- 1Zvitambo Institute for Maternal and Child Health Research, Harare, Zimbabwe
- 2Department of International Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, United States
- 3Centre for Genomics and Child Health, Queen Mary University of London, London, United Kingdom
Background: Children who are stunted (length-for-age Z-score<-2) are at greater risk of infectious morbidity and mortality. Previous studies suggest that stunted children have elevated inflammatory biomarkers, but no studies have characterised their capacity to respond to new infections (i.e., their immune function). We hypothesised that antibacterial immune function would differ between stunted and non-stunted children and relate to their health and environment during early life.
Methods: We enrolled a cross-sectional cohort of 113 HIV-negative children nested within a longitudinal cluster-randomised controlled trial of household-level infant and young child feeding (IYCF) and water, sanitation and hygiene (WASH) interventions in rural Zimbabwe (SHINE; Clinical trials registration: NCT01824940). Venous blood was collected at 18 months of age and cultured for 24 h without antigen or with bacterial antigens: heat-killed Salmonella typhimurium (HKST) or Escherichia coli lipopolysaccharide (LPS). TNFα, IL-6, IL-8, IL-12p70, hepcidin, soluble (s)CD163, myeloperoxidase (MPO) and IFNβ were quantified in culture supernatants by ELISA to determine antigen-specific immune function. The effect of stunting status and early-life exposures (anthropometry, inflammation at 18 months, maternal health during pregnancy, household WASH) on immune function was tested in logit and censored log-normal (tobit) regression models.
Results: Children who were stunted (n = 44) had higher proportions (86.4% vs. 65.2%; 88.6% vs. 73.4%) and concentrations of LPS-specific IL-6 (geometric mean difference (95% CI): 3.46 pg/mL (1.09, 10.80), p = 0.035) and IL-8 (3.52 pg/mL (1.20, 10.38), p = 0.022) than non-stunted children (n = 69). Bacterial antigen-specific pro-inflammatory cytokine concentrations were associated with biomarkers of child enteropathy at 18 months and biomarkers of systemic inflammation and enteropathy in their mothers during pregnancy. Children exposed to the WASH intervention (n = 33) produced higher LPS- (GMD (95% CI): 10.48 pg/mL (1.84, 60.31), p = 0.008) and HKST-specific MPO (5.10 pg/mL (1.77, 14.88), p = 0.003) than children in the no WASH group (n = 80). There was no difference in antigen-specific immune function between the IYCF (n = 55) and no IYCF groups (n = 58).
Conclusions: Antibacterial immune function among 18-month-old children in a low-income setting was shaped by their stunting status and prior exposure to maternal inflammation and household WASH. Heterogeneity in immune function due to adverse exposures in early life could plausibly contribute to infection susceptibility.
Introduction
The first 1,000 days of life (between conception and a child’s second birthday) is a critical window for the development of long-term immune defence against infections (1). Much of the variation between individual immune phenotype and function is known to be non-heritable (2, 3); a range of drivers including infections, maternal health status, microbiome, urbanisation, vaccinations, environmental contaminants (e.g., cigarette smoke) and diet are implicated for healthy children and adults (4, 5). However, empirical studies of how cellular immune function develops in young children are limited in low- to- middle-income countries (LMIC) (1, 6, 7), where the health burden of infections is aggregated (8).
Stunting (height- or length-for-age Z score (LAZ) < -2) is an indicator of chronic undernutrition, can originate in utero and affects 144 million children under 5 years old, predominantly in LMIC (8). Stunted children have high symptomatic and asymptomatic pathogen carriage (9) and are at greater risk of infectious mortality than their non-stunted peers (10). However, linear growth deficits are more likely an indicator than a cause of infectious susceptibility among undernourished children (11). There is some evidence that systemic inflammatory mediators are consistently higher (e.g., 6 weeks to 6 months (12), birth to 12 months (13)) in stunted versus non-stunted children, positively associated with circulating pathogen-associated molecular patterns (PAMPs) and anti-pathogen antibodies (14) but negatively associated with linear growth factors (12, 13, 15). Environmental enteropathy, a condition which develops in the context of asymptomatic enteropathogen carriage, recurrent infection, intestinal damage and inflammation, is thought to underlie the relationship between stunting and systemic inflammation (9, 16), primarily via translocation of PAMP from microbes in the gut into circulation (9, 14). For example, concentrations of lipopolysaccharide (LPS, a component of gram-negative bacteria also termed endotoxin) in plasma samples from a cohort of infants in rural Gambia were found to be twice the upper reference limit for infants in high-income settings and negatively associated with their linear and ponderal growth (14). However, no studies to date have directly assessed whether immune cells from stunted children respond differently to PAMP relative to those of non-stunted children, how these functional differences originate or whether they relate to subsequent infection risk and severity.
The goal of this study was to assess blood immune cell function among 18-month-old children in rural Zimbabwe, which has an estimated 23.5% prevalence of stunting among children under 5 years old (8), high rates of adverse birth outcomes (17) and endemic pathogen exposure (18, 19). We focused on PAMPs derived from two gram-negative bacteria commonly isolated from stool samples collected from children in LMIC (purified LPS from Escherichia coli and whole heat-killed Salmonella typhimurium, HKST) (15, 16) and soluble mediators typically upregulated upon initial PAMP recognition by surface-expressed toll-like receptors (TLR) on innate immune cells. We compared bacterial antigen-specific immune-mediator secretion between children who were stunted versus non-stunted and used longitudinal data on each child, their mother and household environment from pregnancy through to 18 months to identify early-life factors associated with immune heterogeneity. Our data provide a holistic view of the capacity of immune cells to respond to a new bacterial challenge in a cohort of young children exposed to a range of adverse environmental exposures in early life.
Materials and Methods
Study Ethics
This is a sub-study of the Sanitation Hygiene and Infant Nutrition Efficacy trial (SHINE (20); ClinicalTrials.gov, number NCT01824940), a cluster-randomised 2 × 2 factorial trial of the impact of household water, sanitation and hygiene (WASH) and/or infant and young child feeding (IYCF) interventions on linear growth and haemoglobin among children in rural Zimbabwe (19, 21). Ethical approval for SHINE was provided by the Medical Research Council of Zimbabwe and the Institutional Review Board of the Johns Hopkins Bloomberg School of Public Health. All participants provided written informed consent for themselves and their children to participate.
Study Participants
Participants were pregnant women who were permanent rural residents of Chirumanzu or Shurugwi district, Zimbabwe; became pregnant during SHINE enrolment (November 2012-March 2015); and consented to participation during pregnancy. Women were recruited throughout their pregnancies. Prevalence of maternal HIV and urogenital schistosomiasis, adverse birth outcomes and child stunting are high in these communities; however, malaria prevalence is low (17–19). Additional inclusion criteria for this sub-study were as follows: children born to SHINE mothers who completed an 18-month study visit between July 2016 and July 2017; provided a blood sample ≥2 mL to allow for all whole-blood culture assays; and lived in the catchment area of Shurugwi District Hospital or St. Theresa’s Hospital, where trial hubs with laboratory facilities for cell culture were based.
SHINE Interventions
SHINE was cluster-randomised by the catchment area of one to four Village Health Workers (VHW) working for Zimbabwe’s Ministry of Health and Child Care. VHWs made 15 home visits to SHINE participants at approximately monthly intervals between enrolment and 12 months after birth. VHWs delivered interactive behaviour-change interventions within one of four arms: standard of care (SOC): promoted breastfeeding (early initiation, exclusive to 6 months and prolonged), family planning, prevention-of-mother-to-child transmission of HIV services and childhood immunisations; WASH: SOC interventions plus households received a Blair ventilated improved pit latrine, two handwashing stations, water chlorination (WaterGuard, Nelspot, Zimbabwe) and a play space to separate infants from animal faeces; IYCF: SOC interventions plus promoted nutrient-dense, diverse infant diets and provided small-quantity lipid-based nutrient supplementation (SQ-LNS, 20 g/day) for infants from 6 to 18 months; and combined WASH + IYCF: all SOC, WASH and IYCF interventions. Fidelity of intervention delivery was high for all intervention arms: >98% of WASH households received ventilated improved pit latrines and handwashing stations, 92% received play mats and play yards; 80% received ≥80% of the planned deliveries of soap and chlorine solution; and 79% of IYCF households received ≥80% of planned deliveries of SQ-LNS (21). This sub-study recruited children across the four trial arms.
Research nurses visited households at baseline (approximately 2 weeks after enrolment), 32 weeks’ gestation and 1, 3, 6, 12 and 18 months after birth. Maternal anthropometry (height, weight, mid-upper-arm circumference (MUAC)), haemoglobin (HemoCue, Ängelholm, Sweden), Schistosoma haematobium (urinary microscopy), and HIV status (via the Zimbabwe National HIV testing algorithm (22)) were measured, blood and stool samples collected and maternal and household characteristics assessed by questionnaire at baseline. Birthweight (Tanita scales; Amsterdam, Netherlands) and delivery characteristics were recorded at birth. Child anthropometry and symptoms of infection (7-day caregiver recall questionnaire) and blood and stool sampling were done at all postnatal visits. At the 18-month visit, child haemoglobin was measured by HemoCue and median length calculated from three measurements.
Plasma and Stool ELISA
Biomarkers of systemic inflammation (capsular polysaccharide-reactive protein (CRP), sCD14) and environmental enteropathy (intestinal fatty acid-binding protein (IFABP)) were quantified in plasma by enzyme-linked immunosorbent assay (ELISA) (23). Biomarkers of intestinal inflammation and environmental enteropathy (myeloperoxidase (MPO), neopterin, α-1-antitrypsin (AAT)) were measured by stool ELISA (23).
Whole Blood Cultures
Blood was collected from each child by venipuncture at their 18-month visit. Six hundred millilitres of blood was diluted in RPMI 1640 medium supplemented with 1% v/v penicillin–streptomycin (GIBCO, Amarillo, USA) and cultured in 24-well plates for 24 h under three parallel conditions (one culture well/condition; culture conditions for the study were pre-prepared and stored at -80°C until use to minimise inter-plate variation): without stimulus (Media); 0.25 EU/mL ultrapure lipopolysaccharide from E. coli (LPS); and 1 × 108 cells/mL heat-killed Salmonella enterica serovar Typhimurium (HKST; Invivogen, Toulouse, France). Cultures were maintained at 37°C, 6% CO2, using the CO2Gen Compact system (Oxoid, Basingstoke, UK). Cell-free culture supernatants were harvested and stored at -80°C. Supernatants were transported to Zvitambo Institute laboratory, Harare, where TNFα, IL-6, IL-8, IL-12p70, hepcidin, sCD163, MPO (Bio-Techne DuoSet, Abingdon, UK) and IFNβ (PBL VeriKine, Piscataway, USA) concentrations were quantified in duplicate ELISA relative to eight-point standard curves. Mediators were chosen to reflect key domains of innate immune cell function: pro-inflammatory cytokines (TNFα and IL-6) that trigger the liver acute-phase response; IFNβ known for its antiviral and antibacterial action (24); IL-8, a pro-inflammatory cytokine and neutrophil chemoattractant; sCD163, a bacterial sensor shed by activated monocytes/macrophages (25); IL-12p70 which promotes antibacterial/antiviral T-cell function; hepcidin which inhibits cellular iron efflux to regulate iron homeostasis and/or defend against extracellular pathogens (26); and MPO, an enzyme which catalyses bactericidal reactive oxygen species (ROS) production (27). ELISA lower limits of detection were as follows: TNFα—15.6 pg/mL, IL-6—4 pg/mL, IL-8—31.2 pg/mL, IL-12p70—31.2 pg/mL, sCD163—156 pg/ml, hepcidin—3.1 pg/mL, MPO—62.5 pg/mL and IFNβ—50 pg/mL. Concentrations below the assay detection limit were censored at the limit of detection; those greater than the top standard concentration were rerun at higher dilution and multiplied by the dilution factor.
