- 1Institute of Medical Immunology, Martin Luther University Halle-Wittenberg, Halle (Saale), Germany
- 2Fraunhofer Institute for Cell Therapy and Immunology, Leipzig, Germany
- 3Institute of Clinical Immunology, University of Leipzig, Leipzig, Germany
- 4Institute of Cellular Therapeutics, Hannover Medical School, Hannover, Germany
Natural killer (NK) cells belong to the family of innate immune cells with the capacity to recognize and kill tumor cells. Different phenotypes and functional properties of NK cells have been described in tumor patients, which could be shaped by the tumor microenvironment. The discovery of HLA class I-specific inhibitory receptors controlling NK cell activity paved the way to the fundamental concept of modulating immune responses that are regulated by an array of inhibitory receptors, and emphasized the importance to explore the potential of NK cells in cancer therapy. Although a whole range of NK cell-based approaches are currently being developed, there are still major challenges that need to be overcome for improved efficacy of these therapies. These include escape of tumor cells from NK cell recognition due to their expression of inhibitory molecules, immune suppressive signals of NK cells, reduced NK cell infiltration of tumors, an immune suppressive micromilieu and limited in vivo persistence of NK cells. Therefore, this review provides an overview about the NK cell biology, alterations of NK cell activities, changes in tumor cells and the tumor microenvironment contributing to immune escape or immune surveillance by NK cells and their underlying molecular mechanisms as well as the current status and novel aspects of NK cell-based therapeutic strategies including their genetic engineering and their combination with conventional treatment options to overcome tumor-mediated evasion strategies and improve therapy efficacy.
General features of NK cells
Natural killer (NK) cells are cytotoxic innate immune cells that were first described in 1973 by E. Klein and colleagues (1). They originate from multipotent hematopoietic stem cells (HSC) in the bone marrow (BM) and undergo different developmental stages gradually acquiring the expression of distinct surface markers defining the commitment to the lymphoid/NK cell lineage. Maturation of human NK cells is characterized by a loss of CD34 and c-KITC (CD117) expression followed by a sequential upregulation of CD94, CD16 and killer cell immunoglobulin-like receptors (KIRs) (2). NK cells comprise 5-10% of peripheral blood mononuclear cells (PBMCs), but they are also found with a variable frequency in various lymphoid and non-lymphoid tissues including BM, liver, lung, skin, kidney and spleen (3). NK cells have the capacity to form cytoplasmic lytic granules containing perforin and granzymes and produce a large number of cytokines, in particular interferon (IFN)-γ, but also proinflammatory and immune suppressive cytokines, such as tumor necrosis factor (TNF)-α, interleukin (IL)-10, chemokines and various growth factors like granulocyte-macrophage stimulatory factor (GM-CSF), granulocyte-stimulating factor (G-CSF) and IL-3. They exert their cytotoxic activity by distinct mechanisms, including the release of granzymes and perforin, secretion of IFN-γ and TNF-α, the expression of the FasL/Fas or TNF-related apoptosis-inducing ligand (TRAIL)/TRAIL receptors and the antibody-dependent cell-mediated cytotoxicity (ADCC) via Fc receptors (CD16) recognizing antibodies bound to antigen-coated (tumor) cells (4–6). Based on their cytolytic function, NK cells play a key role in the first line of immune defense and are able to directly eliminate tumor or pathogen-infected cells. In this context it is noteworthy that NK cells have safety features, rarely elicit autoimmunity and promote immune homeostasis.
NK cells arise and progressively evolve from a limited diversity to highly differentiated and heterogeneous phenotypes, which are dictated by genetic factors and environmental stimuli, such as pathogen exposure, leading to distinct functions (7). In PBMCs, NK cells are generally subdivided into two major subsets based on their differential expression of CD56: (i) CD56bright, CD94+, CD16- NK cells, which are less abundant in PBMCs, are poorly cytotoxic, but produce high amounts of IL-1β, IFN-γ, IL-2, IL-12, IL-15, IL-18 and TNF-α upon stimulation, extensively proliferate in response to DC-derived cytokines and can extravasate from the circulation into tissues and (ii) CD56dim, CD16+ and KIR+ NK cells, which have a low proliferative capacity, but high cytotoxic activity accounting for most of the circulating NK cells (8, 9). Furthermore, terminally differentiated CD57+ and adaptive NKG2C+CD57+ NK cells exist (10). Also, the discovery of memory-like NK cells being able to mount a robust secondary immune response upon activation has expanded the understanding of this innate immune cell population over the past decade (11, 12).
With the possibility of the in depth characterization of immune cell subpopulations by high-dimensional transcriptional and phenotypic profiling using (single cell) RNA-sequencing (RNA-seq) and mass cytometry an unexpected NK cell diversity was identified across different organs within individual donors regarding their function, maturation and interaction with stromal cells, which also provide a new framework for the analyses of NK cell responses under physiologic and pathophysiologic conditions (13–17). Interestingly, the diversity of NK cells was found both in the immune cell infiltrate of tissues and in peripheral blood (17, 18).
NK cell receptors and NK cell activity
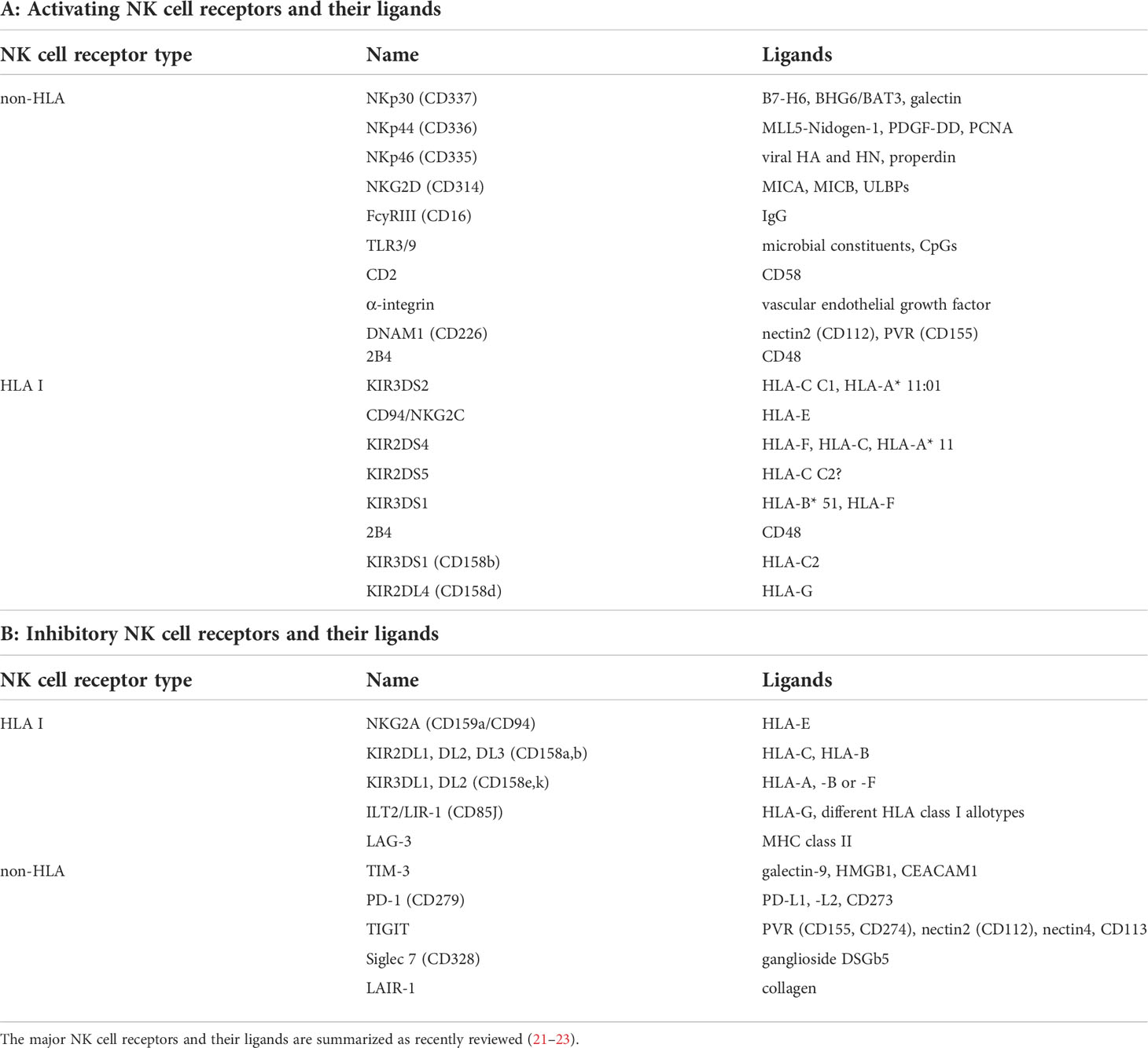
NK cells are tightly regulated by a dynamic balance of transduced signals mediated by the physical interaction with adjacent cells. They express a number of germline-encoded activating and inhibitory receptors as well as cytokine and chemokine receptors on the cell surface, which influence the NK cell function, but knowledge of how these receptors convey signals and affect NK cell biology is still limited. There is evidence of a balance between activating and inhibitory receptors, which control the activity, cell diversity and function of NK cells (19, 20). These constitutively expressed NK cell receptors comprise non-HLA-specific receptors, HLA-specific receptors and homing receptors (20), and recognize their corresponding ligands expressed on the cell surface of target cells such as tumor cells or virus-infected cells (21), as summarized in Table 1.
Next to CD16 (FcγRIIIA), which interacts with Fc fragments of several IgG subclasses, triggering the ADCC (24), the natural cytotoxicity receptors (NCR) NKp30 (CD337), NKp44 (CD336), NKp46 (CD335), NKp80, DNAM-1 (CD226) and NKG2D (CD314) are the major activating receptors and are able to recognize induced self-ligands that are downregulated on healthy cells and highly expressed on tumor cells (25). There are a number of HLA class I-specific activating NK cell receptors (NKR) that recognize the non-classical HLA class I antigens HLA-E and HLA-F or epitopes shared by distinct HLA class I allotypes. For the activating receptor KIR2DS3, the ligand is still unknown. Other NK cell activating receptors include SLAMs, CD18, CD2 and the toll-like receptor (TLR) 3/9 (26, 27).
