
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 03 May 2022
Sec. Inflammation
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.893929
This article is part of the Research Topic New Insights of Immune Cells in Cardiovascular and Metabolic Disorders View all 36 articles
Purpose: To examine the levels of 1-palmitoyl-2-(5-oxovaleroyl)-sn-glycero phosphatidylcholine (POVPC) and 1-palmitoyl-2-glutaroyl-sn-glycero-phosphatidylcholine (PGPC) (the oxidized phosphatidylcholines) in HDL during the course of sepsis and to evaluate their prognostic value.
Materials and Methods: This prospective cohort pilot study enrolled 25 septic patients and 10 healthy subjects from 2020 to 2021. The HDLs were extracted from patient plasmas at day 1, 3 and 7 after sepsis onset and from healthy plasmas (total 81 plasma samples). These HDLs were then subjected to examining POVPC and PGPC by using an ultra-high performance liquid chromatography coupled with tandem mass spectrometry (UHPLC-MS/MS) system. We further measured the levels of 38 plasma cytokines by Luminex and evaluated the correlation of HDL-POVPC level with these cytokines. Patients were further stratified into survivors and non-survivors to analyze the association of HDL-POVPC level with 28-day mortality.
Results: Septic patients exhibited significant increase of HDL-POVPC at day 1, 3 and 7 after sepsis onset (POVPC-D1, p=0.0004; POVPC-D3, p=0.033; POVPC-D7, p=0.004, versus controls). HDL-PGPC was detected only in some septic patients (10 of 25) but not in healthy controls. Septic patients showed a significant change of the plasma cytokines profile. The correlation assay showed that IL-15 and IL-18 levels were positively correlated with HDL-POVPC level, while the macrophage-derived chemokine (MDC) level was negatively correlated with HDL-POVPC level. Furthermore, HDL-POVPC level in non-survivors was significantly increased versus survivors at day 1 and 3 (POVPC-D1, p=0.002; POVPC-D3, p=0.003). Area under ROC curves of POVPC-D1 and POVPC-D3 in predicting 28-day mortality were 0.828 and 0.851. POVPC-D1and POVPC-D3 were the independent risk factors for the death of septic patients (p=0.046 and 0.035).
Conclusions: HDL-POVPC was persistently increased in the course of sepsis. POVPC-D1 and POVPC-D3 were significantly correlated with 28-mortality and might be valuable to predict poor prognosis.
Sepsis, represented as the dysregulated systemic inflammation syndrome in response to infection, is a common pathway to death in infectious diseases. The tissue injuries and the organ failures during sepsis are involved with complex interactions between microbes, immune cells, coagulation cascade, and endothelium (1, 2). Some clinical treatments, including appropriate antibiotic administration and fluid resuscitation, are suggested to improve outcomes of septic patients, but sepsis remains a high mortality rate (3). The reliable diagnostic and prognostic biomarkers are critical for constraining its mortality through guiding precise and effective therapeutic strategies.
Some clinical markers, such as procalcitonin (PCT) and C-reactive protein (CRP), have predictive value for septic prognosis, but their specificity or sensitivity are significantly limited by the clinical and pathogenetic heterogeneities of sepsis (4). Of note, the increasing lipidomic studies indicate that lipid metabolism remodeling plays roles in septic pathophysiology (5). Given that most alterations in cellular function, inflammatory response and energy imbalance during sepsis could lead to the changes in lipid metabolism, the lipids biomarkers show advantages in prognosis of septic patients (6).
High-density lipoprotein (HDL), as a critical mediator in lipid metabolic homeostasis, has notable anti-inflammatory and anti-oxidation roles in septic response (7). The decrease in plasma level of HDL-cholesterol (HDL-C) is correlated with a poor prognosis in septic patients (8–11). Notably, recent studies indicate an adverse transition of HDL to pro-inflammatory properties, in acute inflammatory disorder diseases including sepsis (8, 12, 13). Our previous study further demonstrates that the HDL from patients with sepsis-induced ARDS significantly promotes endothelial barrier dysfunction, which is associated with the remodeling in HDL constitutes, such as the increases of apoC-III, apoE, and serum amyloid A (SAA) (14). We hypothesized that the constitute changes in apoproteins would cause the alterations in lipid constitute and such lipid remodeling might be involved in adverse HDL transition.
Oxidized phospholipids (Ox-PLs) have significant pro-inflammatory function contributing to the pathogenesis of acute and chronic infection diseases (15). 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphatidylcholine (PAPC) is located in lipoproteins and it is oxidatively truncated in the sn-2 fatty acid residues to oxidized PAPC (Ox-PAPCs), including 1-palmitoyl-2-(5-oxovaleroyl)-sn-glycero-phosphatidylcholine (POVPC) and 1-palmitoyl-2glutaroyl-sn-glycero-phosphatidylcholine (PGPC) (16). The ox-PAPCs referenced as active principles of Ox-LDL have been involved in pro-inflammatory effects on the endothelial cells and innate immune cells in the pathogenesis of atherosclerosis and acute lung injury (17). Intriguingly, HDL plays a critical role to prevent Ox-LDL formation due to its anti-oxidation property (18). It has been shown that phospholipids in HDL itself are more susceptible to oxidation (19). However, no study has yet examined the changes in the levels of POVPC and PGPC in HDL during inflammatory response such as sepsis.
Herein, we examined the levels of POVPC and PGPC in plasma HDL from healthy controls and the septic patients at Day 1, 3, and 7 from septic diagnosis. We further conducted the correlation analysis of the level of HDL POVPC to clinical outcome. Our results could shed additional light on developing an early-stage biomarker for septic prognosis.
This study was a prospective cohort pilot study that was conducted at the Department of Pulmonary and Critical Care Medicine and at the emergency department in Beijing Chao-Yang Hospital. All enrolled adult patients with sepsis (ages ≥ 18 years) met the Sepsis-3 criteria: an acute elevation of sequential organ failure assessment (SOFA) scores ≥ 2 points upon suspected or confirmed infections (20). The patients were excluded as follows: patients died within 3 days; patients with diabetes mellitus or hyperthyroidism; patients diagnosed with hypercholesterolemia; patients who received statin drugs; patients who were pregnant. There were 13 patients further excluded because their blood samples were not collected at Day 3 due to clinical conditions. Finally, a total of 25 eligible septic patients and 10 of sex- and age-matched healthy subjects were included, in which 24 of 25 septic patients were infected with bacteria, and only 1 patient was infected with fungi. All the study participants provided informed consent for the study. Our protocol and procedures were approved by the Ethics Committee of Beijing Chao-Yang Hospital (approval No.: 2021-ke-313).
The primary endpoint of interest was the 28-day mortality from diagnosis and the goal was to explore the correlation of HDL POVPC level to the 28-day mortality in these enrolled septic patients. The enter logistic regression was performed to identify the independent risk factors for the 28-day mortality of sepsis.
Demographic characteristics, vital signs and comorbidities at admission, laboratory data, SOFA score, acute physiology, and chronic health evaluation II (APACHE II) score at septic diagnosis, treatments during RICU, including vasopressors administration and continuous renal replacement therapy (CRRT) and survival information were collected.
To track changes of POVPC and PGPC during their pathophysiological processes, whole blood was collected at Day 1 (the day at diagnosis), Day 3, and Day 7 from septic diagnosis as well as from healthy controls at enrollment. At Day 7 of sepsis, only 21 samples were collected due to the death of 4 patients within 7 days. Finally, a total of 81 whole blood samples was collected in the 5-mL EDTA blood collection tubes. The plasmas were obtained after centrifugation at 1800 g for 15 min and immediately stored at -80°C until use. The EDTA plasmas were further subjected into an HDL isolation procedure and the multiplex inflammatory cytokines assays using the 38-cytokine panel MILLIPLEX® MAP (Merck Millipore, HCYTA-60K-PX38).
