- Department of Hepatobiliary Surgery, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China
Objective: This is the first systematic review and meta-analysis to determine the factors that contribute to poor antibody response in organ transplant recipients after receiving the 2-dose severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine.
Method: Data was obtained from Embase, PubMed, Web of Science, Cochrane Library, China National Knowledge Infrastructure (CNKI), and Chinese Biomedical Literature Database (CBM). Studies reporting factors associated with antibody responses to the 2-dose SARS-CoV-2 vaccine in solid organ transplant recipients were included in our study based on the inclusion and exclusion criteria. Two researchers completed the literature search, screening, and data extraction. Randomized models were used to obtain results. Egger’s test was performed to determine publication bias. Sensitivity analysis was performed to determine the stability of the result. The heterogeneity was determined using the Galbraith plot and subgroup analysis.
Results: A total of 29 studies were included in the present study. The factors included living donor, BNT162b2, tacrolimus, cyclosporine, antimetabolite, mycophenolic acid (MPA) or mycophenolate mofetil (MMF), azathioprine, corticosteroids, high-dose corticosteroids, belatacept, mammalian target of rapamycin (mTOR) inhibitor, tritherapy, age, estimated glomerular filtration rate (eGFR), hemoglobin, and tacrolimus level were significantly different. Multivariate analysis showed significant differences in age, diabetes mellitus, MPA or MMF, high-dose corticosteroids, tritherapy, and eGFR.
Conclusion: The possible independent risk factors for negative antibody response in patients with organ transplants who received the 2-dose SARS-CoV-2 vaccine include age, diabetes mellitus, low eGFR, MPA or MMF, high-dose corticosteroids, and triple immunosuppression therapy. mTOR inhibitor can be a protective factor against weak antibody response.
Systematic Review Registration: PROSPERO, identifier CRD42021257965.
Introduction
During the coronavirus disease (COVID-19) pandemic, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccination became important prophylaxis. The antibody response produced by the immunization can protect the body against SARS-CoV-2 (1–3). Previous studies have reported decreased antibody response after the administration of the SARS-CoV-2 vaccine in solid organ transplant recipients compared with that in healthy vaccinators (4, 5) who had a worse prognosis after infection (6–8). Identifying the risk factors associated with weak antibody response after vaccination can help further measures to increase the benefit of vaccination in solid organ transplant recipients. The present study focused on identifying factors that can affect the antibody response to the 2-dose SARS-CoV-2 vaccine in adults with solid organ transplants.
Method
Registration
The protocol of this systematic review and meta-analysis had been reported on the PROSPERO (registration no. CRD42021257965).
Search Strategy
Electronic Databases used for searching included Embase, PubMed, Web of Science, Cochrane Library, China National Knowledge Infrastructure (CNKI), and Chinese Biomedical Literature Database (CBM). Few records were obtained from other sources (Studies were found to potentially meet the inclusion criteria before conducting the systematic search). The search strategy was conducted by combining subject terms with free words. Two investigators designed the search strategy collaboratively.
Eligibility Criteria and Study Selection
Cohort studies, case-control studies, and case series were included in this study. The included population was defined as adult solid organ transplant recipients who received a standard dose of the SARS-CoV-2 vaccine. The inclusion criteria were as follows (1): study type: case-control, cohort studies, and case series (2); population: adult solid organ transplant recipients (3); intervention: 2-dose SARS-CoV-2 vaccine (4) outcome: the serologic results. The exclusion criteria were as follows (1): articles not published in Chinese or English language (2); no relevant factors reported (3); studies including the same population unless different outcomes were reported (4); recipients with a history of COVID19 (5); data not available to conduct the meta-analysis. Two examiners performed the study selection independently, with contradictions decided by negotiating with a third examiner.
Data Collection and Extraction
Two researchers extracted the data separately, and different branches reached the decision together through discussion with a third researcher. Data were extracted from the general information about the study, population characteristics, the intervention method, serological assays, and potential influencing factors. Data that were believed to contain errors were not extracted and included. EndNote X9 was used for reference.
Risk of Bias
Study quality was independently reviewed by two evaluators using the Newcastle Ottawa Scale (NOS), containing 3 main elements: selection, comparability, and outcome. The study quality was defined as follows: 0–3 points indicating low quality; 4–6 points indicating medium quality; 7–9 points indicating high quality. The Egger’s test was applied to assess publication bias, with P < 0.05 representing publication bias.
Data Synthesis
Following the past protocol, a random-effect meta-analysis model was applied irrespective of heterogeneity. Standardized mean difference (SMD) with 95% confidence intervals was calculated for continuous variables, and odds ratio (OR) with 95% confidence intervals was applied for the dichotomous variables. Heterogeneity was evaluated using the Cochran Q test and I-squared, with I² > 50% or P < 0.1 considered to indicate high heterogeneity. Sensitivity analysis based on individual studies was conducted to examine the stability of the results. Subgroup analysis was conducted to explore the source of heterogeneity, which included the country, population, vaccine, and antibody for testing. Studies found to infer heterogeneity according to the Galbraith plot were excluded and then reanalyzed. OR originally reported by multivariate analysis was included in the analysis, but heterogeneity analysis and publication bias assessment was limited. The primary statistical analysis was performed by the StataSE 16 software.
Results
Literature Search and Study Characteristics
The last literature search was performed on October 8, 2021. A total of 1129 records were obtained from electronic databases and other sources. The detailed search strategy is included in Supplementary Material. A total of 64 studies were included in the full-text review after initial screening and excluding duplicate studies. A total of 29 cohort studies (9–37) were finally included in the present study (Figure 1). Among the included studies, heart transplant recipients (HTR), liver transplant recipients (LTR), kidney transplant recipients (KTR), pulmonary transplant recipients (PTR), and other solid organ transplant recipients (SOTR) were involved, wherein KTR predominated. These individuals belonged from 11 different countries and received vaccination with four types of SARS-CoV-2 vaccines. Eventually, a total of 16 dichotomous variables, 11 continuous variables, and 10 OR values obtained from the multivariate analysis were combined in the analysis. The detailed study information and NOS score are present in the Supplemental Material.

Figure 1 Flow chart depicting the study inclusion and exclusion processes. CNKI, China National Knowledge Infrastructure; CBM, Chinese Biomedical Literature Database; other sources, literature collected in other ways before conducting the systemic search.