The primary inhibitory receptors on the cell surface of NK cells represent members of the killer cell immunoglobulin-like receptor (KIR) family, which consists of 14 polymorphic receptors. The different inhibitory KIRs can recognize classical HLA class I antigens, but for KIR2DL5 no ligand has yet been identified. Other inhibitory receptors include NKG2A, a member of the C-type lectin family, which heterodimerizes with CD94 and binds to the HLA-E antigen, the immunoglobulin-like receptor superfamily B member 1 (LILRB1, ILT2, CD85j), the T cell immunoglobulin and mucin domain containing molecule 3 (TIM-3), T cell immunoreceptor with Ig and immunoreceptor tyrosine-based inhibition motif (ITIM) domains (TIGIT) (28–30), CD161, SIGLEC7, SIGLEC9, programed death receptor 1 (PD-1) and lymphocyte-activation gene (LAG-3) (31). These inhibitory receptors regulate the activation status and anti-tumoral immunity of NK cells by suppressing effector functions and augmenting Treg activity (32).
MHC class I molecules are ligands of the inhibitory receptors of NK cells thereby providing signals to self-tolerance resulting in NK cell inactivation and the discrimination between healthy, “self” and “non-self” cells including tumor or virus-infected cells. However, tumor or pathogen-infected cells often lack or downregulate MHC class I surface antigens, which results in an escape from recognition by CD8+ cytotoxic T lymphocytes (CTL). In contrast, these MHC class I-negative cells could be recognized and eliminated by NK cells via the missing self-mechanism (“missing-self recognition”). However, the NK cell activation requires additional signals to induce self changes, e.g. by virus-encoded ligands or ligands upregulated by cellular stress, by DNA damage and alterations of suppressor genes (33) leading to the so-called “induced self-recognition” (34). Activated NK cells can eliminate target cells either directly via NK cell-mediated cytotoxicity or indirectly via proinflammatory cytokine-mediated killing by TNF-α and IFN-γ. In addition to the interaction with tumor and pathogen-infected cells, NK cells could crosstalk with other immune cells, like macrophages, T lymphocytes and different dendritic cell (DC) subpopulations (35, 36). Over the last decade, the functional links between NK cells and myeloid cells have been broadly analyzed. This cooperative interaction triggers the innate and adaptive immune responses by stimulating the survival, maturation and tumor infiltration of DCs leading to “DC editing” (37–39). Vice versa, macrophages could shape NK cell differentiation and function (40).
NK cells as critical players for tumor immune surveillance
The primary role of NK cells is the recognition and elimination of tumor cells or virus-/pathogen-infected cells as the first line of defense against initiation of tumor formation and pathogen invasion without prior sensitization (41). Evidence for this hypothesis is an increased tumor incidence in human and experimental models with impaired NK cell function (42). NK cells are educated and licensed by inhibitory receptors that recognize classical MHC class I molecules, but could recognize MHC class I-deficient cells, which are then eliminated (43). Thus, NK cells are activated by tumor cells due to the decreased expression of MHC class I on tumor cells through the lack of inhibitory signals and by the induction of activating NK cell receptor ligands through their “missing-self” program (44) leading to productive cytotoxic responses. An additional major pathway involved in NK cell-mediated cytotoxicity is the FasL/Fas interaction, which provides a death signal to target cells leading to apoptosis. The activating receptor NKG2D on NK cells recognize the MHC class I-related surface proteins MICA and MICB as well as the UL-16-binding proteins (ULBPs; ULBP1-6), which are often upregulated in e.g. tumor cells countermanding any inhibitory signals and inducing NK cell-mediated cytotoxicity (45, 46).
Composition of the tumor microenvironment and NK cells
Detailed analysis of the tumor microenvironment (TME) in different cancer types demonstrated a complex network of immune effector cells, such as CTL and NK cells, but also immune suppressive cells, like regulatory T cells (Tregs), tumor-associated macrophages (TAMs), regulatory γδ T cells, myeloid-derived suppressor cells (MDSCs), soluble factors, extracellular matrix (ECM) components as well as suppressive molecules expressed on tumor cells. The interaction between the different immune cell subpopulations in the TME with tumor cells is diverse and orchestrated by the presence of specific chemokines and cytokines recruiting different immune suppressive cells into the TME and modulating immune effector cells, which is associated with tumor progression (47). This complex interplay is also shaped by changes in the metabolic activity of immune, stromal and tumor cells (48).
The distribution of NK cells is highly dynamic. Circulating NK cells can migrate into tissues via the expression of a broad number of receptors that control this recruitment (31). In different tissues, NK cells display specific phenotypic and functional features, which are altered by the physiologic and pathophysiologic micro-milieu. To reach the solid tumors, NK cells extravasate from the blood and traverse the ECM and the tumor stroma. In the tumor bed, NK cells are able to control tumor growth and metastasis (49). However, NK cell responsiveness is often reduced by the immune suppressive TME.
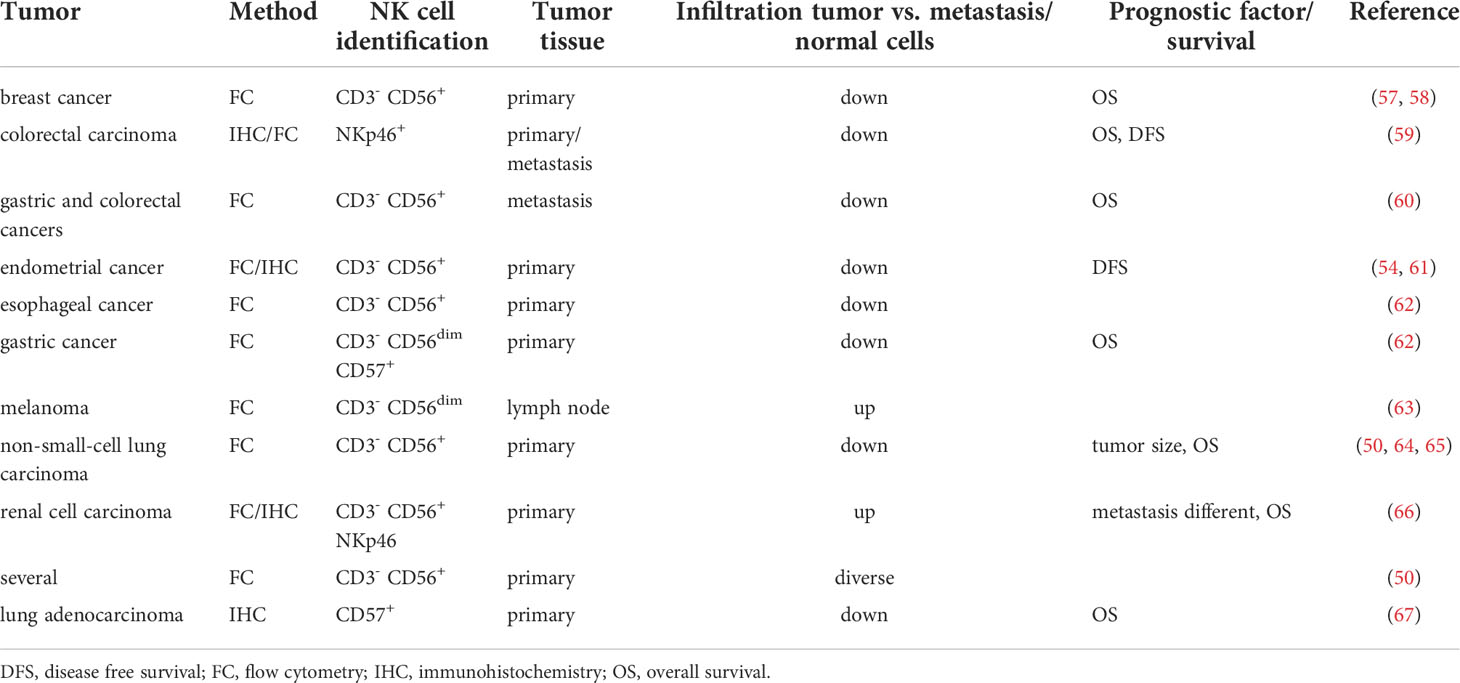
Although NK cells have been demonstrated to infiltrate into primary solid tumors, metastases and even into tumor-draining lymph nodes, the frequencies of NK cells in solid tumors were lower when compared to adjacent tissues and less abundant regarding the numbers of CD4+ and CD8+ T cells and B lymphocytes. The degree of NK cell infiltration in tumors is influenced by several factors (50), such as tumor localization, nature of cancer cells and expression of chemokine receptors/chemokines (51). In addition, NK cells recruited to the tumor core had a reduced cytotoxic potential compared to NK cells from normal tissues and are often associated with an unfavorable condition for survival (52, 53). The clinical relevance of tumor-infiltrating NK cells, e.g. their correlation with the patients’ survival, depends on the expression of ligands for their receptors and is accompanied by a high variability of the different NK cell populations in distinct tumor entities (54). For example, NK cell frequencies are associated with an altered patients’ survival in many tumor entities. NK cells highly infiltrating renal cell carcinoma (RCC) were dysfunctional in ex vivo cultures (14, 55, 56) (Table 2) and showed an increased expression of inhibitory receptors and a downregulation of activating receptors. Furthermore, low numbers of NK cells in head and neck squamous cell carcinoma (HNSCC) were associated with insufficient tumor elimination, while higher numbers of NK cells at the tumor site correlated with an increased patients’ survival. Comparable results were also shown for colorectal carcinoma (CRC), gastric and esophageal cancer. Concerning non-small cell lung cancer (NSCLC), NK cells are less frequent in tumor tissues compared to normal lung epithelium, overexpress NK cell inhibitory receptors and show a CD56bright perforinlow phenotype. The number of NK cells in NSCLC is of clinical relevance and linked to the tumor size, smoking history and a bad patients’ prognosis (31, 68).
Bioinformatics of large RNA-seq datasets from The Cancer Genome Atlas (TCGA) revealed not only a link between NK cell numbers and patients’ survival (31), but also identified a NK cell signature of 13 genes, which makes it possible to determine NK cell abundance across different tumor types and offers novel opportunities for NK cell-based treatment in specific cancer conditions (69, 70). Thus, strategies that increase the recruitment and activation of NK cells in tumors would be a suitable approach to enhance anti-tumor efficacy (71).
