
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 10 June 2022
Sec. Autoimmune and Autoinflammatory Disorders
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.890543
 Kathy Ming Feng1
Kathy Ming Feng1 Wu-Chien Chien2,3,4,5
Wu-Chien Chien2,3,4,5 Yi-Hao Chen1
Yi-Hao Chen1 Chien-An Sun6,7
Chien-An Sun6,7 Chi-Hsiang Chung2,3,4
Chi-Hsiang Chung2,3,4 Jiann-Torng Chen1
Jiann-Torng Chen1 Ching-Long Chen1*
Ching-Long Chen1*Background: Uveitis, a sight-threatening ocular inflammatory state, is associated with autoimmune diseases and systemic inflammation. This prolonged systemic inflammation may cause plaque formation in coronary arteries, subsequently resulting in acute coronary syndrome (ACS).
Methods: This retrospective, population-based study (15-year period) used the Longitudinal Health Insurance Database based on the National Health Insurance Research Database in Taiwan. Chi-square and Student’s t-tests were used to examine differences between the study and comparison cohorts for categorical and continuous variables, respectively. Fine and Gray’s competing risk model was used to determine the hazard ratio of the risk of ACS. Furthermore, the cumulative risk of ACS was determined using Kaplan-Meier analysis.
Results: A total of 1,111 patients with AS and uveitis were enrolled in this study cohort, and 4,444 patients with AS without uveitis were enrolled in the comparison cohort. After adjustment for age, sex, and comorbidities, patients with AS and uveitis demonstrated an increased risk of ACS compared to those without uveitis (adjusted hazard ratio: 1.675, p<0.001). In addition, Kaplan-Meier analysis revealed that patients with AS and uveitis had a significantly higher risk of ACS than those without uveitis (p<0.001). Age, diabetes mellitus, hypertension, hyperlipidemia, chronic obstructive pulmonary disease, asthma, and systemic steroids were significant risk factors for ACS. Both anterior uveitis and posterior segment involvement were associated with an increased risk of ACS in patients with AS. All-cause mortality was higher in the uveitis group (9.81%) than in the non-uveitis group (8.10%) (p=0.015).
Conclusion: Our analysis revealed that uveitis could potentially be a predictor of ACS in patients with AS. However, further prospective controlled studies are required to assess the association between uveitis and ACS in patients with AS.
Ankylosing spondylitis (AS) is a chronic inflammatory arthritis that involves the axial skeleton, belonging to a group of diseases known as spondyloarthritis (1). Usually, AS presents in the third decade of life and occurs more commonly in men (2, 3). An increased risk of cardiovascular morbidity and mortality has been recognized in patients with AS (4, 5). Several studies have reported a higher incidence of acute coronary syndrome (ACS) in patients with AS than in the general population (6, 7). However, the exact underlying mechanism remains unknown. The pathogenesis of atherosclerosis involves immune cell aggregation and deposition of cholesterol in arterial walls (8). Previous studies have shown that inflammatory cytokines, oxidative stress, and activated T-cells can cause endothelial dysfunction and endovascular injury (9–11). Prolonged inflammation may cause the plaque to become unstable with subsequent rupture and thrombus formation, occlusion of vessels, and finally ACS. Thus, immune-mediated inflammatory disorders may contribute to ACS events through systemic inflammation rather than through commonly known risk factors.
Uveitis, or inflammation of the uvea, is the most frequent extra-articular manifestation of AS, followed by psoriasis and inflammatory bowel disease (2). Pro-inflammatory cytokines and chemokines are known for their role in the pathogenesis of uveitis, and studies have demonstrated an increase in cytokines and chemokines not only in aqueous and vitreous samples but also in tears and serum samples from patients with uveitis (12, 13). Thus, uveitis may imply active systemic inflammation in patients with AS. In addition, a cross-sectional study in Norway reported an increased odds ratio for atherosclerosis and hypertension in patients with AS with a history of uveitis (14). As inflammation attracts cytokines and chemokines, which may affect further coronary vascular endothelial injury, uveitis may be considered a risk factor for ACS in patients with AS. However, no study has examined this association. Undoubtedly, the health and economic burden of ACS is high, and ACS has the greatest mortality and disability-adjusted life years worldwide (15, 16). Although ACS mainly occurs in elderly patients, the incidence of ACS in younger patients is increasing (17). The consequences of ACS in younger, active patients can be devastating; hence, it is essential to understand the relationship between patients with AS and uveitis and the risk of ACS.
Taking into account the above-mentioned considerations, we designed a retrospective matched-cohort study that used claims data from the National Health Insurance Research Database (NHIRD), which encompasses a comprehensive longitudinal medical record from almost the entire population of Taiwan (23 million), to evaluate the association between patients with AS and uveitis and the risk of ACS.
This study employed the Longitudinal Health Insurance Database (LHID) from the NHIRD to examine the association between uveitis in patients with AS and subsequent development of ACS in Taiwan over a 15-year period. The claims data contained medical records of outpatients and inpatients and were registered using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes. This NHIRD included around 99% of 23 million Taiwanese citizens and represented the real-world data in Taiwan (18). Patients’ personal data were encrypted before the data were released for research purposes. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki.
We retrospectively conducted a matched-cohort study from January 1, 2000, to December 31, 2015. Patients were selected if they had at least one inpatient claim or more than three outpatient visits with the diagnosis of AS (ICD-9-CM code 720.0). The index date was defined as the date on which the patient was first diagnosed with AS. Patients diagnosed with AS or ACS before the index date were excluded to ensure that all ACS cases were newly diagnosed after AS. Patients aged 20 years and younger, of unknown sex, or without tracking follow-up were excluded. The study population was divided into uveitis (study cohort) and non-uveitis (comparison cohort) groups. The non-uveitis group was randomly matched fourfold with the uveitis group according to sex, age, and index year (under the same exclusion criteria). Furthermore, the uveitis group was divided into the anterior uveitis and posterior segment involvement groups. Patients were tracked until ACS onset or the end of the study period, whichever occurred first. The selection process is illustrated in Figure 1.
