
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 30 May 2022
Sec. Inflammation
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.903498
This article is part of the Research Topic The Immunological Consequences of Regulated Cell Death in Infection and Inflammation View all 12 articles
 Cristiana Barbati1*†
Cristiana Barbati1*† Alessandra Ida Celia1†
Alessandra Ida Celia1† Tania Colasanti1
Tania Colasanti1 Marta Vomero2
Marta Vomero2 Mariangela Speziali1
Mariangela Speziali1 Erisa Putro1
Erisa Putro1 Giorgia Buoncuore1
Giorgia Buoncuore1 Flavia Savino1
Flavia Savino1 Serena Colafrancesco1
Serena Colafrancesco1 Federica Maria Ucci1
Federica Maria Ucci1 Claudia Ciancarella1
Claudia Ciancarella1 Eugenia Balbinot1
Eugenia Balbinot1 Susanna Scarpa3
Susanna Scarpa3 Francesco Natalucci1
Francesco Natalucci1 Greta Pellegrino1
Greta Pellegrino1 Fulvia Ceccarelli1
Fulvia Ceccarelli1 Francesca Romana Spinelli1
Francesca Romana Spinelli1 Claudio Maria Mastroianni4
Claudio Maria Mastroianni4 Fabrizio Conti1
Fabrizio Conti1 Cristiano Alessandri1
Cristiano Alessandri1Autophagy is a homeostatic process responsible for the self-digestion of intracellular components and antimicrobial defense by inducing the degradation of pathogens into autophagolysosomes. Recent findings suggest an involvement of this process in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. However, the role of autophagy in the immunological mechanisms of coronavirus disease 2019 (COVID-19) pathogenesis remains largely unexplored. This study reveals the presence of autophagy defects in peripheral immune cells from COVID-19 patients. The impairment of the autophagy process resulted in a higher percentage of lymphocytes undergoing apoptosis in COVID-19 patients. Moreover, the inverse correlation between autophagy markers levels and peripheral lymphocyte counts in COVID-19 patients confirms how a defect in autophagy might contribute to lymphopenia, causing a reduction in the activation of viral defense. These results provided intriguing data that could help in understanding the cellular underlying mechanisms in COVID-19 infection, especially in severe forms.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a highly transmissible coronavirus that emerged in late 2019 and caused a pandemic of acute respiratory disease, named “coronavirus disease 2019” (COVID-19), which still now threatens human health and public safety (1).
In severe COVID-19 patients, both adaptive and innate immune responses that are critical for antiviral reactions were described as impaired (2).
In particular, lymphopenia (lymphocyte count <1.0 × 109/L)3 and inflammatory cytokine storm are typical abnormalities found in COVID-19 patients, probably associated with disease severity, highlighting the strong SARS-CoV-2 ability in suppressing the adaptive immune responses (3). Recent studies have shown that COVID-19 patients, compared to healthy controls, had significantly lower absolute numbers of total lymphocytes and subsets of CD3+, CD4+, and CD8+ T cells, CD19+ B cells, and CD56+ NK cells (4).
Concerning this, lymphocytes’ survival is known to be closely regulated by autophagy (5). Autophagy is a metabolic process involved in the degradation of intracellular components via lysosomal machinery, by engulfment of damaged proteins and organelles in double-membrane vesicles called autophagosomes (6). Furthermore, the crosstalk between autophagy and apoptosis, crucial for cell survival, has been previously shown (7), and several studies correlated the disruption of autophagy with the contemporary increase of apoptotic cell death in several diseases (8).
In COVID-19 patients, the accumulation of autophagosomes promoted by SARS-CoV-2 infection may exacerbate the processes of apoptotic cell death (9), and higher levels of apoptosis in lymphocytes could be related to lymphopenia detected in severe COVID-19 conditions (10).
To date, the mechanism underlying the kinetics of peripheral lymphocyte changes due to COVID-19 is unclear.
This study sought to investigate the relationship between COVID-19 and the autophagy of circulating peripheral blood mononuclear cells (PBMCs) to better characterize lymphopenia during SARS-CoV-2 infection.
Eighteen COVID-19 patients attending the University Hospital Policlinico Umberto I, Sapienza University of Rome, were enrolled. The study was approved by the local ethics committee (protocol number 0586/20), and informed consent was obtained from each patient. As a control group, twelve age- and sex-matched healthy donors (HDs) were studied. From every participant in the study, a blood sample was collected to purify PBMCs by Ficoll-Hypaque. Sera were obtained by centrifugation at 3,500 rpm for 15 min and then stored at −20°C until use for in vitro treatments.
After counting, isolated PBMCs were cultured on 6-well dishes at a concentration of 2 × 106 PBMCs/ml/well in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS), 2 mM of glutamine, and 50 μg/ml of gentamycin and treated with the following: i) lysosomal inhibitors E64d and pepstatin A (both at 10 μg/ml) for 2 h before the end of culture; ii) 10% COVID-19 or HD sera (as a replacement of FBS) for 24 h, selected by preliminary time-course experiments (11).
On the same day of the blood sample collection and after in vitro treatment with sera, PBMCs were lysed in radioimmunoprecipitation assay (RIPA) buffer (100 mM of Tris-HCl pH 8, 150 mM of NaCl, 1% Triton X-100, 1 mM of MgCl, 25 mM of NaVO4, and protease-inhibitor mixture). Lysates were loaded onto a 15% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) in denaturing conditions. Subsequently, Western blotting was performed, and the membranes were incubated with a rabbit anti-human LC3IIB and a rabbit anti-p62 Abs (1:1,000 diluted in Tris-buffered saline containing milk at 5%) (Cell Signaling, Danvers, MA, USA) (12). Peroxidase-conjugated goat anti-rabbit IgG was used as a secondary Abs, and the reaction was developed using the SuperSignal West Pico Chemiluminescent Substrate (Millipore, Billerica, MA, USA). A rabbit anti-human β-actin Ab was used to ensure the presence of equal amounts of protein. Quantification of protein expression was performed by densitometry analysis.