Impaired NK cell functions due to intrinsic mechanisms
Studies of different tumor entities demonstrated that the function of intra-tumoral NK cells is impaired, which might be due to aging, genetic defects and chronic infections (72–74), but also due to continuous exposure to tumor antigens (75–77). Tumor escape from NK cell-mediated immune surveillance could be due to impaired anti-tumor NK effector mechanisms, such as reduced production of proinflammatory cytokines, e.g. IFN-γ and TNF-α, proliferation and cytotoxicity due to a diminished expression of effector molecules, like perforin and granzymes. Various solid and hematopoietic cancers demonstrated a downregulation of the activating receptors NKp30, NKG2D, NKp46 and CD16 and an increase of soluble NKG2D ligands sMICA/B shed from the tumor cell surface, but high expression levels of the inhibitory receptor CD94/NKG2A, resulting in impaired NK cell cytotoxicity (78). This was associated with a poor prognosis of patients with breast cancer, chronic lymphocytic leukemia (CLL), ovarian cancer and acute myeloid leukemia (AML) (79). The presence of NK cells in the TME and higher expression levels of CD56, CD57, NKp30 or NKp46 at the tumor site were associated with a favorable patients’ prognosis, while low NK cell numbers correlated with an increased risk of cancer recurrence after resection, and a reduced patients’ survival (80). In NSCLC, overexpression of inhibitory NK cell receptors and a reduced number of NK cells was associated with a poor patients’ outcome (64, 81) which was accompanied by a reduced cytotoxicity and promotion of tumor evasion. Next to the distinct expression pattern of NK cell receptors, the programmed death receptor PD1 has been well characterized as an exhaustion marker for T cells, but also for NK cells (82). The same applies to TIGIT, which is also associated with NK cell exhaustion (83). It is noteworthy that actin cytoskeleton remodeling and fragmented mitochondria in the cytoplasm of tumor-infiltrating NK cells can also lead to immune suppression (84, 85).
Impaired NK cell function due to extrinsic mechanisms
It is generally accepted that the TME shapes the innate as well as the adaptive immune responses, which are variable between distinct tumor types due to differences in the composition of infiltrating immune cells and soluble constituents. It is noteworthy that the critical function of NK cells to induce an effective anti-tumor immunity is a successful interaction between NK cells and DCs, and the production of chemokines. Both processes are negatively influenced by unique locoregional characteristics, in particular cellular and soluble components of the TME, which are associated with an immune escape due to a lack effector responses thereby promoting tumor cell metastasis (75–77). The chemokine milieu in the TME consists of reduced expression of CXCL2, CX3CL1, CXCL1 and CXCL8 thereby attracting CD56dim NK cells and an increased CXCL9/10, CCL5 and CXCL19/21 expression driving the homing of CD56bright NK cells toward the stromal compartment (86).
Solid tumors often showed a high oxygen consumption, a low pH in the TME due to higher concentrations of lactate and a disorganized vascularization leading to hypoxia as well as an altered expression of genes involved in the regulation of metabolic processes. An acidic microenvironment (87) and a permanent or transient hypoxia leading to an upregulation of the transcription factor HIF-1α (88) due to the restricted access to nutrients and oxygen mediated by changes in the vascularization have been demonstrated to downregulate the expression of activating NCRs, reduce NK cell cytotoxicity and survival, which downregulates NK cell anti-tumor responses (89). This could be reverted by e.g. the treatment with an inhibitor of HIF-1α (89). In addition, NK cells may not penetrate into solid tumors including in low MHC class I-expressing tumors or once within the tumors become anergic or exhausted. Increased H2O2 levels lead to a decrease in the infiltration of CD56dim NK cells and impaired ADCC. Furthermore, NK cells in tumors can also acquire proangiogenic functions by secretion of vascular endothelial growth factor (VEGF), angiogenin and matrix metalloproteinases (MMPs) (90, 91). Although a proangiogenic NK cell phenotype has been identified, the potential of proangiogenic NK cell-driving tumor progression has not yet been analyzed in detail. However, tumor endothelium might improve NK cell recruitment to the tumor site as an indirect mechanism of targeting myeloid cells affecting NK cell recruitment and function (92).
Several immune suppressive cells, like MDSC, TAMs and Tregs negatively interfere with NK cell activation. This has been attributed to immune modulatory molecules present in the TME, such as indolamine 2, 3-deoxygenase (IDO) activity and transforming growth factor (TGF)-β, which can be secreted by MDSC, Tregs and anti-inflammatory macrophages. Additionally, IL-1β secreted by 6-sulfo LacNAc DCs induces cell apoptosis (93), while Tregs could also suppress NK cells by deprivation of IL-2 (94). Several other factors produced by tumor or tumor-associated cells, like prostaglandin E2, extracellular adenosine, IL-10 and IL-6, further directly or indirectly prevent NK cell activation (95). During infection and tumorigenesis, macrophages can modulate NK cell function by direct cell-to-cell contact or due to secretion of the cytokines IL-18, IL-12 and TGF-β (96). TGF-β modulates NK cell function via a decrease of NKG2D levels and CD16-mediated ADCC in tumors by impairing the cytotoxic potential as demonstrated in in vivo and in vitro co-culture experiments. In addition, TGF-β affects the expression of chemokine receptors thereby preventing NK cell recruitment as well as the NK cell metabolism by inducing a reduced glycolysis and oxidative phosphorylation that inhibits NK cell effector function. NK cell dysfunction has been associated with the inactivation of the glycogen synthase kinase-3 (GSK3). In contrast, IL-15 is chemotactic for NK cells and maintains NK cell activation by suppressing tumor escape mechanisms (97). However, sustained persistence of IL-15 in the TME could induce the expression of the cytokine-inducible SH2-containing protein, an IL-15 inducible IL-15 signaling inhibitor, leading to the degradation of IL-15R. This is associated with a diminished responsiveness of NK cells to IL-15 (98).
Strategies of tumor cells evading NK cell recognition
As described above, NK cells preferably recognize and kill malignant cells. But there exist many different strategies of tumors to directly evade NK cell recognition. On the one hand, these include the prevention of NK cell recruitment into tumors by physical barriers (laminin and collagen) of tumors or by preferential recruitment of immature NK cells via a chemokine gradient. On the other hand, tumors dampen the NK cell activation and effector function by a decreased expression of ligands for the activating NKRs or by generation of soluble activating receptor ligands, which block recognition. In contrast, inhibitory molecules, like the non-classical HLA class I molecules HLA-G and -E, Nectin-4 or PVR and inhibitory immune checkpoint (ICP) ligands, are often overexpressed in tumors thereby impairing not only T cell, but also NK cell responses (20, 21). High levels of HLA-E were found in many solid tumors and its overexpression correlated with a poor prognosis and NK cell exhaustion (99, 100), while the innate immunity is regulated by the engagement of HLA-G with the NK cell receptor KIR2DL4 or ILT2 (101, 102) leading to a reduced cytotoxicity. Many tumors express the MHC class I chain-regulated polypeptide A (MICA) and MICB, known as ligands for the activating receptor NKG2D on NK cells. However, tumors frequently shed MICA and -B thereby removing an activation signal and creating a soluble ligand, which can block the NK cell cognate receptors (103, 104). Thus, classical, non-classical as well as HLA class I-related molecules play a key role in NK cell functionality by either leading to immune escape or immune recognition. Characterization of these immune escape mechanisms represent the rational for the development of NK cell-based immunotherapies.
Different strategies to revert immune surveillance by NK cells-antibody-based approaches
Since NK cell anti-tumor function is frequently impaired in tumor patients, restoring their function is an obvious therapeutic option. Indeed, there exist different approaches to restore the anti-tumor surveillance of NK cells (105). Agents that enhance NK cell function, like immune modulatory drugs, various stimulatory cytokines, STING agonists and TGF-β inhibitors have been recently summarized (106). In addition, a number of mAbs directed against key ICP ligands and their receptors have been designed, which prevent NK cell inactivation by e.g. decreasing inhibitory factors or increasing factors, which boost NK cell function. Recently, a humanized anti-NKGA mAb (monalizumab) has been developed, which exerts in vitro and in vivo anti-tumor efficacy as a single agent or with other therapeutics (107). The inhibition of NKG2A restores the cytotoxic activity against HLA-E-expressing target cells as well as the NK cell-dependent maturation of monocyte-derived DC and reduces the secretion of immune suppressive cytokines.
Major ICP-targeted therapies that affect NK cell-mediated anti-tumor immune responses are the immune checkpoint inhibitors (ICPis) PD1/PD-L1 and CTLA4. PD1 has been shown to be mainly expressed on T, B and myeloid cells, but also on about 25% of NK cells in healthy donors, but the molecular mechanisms leading to PD1 expression have not yet been identified (108). PD1 can also be expressed on tumor infiltrating NK cells of patients with different solid tumors (109). Blockade of PD1/PD-L1 interaction can enhance NK cell activity both in vitro as well as in animal models due to an enhanced ADCC-induced anti-tumor function leading to an increased tumor control. Moreover, NK cells play also a role in response to treatment with agonistic anti-CD137/4-1BB antibodies (Abs) (110). CD137 is expressed on primed NK cells, which upon ligation provides a powerful costimulatory signal (111). The addition of agonistic Abs increased NK cell proliferation and a synergistic effect was found between IL-15 and IL-21 upon CD137 engagement and the presence of APCs. Thus, CD137 triggering contributes to NK cell activation (112). These data suggest that restoring of the NK cell function by co-targeting immune modulatory pathways might be an important therapeutic strategy to prevent tumor immune escape.
Since intra-tumoral activated NK cells are often characterized by overexpression of TIGIT, which competes with the activating NK cell receptor DNAM1, TIGIT blockade might also be a promising approach (113) and has been described to increase patients’ response (114). However, TIGIT and the activating receptor DNAM1 have CD155 as ligand suggesting a complex of CD155-mediated immune regulation via these receptors. Human tumor cells could express both membranous and soluble CD155. The latter binds preferentially and with a higher affinity to DNAM1 thereby inhibiting the DNAM1-mediated anti-tumor activity of NK cells (115). Recent studies also focused on increasing the infiltration and recruitment of NK cells by inhibiting soluble factors secreted by tumor cells, e.g. TGF-β (116). Furthermore, antibodies targeting the proteolytic site of MICA shedding can promote NK cell-driven tumor immunity (117).