Uveitis was the main independent variable of interest. The evaluated covariates included sex, age, diabetes mellitus (DM), hyperlipidemia, hypertension (HTN), asthma, and chronic obstructive pulmonary disease (COPD). The considered medication was systemic steroids. These factors were matched at baseline and considered confounders and adjusted for statistical analysis.
All statistical analyses were performed using SPSS software version 22 (SPSS Inc., Chicago, IL, USA). The differences between the study participants and the comparison cohort for categorical and continuous variables were assessed using the chi-square and t-tests, respectively. A Cox proportional hazards model with Fine and Gray’s competing risk model was used to determine the hazard ratio of risk of ACS based on each variable. Survival analysis was performed using the Kaplan-Meier method with log-rank test. A two-tailed p value <0.05 was considered statistically significant.
This study was approved by the Institutional Review Board of the Tri-Service General Hospital (TSGHIRB: B-110-41), and the need for individual written informed consent was waived. This study was conducted in accordance with the code of ethics of the World Medical Association (Declaration of Helsinki).
As depicted in Figure 1, a total of 19,941 newly diagnosed patients with AS were identified, while 1,905 did not proceed past the exclusion criteria. Among them, 1,111 patients had uveitis (uveitis group), and a total of 18,830 patients had no uveitis during the study period; these patients were matched with a 4 fold-propensity score by sex, age, index date, and comorbidities. Then, 4,444 patients were included in the comparison cohort. These patients were followed up to ascertain the incidence of ACS.
Table 1 shows the baseline characteristics and demographic characteristics of the study population. The mean age was 37.66 ± 19.10 years and 37.71 ± 19.91 years in study and comparison cohort, respectively. Men and women respectively accounted for 55.90% and 44.10% of the study population, while patients in the 20–39 age group constituted 59.68% of the study population. There were no significant differences in age, sex, comorbidities, and CCI-R between the study and comparison cohorts at baseline. At the study endpoint, ACS occurred in 175 (15.75%) patients with uveitis and in 419 (9.43%) patients without uveitis (p<0.001). The mean age at the endpoint was 40.68±20.20 years in the uveitis group and 41.48±20.53 years in the non-uveitis group, which was not significantly different (p=0.142). All-cause mortality was higher in the uveitis group (9.81%) than in the non-uveitis group (8.10%) (p=0.015). In Table S1-1, the mean follow-up time for the study and comparison cohort is 9.82 ± 8.40 years and 9.85 ± 8.55 years, respectively (p= 0.784). Table S1-2 revealed that the average time in developing ACS in patients with AS is 3.01±3.21 years in the uveitis group and 3.75 ± 4.11 years in the non-uveitis group (p<0.001).
