
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol., 23 June 2022
Sec. Alloimmunity and Transplantation
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.912917
 Meiqing Lei1,2†
Meiqing Lei1,2† Yanming Zhang3†
Yanming Zhang3† Wenjing Jiao4†
Wenjing Jiao4† Xiaoli Li5
Xiaoli Li5 Huifen Zhou1
Huifen Zhou1 Qingyuan Wang1
Qingyuan Wang1 Huiying Qiu1
Huiying Qiu1 Xiaowen Tang1
Xiaowen Tang1 Yue Han1
Yue Han1 Chengcheng Fu1
Chengcheng Fu1 Zhengming Jin1
Zhengming Jin1 Suning Chen1
Suning Chen1 Aining Sun1
Aining Sun1 Miao Miao1*
Miao Miao1* Limin Liu1*
Limin Liu1* Depei Wu1*
Depei Wu1*The purpose of this study in severe aplastic anemia (SAA) patients was to compare the feasibility and efficacy of haploidentical hematological stem cell transplantation combined with a single unrelated cord blood (UCB) infusion (Haplo-cord-HSCT) or haplo-identical HSCT (Haplo-HSCT) alone. The five-year graft-versus-host disease (GVHD)-free or failure-free survival (GFFS) was similar between the two groups (72.4 ± 3.4% vs. 65.4 ± 5.2%, P = 0.178); however, the five-year overall survival (OS) was more favorable in the Haplo-cord-HSCT group than that in the Haplo-HSCT group (84.0 ± 2.8% vs. 72.6 ± 4.9%, P = 0.022), as was transplantation-related mortality (16.4% vs. 27.4%, P = 0.039). Multivariate analysis showed that Haplo-cord HSCT was the only independent determinant of increased OS (P = 0.013). Explorative subgroup analysis showed that only an Human leukocyte antigen-A (HLA-A) allele match between UCB and the recipient was a beneficial factor for GFFS in the Haplo-cord-HSCT group (P = 0.011). In the haplo-cord with an HLA-A match (n = 139) or mismatch (n = 32) or Haplo-HSCT groups, a haplo-cord HLA-A allele match was associated with lower I–IV and III–IV acute GVHD. The haplo-cord with an HLA-A match subgroup also had higher five-year OS than the Haplo-HSCT group (85.4 ± 3.0% vs. 72.6 ± 4.9%, P = 0.013), and higher five-year GFFS than the Haplo-cord HLA-A allele mismatch subgroup (76.2 ± 3.6% vs. 56.3 ± 8.8%, P = 0.011). These findings suggest that the coinfusion of a single UCB potentially improves survival of Haplo-HSCT in SAA patients and that an HLA-A allele-matched UCB is the preferred option.
Allogeneic hematological stem cell transplantation (allo-HSCT) from an available matched sibling donor (MSD) is recommended as a first-line treatment for patients with acquired severe aplastic anemia (SAA), particularly in young patients (1). Unfortunately, less than 30% of patients who require an allo-HSCT have a human leukocyte antigen (HLA)-compatible sibling. Fortunately, almost everyone can be matched to at least one related HLA-haploidentical donor (HID). With recent advances in transplant technology, haploidentical HSCT (haplo-HSCT) has become an important alternative treatment for SAA patients who do not have a suitable identical donor and for those who are refractory to immunosuppressive therapy (IST) (2, 3). The overall survival (OS) rate for haplo-HSCT ranges from 67.1% to 89.0% in SAA patients, with this high value comparable to that for MSD transplantation (4–6). Nevertheless, the outcome of a haplo-HSCT in SAA patients is still limited by transplantation-related mortality (TRM) caused by graft failure (GF) or graft-versus-host disease (GVHD) and infections associated with delayed immune reconstitution (7, 8). If a way to reduce the risk of these complications was discovered, the efficacy of haplo-HSCT would be further improved.
In recent years, some experienced transplant centers have been investigating strategies to optimize the haplo-HSCT model, such as haplo-HSCT combined with mesenchymal stem cells (MSCs), umbilical cord blood (UCB), or a post-transplant cyclophosphamide conditioning regimen (9–11). It should be noted that the conditioning regimen, the time and count of UCB infusion, and the mechanism of allo-HSCT success in different hematological diseases are sometimes different (12–15). Until recently, our two studies not only demonstrated that a haplo-cord-HSCT achieved 97.1% donor myeloid engraftment and 81.4 ± 3.3% four-year OS in SAA patients but also that failure-free survival and a health-related quality of life were both better in SAA patients after first-line haplo-cord-HSCT than that achieved by IST (16, 17). However, to the best of our knowledge, there has been no direct comparison of the therapeutic outcomes of haplo-HSCT with or without a UCB infusion in SAA patients. We therefore carried out a multicenter cohort study to retrospectively compare these two treatment modalities in this specific disease.
Between March 2011 and August 2020, 255 consecutive acquired SAA or very SAA (vSAA) patients were enrolled in this study from five transplant centers. All patients met the following diagnosis and management guidelines for SAA or vSAA (1, 18) (1): no MSD or matched unrelated donor (MUD), or not willing to wait for an MUD (2); no response to previous IST [including anti-thymocyte globulin (ATG)/antilymphocyte immunoglobulin (ALG) plus cyclosporine A] (3); having one or more available HIDs, and willing to choose haplo-HSCT as the first-line or alternative treatment (4); transfusion dependent (5); a Karnofsky score of 80−100; and (6) the absence of severe liver, renal, lung, and heart diseases. The patients were classified into two groups based on whether they have received a single UCB infusion before the haploidentical grafts (haplo-cord-HSCT, n = 171 and haplo-HSCT, n = 84). Every patient signed a written, informed consent form prior to participation. The study was approved by our hospital’s Ethics Committee and was carried out in accordance with the Declaration of Helsinki.
The HLA-A, -B, -C, -DRB1, and -DQB1 typing of recipients and donors including their parents, siblings, or children were matched at the allele level using a high-resolution molecular standard technique. HIDs were selected based on matched HLA with a true haploid genetic background, age, gender, health condition, and willingness to donate stem cells. In addition, the donors were excluded if the recipient had donor-specific antibodies (DSAs) against high-expression HLA, with a mean fluorescence intensity >2,000. If a targeted positive donor was the only choice, rituximab and/or plasma exchange were administered to the recipient prior to transplantation.