Data Analysis and Publication Bias
A negative antibody response was defined as an outcome event. The results of the dichotomous variables and the continuous variables are shown in Figures 2 and 3, respectively. The results of risk factors obtained from multivariate logistic regression analysis are shown in Figure 4. Significant differences were found among the dichotomous variables for twelve factors, which included living donor, BNT162b2, tacrolimus, cyclosporine, antimetabolite, mycophenolic acid (MPA)/mycophenolate mofetil (MMF), azathioprine (AZA), corticosteroids (CS), high-dose CS, belatacept, mammalian target of rapamycin (mTOR) inhibitor, and tritherapy. Significant heterogeneity was found in MPA/MMF, CS, mTOR inhibitor, and tritherapy. Egger’s test suggested publication bias for high-dose CS. Significant differences were found for the four continuous factors, which included age, estimated glomerular filtration rate (eGFR), hemoglobin, and tacrolimus level. Nevertheless, significant heterogeneity was found in age, time from transplantation, eGFR, creatinine, and lymphocyte count. Publication bias in body mass index (BMI) was determined by Egger’s test. The combined result from multivariate analysis associated with a negative response, age, diabetes mellitus, MPA/MMF, high-dose CS, and tritherapy showed significant differences. Significant heterogeneity was found in the female gender, age of the patient, and MPA/MMF. The only significant difference was in eGFR for factors associated with the positive response. Both age and time from transplantation showed significant heterogeneity.
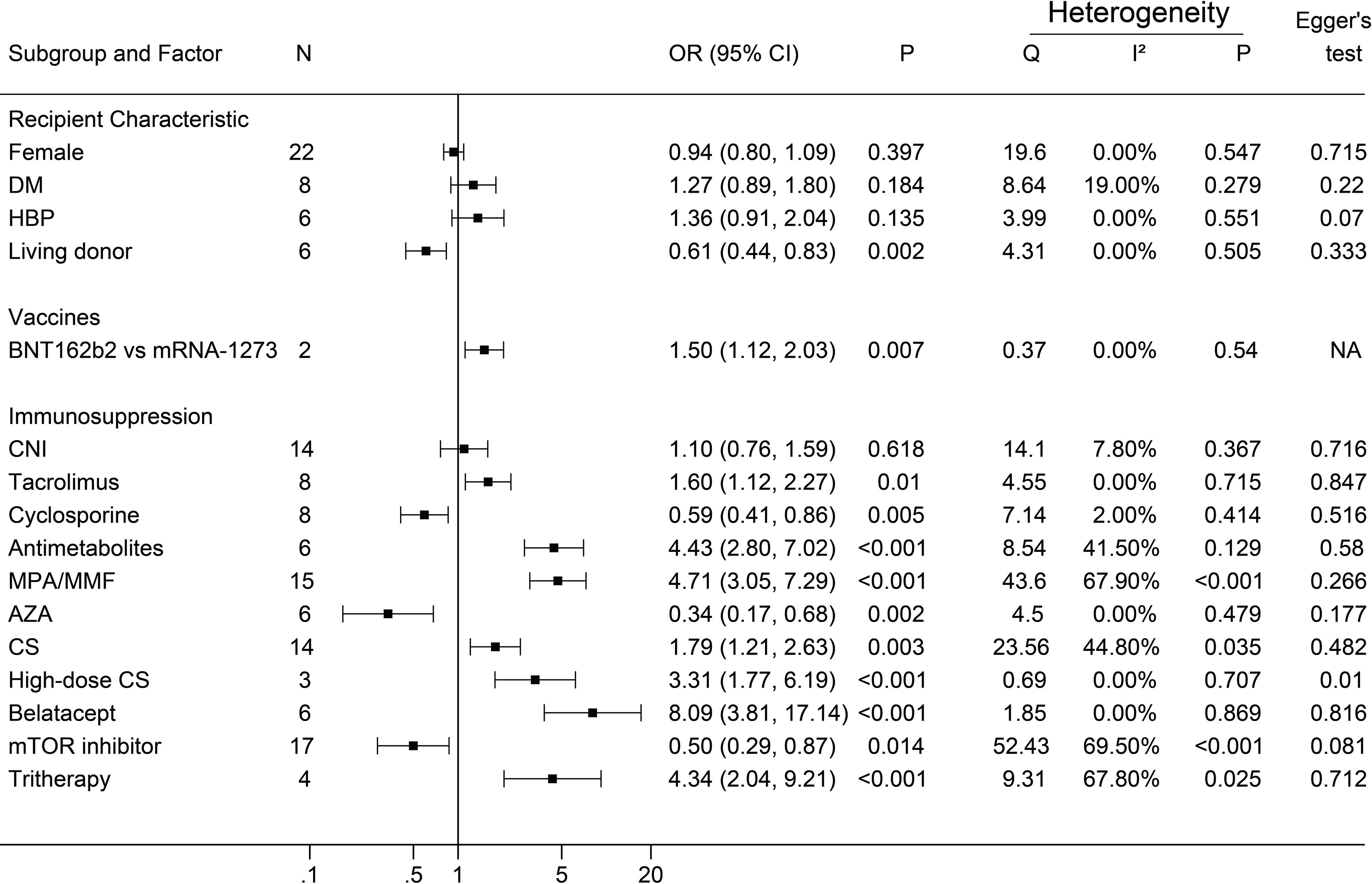
Figure 2 Forest plots of dichotomous factors. The event was a negative antibody response after vaccination. N, The number of studies; DM, diabetes mellitus; HBP, hypertension; CNI, calcineurin inhibitor; MPA/MMF, mycophenolic acid or mycophenolate mofetil; AZA, azathioprine; CS, low-dose corticosteroids; mTOR inhibitor, mammalian target of rapamycin inhibitor; Tritherapy, triple immunosuppressive therapy. NA indicates missing Egger's test.