Figure 2 shows the cumulative risk of ACS in patients with AS with and without uveitis using the Kaplan-Meier method. The result demonstrates that uveitis group had a significantly higher risk of ACS than the non-uveitis group (log-rank test, P <0.001). The results of the Cox proportional hazards regression analysis using Fine and Gray’s competing risk model are shown in Table 2. The adjusted HR (aHR) for ACS in patients with AS and uveitis was 1.64 times that in those without uveitis (p<0.001). Furthermore, AS patients aged 40–59 years and ≥ 60 years had an increased risk for ACS (aHR=1.105 and 1.709, respectively). Surprisingly, no gender preference for ACS development was found. The aHR for DM, hyperlipidemia, HTN, COPD, and asthma were 3.080, 2.931, 3.336, 1.505, and 1.929, respectively. The aHR for CCI_R was 1.197 (P <0.001). Patients using systemic steroids had a higher risk of developing ACS (aHR: 2.017, p<0.001). This result demonstrates a higher risk of ACS in patients with AS with comorbidities than in those without these comorbidities.
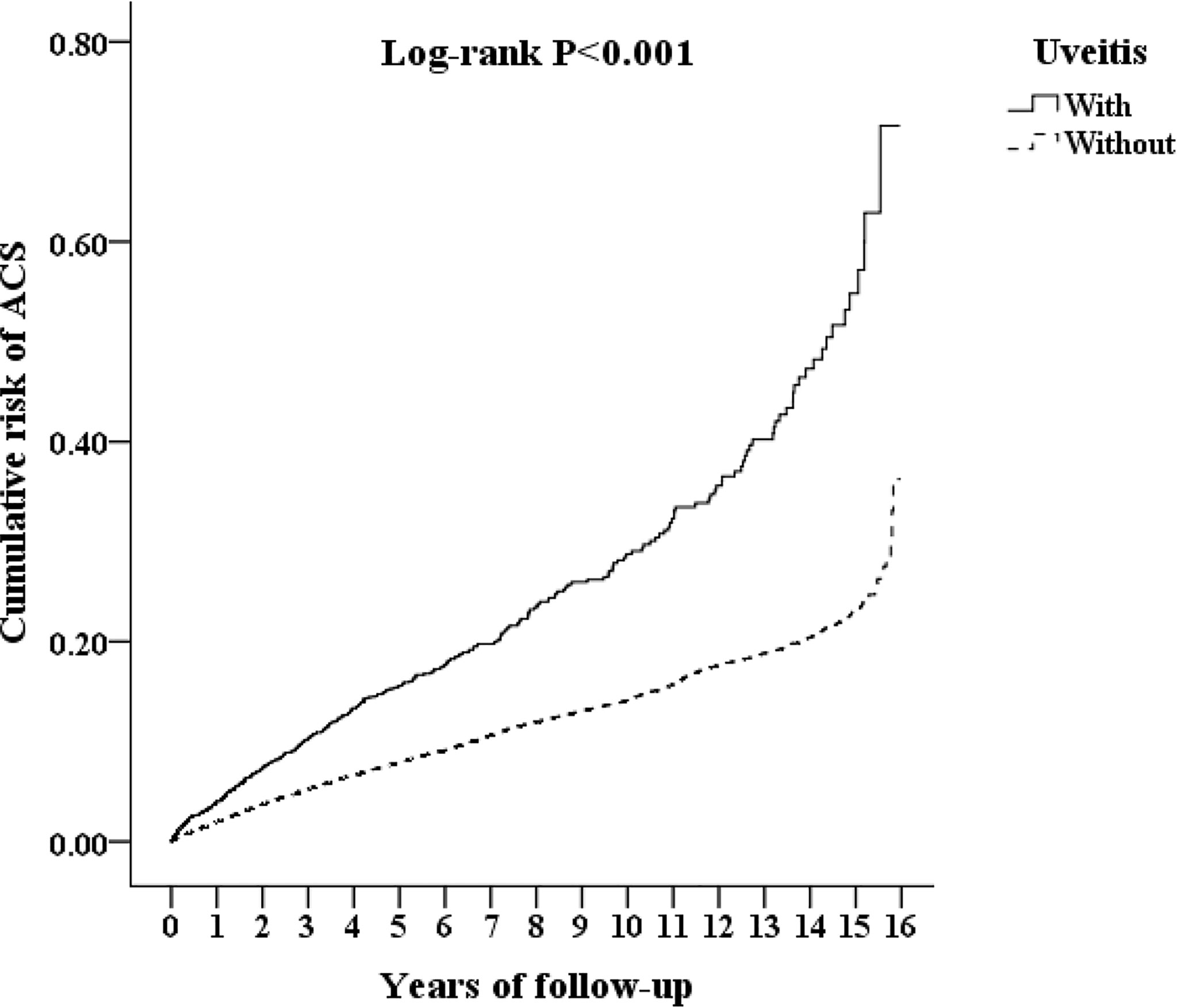
Figure 2 Kaplan-Meier curves for acute coronary syndrome (ACS) in patients with ankylosing spondylitis (AS), corresponding to the uveitis and non-uveitis groups. Line represents the uveitis group and dotted line represents the non-uveitis group.
The results of the stratified analyses of the risk factors associated with the development of ACS are shown in Table 3. The incidence of ACS was 1,593.38 per 100,000 person-years in the uveitis group and 953.40 per 100,000 person-years in the non-uveitis group. After adjustment for age, sex, and comorbidities, patients with AS and uveitis demonstrated an increased risk of ACS compared to those without uveitis (aHR: 1.675, p<0.001). The aHRs for ACS were 1.736 for men and 1.605 for women. Patients with AS and uveitis had an increased risk of ACS regardless of sex. A trend of increasing aHR for ACS was observed in patients with AS and uveitis as patient age increased (20–39 years, aHR: 1.390; 40–59 years, aHR: 1.578; ≥60 years, aHR: 4.188). This might indicate that age is a significant risk factor for ACS in AS patients with uveitis. Compared with the comparison cohort, the study cohort had a greater aHR for ACS in the absence or presence of comorbidities and systemic steroids (DM, 1.548, 3.091; hyperlipidemia, 1.581, 3.017; HTN, 1.52, 3.649; COPD, 1.653, 2.362; asthma, 1.69, 1.829; and systemic steroids, 1.510, 2.035, respectively). In addition, the presence of comorbidities increased the aHR for ACS.
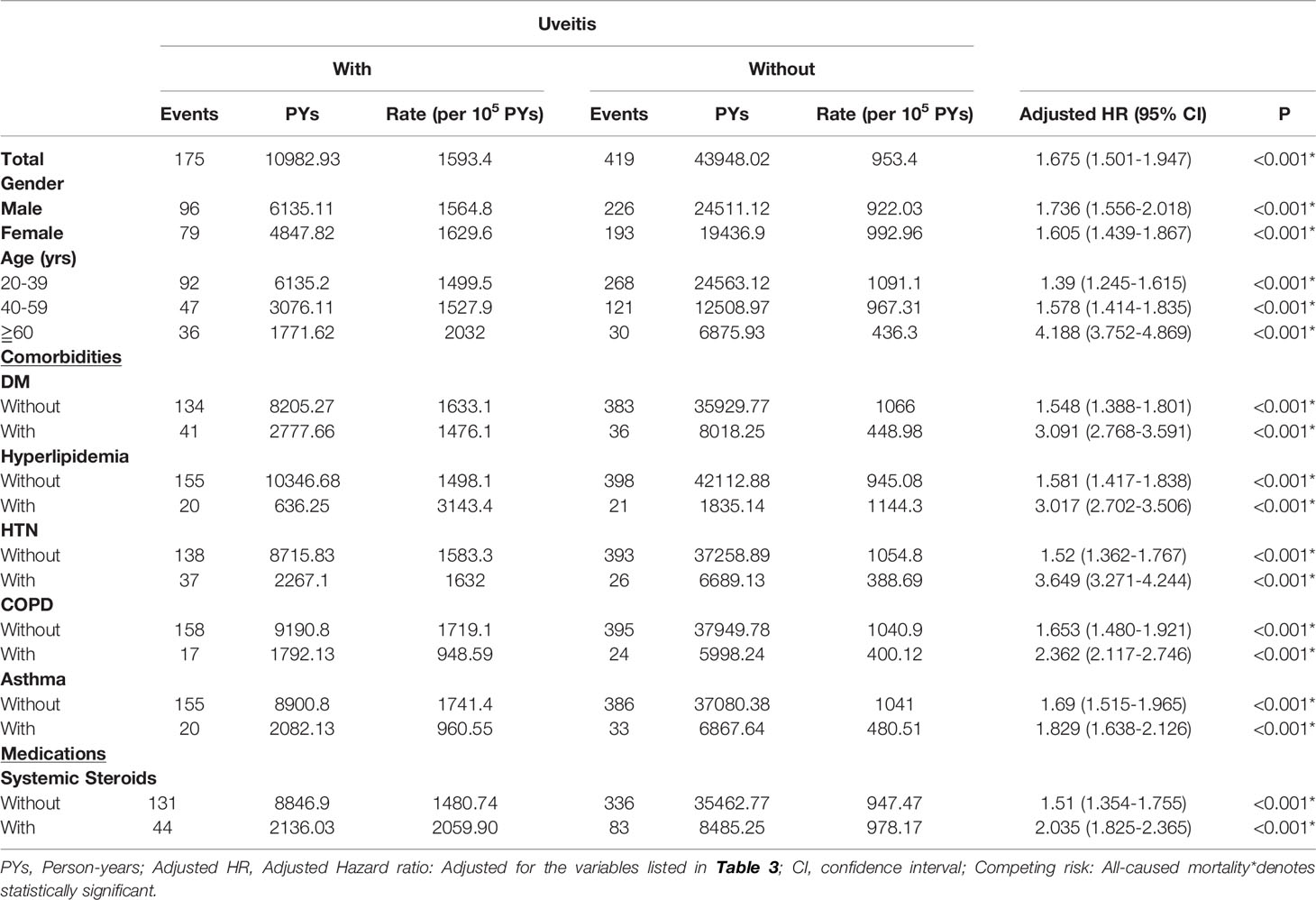
Table 3 Factors of ACS stratified by variables listed using Cox regression with Fine & Gray’s competing risk model.