The HLA-A, -B, and -DRB1 typing of UCB units obtained from the cord blood bank in Shanghai, China was performed. The choice of UCB was according to the treating physician’s discretion, depending on the availability of a suitable UBC and the patient’s preference. As in our previous report (17), UCB units with two or less mismatchings in the HLA-A, -B, and -DRB1 loci were selected as minimum candidates. Priority was given to units with the most closely matched HLA and subsequently to the unit with the highest cell count with a similar degree of matching for the HLA type. The HLA typing and cell count of the selected UCB units were reassessed after rapid thawing at our centers. If no suitable UCB was found according to the above principles, the recipient was placed in the haplo-HSCT group.
The transplant schedule was described as follows: the first day of the stem cell infusion was designated as “day 01” and the second day of infusion as “day 02.” The specific days before the first and last stem cell infusion were designated by a minus (–) sign and plus (+) sign, respectively. All patients were treated with a busulfan (BU)/cyclophosphamide (CY)-based regimen as described in our previous report (17): busulfan (Bu, intravenous, total dose 6.4 mg/kg, days –7, –6), cyclophosphamide (Cy, intravenous, total dose 200 mg/kg, days –5 to –2), and rabbit ATG (rATG, intravenous, total dose 10.0 mg/kg, days –5 to –2) or porcine antihuman lymphocyte immunoglobulin (pALG, intravenous, total dose 80.0 mg/kg, days –5 to –2).
From day –4 to the last day of stem cell collection, hematopoietic stem cells from the HIDs were mobilized using the recombinant human granulocyte colony-stimulating factor (rhG-CSF) at a dose of 10 μg/kg/day. BM grafts were collected via BM aspiration in the surgery room on day 01, with the target count for mononuclear cells (MNCs) set at 2–4 × 108/kg of the recipient’s weight. Peripheral blood (PB) grafts were collected by apheresis using a COBE Spectra device (Gambro BCT, Lakewood, CO, USA) on day 02. The total MNC count from BM and PB were required to achieve 6–8 × 108/kg of the recipient’s weight. If the count of total MNCs was still not at this level, more PBSCs were collected on the next 1–2 days. Unmanipulated grafts from BM and PB were infused into the recipient on the day of collection. In the haplo-cord HSCT group, a single UCB infusion was conducted 8 h prior to haploidentical graft infusion on day 01.
Cyclosporine A, mycophenolate mofetil (MMF), and short-term methotrexate (MTX) were administered for acute GVHD (aGVHD) prophylaxis in the two groups. Once aGVHD or chronic GVHD (cGVHD) occurred, the corresponding treatment was given as described in our previous report (17).
The details of supportive care and post-transplantation surveillance were in line with our previous experience (17).
Neutrophil engraftment was defined as the first day of an absolute neutrophil count (ANC) >0.5 × 109/L on three consecutive days. Platelet engraftment was defined as the first day of a platelet count >20 × 109/L during a week without platelet transfusion. Primary GF was defined as the failure to achieve neutrophil engraftment after HSCT up to day +28, while secondary GF was defined as an ANC <0.5 × 109/L on three consecutive time points after the confirmation of initial complete donor engraftment (19). Delayed platelet recovery was defined as platelet engraftment achieved after more than +30 days. The diagnosis and severity of aGVHD and cGVHD were based on established criteria (20, 21). On the premise of full donor chimerism without relapse or severe GVHD, poor graft function was defined as persistent cytopenia in at least two lineages (platelet <20 × 109/L, neutrophil count <0.5 × 109/L, hemoglobin level <70 g/L) and/or requiring a transfusion beyond +28 days (22). TRM was defined as death related to the transplantation instead of SAA relapse. GVHD-free or failure-free survival (GFFS) was defined as survival without grade III–IV aGVHD, moderate-to-severe cGVHD, or treatment failure including death, primary or secondary GF, and relapse. After transplantation, the recipients’ BM was reexamined monthly for 3 months and every 3–6 months for the following 1–2 years.
The date of the last follow-up for all surviving patients was June 30, 2021. SPSS 22.0 statistical software (IBM, Armonk, NY, USA) was used for the statistical analyses. Continuous and categorical variables of demographic-, disease-, and treatment-related factors were compared using the Mann–Whitney U and Pearson chi-squared tests, respectively. GVHD was estimated as a cumulative incidence, considering early death and GF as competing events. Survival analysis was conducted using the Kaplan–Meier method and log-rank test. Factors with a P-value <0.05 in the univariate analysis were included in a Cox regression multivariate analysis. To examine the impact of a UCB HLA-loci mismatch, the variable of primary interest, a three-group comparison was carried out to construct a Cox proportional hazards model: haplo-cord with an HLA-A allele match vs. haplo-cord with an HLA-A allele mismatch vs. haplo-HSCT. P-values <0.05 were considered statistically significant.
A comparison of the clinical characteristics of the patients and donors (grafts) in the haplo-HSCT and haplo-cord-HSCT groups is shown in Table 1. There were no differences in recipient sex/age, disease status, PNH (paroxysmal nocturnal hemoglobinuria) clone, previous treatment, time from diagnosis to allo-HSCT, donor–recipient relationship, the blood types of donors to recipients, the source of haploidentical graft, the count of MNCs, and CD34+ cells from the haploidentical grafts. Notably, the donors in the haplo-HSCT group were younger than those in the haplo-cord-HSCT group (median age 36.5 vs. 41 years, P = 0.045). The proportion of male donors in the haplo-HSCT group was higher than that in the haplo-cord-HSCT group (75.0% vs. 62.6%; P = 0.048). The median count of total nucleated cells (TNCs) and CD34+ cells in the UCB of the haplo-cord-HSCT group was 1.80 × 107/kg and 0.48× 105/kg of the recipient’s weight, respectively.
A total of 77 and 166 patients (evaluable engraftment) survived for longer than +28 days in the haplo-HSCT and the haplo-cord-HDCT groups, respectively. The median time of neutrophil engraftment in the haplo-HSCT (75/77) and the haplo-cord HSCT (164/166) was 12 (range, 9–27) and 11 (range, 10–20) days (P = 0.381), respectively, with all achieving complete haploidentical chimerism only, without the evidence of UCB or mixed engraftment. The primary GF rates in the haplo-HSCT (2/77) and haplo-cord-HSCT groups (2/166) were 2.6% and 1.2%, respectively (P = 0.801). The corresponding secondary GF rates in the haplo-HSCT (1/77) and haplo-cord-HSCT groups (1/166) were 1.3% and 0.6% (P = 0.534). Of the 6 patients with GF, 5 patients died of GF, while 1 patient with primary GF survived with a dependence on blood transfusion (Table 1). The median time to achieve platelet engraftment was 15 (range, 8–101) days in the haplo-HSCT group and 15 (range, 9–330) days in the haplo-cord-HSCT groups (P = 0.828). The delayed platelet recovery rates in the haplo-HSCT (9/77) and haplo-cord-HSCT groups (12/166) were 11.7% and 7.2%, respectively (P = 0.250). A total of 3 patients in the haplo-HSCT group and 4 in the haplo-cord-HSCT group experienced platelet GF (3.9% vs. 2.4%, P = 0.816), while 1 patient in the haplo-HSCT group and 3 patients in the haplo-cord-HSCT group had poor graft function (1.3% vs. 1.8%, respectively, P = 1.000) (Table 1).
