
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Immunol., 10 June 2022
Sec. Cancer Immunity and Immunotherapy
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.894787
This article is part of the Research TopicThe Mechanism and Novel Strategies of Overcoming Resistance of Hematological Malignancies to CAR T-cell KillingView all 6 articles
Background: Failure to CD19-targeted chimeric antigen receptor T-cell (CAR-T) therapy for patients with relapsed/refractory (R/R) diffuse large B-cell lymphoma (DLBCL), is an emerging clinical problem. There is no consensus on the treatment for these patients and treatment remains empirical.
Case Report: We reported a case of an elderly R/R DLBCL patient who had TP53 mutation and relapsed 12 months after initial response to CAR T-cell therapy. The patient did not respond to salvage chemotherapy with the GDP regimen and could not tolerate any aggressive chemotherapy. Thereafter, the patient was given chidamide and zanubrutinib. After two months of treatment, the patient achieved sustained complete remission. At the last follow-up, the patient remains in radiographic CR 22 months after CAR-T infusion and 10 months after the initiation of the combination treatment.
Conclusion: We report the first successful case of dual inhibition of HDAC and BTK for the treatment of R/R DLBCL after failure to CAR-T cell therapy, which opens a new therapeutic possibility for the future.
CD19-targeted chimeric antigen receptor T cell (CAR-T) immunotherapy has been reported with superior response and long-term survival benefits for patients with relapsed or refractory (R/R) diffuse large B-cell lymphoma (DLBCL) (1–3). Despite these encouraging clinical results, more than half of patients either do not respond to or eventually relapse after CAR-T therapy (4, 5). The prognosis of these patients is usually very poor with a median overall survival (OS) of approximately 6 months (6). They represent a new unmet need in the treatment of this disease.
Unfortunately, there are only a few salvage options currently available and there are no standard options for DLBCL patients after CAR-T failure (7). Although novel drugs, such as selinexor, tafasitamab, polatuzumab, checkpoint inhibitors, loncastuximab tesirine, and bi-specific antibodies, are clinically available, there are no data available on the patients with CAR-T failure. It is unclear whether any of these drugs will lead to durable remissions. CAR-T cells targeting an alternative antigen, administration of 1 or more kinds of CAR-T cells targeting different antigens, bivalent CAR-Ts targeting multiple antigens, and allogeneic CAR-T show favorable responses after CD19-targeted CAR-T failure. However, these CAR-Ts are still in clinical trials and only accessible to a small proportion of patients in a few large centers. Allogeneic stem cell transplantation could also be considered in eligible patients. However, the recipient’s fitness and donor availability may impede its applicability immediately after CAR-T relapse. Therefore, there is an urgent demand for novel drugs and drug combinations to treat these patients.
Histone deacetylases (HDACs), a family of enzymes that remove acetyl groups from histones, are critical epigenetic silencers. Histone deacetylase inhibitors (HDACi) function by interfering with HDACs can reverse transcriptional inhibition of tumor suppressor genes. Recently, several kinds of HDACi were evaluated in clinical trials for B-cell non-Hodgkin’s lymphoma (NHL). In a phase 2 study, mocetinostat, an isotype-selective HDACi, achieves a 18.9% overall response rate (ORR) in 41 R/R DLBCL patients, and nearly 1/3 of these patients reach a stable disease state (8). In a retrospective study, chidamide with PEL regimen (prednisone, etoposide, lenalidomide) in 34 unfit patients with R/R DLBCL achieved an ORR of 73.5%, a complete remission (CR) rate of 32.4%, and a 1-year expected progression free survival (PFS) of 43.0% (9). Bruton’s tyrosine kinase (BTK) plays a vital role in the BCR signaling pathway. BTK inhibitors (BTKi) have confirmed therapeutic activity in B-cell malignancies, with modest activity in DLBCL. Ibrutinib, the first-in-class BTK inhibitor, has shown modest activity with an ORR of 37% in patients with R/R non-germinal center B-cell (non-GCG) DLBCL (10). Zanubrutinib, a second-generation BTKi, produced an ORR of 29% and a CR rate of 17% in R/R DLBCL regardless of molecular subtype (11). In preclinical experiments and case reports, dual inhibition of HDAC and BTK showed a possible synergistic effect for DLBCL (12, 13).
