
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Immunol. , 30 June 2022
Sec. Cytokines and Soluble Mediators in Immunity
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.906078
This article is part of the Research Topic Insights into the functional role of extracellular vesicles with specific cell types following infection and inflammation View all 8 articles
 Somayeh Keshtkar1,2,3
Somayeh Keshtkar1,2,3 Saeede Soleimanian4
Saeede Soleimanian4 Maryam Kaviani3
Maryam Kaviani3 Fatemeh Sabet Sarvestani3
Fatemeh Sabet Sarvestani3 Negar Azarpira1,3*
Negar Azarpira1,3* Zahra Asvar5
Zahra Asvar5 Sara Pakbaz6,7
Sara Pakbaz6,7Extracellular Vesicles (EVs) are a collection of vesicles released from cells that play an important role in intercellular communication. Microbial infections are known as one of the major problems in the medical field. Considering the increasing resistance of strains to routine drug treatments, the need for new therapies seems to be more than ever. Recent studies have shown that the EVs released from immune cells during microbial infections had anti-microbial effects or were able to induce neighbouring cells to display anti-microbial effects. This mini-review aimed to explore the latest studies on immune cell-derived EVs in viral, bacterial, fungal, and parasitic infections. Review of the literature demonstrated that specific cargos in EVs were involved in the fight against pathogenic infections. Additionally, the transport of appropriate bioactive molecules including miRNAs, mRNAs, and proteins via EVs could mediate the anti-microbial process. Thus, it could be a proof-of-principle that therapeutic approaches based on EVs derived from immune cells could offer a promising path forward, which is still in early stages and needs further assessments.
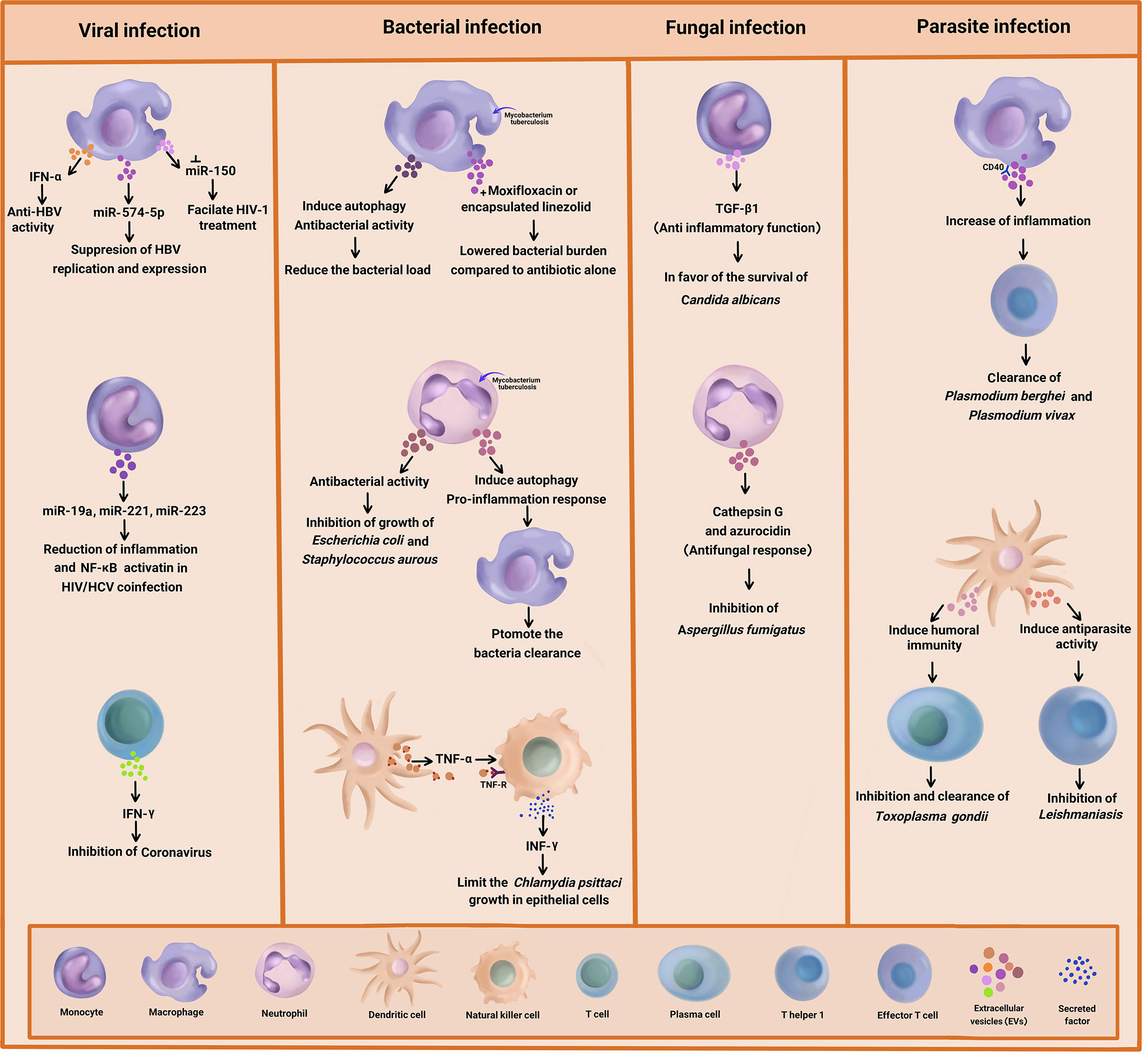
Graphical Abstract Summary of the antimicrobial effect of immune cell-derived extracellular vesicles (EVs) on bacteria, viral, fungal, and parasitic diseases.
