
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 12 July 2022
Sec. Multiple Sclerosis and Neuroimmunology
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.900642
This article is part of the Research Topic Immune Response in Neurodegenerative Diseases, Cerebrovascular Disease, Intracranial and Skull Base Tumors View all 5 articles
 Tao Sun1,2
Tao Sun1,2 Fei Wang1,2
Fei Wang1,2 Yiming He1,2
Yiming He1,2 Bo Mao1,2
Bo Mao1,2 Mengtao Han1,2
Mengtao Han1,2 Han Liu2,3
Han Liu2,3 Peng Zhao1,2
Peng Zhao1,2 Xingang Li1,2*
Xingang Li1,2* Donghai Wang1,2*
Donghai Wang1,2*Atherosclerosis is a chronic inflammatory disease closely associated with immunological activity. Lymph nodes (LNs) are essential secondary lymphoid organs, in which complex immune responses occur. Enlarged LNs are commonly observed around inflamed tissues or tumors; however, their role in atherosclerosis is not well understood. We hypothesized that enlarged pericarotid LNs would be present in symptomatic patients with carotid atherosclerosis. Therefore, we recorded the size of LNs around the carotid artery during surgery in patients undergoing carotid endarterectomy (CEA) for carotid atherosclerotic stenosis. Patients were stratified by enlarged LNs, defined as a diameter ≥ 10mm in the transverse diameters. Demographic and clinical data of participants were measured and analyzed. Hematoxylin and eosin (H&E), Sirius red, DAB-enhanced Perls’ Prussian blue, alizarin red, and immunohistochemistry (IHC) staining were performed for composition identification of plaques or LNs. Symptomatic patients were defined as those presenting with an ipsilateral cerebral ischemic event. Compared with patients with non-enlarged LNs, patients with enlarged LNs were more likely to be symptomatic (22/32, 68.8% versus 9/40, 22.5%, P < 0.001) and use calcium channel blocker drugs (17/32, 53.1% versus 10/40, 25%, P=0.014). In addition, they showed lower body mass index (mean ± SD: 24.00 ± 2.66 versus 25.34 ± 2.56 kg/m2, P=0.034), lower weight (median [interquartile range]: 64 [60.00-76.00] versus 72.5 [65.00-77.50] Kg, P = 0.046) and higher diastolic blood pressure (mean ± SD: 78.94 ± 9.30 versus 73.93 ± 8.84 mmHg, P = 0.022). The plague from patients with enlarged LNs exhibited a lower relative percentage of fibrous tissue (29.49 ± 10.73% versus 34.62 ± 10.33%, P = 0.041). The enlarged LNs remained oval-shaped by visual inspection. Compared to non-enlarged LNs, the predominant changes in enlarged LNs were atrophic lymphatic sinuses and dilated LNs parenchyma. Enlarged LNs contained more germinal centers and lymphocytes. In conclusion, symptomatic patients with carotid atherosclerosis have enlarged pericarotid LNs. The current study supports the conclusion that enlarged LNs with an activated and enhanced adaptive immune response may indicate plaque instability. Pericarotid LNs will be a promising marker of plaque stability and may be a potential therapeutic target in patients with carotid atherosclerosis.
Atherosclerosis is a chronic inflammatory disease closely related to intense immunological activity (1, 2). Accumulation of lipids, vascular inflammation, immune cell activation, foam cell formation, cell apoptosis, and necrosis play important roles in both formation and development of atherosclerotic plaques (3). As the lesion progresses, unstable atherosclerotic plaques tend to rupture and cause ischemic stroke.
As early as 1980, Lemole et al. identified lymphstasis as a critical factor in the genesis of arteriosclerosis (4), but the role of the lymphatic network system, which carries a wide variety of immune cells, in the development of atherosclerosis has only recently been explored. Martel et al. introduced the concept of macrophage reverse cholesterol transport (mRCT) and identified the role of lymphatic vessels in reverse cholesterol transport (5). Subsequent studies have also shown that the lymphatic network is an important way of mobilizing cholesterol from the artery walls (6–8).
Lymph nodes (LNs) are essential secondary lymphoid organs that play an important role in immune responses (9). Enlarged LNs, which are commonly observed around inflamed tissues or tumors, usually indicate a strong inflammatory and immune responses (10, 11). Particular antigens, bacteria and viruses flowing through draining LNs can be effectively cleared, indicating that LNs are an effective filter (9, 12). LNs draining lymph from the arteries may play a more complex role in the course of atherosclerosis. Ox-low-density lipoproteins (LDLs) deposited in plaques can be taken up by DCs and then presented to T cells in draining LNs, thus initiating adaptive immune (2). However, in a healthy artery wall, resident DCs usually exert immune tolerance by silencing T cells (13).
Recent single-cell sequencing has uncovered a new function of LN endothelial cells in scavenging LDLs (14); During the carotid endarterectomy (CEA), enlarged pericarotid LNs were observed in some patients (Figure 1B). In this study, we aimed to explore the relationship between enlarged LNs and recent ischemic symptoms, which helped us improve our understanding of the predictors of plaque vulnerability and better treat this high-risk group of patients with carotid atherosclerosis.
