- 1Department of Ultrasound, The Second Affiliated Hospital of Fujian Medical University, Quanzhou, China
- 2Department of Endocrinology, The Second Affiliated Hospital of Fujian Medical University, Quanzhou, China
- 3Department of Clinical Medicine, Quanzhou Medical College, Quanzhou, China
- 4Department of Ultrasound, Chenggong Hospital, Xiamen University, Xiamen, China
Background: Aspartate aminotransferase-to-platelet ratio index (APRI) and fibrosis-4 index (FIB-4) are the two most widely studied noninvasive markers of liver fibrosis. We aimed to assess the diagnostic accuracy of APRI and FIB-4 for liver fibrosis in patients with autoimmune hepatitis (AIH) using liver biopsy as the reference standard.
Methods: PubMed, EMBASE, Cochrane Library and Web of Science databases were searched for studies (published as of May 1st, 2021) that assessed the diagnostic performance of APRI and FIB-4 for liver fibrosis in AIH. The summary area under receiver operating characteristics curve (AUROC), sensitivity, specificity, diagnostic odds ratios were used to assess the diagnostic accuracy of APRI and FIB-4 for detecting liver fibrosis.
Results: Fourteen studies (including 1015 patients) were selected with 13 studies each evaluating the use of APRI and FIB-4 for detecting different stages of fibrosis in AIH. For prediction of significant fibrosis, advanced fibrosis, and cirrhosis, the summary AUROC value was 0.66 [95% confidence interval (CI): 0.61–0.70], 0.71 (95% CI: 0.67–0.75), and 0.75 (95% CI: 0.71–0.79) for APRI, and the summary AUROC value was 0.75 (95% CI: 0.71–0.79), 0.73 (95% CI: 0.69–0.77) and 0.79 (95% CI: 0.75–0.82) for FIB-4, respectively. The summary sensitivity and specificity for diagnosis of significant fibrosis, advanced fibrosis, and cirrhosis were 90% and 36%, 78% and 55%, and 77% and 61% for APRI, and 70% and 70%, 65% and 70%, and 78% and 65% for FIB-4, respectively.
Conclusions: APRI and FIB-4 showed suboptimal diagnostic performance for identifying liver fibrosis in AIH with mediocre sensitivity and specificity.
Introduction
Autoimmune hepatitis (AIH) is a chronic inflammatory liver disease, which affects all ages, both genders, and all ethnicities (1, 2). The morbidity burden of AIH appears to be increasing across the world (3). In a recent systematic review by Lv et al. (4), the pooled global annual incidence and prevalence of AIH were 1.37 and 17.44 per 100,000 people, respectively. AIH could have a progressive course that gradually develops into cirrhosis, hepatocellular carcinoma, hepatic decompensation, and even death (5–7). In previous studies, approximately 7% of patients with AIH were cirrhotic at the time of diagnosis and these patients showed worse survival outcomes (8, 9). According to the European Association for the Study of the Liver (EASL) guidelines (8), diagnosis of liver fibrosis and cirrhosis is crucial to guide treatment strategies in patients with AIH.
Liver biopsy is considered the “gold standard” for the staging of liver fibrosis; however, it is an invasive procedure with some limitations, such as sampling error, intra- and interobserver variability, and risk of complications (10). Moreover, use of liver biopsy for dynamic evaluation of liver fibrosis stage is unfeasible in clinical practice (11, 12). These factors limit the use of liver biopsy for screening and regular follow-up (13). Therefore, development of alternative, noninvasive methods to stage liver fibrosis in these patients is a key imperative.
In clinical practice, there is a felt-need for a noninvasive method for diagnosis of liver fibrosis that is readily available, low-cost, reliable, and accurate; such a method can also be used for the follow-up monitoring of liver fibrosis (11, 14). Immunosuppressive treatment with corticosteroids alone or in combination with azathioprine is the mainstay of therapy for patients with AIH, which is associated with an excellent prognosis in most cases (15). The presence and extent of fibrosis are associated with the progression of the disease and response to treatment (13); thus, accurate assessment of the degree of liver fibrosis using noninvasive methods is also important for evaluating treatment response of AIH patients during the follow-up.
Currently, serum indices of liver fibrosis based on inexpensive laboratory tests have been developed, including the aspartate aminotransferase to platelet ratio index (APRI) and the fibrosis index based on the four factors (Fibrosis-4 index; FIB-4) (16, 17). In the last two decades, these two serum indices have been extensively studied and their diagnostic value for detecting liver fibrosis assessed in different populations (18–20). Of all the serum noninvasive tests for the evaluation of liver fibrosis, APRI and FIB-4 are the two most widely studied (11, 21).
Several recent meta-analyses have investigated the value of these two serum indices for detection of liver fibrosis. Lin et al. (22) conducted a meta-analysis of studies that investigated the use of APRI for diagnosing liver fibrosis caused by hepatitis C virus (HCV). Xiao et al. (11) conducted a meta-analysis to assess the comparative accuracy of APRI and FIB-4 for the diagnosis of hepatitis B virus-related liver fibrosis. In addition, Xiao et al. (23) recently conducted a meta-analysis to investigate the diagnostic accuracy of APRI and FIB-4 for staging liver fibrosis in patients with nonalcoholic fatty liver disease (NAFLD). Some recent studies that sought to externally validate these two noninvasive models in predicting liver fibrosis in patients with AIH have yielded inconsistent results. To the best of our knowledge, there is just one published meta-analysis that assessed the diagnostic performance of APRI and FIB-4 for the staging of fibrosis in AIH patients (24); however, this study only assessed the accuracy of these two serum indices in predicting advanced fibrosis and cirrhosis. In addition, only a limited number of studies were included in the previous meta-analysis.
Therefore, in the present study, we conducted a systemic review and meta-analysis with the aim to assess the diagnostic accuracy of APRI and FIB-4 for the assessment of liver fibrosis in patients with AIH, using liver biopsy as the reference standard. Moreover, we explored the sources of heterogeneity in results across studies using meta-regression analysis.
Materials and Methods
Search Strategy
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed for reporting this systemic review and meta-analysis (25). An online literature search was conducted on PubMed, EMBASE, the Cochrane Library, and the Web of Science databases for articles published as of May 1st, 2021 using the following keywords: APRI, AST-to-platelet ratio index, AST, platelet, FIB-4, autoimmune hepatitis, liver fibrosis and cirrhosis. In addition, reference lists of the included articles were manually screened to identify other relevant publications.
Study Selection
Studies were included according to the following criteria: (1) studies that assessed the diagnostic value of APRI and/or FIB-4 for the assessment of liver fibrosis in patients with AIH; (2) use of liver biopsy as the reference standard; (3) availability of adequate data to construct at least one 2 × 2 contingency table; and (4) sample size larger than 10 AIH patients, given its prevalence. The exclusion criteria were: (1) review articles, conference abstracts, comments, editorials, case reports, and letters; (2) studies unrelated to the topic; (3) animal or basic research; (4) duplicate publications; and (5) studies not published in English language.
Data Extraction and Quality Assessment
Two investigators (DBT and CYP) independently conducted the literature search, selected the articles for inclusion, and extracted the data. A standardized data extraction format was prepared for this study using Microsoft Excel 2019. Data pertaining to the following variables were extracted from the included studies: number of patients, age, sex, liver biopsy scoring system, number of patients with different stages of liver fibrosis as well as cutoff values, sensitivity, specificity, positive predictive value, negative predictive value and area under the receiver operating characteristic curve (AUROC) values for detecting significant fibrosis, advanced fibrosis, and cirrhosis. In the present study, significant fibrosis, advanced fibrosis, and cirrhosis were defined as stages F2-F4, F3-F4, and F4, respectively, according to the METAVIR or Scheuer scoring system.
The revised Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) tool was used to assess the quality of the studies included in this meta-analysis (26). Study eligibility and quality were independently evaluated by the two researchers (DBT and CYP); discrepancies, if any, were resolved by consensus or by participation of a third investigator (LGR).
Data Synthesis and Analysis
The objective of this meta-analysis was to assess the diagnostic accuracy of APRI and FIB-4 for the assessment of liver fibrosis in patients with AIH. Data extracted from the included studies were used to calculate the summary sensitivity and specificity, positive likelihood ratio (LR), negative LR, diagnostic odds ratios (DORs) and the corresponding 95% confidence intervals (CIs). Using the data, the summary receiver operating characteristic curves of these two serum indices were also constructed, and then the summary AUROC values were obtained. Moreover, the summary sensitivity and specificity and the summary DORs were calculated to further evaluate the accuracy of these two serum indices for predicting liver fibrosis in AIH patients. A random-effect coefficient binary regression model was used to calculate the summary sensitivity and specificity.
All statistical analyses were performed using Stata 15.0 (STATA, College Station, Texas, USA) and Meta-Disc Version 1.4 (Hospital Ramony Cajal, Madrid, Spain).
Assessment of Heterogeneity and Publication Bias
Heterogeneity among the included studies with respect to the diagnostic accuracy of APRI and FIB-4 was assessed using the Cochrane-Q test, and the inconsistency index I2 was also calculated. I2 value > 50% is suggestive of substantial heterogeneity. Moreover, meta-regression and subgroup analyses were conducted to explore the potential sources of heterogeneity using the following covariates: (1) year of publication; (2) region; (3) sample size; (4) median/mean age; and (5) percentage of advanced fibrosis.