The immune system is a complex system consisting of different cell types that reside in multiple organs throughout the body. The connection pathways in the immune system develop by direct contacts between these cells and the release of soluble factors to maintain cellular homeostasis and host defense (1). Raposo et al. introduced a new means of communication based on the release of substances into the extracellular space called Extracellular Vesicles (EVs) (2). More than 50 years ago, Wolf et al. discovered EVs in plasma as “platelet dust” (3). EVs include a heterogeneous group of membrane-bound particles present in all biological fluids. Based on their mechanism of release and size, EVs have been categorized as a) exosomes (diameter < 150 nm), b) microvesicles/shedding particles (150-1000 nm), and c) apoptotic bodies (> 150 nm) (2, 4, 5). In contrast to microvesicles that are secreted by budding from the cell membrane, exosomes originate as intraluminal vesicles in multivesicular endosomes and fuse with the plasma membrane to release into the extracellular space. Exosome biogenesis depends on various critical factors including the site of biogenesis, protein sorting, physicochemical aspects, and transacting mediators. Endosomal Sorting Complex Required for Transport (ESCRT), exosome-bound proteins, annexins, and Rab protein govern membrane transport and fusion, whereas Alix, flotillin, and TSG101 are involved in exosome biogenesis (6–10).
Heterogeneity in size, content and source of EVs can affect on recipient cells responses such as cell survival, death, inflammation and immune response, or assist pathogens to enter and stay in the recipient cell (11). Also, EVs are isolated based on size by gradient ultracentrifugation, coprecipitation, and immunoaffinity enrichment method (12). Moreover, their characterization methods include mass spectrometry, western blot, immunoelectron microscopy image, fluorescence nanoparticle tracking analysis, and polychromatic flow cytometry based on the molecules expressed on their surface (13).
Immune cell-derived EVs are enriched in proteins, especially tetraspanins. Tetraspanins refer to a family of ubiquitous proteins that include CD9, CD63, CD81, and CD82. These proteins on EVs interact with other proteins such as Major Histocompatibility Complex (MHC) molecules and integrins expressed on target cells, eventually leading to prime-specific immune responses (14, 15). They also exhibit special lipid compositions in significant quantities, including sphingomyelin, phosphatidylcholine, and phosphatidylethanolamine (16–19). Immune cell-derived EVs also contain a unique set of enzymes that are involved in lipid metabolism (phospholipase A2, C, and D) (16, 20). Immune cell-derived EVs alter many physiological and pathological processes. In addition, they play important roles in innate and acquired immune responses including antigen presentation, immune cells maturation, differentiation, activation, and suppression, and anti-inflammatory and anti-microbial effects (21–23). Moreover, EVs from host cells can participate in anti-microbial immunity via the production and release of Interferon (IFN), IL-12, granzyme B, perforin, non-coding RNA, etc. (24–26).
The first reports pertaining to functionally active immune cell-derived EVs were provided by Raposo et al. (27). They showed that B cell-derived EVs could stimulate antigen-specific T cell responses via functional peptide–MHC II complexes. Antigen presentation usually needs Antigen Presenting Cells (APCs), which process antigenic peptides and form MHC-peptide complexes. After that, this complex binds to T cell receptors with the synergism of co-stimulatory molecules to activate and proliferate T cells. However, EVs, especially exosomes, make the host’s immune system stronger against invading pathogens without the interaction between APCs and T cells and even without the reprocessing of MHC-peptide complex by the recipient APCs. This results from the fact that they are enriched in efficient carriers of MHC II-peptide complexes and other molecules, allowing robust T cell activation (22, 28–33). What follows includes the investigation of the role of immune cell-derived EVs in combating pathogens.
Generally, a variety of virus-infected cells release EVs that are related to the pathogenesis, diagnostics, and therapeutics of viral diseases. It is noteworthy that immune cell-derived EVs play an important role in the treatment of viral infections. The review of the literature revealed evidence for the EVs derived from monocytes, macrophages, and T cells in the treatment of viral diseases.
Different types of Micro RNA (miRNA) are present in EVs, which are involved in the regulation of cell to cell communication. The cytosol expression of miR-29a and miR-150 was previously reported (1). Further studies indicated strongly upregulated miR-29a and miR-150 in the HIV-Infected macrophages (2). The potential of miR-150 involvement in the HIV/AIDS disease progression and therapy has been elucidated, as well. Accordingly, miR-150 suppression might facilitate HIV-1 treatment (34).
Researchers investigated the role of macrophage-derivedexosomes in the inhibition of DNA replication in Hepatitis BVirus (HBV). They analyzed the expression of different exosomal miRNA and suggested that they were closely related to liver inflammation injury and viral replication (35). In another research, miR-638 was found to target the regulation of a variety of cellular processes such as proliferation, apoptosis, and inflammation (36).
The induction of the macrophages using INF can result in the production of exosomes mediating antiviral activity against HBV. This phenomenon might be associated with the transfer of miR-574-5p from macrophages to HBV-infected hepatocytes, leading to the suppression of HBV replication and expression (37). On the other hand, macrophage-derived exosomes can exert their antiviral properties by transferring IFN-α to infected cells. In this regard, Yao et al. reported that the exosomes derived from macrophages induced anti-HBV activity through the delivery of IFN-α to hepatocytes. The exosomes used T cell immunoglobulin and mucin receptor 1 to enter the hepatocytes (38).
A recent study investigated the reduction of inflammation in Hepatitis C Virus (HCV) treatment amongst HIV/HCV-coinfected individuals. The results indicated that in the coinfected people, miR-19a, miR-221, and miR-223 were upregulated in the monocyte-derived exosomes, which were related to the reduction of plasma inflammation and activation of Nuclear Factor-κB (NF-κB) in the liver (6). Another research showed that miR-221 triggered the anti-inflammatory cascade in monocytes (39) and hindered HIV-1 entry into macrophages by targeting the CD4 viral receptor (40).