As before, PBMC apoptosis was analyzed using a fluorescein isothiocyanate (FITC)-conjugated annexin V (AV) and phycoerythrin (PE)-conjugated propidium iodide (PI) apoptosis detection kit, according to the manufacturer’s protocol (MBL, Woburn, MA, USA). In particular, 1 × 106 PBMCs were stained in AV buffer with AV-FITC and PI-PE (1:100 diluted) for 10 min in the dark. After washing, cells were transferred into fluorescence-activated cell sorting (FACS) tubes and analyzed. The acquisition was performed on a FACSCalibur cytometer (BD, San Jose, CA, USA), and 10,000 events/sample were run. Data were analyzed using the Cell Quest Pro software (11).
Autophagy levels in CD4+, CD8+, CD19+ lymphocytes, and CD14+ cells from COVID-19 were also detected using the Cyto-ID autophagy detection kit (Enzo Life Sciences, Farmingdale, NY, USA). The probe used in this kit consists of cationic amphiphilic tracer dye that stains autophagolysosomes (13). For the immunophenotyping analysis, see the Supplementary Material.
An indirect immunofluorescence assay was developed on freshly isolated PBMCs from both COVID-19 patients and HDs, prepared by the cytospin technique. In detail, after slide preparation, a cell suspension of 0.5 × 106 cells/ml was gently pipetted into the cytofunnel and centrifuged at 1,000 × g for 5 min. The removed slides, after drying at least 2 h at room temperature (RT), were fixed with paraformaldehyde (PFA) at 4% in phosphate-buffered saline (PBS) for 15 min at RT, then treated with PBS-Triton X-100 0.1% for 10 min, and blocked with 3% bovine serum albumin (BSA) in PBS for 30 min at RT. Successively, slides were incubated overnight at 4°C with the following primary antibodies: rabbit anti-human LC3IIB (Cell Signaling; 1:100 diluted) and a mouse anti-human lysosomal-associated membrane protein 1 (LAMP1) (Invitrogen, Carlsbad, CA, USA; 1:100 diluted). The day after, slides were washed three times in PBS, and Tetramethylrhodamine-isothiocyanate (TRITC)–anti-rabbit and FITC–anti-mouse IgG (Sigma Aldrich) were added and incubated for 45 min at RT. After incubation, slides were washed three times in PBS, and the second wash was performed with the addition of Hoechst (Molecular Probes, Eugene, OR, USA) for nuclear staining. Fluorescence was analyzed by a fluorescence microscope (Olympus, Tokyo, Japan; BX52). Image acquisition and processing were conducted by IAS 2000 software. Morphometric analysis of cellular expression was carried out by counting at least 200 cells in different microscopic fields at magnifications of 50× and 100×.
Data are expressed as means ± SD. Results were analyzed with GraphPad Prism 6. The Mann–Whitney test or Student’s t-test was used to compare quantitative variables in different groups, and the chi-square test was used to test a correlation between categorical variables. Spearman’s rank correlation coefficient was applied for the calculation of the correlation between parallel variables in single samples. Values of p < 0.05 were considered statistically significant.
The serological characteristics of COVID-19 patients are summarized in Table 1.
Patients were divided into two groups, high inflammatory profile (HIP) and low inflammatory profile (LIP), according to serological and laboratory features.
Specifically, HIP patients displayed lymphocyte < 1,000 × 109/L with the addition of one of the following serological features: platelets < 100,000 × 109/L, IL-6 > 5.9 pg/ml, C-reactive protein (CRP) > 5,000 mg/ml, D-dimers > 1,000 ng/ml, ferritin > 250 ng/ml, and erythrocyte sedimentation rate (ESR) > 30 mm/h.
LIP patients did not meet the abovementioned criteria.