To assess the influence of potential publication bias on the results of the meta-analysis, a linear regression test of funnel plot asymmetry using Deeks’ plot was conducted.
Results
Search Results
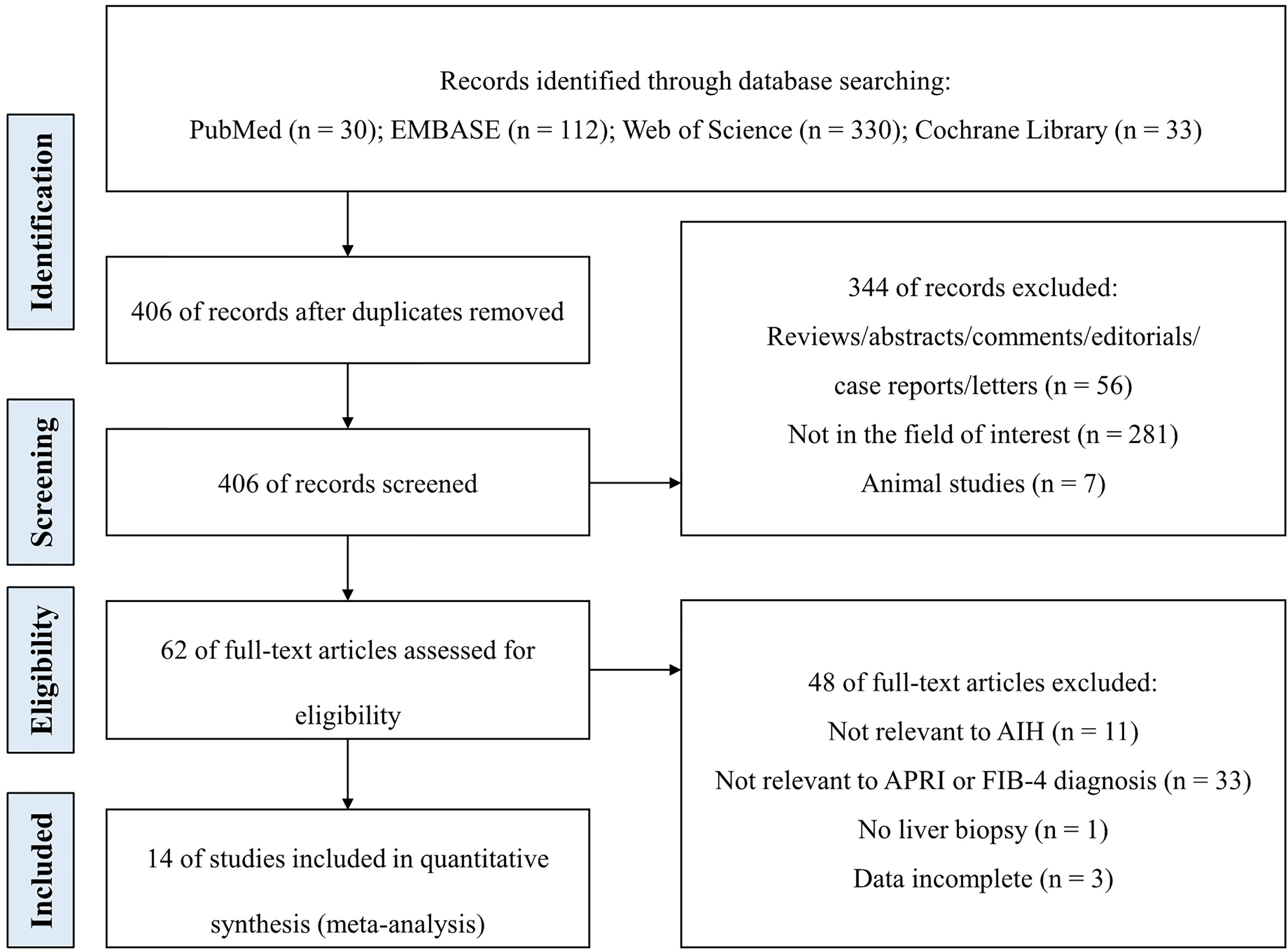
Figure 1 illustrates the study selection process. A total of 505 studies were retrieved on database search using the search strategy. After removal of 99 duplicate publications, titles and abstracts of 406 studies were screened. Of these, 392 studies did not qualify the inclusion criteria. Finally, 14 studies were included in the meta-analysis after full-text review (13, 27–39).
Characteristics of the Included Studies
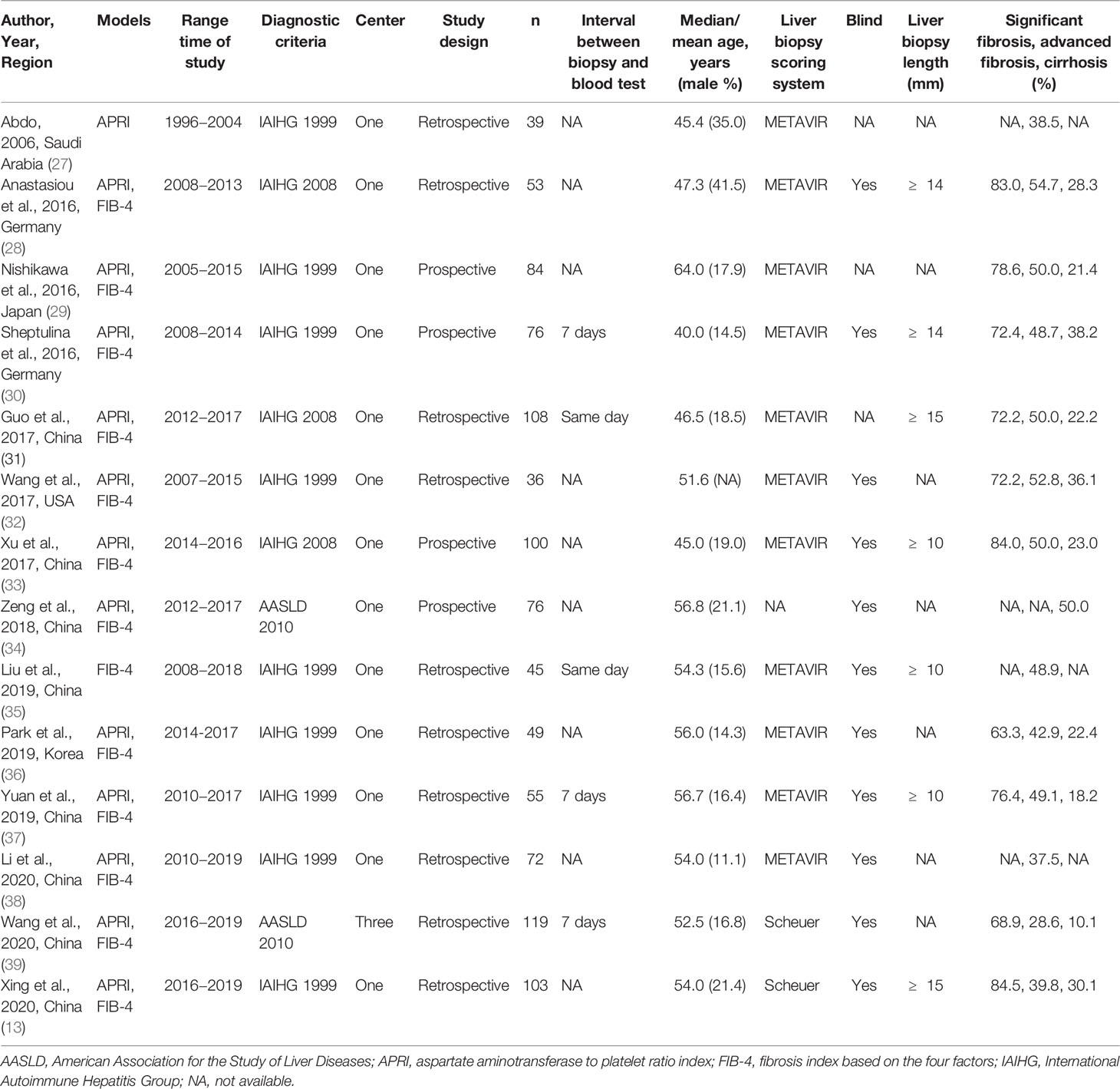
The characteristics of the included studies are summarized in Table 1. In total, thirteen APRI original articles with 2106 AIH patients and thirteen FIB-4 original articles with 2112 AIH patients were selected for evaluation and meta-analysis. The mean age of patients was 51.7 years (range: 40.0–64.0). In 9 (64.3%) studies, the average age of patients with AIH was above fifty years. Male patients accounted for approximately 20.2% (range: 11.1%–41.5%) of all patients. It is worth mentioning that the average proportion of males in the individual studies was much lower than that of females. A total of 7 (50.0%) studies were published between 2018 and 2020, 6 (42.9%) studies were published between 2016 and 2017, and 1 (7.1%) study was published in 2006. Most of the included studies were conducted in Asia (8 in China, 1 in Saudi Arabia, 1 in Japan, and 1 in Korea); 2 studies were conducted in Germany and 1 study was conducted in the USA. With the exception of 1 (7.1%) study that included subjects from three centers, all other studies were single-center studies. Among the included studies, 4 (28.6%) studies were prospective, and 10 (71.4%) studies were retrospective. Eleven studies used the METAVIR score, and 2 of the remaining 3 studies used the Scheuer score to determine the stages of liver fibrosis in AIH. The articles included in the meta-analysis were published in twelve different Science Citation Index journals; the mean impact factor of these journals was 3.160 (range: 0.660–5.742).
