progenitor cells differentiation: canonical WNT signaling promotes the differentiation into hepatocytes, while noncanonical WNT signaling promotes the differentiation into myofibroblast and the consequent progression of liver fibrosis (27). Among the members of noncanonical cascade, WNT11 was reported to have a role in hepatic oncogenesis. In particular, the expression levels of WNT11 were significantly downregulated in human HCC, thus suggesting a tumor-suppressing role for WNT11 (28). Our results are totally in accord with this suggestion being WNT11 expression in CHC-GG 5.8 higher than in CHC-AA and 3.9 times higher than in HCC-AA (Figure 2C). On the other hand, we found that the expression of SFRP, which is a negative modulator of WNT canonical pathway (29), is higher in CHC-GG than in HCC-AA (Figure 2D). Moreover, we suggest that the observed differential expression of WNT11 and SFRP1 which work together to the activation of noncanonical WNT pathway, is related to MERTK polymorphic status.
We are aware that a major criticism of the present work is represented by the small size of human liver samples, due to the lack of a liver biopsy approach in the era of direct-acting antivirals. Nevertheless, due to the magnitude of differential gene expression, our hypotheses appear reasonable.
Certainly, our present data represent a first step, and, in the next future, we are planning to implement the sample size, including tissues from liver diseases with different etiology (viral and metabolic), focusing our attention on the pathways suggested by this preliminary work.
From the clinical perspective, our study strongly suggests that the rs4374383/rs6726639 SNPs of MERTK may represent a useful genetic biomarker to identify those patients with chronic liver diseases who are at high risk of progression and therefore deserve a more rigorous follow-up for HCC surveillance. Unraveling the mechanisms involved in hepatocarcinogenesis have intrinsic translational relevance to identify key pathways for clinical approach.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below:
https://www.ncbi.nlm.nih.gov/, NM_006343.2
https://www.ncbi.nlm.nih.gov/, NM_006343.3
https://www.ncbi.nlm.nih.gov/, NM_004994.3
https://www.ncbi.nlm.nih.gov/, NM_003012.5
https://www.ncbi.nlm.nih.gov/, NM_004626.3.
The study was conducted in accordance with the principles of the Declaration of Helsinki and with local and national laws. The approval was obtained by the Comitato Etico Palermo 1 ID 2014 - AOUP “Paolo Giaccone” of Palermo. The patients/participants provided their written informed consent to participate in this study.
Conceptualization: RP, SG, and AC. Methodology: RZ, CM, GL, and MG. Software: FS and MR. Validation: FS. Formal analysis: FS and MR. Investigation: RP, SG, LM, VC, VM, and SP. Data curation: RP and FS. Writing: RP and SG. Original draft preparation, review and editing: RP, SG, and AC. Supervision, SG and AC. All authors have read and agreed to the published version of the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Sahakyan Y, Lee-Kim V, Bremner KE, Bielecki JM, Krahn MD. Impact of direct-acting antiviral regimens on mortality and morbidity outcomes in patients with chronic hepatitis c: Systematic review and meta-analysis. J Viral Hepat (2021) 28(5):739–54. doi: 10.1111/jvh.13482
2. Zizzo G, Cohen PL. Antibody cross-linking of CD14 activates MerTK and promotes human macrophage clearance of apoptotic neutrophils: the dual role of CD14 at the crossroads between M1 and M2c polarization. Inflammation (2018) 41(6):2206–21. doi: 10.1007/s10753-018-0864-x
3. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature (2008) 454(7203):436–44. doi: 10.1038/nature07205
4. Sica A, Allavena P, Mantovani A. Cancer related inflammation: the macrophage connection. Cancer Lett (2008) 267(2):204–15. doi: 10.1016/j.canlet.2008.03.028
5. Fadok VA, Warner ML, Bratton DL, Henson PM. CD36 is required for phagocytosis of apoptotic cells by human macrophages that use either a phosphatidylserine receptor or the vitronectin receptor (alpha v beta 3). J Immunol (1998) 161(11):6250–7.
