- 1State Key Laboratory of Experimental Hematology, National Clinical Research Center for Blood Diseases, Haihe Laboratory of Cell Ecosystem, Institute of Hematology and Blood Diseases Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Tianjin, China
- 2Department of Hematology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 3Department of Hematology, The Affiliated Yantai Yuhuangding Hospital of Qingdao University, Yantai, China
- 4Bone Marrow Transplantation Center, The First Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, China
We compared the efficacy and safety of porcine anti-lymphocyte globulin (pALG) (n=140) and rabbit anti-thymocyte globulin (rATG) (n=86) in patients with acquired aplastic anemia (AA) receiving hematopoietic stem cell transplantation (HSCT) from matched sibling donors (MSD) in two transplantation centers in China ranging from 2005 to 2020. The groups had similar baseline characteristics except for a higher number of infused mononuclear cells (P<0.001) and a higher proportion of peripheral blood stem cells as graft sources (P=0.003) in the pALG group. The rates of neutrophil engraftment at day 28 (P=1), platelet engraftment at day 28 (P=0.228), bloodstream infection before engraftment (P=0.867), invasive fungal diseases (P=0.362), cytomegalovirus viremia (P=0.667), and graft rejection (P=0.147) were similar in the two groups. A higher cumulative incidence of grades II-IV acute graft versus host disease (aGvHD) at 100 days occurred in the pALG group (19% vs. 8%, P=0.035) while no significant differences in grades III-IV aGvHD (P=0.572), mild to severe chronic GvHD (cGvHD) (P=0.181), and moderate to severe cGvHD (P=0.586) were observed. The actuarial 5-year overall survival (OS), failure-free survival (FFS), and GvHD-free, FFS rates of the pALG group were 87% (95% confidence interval [CI], 82-93), 85% (95% CI, 80-92), and 78% (95% CI, 72-92) versus 91% (95% CI, 86-99) (P=0.33), 88% (95% CI, 82-97) (P=0.428), and 79% (95% CI, 72-90) (P=0.824) in the rATG group, respectively. A busulfan-containing conditioning regimen was the only adverse risk factor for OS and FFS in multivariate analysis. In conclusion, pALG is an alternative to rATG in patients with severe AA receiving MSD-HSCT. A prospective, large-sample study is needed to explore this therapy further.
Introduction
Severe aplastic anemia (SAA) is a disease with a high mortality rate, mainly due to infections or bleeding caused by persistent pancytopenia. Hematopoietic stem cell transplantation (HSCT) is preferred for patients with matched sibling donors (MSD). Many studies have shown significant superiority of MSD-HSCT over immunosuppressive therapy (IST) in terms of overall survival (OS) and failure-free survival (FFS) (1–5). Over the last two decades, further dramatic progress has been made on several fronts to tackle this disease. The incorporation of anti-thymocyte globulin (ATG) into the conditioning regimen was first investigated. Since 1994, the efficacy of ATG in the conditioning regimen of HSCT from MSD for patients with SAA has been confirmed (6). Storb et al. showed that the actual survival rate at three years was 92%, which was higher than the 72% (historical) survival rate, in 39 patients. In addition, a fludarabine (FLU)-based conditioning regimen also showed reduced toxicity and similar survival compared to ATG plus cyclophosphamide (CTX), especially for older patients (7–9). However, among these studies, rabbit-ATG (rATG) was most commonly used. In China, porcine anti-lymphocyte globulin (pALG) is also available, and studies have reported similar efficacy to rATG among patients receiving IST (10–13). The effect and safety of pALG in patients with SAA receiving HSCT have been previously reported in our centers, with limited sample sizes (14, 15). Therefore, we designed this extended retrospective study to evaluate and compare the efficacy and safety of rATG and pALG in patients with acquired SAA undergoing MSD-HSCT in two transplant centers in China.
Patients and Methods
Patients
From 2005 and 2020, a total of 226 patients with acquired AA who consecutively received MSD-HSCT from Institute of Hematology & Blood Diseases Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College and Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology were enrolled in this study. Of these patients evaluated, 140 patients received pALG while 86 patients received rATG. Enrollment criteria included SAA, very SAA or transfusion-dependent non-severe AA defined by guideline (16); voluntary participation in HSCT; absence of severe organs dysfunction. Excluding criteria included underlying inherited marrow failure disorders such as Fanconi anemia; myelodysplastic syndrome; patients with pregnancy, severe organs impairment, or uncontrolled active infection. Patients with paroxysmal nocturnal hemoglobinuria clones were also included in this study. All written informed consent was attained from patients or their relatives. This study was approved by the Ethics Committees of Institute of Hematology & Blood Diseases Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College and Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, respectively.
Procedures of Transplantation
The conditioning regimen, graft-versus-host disease (GvHD) prophylaxis, infection prevention, and surveillance followed those in our previous report (14, 15). In particular, pALG was prepared using thymic cells (as antigens) introduced in swine and separating anti-lymphocyte serum from the swine (Wuhan Institute of Biological Products Co., Ltd.) (11, 17).