The uveitis group (n=1,111) was further grouped into anterior uveitis (n=394) and posterior uveitis (n=717) groups, as shown in Table 4. The overall incidence of ACS reported was 1,445.38 per 100,000 person-years in the anterior uveitis group and 1,676.30 per 100,000 person-years in the posterior uveitis group. The aHRs were 1.665 and 4.893 in the anterior uveitis and posterior uveitis groups, respectively.
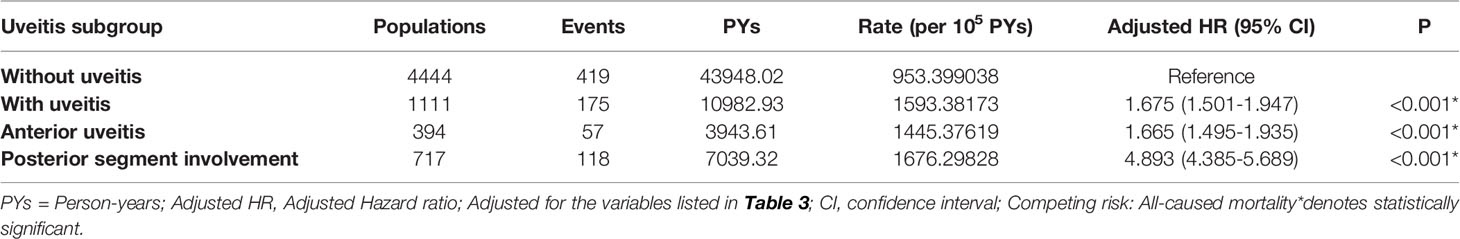
Table 4 Factors of ACS among different uveitis subgroup using Cox regression with Fine & Gray’s competing risk model.
In this 15-year population-based investigation on the correlation between development of ACS and uveitis in patients with AS, we found that patients with AS and uveitis showed a significantly increased risk of ACS compared to those without uveitis, even after adjusting for age, sex, and comorbidities. Similarly, Kaplan-Meier analysis revealed that the cumulative risk of ACS was significantly higher in the uveitis group than that in the non-uveitis group. Uveitis demonstrated a strong association with an increased risk of ACS in both sexes, all ages, and patients with AS with and without DM, hyperlipidemia, HTN, COPD, and asthma. In the subgroups of uveitis, both anterior uveitis and posterior segment involvement demonstrated an increased risk of ACS in patients with AS. This study identified uveitis as a risk factor for ACS in patients with AS and suggests that it may be a potential predictor of ACS in patients with AS.
The prevalence of AS ranges from 9 to 30 per 10,000 people in the general population (3, 19). Although the prevalence may vary from country to country, a male predominance is almost consistently observed (20). The male-to-female ratio was 2.79 in Taiwan and 3.8 in the US (21, 22). Our study population included patients with AS and uveitis, which showed a male-to-female ratio of 1.27, with a male prevalence of 55.90%. In general, the age of onset of AS is usually in the third decade and is consistent with the above studies (1, 2, 19); in this study, 59.67% of patients were aged 20–39 years.
Patients with AS tend to have a higher rate of comorbidities than the general population. The prevalence of HTN, DM, and asthma in patients with AS was 30.7%, 9.8%, and 2.2%, respectively, in the US (23), and the prevalence of HTN, DM and COPD was 25.7%, 7.1%, and 2.71%, respectively, in Spain (24). A systematic review and meta-analysis reported that the pooled prevalence of HTN, DM, hyperlipidemia, asthma, and COPD was 22.8%, 6%, 16.8%, 4.9%, and 1.8%, respectively (25). The prevalence of HTN, DM, hyperlipidemia, asthma, and COPD in our study population was 20.58%, 25.17%, 5.71%, 18.78%, and 15.82%, respectively. The observed differences may be attributed to the study design, environment, and ethnicity. In fact, prevalence of diabetes varies among different ethnicity, which may be because of environmental, lifestyle and genetic factors, and Asian populations have shown to have higher prevalence of diabetes than white populations (26). Since the comorbidities in our study were matched at baseline, the higher cumulative risk of ACS in the uveitis group found in this study was less likely to be caused by the baseline systemic diseases.