A 500 μL of plasma from each subject was used in the procedure of HDL isolation as previously described (21). Briefly, after pre-staining lipoproteins using Sudan 7b to facilitate visualization, the very low-density lipoprotein (VLDL), low-density lipoprotein (LDL), and HDL fractions were separated using a sequential procedure according to their density differences (HDL, ρ = 1.478 g/ml; LDL, ρ = 1.182 g/ml; VLDL, ρ = 1.006 g/ml). The solutions with different densities adjusted by dissolving different qualities of NaBr and NaCl in MilliQ water, were successively layered on serum samples. The layers were centrifuged at 180,000 g with p65a rotor on cp80wx ultracentrifuge (Hitachi) for 12 h to remove VLDL and LDL, and collected purified HDL finally. The purity of HDL was confirmed by Western blot analysis of apoprotein A-1 (apoA-1) and apoprotein B 100 (apoB100) (Supplementary Figure S1).
The POVPC and PGPC lipid standards and the stable isotope-labeled standards were obtained from Sigma-Aldrich (St. Louis, MO). Briefly, the 10 μL of sample was added to 490 μL Calibrator Diluent followed by well vortexed and then 50 μL of the dilution was homogenized with 200 μL of acetonitrile/methanol (1:1) containing internal standards. After centrifuge at 12,000 rpm for 10 min, the supernatant was injected into the LC-MS/MS system. An ultra-high performance liquid chromatography coupled with tandem mass spectrometry (UHPLC-MS/MS) system (UHPLC-QTRAP 6500+) was used to quantitate lipids (Supplementary Figure S2 and Supplementary Table S1). The standard curves for POVPC and PGPC were built by the concentration series of standard solution (Supplementary Figure S3).
Continuous and categorical variables are expressed as interquartile ranges (IQRs) and as numbers and percentages, respectively. For the comparisons between two groups, the Student’s t-test or Mann-Whitney U test was performed for continuous variables, and the Fisher’s exact test was performed for categorical variables. For the comparisons of three groups, the one-way ANOVA (Bonferroni post hoc test) was performed for normally distributed variables and one-way Kruskal–Wallis tests (Dunn’s post hoc test) was performed for non-normally distributed variables. Receiver operating characteristic (ROC) curve was performed for the prediction of 28-day mortality with POVPC, and also compare the sensitivity and specificity with SOFA, C-reactive protein, and lactate; the cutoff value was selected based on the highest Youden’s index in order to maximize both sensitivity and specificity (Youden’s index=sensitivity+specificity-1). The correlation analysis was performed with the Pearson method. Furthermore, enter logistic regression was performed to identify the risk factors for the 28-day mortality (factors with p < 0.05 in univariate logistic regression were in turn subjected to multivariate logistic regression). A 2-sided P < 0.05 was considered statistically significant. SPSS version 24.0 (SPSS, Chicago, IL) statistical software was used in this study.
A total of 25 septic patients and 10 healthy controls were included as indicated by the subject enrollment flowchart (Figure 1). There were 80% of the septic patients with pulmonary infection (20/25) and 20% of patients with abdominal infection (5/25). Moreover, most of the patients (24/25, 96%) had bacterial infection, and only 1 patient had fungal infection. The characteristics of recruited subjects at diagnosis are shown in Table 1. No significant differences between the two groups, in age, sex, and body mass index (BMI), were observed. In laboratory data, septic patients showed significant decreases in the levels of total plasma cholesterol (CHOL, 3.0 vs. 4.7 mmol/L, p = 0.0001), HDL cholesterol (HDL-C, 0.9 vs. 1.5 mmol/L, p = 0.001), and LDL cholesterol (LDL-C, 1.6 vs. 2.7 mmol/L, p = 0.008).
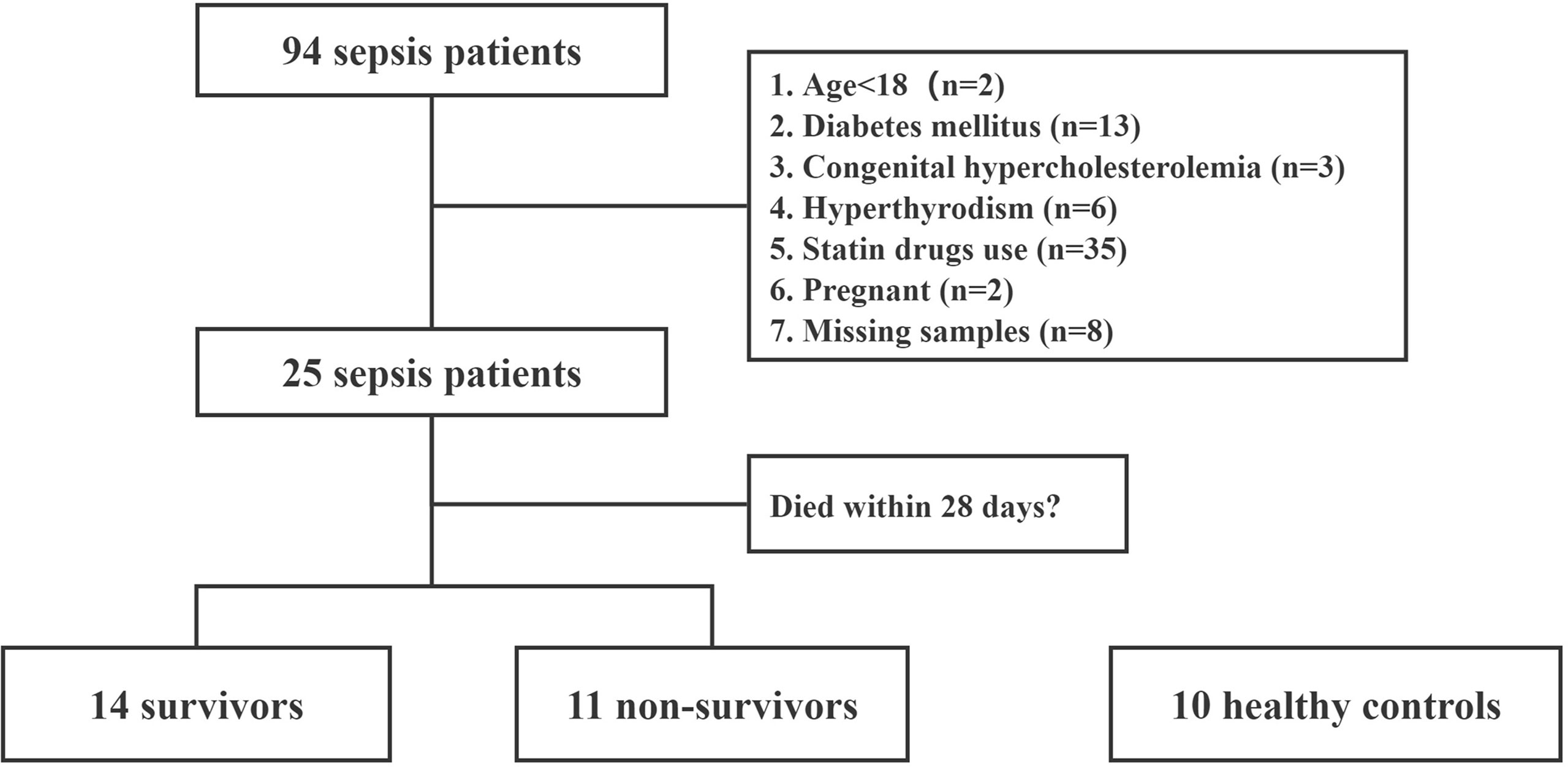
Figure 1 Flowchart of patient enrollment. This is a study flow chart of septic patients along with healthy controls.
POVPC level in HDL from septic patients was significantly increased at Day 1 (POVPC-D1), Day 3 (POVPC-D3), and Day 7 (POVPC-D7) after septic onset (292.0 vs. 224.9 ng/μL, p = 0.0004; 313.7 vs. 224.9 ng/μL, p = 0.033; 288.7 vs. 224.9 ng/μL, p = 0.004, respectively) (Table 1 and Supplementary Tables S2, S3). We also examined the PGPC in HDL. PGPC was not detected in HDL from all healthy controls, and it was only detected in HDL from part of septic patients (10 of 25, Table 1 and Supplementary Tables S2, S3). Furthermore, there was no significant difference among POVPC-D1, POVPC-D3, and POVPC-D7 of all septic patients (p > 0.99 for all these three groups) (Figure 2).