Definitions
Neutrophil and platelet engraftment (18), acute GvHD (aGvHD) (19), and chronic GvHD (cGvHD) (20) were defined according to previously reported criteria. Graft rejection (GR) was defined as less than 5% T-cells of donor origin (21). Primary GR was defined as the failure to achieve neutrophil engraftment after HSCT until day +28. Secondary GR was defined as the absence of graft function after achieving initial full engraftment (22). Transplantation-related mortality (TRM) was defined as death without rejection. Treatment failure after HSCT was defined as death or primary or secondary GR, whichever came first. The FFS was defined as survival without treatment failure. GvHD-free and failure-free survival (GFFS) was defined as survival without grades III-IV aGvHD, moderate to severe cGvHD, or treatment failure (23). OS was defined as the time from treatment to death or the last follow-up.
Statistical Analysis
The objective of this study was to compare major outcomes, including engraftment, infection, GvHD, TRM, and survival, among different ATG groups in patients with AA.
Each patient involved had an electronic-database, outpatient-department, or telephone follow-up. The final follow-up was November 31, 2021. Continuous and categorical variables were compared using the Mann–Whitney U test, chi-square test, or Fisher’s exact test. Median follow-up was calculated using the reverse Kaplan–Meier method. Cumulative incidences of GvHD were compared with the Gray’s test. Death and GR were considered as competing events for GvHD. The probabilities of OS and FFS were estimated using the Kaplan–Meier method and compared between different groups of patients using the log-rank test. Variables with P values ≤ 0.2 in the univariate analysis were entered in multivariate analyses using a Cox proportional hazards model to identify factors impacting OS, FFS, and GFFS of transplant patients. Statistical analyses were performed using the R software packages (R 4.1.2), GraphPad Prism 5, and SPSS 20.0. GraphPad Prism 5 was used to generate figures. All P values were two-sided, and the results were considered statistically significant at P<0.05.
Results
Characteristic of Patients and Donors
As shown in Table 1, 140 and 86 patients were enrolled in the pALG and rATG groups, respectively. There were no significant differences in terms of patient age (P=0.15), patient sex (P=0.857), donor sex (P=0.797), diagnosis (P=0.396), interval from diagnosis to transplantation (P=0.375), conditioning regimen (P=0.415), and dose of CD34+ cells infused (P=0.161) between the two groups, while the pALG group had a higher proportion of peripheral blood stem cells (PBSCs) as a graft source (85% vs. 67.44%, P=0.003), and a higher median dose of infused mononuclear cells (10×108/kg vs. 8×108/kg, P<0.001).
Hematopoietic Recovery
Only patients who survived for >28 days were analyzed for engraftment. There were two early deaths due to respiratory failure and septic shock at day 11 and 15 in the pALG group, while none of the patients in the rATG group suffered early deaths. The neutrophil engraftment rate was 100% at day 28 in the pALG group versus 100% at day 28 in the rATG group; accordingly, the platelet engraftment rate was 96.65% versus 90.7%, respectively (P=0.228). Patients in the pALG group had a faster engraftment of neutrophils and platelets. The median days of neutrophil and platelet engraftment were 12 (range, 7-22) and 12 (range, 7-30) days for patients in the pALG group and 12 (range, 9-23) (P=0.004) and 14 (range, 8-34) days (P=0.001) for patients in the rATG group, respectively (Table 1).
Graft Rejection
Ten patients experienced GR after transplantation (one primary and nine secondary). There was no difference in GR rates between the groups (P=0.147). The median time of secondary GR was 4 months, ranging from 1.2 months to 107 months. Among these patients, all were treated with CTX (50 mg/kg × 2 days) with or without FLU followed by an infusion of frozen PBSCs from the original donor. Eight patients acquired complete blood recovery with donor origin, while two patients received autologous blood recovery.
aGvHD and cGvHD
With regard to aGvHD and cGvHD, although patients in the pALG group mostly received PBSCs as the graft source, there was only a marginally significant difference in the rate of grades II to IV aGvHD between the two groups (P=0.041), whereas rates of grades I to IV aGvHD, grades III to IV aGvHD, mild to severe cGvHD, and moderate to severe cGvHD were similar (Table 1). Figure 1 shows that the cumulative incidences of grades II to IV aGvHD and III to IV aGvHD at 100 days were 19% (95% confidence interval [CI], 6–30) and 8% (95% CI, 0–15) in the pALG group compared to 8% (95% CI, 0–16) (P=0.035) and 6% (95% CI, 0–12) (P=0.572), respectively, in the rATG group. The cumulative incidence of mild-to-severe and moderate-to-severe cGvHD at 5 years was 24% (95% CI, 9–36) and 5% (95% CI, 0–11) in the pALG group versus 13% (95% CI, 0–25) (P=0.181) and 8% (95% CI, 0–16) (P=0.586) in the rATG group.