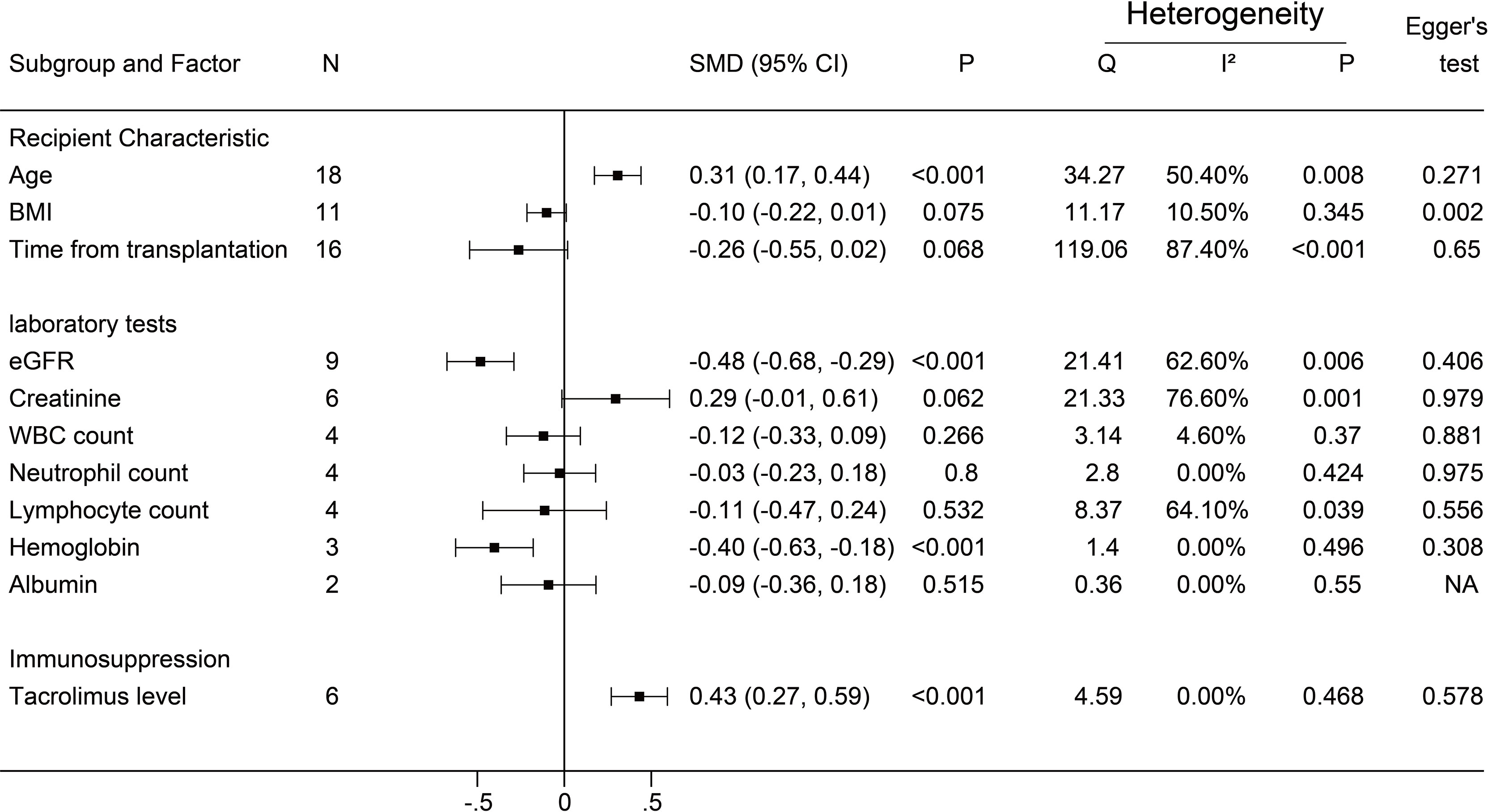
Figure 3 Forest plots of continuous factors.The event was a negative antibody response after vaccination. N, The number of studies; BMI, body mass index; eGFR, estimated glomerular filtration rate; WBC, white blood cells. NA indicates missing Egger's test.
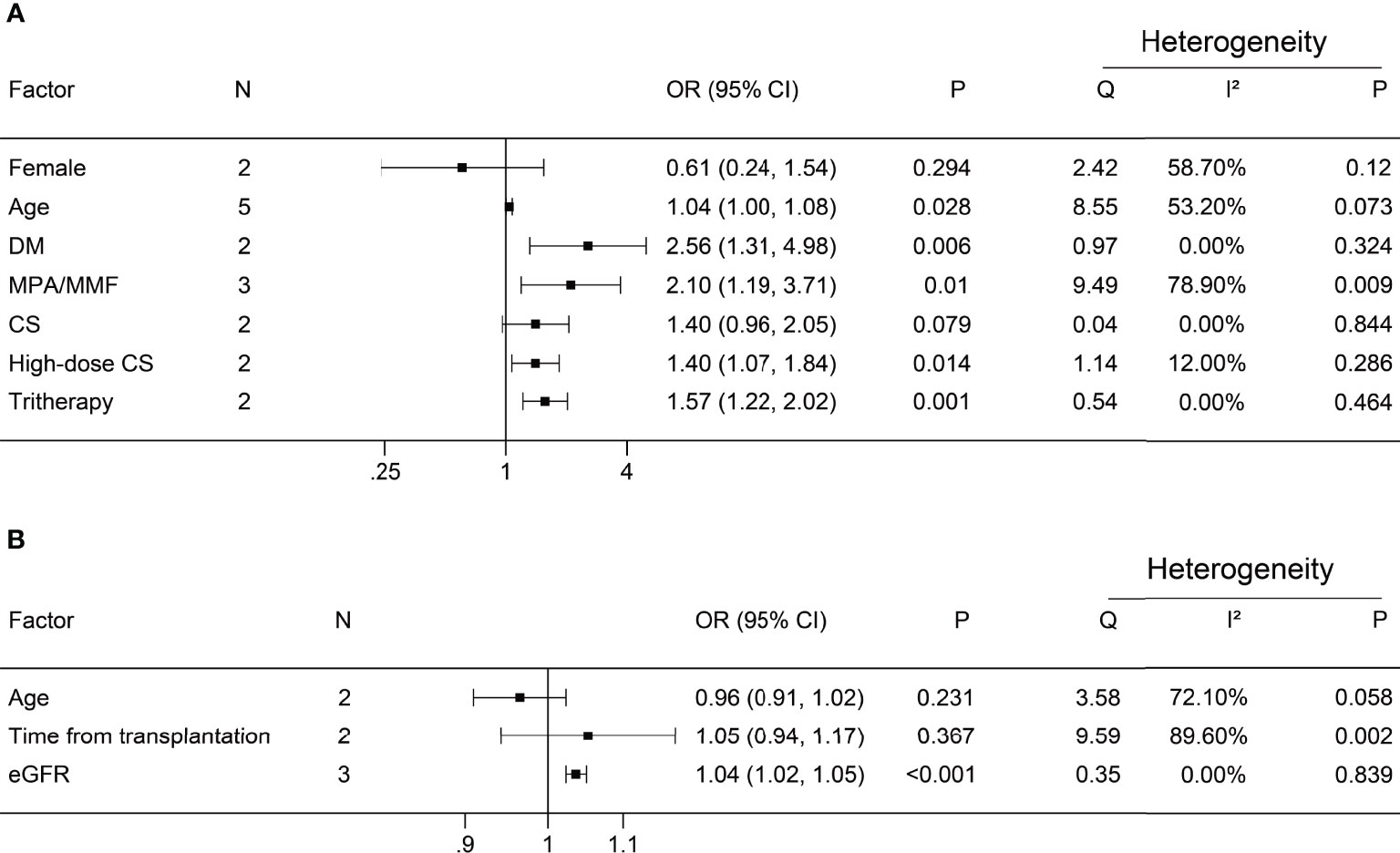
Figure 4 Forest plots of multivariate analysis. (A) Factors associated with a negative antibody response; (B) Factors associated with positive antibody response. N, number of studies; DM, diabetes mellitus; MPA/MMF, mycophenolic acid or mycophenolate mofetil; CS, corticosteroids; tritherapy, triple immunosuppressive therapy; eGFR, estimated glomerular filtration rate.