We here present a case of an elderly R/R DLBCL patient who relapsed after initial response to CAR T-cell therapy and achieved complete remission after treatment with chidamide and zanubrutinib.
A 70-year-old Chinese man with bilateral axillary lymphadenopathy was diagnosed with diffuse large B-cell lymphoma, not otherwise specified through lymph node biopsy 5 years before this study. Immunohistochemical analysis showed the following results for lymphoma cells: CD3 (-), CD10 (-), CD20 (+), BCL2 (+, 80%), BCL6 (+), C-MYC (+, 40%), CyclinD1 (-), MUM1 (+), CD21 (-) and Ki-67 (+, 80%). EBER by in situ hybridization test (ISH) was negative in the lymphoma cells. The fluorescence in situ hybridization test (FISH) yielded negative results for MYC, BCL2/IGH and BCL-6 rearrangement. 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET-CT) revealed multiple lymphadenopathies on both sides of the mediastinum with larynx and stomach involvement. Bone marrow biopsy was negative. A diagnosis of DLBCL, non-GCB subtype with MYC/BCL2 double expression was made. The Ann Arbor stage was IV A and the international prognostic index was 3, suggesting a poor prognosis. The patient then received 6 courses of R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) and achieved the first complete remission (CR). The patient declined autologous stem cell transplantation (auto-SCT). However, 9 months later, the patient experienced his first relapse, developing a single lesion on his tongue. The mass biopsy confirmed the relapse. Two courses of dose-adjusted R-EPOCH (etoposide, doxorubicin, cyclophosphamide, vincristine, prednisone, and rituximab) and two courses of R-DHAP (rituximab, dexamethasone, high-dose cytarabine, and cisplatin) only allowed the patient to achieve stable disease (SD). Therefore, involved field radiotherapy was given and the patient reached his second CR. However, 7 months later, the patient relapsed for the second time, showing bilateral cervical lymphadenopathy confirmed by biopsy. Four courses of L-GemOx (lenalidomide, gemcitabine and oxaliplatin) were given and the patient reached CR as confirmed by PET-CT evaluation. Then, lenalidomide was given as maintenance treatment. However, another 7 months later, the patient relapsed for the third time with a new lung mass confirmed by biopsy as DLBCL, which was CD19 positive.
The patient was then referred to our center and recruited in a clinical trial of CD19-targeted CAR-T therapy with costimulatory 4-1BB endodomain (ClinicalTrials.gov ID: NCT04833504). Pretreatment PET-CT showed a large lung mass with multiple lymph node involvements (Figure 1A). The patient underwent FC regimen lymphodepletion chemotherapy (fludarabine 30 mg/m2 day -5 to -3, cyclophosphamide 500 mg/m2 day -5 to -3) before CAR-T infusion. The patient was infused with a total of 1.1×108 CAR-T cells (2×106 cells per kilogram) on day 0. After CAR-T infusion, the patient suffered from grade 1 cytokine release syndrome (CRS) without immune effector cell-associated neurotoxicity syndrome (ICANS) according to the American Society for transplantation and cellular therapy (ASTCT) guidelines. PET-CT examination confirmed complete metabolic remission 3 months after CAR-T cell therapy (Figure 1B).
