- 1Department of Trauma, University Hospital Zurich, Zurich, Switzerland
- 2Harald Tscherne Research Laboratory, University of Zurich, Zurich, Switzerland
- 3Department of Health Sciences and Technology, Eidgenössische Technische Hochschule (ETH) Zurich, Zurich, Switzerland
- 4Center for Surgical Research, University Hospital Zurich, University Zurich, Zurich, Switzerland
Introduction: Occult hypoperfusion (OH) is defined as persistent lactic acidosis despite normalization of vital parameters following trauma. The aim of this study was to analyze the association of occult hypoperfusion with local circulation and inflammation of injured soft tissue in a porcine polytrauma model.
Methods: This experimental study was performed with male landrace pigs who suffered a standardized polytrauma, including a femoral fracture, blunt chest trauma, liver laceration and a mean arterial pressure (MAP) controlled hemorrhagic shock. One hour after induction of trauma, the animals were resuscitated with retrograde femoral nailing, liver packing and volume replacement. Animals were stratified into Group Norm (normalizing lactate levels after resuscitation) and Group occult hypoperfusion (OH) (persistent lactate levels above 2 mmol/l with normalizing vital parameters after resuscitation). Local circulation (oxygen saturation, hemoglobin amount, blood flow) was measured with optical sensors at the subcutaneous soft tissue at the fractured extremity as well as at the stomach and colon. Local inflammatory parameters [interleukin (IL) 6, 8, 10, and heat shock protein (HSP)] were analyzed in the subcutaneous tissue of the fractured extremity.
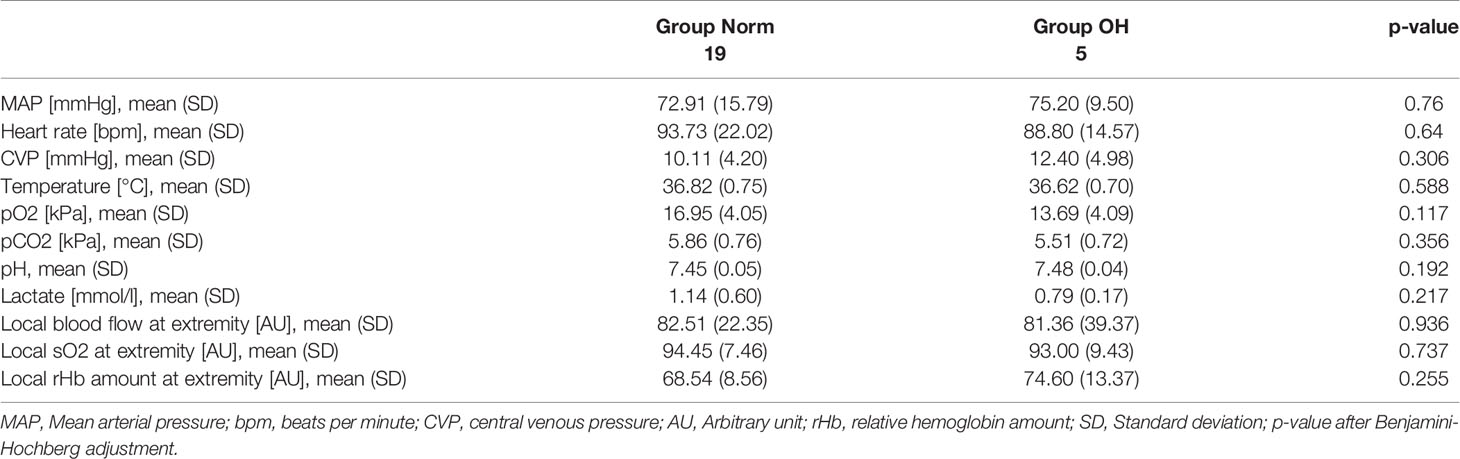
Results: Group Norm (n = 19) and Group OH (n = 5) were comparable in baseline vital and laboratory parameters. The shock severity and total amount of blood loss were comparable among Group Norm and Group OH. Following resuscitation Group OH had significantly lower local relative hemoglobin amount at the injured soft tissue of the fractured extremity when compared with Group Norm (39.4, SD 5.3 vs. 63.9, SD 27.6 A.U., p = 0.031). The local oxygenation was significantly lower in Group OH compared to Group Norm (60.4, SD 4.6 vs. 75.8, SD 12.8, p = 0.049). Local IL-6 in the fatty tissue was significantly higher in Group OH (318.3, SD 326.6 [pg/ml]) when compared with Group Norm (73.9,SD 96.3[pg/ml], p = 0.03). The local circulation at the abdominal organs was comparable in both groups.
Conclusion: OH is associated with decreased local circulation and increased local inflammation at the injured soft tissue of the extremity in polytrauma. OH might reflect the severity of local soft tissue injuries, and guide treatment strategies.
Introduction
The initial assessment and surgical strategy in the treatment of polytrauma patients is based on the physiologic reaction to injuries (1). The early polytrauma management focuses on the stabilization of the triad of acidosis, coagulopathy, and hypothermia. This triad, combined with the soft tissue damage are prominent predictors for mortality in polytrauma patients (1). The physiologic stabilization of the patient represents one guide of the surgical strategy after polytrauma (2). Despite standardized and improved resuscitation, selected polytrauma cases present with persistent lactic acidosis even after responding to resuscitation. The presence of elevated lactate levels during the clinical course has been interpreted as a sign of occult hypoperfusion (OH). OH in trauma is associated with increased risk for infections and other complications (3). The outcome of trauma patients improves if OH is detected and treated early (4). Several different definitions of OH exist, most of which describe a persistent elevated lactate level with concomitant normal vital parameters (3, 5). Risk factors for the development of OH include Injury Severity Score (ISS), Glasgow Coma Scale (GCS) at admission, hypotension, and advanced age (4). OH has only been approximated by systemic measures or analysis of CO2-gaps between central veins and arteries (6). In order to evaluate the association of persistent lactic acidosis and occult hypoperfusion this study aimed to investigate the following research questions in a standardized porcine polytrauma model: What is the effect of OH on the local circulation of the injured soft tissue in polytrauma? Is OH associated with the amount of blood loss during hemorrhagic shock? How is the local inflammatory reaction of the soft tissue affected by OH?