Heterogeneity and Exclusion Criteria
We performed subgroup analyses and Galbraith plots to determine the sources of heterogeneity for dichotomous and continuous variables. In the present study, we found that I² >50% or P <0.1 was necessary for significant heterogeneity. The subgroup analysis based on country, population, vaccine, and antibody is shown in the Supplemental Material. Studies that can cause heterogeneity in results found by the Galbraith plot were excluded. The remaining studies were reanalyzed. Taking the factor MPA/MMF as an example, the results of the Galbraith plot and reanalysis are shown in Figure 5. The summary reanalysis results are shown in Figure 6. The studies that were excluded based on the Galbraith plot were as follows: MPA/MMF = 3 (28, 29, 33), CS = 1 (16), mTOR inhibitor = 3 (13, 15, 17), tritherapy = 1 (28), age = 3 (12, 18, 26), time from transplantation = 5 (11, 17, 21, 28, 35), eGFR = 2 (28, 31), creatinine = 2 (28, 30), and lymphocyte = 1 (34). Subgroup analyses and the Galbraith plot of other factors with significant heterogeneity, which included CS, mTOR inhibitor, tritherapy, age, time from transplantation, eGFR, creatinine, and lymphocyte, are shown in the Supplemental Material.
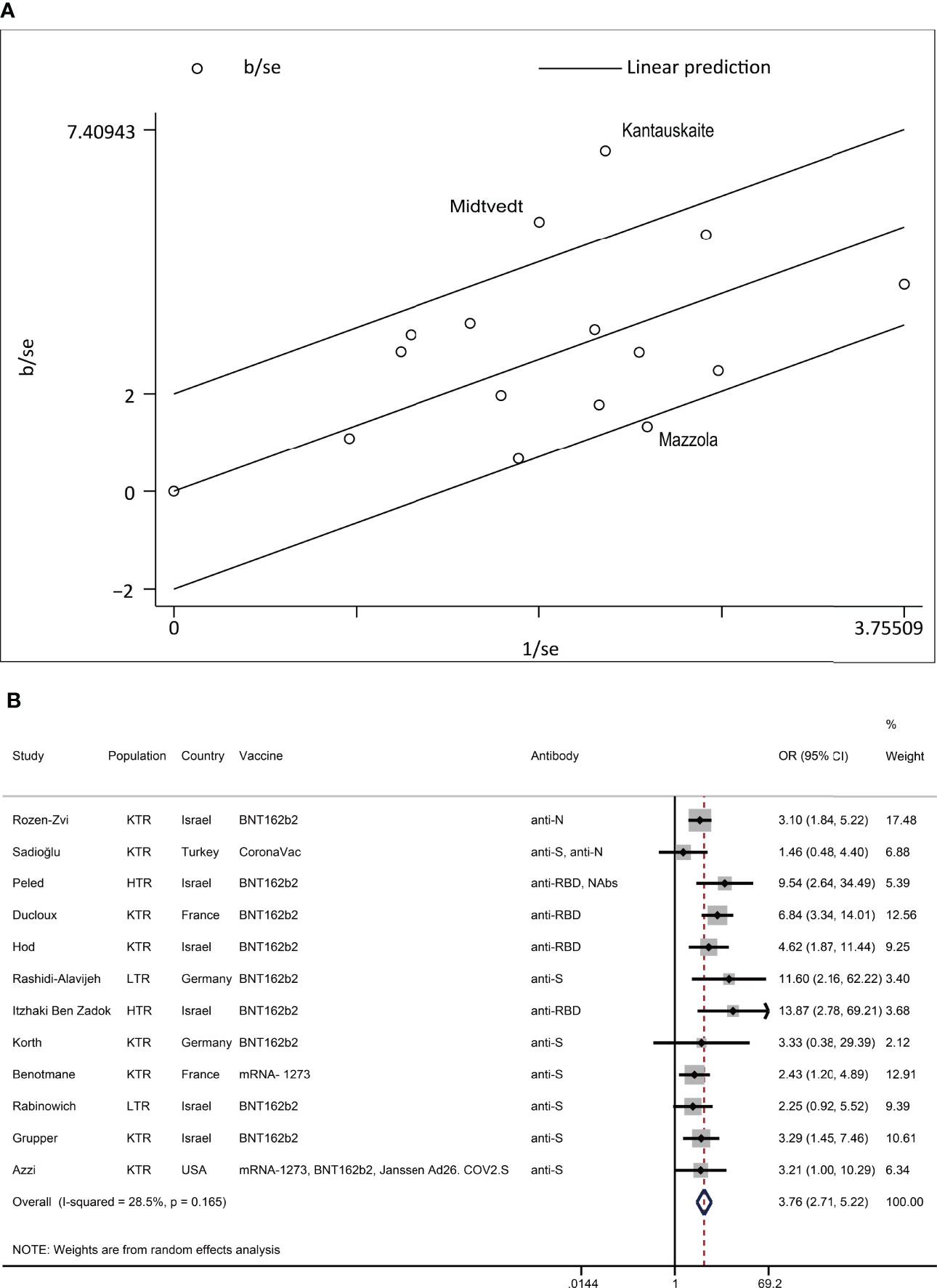
Figure 5 Heterogeneity exploring of MPA/MMF. (A) In the Galbraith plot of MPA/MMF for heterogeneity exploration, hollow circles outside the parallel reference line represent studies that were considered possible sources of heterogeneity. (B) Reanalysis of MPA/MMF after the removal of possible sources of heterogeneity. The event was a negative antibody response after vaccination. N, the number of studies; KTR, kidney transplant recipients; LTR, liver transplant recipients; HTR, heart transplant recipients; anti-S, anti-spike protein antibody; anti-N, anti-nucleocapsid protein antibody; anti-RBD, anti-receptor-binding domain antibody; Nab, neutralizing antibody.