Statistical Analysis
A sample size calculation based on previous estimates was not done as there are no existing comparisons of immune function variables by stunting. The sub-study analysis plan was published prior to data analysis (https://osf.io/rfhve/). SHINE intervention arm analyses were by intention to treat.
Sub-study cohort characteristics were compared to the SHINE cohort and between SHINE arms by generalised estimating equations (means), Somers’ D (medians) and logit regression (proportions).
Log-transformed mediator concentrations were compared between culture conditions using generalised estimating equations to identify mediators for which there was a detectable increase in response to bacterial antigen stimulation above unstimulated cultures. For mediators with a detectable antibacterial response, concentrations in unstimulated culture supernatants were subtracted from those in LPS- and HKST-stimulated culture supernatants from the same child to give antigen-specific mediator concentrations (Δ); antigen-specific concentrations < unstimulated were censored at the mean of the lowest detectable value for the cohort/2. Proportions of children with unstimulated concentrations > ELISA detection limit and proportions of children with antigen-specific concentrations > unstimulated levels were compared between groups by unadjusted logit regression; odds ratios (OR) and 95% confidence intervals (95% CI) are reported.
Censored antigen-specific mediator concentrations were not normally distributed; therefore, censored log-normal (tobit) regression was used to assess the effects of child, maternal and SHINE intervention variables on these outcome measures. We developed separate causal inference models to assess the total effect of four groups of exposure variables (18-minth stunting status; 18-month inflammation; baseline maternal health; SHINE intervention arm) on immune function outcome variables (i.e., antigen-specific immune-mediator concentrations), adjusting for confounders (Supplementary Table 1). Confounder variables were selected for inclusion in each of the adjusted models from a full list of variables available from the SHINE study (Supplementary Table 2) using directed acyclic graphs (DAG) based on existing research literature. Variables with ≥20% missingness and/or ≤10 total events (continuous variables) or events per category (categorical variables) were not included in regression models. The SHINE intervention arm and hub were included in all adjusted models where the exposure was measured after the SHINE baseline visit. We estimated the geometric mean difference (GMD) in immune mediators (pg/mL) by exponentiating unadjusted and adjusted censored log-normal (tobit) regression coefficients and associated 95% CI; results are reported as GMD between case/intervention group and control/no intervention group for categorical exposure variables and geometric mean increase per one-unit increase in exposure for continuous exposure variables.
Sensitivity analyses assessed the modifying effects of exposure to maternal HIV, 18-month anaemia (haemoglobin <10.5 g/dL; predefined per SHINE secondary outcome (20)) and presence of any symptom of infection in the week preceding the 18-month visit on the relationship between immune function and stunting status.
Statistical analyses were performed using Stata version 14 (StataCorp, College Station, TX) and Prism version 9 (GraphPad Software, La Jolla, CA).
Results
Characteristics of the Study Cohort
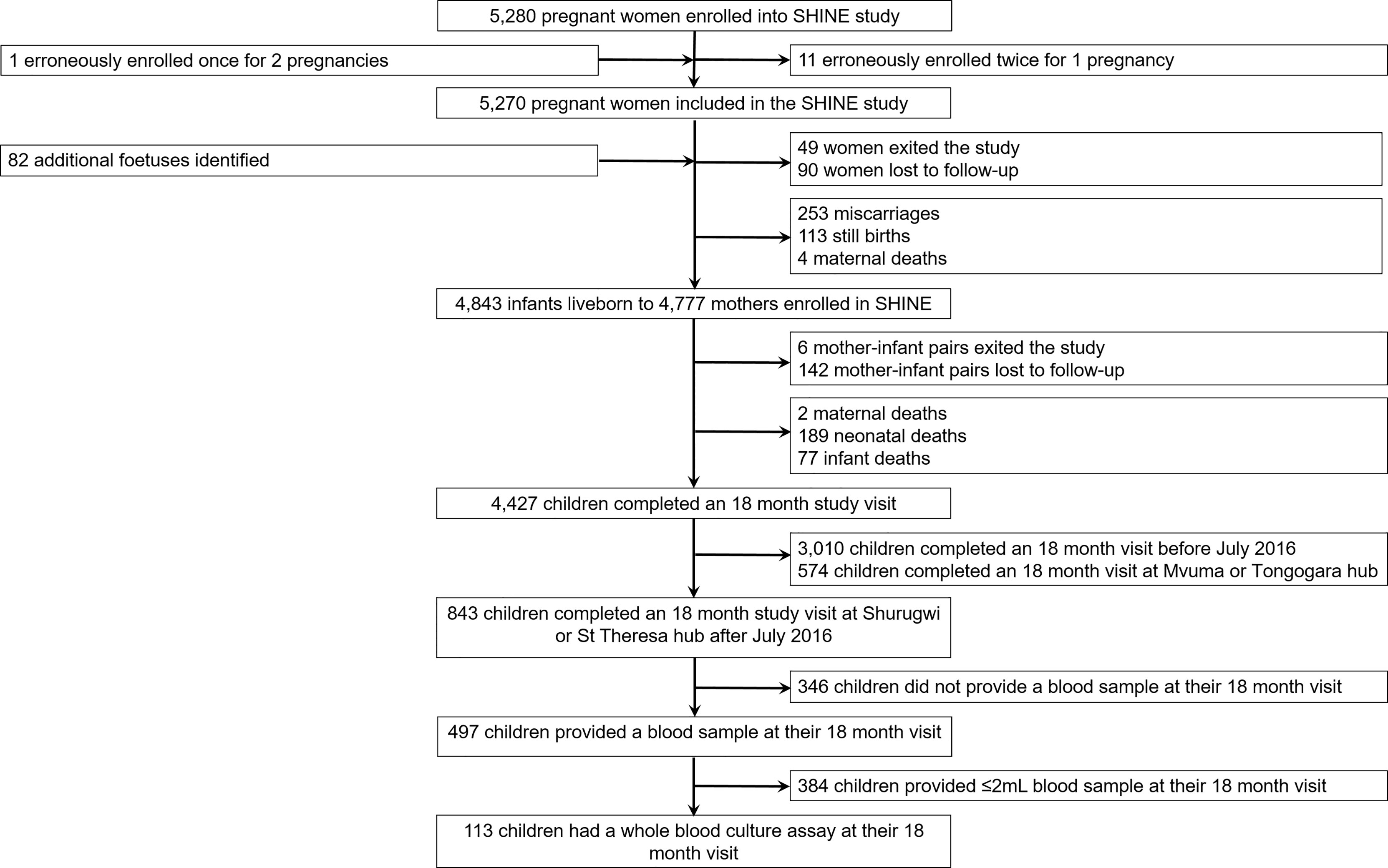
One hundred children (53.1% female; mean age: 18.4 months, standard deviation: 1.6) born to 111 mothers were included in the immune function sub-study of SHINE (Figure 1; Table 1). Characteristics of the children enrolled in the sub-study were broadly comparable to those of the main SHINE trial cohort although the sub-study cohort tended to be healthier, including more institutional deliveries, term and appropriate-for-gestational age births and fewer preterm births and caregiver-reported symptoms of infection (Supplementary Table 2). Six children (5.4%) were born low birthweight (<2.5 kg). All sub-study participants were HIV-negative, but 18 (16.0%) were HIV-exposed, uninfected (HEU). At the 18-month visit, 12 children (10.6%) were anaemic (haemoglobin <10.5 g/dL). Of the 11 (9.7%) children for whom symptoms of infection were reported in the previous 7 days, two had diarrhoea, five had fever and seven had a cough.
Blood Immune Cell Mediator Secretion in Response to Bacterial Antigens
Immune-mediator concentrations and proportions of the cohort producing detectable unstimulated and LPS- and HKST-stimulated mediators in 24h whole-blood cultures are shown in Figure 2. Mediator responses to purified LPS are indicative of cellular activation via TLR4 whereas HKST responses reflect activation via a combination of receptors, primarily TLR2 and TLR4. Cytokines (TNFα, IL-6, IL-8, IL-12p70 and IFNβ) tended to be present at low/undetectable levels in unstimulated cultures, but most participants had detectable MPO, sCD163 and hepcidin in unstimulated cultures, indicative of non-specific mediator production and/or pre-culture plasma concentrations (Figures 2D, G, H). There was evidence that TNFα, IL-6, IL-8 and MPO concentrations were higher in LPS- and HKST-stimulated versus unstimulated cultures (Figures 2A–D), consistent with activation of bacterial antigen-specific responses. Concentrations of IL-12p70 were also higher in HKST-stimulated versus unstimulated cultures despite only 14.2% of children having detectable IL-12p70 (Figure 2E). There was limited evidence for higher production of sCD163, hepcidin or IFNβ in bacterial antigen-stimulated cultures and of IL-12p70 in LPS-stimulated cultures versus unstimulated cultures (Figures 2E–H), suggesting that blood immune cells from participants did not upregulate these mediators upon bacterial antigen challenge in vitro. Based on these data, we went on to explore heterogeneity in antibacterial immune function using ΔTNFα, ΔIL-6, ΔIL-8 and ΔMPO between antigen-stimulated and unstimulated cultures (i.e., antigen-specific mediator levels).