Methods
Study Design
This experimental translational study is based on a previously described standardized porcine polytrauma model (7). Reporting of the results adheres to the ARRIVE Guideline (8).
Ethical Statement and Housing
Animal housing and experimental protocols were approved by the Cantonal Veterinary Office, Zurich, Switzerland, under license no. ZH 138/2017, and were executed in accordance with Swiss Animal Protection Law, following the “The Principles of Laboratory Animal Care”. Housing and experimental procedures also conformed to the European Directive 2010/63/EU of the European Parliament and of the Council on the Protection of vertebrate animals used for scientific purposes (Council of Europe no. 123, Strasbourg 1985) and to the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, National Research Council, National Academy of Sciences, 2011). Castrated male Swiss landrace pigs from a disease-free barrier breeding facility were housed in ventilated rooms at a constant room temperature of 21 ± 3°C and a relative humidity of 50%, with natural daylight. Pigs acclimatize to their surroundings for a minimum of 7 days prior to the experiment. takers. Pigs were fasted for a period of 12 h prior to the experiment, water was available ad libitum.
Animal Care and Monitoring
All pigs were sedated with an intramuscular injection of ketamine (Ketasol®-100 ad us.vet.; Dr. E. Graeub AG, Berne, Switzerland; 15 mg/kg body weight), midazolam (Dormicum®.; Roche Pharma AG, Basel, Switzerland; 0.5 mg/kg body weight) and atropine (Atropinsulfat KA vet 0.1%; Kantonsapotheke, Switzerland; 0.05 mg/kg body weight). Anesthesia was induced by an intravenous administration of propofol (Propofol ®- Lipuro 1%, B. Braun Medical AG; Sempach, Switzerland; 1-2 mg/kg body weight) to achieve relaxation and swallow-reflex diminishment sufficient for intubation. Anesthesia was maintained during the duration of the study with propofol (5-10 mg/kg/h).and Sufentanil (1 μg/kg per hour) under positive pressure ventilation with an FiO2 of 0.3. Following our institutional veterinarian protocol, ventilation was set to maintain an end tidal CO2 (etCO2) of 45-55 mmHg All animals received fluid substitution of 21 ml/h Ringerfundin. Animals were placed in supine position for percutaneous ultrasound guided catheterization of the following vessels: left external jugular vein (6F, Avanti®, Cordis® Corporation, Miami Lakes, FL, USA), right femoral artery (5F, Avanti®, Cordis® Corporation, Miami Lakes, FL, USA) and left femoral vein (HighFlow Dolphin Catheter, 13F, Baxter International, Deerfield IL, USA). Oxygenation and ventilation parameters, inspirational oxygen fraction, and expiratory oxygen fraction, etCO2, positive end-expiratory pressure (PEEP), respiratory frequency, and tidal volume were continuously monitored. Blood gas analysis was performed to regularly assess pressure of oxygen (paO2) and carbon dioxide (paCO2), Arterial blood pressure and central venous pressure (CVP), as well as heart rate was continuously recorded. A suprapubic catheter was placed in the urinary bladder and diuresis was monitored. During the entire experiment, at least two veterinarians, specialized in anesthesiology were present and monitored the narcosis.
Induction of Trauma and Resuscitation
Trauma was induced and resuscitation procedures performed, based on a well-established, previously described standardized large animal protocol (7). Briefly, the polytrauma model included a standardized left-sided femoral shaft fracture, a right sided blunt thoracic trauma, a standardized liver laceration, and mean arterial pressure (MAP)-controlled hemorrhagic shock as follows:
Fractures and associated soft tissue injuries were induced by a bolt gun (Blitz-Kerner, turbocut JOBB GmbH, Germany) with cartridges (9x17; DynamitNobel AG; Troisdorf, Germany) to a custom made metal chisel which was placed on the mid of the left femur shaft. The fracture was verified via fluoroscopy. The blunt chest trauma was introduced with the same bolt gun on the right lateral thorax wall utilizing an additional lead pael (1.0cm thickness) between the chest and the bolt gun. A chest x-ray was performed and in case of pneumothorax a chest tube was inserted in the usual manner. The liver laceration was performed with a cross-like incision through one third of the liver tissue following median laparotomy. Liver packing was performed following 30 seconds of uncontrolled bleeding. Afterwards the animals underwent MAP-controlled hemorrhagic shock for 60 min until they reached a MAP of 25 ± 5 mmHg. During trauma and hemorrhagic shock, FiO2 was reduced to 0.21 and fluid substitution was lowered to 10ml/h.
One hour after induction of trauma animals were resuscitated following the ATLS, AO/OTA, and AWMF-S3 guideline on Treatment of Patients with Severe and Multiple Injuries®) principles (9, 10). Hemorrhagic shock was treated with volume controlled fluid resuscitation (three times the blood loss, Ringerfundin®). Normothermia (38.7–39.8°C) was aimed for with warm pads and blankets. Femoral shaft fractures were treated with retrograde intramedullary nailing utilizing a tailored 120 mm nail (cannulated DFN Ø 10.0 mm, DePuySynthes, Raynham, Massachusetts, United States) in the usual manner. The position of the nail was controlled via fluoroscopy.