It has been reported that miR-19a is involved in the restoration of monocyte immune functions in the coinfection of HIV/HCV (41). miR-223 has been identified as an anti-inflammatory microRNA which acts through the NF-κB pathway (42). The NF-κB pathway has long been regarded as a prototypic pro-inflammatory signalling pathway. Pro- and anti-inflammatory roles of this pathway have been underlined in recent studies. Accordingly, NF-kB exerted anti-inflammatory effects through the direct inhibition of pro-inflammatory genes as well as impacts on the expression or activity of anti-inflammatory cytokines (43).
Up to now, limited studies have been conducted on anti-viral responses through T cell-derived EVs. T cell-derived exosomes could prime Dendritic Cells (DCs) through their cargos, which are genomic and mitochondrial DNA. These factors triggered antiviral responses via the cGAS/STING cytosolic DNA-sensing pathway as well as the expression of Interferon Regulatory Factor 3 (IRF3)-dependent genes (44). T cell-derived exosomes have also been proposed for the treatment of COVID-19 infection. In a single-arm, open-labeled, combined interventional (phase I/II trials) clinical trial (NCT04389385), the safety and efficacy of T cell-derived exosomes in the treatment of COVID-19 infection was explored. In that project, specific T cells from COVID-19 were activated and expanded through exposure with viral peptide fragments and cytokines. The results suggested that mediators like IFN-gamma in the secreted exosomes from T cells might inhibit the coronavirus (45).
Overall, the literature review provided proof-of-principle for the application of immune cell-derived EVs in anti-viral strategies. Accordingly, specific cargos in exosomes were involved in the fight against viral infections, and the transport of appropriate bioactive molecules including miRNAs via exosomes could mediate the antiviral process.
One of the new approaches in the diagnosis, pathogenesis, and treatment of bacterial infections is the use of EVs separated from infected immune cells. EVs released from infected immune cells have shown anti-bacterial effects on pathogenic bacteria. Neutrophil-derived EVs, in particular, are one of the most important innate immune cells for controlling bacterial infections (46). Timar et al. investigated the effects of EVs derived from human Neutrophilic Granulocytes (PMNs) in response to Staphylococcus aurous infection (47). They came to the conclusion that the pre-stimulation of PMNs by various agents induced the release of EVs with different biological properties. Accordingly, only the PMNs stimulated with opsonized particles produced EVs that had the ability to impair bacterial growth. Moreover, the anti-bacterial effect of PMN-derived EVs was correlated to the aggregation of bacteria on their surface, which depended on intact cytoskeleton and metabolic activity within the vesicles. Nonetheless, the anti-bacterial activity of EVs in response to Staphylococcus aurous infection was not limited to these bacteria, as the growth of Escherichia coli was inhibited by neutrophil-derived EVs, as well. However, this anti-microbial activity had no effects on Proteus mirabilis infections, suggesting some levels of specificity on different types of bacteria (32, 47). Interestingly, the anti-bacterial activity of neutrophil-derived EVs depended on particles/bacterial opsonisation through the activation of PLCϒ2 and opsonin receptors as well as the presence of extracellular calcium and Mac-1 integrin complex, independent of the phagocytic process (48, 49).
In another study, EVs derived from neutrophils infected with Mycobacterium tuberculosis (Mtb) activated macrophages and promoted the clearance of intracellular Mtb via enhancing early superoxide anion production and autophagy induction (50). This suggested that EVs acted indirectly by promoting the immune response in neighbouring cells. In an interesting study by Garcia-Martinez et al., EVs released from J774A.1 mice’s macrophage cell line mitigated the bacterial load and production of MCP-1 and TNF-α cytokines in a phosphatidylserine-dependent manner in Mtb-infected macrophages, which suggested the anti-bacterial activity of EVs including exosomes. Furthermore, in vivo results indicated the reduction of the lung bacterial load by macrophage-derived EVs (51).
The mechanism of the anti-bacterial action of EVs is not entirely clear. EVs may be able to capture pathogen derived nucleic acid and protein and transport them to host cell and trigger anti-bacterial immune activity. The results of the research carried out by Cheng and Schorey demonstrated that the transport of Mycobacterium RNA to EVs released from infected macrophage led to the activation of the host RIG-I/MAVS/TBK1/IRF3 RNA sensing signaling and the production of type I INF in recipient cells (52). They showed that the present of pathogen RNA in EVs is dependent on the bacteria’s SecA2 secretion system, suggesting the intercellular transfer of bacterial RNA through host cell-derived EVs may also be perceived for other pathogens that express a SecA2 secretion system such as Staphylococcus, Listeria, and Streptococcus species (53). Moreover, EVs released from Mtb-infected macrophages induced autophagy via LC3-associated pathway and enhanced bacterial killing. Indeed, LC3-associated pathway illustrates an autophagy-dependent antimicrobial pathway in host cells that led to increasing microbial degradation (54). Furthermore, the combination of moxifloxacin and EVs isolated from Mtb-infected macrophages notably lowered the bacterial burden compared to drug treatment alone (52), suggesting a new immunotherapeutic approach to treat drug-resistant Mtb.
Today, drug delivery systems at the nanoscale take up considerable space. Various formulations of nanomedicines have been used to enhance the therapeutic efficacy of chemical and biomolecular medicines. EVs have appeared to be biocompatible vehicles for the delivery of various drugs including antibiotics. Yang et al. disclosed that macrophage-derived EVs with encapsulated linezolid antibiotics were more effective against Methicillin-Resistant Staphylococcus aureus (MRSA) infections in comparison to free linezolid antibiotics (55). Thus, EVs derived from immune cells were found to be biocompatible vehicles for the delivery of various drugs including anti-microbial agents.