Aging, hypertension, and diabetes mellitus are associated with the development of ACS (27, 28) and are also risk factors for ACS in patients with AS. A nationwide study in Taiwan found that patients with AS with hypertension and diabetes had an aHR of 4.36 for ACS compared to patients without these comorbidities (6). A population-based study of the Swedish National Patient Registry compared the mortality of patients with AS with that of the general population and reported a hazard ratio of 1.60 with cardiovascular disease being the major cause of death (29). Furthermore, Backland et al. found that crude mortality in patients with AS was 14.5%, and 40% of cases were attributed to circulatory disease (30). Undeniably, patients with AS are at risk of ACS, and cardiovascular risk factors can only partially explain this excess cardiovascular risk. While the above-mentioned studies are findings for the general AS population, our study population included patients with AS and uveitis and found uveitis to be an independent risk factor for ACS.
Uveitis is the most common extraarticular manifestation of AS (31).The mechanism underlying the relationship between uveitis and ACS is unclear. Elevated interleukin (IL)-6, IL-8, and IL-17A in serum samples of active uveitis suggest that uveitis may be an indicator of disease activity and systemic inflammation in AS (32, 33). Inflammation is also involved in the entire process of atherosclerosis and destabilization of the plaque, ultimately leading to myocardial infarction (8, 34). The cytokines involved in the development of ACS include IL-1, IL-6, tumor necrosis factor alpha)-α, adipokines, chemokines, and interferons (35). Chronic inflammation can cause detrimental effects on endothelial cells in these coronary artery vessels (36). The association between uveitis and ACS may be due to similarities in the shared mechanisms of inflammation. This study reported a higher incidence of ACS in patients with AS and uveitis than in those without uveitis, indicating that uveitis is a new potential risk factor for ACS in patients with AS (aHR= 1.640, p<0.001).
In addition to inflammation, AS has a strong genetic component (37), and HLA-B27 remains the most highly associated gene (2). In addition, genome-wide association studies have found that genes encoding endoplasmic reticulum aminopeptidase (ERAP) and IL-23 receptors are involved in AS. ERAP-1 is involved in the preparation of peptides for binding to HLA class 1 molecules, which are later presented to immune effector cells. The IL-23/IL-17 axis is where IL-23 activates T-helper cells that facilitate the expansion and differentiation of Th17 cells to produce major pro-inflammatory cytokines (2, 38). In animal models, activation of the IL-23/IL-17 pathway and expansion of Th-17 cells contribute to spondyloarthritis-associated uveitis (39). Studies have also suggested an association between the IL-23/IL-17 axis and atherosclerosis (40). Patients with atherosclerosis have significantly increased levels of IL-23 in the plasma, and IL-23 levels are higher in carotid plaques than in nonatherosclerotic vessels (40). IL-17A blockade in apoE-deficient mice shows a reduction in atherosclerosis development and decreases the vulnerability of plaque (41), which suggests a similar pathway of ACS and uveitis in patients with AS.
Our study reported a higher aHR for ACS in the posterior segment involvement group than in the anterior uveitis group (Table 4). Noninfectious uveitis is the major subtype of uveitis in Taiwan (42). While anterior uveitis in spondyloarthropathies has been studied thoroughly, posterior segment manifestations have often been overlooked. However, Rodriguez et al. found that 24% of 29 seronegative spondyloarthropathy patients had retinal vasculitis, 94% had severe vitritis, and 76% had papillitis (43), indicating the importance of posterior segment manifestation in any spondyloarthropathy disease. Very few studies have examined cytokine levels in the different subtypes of uveitis. Using proteomics, Velez et al. analyzed vitreous samples from 15 patients with posterior uveitis, and among them, one AS patient showed upregulation of IL-23 and IL-17R (44). Uveitis and acute coronary syndrome may share a similar IL-23/IL-17 pathway. From the above studies, the posterior uveitis group may have more severe disease inflammation because of the elevation of IL-23 levels; however, further studies are needed to confirm this association. Nonetheless, both anterior uveitis and posterior uveitis were associated with an increased risk of ACS among patients with AS.
Clinically, the treatment of non-infectious uveitis (NIU) included topical steroids or systemic steroids, disease-modifying antirheumatic drugs (DMARDs, such as azathioprine, methotrexate, mycophenolate mofetil, cyclosporine, tacrolimus, cyclophosphamide, or chlorambucil), and biologic agents (such as adalimumab, or Infliximab) (45–47). Uveitis in AS patients belonged to one of NIU. In these patients, topical steroids are the major treatment for acute anterior uveitis. Nevertheless, uveitis with posterior or bilateral involvement, or refractory to topical medication, required systemic treatment such as systemic steroids, DMARDs, or biologic agents (45, 48). Under these conditions, systemic steroids were the first choice of treatment. However, high doses or long-term systemic steroids could cause serious systemic side-effects such as Cushing’s syndrome, hypertension, diabetic mellitus, hyperlipidemia, osteoporosis, …et al. The side effects of systemic steroids such as hypertension and hyperlipidemia could predispose treated patients to cardiovascular disease (49). Consequently, DMARDs or biologic agents were used to control inflammation and spare steroids in high-doses or prolong-used patients (45–48). Consistent with the literature (49), our study also found that using systemic steroids increased the risk of ACS in AS patients with uveitis. Nonetheless, the association of ACS between steroids and AS patients with uveitis still needs to be further investigated.
A strength of this study is the large sample size within a comprehensive database spanning over a 15-year period. This not only provides high statistical power that can best reflect real-world situations, but also minimizes selection bias. In addition, this is the first population-based study to investigate uveitis as a risk factor for acute coronary syndrome in patients with AS. Furthermore, this study matched comorbidities at baseline, which could provide more reliable results for adjusting these variables.