The cumulative incidence of grade I–IV, II–IV, and III–IV aGVHD was not different between the haplo-HSCT and haplo-cord-HSCT groups (41.8 ± 5.5% vs. 38.2 ± 3.8%, P = 0.580, Figure 1A; 34.2 ± 5.3% vs. 32.1 ± 3.6%, P = 0.742, Figure 1B; and 14.1 ± 3.9% vs. 13.3 ± 2.6%, P = 0.880, Figure 1C). The patients who survived for longer than +100 days were evaluated for cGVHD. The five-year cumulative incidence of overall cGVHD tended to be higher in the haplo-HSCT group compared with that in the haplo-cord-HSCT group, although this difference was not statistically significant (37.0 ± 6.2% vs. 27.0 ± 3.7%, P = 0.110, Figure 1D). The five-year cumulative incidence of moderate-to-severe cGVHD after a haplo-HSCT or haplo-cord-HSCT was also similar (8.2 ± 3.5% vs. 10.7 ± 2.5%, respectively, P = 0.529, Figure 1E).
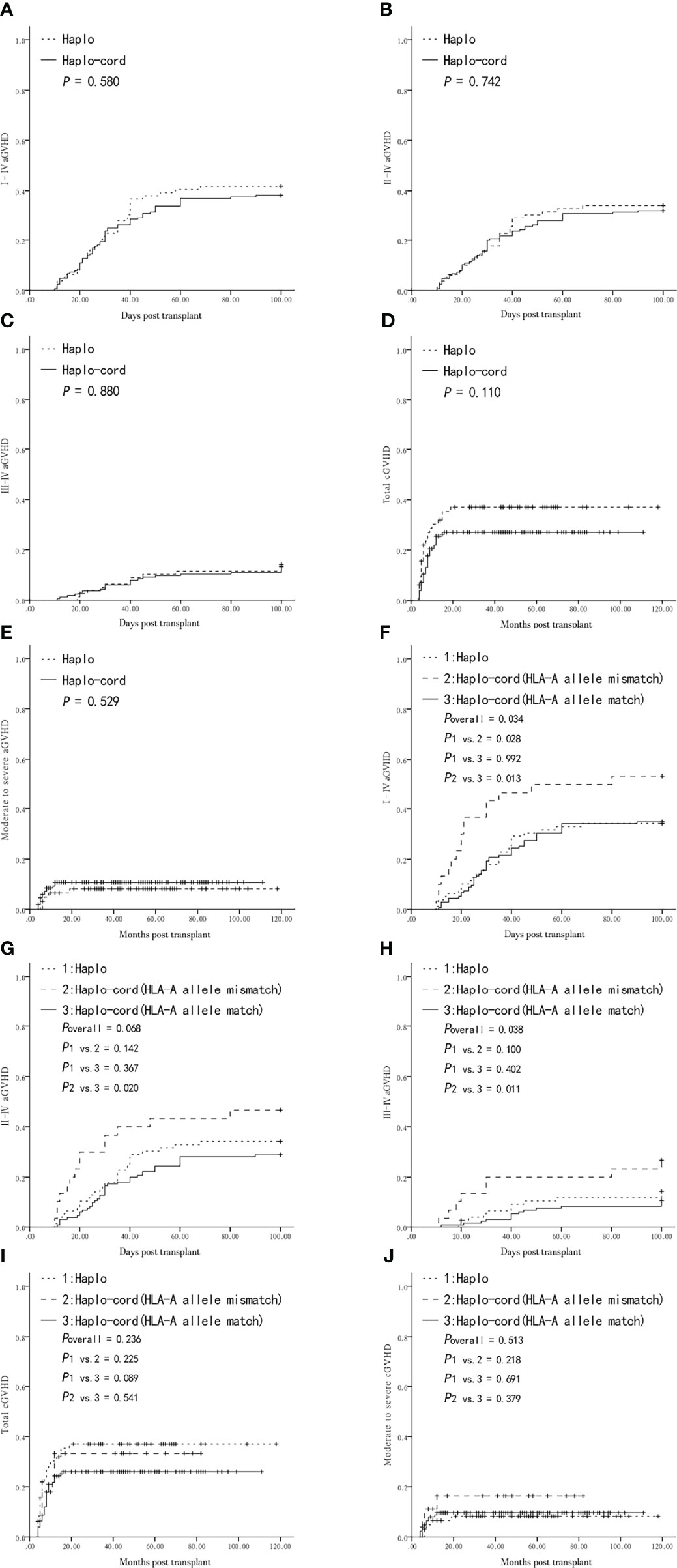
Figure 1 Graft-versus-host-disease (GVHD) after transplantation from Haplo-HSCT or Haplo-cord-HSCT (including subgroups). (A) Grade I–IV acute GVHD (aGVHD) between Haplo-HSCT and Haplo-cord-HSCT group. (B) Grade II–IV aGVHD between the two groups. (C) Grade III–IV aGVHD between the two groups. (D) Total chronic GVHD (cGVHD) between the two groups. (E) Moderate to severe cGVHD between the two groups. (F) Grade I–IV aGVHD among Haplo-HSCT group and Haplo-cord with a HLA-A match or mismatch subgroups. (G) Grade II–IV aGVHD among Haplo-HSCT group and Haplo-cord with a HLA-A match or mismatch subgroups. (H) Grade III–IV aGVHD among Haplo-HSCT group and Haplo-cord with a HLA-A match or mismatch subgroups. (I) Total cGVHD among Haplo-HSCT group and Haplo-cord with a HLA-A match or mismatch subgroups. (J) Moderate to severe cGVHD among Haplo-HSCT group and Haplo-cord with a HLA-A match or mismatch subgroups.