More recently, it was demonstrated that EVs released from Chlamydia psittaci-infected DCs were strongly able to induce INF-γ production and secretion in natural killer cells through a TNF-α/TNF receptor interaction (56). The combination of EVs with INF-γ and TNF-α released from infected DCs and neighbouring NK cells limited the C. psittaci growth in infected epithelial cells and attenuated the subversion bacterial resistance to apoptosis. Overall, that study emphasized that the induction of pro-inflammatory cytokines by EVs derived from host immune cells could further activate the anti-bacterial defense (56). A similar mechanism was also applied by the host to deliver cytokines for the activation of immune response in combat with infections. Accordingly, DC-derived EVs transferred the pro-inflammatory cytokine TNF-α and activated the neighbouring epithelial cells, leading to the release of additional inflammatory cytokines and chemokines as well as the promotion of the innate immune response. All in all, these limited studies highlighted the role of immune cell-derived EVs in response to bacterial infections. Yet, more detailed studies in this area are warranted.
The immune system is concomitantly involved with infectious agents such as fungi and parasites to generate exosomes that are applicable in defense against these pathogens. Hence, immune cell-derived EVs could be considered a valuable treatment target against these infectious agents. In this regard, investigations were done on Aspergillus fumigatus and Candida albicans, as two clinically relevant’ human fungal pathogens, to explore the host’s immune response (57). Based on the findings, anti-fungal responses from neutrophil-produced EVs were shown in response to A. fumigatus infection. Indeed, A. fumigatus stimulates EV produced by human neutrophils and utilizing these EVs in vitro led to the elimination of fungal hyphae (58, 59). As such, during infection with opsonized A.fumigatus, fresh human polymorph nuclear granulocytes(PMNs) were extracted, and then EV release by human neutrophils were collected and co-incubated with fungi for 4 to 6 hour at a different multiplicity of infection (MOI) to one PMNs to optimize minimal cell death in the PMN population (60). Accordingly, the EVs could attach to and enter fungal conidia and inhibit their growth (57). Surprisingly, anti-fungal cargo proteins including cathepsin G and azurocidin were identified in these anti-fungal EVs through mass spectrometry-based proteomic analysis. Notably, these productions from the host were specifically affirmed against a mutant strain of A. fumigatus (61). Conversely, a recent study found that monocyte-derived EVs presented anti-inflammatory functions in the context of C. albicans infection. TGF-β1-transporting EVs, as anti-inflammatory vesicles, were released via interaction between Complement Receptor 3 (CR3, also known as CD11b/CD18) on monocytes and soluble β-glucan produced from C. albicans (62). In addition, TGF-β1 led to the development of immune modulation, favoring the survival of C. albicans commensalism (63). Inconsistent with the anti-fungal role of EVs in A. fumigatus infection, these EVs appeared to provide an opportunity for C. albicans to be a human commensal. It is noteworthy that the lipid-enclosed Amphotericin B (AmBisome) was utilized instead of amphotericin B, which enhanced the uptake and decreased the off-target effects (64). This technique is classically similar to transfer via EVs. Overall, these findings proved a promising path forward to target exosomes for fungal pathogens of interest (65, 66).
Plasmodium berghei, as a rodent malaria parasite, leads to the secretion of plasma cell-derived EVs and stimulates antigen-presenting cells through CD40, which generates an inflammatory response that causes the initiation of effector T cells activity (67). Therefore, macrophage induction triggers the parasite clearance. Evidence has indicated that the distinct properties of EVs make them of interest for new drugs and treatment prospects in parasitic infections. Hence, attention has to be paid to the potential of exosomes as a therapeutic agent and vaccine target in parasitic diseases.
Conversely, during acute Plasmodium vivax infection in humans, the presence of increased circulating immune cell-derived EVs might be important on the acute inflammatory signs of malaria vivax (68, 69). Besides, EVs derived from Toxoplasma gondii antigen-pulsed DCs could be candidate as an effective vaccine against toxoplasmosis, because these pulsed DCs were able to induce humoral immunity against the parasite (70). This was further supported by a study performed on cutaneous leishmaniasis, which indicated that protective Th1 responses were mediated by DC-derived EVs (71). Similar results were also obtained regarding common livestock parasites, which showed that the EVs derived from parasite antigen-loaded DCs played an important role in protection against the infection (72). This could provide a proof-of-principle that therapeutic approaches based on EVs derived from immune cells offer a promising path forward.
Considering the results of earlier studies pertaining to the release of EVs from parasites or parasitized cells, utilizing suitable strategies against the function of these EVs can be a therapeutic approach. Accordingly, there are particular interactions between parasite-derived EVs and the immune system (73). Another support for the therapeutic application of exosomes in parasitic diseases comes from a study by Chappuis et al. (74), which described challenges in the treatment of visceral leishmaniosis including drug resistance and variable responses to treatment regimens, leading to a long sought treatment. Nonetheless, leishmania exosomes were found to affect the innate and adaptive immune responses (75, 76). In this regard, designing immune cell-derived EVs containing the related drugs or specific cargoes may lead to the activation and induction of immune responses, inducing protection against parasitic diseases. This technique can be used to treat such infections, and such an insight will be highly valuable.
The studies reported in this mini-review demonstrated that the EVs released by immune cells were able to invade various pathogens including bacterial, viral, and fungal infections. However, unanswered questions have remained about the antimicrobial effects of EVs including their exact mechanism of action. On the other hand, the exact cargos of EVs produced by each immune cell are changeable depending on the pathophysiology of the microenvironment. Thus, valuable information can be gained through a close evaluation of the role of EVs during infections. Hopefully, the years to come will witness a change in the use of EVs as both diagnostic and therapeutic agents for the treatment of infectious diseases.
