- Department of Endocrinology and Metabolism, Institute of Endocrinology, NHC Key Laboratory of Diagnosis and Treatment of Thyroid Diseases, The First Affiliated Hospital of China Medical University, Shenyang, China
Objective: The aim of the research is to study the association between the serum levels of autoantibodies against one important epitope (168FMILPVGAANFREAMR183, designated as P6) of α-enolase (ENO1-P6Abs) and miscarriage among euthyroid females with thyroid autoimmunity (TAI).
Methods: Anti-ENO1-P6 total IgG was investigated in 432 euthyroid women, and its four subclasses were analyzed in 184 euthyroid women. The serum FT4, TSH, TgAb, and TPOAb levels were determined using an electrochemiluminescence immunoassay. The serum ENO1-P6Ab and anti-protein disulfide isomerase A3 autoantibody (PDIA3Ab) levels were determined using an enzyme-linked immunosorbent assay.
Results: The serum levels of anti-ENO1-P6 total IgG, IgG2, IgG3, and IgG4 were significantly higher in euthyroid TAI females than in non-TAI controls. Additionally, anti-ENO1-P6 total IgG and its 4 subtypes were all markedly higher in euthyroid TAI females with pregnancy loss than those without miscarriage. Moreover, logistic regression analysis showed that highly expressed anti-ENO1-P6 total IgG, IgG1, IgG2, and IgG3 subtypes in the serum were all independent risk factors for euthyroid TAI-related miscarriage, and its IgG1 was also for non-TAI-related abortion. According to the trend test, the prevalence of miscarriage was increased in a titer-dependent manner with the raised levels of serum anti-ENO1-P6 total IgG and IgG1, IgG2, and IgG3 subtypes among euthyroid TAI females. The receiver operating characteristic curve analysis of anti-ENO1-P6 total IgG and IgG1, IgG2, and IgG3 subclass expressions in the serum for miscarriage prediction in euthyroid TAI females exhibited that the total areas under the curves were 0.773 ± 0.041, 0.761 ± 0.053, 0.827 ± 0.043, and 0.760 ± 0.050, respectively (all P <0.0001). Their corresponding optimal cut-off OD450 values were 0.68 (total IgG), 0.26 (IgG1), 0.97 (IgG2), and 0.48 (IgG3), with sensitivities of 70.8, 87.5, 83.3, and 85.4%, and specificities of 70.8, 59.1, 77.3, and 56.8%, respectively. There was an additive interaction between serum anti-ENO1-P6 and anti-PDIA3 total IgGs on the development of miscarriage (RERI = 23.6, AP = 0.79, SI = 5.37).
Conclusion: The highly expressed ENO1-P6Abs may be important risk factors for euthyroid TAI-related miscarriage. The serum levels of ENO1-P6Abs may become good predictive markers for pregnancy loss in euthyroid TAI females, especially its IgG2 subclass expression.
Introduction
Thyroid autoimmunity (TAI) is the most common autoimmune thyroid disease, which is usually characterized by high expression of anti-thyroperoxidase autoantibody (TPOAb) and anti-thyroglobulin autoantibody (TgAb) and local lymphocyte infiltration. The pathogenesis of the disease is still unclear in which genetic susceptibility, endogenous, and environmental factors work together (1). According to the Thyroid Disease, Iodine status, and Diabetes National Epidemiological (TIDE) survey in 31 Chinese provinces from 2015 to 2017, the prevalence of TAI was 20.35% in women and 8.16% in men (2). It has been found that TAI does not only affect the thyroid but also causes extra-thyroidal injuries (3). Stagnaro-Green et al. have previously reported that 17% of TAI women suffered from spontaneous abortion, whereas only 8.4% of women without TAI had miscarriage (4). Many of the later studies have demonstrated the raised risk of miscarriage in euthyroid TAI women undergoing either natural conception or in vitro fertilization (IVF) procedures due to subfertility (5–8). Although not clearly known, the potential mechanisms of spontaneous abortion in TAI women have been attributed to the direct effects of the classical anti-thyroid autoantibodies (ATA), high serum levels of thyrotropin (TSH), cellular immunity, and concurrent non-organ specific autoantibodies (NOSA) (9, 10). There is a lack of direct evidence about the effects of ATA on the occurrence of miscarriage among TAI. Previous studies analyzed the relationship between serum ATA titers and the risk of pregnancy loss in women, but they did not find the presence of titer-dependence (4, 11). The levothyroxine (LT4) treatment of euthyroid TAI women did not increase the live birth rate (12). No significant difference was found in the miscarriage rate between the LT4-treated group and the controls (17.1% vs. 19.9%) (13). Anti-protein disulfide isomerase A3 autoantibody (PDIA3Ab) is a NOSA. We have recently found that serum PDIA3Ab expression is independently associated with TAI-related miscarriage (14). It has been noticed that not only one NOSA can contribute to the development of spontaneous abortion in TAI women. Therefore, NOSA in the pathogenesis of pregnancy loss among euthyroid TAI women needs to be focused on.
α-enolase (ENO1) has been known as a multi-functional protein, such as a SUPER molecule on the surfaces of many eukaryotic cells, a c-myc promoter binding protein in the nuclei, and the key glycolytic enzyme in the cytosol (15, 16). The SUPER refers to Surface-exposed (during apoptotic cell death), Ubiquitously expressed, Protease sensitive, Evolutionary-conserved, and Resident normally in viable cells (15). ENO1 protein is widely distributed in various organs, such as the thyroid, brain, placenta, and uterus (17–20). Anti-ENO1 autoantibodies (ENO1Abs) have been found in a few autoimmune and inflammatory disorders (16). Their expression has also been found to be associated with unexplained recurrent miscarriage (uRM), endometriosis, premature ovarian failure, and tubal factor infertility (21–24). In our previous study, the increased serum levels of ENO1Abs were observed in mice with experimental autoimmune thyroiditis (EAT) induced only by Tg immunization (18). Those studies suggest that ENO1Abs may be involved in the development of TAI-related pregnancy loss. There are only a few studies on disease-specific epitopes (DSEs) of ENO1 as an autoantigen, and no investigation has been reported on either miscarriage or TAI. It is necessary to identify the responsible autoantigen epitopes, which can help better understand its pathogenesis and explore new predictive markers and therapeutic targets for the disease.
In the previous pilot study, we identified 18 potential immunodominant epitopes designated as P1–18 through the IEDB, ABCpred, APCpred, BCpred, Bepipred 2.0, DiscoTope2.0, and Ellipro databases. The P6 epitope refers to the amino acid (aa) 168–183 (i.e., FMILPVGAANFREAMR) in the ENO1 protein. The aa153–169 is involved in catalyzing the conversion of 2-phospho-D-glycerate (PGA) to phosphoenolpyruvate (PEP) during the glycolysis process (25). When the aa sequences of ENO1 from different species are compared, the aa168–175 region is highly conserved (25). Thus, we investigated the relationship between the expression of ENO1-P6Abs and the occurrence of pregnancy loss among euthyroid TAI females in this cohort study.
Materials and Methods
Study Design and Participants
All the participants in this retrospective investigation were recruited from the Subclinical Hypothyroid in Early Pregnancy (SHEP) study. The SHEP project was a large-scale population-based study involving 19 hospitals in the Liaoning Province of China to study the effects of thyroid abnormalities on pregnancy outcomes and the benefits of LT4 treatments (5, 26–29). The study was approved by the Medical Ethics Committee of China Medical University ([2012]2011-32-4). All participants completed consent forms when enrolled in the trial. All participants were asked to fill out the questionnaires about their personal information on the first visit, which included age, gestational age, alcohol use, smoking, residential city, educational background, family income, and history related to medication, diseases, reproduction, and family members. Height and weight measurements were performed by physicians, and body mass index (BMI) was calculated by weight/height2 (kg/m2). On the day of enrollment, all individuals underwent an ultrasound examination to confirm the presence of an ongoing intrauterine pregnancy. TAI was confirmed in those pregnant women with raised serum TPOAb (>34 IU/ml) and/or TgAb (>115 IU/ml) levels, for which reference ranges were provided by the manufacturer (Roche Diagnostics, Switzerland).
The inclusion criteria for this study were as follows: residing in the same region for at least 5 years; age 19–45 years; during the 4–12 weeks of pregnancy; urine iodine at 100–300 g/L (relatively sufficient iodine nutrition) (30); serum ferritin (SF) of ≥20 μg/L (no iron deficiency) (31), serum free thyroxine (FT4) and TSH within the trimester-specific reference ranges of 12.35–20.71 pmol/L and 0.14–4.87 mIU/L, respectively, according to our previous study (32). The exclusion criteria for this study were as follows: participants with thyroid diseases except TAI; intake of any drugs affecting thyroid functions; with a known personal history of inherited, other chronic or autoimmune inflammatory diseases (e.g., diabetes, hypertension, and antiphospholipid syndrome); with placenta previa, preterm labor, and placental abruption during pregnancy; or undergoing artificial abortion. The pregnancy outcome focused on by this study was miscarriage, which either occurred during this pregnancy or in the last 5 years. Following the inclusion and exclusion criteria, a total of 48 TAI women who experienced spontaneous pregnancy loss were eventually recruited into this study as the TAI-miscarriage group. The other three following groups were acquired through 1:2 or 1:1 random sampling using propensity score matching (PSM) for residential city, educational background, and family income based on the TAI-miscarriage group, respectively (Figure 1). The serum samples left were not enough for the examination of IgG subclasses in some participants, so 1:1 random sampling was performed. The TAI-non-miscarriage group referred to TAI women without pregnancy loss (N = 96 for anti-ENO1-P6 total IgG, and N = 44 for its IgG subclasses). Non-TAI-miscarriage group consisted of non-TAI women complicated by spontaneous pregnancy loss (N = 96 for anti-ENO1-P6 total IgG, and N = 46 for its IgG subclasses). The non-TAI-non-miscarriage group was made of non-TAI women without pregnancy loss (N = 192 for anti-ENO1-P6 total IgG, N = 46 for its IgG subclasses). Finally, anti-ENO1-P6 total IgG was investigated in 432 euthyroid women, while anti-ENO1-P6 IgG subclasses were analyzed in 184 euthyroid women. Among the TAI-miscarriage women for the analysis of anti-ENO1-P6 total IgG, 10.4% of females underwent a second-trimester miscarriage while the others had spontaneous abortions only in the first trimester. The TAI-miscarriage women for the analysis of anti-ENO1-P6 IgG subclasses were the same as those for the total IgG. Among the non-TAI women for the analysis of anti-ENO1-P6 total IgG, 12.5% experienced a miscarriage in the second trimester, while the others underwent pregnancy losses only in the first trimester. Among those non-TAI women for anti-ENO1-P6 IgG subclass analysis, 8.7% experienced a miscarriage in the second trimester, while the others underwent pregnancy losses only in the first trimester.