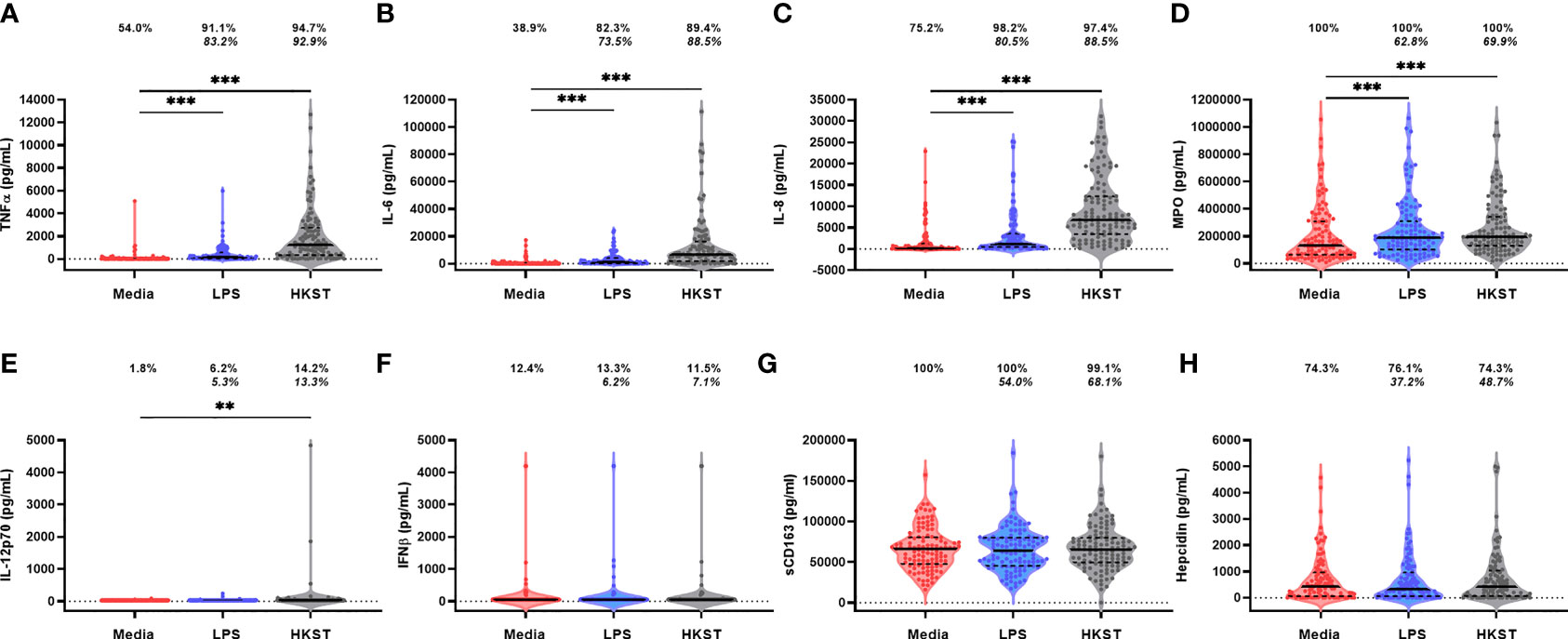
Figure 2 Soluble mediator production by blood immune cells from 18-month-old Zimbabwean children in response to in vitro bacterial antigen challenge. Violin plots (median and interquartile range indicated) of (A) TNFα; (B) IL-6; (C) IL-8; (D) MPO; (E) IL-12p70; (F) IFNβ; (G) sCD163; and (H) hepcidin concentrations (pg/mL) present in supernatants from parallel 24h culture of whole blood samples from Zimbabwean children with culture media only (unstimulated; red), LPS (blue) and HKST (grey). Proportions indicate participants with mediator concentration > ELISA limit of detection; proportions in italics indicate participants with mediator concentration > unstimulated. Log-transformed mediator concentrations were compared between culture conditions using generalised estimating equations (n = 113); **p < 0.01, ***p < 0.001.
Immune Function of Stunted Versus Non-Stunted Children at 18 Months
Forty-four children (38.9%) were stunted at the 18-month visit consistent with prevalence of stunting in the SHINE cohort (33.2%; Supplementary Table 2). We hypothesised that stunting status is associated with antibacterial immune function. In causal inference models, we adjusted for the SHINE arm and hub, infant sex due to its impact on immune development (28) and because boys had poorer linear growth than girls in SHINE (21) and birthweight, which was associated with both 18-month stunting and immune function. The stunted and non-stunted groups had comparable proportions of children with detectable TNFα, IL-6, IL-8 and MPO in unstimulated cultures (Figure 3A). Comparisons of other unstimulated mediator proportions and concentrations by stunting status are provided in Supplementary Figure 1 and Supplementary Table 3.
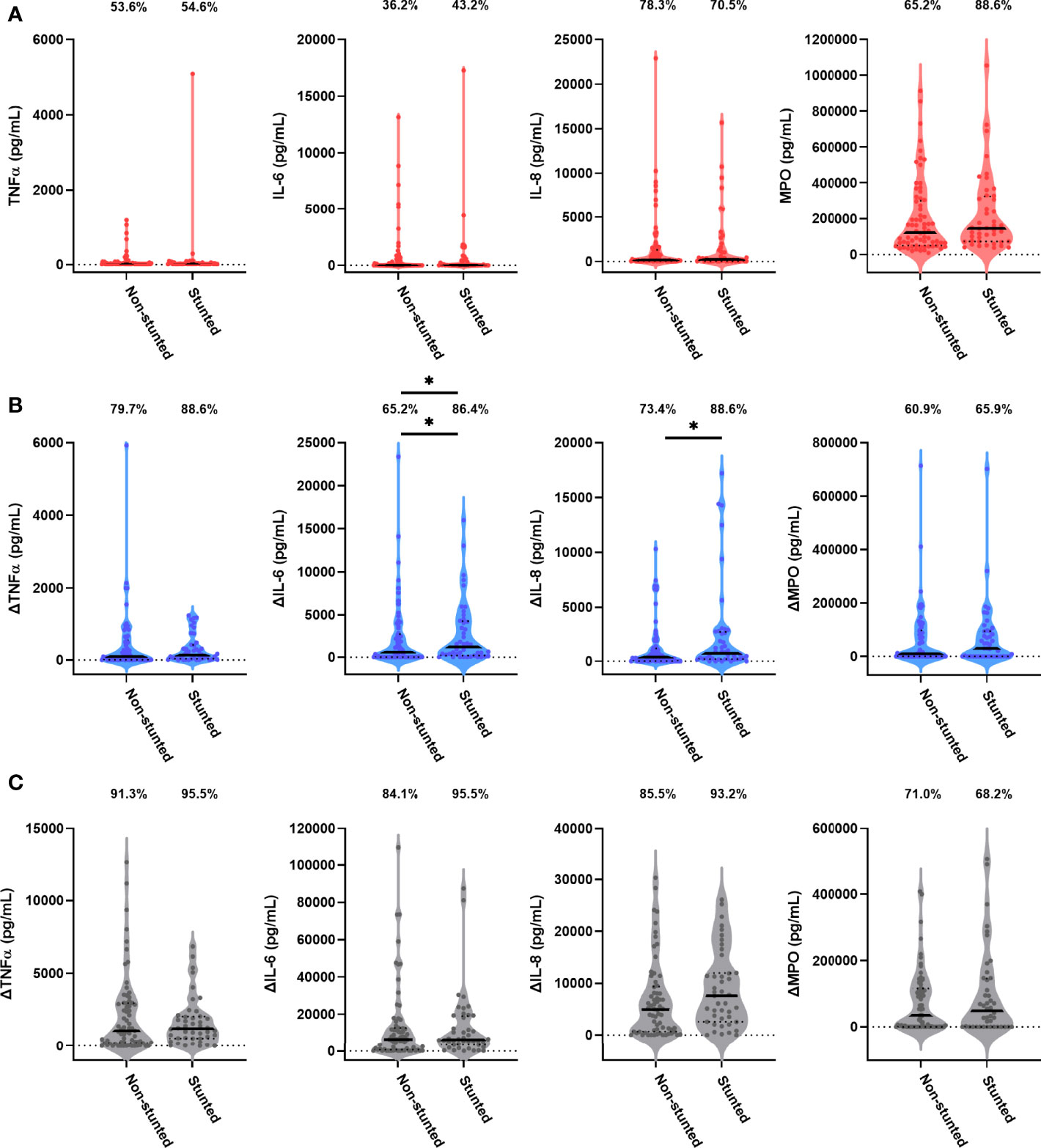
Figure 3 Unstimulated and bacterial antigen-stimulated immune-mediator production by blood immune cells from stunted versus non-stunted children. Violin plots (median and interquartile range indicated) of (A) unstimulated (red), (B) LPS-stimulated (blue) and (C) HKST-stimulated (grey) TNFα, IL-6, IL-8 and MPO concentrations in 24h culture supernatants. LPS- and HKST-specific concentrations (Δ) were calculated for each child by subtracting concentrations present in matched unstimulated culture supernatants. Proportions indicate participants with mediator concentration > ELISA limit of detection (A) and participants with antigen-stimulated mediator concentration > unstimulated (B, C). Proportions of children with detectable mediator levels and mediator concentrations were compared by stunting status (stunted n = 44 versus non-stunted n = 69) via multinomial logit regression and censored log-normal (tobit) regression, respectively (unadjusted analyses indicated; full results in Table 2); *p < 0.05.
Higher proportions of children who were stunted versus non-stunted at 18 months had detectable LPS- and HKST-specific mediators, and concentrations of all antigen-specific mediators were higher in stunted versus non-stunted children (Figures 3B, C). This was most evident for proportions of children producing detectable LPS-specific IL-6 (logit regression OR: 3.38, 95% CI: 1.17, 9.75; p = 0.024) and for concentrations of LPS-specific IL-6 and LPS-specific IL-8 in unadjusted models (Figure 3B; Table 2). The stunted group produced 3.46 pg/mL (95% CI: 1.09, 10.80; p = 0.035) more IL-6 and 3.52 pg/mL (95% CI: 1.20, 10.38; p = 0.022) more IL-8 in response to LPS than the non-stunted group. The magnitude of the difference in LPS-specific IL-6 and IL-8 concentrations between stunted and non-stunted children was reduced in adjusted models (Table 2). These data are the first evidence that stunted children have a distinct TLR4-mediated response to LPS compared to non-stunted children; we did not find evidence for a difference in responses to HKST which immune cells detect via multiple receptors.
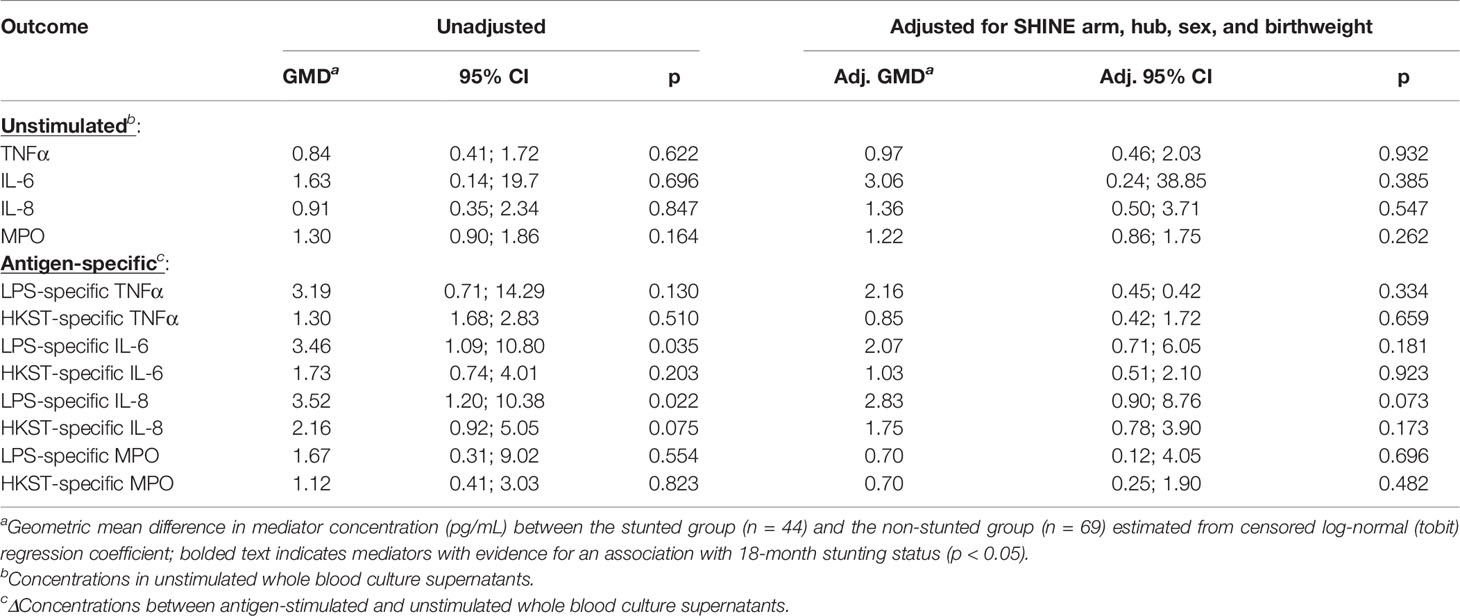
Table 2 Censored log-normal (tobit) regression analysis of the relationship between child stunting status and immune-mediator production at 18 months of age.