The prevalence of liver fibrosis in patients with AIH across studies is shown in Table 1, and Figure 2 displays the distribution of liver fibrosis stages in each included study as well as the region-wise overall prevalence of fibrosis stages. The total prevalence of significant fibrosis, advanced fibrosis, and cirrhosis was 75.6% (range: 63.3%–84.5%), 45.5% (range: 28.6%–54.7%), and 27.3% (range: 10.1%–50.0%).
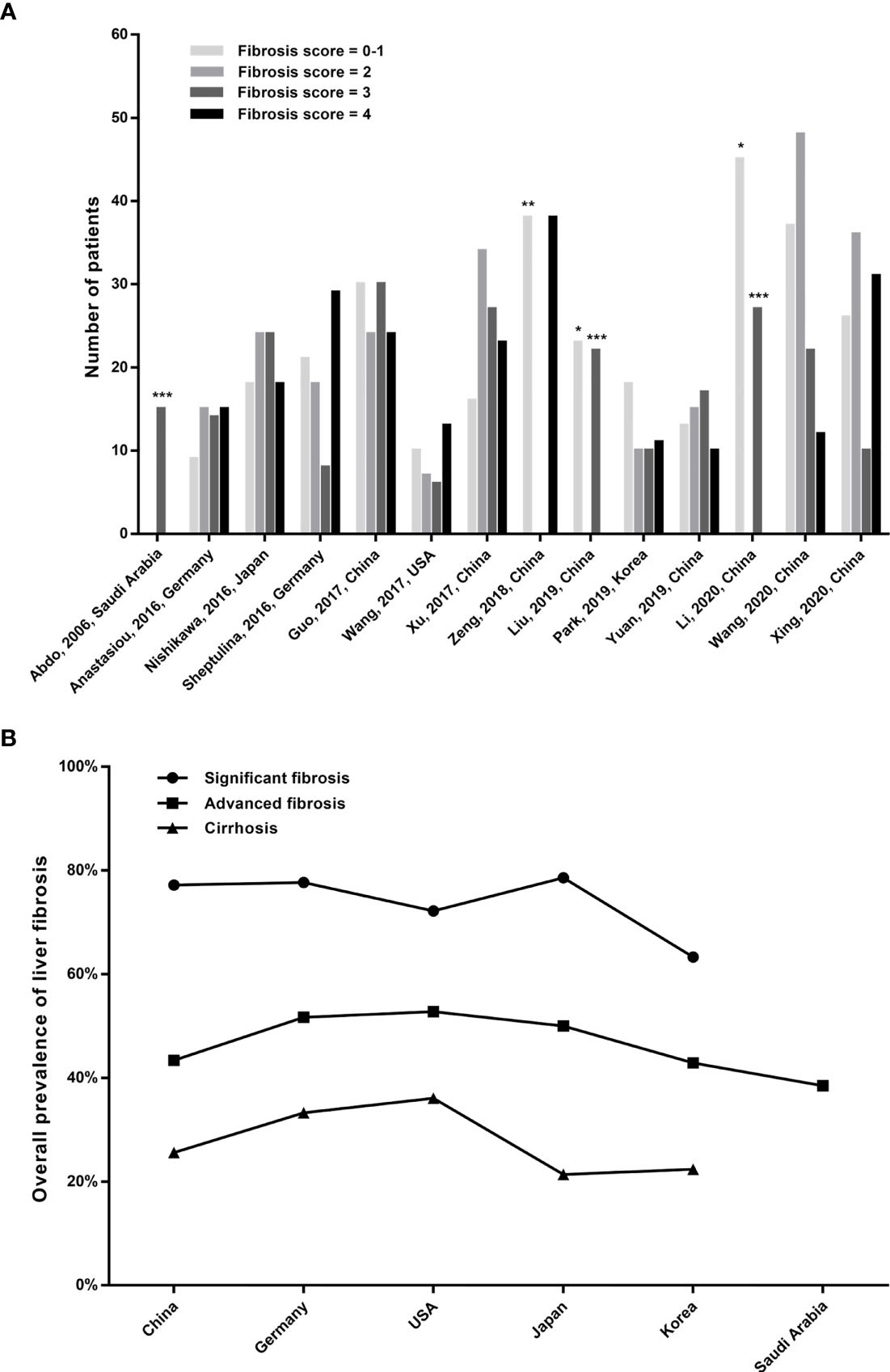
Figure 2 (A) Distribution of liver fibrosis stages in AIH patients of the included studies. *The columns represent fibrosis stage of 0-2; **the column represents fibrosis stage of 0-3; and ***the columns represent fibrosis stage of 3-4. (B) The overall prevalence of fibrosis stages between the included regions.
Diagnostic Accuracy for Significant Fibrosis
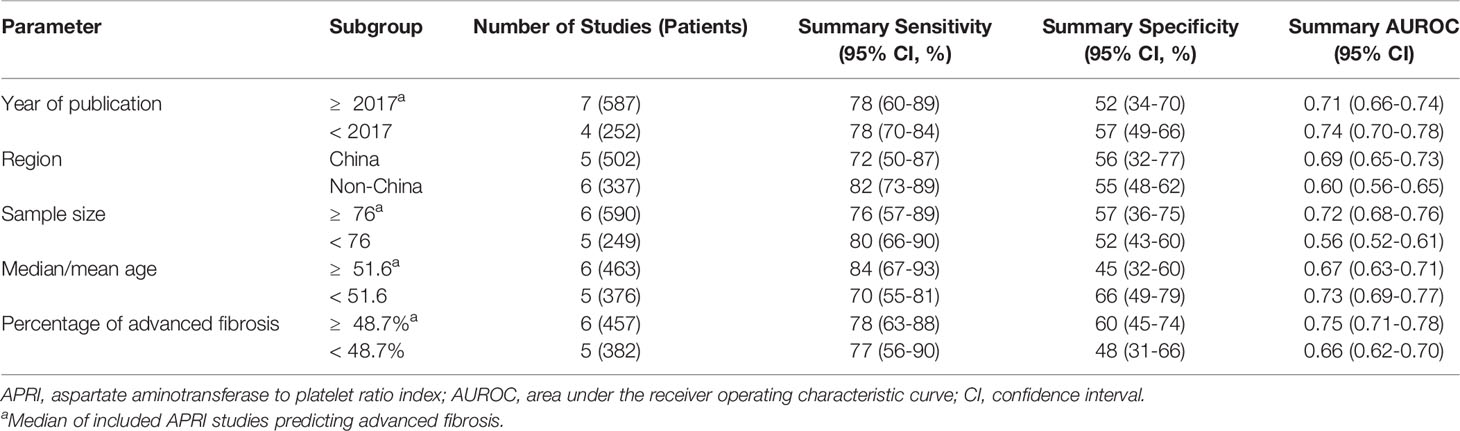
A total of six studies (508 patients) investigated the diagnostic performance of APRI and FIB-4 for the detection of significant fibrosis (Table 2). The mean AUROC values of APRI and FIB-4 for diagnosing significant fibrosis were 0.580 (range: 0.499–0.635) and 0.662 (range: 0.560–0.750), respectively. Results were then combined, the summary AUROC value of FIB-4 for diagnosing significant fibrosis was 0.75 (95% CI: 0.71–0.79), while the summary AUROC value of APRI was 0.66 (95% CI: 0.61–0.70) (Table 3). APRI showed a good summary sensitivity (90%), but had a poor summary specificity (36%). The summary sensitivity and specificity of FIB-4 for predicting significant fibrosis in AIH patients were both 70%. Moreover, the summary DORs of APRI and FIB-4 for diagnosing significant fibrosis were 5 (95% CI: 2–10) and 5 (95% CI: 3–8), respectively. Notably, the cutoff values varied among the included studies. A total of five studies had simultaneously reported the cutoff values of APRI and FIB-4 for the diagnosis of significant fibrosis. The average cutoff values of APRI and FIB-4 for predicting significant fibrosis were 0.89 (range: 0.27–1.55) and 2.90 (range: 1.28–5.07), respectively.