Measurements of Local Circulation
Local tissue perfusion and microcirculation at the fracture site was assessed using an O2C (Oxygen to see, LEA Medizintechnik GMBH, Giessen, Germany) device. White light spectrometry and laser spectroscopy were used to screen the capillary-venous part of the vascular system (penetration depth of 4–8 mm). Relative local blood flow (flow), flow velocity, local oxygen saturation (sO2) and relative local hemoglobin concentration (rHB) were measured one the vastus lateralis muscle adjacent to the femoral fracture. For this, a 3 cm incision was made on the left lateral thigh and the subcutaneous tissue was exposed by preparation of subcutaneous tissue and incision of the fascia. The measuring probe was placed on the subcutaneous tissue for 1 min above the fracture and measurements were continuously taken every second. Local circulation measurements were further taken at the stomach, and the colon. Data are presented in arbitrary units (A.U.). The reason for the introduction of “Arbitrary Units” is based on the origin of the values. The measured signals are electrical values of frequencies and amplitudes, so that the unit would be a combination of electrical units. Therefore usually a new unit for blood flow is introduced. To calculate the blood flow in ml/min, it would be necessary to compare the electrical signals with a method that measures the blood flow in ml/min (e.g. plethysmography, microspheres) for each organ (or organs with similar optical properties). Then the arbitrary units can be converted in ml/min. This “calibration” has to be done at the measured organ, as there is no artificial model at the moment that simulates tissue in a realistic way.
Local Inflammation
Local inflammation was measured 6 hours after induction of trauma. Quantikine porcine immunoassay kits from R&D Systems (Minneapolis, MN) were used for analyses of IL-6, IL-8, and IL-10 according to the manufacturer’s protocol. Prior termination of the experiment local soft tissue including fatty tissue and muscle tissue were taken at the fracture site and stored in RNAlater solution (RNAlater Solutions for RNA Stabilization and Storage, ThermoFisher). After shredding of the tissue, RNA extraction was performed using the RNeasy Mini Kit (Qiagen, Hombrechtikon, Switzerland) according to the manufacturer’s protocol. PCR analysis for HSP70, IL-6, IL-8, and IL-10 was performed using the following primer (F forward, R reverse): IL-6 F: 5-GAATCCAGACAAAGCCACCA-3, R: 5-GTGCCCCAGCTACATTATCC-3; IL-8/CXCL8, F: 5-CTTCCAAACTGGCTGTTGCC-3, R: 5-GTTGTTGTTGCTTCTCAGTTCTCT-3; IL-10, F: 5-CGGCGCTGTCATCAATTTCT-3, R: 5-CGGGAACCTTGGAGCAGATT-3; HSP70, F: 5-GCCCTGAATCCGCAGAATA-3, R: 5-TCCCC ACGGTAGGAAACG-3.
Statistical Methods and Stratification of Animals
Continuous variables are presented with mean and standard deviation (SD), or standard error of the mean (SEM) in graphical presentation, categorical variables as count and percentage. Experimental subjects were stratified according to persistent lactic acidosis as defined by persistent elevated serum lactate level of >2mmol/l (Group OH) despite responding vital signs to resuscitation during the entire observation time. Distribution of data was visualized with histogram and qq-plots. Group comparison on normally distributed continuous variables were performed using the student’s t-test, for non-normal distributed variables the Mann-Whitney U test was utilized; for comparisons on categorical variables the Fisher exact test was used. Adjustment for multiple testing with a false discovery rate at 1% was performed with the Benjamini-Hochberg procedure. The p-value reported is the adjusted value. All statistical analyses were performed using R (R Core Team (2021). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/.). GraphPad Prism version 9 for macOS was utilized to print the graphs (GraphPad Software, San Diego, California USA, www.graphpad.com).
Results
This experiment included 27 animals. Out of these 24 (88.9%) survived the experiment and were stratified into Group Norm (n=19, 79.2%) and Group OH (n= 5, 20.8%, Figure 1). 3 animals died prematurely and were excluded from analyses.

Figure 1 Flowchart and stratification according to occult hypoperfusion (OH) as defined by persistent elevated lactate levels despite responding vital parameters following resuscitation.
Baseline Values
The animals had a mean weight of 50.7kg (SD 4.5kg) representing adolescent animals. At baseline the animals had a MAP of 73.3mmHg (SD 14.7mmHg), a heart rate of 91.5bpm (SD 17.0bpm), and a CVP of 10.1mmHg (SD 3.7mmHg). Baseline lactate level was 1.1mmol/l (SD 0.6mmol/l), base excess 6.3mmol/l (SD 2.4mmol/l), and the temperature 37.0°C (SD 0.7°C). The baseline hemoglobin was 9.7g/dl (SD 0.9g/dl), and the calcium 1.34mg/dl (SD 0.06mg/dl). The animals were comparable in baseline values and characteristics (Table 1).
Severity of Trauma: Course of MAP and Lactate During Trauma and After Resuscitation
During the hemorrhagic shock, the animals lost a mean of 991.8ml (SD 303.8ml) of blood, and had a MAP of 25mmHg (SD 5mmHg) and a mean heart rate of 94.0bpm (SD 18.9bpm). The lactate level increased to 5.9mmol/l (SD 1.4mmol/l), the temperature was 37.4°C (SD 0.6°C).
Lactate level increased to 6.03mmol/l (SD 0.75mmol/l) in Group OH and remained at a mean of 3.44 mmol/l (SD 1.97mmol/l). The lactate level decreased in Group Norm following resuscitation to baseline values. The temperature remained comparable in both groups throughout the experiment as did the pCO2 levels (Figure 2).