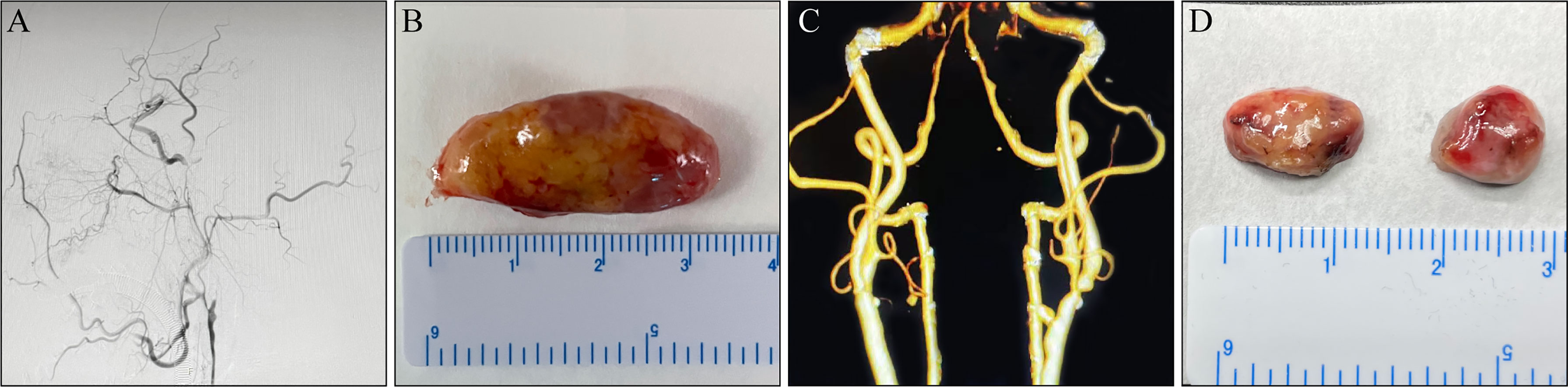
Figure 1 Macroscopic lymph nodes (LNs) morphology. (A, B) A 65-year-old man complaining of alalia for 1 month was hospitalized. (A) Digital subtraction angiogram (DSA) before carotid endarterectomy (CEA) revealed a left internal carotid artery (ICA) with complete occlusion. (B) An enlarged LN around the carotid artery. (C, D) A 74-year-old man was admitted to hospital owing to “carotid artery stenosis found on physical examination for 3 months”. (C) DSA before CEA revealed a left ICA with severe stenosis. (D) Non-enlarged LNs around the carotid artery.
This study was approved by the ethics committee of our hospital. Informed consent was obtained from all patients prior to the procedure. Patients undergoing CEA for carotid atherosclerotic stenosis or occlusion in our department were enrolled consecutively in the study between December 2020 and January 2022. All patients were clearly diagnosed and other etiologies were excluded by Doppler ultrasound, computed tomography angiography (CTA), magnetic resonance imaging or angiography, and digital subtraction angiography (DSA). The minimum age limit was 18. Patients with neck tumors, a history of neck radiation, lymphoma, and lymph node tuberculosis were excluded. LN size around the carotid artery, including longitudinal and transversal diameters, was recorded during the operation. If there is more than one lymph node around the carotid artery, the largest one is recorded. Enlarged LNs were defined as those with transverse diameters ≥ 10 mm (15, 16). Symptomatic patients had a history of amaurosis fugax, transient ischemic attack, or ischemic stroke ipsilateral to the extracted plaques within six months before the endarterectomy procedure. Conversely, asymptomatic patients did not have a history of cerebrovascular events (17). Data on venous blood samples, and the clinical and demographic characteristics of the participants were gathered from patients before CEA.
For this study, 72 formalin-fixed paraffin-embedded blocks of carotid artery specimens met the inclusion criteria. All samples exhibited morphological characteristics of atherosclerotic plaques such as a necrotic core, connective tissue, and the presence of at least a portion of the fibrous cap in sectioned slides. Hematoxylin and eosin (H&E) staining was performed according to standard procedures (18). Macrophages were identified in consecutive sections by immunohistochemistry (IHC) using primary antibodies against CD68 (Cell Signaling Technology, 76437T) (19). Picrosirius red staining was used to detect collagen fibers (19). DAB-enhanced Perls’ Prussian blue staining was used to evaluate the presence of intraplaque hemorrhage (19). Alizarin red staining was used to evaluate calcium deposition (19). Quantification of staining was documented as the threshold area divided by the lesion area using ImageJ software.
H&E staining and IHC were performed on formalin-fixed and paraffin-embedded LNs, which included nine enlarged and five non-enlarged LNs. All LNs were dissected transversely in the middle of the longitudinal axis and serially sectioned. The primary antibodies used were anti-CD20 (Cell Signaling Technology, 48750T, 1: 100) for identifying B cells, anti-CD68 (Cell Signaling Technology, 76437T) for identifying macrophages, anti-CD3 (Servicebio, GB11014) for identifying T cells, and anti-Ki67(Cell Signaling Technology, 9449T) for identifying proliferating cells (18). Diluents without primary antibodies were used as negative controls. Staining was visualized using the Dako REAL™ EnVision™ Detection System followed by counterstaining with hematoxylin. Images were captured using a digital camera under a light microscope (VS120; Olympus). The number of germinal centers (GCs) observed was counted in one HE-stained section. The proportions of CD68- and Ki67-positive cells in the LNs were calculated as positive cells versus total cells in at least five randomly selected areas under ×1000 magnification of microscopic fields.
In this study, all statistical analyses were performed using the SPSS software version (version 25.0; SPSS, Inc., Chicago, IL, USA). Normally distributed continuous variables were expressed as the mean ± standard deviation, and were analyzed using the Student’s t-test. Abnormally distributed continuous variables were expressed as median (interquartile range [IQR]) and analyzed by the Mann-Whitney U test. Categorical variables were described as percentages, and were analyzed using the chi-square test or Fisher’s exact test. Statistical significance was set at P < 0.05.