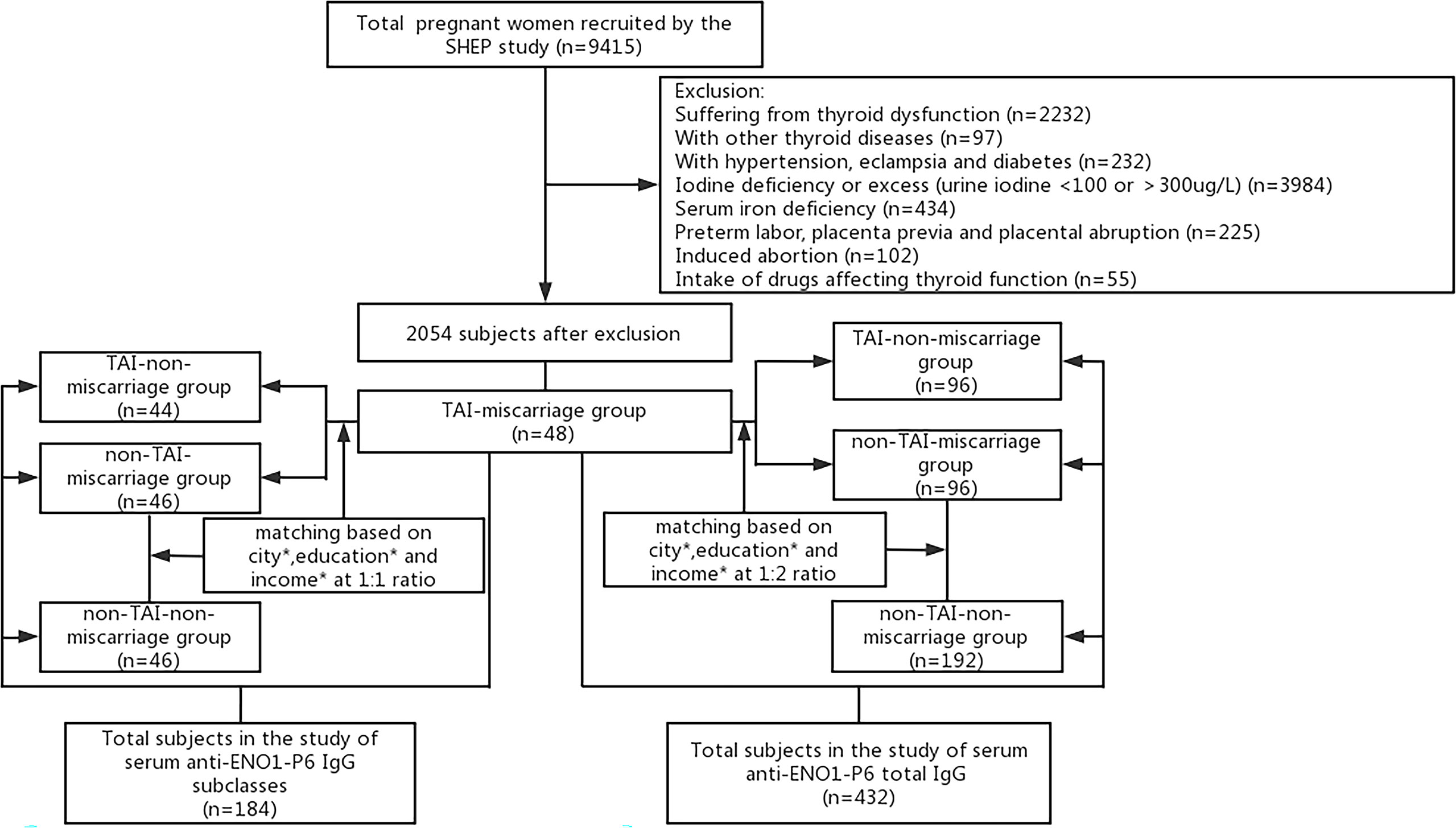
Figure 1 Flowchart of the inclusion and exclusion of participants in this study. *Residential city, educational background, family income.
Sample Collection and Laboratory Analysis
During the early pregnancy, blood and urine samples from each subject were collected in the morning following an overnight fast. The specimens were kept at −80°C until further examination. The serum FT4, TSH, TgAb, TPOAb, and SF levels, and urinary creatinine concentration were determined using an electrochemiluminescence immunoassay with the Cobas Elesys 601 (Roche Diagnostics, Switzerland) (32, 33). The ammonium persulfate method, based on the Sandell–Kolthoff reaction, was used to detect urinary iodine concentration (33). The ratio of urinary iodine to creatinine (UI/Cr) was then calculated.
Determination of ENO1-P6Abs and PDIA3Abs by ELISA
The serum anti-ENO1-P6 total IgG and its 4 subclass levels were measured by ELISA as previously described with slight modification (18). The recombinant ENO1-P6 peptide (FMILPVGAANFREAMR) was synthesized by Sangon Biotech (Shanghai, China). The microtiter plates were coated with recombinant ENO1-P6 peptide at either 10 ng per well for the examination of total IgG or 50 ng per well for the test of IgG subclasses. After incubation at 4°C overnight, the plates were further blocked with 1% bovine serum albumin (Sigma-Aldrich). Then they were incubated with the serum samples at a 1:250 dilution for total IgG measurement and at 1:50 for IgG subclasses for 2 h at 37 °C. After washing, the microtiter plates were further incubated with the HRP-labeled goat anti-human total IgG (Bioss, China) or HRP-labeled mouse anti-human IgG subclasses (Beijing Bersee, China), which were all diluted at 1:5,000. The enzymatic reaction was examined with TMB and terminated by hydrochloric acid. Finally, the absorbance values were measured at 450 nm using a microplate reader (Bio-Rad 680, Bio-Rad, CA, USA). The intra-assay coefficients of variation (CVs) were 2.7–9.5% (total IgG), 1.7–2.2% (IgG1), 1.2–5.8% (IgG2), 5.6–6.6% (IgG3), and 9.2–11.8% (IgG4), and the inter-assay CVs were 4.5–16.2% (total IgG), 0.2–11.3% (IgG1), 5.9–13.3% (IgG2), 0.3–10.9% (IgG3), and 6.3–11.9% (IgG4), respectively. The serum PDIA3Ab levels have been determined using ELISA as described in our previous study (14).
Statistical Analysis
All statistical analyses were performed using SPSS software (version 23.0, IBM Corporation, NY, USA) and the R statistical programming language. The Kruskal–Wallis test with post-hoc Bonferroni correction was adopted for the comparisons among the 4 groups, while the Mann–Whitney U test was used for the comparison between 2 groups. The frequencies of categorical variables were compared using the chi-square test (or Fisher’s exact test if required). The relationships between anti-ENO1-P6 total IgG and serum FT4, TSH, TPOAb, and TgAb levels were assessed by Spearman correlation analysis (SCA). Binary logistic regression analysis (LRA) was used to evaluate the independent risk variables for miscarriage. Receiver operating characteristic (ROC) curve analysis was used to determine the optimal cut-off values for serum ENO1-P6Abs. The trend test was used to examine the titer-dependent associations between serum TPOAb, TgAb, and ENO1-P6Ab levels and miscarriage prevalence in euthyroid TAI women. Logistic regression was used to estimate the multiplicative interaction. Statistical significance was determined by P <0.05. The calculation of the relative excess risk due to interaction (RERI), attributable proportion due to interaction (AP), and synergy index (SI) through the R package was used to analyze the additive interaction (34).
Results
Baseline Characteristics of Subjects
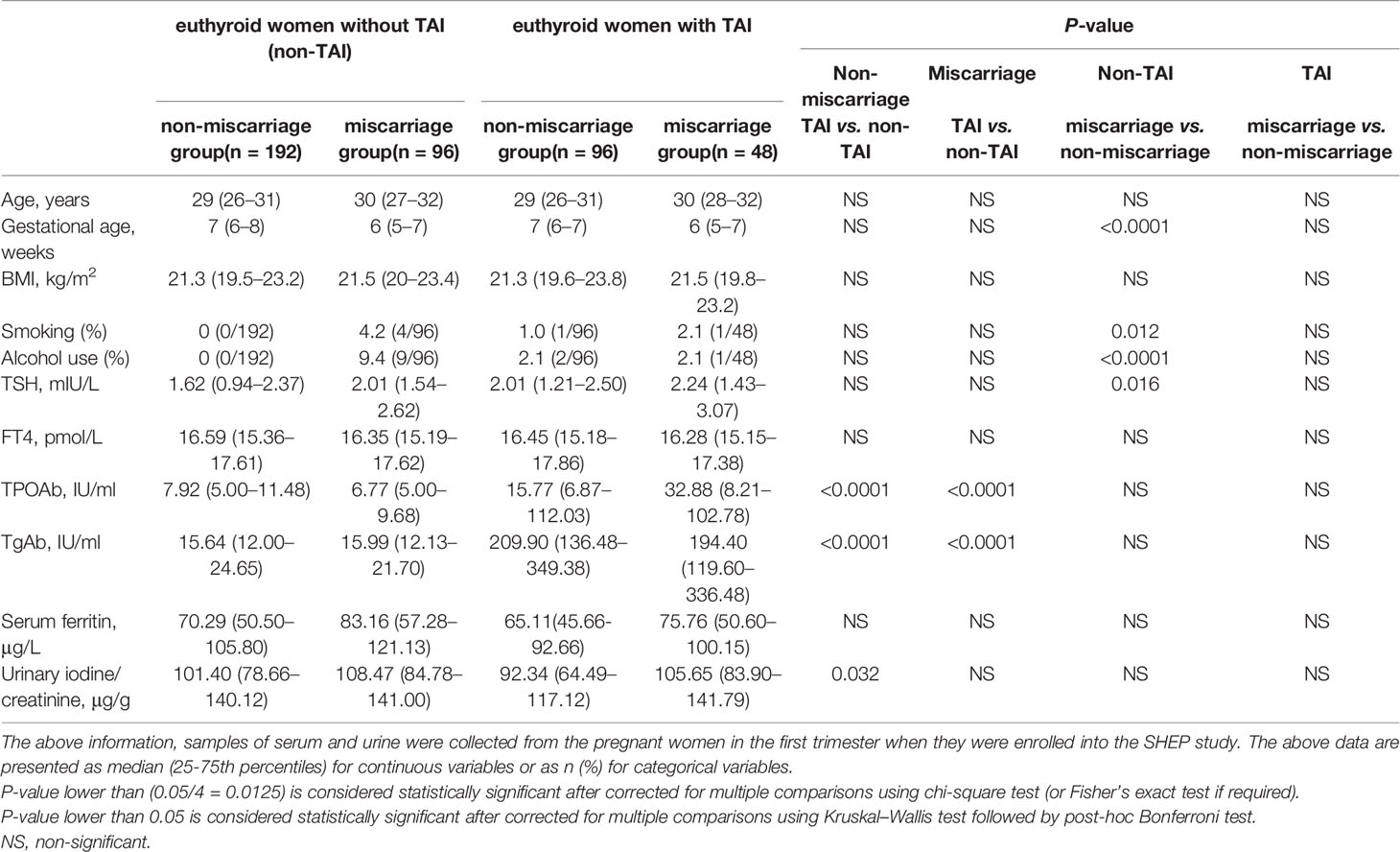
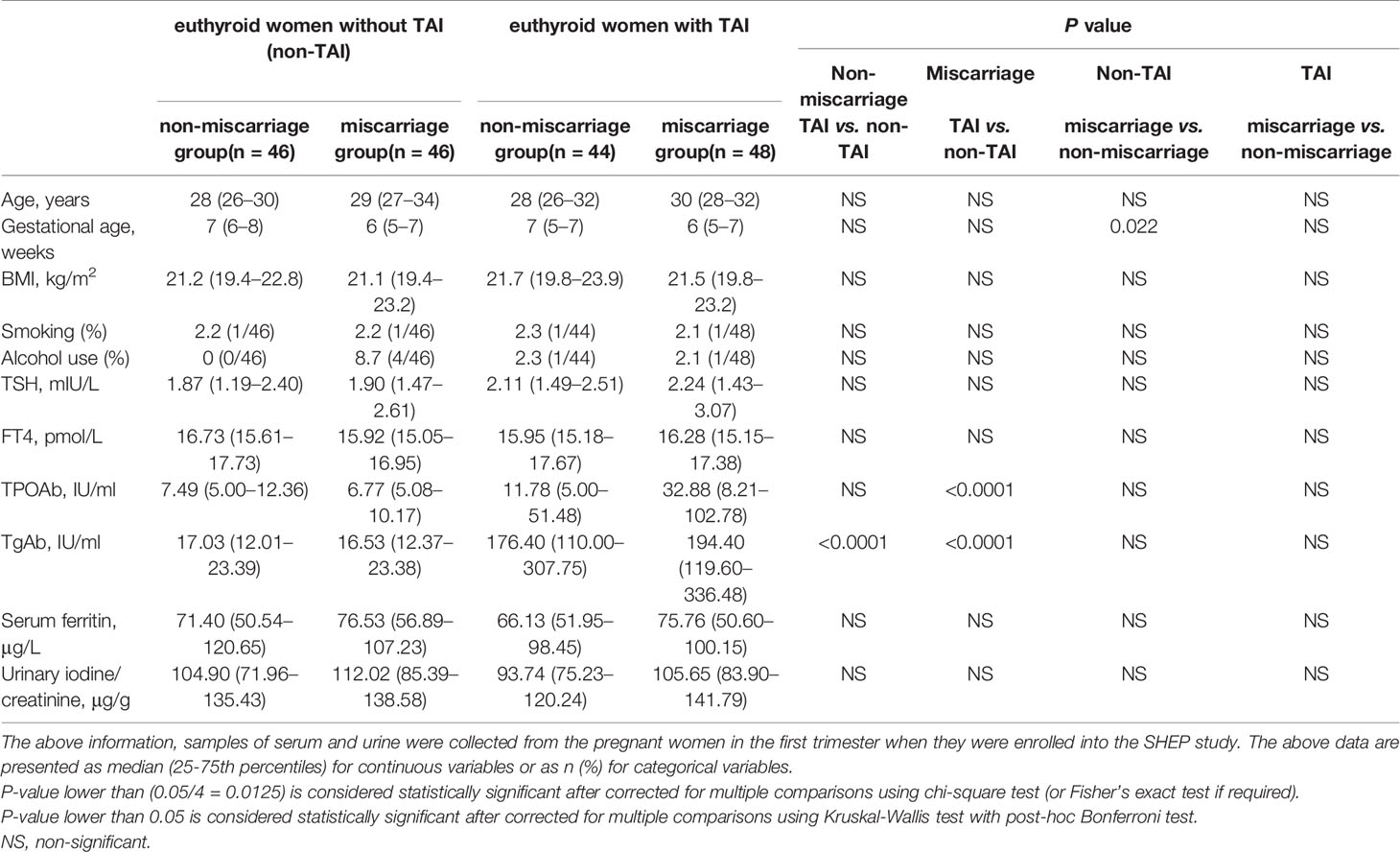
The baseline characteristics of the cohort are shown in Tables 1, 2. There were no significant differences in age, BMI, serum FT4, and SF levels (Table 1). Among the euthyroid non-TAI women, the percentage of those with a history of smoking, drinking and the serum TSH level were significantly higher, and the gestational age was markedly lower in the ones with than without miscarriage (Table 1). Nonetheless, they were not significantly different between the miscarriage and non-miscarriage groups with TAI. Among the groups of subjects enrolled in the study of anti-ENO1-P6 IgG subclasses, only the gestational age was pronouncedly lower in the non-TAI women with than in women without miscarriage (Table 2).