All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors would like to thank Ms. A. Keivanshekouh at the Research Consultation Center (RCC) of Shiraz University of Medical Sciences for improving the use of English in the manuscript.
1. Wen C, Seeger RC, Fabbri M, Wang L, Wayne AS, Jong AY. Biological Roles and Potential Applications of Immune Cell-Derived Extracellular Vesicles. J Extracell Vesicles (2017) 6(1):1400370. doi: 10.1080/20013078.2017.1400370
2. Raposo G, Stoorvogel W. Extracellular Vesicles: Exosomes, Microvesicles, and Friends. J Cell Biol (2013) 200(4):373–83. doi: 10.1083/jcb.201211138
3. Wolf P. The Nature and Significance of Platelet Products in Human Plasma. Br J Haematol (1967) 13(3):269–88. doi: 10.1111/j.1365-2141.1967.tb08741.x
4. Akbari A, Jabbari N, Sharifi R, Ahmadi M, Vahhabi A, Seyedzadeh SJ, et al. Free and Hydrogel Encapsulated Exosome-Based Therapies in Regenerative Medicine. Life Sci (2020) 249:117447. doi: 10.1016/j.lfs.2020.117447
5. Keshtkar S, Azarpira N, Ghahremani MH. Mesenchymal Stem Cell-Derived Extracellular Vesicles: Novel Frontiers in Regenerative Medicine. Stem Cell Res Ther (2018) 9(1):1–9. doi: 10.1186/s13287-018-0791-7
6. Pegtel DM, Gould SJ. Exosomes. Annu Rev Biochem (2019) 88:487–514. doi: 10.1146/annurev-biochem-013118-111902
7. Williams RL, Urbé S. The Emerging Shape of the ESCRT Machinery. Nat Rev Mol Cell Biol (2007) 8(5):355–68. doi: 10.1038/nrm2162
8. Palanisamy V, Sharma S, Deshpande A, Zhou H, Gimzewski J, Wong DT. Nanostructural and Transcriptomic Analyses of Human Saliva Derived Exosomes. PloS One (2010) 5(1):e8577. doi: 10.1371/journal.pone.0008577
9. Kalluri R. The Biology and Function of Exosomes in Cancer. J Clin Invest (2016) 126(4):1208–15. doi: 10.1172/JCI81135
10. Keshtkar S, Kaviani M, Soleimanian S, Azarpira N, Asvar Z, Pakbaz S. Stem Cell-Derived Exosome as Potential Therapeutics for Microbial Diseases. Front Microbiol (2022) 12:786111. doi: 10.3389/fmicb.2021.786111
11. Spencer N, Yeruva L. Role of Bacterial Infections in Extracellular Vesicles Release and Impact on Immune Response. Biomed J (2021) 44(2):157–64. doi: 10.1016/j.bj.2020.05.006
12. de Lima Kaminski V, Ellwanger JH, Chies JAB. Extracellular Vesicles in Host-Pathogen Interactions and Immune Regulation—Exosomes as Emerging Actors in the Immunological Theater of Pregnancy. Heliyon (2019) 5(8):e02355. doi: 10.1016/j.heliyon.2019.e02355
13. Martins S, Kuczera D, Lötvall J, Bordignon J, Alves LR. Characterization of Dendritic Cell-Derived Extracellular Vesicles During Dengue Virus Infection. Front Microbiol (2018) 9:1792. doi: 10.3389/fmicb.2018.01792
14. Kleijmeer MJ, Stoorvogel W, Griffith JM, Yoshie O, Geuze HJ. Selective Enrichment of Tetraspan Proteins on the Internal Vesicles of Multivesicular Endosomes and on Exosomes Secreted by Human B-Lymphocytes. J Biol Chem (1998) 273(32):20121–7. doi: 10.1074/jbc.273.32.20121
15. Perez-Hernandez D, Gutiérrez-Vázquez C, Jorge I, López-Martín S, Ursa A, Sánchez-Madrid F, et al. The Intracellular Interactome of Tetraspanin-Enriched Microdomains Reveals Their Function as Sorting Machineries Toward Exosomes. J Biol Chem (2013) 288(17):11649–61. doi: 10.1074/jbc.M112.445304
16. Subra C, Grand D, Laulagnier K, Stella A, Lambeau G, Paillasse M, et al. Exosomes Account for Vesicle-Mediated Transcellular Transport of Activatable Phospholipases and Prostaglandins [s]. J Lipid Res (2010) 51(8):2105–20. doi: 10.1194/jlr.M003657
17. Llorente A, Skotland T, Sylvänne T, Kauhanen D, Róg T, Orłowski A, et al. Molecular Lipidomics of Exosomes Released by PC-3 Prostate Cancer Cells. Biochim Biophys Acta (BBA)-Mol Cell Biol Lipids (2013) 1831(7):1302–9. doi: 10.1016/j.bbalip.2013.04.011
18. Laulagnier K, Motta C, Hamdi S, Roy S, Fauvelle F, Pageaux J-F, et al. Mast Cell-and Dendritic Cell-Derived Exosomes Display a Specific Lipid Composition and an Unusual Membrane Organization. Biochem J (2004) 380(1):161–71. doi: 10.1042/bj20031594
19. Record M, Carayon K, Poirot M, Silvente-Poirot S. Exosomes as New Vesicular Lipid Transporters Involved in Cell–Cell Communication and Various Pathophysiologies. Biochim Biophys Acta (BBA)-Mol Cell Biol Lipids (2014) 1841(1):108–20. doi: 10.1016/j.bbalip.2013.10.004
20. Esser J, Gehrmann U, D'Alexandri FL, Hidalgo-Estévez AM, Wheelock CE, Scheynius A, et al. Exosomes From Human Macrophages and Dendritic Cells Contain Enzymes for Leukotriene Biosynthesis and Promote Granulocyte Migration. J Allergy Clin Immunol (2010) 126(5):1032–40. e4. doi: 10.1016/j.jaci.2010.06.039
21. Zhang B, Yin Y, Lai RC, Lim SK. Immunotherapeutic Potential of Extracellular Vesicles. Front Immunol (2014) 5:518. doi: 10.3389/fimmu.2014.00518
22. Rybak K, Robatzek S. Functions of Extracellular Vesicles in Immunity and Virulence. Plant Physiol (2019) 179(4):1236–47. doi: 10.1104/pp.18.01557