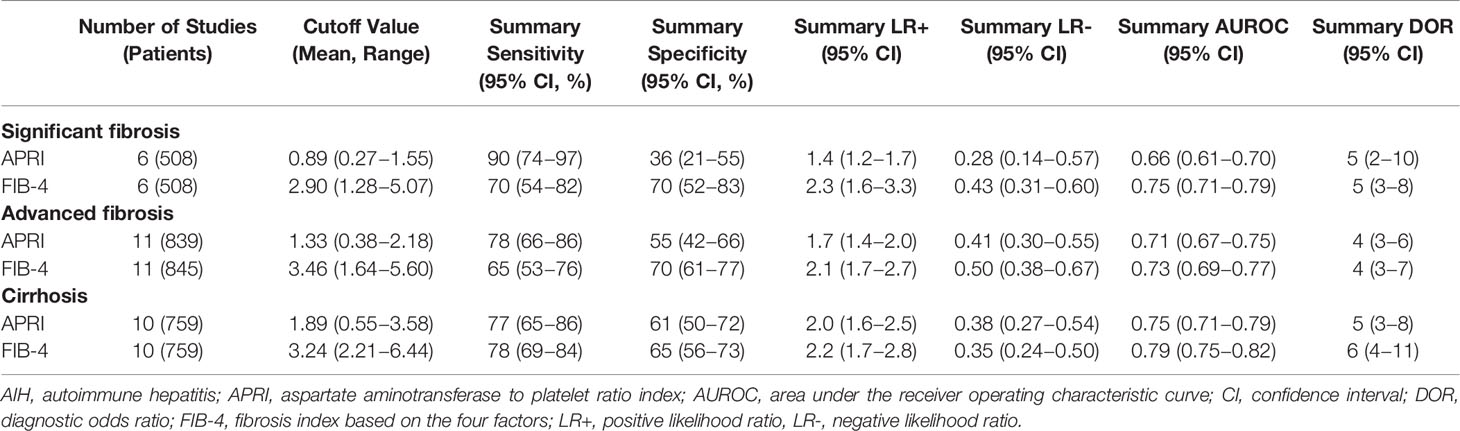
Table 3 Meta-analysis results of APRI and FIB-4 for prediction of significant fibrosis, advanced fibrosis and cirrhosis in AIH.
Diagnostic Accuracy for Advanced Fibrosis
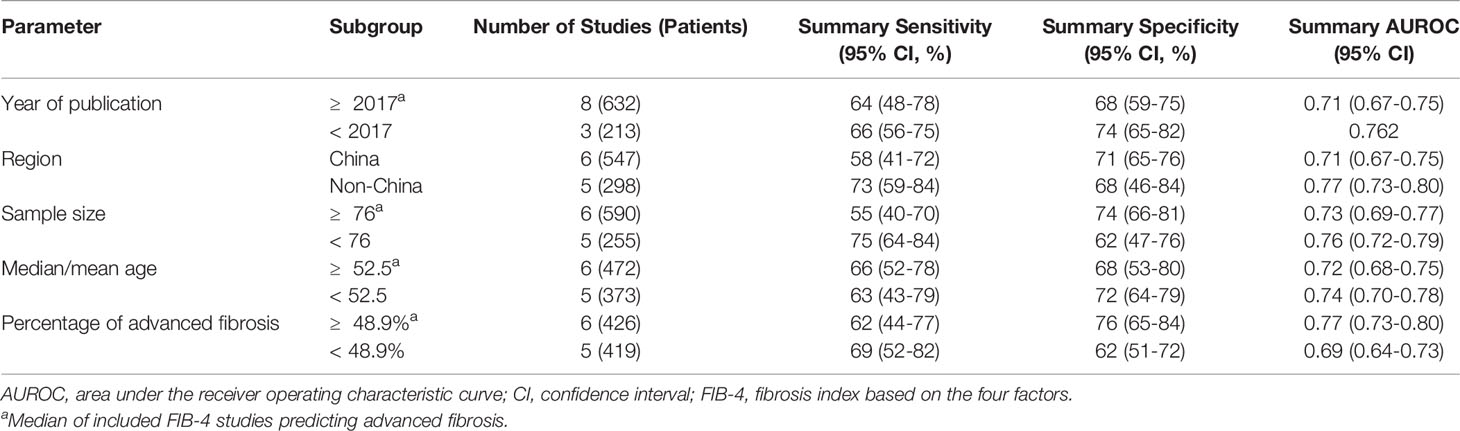
Twelve studies (with 884 AIH patients) examined the ability of these two serum indices in predicting advanced fibrosis. Among these studies, 11 (839 patients) and 11 (845 patients) studies investigated the diagnostic performance of APRI and FIB-4 for advanced fibrosis. The summary sensitivity of APRI for detecting advanced fibrosis exceeded 75%; however, the summary specificity of APRI was mediocre (Figure 3). The summary sensitivity and specificity of FIB-4 for detecting advanced fibrosis were 65% and 70%, respectively. The summary specificity of FIB-4 in predicting advanced fibrosis was greater than that of APRI (70% vs. 55%), but the summary sensitivity was lower than that of APRI (65% vs. 78%). The summary AUROC values of APRI and FIB-4 for diagnosing advanced fibrosis were 0.71 (95% CI: 0.67–0.75) and 0.73 (95% CI: 0.69–0.77), respectively (Figure 4). The mean AUROC values of APRI and FIB-4 for diagnosing advanced fibrosis were 0.619 (range: 0.434–0.728) and 0.690 (range: 0.522–0.793), respectively. The summary DORs of APRI and FIB-4 for predicting advanced fibrosis were 4 (95% CI: 3–6) and 4 (95% CI: 3–7), respectively (Table 3).
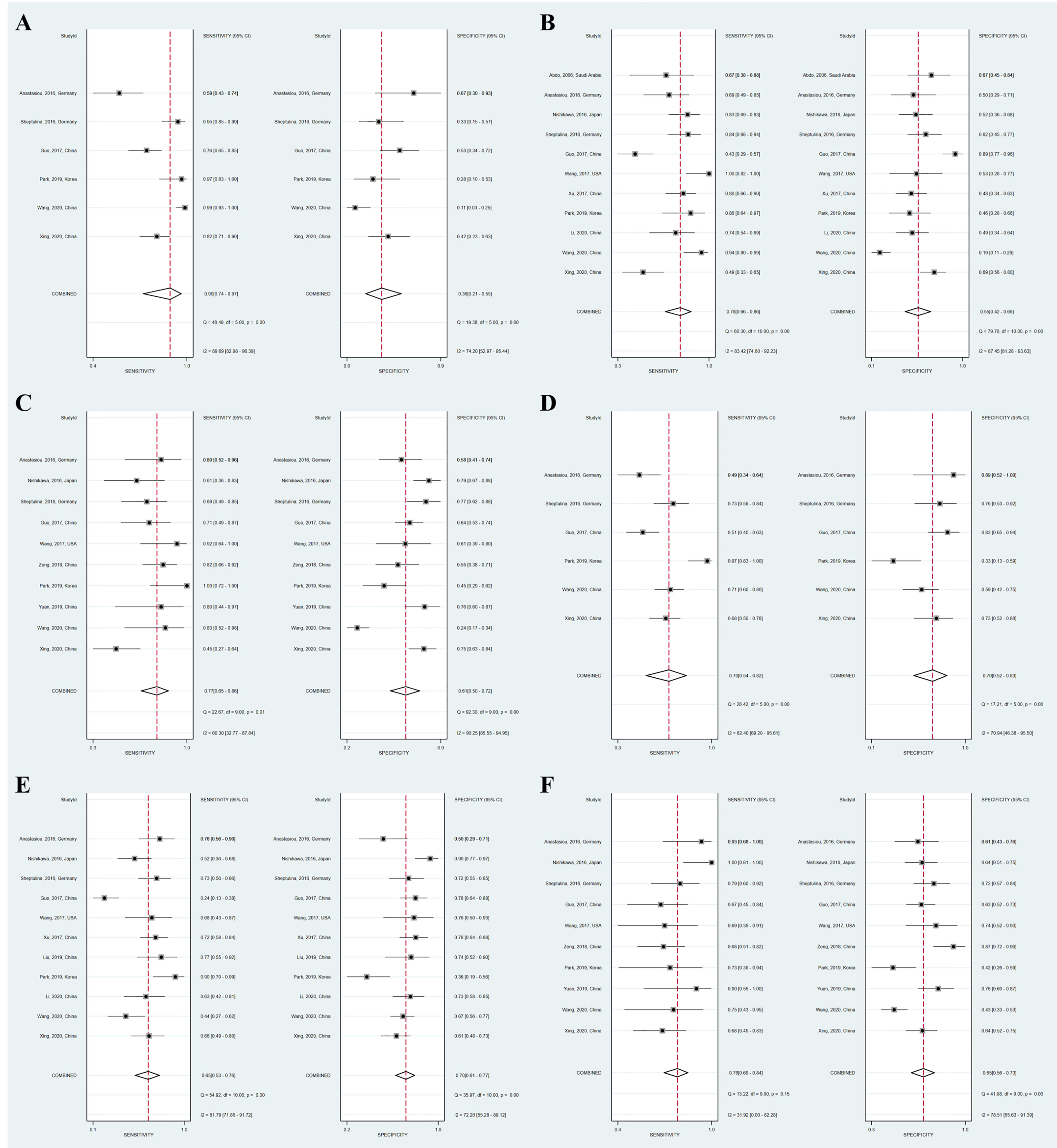
Figure 3 Coupled forest plots of the summary sensitivity and specificity of APRI and FIB-4 for the prediction of liver fibrosis in AIH patients. (A) APRI for detecting significant fibrosis; (B) APRI for detecting advanced fibrosis; (C) APRI for detecting cirrhosis; (D) FIB-4 for detecting significant fibrosis; (E) FIB-4 for detecting advanced fibrosis; (F) FIB-4 for detecting cirrhosis.