Basal autophagy and apoptosis in PBMCs from COVID-19 patients and HDs were evaluated, and our results showed significantly higher LC3IIB levels in COVID-19 patients compared to HDs (p < 0.0001) (Figures 1A, B), presuming an upregulation of autophagy. In addition, p62 levels were significantly higher in COVID-19 patients than in HDs (p < 0.0001) (Figures 1A, C), underlining an accumulation of p62 due to an autophagy impairment in these patients. Furthermore, freshly isolated PBMC apoptosis was higher in COVID-19 patients in comparison to HDs (p = 0.0186) (Figure 1D).
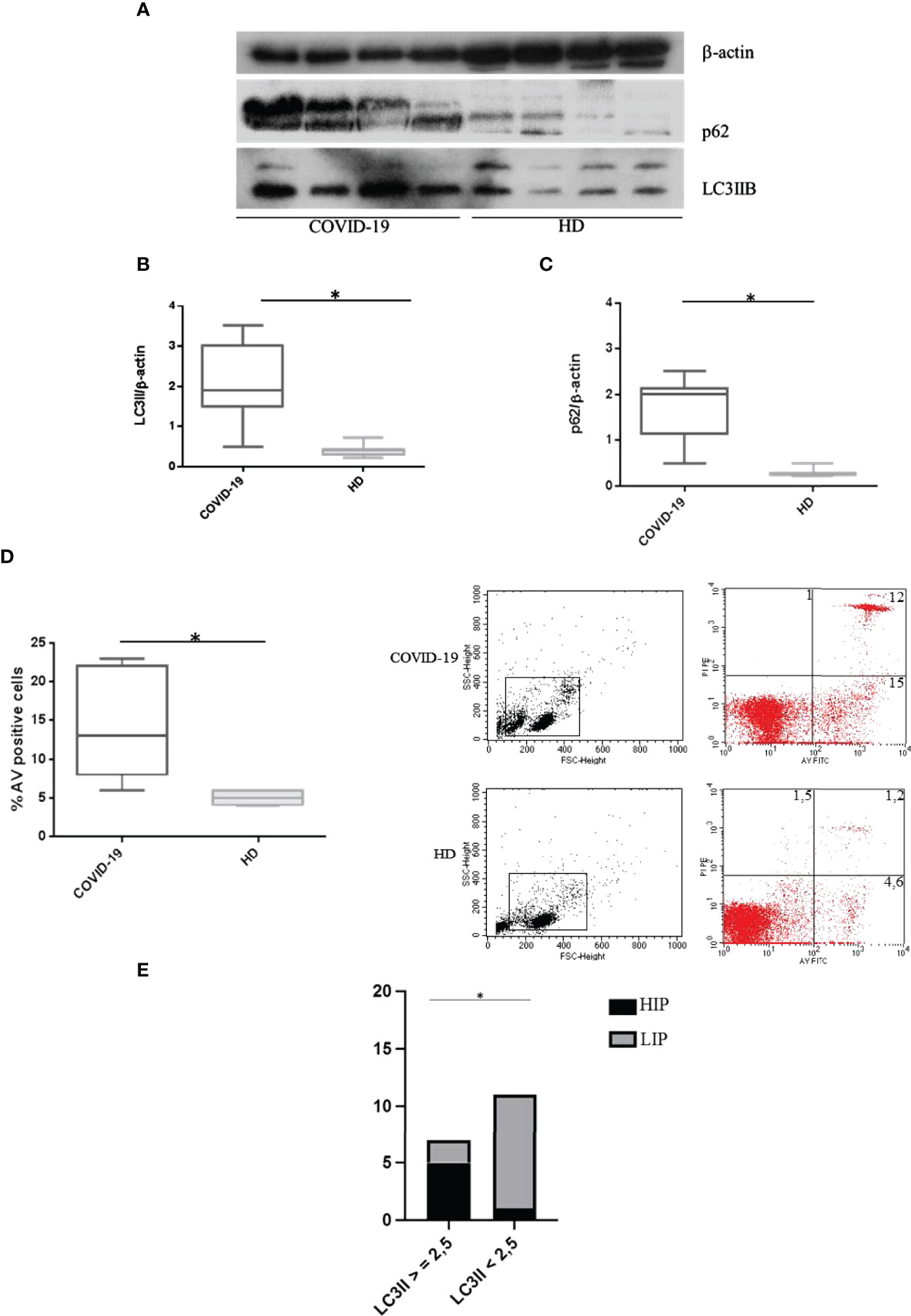
Figure 1 Spontaneous autophagy and apoptosis in COVID-19 patients and HDs. (A) LC3IIB and p62 Western blotting analysis of PBMC lysates (30 μg/lane) from 4 COVID-19 patients and 4 HDs. Blots shown are representative of independent experiments performed on COVID-19 patients (n = 18) and HDs (n = 12). Quantification of LC3IIB (B) and p62 (C) levels relative to β-actin is also shown (mean with range is presented) (Mann–Whitney test). (D) Flow cytometry analysis of PBMC apoptosis. Data are referred to as AV-positive cells and are presented as independent experiments performed in PBMC from COVID-19 patients (n = 18) and HDs (n = 12) (Mann–Whitney test). (E) Contingency: prospective data (chi-square test) between LC3IIB level and HIP/LIP patients. Values are expressed as means ± SD. *p < 0.05. COVID-19, coronavirus disease 2019; HDs, healthy donors; PBMC, peripheral blood mononuclear cell; AV, annexin V; HIP, high inflammatory profile; LIP, low inflammatory profile.
Interestingly, we observed that patients showing high levels of LC3IIB (>2.5) were classified as HIP (Figure 1E).
Moreover, COVID-19 immunophenotyping studies revealed higher levels of autophagolysosome formation in CD4+ and CD8+ cells compared to CD14+ cells (p = 0.04 and p = 0.0007, respectively); however, the autophagy level in CD19+ cells was not higher as compared to CD14+ cells (Figure 1A—Supplementary Material).
According to these results, we hypothesized an autophagy dysfunction and/or blockade in PBMCs from COVID-19 patients. Thus, we assessed in vitro experiments with lysosomal inhibitors E64d and pepstatin A. In PBMCs from patients affected by COVID-19, LC3IIB levels did not change in the presence of lysosomal inhibitors, compared to untreated cells (p > 0.05) (Figures 2A, B). As previously demonstrated (11), we observed an increase in LC3IIB levels in HD PBMCs treated with lysosomal inhibitors versus untreated (p = 0.0286) (Figures 2A, B). Concurrently, p62 levels did not change in COVID-19 PBMCs treated with lysosomal inhibitors but increased in treated PBMCs from HDs, compared to untreated in both conditions (p > 0.05 and p = 0.0286, respectively) (Figures 2A, C). This result explains how a blocked mechanism does not undergo modifications under further inhibitory conditions. So PBMCs from COVID-19 patients, in which the mechanism is blocked, contrary to HD PBMCs, do not respond to inhibitors, and neither LC3IIB nor p62 nor apoptosis changed under E64d/pepstatin A treatment.

Figure 2 Effects of lysosomal inhibitors E64d and pepstatin A on PBMC autophagy. (A) LC3IIB and p62 Western blotting analysis of PBMC lysate (30 μg/lane) from 2 representative COVID-19 patients and 2 HDs (10 independent experiments). Where indicated, cells were treated with the lysosomal inhibitors E64d and pepstatin A indicated as “ini.” Densitometry analysis of LC3IIB (B) and p62 (C) levels relative to β-actin is also shown (Mann–Whitney test). Values are expressed as means ± SD. *p < 0.05. PBMC, peripheral blood mononuclear cell; COVID-19, coronavirus disease 2019.
However, we observed variability in p62 expression levels among patients in the COVID-19 cohort (Figure 3). In detail, LIP patients were susceptible to E64d/pepstatin A treatment (p < 0.0001 for autophagy, p = 0.002 for apoptosis, versus untreated) (i.e., pz 2 in Western blotting of Figures 3A–C), while HIP patients did not respond to autophagy inhibitors (p > 0.05) [i.e., pz 1 in (Figure 3A–C)].