6. Platt N, Da Silva RP, Gordon S. Recognizing death: the phagocytosis of apoptotic cells. Trends Cell Biol (1998) 8(9):365–72. doi: 10.1016/S0962-8924(98)01329-4
7. Patin E, Kutalik Z, Guergnon J, Bibert S, Nalpas B, Jouanguy E, et al. Genome-wide association study identifies variants associated with progression of liver fibrosis from HCV infection. Gastroenterology (2012) 143(5):1244–52. doi: 10.1053/j.gastro.2012.07.097
8. Kuang DM, Zhao Q, Peng C, Xu J, Zhang JP, Wu C, et al. Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1. J Exp Med (2009) 206(6):1327–37. doi: 10.1084/jem.20082173
9. Di Marco V, Calvaruso V, Pipitone RM, Grimaudo S, Ferraro D, et al. rs4374383single nucleotide polymorphism of MERTK gene influence the development of hepatocellular carcinoma (HCC) in patients with HCV cirrhosis. Digest Liver Dis (2013) 45:e367–71. doi: 10.1016/j.dld.2013.09.006
10. Bronte F, Pipitone RM, Grimaudo S, Ferraro D, et al. The risk of of hepatocellular carcinoma in HBV cirrhosis is affected by polymorphisms of the MERTK gene. Digest Liver Dis (2014) 465:e136–41. doi: 10.1016/j.dld.2014.08.019
11. Petta S, Valenti L, Marra F, Grimaudo S, Tripodo C, Bugianesi E, et al. MERTK rs4374383 polymorphism affects the severity of fibrosis in nonalcoholic fatty liver disease”. J Hepatol (2016) 64:682–90. doi: 10.1016/j.jhep.2015.10.016
12. Huang D, Ovcharenko I. Identifying causal regulatory SNPs in ChIP-seq enhancers. Nucleic Acids Res (2015) 43(1):225–36. doi: 10.1093/nar/gku1318
13. Ensemble.org Available at: http://www.ensembl.org/Homo_sapiens/Variation/HighLD?db=core;r=2:111995020-111996020;v=rs6726639;vdb=variation;vf=184537024;second_variant_name=rs4374383 (Accessed 30 July 2021).
14. Cavalli M, Pan G, Nord H, Wallén Arzt E, Wallerman O, Wadelius C, et al. Genetic prevention of hepatitis c virus-induced liver fibrosis by allele-specific downregulation of MERTK. Hepatol Res (2016) 30. doi: 10.1111/hepr.12810
15. R Core Team. R: A language and environment for statistical computing, r foundation for statistical computing (2020). Available at: https://www.R-project.org/.