The haplo-cord-HSCT group was divided into haplo-cord with HLA-A allele mismatched and matched subgroups, for comparison with the haplo-HSCT group. The cumulative incidence of grade I–IV aGVHD was higher in the haplo-cord with HLA-A allele mismatch subgroup than that in the haplo-cord with HLA-A allele matched or haplo-HSCT groups (53.3 ± 9.1% vs. 34.8 ± 4.1% vs. 34.2 ± 5.3%, overall P = 0.034, Figure 1F). Pairwise comparison showed that the difference was only statistically significant in the haplo-cord with HLA-A allele mismatch group vs. the haplo-cord with HLA-A allele match group (P = 0.013, Figure 1F). There was no significant difference in the cumulative incidence of grade II–IV aGVHD between the haplo-cord with HLA-A allele mismatch, haplo-HSCT, and haplo-cord with HLA-A allele match groups (46.7 ± 9.1% vs.34.2 ± 5.3% vs. 28.9 ± 3.9%, overall P = 0.068, respectively Figure 1G). The cumulative incidence of III–IV aGVHD was higher in the haplo-cord with HLA-A allele mismatch group than that in the haplo-HSCT or haplo-cord with HLA-A allele match groups (26.7 ± 8.1% vs. 14.1 ± 3.9% vs. 10.2 ± 2.6%, respectively, overall P = 0.038, Figure 1H). Pairwise comparison showed that the difference in grade III–IV aGVHD was only statistically significant in the haplo-cord with HLA-A allele mismatch group vs. the haplo-cord with HLA-A allele match pair group (P = 0.011, Figure 1H). There was no difference in the five-year cumulative incidence of overall cGVHD and moderate-to-severe cGVHD between the three subgroups (overall P = 0.236, Figure 1I; overall P = 0.513, Figure 1J).
There was also no difference in I–IV, II–IV, and III–IV aGVHD and total and moderate-to-severe cGVHD between the haplo-cord-HSCT subgroups grouped by matching or non-matching of the HLA-A antigen, HLA-B antigen and allele, and HLA-DRB1 antigen and allele (P > 0.05, Supplemental Tables 1, 2) and between the haplo-cord-HSCT subgroups according to the counts of TNCs and CD34 + cells (greater or less than the median). In addition, no differences in the five types of GVHD mentioned above were observed among the haplo-cord-HSCT subgroups according to the degree of UCB and recipient HLA-matching (4/6, 5/6, or 6/6) (P > 0.05, Supplemental Tables 1, 2).
In the entire cohort, the use of low-dose PTCy was a significantly protective factor for grade I–IV aGVHD and total cGVHD. the choice of haplo-cord-HSCT was another protective factor for total cGVHD in multivariate analysis (Table 2). Multivariate analysis showed that the matching of the HLA-A allele between the UCB and the recipient pair was only associated with lower I–IV and III–IV aGVHD in the haplo-cord-HSCT group (both P = 0.013) (Table 3).
The incidence rate of bacterial and fungal infections was 64.3% in the haplo-HSCT group and 59.1% in the haplo-cord-HSCT (P = 0.422) (Table 1). The incidence of cytomegalovirus (CMV) viremia in the haplo-HSCT group was not different between the two groups (P = 0.779) (Table 1). Of the 84 patients in the haplo-HSCT group, 14 (16.7%) experienced EBV reactivation, while of the 171 patients in the haplo-cord-HSCT group, 24 (14.9%) experienced EBV viremia. After treatment with rituximab, the majority of patients with EBV viremia had a full recovery. However, one patient in the haplo-HSCT group finally died of EBV-associated post-transplant lymphoproliferative disease at day +395 (Table 1).
The median follow-up time in the living patients was 62 months (range, 11–156) in the haplo-HSCT group and 56 months (range, 12–117) in the haplo-cord-HSCT group (P = 0.407). No case had a recurrence of SAA during the follow-up period. The rate of TRM was higher in the haplo-HSCT group than in the haplo-cord-HSCT group (27.4% vs. 16.4%, P = 0.039). Further analysis showed that the prevalence of TRM caused by infection was higher in the haplo-HSCT group than that in the haplo-cord-HSCT group (13.7% vs. 4.1%, P = 0.016) (Table 1).
The five-year probability of OS in the haplo-cord-HSCT group was higher than that in the haplo-HSCT group (84.0 ± 2.8% vs. 72.6 ± 4.9%, P = 0.022, Figure 2A). However, the five-year GFFS was not significantly different between the two groups (72.4 ± 3.4% vs. 65.4 ± 5.2%, P = 0.178, Figure 2B). Multivariate analysis showed that the choice of a haplo-cord-HSCT was associated with a longer OS (P = 0.013), whereas no beneficial factor for GFFS was identified (P > 0.05) (Table 2).
