- 1Departments of Neurosurgery, and National Clinical Research Center of Geriatric Disorders, Xiangya Hospital, Central South University, Changsha, China
- 2Department of Neonatology, Yale School of Medicine, New Haven, CT, United States
- 3Department of Neurosurgery, Yale School of Medicine, New Haven, CT, United States
Epilepsy accounts for a significant proportion of the burden of neurological disorders. Neuroinflammation acting as the inflammatory response to epileptic seizures is characterized by aberrant regulation of inflammatory cells and molecules, and has been regarded as a key process in epilepsy where mTOR signaling serves as a pivotal modulator. Meanwhile, accumulating evidence has revealed that non-coding RNAs (ncRNAs) interfering with mTOR signaling are involved in neuroinflammation and therefore articipate in the development and progression of epilepsy. In this review, we highlight recent advances in the regulation of mTOR on neuroinflammatory cells and mediators, and feature the progresses of the interaction between ncRNAs and mTOR in epileptic neuroinflammation.
Introduction
Epilepsy, characterized by recurrent spontaneous seizures, is one of the most common neurological diseases affecting over 50 million people worldwide (1). Epileptic seizures can be traced to a broad spectrum of factors such as brain abnormalities and trauma (2). Extremely complicated pathophysiological processes are involved in epilepsy in which aberrant regulation of inflammatory cells and molecules have emerged to act as important mediators of epileptogenesis and epileptic injury, which is considered as an epileptic factor independent of etiology (3–5). Epileptogenesis is commonly associated with persistent inflammatory in the brain microenvironment (3). Conversely, seizures contribute to a cascade of inflammatory events as well leading the activation of pro-inflammatory molecules, glial proliferation and phenotypic transformation, breakdown of the blood-brain barrier (BBB), and subsequential neural damages (6).
The mammalian target of the rapamycin signaling (mTOR), as the key regulator of protein synthesis and autophagy, plays critical roles in neurophysiological processes such as neural development and brain circuit formation, as well as the continuum of neurological disorders (7–12). Recently, dysregulation of mTOR signaling has been implicated in infection and inflammatory progresses and acts as an intriguing component of the complex mechanism involved in epileptic neuroinflammation (9, 13). Non-coding RNAs (ncRNAs), defined as the RNAs that are not translated into functional proteins, have been demonstrated to affect gene translation and interfere with signaling pathways in diverse biological contexts, such as neuronal disorders, cancer and immune responses (14–16). In addition, ncRNAs has been found to be involved in neuroinflammation and participate in the initiation and progression of epilepsy by evidence accumulated over the past decade (2, 17–20), which provide their potentials of acting as biomarkers and therapeutic targets. In this review, we provide an overview of the current understanding on the coordination of mTOR signaling and ncRNAs in the regulation of epileptic neuroinflammation, and its potential contribution to the development and progression of epileptic seizures.
Overview of mTOR Signaling
mTOR was originally identified as the target of rapamycin that consists of the catalytic subunits of two distinct multiprotein complexes: mTOR complex 1 (mTORC1) and 2 (mTORC2). mTORC1 is composed of mTOR, regulatory associated protein of mammalian target of rapamycin (Raptor), mammalian lethal with Sec13 protein 8 (mLST8), DEP domain containing mTOR-interacting protein (DEPTOR), and proline-rich AKT substrate of 40 kD (PRAS40). mTORC1 balances anabolic and catabolic processes by the stimulation of biosynthetic processes that produce proteins, nucleotides, and lipids required for cell growth and proliferation, and suppression of autophagic pathway (21, 22). mTORC1 integrates both intracellular and extracellular signaling including growth factors, energy, oxygen, and amino acid levels (23), and regulates a broad spectrum of downstream effectors though S6 kinases (S6K) and eIF4E-binding protein 1 (4EBP1) (24, 25). mTORC2 is composed of mTOR, rapamycin-insensitive companion of mTOR (Rictor), mLST8, DEPTOR, and the regulatory subunits mSin1 and Protor1/2. mTOC2 senses insulin, growth factors, etc., and phosphorylate mainly AGC kinases. mTORC2 is essential for cell survival and the maintenance of the actin cytoskeleton (26, 27).
mTOR and Epileptic Neuroinflammation
Neuroinflammation in Epilepsy
Neuroinflammation as one of the most important pathophysiological traits of epilepsy is the inflammatory response to epileptic seizures within the brain. Although the aspects of neuroinflammation can vary due to the diverse types of seizure, the main context and course are shared. Epileptic neuroinflammation commonly presents abnormal activations of resident central nervous system (CNS) glia (microglia and astrocytes) and endothelial cells, recruits of peripherally derived immune cells via the disruption of BBB, and the increased releases of inflammatory cytokines, chemokines, reactive oxygen species, and secondary messengers. Moreover, epileptic neuroinflammation can lead to the consequences of edema, neural damage, or even cell death, and acts as a “second hit” to trigger or worsen epilepsy.
Microglia cells are regarded as resident macrophages of brain and are able to polarize to pro-inflammatory M1 or anti-inflammatory M2 phenotypes with distinct physiopathological functions. The hyperactivation of microglial is primarily found in epileptic neuroinflammation with releasing of high-mobility group box-1 (HMGB1), a pro-inflammatory mediator (28, 29). Glia cells and astrocytes also respond to inflammatory signals and crosstalk with other cells, such as microglia, neurons, endothelial cells, and peripheral immune cells (30, 31). Glia cells along with other cells in the brain such as neurons and endothelial cells express pro-inflammatory mediators during neuroinflammation in various neurological disorders. Reactive astrocytes triggered by seizures are important cellular components involved in both pro-inflammatory and anti-inflammatory processes in neuroinflammation (32–34). They can release a wide range of inflammatory cytokines and chemokines that complement cascade components (35). Overreactions of astrocytes have been reported to disturb synaptic activity and linked to neuronal loss in epileptic inflammation (36, 37).
BBB, an important structure consisting of capillary walls and astrocytes, is temporally and anatomically associated with epilepsy (38, 39). The disruption of BBB structure and permeability induced by neuroinflammation promotes peripheral immune cells and inflammatory mediators to enter the brain microenvironment. As a result, the infiltration of peripheral immune cells such as monocytes and T cells can impair BBB as well (40). Monocytes have been gradually studied as potential treatment targets for epilepsy. Monocytes can migrate via the bloodstream to the brain though disrupted BBB to sustain seizure activity and promote neuroinflammation by differentiation into macrophages and microglia-like cells (41–44). C-C motif chemokine ligand 2 (CCL2) and its receptor CCR2 are essential for the process (45). Lymphocytes that participate in the adaptive immune system are recruited to the brain in epileptic neuroinflammation as well (46–48). Pro-inflammatory CD4+ and CD8+ T cells have been observed to infiltrate the brain in seizure animals (49, 50). Interestingly, regulatory T cells (Tregs), a type of tissue-resident lymphocytes that participate in the negative regulation of immune responses, are less common in the normal brain but significantly accumulate in neuroinflammation (51).
Proinflammatory molecules, such as cytokines, chemokines, and growth factors, are important mediators in epileptic neuroinflammation, which regulate onset and development of seizures. In addition, the inflammatory mediators can cause secondary damage and contribute to repetitive seizures (4, 52–55). Proinflammatory Interleukin (IL)-1, released from glial cells and white cells, plays a crucial role in neuroinflammation (56). In seizure models, the concentration and activation are significantly increased, which enhance the neuronal excitability and thus exacerbate neuronal hyperactivity and excitotoxicity (34, 45, 57). Tumor necrosis factor alpha (TNF-α), a pro-inflammatory cytokine primarily released from microglia, has recently reported to be involved in the etiology of epilepsy and attenuate aberrant neurogenesis (4, 58, 59). Chemokines secreted from astrocytes, microglia, and endothelial cells contribute to seizures and epileptogenic progression (32, 57) and have been suggested to further recruit more inflammatory cells. Hyperexpression of CCL2 in epilepsy is suggested to increase seizure-induced IL-1β production and neuronal cell death (30, 45). Recent studies show that interference with CCL2 signaling effectively inhibits seizures (60). Danger-associated molecular products (DAMPs) such as HMGB1, complements, ATP, reactive oxygen species (ROS) are cell-derived molecules that are released during tissue damage. The endogenous signals are recognized by toll-like receptors (TLRs) that belong to innate immunity and are vital in neuroinflammation, and the receptor for advanced glycation end products (RAGE) as well (4, 61–65).
mTOR and Inflammatory Cells
mTOR signaling is a critical regulator of the function of immune cells. Previous studies have demonstrated that mTORC1 signaling and the protein levels of inflammatory mediators in microglia are upregulated (66). Inhibition of mTOR decreased the formation of autophagosomes and production of lipopolysaccharides (LPS)-induced proinflammatory cytokines in microglia (67, 68). In neuroinflammation, mTOR can modulate microglia polarization and thus function though metabolic reprogramming (67, 69). Suppression of mTOR activity is suggested to attenuate the microglial activation and therefore alleviate seizure severity (70). However, another study indicated that mTOR-deficient microglia in status epilepticus reduces induction of inflammation and thus increases seizure susceptibility (71). In addition, mTOR activation could promote the growth of neurites by promoting the releases of astrocyte-derived neurotrophic factors (72). Inhibition of mTOR pathway by rapamycin mitigates astrocyte migration, proliferation, and production of inflammatory mediators (73).
In epileptic neuroinflammation, mTOR hyperactivation leads to the disruption of BBB facilitating the infiltration of peripheral immune cells (74, 75). Meanwhile, mTOR advocates the development of monocyte and macrophage in the bone marrow by reducing macrophage colony-stimulating factor receptor CD115 expression and stimulates monocytes to enter M2-like macrophages (76, 77). Interestingly, monocytes infiltration can upregulate microglia activation after seizure induction (45). mTOR also regulates differentiation and activation of T cells and T helper (Th) cells. mTOR inhibition decreases effector molecules in both CD4+ and CD8+ cells (78) and maintain the quiescence state of naïve cells (79, 80). mTORC1 has been reported to coordinate metabolic programs to selectively regulate Th1 and Th17 differentiation (81, 82). While mTORC2 promotes the differentiation of Th2 cells (81, 83). Interestingly, the inhibition of mTOR by rapamycin can promote the development of regulatory Treg cells from naive T cells and improve the quality of memory CD8+ T cells, mainly due to the enhancement of autophagy activity (84, 85).
mTOR and Neuroinflammatory Mediators
Cytokines and Chemokines
mTOR regulates a number of inflammatory cytokines and chemokines expression in epileptic neuroinflammation. IL-1β, for example, has been shown to be upregulated in mesial temporal lobe epilepsy (MTLE). Inhibition of mTOR activity by rapamycin blocks secretion of the IL-1β though the activation of autophagy, which could block the differentiation of Th17 cells (86–88). IL-17, mainly derived from Th17 cells, is also increased though mTOR activation in epilepsy, which leads excessive pro-inflammatory cytokine expression and maintain the chronic inflammation (48, 89). mTOR can also modulates IL-17 expression indirectly via several pathways, including signal transducer and activator of transcription 3 (STAT3) and hypoxia-inducible factor (HIF)-1α, which is suggested to be induced by IL-1β (90–92). Of note,
IL-17 can activate mTOR pathway and inhibit autophagy conversely (93, 94). In addition, transforming growth factor-β (TGF-β) released by reactive astrocytes is considered as an epileptogenic factor, which can preserve cellular metabolism in T cells and maintain response and promote neuron autophagy in a mTOR-dependent manner in neuroinflammation (95–97). Moreover, mTOR has been demonstrated to stabilize TNF-α mRNA released from microglia (67).