In addition, Abs directed against inhibitory KIRs are potential therapeutic candidates, which might have fewer side effects compared to other therapeutic approaches. Recently, a humanized anti-NKG2A mAb monalizumab has been developed, which is explored in clinical trials (NCT02643550, NCT02921685). Other trials are addressing IPH4102 as an anti-KIR3DL2 (NCT02593045), lirilumab as an anti-KIR2DL1-3 (NCT01687387) antibody as well as different Abs directed against the PD1/PD-L1 axis.
Benefit and limitation in clinical NK cell-based immunotherapies – Adoptive cell transfer-based approaches
The translation of in vitro and in situ results of modulating NK cell activity and function into clinical concepts has been challenging and was investigated in a number of clinical trials. Over the past decades, considerable progress has been made in NK cell-based immunotherapies in haploidentical stem cell transplantation (haploSCT) or in the non-transplant setting, since allogeneic NK cells contribute to the graft versus leukemia/tumor effect (GvL/GvT) with generally no or only marginal graft versus host disease (GvHD) compared to allogeneic T cells (118–120). There are several sources for NK cells. They can be obtained from (i) healthy donors via leukapheresis followed by immunomagnetic purification (CD3-depleted, CD56-enriched), (ii) cord blood or (iii) induced pluripotent stem cell (iPSC) and administered unstimulated or cytokine-activated and expanded, respectively. After the first clinical trials in 2004 and 2005 using IL-2 activated donor NK cells, performed in parallel in Europe and the USA (121, 122), multiple clinical trials over the last 1.5 decades showed safety and feasibility of adoptive NK cell transfer for various hematological and oncological diseases, respectively (123). Despite the overall clinical benefit regarding GvL/GvT effect without GvHD, adoptive NK cell therapies are hampered by tumor immune escape mechanism, such as blocking of NKG2D by soluble MICA (124), exhaustion of NK cells in the immune-suppressive tumor microenvironment (125) and limited persistence of NK cells. In addition to the historical use of IL-2 for both ex vivo expansion of the NK cells during manufacturing and in vivo therapy, stimulation of NK cells with IL-12, IL-15, IL-18 and IL-21 enhanced cytotoxicity, successfully generated donor memory-like NK cells with enhanced persistence and improved anti-leukemia response. This could be demonstrated impressively in 4/8 pediatric patients with AML in a current clinical trial (126). Cytokine combinations are increasingly used for optimized manufacturing protocols (127). Nevertheless, the optimal cytokine cocktail after adoptive NK cell transfer to improve cell expansion remains still unclear. Very recently, it has been shown in clinical trials that systemic IL-15 resulted in reduced clinical activity (128). The authors hypothesized that IL-15 promotes recipients CD8+ T cell activation that finally leads to donor NK cell rejection.
Other trials are using NK cell subpopulations. Especially, cytomegalovirus (CMV) infection is one powerful stimulus promoting the functionality and phenotype of NK cells expressing the HLA-specific activating receptor CD94/NKG2C (129). Therefore, clinical protocols are currently developed based on the mechanisms underlying the generation of adoptive NK cells that involve NKG2C triggering to efficiently expand NKG2C+ NK cells for therapy. Interestingly, adoptive NK cells appear to be resistant to MDSC and Treg suppression thereby providing them with a further advantage compared to CAR T cells for their use as therapeutics. Another benefit is the availability of NK cells for therapy from distinct sources. Multiple other approaches are ongoing to restore NK cell activity and reach long-lasting effects. These include the blockade of inhibitory receptors, blocking soluble activating receptors, combinational therapies with immune checkpoint inhibitors (130) and genetic engineering of the NK cells (131). In addition, cytokine-activated NK cells with upregulated NCRs and NKG2D are partly able to overcome tumor immune escape by restoring NKG2D-mediated NK cell cytotoxicity via scavenging of plasma MICA as demonstrated for neuroblastoma and head and neck cancer, respectively (124, 132).
Engineered NK cell-based immunotherapies
Genetic modification of immune effector cells has been demonstrated to be a promising strategy for the treatment of advanced cancers refractory to conventional therapies. In particular, chimeric antigen receptor (CAR) targeting cell surface antigens provide a suitable tool to increase the efficacy of effector cells. CARs are genetically engineered proteins composed of an extracellular domain specific for the respective/selected target antigen, a transmembrane domain and an intracellular signaling domain responsible for the transduction of the activating signal. During the last two decades, the CAR technology has been developed as next generation immunotherapeutic approach reaching impressive clinical results in two hematological disorders, the acute lymphoblastic leukemia and diffuse large B cell lymphoma. This led to more than 800 clinical trials worldwide (clinicaltrials.gov) (133) as well as to five approved CAR T cell products, the first four targeting CD19 and the last one directed against BCMA, respectively (134). In a similar way, engineered CAR NK cells redirected against several cancer epitopes including hematological and tumor targets resulted in improved NK cell cytotoxicity (135–138). Moreover, in addition to use the intracellular CAR T cell signaling, DAP10 and DAP12 give rise for more improvement for CAR NK cell cytotoxicity (139). There is a clear advantage of CAR NK cells over CAR T cells, since NK cells can be obtained from allogeneic donors, do not induce a cytokine storm, persist for more than one year and can be applied to the patients without development of GvHD and thus represent an “off the shelf” product for the treatment of patients (105, 137, 140). Another challenge is to overcome the high manufacturing costs of personalized autologous CAR T cell products by using one allogeneic CAR NK cell product for multiple applications in various patients. To date, more than 35 clinical trials using CAR NK cells are conducted (clinicaltrials.gov) against several cancer epitopes, such as CD19, CD19/22, CD33, CD7, HER2, MUC1, PDL1, NKG2D ligand, BCMA, ROBO1, PSMA, mesothelin and others using CAR NK cells from different sources, primary human NK cells, cord-blood derived and iPSC-derived NK cells as well as CARs from the cell line NK92 (141, 142).
While most of the trials are performed in China and USA, currently a phase I trial, CAR2BRAIN using lentiviral transduced CAR NK92 cells redirected against the human epidermal growth factor 2 (HER2, ErbB2) for treatment of recurrent patients with glioblastoma is conducted in Europe, in Frankfurt, Germany. The very well recognized study of Katy Rezvani, USA, employed cord-blood derived CAR NK cells redirected against CD19 for B cell malignancies. The promising results showed clinical responses in 8 out of 11 patients with no sign of cytokine release syndrome or neurotoxicity (143). Next to conventional CARs, additional genetic modifications are currently explored to enhance NK cell activity and homing into the tumor. Preclinical studies demonstrated an improvement of tumor cell infiltration through transgene expression of chemokine or adhesion receptors (144). Furthermore, the integration of the autocrine growth factor IL-15 as a down-stream cassette has been used, which led to an improved life span and persistence of those CAR NK cells in all patients (145). The overall cytokine and chemokine profile clearly differ between CAR T and CAR NK cells and supports the observation that allogeneic CAR NK cells do not contribute to any severe side effects, like cytokine release syndrome and toxicity. Nevertheless, NK cells are considered hard-to-engineer and hard-to expand compared to T cells. Recently, a novel viral envelope derived from the baboon endogenous virus (BaEV) showed superior efficacy as compared to other lentiviral envelope proteins to successfully genetically manipulate human NK cells (146, 147).
Finally, the question arises how to further improve both anti-tumoral activity, cytotoxicity and homing of CAR NK cells in the TME, which led to combinational therapies with ICPis. In the end of 2021, two clinical trials were started: (i) a phase II study using irradiated PD-L1 CAR-NK cells plus pembrolizumab for recurrent/metastatic gastric or head and neck cancer (NCT04847466) and (ii) FT576 (iPSC derived CAR NK cells) as monotherapy and in combination with daratumumab in subjects with relapsed/refractory multiple myeloma (NCT05182073), respectively.