16. Rothlin CV, Carrera-Silva EA, Bosurgi L, Ghosh S. TAM receptor signaling in immune homeostasis. Annu Rev Immunol. (2015) 33:355–91. doi: 10.1146/annurevimmunol-032414-112103
17. Behrens EM, Gadue P, Gong SY, Garrett S, Stein PL, Cohen PL. The mer receptor tyrosine kinase: expression and function suggest a role in innate immunity. Eur J Immunol (2003) 33(8):2160–7. doi: 10.1002/eji.200324076
18. Zizzo G, Hilliard BA, Monestier M, Cohen PL. Efficient clearance of early apoptotic cells by human macrophages requires M2c polarization and MerTK induction. J Immunol (2012) 189(7):3508–20. doi: 10.4049/jimmunol.1200662
19. Smith W, Feldmann M, Londei M. Human macrophages induced in vitro by macrophage colony stimulating factor are deficient in IL-12 production. Eur J Immunol (1998) 28(8):2498–507. doi: 10.1002/(SICI)1521-4141(199808)28:08<2498::AID-IMMU2498>3.0.CO;2-Q
20. Braunger J, Schleithoff L, Schulz AS, Kessler H, Lammers R, Ullrich A, et al. Intracellular signaling of the Ufo/Axl receptor tyrosine kinase is mediated mainly by a multi-substrate docking-site. Oncogene (1997) 14(22):2619–31. doi: 10.1038/sj.onc.1201123
21. Rothlin CV, Ghosh S, Zuniga EI, Oldstone MB, Lemke G. TAM receptors are pleiotropic inhibitors of the innate immune response. Cell (2007) 131(6):1124–36. doi: 10.1016/j.cell.2007.10.034
22. Linger RM, Keating AK, Earp HS, Graham DK. TAM receptor tyrosine kinases: biologic functions, signaling, and potential therapeutic targeting in human cancer. Adv Cancer Res (2008) 100:35–83. doi: 10.1016/S0065-230X(08)00002-X
23. Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol (2002) 23(11):549–55. doi: 10.1016/s1471-4906(02)02302-5
24. Pollard JW. Tumor-educated macrophages promote tumor progression and metastasis. Nat Rev Cancer (2004) 4(1):71–8. doi: 10.1038/nrc1256
25. Scheu C, Badaru IA, Costache R, Caruntu C, Mihai GL, Didilescu AC, et al. The role of matrix metalloproteinases in the epithelial-mesenchymal transition of hepatocellular carcinoma. Anal Cell Pathol (2019) 26:9423907. doi: 10.1155/2019/9423907
26. Lichtinghagen R, Michels D, Haberkorn CI, Arndt B, Bahr M, Flemming P, et al. Matrix metalloproteinase (MMP)-2, MMP-7, and tissue inhibitor of metalloproteinase-1 are closely related to the fibroproliferative process in the liver during chronic hepatitis c. J Hepatol (2001) 34(2):239–47. doi: 10.1016/s0168-8278(00)00037-4
27. Xu Y, Fan W, Xu W, Jiang S, Chen G, Liu C, et al. Yiguanjian decoction enhances fetal liver stem/progenitor cell-mediated repair of liver cirrhosis through regulation of macrophage activation state. World J Gastroenterol (2018) 24(42):4759–72. doi: 10.3748/wjg.v24.i42.4759
28. Takashi T, Han Chu L, Hironori K, Jack RW, Miran K. Noncanonical Wnt11 inhibits hepatocellular carcinoma cell proliferation and migration. Mol Cancer Res (2010) 8(2):254–65. doi: 10.1158/1541-7786.MCR09-0238
Keywords: Mer Tyrosine Kinase polymorphism (MERTK polymorphism), liver fibrosis, hepatocellular carcinoma, matrix metallopeptidase, WNT gene family pathway (WNT pathway)
Citation: Pipitone RM, Calvaruso V, Di Marco L, Di Salvo F, Gaggianesi M, Lupo G, Zito R, La Mantia C, Ramazzotti M, Petta S, Di Marco V, Craxì A and Grimaudo S (2022) Mer Tyrosine Kinase (MERTK) modulates liver fibrosis progression and hepatocellular carcinoma development. Front. Immunol. 13:926236. doi: 10.3389/fimmu.2022.926236
Received: 22 April 2022; Accepted: 05 July 2022;
Published: 08 August 2022.
Edited by:
Gaetano Zizzo, ASST Ovest Milanese, ItalyReviewed by:
Xiaobo Wang, Columbia University, United StatesCopyright © 2022 Pipitone, Calvaruso, Di Marco, Di Salvo, Gaggianesi, Lupo, Zito, La Mantia, Ramazzotti, Petta, Di Marco, Craxì and Grimaudo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rosaria Maria Pipitone, cm9zYXJpYW1hcmlhLnBpcGl0b25lQHVuaXBhLml0; Stefania Grimaudo, c3RlZmFuaWEuZ3JpbWF1ZG9AdW5pcGEuaXQ=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.