Danger-Associated Molecular Products
DAMPs have been shown to initiate proinflammatory immune responses from nonneuronal glial cells and contribute to the chronic neuroinflammation present in seizures accelerating the degeneration of neurons. HMGB1, a representative DAMPs, is secreted by immune cells as a cytokine mediator of inflammation involved in epileptic neuroinflammation (98–101). mTOR acting as the downstream effector is suggested to be implicated in HMGB1-induced maturation and antigen-presenting ability of dendritic cells and secretion of proinflammatory cytokines (102). In addition, nitric oxide (NO) is a neurotransmitter that regulates the differentiation and proliferation of neurons, and is proven to be involved in the anti-seizure effects of morphine and thiamine (103–105). mTOR can promote the release of inducible nitric oxide synthase (iNOS) as well as iNOS mRNA stability in astrocytes (106, 107), which in turn can activate mTOR pathway by a reversible nitrosylation of tuberous sclerosis complex (TSC) 2 (108). Moreover, excessive ROS in neurons has been found to be associated with epileptic injury (109). mTOR serves as a modulator of ROS production as well as an important effector in response to ROS (67, 110, 111).
mTOR-Targeted Epilpesy Therapy
Drugs targeting mTOR have been widely studied, especially for the treatment of refractory epilepsy, which present promising anti-seizure effects in both lab investigation and clinical trials. Recently, Everolimus, a rapamycin derivative, is approved by FDA for the treatment of tuberous sclerosis complex-associated partial-onset seizures. However, clinical evidence indicates that solo inhibition of mTOR activity isn’t fully effective to cure epilepsy and epilepsy associated pathophysiological changes, and can be associated with serious adverse events, such as protein synthesis disorder (112), suggesting other unknow molecular mechanisms are involved in epileptogenesis (113), and more precise targeting of specific molecules in mTOR pathway is required to reduce side effects.
mTOR Related Non-Coding RNA
Non-coding RNAs (ncRNAs) consist of intronic RNAs, microRNAs (miRNAs), long noncoding RNAs (lncRNAs), circular RNAs (circRNAs), and extracellular RNAs (114). ncRNAs can exert biological effects via directly targeting mTOR, targeting other components of mTOR complex or targeting key upstream regulators of mTOR. Conversely, mTOR can also regulate ncRNA biogenesis. For example, Raptor, an essential component of mTORC1 controls miRNA biogenesis though Drosha, a RNase processing primary miRNA (pri-miRNA) to precursor miRNA (115). GSK3β, a downstream molecule of mTOR, directly increases Drosha activity and mature miRNAs accumulation (116). Studies also suggested mTOR regulation of miRNA expression through its upstream enhancer or transcription factor (117, 118). In epilepsy, ncRNAs can regulate inflammatory cells and mediators by interacting with mTOR signaling during the intricate neuroinflammatory processes. Here, we focus on the functions of three main categories of ncRNAs (miRNAs, lncRNAs, and circRNAs) on epileptic neuroinflammation, as well as the interrelation with mTOR signaling.
miRNAs
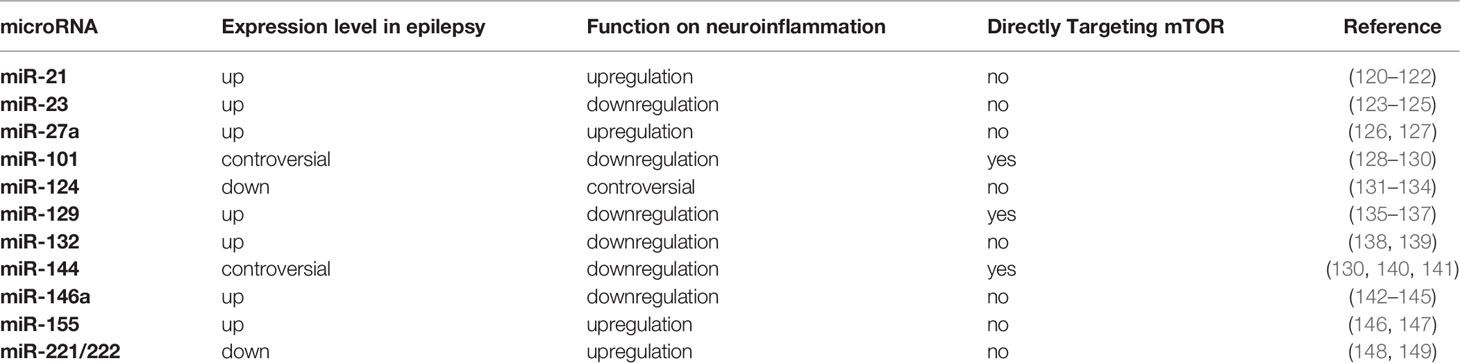
miRNAs, a major class of functional noncoding RNAs, have emerged as important post-transcriptional regulators of gene expression and provided a completely new manner in manipulating gene expression (114). miRNAs are short noncoding RNAs (20-23 nucleotides) that recognize partially complementary target sequences in selected mRNAs, and predominantly inhibit protein expression by either destabilizing their mRNA targets or inhibiting protein translation. A single miRNA can have multiple targets and have diverse effects on gene translation, which forms a complex regulatory signaling network (119). Series of studies have demonstrated that miRNAs mediate neuroinflammation and play a crucial role in the development and progression of epilepsy via mTOR pathway (see Table 1).
miR-21-5p is an inflammatory miRNA increased in epilepsy. Recent studies have suggested that miR-21-5p can modulate the proliferation and apoptosis of astrocytes in neuroinflammation by targeting phosphatase and tensin homolog (PTEN)-mTOR signaling. Inhibition of miR-21-5p has the potential of preventing neuronal damage in stages of epileptogenesis (120, 121, 150). mTOR, in turn, can enhance the expression of miR-21 though transcription factor STAT3, a direct substrate of mTOR (151, 152). Meanwhile, miR-21-5p has been reported to decrease in hippocampal sclerosis (HS) and focal cortical dysplasia (FCD) (122, 153). Further research on miR-21-5p would be appreciated. miR-23 has been reported to interact with mTOR signaling and contribute to temporal lobe epilepsy (TLE) (123, 124). miR-23a can reactivate the AKT/mTOR pathway and suppresses neuroinflammation (154), while miR-23b suppresses IL-17-associated autoimmune inflammation and alleviates brain injury by interacting with AKT/mTOR pathway on negative regulation of inositol polyphosphate multikinase (IPMK) in pediatric refractory epilepsy (48, 125, 155). miR-27 acting as a regulator of phosphoinositide 3-kinase (PI3K)/AKT/mTOR axis widely participates in metabolic and inflammatory processes (156–158). miR-27a has been shown to be upregulated in epilepsy patients and inhibition of miR-27a alleviates the inflammatory response (126). Recently, miR-27a has been suggested as a potential serum biomarker for diagnosis and mechanistic links to underlying pathomechanisms in adult TLE (127). In addition, miR-124 is one of the most abundant miRNA identified in the CNS (159), which has been reported controversially in the mediation of epilepsy. miR-124 presents the inhibition of susceptibility of epileptic seizures via regulating cAMP-response element-binding protein1 (CREB1) or neuron restrictive silencer factor (NRSF) (131, 132). However, other studies have shown that miR-124 can promote epilepsy via enhancing microglia activation and inflammatory cytokines (132). By interacting with mTOR, miR-124 conducts mostly negative regulations on neuroinflammation (133, 160). miR-124 derived from microglia promotes the anti-inflamed M2 polarization and inhibits neuroinflammation via the suppression of mTOR signaling (134). Moreover, miR-146a has been widely studied in metabolic dysfunction and neuroinflammatory response (142). miR-146a is persistently expressed in reactive astrocytes to protect the CNS from neuroinflammatory damage in epilepsy (143, 161). Interestingly, miR-146a are upregulated commonly associated with IL-1β in epilepsy (162). A possible explanation is that enhanced miR-146a can upregulate IL-β by downregulating complement factor H (CFH), and facilitates miR-146a-CFH-IL-1β feedback loop that maintains chronic inflammation (144). miR-146a can suppress apoptosis and thus regulate the function of macrophage in neuroinflammation through mTOR pathway (163, 164), while mTORC1 can reactivate miR-146 micro-ribonucleoproteins (miRNPs) to restrict proinflammatory cytokine production in amyloid beta-exposed glial cells (165). In addition, miR-146a has been observed in the serum of both epilepsy patients and animal models (145, 166), offering the potentials of biomarker for both diagnosis and prognosis. Finally, miR-155 tag single-nucleotide polymorphisms (SNPs) are genetic susceptibility factors for epilepsy, where miR-155 may positively participate in the neuroinflammatory process (146, 167). For example, under the exposition of LPS, microglia increase the releases of nitric oxide and inflammatory cytokines by upregulating miR-155 (168). mTOR signaling and miR-155 could be directly targeted to each other. mTOR related epileptic disorders, such as TSC, display high expression of miR-155 and oxidative stress markers (169), while miR-155 can regulate mTOR activation controlling estrogen receptor function (147).
Additional microRNAs have been revealed to be involved in epileptic neuroinflammation with the interaction with mTOR signaling. Altered expression of miR-144 has been reported in TLE (170), which may modulate endothelial cells and macrophages in neuroinflammation by directly interacting with the 3’ untranslated regions (UTRs) of mTOR (140, 141, 171, 172). Astrocyte-derived miR-221/222 signals mTOR pathway leading the regulation of immune responses and autophagy through cytokines and cell adhesion molecules in epileptic neuroinflammation (148, 149). Moreover, miR-132 increases in astrocytes in TLE and promotes the activations of molecules in PI3K/mTOR pathway, such as AKT, TSC2, mTOR (138, 173, 174), which decrease the expression of inflammatory mediators including IL-1β、TGF-β、CCL2 (139).
LncRNA
LncRNAs are large and heterogeneous ncRNAs with more than 200 nucleotides in length, which regulate gene expression at transcriptional, post-transcriptional, chromatin remodeling and epigenetic levels and act as competing endogenous RNAs (ceRNAs) with miRNAs (175, 176). Diverse functions of lncRNAs have been identified in epileptic neuroinflammation.
Pro-Neuroinflammatory lncRNAs
LncRNA ILF3-AS1 is a newly discovered lncRNA in epilepsy which promotes the expression of inflammatory cytokines by targeting miR-212 in TLE (177). However, upregulation of ILF3-AS1 could have a protective effect of alleviating hypoxic injury via activation of PI3K/AKT signaling pathway (178). ZFAS1 has been reported to be upregulated in epilepsy which promotes neuronal apoptosis and neuroinflammation (179). The inhibition of PI3K/AKT pathway can silence ZFAS1 leading the downregulation of epilepticus-induced neural autophagy (180). TUG1 positively regulates inflammatory processes in many neurological diseases such as epilepsy, cognitive impairment, and ischemic stroke (181, 182). In epilepticus, downregulation of TUG1 relieves neuronal apoptosis and the releases of inflammatory cytokines via targeting the miR-421/mTOR and miR-199a-3p/mTOR axes (183–185). The nuclear paraspeckle assembly transcript 1 (NEAT1) has been demonstrated to regulate both neural activity and inflammation though interacting with mTOR pathway in epilepsy (186, 187). It binds epilepsy-associated potassium channel-interacting proteins and subsequently induces neuronal hyperexcitation contributing seizure initiation (188). Meanwhile, NEAT1 promotes IL-6, cyclooxygenase (COX)-2, and TNF-α expression by targeting miR-129-5p and activating the Notch signaling pathway in epilepsy (136). In addition, excessive NEAT1 can impair the integrity and augments the permeability of BBB, worsening neuroinflammation (189). Moreover, H19 has been found to activate astrocytes and microglia, and prompt inflammatory responses and neuron apoptosis in epileptic seizures via the binding and suppression of miRNA let-7b and blocking mTORC1-mediated 4EBP1 phosphorylation (190–193).