23. Kolonics F, Szeifert V, Timár CI, Ligeti E, Lőrincz ÁM. The Functional Heterogeneity of Neutrophil-Derived Extracellular Vesicles Reflects the Status of the Parent Cell. Cells (2020) 9(12):2718. doi: 10.3390/cells9122718
24. Cai C, Koch B, Morikawa K, Suda G, Sakamoto N, Rueschenbaum S, et al. Macrophage-Derived Extracellular Vesicles Induce Long-Lasting Immunity Against Hepatitis C Virus Which is Blunted by Polyunsaturated Fatty Acids. Front Immunol (2018) 9:723. doi: 10.3389/fimmu.2018.00723
25. Peters PJ, Borst J, Oorschot V, Fukuda M, Krähenbühl O, Tschopp J, et al. Cytotoxic T Lymphocyte Granules are Secretory Lysosomes, Containing Both Perforin and Granzymes. J Exp Med (1991) 173(5):1099–109. doi: 10.1084/jem.173.5.1099
26. Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-Mediated Transfer of mRNAs and microRNAs is a Novel Mechanism of Genetic Exchange Between Cells. Nat Cell Biol (2007) 9(6):654–9. doi: 10.1038/ncb1596
27. Tetta C, Ghigo E, Silengo L, Deregibus MC, Camussi G. Extracellular Vesicles as an Emerging Mechanism of Cell-to-Cell Communication. Endocrine (2013) 44(1):11–9. doi: 10.1007/s12020-012-9839-0
28. Segura E, Amigorena S, Théry C. Mature Dendritic Cells Secrete Exosomes With Strong Ability to Induce Antigen-Specific Effector Immune Responses. Blood Cells Mol Dis (2005) 35(2):89–93. doi: 10.1016/j.bcmd.2005.05.003
29. Chaput N, Théry C. editors. Exosomes: Immune Properties and Potential Clinical Implementations. Semin Immunopathol (2011) 33(5):419–40. doi: 10.1007/s00281-010-0233-9
30. Robbins PD, Morelli AE. Regulation of Immune Responses by Extracellular Vesicles. Nat Rev Immunol (2014) 14(3):195–208. doi: 10.1038/nri3622
31. Tang N, Sun B, Gupta A, Rempel H, Pulliam L. Monocyte Exosomes Induce Adhesion Molecules and Cytokines via Activation of NF-κb in Endothelial Cells. FASEB J (2016) 30(9):3097–106. doi: 10.1096/fj.201600368RR
32. Brakhage AA, Zimmermann A-K, Rivieccio F, Visser C, Blango MG. Host-Derived Extracellular Vesicles for Antimicrobial Defense. microLife (2021) 2:Uqab003. doi: 10.1093/femsml/uqab003
33. Segura E, Nicco C, Lombard B, Véron P, Raposo G, Batteux F, et al. ICAM-1 on Exosomes From Mature Dendritic Cells is Critical for Efficient Naive T-Cell Priming. Blood (2005) 106(1):216–23. doi: 10.1182/blood-2005-01-0220
34. Munshi SU, Panda H, Holla P, Rewari BB, Jameel S. MicroRNA-150 is a Potential Biomarker of HIV/AIDS Disease Progression and Therapy. PloS One (2014) 9(5):e95920. doi: 10.1371/journal.pone.0095920
35. Lu W, Bai L, Chen Y. The Role of Macrophage-Derived Exosomes in Liver Diseases. Infect Dis Imm (2022) 2(1):34–41. doi: 10.1097/ID9.0000000000000034
36. Chong ZX, Yeap SK, Ho WY. Dysregulation of miR-638 in the Progression of Cancers. Pathol Res Pract (2021) 220:153351. doi: 10.1016/j.prp.2021.153351
37. Wu W, Wu D, Yan W, Wang Y, You J, Wan X, et al. Interferon-Induced Macrophage-Derived Exosomes Mediate Antiviral Activity Against Hepatitis B Virus Through miR-574-5p. J Infect Dis (2021) 223(4):686–98. doi: 10.1093/infdis/jiaa399
38. Yao Z, Qiao Y, Li X, Chen J, Ding J, Bai L, et al. Exosomes Exploit the Virus Entry Machinery and Pathway to Transmit Alpha Interferon-Induced Antiviral Activity. J Virol (2018) 92(24):e01578–18. doi: 10.1128/JVI.01578-18
39. Liu C-W, Sung H-C, Lin S-R, Wu C-W, Lee C-W, Lee I-T, et al. Resveratrol Attenuates ICAM-1 Expression and Monocyte Adhesiveness to TNF-α-Treated Endothelial Cells: Evidence for an Anti-Inflammatory Cascade Mediated by the miR-221/222/AMPK/p38/NF-κb Pathway. Sci Rep (2017) 7(1):1–14. doi: 10.1038/srep44689
40. Lodge R, Barbosa JAF, Lombard-Vadnais F, Gilmore JC, Deshiere A, Gosselin A, et al. Host microRNAs-221 and-222 Inhibit HIV-1 Entry in Macrophages by Targeting the CD4 Viral Receptor. Cell Rep (2017) 21(1):141–53. doi: 10.1016/j.celrep.2017.09.030
41. Sun B, Abadjian L, Monto A, Freasier H, Pulliam L. HCV Cure in HIV Coinfection Dampens Inflammation and Improves Cognition Through Multiple Mechanisms. J Infect Dis (2020) 222:396–406. doi: 10.1093/infdis/jiaa109
42. Ye D, Zhang T, Lou G, Liu Y. Role of miR-223 in the Pathophysiology of Liver Diseases. Exp Mol Med (2018) 50(9):1–12. doi: 10.1038/s12276-018-0153-7
43. Lawrence T. The Nuclear Factor NF-κb Pathway in Inflammation. Cold Spring Harbor Perspect Biol (2009) 1(6):a001651. doi: 10.1101/cshperspect.a001651
44. Torralba D, Baixauli F, Villarroya-Beltri C, Fernández-Delgado I, Latorre-Pellicer A, Acín-Pérez R, et al. Priming of Dendritic Cells by DNA-Containing Extracellular Vesicles From Activated T Cells Through Antigen-Driven Contacts. Nat Commun (2018) 9(1):1–17. doi: 10.1038/s41467-018-05077-9
45. Dutta A, Paul S. Multimodal Role of Exosomes in COVID-19 Transmission, Diagnosis, and Therapy. J Mol Sci (2020) NCT04389385.