Comparisons of Serum ENO1-P6Abs
The serum anti-ENO1-P6 total IgG, IgG2, IgG3, and IgG4 subclass levels were significantly higher in euthyroid TAI females than non-TAI ones. Additionally, the serum anti-ENO1-P6 total IgG and its 4 subclass levels were markedly raised, especially IgG2, in the TAI-miscarriage group compared with those of the TAI-non-miscarriage group. Furthermore, they were markedly higher in the TAI-miscarriage group than the non-TAI miscarriage group except serum anti-ENO1-P6 IgG1 level. Interestingly, only the latter was significantly higher in non-TAI women with miscarriage than in women without miscarriage. These findings indicate that the highly expressed ENO1-P6Abs in the serum were associated with the development of TAI-related miscarriage (Figures 2, 3).
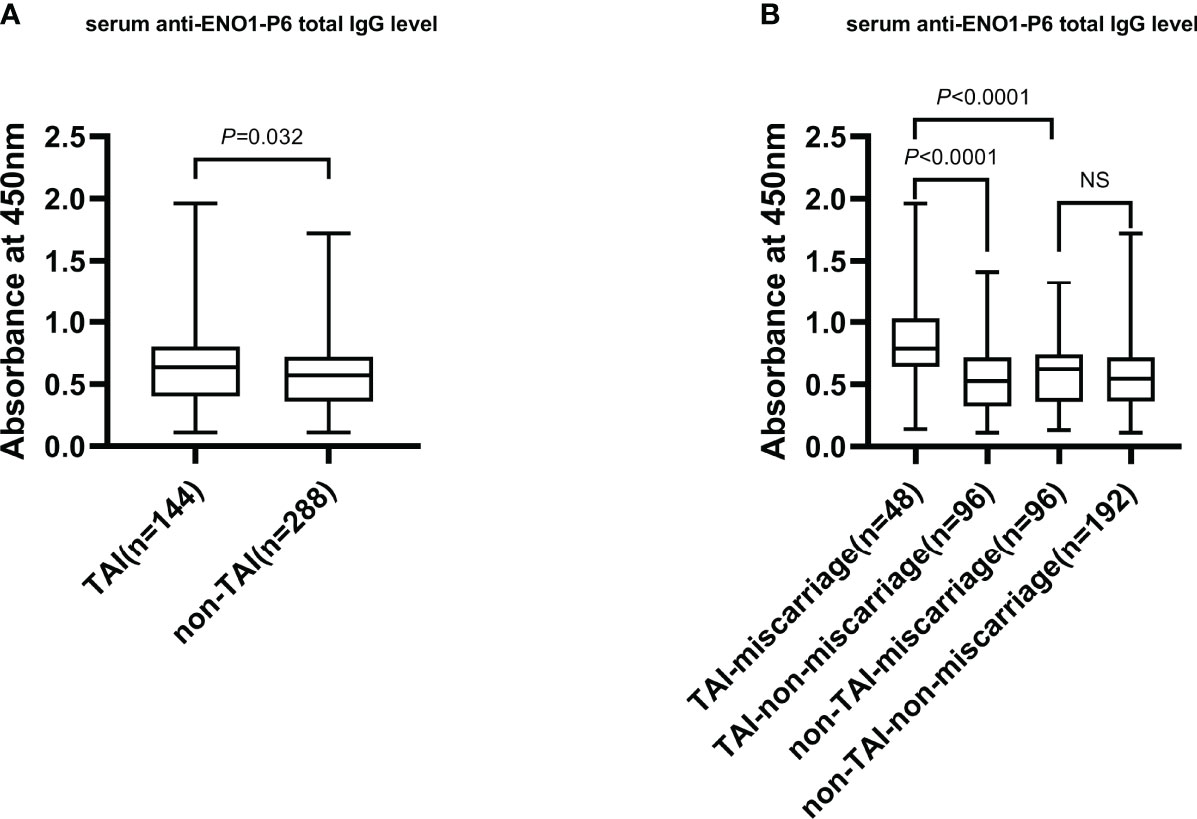
Figure 2 The serum total IgG level against ENO1-P6 epitope in all participants. The serum level of anti-ENO1-P6 total IgG was measured by ELISA, and reflected by the absorbance at 450 nm. Box plots are used to show the data, with boxes representing the 25th and 75th percentiles, bars representing the medians, and whiskers representing the minimum and maximum values. The Mann–Whitney U test (A) and the Kruskal–Wallis test followed by Bonferroni post-hoc analysis (B) were used to compare the results between the groups. NS, non-significant.
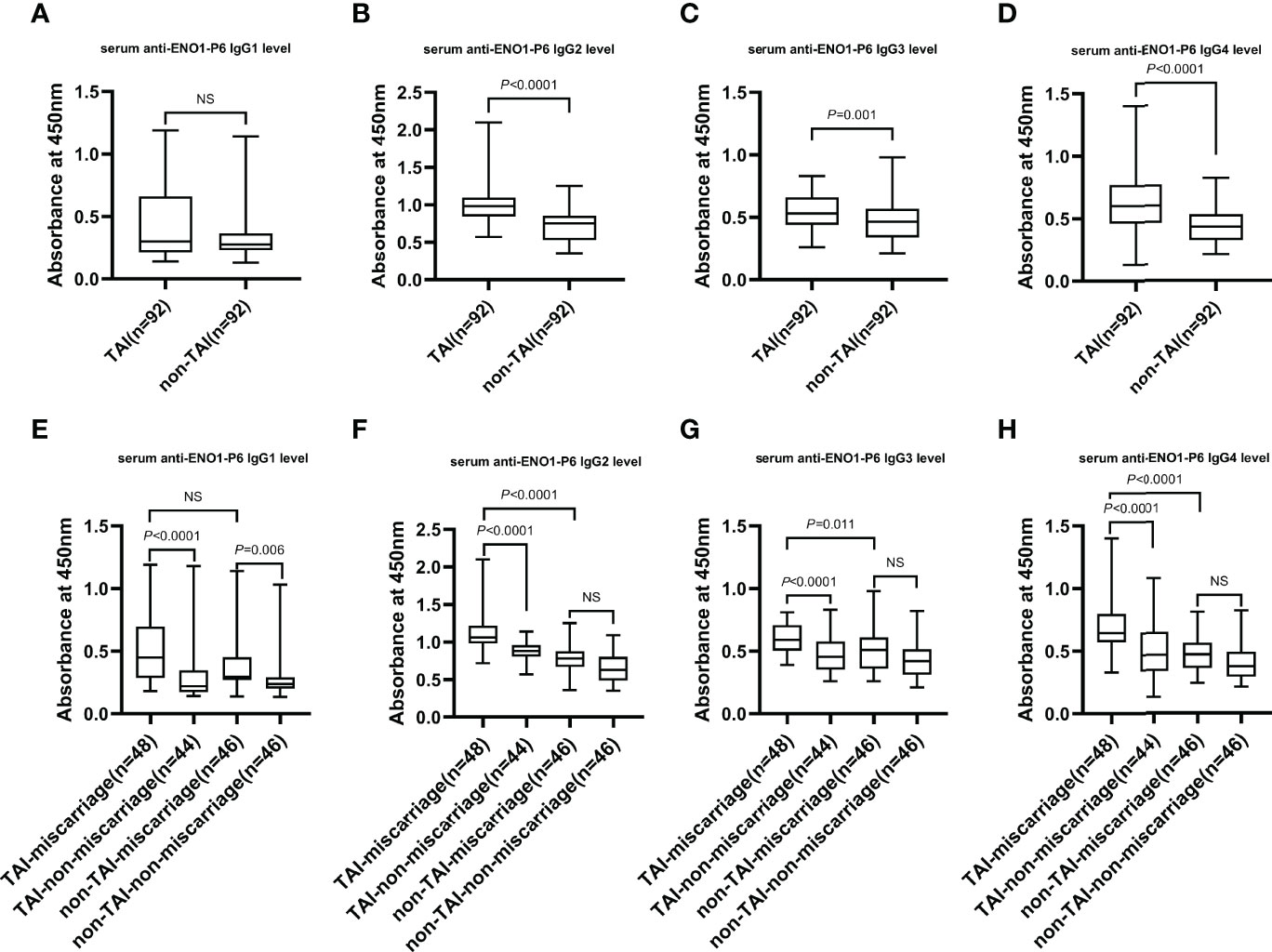
Figure 3 The serum levels of IgG subclasses against the ENO1-P6 epitope in all the participants. Serum anti-ENO1-P6 IgG subclass levels were determined by ELISA and reflected by the absorbance at 450 nm. Box plots are used to show the data, with boxes representing the 25th and 75th percentiles, bars representing the medians, and whiskers representing the minimum and maximum values. The Mann–Whitney U test (A–D) and the Kruskal-Wallis test followed by Bonferroni post-hoc analysis (E–H) were used to compare the results between the different groups. NS, non-significant.
Correlations of Anti-ENO1-P6 Total IgG With FT4, TSH, TPOAb, and TgAb in the Sera of Euthyroid TAI Women
The associations between serum FT4, TSH, TPOAb, TgAb, and anti-ENO1-P6 total IgG in euthyroid TAI women with and without pregnancy loss were analyzed by SCA, respectively. There was a weak correlation between serum anti-ENO1-P6 total IgG and TPOAb levels in TAI females with pregnancy loss but not in those without miscarriage (Table 3). These findings suggest that the predictive role of TPOAb expression in the occurrence of pregnancy loss reported in previous studies may be due to the concurrent existence of ENO1-P6Abs.

Table 3 Spearman correlation analysis of anti-ENO1-P6 total IgG expression with the levels of FT4, TSH, TPOAb, and TgAb among euthyroid TAI women.