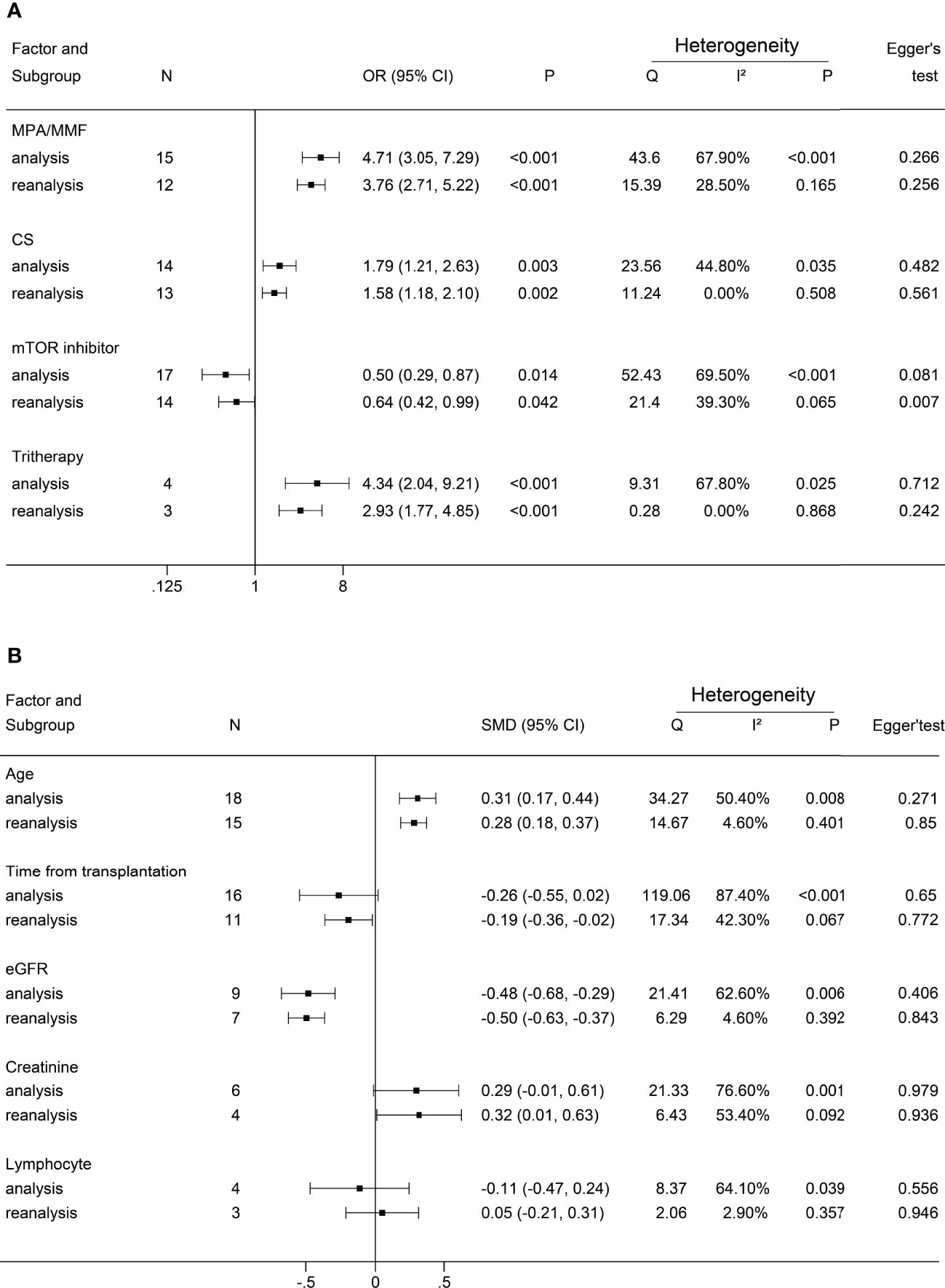
Figure 6 Forest plots of reanalysis. (A) reanalysis of dichotomous factors. (B) reanalysis of continuous factors. The event was a negative antibody response after vaccination. MPA/MMF, mycophenolic acid or mycophenolate mofetil; CS, corticosteroids; mTOR inhibitor, mammalian target of rapamycin inhibitor; Tritherapy, triple immunosuppressive therapy; eG.
Sensitivity Analysis
Sensitivity analysis of each factor showed that no individual studies changed the final results. The factors associated with multivariate analysis were not included in sensitivity analyses for a few included studies. The sensitivity analysis results are shown in the Supplemental Material.
Discussion
To the best of our knowledge, this is the first systemic review and meta-analysis to determine the risk factors of negative antibody response to the 2-dose SARS-CoV-2 vaccine in adult recipients of solid organs. The significant risk factors associated with negative antibody response included age, diabetes mellitus, MPA or MMF, high-dose corticosteroids, and triple immunosuppression therapy.