Anti-Neuroinflammatory lncRNAs
Brain-derived neurotrophic factor (BDNF) antisense RNA (BDNF-AS) acts a natural antisense lncRNA of BDNF, a canonical nerve growth factor to support the survival of neuronal population and has been suggested as a potential target of epilepsy (194, 195). The regulation of BDNF-AS on seizure activities probably attribute to its activation of mTOR by inhibiting ribonuclease inhibitor 1 (RNH1)-mediated mTOR mRNA decay, as well as the controlling on the production of BDNF related inflammatory cytokines, such as TNF-α, IL-2, IL-6 (196, 197) and MALAT1, participates in the anti-inflammatory processes and regulates autophagy in endothelial cells and neurons by interacting with mTOR pathway in epilepsy (198–201). MALAT1 can induce splicing factor Ser/Arg-rich splicing factor 1 (SRSF1) and thus modulates the alternative splicing of S6K1 to activate mTOR signaling (202). It is also noteworthy that MALAT1 can also bind to miR-101 to upregulate PI3K/AKT pathway in epilepsy (203, 204). UCA1 has been found to be differentially hypermethylated in TLE as well (205). By regulating miR-203, UCA1 decreases IL-1β, IL-6, TNF-α, and Cox-2 levels via miR-499b-5p in epilepsy (206, 207). In addition, UCA1 might implement the anti-inflammatory function by though AKTmTOR pathway by directly targeting TLR4 (207–209).
CircRNAs
CircRNA is a closed non-coding RNA which, unlike linear RNA, form a closed-loop structure with a direct ligation of the 3’ and 5’ ends (210). CircRNAs are more stable and abundant than the corresponding linear mRNAs in plasma leading their potentials to be disease biomarkers (211). It also functions as ceRNA to modulate the expression of genes (212). A growing body of studies has uncovered the diverse functions of circRNAs on gene translation and interacting with cellular signaling, such as mTOR pathway (213–218).
Recent studies have assessed the pattern of circRNAs expression in epilepsy (219, 220). For example, circHivep2 can significantly inhibit microglial activation and the expression of inflammatory cytokines in kainic acid (KA)-induced epilepsy (221). It is increasingly recognized that activated astrocytes play a key role in neuronal damages. Similarly, circIgf1r is upregulated in astrocytes in epilepsy and silencing of circIgf1r could play a protective role in neuronal injury by converting reactive astrocytes from Neurotoxic A1 states to A2 phenotype (222). In addition, circRNAs can be cooperate with miRNAs in the development of epilepsy. CircUBQLN, for instance, can directly target mTOR signaling to attenuate oxidative stress in epilepsy via reymark(223) the combination with miR-155.
Conclusion
Neuroinflammation as a major hallmark of seizure plays a key role in the initiation and exacerbation of epilepsy, especially in refractory epilepsy. The of molecular mechanisms underlying epileptic neuroinflammation would largely extend our understanding of epilepsy and benefit the development of targeted therapies. Accumulating evidence has indicated that ncRNAs coordinated with mTOR signaling are essential for regulating the context and course of neuroinflammation and corresponding pathophysiological traits. Moreover, ncRNAs and mTOR have emerged as potential biomarkers, as well as therapeutic targets in the management of epilepsy. However, since only a small fraction of annotated ncRNAs is well characterized, and the interaction of ncRNAs and mTOR signaling in epileptic neuroinflammation requires further studies where functional screening and identification of ncRNAs interoperating in the intricate regulation network would be appreciated.
Author Contributions
LZ and CZ conceived the review. CZ wrote the draft. All authors contribute to reviewing and editing.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor WP declared a shared parent affiliation with the authors at the time of review.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Thijs RD, Surges R, O'Brien TJ, Sander JW. Epilepsy in Adults. Lancet (2019) 393(10172):689–701. doi: 10.1016/s0140-6736(18)32596-0
2. Pitkänen A, Löscher W, Vezzani A, Becker AJ, Simonato M, Lukasiuk K, et al. Advances in the Development of Biomarkers for Epilepsy. Lancet Neurol (2016) 15(8):843–56. doi: 10.1016/s1474-4422(16)00112-5
3. Rana A, Musto AE. The Role of Inflammation in the Development of Epilepsy. J Neuroinflamm (2018) 15(1):144. doi: 10.1186/s12974-018-1192-7
4. Vezzani A, Balosso S, Ravizza T. Neuroinflammatory Pathways as Treatment Targets and Biomarkers in Epilepsy. Nat Rev Neurol (2019) 15(8):459–72. doi: 10.1038/s41582-019-0217-x
5. Alvim MKM, Morita-Sherman ME, Yasuda CL, Rocha NP, Vieira É L, Pimentel-Silva LR, et al. Inflammatory and Neurotrophic Factor Plasma Levels are Related to Epilepsy Independently of Etiology. Epilepsia (2021) 62(10):2385–94. doi: 10.1111/epi.17023
6. Vezzani A, Lang B, Aronica E. Immunity and Inflammation in Epilepsy. Cold Spring Harbor Perspect Med (2015) 6(2):a022699–a022699. doi: 10.1101/cshperspect.a022699
7. Lipton JO, Sahin M. The Neurology of mTOR. Neuron (2014) 84(2):275–91. doi: 10.1016/j.neuron.2014.09.034
8. Zeng LH, Rensing NR, Wong M. The Mammalian Target of Rapamycin Signaling Pathway Mediates Epileptogenesis in a Model of Temporal Lobe Epilepsy. J Neurosci (2009) 29(21):6964–72. doi: 10.1523/jneurosci.0066-09.2009
9. Saxton RA, Sabatini DM. mTOR Signaling in Growth, Metabolism, and Disease. Cell (2017) 169(2):361–71. doi: 10.1016/j.cell.2017.03.035
10. Zhang L, Huang T, Teaw S, Nguyen LH, Hsieh LS, Gong X, et al. Filamin A Inhibition Reduces Seizure Activity in a Mouse Model of Focal Cortical Malformations. Sci Transl Med (2020) 12(531). doi: 10.1126/scitranslmed.aay0289
11. Nguyen LH, Xu Y, Mahadeo T, Zhang L, Lin TV, Born HA, et al. Expression of 4E-BP1 in Juvenile Mice Alleviates mTOR-Induced Neuronal Dysfunction and Epilepsy. Brain (2021) 145(4):1310–25. doi: 10.1093/brain/awab390
12. Crino PB. mTOR: A Pathogenic Signaling Pathway in Developmental Brain Malformations. Trends Mol Med (2011) 17(12):734–42. doi: 10.1016/j.molmed.2011.07.008
13. Bordon Y. Infectious Disease: Hushing mTOR Boosts Immunity to Pathogens. Nat Rev Immunol (2013) 13(12):847. doi: 10.1038/nri3562
14. Winkle M, El-Daly SM, Fabbri M, Calin GA. Noncoding RNA Therapeutics - Challenges and Potential Solutions. Nat Rev Drug Discovery (2021) 20(8):629–51. doi: 10.1038/s41573-021-00219-z
15. Slack FJ, Chinnaiyan AM. The Role of Non-Coding RNAs in Oncology. Cell (2019) 179(5):1033–55. doi: 10.1016/j.cell.2019.10.017
16. Lin Z, Pang K, Li H, Zhang X, Wan J, Zheng T, et al. Characterization of Immune-Related Long Non-Coding RNAs to Construct a Novel Signature and Predict the Prognosis and Immune Landscape of Soft Tissue Sarcoma. Front Cell Dev Biol (2021) 9:709241. doi: 10.3389/fcell.2021.709241
17. Henshall DC, Hamer HM, Pasterkamp RJ, Goldstein DB, Kjems J, Prehn JHM, et al. MicroRNAs in Epilepsy: Pathophysiology and Clinical Utility. Lancet Neurol (2016) 15(13):1368–76. doi: 10.1016/s1474-4422(16)30246-0
18. Tripathi S, Shree B, Mohapatra S, Swati A, Basu, Sharma V. The Expanding Regulatory Mechanisms and Cellular Functions of Long Non-Coding RNAs (lncRNAs) in Neuroinflammation. Mol Neurobiol (2021) 58(6):2916–39. doi: 10.1007/s12035-020-02268-8
19. Shao Y, Chen Y. Pathophysiology and Clinical Utility of Non-Coding RNAs in Epilepsy. Front Mol Neurosci (2017) 10:249. doi: 10.3389/fnmol.2017.00249
20. Ma Y. The Challenge of microRNA as a Biomarker of Epilepsy. Curr Neuropharmacol (2018) 16(1):37–42. doi: 10.2174/1570159x15666170703102410
21. Ben-Sahra I, Manning BD. Mtorc1 Signaling and the Metabolic Control of Cell Growth. Curr Opin Cell Biol (2017) 45:72–82. doi: 10.1016/j.ceb.2017.02.012
22. Rabanal-Ruiz Y, Otten EG, Korolchuk VI. Mtorc1 as the Main Gateway to Autophagy. Essays Biochem (2017) 61(6):565–84. doi: 10.1042/ebc20170027
23. Saxton RA, Sabatini DM. mTOR Signaling in Growth, Metabolism, and Disease. Cell (2017) 168(6):960–76. doi: 10.1016/j.cell.2017.02.004
24. Ma XM, Blenis J. Molecular Mechanisms of mTOR-Mediated Translational Control. Nat Rev Mol Cell Biol (2009) 10(5):307–18. doi: 10.1038/nrm2672
25. Hartman NW, Lin TV, Zhang L, Paquelet GE, Feliciano DM, Bordey A. Mtorc1 Targets the Translational Repressor 4E-BP2, But Not S6 Kinase 1/2, to Regulate Neural Stem Cell Self-Renewal In Vivo. Cell Rep (2013) 5(2):433–44. doi: 10.1016/j.celrep.2013.09.017
26. Huang W, Zhu PJ, Zhang S, Zhou H, Stoica L, Galiano M, et al. Mtorc2 Controls Actin Polymerization Required for Consolidation of Long-Term Memory. Nat Neurosci (2013) 16(4):441–8. doi: 10.1038/nn.3351
27. Jacinto E, Loewith R, Schmidt A, Lin S, Rüegg MA, Hall A, et al. Mammalian TOR Complex 2 Controls the Actin Cytoskeleton and is Rapamycin Insensitive. Nat Cell Biol (2004) 6(11):1122–8. doi: 10.1038/ncb1183
28. Aronica E, Bauer S, Bozzi Y, Caleo M, Dingledine R, Gorter JA, et al. Neuroinflammatory Targets and Treatments for Epilepsy Validated in Experimental Models. Epilepsia (2017) 58 Suppl 3(Suppl 3):27–38. doi: 10.1111/epi.13783