Baseline clinical, biochemical, and demographic characteristics of the study participants are shown in Table 1. No significant differences in age, sex, systolic blood pressure, pulse pressure, serum low-density lipoprotein cholesterol, cholesterol, or triglyceride levels were found between patients with enlarged and non-enlarged LNs (P > 0.05). Compared with patients without enlarged LNs, patients with enlarged LNs were more likely to be symptomatic (68.8% vs. 22.5%, P < 0.001) and more likely to use calcium channel blocker drugs (53.1% vs. 25%, P = 0.014). In addition, they showed lower BMI values (mean ± SD: 24.00 ± 2.66 versus 25.34 ± 2.56 kg/m2, P = 0.034), lower weight (median [interquartile range]: 64 [60.00-76.00] versus 72.5 [65.00-77.50] Kg, P = 0.046), and higher diastolic blood pressure (mean ± SD: 78.94 ± 9.30 versus 73.93 ± 8.84 mmHg, P = 0.022).
The plagues from patients with enlarged LNs exhibited a lower relative percentage of fibrous tissue (29.49 ± 10.73% vs. 34.62 ± 10.33%, P = 0.041, Table 2). There were no significant differences in incidences of intraplaque hemorrhage or plaque calcification between patients with enlarged and non-enlarged LNs (P > 0.05, Table 2). Macrophage infiltration was present in all plaques, mainly in the shoulder region (Figures 2I–L). No difference was observed in the relative percentage of the histological components of atherosclerosis including fibrosis, plaque hemorrhage, and calcium between the two groups (P > 0.05, Table 2).
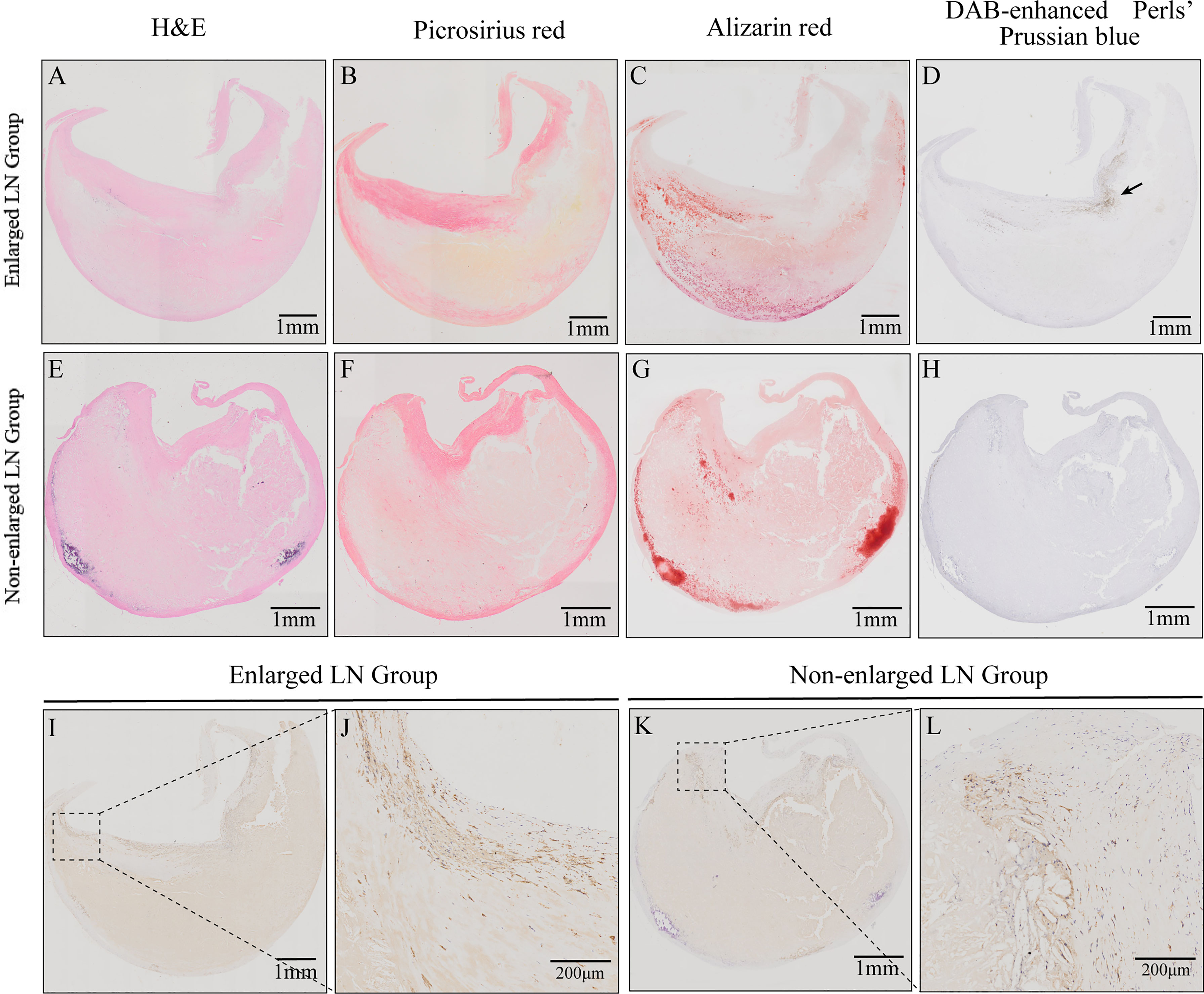
Figure 2 Histological characteristics of carotid atherosclerotic plaques from patients with enlarged or non-enlarged lymph nodes (LNs). (A, E) Hematoxylin and eosin (H&E) staining. (B, F) Relative content of collagen fibers and thickness of fiber cap. (C, G) Relative content of calcification and calcium morphology. (D, H) Intraplaque hemorrhage (arrow). (I–L) Immunohistochemistry (IHC) staining revealed macrophages and their infiltration in the shoulder regions.