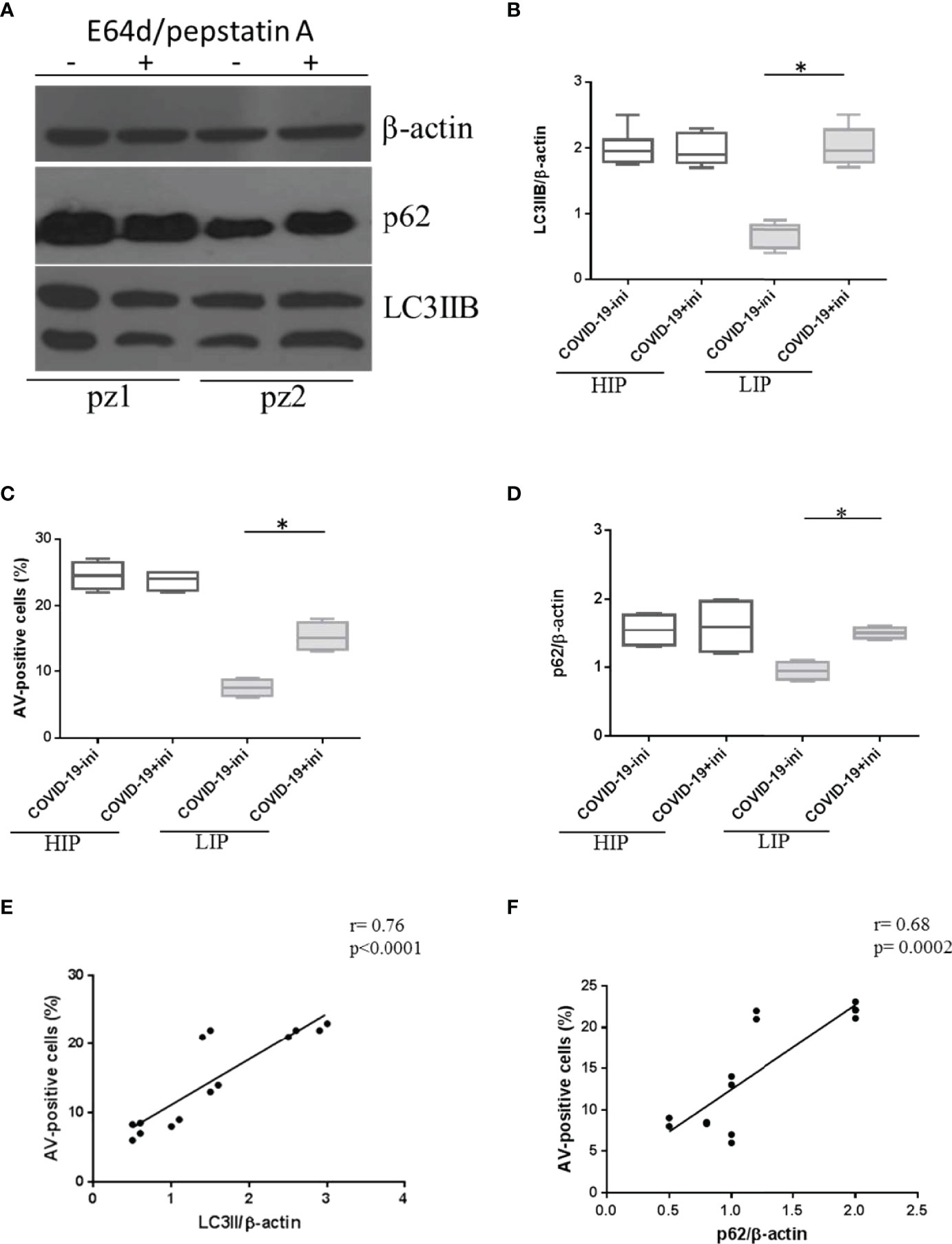
Figure 3 Expression level of autophagy and apoptosis markers in HIP and LIP COVID-19 patients treated with lysosomal inhibitors. (A) LC3IIB and p62 Western blotting analysis of PBMC lysate (30 μg/lane) from 2 representative COVID-19 patients (1 HIP indicated as pz1 and 1 LIP indicated as pz2 of the 18 analyzed). Densitometry analysis of LC3IIB (B) and p62 (D) levels relative to β-actin is also shown (Mann–Whitney test). (C) Flow cytometry analysis of PBMC apoptosis. Data are referred to as AV-positive cells and are presented as independent experiments performed in PBMCs from COVID-19 patients (n = 18) (Student’s t-test). Where indicated, cells were treated with the lysosomal inhibitors E64d and pepstatin A indicated as “ini.” Values are expressed as means ± SD. *p < 0.05. (E, F) Correlation and linear regression analysis of apoptosis and LC3IIB and p62 levels in PBMCs from COVID-19 patients. HIP, high inflammatory profile; LIP, low inflammatory profile; PBMC, peripheral blood mononuclear cell; COVID-19, coronavirus disease 2019; AV, annexin V.
As expected, in HIP patients displaying a basal block of autophagy and higher levels of apoptosis, the amount of p62 in the presence of lysosomal inhibitors versus untreated did not change (p > 0.05) (Figure 3D); on the contrary, LIP patients presenting basal levels of autophagy and apoptosis, similar to HDs, showed an accumulation of p62 under lysosomal inhibitors treatment (p = 0.0008) (Figure 3D).
In addition, a positive correlation between autophagy markers and apoptosis was observed in PBMCs from COVID-19 patients (Figures 3E, F), leading us to speculate that autophagy hijacking in PBMCs from COVID-19 patients is directly involved in cell death.
To confirm the alteration in the autophagy process, immunofluorescence analysis was also used to verify the expression levels of the autophagosome marker LC3IIB and the lysosome marker LAMP1. Specifically, the presence of intracellular autophagolysosomes indicating the ongoing PBMC autophagy was assessed by the detection of colocalization between LC3IIB and LAMP1, revealed by the yellow color in immunofluorescence analysis (Figure 4).