46. Kolaczkowska E, Kubes P. Neutrophil Recruitment and Function in Health and Inflammation. Nat Rev Immunol (2013) 13(3):159–75. doi: 10.1038/nri3399
47. Timár CI, Lőrincz ÁM, Csépányi-Kömi R, Vályi-Nagy A, Nagy G, Buzás EI, et al. Antibacterial Effect of Microvesicles Released From Human Neutrophilic Granulocytes. Blood J Am Soc Hematol (2013) 121(3):510–8. doi: 10.1182/blood-2012-05-431114
48. Lőrincz ÁM, Schütte M, Timár CI, Veres DS, Kittel Á, McLeish KR, et al. Functionally and Morphologically Distinct Populations of Extracellular Vesicles Produced by Human Neutrophilic Granulocytes. J Leuk Biol (2015) 98(4):583–9. doi: 10.1189/jlb.3VMA1014-514R
49. Lőrincz ÁM, Szeifert V, Bartos B, Szombath D, Mócsai A, Ligeti E. Different Calcium and Src Family Kinase Signaling in Mac-1 Dependent Phagocytosis and Extracellular Vesicle Generation. Front Immunol (2019) 10:2942. doi: 10.3389/fimmu.2019.02942
50. Kolonics F, Kajdácsi E, Farkas VJ, Veres DS, Khamari D, Kittel Á, et al. Neutrophils Produce Proinflammatory or Anti-Inflammatory Extracellular Vesicles Depending on the Environmental Conditions. J Leuk Biol (2021) 109(4):793–806. doi: 10.1002/JLB.3A0320-210R
51. García-Martínez M, Vázquez-Flores L, Álvarez-Jiménez VD, Castañeda-Casimiro J, Ibáñez-Hernández M, Sánchez-Torres LE, et al. Extracellular Vesicles Released by J774A. 1 Macrophages Reduce the Bacterial Load in Macrophages and in an Experimental Mouse Model of Tuberculosis. Int J Nanomed (2019) 14:6707. doi: 10.2147/IJN.S203507
52. Cheng Y, Schorey JS. Extracellular Vesicles Deliver Mycobacterium RNA to Promote Host Immunity and Bacterial Killing. EMBO Rep (2019) 20(3):e46613. doi: 10.15252/embr.201846613
53. Green ER, Mecsas J. Bacterial Secretion Systems: An Overview. Microbiology Spectrum (2016) 4(1):4.1.13. doi: 10.1128/9781555819286.ch8
54. Mitchell G, Isberg RR. Innate Immunity to Intracellular Pathogens: Balancing Microbial Elimination and Inflammation. Cell Host Microbe (2017) 22(2):166–75. doi: 10.1016/j.chom.2017.07.005
55. Yang X, Shi G, Guo J, Wang C, He Y. Exosome-Encapsulated Antibiotic Against Intracellular Infections of Methicillin-Resistant Staphylococcus Aureus. Int J Nanomed (2018) 13:8095. doi: 10.2147/IJN.S179380
56. Radomski N, Karger A, Franzke K, Liebler-Tenorio E, Jahnke R, Matthiesen S, et al. Chlamydia Psittaci-Infected Dendritic Cells Communicate With NK Cells via Exosomes to Activate Antibacterial Immunity. Infect Immun (2019) 88(1):e00541–19. doi: 10.1128/IAI.00541-19
57. Shopova IA, Belyaev I, Dasari P, Jahreis S, Stroe MC, Cseresnyés Z, et al. Human Neutrophils Produce Antifungal Extracellular Vesicles Against Aspergillus Fumigatus. MBio (2020) 11(2):e00596–20. doi: 10.1128/mBio.00596-20
58. Ermert D, Zychlinsky A, Urban C. Fungal and Bacterial Killing by Neutrophils. (2009) Host-Pathogen Interactions: Springer, 293–312. doi: 10.1007/978-1-59745-204-5_21
59. Gazendam RP, van de Geer A, Roos D, van den Berg TK, Kuijpers TW. How Neutrophils Kill Fungi. Immunol Rev (2016) 273(1):299–311. doi: 10.1111/imr.12454
60. Gazendam RP, van Hamme JL, Tool AT, Hoogenboezem M, van den Berg JM, Prins JM, et al. Human Neutrophils Use Different Mechanisms to Kill Aspergillus Fumigatus Conidia and Hyphae: Evidence From Phagocyte Defects. J Immunol (2016) 196(3):1272–83. doi: 10.4049/jimmunol.1501811
61. Blango MG, Kniemeyer O, Brakhage AA. Conidial Surface Proteins at the Interface of Fungal Infections. PloS Pathog (2019) 15(9):e1007939. doi: 10.1371/journal.ppat.1007939
62. Halder LD, Jo EA, Hasan MZ, Ferreira-Gomes M, Krüger T, Westermann M, et al. Immune Modulation by Complement Receptor 3-Dependent Human Monocyte TGF-β1-Transporting Vesicles. Nat Commun (2020) 11(1):1–19. doi: 10.1038/s41467-020-16241-5