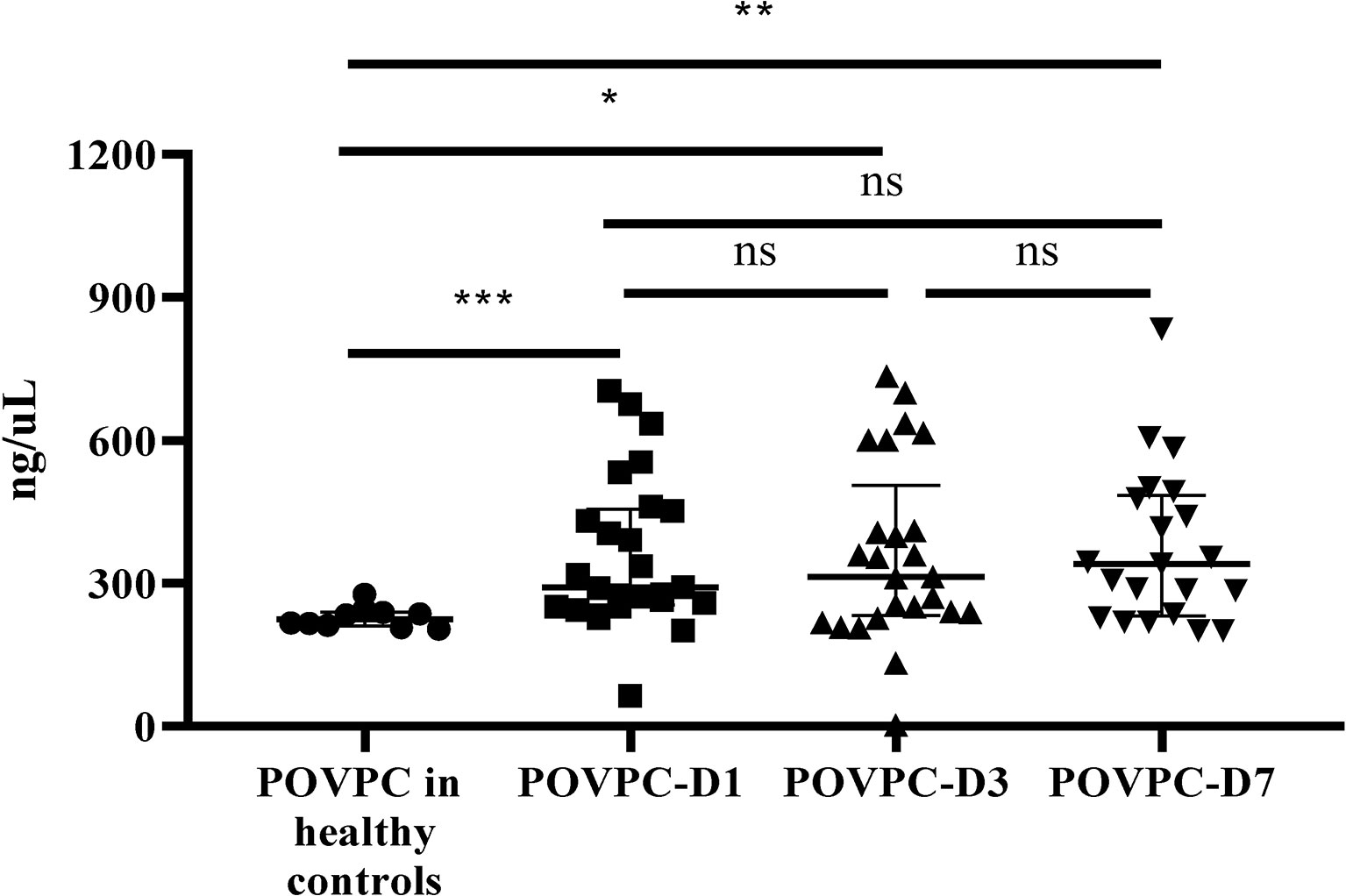
Figure 2 The persistent increase of HDL POVPC in septic patients vs. healthy controls. The difference in HDL POVPC between healthy controls and septic patients, and the increased levels were persistent in the course of sepsis. Data were presented in median with interquartile range. N=10 for healthy controls, n=14 for survivors, n=11 for non-survivors (D1 and D3), n=7 for non-survivors (D7); POVPC, 1-palmitoyl-2- (5-oxovaleroyl) -sn-glycero-phosphatidylcholine; D1, Day 1; D3, Day 3; D7, Day 7. *p<0.05; **p<0.01; ***p<0.001; ns, not significant.
Patients were subgrouped into survivors and non-survivors according to the 28-day mortality (the primary outcome). The comparisons of clinical characteristics between two groups are shown in Table 2. Non-survivors exhibited higher levels of C reactive protein (CRP), serum lactate, and SOFA score (210.9 vs. 119.1 mg/L, p = 0.025; 5.2 vs. 2.1 mmol/L, p = 0.048; 11 vs. 7.5, p = 0.015, respectively). Non-survivors also displayed a decrease in the level of plasma HDL-C vs. survivors, although the difference failed to achieve the statistical significance (0.8 vs. 1.0 mmol/L, p = 0.067). Of note, non-survivors showed a significantly increased level of POVPC in HDL at Day 1 and Day 3 after septic onset (POVPC-D1: 451 vs. 270 ng/μL, p = 0.002; POVPC-D3: 406.4 vs. 239.5 ng/μL, p = 0.003). There were no significant differences between the septic survivors and non-survivors in ventilator-free days, ICU days, and the complications and treatments (Table 3). Moreover, there is also no significant difference among POVPC-D1, POVPC-D3, and POVPC-D7 in septic survivors or non-survivors (survivors: POVPC-D1 vs. D3, p > 0.99; POVPC-D1 vs. D7, p > 0.99; POVPC-D3 vs. D7, p = 0.46; non-survivors: p > 0.99 for all these three groups) (Figure 3).
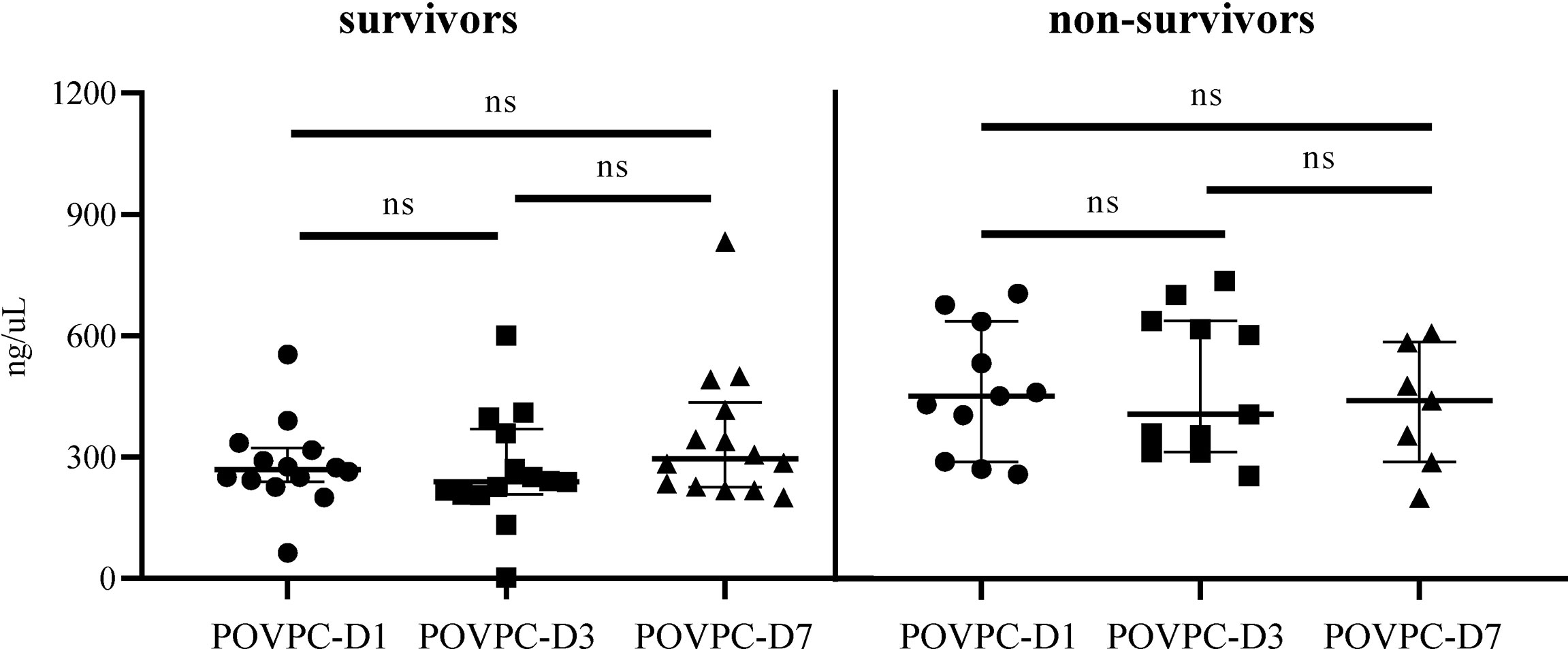
Figure 3 The difference in HDL POVPC of septic survivors and non-survivors at different time points. The difference in HDL POVPC of septic survivors and non-survivors at Day 1, Day 3, and Day 7. POVPC, 1-palmitoyl-2- (5-oxovaleroyl)-sn-glycero-phosphatidylcholine; D1, Day 1; D3, Day 3; D7, Day 7; ns, not significant.