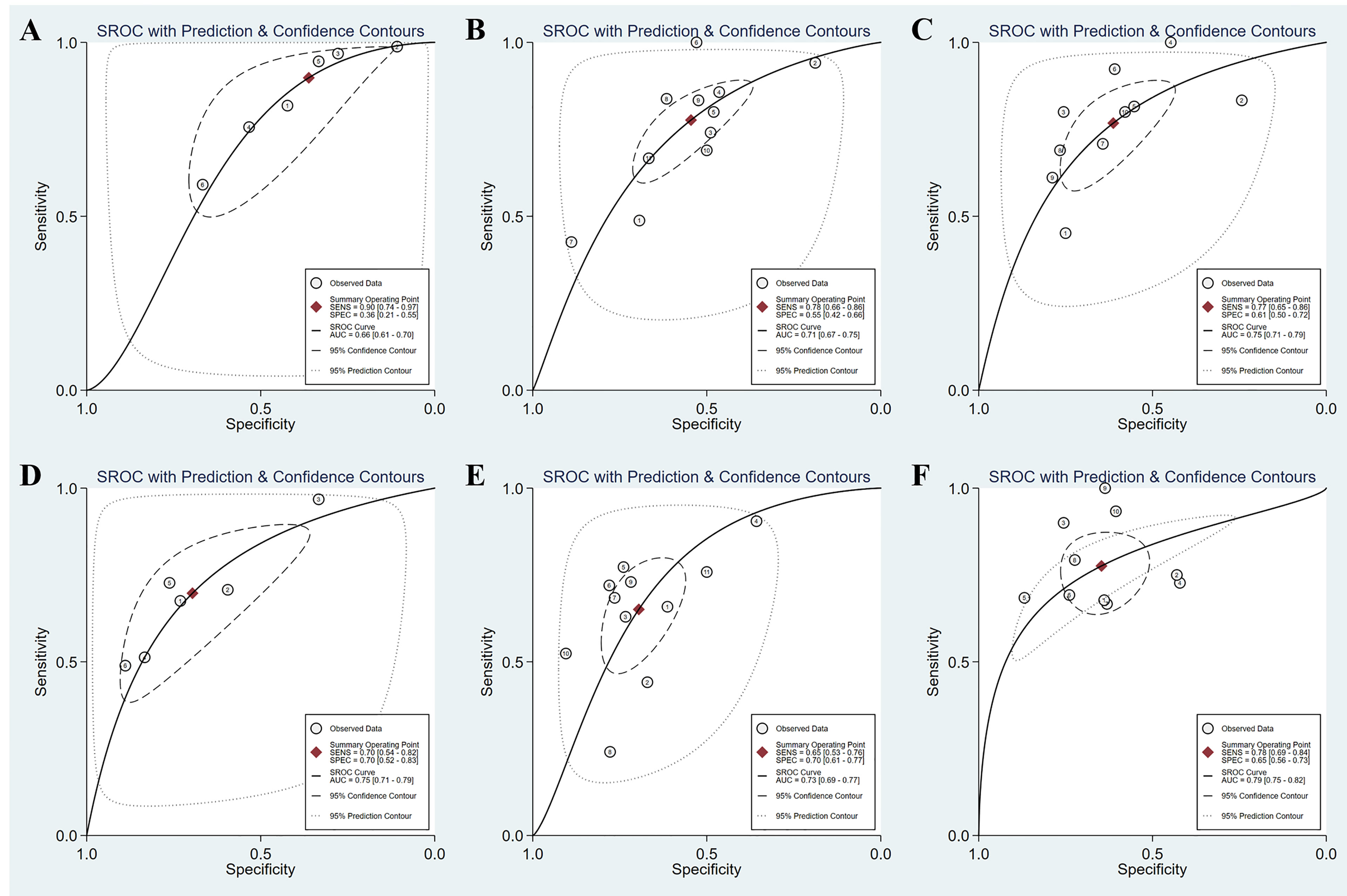
Figure 4 SROC curves of the diagnostic performance of APRI and FIB-4 for the prediction of liver fibrosis in AIH patients. (A) APRI for detecting significant fibrosis; (B) APRI for detecting advanced fibrosis; (C) APRI for detecting cirrhosis; (D) FIB-4 for detecting significant fibrosis; (E) FIB-4 for detecting advanced fibrosis; (F) FIB-4 for detecting cirrhosis.
Diagnostic Accuracy for Cirrhosis
Ten (759 patients) studies had also examined the diagnostic performance of APRI and FIB-4 for predicting cirrhosis in AIH. The summary sensitivities of APRI and FIB-4 for detecting cirrhosis were 77% (95% CI: 65%–86%) and 78% (95% CI: 69%–84%), respectively (Table 3). The summary specificities of APRI and FIB-4 for detecting cirrhosis were 61% (95% CI: 50%–72%) and 65% (95% CI: 56%–73%), respectively. Moreover, the summary AUROC values of APRI and FIB-4 for the diagnosis of cirrhosis were 0.75 (95% CI: 0.71–0.79) and 0.79 (95% CI: 0.75–0.82), respectively. We found that both APRI and FIB-4 had greater summary AUROC values in detecting cirrhosis than detecting significant fibrosis and advanced fibrosis. The mean AUROC values of APRI and FIB-4 for diagnosing cirrhosis were 0.644 (range: 0.380–0.798) and 0.732 (range: 0.535−0.881), respectively (Table 2). In addition, the summary DORs of APRI and FIB-4 were 5 (95% CI: 3–8) and 6 (95% CI: 4–11), respectively. The summary positive LR values of APRI and FIB-4 for the diagnosis of cirrhosis were 2.0 (95% CI: 1.6–2.5) and 2.2 (95% CI: 1.7–2.8), respectively.
Heterogeneity and Publication Bias
We observed substantial heterogeneity in several groups (Figure 3). Substantial heterogeneity was observed with respect to the summary sensitivity (I2 = 89.69%, 83.42%, and 60.30%) and summary specificity (I2 = 74.20%, 87.45%, and 90.25%) of APRI for detecting significant fibrosis, advanced fibrosis, and cirrhosis, respectively. Similarly, substantial heterogeneity was observed with respect to the summary sensitivity (I2 = 82.40% and 81.79%) and specificity (I2 = 70.94% and 72.20%) of FIB-4 for detecting significant fibrosis and advanced fibrosis, respectively. Substantial heterogeneity was also observed with regard to the summary specificity (I2 = 78.51%), but not with regard to the summary sensitivity, when FIB-4 was used to detect cirrhosis. The conduct of meta-regression analyses is limited by the number of studies. In groups of larger than ten studies, methodological heterogeneity can be examined. The accuracy of APRI for the diagnosis of advanced fibrosis was not affected by the median/mean age [≥ 51.6 (median of all included studies) vs. < 51.6] (P = 0.83), sample size [≥ 76 (median of all included studies) vs. < 76] (P = 0.49), year of publication [≥ 2017 (median of all included studies) vs. < 2017] (P = 0.30), region (China vs. Non-China) (P = 0.50), or percentage of advanced fibrosis [≥ 48.7% (median of all included studies) vs. < 48.7%] (P = 0.18). Moreover, the diagnostic accuracy of FIB-4 for advanced fibrosis was not affected by the following factors: sample size [≥ 76 (median of all included studies) vs. < 76] (P = 0.91), year of publication [≥ 2017 (median of all included studies) vs. < 2017] (P = 0.83), median/mean age [≥ 52.5 (median of all included studies] vs. < 52.5] (P = 0.50), percentage of advanced fibrosis [≥ 48.9% (median of all included studies) vs. < 48.9%] (P = 0.49), or region (China vs. Non-China) (P = 0.35). Results of subgroup analyses of APRI and FIB-4 for the diagnosis of advanced fibrosis are shown in Tables 4, 5.
Figure 5 illustrates the Deeks’ funnel plots of these two serum indices. We found no significant effect of publication bias on the meta-analysis of the diagnostic value of APRI for detecting advanced fibrosis and cirrhosis (P = 0.28 and 0.09). Similarly, no significant effect of publication bias was observed on the meta-analysis of the value of FIB-4 in detecting advanced fibrosis and cirrhosis (P = 0.22 and 0.89). Owing to the small number of included studies (i.e., < 10 studies), we did not further assess the effect of potential publication bias on the diagnostic value of APRI and FIB-4 for detecting significant fibrosis.