Morphologically, the enlarged LNs were oval (transverse diameter, mean ± SD:13.12 ± 2.23 mm), whereas the non-enlarged LNs (transverse diameter, mean ± SD: 7.28 ± 1.34 mm) were round or oval (Figure 1). The predominant changes in enlarged LNs were atrophic lymphatic sinuses and dilated parenchyma (Figures 3A, B). Compared with non-enlarged LNs, enlarged LNs contained more and larger GCs (Figures 3A, B, K). The proportion of Ki67 positive cells was increased in the cortex of enlarged LNs compared with that of non-enlarged LNs (Figures 3C, D, L). The proportion of Ki67-positive cells was similar regarding GCs between the two (Figures 3C, D, M). CD68-positive macrophages were mainly infiltrated in the lymphoid sinus (Figures 3E, F). The proportion of CD68-positive macrophages showed no apparent changes in the cortex excluding sinus of enlarged LNs, but was increased slightly in the GCs (Figures 3E, F, N, O). The number of CD20-positive B cells and CD3-positive T cells increased in enlarged LNs (Figures 3G–J).
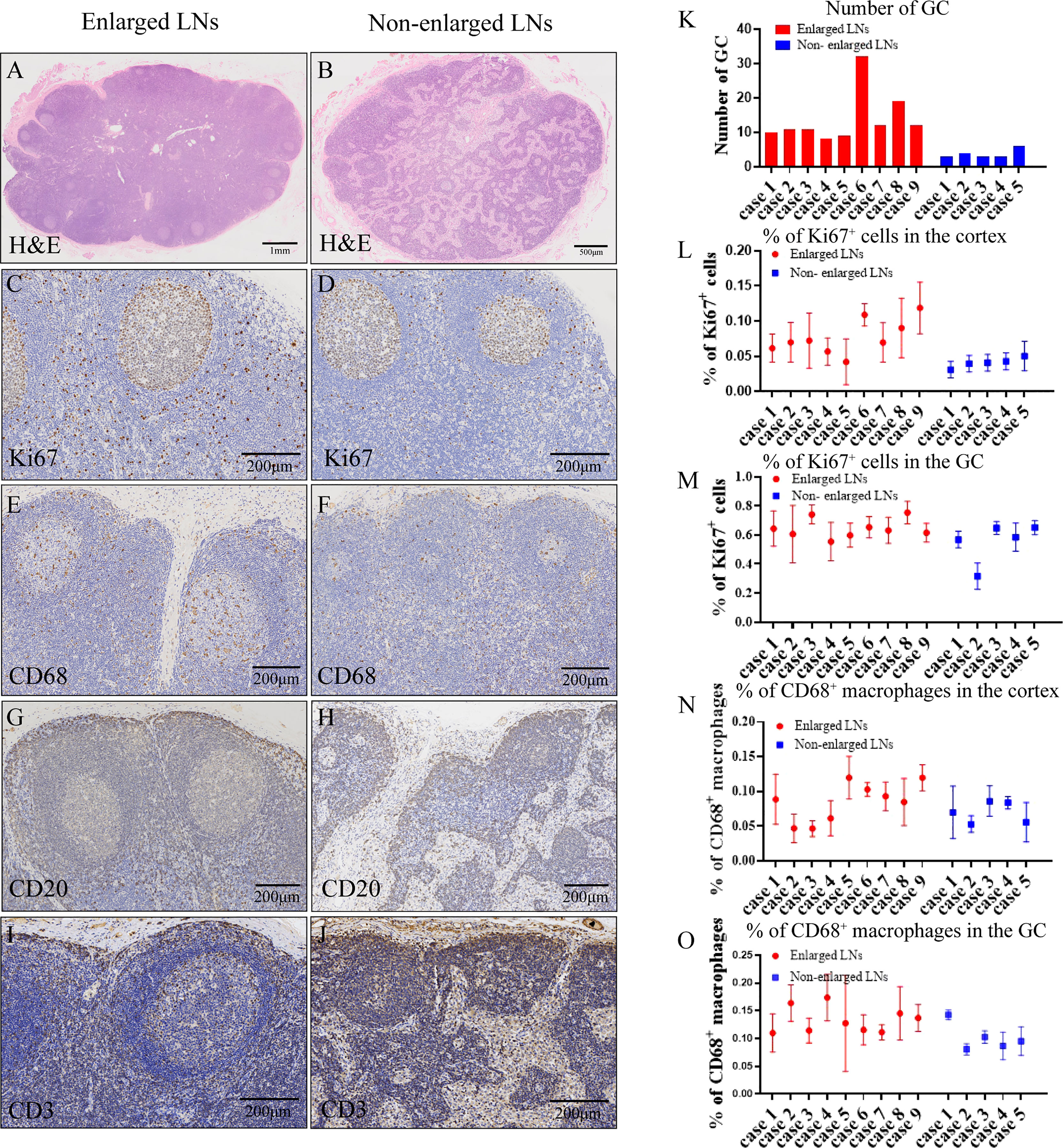
Figure 3 Histological characteristics of lymph nodes. (A, B, K) Hematoxylin and eosin (H&E) staining (A, B) showing microscopic characteristics of lymph nodes (LNs) and number (K) of germinal centers (GCs) in one HE-stained section. (C, D, L, M) Immunohistochemistry (IHC) staining of Ki67 (C, D) and proportion of Ki67-positive cells (L, M) in the cortex or GC of enlarged and non-enlarged LNs. (E, F, N, O) IHC staining of CD68 (E, F) and proportion of CD68-positive macrophages (N, O) in the cortex or GC of enlarged and non-enlarged LNs. (G, H) IHC staining showing the increased CD20-positive B cells in the enlarged LNs. (I, J) IHC staining showing the increased CD3-positive T cells in the enlarged LNs.