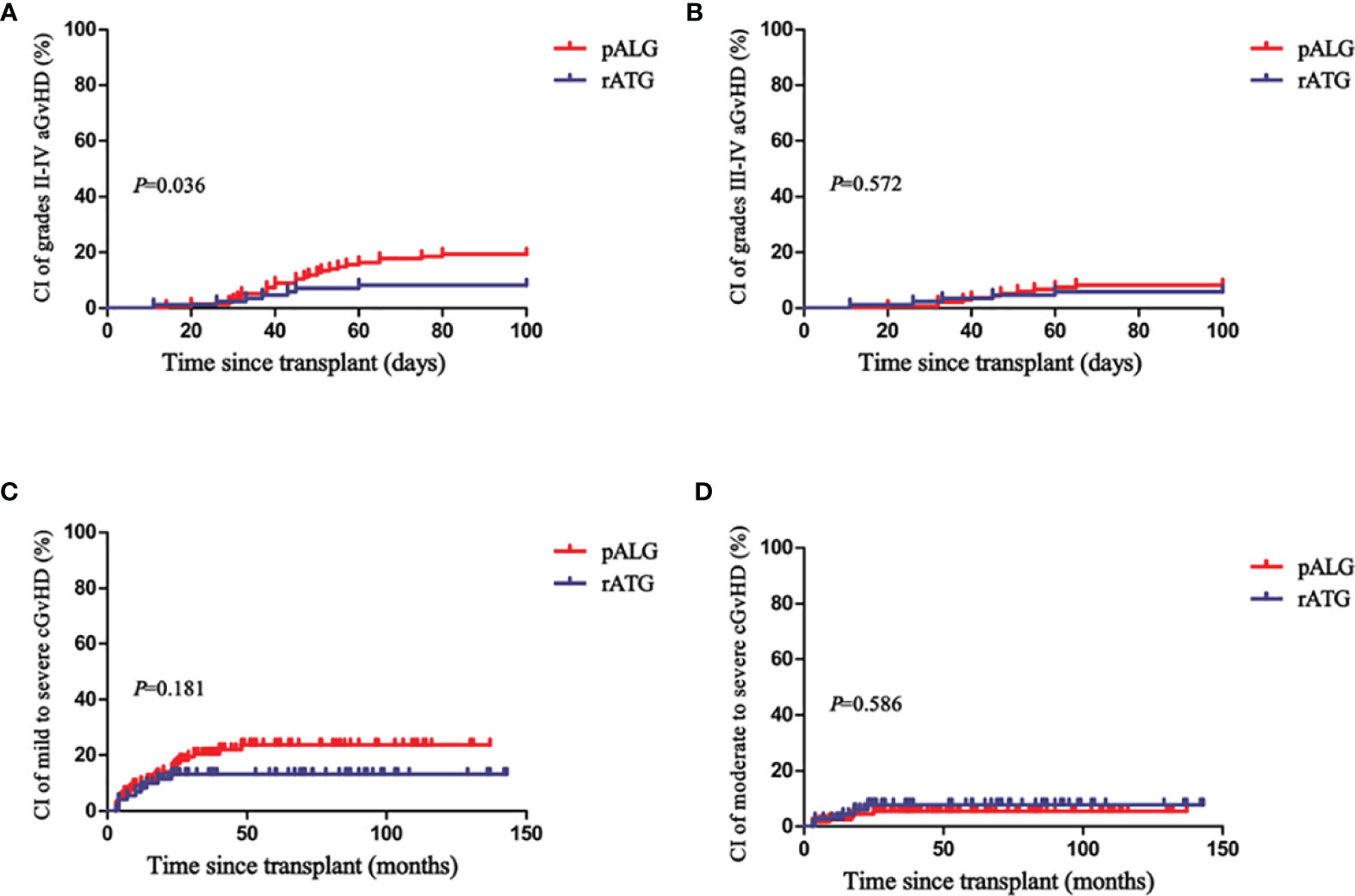
Figure 1 Cumulative incidences (CI) of grades II-IV aGvHD (A), grades III-IV aGvHD (B), mild to severe cGvHD (C), and moderate to severe cGvHD (D) in the pALG and rATG groups.
Infections
There were no statistical differences in bloodstream infections before engraftment (P=0.867), invasive fungal diseases (P=0.362), or cytomegalovirus viremia (P=0.667) between the two groups (Table 1).
Deaths
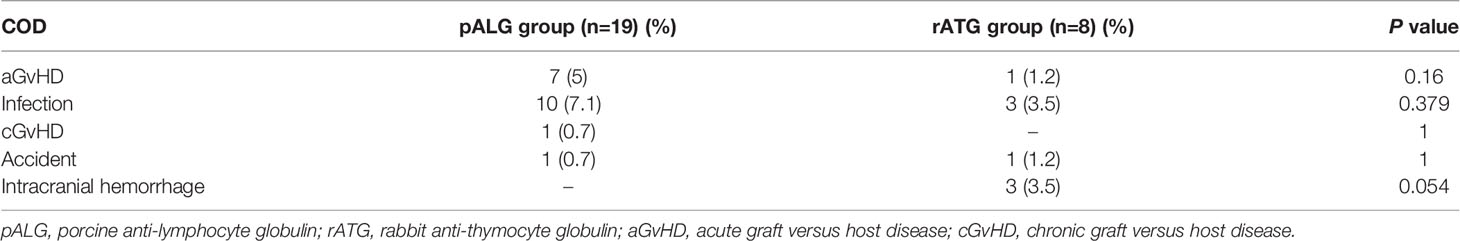
With a median follow-up of 62 months (range, 7-190 months) and 79 months (range, 3-169 months), 19 and 8 deaths occurred in the pALG and rATG groups (P=0.454), respectively. The primary causes of death (COD) are listed in Table 2. The leading COD was infection (n=13), followed by aGvHD (n=8). Remarkably, four patients died from invasive fungal diseases of the lung (n=3) or brain (n=1) before 2010. Secondary COD followed by aGvHD included infections (n=4), organ failure (n=3), and gastrointestinal bleeding (n=1). More patients receiving busulfan (BU)-containing regimens suffered COD owing to aGvHD (11.1% vs. 1.7%, P=0.009), whereas COD caused by infection between the two conditioning groups was similar (6.1% vs. 2.2%, P=0.468).