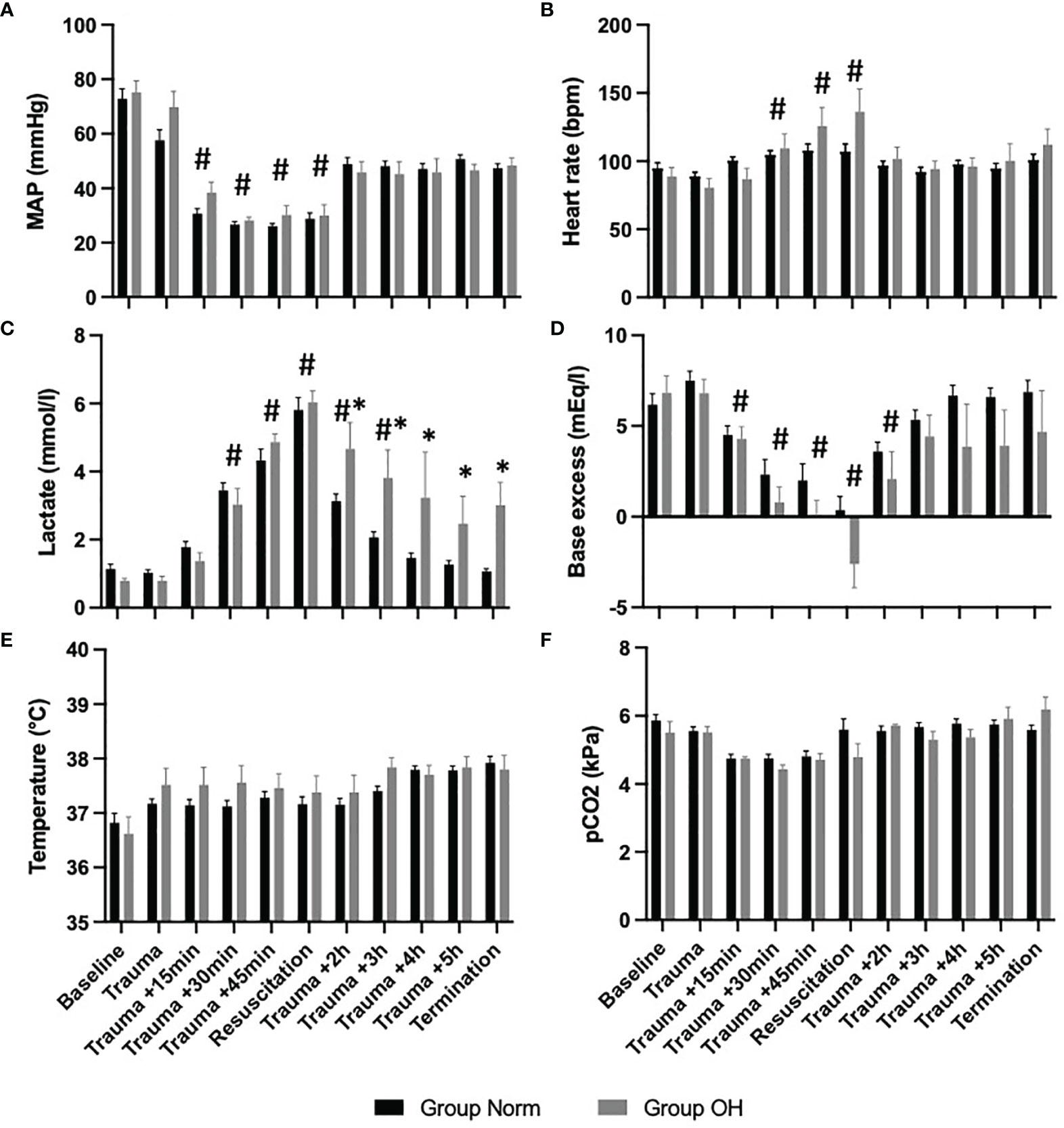
Figure 2 The course of vital parameters, and measures of arterial blood gas analysis during the observational period, presented as mean and standard error of the mean #represents statistical significant difference to baseline, * represents statistical difference among Group Norm and Group OH at the respected timepoint Statistical significance after Benjamini-Hochberg adjustment set at p < 005. (A) The course of MAP controlled hemorrhagic shock The MAP decreased significantly compared with baseline values, however, the MAP remained comparable among both groups. (B) Significant increase of heart rate (compared with baseline) during hemorrhagic shock and return to comparable values. (C) The lactate value increased significantly in both Groups when compared to baseline values After the initiation of resuscitation, the lactate value decreased in Group Norm. in Group OH the lactate value remained significantly higher compared to baseline and to Group Norm. (D) Base excess decreased significantly in both groups, and remained lower in Group OH (not statistically significant). (E) The Temperature was comparable during the experiment, and the animals were warmed throughout. (F) The pCO2 level was utilized for ventilation control and remained comparable during the experiment and among both groups.
Occult Hypoperfusion Is Associated With Reduced Local Circulation in the Soft Tissue of the Extremity
Local soft tissue circulation measurements on the hind extremities have shown a significantly reduced microcirculation in Group OH when compared with Group Norm, with local SpO2 in the vastus muscle being significantly lower (Group OH: 60.4, SD 4.6 A.U. versus Group Norm 75.8 SD 12.8 A.U., p=0.049). Local Hb values were also significantly reduced in the OH group (Group OH: 39.4, SD 5.3 A.U. versus Group Norm 63.9, SD 27.6 A.U., p=0.031). No differences were found between the groups in the microcirculation of the abdominal organs, such as the stomach, colon or liver (Table 2).