Ischemic stroke is a common cause of significant morbidity, mortality, and disability-adjusted life-years (DALYs) worldwide (20). The underlying pathological process is atherosclerosis, a chronic disorder of the intimal layer of large-and medium-sized arteries associated with inflammation and immunity (21). Significant stenosis of the carotid artery, rupture of unstable plaques, and subsequent thrombosis result in transient ischemic attacks (TIAs) or ischemic strokes (21, 22). Currently, the percentage of carotid stenosis based on angiographic measurements remains a major criterion for risk stratification in patients with carotid artery stenosis (23). This method has also been validated in randomized clinical trials (RCT) and meta-analyses, which demonstrated that CEAs reduce the risk of future stroke in symptomatic patients with carotid stenosis (24, 25). However, this method does not provide information about plaque composition, plaque stability, inflammation, intraplaque hemorrhage (IPH), ulceration, and calcification. It is widely recognized that plaque vulnerability is more important than degree of stenosis in evaluating the risk of stroke. Many attempts have been made to identify patients with unstable atherosclerotic plaques to prevent stroke. Some researchers are developing advanced imaging systems that can identify plaque components, such as high-resolution magnetic resonance imaging, ultrasonography, and CT angiography (22, 23). Others search for plasma biomarkers such as C-reactive protein (CRP), interleukin-6 and P-selectin (26). Clinical studies and meta-analyses have confirmed the feasibility of these markers and their effectiveness in risk assessment. Nevertheless, peripheral carotid LNs, which are closely associated with plaque inflammation and immune responses, have received little attention. Our study focused on lymph nodes around the atherosclerotic carotid artery. We found that enlarged LNs around the carotid artery in patients with carotid atherosclerosis suggest recent ischemic symptoms.
LNs are mainly composed of the cortex, paracortical cortex and medulla. These are indispensable secondary lymphoid organs. They also generate a highly specialized microenvironment in response to effective immune responses and play an important role in immune initiation and efficacy (14, 27–29). In the body’s response to diseases, such as infections and tumors, enlarged LNs can be seen as a sign that the body’s immune function is activated or expanded. Pericarotid LNs, which drain lymph fluid from the plaque, are likely to indicate plaque inflammation and a strong immune response, thereby reflecting plaque instability. The results of our study support this hypothesis. Among patients with carotid atherosclerosis, we found that a majority of patients with recent ischemic symptoms, which suggest plaque vulnerability, had enlarged pericarotid LNs, and there was a strong statistical significance between the two (P < 0.001). Our analysis of plaque composition also supported this view. We found that plagues from patients with non-enlarged LNs were more likely to be fibrous (Table 2), suggesting that these plaques were more stable (30). Previous animal studies have reported the expansion of LNs draining the atherosclerotic aorta in aged atherosclerotic Apoe-/- mice (21). To the best of our knowledge, this is the first study to demonstrate this phenomenon in humans.
Through statistical analysis, we founded that patients with enlarged LNs had lower BMI and lower body weight. Some scholars have conducted a retrospective study on female mammography (31) and founded that the longitudinal and transverse diameters of axillary LN increased with increasing BMI. The author attributed the increase in LN size to expansion of the LN hilum caused by fat infiltration. This adds to the evidence that enlarged pericarotid LNs are associated with plaque formation rather than obesity. Meanwhile, lower systolic blood pressure in patients without enlarged LNs may partly explain the stiffer aorta in these patients (32); however, the differences in systolic blood pressure and pulse pressure were not statistically significant. Calcium channel blockers (CCB) are commonly used in patients with enlarged LNs. However, a literature review found no evidence of a relationship between CCB and enlarged LNs. Therefore, our result may not be of practical significance.
Macroscopic examination revealed that the non-enlarged LN was round or oval in shape, but the enlarged LN remained oval in shape, and the hilum was not obvious. Microscopic observation revealed that LN enlargement mainly result from an increase in the number of cells. First, an increase in the number of cells in the LNs may result from an increase in the influx of cells into the LNs. Lymphatic vessels carrying various types of immune cells have been found in the adventitia of atherosclerotic arteries (33). The establishment and development of the lymphatic system can reduce the deposition of lipids and immune cells in arteries (34). These immune cells travel along the lymphatic vessels into the draining LNs, resulting in increased cell numbers in the LNs. Second, this may result from increased cell proliferation in LNs. We observed an increased proportion of Ki67-positive cells in the LN cortex. Although there was no difference in the proportion of Ki67-positive cells in the LN GCs, the number and volume of GCs increased. Finally, impairment of lymphocyte export also contributed to increased cell numbers in the LNs. Tay et al. suggested that disruption of lymphocyte output and interception signals in Apoe-/- mouse LNs leads to the deposition of lymphocytes, resulting in LNs enlargement (35). In our study, CD20+ B cells and CD3+ T cells were found to accumulate in the cortex and subcapsular sinuses of the LNs. In summary, increased cellular input, decreased output, and enhanced cellular proliferation contribute to the appearance of enlarged LNs.
GCs are transient histological microstructures formed within the follicles of secondary lymphoid tissues in response to foreign pathogens (36). GCs are the sites of B cell selection and maturation and play a vital role in the activation and development of immune system function (37). In our study, we found that the number and volume of GCs in enlarged LNs were higher than those in non-enlarged LNs. At the same time, a large number of proliferating B cells in the GC also suggest that a strong immune response was occurring around the carotid artery in patients with enlarged LNs. B cells are found in the adventitia of atherosclerotic arteries (38, 39), and these cells even form small lymphoid follicles (40, 41). The transfer of spleen B cells into splenectomized Apoe-/- mice alleviates the development of atherosclerosis (42), suggesting that B cells, and consequently humoral immunity, have a protective effect against atherosclerosis. In summary, we think that enlarged pericarotid LNs indicate robust antiatherogenic response.
T cells, another important immune cell in adaptive immunity, are key modulators of atherosclerosis. T cell subpopulations differ at different stages of atherosclerosis, in both plaque and circulation (43). Furthermore, different subpopulations of T cells have different effects on atherosclerosis progression through immune activation, immune suppression, or by helping B cells produce antibodies (44). T cells in pericarotid LNs may play a supporting role, but the specific role still needs to be further explored.