Conclusions
The enhancement of NK cell activity represents an important approach to control cancer growth. The increased understanding of the NK cell biology has led to the development of NK cell-based strategies to control tumors. New ways to enhance the NK cell targeting, their activation and cytolytic function are required, since the NK cells are becoming dysfunctional in the immune suppressive TME. Despite the potential of NK cell-based therapies it has become obvious that for the design of effective strategies using NK cells in the clinics, a detailed knowledge of NK cell receptors, NK cell subpopulations, tissue-specific NK cells and memory-like NK cells is required. Furthermore, the NK cell heterogeneity might influence the efficacy of NK cell-based therapies. Some preclinical and clinical studies suggest multifaceted opportunities of the implementation of NK cells for the treatment of cancer patients using combination therapies, which will lead to further clinical advances.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
Ab, antibody; ADCC, antibody-dependent cell-mediated cytotoxicity; ALL, acute lymphoblastic leukemia; BM, bone marrow; CAR; chimeric antigen receptor; CLL, chronic lymphocytic leukemia; CMV, cytomegalovirus; CR, complete revision; DC, dendritic cell; DFS, disease free survival; ECM, extracellular matrix; EGF-R, epidermal growth factor receptor; EV, extracellular vesicle; FC, flow cytometry; FDA, Food and Drug Administration; G-CSF, granulocyte-stimulating factor; GM-CSF, granulocyte-macrophage stimulating factor; GMP, good medical practice; Had, graft-versus-host disease; HLA, human leukocyte antigen; HSC, hematopoietic stem cells; HSCT, hematopoietic stem cell transplantation; ICPi, immune checkpoint inhibitor; IDO, indolamine 2, 3-deoxygenase; IFN, interferon; IL, interleukin; iPSC, induced pluripotent stem cell; KIR, killer cell immunoglobulin-like receptors; LAG, lymphocyte-activation gene; mAb, monoclonal antibody; MDSC, myeloid-derived suppressor cell; MHC, major histocompatibility complex; MIC, MHC class I-related; MMP, matrix metalloproteinase; NCR, natural killer receptor; NK, natural killer; OS, overall survival; PBMNC, peripheral blood mononuclear cell; PD1, programmed death; PD-L1, programmed death ligand 1; RNA-seq, RNA-sequencing; TAM, tumor-associated macrophages; TCR, T cell receptor; TGF-β, transforming growth factor β; TIGIT, T cell immunoglobulin and immunoreceptor tyrosine-based inhibitory motif; TMB, tumor mutational burden; TME, tumor microenvironment; TNF; tumor necrosis factor; TRAIL, TNF-related apoptosis inducing ligand; Treg, regulatory T cell; ULBP, UL-16 binding protein; VEGF, vascular endothelial growth factor
Glossary
References
1. Kiessling R, Klein E, Wigzell H. "Natural" killer cells in the mouse. i. cytotoxic cells with specificity for mouse moloney leukemia cells. specificity and distribution according to genotype. Eur J Immunol (1975) 5(2):112–7. doi: 10.1002/eji.1830050208
2. Montaldo E, Del Zotto G, Della Chiesa M, Mingari MC, Moretta A, De Maria A, et al. Human NK cell receptors/markers: a tool to analyze NK cell development, subsets and function. Cytometry A (2013) 83(8):702–13. doi: 10.1002/cyto.a.22302
3. Cherrier DE, Serafini N, Di Santo JP. Innate lymphoid cell development: A T cell perspective. Immunity (2018) 48(6):1091–103. doi: 10.1016/j.immuni.2018.05.010
4. Cantoni C, Wurzer H, Thomas C, Vitale M. Escape of tumor cells from the NK cell cytotoxic activity. J Leukoc Biol (2020) 108(4):1339–60. doi: 10.1002/JLB.2MR0820-652R
5. Voskoboinik I, Whisstock JC, Trapani JA. Perforin and granzymes: function, dysfunction and human pathology. Nat Rev Immunol (2015) 15(6):388–400. doi: 10.1038/nri3839
6. Takeda K, Hayakawa Y, Smyth MJ, Kayagaki N, Yamaguchi N, Kakuta S, et al. Involvement of tumor necrosis factor-related apoptosis-inducing ligand in surveillance of tumor metastasis by liver natural killer cells. Nat Med (2001) 7(1):94–100. doi: 10.1038/83416
7. Della Chiesa M, Marcenaro E, Sivori S, Carlomagno S, Pesce S, Moretta A. Human NK cell response to pathogens. Semin Immunol (2014) 26(2):152–60. doi: 10.1016/j.smim.2014.02.001
8. Romagnani C, Juelke K, Falco M, Morandi B, D'Agostino A, Costa R, et al. CD56brightCD16- killer ig-like receptor- NK cells display longer telomeres and acquire features of CD56dim NK cells upon activation. J Immunol (2007) 178(8):4947–55. doi: 10.4049/jimmunol.178.8.4947
9. Del Zotto G, Marcenaro E, Vacca P, Sivori S, Pende D, Della Chiesa M, et al. Markers and function of human NK cells in normal and pathological conditions. Cytometry B Clin Cytom (2017) 92(2):100–14. doi: 10.1002/cyto.b.21508
10. Kobyzeva PA, Streltsova MA, Erokhina SA, Kanevskiy LM, Telford WG, Sapozhnikov AM, et al. CD56(dim) CD57(-) NKG2C(+) NK cells retaining proliferative potential are possible precursors of CD57(+) NKG2C(+) memory-like NK cells. J Leukoc Biol (2020) 108(4):1379–95. doi: 10.1002/JLB.1MA0720-654RR
11. O'Sullivan TE, Sun JC, Lanier LL. Natural killer cell memory. Immunity (2015) 43(4):634–45. doi: 10.1016/j.immuni.2015.09.013
12. Lam VC, Lanier LL. NK cells in host responses to viral infections. Curr Opin Immunol (2017) 44:43–51. doi: 10.1016/j.coi.2016.11.003
13. Horowitz A, Strauss-Albee DM, Leipold M, Kubo J, Nemat-Gorgani N, Dogan OC, et al. Genetic and environmental determinants of human NK cell diversity revealed by mass cytometry. Sci Transl Med (2013) 5(208):208ra145. doi: 10.1126/scitranslmed.3006702
14. Wu SY, Fu T, Jiang YZ, Shao ZM. Natural killer cells in cancer biology and therapy. Mol Cancer (2020) 19(1):120. doi: 10.1158/1557-3125.HIPPO19-IA12
15. Crinier A, Milpied P, Escaliere B, Piperoglou C, Galluso J, Balsamo A, et al. High-dimensional single-cell analysis identifies organ-specific signatures and conserved NK cell subsets in humans and mice. Immunity (2018) 49(5):971–86.e5. doi: 10.1016/j.immuni.2018.09.009
16. Jameson G, Robinson MW. Insights into human intrahepatic NK cell function from single cell RNA sequencing datasets. Front Immunol (2021) 12:649311. doi: 10.3389/fimmu.2021.649311
17. Smith SL, Kennedy PR, Stacey KB, Worboys JD, Yarwood A, Seo S, et al. Diversity of peripheral blood human NK cells identified by single-cell RNA sequencing. Blood Adv (2020) 4(7):1388–406. doi: 10.1182/bloodadvances.2019000699
18. Dogra P, Rancan C, Ma W, Toth M, Senda T, Carpenter DJ, et al. Tissue determinants of human NK cell development, function, and residence. Cell (2020) 180(4):749–63.e13. doi: 10.1016/j.cell.2020.01.022
19. Martinet L, Smyth MJ. Balancing natural killer cell activation through paired receptors. Nat Rev Immunol (2015) 15(4):243–54. doi: 10.1038/nri3799
20. Sivori S, Vacca P, Del Zotto G, Munari E, Mingari MC, Moretta L. Human NK cells: surface receptors, inhibitory checkpoints, and translational applications. Cell Mol Immunol (2019) 16(5):430–41. doi: 10.1038/s41423-019-0206-4
21. Sivori S, Della Chiesa M, Carlomagno S, Quatrini L, Munari E, Vacca P, et al. Inhibitory receptors and checkpoints in human NK cells, implications for the immunotherapy of cancer. Front Immunol (2020) 11:2156. doi: 10.3389/fimmu.2020.02156
22. Zhang C, Liu Y. Targeting NK cell checkpoint receptors or molecules for cancer immunotherapy. Front Immunol (2020) 11:1295. doi: 10.3389/fimmu.2020.01295
23. Manion BA. Acute renal failure secondary to hemorrhagic compartment syndrome and subsequential rhabdomyolysis. ANNA J (1988) 15(3):188194.
24. Seidel UJ, Schlegel P, Lang P. Natural killer cell mediated antibody-dependent cellular cytotoxicity in tumor immunotherapy with therapeutic antibodies. Front Immunol (2013) 4:76. doi: 10.3389/fimmu.2013.00076
25. Lanier LL. NKG2D receptor and its ligands in host defense. Cancer Immunol Res (2015) 3(6):575–82. doi: 10.1158/2326-6066.CIR-15-0098
26. Sivori S, Carlomagno S, Pesce S, Moretta A, Vitale M, Marcenaro E. TLR/NCR/KIR: Which one to use and when? Front Immunol (2014) 5:105. doi: 10.3389/fimmu.2014.00105
27. Barrow AD, Martin CJ, Colonna M. The natural cytotoxicity receptors in health and disease. Front Immunol (2019) 10:909. doi: 10.3389/fimmu.2019.00909
28. Tan S, Xu Y, Wang Z, Wang T, Du X, Song X, et al. Tim-3 hampers tumor surveillance of liver-resident and conventional NK cells by disrupting PI3K signaling. Cancer Res (2020) 80(5):1130–42. doi: 10.1158/0008-5472.CAN-19-2332
29. Meng F, Li L, Lu F, Yue J, Liu Z, Zhang W, et al. Overexpression of TIGIT in NK and T cells contributes to tumor immune escape in myelodysplastic syndromes. Front Oncol (2020) 10:1595. doi: 10.3389/fonc.2020.01595
30. Dougall WC, Kurtulus S, Smyth MJ, Anderson AC. TIGIT and CD96: new checkpoint receptor targets for cancer immunotherapy. Immunol Rev (2017) 276(1):112–20. doi: 10.1111/imr.12518
31. Cozar B, Greppi M, Carpentier S, Narni-Mancinelli E, Chiossone L, Vivier E. Tumor-infiltrating natural killer cells. Cancer Discovery (2021) 11(1):34–44. doi: 10.1158/2159-8290.CD-20-0655