To examine if the POVPC level would be correlated with the clinical conditions at the septic onset, we investigated the correlations of POVPC level with SOFA score, CRP, and lactate level. There were no statistical correlations between POVPC-D1 and these indexes (SOFA: r = 0.035, p = 0.868; CRP: r = -0.048, p = 0.818; lactate: r = -0.156, p = 0.458, respectively) (Figures 4A–C). Notably, there was a significant positive correlation between POVPC-D1 and POVPC-D3, suggesting an unreversible remodeling of HDL lipids (r = 0.834, p < 0.0001) (Figure 4D).
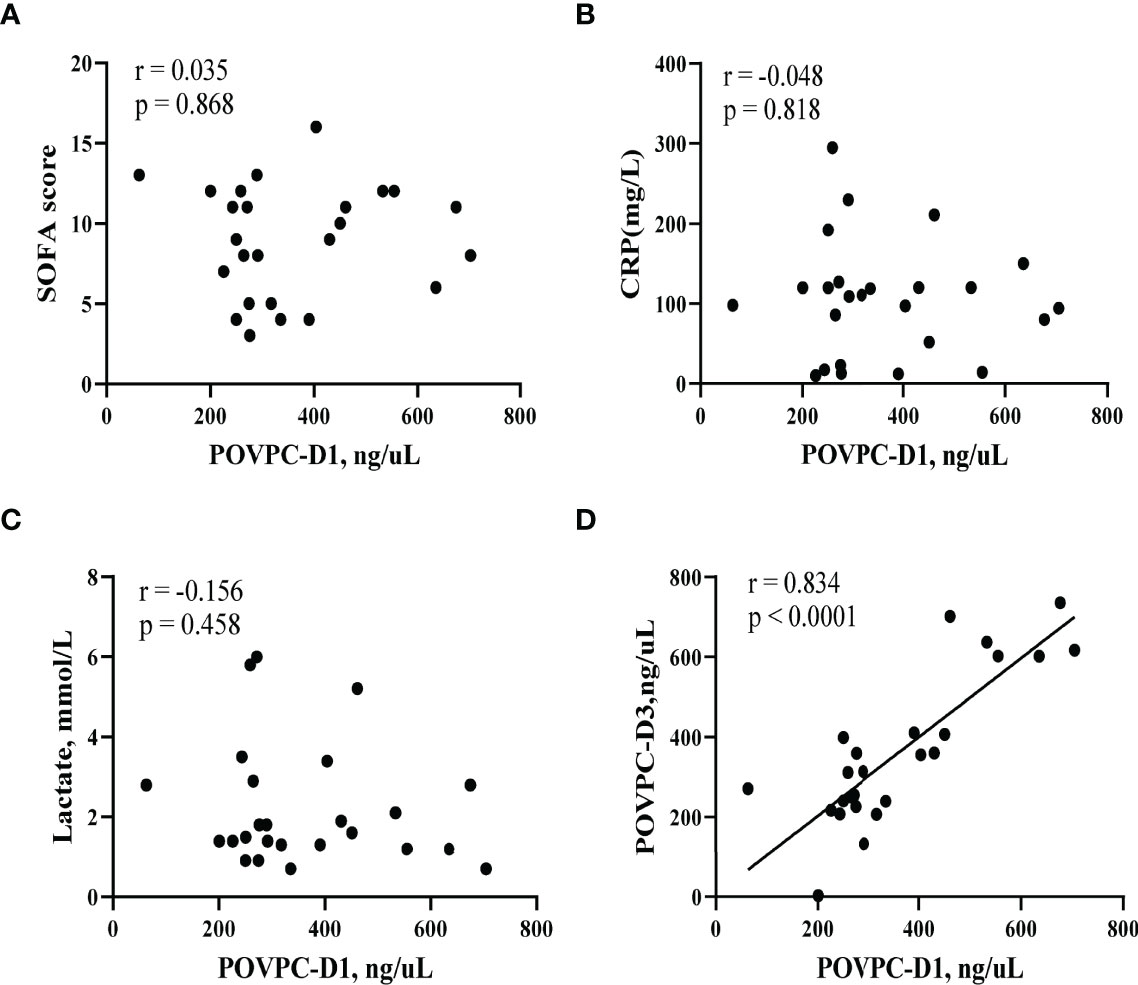
Figure 4 Correlation of HDL POVPC level with SOFA score and CRP and lactate levels. Scatter diagram for (A) correlation analysis between HDL POVPC-D1 and SOFA; (B) correlation analysis between HDL POVPC-D1 and CRP; (C) correlation analysis between HDL POVPC-D1 and lactate; (D) correlation analysis between HDL POVPC-D1 and HDL POVPC-D3. POVPC, 1-palmitoyl-2-(5-oxovaleroyl)-sn-glycero-phosphatidylcholine; POVPC-D1, POVPC at Day 1; POVPC-D3, POVPC at Day 3; HDL, high-density lipoprotein; SOFA, sequential organ failure assessment; CRP, C reactive protein.
To examine the correlation between HDL-POVPC level and inflammatory conditions during sepsis, we measured the levels of 38 plasma cytokines by Luminex (Supplementary Tables S4–S6) and further evaluated the correlation of HDL-POVPC level with these cytokines at Day 1 and Day 3 after septic onset (Table 4). The correlation assay only showed that the IL-15 level was positively correlated with the level of POVPC at Day 1 (r = 0.44, p = 0.03). At Day 3, IL-15 and IL-18 levels were positively correlated with HDL-POVPC level (IL-15: r = 0.42, p = 0.04; IL-18: r = 0.53, p = 0.01), while the macrophage-derived chemokine (MDC) level was negatively correlated with HDL-POVPC level (r = -0.62, p = 0.001) (Table 4). In addition, compared with healthy controls, septic patients showed a significant change of the plasma cytokines profile at Day 1 and Day 3, in which the levels of IL-15, IL-18, and MDC were markedly altered at both Day 1 and Day 3 (Supplementary Tables S4, S5). Furthermore, compared to survivors, non-survivors exhibited increased levels in IL-18, MCP-1, M-CSF, PDGF-AA, and PDGF-AB/BB; the IL-6, IL-8, and M-CSF were increased only at Day 3 and RANTES was decreased only at Day 1 (Supplementary Table S6). These results suggested that HDL-POVPC level was not predominantly correlated with inflammatory conditions during the course of sepsis.
The ROC curves for HDL POVPC-D1 and POVPC-D3, SOFA score, plasma CRP, and lactate levels were plotted to compare the accuracy and specificity for the prediction of 28-day mortality. The area under the curve (AUC) for POVPC-D1 was 0.828 (p = 0.004, 95% CI: 0.677-0.998) (Figure 5A), for POVPC-D3 was 0.851 (p = 0.003, 95% CI: 0.702-1) (Figure 5B), for SOFA score was 0.757 (p = 0.031, 95% CI: 0.565-0.948), for CRP was 0.766 (p = 0.025, 95% CI: 0.578-0.955), and for lactate was 0.734 (p = 0.049, 95% CI: 0.521-0.946) (Figure 5C), suggesting the better predictive values of POVPC-D1/3 vs. other indexes (Figure 5).