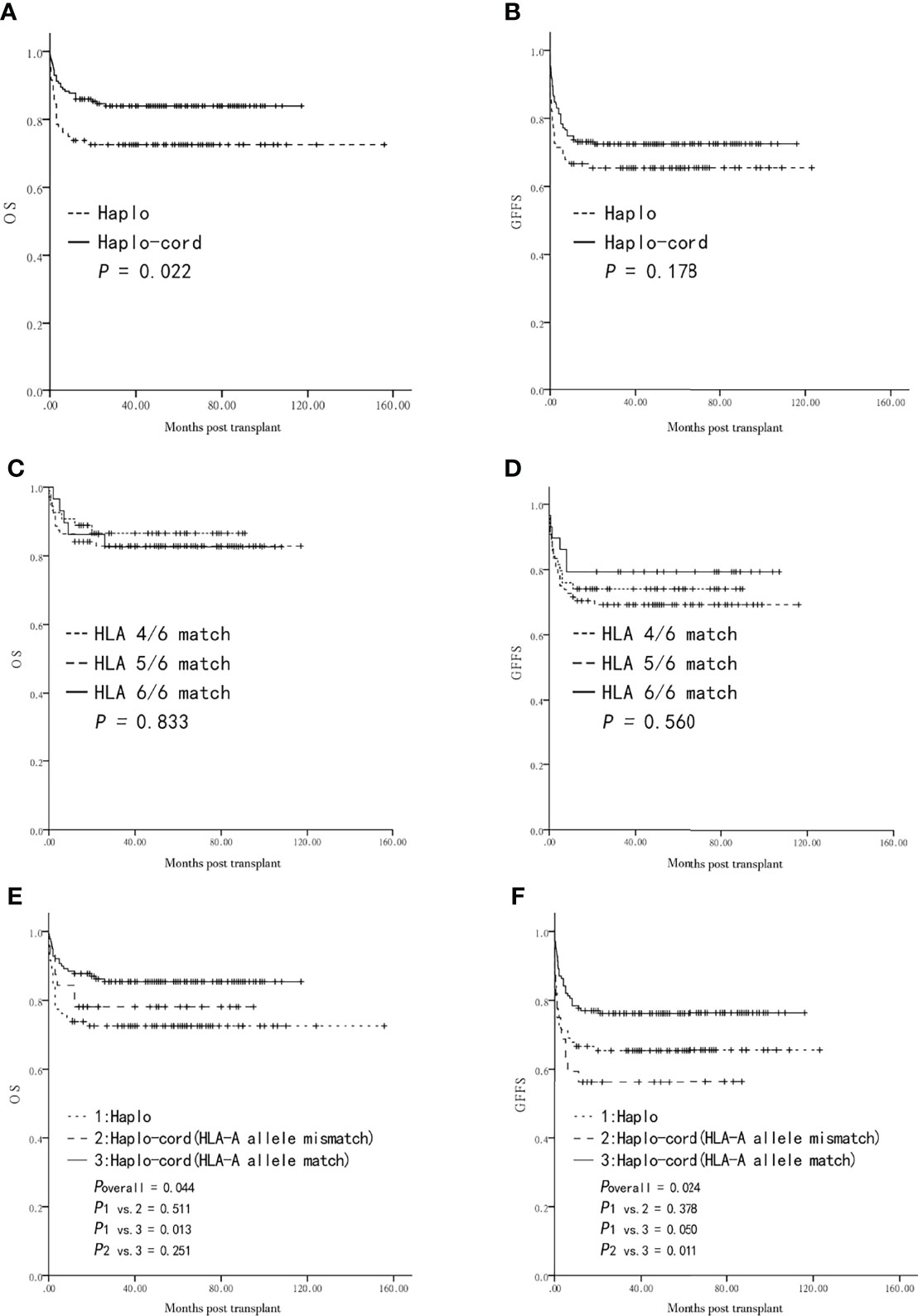
Figure 2 Overall survival (OS) and GVHD-free and failure-free survival (GFFS) after transplantation from Haplo-HSCT or Haplo-cord-HSCT (including subgroups). (A) OS between the two groups. (B) GFFS between the two groups. (C) OS among HLA 4, 5, 6 Haplo-cord-HSCT subgroups. (D) GFFS among HLA 4, 5, 6 Haplo-cord-HSCT subgroups. (E) OS among Haplo-HSCT group and Haplo-cord with a HLA-A match or mismatch subgroups. (F) GFFS among Haplo-HSCT group and Haplo-cord with a HLA-A match or mismatch subgroups.
The UCB-related variables were then subjected to the univariate and multivariate analyses of the survival haplo-cord-HSCT group. The five-year probabilities of OS and GFFS were similar in the haplo-cord-HSCT subgroups according to the degree of HLA-matching (4/6, 5/6, 6/6) (86.5 ± 4.8% vs. 82.9 ± 4.0% vs. 82.6 ± 7.1%, P = 0.833, Figure 2C; 74.1 ± 6.0% vs. 69.2 ± 4.9% vs. 79.3 ± 7.5%, P = 0.560, Figure 2D).
Furthermore, no differences in the five-year OS and GFFS were observed between the matched and mismatched groups for the HLA-A antigen, HLA-B antigen, HLA-B allele, HLA-DRB1 antigen, and HLA-DRB1 allele (P > 0.05) (Table 4). Similarly, the OS and GFFS were not affected by the count of TNCs or CD34+ cells, sex (male or female), or blood type (match or mismatch) (P > 0.05) (Table 4).
Further survival comparison was performed for the haplo-cord with an HLA-A allele match, the haplo-cord with an HLA-A allele mismatch, and the haplo-HSCT groups. The five-year probabilities of OS and GFFS were higher in the former than in the latter two subgroups (85.4 ± 3.0% vs. 78.1 ± 7.3% vs. 72.6 ± 4.9%, overall P = 0.044, Figure 2E; 76.2 ± 3.6% vs. 56.3 ± 8.8% vs. 65.4 ± 5.2%, overall P = 0.024, Figure 2F). Pairwise comparison showed that OS was significantly different only in the haplo-cord with HLA-A allele match vs. the haplo-HSCT pairing (P = 0.013, Figure 2E), while GFFS was significantly different only in the haplo-cord with HLA-A allele match vs. the haplo-cord with HLA-A allele mismatch pairing (P = 0.011, Figure 2F).
In the multivariate analysis, a match between the UCB and the recipient pair for the HLA-A allele was only associated with a better GFFS in the haplo-cord-HSCT group (P = 0.013, Table 4).
Several single-arm studies have demonstrated the feasibility and effectiveness of haplo-cord-HSCT in SAA and other non-neoplastic and malignant hematologic disorders (13–15, 17). However, this approach for SAA is currently considered investigational and requires further study before firm recommendations can be made. In this double-arm multicenter study, we not only confirmed that OS is significantly increased in SAA patients receiving a haplo-cord-HSCT rather than a haplo-HSCT but also investigated the impact of UCB-related characteristics on outcomes using explorative subgroup analysis.
During the process of engraftment, we observed a similar median time of neutrophil and platelet engraftment between the haplo-HSCT and the haplo-cord-HSCT groups. Primary and secondary GF were also similar and low in both groups. The low incidence of GF can be explained by the following factors. First, because DSA is an important factor for GF in haplo-HSCT (23, 24), we attempted to avoid donors targeted by DSA or alternatively decreased the positive degree of DSA prior to transplantation. Second, all patients received the myeloablative conditioning regimen. Third, we ensured that repeated blood transfusions were minimized and also shortened the interval from diagnosis to transplantation. It is noteworthy that chimerism in the haplo-cord HSCT group all involved related haplografts without the evidence of UCB or mixed engraftment. This may be related to the following factors. First, because the majority of patients in our study were adults, the count of CD34+ cells was <0.5 × 105 cells of the recipient’s weight, which was less than the CD34+ cell dose criteria in a single UCB unit (25). Second, in our protocol, the count of CD34+ cells relative to the recipient’s weight was more than 1 log lower in the UCB graft than in the haploidentical graft. Third, the unmanipulated haploidentical graft and conditioning regime with ATG were also different from the previous haplo-cord-HSCT protocol designed by foreign research teams (26–28). In patients with a successful engraftment, the incidence of aGVHD and moderate-to-severe cGVHD was similar between the haplo-HSCT and haplo-cord-HSCT groups, while the overall cGVHD in the haplo-cord-HSCT group tended to be lower than that observed in the haplo-HSCT group. These findings are consistent with those reported by an earlier study (15). This may be because UCB contains MSCs and CD4+CD25+ Tregs (29, 30), which have an immune regulatory role on the hematopoietic microenvironment and prevention of GVHD.