29. Maroso M, Balosso S, Ravizza T, Liu J, Aronica E, Iyer AM, et al. Toll-Like Receptor 4 and High-Mobility Group Box-1 are Involved in Ictogenesis and can be Targeted to Reduce Seizures. Nat Med (2010) 16(4):413–9. doi: 10.1038/nm.2127
30. Broekaart DWM, Anink JJ, Baayen JC, Idema S, de Vries HE, Aronica E, et al. Activation of the Innate Immune System is Evident Throughout Epileptogenesis and is Associated With Blood-Brain Barrier Dysfunction and Seizure Progression. Epilepsia (2018) 59(10):1931–44. doi: 10.1111/epi.14550
31. Linnerbauer M, Wheeler MA, Quintana FJ. Astrocyte Crosstalk in CNS Inflammation. Neuron (2020) 108(4):608–22. doi: 10.1016/j.neuron.2020.08.012
32. Mukherjee S, Arisi GM, Mims K, Hollingsworth G, O'Neil K, Shapiro LA. Neuroinflammatory Mechanisms of Post-Traumatic Epilepsy. J Neuroinflamm (2020) 17(1):193–3. doi: 10.1186/s12974-020-01854-w
33. Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, et al. Neurotoxic Reactive Astrocytes are Induced by Activated Microglia. Nature (2017) 541(7638):481–7. doi: 10.1038/nature21029
34. Akin D, Ravizza T, Maroso M, Carcak N, Eryigit T, Vanzulli I, et al. IL-1β is Induced in Reactive Astrocytes in the Somatosensory Cortex of Rats With Genetic Absence Epilepsy at the Onset of Spike-and-Wave Discharges, and Contributes to Their Occurrence. Neurobiol Dis (2011) 44(3):259–69. doi: 10.1016/j.nbd.2011.05.015
35. Giovannoni F, Quintana FJ. The Role of Astrocytes in CNS Inflammation. Trends Immunol (2020) 41(9):805–19. doi: 10.1016/j.it.2020.07.007
36. Devinsky O, Vezzani A, Najjar S, De Lanerolle NC, Rogawski MA. Glia and Epilepsy: Excitability and Inflammation. Trends Neurosci (2013) 36(3):174–84. doi: 10.1016/j.tins.2012.11.008
37. Nikolic L, Nobili P, Shen W, Audinat E. Role of Astrocyte Purinergic Signaling in Epilepsy. Glia (2020) 68(9):1677–91. doi: 10.1002/glia.23747
38. Rüber T, David B, Lüchters G, Nass RD, Friedman A, Surges R, et al. Evidence for Peri-Ictal Blood-Brain Barrier Dysfunction in Patients With Epilepsy. Brain (2018) 141(10):2952–65. doi: 10.1093/brain/awy242
39. Heinemann U, Kaufer D, Friedman A. Blood-Brain Barrier Dysfunction, Tgfβ Signaling, and Astrocyte Dysfunction in Epilepsy. Glia (2012) 60(8):1251–7. doi: 10.1002/glia.22311
40. Oby E, Janigro D. The Blood-Brain Barrier and Epilepsy. Epilepsia (2006) 47(11):1761–74. doi: 10.1111/j.1528-1167.2006.00817.x
41. Djukic M, Mildner A, Schmidt H, Czesnik D, Brück W, Priller J, et al. Circulating Monocytes Engraft in the Brain, Differentiate Into Microglia and Contribute to the Pathology Following Meningitis in Mice. Brain (2006) 129(Pt 9):2394–403. doi: 10.1093/brain/awl206
42. Bosco DB, Tian DS, Wu LJ. Neuroimmune Interaction in Seizures and Epilepsy: Focusing on Monocyte Infiltration. FEBS J (2020) 287(22):4822–37. doi: 10.1111/febs.15428
43. Varvel NH, Neher JJ, Bosch A, Wang W, Ransohoff RM, Miller RJ, et al. Infiltrating Monocytes Promote Brain Inflammation and Exacerbate Neuronal Damage After Status Epilepticus. Proc Natl Acad Sci U.S.A. (2016) 113(38):E5665–74. doi: 10.1073/pnas.1604263113
44. Librizzi L, Vila Verde D, Colciaghi F, Deleo F, Regondi MC, Costanza M, et al. Peripheral Blood Mononuclear Cell Activation Sustains Seizure Activity. Epilepsia (2021) 62(7):1715–28. doi: 10.1111/epi.16935
45. Tian DS, Peng J, Murugan M, Feng LJ, Liu JL, Eyo UB, et al. Chemokine CCL2-CCR2 Signaling Induces Neuronal Cell Death via STAT3 Activation and IL-1β Production After Status Epilepticus. J Neurosci (2017) 37(33):7878–92. doi: 10.1523/jneurosci.0315-17.2017
46. Ravizza T, Gagliardi B, Noé F, Boer K, Aronica E, Vezzani A. Innate and Adaptive Immunity During Epileptogenesis and Spontaneous Seizures: Evidence From Experimental Models and Human Temporal Lobe Epilepsy. Neurobiol Dis (2008) 29(1):142–60. doi: 10.1016/j.nbd.2007.08.012
47. Bauer J, Becker AJ, Elyaman W, Peltola J, Rüegg S, Titulaer MJ, et al. Innate and Adaptive Immunity in Human Epilepsies. Epilepsia (2017) 58 Suppl 3(Suppl Suppl 3):57–68. doi: 10.1111/epi.13784
48. Kumar P, Shih DCW, Lim A, Paleja B, Ling S, Li Yun L, et al. Pro-Inflammatory, IL-17 Pathways Dominate the Architecture of the Immunome in Pediatric Refractory Epilepsy. JCI Insight (2019) 5(8):e126337. doi: 10.1172/jci.insight.126337
49. Silverberg J, Ginsburg D, Orman R, Amassian V, Durkin HG, Stewart M. Lymphocyte Infiltration of Neocortex and Hippocampus After a Single Brief Seizure in Mice. Brain Behav Immun (2010) 24(2):263–72. doi: 10.1016/j.bbi.2009.10.006
50. Geis C, Planagumà J, Carreño M, Graus F, Dalmau J. Autoimmune Seizures and Epilepsy. J Clin Invest (2019) 129(3):926–40. doi: 10.1172/jci125178
51. Mohammad K, Dakik P, Medkour Y, Mitrofanova D, Titorenko VI. Quiescence Entry, Maintenance, and Exit in Adult Stem Cells. Int J Mol Sci (2019) 20(9):2158. doi: 10.3390/ijms20092158
52. Kothur K, Bandodkar S, Wienholt L, Chu S, Pope A, Gill D, et al. Etiology is the Key Determinant of Neuroinflammation in Epilepsy: Elevation of Cerebrospinal Fluid Cytokines and Chemokines in Febrile Infection-Related Epilepsy Syndrome and Febrile Status Epilepticus. Epilepsia (2019) 60(8):1678–88. doi: 10.1111/epi.16275
53. de Vries EE, van den Munckhof B, Braun KP, van Royen-Kerkhof A, de Jager W, Jansen FE. Inflammatory Mediators in Human Epilepsy: A Systematic Review and Meta-Analysis. Neurosci Biobehav Rev (2016) 63:177–90. doi: 10.1016/j.neubiorev.2016.02.007
54. van Vliet EA, Aronica E, Vezzani A, Ravizza T. Review: Neuroinflammatory Pathways as Treatment Targets and Biomarker Candidates in Epilepsy: Emerging Evidence From Preclinical and Clinical Studies. Neuropathol Appl Neurobiol (2018) 44(1):91–111. doi: 10.1111/nan.12444
55. Rojas A, Jiang J, Ganesh T, Yang MS, Lelutiu N, Gueorguieva P, et al. Cyclooxygenase-2 in Epilepsy. Epilepsia (2014) 55(1):17–25. doi: 10.1111/epi.12461
56. Paré A, Mailhot B, Lévesque SA, Juzwik C, Ignatius Arokia Doss PM, Lécuyer MA, et al. IL-1β Enables CNS Access to CCR2(hi) Monocytes and the Generation of Pathogenic Cells Through GM-CSF Released by CNS Endothelial Cells. Proc Natl Acad Sci U.S.A. (2018) 115(6):E1194–e1203. doi: 10.1073/pnas.1714948115
57. Arisi GM, Foresti ML, Katki K, Shapiro LA. Increased CCL2, CCL3, CCL5, and IL-1β Cytokine Concentration in Piriform Cortex, Hippocampus, and Neocortex After Pilocarpine-Induced Seizures. J Neuroinflamm (2015) 12:129. doi: 10.1186/s12974-015-0347-z
58. Welser-Alves JV, Milner R. Microglia are the Major Source of TNF-α and TGF-β1 in Postnatal Glial Cultures; Regulation by Cytokines, Lipopolysaccharide, and Vitronectin. Neurochem Int (2013) 63(1):47–53. doi: 10.1016/j.neuint.2013.04.007
59. Matsuda T, Murao N, Katano Y, Juliandi B, Kohyama J, Akira S, et al. TLR9 Signalling in Microglia Attenuates Seizure-Induced Aberrant Neurogenesis in the Adult Hippocampus. Nat Commun (2015) 6:6514. doi: 10.1038/ncomms7514
60. Cerri C, Genovesi S, Allegra M, Pistillo F, Püntener U, Guglielmotti A, et al. The Chemokine CCL2 Mediates the Seizure-Enhancing Effects of Systemic Inflammation. J Neurosci (2016) 36(13):3777–88. doi: 10.1523/jneurosci.0451-15.2016
61. Lehnardt S. Innate Immunity and Neuroinflammation in the CNS: The Role of Microglia in Toll-Like Receptor-Mediated Neuronal Injury. Glia (2010) 58(3):253–63. doi: 10.1002/glia.20928
62. Zindel J, Kubes P. DAMPs, PAMPs, and LAMPs in Immunity and Sterile Inflammation. Annu Rev Pathol (2020) 15:493–518. doi: 10.1146/annurev-pathmechdis-012419-032847
63. Tan TH, Perucca P, O'Brien TJ, Kwan P, Monif M. Inflammation, Ictogenesis, and Epileptogenesis: An Exploration Through Human Disease. Epilepsia (2021) 62(2):303–24. doi: 10.1111/epi.16788
64. Terrone G, Balosso S, Pauletti A, Ravizza T, Vezzani A. Inflammation and Reactive Oxygen Species as Disease Modifiers in Epilepsy. Neuropharmacology (2020) 167:107742. doi: 10.1016/j.neuropharm.2019.107742
65. Tang D, Kang R, Coyne CB, Zeh HJ, Lotze MT. PAMPs and DAMPs: Signal 0s That Spur Autophagy and Immunity. Immunol Rev (2012) 249(1):158–75. doi: 10.1111/j.1600-065X.2012.01146.x
66. Keane L, Antignano I, Riechers SP, Zollinger R, Dumas AA, Offermann N, et al. mTOR-Dependent Translation Amplifies Microglia Priming in Aging Mice. J Clin Invest (2021) 131(1):e132727. doi: 10.1172/jci132727
67. Hu Y, Mai W, Chen L, Cao K, Zhang B, Zhang Z, et al. mTOR-Mediated Metabolic Reprogramming Shapes Distinct Microglia Functions in Response to Lipopolysaccharide and ATP. Glia (2020) 68(5):1031–45. doi: 10.1002/glia.23760
68. Ye X, Zhu M, Che X, Wang H, Liang X-J, Wu C, et al. Lipopolysaccharide Induces Neuroinflammation in Microglia by Activating the MTOR Pathway and Downregulating Vps34 to Inhibit Autophagosome Formation. J Neuroinflamm (2020) 17(1):18–8. doi: 10.1186/s12974-019-1644-8
69. Xu X, Gao W, Li L, Hao J, Yang B, Wang T, et al. Annexin A1 Protects Against Cerebral Ischemia-Reperfusion Injury by Modulating Microglia/Macrophage Polarization via FPR2/ALX-Dependent AMPK-mTOR Pathway. J Neuroinflamm (2021) 18(1):119. doi: 10.1186/s12974-021-02174-3
70. Yang M-T, Lin Y-C, Ho W-H, Liu C-L, Lee W-T. Everolimus is Better Than Rapamycin in Attenuating Neuroinflammation in Kainic Acid-Induced Seizures. J Neuroinflamm (2017) 14(1):15–5. doi: 10.1186/s12974-017-0797-6