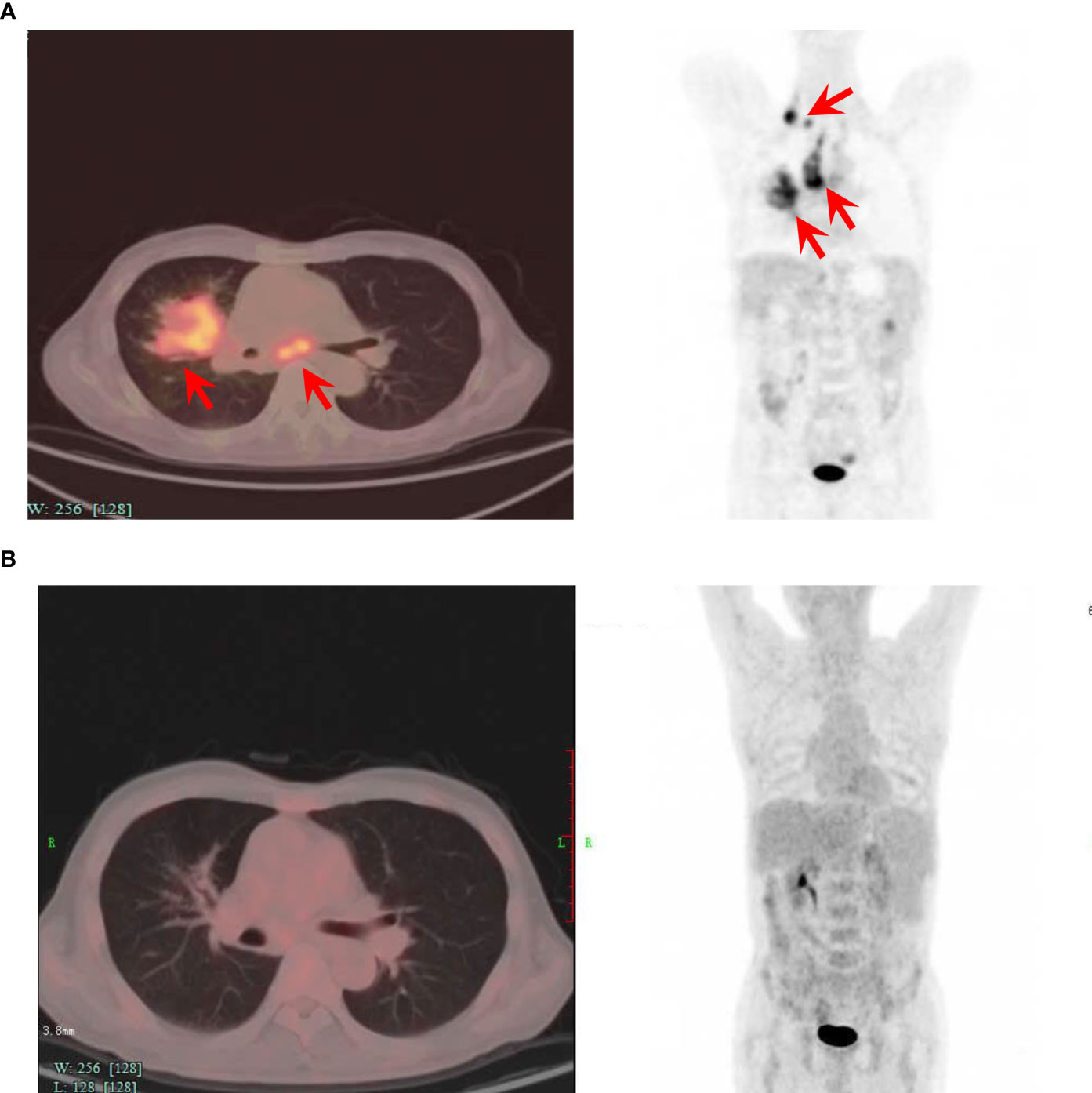
Figure 1 Positron-emission tomography-computed tomography before and 3 months after CAR-T cell infusion. (A) PET-CT image of the patient before the CAR-T cell infusion, showing lymphoma invasion. (B) PET-CT image of the patient 3 months after the CAR-T cell infusion, showing no lesions and complete remission. The axial image (left) and coronal image (right) are shown. Red arrows indicate lymphoma lesions.
Unfortunately, 12 months after CAR-T infusion, the patient relapsed for the fourth time with lung mass (Figure 2A), which was confirmed by biopsy. Notably, immunohistochemical analysis showed that CD19 was still positive. Circulating tumor DNA (ctDNA) of DLBCL (a 49 cancer-related gene panel, Supplementary File 1) performed with next-generation sequencing (Illumina, Wuhan Sitaide Medical Laboratory) detected the mutations in TP53 (c.524G>A, p.R175H, 47.60%) and NOTCH1 (c.3539G>A, p.C1180Y, 35.02%), without mutations in MYD88, CD79b, EP300 or CREBBP. During the salvage chemotherapy with two courses of GDP regimen, the patient suffered from grade IV bone marrow suppression and infective fever. However, the patient failed to achieve any remission and lung lesion progressed with increasing pleural effusion (Figure 2B). His chest distress and shortness of breath worsened, and he could not tolerate any further aggressive chemotherapy. Thereafter, He was given oral chidamide 30 mg twice a week and oral zanubrutinib (a novel BTK inhibitor) 160mg twice daily as salvage therapy. Surprisingly, his symptoms were relieved after 2 weeks of this treatment, and a CT scan 2 months later showed significant remission of lung lesions (Figure 2C). This regimen was well tolerated with only grade 1 leukopenia. Peripheral blood CAR-T cells of the entire course management were monitored of this patient (Figure 2D). There were no CAR-T cells by flow cytometry and CAR copies by ddPCR detected at relapse after CAR-T therapy and there was no CAR-T cell expansion at the remission of this regimen. At the last follow-up, the patient remains in radiographic CR at 22 months after CAR-T infusion and 10 months after the initiation of chidamide and zanubrutinib. The timeline of diagnosis and treatment was shown in Figure 2E.