This study had several limitations that should be addressed. First, misclassifications of the subtype of uveitis and cardiovascular outcomes may be inevitable; however, the National Health Administration in Taiwan checks charts to ensure that patients have appropriate claims and treatment. Second, the LHID only included the population of Taiwan, thus, this result needed to be validated and extrapolated by a further study in a much bigger group. Third, due to that our research was a retrospective study by analyzing the fully anonymized database, we could not obtain other confounding variables (such as dietary habits, physical activity, smoking habits, environmental factors, regional difference, body mass index, and laboratory data). Hence, the interferences of these confounding variables needed a further prospective study to determine these.
In conclusion, this study demonstrated that uveitis is an independent risk factor for ACS in patients with AS. In patients with AS and uveitis, a trend of specific comorbidities showed a higher risk of ACS. The results of this study will allow ophthalmologists, rheumatologists, or any medical doctor to be aware of the risk of ACS when monitoring patients with AS and uveitis.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Institutional Review Board of the Tri-Service General Hospital (TSGHIRB: B-110-41). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
KF, W-CC, Y-HC, J-TC, and C-LC: study design and manuscript writing. W-CC, C-AS, C-HC, and C-LC: data extracting and statistical analysis. K-MF, Y-HC, C-AS, J-TC, and C-LC: data checking. All authors contributed to the article and approved submitted version.
This study was funded by the Tri-Service General Hospital Research Foundation (TSGH-D-110112 and VTA111-V1-1-2) and by the Ministry of Science and Technology (MOST 107-2314-B-016-030). The sponsors have no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to acknowledge the Health and Welfare Data Science Center, Ministry of Health and Welfare (HWDC, MOHW), Taiwan, for providing the National Health Insurance Research Database. In addition, the authors would like to thank Professor Chin-Sheng Lin, from the Tri-Service General Hospital, for his advice and suggestions to our study.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.890543/full#supplementary-material
1. Taurog JD, Chhabra A, Colbert RA. Ankylosing Spondylitis and Axial Spondyloarthritis. N Engl J Med (2016) 374(26):2563–74. doi: 10.1056/NEJMra1406182
2. Sieper J, Poddubnyy D. Axial Spondyloarthritis. Lancet (2017) 390(10089):73–84. doi: 10.1016/s0140-6736(16)31591-4
3. Ward MM, Deodhar A, Gensler LS, Dubreuil M, Yu D, Khan MA, et al. 2019 Update of the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network Recommendations for the Treatment of Ankylosing Spondylitis and Nonradiographic Axial Spondyloarthritis. Arthritis Care Res (Hoboken) (2019) 71(10):1285–99. doi: 10.1002/acr.24025
4. Castaneda S, Nurmohamed MT, Gonzalez-Gay MA. Cardiovascular Disease in Inflammatory Rheumatic Diseases. Best Pract Res Clin Rheumatol (2016) 30(5):851–69. doi: 10.1016/j.berh.2016.10.006
5. Liew JW, Ramiro S, Gensler LS. Cardiovascular Morbidity and Mortality in Ankylosing Spondylitis and Psoriatic Arthritis. Best Pract Res Clin Rheumatol (2018) 32(3):369–89. doi: 10.1016/j.berh.2019.01.002
6. Chou CH, Lin MC, Peng CL, Wu YC, Sung FC, Kao CH, et al. A Nationwide Population-Based Retrospective Cohort Study: Increased Risk of Acute Coronary Syndrome in Patients With Ankylosing Spondylitis. Scand J Rheumatol (2014) 43(2):132–6. doi: 10.3109/03009742.2013.822097
7. Bengtsson K, Forsblad-d'Elia H, Lie E, Klingberg E, Dehlin M, Exarchou S, et al. Are Ankylosing Spondylitis, Psoriatic Arthritis and Undifferentiated Spondyloarthritis Associated With an Increased Risk of Cardiovascular Events? A Prospective Nationwide Population-Based Cohort Study. Arthritis Res Ther (2017) 19(1):102. doi: 10.1186/s13075-017-1315-z
8. Wang H, Liu Z, Shao J, Lin L, Jiang M, Wang L, et al. Immune and Inflammation in Acute Coronary Syndrome: Molecular Mechanisms and Therapeutic Implications. J Immunol Res (2020) 2020:4904217. doi: 10.1155/2020/4904217
9. Niessner A, Sato K, Chaikof EL, Colmegna I, Goronzy JJ, Weyand CM. Pathogen-Sensing Plasmacytoid Dendritic Cells Stimulate Cytotoxic T-Cell Function in the Atherosclerotic Plaque Through Interferon-Alpha. Circulation (2006) 114(23):2482–9. doi: 10.1161/CIRCULATIONAHA.106.642801
10. Rho YH, Chung CP, Oeser A, Solus JF, Gebretsadik T, Shintani A, et al. Interaction Between Oxidative Stress and High-Density Lipoprotein Cholesterol Is Associated With Severity of Coronary Artery Calcification in Rheumatoid Arthritis. Arthritis Care Res (Hoboken) (2010) 62(10):1473–80. doi: 10.1002/acr.20237
11. Montecucco F, Mach F. Common Inflammatory Mediators Orchestrate Pathophysiological Processes in Rheumatoid Arthritis and Atherosclerosis. Rheumatol (Oxford) (2009) 48(1):11–22. doi: 10.1093/rheumatology/ken395
12. Carreno E, Portero A, Herreras JM, Garcia-Vazquez C, Whitcup SM, Stern ME, et al. Cytokine and Chemokine Tear Levels in Patients With Uveitis. Acta Ophthalmol (2017) 95(5):e405–e14. doi: 10.1111/aos.13292
13. Valentincic NV, de Groot-Mijnes JD, Kraut A, Korosec P, Hawlina M, Rothova A. Intraocular and Serum Cytokine Profiles in Patients With Intermediate Uveitis. Mol Vis (2011) 17:2003–10.