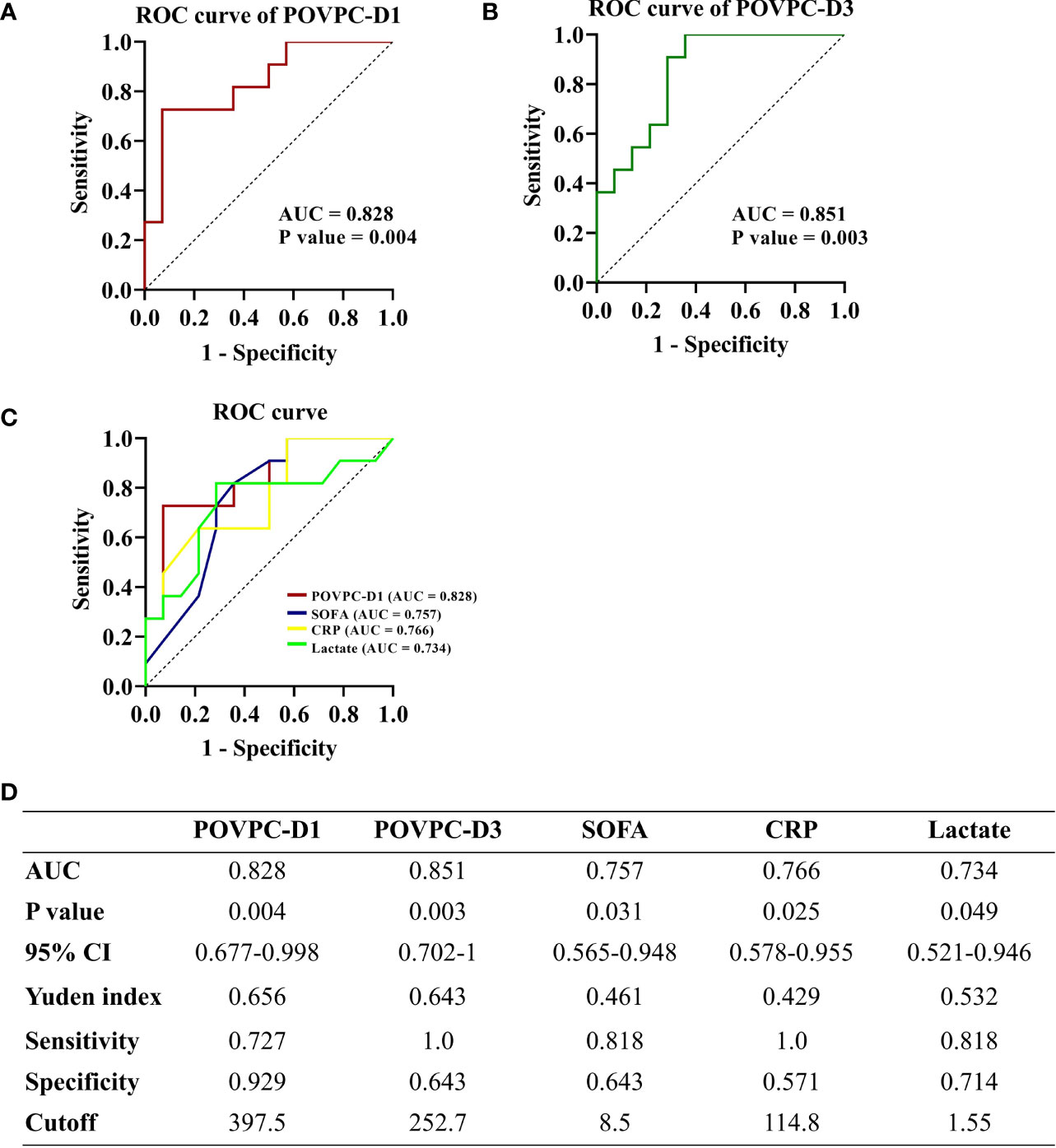
Figure 5 Predictive ability of HDL POVPC for 28-day mortality of septic patients. ROC curve for HDL POVPC-D1 and POVPC-D3 in diagnosis of the sepsis (A, B). ROC curves for POVPC-D1, SOFA score, CRP, and lactate in prediction of the 28-day mortality in septic patients (C), and the Youden’s index in order to maximize both sensitivity and specificity (D). ROC, receiver operating characteristic; AUC, area under the curve; CI, confidence interval; HDL, high-density lipoprotein; SOFA, sequential organ failure assessment; CRP, C-reaction protein; POVPC, 1-palmitoyl-2-(5-oxovaleroyl)-sn-glycero-phosphatidylcholine; D1, Day 1; D3, Day 3.
We further selected cutoff values based on the highest Youden’s index in order to maximize both sensitivity and specificity (Youden’s index=sensitivity+specificity-1). A POVPC-D1 cutoff value of 397.5 ng/μL had a sensitivity of 72.7% and a specificity of 92.9%. A POVPC-D3 cutoff value of 252.7 ng/μL had a sensitivity of 100.0% and a specificity of 64.3%. Septic patients were regrouped by these cutoff values to validate their prediction efficacy for 28-day mortality, as well as the severity at sepsis onset, including SOFA score, APACHE II score, and serum CRP and lactate levels. The POVPC-D1 and POVPC-D3 displayed a predictive efficacy for 28-day mortality, but not the sepsis onset severity (POVPC-D1: p = 0.002; POVPC-D3, p = 0.001) (Table 5), suggesting unique clinical merits of HDL POVPC as a biomarker for prognosis.
Enter logistic regression was performed to identify the risk factors for the 28-day mortality, factors with p<0.05 in univariate logistic regression were in turn subjected to multivariate logistic regression. The univariate logistic regression analysis showed POVPC-D1, POVPC-D3, SOFA, and CRP were significantly associated with 28-day mortality of septic patients (OR: 1.011, 95% CI: 1.002-1.02, p = 0.017; OR: 1.009, 95% CI: 1.001-1.016, p = 0.018; OR: 1.418, 95% CI: 1.035-1.943, p = 0.03; OR: 1.018, 95% CI: 1.001-1.036, p = 0.04). We constructed two multivariate binary regression models, Model I included CRP, SOFA score, and POVPC-D1, Model II included CRP, SOFA score, and POVPC-D3. The goodness-of-fit for these two regression models was evaluated with the Hosmer Lemeshow test. For Model I, the p value of the Hosmer Lemeshow goodness-of-fit test was 0.959; for Model II, the p value was 0.648; both p values were greater than 0.05, which indicated these two models were a good fit. The -2log likelihood value of Model I was 10.06, and the Nagelkerke R Square of Model I was 0.83; for Model II they were 13.38 and 0.76, respectively. These results suggested that our fitted logistic regression models were reliable. Multivariate logistic regression results confirmed both POVPC-D1 and POVPC-D3 were significantly associated with 28-day mortality of septic patients (POVPC-D1: OR=1.018, 95% CI: 1.0-1.036, p = 0.046; POVPC-D3: OR=1.012, 95% CI: 1.001-1.023, p = 0.035) (Table 6).