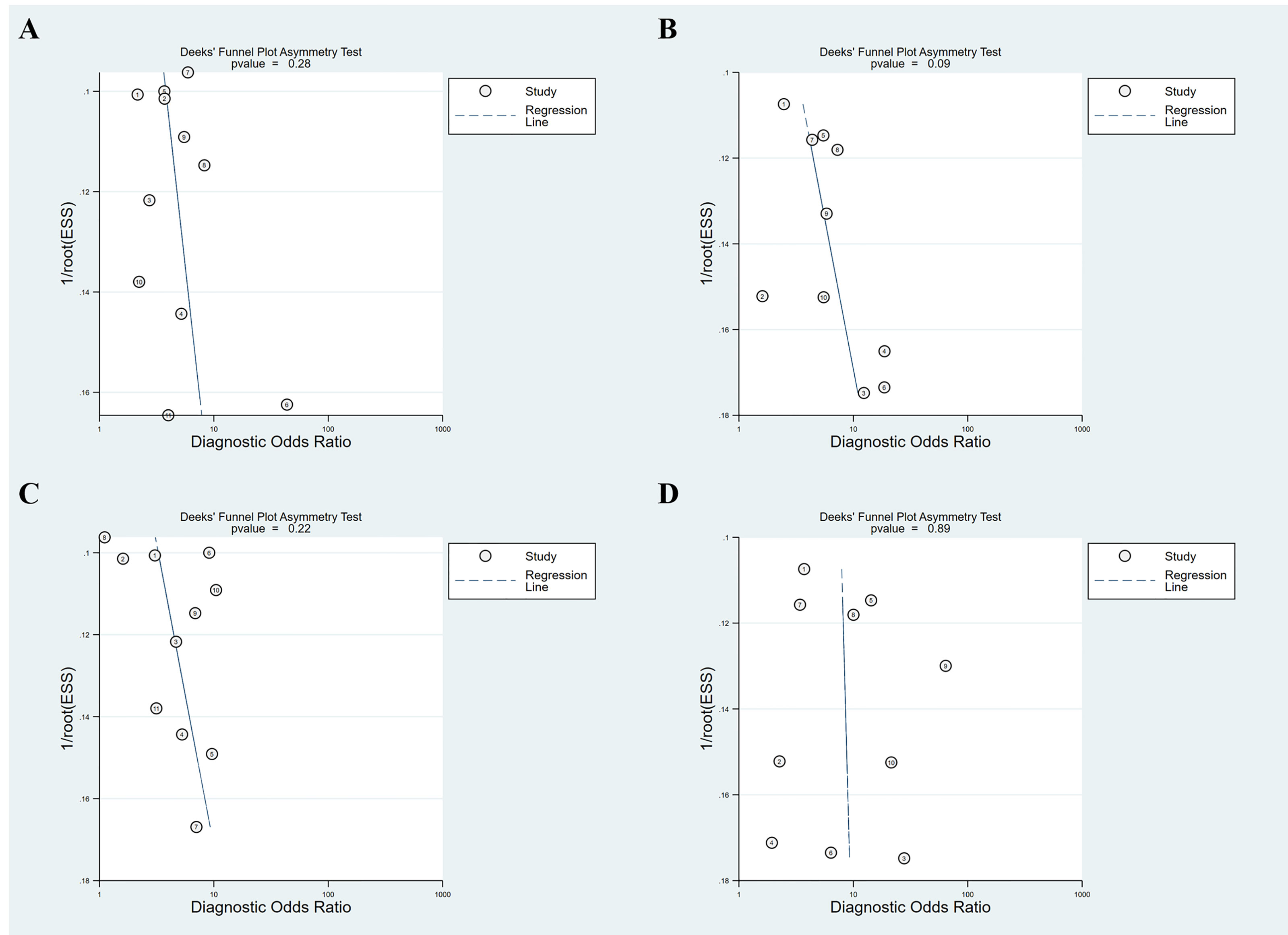
Figure 5 Deeks’ funnel plot asymmetry test for publication bias. (A) APRI for detecting advanced fibrosis; (B) APRI for detecting cirrhosis; (C) FIB-4 for detecting advanced fibrosis; (D) FIB-4 for detecting cirrhosis.
Discussion
In this study, we performed a meta-analysis of available data pertaining to the diagnostic accuracy of APRI and FIB-4 for detecting different stages of liver fibrosis in AIH patients. Finally, a total of 13 APRI original articles with 2106 AIH patients and 13 FIB-4 original articles with 2112 AIH patients were systematic reviewed. Our results suggest a suboptimal diagnostic performance of APRI and FIB-4 for identifying significant fibrosis, advanced fibrosis, and cirrhosis in patients with AIH, with mediocre sensitivity and specificity.
Accurate diagnosis of liver fibrosis stage, particularly by noninvasive methods, plays a critical role in the assessment of disease progression in patients with AIH (24). Until now, there is no clear consensus about the diagnostic value of APRI and FIB-4 in predicting liver fibrosis in patients with AIH. A systematic review by Wu et al. (24) investigated the diagnostic performance of several noninvasive methods (including imaging techniques and serum indices) for evaluating liver fibrosis in patients with AIH. The reported summary AUROC of APRI and FIB-4 were 0.74 and 0.76 for the diagnosis of advanced fibrosis; 0.75 and 0.66 for the diagnosis of cirrhosis, respectively. The summary AUROC values of APRI and FIB-4 for the diagnosis of advanced fibrosis in that study were similar to our results; however, the summary AUROC value of FIB-4 for the diagnosis of cirrhosis in the previous study was significantly lower. This may be related to the inclusion of fewer studies with regard to the APRI (n = 8) and FIB-4 (n = 6) in the study by Wu et al. Moreover, Wu et al. did not report the summary AUROC values of APRI and FIB-4 for diagnosing significant fibrosis. In contrast, a total of 13 APRI original articles and 13 FIB-4 original articles were included in our study. Therefore, our study provides a more comprehensive analysis of the summary diagnostic accuracy of APRI and FIB-4 for detecting significant fibrosis, advanced fibrosis, and cirrhosis in AIH.
In the prediction of significant fibrosis, our results showed that FIB-4 had a summary sensitivity and specificity of 70% (95% CI: 54%−82%) and 70% (95% CI: 52%−83%), respectively, and a summary AUROC of 0.75 (95% CI: 0.71−0.79), while for APRI, the summary sensitivity was 90% (95% CI: 74%−97%), the summary specificity was 36% (95% CI: 21%−55%), and the summary AUROC was 0.66 (95% CI: 0.61−0.70). We found that the summary AUROC of FIB-4 for predicting significant fibrosis was higher than that of APRI. Moreover, APRI displayed a good summary sensitivity for diagnosis of significant fibrosis, but had a poor summary specificity. The summary AUROC values of FIB-4 in our study were slightly higher than that of APRI for diagnosing advanced fibrosis and cirrhosis in AIH patients (advanced fibrosis: 0.73 vs. 0.71; cirrhosis: 0.79 vs. 0.75). This suggests that FIB-4 may be a better than APRI for detecting liver fibrosis in AIH patients; however, APRI seems to be less accurate for the assessment of significant fibrosis, advanced fibrosis, and cirrhosis in AIH. In a recent multicenter prospective study by Duan et al. (19), the AUROC values of APRI and FIB-4 were 0.665 and 0.674 for diagnosing significant fibrosis, 0.670 and 0.671 for diagnosing advanced fibrosis, and 0.616 and 0.631 for diagnosing cirrhosis, in patients with chronic hepatitis B (CHB). The AUROC values of APRI and FIB-4 for the diagnosis of liver fibrosis in CHB in that study were comparable, but both were relatively lower than our present results in AIH. In the study by Wai et al. (16), the AUROC values of APRI for detecting significant fibrosis and cirrhosis in patients with chronic hepatitis C (CHC) were high (training cohort: 0.80 and 0.89; validation cohort: 0.88 and 0.94). Subsequently, two systematic reviews (22, 40) investigated the performance of APRI in predicting liver fibrosis, mainly in HCV patients. The results of previous studies demonstrated that APRI may not have great diagnostic value as initially described in HCV-infected or HCV and human immunodeficiency virus (HIV) coinfected patients. Given the prevalence of AIH, the number of original articles published on APRI and FIB-4 for evaluating different stages of liver fibrosis is limited. Thus, large scale and multicenter studies are required to further assess the comparative accuracy of APRI and FIB-4 for detecting significant fibrosis, advanced fibrosis, and cirrhosis in AIH.
Since APRI and FIB-4 were proposed as markers of liver fibrosis, they have received considerable attention for the detection of liver fibrosis induced by various causes (11). To date, several meta-analyses have investigated the diagnostic accuracy of APRI and/or FIB-4 for detecting liver fibrosis in different populations such as patients with chronic viral hepatitis and NAFLD. Of interest, in the meta-analysis by Xu et al. (21), the summary AUROC values of APRI and FIB-4 for detecting hepatitis B-related significant fibrosis were both 0.75. Similarly, in our previous study, the summary AUROC values of APRI and FIB-4 were 0.76 and 0.75, respectively, for prediction of significant fibrosis, 0.74 and 0.77, respectively, for prediction of advanced fibrosis, and 0.77 and 0.82, respectively, for prediction of cirrhosis (41). In the study by Lin et al. (22), the summary AUROC values for APRI for predicting significant fibrosis, advanced fibrosis, and cirrhosis in CHC patients were 0.77, 0.80, and 0.83, respectively. Moreover, a meta-analysis conducted by Xiao et al. (23) investigated the accuracy of these two serum indices in NAFLD patients; the reported summary AUROC values of APRI and FIB-4 were 0.76 and 0.73 for detecting significant fibrosis; 0.77 and 0.84 for detecting advanced fibrosis, respectively. In addition, APRI had a summary AUROC value of 0.76 for detecting cirrhosis. These two serum indices displayed reliable accuracy for assessing cirrhosis or advanced fibrosis in patients with chronic viral hepatitis or NAFLD.