Due to the low number of children who were HIV-exposed uninfected, anaemic and/or had symptoms of infection at 18 months of age, we investigated the modifying effects of these characteristics on the association between immune function and stunting status in sensitivity analyses (Supplementary Figures 2, 3). We found evidence that GMD in LPS-specific IL-6 and IL-8 between stunted and non-stunted children was higher when HIV-exposed (GMD (95% CI): ΔIL-6: 3.56 (1.15, 11.1), p = 0.028; ΔIL-8: 4.62 (1.46, 14.73), p = 0.035) and anaemic children (ΔIL-6: 4.62 (1.46, 14.73); p = 0.009; ΔIL-8: 4.18 (1.32, 13.33), p = 0.015) were excluded, but lower when symptomatic children were excluded (ΔIL-6: 3.13 (0.95, 10.28), p = 0.061; ΔIL-8: 3.06 (1.12, 9.30), p = 0.048). These data support the hypothesis that HIV status, anaemia and infectious symptoms modify how stunted children respond to bacterial antigens in the small number of affected children in our cohort; future studies powered to compare these clinical groups are required.
Relationship Between Anthropometry and Antibacterial Immune Function at 18 Months
Given that stunting is characterised clinically as low height-for-age, we investigated whether immune function was associated with age-adjusted measures of anthropometry across both the stunted and non-stunted groups. There was limited evidence for a continuous relationship between any bacterial antigen-specific mediators and 18-month anthropometry (LAZ, weight-for-height, weight-for-age, MUAC or head circumference Z scores) before or after adjustment for the SHINE arm, hub, infant sex and maternal HIV status (p > 0.05; data not shown). However, antigen-specific MPO concentrations increased per 100-g increase in birthweight across the cohort (n = 111; GMD (95% CI) LPS: 0.14 (0.02, 1.03), p = 0.053; HKST: 0.29 (0.09, 0.93), p = 0.037); there was no evidence for an association between birthweight and antigen-specific TNFα, IL-6 or IL-8 (Supplementary Figure 4). We did not find evidence for an association between LAZ at 3 (n = 92), 6 (n = 95) or 12 months (n = 91) and any antigen-specific immune mediators (p > 0.05; data not shown). Thus, differences in LPS-specific IL-6 and IL-8 between stunted and non-stunted children were not solely explained by a continuous relationship between immune function and children’s current or preceding anthropometry. We went on to explore alternative, non-anthropometric characteristics of stunting that could contribute to immune heterogeneity in this cohort.
Relationship Between Inflammation and Antibacterial Immune Function at 18 Months
Biomarkers of systemic inflammation (plasma CRP, sCD14) and intestinal inflammation (stool MPO and neopterin) are non-specific indicators of immune cell activation, which can be elevated as a result of infection and/or microbial carriage in peripheral tissues; these mediators are also associated with translocation of PAMPs into circulation as a result of intestinal damage (plasma IFABP, stool AAT) and environmental enteropathy (9). We therefore investigated whether there was an association between inflammatory biomarker concentrations and antigen-specific immune function at 18 months of age. Children enrolled in the sub-study had similar concentrations of all systemic inflammatory and enteropathy biomarkers to the SHINE cohort (Supplementary Table 2) with the exception of plasma IFABP (median, interquartile range: 314.8 μg/mL (142.2; 588.5) versus 211.7 mg/mL (112.6; 400.3), p = 0.006).
We did not find evidence that plasma CRP or sCD14 at 18 months was associated with antigen-specific immune function (p > 0.05; Supplementary Table 4). However, biomarkers of enteropathy and intestinal damage were associated with higher concentration of antigen-specific immune mediators. The strongest association was with LPS-specific MPO, which increased by 0.44 pg/mL (95% CI: 1.06, 4.85, p = 0.035) in culture supernatants per 1-nmol/mL increase in stool neopterin concentration; this association was strengthened after adjustment for the SHINE arm, hub, child sex and maternal HIV status (adj. GMD (95% CI): 2.48 (1.19, 5.15), p = 0.015). There was also evidence for higher antigen-specific pro-inflammatory cytokine concentrations per unit increase in other biomarkers of environmental enteropathy (Supplementary Table 4): LPS-specific TNFα (GMD (95% CI), AAT: 0.41 (0.17, 0.98), p = 0.044; adj. GMD (95% CI): 0.36 (0.16, 0.81), p = 0.013), HKST-specific TNFα (GMD (95% CI), IFABP: 0.36 (0.17, 0.77), p = 0.009; adj. GMD (95%CI): 0.44 (0.23, 0.81), p = 0.009), HKST-specific IL-6 (GMD (95% CI), IFABP: 0.41 (0.18, 0.96), p = 0.040; not significant in adjusted models) and LPS-specific IL-8 (GMD (95%CI): 0.53 (0.27, 0.97), p = 0.062; adj. GMD (95% CI): 0.44 (0.24, 0.82), p = 0.009). These data suggest that concurrent enteropathy drives a heightened capacity to generate pro-inflammatory cytokine and pro-ROS responses to a new bacterial antigen stimulus in vitro.
Relationship Between Maternal Health During Pregnancy and Infant Immune Function
Maternal health may contribute to development of infant immune function since maternal infections, inflammation and nutritional state during pregnancy influence the in utero environment, birthweight and breastmilk composition; to investigate this, we used baseline data collected from mothers during their pregnancies (Table 3). Mothers of children enrolled in the sub-study were taller (mean ± standard deviation: 161 ± 7 versus 160 ± 9 cm), and a slightly lower proportion were married (95.0% versus 95.2%) compared to the SHINE cohort; other maternal characteristics, including levels of systemic and intestinal inflammatory biomarkers, were similar (Supplementary Table 2). Eighteen mothers (16.2% versus 15% in SHINE) were HIV-positive, and of those with available data on treatment, all were taking ART and all but one were taking prophylactic cotrimoxazole during pregnancy. Twelve mothers (13.0%) had detectable S. haematobium ova in their urine.
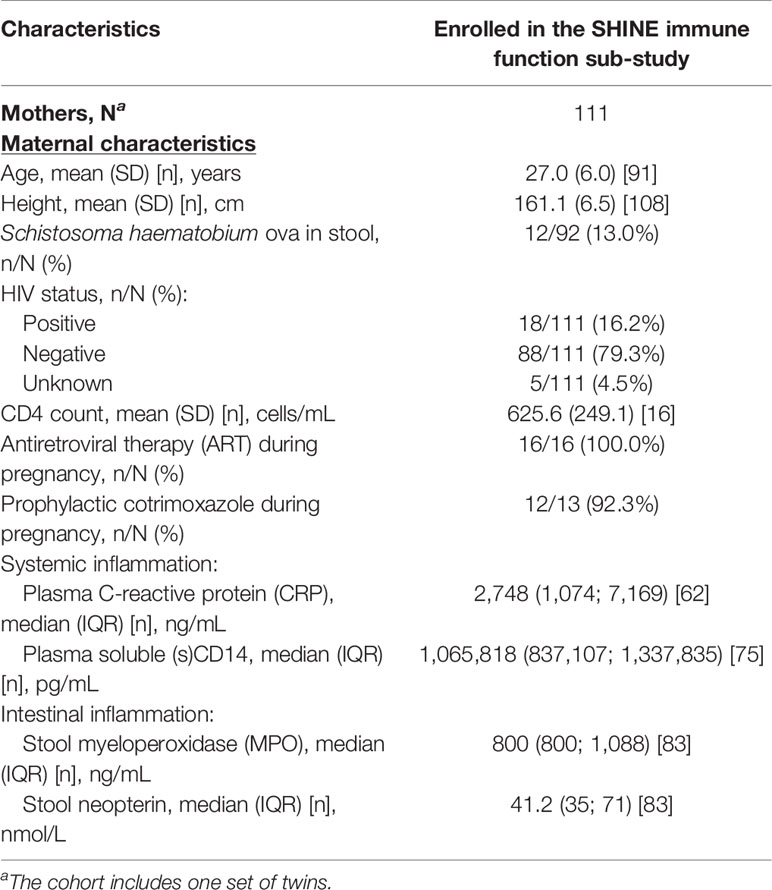
Table 3 Baseline household and maternal characteristics of the SHINE immune function sub-study cohort.
There was limited evidence for an association between maternal age, anthropometry (height, MUAC), HIV status or S. haematobium infection status at baseline and the antigen-specific immune function of their children at 18 months of age (p > 0.05; data not shown). However, maternal plasma sCD14 concentrations were associated with higher LPS- and HKST-specific TNFα (GMD (95% CI), LPS: 20.90 (1.75, 247.00), p = 0.016; HKST: 7.92 (1.99, 31.80), p = 0.003), LPS-specific IL-6 (GMD (95% CI): 7.69 (1.36, 43.80), p = 0.021) and HKST-specific IL-8 (GMD (95% CI): 4.44 (1.06, 18.72), p = 0.041). Adjustment for the SHINE arm, maternal age, HIV status and S. haematobium infection status weakened the association between maternal sCD14 and 18-month antigen-specific immune mediators; evidence for an association with 18-month HKST-specific TNFα was retained in adjusted models (adj. GMD (95% CI): 7.10 (1.51, 33.10), p = 0.013). Higher plasma CRP (GMD (95% CI): 1.80 (1.08, 2.97), p = 0.023; adj. GMD (95% CI): 1.73 (1.03, 2.92), p = 0.037) and stool neopterin (GMD (95% CI): 2.48 (1.40, 4.44), p = 0.002; adj. GMD (95% CI): 2.32 (1.32, 4.10), p = 0.003) concentrations during pregnancy were also associated with higher HKST-specific IL-8 responses in children at 18 months in both adjusted and unadjusted models. Thus, maternal inflammation and enteropathy during pregnancy influence the antibacterial immune function of their children at 18 months.
Impact of Household WASH and IYCF Interventions on Antibacterial Immune Function
The rationale behind the SHINE trial was that improved household WASH and IYCF during early life would improve child linear growth and reduce stunting; we hypothesised that the SHINE interventions might also influence antibacterial immune cell function. During pregnancy and prior to initiation of the SHINE interventions, WASH access and behaviours were limited and only a minority of households met the minimum dietary diversity scores (Supplementary Table 2), as is typical of rural households in this region. Compared to the larger SHINE cohort, households of children enrolled in the sub-study were similar across WASH indices with the exception of fewer households having access to handwashing stations with rubbing agent (0% versus 0.7%; Supplementary Table 2). Similar proportions of households in the sub-study and SHINE cohorts met minimum dietary diversity scores at baseline (35.4% versus 39.7%; Supplementary Table 2).
Consistent with SHINE primary outcome measures (19, 21), we did not identify a significant interaction between the effects of the SHINE WASH and IYCF interventions on unstimulated or antigen-specific outcome variables (Supplementary Table 5); we therefore compared WASH (i.e., children exposed to the SHINE WASH or WASH+IYCF arm) to no WASH (i.e., children exposed to the SHINE IYCF or SOC arm; Supplementary Table 6) and IYCF (i.e., children exposed to the SHINE IYCF or WASH+IYCF arm) to no IYCF (i.e., children exposed to the SHINE WASH or SOC arm; Supplementary Table 7). The WASH arm had a higher proportion of stunted children at 18 months than the no WASH arm (51.5% versus 33.8%; p = 0.069; Supplementary Table 6). We therefore included stunting status with the SHINE hub in adjusted tobit regression of immune function by the SHINE arm.