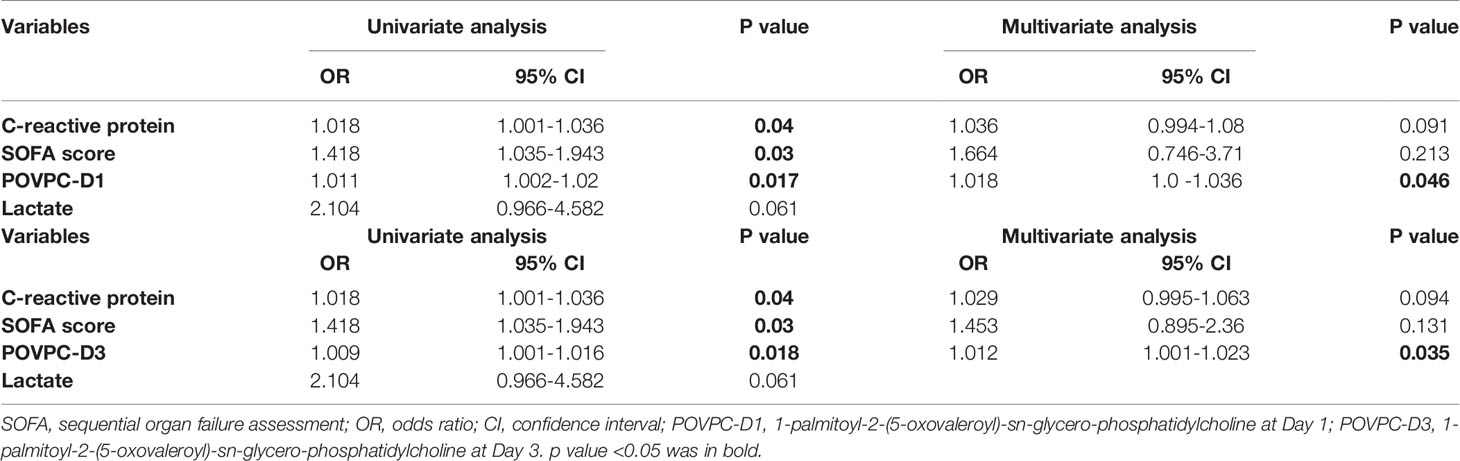
Table 6 Univariate and multivariate logistic regression analysis of independent risk factors for 28-day mortality in septic patients.
Our study, for the first time, confirmed the marked increase of POVPC level in the HDL particle during the course of sepsis. We further indicated that the HDL-POVPC levels at Day 1 and Day 3 after septic onset were independent risk factors for poor prognosis of septic patients. POVPC-D1: 397.5 ng/μL and POVPC-3: 252.7 ng/μL were significantly correlated with the 28-day mortality of septic patients.
The Ox-PAPCs, including POVPC and PGPC have been involved in the chronic atherosclerotic pathogenesis, for decades, by modulating inflammatory response, endothelial barrier function, thrombosis, and angiogenesis (22, 23). Recently, POVPC was detected in the plasma of septic and ALI patients and the increase in POVPC generation was observed in severe ALI with marked vascular leakage and enhanced inflammation (24–26). In this study, we indicated a sustained increase of POVPC in the HDL particle from septic non-survivors at Day 1 and 3 and the HDL-POVPC levels were positively correlated with some key pro-inflammatory cytokines. The HDL-POVPC level at Day 7 failed to achieve significance, which might be due to the complicated conditions after several days of treatments intervene. Given the importance of HDL in anti-inflammation, these observations for the first time suggested that the Ox-PAPCs in HDL, other than in LDL, likely contribute to deregulated inflammation in sepsis. Moreover, our results exhibited a persistent increase of HDL POVPC from Day 1 to Day 7 during the course of sepsis, implying an unreversible remodeling of HDL lipids. Interestingly, we also detected the POVPC in plasma HDL from healthy controls (Table 1). A previous study showed an age-dependent increase of POVPC production, suggesting an association between oxidative HDL remodeling and aging (26). In our study, the constitute of HDL-POVPC in healthy controls is likely due to their elder ages (71 [59~82.5] years old).
Previous studies have demonstrated the contribution of Ox-PAPC to the deleterious function of oxidized LDL in atherosclerosis and the oxidized LDL has been found to be increased in patients with severe sepsis (27, 28). Of note, the dysfunction of HDL was associated with the increase of oxidized LDL, owing to its essential role in preventing the formation of the LDL-derived oxidized phospholipids as well as inactivating formed oxidized phospholipids by enzymatic and non-enzymatic mechanisms (29, 30). The oxidation degree of HDL itself would be more sensitive than that of LDL in response to septic disorder. Consistently, our results showed the persistent increase of HDL-POVPC in septic patients at Day 1 and 3, which was significantly correlated with pro-inflammatory cytokines and exhibited better predictive abilities for 28-day mortality of septic patients than SOFA, CRP, and lactate. These observations suggested the significant role of HDL-POVPC in sepsis. Thus, we hypothesize that the higher levels of oxidized LDL could be associated with the enhanced HDL-oxidation. These pilot results also imply that HDL-POVPC could be a potential biomarker of sepsis outcome.
The SOFA score, plasma CRP, and lactate levels normally present during the clinical conditions at septic onset include inflammatory stress and organ injuries. Of note, our correlation analyses failed to show significant correlation between HDL-POVPC levels and these indexes, although they were associated with poor prognosis of septic patients. This might be due to that HDL-POVPC represents the dysfunction of HDL including anti-inflammatory and endothelial protection. Our further correlation analysis showed HDL-POVPC levels were significantly correlated with several critical inflammatory cytokines including IL-15, IL-18, and MDC which were dramatically changed in septic patients, although HDL-POVPC was not predominantly correlated with inflammatory conditions. Notably, IL-18 level was dramatically increased in septic non-survivors vs. survivors at both Day 1 and Day 3. This is consistent with previous findings that the increase of plasma IL-18 level has been shown as a biomarker for adverse outcomes of sepsis (31, 32). The positive correlation of HDL-POVPC levels with IL-18 endorsed the prognostic value of HDL-POVPC for sepsis. In addition, MDC, as a CC chemokine involved in dendritic cell and lymphocyte homing, can ameliorate systemic tissue inflammation. The decrease of MDC level is associated with poor outcome of sepsis (33). Herein, our results showed HDL-POVPC was negatively associated with MDC that was significantly decreased in septic patients. These observations suggested the unique contribution of HDL-POVPC in inflammatory disorder, which might be associated with poor prognosis of sepsis.
Sepsis is involved with complex interactions between microbes, immune cells, and endothelium. It has been shown the truncated Ox-PAPCs, including POVPC and PGPC, can induce vascular leaking and exacerbate inflammation, due to their direct pro-inflammatory effects on both endothelial cell and macrophages (24, 34, 35). The deleterious effects of POVPC on endothelial function have been highlighted for decades in cardiovascular diseases (36–38). Therefore, the variety of POVPC function could be an explanation for why HDL POVPC was not significantly correlated with these SOFA scores, plasma CRP, and other pro-inflammatory cytokines enhanced in septic patients. Our finding further suggested the deleterious effects of HDL-POVPC on endothelial barrier should be considered in clinical therapeutic strategy for septic patients, such as respiratory disorders and vascular leaking.
It has been shown that a variety of HDL protective functions (anti-oxidative, anti-inflammatory, and vasoprotective effects) were impaired upon the pathological processes with oxidative stress, especially in acute sepsis (7, 39). The oxidative modification of HDL apoproteins, such as the apoprotein A-I, has been proposed as a key molecular mechanism leading to HDL dysfunction (40). However, recent evidence indicates oxidized phospholipids (Ox-PLs) in HDL can modify apoproteins via direct cross-linking leading to their dysfunction (41). Oxidized phospholipids could be more sensitive than apoprotein modification to represent HDL dysfunction. Consistently, in our study, the HDL-POVPC seemed to be more efficient to predict 28-day mortality than SOFA score and plasma CRP and lactate levels indicated by ROC curve (AUC: POVPC-D1, 0.828 and POVPC-D3, 0.851 vs. SOFA, 0.757, CRP 0.766, and lactate, 0.734). Furthermore, we evaluated the cutoff point of POVPC levels for prognostic prediction in septic patients: POVPC-D1: 397.5 ng/μL with a sensitivity of 72.7% and a specificity of 92.9% and POVPC-D3: 252.7 ng/μL with a sensitivity of 100.0% and a specificity of 64.3%. The validation cohort with larger sample sizes is worthy of a study to evaluate the predictive merits of POVPC for sepsis.
The decrease of HDL-C level has become a poor prognostic factor for sepsis, owing to its dramatic decrease in these patients (42–44). Our septic cohort also showed significantly decreased levels of HDL-C at admission vs. healthy controls (0.9 vs. 1.5 mmol/L, p = 0.001), but there was no significant difference between survivors and non-survivors. This inconsistency might be due to the limited sample size, as indicated by the obvious group variation (survivors: 1.0 (0.8~1.3); non-survivors: 0.8 (0.3~1.0) mmol/L). In contrast, this cohort exhibited significant difference in HDL POVPC levels between survivors and non-survivors, suggesting that the HDL quality remodeling seemed to be more sensitive than quantity change in the course of sepsis.