Although the five-year GFFS and relapse of the patients in the haplo-cord-HSCT and the haplo-HSCT groups was not statistically different, we found a significant difference in five-year OS and TRM between the two groups, both favoring the haplo-cord-HSCT group. Multivariate analysis showed that the most significant factor affecting OS was the treatment group, rather than the age and sex of the donor and the time from diagnosis to HSCT. Notably, survival in the haplo-HSCT group was relatively low compared to that described in another recent report (nine-year OS of 85.4%) (31). This may be because the proportion of young patients (<18 years old) was up to 50% in that study, whereas it was only 38% (<20 years old) in our study. In addition, this may be related to their coming from different multicenters. Among the causes of TRM, infection-related mortality was lower in the haplo-cord-HSCT group than in the haplo-HSCT group. Previous studies explored the relation between infection and GVHD (32, 33). Nevertheless, the limited number of infectious deaths does not allow determining the association between infectious deaths and cGVHD and comparing it between the two groups in the present study. A large, population-based analysis including the control of confounding factors is worthy of further study.
Our previous study has demonstrated that no differences in OS and GFFS were found between SAA subgroups after haplo-cord HSCT with 5/10 or 6/10–9/10 HLA-matched HIDs (17). Based on this, the improved survival in haplo-cord-HSCT group may have been affected by other factors, especially the coinfusion of UCB, which has seldom been the focus of previous studies. We next examined whether some characteristics of UCB were associated with survival outcomes, such as the degree of HLA matching, the locus of HLA disparity, and the number of CD34+ cells and TNCs. The results of our univariate analysis showed that an HLA-A allele match between the UCB and the recipient was the only beneficial factor for GFFS. Multivariate analysis also determined that the HLA-A allele match was a beneficial factor for GFFS and grade I–IV and III–IV aGVHD. Therefore, sharing the same HLA-A allele as the UCB in a haplo-cord-HSCT for SAA improves the possibility of GFFS by decreasing the incidence of grade III–IV aGVHD. Similar findings have been reported in a previous study (34). Furthermore, a low incidence of GVHD is often associated with an improvement in the health-related quality of life in patients following an allo-HSCT (16, 35). Although previous studies have reported that the degree of HLA matching and the number of CD34+ cells were important factors for UCB transplantation in pediatric patients with hematological diseases (36), no differences in OS and GFFS were found in our study between the haplo-cord HSCT subgroups with high or low TNCs and CD34+ cells and among subgroups with 4/6, 5/6, and 6/6 matching. As mentioned above, these inconsistent outcomes may be due to differences in the conditioning regime, grafts, and diseases in the different studies.
To examine the impact of an HLA-A allele match or mismatch between the UCB and the recipient on outcomes within and outside the haplo-cord group, we then performed a comparison between these two corresponding groups and the haplo-HSCT group. On the one hand, the incidence of GVHD was lower in the haplo-cord with the HLA allele match group than that observed in the haplo-cord with HLA allele mismatch and haplo-HSCT groups. No significant difference was found between the latter two groups. On the other hand, the OS and GFFS rates were higher in the haplo-cord with the HLA allele match group than that in the haplo-cord with HLA allele mismatch and haplo-HSCT groups, with no significant difference between the latter two groups. These preliminary results suggest that when an SAA patient undergoes a haplo-HSCT, the combination of UCB with an HLA-A allele match with the recipient should be preferred, while the option with an HLA-A allele mismatch is still worth considering. A future prospective study in a larger number of patients is required to confirm these conclusions.
In conclusion, this multiple-centered cohort study in SAA patients had two major findings (1): preliminary data showed that a haplo-cord-HSCT had a better OS and lower TRM compared with that observed for a haplo-HSCT alone, and (2) on the basis of at least an HLA 4/6 match between the UCB and the recipient pair, UCB’ selection should primarily consider the degree of HLA-A allele match, rather than the count of TNCs/CD 34+ cells, sex, and blood type. These algorithms may be helpful for UCB selection in SAA patients undergoing a haplo-cord-HSCT.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
M-QL, Y-MZ, and W-JJ wrote the manuscript and performed the analysis. D-PW, MM, and L-ML designed the protocol. All authors contributed patients, provided clinical and laboratory data, and revised and corrected the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
This work was supported partially by grants from the National Key R&D Program of China (2016YFC0902800, 2017YFA0104502, and 2017ZX09304021), the Innovation Capability Development Project of Jiangsu Province (BM2015004), the Jiangsu Provincial Key Medical Center (YXZXA2016002), the Jiangsu Medical Outstanding Talents Project (JCRCA2016002), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), the Science Foundation of Suzhou (No.SKY2021040), and the Open Project of Jiangsu Biobank of Clinical Resources, No. SBK202003003. The authors would like to express their gratitude to EditSprings (https://www.editsprings.com/) for the expert linguistic services provided.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.912917/full#supplementary-material