Children in the WASH arm had lower unstimulated MPO concentrations than those in the no WASH arm (Figure 4A; Table 4). Neither proportions nor concentrations of other mediators in unstimulated cultures differed by the WASH arm (Figure 4A; Table 4; Supplementary Table 8). Higher proportions of children in the WASH versus no WASH arm had detectable bacterial antigen-specific responses; this effect was strongest for LPS-specific MPO (logit regression OR (95% CI): 3.67 (1.28, 10.59), p = 0.016) and HKST-specific MPO (OR (95% CI): 4.35 (1.42, 13.33), p = 0.010; Figures 4B, C). LPS- and HKST-specific IL-6 and MPO and HKST-specific TNFα and IL-8 concentrations were also higher in the WASH versus no WASH arm in unadjusted models (Figures 4B, C). This pattern was retained for LPS- and HKST-specific MPO after adjustment for the SHINE hub and stunting status (Table 4), suggesting that the WASH intervention had a greater total effect on antibacterial MPO production than on pro-inflammatory cytokines.
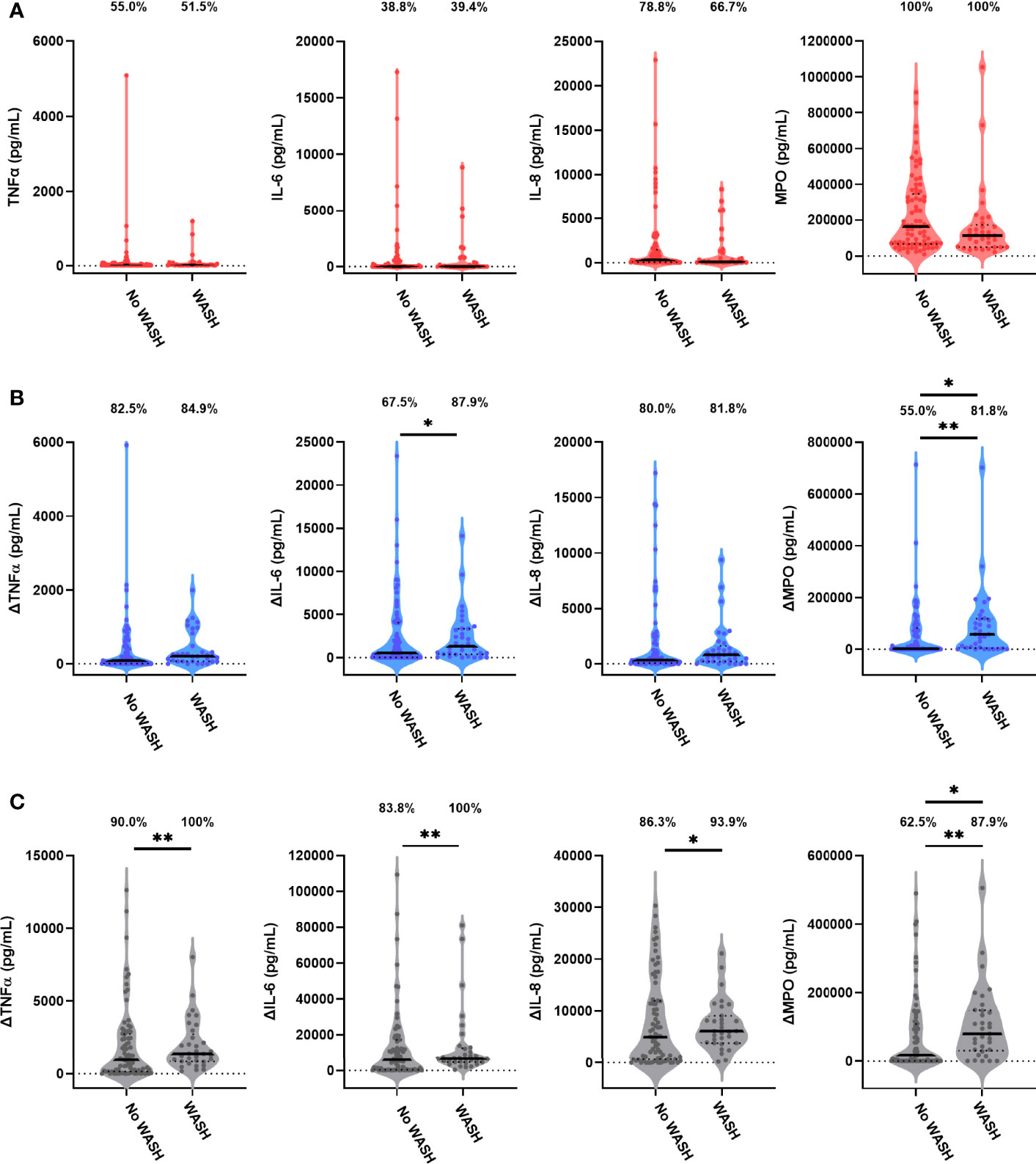
Figure 4 Unstimulated and bacterial antigen-stimulated immune-mediator production by blood immune cells from children exposed versus not exposed to a household WASH intervention. Violin plots (median and interquartile range indicated) of (A) unstimulated (red), (B) LPS-stimulated (blue) and (C) HKST-stimulated (grey) TNFα, IL-6, IL-8 and MPO concentrations in 24h culture supernatants. LPS- and HKST-specific concentrations (Δ) were calculated for each child by subtracting concentrations present in matched unstimulated culture supernatants. Proportions indicate participants with mediator concentration > ELISA limit of detection (A) and participants with antigen-stimulated mediator concentration > unstimulated (B, C). Proportions of children with detectable mediator levels and mediator concentrations were compared by exposure to the SHINE WASH intervention (WASH n = 33, no WASH n = 80) via multinomial logit regression and censored log-normal (tobit) regression, respectively (unadjusted analyses indicated; full results in Table 4); *p < 0.05, **p < 0.01.
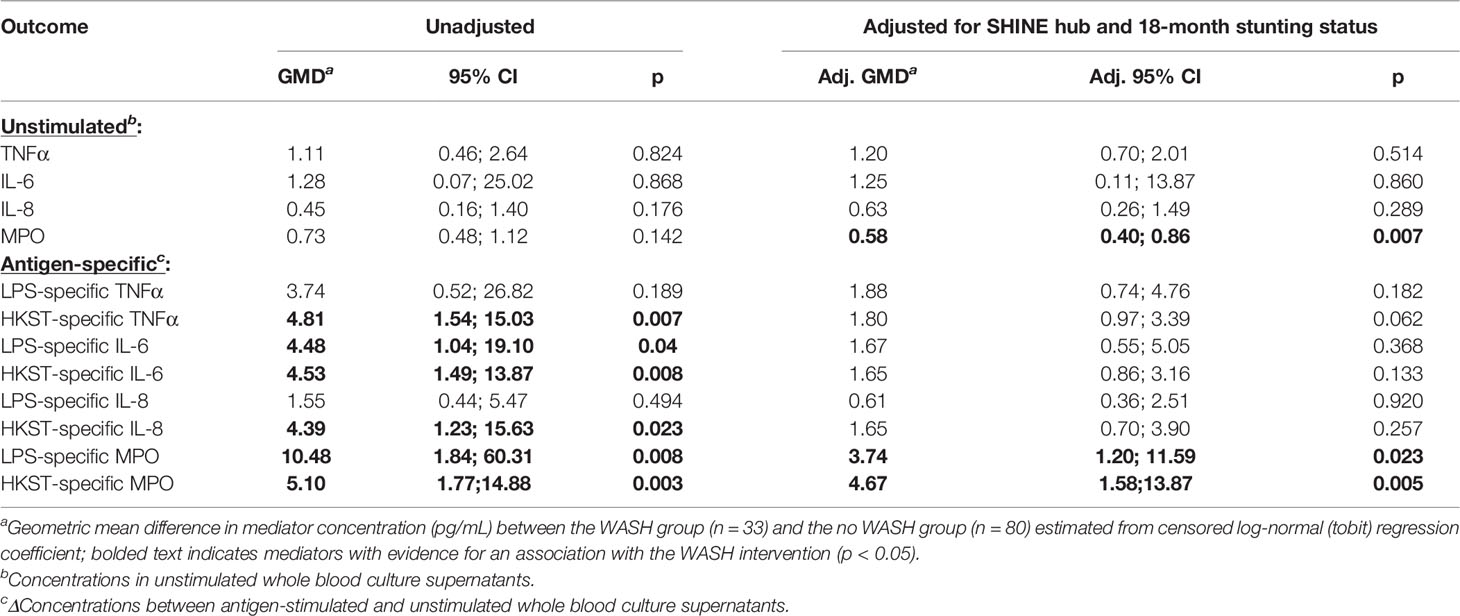
Table 4 Censored log-normal (tobit) regression analysis of the relationship between WASH intervention arm and immune-mediator production at 18 months of age.
We found no evidence for an effect of the SHINE IYCF intervention on proportions or concentrations of unstimulated or bacterial antigen-specific mediators (Figure 5; Table 5; Supplementary Table 8).