Our study, for the first time, showed a marked increase of POVPC level in the HDL particle from septic patients and such increase is persistent during the course of sepsis. We further indicated that the POVPC levels at Day 1 and Day 3 after sepsis onset were independent risk factors for poor prognosis of septic patients. POVPC-D1: 397.5 ng/μL and POVPC-3: 252.7 ng/μL were significantly correlated with the 28-day mortality of septic patients. Our finding further endorses the necessity to explore the quality remodeling of HDL in response to septic stress. Future studies embracing larger sample sizes is required to provide solid evidence for the predictive merits of POVPC for sepsis.
Several limitations should be considered in our present research. First, our study was carried out at a single center, results cannot be extrapolated. Second, our sample size was relatively small, which may have affected our results. The outcome in regression models for the prognostic value of POVPC levels was rather low, which limited generalizability of our results. Third, our recruited septic patients were predominantly infected with bacteria, thus our results may not be suitable for viral infected septic patients.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by The Ethics Committee of Beijing ChaoYang Hospital (2021-ke-313). The patients/participants provided their written informed consent to participate in this study.
ZHL: Formal analysis, writing-original draft. XS and ZTL: Data processing. BP Writing-review and editing. YM: Conceptualization and design. JJ: Writing-review and editing. All authors contributed to the article and approved the submitted version.
This work was supported by Reform and Development Program of Beijing Institute of Respiratory Medicine (2021).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We appreciate the doctors and nurses in the department of Pulmonary and Critical Care Medicine and Emergency of Beijing ChaoYang Hospital for their help during the present study.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.893929/full#supplementary-material
HDL (-C), high-density lipoprotein (-cholesterol); LDL (-C), low-density lipoprotein (-cholesterol); VLDL (-C), very low-density lipoprotein (-cholesterol); CHOL, cholesterol; Ox-PLs, oxidized phospholipids; UHPLC-MS/MS, ultra-high performance liquid chromatography coupled to tandem mass spectrometry system; PAPC, 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphatidylcholine; Ox-PAPCs, oxidized PAPCs; POVPC, 1-palmitoyl-2-(5-oxovaleroyl)-sn-glycero-phosphatidylcholine; PGPC, 1-palmitoyl-2-glutaroyl-sn-glycero-phosphatidylcholine; D1, at Day 1; D3, at Day 3; D7, at Day 7; Ox-LDL, oxidized low-density lipoprotein; apoA-1, apoprotein A-1; apoB100, apoprotein B 100; BMI, body mass index; PCT, procalcitonin; CRP, C-reactive protein; SOFA score, Sequential organ failure assessment score; APACHE II score, acute physiology and chronic health evaluation II score; CRRT, continuous renal replacement therapy; IQRs, interquartile ranges; ROC, receiver operating characteristic; AUC, area under the curve; ALI, acute lung injury.
1. Rhee C, Dantes R, Epstein L, Murphy DJ, Seymour CW, Iwashyna TJ, et al. Incidence and Trends of Sepsis in Us Hospitals Using Clinical Vs Claims Data, 2009-2014. Jama-J Am Med Assoc (2017) 318(13):1241–9. doi: 10.1001/jama.2017.13836
2. Cohen J, Vincent J-L, Adhikari NKJ, Machado FR, Angus DC, Calandra T, et al. Sepsis: A Roadmap for Future Research. Lancet Infect Dis (2015) 15(5):581–614. doi: 10.1016/s1473-3099(15)70112-x
3. Kahn JM, Davis BS, Yabes JG, Chang CH, Chong DH, Hershey TB, et al. Association Between State-Mandated Protocolized Sepsis Care and in-Hospital Mortality Among Adults With Sepsis. JAMA (2019) 322(3):240–50. doi: 10.1001/jama.2019.9021
4. Kataria Y, Remick D. Sepsis Biomarkers. Methods Mol Biol (Clifton NJ) (2021) 2321:177–89. doi: 10.1007/978-1-0716-1488-4_16
5. Amunugama K, Pike DP, Ford DA. The Lipid Biology of Sepsis. J Lipid Res (2021) 62:100090. doi: 10.1016/j.jlr.2021.100090
6. Mecatti GC, Messias MCF, de Oliveira Carvalho P. Lipidomic Profile and Candidate Biomarkers in Septic Patients. Lipids Health Dis (2020) 19(1):68. doi: 10.1186/s12944-020-01246-2
7. Chiesa ST, Charakida M. High-Density Lipoprotein Function and Dysfunction in Health and Disease. Cardiovasc Drugs Ther (2019) 33(2):207–19. doi: 10.1007/s10557-018-06846-w
8. Tanaka S, Diallo D, Delbosc S, Genève C, Zappella N, Yong-Sang J, et al. High-Density Lipoprotein (Hdl) Particle Size and Concentration Changes in Septic Shock Patients. Ann Intensive Care (2019) 9(1):68. doi: 10.1186/s13613-019-0541-8
9. Chien JY, Jerng JS, Yu CJ, Yang PC. Low Serum Level of High-Density Lipoprotein Cholesterol Is a Poor Prognostic Factor for Severe Sepsis. Crit Care Med (2005) 33(8):1688–93. doi: 10.1097/01.Ccm.0000171183.79525.6b
10. Tanaka S, Labreuche J, Drumez E, Harrois A, Hamada S, Vigue B, et al. Low Hdl Levels in Sepsis Versus Trauma Patients in Intensive Care Unit. Ann Intensive Care (2017) 7:1–16. doi: 10.1186/s13613-017-0284-3
11. Yang L, Luo Z, Shi X, Pang B, Ma Y, Jin J. Different Value of Hdl-C in Predicting Outcome of Ards Secondary to Bacterial and Viral Pneumonia: A Retrospective Observational Study. Heart Lung (2021) 50(1):206–13. doi: 10.1016/j.hrtlng.2020.09.019
12. Tanaka S, Couret D, Tran-Dinh A, Duranteau J, Montravers P, Schwendeman A, et al. High-Density Lipoproteins During Sepsis: From Bench to Bedside. Crit Care (2020) 24(1):134. doi: 10.1186/s13054-020-02860-3
13. Guirgis FW, Dodani S, Moldawer L, Leeuwenburgh C, Bowman J, Kalynych C, et al. Exploring the Predictive Ability of Dysfunctional High-Density Lipoprotein for Adverse Outcomes in Emergency Department Patients With Sepsis: A Preliminary Investigation. Shock (2017) 48(5):539–44. doi: 10.1097/SHK.0000000000000887
14. Yang L, Liu S, Han S, Hu Y, Wu Z, Shi X, et al. The Hdl From Septic-Ards Patients With Composition Changes Exacerbates Pulmonary Endothelial Dysfunction and Acute Lung Injury Induced by Cecal Ligation and Puncture (Clp) in Mice. Respir Res (2020) 21(1):293. doi: 10.1186/s12931-020-01553-3
15. Nie J, Yang J, Wei YQ, Wei XW. The Role of Oxidized Phospholipids in the Development of Disease. Mol Aspects Med (2020) 76:1–8. doi: 10.1016/j.mam.2020.100909
16. Fu P, Birukov KG. Oxidized Phospholipids in Control of Inflammation and Endothelial Barrier. Transl Res (2009) 153(4):166–76. doi: 10.1016/j.trsl.2008.12.005
17. Berliner JA, Leitinger N, Tsimikas S. The Role of Oxidized Phospholipids in Atherosclerosis. J Lipid Res (2009) 50(Suppl):S207–12. doi: 10.1194/jlr.R800074-JLR200