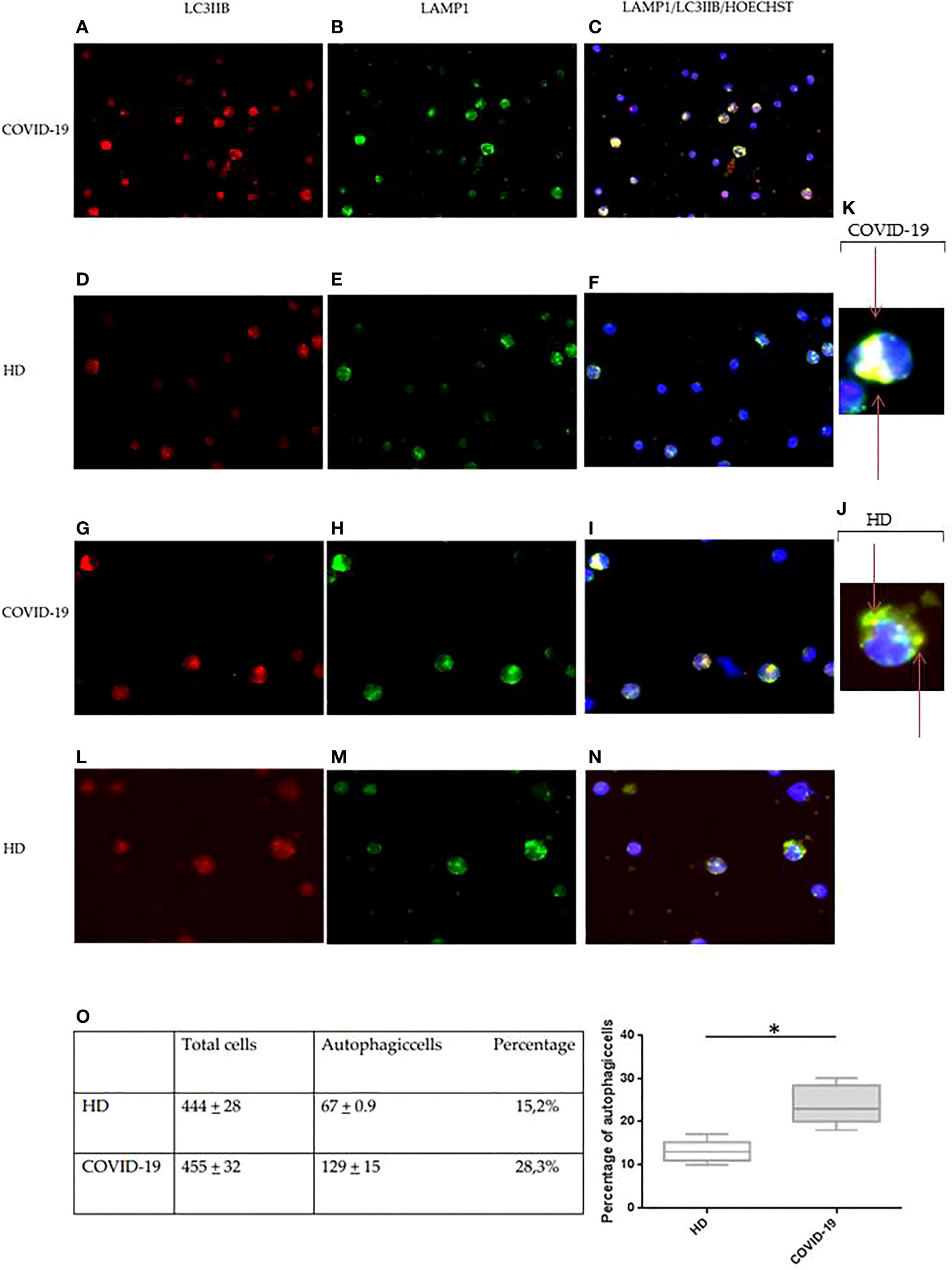
Figure 4 Immunofluorescence analysis of autophagy in PBMCs from COVID-19 patients and HDs. LC3IIB (red fluorescence) and LAMP1 (green fluorescence) expression in PBMCs from COVID-19 patients (A, B, G, H) and HDs (D, E, L, M). Intracellular autophagolysosome formation detection by colocalization between LC3IIB and LAMP1 (yellow fluorescence) in PBMCs from COVID-19 patients (C, I) and HDs (F, N). (A–F) Magnification, 50×. (G-I, L-N) Magnification, 100×. (J, K) Magnification, x200. (O) Table of total cells and autophagic cells percentage and analysis of the percentage of autophagic cells in COVID-19 and HD PBMCs (Student’s t-test). Values are expressed as means ± SD. *p < 0.05. PBMC, peripheral blood mononuclear cell; COVID-19, coronavirus disease 2019; HDs, healthy donors.
In PBMCs from COVID-19 and HDs, the expression of LC3IIB and LAMP1 was diffusely detectable (Figure 4). However, the expression of both LC3IIB puncta and LAMP1 was more regularly distributed in PBMCs from HDs (Figures 4D, E, L, M) as compared to PBMCs from COVID-19 (Figures 4A, B, G, H).
Additionally, the aberrant autophagolysosome formation was commonly observed in PBMCs from COVID-19 patients (Figures 4C, I), showing a diffuse pattern, while autophagolysosomes of PBMCs from HDs had the typical dotted pattern (Figures 4F, N). This result confirmed an alteration of the degradation cellular mechanism.
The autophagic cells and the total stained cells were then counted in 10 different selected fields per specimen, and autophagic cells resulted from 28.1% in COVID-19 PBMCs and 15.2% in HD PBMCs (p = 0.0001) (Figure 4O).
Our findings suggest the presence of soluble factors able to hijack PBMCs autophagy and apoptosis in COVID-19 patients’ sera. To confirm this, we conducted in vitro studies treating PBMCs from HDs with COVID-19 patients’ sera for 24 h. For this set of experiments, we chose sera from a pool of COVID-19 patients classified as HIP or LIP.
Interestingly, sera from COVID-19 patients were able to modulate autophagy and apoptosis based on their inflammatory profile (Figure 5). PBMCs from HDs in vitro cultured with sera from COVID-19 patients classified as HIP, displayed high levels of apoptosis and a block of autophagy, as confirmed by LC3IIB and p62 accumulation (p = 0.02, p = 0.002, and p = 0.0007 versus untreated, for apoptosis, LC3IIB, and p62 levels, respectively). In contrast, sera from patients classified as LIP were not able to modulate apoptosis and autophagy in PBMCs from HDs in vitro (p > 0.05 for all parameters) (Figures 5A–D).