Investigation of Independent Risk Factors for Miscarriage Among Euthyroid Women
Increased maternal age, obesity, iron deficiency, thyroid dysfunction, abnormal iodine nutritional status, smoking and drinking have been reported as potential risk factors for miscarriage in previous studies (6, 31, 35–37). In this study, the number of subjects with a smoking and alcohol use history were both too small to be included in the LRA. When ENO1-P6Ab expression was not included, the LRA showed that age, BMI, gestational age, UI/Cr, serum FT4, TSH, SF, TPOAb, and TgAb levels were not independently related to pregnancy loss in those euthyroid TAI females (Table 4). However, both the older age and the smaller gestational age were related to a raised risk of pregnancy loss in the euthyroid non-TAI females (Table 5). To investigate the independent relationships between the expression of ENO1-P6Abs and miscarriage among euthyroid TAI and non-TAI females, serum anti-ENO1-P6 total IgG was further included in Models 1–3 of LRA after natural logarithm-transformation and quartile stratification of the absorbance at 450 nm, respectively (Figure 4). Afterwards, serum levels of anti-ENO1-P6 IgG1-4 were included and analyzed in the LRA after quartile stratification of the absorbances at 450 nm (Figure 5). Not only the upraised serum total IgG but also the IgG1, IgG2, and IgG3 subclasses against ENO1-P6 were independently associated with an increased risk of pregnancy loss in euthyroid TAI females, while high serum anti-ENO1-P6 IgG1 expression was still independently related to a raised risk of miscarriage in euthyroid women without TAI. These findings further indicate that the expression of ENO1-P6Abs is an important and independent risk factor for pregnancy loss among euthyroid TAI females.
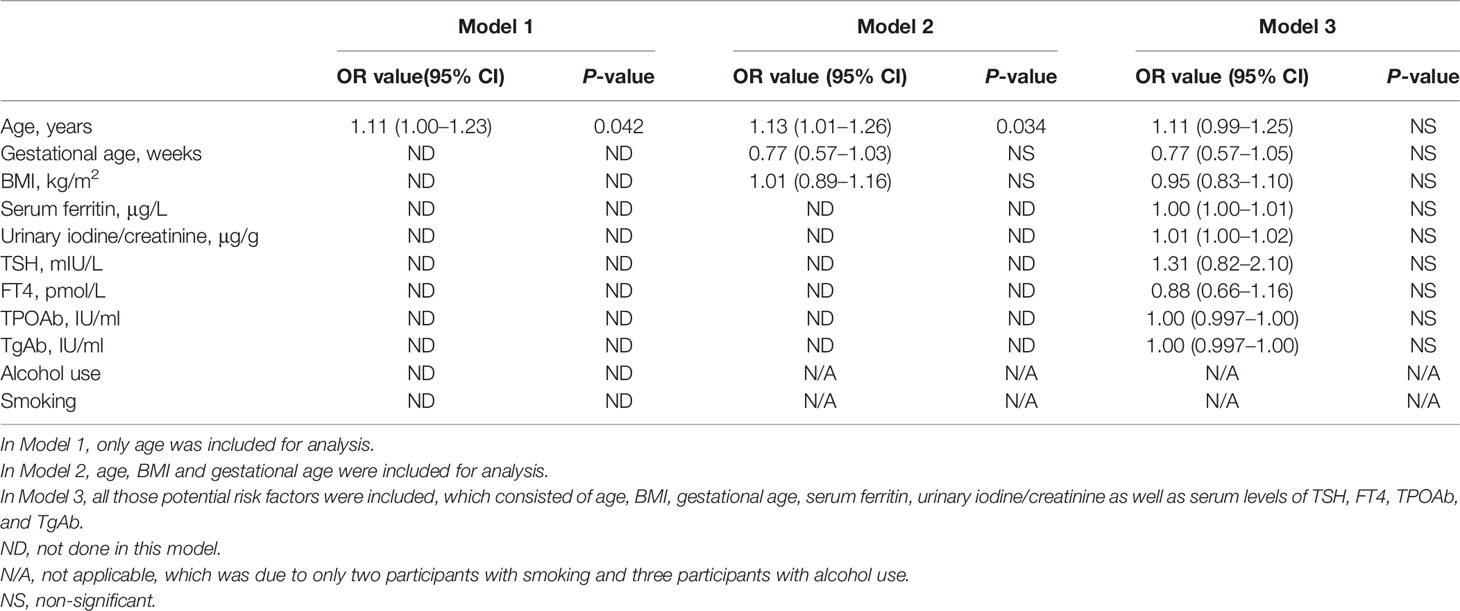
Table 4 Binary logistic regression analysis of the potential risk factors for miscarriage without ENO1-P6Abs included among the euthyroid TAI women.
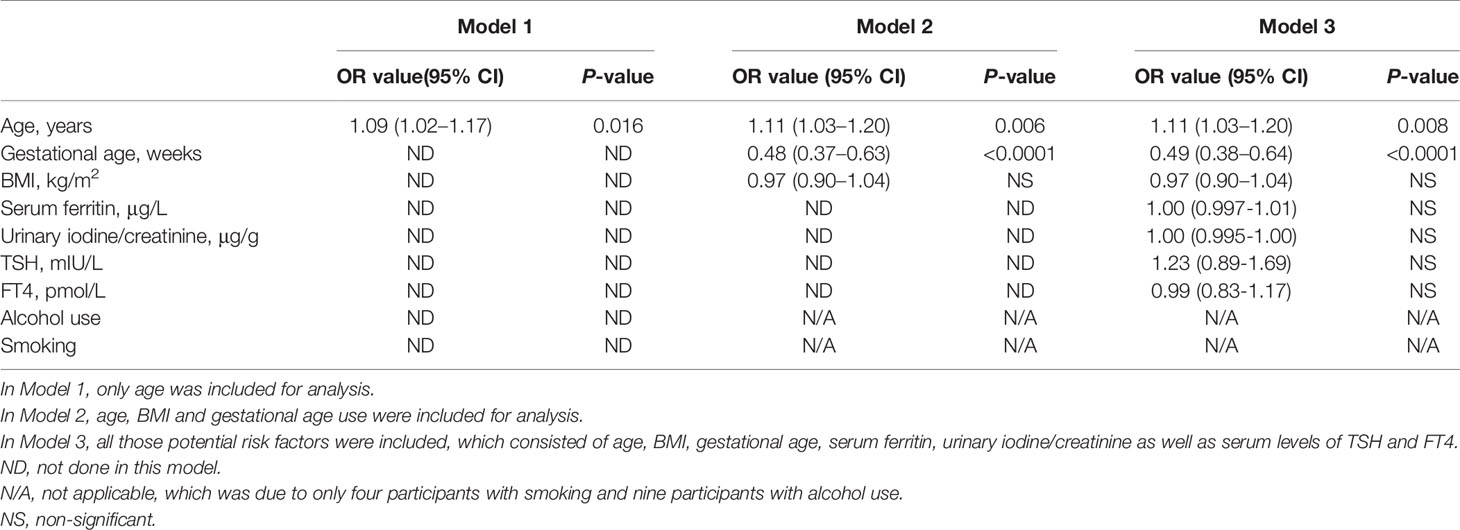
Table 5 Binary logistic regression analysis of the potential risk factors for miscarriage without ENO1-P6Abs included among the euthyroid women without TAI. .
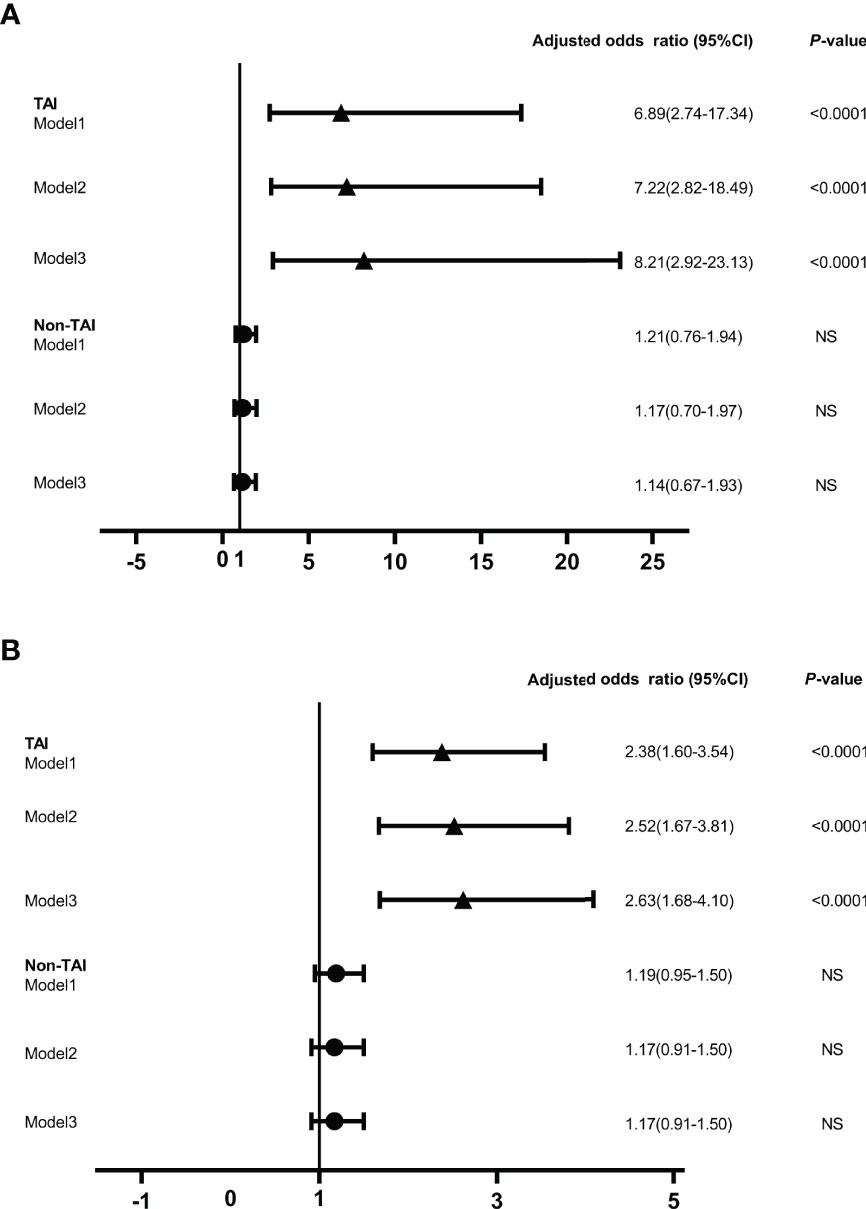
Figure 4 The odds ratios of serum anti-ENO1-P6 total IgG level associated with miscarriage in the euthyroid TAI females (n = 144) and non-TAI females (n = 288). Binary logistic regression analysis was conducted in Models 1–3 following the adjustment of the potential covariates. NS, non-significant. Model 1. Age was adjusted. Model 2. Age, BMI and gestational age were adjusted. Model 3. Age, BMI, gestational age, UI/Cr, serum ferritin, TSH, FT4, TPOAb, and TgAb levels (only in euthyroid TAI women) were adjusted. The serum level of anti-ENO1-P6 total IgG was included in the binary logistic regression analysis after natural logarithm-transformation (A) and quartile stratifications of absorbance at 450 nm (B).