63. Konkel JE, Chen W. Balancing Acts: The Role of TGF-β in the Mucosal Immune System. Trends Mol Med (2011) 17(11):668–76. doi: 10.1016/j.molmed.2011.07.002
64. Walker L, Sood P, Lenardon MD, Milne G, Olson J, Jensen G, et al. The Viscoelastic Properties of the Fungal Cell Wall Allow Traffic of AmBisome as Intact Liposome Vesicles. MBio (2018) 9(1):e02383–17. doi: 10.1128/mBio.02383-17
65. Rizzo J, Rodrigues ML, Janbon G. Extracellular Vesicles in Fungi: Past, Present, and Future Perspectives. Front Cell Infect Microbiol (2020) 10:346. doi: 10.3389/fcimb.2020.00346
66. Andriantsitohaina R, Papon N. Extracellular Vesicles: New Bullets to Fight Fungal Infections. Trends Cell Biol (2020) 30(8):589–90. doi: 10.1016/j.tcb.2020.05.008
67. Couper KN, Barnes T, Hafalla JC, Combes V, Ryffel B, Secher T, et al. Parasite-Derived Plasma Microparticles Contribute Significantly to Malaria Infection-Induced Inflammation Through Potent Macrophage Stimulation. PloS Pathog (2010) 6(1):e1000744. doi: 10.1371/journal.ppat.1000744
68. Campos FM, Franklin BS, Teixeira-Carvalho A, de Paula SC, Fontes CJ, Brito CF, et al. Augmented Plasma Microparticles During Acute Plasmodium Vivax Infection. Malaria J (2010) 9(1):1–8. doi: 10.1186/1475-2875-9-327
69. Anstey NM, Handojo T, Pain MC, Kenangalem E, Tjitra E, Price RN, et al. Lung Injury in Vivax Malaria: Pathophysiological Evidence for Pulmonary Vascular Sequestration and Posttreatment Alveolar-Capillary Inflammation. J Infect Dis (2007) 195(4):589–96. doi: 10.1086/510756
70. Beauvillain C, Ruiz S, Guiton R, Bout D, Dimier-Poisson I. A Vaccine Based on Exosomes Secreted by a Dendritic Cell Line Confers Protection Against T. Gondii Infection in Syngeneic and Allogeneic Mice. Microbes Infect (2007) 9(14-15):1614–22. doi: 10.1016/j.micinf.2007.07.002
71. Schnitzer JK, Berzel S, Fajardo-Moser M, Remer KA, Moll H. Fragments of Antigen-Loaded Dendritic Cells (DC) and DC-Derived Exosomes Induce Protective Immunity Against Leishmania Major. Vaccine (2010) 28(36):5785–93. doi: 10.1016/j.vaccine.2010.06.077
72. del Cacho E, Gallego M, Lee SH, Lillehoj HS, Quilez J, Lillehoj EP, et al. Induction of Protective Immunity Against Eimeria Tenella, Eimeria Maxima, and Eimeria Acervulina Infections Using Dendritic Cell-Derived Exosomes. Infect Immun (2012) 80(5):1909–16. doi: 10.1128/IAI.06413-11
73. Coakley G, McCaskill JL, Borger JG, Simbari F, Robertson E, Millar M, et al. Extracellular Vesicles From a Helminth Parasite Suppress Macrophage Activation and Constitute an Effective Vaccine for Protective Immunity. Cell Rep (2017) 19(8):1545–57. doi: 10.1016/j.celrep.2017.05.001
74. Chappuis F, Sundar S, Hailu A, Ghalib H, Rijal S, Peeling RW, et al. Visceral Leishmaniasis: What are the Needs for Diagnosis, Treatment and Control? Nat Rev Microbiol (2007) 5(11):873–82. doi: 10.1038/nrmicro1748
75. Silverman JM, Clos J, Horakova E, Wang AY, Wiesgigl M, Kelly I, et al. Leishmania Exosomes Modulate Innate and Adaptive Immune Responses Through Effects on Monocytes and Dendritic Cells. J Immunol (2010) 185(9):5011–22. doi: 10.4049/jimmunol.1000541
Keywords: extracellular vesicles, immune cell, microbial infection, anti-microbial, pathogens
Citation: Keshtkar S, Soleimanian S, Kaviani M, Sarvestani FS, Azarpira N, Asvar Z and Pakbaz S (2022) Immune Cell-Derived Extracellular Vesicles in the Face of Pathogenic Infections. Front. Immunol. 13:906078. doi: 10.3389/fimmu.2022.906078
Received: 28 March 2022; Accepted: 31 May 2022;
Published: 30 June 2022.
Edited by:
Yang Jin, Boston University, United StatesReviewed by:
Caroline N. Jones, The University of Texas at Dallas, United StatesCopyright © 2022 Keshtkar, Soleimanian, Kaviani, Sarvestani, Azarpira, Asvar and Pakbaz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Negar Azarpira, bmVnYXJhemFycGlyYUBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.