The use of immunosuppressants is one of the most important reasons for anergy to vaccination. Our results showed that MPA/MMF, as a representative among antimetabolites, is an independent risk factor for negative antibody response after immunization. The study showed that the administration of MPA/MMF in patients with systemic lupus erythematosus (SLE) reduced the serologic response to the SARS-CoV-2 vaccine (38), which indicated that MMF/MPA weakened the humoral response after vaccination regardless of the receiver. Furthermore, in liver transplant recipients, mycophenolate not only resulted in a poor prognosis of COVID19 but also increased the risk of severe COVID-19 (39). These results implied that MMF/MPA should be used with caution during the COVID-19 pandemic. Corticosteroids are another widely used immunosuppressant that can reduce the antibody response after vaccination in solid organ transplant recipients with high doses versus low doses. Even though the result of dichotomous variables was different from the results of multivariate analysis, we believe that low-dose CS cannot increase the risk of nonresponse to vaccination because of the reliability of the multivariate analysis. Although reduced vaccine response with triple immunosuppressive therapy is dangerous, it is difficult to balance the immunostability and immune response when solid organ transplant recipients take triple immunosuppressants to keep their immune system silent.
Surprisingly, our study suggested another immunosuppressant, mTOR inhibitor, which did not increase the risk of vaccine ineffectiveness. This result was consistent with the multiple-logistic-regression results of Stumpf et al. and Ducloux et al. (17, 19). However, these results are difficult to synthesize. According to our results, dichotomous variables showed risk factors, whereas the opposite was found for cyclosporine. The results of the dichotomous variables (tacrolimus) were consistent with the continuous variables (tacrolimus level), which implied that tacrolimus can be negatively correlated with humoral immune response. The results show that belatacept can be associated with negative antibody response. Terrec et al. reported studies on COVID-19 infection and vaccine response after belatacept conversion in renal transplant recipients and concluded that renal transplant recipients receiving belatacept have a poorer response to the SARS-CoV-2 vaccine. This is consistent with our results, which suggested a delay in belatacept conversion because it can increase the risk of opportunistic infections (40).
The general characteristics of solid organ transplant recipients help to identify the high-risk population. Age is a risk factor for negative antibody response to SARS-CoV-2 vaccination. Many studies reported a late and weak response to the vaccine in elderly vaccinees than in younger vaccinees (41, 42), which is consistent with our results. However, we did not find a significant result for age in the multivariate analysis associated with positive antibody response. Diabetes mellitus is considered to be associated with negative antibody response because the multivariate analysis is more reliable. Moreover, meta-analysis results also indicated a negative correlation between diabetes mellitus prevalence and humoral response rate to the SARS-CoV-2 vaccine in other populations (43). Hypertension, another prevalent disease, was not a significant risk factor in our results. Living donors can be a protective factor for negative antibody responses, whereas administration of the BNT162b2 vaccine (compared to mRNA-1273) can be a risk factor. The result of multiple logistic regression also showed that BNT162b2 was an essential factor responsible for the failure of the antibody response in a previous study (19). The gender (female), BMI, and time from transplantation were not significant factors, which implied that they did not affect the antibody response.
The laboratory results from our study suggested that eGFR correlated with vaccine antibody response, which was shown by continuous variables and multivariate analyses. This indicated that patients with better renal function can react to the SARS-CoV-2 vaccine better. Unexpectedly, the results shown by hemoglobin indicated that it can be positively correlated with the antibody response. The reanalysis for creatinine showed a significant difference. However, we considered that the evidence for its correlation with the vaccine-antibody response was insufficient without the support of multivariate analysis (12, 32). However, according to our results, white blood cell (WBC) count, neutrophils count, lymphocytes count, and albumin did not show significance.
For heterogeneity determination, the subgroup analysis was unable to identify the causes of heterogeneity, which suggested that such subgroupings such as country, type of vaccine, antibody response, and type of transplantation may not be appropriate, and heterogeneity was because of a combination of multiple factors. After excluding the studies that caused heterogeneity found by the Galbraith plot, the heterogeneity of many factors became non-significant, and the results were also unchanged. However, the heterogeneity of several factors, such as mTOR inhibitor, time from transplantation, and creatinine levels, were significant after reanalysis, and the creatinine results changed, which suggested further investigating.
Limitations
We did not limit the graft type of the included population because of the similarity of immune status and immunosuppressive agents in solid organ transplant recipients and also to include more studies to expand the conclusions. This led to heterogeneity. We selected the 2-dose-vaccine studies for intervention. However, studies have been performed to show that an increased dose can improve the immune response in solid organ transplant recipients. This was not included in our study (44, 45). We only considered the outcomes of antibody responses to the vaccine. We did not include cellular immune responses in the study; however, studies have reported that cellular immune responses were an equally important factor (46, 47). Among the factors included in the analysis, when constitutional ratios were not available, we considered calcineurin inhibitor (CNI) and antimetabolites, a class of drugs in general, as a variable. This can also contribute to the difficulty in interpreting some of our results.
Certain factors, such as eGFR and belatacept, were almost exclusively reported in renal transplantation studies. Some of the studies that fulfilled the inclusion criteria contained brief methods or process descriptions, which can increase the risk of errors. When potentially incorrect data were found, we excluded those parts of the data rather than the entire study. While determining heterogeneity, we identified that the study by Kantauskaite et al. was a source of heterogeneity in more than one factor. Therefore, we possibly had unreasonable inclusion and exclusion criteria for the study.
Conclusions
Old age, diabetes mellitus, MPA or mycophenolate, high-dose corticosteroids, and triple immunosuppression therapy are important risk factors for negative antibody response in solid organ transplant recipients administered with a 2-dose SARS-CoV-2 vaccine. BNT162b2, antimetabolites, belatacept, and tacrolimus can be associated with weak antibody responses to the SARS-CoV-2 vaccine in solid organ transplant recipients, whereas the factor living donors showed opposite results. The administration of low-dose CS and mTOR inhibitor do not increase the risk of negative humoral response in solid organ transplant recipients receiving a 2-dose SARS-CoV-2 vaccine. In general, during the COVID-19 pandemic, MMF/MPA, high-dose CS, and triple immunosuppressants should be limited. For the population with risk factors, such as diabetes mellitus, old age, and low eGFR, the SARS-CoV-2 vaccine should be enhanced and more attention should be paid to preventing COVID-19 infection while keeping the vaccine safe. Compared with BNT162b2, the mRNA-1273 vaccine can be a better choice for solid organ transplant recipients. More advanced and comprehensive studies are required in the future.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
Study design and registration: KZ and DP. Search strategy design: TL and TM. literature search: YW. Study selection: HY, YL, and KZ. Data collection and extraction: KZ, DP, and SX. Data analysis and writing: KZ, DP, and ZH. Arrangement and supervision: ZW. All authors contributed to the article and approved the submitted version.