Macrophages play a key role in atherosclerosis progression. Monocyte-derived macrophages phagocytize lipoproteins and develop into lipid-rich foam cells, resulting in the formation of a necrotic core (45, 46). Consistent with previous reports (47), we also found macrophage infiltration in the shoulder region of atherosclerotic plaque. But there is no significant difference between the two (Figures 2I–L). Ox-LDL deposited in the intima of artery initiates immunity by interacting with macrophages to remove local cell debris, produce cytokines, and promote local inflammatory responses (48). However, lymphatic reverse cholesterol transport (RCT), which is carried by macrophages, reduces the accumulation of cholesterol in arteries (5). Macrophages play a bidirectional regulatory role in atherosclerosis. In our study, most macrophages are found in the lymphatic sinuses, including subcapsular sinus, cortical sinus, and medullary sinus. These macrophages may come from the draining lymphatic vessels, or they may settle here themselves (9). Recent single-cell sequencing revealed an unanticipated function of LN endothelial cells in scavenging ox-LDL (14). Phagocytosis may occur in pericarotid LNs, in which lymphatic endothelial cells remove modified LDLs unloaded by the macrophages.
This study has several limitations. First, it was not a prospective study. Thus, the chronological relationship between the enlarged LNs and ischemic events could not be determined. Second, the mechanistic pathways of the association between enlarged LNs and plaque development were not assessed. Therefore, no causal relationships were observed. Third, the present analysis only looked at the correlation between enlarged LNs and ischemic symptoms, but did not mention complication and follow-up. Fourth, the criteria for enlarged LNs are mainly applicable to tumors. More precise and specialized criteria are required for carotid atherosclerotic diseases. Finally, the sample size may limit the generalizability of the results.
In conclusion, the current study demonstrated that enlarged pericarotid LNs suggest recent ischemic symptoms and may be a sign of plaque destabilization in patients with carotid atherosclerosis. Adaptive immune responses are activated and reinforced in enlarged pericarotid LNs that drain carotid plaque. Further studies are necessary to explore the potential mechanism between pericarotid LNs and plaque stability and to understand the lymphatic system as a potential therapeutic target in patients with carotid atherosclerosis.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by the Ethics Committee of the Qilu Hospital, Shandong University. The patients/participants provided their written informed consent to participate in this study.
TS, XL and DW conceived and designed the study. TS, PZ, FW, YH, MH, HL and DW were responsible for patient care and treatment, clinical oversight, and clinical data collection. TS, HL and BM collected and characterized samples. TS and FW conducted data analysis. TS and DW wrote the manuscript. XL and DW modified and revised the manuscript. PZ, XL, and DW supervised the study. All authors contributed to the article and approved the submitted version.
This work was supported by the Clinical Practical New Technology Development Foundation of Qilu Hospital (grant 2019-7), and the crosswise tasks (contract number: 11691806 and 6010120062).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to thank Editage (www.editage.cn) for English language editing.
1. Wu MY, Li CJ, Hou MF, Chu PY. New Insights Into the Role of Inflammation in the Pathogenesis of Atherosclerosis. Int J Mol Sci (2017) 18:2034–51. doi: 10.3390/ijms18102034
2. Hansson GK, Hermansson A. The Immune System in Atherosclerosis. Nat Immunol (2011) 12:204–12. doi: 10.1038/ni.2001
3. Schaftenaar F, Frodermann V, Kuiper J, Lutgens E. Atherosclerosis: The Interplay Between Lipids and Immune Cells. Curr Opin Lipidol (2016) 27:209–15. doi: 10.1097/MOL.0000000000000302
4. Lemole GM. The Role of Lymphstasis in Atherogenesis. Ann Thorac Surg (1981) 31:290–3. doi: 10.1016/s0003-4975(10)60949-6
5. Martel C, Li W, Fulp B, Platt AM, Gautier EL, Westerterp M, et al. Lymphatic Vasculature Mediates Macrophage Reverse Cholesterol Transport in Mice. J Clin Invest (2013) 123:1571–9. doi: 10.1172/JCI63685
6. Milasan A, Farhat M, Martel C. Extracellular Vesicles as Potential Prognostic Markers of Lymphatic Dysfunction. Front Physiol (2020) 11:476. doi: 10.3389/fphys.2020.00476
7. Milasan A, Smaani A, Martel C. Early Rescue of Lymphatic Function Limits Atherosclerosis Progression in Ldlr(-/-) Mice. Atherosclerosis (2019) 283:106–19. doi: 10.1016/j.atherosclerosis.2019.01.031