71. Zhao XF, Liao Y, Alam MM, Mathur R, Feustel P, Mazurkiewicz JE, et al. Microglial mTOR is Neuronal Protective and Antiepileptogenic in the Pilocarpine Model of Temporal Lobe Epilepsy. J Neurosci (2020) 40(40):7593–608. doi: 10.1523/jneurosci.2754-19.2020
72. Hsieh LS, Wen JH, Nguyen LH, Zhang L, Getz SA, Torres-Reveron J, et al. Ectopic HCN4 Expression Drives mTOR-Dependent Epilepsy in Mice. Sci Transl Med (2020) 12(570):eabc1492. doi: 10.1126/scitranslmed.abc1492
73. Li CY, Li X, Liu SF, Qu WS, Wang W, Tian DS. Inhibition of mTOR Pathway Restrains Astrocyte Proliferation, Migration and Production of Inflammatory Mediators After Oxygen-Glucose Deprivation and Reoxygenation. Neurochem Int (2015) 83(84):9–18. doi: 10.1016/j.neuint.2015.03.001
74. Van Skike CE, Jahrling JB, Olson AB, Sayre NL, Hussong SA, Ungvari Z, et al. Inhibition of mTOR Protects the Blood-Brain Barrier in Models of Alzheimer's Disease and Vascular Cognitive Impairment. Am J Physiol Heart Circ Physiol (2018) 314(4):H693–h703. doi: 10.1152/ajpheart.00570.2017
75. Zhang L, Huang T, Teaw S, Bordey A. Hypervascularization in mTOR-Dependent Focal and Global Cortical Malformations Displays Differential Rapamycin Sensitivity. Epilepsia (2019) 60(6):1255–65. doi: 10.1111/epi.15969
76. Nowak W, Grendas LN, Sanmarco LM, Estecho IG, Arena Á R, Eberhardt N, et al. Pro-Inflammatory Monocyte Profile in Patients With Major Depressive Disorder and Suicide Behaviour and How Ketamine Induces Anti-Inflammatory M2 Macrophages by NMDAR and mTOR. EBioMedicine (2019) 50:290–305. doi: 10.1016/j.ebiom.2019.10.063
77. Zhao Y, Shen X, Na N, Chu Z, Su H, Chao S, et al. mTOR Masters Monocyte Development in Bone Marrow by Decreasing the Inhibition of STAT5 on IRF8. Blood (2018) 131(14):1587–99. doi: 10.1182/blood-2017-04-777128
78. Howden AJM, Hukelmann JL, Brenes A, Spinelli L, Sinclair LV, Lamond AI, et al. Quantitative Analysis of T Cell Proteomes and Environmental Sensors During T Cell Differentiation. Nat Immunol (2019) 20(11):1542–54. doi: 10.1038/s41590-019-0495-x
79. Yang K, Blanco DB, Chen X, Dash P, Neale G, Rosencrance C, et al. Metabolic Signaling Directs the Reciprocal Lineage Decisions of αβ and γδ T Cells. Sci Immunol (2018) 3(25):eaas9818. doi: 10.1126/sciimmunol.aas9818
80. Yang K, Chi H. mTOR and Metabolic Pathways in T Cell Quiescence and Functional Activation. Semin Immunol (2012) 24(6):421–8. doi: 10.1016/j.smim.2012.12.004
81. Delgoffe GM, Pollizzi KN, Waickman AT, Heikamp E, Meyers DJ, Horton MR, et al. The Kinase mTOR Regulates the Differentiation of Helper T Cells Through the Selective Activation of Signaling by Mtorc1 and Mtorc2. Nat Immunol (2011) 12(4):295–303. doi: 10.1038/ni.2005
82. Yang K, Shrestha S, Zeng H, Karmaus PW, Neale G, Vogel P, et al. T Cell Exit From Quiescence and Differentiation Into Th2 Cells Depend on Raptor-Mtorc1-Mediated Metabolic Reprogramming. Immunity (2013) 39(6):1043–56. doi: 10.1016/j.immuni.2013.09.015
83. Heikamp EB, Patel CH, Collins S, Waickman A, Oh MH, Sun IH, et al. The AGC Kinase SGK1 Regulates TH1 and TH2 Differentiation Downstream of the Mtorc2 Complex. Nat Immunol (2014) 15(5):457–64. doi: 10.1038/ni.2867
84. Valmori D, Tosello V, Souleimanian NE, Godefroy E, Scotto L, Wang Y, et al. Rapamycin-Mediated Enrichment of T Cells With Regulatory Activity in Stimulated CD4+ T Cell Cultures is Not Due to the Selective Expansion of Naturally Occurring Regulatory T Cells But to the Induction of Regulatory Functions in Conventional CD4+ T Cells. J Immunol (2006) 177(2):944–9. doi: 10.4049/jimmunol.177.2.944
85. Xu X, Araki K, Li S, Han J-H, Ye L, Tan WG, et al. Autophagy is Essential for Effector CD8(+) T Cell Survival and Memory Formation. Nat Immunol (2014) 15(12):1152–61. doi: 10.1038/ni.3025
86. Xiao Z, Peng J, Gan N, Arafat A, Yin F. Interleukin-1β Plays a Pivotal Role via the PI3K/Akt/mTOR Signaling Pathway in the Chronicity of Mesial Temporal Lobe Epilepsy. Neuroimmunomodulation (2016) 23(5-6):332–44. doi: 10.1159/000460254
87. Harris J, Hartman M, Roche C, Zeng SG, O'Shea A, Sharp FA, et al. Autophagy Controls IL-1beta Secretion by Targeting Pro-IL-1beta for Degradation. J Biol Chem (2011) 286(11):9587–97. doi: 10.1074/jbc.M110.202911
88. Deason K, Troutman TD, Jain A, Challa DK, Mandraju R, Brewer T, et al. BCAP Links IL-1R to the PI3K-mTOR Pathway and Regulates Pathogenic Th17 Cell Differentiation. J Exp Med (2018) 215(9):2413–28. doi: 10.1084/jem.20171810
89. Jin W, Dong C. IL-17 Cytokines in Immunity and Inflammation. Emerg Microbes Infect (2013) 2(9):e60–0. doi: 10.1038/emi.2013.58
90. Ren W, Yin J, Duan J, Liu G, Tan B, Yang G, et al. Mtorc1 Signaling and IL-17 Expression: Defining Pathways and Possible Therapeutic Targets. Eur J Immunol (2016) 46(2):291–9. doi: 10.1002/eji.201545886
91. Wyszynski RW, Gibbs BF, Varani L, Iannotta D, Sumbayev VV. Interleukin-1 Beta Induces the Expression and Production of Stem Cell Factor by Epithelial Cells: Crucial Involvement of the PI-3k/mTOR Pathway and HIF-1 Transcription Complex. Cell Mol Immunol (2016) 13(1):47–56. doi: 10.1038/cmi.2014.113
92. Zhang L, Feliciano DM, Huang T, Zhang S, Bordey A. Hypoxia-Inducible Factor-1a Contributes to Dendritic Overgrowth in Tuberous Sclerosis. Neurosci Lett (2016) 612:43–7. doi: 10.1016/j.neulet.2015.11.038
93. Varshney P, Saini N. PI3K/AKT/mTOR Activation and Autophagy Inhibition Plays a Key Role in Increased Cholesterol During IL-17A Mediated Inflammatory Response in Psoriasis. Biochim Biophys Acta Mol Basis Dis (2018) 1864(5 Pt A):1795–803. doi: 10.1016/j.bbadis.2018.02.003
94. Zhou X, Li J, Zhou Y, Yang Z, Yang H, Li D, et al. Down-Regulated ciRS-7/Up-Regulated miR-7 Axis Aggravated Cartilage Degradation and Autophagy Defection by PI3K/AKT/mTOR Activation Mediated by IL-17A in Osteoarthritis. Aging (2020) 12(20):20163–83. doi: 10.18632/aging.103731
95. Gabriel SS, Tsui C, Chisanga D, Weber F, Llano-León M, Gubser PM, et al. Transforming Growth Factor-β-Regulated mTOR Activity Preserves Cellular Metabolism to Maintain Long-Term T Cell Responses in Chronic Infection. Immunity (2021) 54(8):1698–714.e5. doi: 10.1016/j.immuni.2021.06.007
96. Tripathi P, Rodriguez-Muela N, Klim JR, de Boer AS, Agrawal S, Sandoe J, et al. Reactive Astrocytes Promote ALS-Like Degeneration and Intracellular Protein Aggregation in Human Motor Neurons by Disrupting Autophagy Through TGF-β1. Stem Cell Rep (2017) 9(2):667–80. doi: 10.1016/j.stemcr.2017.06.008
97. Weissberg I, Wood L, Kamintsky L, Vazquez O, Milikovsky DZ, Alexander A, et al. Albumin Induces Excitatory Synaptogenesis Through Astrocytic TGF-β/ALK5 Signaling in a Model of Acquired Epilepsy Following Blood-Brain Barrier Dysfunction. Neurobiol Dis (2015) 78:115–25. doi: 10.1016/j.nbd.2015.02.029
98. Xue J, Suarez JS, Minaai M, Li S, Gaudino G, Pass HI, et al. HMGB1 as a Therapeutic Target in Disease. J Cell Physiol (2021) 236(5):3406–19. doi: 10.1002/jcp.30125
99. Pauletti A, Terrone G, Shekh-Ahmad T, Salamone A, Ravizza T, Rizzi M, et al. Targeting Oxidative Stress Improves Disease Outcomes in a Rat Model of Acquired Epilepsy. Brain (2019) 142(7):e39. doi: 10.1093/brain/awz130
100. Dulmovits BM, Tang Y, Papoin J, He M, Li J, Yang H, et al. HMGB1-Mediated Restriction of EPO Signaling Contributes to Anemia of Inflammation. Blood (2022) 139(21):3181–93. doi: 10.1182/blood.2021012048
101. Chen H, Li N, Zhan X, Zheng T, Huang X, Chen Q, et al. Capsaicin Protects Against Lipopolysaccharide-Induced Acute Lung Injury Through the HMGB1/NF-κb and PI3K/AKT/mTOR Pathways. J Inflammation Res (2021) 14:5291–304. doi: 10.2147/jir.S309457
102. Song X, Zhang H, Zhao Y, Lin Y, Tang Q, Zhou X, et al. HMGB1 Activates Myeloid Dendritic Cells by Up-Regulating mTOR Pathway in Systemic Lupus Erythematosus. Front Med (Lausanne) (2021) 8:636188. doi: 10.3389/fmed.2021.636188
103. Tripathi MK, Kartawy M, Amal H. The Role of Nitric Oxide in Brain Disorders: Autism Spectrum Disorder and Other Psychiatric, Neurological, and Neurodegenerative Disorders. Redox Biol (2020) 34:101567. doi: 10.1016/j.redox.2020.101567
104. Meskinimood S, Rahimi N, Faghir-Ghanesefat H, Gholami M, Sharifzadeh M, Dehpour AR. Modulatory Effect of Opioid Ligands on Status Epilepticus and the Role of Nitric Oxide Pathway. Epilepsy Behav (2019) 101(Pt A):106563. doi: 10.1016/j.yebeh.2019.106563
105. Taskiran AS, Ergul M. The Modulator Action of Thiamine Against Pentylenetetrazole-Induced Seizures, Apoptosis, Nitric Oxide, and Oxidative Stress in Rats and SH-SY5Y Neuronal Cell Line. Chem Biol Interact (2021) 340:109447. doi: 10.1016/j.cbi.2021.109447
106. Lisi L, Laudati E, Navarra P, Dello Russo C. The mTOR Kinase Inhibitors Polarize Glioma-Activated Microglia to Express a M1 Phenotype. J Neuroinflamm (2014) 11:125. doi: 10.1186/1742-2094-11-125
107. Lisi L, Navarra P, Feinstein DL, Dello Russo C. The mTOR Kinase Inhibitor Rapamycin Decreases iNOS mRNA Stability in Astrocytes. J Neuroinflamm (2011) 8(1):1. doi: 10.1186/1742-2094-8-1
108. Lopez-Rivera E, Jayaraman P, Parikh F, Davies MA, Ekmekcioglu S, Izadmehr S, et al. Inducible Nitric Oxide Synthase Drives mTOR Pathway Activation and Proliferation of Human Melanoma by Reversible Nitrosylation of TSC2. Cancer Res (2014) 74(4):1067–78. doi: 10.1158/0008-5472.Can-13-0588