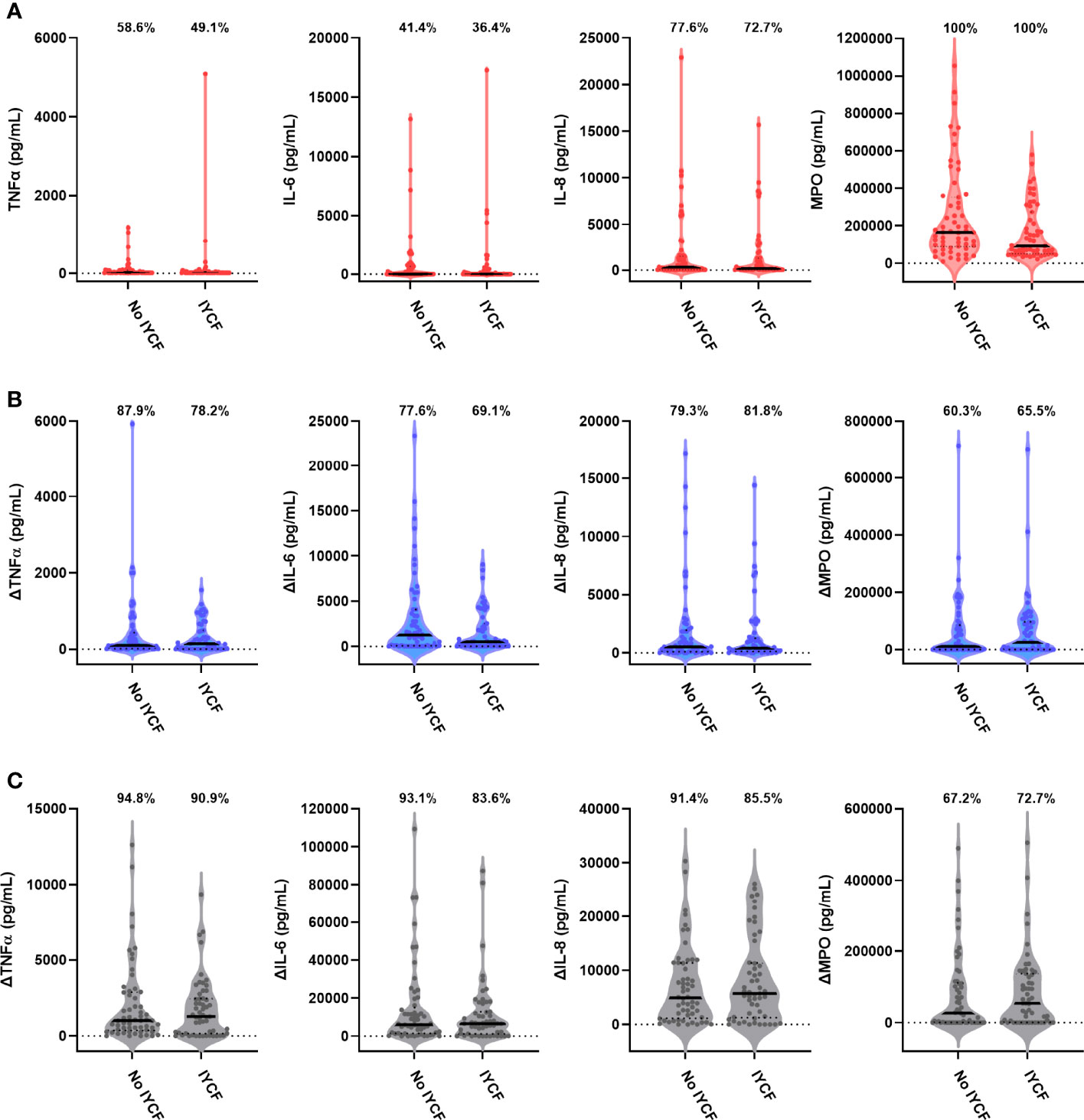
Figure 5 Unstimulated and bacterial antigen-stimulated immune-mediator production by blood immune cells from children exposed versus not exposed to a household IYCF intervention. Violin plots (median and interquartile range indicated) of (A) unstimulated (red), (B) LPS-stimulated (blue) and (C) HKST-stimulated (grey) TNFα, IL-6, IL-8 and MPO concentrations in 24h whole blood culture supernatants. LPS- and HKST-specific concentrations (Δ) were calculated for each child by subtracting concentrations present in matched unstimulated culture supernatants. Proportions indicate participants with mediator concentration > ELISA limit of detection (A) and participants with antigen-stimulated mediator concentration > unstimulated (B, C). Proportions of children with detectable mediator levels and mediator concentrations were compared between children exposed to the SHINE household IYCF intervention (n = 55) and those not exposed to the IYCF intervention (n = 58) by multinomial logit regression and censored log-normal (tobit) regression, respectively (unadjusted analyses indicated; full results in Table 5).
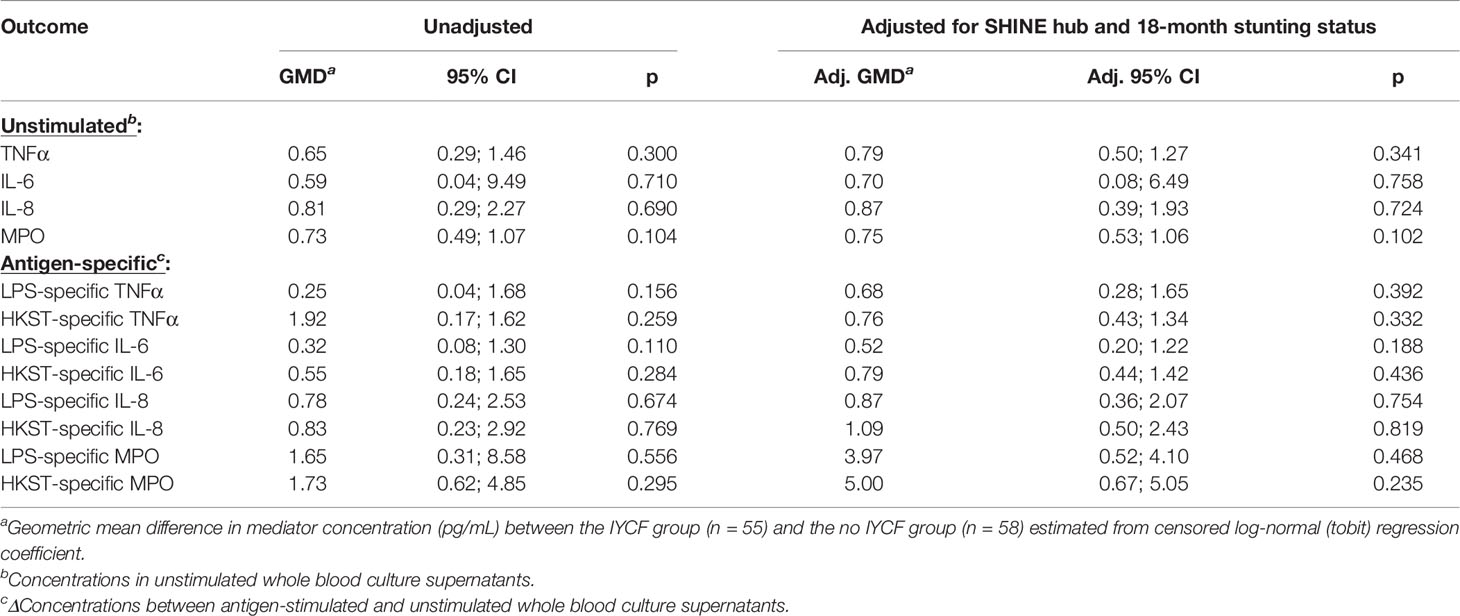
Table 5 Censored log-normal (tobit) regression analysis of the relationship between IYCF intervention arm and immune-mediator production at 18 months of age.
Discussion
In this study, we directly assessed the capacity for blood immune cells from 113 children in rural Zimbabwe to respond to bacterial antigen challenge in vitro—a model for how each child might respond to a newly acquired bacterial infection. Antibacterial immune function was highly heterogeneous even among this predominantly healthy HIV-negative cohort. We found evidence that stunting status and environmental enteropathy at 18 months of age, maternal inflammation during pregnancy, birthweight and exposure to improved household WASH contributed to inter-individual variation that could plausibly contribute to differences in infectious susceptibility.
Stunting is a multifactorial syndrome arising among children exposed to a marginal diet, recurrent infections, chronic inflammation and impaired gut function. In support of our hypothesis that stunting alters antibacterial immune function, higher proportions of stunted children responded to bacterial antigen challenge and these children produced higher concentrations of LPS-specific pro-inflammatory cytokines (IL-6 and IL-8) than children who were non-stunted. Epigenetic analyses of 8 stunted and 11 non-stunted Bangladeshi children have previously identified a histone-3-lysine-4 methylation signature on blood immune cells that could putatively affect their function (29). However, this is the first study to our knowledge to directly characterise the relationship between antibacterial immune cell function and stunting (6, 7). Stunting and wasting are indicators of undernutrition which frequently coexist; wasting tends to be more acute and reversible than stunting (30). Several small studies have previously assessed immune cell function in wasted children, among whom cytokine responses to in vitro activation tended to be lower compared to well-nourished controls (31, 32) or to their own responses after nutritional recovery (32, 33). More recently, a study among severely unwell 2–23-month-old children in Kenya and Uganda with a range of wasting severities found evidence that hospitalised children had consistently higher LPS-specific pro-inflammatory mediator concentrations within 48 h of admission (n = 43), on the day of discharge (n = 60) and, for survivors, 6 months after admission (n = 51) than healthy community controls (n = 41) (34). In contrast to LPS (a TLR4 ligand), mediator responses to TLR7/8 ligands and Staphylococcal enterotoxin B, which activates innate and adaptive immune cells by binding MHC-II and T-cell receptor molecules, were lower in unwell children versus controls (34). Our observations among stunted children are consistent with those for TLR4-driven mediator responses among wasted children, implicating priming of the pro-inflammatory innate immune response to gram-negative bacteria among children with both acute and chronic forms of undernutrition. Our study recruited children from the community which minimised immune heterogeneity due to severe symptomatic infections, associated clinical complications and antibiotic treatment which are common among hospital inpatients. It is plausible that the differences we observed in antigen-specific mediator responses are due to differences in circulating immune cell composition between stunted and non-stunted children; to our knowledge, there are no published studies comparing blood immunophenotypes between stunted and non-stunted children from the same community. We did not localise the cellular source of supernatant mediators; however, our choice of short culture time, PAMPs and ELISA favours assessment of innate cell types, particularly monocytes. The expression of TLR2 and TLR4, the primary receptors for LPS and HKST, could also be influential. For example, low LPS-specific TNFα and IL-6 production was related to low monocyte TLR4 expression among 20 uninfected infants born preterm in Austria (35). A more granular evaluation of cellular antibacterial immune responses in stunting is warranted.
Immune function was not associated with LAZ at 3, 6, 12 or 18 months or with the IYCF intervention, which improved linear growth and reduced stunting prevalence in the SHINE trial (19, 21). In contrast to severely unwell children in Kenya and Uganda, among whom MUAC was positively associated with LPS-specific responses at hospital discharge (34), we did not identify an association between antigen-specific immune function and MUAC or with other indicators of concurrent wasting (WHZ, WAZ); this may reflect the fact that our cohort was largely within healthy WHZ and WAZ ranges. Thus, our data implicate factors besides anthropometry and feeding-based interventions (such as IYCF) in the differences in immune function between stunted and non-stunted children. Enteropathogen carriage is an in vivo source of PAMPs, greater among children with slower growth velocities and associated with systemic and intestinal inflammation (13, 16, 36). Circulating LPS (also termed endotoxin) is the most studied PAMP in undernourished cohorts and widely used as an indicator of increased translocation of commensal bacteria and enteropathogens from the gut (9). The LPS-TLR4 pathway is also a means by which circulating monocyte function is modified in gram-negative bacterial sepsis (37), which may contribute to the infectious susceptibility that characterises post-sepsis convalescence. Consistent with pathogen carriage contributing to higher antibacterial mediator production, the effect of stunting status on LPS-specific pro-inflammatory cytokines was reduced when the small number children with recently reported symptoms of infection were excluded in sensitivity analyses. Asymptomatic pathogen carriage and LPS concentrations were not assessed in this study but could also plausibly underlie a more primed circulating immune cell response to PAMP. We found some evidence for this in the association between 18-month biomarkers of environmental enteropathy (stool neopterin, MPO and AAT, plasma IFABP), particularly between LPS-specific MPO and stool neopterin. Stool MPO and plasma IFABP were also associated with seroconversion in response to the oral rotavirus vaccine in the SHINE rotavirus sub-study (38). Consistent with humoral responses to the rotavirus vaccine (38), we did not find evidence for an association between antigen-specific immune-mediator concentrations and plasma sCD14, a biomarker of monocyte activation frequently used as an indirect indicator of microbial translocation. Thus, antibacterial functions of circulating immune cells appear to be more influenced by intestinal than systemic inflammation at 18 months; this could reflect age-related changes in the gut to limit systemic inflammation in the context of microbial colonisation and recurrent infections at earlier ages. In the SHINE trial, enteropathy biomarkers changed significantly between the 1- and 18-month visits (23); stool MPO and neopterin tended to be lower at 18 months, indicative of reduced innate immune cell accumulation in the lamina propria in older children. In a Zambian cohort of children under 5 years of age, stunting was associated with cumulative adaptation of the gastrointestinal tract to reduce LPS translocation with age (9).