8. Rademakers T, van der Vorst EP, Daissormont IT, Otten JJ, Theodorou K, Theelen TL, et al. Adventitial Lymphatic Capillary Expansion Impacts on Plaque T Cell Accumulation in Atherosclerosis. Sci Rep (2017) 7:45263. doi: 10.1038/srep45263
9. Jalkanen S, Salmi M. Lymphatic Endothelial Cells of the Lymph Node. Nat Rev Immunol (2020) 20:566–78. doi: 10.1038/s41577-020-0281-x
10. Dieterich LC, Detmar M. Tumor Lymphangiogenesis and New Drug Development. Adv Drug Delivery Rev (2016) 99:148–60. doi: 10.1016/j.addr.2015.12.011
11. Dieterich LC, Seidel CD, Detmar M. Lymphatic Vessels: New Targets for the Treatment of Inflammatory Diseases. Angiogenesis (2014) 17:359–71. doi: 10.1007/s10456-013-9406-1
12. Butler MG, Isogai S, Weinstein BM. Lymphatic Development. Birth Defects Res C Embryo Today (2009) 87:222–31. doi: 10.1002/bdrc.20155
13. Niessner A, Weyand CM. Dendritic Cells in Atherosclerotic Disease. Clin Immunol (2010) 134:25–32. doi: 10.1016/j.clim.2009.05.006
14. Fujimoto N, He Y, D’Addio M, Tacconi C, Detmar M, Dieterich LC. Single-Cell Mapping Reveals New Markers and Functions of Lymphatic Endothelial Cells in Lymph Nodes. PloS Biol (2020) 18:e3000704. doi: 10.1371/journal.pbio.3000704
15. Saleh SO, Hojaij FC, Itezeroti AM, Camargo CP, Saleh KS, Andrade MFC, et al. Level Vi Lymph Nodes: An Anatomic Study of Lymph Nodes Located Between the Recurrent Laryngeal Nerve and the Right Common Carotid Artery. Rev Col Bras Cir (2018) 45:e1972. doi: 10.1590/0100-6991e-20181972
16. Nishino M, Hatabu H, Ricciuti B, Vaz V, Michael K, Awad MM. Axillary Lymphadenopathy After Coronavirus Disease 2019 Vaccinations in Patients With Thoracic Malignancy: Incidence, Predisposing Factors, and Imaging Characteristics. J Thorac Oncol (2021) 17:154–9. doi: 10.1016/j.jtho.2021.08.761
17. Ganji M, Nardi V, Prasad M, Jordan KL, Bois MC, Franchi F, et al. Carotid Plaques From Symptomatic Patients are Characterized by Local Increase in Xanthine Oxidase Expression. Stroke (2021) 52:2792–801. doi: 10.1161/STROKEAHA.120.032964
18. Liu Q, Shi Y, Cai J, Duan Y, Wang R, Zhang H, et al. Pathological Changes in the Lungs and Lymphatic Organs of 12 Covid-19 Autopsy Cases. Natl Sci Rev (2020) 7:1868–78. doi: 10.1093/nsr/nwaa247
19. Jiang X, Wang F, Wang Y, Gistera A, Roy J, Paulsson-Berne G, et al. Inflammasome-Driven Interleukin-1alpha and Interleukin-1beta Production in Atherosclerotic Plaques Relates to Hyperlipidemia and Plaque Complexity. JACC Basic Transl Sci (2019) 4:304–17. doi: 10.1016/j.jacbts.2019.02.007
20. Feigin VL, Nichols E, Alam T, Bannick MS, Beghi E, Blake N, et al. Global, Regional, and National Burden of Neurological Disorders, 1990–2016: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet Neurol (2019) 18:459–80. doi: 10.1016/s1474-4422(18)30499-x
21. Wolf D, Ley K. Immunity and Inflammation in Atherosclerosis. Circ Res (2019) 124:315–27. doi: 10.1161/CIRCRESAHA.118.313591
22. Brinjikji W, Huston J 3rd, Rabinstein AA, Kim GM, Lerman A, Lanzino G. Contemporary Carotid Imaging: From Degree of Stenosis to Plaque Vulnerability. J Neurosurg (2016) 124:27–42. doi: 10.3171/2015.1.JNS142452
23. Gupta A, Marshall RS. Moving Beyond Luminal Stenosis: Imaging Strategies for Stroke Prevention in Asymptomatic Carotid Stenosis. Cerebrovasc Dis (2015) 39:253–61. doi: 10.1159/000381108
24. European Carotid Surgery Trialists’ Collaborative Group Randomised Trial of Endarterectomy for Recently Symptomatic Carotid Stenosis: Final Results of the MRC European Carotid Surgery Trialists’ Collaborative Group (ECST). Lancet (1998) 351:1379–87. doi: 10.1016/s0140-6736(97)09292-1
25. Isabel C, Calvet D, Mas JL. Stroke Prevention. Presse Med (2016) 45:e457–71. doi: 10.1016/j.lpm.2016.10.009
26. Zhu Y, Xian X, Wang Z, Bi Y, Chen Q, Han X, et al. Research Progress on the Relationship Between Atherosclerosis and Inflammation. Biomolecules (2018) 8:80. doi: 10.3390/biom8030080
27. Takeda A, Hollmen M, Dermadi D, Pan J, Brulois KF, Kaukonen R, et al. Single-Cell Survey of Human Lymphatics Unveils Marked Endothelial Cell Heterogeneity and Mechanisms of Homing for Neutrophils. Immunity (2019) 51:561–572.e565. doi: 10.1016/j.immuni.2019.06.027
28. Girard JP, Moussion C, Forster R. Hevs, Lymphatics and Homeostatic Immune Cell Trafficking in Lymph Nodes. Nat Rev Immunol (2012) 12:762–73. doi: 10.1038/nri3298
29. Qi H, Kastenmuller W, Germain RN. Spatiotemporal Basis of Innate and Adaptive Immunity in Secondary Lymphoid Tissue. Annu Rev Cell Dev Biol (2014) 30:141–67. doi: 10.1146/annurev-cellbio-100913-013254
30. Adiguzel E, Ahmad PJ, Franco C, Bendeck MP. Collagens in the Progression and Complications of Atherosclerosis. Vasc Med (2009) 14:73–89. doi: 10.1177/1358863X08094801