109. Olowe R, Sandouka S, Saadi A, Shekh-Ahmad T. Approaches for Reactive Oxygen Species and Oxidative Stress Quantification in Epilepsy. Antioxid (Basel Switzerland) (2020) 9(10):990. doi: 10.3390/antiox9100990
110. McElroy PB, Liang LP, Day BJ, Patel M. Scavenging Reactive Oxygen Species Inhibits Status Epilepticus-Induced Neuroinflammation. Exp Neurol (2017) 298(Pt A):13–22. doi: 10.1016/j.expneurol.2017.08.009
111. Zhao Y, Hu X, Liu Y, Dong S, Wen Z, He W, et al. ROS Signaling Under Metabolic Stress: Cross-Talk Between AMPK and AKT Pathway. Mol Cancer (2017) 16(1):79. doi: 10.1186/s12943-017-0648-1
112. French JA, Lawson JA, Yapici Z, Ikeda H, Polster T, Nabbout R, et al. Adjunctive Everolimus Therapy for Treatment-Resistant Focal-Onset Seizures Associated With Tuberous Sclerosis (EXIST-3): A Phase 3, Randomised, Double-Blind, Placebo-Controlled Study. Lancet (2016) 388(10056):2153–63. doi: 10.1016/S0140-6736(16)31419-2
113. Zhang L, Bartley CM, Gong X, Hsieh LS, Lin TV, Feliciano DM, et al. MEK-ERK1/2-Dependent FLNA Overexpression Promotes Abnormal Dendritic Patterning in Tuberous Sclerosis Independent of mTOR. Neuron (2014) 84(1):78–91. doi: 10.1016/j.neuron.2014.09.009
114. Matsui M, Corey DR. Non-Coding RNAs as Drug Targets. Nat Rev Drug Discovery (2017) 16(3):167–79. doi: 10.1038/nrd.2016.117
115. Ye P, Liu Y, Chen C, Tang F, Wu Q, Wang X, et al. An Mtorc1-Mdm2-Drosha Axis for miRNA Biogenesis in Response to Glucose- and Amino Acid-Deprivation. Mol Cell (2015) 57(4):708–20. doi: 10.1016/j.molcel.2014.12.034
116. Fletcher CE, Godfrey JD, Shibakawa A, Bushell M, Bevan CL. A Novel Role for GSK3β as a Modulator of Drosha Microprocessor Activity and MicroRNA Biogenesis. Nucleic Acids Res (2017) 45(5):2809–28. doi: 10.1093/nar/gkw938
117. Sun Y, Ge Y, Drnevich J, Zhao Y, Band M, Chen J. Mammalian Target of Rapamycin Regulates miRNA-1 and Follistatin in Skeletal Myogenesis. J Cell Biol (2010) 189(7):1157–69. doi: 10.1083/jcb.200912093
118. Shen C, Houghton PJ. The mTOR Pathway Negatively Controls ATM by Up-Regulating miRNAs. Proc Natl Acad Sci (2013) 110(29):11869–74. doi: 10.1073/pnas.1220898110
119. Ebert MS, Sharp PA. Roles for microRNAs in Conferring Robustness to Biological Processes. Cell (2012) 149(3):515–24. doi: 10.1016/j.cell.2012.04.005
120. Tang C, Gu Y, Wang H, Wu H, Wang Y, Meng Y, et al. Targeting of microRNA-21-5p Protects Against Seizure Damage in a Kainic Acid-Induced Status Epilepticus Model via PTEN-mTOR. Epilepsy Res (2018) 144:34–42. doi: 10.1016/j.eplepsyres.2018.05.001
121. Olivieri F, Prattichizzo F, Giuliani A, Matacchione G, Rippo MR, Sabbatinelli J, et al. miR-21 and miR-146a: The microRNAs of Inflammaging and Age-Related Diseases. Ageing Res Rev (2021) 70:101374. doi: 10.1016/j.arr.2021.101374
122. Chak K, Roy-Chaudhuri B, Kim HK, Kemp KC, Porter BE, Kay MA. Increased Precursor microRNA-21 Following Status Epilepticus can Compete With Mature microRNA-21 to Alter Translation. Exp Neurol (2016) 286:137–46. doi: 10.1016/j.expneurol.2016.10.003
123. Song YJ, Tian XB, Zhang S, Zhang YX, Li X, Li D, et al. Temporal Lobe Epilepsy Induces Differential Expression of Hippocampal miRNAs Including Let-7e and miR-23a/B. Brain Res (2011) 1387:134–40. doi: 10.1016/j.brainres.2011.02.073
124. Zhu X, Yao Y, Liu Y, Zhou R, Zhang W, Hu Q, et al. Regulation of ADAM10 by MicroRNA-23a Contributes to Epileptogenesis in Pilocarpine-Induced Status Epilepticus Mice. Front Cell Neurosci (2019) 13:180. doi: 10.3389/fncel.2019.00180
125. Hu L, Zhang H, Wang B, Ao Q, Shi J, He Z. MicroRNA-23b Alleviates Neuroinflammation and Brain Injury in Intracerebral Hemorrhage by Targeting Inositol Polyphosphate Multikinase. Int Immunopharmacol (2019) 76:105887. doi: 10.1016/j.intimp.2019.105887
126. Lu J, Zhou N, Yang P, Deng L, Liu G. MicroRNA-27a-3p Downregulation Inhibits Inflammatory Response and Hippocampal Neuronal Cell Apoptosis by Upregulating Mitogen-Activated Protein Kinase 4 (MAP2K4) Expression in Epilepsy: In Vivo and In Vitro Studies. Med Sci Monit (2019) 25:8499–508. doi: 10.12659/msm.916458
127. Raoof R, Bauer S, El Naggar H, Connolly NMC, Brennan GP, Brindley E, et al. Dual-Center, Dual-Platform microRNA Profiling Identifies Potential Plasma Biomarkers of Adult Temporal Lobe Epilepsy. EBioMedicine (2018) 38:127–41. doi: 10.1016/j.ebiom.2018.10.068
128. Geng J, Zhao H, Liu X, Geng J, Gao Y, He B. MiR-101a-3p Attenuated Pilocarpine-Induced Epilepsy by Downregulating C-FOS. Neurochem Res (2021) 46(5):1119–28. doi: 10.1007/s11064-021-03245-w
129. Saika R, Sakuma H, Noto D, Yamaguchi S, Yamamura T, Miyake S. MicroRNA-101a Regulates Microglial Morphology and Inflammation. J Neuroinflamm (2017) 14(1):109. doi: 10.1186/s12974-017-0884-8
130. Alsharafi WA, Xiao B, Abuhamed MM, Luo Z. miRNAs: Biological and Clinical Determinants in Epilepsy. Front Mol Neurosci (2015) 8:59. doi: 10.3389/fnmol.2015.00059
131. Wang W, Wang X, Chen L, Zhang Y, Xu Z, Liu J, et al. The microRNA miR-124 Suppresses Seizure Activity and Regulates CREB1 Activity. Expert Rev Mol Med (2016) 18:e4. doi: 10.1017/erm.2016.3
132. Brennan GP, Dey D, Chen Y, Patterson KP, Magnetta EJ, Hall AM, et al. Dual and Opposing Roles of MicroRNA-124 in Epilepsy Are Mediated Through Inflammatory and NRSF-Dependent Gene Networks. Cell Rep (2016) 14(10):2402–12. doi: 10.1016/j.celrep.2016.02.042
133. Zhao J, He Z, Wang J. MicroRNA-124: A Key Player in Microglia-Mediated Inflammation in Neurological Diseases. Front Cell Neurosci (2021) 15:771898. doi: 10.3389/fncel.2021.771898
134. Huang S, Ge X, Yu J, Han Z, Yin Z, Li Y, et al. Increased miR-124-3p in Microglial Exosomes Following Traumatic Brain Injury Inhibits Neuronal Inflammation and Contributes to Neurite Outgrowth via Their Transfer Into Neurons. FASEB J (2018) 32(1):512–28. doi: 10.1096/fj.201700673R
135. Liu AH, Wu YT, Wang YP. MicroRNA-129-5p Inhibits the Development of Autoimmune Encephalomyelitis-Related Epilepsy by Targeting HMGB1 Through the TLR4/NF-kB Signaling Pathway. Brain Res Bull (2017) 132:139–49. doi: 10.1016/j.brainresbull.2017.05.004
136. Wan Y, Yang ZQ. LncRNA NEAT1 Affects Inflammatory Response by Targeting miR-129-5p and Regulating Notch Signaling Pathway in Epilepsy. Cell Cycle (2020) 19(4):419–31. doi: 10.1080/15384101.2020.1711578
137. Alcantara Llaguno S, Sun D, Pedraza AM, Vera E, Wang Z, Burns DK, et al. Cell-Of-Origin Susceptibility to Glioblastoma Formation Declines With Neural Lineage Restriction. Nat Neurosci (2019) 22(4):545–55. doi: 10.1038/s41593-018-0333-8
138. Mziaut H, Henniger G, Ganss K, Hempel S, Wolk S, McChord J, et al. MiR-132 Controls Pancreatic Beta Cell Proliferation and Survival Through Pten/Akt/Foxo3 Signaling. Mol Metab (2020) 31:150–62. doi: 10.1016/j.molmet.2019.11.012
139. Korotkov A, Broekaart DWM, Banchaewa L, Pustjens B, van Scheppingen J, Anink JJ, et al. microRNA-132 is Overexpressed in Glia in Temporal Lobe Epilepsy and Reduces the Expression of Pro-Epileptogenic Factors in Human Cultured Astrocytes. Glia (2020) 68(1):60–75. doi: 10.1002/glia.23700
140. Wang Z, Yuan B, Fu F, Huang S, Yang Z. Hemoglobin Enhances miRNA-144 Expression and Autophagic Activation Mediated Inflammation of Microglia via mTOR Pathway. Sci Rep (2017) 7(1):11861. doi: 10.1038/s41598-017-12067-2
141. Li J, Cai SX, He Q, Zhang H, Friedberg D, Wang F, et al. Intravenous miR-144 Reduces Left Ventricular Remodeling After Myocardial Infarction. Basic Res Cardiol (2018) 113(5):36. doi: 10.1007/s00395-018-0694-x
142. Kim SJ, Russell AE, Wang W, Gemoets DE, Sarkar SN, Simpkins JW, et al. miR-146a Dysregulates Energy Metabolism During Neuroinflammation. J Neuroimmune Pharmacol (2021). doi: 10.1007/s11481-021-09999-y
143. Aronica E, Fluiter K, Iyer A, Zurolo E, Vreijling J, van Vliet EA, et al. Expression Pattern of miR-146a, an Inflammation-Associated microRNA, in Experimental and Human Temporal Lobe Epilepsy. Eur J Neurosci (2010) 31(6):1100–7. doi: 10.1111/j.1460-9568.2010.07122.x
144. Li TR, Jia YJ, Ma C, Qiu WY, Wang Q, Shao XQ, et al. The Role of the microRNA-146a/Complement Factor H/interleukin-1β-Mediated Inflammatory Loop Circuit in the Perpetuate Inflammation of Chronic Temporal Lobe Epilepsy. Dis Model Mech (2018) 11(3):dmm031708. doi: 10.1242/dmm.031708
145. Leontariti M, Avgeris M, Katsarou MS, Drakoulis N, Siatouni A, Verentzioti A, et al. Circulating miR-146a and miR-134 in Predicting Drug-Resistant Epilepsy in Patients With Focal Impaired Awareness Seizures. Epilepsia (2020) 61(5):959–70. doi: 10.1111/epi.16502
146. Tao H, Cui L, Li Y, Zhou X, Ma G, Yao L, et al. Association of Tag SNPs and Rare CNVs of the MIR155HG/miR-155 Gene With Epilepsy in the Chinese Han Population. BioMed Res Int (2015) 2015:837213. doi: 10.1155/2015/837213
147. Martin EC, Rhodes LV, Elliott S, Krebs AE, Nephew KP, Flemington EK, et al. microRNA Regulation of Mammalian Target of Rapamycin Expression and Activity Controls Estrogen Receptor Function and RAD001 Sensitivity. Mol Cancer (2014) 13:229. doi: 10.1186/1476-4598-13-229