Funding
The present study was funded by The National Natural Science Foundation of China (No. 82170666 and No. 81873592).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors thank Xin Ye from The First Affiliated Hospital of Chongqing Medical University, Chongqing, China, for her support and for providing some of the references although she was not a participant in our original plan. FR, estimated glomerular filtration rate.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.888385/full#supplementary-material
References
1. Corbett KS, Nason MC, Flach B, Gagne M, O'Connell S, Johnston TS, et al. Immune Correlates of Protection by mRNA-1273 Vaccine Against SARS-CoV-2 in Nonhuman Primates. Science (2021) 373(6561):eabj0299. doi: 10.1126/science.abj0299
2. He X, Chandrashekar A, Zahn R, Wegmann F, Yu J, Mercado NB, et al. Low-Dose Ad26.Cov2.S Protection Against SARS-CoV-2 Challenge in Rhesus Macaques. Cell (2021) 184(13):3467–73.e11. doi: 10.1016/j.cell.2021.05.040
3. McMahan K, Yu J, Mercado NB, Loos C, Tostanoski LH, Chandrashekar A, et al. Correlates of Protection Against SARS-CoV-2 in Rhesus Macaques. Nature (2021) 590(7847):630–4. doi: 10.1038/s41586-020-03041-6
4. Havlin J, Svorcova M, Dvorackova E, Lastovicka J, Lischke R, Kalina T, et al. Immunogenicity of Bnt162b2 mRNA Covid-19 Vaccine and SARS-CoV-2 Infection in Lung Transplant Recipients. J Heart Lung Transplant (2021) 40(8):754–8. doi: 10.1016/j.healun.2021.05.004
5. Sattler A, Schrezenmeier E, Weber UA, Potekhin A, Bachmann F, Straub-Hohenbleicher H, et al. Impaired Humoral and Cellular Immunity After SARS-CoV-2 Bnt162b2 (Tozinameran) Prime-Boost Vaccination in Kidney Transplant Recipients. J Clin Invest (2021) 131(14):e150175. doi: 10.1172/jci150175
6. Ao G, Wang Y, Qi X, Nasr B, Bao M, Gao M, et al. The Association Between Severe or Death Covid-19 and Solid Organ Transplantation: A Systematic Review and Meta-Analysis. Transplant Rev (Orlando) (2021) 35(3):100628. doi: 10.1016/j.trre.2021.100628
7. Ahmed F, Abid M, Maniya T, Usman MS, Fudim M. Incidence and Prognosis of Covid-19 Amongst Heart Transplant Recipients: A Systematic Review and Meta-Analysis. Eur J Prev Cardiol (2022) 29(6):e224–6. doi: 10.1093/eurjpc/zwab175
8. Caillard S, Anglicheau D, Matignon M, Durrbach A, Greze C, Frimat L, et al. An Initial Report From the French Sot Covid Registry Suggests High Mortality Due to Covid-19 in Recipients of Kidney Transplants. Kidney Int (2020) 98(6):1549–58. doi: 10.1016/j.kint.2020.08.005
9. Boyarsky BJ, Werbel WA, Avery RK, Tobian AAR, Massie AB, Segev DL, et al. Antibody Response to 2-Dose SARS-CoV-2 mRNA Vaccine Series in Solid Organ Transplant Recipients. Jama (2021) 325(21):2204–6. doi: 10.1001/jama.2021.7489
10. Bertrand D, Hanoy M, Edet S, Lemée V, Hamzaoui M, Laurent C, et al. Antibody Response to SARS-CoV-2 mRNA Bnt162b2 Vaccine in Kidney Transplant Recipients and in-Centre and Satellite Centre Haemodialysis Patients. Clin Kidney J (2021) 14(9):2127–8. doi: 10.1093/ckj/sfab100
11. Rozen-Zvi B, Yahav D, Agur T, Zingerman B, Ben-Zvi H, Atamna A, et al. Antibody Response to SARS-CoV-2 mRNA Vaccine Among Kidney Transplant Recipients: A Prospective Cohort Study. Clin Microbiol Infect (2021) 27(8):1173 e1– e4. doi: 10.1016/j.cmi.2021.04.028
12. Sadioğlu RE, Demir E, Evren E, Aktar M, Şafak S, Artan AS, et al. Antibody Response to Two Doses of Inactivated SARS-CoV-2 Vaccine (Coronavac) in Kidney Transplant Recipients. Transpl Infect Dis (2021) 23(6):e13740. doi: 10.1111/tid.13740
13. Peled Y, Ram E, Lavee J, Sternik L, Segev A, Wieder-Finesod A, et al. Bnt162b2 Vaccination in Heart Transplant Recipients: Clinical Experience and Antibody Response. J Heart Lung Transplant (2021) 40(8):759–62. doi: 10.1016/j.healun.2021.04.003
14. Herrera S, Colmenero J, Pascal M, Escobedo M, Castel MA, Sole-González E, et al. Cellular and Humoral Immune Response After mRNA-1273 SARS-CoV-2 Vaccine in Liver and Heart Transplant Recipients. Am J Transplant (2021) 21(12):3971–9. doi: 10.1111/ajt.16768
15. Shostak Y, Shafran N, Heching M, Rosengarten D, Shtraichman O, Shitenberg D, et al. Early Humoral Response Among Lung Transplant Recipients Vaccinated With Bnt162b2 Vaccine. Lancet Respir Med (2021) 9(6):e52–e3. doi: 10.1016/s2213-2600(21)00184-3
16. Rabinowich L, Shibolet O, Katchman H. Effectiveness of SARS-CoV-2 Vaccination in Liver Transplanted Patients: The Debate Is Open! J Hepatol (2022) 76(1):239–40. doi: 10.1016/j.jhep.2021.07.034