Since both stunting and immune development have in utero origins, a strength of this study was the availability of longitudinal data on each child’s early life with which to explore factors that could contribute to the heterogeneity in immune function we observed at 18 months. Direct indicators of baseline maternal health were not associated with 18-month immune function. However, antigen-specific MPO was higher among children with higher birthweight, indicating that children born smaller may have a lower capacity to produce this enzyme even when born within the healthy birthweight range. Furthermore, children born to mothers with higher concentrations of systemic (CRP and sCD14) and intestinal (neopterin) inflammatory biomarkers during pregnancy had higher pro-inflammatory cytokine responses to bacterial antigens at the 18-month visit. The most consistent positive relationship was with maternal sCD14, an LPS receptor/TLR4 co-receptor expressed by circulating monocytes that is shed upon their activation (39). We did not find evidence for an association between 18-month concentrations of this biomarker and immune function in children, suggesting that history of exposure to sCD14 in utero has more influence on a child’s capacity to produce pro-inflammatory cytokines in response to bacteria than their concurrent circulating sCD14 concentrations. In utero exposure to maternal monocyte activation could prime responses to gram-negative bacteria via a variety of mechanisms. For example, since monocytes are early responders to circulating PAMPs in vivo (39), sCD14 could be an indicator of microbial translocation during pregnancy with implications for in utero growth; among the 207 SHINE mothers for whom stool samples were analysed by metagenomic sequencing, microbiome taxa and metabolic genes were predictive of birthweight (40). We adjusted analyses by maternal HIV and S. haematobium but did not screen for other potentially influential pathogens during pregnancy. Maternal sCD14 could also reduce infant LPS exposure prior to weaning; radiolabelled sCD14 from rat dams persists in the small intestine and blood of their pups up to 8 h after breastmilk ingestion (41).
In light of the relationships identified between antibacterial immune function and child stunting, enteropathy and maternal inflammation during pregnancy, all plausibly mediated through enteropathogen exposure, we went on to characterise the effects of household WASH. WASH is posited as an intervention to reduce child stunting via reducing enteropathogen exposure (42); however, the SHINE WASH intervention did not improve linear growth at 18 months in the main trial (19, 21) nor did it effect diarrhoea or enteropathy biomarkers (21, 23, 38), suggesting that more transformative WASH (e.g., piped water, improved housing, managed sanitation) is required to sufficiently reduce enteropathogen exposure for meaningful impacts on child growth outcomes (43). However, since immune cells are direct responders to PAMPs, we hypothesised that they would be more sensitive to small changes in enteropathogen exposure than growth outcomes or indirect biomarker measures. Accordingly, exposure to the WASH intervention was associated with higher proportions and concentrations of LPS- and HKST-specific MPO. MPO is more abundantly expressed in neutrophils than other blood immune cells and catalyses production of ROS, a potent microbicidal response to infection (27). Whilst we did not directly measure the ability of blood leukocytes to kill bacteria, these data suggest that exposure to improved household WASH in early life may increase neutrophil-mediated antibacterial defence. The observation that children exposed to the WASH intervention had increased cellular immunoreactivity to bacterial PAMPs is consistent with findings for humoral immune function in the SHINE rotavirus sub-study where there was a significant increase in seroconversion to the oral rotavirus vaccine among children in the WASH (n = 109) versus no WASH arm (n = 219) (44).
Our results should be interpreted in the context of several study limitations. Most notably, we did not have preexisting data on immune function in stunted versus non-stunted children with which to estimate sample size; given the heterogeneity in mediator concentrations, a larger sample size would have given greater power to detect differences in causal inference models. Cross-sectional assessment meant that longevity and clinical relevance of the pg/mL magnitude differences observed in immune function variables are unclear and longitudinal assessment is warranted (1, 6); production of higher concentrations of pro-inflammatory cytokines could benefit stunted children by promoting more effective clearance of bacterial infection but could also exacerbate immunopathology. For example, biomarker studies among severely wasted children requiring hospital admission indicate that pro-inflammatory cytokine concentrations are higher in plasma from children who die versus survive (45, 46) and nutritional recovery is slower among children with systemic inflammation (47). Inclusion of SHINE participants in this sub-study was based on blood sample volume and completion of an 18-month follow-up visit, meaning that our cohort reflects a healthier group of children than that reflected by the SHINE cohort as a whole and differences were evident between the intervention groups. Thus, further studies of the interrelationships between immune function, child growth and more direct measures of enteropathogen exposure and microbial translocation are required, particularly among children most vulnerable to adverse infectious outcomes.
In conclusion, this study provides proof of principle that stunted children have distinct antibacterial immune function to non-stunted children which is shaped by indicators of maternal and child enteropathogen exposure and inflammation to a greater extent than their anthropometry alone. A pertinent outstanding question is whether the differences that we identify in immune function are adaptive, benefitting health and survival in the context of adverse exposures, or deleterious, increasing risk of acquiring and succumbing to infection; answering this question will rely on combining longitudinal immune function assessment with clinical outcome measures among undernourished children (1, 6). Our results also demonstrate how assays of immune cell function could provide a sensitive tool with which to evaluate interventions targeting infectious morbidity and mortality among children growing up in LMIC.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Medical Research Council of Zimbabwe Institutional Review Board of the Johns Hopkins Bloomberg School of Public Health. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author Contributions
KM and JT contributed equally to this work. KM assisted CB to develop the laboratory assays and managed laboratory operations for the sub-study with support from SR. JT undertook statistical analyses supervised by CB and with support from RN and BC. FM and CC performed whole blood cultures in field laboratories. SR, MG, and PM performed ELISA. NT managed field operations, KM managed the laboratory, FM supervised data collection nurses, BM supervised the field data supervisors, and RN developed and managed information technology for the main SHINE trial. JH conceived of SHINE and secured funding for the main trial. AP directed clinical and laboratory aspects of SHINE and secured funding for the sub-study. CB designed and managed the sub-study and prepared the first draft of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The SHINE trial was funded by the Bill and Melinda Gates Foundation (OPP1021542 and OPP1143707), the United Kingdom Department for International Development (DFID/UKAID), Wellcome Trust (093768/Z/10/Z and 108065/Z/15/Z), Swiss Agency for Development and Cooperation, and UNICEF (PCA-2017-0002). CB is funded by a Sir Henry Dale Postdoctoral Research Fellowship from the Wellcome Trust and The Royal Society (206225/Z/17/Z). Open Access Publication was funded via Wellcome.
Author Disclaimer
The study funders were not involved in data collection, analysis, interpretation, or manuscript preparation.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors are grateful to the SHINE trial participants and the SHINE trial team (all members listed here: https://doi.org/10.1093/cid/civ844). They acknowledge the Ministry of Health and Child Care staff in Chirumanzu and Shurugwi districts and Midlands province for their roles in operationalization of SHINE study protocols. We acknowledge Stephen Moyo, Philippa Rambanepasi, and Virginia Sauramba who respectively managed logistics, finance and compliance for the sub-study. We also acknowledge the Prendergast research group members past and present for the helpful discussion.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.899296/full#supplementary-material
References
1. Bourke CD, Berkley JA, Prendergast AJ. Immune Dysfunction as a Cause and Consequence of Malnutrition. Trends Immunol (2016) 37:386–98. doi: 10.1016/j.it.2016.04.003
2. Brodin P, Jojic V, Gao T, Bhattacharya S, Angel CJ, Furman D, et al. Variation in the Human Immune System is Largely Driven by non-Heritable Influences. Cell (2015) 160:37–47. doi: 10.1016/j.cell.2014.12.020
3. Yan Z, Maecker HT, Brodin P, Nygaard UC, Lyu SC, Davis MM, et al. Aging and CMV Discordance are Associated With Increased Immune Diversity Between Monozygotic Twins. Immun Ageing (2021) 18:5. doi: 10.1186/s12979-021-00216-1
4. Brodin P, Davis MM. Human Immune System Variation. Nat Rev Immunol (2017) 17:21–9. doi: 10.1038/nri.2016.125
5. Temba GS, Kullaya V, Pecht T, Mmbaga BT, Aschenbrenner AC, Ulas T, et al. Urban Living in Healthy Tanzanians is Associated With an Inflammatory Status Driven by Dietary and Metabolic Changes. Nat Immunol (2021) 22:287–300. doi: 10.1038/s41590-021-00867-8
6. Bourke CD, Jones KDJ, Prendergast AJ. Current Understanding of Innate Immune Cell Dysfunction in Childhood Undernutrition. Front Immunol (2019) 10:1728–8. doi: 10.3389/fimmu.2019.01728
7. Rytter MJ, Kolte L, Briend A, Friis H, Christensen VB. The Immune System in Children With Malnutrition–a Systematic Review. PloS One (2014) 9:e105017. doi: 10.1371/journal.pone.0105017
8. UNICEF WHO, Group WB. Levels and Trends in Child Malnutrition; Joint Child Malnutrition Estimates; Key Findings of the 2020 Edition. (2020). Available at: https://www.who.int/publications/i/item/9789240003576