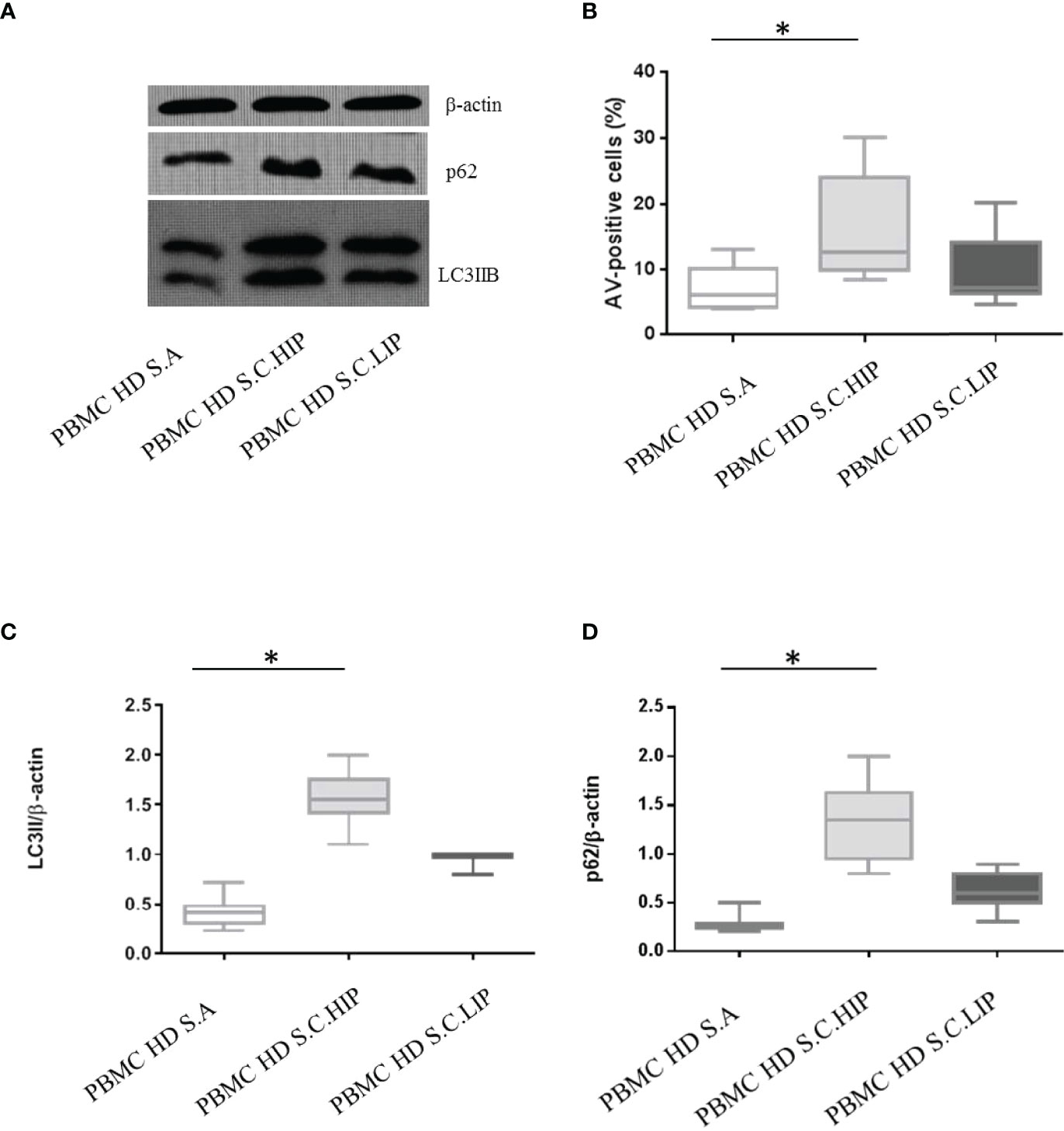
Figure 5 Effects of sera from patients with COVID-19 on HD PBMC autophagy and apoptosis. (A) LC3IIB and p62 Western blotting analysis of PBMC lysate (30 μg/lane) from 1 representative HDs in vitro treated with autologous sera (S.A), with sera from 1 HIP COVID-19 patients (S.C.HIP) and from 1 COVID-19 classified as LIP (S.C.LIP) (B) Flow cytometry analysis of PBMC apoptosis. Data are referred to as AV-positive cells and are presented as 10 independent experiments performed on HDs in vitro treated with sera from HIP COVID-19 patients and COVID-19 classified as LIP (Student’s t-test). Densitometry analysis of LC3IIB (C) and p62 (D) levels relative to β-actin is also shown (10 independent experiments) (Mann–Whitney test). Values are expressed as means ± SD. *p < 0.05. PBMC, peripheral blood mononuclear cell; HD, healthy donor; COVID-19, coronavirus disease 2019; HIP, high inflammatory profile; LIP, low inflammatory profile.
Finally, we observed a significant correlation between lymphopenia and autophagic/apoptotic markers in COVID-19 PBMCs. In particular, peripheral lymphocyte count negatively correlated with LC3IIB and p62 levels and with cell apoptosis rate (Figures 6A–C).
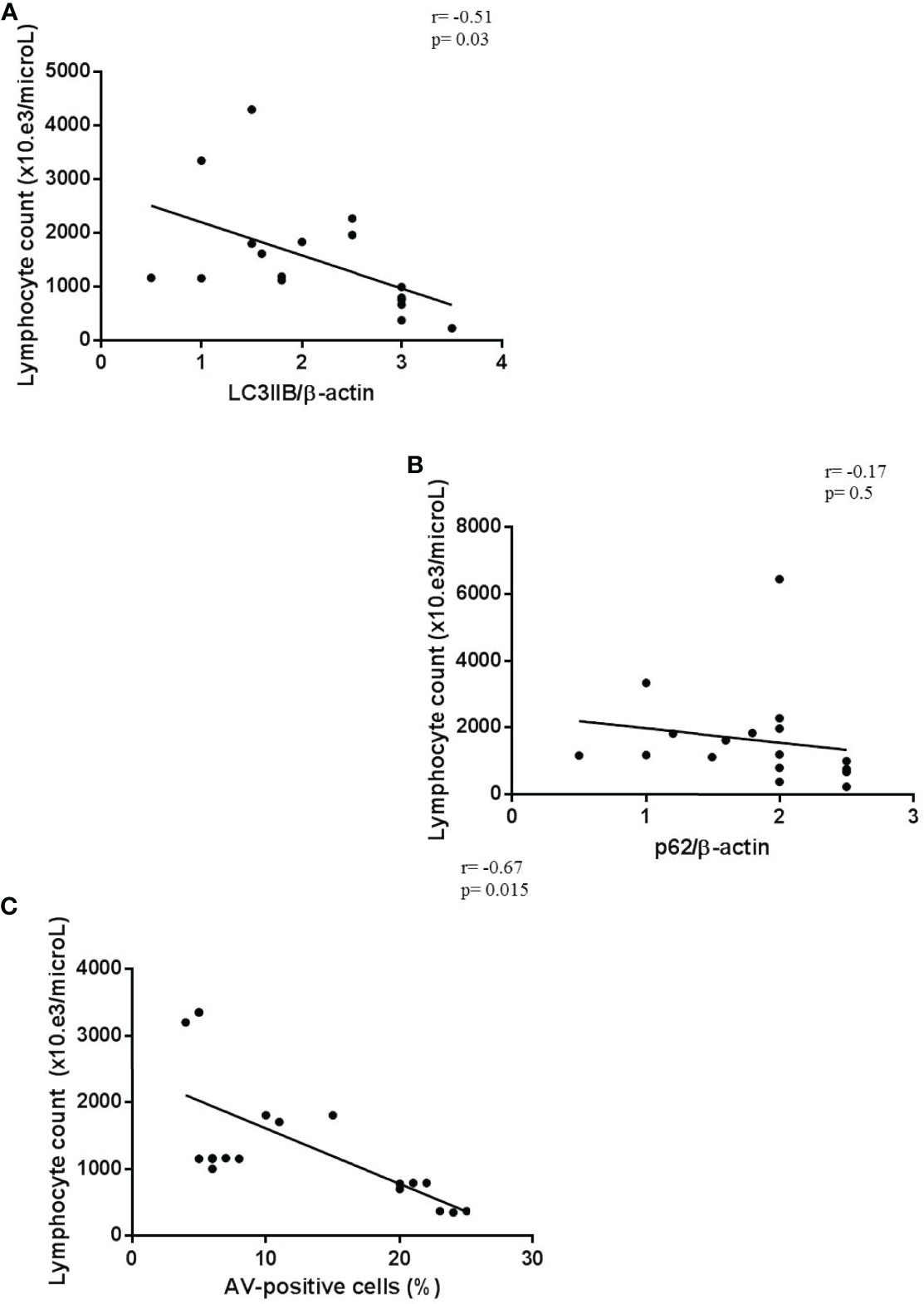
Figure 6 Correlation between lymphopenia and autophagy/apoptotic markers. (A–C) Correlation and linear regression analysis of lymphocyte count and LC3IIB and p62 levels, and apoptosis in PBMCs from COVID-19 patients (n = 18) (Spearman’s rank correlation). PBMC, peripheral blood mononuclear cell; COVID-19, coronavirus disease 2019.
These results suggest that in these patients the inflammatory condition could interfere with the major mechanism of cellular survival, which is autophagy, leading to lymphocyte death by apoptosis.
In our study, we firstly showed a block of autophagy in PBMCs from COVID-19 patients who displayed high expression of LC3IIB and p62 levels. These results were strengthened by experiments under lysosomal inhibition conditions, in which lysosomal proteases did not affect autophagy levels in HIP COVID-19 PBMCs but affected autophagy only in those patients showing a LIP.
It is well known that autophagy is a mechanism involved in the most important steps of the immune response, such as intracellular pathogen sensing, lymphocyte development, homeostasis, and survival (14). Although autophagy is a cell survival mechanism, it is also linked to cell death, through the interaction with apoptosis-related proteins (15).
In the present study, patients with a HIP showed a block of autophagy and a concomitant high percentage of PBMCs undergoing apoptosis. This could mean that cellular death is the direct consequence of the aberration of the survival mechanism. This hypothesis is supported by the increased apoptosis and the block of autophagy in PBMCs from HDs in vitro treated with COVID-19 sera showing HIP.