Survival
The actuarial 5-year OS, FFS, and GFFS rates of the pALG group were 87% (95% CI, 82-93), 85% (95% CI, 80-92), and 78% (95% CI, 72-92) compared to 91% (95% CI, 86-99) (P=0.33), 88% (95% CI, 82-97) (P=0.428), and 79% (95% CI, 72-90) (P=0.824) of the rATG group, respectively (Figure 2). In the subgroup analysis, the actuarial 5-year OS rates of patients aged<20 years, 20-40 years, and >40 years were 91% (95% CI, 86-100), 88% (95% CI, 83-95), and 83% (95% CI, 72-100) (P=0.42) (Figure 3), respectively.
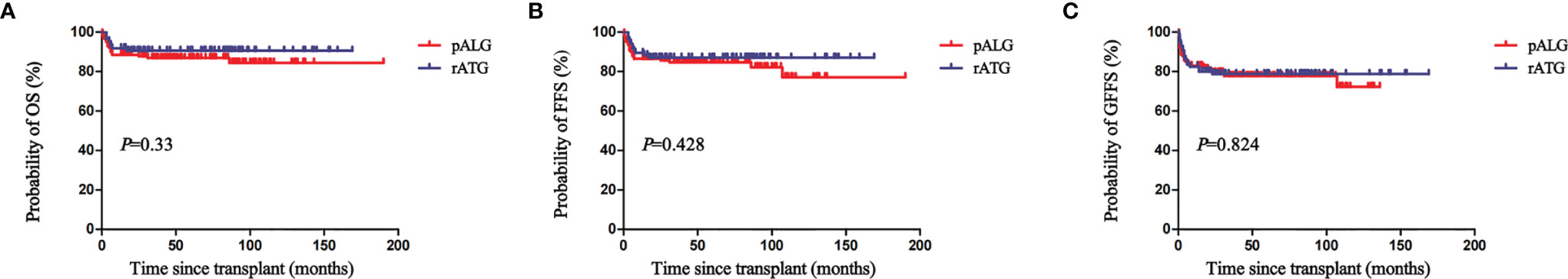
Figure 2 The actuarial 5-year overall survival (OS) (A), failure-free survival (FFS) (B), and GvHD-free, failure-free survival (GFFS) (C) rates of patients in the pALG and rATG groups.
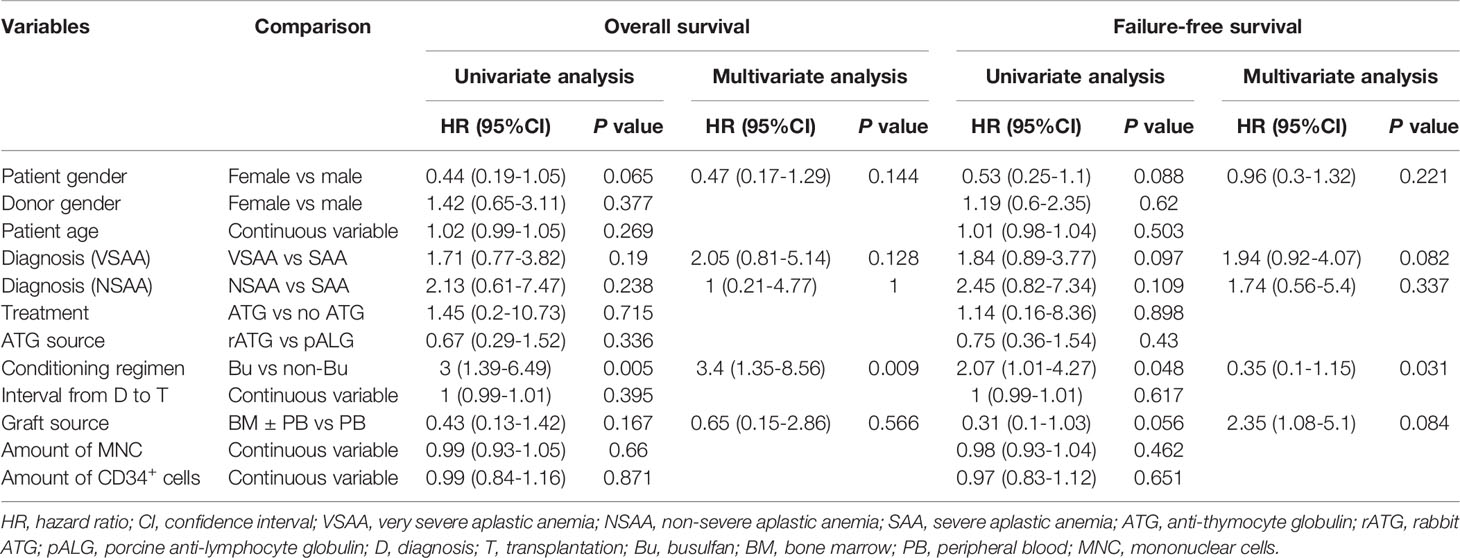
As shown in Table 3, in univariate and multivariate analysis, a BU-containing regimen was the only adverse risk factor of OS and FFS.