32. Shibuya A, Shibuya K. DNAM-1 versus TIGIT: competitive roles in tumor immunity and inflammatory responses. Int Immunol (2021) 33(12):687–92. doi: 10.1093/intimm/dxab085
33. Ljunggren HG, Karre K. In search of the 'missing self': MHC molecules and NK cell recognition. Immunol Today (1990) 11(7):237–44. doi: 10.1016/0167-5699(90)90097-S
34. Diefenbach A, Raulet DH. Strategies for target cell recognition by natural killer cells. Immunol Rev (2001) 181:170–84. doi: 10.1034/j.1600-065X.2001.1810114.x
35. Rabinovich BA, Li J, Shannon J, Hurren R, Chalupny J, Cosman D, et al. Activated, but not resting, T cells can be recognized and killed by syngeneic NK cells. J Immunol (2003) 170(7):3572–6. doi: 10.4049/jimmunol.170.7.3572
36. Bellora F, Castriconi R, Dondero A, Reggiardo G, Moretta L, Mantovani A, et al. The interaction of human natural killer cells with either unpolarized or polarized macrophages results in different functional outcomes. Proc Natl Acad Sci U.S.A. (2010) 107(50):21659–64. doi: 10.1073/pnas.1007654108
37. Ferlazzo G, Morandi B. Cross-talks between natural killer cells and distinct subsets of dendritic cells. Front Immunol (2014) 5:159. doi: 10.3389/fimmu.2014.00159
38. Zhou J, Zhang S, Guo C. Crosstalk between macrophages and natural killer cells in the tumor microenvironment. Int Immunopharmacol (2021) 101(Pt B):108374. doi: 10.1016/j.intimp.2021.108374
39. Russo E, Laffranchi M, Tomaipitinca L, Del Prete A, Santoni A, Sozzani S, et al. NK cell anti-tumor surveillance in a myeloid cell-shaped environment. Front Immunol (2021) 12:787116. doi: 10.3389/fimmu.2021.787116
40. Ferlazzo G, Tsang ML, Moretta L, Melioli G, Steinman RM, Munz C. Human dendritic cells activate resting natural killer (NK) cells and are recognized via the NKp30 receptor by activated NK cells. J Exp Med (2002) 195(3):343–51. doi: 10.1084/jem.20011149
41. Prager I, Watzl C. Mechanisms of natural killer cell-mediated cellular cytotoxicity. J Leukoc Biol (2019) 105(6):1319–29. doi: 10.1002/JLB.MR0718-269R
42. Dewan MZ, Terunuma H, Takada M, Tanaka Y, Abe H, Sata T, et al. Role of natural killer cells in hormone-independent rapid tumor formation and spontaneous metastasis of breast cancer cells in vivo. Breast Cancer Res Treat (2007) 104(3):267–75. doi: 10.1007/s10549-006-9416-4
43. Yokoyama WM, Kim S. How do natural killer cells find self to achieve tolerance? Immunity (2006) 24(3):249–57. doi: 10.1016/j.immuni.2006.03.006
44. Sathe P, Delconte RB, Souza-Fonseca-Guimaraes F, Seillet C, Chopin M, Vandenberg CJ, et al. Innate immunodeficiency following genetic ablation of Mcl1 in natural killer cells. Nat Commun (2014) 5:4539. doi: 10.1038/ncomms5539
45. Maurer S, Ferrari de Andrade L. NK cell interaction with platelets and myeloid cells in the tumor milieu. Front Immunol (2020) 11:608849. doi: 10.3389/fimmu.2020.608849
46. Deng W, Gowen BG, Zhang L, Wang L, Lau S, Iannello A, et al. Antitumor immunity. a shed NKG2D ligand that promotes natural killer cell activation and tumor rejection. Science (2015) 348(6230):136–9. doi: 10.1126/science.1258867
47. Saxena S, Singh RK. Chemokines orchestrate tumor cells and the microenvironment to achieve metastatic heterogeneity. Cancer Metastasis Rev (2021) 40(2):447–76. doi: 10.1007/s10555-021-09970-6
48. Kaymak I, Williams KS, Cantor JR, Jones RG. Immunometabolic interplay in the tumor microenvironment. Cancer Cell (2021) 39(1):28–37. doi: 10.1016/j.ccell.2020.09.004
49. Morvan MG, Lanier LL. NK cells and cancer: you can teach innate cells new tricks. Nat Rev Cancer (2016) 16(1):7–19. doi: 10.1038/nrc.2015.5
50. Carrega P, Bonaccorsi I, Di Carlo E, Morandi B, Paul P, Rizzello V, et al. CD56(bright)perforin(low) noncytotoxic human NK cells are abundant in both healthy and neoplastic solid tissues and recirculate to secondary lymphoid organs via afferent lymph. J Immunol (2014) 192(8):3805–15. doi: 10.4049/jimmunol.1301889
51. Gregoire C, Chasson L, Luci C, Tomasello E, Geissmann F, Vivier E, et al. The trafficking of natural killer cells. Immunol Rev (2007) 220:169–82. doi: 10.1111/j.1600-065X.2007.00563.x
52. Chiu J, Ernst DM, Keating A. Acquired natural killer cell dysfunction in the tumor microenvironment of classic Hodgkin lymphoma. Front Immunol (2018) 9:267. doi: 10.3389/fimmu.2018.00267
53. Chan IS, Knutsdottir H, Ramakrishnan G, Padmanaban V, Warrier M, Ramirez JC, et al. Cancer cells educate natural killer cells to a metastasis-promoting cell state. J Cell Biol (2020) 219(9) 219(9):e202001134. doi: 10.1083/jcb.202001134
54. Versluis MAC, Marchal S, Plat A, de Bock GH, van Hall T, de Bruyn M, et al. The prognostic benefit of tumour-infiltrating natural killer cells in endometrial cancer is dependent on concurrent overexpression of human leucocyte antigen-e in the tumour microenvironment. Eur J Cancer (2017) 86:285–95. doi: 10.1016/j.ejca.2017.09.008
55. Ziblat A, Iraolagoitia XLR, Nunez SY, Torres NI, Secchiari F, Sierra JM, et al. Circulating and tumor-infiltrating NK cells from clear cell renal cell carcinoma patients exhibit a predominantly inhibitory phenotype characterized by overexpression of CD85j, CD45, CD48 and PD-1. Front Immunol (2021) 12:681615. doi: 10.3389/fimmu.2021.681615
56. Guillerey C. NK cells in the tumor microenvironment. Adv Exp Med Biol (2020) 1273:69–90. doi: 10.1007/978-3-030-49270-0_4
57. Mamessier E, Sylvain A, Thibult ML, Houvenaeghel G, Jacquemier J, Castellano R, et al. Human breast cancer cells enhance self tolerance by promoting evasion from NK cell antitumor immunity. J Clin Invest (2011) 121(9):3609–22. doi: 10.1172/JCI45816
58. Bouzidi L, Triki H, Charfi S, Kridis WB, Derbel M, Ayadi L, et al. Prognostic value of natural killer cells besides tumor-infiltrating lymphocytes in breast cancer tissues. Clin Breast Cancer (2021) 21(6):e738–47. doi: 10.1016/j.clbc.2021.02.003
59. Halama N, Braun M, Kahlert C, Spille A, Quack C, Rahbari N, et al. Natural killer cells are scarce in colorectal carcinoma tissue despite high levels of chemokines and cytokines. Clin Cancer Res (2011) 17(4):678–89. doi: 10.1158/1078-0432.CCR-10-2173
60. Gulubova M, Manolova I, Kyurkchiev D, Julianov A, Altunkova I. Decrease in intrahepatic CD56+ lymphocytes in gastric and colorectal cancer patients with liver metastases. APMIS (2009) 117(12):870–9. doi: 10.1111/j.1600-0463.2009.02547.x
61. Degos C, Heinemann M, Barrou J, Boucherit N, Lambaudie E, Savina A, et al. Endometrial tumor microenvironment alters human NK cell recruitment, and resident NK cell phenotype and function. Front Immunol (2019) 10:877. doi: 10.3389/fimmu.2019.00877
62. Izawa S, Kono K, Mimura K, Kawaguchi Y, Watanabe M, Maruyama T, et al. H(2)O(2) production within tumor microenvironment inversely correlated with infiltration of CD56(dim) NK cells in gastric and esophageal cancer: possible mechanisms of NK cell dysfunction. Cancer Immunol Immunother (2011) 60(12):1801–10. doi: 10.1007/s00262-011-1082-7
63. Ali TH, Pisanti S, Ciaglia E, Mortarini R, Anichini A, Garofalo C, et al. Enrichment of CD56(dim)KIR + CD57 + highly cytotoxic NK cells in tumour-infiltrated lymph nodes of melanoma patients. Nat Commun (2014) 5:5639. doi: 10.1038/ncomms6639
64. Platonova S, Cherfils-Vicini J, Damotte D, Crozet L, Vieillard V, Validire P, et al. Profound coordinated alterations of intratumoral NK cell phenotype and function in lung carcinoma. Cancer Res (2011) 71(16):5412–22. doi: 10.1158/0008-5472.CAN-10-4179
65. Esendagli G, Bruderek K, Goldmann T, Busche A, Branscheid D, Vollmer E, et al. Malignant and non-malignant lung tissue areas are differentially populated by natural killer cells and regulatory T cells in non-small cell lung cancer. Lung Cancer (2008) 59(1):32–40. doi: 10.1016/j.lungcan.2007.07.022
66. Remark R, Alifano M, Cremer I, Lupo A, Dieu-Nosjean MC, Riquet M, et al. Characteristics and clinical impacts of the immune environments in colorectal and renal cell carcinoma lung metastases: influence of tumor origin. Clin Cancer Res (2013) 19(15):4079–91. doi: 10.1158/1078-0432.CCR-12-3847
67. Takanami I, Takeuchi K, Giga M. The prognostic value of natural killer cell infiltration in resected pulmonary adenocarcinoma. J Thorac Cardiovasc Surg (2001) 121(6):1058–63. doi: 10.1067/mtc.2001.113026
68. Jin S, Deng Y, Hao JW, Li Y, Liu B, Yu Y, et al. NK cell phenotypic modulation in lung cancer environment. PLoS One (2014) 9(10):e109976. doi: 10.1371/journal.pone.0109976
69. Mandal R, Senbabaoglu Y, Desrichard A, Havel JJ, Dalin MG, Riaz N, et al. The head and neck cancer immune landscape and its immunotherapeutic implications. JCI Insight (2016) 1(17):e89829. doi: 10.1172/jci.insight.89829
70. Cursons J, Souza-Fonseca-Guimaraes F, Foroutan M, Anderson A, Hollande F, Hediyeh-Zadeh S, et al. A gene signature predicting natural killer cell infiltration and improved survival in melanoma patients. Cancer Immunol Res (2019) 7(7):1162–74. doi: 10.1158/2326-6066.CIR-18-0500