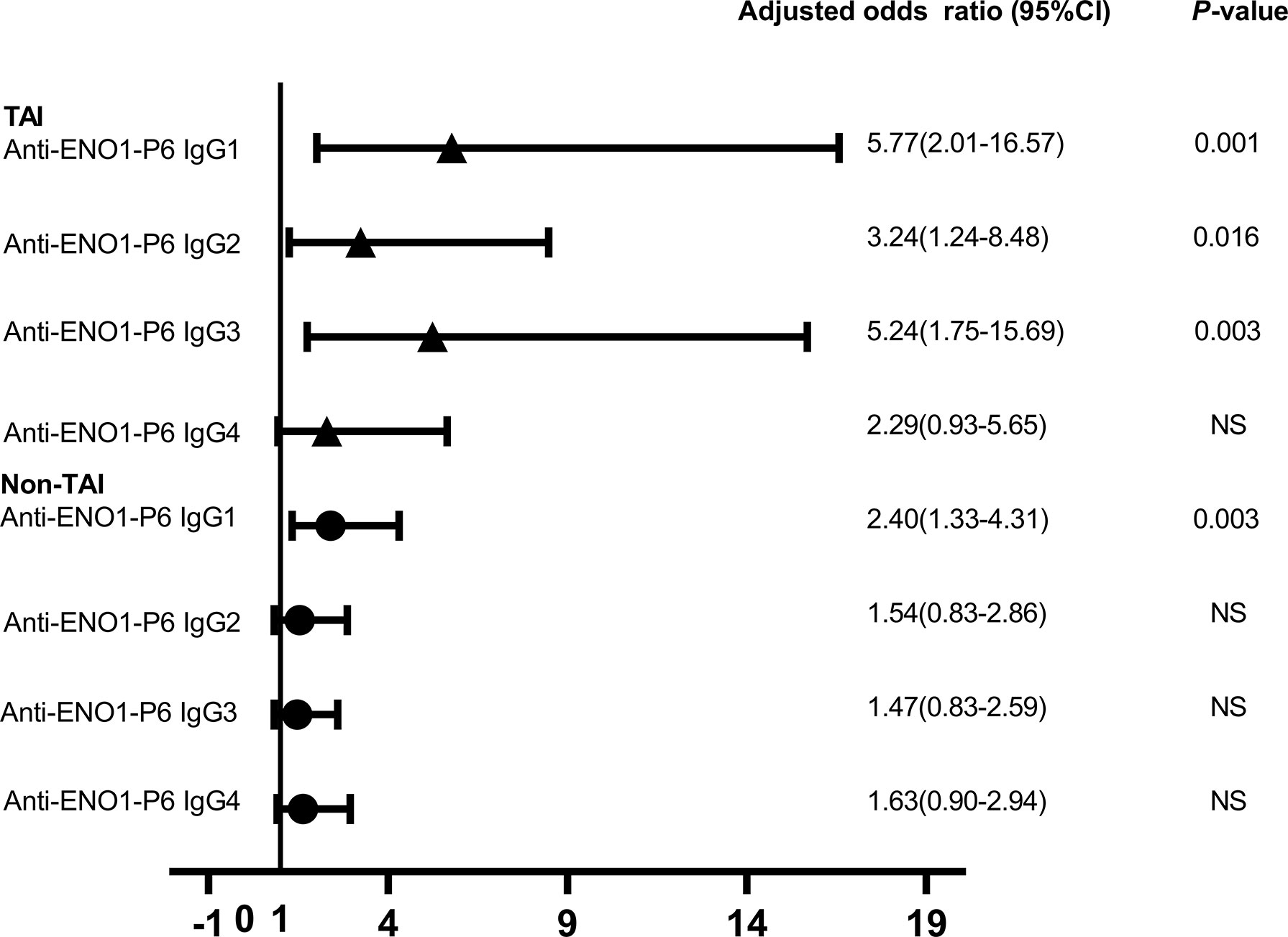
Figure 5 The odds ratios of serum anti-ENO1-P6 IgG subclass levels associated with the miscarriage in the euthyroid TAI females (n = 92) and those non-TAI females (n = 92). Binary logistic regression analysis was performed after adjusting for age, BMI, gestational age, UI/Cr, serum ferritin, TSH, FT4, TPOAb, and TgAb levels (only in euthyroid TAI women). NS, non-significant. Serum levels of anti-ENO1-P6 IgG subclasses after quartile stratifications of absorbance at 450 nm were included in the logistic regression analysis.
The Predictive Value of Serum ENO1-P6Abs in Euthyroid TAI Women
Based on the above findings, ROC curves and titer-dependence trend analysis were used to further explore the predictive values of serum ENO1-P6Ab expressions in the occurrence of pregnancy loss among euthyroid TAI females. The area under the ROC curve (AUC) of anti-ENO1-P6 IgG2 was the largest (0.827 ± 0.043, Figure 6). Based on the largest Youden index (0.416, 0.466, 0.606, and 0.422), the corresponding cut-off OD450 values of serum ENO1-P6Abs were 0.68 (total IgG), 0.26 (IgG1), 0.97 (IgG2), and 0.48 (IgG3), respectively (Figure 6). After adjusting for age, gestational age, BMI, UI/Cr, serum FT4, TSH, SF, TPOAb, and TgAb levels, it was determined that each 0.1 OD value increase in serum anti-ENO1-P6 total IgG, IgG1, IgG2, and IgG3 subclass levels above the corresponding cut-off values (0.68, 0.26, 0.97, and 0.48) was associated with the 45.4% (total IgG), 19.5% (IgG1), 307% (IgG2), and 221% (IgG3) higher risks of pregnancy loss in euthyroid TAI females, respectively. Additionally, each of those Ab levels was classified into 4 grades for titer-dependence trend analysis based on either the quartiles or upper limits of reference ranges. The prevalence of miscarriage was significantly raised with the increased serum anti-ENO1-P6 total IgG, IgG1, IgG2, IgG3, and IgG4 levels in euthyroid TAI women (Figures 6F–J). However, it did not show a titer-dependent change with the increase in serum TPOAb or TgAb levels (Figure 7). The above results indicate that serum ENO1-P6Abs can become important prediction factors for the occurrence of pregnancy loss in euthyroid TAI females, and especially its IgG2 subclass, may exhibit optimistic sensitivity and specificity. Interestingly, when the anti-ENO1-P6 IgG4 subclass was separately analyzed, the P-values of the ROC and titer-dependent trend analyses were both less than 0.05 (Figure 6). However, when all the four IgG subclasses were included in the LRA, anti-ENO1-P6 IgG4 was not an independent risk factor for miscarriage in euthyroid TAI females (Figure 5).

Figure 6 Relationship between anti-ENO1-P6 total IgG and IgG1, IgG2, IgG3, and IgG4 subclass expressions in the serum and the development of miscarriage in euthyroid TAI females. The ROC curve of the anti-ENO1-P6 total IgG and IgG1, IgG2, IgG3, and IgG4 subclass expressions for miscarriage prediction (A–E), and the titer-dependent association between serum anti-ENO1-P6 total IgG and IgG1, IgG2, IgG3, and IgG4 subclass levels after quartile stratifications and the prevalence of miscarriage (F–J). NS, non-significant. The serum levels of ENO1-P6Abs were determined by ELISA, and reflected by the absorbance at 450 nm.
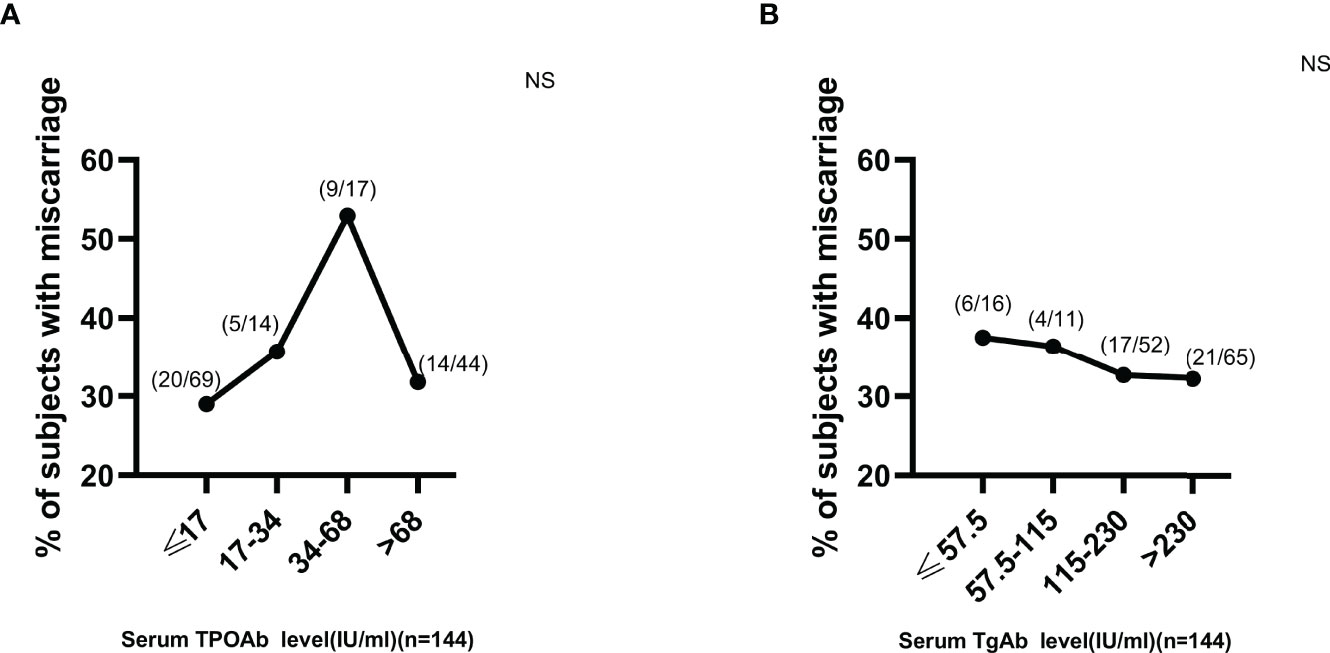
Figure 7 No titer-dependent effect of either serum TPOAb or TgAb expression on miscarriage development in euthyroid TAI females. NS, non-significant. The manufacturer (Roche Diagnostics, Switzerland) provided the reference ranges for serum TPOAb (≤34 IU/ml) and TgAb (≤115 IU/ml).
The Correlation of Serum Anti-ENO1-P6 and Anti-PDIA3 Total IgGs in Euthyroid TAI Women
We have recently found that serum PDIA3Ab expression was also independently associated with TAI-related miscarriage (14). It was further investigated whether there was any interaction between anti-ENO1-P6 and anti-PDIA3 total IgG expressions in TAI-related miscarriage. Thirty-six TAI women with miscarriage and 66 matched TAI women without miscarriage who had been tested for both the two Abs in the sera were recruited in this pilot study. Serum anti-ENO1-P6 and anti-PDIA3 total IgGs were both included with the above potential risk factors in Models 1–3 of LRA after natural logarithm-transformation. Both of them were independent risk factors for spontaneous abortion in euthyroid TAI females (Supplementary Table 1). According to corresponding cut-off values of anti-ENO1-P6 and anti-PDIA3 total IgGs (14), the serum levels of two Abs were converted to binary variables. The binary variables were used to analyze interactions. No multiplicative interaction between anti-ENO1-P6 and anti-PDIA3 total IgGs was observed (OR = 2.27, 95% CI = 0.26, 19.88, P = 0.458). However, there was an additive interaction between anti-ENO1-P6 and anti-PDIA3 total IgGs on the development of miscarriage (RERI = 23.6, 95% CI = −18.1, 65.3; AP = 0.79, 95% CI = 0.47, 1.11; SI = 5.37, 95% CI = 1.03, 27.87). SCA was used to analyze the association between the serum expressions of anti-ENO1-P6 and anti-PDIA3 total IgGs among euthyroid TAI females with and without pregnancy loss. The results showed that there was a positive correlation between anti-ENO1-P6 and anti-PDIA3 total IgG levels in the TAI-miscarriage group (P = 0.027; rs = 0.368), but not in the TAI-non-miscarriage group (P = 0.317; rs = 0.125). These results indicate that both ENO1-P6Ab and PDIA3Ab are independent risk factors for pregnancy loss among euthyroid TAI women. Our previous studies have suggested that they may be involved in the development of extrathyroidal damage (e.g., encephalopathy) through different mechanisms in TAI individuals (18, 38), which needs to be further investigated for the occurrence of miscarriage.