14. Berg IJ, Semb AG, van der Heijde D, Kvien TK, Hisdal J, Olsen IC, et al. Uveitis Is Associated With Hypertension and Atherosclerosis in Patients With Ankylosing Spondylitis: A Cross-Sectional Study. Semin Arthritis Rheum (2014) 44(3):309–13. doi: 10.1016/j.semarthrit.2014.05.017
15. Kim J, Lee E, Lee T, Sohn A. Economic Burden of Acute Coronary Syndrome in South Korea: A National Survey. BMC Cardiovasc Disord (2013) 13:55. doi: 10.1186/1471-2261-13-55
16. Makki N, Brennan TM, Girotra S. Acute Coronary Syndrome. J Intensive Care Med (2015) 30(4):186–200. doi: 10.1177/0885066613503294
17. Sawada H, Ando H, Takashima H, Waseda K, Shimoda M, Ohashi H, et al. Epidemiological Features and Clinical Presentations of Acute Coronary Syndrome in Young Patients. Intern Med (2020) 59(9):1125–31. doi: 10.2169/internalmedicine.4138-19
18. Hsieh CY, Su CC, Shao SC, Sung SF, Lin SJ, Kao Yang YH, et al. Taiwan's National Health Insurance Research Database: Past and Future. Clin Epidemiol (2019) 11:349–58. doi: 10.2147/clep.S196293
19. Dean LE, Jones GT, MacDonald AG, Downham C, Sturrock RD, Macfarlane GJ. Global Prevalence of Ankylosing Spondylitis. Rheumatol (Oxford) (2014) 53(4):650–7. doi: 10.1093/rheumatology/ket387
20. Exarchou S, Lindstrom U, Askling J, Eriksson JK, Forsblad-d'Elia H, Neovius M, et al. The Prevalence of Clinically Diagnosed Ankylosing Spondylitis and Its Clinical Manifestations: A Nationwide Register Study. Arthritis Res Ther (2015) 17:118. doi: 10.1186/s13075-015-0627-0
21. Chen HH, Chen TJ, Chen YM, Ying-Ming C, Chen DY. Gender Differences in Ankylosing Spondylitis-Associated Cumulative Healthcare Utilization: A Population-Based Cohort Study. Clinics (Sao Paulo) (2011) 66(2):251–4. doi: 10.1590/s1807-59322011000200012
22. Wright KA, Crowson CS, Michet CJ, Matteson EL. Time Trends in Incidence, Clinical Features, and Cardiovascular Disease in Ankylosing Spondylitis Over Three Decades: A Population-Based Study. Arthritis Care Res (Hoboken) (2015) 67(6):836–41. doi: 10.1002/acr.22512
23. Walsh JA, Song X, Kim G, Park Y. Evaluation of the Comorbidity Burden in Patients With Ankylosing Spondylitis Using a Large Us Administrative Claims Data Set. Clin Rheumatol (2018) 37(7):1869–78. doi: 10.1007/s10067-018-4086-2
24. Fernandez-Carballido C, Martin-Martinez MA, Garcia-Gomez C, Castaneda S, Gonzalez-Juanatey C, Sanchez-Alonso F, et al. Impact of Comorbidity on Physical Function in Patients With Ankylosing Spondylitis and Psoriatic Arthritis Attending Rheumatology Clinics: Results From a Cross-Sectional Study. Arthritis Care Res (Hoboken) (2020) 72(6):822–8. doi: 10.1002/acr.23910
25. Zhao SS, Robertson S, Reich T, Harrison NL, Moots RJ, Goodson NJ. Prevalence and Impact of Comorbidities in Axial Spondyloarthritis: Systematic Review and Meta-Analysis. Rheumatol (Oxford) (2020) 59(Suppl4):iv47–57. doi: 10.1093/rheumatology/keaa246
26. Cheng YJ, Kanaya AM, Araneta MRG, Saydah SH, Kahn HS, Gregg EW, et al. Prevalence of Diabetes by Race and Ethnicity in the United States, 2011-2016. JAMA (2019) 322(24):2389–98. doi: 10.1001/jama.2019.19365
27. Pagidipati NJ, Peterson ED. Acute Coronary Syndromes in Women and Men. Nat Rev Cardiol (2016) 13(8):471–80. doi: 10.1038/nrcardio.2016.89
28. Smith SC Jr., Benjamin EJ, Bonow RO, Braun LT, Creager MA, Franklin BA, et al. Aha/Accf Secondary Prevention and Risk Reduction Therapy for Patients With Coronary and Other Atherosclerotic Vascular Disease: 2011 Update: A Guideline From the American Heart Association and American College of Cardiology Foundation. Circulation (2011) 124(22):2458–73. doi: 10.1161/CIR.0b013e318235eb4d
29. Exarchou S, Lie E, Lindstrom U, Askling J, Forsblad-d'Elia H, Turesson C, et al. Mortality in Ankylosing Spondylitis: Results From a Nationwide Population-Based Study. Ann Rheum Dis (2016) 75(8):1466–72. doi: 10.1136/annrheumdis-2015-207688
30. Bakland G, Gran JT, Nossent JC. Increased Mortality in Ankylosing Spondylitis Is Related to Disease Activity. Ann Rheum Dis (2011) 70(11):1921–5. doi: 10.1136/ard.2011.151191
31. El Maghraoui A. Extra-Articular Manifestations of Ankylosing Spondylitis: Prevalence, Characteristics and Therapeutic Implications. Eur J Intern Med (2011) 22(6):554–60. doi: 10.1016/j.ejim.2011.06.006
32. Kramer M, Monselise Y, Bahar I, Cohen Y, Weinberger D, Goldenberg-Cohen N. Serum Cytokine Levels in Active Uveitis and Remission. Curr Eye Res (2007) 32(7-8):669–75. doi: 10.1080/02713680701523147