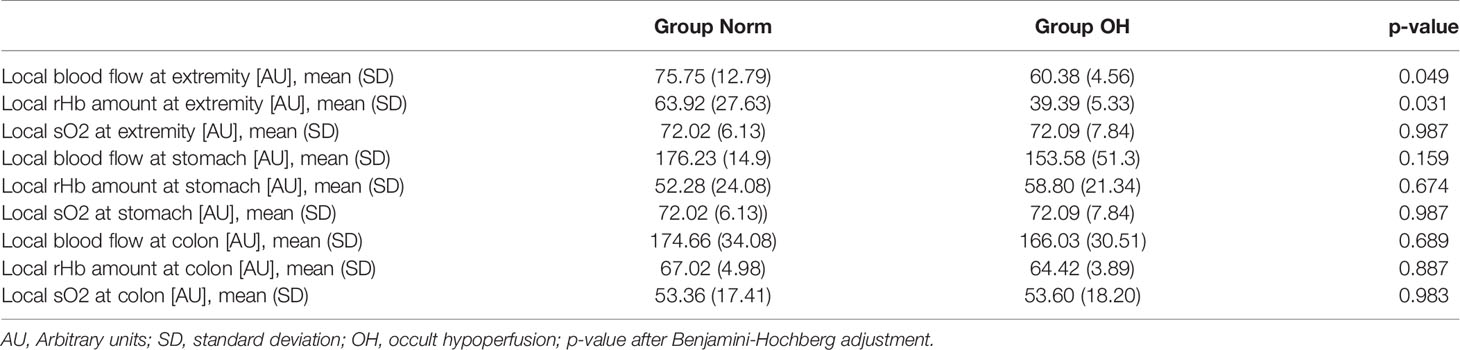
Table 2 Occult hypoperfusion is associated with reduced local circulation in injured soft tissue 6 hours after trauma.
Occult Hypoperfusion Is Associated With an Increased Local Inflammatory Response
The analysis of local soft tissue inflammation revealed a significantly higher concentration of pro-inflammatory cytokines in animals in Group OH when compared with Group Norm. Interleukin-6 was approximately 3 times higher in the OH group when compared to the Norm group (Group OH: 318.3, SD 326.6 pg/ml versus Group Norm 73.9. SD 96.3 pg/ml, p=0.03). Similarly, IL-8 has shown 4 times higher values (Group OH: 442.9, SD 687.6 pg/ml versus Group Norm 72.3, SD121.3 pg/ml, p=0.056). Interleukin-10 and HSP were comparable between the groups (Table 3).
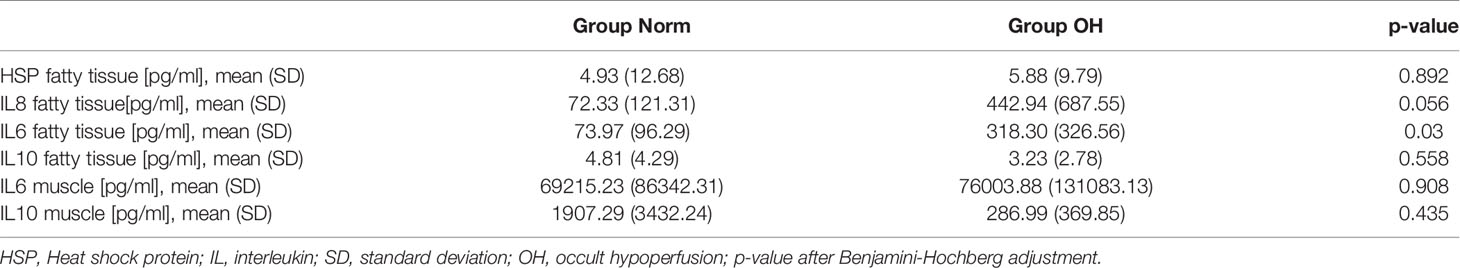
Table 3 Occult hypoperfusion is associated with increased local inflammatory response of the injured soft tissue at the fracture.
Discussion
The aim of this study was to analyze the association of OH on the local microcirculation and the local inflammatory of the injured soft tissue reaction following a standardized porcine polytrauma model and found the following points:
• Persistent elevated lactate levels can be present despite standardized trauma and resuscitation protocols.
• Animals in Group OH show reduced local circulation at the injured extremity when compared with Group Norm.
• The local inflammatory reaction of the soft tissue in the injured extremity is significantly higher Group OH when compared with Group Norm.