71. Melero I, Rouzaut A, Motz GT, Coukos G. T-Cell and NK-cell infiltration into solid tumors: a key limiting factor for efficacious cancer immunotherapy. Cancer Discovery (2014) 4(5):522–6. doi: 10.1158/2159-8290.CD-13-0985
72. Camous X, Pera A, Solana R, Larbi A. NK cells in healthy aging and age-associated diseases. J BioMed Biotechnol (2012) 2012:195956. doi: 10.1155/2012/195956
73. Krneta T, Gillgrass A, Chew M, Ashkar AA. The breast tumor microenvironment alters the phenotype and function of natural killer cells. Cell Mol Immunol (2016) 13(5):628–39. doi: 10.1038/cmi.2015.42
74. Parisi L, Bassani B, Tremolati M, Gini E, Farronato G, Bruno A. Natural killer cells in the orchestration of chronic inflammatory diseases. J Immunol Res (2017) 2017:4218254. doi: 10.1155/2017/4218254
75. Mantovani S, Varchetta S, Mele D, Donadon M, Torzilli G, Soldani C, et al. An anti-MICA/B antibody and IL-15 rescue altered NKG2D-dependent NK cell responses in hepatocellular carcinoma. Cancers (Basel) (2020) 12(12):3583. doi: 10.3390/cancers12123583
76. Easom NJW, Stegmann KA, Swadling L, Pallett LJ, Burton AR, Odera D, et al. IL-15 overcomes hepatocellular carcinoma-induced NK cell dysfunction. Front Immunol (2018) 9:1009. doi: 10.3389/fimmu.2018.01009
77. Morimoto T, Nakazawa T, Matsuda R, Nishimura F, Nakamura M, Yamada S, et al. Evaluation of comprehensive gene expression and NK cell-mediated killing in glioblastoma cell line-derived spheroids. Cancers (Basel) (2021) 13(19):4896. doi: 10.3390/cancers13194896
78. Glasner A, Ghadially H, Gur C, Stanietsky N, Tsukerman P, Enk J, et al. Recognition and prevention of tumor metastasis by the NK receptor NKp46/NCR1. J Immunol (2012) 188(6):2509–15. doi: 10.4049/jimmunol.1102461
79. Dhar P, Wu JD. NKG2D and its ligands in cancer. Curr Opin Immunol (2018) 51:55–61. doi: 10.1016/j.coi.2018.02.004
80. Hu Z, Xu X, Wei H. The adverse impact of tumor microenvironment on NK-cell. Front Immunol (2021) 12:633361. doi: 10.3389/fimmu.2021.633361
81. He Y, Bunn PA, Zhou C, Chan D. KIR 2D (L1, L3, L4, S4) and KIR 3DL1 protein expression in non-small cell lung cancer. Oncotarget (2016) 7(50):82104–11. doi: 10.18632/oncotarget.13486
82. Pesce S, Greppi M, Grossi F, Del Zotto G, Moretta L, Sivori S, et al. PD/1-PD-Ls checkpoint: Insight on the potential role of NK cells. Front Immunol (2019) 10:1242. doi: 10.3389/fimmu.2019.01242
83. Yu L, Liu X, Wang X, Yan F, Wang P, Jiang Y, et al. TIGIT(+) TIM-3(+) NK cells are correlated with NK cell exhaustion and disease progression in patients with hepatitis b virusrelated hepatocellular carcinoma. Oncoimmunology (2021) 10(1):1942673. doi: 10.1080/2162402X.2021.1942673
84. Zheng X, Qian Y, Fu B, Jiao D, Jiang Y, Chen P, et al. Mitochondrial fragmentation limits NK cell-based tumor immunosurveillance. Nat Immunol (2019) 20(12):1656–67. doi: 10.1038/s41590-019-0511-1
85. Al Absi A, Wurzer H, Guerin C, Hoffmann C, Moreau F, Mao X, et al. Actin cytoskeleton remodeling drives breast cancer cell escape from natural killer-mediated cytotoxicity. Cancer Res (2018) 78(19):5631–43. doi: 10.1158/0008-5472.CAN-18-0441
86. Castriconi R, Carrega P, Dondero A, Bellora F, Casu B, Regis S, et al. Molecular mechanisms directing migration and retention of natural killer cells in human tissues. Front Immunol (2018) 9:2324. doi: 10.3389/fimmu.2018.02324
87. Huber V, Camisaschi C, Berzi A, Ferro S, Lugini L, Triulzi T, et al. Cancer acidity: An ultimate frontier of tumor immune escape and a novel target of immunomodulation. Semin Cancer Biol (2017) 43:74–89. doi: 10.1016/j.semcancer.2017.03.001
88. Palazon A, Goldrath AW, Nizet V, Johnson RS. HIF transcription factors, inflammation, and immunity. Immunity (2014) 41(4):518–28. doi: 10.1016/j.immuni.2014.09.008
89. Ni J, Wang X, Stojanovic A, Zhang Q, Wincher M, Buhler L, et al. Single-cell RNA sequencing of tumor-infiltrating NK cells reveals that inhibition of transcription factor HIF-1alpha unleashes NK cell activity. Immunity (2020) 52(6):1075–87.e8. doi: 10.1016/j.immuni.2020.05.001
90. Albini A, Bruno A, Noonan DM, Mortara L. Contribution to tumor angiogenesis from innate immune cells within the tumor microenvironment: Implications for immunotherapy. Front Immunol (2018) 9:527. doi: 10.3389/fimmu.2018.00527
91. Bassani B, Baci D, Gallazzi M, Poggi A, Bruno A, Mortara L. Natural killer cells as key players of tumor progression and angiogenesis: Old and novel tools to divert their pro-tumor activities into potent anti-tumor effects. Cancers (Basel) (2019) 11(4):461. doi: 10.3390/cancers11040461
92. Thompson TW, Kim AB, Li PJ, Wang J, Jackson BT, Huang KTH, et al. Endothelial cells express NKG2D ligands and desensitize antitumor NK responses. Elife (2017) 6:e30881. doi: 10.7554/eLife.30881
93. Elkabets M, Ribeiro VS, Dinarello CA, Ostrand-Rosenberg S, Di Santo JP, Apte RN, et al. IL-1beta regulates a novel myeloid-derived suppressor cell subset that impairs NK cell development and function. Eur J Immunol (2010) 40(12):3347–57. doi: 10.1002/eji.201041037
94. Sitrin J, Ring A, Garcia KC, Benoist C, Mathis D. Regulatory T cells control NK cells in an insulitic lesion by depriving them of IL-2. J Exp Med (2013) 210(6):1153–65. doi: 10.1084/jem.20122248
95. Krneta T, Gillgrass A, Poznanski S, Chew M, Lee AJ, Kolb M, et al. M2-polarized and tumor-associated macrophages alter NK cell phenotype and function in a contact-dependent manner. J Leukoc Biol (2017) 101(1):285–95. doi: 10.1189/jlb.3A1215-552R
96. Brownlie D, Doughty-Shenton D, Yh Soong D, Nixon C, OC N, MC L, et al. Metastasis-associated macrophages constrain antitumor capability of natural killer cells in the metastatic site at least partially by membrane bound transforming growth factor beta. J Immunother Cancer (2021) 9(1):e001740. doi: 10.1136/jitc-2020-001740
97. Allavena P, Giardina G, Bianchi G, Mantovani A. IL-15 is chemotactic for natural killer cells and stimulates their adhesion to vascular endothelium. J Leukoc Biol (1997) 61(6):729–35. doi: 10.1002/jlb.61.6.729
98. Delconte RB, Kolesnik TB, Dagley LF, Rautela J, Shi W, Putz EM, et al. CIS is a potent checkpoint in NK cell-mediated tumor immunity. Nat Immunol (2016) 17(7):816–24. doi: 10.1038/ni.3470
99. Zhen ZJ, Ling JY, Cai Y, Luo WB, He YJ. Impact of HLA-e gene polymorphism on HLA-e expression in tumor cells and prognosis in patients with stage III colorectal cancer. Med Oncol (2013) 30(1):482. doi: 10.1007/s12032-013-0482-2
100. Borst L, van der Burg SH, van Hall T. The NKG2A-HLA-E axis as a novel checkpoint in the tumor microenvironment. Clin Cancer Res (2020) 26(21):5549–56. doi: 10.1158/1078-0432.CCR-19-2095
101. Liu L, Wang L, Zhao L, He C, Wang G. The role of HLA-G in tumor escape: Manipulating the phenotype and function of immune cells. Front Oncol (2020) 10:597468. doi: 10.3389/fonc.2020.597468
102. Jacquier A, Dumont C, Carosella ED, Rouas-Freiss N, LeMaoult J. Cytometry-based analysis of HLA-G functions according to ILT2 expression. Hum Immunol (2020) 81(4):168–77. doi: 10.1016/j.humimm.2020.02.001
103. Raulet DH, Gasser S, Gowen BG, Deng W, Jung H. Regulation of ligands for the NKG2D activating receptor. Annu Rev Immunol (2013) 31:413–41. doi: 10.1146/annurev-immunol-032712-095951
104. Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, et al. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science (1999) 285(5428):727–9. doi: 10.1126/science.285.5428.727
105. Myers JA, Miller JS. Exploring the NK cell platform for cancer immunotherapy. Nat Rev Clin Oncol (2021) 18(2):85–100. doi: 10.1038/s41571-020-0426-7
106. Maskalenko NA, Zhigarev D, Campbell KS. Harnessing natural killer cells for cancer immunotherapy: dispatching the first responders. Nat Rev Drug Discovery (2022). doi: 10.1038/s41573-022-00413-7
107. Andre P, Denis C, Soulas C, Bourbon-Caillet C, Lopez J, Arnoux T, et al. Anti-NKG2A mAb is a checkpoint inhibitor that promotes anti-tumor immunity by unleashing both T and NK cells. Cell (2018) 175(7):1731–43.e13. doi: 10.1016/j.cell.2018.10.014
108. Pesce S, Greppi M, Tabellini G, Rampinelli F, Parolini S, Olive D, et al. Identification of a subset of human natural killer cells expressing high levels of programmed death 1: A phenotypic and functional characterization. J Allergy Clin Immunol (2017) 139(1):335–46.e3. doi: 10.1016/j.jaci.2016.04.025
109. Vari F, Arpon D, Keane C, Hertzberg MS, Talaulikar D, Jain S, et al. Immune evasion via PD-1/PD-L1 on NK cells and monocyte/macrophages is more prominent in Hodgkin lymphoma than DLBCL. Blood (2018) 131(16):1809–19. doi: 10.1182/blood-2017-07-796342
110. Vidard L, Dureuil C, Baudhuin J, Vescovi L, Durand L, Sierra V, et al. CD137 (4-1BB) engagement fine-tunes synergistic IL-15- and IL-21-Driven NK cell proliferation. J Immunol (2019) 203(3):676–85. doi: 10.4049/jimmunol.1801137