Discussion and Conclusion
MSD-HSCT, which promotes effective and fast recovery of blood counts, is the preferred treatment for young patients with SAA. As SAA is a nonmalignant disease, it is recommended to sustain engraftment and minimize GvHD by modifying the conditioning regimen. High-dose CTX plus ATG is the standard conditioning regimen for SAA patients undergoing MSD-HSCT (24, 25). The addition of ATG to CTX reduces GR and GvHD rates (6). In different multivariate analyses, a conditioning regimen without ATG was a negative risk factor for survival in patients with SAA who received HSCT (26–28).
However, the mechanisms of ATG in conditioning are not well understood. It plays a role in suppressing recipient T cells to promote engraftment, as well as donor-activating T cells to reduce GvHD (29). There are three types of ATG worldwide. In vivo studies have demonstrated that the immunosuppressive effect of rATG was stronger than that of horse ATG (hATG) in SAA (30, 31); on the other hand, more infections and lower rates of aGvHD were related to rATG for patients with SAA receiving HSCT (32, 33). In China, no hATG or pALG has been approved by the China Food and Drug Administration as a drug in the conditioning regimen for transplantation. Several studies have demonstrated comparable outcomes between pALG and rATG as IST in patients with SAA (10–13). Previously, another study in IST has demonstrated that compared to pALG, r-ATG exhibited a stronger and prolonged inhibition effect on the CD4+ T cell subset while a subset of CD4+ T cells played a role in hematopoietic recovery (12).
Consistent with our previous study (14), we found no differences in the rates of neutrophil engraftment (P=1), platelet engraftment (P=0.228), bloodstream infections (P=0.867), invasive fungal diseases (P=0.362), cytomegalovirus viremia (P=0.667), or GR (P=0.147) between the two groups. Patients in the pALG group experienced faster recovery of neutrophils (P=0.004) and platelet (P=0.001). Meanwhile, a higher cumulative incidence of grades II-IV aGvHD at 100 days occurred in the pALG group (19% vs. 8%, P=0.036), while no differences were observed in the cumulative incidence of grades III-IV aGvHD (P=0.572), mild to severe cGvHD (P=0.181), and moderate to severe cGvHD (P=0.586). Schrezenmeier et al. reported the median days of neutrophil and platelet engraftment were 13 and 19 days in 134 PB recipients compared to 19 and 25 days in 558 bone marrow (BM)recipients of MSD-HSCT for SAA (34). Bacigalupo et al. have compared the efficacy of PB (n=723) with BM (n=1138) as graft sources for patients with AA receiving MSD-HSCT. They demonstrated that the median days of neutrophil and platelet engraftment in PB patients were 15 (5-68) and 15 (5-68) days versus 20 (3-156) and 27 (4-305) days in BM patients. Grades II to IV aGvHD of PB patients was higher than that of BM patients (17% vs. 11%, P=0.001) (26). Therefore, we should notice that a higher proportion of PB (P=0.003) as a graft source and a higher amount of infused MNC (P<0.001) in the pALG group may lead to faster recovery in the WBC and PLT engraftment as well as a higher rate of grades II to IV aGvHD. Even so, the actuarial 5-year OS, FFS, and GFFS rates between our two groups were similar.
In our study, we applied a non-myeloablative conditioning regimen consisting of FLU, a reduced dose of CTX, and ATG in patients with acquired AA. Several studies have reported similar efficacy of FLU-based conditioning regimens for SAA compared with a standard dose of CTX plus ATG conditioning regimen, especially for patients older than 30 years (7–9). Usually, a dose of BU 6.4 mg/kg was added to patients with a high risk of graft failure, for instance, patients with long intervals from diagnosis to transplantation or heavy blood cell transfusion. Based on the intensity of conditioning (35), this is defined as reduced-intensity conditioning. Only one patient in our study experienced primary GR. Although we demonstrated that a BU-containing conditioning regimen was an adverse predictor of OS, and FFS, these results should be interpreted with critical caution. As we know, the interval from diagnosis to transplantation and heavy transfusions before transplantation are associated with poor outcomes in patients with SAA, which may impact these results as well (26, 27, 36). Meanwhile, enhancing the intensity of the conditioning regimen may improve engraftment at the cost of more toxicity, as revealed by a meta-analysis (37). In our study, we found that more patients receiving a BU-containing conditioning regimen died of aGvHD (P=0.009). Notably, none of our patients with GR died, and most of them were successfully salvaged by the original donors’ PBSC infusion. In the subgroup analysis, there was no difference in OS among patient age groups, which indicated that this regimen may be applied to older patients (8). Therefore, these results indicate that a fludarabine-based conditioning regimen was effective for patients with SAA undergoing MSD-HSCT, independent of age.
Our study had several limitations. First, it was a retrospective study with unavoidable bias. Notably, our enrolled patients had relatively similar basic characteristics to minimize the effect of potential bias. Second, our data on the rates of full immune reconstitution at different times between the two groups was incomplete. In the future, we could use this as a useful secondary endpoint in prospective studies. Third, longer follow-up is necessary as the significant difference in cGvHD rate after PB and BM allografts was most obvious with follow-ups of more than 6 to 7 years (34).
In summary, our study showed that pALG is an alternative treatment for patients with SAA undergoing HSCT from an MSD. Its safety and efficacy were similar to those of rATG. A prospective, large-sample study is needed to validate our findings.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committees of Institute of Hematology & Blood Diseases Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College and Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author Contributions
SF and YcZ contributed to the study design and manuscript review. YfZ, XC, LinL, YL, LiL, GY, and YN contributed to data collection and analysis. YfZ wrote the manuscript, and YfZ, XC, and LinL performed statistical analyses. XC, AP, DY, RZ, QM, WZ, YH, JW, EJ, and MH contributed to disease treatment and data collection. All authors have contributed to the manuscript and approved the submitted version.