The identification of multiple injured patients with insufficient response to resuscitation is required to choose an appropriate treatment strategy. Studies have reported a negative outcomes in patients with persistent occult hypoperfusion (OH) (11). There still is, however, a lack of consensus on the definition of OH: Some studies defined OH based on the pH level (pH < 7.25) (12) others described significant hemorrhage despite hemodynamic stability to be associated with occult hypoperfusion (13). A base deficit of less than -4.5mmol/l was further a proposed threshold for OH (14). However, most articles define occult hypoperfusion based on persistent elevated lactate levels, despite normal vital signs (15–17). The range of the cut-off for persistent lactic acidosis varies from 2mmol/l (18), to 2.4 mmol/l (19), 2.5 mmol/l (16), and 3mmol/l (20). The present study has chosen the cut-off of 2mmol/l since this value is clinically one relevant factor in distinguishing between stable and unstable trauma patients (1, 2). This is the first study that provides some evidence for the development of OH despite standardized trauma and standardized treatment protocols. The pathophysiologic cause for OH remains subject of investigations and is hypothesized to be the result of multifactorial pathways. If the oxygen supply decreases other mechanisms attempt to restore oxygenation (21). Hypoxia can induced inflammation and vice versa (22). This association is based on experimental designs that assessed toll-like receptors (TLRs) in kidney or lung transplantation, and the resulting insulin resistance following macrophage migration in adipose tissue (22). The present study provides new data that shows the association of OH with increased inflammation in acute injured soft tissue in a polytrauma model. Ischemia and reperfusion injuries result in sterile inflammation following activation of TLRs, recruitment and activation of immune cells of the innate and adaptive immune system and activation of the complement system (23). This clinically very relevant association has been discussed following biomolecular and histopathologic studies. The present investigation provides a translational approach that introduces standardized quantification methods for OH and local hypoperfusion that might be feasible for routine clinical use. Changes in local circulation depend further on the specific organ of interest (24). The relevance of ischemia-reperfusion injury is further associated with local circulation and represents a challenging condition in trauma patients (25). It has further been shown that the local soft tissue injury serves as a relevant factor for the prediction of mortality in severely injured patients (1, 2). OH and altered microcirculation might be utilized to quantify the severity of the local soft tissue damage. OH might further be associated with persistent centralization. The present study showed that the abdominal organs are not as severely affected by circulatory changes during OH when compared with the extremity. The centralization appears to persist during OH and is an increased risk for complications and mortality (26).
Shock and resuscitation have a substantial impact on the local circulation and the local inflammation (27). Systemic inflammatory response is associated with the severity of trauma and skeletal muscle oxygenation and represents a relevant factor for resuscitation strategies and success (28). The release of damage-associated molecular patterns (DAMPs) following trauma are associated with increased inflammation. Systemic inflammation is increased in polytrauma and the damaged local soft tissue might be responsible for the inflammatory reaction (29). The elevated local inflammation impairs the coagulatory system, increases vascular leakage by damaging endothelium and might therefore subsequently damage remote organs (30).
The presented results are in accordance with previously published data that have shown an adequate response to resuscitation within 30 minutes (31). The local circulatory flow rate correlates with the MAP, and following hemorrhagic shock rHB and local O2 measures decreased significantly (27). These data are comparable to the present experiment that shows an effect of the trauma and the hemorrhage to the local circulation. But the present experiment goes one step further, by providing data on an even further effect of OH on the local circulation and inflammation. The systemic response to severe trauma has been shown (31), the effect of polytrauma on local circulation can be quantified (27), and following our results, the severity of decreased local circulation or increased local inflammation of injured soft tissue is further associated with OH.
Experimental studies allow detection of these markers, however, the transfer to human situation remains challenging (32). The present study allows the association of OH, decreased local circulation, and increased local inflammation and might therefore support the translation of these results to the human situation. The quantification of the local tissue injury is relevant for the risk for complications and for the treatment strategy (33). The local inflammatory response is time-dependent and therefore provides an association of OH and increased local inflammation (34).
Strengths and Limitations
This study is conducted as an experimental study. The strengths of this study are the standardized induction of trauma, and the standardized resuscitation methods. The included study subjects were comparable in baseline characteristics and the observation time were standardized. These conditions are rarely found in clinical trials. OH might be the result of the local microcirculation. Following the present study, we provide data that show a certain association of OH and local microcirculation. Further, this association was measured in a standardized and reproducible manner. However, the reported data cannot prove a cause-relation effect that provides evidence whether the decreased local circulation causes OH or vice versa. Based on the standardized trauma and standardized resuscitation the effect should be investigated on an epigenetic level, since it might be based on an individual reaction to the injury. One might argue that the translation of our results to routine clinical practice might be lacking, however, large animals mirror human physiology better when compared with rodents (35). For those reasons, a certain translation of our results might be feasible. This experiment was designed to analyze the short term effect of trauma on inflammation and circulation. Therefore, long term complications, infections, or mortality were not observed. Albeit this shortcoming, we believe that the presented results might improve the pathophysiologic understanding of OH and its association with decreased local circulation at the extremity and increased local inflammation.
Conclusion
OH is associated with decreased local perfusion and increased local inflammation at the injured soft tissue and might serve as a measure for severity of local soft tissue injuries, guide treatment strategies, and measure treatment success.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
Animal housing and experimental protocols were reviewed and approved by the Cantonal Veterinary Office, Zurich, Switzerland, under license no. ZH 138/2017, and were executed in accordance with Swiss Animal Protection Law, following the “The Principles of Laboratory Animal Care”. Housing and experimental procedures also conformed to the European Directive 2010/63/EU of the European Parliament and of the Council on the Protection of vertebrate animals used for scientific purposes (Council of Europe no. 123, Strasbourg 1985) and to the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, National Research Council, National Academy of Sciences, 2011).
Author Contributions
SH and YK contributed equally to this work, conducted the research, collected the data, analysed and interpreted the data, wrote the original draft and critically reviewed the manuscript. MPJT performed the study, was involved in funding acquisition, collected data, interpreted the data and critically reviewed the manuscript. HT interpreted the data, critically reviewed the manuscript. MW and NC conducted the study, performed the anaesthesia on animals, were in charge of housing and animal welfare, collected the data, critically reviewed the manuscript. H-CP and RP supervised the study, were involved in funding acquisition, critically reviewed the manuscript. RP conducted the study, supervised data collection, interpreted the data, developed the study idea, wrote the original draft critically reviewed the manuscript.