111. Lin W, Voskens CJ, Zhang X, Schindler DG, Wood A, Burch E, et al. Fc-dependent expression of CD137 on human NK cells: insights into "agonistic" effects of anti-CD137 monoclonal antibodies. Blood (2008) 112(3):699–707. doi: 10.1182/blood-2007-11-122465
112. Etxeberria I, Glez-Vaz J, Teijeira A, Melero I. New emerging targets in cancer immunotherapy: CD137/4-1BB costimulatory axis. ESMO Open (2020) 4(Suppl 3):e000733. doi: 10.1136/esmoopen-2020-000733
113. Judge SJ, Darrow MA, Thorpe SW, Gingrich AA, O'Donnell EF, Bellini AR, et al. Analysis of tumor-infiltrating NK and T cells highlights IL-15 stimulation and TIGIT blockade as a combination immunotherapy strategy for soft tissue sarcomas. J Immunother Cancer (2020) 8(2):e001355. doi: 10.1136/jitc-2020-001355
114. Zhang Q, Bi J, Zheng X, Chen Y, Wang H, Wu W, et al. Blockade of the checkpoint receptor TIGIT prevents NK cell exhaustion and elicits potent anti-tumor immunity. Nat Immunol (2018) 19(7):723–32. doi: 10.1038/s41590-018-0132-0
115. Okumura G, Iguchi-Manaka A, Murata R, Yamashita-Kanemaru Y, Shibuya A, Shibuya K. Tumor-derived soluble CD155 inhibits DNAM-1-mediated antitumor activity of natural killer cells. J Exp Med (2020) 217(4):1. doi: 10.1084/jem.20191290
116. Shaim H, Shanley M, Basar R, Daher M, Gumin J, Zamler DB, et al. Targeting the alphav integrin/TGF-beta axis improves natural killer cell function against glioblastoma stem cells. J Clin Invest (2021) 131(14):e142116. doi: 10.1172/JCI142116
117. Ferrari de Andrade L, Tay RE, Pan D, Luoma AM, Ito Y, Badrinath S, et al. Antibody-mediated inhibition of MICA and MICB shedding promotes NK cell-driven tumor immunity. Science (2018) 359(6383):1537–42. doi: 10.1126/science.aao0505
118. Olson JA, Leveson-Gower DB, Gill S, Baker J, Beilhack A, Negrin RS, et al. NK cells mediate reduction of GVHD by inhibiting activated, alloreactive T cells while retaining GVT effects. Blood (2010) 115(21):4293–301. doi: 10.1182/blood-2009-05-222190
119. Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science (2002) 295(5562):2097–100. doi: 10.1126/science.1068440
120. Velardi A, Ruggeri L, Mancusi A, Aversa F, Christiansen FT. Natural killer cell allorecognition of missing self in allogeneic hematopoietic transplantation: a tool for immunotherapy of leukemia. Curr Opin Immunol (2009) 21(5):525–30. doi: 10.1016/j.coi.2009.07.015
121. Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood (2005) 105(8):3051–7. doi: 10.1182/blood-2004-07-2974
122. Koehl U, Sorensen J, Esser R, Zimmermann S, Gruttner HP, Tonn T, et al. IL-2 activated NK cell immunotherapy of three children after haploidentical stem cell transplantation. Blood Cells Mol Dis (2004) 33(3):261–6. doi: 10.1016/j.bcmd.2004.08.013
123. Koehl U, Kalberer C, Spanholtz J, Lee DA, Miller JS, Cooley S, et al. Advances in clinical NK cell studies: Donor selection, manufacturing and quality control. Oncoimmunology (2016) 5(4):e1115178. doi: 10.1080/2162402X.2015.1115178
124. Kloess S, Huenecke S, Piechulek D, Esser R, Koch J, Brehm C, et al. IL-2-activated haploidentical NK cells restore NKG2D-mediated NK-cell cytotoxicity in neuroblastoma patients by scavenging of plasma MICA. Eur J Immunol (2010) 40(11):3255–67. doi: 10.1002/eji.201040568
125. Merino AM, Kim H, Miller JS, Cichocki F. Unraveling exhaustion in adaptive and conventional NK cells. J Leukoc Biol (2020) 108(4):1361–8. doi: 10.1002/JLB.4MR0620-091R
126. Bednarski JJ, Zimmerman C, Berrien-Elliott MM, Foltz JA, Becker-Hapak M, Neal CC, et al. Donor memory-like NK cells persist and induce remissions in pediatric patients with relapsed AML after transplant. Blood (2022) 139(11):1670–83. doi: 10.1182/blood.2021013972
127. Granzin M, Wagner J, Kohl U, Cerwenka A, Huppert V, Ullrich E. Shaping of natural killer cell antitumor activity by ex vivo cultivation. Front Immunol (2017) 8:458. doi: 10.3389/fimmu.2017.00458
128. Berrien-Elliott MM, Becker-Hapak M, Cashen AF, Jacobs M, Wong P, Foster M, et al. Systemic IL-15 promotes allogeneic cell rejection in patients treated with natural killer cell adoptive therapy. Blood (2022) 139(8):1177–83. doi: 10.1182/blood.2021011532
129. Grutza R, Moskorz W, Senff T, Backer E, Lindemann M, Zimmermann A, et al. NKG2C(pos) NK cells regulate the expansion of cytomegalovirus-specific CD8 T cells. J Immunol (2020) 204(11):2910–7. doi: 10.4049/jimmunol.1901281
130. Chauhan SKS, Koehl U, Kloess S. Harnessing NK cell checkpoint-modulating immunotherapies. Cancers (Basel) (2020) 12(7):1807. doi: 10.3390/cancers12071807
131. Peng X, Chen L, Chen L, Wang B, Wang Y, Zhan X, et al. Chimeric antigen receptor-natural killer cells: Novel insight into immunotherapy for solid tumors (Review). Exp Ther Med (2021) 21(4):340. doi: 10.3892/etm.2021.9771
132. Kloss S, Chambron N, Gardlowski T, Arseniev L, Koch J, Esser R, et al. Increased sMICA and TGFbeta1 levels in HNSCC patients impair NKG2D-dependent functionality of activated NK cells. Oncoimmunology (2015) 4(11):e1055993. doi: 10.1080/2162402X.2015.1055993
133. Moreno-Cortes E, Forero-Forero JV, Lengerke-Diaz PA, Castro JE. Chimeric antigen receptor T cell therapy in oncology - pipeline at a glance: Analysis of the ClinicalTrials.gov database. Crit Rev Oncol Hematol (2021) 159:103239. doi: 10.1016/j.critrevonc.2021.103239
134. Vucinic V, Quaiser A, Luckemeier P, Fricke S, Platzbecker U, Koehl U. Production and application of CAR T cells: Current and future role of Europe. Front Med (Lausanne) (2021) 8:713401. doi: 10.3389/fmed.2021.713401
135. Hosseini M, Habibi Z, Hosseini N, Abdoli S, Rezaei N. Preclinical studies of chimeric antigen receptor-modified natural killer cells in cancer immunotherapy: a review. Expert Opin Biol Ther (2022) 22(3):349–66. doi: 10.1080/14712598.2021.1983539
136. Marofi F, Rahman HS, Thangavelu L, Dorofeev A, Bayas-Morejon F, Shirafkan N, et al. Renaissance of armored immune effector cells, CAR-NK cells, brings the higher hope for successful cancer therapy. Stem Cell Res Ther (2021) 12(1):200. doi: 10.1186/s13287-021-02251-7
137. Teng KY, Mansour AG, Zhu Z, Li Z, Tian L, Ma S, et al. Off-the-Shelf prostate stem cell antigen-directed chimeric antigen receptor natural killer cell therapy to treat pancreatic cancer. Gastroenterology (2022) 162(4):1319–33. doi: 10.1053/j.gastro.2021.12.281
138. Karvouni M, Vidal-Manrique M, Lundqvist A, Alici E. Engineered NK cells against cancer and their potential applications beyond. Front Immunol (2022) 13:825979. doi: 10.3389/fimmu.2022.825979
139. Oberschmidt O, Kloess S, Koehl U. Redirected primary human chimeric antigen receptor natural killer cells as an "Off-the-Shelf immunotherapy" for improvement in cancer treatment. Front Immunol (2017) 8:654. doi: 10.3389/fimmu.2017.00654
140. Morgan MA, Buning H, Sauer M, Schambach A. Use of cell and genome modification technologies to generate improved "Off-the-Shelf" CAR T and CAR NK cells. Front Immunol (2020) 11:1965. doi: 10.3389/fimmu.2020.01965
141. Mitwasi N, Feldmann A, Arndt C, Koristka S, Berndt N, Jureczek J, et al. "UniCAR"-modified off-the-shelf NK-92 cells for targeting of GD2-expressing tumour cells. Sci Rep (2020) 10(1):2141. doi: 10.1038/s41598-020-59082-4
142. Albinger N, Hartmann J, Ullrich E. Current status and perspective of CAR-T and CAR-NK cell therapy trials in Germany. Gene Ther (2021) 28(9):513–27. doi: 10.1038/s41434-021-00246-w
143. Liu E, Marin D, Banerjee P, Macapinlac HA, Thompson P, Basar R, et al. Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors. N Engl J Med (2020) 382(6):545–53. doi: 10.1056/NEJMoa1910607
144. Bonanni V, Sciume G, Santoni A, Bernardini G. Bone marrow NK cells: Origin, distinctive features, and requirements for tissue localization. Front Immunol (2019) 10:1569. doi: 10.3389/fimmu.2019.01569
145. Mantesso S, Geerts D, Spanholtz J, Kucerova L. Genetic engineering of natural killer cells for enhanced antitumor function. Front Immunol (2020) 11:607131. doi: 10.3389/fimmu.2020.607131
146. Bari R, Granzin M, Tsang KS, Roy A, Krueger W, Orentas R, et al. Corrigendum: A distinct subset of highly proliferative and lentiviral vector (LV)-transducible NK cells define a readily engineered subset for adoptive cellular therapy. Front Immunol (2019) 10:2784. doi: 10.3389/fimmu.2019.02784
Keywords: NK cells, immune escape, immunotherapy, tumor, HLA
Citation: Seliger B and Koehl U (2022) Underlying mechanisms of evasion from NK cells as rationale for improvement of NK cell-based immunotherapies. Front. Immunol. 13:910595. doi: 10.3389/fimmu.2022.910595
Received: 01 April 2022; Accepted: 20 July 2022;
Published: 12 August 2022.
Edited by:
Dagmar Stoiber, Karl Landsteiner University of Health Sciences, AustriaReviewed by:
Lorenzo Moretta, Bambino Gesù Children’s Hospital (IRCCS), ItalyMark W. Lowdell, Royal Free London NHS Foundation Trust, United Kingdom
Copyright © 2022 Seliger and Koehl. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Barbara Seliger, YmFyYmFyYS5zZWxpZ2VyQHVrLWhhbGxlLmRl
 Barbara Seliger
Barbara Seliger Ulrike Koehl
Ulrike Koehl