Discussion
TAI is the most prevalent autoimmune disorder, affecting up to 20.35% of women (2). There was a 2–3 times higher prevalence rate of pregnancy loss among euthyroid TAI women than non-TAI women (5). However, it is still unknown why TAI women are more prone to miscarriage. TPOAb and TgAb are important markers for the existence of TAI. Some studies have examined the relationship between the expression of ATA and pregnancy loss among TAI females. However, the direct and independent relationship between the serum ATA levels and the occurrence of pregnancy loss has not been demonstrated yet (4, 5, 11, 39–43). In a previous study, LRA indicated that TPOAb positivity was not associated with a raised risk of poor pregnancy outcomes in euthyroid women (41). The current study also systematically investigated the potential risk factors for pregnancy loss among euthyroid TAI women, namely, age, gestational age, BMI, UI/Cr, SF, serum TSH, FT4, TPOAb, and TgAb levels. Only age and gestational age were independently related to the occurrence of pregnancy loss in euthyroid non-TAI women. There was no independent relationship between the risk of pregnancy loss and the above factors among euthyroid TAI women. In previous studies, no titer-dependent association was found between the serum levels of ATA and pregnancy loss (4, 11). This study did not show any independent correlation of serum TPOAb or TgAb expression with the occurrence of miscarriage or any titer-dependent relationship between them in euthyroid TAI women.
There may be complex mechanisms for TAI-related miscarriage. Although the specific mechanisms have not been clearly known yet, the abnormal activation of the maternal immune system may result in pregnancy loss (9, 10, 44). NOSA has received much attention besides T, NK cell abnormalities, and concurrent autoimmune diseases (9, 45). Liu et al. found no significant difference in the proportions of peripheral CD3+T, CD4+T, CD8+T, NK, and B cells between recurrent miscarriage patients with and without TAI (46). The findings indicate that there may be a mild correlation between abnormal cellular immunity and TAI-related miscarriage. It has been reported that euthyroid TAI women have one or more NOSA expressed in their sera (47). One of the important NOSA related to miscarriage is antiphospholipid Abs (aPLs) (48). APLs are a family of autoantibodies that recognize various combinations of phospholipids such as cardiolipin, phosphatidylserine, phosphatidylethanolamine, and phospholipid-binding proteins. The latter mainly include β2 glycoprotein-I, prothrombin or annexin-V, and protein-Z. These Abs have been linked to a possible contribution to reproductive failure (49). Anticardiolipin Abs, anti-β2-glycoprotein I Abs, and lupus anticoagulant are usually determined to screen for antiphospholipid syndrome (50). However, aPLs have been found to explain only a tiny part of the occurrence of miscarriage in patients with polyautoimmunity (51). Besides, another study has reported that patients with aPLs alone had higher percentages of spontaneous pregnancies and live births when compared with those positive for anti-thyroid antibodies alone or with aPLs (52). Antinuclear Abs (ANAs) are indeed common in NOSA under the conditions of many autoimmune diseases. The prevalence of ANA by indirect immunofluorescence has been reported as 5.92% in China (53). The frequency of serum ANA positivity has been found to be significantly higher in the patients with recurrent miscarriage (20.6%) than in the control females (6.7%) (54). One study has compared the prevalence of NOSA (ANA, antiphospholipid antibodies, anti-dsDNA, anti-ssDNA, anti-histone, and anti-Scl70 autoantibodies) between TAI and non-TAI women, who all presented a history of recurrent pregnancy loss or unexplained infertility (47). The total prevalence of anti-dsDNA, anti-ssDNA, anti-histone, and anti-Scl70 was significantly higher in women with TAI (35.2%) than in those without TAI (11.4%). However, there was no significant difference in the positive prevalence of either aPLs or ANA between women with TAI (aPLs 46.3%, ANA 29.6%) and those without TAI (aPLs 43.6%, ANA 28.0%) (47). Another study has found that the positivity rate of thyroid Abs in women with recurrent miscarriage was increased, while the expression of ANA was not detectable (55). Both studies have indicated that ANA may not be the main specific factor contributing to TAI-related miscarriage. Although we have recently found that PDIA3Ab may be involved in TAI-related miscarriage, there are rare studies on the relationships between NOSA expressions and this disease. In this investigation, we focused on the role of another NOSA in the development of TAI-related miscarriage.
ENO1 is a multifunctional protein located in the cellular nucleus, cytoplasm, and surface that plays a vital role in glucose metabolism and inflammatory responses (15, 16, 56). ENO1 has also been known as a SUPER protein and is expressed on the surface of early apoptotic cells, which can trigger an autoimmune response (15, 16, 57). ENO1Abs have been reported in various autoimmune diseases, such as Hashimoto’s encephalopathy (HE), Behcet’s disease, rheumatoid arthritis (RA), multiple sclerosis, cancer-associated retinopathy (CAR), and lupus nephritis, and its positivity prevalence in the serum is found to be about 6–83% (16, 18, 58–63). Our previous study found that ENO1 protein was expressed in thyroid tissue, and serum ENO1Ab levels were significantly increased in the mice with EAT established by only Tg immunization (18). It further indicates that ENO1Abs can be induced in situ, which may be due to increased thyrocyte apoptosis and/or intermolecular epitope diffusion in the development of TAI but not caused by the other co-occurrent autoimmune diseases (64, 65).
Additionally, ENO1 expression has been found in placental and decidua tissues (19, 21, 66). It was significantly decreased in the placentas of uRM patients compared with that of normal pregnant women (19). A recent study has reported that serum ENO1Abs were significantly increased in uRM patients and could inhibit the production of β-hCG and progesterone from trophoblast cells cultivated in vitro, which may result in the occurrence of miscarriage (21). ENO1Abs have been considered a kind of anti-endometrial Ab existing in the sera of endometriosis patients and are potentially responsible for autoimmune premature ovarian failure (22, 23). ENO1Ab expression was also found prevalently in tubal factor infertility patients and was associated with IVF implantation failure (24). All those studies suggest that ENO1Abs play an important role in the occurrence of miscarriage, and involved in TAI-related miscarriage.
Since ENO1Abs have been found in various autoimmune diseases, the DSEs need to be clarified. However, they have been investigated in only a few studies. ENO1Abs in the sera of HE patients have been shown to specifically recognize the amino-terminal epitopes (aa1-157) from ENO1 (67). The epitope aa26-40 of ENO1 was specifically recognized by the serum ENO1Abs in RA patients (59). The epitopes aa31–38, aa176–183, and aa421–428 of ENO1 were mainly recognized by ENO1Abs from both CAR patients and healthy controls, while epitope aa56–63 was recognized only by those from CAR patients (62). The epitopes recognized by ENO1Abs separated from the sera and renal biopsy samples of lupus nephritis patients have been investigated. Its IgG2 subtype specifically recognized epitope aa170–182 and its IgG4 subtype recognized aa 98–165 (58). Another two linear regions of ENO1, aa53–87 and aa207–238, were specifically recognized by ENO1Abs from endometriosis patients (22). However, the target epitopes of ENO1 specific to miscarriage due to various causes have not been investigated yet.
In this study, we have performed a series of analyses to explore whether the Abs against the epitope aa 168–183 of the ENO1 protein (P6) were involved in the development of TAI-related miscarriage. This epitope was achieved by bioinformatics analysis using the IEDB, BCpred, and Ellipro databases. We further found that the serum levels of total IgG and IgG2, IgG3, and IgG4 subclasses against ENO1-P6 epitope were markedly higher in TAI females than in non-TAI controls, and especially raised among euthyroid TAI females with miscarriage. LRA showed that either anti-ENO1-P6 total IgG or IgG1, IgG2, and IgG3 subtypes were independent risk factors for pregnancy loss among euthyroid TAI patients, whereas their IgG1 subtype was also independently associated with pregnancy loss among non-TAI women. The serum levels of anti-ENO1-P6 total IgG and IgG1, IgG2, and IgG3 subclass exhibited titer-dependent positive associations with the prevalence of pregnancy loss among euthyroid TAI women. The findings indicate that ENO1-P6Abs may play a critical role in the pathogenesis of TAI-related pregnancy loss and may become the predictive markers for TAI-related miscarriage. The ROC curve analyses suggest that serum ENO1-P6 IgG2 is an optimistic predictive marker based on its specificity and sensitivity. It was found that every 0.1 OD value higher anti-ENO1-P6 IgG2 level above the corresponding cut-off value (i.e., 0.97) was related to a 307% higher risk of miscarriage in euthyroid TAI women. Besides, serum TPOAb levels in euthyroid TAI females with pregnancy loss were indeed positively correlated with the expression of serum ENO1-P6-specific total IgG. This suggests that the raised risk of pregnancy loss among TPOAb-positive women may be due to the presence of ENO1-P6Abs besides PDIA3Ab (14).
At present, the precise biological functions of the ENO1-P6 epitope have not been fully understood. According to a previous study (25), the P6 epitope may be involved in maintaining the glycolytic enzymatic activity of ENO1. As a glycolytic enzyme, the loop (aa153–169) of ENO1 donates a proton to the phosphoryl of PGA, and the proton shared by Glu168 and Glu211 forms a hydrogen bond with the PGA hydroxyl group (25). It indicates the ENO1-P6 epitope participates in the conversion of PGA to PEP in the glycolytic pathway. It is well known that glycolysis is the main energy source to support normal placental development and successful pregnancy in the first trimester when the placenta has to endure the conditions of physiologically low oxygen (68, 69). Therefore, we infer that the direct binding of ENO1-P6Abs to the epitope may inhibit the glycolysis process, reduce the energy supply to the placenta and the embryo, and finally contribute to the occurrence of miscarriage. In addition, the increased serum anti-ENO1-P6 IgG1, IgG2, and IgG3 may bind with ENO1-P6 epitope on the trophoblasts, and cause their damages since all these 3 subclasses have strong binding and activating capacities to complement system and FcR fragments on the lymphocytes (70). Previous studies have been found that ENO1Abs damaged the thyrocytes through antibody-dependent cell-mediated cytotoxicity (ADCC) and injured the pancreatic ductal adenocarcinoma cells through complement-dependent cytotoxicity (CDC) (18, 71). Previous studies have indicated that uncontrolled complement activation may lead to fetal loss (72). Also, abnormal expression of complement regulators and antibodies to trophoblast surface antigens may be implicated in excess complement activation among females with recurrent spontaneous abortions (73). In autoimmune and inflammatory diseases, ENO1Abs may result in endothelial damage by generating immune complexes, activating the complement cascade, and inhibiting the binding of plasminogen to ENO1 so as to disrupt the intravascular and pericellular fibrinolytic systems and result in cell death (16). Bruschi et al. found that IgG2 was the predominant subtype of the ENO1Abs in the serum and glomerulus of LN patients (58). Thus, we deduce that the ENO1-P6Abs may also damage trophoblast cells through ADCC and/or CDC. The specific mechanisms will be further explored in the future, which may bring new therapeutic strategies for the occurrence of TAI-related pregnancy loss.
However, there are several limitations to this cohort study. This is a retrospective study with a relatively small number of subjects. A larger-sample prospective investigation needs to be performed in the future. The mechanism related to the production of ENO1Abs has not been known yet, which will be further investigated in our future work. Additionally, we did not explore the related mechanisms for ENO1-P6Abs to be involved in the development of TAI-related miscarriage in this study. However, we have designed a series of experimental studies for them. The ENO1Abs may interfere with the enzymatic activity and other physiological functions of ENO1 in the tissue cells and mediate cell injury via antibody-dependent cytotoxicity (18, 21). The placenta and decidua tissues have been collecting in the newly enrolled participants. We would use western blot and an ENO1 activity assay kit (Abcam) to measure the level and activity of ENO1 in the homogenates of those tissues in the future. It may help explain whether the ENO1-P6Abs affect miscarriage by directly inhibiting ENO1 enzymatic activity. We admit that ENO1-P6Abs are not the sole Abs involved in the pathogenesis of TAI-related pregnancy loss, since another potentially pathogenic Ab (i.e., PDIA3Ab) has been recently found (14). However, this study has shown that they are both independent risk factors for the occurrence of TAI-related miscarriage with synergistic additive effects. Our previous studies have suggested that the target autoantigens and potential mechanisms are different for ENO1Abs and PDIA3Abs (14, 38). Although this study did not directly determine some other NOSA, including ANA and aPLs, and those participants with a known personal history of other chronic or autoimmune inflammatory diseases, including ANA or aPLs positivity, had been excluded from this study, we would conduct a larger-scale prospective study to further observe the relationship between the expressions of all known NOSA, including ANA and multiple aPLs, and the occurrence of miscarriage in euthyroid TAI women in the future, so as to establish a biomarker panel for better predicting their pregnancy loss risk.