Funding
This study was supported by the AO Grant S-16–133T: Effects of standard reaming and RIA techniques on local soft tissue and systemic homeostasis in a porcine trauma model.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Pape H-C, Giannoudis PV, Krettek C, Trentz O. Timing of Fixation of Major Fractures in Blunt Polytrauma: Role of Conventional Indicators in Clinical Decision Making. J Orthop Trauma (2005) 19(8):551–62. doi: 10.1097/01.bot.0000161712.87129.80
2. Halvachizadeh S, Baradaran L, Cinelli P, Pfeifer R, Sprengel K, Pape H-C. How to Detect a Polytrauma Patient at Risk of Complications: A Validation and Database Analysis of Four Published Scales. PloS One (2020) 15(1):e0228082. doi: 10.1371/journal.pone.0228082
3. Claridge JA, Crabtree TD, Pelletier SJ, Butler K, Sawyer RG, Young JS. Persistent Occult Hypoperfusion Is Associated With a Significant Increase in Infection Rate and Mortality in Major Trauma Patients. J Trauma (2000) 48(1):8–14. discussion 14–5. doi: 10.1097/00005373-200001000-00003
4. Blow O, Magliore L, Claridge JA, Butler K, Young JS. The Golden Hour and the Silver Day: Detection and Correction of Occult Hypoperfusion Within 24 Hours Improves Outcome From Major Trauma. J Trauma (1999) 47(5):964–9. doi: 10.1097/00005373-199911000-00028
5. Martin JT, Alkhoury F, O’Connor JA, Kyriakides TC, Bonadies JA. “Normal” Vital Signs Belie Occult Hypoperfusion in Geriatric Trauma Patients. Am Surg (2010) 76(1):65–9. doi: 10.1177/000313481007600113
6. Silbert BI, Litton E, Ho KM. Central Venous-to-Arterial Carbon Dioxide Gradient as a Marker of Occult Tissue Hypoperfusion After Major Surgery. Anaesth Intensive Care (2015) 43(5):628–34. doi: 10.1177/0310057X1504300512
7. Hildebrand F, Weuster M, Mommsen P, Mohr J, Fröhlich M, Witte I, et al. A Combined Trauma Model of Chest and Abdominal Trauma With Hemorrhagic Shock–Description of a New Porcine Model. Shock (2012) 38(6):664–70. doi: 10.1097/SHK.0b013e3182709c90
8. Percie du Sert N, Hurst V, Ahluwalia A, Alam S, Avey MT, Baker M, et al. The ARRIVE Guidelines 20: Updated Guidelines for Reporting Animal Research. PloS Biol (2020) 18(7):e3000410. doi: 10.1371/journal.pbio.3000410
9. ATLS Subcommittee. American College of Surgeons’ Committee on Trauma, International ATLS Working Group Advanced Trauma Life Support (ATLS®): The Ninth Edition. J Trauma Acute Care Surg (2013) 74(5):1363–6. doi: 10.1097/TA.0b013e31828b82f5
10. Bouillon B, Marzi I. The Updated German “Polytrauma - Guideline”: An Extensive Literature Evaluation and Treatment Recommendation for the Care of the Critically Injured Patient. Eur J Trauma Emerg Surg (2018) 44(Suppl 1):1. doi: 10.1007/s00068-018-0949-0
11. Meregalli A, Oliveira RP, Friedman G. Occult Hypoperfusion is Associated With Increased Mortality in Hemodynamically Stable, High-Risk, Surgical Patients. Crit Care (2004) 8(2):R60–5. doi: 10.1186/cc2423
12. Miami Trauma Clinical Trials Group. Splanchnic Hypoperfusion-Directed Therapies in Trauma: A Prospective, Randomized Trial. Am Surg (2005) 71(3):252–60.
13. Brown CVR, Velmahos GC, Neville AL, Rhee P, Salim A, Sangthong B, et al. Hemodynamically “Stable” Patients With Peritonitis After Penetrating Abdominal Trauma: Identifying Those Who Are Bleeding. Arch Surg (2005) 140(8):767–72. doi: 10.1001/archsurg.140.8.767
14. Arnold TDW, Miller M, van Wessem KP, Evans JA, Balogh ZJ. Base Deficit From the First Peripheral Venous Sample: A Surrogate for Arterial Base Deficit in the Trauma Bay. J Trauma (2011) 71(4):793–7. discussion 797. doi: 10.1097/TA.0b013e31822ad694
15. Salottolo KM, Mains CW, Offner PJ, Bourg PW, Bar-Or D. A Retrospective Analysis of Geriatric Trauma Patients: Venous Lactate Is a Better Predictor of Mortality Than Traditional Vital Signs. Scand J Trauma Resusc Emerg Med (2013) 21:7. doi: 10.1186/1757-7241-21-7
16. Bar-Or D, Salottolo KM, Orlando A, Mains CW, Bourg P, Offner PJ. Association Between a Geriatric Trauma Resuscitation Protocol Using Venous Lactate Measurements and Early Trauma Surgeon Involvement and Mortality Risk. J Am Geriatr Soc (2013) 61(8):1358–64. doi: 10.1111/jgs.12365
17. Strnad M, Lesjak VB, Vujanović V, Pelcl T, Križmarić M. Predictors of Mortality and Prehospital Monitoring Limitations in Blunt Trauma Patients. BioMed Res Int (2015) 2015:983409. doi: 10.1155/2015/983409
18. Caputo N, Reilly J, Kanter M, West J. A Retrospective Analysis of the Respiratory Adjusted Shock Index to Determine the Presence of Occult Shock in Trauma Patients. J Trauma Acute Care Surg (2018) 84(4):674–8. doi: 10.1097/TA.0000000000001761