148. Kan AA, van Erp S, Derijck AA, de Wit M, Hessel EV, O'Duibhir E, et al. Genome-Wide microRNA Profiling of Human Temporal Lobe Epilepsy Identifies Modulators of the Immune Response. Cell Mol Life Sci (2012) 69(18):3127–45. doi: 10.1007/s00018-012-0992-7
149. Xu J, Su Y, Xu A, Fan F, Mu S, Chen L, et al. miR-221/222-Mediated Inhibition of Autophagy Promotes Dexamethasone Resistance in Multiple Myeloma. Mol Ther (2019) 27(3):559–70. doi: 10.1016/j.ymthe.2019.01.012
150. Kim ST, Cristescu R, Bass AJ, Kim KM, Odegaard JI, Kim K, et al. Comprehensive Molecular Characterization of Clinical Responses to PD-1 Inhibition in Metastatic Gastric Cancer. Nat Med (2018) 24(9):1449–58. doi: 10.1038/s41591-018-0101-z
151. Bornachea O, Santos M, Martínez-Cruz AB, García-Escudero R, Dueñas M, Costa C, et al. EMT and Induction of miR-21 Mediate Metastasis Development in Trp53-Deficient Tumours. Sci Rep (2012) 2:434. doi: 10.1038/srep00434
152. Iliopoulos D, Jaeger SA, Hirsch HA, Bulyk ML, Struhl K. STAT3 Activation of miR-21 and miR-181b-1 via PTEN and CYLD are Part of the Epigenetic Switch Linking Inflammation to Cancer. Mol Cell (2010) 39(4):493–506. doi: 10.1016/j.molcel.2010.07.023
153. Srivastava A, Dixit AB, Paul D, Tripathi M, Sarkar C, Chandra PS, et al. Comparative Analysis of Cytokine/Chemokine Regulatory Networks in Patients With Hippocampal Sclerosis (HS) and Focal Cortical Dysplasia (FCD). Sci Rep (2017) 7(1):15904. doi: 10.1038/s41598-017-16041-w
154. Li Z, Xu R, Zhu X, Li Y, Wang Y, Xu W. MicroRNA-23a-3p Improves Traumatic Brain Injury Through Modulating the Neurological Apoptosis and Inflammation Response in Mice. Cell Cycle (2020) 19(1):24–38. doi: 10.1080/15384101.2019.1691763
155. Zhang Y, Han JJ, Liang XY, Zhao L, Zhang F, Rasouli J, et al. miR-23b Suppresses Leukocyte Migration and Pathogenesis of Experimental Autoimmune Encephalomyelitis by Targeting Ccl7. Mol Ther (2018) 26(2):582–92. doi: 10.1016/j.ymthe.2017.11.013
156. Chen T, Zhang Y, Liu Y, Zhu D, Yu J, Li G, et al. MiR-27a Promotes Insulin Resistance and Mediates Glucose Metabolism by Targeting PPAR-γ-Mediated PI3K/AKT Signaling. Aging (Albany NY) (2019) 11(18):7510–24. doi: 10.18632/aging.102263
157. Zuckermann M, Hovestadt V, Knobbe-Thomsen CB, Zapatka M, Northcott PA, Schramm K, et al. Somatic CRISPR/Cas9-Mediated Tumour Suppressor Disruption Enables Versatile Brain Tumour Modelling. Nat Commun (2015) 6:7391. doi: 10.1038/ncomms8391
158. Cai C, Min S, Yan B, Liu W, Yang X, Li L, et al. MiR-27a Promotes the Autophagy and Apoptosis of IL-1β Treated-Articular Chondrocytes in Osteoarthritis Through PI3K/AKT/mTOR Signaling. Aging (Albany NY) (2019) 11(16):6371–84. doi: 10.18632/aging.102194
159. Ponomarev ED, Veremeyko T, Barteneva N, Krichevsky AM, Weiner HL. MicroRNA-124 Promotes Microglia Quiescence and Suppresses EAE by Deactivating Macrophages via the C/EBP-α–PU.1 Pathway. Nat Med (2011) 17(1):64–70. doi: 10.1038/nm.2266
160. Ponomarev ED, Veremeyko T, Barteneva N, Krichevsky AM, Weiner HL. MicroRNA-124 Promotes Microglia Quiescence and Suppresses EAE by Deactivating Macrophages via the C/EBP-α-PU.1 Pathway. Nat Med (2011) 17(1):64–70. doi: 10.1038/nm.2266
161. Yang B, Yang R, Xu B, Fu J, Qu X, Li L, et al. miR-155 and miR-146a Collectively Regulate Meningitic Escherichia Coli Infection-Mediated Neuroinflammatory Responses. J Neuroinflamm (2021) 18(1):114. doi: 10.1186/s12974-021-02165-4
162. Omran A, Peng J, Zhang C, Xiang QL, Xue J, Gan N, et al. Interleukin-1β and microRNA-146a in an Immature Rat Model and Children With Mesial Temporal Lobe Epilepsy. Epilepsia (2012) 53(7):1215–24. doi: 10.1111/j.1528-1167.2012.03540.x
163. Runtsch MC, Nelson MC, Lee SH, Voth W, Alexander M, Hu R, et al. Anti-Inflammatory microRNA-146a Protects Mice From Diet-Induced Metabolic Disease. PLoS Genet (2019) 15(2):e1007970. doi: 10.1371/journal.pgen.1007970
164. Huang W, Tian SS, Hang PZ, Sun C, Guo J, Du ZM. Combination of microRNA-21 and microRNA-146a Attenuates Cardiac Dysfunction and Apoptosis During Acute Myocardial Infarction in Mice. Mol Ther Nucleic Acids (2016) 5(3):e296. doi: 10.1038/mtna.2016.12
165. De D, Mukherjee I, Guha S, Paidi RK, Chakrabarti S, Biswas SC, et al. Rheb-mTOR Activation Rescues Aβ-Induced Cognitive Impairment and Memory Function by Restoring miR-146 Activity in Glial Cells. Mol Ther Nucleic Acids (2021) 24:868–87. doi: 10.1016/j.omtn.2021.04.008
166. Roncon P, Soukupovà M, Binaschi A, Falcicchia C, Zucchini S, Ferracin M, et al. MicroRNA Profiles in Hippocampal Granule Cells and Plasma of Rats With Pilocarpine-Induced Epilepsy–Comparison With Human Epileptic Samples. Sci Rep (2015) 5:14143. doi: 10.1038/srep14143
167. O'Connell RM, Kahn D, Gibson WSJ, Round JL, Scholz RL, Chaudhuri AA, et al. MicroRNA-155 Promotes Autoimmune Inflammation by Enhancing Inflammatory T Cell Development. Immunity (2010) 33(4):607–19. doi: 10.1016/j.immuni.2010.09.009
168. Cardoso AL, Guedes JR, Pereira de Almeida L, Pedroso de Lima MC. miR-155 Modulates Microglia-Mediated Immune Response by Down-Regulating SOCS-1 and Promoting Cytokine and Nitric Oxide Production. Immunology (2012) 135(1):73–88. doi: 10.1111/j.1365-2567.2011.03514.x
169. Zimmer TS, Ciriminna G, Arena A, Anink JJ, Korotkov A, Jansen FE, et al. Chronic Activation of Anti-Oxidant Pathways and Iron Accumulation in Epileptogenic Malformations. Neuropathol Appl Neurobiol (2020) 46(6):546–63. doi: 10.1111/nan.12596
170. Gorter JA, Iyer A, White I, Colzi A, van Vliet EA, Sisodiya S, et al. Hippocampal Subregion-Specific microRNA Expression During Epileptogenesis in Experimental Temporal Lobe Epilepsy. Neurobiol Dis (2014) 62:508–20. doi: 10.1016/j.nbd.2013.10.026
171. Siddiqui MR, Akhtar S, Shahid M, Tauseef M, McDonough K, Shanley TP. miR-144-Mediated Inhibition of ROCK1 Protects Against LPS-Induced Lung Endothelial Hyperpermeability. Am J Respir Cell Mol Biol (2019) 61(2):257–65. doi: 10.1165/rcmb.2018-0235OC
172. Spinelli M, Boucard C, Ornaghi S, Schoeberlein A, Irene K, Coman D, et al. Preimplantation Factor Modulates Oligodendrocytes by H19-Induced Demethylation of NCOR2. JCI Insight (2021) 6(20):e132335. doi: 10.1172/jci.insight.132335
173. Wang Y, Han B, Wang Y, Wang C, Zhang H, Xue J, et al. Mesenchymal Stem Cell-Secreted Extracellular Vesicles Carrying TGF-β1 Up-Regulate miR-132 and Promote Mouse M2 Macrophage Polarization. J Cell Mol Med (2020) 24(21):12750–64. doi: 10.1111/jcmm.15860
174. Li S, Xu JJ, Zhang QY. MicroRNA-132-3p Inhibits Tumor Malignant Progression by Regulating Lysosomal-Associated Protein Transmembrane 4 Beta in Breast Cancer. Cancer Sci (2019) 110(10):3098–109. doi: 10.1111/cas.14164
175. Statello L, Guo CJ, Chen LL, Huarte M. Gene Regulation by Long non-Coding RNAs and its Biological Functions. Nat Rev Mol Cell Biol (2021) 22(2):96–118. doi: 10.1038/s41580-020-00315-9
176. Dykes IM, Emanueli C. Transcriptional and Post-Transcriptional Gene Regulation by Long Non-Coding RNA. Genomics Proteomics Bioinf (2017) 15(3):177–86. doi: 10.1016/j.gpb.2016.12.005
177. Cai X, Long L, Zeng C, Ni G, Meng Y, Guo Q, et al. LncRNA ILF3-AS1 Mediated the Occurrence of Epilepsy Through Suppressing Hippocampal miR-212 Expression. Aging (Albany NY) (2020) 12(9):8413–22. doi: 10.18632/aging.103148
178. Zhang JY, Yang Z, Fang K, Shi ZL, Ren DH, Sun J. Long Noncoding RNA ILF3-AS1 Regulates Myocardial Infarction via the miR-212-3p/SIRT1 Axis and PI3K/Akt Signaling Pathway. Eur Rev Med Pharmacol Sci (2020) 24(5):2647–58. doi: 10.26355/eurrev_202003_20534
179. He C, Su C, Zhang W, Zhou Q, Shen X, Yang J, et al. Modulatory Potential of LncRNA Zfas1 for Inflammation and Neuronal Apoptosis in Temporal Lobe Epilepsy. Yonsei Med J (2021) 62(3):215–23. doi: 10.3349/ymj.2021.62.3.215
180. Ghafouri-Fard S, Kamali MJ, Abak A, Shoorei H, Taheri M. LncRNA ZFAS1: Role in Tumorigenesis and Other Diseases. BioMed Pharmacother (2021) 142:111999. doi: 10.1016/j.biopha.2021.111999
181. Wang J, Niu Y, Tao H, Xue M, Wan C. Knockdown of lncRNA TUG1 Inhibits Hippocampal Neuronal Apoptosis and Participates in Aerobic Exercise-Alleviated Vascular Cognitive Impairment. Biol Res (2020) 53(1):53. doi: 10.1186/s40659-020-00320-4
182. Bao MH, Szeto V, Yang BB, Zhu SZ, Sun HS, Feng ZP. Long non-Coding RNAs in Ischemic Stroke. Cell Death Dis (2018) 9(3):281. doi: 10.1038/s41419-018-0282-x
183. Yang B, Liang RS, Wu XY, Lin YJ. LncRNA TUG1 Inhibits Neuronal Apoptosis in Status Epilepticus Rats via Targeting the miR-421/mTOR Axis. Cell Signal (2020) 76:109787. doi: 10.1016/j.cellsig.2020.109787
184. Wang H, Liao S, Li H, Chen Y, Yu J. Long Non-Coding RNA TUG1 Sponges Mir-145a-5p to Regulate Microglial Polarization After Oxygen-Glucose Deprivation. Front Mol Neurosci (2019) 12:215. doi: 10.3389/fnmol.2019.00215
185. Li C, Zheng X, Liu P, Li M. Clinical Value of lncRNA TUG1 in Temporal Lobe Epilepsy and its Role in the Proliferation of Hippocampus Neuron via Sponging miR-199a-3p. Bioengineered (2021) 12(2):10666–73. doi: 10.1080/21655979.2021.2001904
186. Yu H, Xu A, Wu B, Wang M, Chen Z. Long Noncoding RNA NEAT1 Promotes Progression of Glioma as a ceRNA by Sponging miR-185-5p to Stimulate DNMT1/mTOR Signaling. J Cell Physiol (2021) 236(1):121–30. doi: 10.1002/jcp.29644