Overall, APRI and FIB-4 did not show a high predictive accuracy for liver fibrosis in patients with AIH. In the present study, we did not assess the treatment history of the included AIH patients. Azathioprine, one of the main drugs for AIH treatment, can cause bone marrow suppression leading to thrombocytopenia (42). Both APRI and FIB-4 include platelet count. Therefore, treatment with azathioprine may have contributed to the suboptimal accuracy of these two serum indices in staging liver fibrosis in patients with AIH. Additionally, although liver biopsy is the gold standard for identifying the histological stage, potential errors in the fibrosis staging of the liver biopsies due to collapse/bridging necrosis may also have influenced the diagnostic accuracy of these tests in patients with an acute presentation of AIH.
Nevertheless, as the two most widely studied serum indices for evaluating liver fibrosis, APRI and FIB-4 offer many advantages, including wide availability, no extra costs, good reproducibility, and high applicability (43, 44). Furthermore, these two common serum indices are simple to use, and no particular expertise is required in their interpretation (45). APRI and FIB-4 tests can also be performed in an outpatient setting (45). Therefore, APRI and FIB-4 still can be considered as a choice for the diagnosis of liver fibrosis in AIH patients, especially in resource-constrained settings. For example, APRI and FIB-4 were recommended by the World Health Organization (WHO) as the preferred noninvasive tests for detection of liver fibrosis in resource-constrained settings (46). Moreover, the components of APRI and FIB-4 are routine biochemical or hematological tests that are readily available, so that these two serum indices can also be used in epidemiological research (47).
Several limitations of our meta-analysis should be acknowledged. First, only original articles published in English language were included in the meta-analysis, which may have introduced an element of bias. Second, we did not evaluate the treatment history of AIH patients. The severity of liver fibrosis may be impacted by this factor. Third, there was considerable variability among the included studies with respect to the reported cutoff values, which is likely attributable to differences in the study population as well as the variable distribution of severity. Thus, more studies are needed. Fourth, of the fourteen included studies, eight were from China, so the generalization of the present meta-analysis findings may be relatively limited. Moreover, due to the different genetic backgrounds of AIH patients with different geographic locations (48, 49), it is of great importance to investigate the performance of APRI and FIB-4 for diagnosis of liver fibrosis in patients with AIH in multicenter studies. Therefore, multicenter prospective studies are still warranted in the future. Finally, there are some intrinsic limitations in our meta-analysis, including the possibility of heterogeneity and inability to identify optimal thresholds.
In conclusion, our meta-analysis demonstrated suboptimal diagnostic performance of APRI and FIB-4 for identifying liver fibrosis in AIH patients, with mediocre sensitivity and specificity. Despite the suboptimal diagnostic accuracy of these two serum indices, their convenience of use, low cost and wide availability make them an important option for evaluation of liver fibrosis in AIH patients, especially in resource-constrained settings. Moreover, APRI and FIB-4 can also be used in epidemiological research, considering that the component parameters used are readily available. Future studies should explore novel noninvasive methods (e.g., ultrasound elastography and magnetic resonance elastography) with improved diagnostic accuracy for liver fibrosis in patients with AIH.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author Contributions
Search of the literature and data extraction BD and CY. Drafted the manuscript BD. Performed the statistical analysis BD, CY and XY. Conceived of and supervised the study GL. All the authors edited the manuscript and provided intellectual input.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Komori A. Recent Updates on the Management of Autoimmune Hepatitis. Clin Mol Hepatol (2021) 27(1):58–69. doi: 10.3350/cmh.2020.0189
2. Mack CL, Adams D, Assis DN, Kerkar N, Manns MP, Mayo MJ, et al. Diagnosis and Management of Autoimmune Hepatitis in Adults and Children: 2019 Practice Guidance and Guidelines From the American Association for the Study of Liver Diseases. Hepatology (2020) 72(2):671–722. doi: 10.1002/hep.31065
4. Lv T, Li M, Zeng N, Zhang J, Li S, Chen S, et al. Systematic Review and Meta-Analysis on the Incidence and Prevalence of Autoimmune Hepatitis in Asian, European, and American Population. J Gastroenterol Hepatol (2019) 34(10):1676–84. doi: 10.1111/jgh.14746
5. Czaja AJ, Carpenter HA. Progressive Fibrosis During Corticosteroid Therapy of Autoimmune Hepatitis. Hepatology (2004) 39(6):1631–8. doi: 10.1002/hep.20235
6. Carbone M, Neuberger JM. Autoimmune Liver Disease, Autoimmunity and Liver Transplantation. J Hepatol (2014) 60(1):210–23. doi: 10.1016/j.jhep.2013.09.020
7. Granito A, Muratori L, Lalanne C, Quarneti C, Ferri S, Guidi M, et al. Hepatocellular Carcinoma in Viral and Autoimmune Liver Diseases: Role of CD4+ CD25+ Foxp3+ Regulatory T Cells in the Immune Microenvironment. World J Gastroenterol (2021) 27(22):2994–3009. doi: 10.3748/wjg.v27.i22.2994
8. European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Autoimmune Hepatitis. J Hepatol (2015) 63(4):971–1004. doi: 10.1016/j.jhep.2015.06.030
9. Migita K, Watanabe Y, Jiuchi Y, Nakamura Y, Saito A, Yagura M, et al. Evaluation of Risk Factors for the Development of Cirrhosis in Autoimmune Hepatitis: Japanese NHO-AIH Prospective Study. J Gastroenterol (2011) 46 Suppl 1:56–62. doi: 10.1007/s00535-010-0337-y
10. Chinese Society of Hepatology, Chinese Medical Association; Chinese Society of Gastroenterology, Chinese Medical Association; Chinese Society of Infectious Diseases, Chinese Medical Association. Consensus on the Diagnosis and Treatment of Hepatic Fibrosis (2019). J Dig Dis (2020) 21(3):127–38. doi: 10.1111/1751-2980.12854
11. Xiao G, Yang J, Yan L. Comparison of Diagnostic Accuracy of Aspartate Aminotransferase to Platelet Ratio Index and Fibrosis-4 Index for Detecting Liver Fibrosis in Adult Patients With Chronic Hepatitis B Virus Infection: A Systemic Review and Meta-Analysis. Hepatology (2015) 61(1):292–302. doi: 10.1002/hep.27382
12. Janik MK, Kruk B, Szczepankiewicz B, Kostrzewa K, Raszeja-Wyszomirska J, Górnicka B, et al. Measurement of Liver and Spleen Stiffness as Complementary Methods for Assessment of Liver Fibrosis in Autoimmune Hepatitis. Liver Int (2021) 41(2):348–56. doi: 10.1111/liv.14726
13. Xing X, Yan Y, Shen Y, Xue M, Wang X, Luo X, et al. Liver Fibrosis With Two-Dimensional Shear-Wave Elastography in Patients With Autoimmune Hepatitis. Expert Rev Gastroenterol Hepatol (2020) 14(7):631–8. doi: 10.1080/17474124.2020.1779589
14. Li Y, Huang YS, Wang ZZ, Yang ZR, Sun F, Zhan SY, et al. Systematic Review With Meta-Analysis: The Diagnostic Accuracy of Transient Elastography for the Staging of Liver Fibrosis in Patients With Chronic Hepatitis B. Aliment Pharmacol Ther (2016) 43(4):458–69. doi: 10.1111/apt.13488
15. Granito A, Muratori P, Ferri S, Pappas G, Quarneti C, Lenzi M, et al. Diagnosis and Therapy of Autoimmune Hepatitis. Mini Rev Med Chem (2009) 9(7):847–60. doi: 10.2174/138955709788452676
16. Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, et al. A Simple Noninvasive Index Can Predict Both Significant Fibrosis and Cirrhosis in Patients With Chronic Hepatitis C. Hepatology (2003) 38(2):518–26. doi: 10.1053/jhep.2003.50346
17. Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, et al. Development of a Simple Noninvasive Index to Predict Significant Fibrosis in Patients With HIV/HCV Coinfection. Hepatology (2006) 43(6):1317–25. doi: 10.1002/hep.21178
18. Dong M, Wu J, Yu X, Li J, Yang S, Qi X, et al. Validation and Comparison of Seventeen Noninvasive Models for Evaluating Liver Fibrosis in Chinese Hepatitis B Patients. Liver Int (2018) 38(9):1562–70. doi: 10.1111/liv.13688