The findings from this study have revealed that the expressions of ENO1-P6Abs are important independent risk factors for pregnancy loss among euthyroid TAI women. ENO1-P6Abs, especially its IgG2 subclass, may be used as a new predictive biomarker for TAI-related miscarriage. Based on the previous studies, it can be deduced that ENO1-P6Abs may contribute to the risk of pregnancy loss in euthyroid TAI females through some complex mechanisms, potentially including interference with the glycolysis process, injury of trophoblast cells, and inhibition of pregnancy-related hormone production. Experimental studies on the potential mechanisms have been designed and will be completed in the near future. As a relatively specific antigenic epitope, ENO1-P6 and its Abs may become new targets for the intervention of TAI-related miscarriage.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by Medical Ethics Committee of China Medical University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
XH—formal analysis, investigation, and writing-original draft. YL—methodology and investigation. HW—investigation, data curation, and project administration. WS—methodology and investigation. YL—methodology and investigation. ZS—resources, supervision, and funding acquisition. WT—resources and supervision. JL—conceptualization, methodology, writing—review & editing, supervision, and funding acquisition.
Funding
This work was supported by the National Nature Science Foundation of China (grant numbers 81771741 and 81570709), the Distinguished Professor at Educational Department of Liaoning Province (grant number No. [2014]187), and the National Science and Technology Support Program (grant number 2014BA106B02).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.890502/full#supplementary-material
References
1. Effraimidis G, Wiersinga WM. Mechanisms in Endocrinology: Autoimmune Thyroid Disease: Old and New Players. Eur J Endocrinol (2014) 170(6):R241–52. doi: 10.1530/EJE-14-0047
2. Li Y, Teng D, Ba J, Chen B, Du J, He L, et al. Efficacy and Safety of Long-Term Universal Salt Iodization on Thyroid Disorders: Epidemiological Evidence From 31 Provinces of Mainland China. Thyroid (2020) 30(4):568–79. doi: 10.1089/thy.2019.0067
3. Fröhlich E, Wahl R. Thyroid Autoimmunity: Role of Anti-Thyroid Antibodies in Thyroid and Extra-Thyroidal Diseases. Front Immunol (2017) 8:521. doi: 10.3389/fimmu.2017.00521
4. Stagnaro-Green A, Roman SH, Cobin RH, el-Harazy E, Alvarez-Marfany M, Davies TF. Detection of at-Risk Pregnancy by Means of Highly Sensitive Assays for Thyroid Autoantibodies. Jama (1990) 264(11):1422–5. doi: 10.1001/jama.264.11.1422
5. Liu H, Shan Z, Li C, Mao J, Xie X, Wang W, et al. Maternal Subclinical Hypothyroidism, Thyroid Autoimmunity, and the Risk of Miscarriage: A Prospective Cohort Study. Thyroid (2014) 24(11):1642–9. doi: 10.1089/thy.2014.0029
6. Poppe K, Velkeniers B, Glinoer D. The Role of Thyroid Autoimmunity in Fertility and Pregnancy. Nat Clin Pract Endocrinol Metab (2008) 4(7):394–405. doi: 10.1038/ncpendmet0846
7. Thangaratinam S, Tan A, Knox E, Kilby MD, Franklyn J, Coomarasamy A. Association Between Thyroid Autoantibodies and Miscarriage and Preterm Birth: Meta-Analysis of Evidence. BMJ (2011) 342:d2616. doi: 10.1136/bmj.d2616
8. Toulis KA, Goulis DG, Venetis CA, Kolibianakis EM, Negro R, Tarlatzis BC, et al. Risk of Spontaneous Abortion in Euthyroid Women With Thyroid Autoimmunity Undergoing IVF: A Meta-Analysis. Eur J Endocrinol (2010) 162(4):643–52. doi: 10.1530/EJE-09-0850
9. Lin C, Xiang Y, Li J. The Contribution of Thyroid Autoimmunity to Miscarriage in Euthyroid Women. Minerva Med (2016) 107(3):173–81.
10. Wang JW, Liao XX, Li T. Thyroid Autoimmunity in Adverse Fertility and Pregnancy Outcomes: Timing of Assisted Reproductive Technology in AITD Women. J Transl Int Med (2021) 9(2):76–83. doi: 10.2478/jtim-2021-0001
11. Iijima T, Tada H, Hidaka Y, Mitsuda N, Murata Y, Amino N. Effects of Autoantibodies on the Course of Pregnancy and Fetal Growth. Obstet Gynecol (1997) 90(3):364–9. doi: 10.1016/s0029-7844(97)00283-4
12. Dhillon-Smith RK, Middleton LJ, Sunner KK, Cheed V, Baker K, Farrell-Carver S, et al. Levothyroxine in Women With Thyroid Peroxidase Antibodies Before Conception. N Engl J Med (2019) 380(14):1316–25. doi: 10.1056/NEJMoa1812537
13. Wang X, Zhang Y, Tan H, Bai Y, Zhou L, Fang F, et al. Effect of Levothyroxine on Pregnancy Outcomes in Women With Thyroid Autoimmunity: A Systematic Review With Meta-Analysis of Randomized Controlled Trials. Fertil Steril (2020) 114(6):1306–14. doi: 10.1016/j.fertnstert.2020.06.034
14. Yang Z, Wang H, Liu Y, Feng Y, Xiang Y, Li J, et al. The Expression of Anti-Protein Disulfide Isomerase A3 Autoantibody Is Associated With the Increased Risk of Miscarriage in Euthyroid Women With Thyroid Autoimmunity. Int Immunopharmacol (2022) 104:108507. doi: 10.1016/j.intimp.2021.108507
15. Díaz-Ramos A, Roig-Borrellas A, García-Melero A, López-Alemany R. α-Enolase, a Multifunctional Protein: Its Role on Pathophysiological Situations. J BioMed Biotechnol (2012) 2012:156795. doi: 10.1155/2012/156795
16. Terrier B, Degand N, Guilpain P, Servettaz A, Guillevin L, Mouthon L. Alpha-Enolase: A Target of Antibodies in Infectious and Autoimmune Diseases. Autoimmun Rev (2007) 6(3):176–82. doi: 10.1016/j.autrev.2006.10.004
17. Liu Y, Liao L, An C, Wang X, Li Z, Xu Z, et al. α-Enolase Lies Downstream of mTOR/Hif1α and Promotes Thyroid Carcinoma Progression by Regulating Cst1. Front Cell Dev Biol (2021) 9:670019. doi: 10.3389/fcell.2021.670019
18. Lu Y, Qin J, Xiang Y, Sun R, Feng Y, Zhang H, et al. Experimental Evidence for Alpha Enolase as One Potential Autoantigen in the Pathogenesis of Both Autoimmune Thyroiditis and Its Related Encephalopathy. Int Immunopharmacol (2020) 85:106563. doi: 10.1016/j.intimp.2020.106563
19. Gharesi-Fard B, Zolghadri J, Kamali-Sarvestani E. Alteration in the Expression of Proteins in Unexplained Recurrent Pregnancy Loss Compared With in the Normal Placenta. J Reprod Dev (2014) 60(4):261–7. doi: 10.1262/jrd.2013-096
20. Reiss NA. Ontogeny and Estrogen Responsiveness of Creatine Kinase and Glycolytic Enzymes in Brain and Uterus of Rat. Neurosci Lett (1988) 84(2):197–202. doi: 10.1016/0304-3940(88)90407-7
21. Ye Y, Kuhn C, Kösters M, Arnold GJ, Ishikawa-Ankerhold H, Schulz C, et al. Anti α-Enolase Antibody is a Novel Autoimmune Biomarker for Unexplained Recurrent Miscarriages. EBioMedicine (2019) 41:610–22. doi: 10.1016/j.ebiom.2019.02.027
22. Walter M, Berg H, Leidenberger FA, Schweppe KW, Northemann W. Autoreactive Epitopes Within the Human Alpha-Enolase and Their Recognition by Sera From Patients With Endometriosis. J Autoimmun (1995) 8(6):931–45. doi: 10.1016/s0896-8411(95)80027-1
23. Sundblad V, Bussmann L, Chiauzzi VA, Pancholi V, Charreau EH. Alpha-Enolase: A Novel Autoantigen in Patients With Premature Ovarian Failure. Clin Endocrinol (2006) 65(6):745–51. doi: 10.1111/j.1365-2265.2006.02661.x
24. Sarapik A, Haller-Kikkatalo K, Utt M, Teesalu K, Salumets A, Uibo R. Serum Anti-Endometrial Antibodies in Infertile Women - Potential Risk Factor for Implantation Failure. Am J Reprod Immunol (2010) 63(5):349–57. doi: 10.1111/j.1600-0897.2010.00808.x
25. Pancholi V. Multifunctional Alpha-Enolase: Its Role in Diseases. Cell Mol Life Sci (2001) 58(7):902–20. doi: 10.1007/pl00000910
26. Gong X, Liu A, Li Y, Sun H, Li Y, Li C, et al. The Impact of Isolated Maternal Hypothyroxinemia During the First and Second Trimester of Gestation on Pregnancy Outcomes: An Intervention and Prospective Cohort Study in China. J Endocrinol Invest (2019) 42(5):599–607. doi: 10.1007/s40618-018-0960-7
27. Zhang X, Yao B, Li C, Mao J, Wang W, Xie X, et al. Reference Intervals of Thyroid Function During Pregnancy: Self-Sequential Longitudinal Study Versus Cross-Sectional Study. Thyroid (2016) 26(12):1786–93. doi: 10.1089/thy.2016.0002
28. Hou Y, Liu A, Li J, Wang H, Yang Y, Li Y, et al. Different Thyroidal Responses to Human Chorionic Gonadotropin Under Different Thyroid Peroxidase Antibody and/or Thyroglobulin Antibody Positivity Conditions During the First Half of Pregnancy. Thyroid (2019) 29(4):577–85. doi: 10.1089/thy.2018.0097
29. Li J, Liu A, Liu H, Li C, Wang W, Han C, et al. Maternal TSH Levels at First Trimester and Subsequent Spontaneous Miscarriage: A Nested Case-Control Study. Endocr Connect (2019) 8(9):1288–93. doi: 10.1530/EC-19-0316
30. WHO, UNICEF, ICCIDD. Assessment of Iodine Deficiency Disorders and Monitoring Their Elimination. 3rd ed. Geneva: World health Organization (2007).
31. Breymann C, Bian XM, Blanco-Capito LR, Chong C, Mahmud G, Rehman R. Expert Recommendations for the Diagnosis and Treatment of Iron-Deficiency Anemia During Pregnancy and the Postpartum Period in the Asia-Pacific Region. J Perinat Med (2011) 39(2):113–21. doi: 10.1515/jpm.2010.132
32. Li C, Shan Z, Mao J, Wang W, Xie X, Zhou W, et al. Assessment of Thyroid Function During First-Trimester Pregnancy: What is the Rational Upper Limit of Serum TSH During the First Trimester in Chinese Pregnant Women? J Clin Endocrinol Metab (2014) 99(1):73–9. doi: 10.1210/jc.2013-1674
33. Li C, Peng S, Zhang X, Xie X, Wang D, Mao J, et al. The Urine Iodine to Creatinine as an Optimal Index of Iodine During Pregnancy in an Iodine Adequate Area in China. J Clin Endocrinol Metab (2016) 101(3):1290–8. doi: 10.1210/jc.2015-3519
34. Stevenson M, Sergeant E, Nunes T, Heuer C, Marshall J, Sanchez J, et al. Epir: Tools for the Analysis of Epidemiological Data (2022). Available at: https://CRAN.R-project.org/package=epiR.