Funding
The authors disclose receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the CAMS Innovation Fund for Medical Sciences (CIFMS) (grant numbers 2021-1-I2M-017 and 2021-I2M-C&T-B-080) and the Youth Program of the National Natural Science Foundation of China (grant number 81900182).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We are grateful to the patients and their family members for their trust and our staffs for their efforts in caring for our patients.
References
1. Doney K, Leisenring W, Storb R, Appelbaum FR. Primary Treatment of Acquired Aplastic Anemia: Outcomes With Bone Marrow Transplantation and Immunosuppressive Therapy. Seattle Bone Marrow Transplant Team. Ann Intern Med (1997) 126(2):107–15. doi: 10.7326/0003-4819-126-2-199701150-00003
2. Bacigalupo A, Brand R, Oneto R, Bruno B, Socié G, Passweg J, et al. Treatment of Acquired Severe Aplastic Anemia: Bone Marrow Transplantation Compared With Immunosuppressive Therapy–The European Group for Blood and Marrow Transplantation Experience. Semin Hematol (2000) 37(1):69–80. doi: 10.1016/s0037-1963(00)90031-3
3. Locasciulli A, Oneto R, Bacigalupo A, Socié G, Korthof E, Bekassy A, et al. Outcome of Patients With Acquired Aplastic Anemia Given First Line Bone Marrow Transplantation or Immunosuppressive Treatment in the Last Decade: A Report From the European Group for Blood and Marrow Transplantation (EBMT). Haematologica (2007) 92(1):11–8. doi: 10.3324/haematol.10075
4. Yoshida N, Kobayashi R, Yabe H, Kosaka Y, Yagasaki H, Watanabe K, et al. First-Line Treatment for Severe Aplastic Anemia in Children: Bone Marrow Transplantation From a Matched Family Donor Versus Immunosuppressive Therapy. Haematologica (2014) 99(12):1784–91. doi: 10.3324/haematol.2014.109355
5. Dufour C, Pillon M, Sociè G, Rovò A, Carraro E, Bacigalupo A, et al. Outcome of Aplastic Anaemia in Children. A Study by the Severe Aplastic Anaemia and Paediatric Disease Working Parties of the European Group Blood and Bone Marrow Transplant. Br J Haematol (2015) 169(4):565–73. doi: 10.1111/bjh.13297
6. Storb R, Etzioni R, Anasetti C, Appelbaum FR, Buckner CD, Bensinger W, et al. Cyclophosphamide Combined With Antithymocyte Globulin in Preparation for Allogeneic Marrow Transplants in Patients With Aplastic Anemia. Blood (1994) 84(3):941–9. doi: 10.1182/blood.V84.3.941.bloodjournal843941
7. Maury S, Bacigalupo A, Anderlini P, Aljurf M, Marsh J, Socie G, et al. Improved Outcome of Patients Older Than 30 Years Receiving HLA-Identical Sibling Hematopoietic Stem Cell Transplantation for Severe Acquired Aplastic Anemia Using Fludarabine-Based Conditioning: A Comparison With Conventional Conditioning Regimen. Haematologica (2009) 94(9):1312–5. doi: 10.3324/haematol.2009.006916
8. Shin SH, Jeon YW, Yoon JH, Yahng SA, Lee SE, Cho BS, et al. Comparable Outcomes Between Younger (≦̸40 Years) and Older (>40 Years) Adult Patients With Severe Aplastic Anemia After HLA-Matched Sibling Stem Cell Transplantation Using Fludarabine-Based Conditioning. Bone Marrow Transplant (2016) 51(11):1456–63. doi: 10.1038/bmt.2016.171
9. Bejanyan N, Kim S, Hebert KM, Kekre N, Abdel-Azim H, Ahmed I, et al. Choice of Conditioning Regimens for Bone Marrow Transplantation in Severe Aplastic Anemia. Blood Adv (2019) 3(20):3123–31. doi: 10.1182/bloodadvances.2019000722
10. Wei J, Huang Z, Guo J, Zhang Y, Wang C, Zhu X, et al. Porcine Antilymphocyte Globulin (P-ALG) Plus Cyclosporine A (CsA) Treatment in Acquired Severe Aplastic Anemia: A Retrospective Multicenter Analysis. Ann Hematol (2015) 94(6):955–62. doi: 10.1007/s00277-015-2308-0
11. Chen M, Liu C, Zhuang J, Zou N, Xu Y, Zhang W, et al. Long-Term Follow-Up Study of Porcine Anti-Human Thymocyte Immunoglobulin Therapy Combined With Cyclosporine for Severe Aplastic Anemia. Eur J Haematol (2016) 96(3):291–6. doi: 10.1111/ejh.12590
12. Ma X, Wang J, Zhang W, Cao X, Chen Y, He A, et al. Comparison of Porcine Anti-Human Lymphocyte Globulin and Rabbit Anti-Human Thymocyte Globulin in the Treatment of Severe Aplastic Anemia: A Retrospective Single-Center Study. Eur J Haematol (2016) 96(3):260–8. doi: 10.1111/ejh.12584