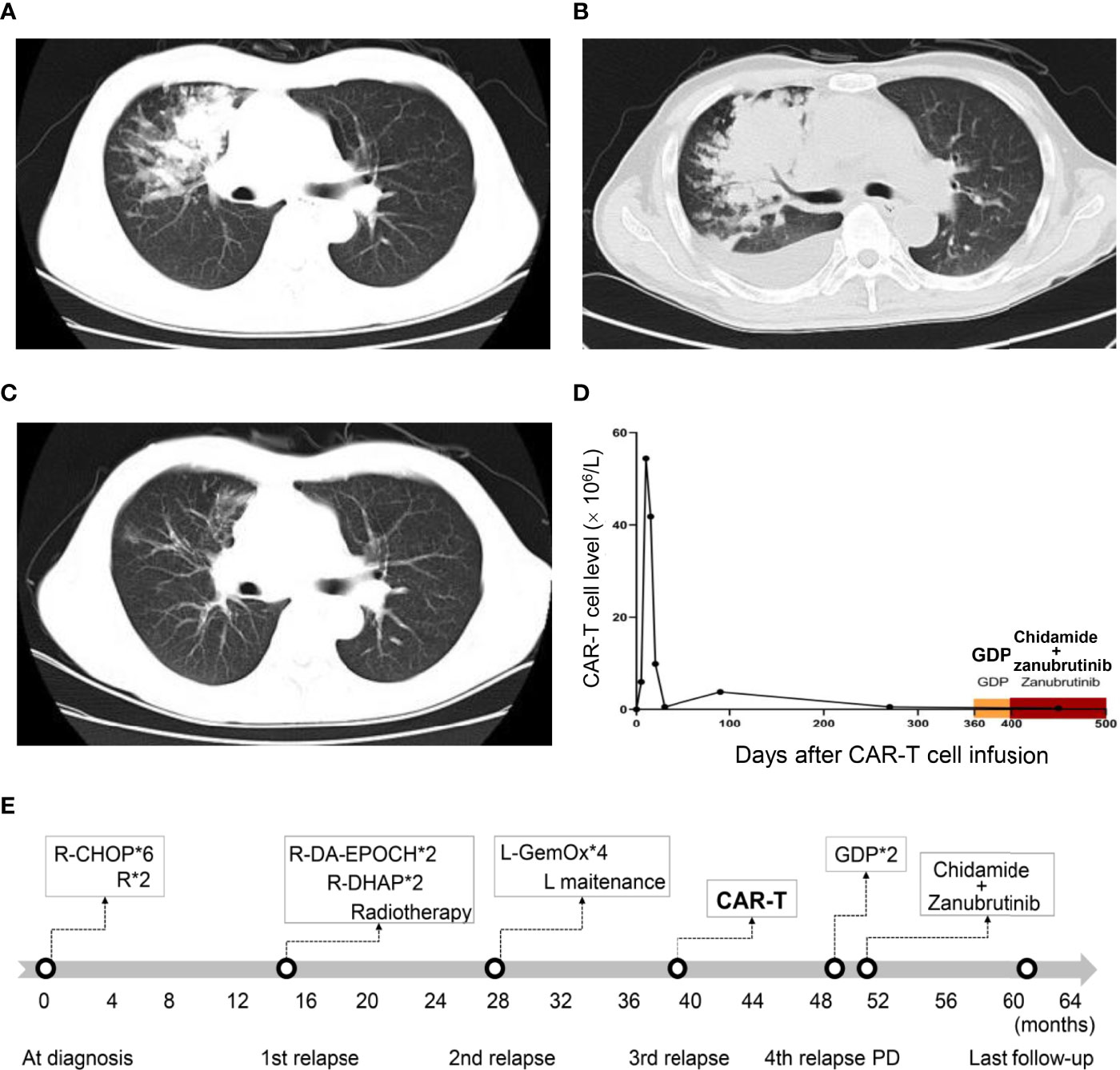
Figure 2 Computed tomography and CAR-T cell monitoring after CAR-T infusion. (A) Axial CT image at relapse after CAR-T therapy. (B) Axial CT image before the combination treatment of chidamide and zanubrutinib, showing disease progression. (C) Axial CT image 2 months after the combination treatment of chidamide and zanubrutinib, showing disease remission. (D) CAR-T cell monitoring after CAR-T cell infusion. (E)Timeline of diagnosis and treatment. “*” means “times” (or multiply).