In PBMCs from COVID-19 patients, at a molecular level, the interplay between lymphocyte autophagy and apoptosis has never been investigated, but our results indicate cytokines or circulating proinflammatory molecules as a possible main culprit. In literature, it is known that many circulating cytokines, such as IL-6, TNF-alpha, and BlyS, as in autoimmune/inflammatory diseases, could be involved in the regulation of this process (16, 17), causing a cytokine storm that could contribute to a more severe COVID-19 disease also by exhaustion of lymphocytes (18). Data from the present study suppose a possible effect of the virus on lymphocyte autophagy dysregulation, likely as a result of a cytokine storm (19).
To strengthen our hypothesis on the autophagy blockade in circulating lymphocytes from COVID-19 patients, the analysis of the autophagolysosome formation level in the PBMC subpopulations, which is significantly higher in lymphocytes than in monocytes, contributes to lymphocyte death. In this regard, we observed also a strong positive correlation between autophagy markers and apoptosis.
In addition, the indirect correlation between autophagy/apoptotic markers and lymphocyte count demonstrates the interaction between autophagy block and the concomitant apoptosis increase with the decrease in circulating lymphocytes. Moreover, lymphocytes are crucial in the maintenance of immune homeostasis and inflammatory response; thus, the understanding of the mechanism of reduced blood lymphocyte levels could provide an additional strategy for the treatment of COVID-19 (20).
In our previous study, we speculated the direct infection of the virus on lymphocytes, resulting in death due to lymphocyte expression of the coronavirus receptor ACE2 (19). In accordance with our hypothesis, Tan et al. added the direct destruction of lymphatic organs by the virus as a possible cause of lymphocyte decline, with the inflammatory cytokine storm that leads to lymphocyte apoptosis (20). The authors concluded that lymphopenia is an indicator of the severity of COVID-19 hospitalized patients and suggested including the evaluation of blood lymphocyte percentage in the guidelines for the diagnosis of COVID-19 (21). Our results reinforce this suggestion and add new knowledge on the mechanisms underlying lymphopenia in COVID-19 patients.
Considering these results, drugs targeting autophagy could represent an important issue, worthy to be considered as a new therapeutic strategy in the context of COVID-19. Although additional studies are needed to confirm our hypotheses, since autophagy and apoptosis are usually involved in many disease conditions, this study provides intriguing data to better understand the mechanisms underlying COVID-19 and causing the disease progression.
The strength of this study is the use of different experimental approaches to confirm a block of autophagy in PBMCs from COVID-19 patients. Despite the promising information obtained from the analysis of COVID-19 PBMC homeostasis, limitations are present. The number of patients is small due to the difficult enrollment during the pandemic period. In addition, we do not know the patients’ therapy that could interfere with the investigated mechanisms.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The study was approved by the ethics committee of Sapienza University of Rome (protocol number 0586/20). The patients/participants provided their written informed consent to participate in this study.