1. Killick SB, Bown N, Cavenagh J, Dokal I, Foukaneli T, Hill A, et al. Guidelines for the Diagnosis and Management of Adult Aplastic Anaemia. Br J Haematol (2016) 172:187–207. doi: 10.1111/bjh.13853
2. Xu LP, Xu ZL, Wang FR, Mo XD, Han TT, Han W, et al. Unmanipulated Haploidentical Transplantation Conditioning With Busulfan, Cyclophosphamide and Anti-Thymoglobulin for Adult Severe Aplastic Anaemia. Bone Marrow Transplant (2018) 53:188–92. doi: 10.1038/bmt.2017.237
3. Gao L, Li Y, Zhang Y, Chen X, Gao L, Zhang C, et al. Long-Term Outcome of HLA-Haploidentical Hematopoietic SCT Without In Vitro T-Cell Depletion for Adult Severe Aplastic Anemia After Modified Conditioning and Supportive Therapy. Bone Marrow Transplant (2014) 49:519–24. doi: 10.1038/bmt.2013.224
4. Esteves I, Bonfim C, Pasquini R, Funke V, Pereira NF, Rocha V, et al. Haploidentical BMT and Post-Transplant Cy for Severe Aplastic Anemia: A Multicenter Retrospective Study. Bone Marrow Transplant (2015) 50:685–9. doi: 10.1038/bmt.2015.20
5. Xu LP, Wang SQ, Wu DP, Wang JM, Gao SJ, Jiang M, et al. Haplo-Identical Transplantation for Acquired Severe Aplastic Anaemia in a Multicentre Prospective Study. Br J Haematol (2016) 175:265–74. doi: 10.1111/bjh.14225
6. Xu LP, Jin S, Wang SQ, Xia LH, Bai H, Gao SJ, et al. Upfront Haploidentical Transplant for Acquired Severe Aplastic Anemia: Registry-Based Comparison With Matched Related Transplant. J Hematol Oncol (2017) 10:25. doi: 10.1186/s13045-017-0398-y
7. Ciceri F, Lupo-Stanghellini MT. And Korthof ET: Haploidentical Transplantation in Patients With Acquired Aplastic Anemia. Bone Marrow Transplant (2013) 48:183–5. doi: 10.1038/bmt.2012.231
8. Xu LP, Liu KY, Liu DH, Han W, Chen H, Chen YH, et al. A Novel Protocol for Haploidentical Hematopoietic SCT Without In Vitro T-Cell Depletion in the Treatment of Severe Acquired Aplastic Anemia. Bone Marrow Transplant (2012) 47:1507–12. doi: 10.1038/bmt.2012.79
9. Liu Z, Zhang Y, Xiao H, Yao Z, Zhang H, Liu Q, et al. Cotransplantation of Bone Marrow-Derived Mesenchymal Stem Cells in Haploidentical Hematopoietic Stem Cell Transplantation in Patients With Severe Aplastic Anemia: An Interim Summary for a Multicenter Phase II Trial Results. Bone Marrow Transplant (2017) 52:704–10. doi: 10.1038/bmt.2016.347
10. Purev E, Aue G, Vo P, Kotecha R, Wilder JS, McDuffy E, et al. Excellent Outcomes of Combined Haploidentical and Cord-Blood (Haplo-Cord) Transplantation and HLA-Matched Sibling (Matched-Sib) Donor Transplantation for High-Risk Patients With Severe Aplastic Anemia (SAA) Refractory to Immunosuppressive Therapy. Blood (2016) 128:4689-9. doi: 10.1182/blood.V128.22.4689.4689
11. Zhang XH, Chen J, Han MZ, Huang H, Jiang EL, Jiang M, et al. The Consensus From The Chinese Society of Hematology on Indications, Conditioning Regimens and Donor Selection for Allogeneic Hematopoietic Stem Cell Transplantation: 2021 Update. J Hematol Oncol (2021) 14:145. doi: 10.1186/s13045-021-01159-2
12. Li H, Li X, Chen Y, Li D, Chen X, Zhu Z, et al. Sequential Transplantation of Haploidentical Stem Cell and Unrelated Cord Blood With Using ATG/PTCY Increases Survival of Relapsed/Refractory Hematologic Malignancies. Front Immunol (2021) 12:733326. doi: 10.3389/fimmu.2021.733326
13. Ke P, Bao XB, Hu XH, Zhuang J, Wu XJ, Liu YJ, et al. Myeloablative Conditioning Regimens With Combined of Haploidentical and Cord Blood Transplantation for Myelodysplastic Syndrome Patients. Bone Marrow Transplant (2018) 53:162–8. doi: 10.1038/bmt.2017.229
14. Wang J, Wang Z, Wei W, Zhang W, Zhang T, Cheng H, et al. Cord Haploidentical Non-In Vitro T Cell Depletion Allogeneic Hematopoietic Stem Cell Transplantation Reduces Relapse of Refractory Acute Leukemia. Biol Blood Marrow Transplant (2019) 25:121–8. doi: 10.1016/j.bbmt.2018.09.002
15. Tang X, Yu Z, Ping L, Lu W, Jing Y. And Cao X: Improved Outcomes Using Unmanipulated Haploidentical Hematopoietic Stem Cells Combined With Third-Party Umbilical Cord Blood Transplantation for non-Malignant Diseases in Children: The Experience of a Single Center. Pediatr Transplant (2021) 25:e13995. doi: 10.1111/petr.13995
16. Liu L, Zhang Y, Jiao W, Zhou H, Wang Q, Jin S, et al. Comparison of Efficacy and Health-Related Quality of Life of First-Line Haploidentical Hematopoietic Stem Cell Transplantation With Unrelated Cord Blood Infusion and First-Line Immunosuppressive Therapy for Acquired Severe Aplastic Anemia. Leukemia (2020) 34:3359–69. doi: 10.1038/s41375-020-0933-7
17. Liu L, Zhang Y, Jiao W, Zhou H, Wang Q, Qiu H, et al. Combination of Haploidentical Haematopoietic Stem Cell Transplantation With an Unrelated Cord-Blood Unit in Patients With Severe Aplastic Anemia: A Report of 146 Cases. Bone Marrow Transplant (2020) 55:2017-25. doi: 10.1038/s41409-020-0874-9
18. Camitta BM, Thomas ED, Nathan DG, Santos G, Gordon-Smith EC, Gale RP, et al. Severe Aplastic Anemia: A Prospective Study of the Effect of Early Marrow Transplantation on Acute Mortality. Blood (1976) 48:63–70. doi: 10.1182/blood.V48.1.63.63
19. Kako S, Yamazaki H, Ohashi K, Ozawa Y, Ota S, Kanda Y, et al. Mixed Chimerism and Secondary Graft Failure in Allogeneic Hematopoietic Stem Cell Transplantation for Aplastic Anemia. Biol Blood Marrow Transplant (2020) 26:445–50. doi: 10.1016/j.bbmt.2019.10.004
20. Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant (1994) 1995) 15:825–8.
21. Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-Versus-Host Disease: I. Diagnosis and Staging Working Group Report. Biol Blood Marrow Transplant (2005) 11:945–56. doi: 10.1016/j.bbmt.2005.09.004
22. Kong Y, Chang YJ, Wang YZ, Chen YH, Han W, Wang Y, et al. Association of an Impaired Bone Marrow Microenvironment With Secondary Poor Graft Function After Allogeneic Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant (2013) 19:1465–73. doi: 10.1016/j.bbmt.2013.07.014
23. Wang L. [Donor-Specific Antibodies in Allogeneic Hematopoietic Stem Cell Transplantation-Review]. Zhongguo Shi Yan Xue Ye Xue Za Zhi (2019) 27:629–32. doi: 10.19746/j.cnki.issn1009-2137.2019.02.052
24. Ozdemir ZN, Civriz Bozdag S. Graft Failure After Allogeneic Hematopoietic Stem Cell Transplantation. Transfus Apher Sci (2018) 57:163–7. doi: 10.1016/j.transci.2018.04.014
25. Politikos I, Davis E, Nhaissi M, Wagner JE, Brunstein CG, Cohen S, et al. Guidelines for Cord Blood Unit Selection. Biol Blood Marrow Transplant (2020) 26:2190–6. doi: 10.1016/j.bbmt.2020.07.030
26. Sebrango A, Vicuna I, de Laiglesia A, Millan I, Bautista G, Martin-Donaire T, et al. Haematopoietic Transplants Combining a Single Unrelated Cord Blood Unit and Mobilized Haematopoietic Stem Cells From an Adult HLA-Mismatched Third Party Donor. Comparable Results to Transplants From HLA-Identical Related Donors in Adults With Acute Leukaemia and Myelodysplastic Syndromes. Best Pract Res Clin Haematol (2010) 23:259–74. doi: 10.1016/j.beha.2010.05.002
27. Fernandez MN, Regidor C, Cabrera R, Garcia-Marco JA, Fores R, Sanjuan I, et al. Unrelated Umbilical Cord Blood Transplants in Adults: Early Recovery of Neutrophils by Supportive Co-Transplantation of a Low Number of Highly Purified Peripheral Blood CD34(+) Cells From an HLA-Haploidentical Donor. Exp Hematol (2003) 31:535–44. doi: 10.1016/S0301-472x(03)00067-5
28. Magro E, Regidor C, Cabrera R, Sanjuan I, Fores R, Garcia-Marco JA, et al. Early Hematopoietic Recovery After Single Unit Unrelated Cord Blood Transplantation in Adults Supported by Co-Infusion of Mobilized Stem Cells From a Third Party Donor. Haematologica (2006) 91:640–8.
29. Kellner JN, Delemarre EM, Yvon E, Nierkens S, Boelens JJ, McNiece I, et al. Third Party, Umbilical Cord Blood Derived Regulatory T-Cells for Prevention of Graft Versus Host Disease in Allogeneic Hematopoietic Stem Cell Transplantation: Feasibility, Safety and Immune Reconstitution. Oncotarget (2018) 9:35611–22. doi: 10.18632/oncotarget.26242
30. Wu QL, Liu XY, Nie DM, Zhu XX, Fang J, You Y, et al. Umbilical Cord Blood-Derived Mesenchymal Stem Cells Ameliorate Graft-Versus-Host Disease Following Allogeneic Hematopoietic Stem Cell Transplantation Through Multiple Immunoregulations. J Huazhong Univ Sci Technolog Med Sci (2015) 35:477–84. doi: 10.1007/s11596-015-1456-8
31. Xu L-P, Xu Z-L, Wang S-Q, Wu D-P, Gao S-J, Yang J-M, et al. Long-Term Follow-Up of Haploidentical Transplantation in Relapsed/Refractory Severe Aplastic Anemia: A Multicenter Prospective Study. Sci Bull (2022) 67:963–70. doi: 10.1016/j.scib.2022.01.024
32. Poutsiaka DD, Munson D, Price LL, Chan GW, Snydman DR. Blood Stream Infection (BSI) and Acute GVHD After Hematopoietic SCT (HSCT) are Associated. Bone Marrow Transplant (2011) 46:300–7. doi: 10.1038/bmt.2010.112
33. Dix D, Cellot S, Price V, Gillmeister B, Ethier MC, Johnston DL, et al. Association Between Corticosteroids and Infection, Sepsis, and Infectious Death in Pediatric Acute Myeloid Leukemia (AML): Results From the Canadian Infections in AML Research Group. Clin Infect Dis (2012) 55:1608–14. doi: 10.1093/cid/cis774
34. Yokoyama H, Morishima Y, Fuji S, Uchida N, Takahashi S, Onizuka M, et al. Impact of HLA Allele Mismatch at HLA-A, -B, -C, and-DRB1 in Single Cord Blood Transplantation. Biol Blood Marrow Transplant (2020) 26:519–28. doi: 10.1016/j.bbmt.2019.11.001
35. Mo XD, Xu LP, Liu DH, Chen YH, Han W, Zhang XH, et al. Patients Receiving HLA-Haploidentical/Partially Matched Related Allo-HSCT can Achieve Desirable Health-Related QoL That is Comparable to That of Patients Receiving HLA-Identical Sibling Allo-HSCT. Bone Marrow Transplant (2012) 47:1201–5. doi: 10.1038/bmt.2011.250
36. Wagner JE, Barker JN, DeFor TE, Baker KS, Blazar BR, Eide C, et al. Transplantation of Unrelated Donor Umbilical Cord Blood in 102 Patients With Malignant and Nonmalignant Diseases: Influence of CD34 Cell Dose and HLA Disparity on Treatment-Related Mortality and Survival. Blood (2002) 100:1611–8. doi: 10.1182/blood-2002-01-0294
Keywords: Severe aplastic anemia, Haploidentical donor, hematopoietic stem cell transplant, unrelated cord blood, Comparision
Citation: Lei M, Zhang Y, Jiao W, Li X, Zhou H, Wang Q, Qiu H, Tang X, Han Y, Fu C, Jin Z, Chen S, Sun A, Miao M, Liu L and Wu D (2022) Comparison of Haploidentical Hematopoietic Stem Cell Transplant With or Without Unrelated Cord Blood Infusion in Severe Aplastic Anemia: Outcomes of a Multicenter Study. Front. Immunol. 13:912917. doi: 10.3389/fimmu.2022.912917
Received: 05 April 2022; Accepted: 18 May 2022;
Published: 23 June 2022.
Edited by:
Robert James Hayashi, Washington University in St. Louis, United StatesReviewed by:
Xiao-Dong Mo, Peking University People’s Hospital, ChinaCopyright © 2022 Lei, Zhang, Jiao, Li, Zhou, Wang, Qiu, Tang, Han, Fu, Jin, Chen, Sun, Miao, Liu and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Depei Wu, d3VkZXBlaUBzdWRhLmVkdS5jbg==; Limin Liu, bGltaW5saXUyMDA2QDE2My5jb20=; Miao Miao, bW04NTEyNEBzaW5hLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.