Loss of tumor antigens and CAR-T cell exhaustion contributed to the adaptive resistance to CAR-T immunotherapy (14). Following CAR-T, CD19 loss occurs in approximately 30% of cases at time of progression (6), however, its mechanism is unclear. CAR-T cell exhaustion could partially attribute to epigenetic dysregulation, such as histone acetylation modification. The pan-HDAC inhibitor (LAQ824) can decrease T regulatory cell populations and enhance adoptive T cell therapy survival, which significantly improves the antitumor immune activity in recipient mice (15). The class I HDACi (entinostat) enhances the proliferative and functional capacity of CD8+ T cells and sensitizes tumor cells to T-cell recognition, which improves the antitumor effect (16). The HDACi (LBH589 and SAHA) partially reversed the resistance of lymphoma cells to CD19-targeted CAR-T cells (17). BTK inhibitors inhibit AKT signaling, reduce cell terminal differentiation, increase the proportion of memory CAR-T cells, and consequently, increase the efficiency of CD19-targeted CAR-T cell therapy (18). For our case, CD19 was persistently positive, so CAR-T cell exhaustion might be the primary cause. Since we did not observe a significant expansion of CAR-T cells, the antitumor effect of dual inhibition of HDAC and BTK therapy might not rely on promoting T-cell clonal expansion or enhancing the efficacy of CAR-T cells.
Chidamide, which selectively inhibits the activity of HDAC1, HDAC2, HDAC3, and HDAC10, has been approved by the Chinese FDA to treat R/R peripheral T-cell lymphomas. It is the only orally active HDACi available in the Chinese market (19). Chidamide has been used in combined treatments for newly diagnosed and R/R DLBCL in many clinical trials. Zanubrutinib, a potent and selective BTK inhibitor, has been approved for the treatment of R/R chronic lymphocytic leukemia and mantle cell lymphoma with a trend toward better responses and less toxicity, particularly cardiovascular toxicities (20, 21). We gave this patient chidamide and zanubrutinib, mainly based on the accessibility and lower off-target toxicities. The regimen was well tolerated and the patient again achieved remission. Similarly, it was reported that combination treatment with chidamide and ibrutinib produced complete remission in a patient with primary refractory DLBCL (12). Nevertheless, the mechanism underlying the synergistic mechanism remains unclear.
There are connections between the BTK signaling pathway and HDACs. HDACi restores the expression of BTK-targeting miRNA, resulting in decreased signaling of the BTK pathway and ultimately apoptosis (22). HDAC6 inhibition acetylates and abrogates the effects of MYD88, leading to cell cycle arrest and cell apoptosis (23). Tonic HDAC6 inhibition upregulates the IRE1 pathway of the unfolded protein response, leading to up-regulation of the BCR pathway (24). In preclinical models, HDACi transcriptionally downregulates MyD88 transcription, moreover, combining ibrutinib with panobinostat (a HDACi) resulted in synergistic inhibition of NF-κB activity and caused regression of DLBCL xenografts (13). In this way, dual inhibition of HDAC and BTK could theoretically have a synergistic anti-lymphoma effect. Many DLBCL patients harbor NOTCH1 mutations, termed N1 genetic subtypes (25). NOTCH1 promotes the activation of the NF-κB signaling pathway and PI3K-AKT-mTOR, which plays a vital role in promoting cell growth and accelerating cell apoptosis in lymphoma cells. Combination treatment with ibrutinib and enzastaurin significantly reduces the expression of NOTCH1, which dramatically inhibits DLBCL cell proliferation (26). TP53 mutations, occurring in approximately 20-30% of DLBCL patients, are associated with poor prognosis and therapy refractoriness. There is no standard therapy for the treatment of DLBCL with TP53 mutation. HDAC1 and HDAC2 can restore the expression of mutant TP53, and HDACi can reduce the expression of mutant TP53 (27). Chidamide has a therapeutic effect on DLBCL by upregulating the surface expression of the CD20 antigen and inhibiting the expression of mutant TP53 (28). Our patient did not have mutations in CREBBP, EP300, or MYD88(L265P), which have been reported as targets for HDCA inhibitors or BTK inhibitors, respectively. However, our patient had TP53 and NOTCH1 mutations, which we considered may be the targets of dual inhibition of HDAC and BTK. The precise underlying mechanism of dual inhibition of HDAC and BTK is likely to be a promising direction for further research.