9. Amadi B, Zyambo K, Chandwe K, Besa E, Mulenga C, Mwakamui S, et al. (2021). Adaptation of the Small Intestine to Microbial Enteropathogens in Zambian Children With Stunting. Nat Microbiol (2021) 6:445–54. doi: 10.1038/s41564-020-00849-w
10. Olofin I, McDonald CM, Ezzati M, Flaxman S, Black RE, Fawzi WW, et al. For the Nutrition Impact Model, Associations of Suboptimal Growth With All-Cause and Cause-Specific Mortality in Children Under Five Years: A Pooled Analysis of Ten Prospective Studies. PloS One (2013) 8:e64636. doi: 10.1371/journal.pone.0064636
11. Leroy JL, Frongillo EA. Perspective: What Does Stunting Really Mean? A Crit Rev Evidence Adv Nutr (2019) 10:196–204. doi: 10.1093/advances/nmy101
12. Syed S, Manji KP, McDonald CM, Kisenge R, Aboud S, Sudfeld C, et al. Biomarkers of Systemic Inflammation and Growth in Early Infancy are Associated With Stunting in Young Tanzanian Children. Nutrients (2018) 10(9):1158. doi: 10.3390/nu10091158
13. Prendergast AJ, Rukobo S, Chasekwa B, Mutasa K, Ntozini R, Mbuya MNN, et al. Stunting is Characterized by Chronic Inflammation in Zimbabwean Infants. PloS One (2014) 9:e86928–8. doi: 10.1371/journal.pone.0086928
14. Campbell DI, Elia M, Lunn PG. Growth Faltering in Rural Gambian Infants Is Associated With Impaired Small Intestinal Barrier Function, Leading to Endotoxemia and Systemic Inflammation. J Nutr (2003) 133:1332–8. doi: 10.1093/jn/133.5.1332
15. Syed S, Iqbal NT, Sadiq K, Ma JZ, Akhund T, Xin W, et al. Serum Anti-Flagellin and Anti-Lipopolysaccharide Immunoglobulins as Predictors of Linear Growth Faltering in Pakistani Infants at Risk for Environmental Enteric Dysfunction. PloS One (2018) 13:e0193768. doi: 10.1371/journal.pone.0193768
16. Kosek MN, Investigators M-EN. Causal Pathways From Enteropathogens to Environmental Enteropathy: Findings From the MAL-ED Birth Cohort Study. EBioMedicine (2017) 18:109–17. doi: 10.1016/j.ebiom.2017.02.024
17. Chasekwa B, Ntozini R, Church JA, Majo FD, Tavengwa N, Mutasa B, et al. Prevalence, Risk Factors and Short-Term Consequences of Adverse Birth Outcomes in Zimbabwean Pregnant Women: A Secondary Analysis of a Cluster-Randomized Trial. Int J Epidemiol (2021) dyab248. doi: 10.1093/ije/dyab248
18. Murenjekwa W, Makasi R, Ntozini R, Chasekwa B, Mutasa K, Moulton LH, et al. Determinants of Urogenital Schistosomiasis Among Pregnant Women and its Association With Pregnancy Outcomes, Neonatal Deaths, and Child Growth. J Infect Dis (2021) 223:1433–44. doi: 10.1093/infdis/jiz664
19. Prendergast AJ, Chasekwa B, Evans C, Mutasa K, Mbuya MNN, Stoltzfus RJ, et al. Independent and Combined Effects of Improved Water, Sanitation, and Hygiene, and Improved Complementary Feeding, on Stunting and Anaemia Among HIV-Exposed Children in Rural Zimbabwe: A Cluster-Randomised Controlled Trial. Lancet Child Adolesc Health (2019) 3:77–90. doi: 10.1016/S2352-4642(18)30340-7
20. Humphrey JH, Jones AD, Manges A, Mangwadu G, Maluccio JA, Mbuya MNN, et al. The Sanitation Hygiene Infant Nutrition Efficacy (SHINE) Trial: Rationale, Design, and Methods. Clin Infect Dis (2015) 61:S685–702. doi: 10.1093/cid/civ844
21. Humphrey JH, Mbuya MNN, Ntozini R, Moulton LH, Stoltzfus RJ, Tavengwa NV, et al. Independent and Combined Effects of Improved Water, Sanitation, and Hygiene, and Improved Complementary Feeding, on Child Stunting and Anaemia in Rural Zimbabwe: A Cluster-Randomised Trial. Lancet Global Health (2019) 7:e132–47. doi: 10.1016/S2214-109X(18)30374-7
22. Gregson S, Moorhouse L, Dadirai T, Sheppard H, Mayini J, Beckmann N, et al. Comprehensive Investigation of Sources of Misclassification Errors in Routine HIV Testing in Zimbabwe. J Int AIDS Soc (2021) 24:e25700–0. doi: 10.1002/jia2.25700
23. Gough EK, Moulton LH, Mutasa K, Ntozini R, Stoltzfus RJ, Majo FD, et al. For the Sanitation Hygiene Infant Nutrition Efficacy Trial, Effects of Improved Water, Sanitation, and Hygiene and Improved Complementary Feeding on Environmental Enteric Dysfunction in Children in Rural Zimbabwe: A Cluster-Randomized Controlled Trial. PloS Neglected Trop Dis (2020) 14:e0007963. doi: 10.1371/journal.pntd.0007963
24. Robinson N, McComb S, Mulligan R, Dudani R, Krishnan L, Sad S. Type I Interferon Induces Necroptosis in Macrophages During Infection With Salmonella Enterica Serovar Typhimurium. Nat Immunol (2012) 13:954–62. doi: 10.1038/ni.2397
25. Fabriek BO, van Bruggen R, Deng DM, Ligtenberg AJ, Nazmi K, Schornagel K, et al. The Macrophage Scavenger Receptor CD163 Functions as an Innate Immune Sensor for Bacteria. Blood (2009) 113:887–92. doi: 10.1182/blood-2008-07-167064
26. Michels K, Nemeth E, Ganz T, Mehrad B. Hepcidin and Host Defense Against Infectious Diseases. PloS Pathog (2015) 11:e1004998. doi: 10.1371/journal.ppat.1004998
27. Klebanoff SJ. Myeloperoxidase: Friend and Foe. J leukocyte Biol (2005) 77:598–625. doi: 10.1189/jlb.1204697
28. Klein SL, Flanagan KL. Sex Differences in Immune Responses. Nat Rev Immunol (2016) 16:626–38. doi: 10.1038/nri.2016.90
29. Uchiyama R, Kupkova K, Shetty SJ, Linford AS, Pray-Grant MG, Wagar LE, et al. Histone H3 Lysine 4 Methylation Signature Associated With Human Undernutrition. Proc Natl Acad Sci (2018) 115:E11264–73. doi: 10.1073/pnas.1722125115
30. Mertens A, Benjamin-Chung J, Colford JM, Hubbard AE, van der Laan MJ, Coyle J, et al. Consortium, Child Wasting and Concurrent Stunting in Low- and Middle-Income Countries. medRxiv (2021) 2020:06.09.20126979. doi: 10.1101/2020.06.09.20126979
31. Rodríguez L, Graniel J, Ortiz R. Effect of Leptin on Activation and Cytokine Synthesis in Peripheral Blood Lymphocytes of Malnourished Infected Children. Clin Exp Immunol (2007) 148:478–85. doi: 10.1111/j.1365-2249.2007.03361.x
32. Palacio A, Lopez M, Perez-Bravo F, Monkeberg F, Schlesinger L. Leptin Levels are Associated With Immune Response in Malnourished Infants. J Clin Endocrinol Metab (2002) 87:3040–6. doi: 10.1210/jcem.87.7.8636
33. Hughes SM, Amadi B, Mwiya M, Nkamba H, Tomkins A, Goldblatt D. Dendritic Cell Anergy Results From Endotoxemia in Severe Malnutrition. J Immunol (2009) 183:2818–26. doi: 10.4049/jimmunol.0803518
34. Uebelhoer LS, Gwela A, Thiel B, Nalukwago S, Mukisa J, Lwanga C, et al. Toll-Like Receptor-Induced Immune Responses During Early Childhood and Their Associations With Clinical Outcomes Following Acute Illness Among Infants in Sub-Saharan Africa. Front Immunol (2022) 12:748996. doi: 10.3389/fimmu.2021.748996
35. Förster-Waldl E, Sadeghi K, Tamandl D, Gerhold B, Hallwirth U, Rohrmeister K, et al. Monocyte Toll-Like Receptor 4 Expression and LPS-Induced Cytokine Production Increase During Gestational Aging. Pediatr Res (2005) 58:121–4. doi: 10.1203/01.PDR.0000163397.53466.0F
36. MAL-ED Network Investigators. Relationship Between Growth and Illness, Enteropathogens and Dietary Intakes in the First 2 Years of Life: Findings From the MAL-ED Birth Cohort Study. BMJ Global Health (2017) 2:e000370. doi: 10.1136/bmjgh-2017-000370
37. Shalova IN, Lim JY, Chittezhath M, Zinkernagel AS, Beasley F, Hernandez-Jimenez E, et al. Human Monocytes Undergo Functional Re-Programming During Sepsis Mediated by Hypoxia-Inducible Factor-1alpha. Immunity (2015) 42:484–98. doi: 10.1016/j.immuni.2015.02.001
38. Church JA, Rukobo S, Govha M, Gough EK, Chasekwa B, Lee B, et al. Associations Between Biomarkers of Environmental Enteric Dysfunction and Oral Rotavirus Vaccine Immunogenicity in Rural Zimbabwean Infants. eClinicalMedicine (2021) 41:101173. doi: 10.1016/j.eclinm.2021.101173
39. Patel AA, Zhang Y, Fullerton JN, Boelen L, Rongvaux A, Maini AA, et al. The Fate and Lifespan of Human Monocyte Subsets in Steady State and Systemic Inflammation. J Exp Med (2017) 214:1913–23. doi: 10.1084/jem.20170355
40. Gough EK, Edens TJ, Geum HM, Baharmand I, Gill SK, Robertson RC, et al. Maternal Fecal Microbiome Predicts Gestational Age, Birth Weight and Neonatal Growth in Rural Zimbabwe. EBioMedicine (2021) 68:103421. doi: 10.1016/j.ebiom.2021.103421
41. Ward TL, Spencer WJ, Davis LDR, Harrold J, Mack DR, Altosaar I. Ingested Soluble CD14 From Milk is Transferred Intact Into the Blood of Newborn Rats. Pediatr Res (2014) 75:252–8. doi: 10.1038/pr.2013.225
42. UNICEF. Conceptual Framework on Maternal and Child Nutrition. (2021). Available at: https://www.unicef.org/media/113291/file/UNICEF%20Conceptual%20Framework.pdf
43. Cumming O, Arnold BF, Ban R, Clasen T, Esteves Mills J, Freeman MC, et al. The Implications of Three Major New Trials for the Effect of Water, Sanitation and Hygiene on Childhood Diarrhea and Stunting: A Consensus Statement. BMC Med (2019) 17:173–3. doi: 10.1186/s12916-019-1410-x
44. Church JA, Rukobo S, Govha M, Lee B, Carmolli MP, Chasekwa B, et al. The Impact of Improved Water, Sanitation, and Hygiene on Oral Rotavirus Vaccine Immunogenicity in Zimbabwean Infants: Substudy of a Cluster-Randomized Trial. Clin Infect Dis (2019) 69:2074–81. doi: 10.1093/cid/ciz140
45. Attia S, Versloot CJ, Voskuijl W, van Vliet SJ, Di Giovanni V, Zhang L, et al. Mortality in Children With Complicated Severe Acute Malnutrition is Related to Intestinal and Systemic Inflammation: An Observational Cohort Study. Am J Clin Nutr (2016) 104:1441–9. doi: 10.3945/ajcn.116.130518
46. Njunge JM, Gwela A, Kibinge NK, Ngari M, Nyamako L, Nyatichi E, et al. Biomarkers of Post-Discharge Mortality Among Children With Complicated Severe Acute Malnutrition. Sci Rep (2019) 9:5981. doi: 10.1038/s41598-019-42436-y
Keywords: Immune Function, Zimbabwe, Malnutrition, Stunting, Inflammation, Maternal and Child Health, Pregnancy, Immune Cells
Citation: Mutasa K, Tome J, Rukobo S, Govha M, Mushayanembwa P, Matimba FS, Chiorera CK, Majo FD, Tavengwa NV, Mutasa B, Chasekwa B, Humphrey JH, Ntozini R, Prendergast AJ and Bourke CD (2022) Stunting Status and Exposure to Infection and Inflammation in Early Life Shape Antibacterial Immune Cell Function Among Zimbabwean Children. Front. Immunol. 13:899296. doi: 10.3389/fimmu.2022.899296
Received: 18 March 2022; Accepted: 11 May 2022;
Published: 13 June 2022.
Edited by:
Bisheng Zhou, University of Illinois at Chicago, United StatesReviewed by:
Osaretin Emmanuel Asowata, Population Council, United StatesSuzanna Attia, University of Kentucky, United States
Copyright © 2022 Mutasa, Tome, Rukobo, Govha, Mushayanembwa, Matimba, Chiorera, Majo, Tavengwa, Mutasa, Chasekwa, Humphrey, Ntozini, Prendergast and Bourke. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Claire D. Bourke, Yy5ib3Vya2VAcW11bC5hYy51aw==
†These authors have contributed equally to this work and share first authorship
 Kuda Mutasa1†
Kuda Mutasa1† Andrew J. Prendergast
Andrew J. Prendergast Claire D. Bourke
Claire D. Bourke