13. Chen M, Liu C, Qiao X, Zhou D, Zhuang J, Han B. Comparative Study of Porcine Anti-Human Lymphocyte Immunoglobulin and Rabbit Anti-Human Thymocyte Immunoglobulin as a First-Line Treatment of Acquired Severe Aplastic Anemia. Leuk Res (2018) 65:55–60. doi: 10.1016/j.leukres.2018.01.001
14. Chen X, Wei J, Huang Y, He Y, Yang D, Zhang R, et al. Effect of Antithymocyte Globulin Source on Outcomes of HLA-Matched Sibling Allogeneic Hematopoietic Stem Cell Transplantation for Patients With Severe Aplastic Anemia. Biol Blood Marrow Transplant (2018) 24(1):86–90. doi: 10.1016/j.bbmt.2017.10.007
15. Li L, Li Y, Lin L, Yin J, Xu J, Wei J, et al. Outcomes of Allogeneic Haematopoietic Stem Cell Transplantation for Patients With Severe Aplastic Anaemia Using the Porcine Antilymphocyte Globulin-Containing Conditioning Regimen. Ann Hematol (2020) 99(8):1863–71. doi: 10.1007/s00277-020-04111-5
16. Killick SB, Bown N, Cavenagh J, Dokal I, Foukaneli T, Hill A, et al. Guidelines for the Diagnosis and Management of Adult Aplastic Anaemia. Br J Haematol (2016) 172(2):187–207. doi: 10.1111/bjh.13853
17. Bing H, Siyi Y, Wei Z, Jian L, Minghui D, Li J, et al. The Use of Anti-Human T Lymphocyte Porcine Immunoglobulin and Cyclosporine a to Treat Patients With Acquired Severe Aplastic Anemia. Acta Haematol (2010) 124(4):245–50. doi: 10.1159/000321790
18. Liu L, Zhang Y, Jiao W, Zhou H, Wang Q, Jin S, et al. Comparison of Efficacy and Health-Related Quality of Life of First-Line Haploidentical Hematopoietic Stem Cell Transplantation With Unrelated Cord Blood Infusion and First-Line Immunosuppressive Therapy for Acquired Severe Aplastic Anemia. Leukemia (2020) 34(12):3359–69. doi: 10.1038/s41375-020-0933-7
19. Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant (1995) 15(6):825–8.
20. Shulman HM, Sullivan KM, Weiden PL, McDonald GB, Striker GE, Sale GE, et al. Chronic Graft-Versus-Host Syndrome in Man. A Long-Term Clinicopathologic Study of 20 Seattle Patients. Am J Med (1980) 69(2):204–17. doi: 10.1016/0002-9343(80)90380-0
21. Baron F, Sandmaier BM. Chimerism and Outcomes After Allogeneic Hematopoietic Cell Transplantation Following Nonmyeloablative Conditioning. Leukemia (2006) 20(10):1690–700. doi: 10.1038/sj.leu.2404335
22. Chen J, Pang A, Zhao Y, Liu L, Ma R, Wei J, et al. Primary Graft Failure Following Allogeneic Hematopoietic Stem Cell Transplantation: Risk Factors, Tre Atment and Outcomes. Hematology (2022) 27(1):293–9. doi: 10.1080/16078454.2022.2042064
23. Holtan SG, DeFor TE, Lazaryan A, Bejanyan N, Arora M, Brunstein CG, et al. Composite End Point of Graft-Versus-Host Disease-Free, Relapse-Free Survival After Allogeneic Hematopoietic Cell Transplantation. Blood (2015) 125(8):1333–8. doi: 10.1182/blood-2014-10-609032
24. Storb R, Leisenring W, Anasetti C, Appelbaum FR, Buckner CD, Bensinger WI, et al. Long-Term Follow-Up of Allogeneic Marrow Transplants in Patients With Aplastic Anemia Conditioned by Cyclophosphamide Combined With Antithymocyte Globulin. Blood (1997) 89(10):3890–1. doi: 10.1182/blood.V89.10.3890.3890_3890_3890
25. Kahl C, Leisenring W, Joachim Deeg H, Chauncey TR, Flowers MED, Martin PJ, et al. Cyclophosphamide and Antithymocyte Globulin as a Conditioning Regimen for Allogeneic Marrow Transplantation in Patients With Aplastic Anaemia: A Long-Term Follow-Up. Br J Haematol (2005) 130(5):747–51. doi: 10.1111/j.1365-2141.2005.05667.x
26. Bacigalupo A, Socié G, Schrezenmeier H, Tichelli A, Locasciulli A, Fuehrer M, et al. Bone Marrow Versus Peripheral Blood as the Stem Cell Source for Sibling Transplants in Acquired Aplastic Anemia: Survival Advantage for Bone Marrow in All Age Groups. Haematologica (2012) 97(8):1142–8. doi: 10.3324/haematol.2011.054841
27. Bacigalupo A, Socie G, Hamladji RM, Aljurf M, Maschan A, Kyrcz-Krzemien S, et al. Current Outcome of HLA Identical Sibling Versus Unrelated Donor Transplants in Severe Aplastic Anemia: An EBMT Analysis. Haematologica (2015) 100(5):696–702. doi: 10.3324/haematol.2014.115345