Our case has several limitations. First, the potential synergistic mechanism of the dual inhibitors was not well clarified. Second, the 10 months of follow-up period is relatively short, so whether dual inhibitor treatment will lead to long-term remission is unclear. However, we reported the first successful case of dual inhibition of HDAC and BTK for the treatment of R/R DLBCL after failure to CAR-T cell therapy, which opens a new therapeutic possibility for the future.
In conclusion, dual inhibition of HDAC and BTK may serve as a salvage therapeutic strategy for DLBCL patients relapsed or refractory to CAR-T therapy, which deserves further investigation in a large population.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by the Human Ethics Committee of the Second Affiliated Hospital, School of Medicine, Zhejiang University, China (SAHZ2022-0251). The patients/participants provided their written informed consent to participate in this study.
Conceptualization: XY and HL; Data curation: WZ and ST; Formal analysis: WM; Funding acquisition: HL; Resources: XY and HL; Supervision: XY and HL; Validation: WZ and ST; Writing – original draft: WZ; Writing – review and editing: XY and HL. All authors contributed to the article and approved the submitted version.
This work was supported by the Translational Research Grant of National Clinical Research Center for Hematologic Diseases (NCRCH) (No. 2020ZKZC01), Zhejiang Provincial Natural Science Foundation (No.LY18H160008) and the Lymphoma Research Fund of China Anti-Cancer Association.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.894787/full#supplementary-material
Supplementary Table 1 | A 49 cancer-related gene panel of circulating free DNA of DLBCL.
Auto-SCT, Autologous stem cell transplantation; ASTCT, American Society for transplantation and cellular therapy; BTK, Bruton’s tyrosine kinase; BTKi, Bruton’s tyrosine kinase inhibitors; CAR-T, Chimeric antigen receptor T cell; CRS, Cytokine release syndrome; CR, complete remission; HDACs, Histone deacetylases; HDACi, Histone deacetylase inhibitors; ICANS, Immune effector cell-associated neurotoxicity syndrome; NHL, Non-Hodgkin’s lymphoma; Non-GCG, non-germinal center B-cell; ORR, Overall response rate; OS, Overall survival; PET-CT, positron emission tomography-computed tomography; PFS, Progression free survival; R/R DLBCL, Relapsed or refractory diffuse large B-cell lymphoma; SD, Stable disease.
1. Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. New Engl J Med (2017) 377(26):2531–44. doi: 10.1056/NEJMoa1707447
2. Schuster SJ, Svoboda J, Chong EA, Nasta SD, Mato AR, Anak Ö, et al. Chimeric Antigen Receptor T Cells in Refractory B-Cell Lymphomas. New Engl J Med (2017) 377(26):2545–54. doi: 10.1056/NEJMoa1708566
3. Liu H, Lei W, Zhang C, Yang C, Wei J, Guo Q, et al. CD19-Specific CAR T Cells That Express a PD-1/CD28 Chimeric Switch-Receptor Are Effective in Patients With PD-L1-Positive B-Cell Lymphoma. Clin Cancer Res (2021) 27(2):473–84. doi: 10.1158/1078-0432.CCR-20-1457
4. Locke FL, Ghobadi A, Jacobson CA, Miklos DB, Lekakis LJ, Oluwole OO, et al. Long-Term Safety and Activity of Axicabtagene Ciloleucel in Refractory Large B-Cell Lymphoma (ZUMA-1): A Single-Arm, Multicentre, Phase 1-2 Trial. Lancet Oncol (2019) 20(1):31–42. doi: 10.1016/S1470-2045(18)30864-7
5. Schuster SJ, Tam CS, Borchmann P, Worel N, McGuirk JP, Holte H, et al. Long-Term Clinical Outcomes of Tisagenlecleucel in Patients With Relapsed or Refractory Aggressive B-Cell Lymphomas (JULIET): A Multicentre, Open-Label, Single-Arm, Phase 2 Study. Lancet Oncol (2021) 22(10):1403–15. doi: 10.1016/S1470-2045(21)00375-2
6. Spiegel JY, Dahiya S, Jain MD, Tamaresis J, Nastoupil LJ, Jacobs MT, et al. Outcomes of Patients With Large B-Cell Lymphoma Progressing After Axicabtagene Ciloleucel Therapy. Blood (2021) 137(13):1832–5. doi: 10.1182/blood.2020006245
7. Logue JM, Chavez JC. How to Sequence Therapies in Diffuse Large B-Cell Lymphoma Post-CAR-T Cell Failure. Curr Treat options Oncol (2021) 22(12):112. doi: 10.1007/s11864-021-00906-4
8. Batlevi CL, Crump M, Andreadis C, Rizzieri D, Assouline SE, Fox S, et al. A Phase 2 Study of Mocetinostat, a Histone Deacetylase Inhibitor, in Relapsed or Refractory Lymphoma. Br J Haematol (2017) 178(3):434–41. doi: 10.1111/bjh.14698.
9. Wang Y, Xue H, Song W, Xiao S, Jing F, Dong T, et al. Chidamide With PEL Regimen (Prednisone, Etoposide, Lenalidomide) for Elderly or Frail Patients With Relapsed/Refractory Diffuse Large B-Cell Lymphoma -Results of a Single Center, Retrospective Cohort in China. Hematol Oncol. doi: 10.1002/hon.2979.
10. Wilson WH, Young RM, Schmitz R, Yang Y, Pittaluga S, Wright G, et al. Targeting B Cell Receptor Signaling With Ibrutinib in Diffuse Large B Cell Lymphoma. Nat Med (2015) 21(8):922–6. doi: 10.1038/nm.3884
11. Yang H, Xiang B, Song Y, Zhang H, Zhao W, Zou D-H, et al. Zanubrutinib Monotherapy for Relapsed or Refractory Non-Germinal Center Diffuse Large B-Cell Lymphoma. Blood Adv (2021) 6(6):1629–36. doi: 10.1182/bloodadvances.2020003698
12. Tian C, Chen Z, Li Y. Chidamide Combined With Ibrutinib Improved the Prognosis of Primary Bone Marrow Diffuse Large B Cell Lymphoma. J Int Med Res (2020) 48(7):300060520936053. doi: 10.1177/0300060520936053
13. Mondello P, Brea EJ, De Stanchina E, Toska E, Chang AY, Fennell M, et al. Panobinostat Acts Synergistically With Ibrutinib in Diffuse Large B Cell Lymphoma Cells With MyD88 L265P Mutations. JCI Insight (2017) 2(6):e90196. doi: 10.1172/jci.insight.90196
14. Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell (2017) 168(4):707–23. doi: 10.1016/j.cell.2017.01.017
15. Lisiero DN, Soto H, Everson RG, Liau LM, Prins RM. The Histone Deacetylase Inhibitor, LBH589, Promotes the Systemic Cytokine and Effector Responses of Adoptively Transferred CD8+ T Cells. J Immunother Cancer (2014) 2:8. doi: 10.1186/2051-1426-2-8
16. McCaw TR, Li M, Starenki D, Liu M, Cooper SJ, Arend RC, et al. Histone Deacetylase Inhibition Promotes Intratumoral CD8 T-Cell Responses, Sensitizing Murine Breast Tumors to Anti-PD1. Cancer Immunol Immunother (2019) 68(12):2081–94. doi: 10.1007/s00262-019-02430-9
17. Torres-Collado AX, Jazirehi AR. Overcoming Resistance of Human Non-Hodgkin's Lymphoma to CD19-CAR CTL Therapy by Celecoxib and Histone Deacetylase Inhibitors. Cancers (Basel) (2018) 10(6):200. doi: 10.3390/cancers10060200.