33. Jawad S, Liu B, Agron E, Nussenblatt RB, Sen HN. Elevated Serum Levels of Interleukin-17a in Uveitis Patients. Ocul Immunol Inflammation (2013) 21(6):434–9. doi: 10.3109/09273948.2013.815786
34. Willerson JT. Systemic and Local Inflammation in Patients With Unstable Atherosclerotic Plaques. Prog Cardiovasc Dis (2002) 44(6):469–78. doi: 10.1053/pcad.2002.123782
35. Mourouzis K, Oikonomou E, Siasos G, Tsalamadris S, Vogiatzi G, Antonopoulos A, et al. Pro-Inflammatory Cytokines in Acute Coronary Syndromes. Curr Pharm Des (2020) 26(36):4624–47. doi: 10.2174/1381612826666200413082353
36. Ungprasert P, Srivali N, Kittanamongkolchai W. Risk of Coronary Artery Disease in Patients With Ankylosing Spondylitis: A Systematic Review and Meta-Analysis. Ann Transl Med (2015) 3(4):51. doi: 10.3978/j.issn.2305-5839.2015.02.05
37. Tsui FW, Tsui HW, Akram A, Haroon N, Inman RD. The Genetic Basis of Ankylosing Spondylitis: New Insights Into Disease Pathogenesis. Appl Clin Genet (2014) 7:105–15. doi: 10.2147/TACG.S37325
38. Simone D, Al Mossawi MH, Bowness P. Progress in Our Understanding of the Pathogenesis of Ankylosing Spondylitis. Rheumatol (Oxford) (2018) 57(suppl_6):vi4–9. doi: 10.1093/rheumatology/key001
39. Zhong Z, Su G, Kijlstra A, Yang P. Activation of the Interleukin-23/Interleukin-17 Signalling Pathway in Autoinflammatory and Autoimmune Uveitis. Prog Retin Eye Res (2021) 80:100866. doi: 10.1016/j.preteyeres.2020.100866
40. Abbas A, Gregersen I, Holm S, Daissormont I, Bjerkeli V, Krohg-Sorensen K, et al. Interleukin 23 Levels Are Increased in Carotid Atherosclerosis: Possible Role for the Interleukin 23/Interleukin 17 Axis. Stroke (2015) 46(3):793–9. doi: 10.1161/STROKEAHA.114.006516
41. Erbel C, Chen L, Bea F, Wangler S, Celik S, Lasitschka F, et al. Inhibition of Il-17a Attenuates Atherosclerotic Lesion Development in Apoe-Deficient Mice. J Immunol (2009) 183(12):8167–75. doi: 10.4049/jimmunol.0901126
42. Hsu YR, Huang JC, Tao Y, Kaburaki T, Lee CS, Lin TC, et al. Noninfectious Uveitis in the Asia-Pacific Region. Eye (Lond) (2019) 33(1):66–77. doi: 10.1038/s41433-018-0223-z
43. Rodriguez A, Akova YA, Pedroza-Seres M, Foster CS. Posterior Segment Ocular Manifestations in Patients With Hla-B27-Associated Uveitis. Ophthalmology (1994) 101(7):1267–74. doi: 10.1016/s0161-6420(94)31179-1
44. Velez G, Roybal CN, Colgan D, Tsang SH, Bassuk AG, Mahajan VB. Precision Medicine: Personalized Proteomics for the Diagnosis and Treatment of Idiopathic Inflammatory Disease. JAMA Ophthalmol (2016) 134(4):444–8. doi: 10.1001/jamaophthalmol.2015.5934
45. Rosenbaum JT, Bodaghi B, Couto C, Zierhut M, Acharya N, Pavesio C, et al. New Observations and Emerging Ideas in Diagnosis and Management of Non-Infectious Uveitis: A Review. Semin Arthritis Rheum (2019) 49(3):438–45. doi: 10.1016/j.semarthrit.2019.06.004
46. Neri P, Lettieri M, Fortuna C, Zucchi M, Manoni M, Celani S, et al. Adalimumab (Humira™) in Ophthalmology: A Review of the Literature. Middle East Afr J Ophthalmol (2010) 17(4):290–6. doi: 10.4103/0974-9233.71588
47. Neri P, Zucchi M, Allegri P, Lettieri M, Mariotti C, Giovannini A. Adalimumab (Humira™): A Promising Monoclonal Anti-Tumor Necrosis Factor Alpha in Ophthalmology. Int Ophthalmol (2011) 31(2):165–73. doi: 10.1007/s10792-011-9430-3
48. Pleyer U, Neri P, Deuter C. New Pharmacotherapy Options for Noninfectious Posterior Uveitis. Int Ophthalmol (2021) 41(6):2265–81. doi: 10.1007/s10792-021-01763-8
Keywords: acute coronary syndrome, ankylosing spondylitis, uveitis, cardiovascular disease, epidemiology
Citation: Feng KM, Chien W-C, Chen Y-H, Sun C-A, Chung C-H, Chen J-T and Chen C-L (2022) Increased Risk of Acute Coronary Syndrome in Ankylosing Spondylitis Patients With Uveitis: A Population-Based Cohort Study. Front. Immunol. 13:890543. doi: 10.3389/fimmu.2022.890543
Received: 06 March 2022; Accepted: 18 May 2022;
Published: 10 June 2022.
Edited by:
Michele Maria Luchetti Gentiloni, Marche Polytechnic University, ItalyReviewed by:
Piergiorgio Neri, Cleveland Clinic Abu Dhabi, United Arab EmiratesCopyright © 2022 Feng, Chien, Chen, Sun, Chung, Chen and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ching-Long Chen, ZG9jMzA4ODFAbWFpbC5uZG1jdHNnaC5lZHUudHc=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.