28. Samarasinghe S, Clesham K, Iacobelli S, Sbianchi G, Knol C, Hamladji RM, et al. Impact of T-Cell Depletion Strategies on Outcomes Following Hematopoietic Stem Cell Transplantation for Idiopathic Aplastic Anemia: A Study on Behalf of the European Blood and Marrow Transplant Severe Aplastic Anemia Working Party. Am J Hematol (2019) 94(1):80–6. doi: 10.1002/ajh.25314
29. Deeg HJ, Anasetti C, Petersdorf E, Storb R, Doney K, Hansen JA, et al. Cyclophosphamide Plus ATG Conditioning is Insufficient for Sustained Hematopoietic Reconstitution in Patients With Severe Aplastic Anemia Transplanted With Marrow From HLA-A, B, DRB Matched Unrelated Donors. Blood (1994) 83(11):3417–8. doi: 10.1182/blood.V83.11.3417.bloodjournal83113417
30. Scheinberg P, Nunez O, Weinstein B, Scheinberg P, Biancotto A, Wu CO, et al. Horse Versus Rabbit Antithymocyte Globulin in Acquired Aplastic Anemia. N Engl J Med (2011) 365(5):430–8. doi: 10.1056/NEJMoa1103975
31. Feng X, Scheinberg P, Biancotto A, Rios O, Donaldson S, Wu C, et al. In Vivo Effects of Horse and Rabbit Antithymocyte Globulin in Patients With Severe Aplastic Anemia. Haematologica (2014) 99(9):1433–40. doi: 10.3324/haematol.2014.106542
32. Atta EH, de Sousa AM, Schirmer MR, Bouzas LF, Nucci M, Abdelhay E. Different Outcomes Between Cyclophosphamide Plus Horse or Rabbit Antithymocyte Globulin for HLA-Identical Sibling Bone Marrow Transplant in Severe Aplastic Anemia. Biol Blood Marrow Transplant (2012) 18(12):1876–82. doi: 10.1016/j.bbmt.2012.07.004
33. Kekre N, Zhang Y, Zhang MJ, Carreras J, Ahmed P, Anderlini P, et al. Effect of Antithymocyte Globulin Source on Outcomes of Bone Marrow Transplantation for Severe Aplastic Anemia. Haematologica (2017) 102(7):1291–8. doi: 10.3324/haematol.2017.164459
34. Schrezenmeier H, Passweg JR, Marsh JCW, Bacigalupo A, Bredeson CN, Bullorsky E, et al. Worse Outcome and More Chronic GVHD With Peripheral Blood Progenitor Cells Than Bone Marrow in HLA-Matched Sibling Donor Transplants for Young Patients With Severe Acquired Aplastic Anemia. Blood (2007) 110(4):1397–400. doi: 10.1182/blood-2007-03-081596
35. Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V, et al. Defining the Intensity of Conditioning Regimens: Working Definitions. Biol Blood Marrow Transplant (2009) 15(12):1628–33. doi: 10.1016/j.bbmt.2009.07.004
36. Lee SE, Yahng SA, Cho BS, Eom KS, Kim YJ, Kim HJ, et al. Impact of Pretransplant Red Cell Transfusion on Outcome After Allogeneic Stem Cell Transplantation in Adult Patients With Severe Aplastic Anemia. Bone Marrow Transplant (2016) 51(10):1323–9. doi: 10.1038/bmt.2016.140
37. ElGohary G, El Fakih R, de Latour R, Risitano A, Marsh J, Schrezenmeier H, et al. Haploidentical Hematopoietic Stem Cell Transplantation in Aplastic Anemia: A Systematic Review and Meta-Analysis of Clinical Outcome on Behalf of the Severe Aplastic Anemia Working Party of the European Group for Blood and Marrow Transplantation (SAAWP of EBMT). Bone Marrow Transplant (2020) 55(10):1906–17. doi: 10.1038/s41409-020-0897-2
Keywords: rabbit, porcine, aplastic anemia, stem cell transplantation, matched sibling donors
Citation: Zhang Y, Chen X, Li L, Li Y, Lin L, Cao Y, Wang N, Yang D, Pang A, Zhang R, Ma Q, Zhai W, He Y, Wei J, Jiang E, Han MZ, Zhang Y and Feng S (2022) Retrospective Comparison of Efficacy and Safety of Rabbit Anti-Thymocyte Globulin and Porcine Anti-Lymphocyte Globulin in Patients With Acquired Aplastic Anemia Undergoing Hematopoietic Stem Cell Transplantation From Matched Sibling Donors. Front. Immunol. 13:889784. doi: 10.3389/fimmu.2022.889784
Received: 04 March 2022; Accepted: 04 May 2022;
Published: 01 June 2022.
Edited by:
Emmanuel Katsanis, University of Arizona, United StatesCopyright © 2022 Zhang, Chen, Li, Li, Lin, Cao, Wang, Yang, Pang, Zhang, Ma, Zhai, He, Wei, Jiang, Han, Zhang and Feng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sizhou Feng, ZG9jdG9yX3N6aGZlbmdAMTYzLmNvbQ==; c3pmZW5nQGloY2Ftcy5hYy5jbg==; Yicheng Zhang, eWN6aGFuZ0B0amgudGptdS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
 Yuanfeng Zhang
Yuanfeng Zhang Xin Chen1†
Xin Chen1† Lin Li
Lin Li Yun Li
Yun Li Li Lin
Li Lin Yang Cao
Yang Cao Na Wang
Na Wang Donglin Yang
Donglin Yang Aiming Pang
Aiming Pang MingZhe Han
MingZhe Han Yicheng Zhang
Yicheng Zhang