18. Ruella M, Kenderian SS, Shestova O, Fraietta JA, Qayyum S, Zhang Q, et al. The Addition of the BTK Inhibitor Ibrutinib to Anti-CD19 Chimeric Antigen Receptor T Cells (CART19) Improves Responses Against Mantle Cell Lymphoma. Clin Cancer Res (2016) 22(11):2684–96. doi: 10.1158/1078-0432.CCR-15-1527
19. Shi Y, Jia B, Xu W, Li W, Liu T, Liu P, et al. Chidamide in Relapsed or Refractory Peripheral T Cell Lymphoma: A Multicenter Real-World Study in China. J Hematol Oncol (2017) 10(1):69. doi: 10.1186/s13045-017-0439-6
20. Zhou K, Zou D, Zhou J, Hu J, Yang H, Zhang H, et al. Zanubrutinib Monotherapy in Relapsed/Refractory Mantle Cell Lymphoma: A Pooled Analysis of Two Clinical Trials. J Hematol Oncol (2021) 14(1):167. doi: 10.1186/s13045-021-01174-3
21. Xu W, Yang S, Zhou K, Pan L, Li Z, Zhou J, et al. Treatment of Relapsed/Refractory Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma With the BTK Inhibitor Zanubrutinib: Phase 2, Single-Arm, Multicenter Study. J Hematol Oncol (2020) 13(1):48. doi: 10.1186/s13045-020-00884-4
22. Bottoni A, Rizzotto L, Lai T-H, Liu C, Smith LL, Mantel R, et al. Targeting BTK Through microRNA in Chronic Lymphocytic Leukemia. Blood (2016) 128(26):3101–12. doi: 10.1182/blood-2016-07-727750
23. New M, Sheikh S, Bekheet M, Olzscha H, Thezenas M-L, Care MA, et al. TLR Adaptor Protein MYD88 Mediates Sensitivity to HDAC Inhibitors via a Cytokine-Dependent Mechanism. Cancer Res (2016) 76(23):6975–87. doi: 10.1158/0008-5472.CAN-16-0504
24. Amengual JE, Prabhu SA, Lombardo M, Zullo K, Johannet PM, Gonzalez Y, et al. Mechanisms of Acquired Drug Resistance to the HDAC6 Selective Inhibitor Ricolinostat Reveals Rational Drug-Drug Combination With Ibrutinib. Clin Cancer Res (2017) 23(12):3084–96. doi: 10.1158/1078-0432.CCR-16-2022
25. Schmitz R, Wright GW, Huang DW, Johnson CA, Phelan JD, Wang JQ, et al. Genetics and Pathogenesis of Diffuse Large B-Cell Lymphoma. N Engl J Med (2018) 378(15):1396–407. doi: 10.1056/NEJMoa1801445
26. He Y, Li J, Ding N, Wang X, Deng L, Xie Y, et al. Combination of Enzastaurin and Ibrutinib Synergistically Induces Anti-Tumor Effects in Diffuse Large B Cell Lymphoma. J Exp Clin Cancer Res (2019) 38(1):86. doi: 10.1186/s13046-019-1076-4
27. Stojanovic N, Hassan Z, Wirth M, Wenzel P, Beyer M, Schafer C, et al. HDAC1 and HDAC2 Integrate the Expression of P53 Mutants in Pancreatic Cancer. Oncog (2017) 36(13):1804–15. doi: 10.1038/onc.2016.344
Keywords: diffuse large B-cell lymphoma, chimeric antigen receptor T cell, chidamide, zanubrutinib, histone deacetylase inhibitor, Bruton’s tyrosine kinase inhibitor
Citation: Zhu W, Tao S, Miao W, Liu H and Yuan X (2022) Case Report: Dual Inhibition of HDAC and BTK for Diffuse Large B-Cell Lymphoma After Failure to CD19-Targeted CAR-T Therapy. Front. Immunol. 13:894787. doi: 10.3389/fimmu.2022.894787
Received: 12 March 2022; Accepted: 16 May 2022;
Published: 10 June 2022.
Edited by:
Min Xiao, Huazhong University of Science and Technology, ChinaReviewed by:
Zhanglin Zhang, The First Affiliated Hospital of Nanchang University, ChinaCopyright © 2022 Zhu, Tao, Miao, Liu and Yuan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui Liu, c3lsZW5Aemp1LmVkdS5jbg==; Xianggui Yuan, WXVhbnhnQHpqdS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.