31. diFlorio Alexander RM, Haider SJ, MacKenzie T, Goodrich ME, Weiss J, Onega T. Correlation Between Obesity and Fat-Infiltrated Axillary Lymph Nodes Visualized on Mammography. Br J Radiol (2018) 91:20170110. doi: 10.1259/bjr.20170110
32. Hermeling E, Hoeks AP, Winkens MH, Waltenberger JL, Reneman RS, Kroon AA, et al. Noninvasive Assessment of Arterial Stiffness Should Discriminate Between Systolic and Diastolic Pressure Ranges. Hypertension (2010) 55:124–30. doi: 10.1161/HYPERTENSIONAHA.109.143867
33. Kholova I, Dragneva G, Cermakova P, Laidinen S, Kaskenpaa N, Hazes T, et al. Lymphatic Vasculature is Increased in Heart Valves, Ischaemic and Inflamed Hearts and in Cholesterol-Rich and Calcified Atherosclerotic Lesions. Eur J Clin Invest (2011) 41:487–97. doi: 10.1111/j.1365-2362.2010.02431.x
34. Wilhelm AJ, Zabalawi M, Owen JS, Shah D, Grayson JM, Major AS, et al. Apolipoprotein a-i Modulates Regulatory T Cells in Autoimmune Ldlr(-/-), Apoa-i(-/-)Mice. J Biol Chem (2010) 285:36158–69. doi: 10.1074/jbc.M110.134130
35. Tay MHD, Lim SYJ, Leong YFI, Thiam CH, Tan KW, Torta FT, et al. Halted Lymphocyte Egress via Efferent Lymph Contributes to Lymph Node Hypertrophy During Hypercholesterolemia. Front Immunol (2019) 10:575. doi: 10.3389/fimmu.2019.00575
36. Holmes AB, Corinaldesi C, Shen Q, Kumar R, Compagno N, Wang Z, et al. Single-Cell Analysis of Germinal-Center B Cells Informs on Lymphoma Cell of Origin and Outcome. J Exp Med (2020) 217:e20200483. doi: 10.1084/jem.20200483
37. Gars E, Butzmann A, Ohgami R, Balakrishna JP, O’Malley DP. The Life and Death of the Germinal Center. Ann Diagn Pathol (2020) 44:151421. doi: 10.1016/j.anndiagpath.2019.151421
38. Lahoute C, Herbin O, Mallat Z, Tedgui A. Adaptive Immunity in Atherosclerosis: Mechanisms and Future Therapeutic Targets. Nat Rev Cardiol (2011) 8:348–58. doi: 10.1038/nrcardio.2011.62
39. Aubry MC, Riehle DL, Edwards WD, Maradit-Kremers H, Roger VL, Sebo TJ, et al. B-Lymphocytes in Plaque and Adventitia of Coronary Arteries in Two Patients With Rheumatoid Arthritis and Coronary Atherosclerosis: Preliminary Observations. Cardiovasc Pathol (2004) 13:233–6. doi: 10.1016/j.carpath.2004.02.005
40. Watanabe M SA, Sasaki Y YM, Tanaka-Shintani M, Shintaku M, Ishikawa Y. Distribution of Inflammatory Cells in Adventitia Changed With Dvancing Atherosclerosis of Human Coronary Artery. J Atheroscler Thromb (2007) 14:325–31. doi: 10.5551/jat.E489
41. Grabner R, Lotzer K, Dopping S, Hildner M, Radke D, Beer M, et al. Lymphotoxin Beta Receptor Signaling Promotes Tertiary Lymphoid Organogenesis in the Aorta Adventitia of Aged Apoe(-/-) Mice. J Exp Med (2009) 206:233–48. doi: 10.1084/jem.20080752
42. Caligiuri G. Protective Immunity Against Atherosclerosis Carried by B Cells of Hypercholesterolemic Mice. J Clin Invest (2002) 109:745–53. doi: 10.1172/jci7272
43. Poznyak AV, Bezsonov EE, Popkova TV, Starodubova AV, Orekhov AN. Immunity in Atherosclerosis: Focusing on T and B Cells. Int J Mol Sci (2021) 22:8379. doi: 10.3390/ijms22168379
44. Roy P, Orecchioni M, Ley K. How the Immune System Shapes Atherosclerosis: Roles of Innate and Adaptive Immunity. Nat Rev Immunol (2021) 22:251-265. doi: 10.1038/s41577-021-00584-1
45. Tabas I, Lichtman AH. Monocyte-Macrophages and T Cells in Atherosclerosis. Immunity (2017) 47:621–34. doi: 10.1016/j.immuni.2017.09.008
46. Cho KY, Miyoshi H, Kuroda S, Yasuda H, Kamiyama K, Nakagawara J, et al. The Phenotype of Infiltrating Macrophages Influences Arteriosclerotic Plaque Vulnerability in the Carotid Artery. J Stroke Cerebrovasc Dis (2013) 22:910–8. doi: 10.1016/j.jstrokecerebrovasdis.2012.11.020
47. Schneiderman J, Schaefer K, Kolodgie FD, Savion N, Kotev-Emeth S, Dardik R, et al. Leptin Locally Synthesized in Carotid Atherosclerotic Plaques Could be Associated With Lesion Instability and Cerebral Emboli. J Am Heart Assoc (2012) 1:e001727. doi: 10.1161/JAHA.112.001727
Keywords: carotid atherosclerosis, pericarotid lymph nodes, ischemic symptoms, adaptive immune response, plaque instability
Citation: Sun T, Wang F, He Y, Mao B, Han M, Liu H, Zhao P, Li X and Wang D (2022) Enlarged Pericarotid Lymph Nodes Suggest Recent Ischemic Symptoms in Patients with Carotid Atherosclerosis. Front. Immunol. 13:900642. doi: 10.3389/fimmu.2022.900642
Received: 21 March 2022; Accepted: 23 June 2022;
Published: 12 July 2022.
Edited by:
Jianwei Pan, Zhejiang University, ChinaReviewed by:
Ya-bo Huang, The First Affiliated Hospital of Soochow University, ChinaCopyright © 2022 Sun, Wang, He, Mao, Han, Liu, Zhao, Li and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xingang Li, bGl4Z0BzZHUuZWR1LmNu; Donghai Wang, ZHJ3YW5nZG9uZ2hhaUAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.