19. Duan WJ, Wang XZ, Ma AL, Shang J, Nan YM, Gao ZL, et al. Multicenter Prospective Study to Validate a New Transient Elastography Device for Staging Liver Fibrosis in Patients With Chronic Hepatitis B. J Dig Dis (2020) 21(9):519–25. doi: 10.1111/1751-2980.12924
20. Yunihastuti E, Wicaksana B, Wiraguna A, Hidayah AJ, Amelia F, Natali V, et al. Diagnostic Performance of APRI and FIB-4 for Confirming Cirrhosis in Indonesian HIV/HCV Co-Infected Patients. BMC Infect Dis (2020) 20(1):372. doi: 10.1186/s12879-020-05069-5
21. Xu XY, Wang WS, Zhang QM, Li JL, Sun JB, Qin TT, et al. Performance of Common Imaging Techniques vs Serum Biomarkers in Assessing Fibrosis in Patients With Chronic Hepatitis B: A Systematic Review and Meta-Analysis. World J Clin Cases (2019) 7(15):2022–37. doi: 10.12998/wjcc.v7.i15.2022
22. Lin ZH, Xin YN, Dong QJ, Wang Q, Jiang XJ, Zhan SH, et al. Performance of the Aspartate Aminotransferase-to-Platelet Ratio Index for the Staging of Hepatitis C-Related Fibrosis: An Updated Meta-Analysis. Hepatology (2011) 53(3):726–36. doi: 10.1002/hep.24105
23. Xiao G, Zhu S, Xiao X, Yan L, Yang J, Wu G. Comparison of Laboratory Tests, Ultrasound, or Magnetic Resonance Elastography to Detect Fibrosis in Patients With Nonalcoholic Fatty Liver Disease: A Meta-Analysis. Hepatology (2017) 66(5):1486–501. doi: 10.1002/hep.29302
24. Wu S, Yang Z, Zhou J, Zeng N, He Z, Zhan S, et al. Systematic Review: Diagnostic Accuracy of non-Invasive Tests for Staging Liver Fibrosis in Autoimmune Hepatitis. Hepatol Int (2019) 13(1):91–101. doi: 10.1007/s12072-018-9907-5
25. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PloS Med (2009) 6(7):e1000100. doi: 10.1371/journal.pmed.1000100
26. Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: A Revised Tool for the Quality Assessment of Diagnostic Accuracy Studies. Ann Intern Med (2011) 155(8):529–36. doi: 10.7326/0003-4819-155-8-201110180-00009
27. Abdo AA. Clinical Presentation, Response to Therapy, and Predictors of Fibrosis in Patients With Autoimmune Hepatitis in Saudi Arabia. Saudi J Gastroenterol (2006) 12(2):73–6. doi: 10.4103/1319-3767.27849
28. Anastasiou O E, Büchter M A, Baba H, Korth J, Canbay A, Gerken G, et al. Performance and Utility of Transient Elastography and non-Invasive Markers of Liver Fiibrosis in Patients With Autoimmune Hepatitis: A Single Centre Experience. Hepat Mon (2016) 16(11):e40737. doi: 10.5812/hepatmon.40737
29. Nishikawa H, Enomoto H, Iwata Y, Hasegawa K, Nakano C, Takata R, et al. Clinical Significance of Serum Wisteria Floribunda Agglutinin Positive Mac-2-Binding Protein Level and High-Sensitivity C-Reactive Protein Concentration in Autoimmune Hepatitis. Hepatol Res (2016) 46(7):613–21. doi: 10.1111/hepr.12596
30. Sheptulina A, Shirokova E, Nekrasova T, Blum H, Ivashkin V. Platelet Count to Spleen Diameter Ratio non-Invasively Identifies Severe Fibrosis and Cirrhosis in Patients With Autoimmune Hepatitis. J Gastroenterol Hepatol (2016) 31(12):1956–62. doi: 10.1111/jgh.13407
31. Guo L, Zheng L, Hu L, Zhou H, Yu L, Liang W. Transient Elastography (FibroScan) Performs Better Than non-Invasive Markers in Assessing Liver Fibrosis and Cirrhosis in Autoimmune Hepatitis Patients. Med Sci Monit (2017) 23:5106–12. doi: 10.12659/msm.907300
32. Wang J, Malik N, Yin M, Smyrk TC, Czaja AJ, Ehman RL, et al. Magnetic Resonance Elastography Is Accurate in Detecting Advanced Fibrosis in Autoimmune Hepatitis. World J Gastroenterol (2017) 23(5):859–68. doi: 10.3748/wjg.v23.i5.859
33. Xu Q, Sheng L, Bao H, Chen X, Guo C, Li H, et al. Evaluation of Transient Elastography in Assessing Liver Fibrosis in Patients With Autoimmune Hepatitis. J Gastroenterol Hepatol (2017) 32(3):639–44. doi: 10.1111/jgh.13508
34. Zeng T, Yu J, Tan L, Wu Y, Tian Y, Wu Q, et al. Noninvasive Indices for Monitoring Disease Course in Chinese Patients With Autoimmune Hepatitis. Clin Chim Acta (2018) 486:135–41. doi: 10.1016/j.cca.2018.07.030
35. Liu L, Cao J, Zhong Z, Guo Z, Jiang Y, Bai Y, et al. Noninvasive Indicators Predict Advanced Liver Fibrosis in Autoimmune Hepatitis Patients. J Clin Lab Anal (2019) 33(7):e22922. doi: 10.1002/jcla.22922
36. Park DW, Lee YJ, Chang W, Park JH, Lee KH, Kim YH, et al. Diagnostic Performance of a Point Shear Wave Elastography (pSWE) for Hepatic Fibrosis in Patients With Autoimmune Liver Disease. PloS One (2019) 14(3):e0212771. doi: 10.1371/journal.pone.0212771
37. Yuan X, Duan SZ, Cao J, Gao N, Xu J, Zhang L. Noninvasive Inflammatory Markers for Assessing Liver Fibrosis Stage in Autoimmune Hepatitis Patients. Eur J Gastroenterol Hepatol (2019) 31(11):1467–74. doi: 10.1097/MEG.0000000000001437
38. Li X, Xu H, Gao P. Red Blood Cell Distribution Width-to-Platelet Ratio and Other Laboratory Indices Associated With Severity of Histological Hepatic Fibrosis in Patients With Autoimmune Hepatitis: A Retrospective Study at a Single Center. Med Sci Monit (2020) 26:e927946. doi: 10.12659/MSM.927946
39. Wang H, Wang J, Xia J, Yan X, Feng Y, Li L, et al. Red Cell Distribution Width to Platelet Ratio Predicts Liver Fibrosis in Patients With Autoimmune Hepatitis. Med (Baltimore) (2020) 99(34):e21408. doi: 10.1097/MD.0000000000021408
40. Shaheen AA, Myers RP. Diagnostic Accuracy of the Aspartate Aminotransferase-to-Platelet Ratio Index for the Prediction of Hepatitis C-Related Fibrosis: A Systematic Review. Hepatology (2007) 46:912–21. doi: 10.1002/hep.21835
41. Dong B, Lyu G, Chen Y, Lin G, Wang H, Qin R, et al. Comparison of Two-Dimensional Shear Wave Elastography, Magnetic Resonance Elastography, and Three Serum Markers for Diagnosing Fibrosis in Patients With Chronic Hepatitis B: A Meta-Analysis. Expert Rev Gastroenterol Hepatol (2021) 15(9):1077–89. doi: 10.1080/17474124.2021.1880894
42. Doycheva I, Watt KD, Gulamhusein AF. Autoimmune Hepatitis: Current and Future Therapeutic Options. Liver Int (2019) 39(6):1002–13. doi: 10.1111/liv.14062
43. Khare S, Arora A, Sharma P, Dhawan S, Bansal N, Singla V, et al. Performance of non-Invasive Blood Parameters for Ruling Out Significant Liver Fibrosis in Patients With Chronic Hepatitis B. J Clin Transl Hepatol (2020) 8(2):143–9. doi: 10.14218/JCTH.2020.00002
44. Castera L. Hepatitis B: Are non-Invasive Markers of Liver Fibrosis Reliable? Liver Int (2014) 34 Suppl 1:91–6. doi: 10.1111/liv.12393
45. World Health Organization. Guidelines for the Prevention, Care and Treatment of Persons With Chronic Hepatitis B Infection. (2015). Available at: https://www.who.int/publications/i/item/9789241549059
46. World Health Organization. Guidelines for the Screening, Care and Treatment of Persons With Chronic Hepatitis C Infection. Geneva: World Health Organization. (2016). Available at: https://www.who.int/publications/i/item/9789241549615
47. Kim WR, Berg T, Asselah T, Flisiak R, Fung S, Gordon SC, et al. Evaluation of APRI and FIB-4 Scoring Systems for Non-Invasive Assessment of Hepatic Fibrosis in Chronic Hepatitis B Patients. J Hepatol (2016) 64(4):773–80. doi: 10.1016/j.jhep.2015.11.012
48. Liberal R, de Boer YS, Andrade RJ, Bouma G, Dalekos GN, Floreani A, et al. Expert Clinical Management of Autoimmune Hepatitis in the Real World. Aliment Pharmacol Ther (2017) 45(5):723–32. doi: 10.1111/apt.13907
Keywords: autoimmune hepatitis, liver fibrosis, noninvasive methods, APRI, FIB-4
Citation: Dong B, Chen Y, Lyu G and Yang X (2022) Aspartate Aminotransferase to Platelet Ratio Index and Fibrosis-4 Index for Detecting Liver Fibrosis in Patients With Autoimmune Hepatitis: A Meta-Analysis. Front. Immunol. 13:892454. doi: 10.3389/fimmu.2022.892454
Received: 09 March 2022; Accepted: 21 April 2022;
Published: 18 May 2022.
Edited by:
Alessandro Granito, University of Bologna, ItalyReviewed by:
Linda Beenet, University of California, Los Angeles, United StatesJiezuan Yang, Zhejiang University, China
Copyright © 2022 Dong, Chen, Lyu and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guorong Lyu, Z3Vvcm9uZ2x5dUBmam11LmVkdS5jbg==
 Bingtian Dong
Bingtian Dong Yuping Chen
Yuping Chen Guorong Lyu
Guorong Lyu Xiaocen Yang4
Xiaocen Yang4