187. Huang S, Xu Y, Ge X, Xu B, Peng W, Jiang X, et al. Long Noncoding RNA NEAT1 Accelerates the Proliferation and Fibrosis in Diabetic Nephropathy Through Activating Akt/mTOR Signaling Pathway. J Cell Physiol (2019) 234(7):11200–7. doi: 10.1002/jcp.27770
188. Barry G, Briggs JA, Hwang DW, Nayler SP, Fortuna PR, Jonkhout N, et al. The Long non-Coding RNA NEAT1 is Responsive to Neuronal Activity and is Associated With Hyperexcitability States. Sci Rep (2017) 7:40127. doi: 10.1038/srep40127
189. Wang C, Yang Y, Cong L, Jiang Y, Du N, Zhang H. Implication of Long non-Coding RNA NEAT1 in the Pathogenesis of Bacterial Meningitis-Induced Blood-Brain Barrier Damage. Microvasc Res (2021) 138:104225. doi: 10.1016/j.mvr.2021.104225
190. Wu ZR, Yan L, Liu YT, Cao L, Guo YH, Zhang Y, et al. Inhibition of Mtorc1 by lncRNA H19 via Disrupting 4E-BP1/Raptor Interaction in Pituitary Tumours. Nat Commun (2018) 9(1):4624. doi: 10.1038/s41467-018-06853-3
191. Han CL, Ge M, Liu YP, Zhao XM, Wang KL, Chen N, et al. Long non-Coding RNA H19 Contributes to Apoptosis of Hippocampal Neurons by Inhibiting Let-7b in a Rat Model of Temporal Lobe Epilepsy. Cell Death Dis (2018) 9(6):617. doi: 10.1038/s41419-018-0496-y
192. Han CL, Liu YP, Guo CJ, Du TT, Jiang Y, Wang KL, et al. The lncRNA H19 Binding to Let-7b Promotes Hippocampal Glial Cell Activation and Epileptic Seizures by Targeting Stat3 in a Rat Model of Temporal Lobe Epilepsy. Cell Prolif (2020) 53(8):e12856. doi: 10.1111/cpr.12856
193. Han CL, Ge M, Liu YP, Zhao XM, Wang KL, Chen N, et al. LncRNA H19 Contributes to Hippocampal Glial Cell Activation via JAK/STAT Signaling in a Rat Model of Temporal Lobe Epilepsy. J Neuroinflamm (2018) 15(1):103. doi: 10.1186/s12974-018-1139-z
194. Wang X, Hu Z, Zhong K. The Role of Brain-Derived Neurotrophic Factor in Epileptogenesis: An Update. Front Pharmacol (2021) 12:758232. doi: 10.3389/fphar.2021.758232
195. Ghafouri-Fard S, Khoshbakht T, Taheri M, Ghanbari M. A Concise Review on the Role of BDNF-AS in Human Disorders. BioMed Pharmacother (2021) 142:112051. doi: 10.1016/j.biopha.2021.112051
196. Lin X, Dinglin X, Cao S, Zheng S, Wu C, Chen W, et al. Enhancer-Driven lncRNA BDNF-AS Induces Endocrine Resistance and Malignant Progression of Breast Cancer Through the RNH1/TRIM21/mTOR Cascade. Cell Rep (2020) 31(10):107753. doi: 10.1016/j.celrep.2020.107753
197. Xu L, Zhang Z, Xie T, Zhang X, Dai T. Inhibition of BDNF-AS Provides Neuroprotection for Retinal Ganglion Cells Against Ischemic Injury. PLoS One (2016) 11(12):e0164941. doi: 10.1371/journal.pone.0164941
198. Li Z, Li J, Tang N. Long Noncoding RNA Malat1 is a Potent Autophagy Inducer Protecting Brain Microvascular Endothelial Cells Against Oxygen-Glucose Deprivation/Reoxygenation-Induced Injury by Sponging miR-26b and Upregulating ULK2 Expression. Neuroscience (2017) 354:1–10. doi: 10.1016/j.neuroscience.2017.04.017
199. Zhang X, Tang X, Liu K, Hamblin MH, Yin KJ. Long Noncoding RNA Malat1 Regulates Cerebrovascular Pathologies in Ischemic Stroke. J Neurosci (2017) 37(7):1797–806. doi: 10.1523/jneurosci.3389-16.2017
200. Cremer S, Michalik KM, Fischer A, Pfisterer L, Jaé N, Winter C, et al. Hematopoietic Deficiency of the Long Noncoding RNA MALAT1 Promotes Atherosclerosis and Plaque Inflammation. Circulation (2019) 139(10):1320–34. doi: 10.1161/circulationaha.117.029015
201. Zhu Y, Yang T, Duan J, Mu N, Zhang T. MALAT1/miR-15b-5p/MAPK1 Mediates Endothelial Progenitor Cells Autophagy and Affects Coronary Atherosclerotic Heart Disease via mTOR Signaling Pathway. Aging (Albany NY) (2019) 11(4):1089–109. doi: 10.18632/aging.101766
202. Malakar P, Shilo A, Mogilevsky A, Stein I, Pikarsky E, Nevo Y, et al. Long Noncoding RNA MALAT1 Promotes Hepatocellular Carcinoma Development by SRSF1 Upregulation and mTOR Activation. Cancer Res (2017) 77(5):1155–67. doi: 10.1158/0008-5472.Can-16-1508
203. Shao J, Zhang Q, Wang P, Wang Z. LncRNA MALAT1 Promotes Breast Cancer Progression by Sponging Mir101-3p to Mediate mTOR/PKM2 Signal Transmission. Am J Transl Res (2021) 13(9):10262–75.
204. Wu Q, Yi X. Down-Regulation of Long Noncoding RNA MALAT1 Protects Hippocampal Neurons Against Excessive Autophagy and Apoptosis via the PI3K/Akt Signaling Pathway in Rats With Epilepsy. J Mol Neurosci (2018) 65(2):234–45. doi: 10.1007/s12031-018-1093-3
205. Miller-Delaney SF, Bryan K, Das S, McKiernan RC, Bray IM, Reynolds JP, et al. Differential DNA Methylation Profiles of Coding and non-Coding Genes Define Hippocampal Sclerosis in Human Temporal Lobe Epilepsy. Brain (2015) 138(Pt 3):616–31. doi: 10.1093/brain/awu373
206. Yu Q, Zhao MW, Yang P. LncRNA UCA1 Suppresses the Inflammation Via Modulating miR-203-Mediated Regulation of MEF2C/NF-κb Signaling Pathway in Epilepsy. Neurochem Res (2020) 45(4):783–95. doi: 10.1007/s11064-019-02952-9
207. Zhao YJ, Chen YE, Zhang HJ, Gu X. LncRNA UCA1 Remits LPS-Engendered Inflammatory Damage Through Deactivation of miR-499b-5p/TLR4 Axis. IUBMB Life (2021) 73(2):463–73. doi: 10.1002/iub.2443
208. Hu Y, Lou J, Mao YY, Lai TW, Liu LY, Zhu C, et al. Activation of MTOR in Pulmonary Epithelium Promotes LPS-Induced Acute Lung Injury. Autophagy (2016) 12(12):2286–99. doi: 10.1080/15548627.2016.1230584
209. Zhang Z, Li H, Cui Z, Zhou Z, Chen S, Ma J, et al. Long non-Coding RNA UCA1 Relieves Cardiomyocytes H9c2 Injury Aroused by Oxygen-Glucose Deprivation via Declining miR-122. Artif Cells Nanomed Biotechnol (2019) 47(1):3492–9. doi: 10.1080/21691401.2019.1652630
210. Lasda E, Parker R. Circular RNAs: Diversity of Form and Function. RNA (2014) 20(12):1829–42. doi: 10.1261/rna.047126.114
211. Li Y, Zheng Q, Bao C, Li S, Guo W, Zhao J, et al. Circular RNA is Enriched and Stable in Exosomes: A Promising Biomarker for Cancer Diagnosis. Cell Res (2015) 25(8):981–4. doi: 10.1038/cr.2015.82
212. Wang Z, Xu P, Chen B, Zhang Z, Zhang C, Zhan Q, et al. Identifying circRNA-associated-ceRNA Networks in the Hippocampus of Aβ1-42-Induced Alzheimer's Disease-Like Rats Using Microarray Analysis. Aging (2018) 10(4):775–88. doi: 10.18632/aging.101427
213. Chen LL. The Expanding Regulatory Mechanisms and Cellular Functions of Circular RNAs. Nat Rev Mol Cell Biol (2020) 21(8):475–90. doi: 10.1038/s41580-020-0243-y
214. Zhong Y, Du Y, Yang X, Mo Y, Fan C, Xiong F, et al. Circular RNAs Function as ceRNAs to Regulate and Control Human Cancer Progression. Mol Cancer (2018) 17(1):79. doi: 10.1186/s12943-018-0827-8
215. Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK, et al. Natural RNA Circles Function as Efficient microRNA Sponges. Nature (2013) 495(7441):384–8. doi: 10.1038/nature11993
216. Du WW, Yang W, Chen Y, Wu ZK, Foster FS, Yang Z, et al. Foxo3 Circular RNA Promotes Cardiac Senescence by Modulating Multiple Factors Associated With Stress and Senescence Responses. Eur Heart J (2017) 38(18):1402–12. doi: 10.1093/eurheartj/ehw001
217. Ashwal-Fluss R, Meyer M, Pamudurti NR, Ivanov A, Bartok O, Hanan M, et al. circRNA Biogenesis Competes With pre-mRNA Splicing. Mol Cell (2014) 56(1):55–66. doi: 10.1016/j.molcel.2014.08.019
218. Lee WJ, Moon J, Jeon D, Kim TJ, Yoo JS, Park DK, et al. Possible Epigenetic Regulatory Effect of Dysregulated Circular RNAs in Epilepsy. PLoS One (2018) 13(12):e0209829. doi: 10.1371/journal.pone.0209829
219. Gray LG, Mills JD, Curry-Hyde A, Devore S, Friedman D, Thom M, et al. Identification of Specific Circular RNA Expression Patterns and MicroRNA Interaction Networks in Mesial Temporal Lobe Epilepsy. Front Genet (2020) 11:564301. doi: 10.3389/fgene.2020.564301
220. Gomes-Duarte A, Bauer S, Venø MT, Norwood BA, Henshall DC, Kjems J, et al. Enrichment of Circular RNA Expression Deregulation at the Transition to Recurrent Spontaneous Seizures in Experimental Temporal Lobe Epilepsy. Front Genet (2021) 12:627907. doi: 10.3389/fgene.2021.627907
221. Xiaoying G, Guo M, Jie L, Yanmei Z, Ying C, Shengjie S, et al. CircHivep2 Contributes to Microglia Activation and Inflammation via miR-181a-5p/SOCS2 Signalling in Mice With Kainic Acid-Induced Epileptic Seizures. J Cell Mol Med (2020) 24(22):12980–93. doi: 10.1111/jcmm.15894
222. Shao L, Jiang GT, Yang XL, Zeng ML, Cheng JJ, Kong S, et al. Silencing of Circigf1r Plays a Protective Role in Neuronal Injury via Regulating Astrocyte Polarization During Epilepsy. FASEB J (2021) 35(2):e21330. doi: 10.1096/fj.202001737RR
Keywords: epilepsy, neuroinflammation, mTOR, non-coding RNA, neural damage
Citation: Zeng C, Hu J, Chen F, Huang T and Zhang L (2022) The Coordination of mTOR Signaling and Non-Coding RNA in Regulating Epileptic Neuroinflammation. Front. Immunol. 13:924642. doi: 10.3389/fimmu.2022.924642
Received: 20 April 2022; Accepted: 16 June 2022;
Published: 11 July 2022.
Edited by:
Weijun Peng, Central South University, ChinaReviewed by:
David Millrine, University of Dundee, United KingdomYajing Mi, Xi’an Medical University, China
Copyright © 2022 Zeng, Hu, Chen, Huang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fenghua Chen, eHlzd2NmaEBjc3UuZWR1LmNu; Tianxiang Huang, aHR4MjkxQDE2My5jb20=; Longbo Zhang, bG9uZ2JvLnpoYW5nQHlhbGUuZWR1
 Chudai Zeng
Chudai Zeng Jason Hu
Jason Hu Fenghua Chen
Fenghua Chen Tianxiang Huang1*
Tianxiang Huang1* Longbo Zhang
Longbo Zhang