18. Lee S, Birukov K, Romanoski C, Springstead J, Lusis A, Berliner J. Role of Phospholipid Oxidation Products in Atherosclerosis. Circ Res (2012) 111(6):778–99. doi: 10.1161/circresaha.111.256859
19. Martin SS, Jones SR, Toth PP. High-Density Lipoprotein Subfractions: Current Views and Clinical Practice Applications. Trends Endocrinol Metab (2014) 25(7):329–36. doi: 10.1016/j.tem.2014.05.005
20. Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). Jama (2016) 315(8):801–10. doi: 10.1001/jama.2016.0287
21. Bronzert TJ, Brewer HB Jr. New Micromethod for Measuring Cholesterol in Plasma Lipoprotein Fractions. Clin Chem (1977) 23(11):2089–98. doi: 10.1093/clinchem/23.11.2089
22. Que X, Hung M, Yeang C, Gonen A, Prohaska T, Sun X, et al. Oxidized Phospholipids Are Proinflammatory and Proatherogenic in Hypercholesterolaemic Mice. Nature (2018) 558(7709):301–6. doi: 10.1038/s41586-018-0198-8
23. Karki P, Birukov KG. Oxidized Phospholipids in Healthy and Diseased Lung Endothelium. Cells (2020) 9(4):1–21. doi: 10.3390/cells9040981
24. Birukova AA, Starosta V, Tian X, Higginbotham K, Koroniak L, Berliner JA, et al. Fragmented Oxidation Products Define Barrier Disruptive Endothelial Cell Response to Oxpapc. Trans Res J Lab Clin Med (2013) 161(6):495–504. doi: 10.1016/j.trsl.2012.12.008
25. Stamenkovic A, Pierce GN, Ravandi A. Oxidized Lipids: Not Just Another Brick in the Wall. Can J Physiol Pharmacol (2019) 97(6):473–85. doi: 10.1139/cjpp-2018-0490
26. Ke YB, Karki P, Kim J, Son S, Berdyshev E, Bochkov VN, et al. Elevated Truncated Oxidized Phospholipids as a Factor Exacerbating Ali in the Aging Lungs. FASEB J (2019) 33(3):3887–900. doi: 10.1096/fj.201800981R
27. Behnes M, Brueckmann M, Liebe V, Liebetrau C, Lang S, Putensen C, et al. Levels of Oxidized Low-Density Lipoproteins Are Increased in Patients With Severe Sepsis. J Crit Care (2008) 23(4):537–41. doi: 10.1016/j.jcrc.2008.09.002
28. Bavunoglu I, Genc H, Konukoglu D, Cicekci H, Sozer V, Gelisgen R, et al. Oxidative Stress Parameters and Inflammatory and Immune Mediators as Markers of the Severity of Sepsis. J Infect Dev Ctries (2016) 10(10):1045–52. doi: 10.3855/jidc.7585
29. Kontush A, Chantepie S, Chapman MJ. Small, Dense Hdl Particles Exert Potent Protection of Atherogenic Ldl Against Oxidative Stress. Arterioscler Thromb Vasc Biol (2003) 23(10):1881–8. doi: 10.1161/01.ATV.0000091338.93223.E8
30. Navab M, Berliner JA, Subbanagounder G, Hama S, Lusis AJ, Castellani LW, et al. Hdl and the Inflammatory Response Induced by Ldl-Derived Oxidized Phospholipids. Arterioscler Thromb Vasc Biol (2001) 21(4):481–8. doi: 10.1161/01.atv.21.4.481
31. Wu Q, Xiao Z, Pu Y, Zhou J, Wang D, Huang Z, et al. Tni and Il-18 Levels Are Associated With Prognosis of Sepsis. Postgrad Med J (2019) 95(1123):240–4. doi: 10.1136/postgradmedj-2018-136371
32. Eidt MV, Nunes FB, Pedrazza L, Caeran G, Pellegrin G, Melo DAS, et al. Biochemical and Inflammatory Aspects in Patients With Severe Sepsis and Septic Shock: The Predictive Role of Il-18 in Mortality. Clin Chim Acta (2016) 453:100–6. doi: 10.1016/j.cca.2015.12.009
33. Matsukawa A, Hogaboam CM, Lukacs NW, Lincoln PM, Evanoff HL, Kunkel SL. Pivotal Role of the Cc Chemokine, Macrophage-Derived Chemokine, in the Innate Immune Response. J Immunol (2000) 164(10):5362–8. doi: 10.4049/jimmunol.164.10.5362
34. Yan FX, Li HM, Li SX, He SH, Dai WP, Li Y, et al. The Oxidized Phospholipid Povpc Impairs Endothelial Function and Vasodilation Via Uncoupling Endothelial Nitric Oxide Synthase. J Mol Cell Cardiol (2017) 112:40–8. doi: 10.1016/j.yjmcc.2017.08.016
35. Zou B, Goodwin M, Saleem D, Jiang W, Tang J, Chu Y, et al. A Highly Conserved Host Lipase Deacylates Oxidized Phospholipids and Ameliorates Acute Lung Injury in Mice. eLife (2021) 10:1–22. doi: 10.7554/eLife.70938
36. Fruhwirth G, Moumtzi A, Loidl A, Ingolic E, Hermetter A. The Oxidized Phospholipids Povpc and Pgpc Inhibit Growth and Induce Apoptosis in Vascular Smooth Muscle Cells. Biochim Biophys Acta (2006) 1761(9):1060–9. doi: 10.1016/j.bbalip.2006.06.001
37. Subbanagounder G, Leitinger N, Schwenke DC, Wong JW, Lee H, Rizza C, et al. Determinants of Bioactivity of Oxidized Phospholipids. Specific Oxidized Fatty Acyl Groups at the Sn-2 Position. Arterioscler Thromb Vasc Biol (2000) 20(10):2248–54. doi: 10.1161/01.atv.20.10.2248
38. Li Y, Zhang Y-X, Ning D-S, Chen J, Li S-X, Mo Z-W, et al. Simvastatin Inhibits Povpc-Mediated Induction of Endothelial-To-Mesenchymal Cell Transition. J Lipid Res (2021) 62:100066. doi: 10.1016/j.jlr.2021.100066
39. Camont L, Lhomme M, Rached F, Le Goff W, Negre-Salvayre A, Salvayre R, et al. Small, Dense High-Density Lipoprotein-3 Particles Are Enriched in Negatively Charged Phospholipids: Relevance to Cellular Cholesterol Efflux, Antioxidative, Antithrombotic, Anti-Inflammatory, and Antiapoptotic Functionalities. Arterioscler Thromb Vasc Biol (2013) 33(12):2715–23. doi: 10.1161/ATVBAHA.113.301468
40. Huang Y, DiDonato JA, Levison BS, Schmitt D, Li L, Wu Y, et al. An Abundant Dysfunctional Apolipoprotein A1 in Human Atheroma. Nat Med (2014) 20(2):193–203. doi: 10.1038/nm.3459
41. Gao D, Ashraf MZ, Zhang L, Kar N, Byzova TV, Podrez EA. Cross-Linking Modifications of Hdl Apoproteins by Oxidized Phospholipids: Structural Characterization, in Vivo Detection, and Functional Implications. J Biol Chem (2020) 295(7):1973–84. doi: 10.1074/jbc.RA119.008445
42. Chien YF, Chen CY, Hsu CL, Chen KY, Yu CJ. Decreased Serum Level of Lipoprotein Cholesterol Is a Poor Prognostic Factor for Patients With Severe Community-Acquired Pneumonia That Required Intensive Care Unit Admission. J Crit Care (2015) 30(3):506–10. doi: 10.1016/j.jcrc.2015.01.001
43. Cirstea M, Walley KR, Russell JA, Brunham LR, Genga KR, Boyd JH. Decreased High-Density Lipoprotein Cholesterol Level Is an Early Prognostic Marker for Organ Dysfunction and Death in Patients With Suspected Sepsis. J Crit Care (2017) 38:289–94. doi: 10.1016/j.jcrc.2016.11.041
Keywords: sepsis, high-density lipoprotein (HDL), oxidized phospholipids, 1-palmitoyl-2-(5-oxovaleroyl)-sn-glycero-phosphatidylcholine (POVPC), 1-palmitoyl-2glutaroyl-sn-glycero-phosphatidylcholine (PGPC)
Citation: Li Z, Luo Z, Shi X, Pang B, Ma Y and Jin J (2022) The Levels of Oxidized Phospholipids in High-Density Lipoprotein During the Course of Sepsis and Their Prognostic Value. Front. Immunol. 13:893929. doi: 10.3389/fimmu.2022.893929
Received: 11 March 2022; Accepted: 04 April 2022;
Published: 03 May 2022.
Edited by:
Xiangwei Xiao, University of Pittsburgh, United StatesReviewed by:
Xunde Xian, Peking University, ChinaCopyright © 2022 Li, Luo, Shi, Pang, Ma and Jin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiawei Jin, amlhd2VpamluQGNjbXUuZWR1LmNu; Yingmin Ma, bWEueWluZ21pbkAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.