19. Schulman AM, Claridge JA, Carr G, Diesen DL, Young JS. Predictors of Patients Who Will Develop Prolonged Occult Hypoperfusion Following Blunt Trauma. J Trauma (2004) 57(4):795–800. doi: 10.1097/01.TA.0000140835.65944.54
20. Radowsky JS, DuBose JJ, Scalea TM, Miller C, Floccare DJ, Sikorski RA, et al. Handheld Tissue Oximetry for the Prehospital Detection of Shock and Need for Lifesaving Interventions: Technology in Search of an Indication? Air Med J (2019) 38(4):276–80. doi: 10.1016/j.amj.2019.03.014
22. Eltzschig HK, Carmeliet P. Hypoxia and Inflammation. N Engl J Med (2011) 364(7):656–65. doi: 10.1056/NEJMra0910283
23. Eltzschig HK, Eckle T. Ischemia and Reperfusion—From Mechanism to Translation. Nat Med (2011) 17(11):1391–401. doi: 10.1038/nm.2507
24. Halvachizadeh S, Mica L, Kalbas Y, Lipiski M, Canic M, Teuben M, et al. Zone-Dependent Acute Circulatory Changes in Abdominal Organs and Extremities After Resuscitative Balloon Occlusion of the Aorta (REBOA): An Experimental Model. Eur J Med Res (2021) 26(1):10. doi: 10.1186/s40001-021-00485-y
25. Sönmez TT, Al-Sawaf O, Brandacher G, Kanzler I, Tuchscheerer N, Tohidnezhad M, et al. A Novel Laser-Doppler Flowmetry Assisted Murine Model of Acute Hindlimb Ischemia-Reperfusion for Free Flap Research. PloS One (2013) 8(6):e66498. doi:10.1371/journal.pone.0066498
26. Horst K, Hildebrand F, Pfeifer R, Hübenthal S, Almahmoud K, Sassen M, et al. Impact of Haemorrhagic Shock Intensity on the Dynamic of Alarmins Release in Porcine Poly-Trauma Animal Model. Eur J Trauma Emerg Surg (2016) 42(1):67–75. doi: 10.1007/s00068-015-0504-1
27. Qiao Z, Horst K, Teuben M, Greven J, Yin L, Kalbas Y, et al. Analysis of Skeletal Muscle Microcirculation in a Porcine Polytrauma Model With Haemorrhagic Shock. J Orthopaedic Research® (2018) 36(5):1377–82. doi: 10.1002/jor.23759
28. Dutton RP. Resuscitation From Traumatic Shock. Curr Opin Anaesthesiol (2001) 14(2):217–20. doi: 10.1097/00001503-200104000-00014
29. Pfeifer R, Lichte P, Schreiber H, Sellei RM, Dienstknecht T, Sadeghi C, et al. Models of Hemorrhagic Shock: Differences in the Physiological and Inflammatory Response. Cytokine (2013) 61(2):585–90. doi: 10.1016/j.cyto.2012.10.022
30. Pfeifer R, Kobbe P, Darwiche SS, Billiar TR, Pape H-C. Role of Hemorrhage in the Induction of Systemic Inflammation and Remote Organ Damage: Analysis of Combined Pseudo-Fracture and Hemorrhagic Shock. J Orthop Res (2011) 29(2):270–4. doi: 10.1002/jor.21214
31. Horst K, Simon TP, Pfeifer R, Teuben M, Almahmoud K, Zhi Q, et al. Characterization of Blunt Chest Trauma in a Long-Term Porcine Model of Severe Multiple Trauma. Sci Rep (2016) 6:1–13. doi: 10.1038/srep39659
32. Relja B, Land WG. Damage-Associated Molecular Patterns in Trauma. Eur J Trauma Emerg Surg (2020) 46(4):751–75. doi: 10.1007/s00068-019-01235-w
33. Pfeifer R, Kalbas Y, Coimbra R, Leenen L, Komadina R, Hildebrand F, et al. Indications and Interventions of Damage Control Orthopedic Surgeries: An Expert Opinion Survey. Eur J Trauma Emerg Surg (2020), 47:2081–92. doi: 10.1007/s00068-020-01386-1
34. Horst K, Greven J, Lüken H, Zhi Q, Pfeifer R, Simon TP, et al. Trauma Severity and Its Impact on Local Inflammation in Extremity Injury-Insights From a Combined Trauma Model in Pigs. Front Immunol (2019) 10:3028. doi: 10.3389/fimmu.2019.03028
Keywords: local inflammation, porcine model, standardized polytrauma, occult hypoperfusion, persistent lactic acidosis, treat, perfusion in polytrauma
Citation: Halvachizadeh S, Kalbas Y, Teuben MPJ, Teuber H, Cesarovic N, Weisskopf M, Cinelli P, Pape H-C and Pfeifer R (2022) Effects of Occult Hypoperfusion on Local Circulation and Inflammation - An Analysis in a Standardized Polytrauma Model. Front. Immunol. 13:894270. doi: 10.3389/fimmu.2022.894270
Received: 11 March 2022; Accepted: 25 April 2022;
Published: 21 June 2022.
Edited by:
Tom E. Mollnes, University of Oslo, NorwayReviewed by:
Matthias Fröhlich, Universitiy Witten/Herdecke, GermanyErik Waage Nielsen, Nord University, Norway
Copyright © 2022 Halvachizadeh, Kalbas, Teuben, Teuber, Cesarovic, Weisskopf, Cinelli, Pape and Pfeifer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sascha Halvachizadeh, U2FzY2hhLkhhbHZhY2hpemFkZWhAdXN6LmNo
 Sascha Halvachizadeh
Sascha Halvachizadeh Yannik Kalbas1,2
Yannik Kalbas1,2 Michel Paul Johan Teuben
Michel Paul Johan Teuben Nikola Cesarovic
Nikola Cesarovic Miriam Weisskopf
Miriam Weisskopf Paolo Cinelli
Paolo Cinelli Roman Pfeifer
Roman Pfeifer