35. Stagnaro-Green A, Abalovich M, Alexander E, Azizi F, Mestman J, Negro R, et al. American Thyroid Association Taskforce on Thyroid Disease During Pregnancy and Postpartum. Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and Postpartum. Thyroid (2011) 21(10):1081–125. doi: 10.1089/thy.2011.0087
36. Hong Li Y, Marren A. Recurrent Pregnancy Loss: A Summary of International Evidence-Based Guidelines and Practice. Aust J Gen Pract (2018) 47(7):432–6. doi: 10.31128/AJGP-01-18-4459
37. Ashoor G, Maiz N, Rotas M, Jawdat F, Nicolaides KH. Maternal Thyroid Function at 11 to 13 Weeks of Gestation and Subsequent Fetal Death. Thyroid (2010) 20(9):989–93. doi: 10.1089/thy.2010.0058
38. Yang W, Xiang Y, Zhang H, Shan Z, Li J, Teng W. The Role of Protein Disulphide-Isomerase A3 as Autoantigen in the Pathogenesis of Autoimmune Thyroiditis and Related Brain Damage in Adult Mice. Clin Immunol (2020) 212:108350. doi: 10.1016/j.clim.2020.108350
39. Negro R, Mangieri T, Coppola L, Presicce G, Casavola EC, Gismondi R, et al. Levothyroxine Treatment in Thyroid Peroxidase Antibody-Positive Women Undergoing Assisted Reproduction Technologies: A Prospective Study. Hum Reprod (2005) 20(6):1529–33. doi: 10.1093/humrep/deh843
40. Grossmann M, Hoermann R, Francis C, Hamilton EJ, Tint A, Kaitu'u-Lino T, et al. Measuring Thyroid Peroxidase Antibodies on the Day Nulliparous Women Present for Management of Miscarriage: A Descriptive Cohort Study. Reprod Biol Endocrinol (2013) 11:40. doi: 10.1186/1477-7827-11-40
41. Yuan N, Sun J, Li Z, Chai S, Zhang X, Ji L. Relationship Between Anti-Thyroid Peroxidase Antibody Positivity and Pregnancy-Related and Fetal Outcomes in Euthyroid Women: A Single-Center Cohort Study. BMC Pregnancy Childbirth (2020) 20(1):491. doi: 10.1186/s12884-020-03176-4
42. Zhang Y, Sun W, Zhu S, Huang Y, Huang Y, Gao Y, et al. The Impact of Thyroid Function and TPOAb in the First Trimester on Pregnancy Outcomes: A Retrospective Study in Peking. J Clin Endocrinol Metab (2020) 105(3):dgz167. doi: 10.1210/clinem/dgz167
43. Bagis T, Gokcel A, Saygili ES. Autoimmune Thyroid Disease in Pregnancy and the Postpartum Period: Relationship to Spontaneous Abortion. Thyroid (2001) 11(11):1049–53. doi: 10.1089/105072501753271743
44. Ander SE, Diamond MS, Coyne CB. Immune Responses at the Maternal-Fetal Interface. Sci Immunol (2019) 4(31):eaat6114. doi: 10.1126/sciimmunol.aat6114
45. Twig G, Shina A, Amital H, Shoenfeld Y. Pathogenesis of Infertility and Recurrent Pregnancy Loss in Thyroid Autoimmunity. J Autoimmun (2012) 38(2-3):J275–81. doi: 10.1016/j.jaut.2011.11.014
46. Liu S, Xu F, Wei H, Huang C, Chen X, Lian R, et al. The Correlation of Thyroid Autoimmunity and Peripheral and Uterine Immune Status in Women With Recurrent Miscarriage. J Reprod Immunol (2020) 139:103118. doi: 10.1016/j.jri.2020.103118
47. Kim NY, Cho HJ, Kim HY, Yang KM, Ahn HK, Thornton S, et al. Thyroid Autoimmunity and its Association With Cellular and Humoral Immunity in Women With Reproductive Failures. Am J Reprod Immunol (2011) 65(1):78–87. doi: 10.1111/j.1600-0897.2010.00911.x
48. Rodrigues VO, Soligo A, Pannain GD. Antiphospholipid Antibody Syndrome and Infertility. Rev Bras Ginecol Obstet (2019) 41(10):621–7. doi: 10.1055/s-0039-1697982
49. Shoenfeld Y, Blank M. Autoantibodies Associated With Reproductive Failure. Lupus (2004) 13(9):643–8. doi: 10.1191/0961203304lu2009oa
50. Hu Z, Jing X, Liu J, Li M, Ye Y, Chen Y. Simultaneous Quantification of Anticardiolipin IgG and IgM by Time Resolved Fluoroimmunoassay. PloS One (2016) 11(9):e0163682. doi: 10.1371/journal.pone.0163682
51. Cellini M, Santaguida MG, Stramazzo I, Capriello S, Brusca N, Antonelli A, et al. Recurrent Pregnancy Loss in Women With Hashimoto's Thyroiditis With Concurrent Non-Endocrine Autoimmune Disorders. Thyroid (2020) 30(3):457–62. doi: 10.1089/thy.2019.0456
52. De Carolis C, Greco E, Guarino MD, Perricone C, Dal Lago A, Giacomelli R, et al. Anti-Thyroid Antibodies and Antiphospholipid Syndrome: Evidence of Reduced Fecundity and of Poor Pregnancy Outcome in Recurrent Spontaneous Aborters. Am J Reprod Immunol (2004) 52(4):263–6. doi: 10.1111/j.1600-0897.2004.00215.x
53. Guo YP, Wang CG, Liu X, Huang YQ, Guo DL, Jing XZ, et al. The Prevalence of Antinuclear Antibodies in the General Population of China: A Cross-Sectional Study. Curr Ther Res Clin Exp (2014) 76:116–9. doi: 10.1016/j.curtheres.2014.06.004
54. Cavalcante MB, Cavalcante CTMB, Sarno M, da Silva ACB, Barini R. Antinuclear Antibodies and Recurrent Miscarriage: Systematic Review and Meta-Analysis. Am J Reprod Immunol (2020) 83(3):e13215. doi: 10.1111/aji.13215
55. Roberts J, Jenkins C, Wilson R, Pearson C, Franklin IA, MacLean MA, et al. Recurrent Miscarriage Is Associated With Increased Numbers of CD5/20 Positive Lymphocytes and an Increased Incidence of Thyroid Antibodies. Eur J Endocrinol (1996) 134(1):84–6. doi: 10.1530/eje.0.1340084
56. Seki SM, Gaultier A. Exploring Non-Metabolic Functions of Glycolytic Enzymes in Immunity. Front Immunol (2017) 8:1549. doi: 10.3389/fimmu.2017.01549
57. Cappello P, Principe M, Bulfamante S, Novelli F. Alpha-Enolase (ENO1), a Potential Target in Novel Immunotherapies. Front Biosci (2017) 22:944–59. doi: 10.2741/4526
58. Bruschi M, Sinico RA, Moroni G, Pratesi F, Migliorini P, Galetti M, et al. Glomerular Autoimmune Multicomponents of Human Lupus Nephritis In Vivo: α-Enolase and Annexin AI. J Am Soc Nephrol (2014) 25(11):2483–98. doi: 10.1681/ASN.2013090987
59. Gerstner C, Dubnovitsky A, Sandin C, Kozhukh G, Uchtenhagen H, James EA, et al. Functional and Structural Characterization of a Novel HLA-DRB1*04:01-Restricted α-Enolase T Cell Epitope in Rheumatoid Arthritis. Front Immunol (2016) 7:494. doi: 10.3389/fimmu.2016.00494
60. Prado LL, Goncalves CR, Viana VT, Saad CGS, Bonfa E. Anti-Alpha-Enolase Antibodies in Behçet's Disease: A Marker of Mucocutaneous and Articular Disease Activity? Clin Exp Rheumatol (2018) 36(6 Suppl 115):28–32.
61. Lefranc D, Almeras L, Dubucquoi S, de Seze J, Vermersch P, Prin L. Distortion of the Self-Reactive IgG Antibody Repertoire in Multiple Sclerosis as a New Diagnostic Tool. J Immunol (2004) 172(1):669–78. doi: 10.4049/jimmunol.172.1.669
62. Adamus G, Amundson D, Seigel GM, Machnicki M. Anti-Enolase-Alpha Autoantibodies in Cancer-Associated Retinopathy: Epitope Mapping and Cytotoxicity on Retinal Cells. J Autoimmun (1998) 11(6):671–7. doi: 10.1006/jaut.1998.0239
63. Pratesi F, Moscato S, Sabbatini A, Chimenti D, Bombardieri S, Migliorini P. Autoantibodies Specific for Alpha-Enolase in Systemic Autoimmune Disorders. J Rheumatol (2000) 27(1):109–15.
64. Kotani T, Aratake Y, Hirai K, Fukazawa Y, Sato H, Ohtaki S. Apoptosis in Thyroid Tissue From Patients With Hashimoto's Thyroiditis. Autoimmunity (1995) 20(4):231–6. doi: 10.3109/08916939508995700
65. McLachlan SM, Rapoport B. Thyroid Autoantibodies Display Both "Original Antigenic Sin" and Epitope Spreading. Front Immunol (2017) 8:1845. doi: 10.3389/fimmu.2017.01845
66. Zeng L, Yang K, Liu L, Zhang T, Liu H, Tan Z, et al. Systematic Biological and Proteomics Strategies to Explore the Regulation Mechanism of Shoutai Wan on Recurrent Spontaneous Abortion's Biological Network. J Ethnopharmacol (2020) 263:113156. doi: 10.1016/j.jep.2020.113156
67. Fujii A, Yoneda M, Ito T, Yamamura O, Satomi S, Higa H, et al. Autoantibodies Against the Amino Terminal of Alpha-Enolase are a Useful Diagnostic Marker of Hashimoto's Encephalopathy. J Neuroimmunol (2005) 162(1-2):130–6. doi: 10.1016/j.jneuroim.2005.02.004
68. Pringle KG, Kind KL, Sferruzzi-Perri AN, Thompson JG, Roberts CT. Beyond Oxygen: Complex Regulation and Activity of Hypoxia Inducible Factors in Pregnancy. Hum Reprod Update (2010) 16(4):415–31. doi: 10.1093/humupd/dmp046
69. Burton GJ, Jauniaux E, Murray AJ. Oxygen and Placental Development; Parallels and Differences With Tumour Biology. Placenta (2017) 56:14–8. doi: 10.1016/j.placenta.2017.01.130
70. Vidarsson G, Dekkers G, Rispens T. IgG Subclasses and Allotypes: From Structure to Effector Functions. Front Immunol (2014) 5:520. doi: 10.3389/fimmu.2014.00520
71. Cappello P, Rolla S, Chiarle R, Principe M, Cavallo F, Perconti G, et al. Vaccination With ENO1 DNA Prolongs Survival of Genetically Engineered Mice With Pancreatic Cancer. Gastroenterology (2013) 144(5):1098–106. doi: 10.1053/j.gastro.2013.01.020
72. Caucheteux SM, Kanellopoulos-Langevin C, Ojcius DM. At the Innate Frontiers Between Mother and Fetus: Linking Abortion With Complement Activation. Immunity (2003) 18(2):169–72. doi: 10.1016/s1074-7613(03)00028-1
Keywords: α-enolase, autoantibodies, epitope, miscarriage, thyroid autoimmunity
Citation: He X, Liu Y, Wang H, Sun W, Lu Y, Shan Z, Teng W and Li J (2022) A Predictive Role of Autoantibodies Against the Epitope aa168–183 of ENO1 in the Occurrence of Miscarriage Related to Thyroid Autoimmunity. Front. Immunol. 13:890502. doi: 10.3389/fimmu.2022.890502
Received: 06 March 2022; Accepted: 02 May 2022;
Published: 30 May 2022.
Edited by:
Anette S. B. Wolff, Haukeland University Hospital, NorwayReviewed by:
Anna Ghirardello, University of Padua, ItalyAlessandro Rolfo, University of Turin, Italy
Copyright © 2022 He, Liu, Wang, Sun, Lu, Shan, Teng and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Li, bGlqaW5nZW5kb2NyaW5lQDEyNi5jb20=; amxpMjNAY211LmVkdS5jbg==; orcid.org/0000-0002-3681-4095
†Present address: Yihan Lu, Department of International Clinic, The Second Hospital of Dalian Medical University, Dalian, China
‡These authors have contributed equally to this work
 Xiaoqing He
Xiaoqing He Yifu Liu‡
Yifu Liu‡ Jing Li
Jing Li