All authors have made a substantial, direct, and intellectual contribution to the work and approved the final manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.903498/full#supplementary-material
COVID-19, coronavirus disease 2019; FBS, fetal bovine serum; HDs, healthy donors; HIP, high inflammatory profile; LIP, low inflammatory profile; PBMCs, peripheral blood mononuclear cells; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2
1. Chakraborty I, Maity P. COVID-19 Outbreak: Migration, Effects on Society, Global Environment and Prevention. Sci Total Environ (2020) 728:138882. doi: 10.1016/j.scitotenv.2020.138882
2. Lucas C, Wong P, Klein J, Castro TBR, Silva J, Sundaram M, et al. Longitudinal Analyses Reveal Immunological Misfiring in Severe COVID-19. Nature (2020) 584(7821):463–9. doi: 10.1038/s41586-020-2588-y
3. Fathi N, Rezaei N. Lymphopenia in COVID-19: Therapeutic Opportunities. Cell Biol Int (2020) 44:1792–7. doi: 10.1002/cbin.11403
4. Hasichaolu, Zhang X, Li X, Li X, Li D. Circulating Cytokines and Lymphocyte Subsets in Patients Who Have Recovered From COVID-19. BioMed Res Int (2020) 2020:7570981. doi: 10.1155/2020/7570981
5. Cui B, Lin H, Yu J, Yu J, Hu Z. Autophagy and the Immune Response. Adv Exp Med Biol (2019) 1206:595–634. doi: 10.1007/978-981-15-0602-4_27
6. Mizushima N, Ohsumi Y, Yoshimori T. Autophagosome Formation in Mammalian Cells. Cell Struct Funct (2002) 27:421–9. doi: 10.1247/csf.27.421
7. Su M, Mei Y, Sinha S. Role of the Crosstalk Between Autophagy and Apoptosis in Cancer. J Oncol (2013) 2013:102735. doi: 10.1155/2013/102735
8. Liu Y, Levine B. Autosis and Autophagic Cell Death: The Dark Side of Autophagy. Cell Death Differ (2015) 22:367–76. doi: 10.1038/cdd.2014.143
9. Shojaei S, Suresh M, Klionsky DJ, Labouta HI, Ghavami S. Autophagy and SARS-CoV-2 Infection: Apossible Smart Targeting of the Autophagy Pathway. Virulence (2020) 11:32567972. doi: 10.1080/21505594.2020.1780088
10. Zaboli E, Majidi H, Alizadeh-Navaei R, Hedayatizadeh-Omran A, Asgarian-Omran H, Vahedi Larijani L, et al. Lymphopenia and Lung Complications in Patients With Coronavirus Disease-2019 (COVID-19): A Retrospective Study Based on Clinical Data. J Med Virol (2021) 93(9):5425–31. doi: 10.1002/jmv.27060
11. Alessandri C, Barbati C, Vacirca D, Piscopo P, Confaloni A, Sanchez M, et al. T Lymphocytes From Patients With Systemic Lupus Erythematosus are Resistant to Induction of Autophagy. FASEB J (2012) 26:4722–32. doi: 10.1096/fj.12-206060
12. Klionsky DJ, Abdel-Aziz AK, Abdelfatah S, Abdellatif M, Abdoli A, Abel S, et al. Guidelines for the Use and Interpretation of Assays for Monitoring Autophagy (4th Edition)1. Autophagy (2021) 17:1–382. doi: 10.1080/15548627.2020.1797280
13. Wang F, Li B, Schall N, Wilhelm M, Muller S. Assessing Autophagy in Mouse Models and Patients With Systemic Autoimmune Diseases. Cells (2017) 6(3):16. doi: 10.3390/cells6030016
14. Puleston DJ, Simon AK. Autophagy in the Immune System. Immunology (2014) 141:1–8. doi: 10.1111/imm.12165
15. Yonekawa T, Thorburn A. Autophagy and Cell Death. Essays Biochem (2013) 55:105–17. doi: 10.1042/bse0550105
16. Barbati C, Colasanti T, Vomero M, Ceccarelli F, Celia AI, Perricone C, et al. Up-Regulation of Autophagy by Etanercept Treatment Results in TNF-Induced Apoptosis Reduction in EA.hy926 Endothelial Cell Line. Clin Exp Rheumatol (2021) 39:606–11.
17. Liao YC, Liang WG, Chen FW, Hsu JH, Yang JJ, Chang M.S. IL-19 Induces Production of IL-6 and TNF-Alpha and Results in Cell Apoptosis Through TNF-Alpha. J Immunol (2002) 169:4288–97. doi: 10.4049/jimmunol.169.8.4288
18. Yang L, Xie X, Tu Z, Fu J, Xu D, Zhou Y. The Signal Pathways and Treatment of Cytokine Storm in COVID-19. Signal Transduct Target Ther (2021) 6:255. doi: 10.1038/s41392-021-00679-0. Erratum in: Signal Transduct Target Ther. 2021 Aug 31;6(1):326.
19. Vomero M, Barbati C, Colasanti T, Celia AI, Speziali M, Ucci FM, et al. Autophagy Modulation in Lymphocytes From COVID-19 Patients: New Therapeutic Target in SARS-COV-2 Infection. Front Pharmacol (2020) 11:569849. doi: 10.3389/fphar.2020.569849
Keywords: autophagy, apoptosis, inflammation, lymphocytes, SARS-CoV-2, COVID-19
Citation: Barbati C, Celia AI, Colasanti T, Vomero M, Speziali M, Putro E, Buoncuore G, Savino F, Colafrancesco S, Ucci FM, Ciancarella C, Balbinot E, Scarpa S, Natalucci F, Pellegrino G, Ceccarelli F, Spinelli FR, Mastroianni CM, Conti F and Alessandri C (2022) Autophagy Hijacking in PBMC From COVID-19 Patients Results in Lymphopenia. Front. Immunol. 13:903498. doi: 10.3389/fimmu.2022.903498
Received: 24 March 2022; Accepted: 26 April 2022;
Published: 30 May 2022.
Edited by:
Bart Tummers, St. Jude Children’s Research Hospital, United StatesReviewed by:
Qingjun Pan, Affiliated Hospital of Guangdong Medical University, ChinaCopyright © 2022 Barbati, Celia, Colasanti, Vomero, Speziali, Putro, Buoncuore, Savino, Colafrancesco, Ucci, Ciancarella, Balbinot, Scarpa, Natalucci, Pellegrino, Ceccarelli, Spinelli, Mastroianni, Conti and Alessandri. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cristiana Barbati, Y3Jpc3RpYW5hLmJhcmJhdGkxQGdtYWlsLmNvbQ==
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.