17. Ducloux D, Colladant M, Chabannes M, Bamoulid J, Courivaud C. Factors Associated With Humoral Response After Bnt162b2 mRNA Covid-19 Vaccination in Kidney Transplant Patients. Clin Kidney J (2021) 14(10):2270–2. doi: 10.1093/ckj/sfab125
18. Hall VG, Ferreira VH, Ierullo M, Ku T, Marinelli T, Majchrzak-Kita B, et al. Humoral and Cellular Immune Response and Safety of Two-Dose SARS-CoV-2 mRNA-1273 Vaccine in Solid Organ Transplant Recipients. Am J Transplant (2021) 21(12):3980–9. doi: 10.1111/ajt.16766
19. Stumpf J, Siepmann T, Lindner T, Karger C, Schwöbel J, Anders L, et al. Humoral and Cellular Immunity to SARS-CoV-2 Vaccination in Renal Transplant Versus Dialysis Patients: A Prospective, Multicenter Observational Study Using mRNA-1273 or Bnt162b2 mRNA Vaccine. Lancet Reg Health Eur (2021) 9:100178. doi: 10.1016/j.lanepe.2021.100178
20. Hod T, Ben-David A, Olmer L, Levy I, Ghinea R, Mor E, et al. Humoral Response of Renal Transplant Recipients to the BNT162b2 SARS-CoV-2 mRNA Vaccine Using Both RBD IgG and Neutralizing Antibodies. Transplantation (2021) 105(11):e234–43. doi: 10.1097/tp.0000000000003889
21. Rashidi-Alavijeh J, Frey A, Passenberg M, Korth J, Zmudzinski J, Anastasiou OE, et al. Humoral Response to SARS-CoV-2 Vaccination in Liver Transplant Recipients-A Single-Center Experience. Vaccines (Basel) (2021) 9(7):738. doi: 10.3390/vaccines9070738
22. Noble J, Langello A, Bouchut W, Lupo J, Lombardo D, Rostaing L. Immune Response Post-SARS-CoV-2 mRNA Vaccination in Kidney-Transplant Recipients Receiving Belatacept. Transplantation (2021) 105(11):e259–60. doi: 10.1097/tp.0000000000003923
23. Ou MT, Boyarsky BJ, Chiang TPY, Bae S, Werbel WA, Avery RK, et al. Immunogenicity and Reactogenicity After SARS-CoV-2 mRNA Vaccination in Kidney Transplant Recipients Taking Belatacept. Transplantation (2021) 105(9):2119–23. doi: 10.1097/TP.0000000000003824
24. Marinaki S, Adamopoulos S, Degiannis D, Roussos S, Pavlopoulou ID, Hatzakis A, et al. Immunogenicity of SARS-CoV-2 Bnt162b2 Vaccine in Solid Organ Transplant Recipients. Am J Transplant (2021) 21(8):2913–5. doi: 10.1111/ajt.16607
25. Holden IK, Bistrup C, Nilsson AC, Hansen JF, Abazi R, Davidsen JR, et al. Immunogenicity of SARS-CoV-2 mRNA Vaccine in Solid Organ Transplant Recipients. J Intern Med (2021) 290(6):1264–7. doi: 10.1111/joim.13361
26. Itzhaki Ben Zadok O, Shaul AA, Ben-Avraham B, Yaari V, Ben Zvi H, Shostak Y, et al. Immunogenicity of the Bnt162b2 mRNA Vaccine in Heart Transplant Recipients - a Prospective Cohort Study. Eur J Heart Fail (2021) 23(9):1555–9. doi: 10.1002/ejhf.2199
27. Korth J, Jahn M, Dorsch O, Anastasiou OE, Sorge-Hadicke B, Eisenberger U, et al. Impaired Humoral Response in Renal Transplant Recipients to SARS-CoV-2 Vaccination With Bnt162b2 (Pfizer-Biontech). Viruses (2021) 13(5):756. doi: 10.3390/v13050756
28. Kantauskaite M, Müller L, Kolb T, Fischer S, Hillebrandt J, Ivens K, et al. Intensity of Mycophenolate Mofetil Treatment Is Associated With an Impaired Immune Response to SARS-CoV-2 Vaccination in Kidney Transplant Recipients. Am J Transplant (2021) 22(2):634–9. doi: 10.1111/ajt.16851
29. Midtvedt K, Tran T, Parker K, Marti HP, Stenehjem AE, Goransson LG, et al. Low Immunization Rate in Kidney Transplant Recipients Also After Dose 2 of the Bnt162b2 Vaccine: Continue to Keep Your Guard Up! Transplantation (2021) 105(8):e80–e1. doi: 10.1097/TP.0000000000003856
30. Benotmane I, Gautier-Vargas G, Cognard N, Olagne J, Heibel F, Braun-Parvez L, et al. Low Immunization Rates Among Kidney Transplant Recipients Who Received 2 Doses of the mRNA-1273 SARS-CoV-2 Vaccine. Kidney Int (2021) 99(6):1498–500. doi: 10.1016/j.kint.2021.04.005
31. Rabinowich L, Grupper A, Baruch R, Ben-Yehoyada M, Halperin T, Turner D, et al. Low Immunogenicity to SARS-CoV-2 Vaccination Among Liver Transplant Recipients. J Hepatol (2021) 75(2):435–8. doi: 10.1016/j.jhep.2021.04.020
32. Crespo M, Barrilado-Jackson A, Padilla E, Eguia J, Echeverria-Esnal D, Cao H, et al. Negative Immune Responses to Two-Dose mRNA COVID-19 Vaccines in Renal Allograft Recipients Assessed With Simple Antibody and Interferon Gamma Release Assay Cellular Monitoring. Am J Transplant (2021) 22(3):786–800. doi: 10.1111/ajt.16854
33. Mazzola A, Todesco E, Drouin S, Hazan F, Marot S, Thabut D, et al. Poor Antibody Response After Two Doses of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Vaccine in Transplant Recipients. Clin Infect Dis (2022) 74(6):1093–1096. doi: 10.1093/cid/ciab580
34. Grupper A, Rabinowich L, Schwartz D, Schwartz IF, Ben-Yehoyada M, Shashar M, et al. Reduced Humoral Response to mRNA SARS-CoV-2 Bnt162b2 Vaccine in Kidney Transplant Recipients Without Prior Exposure to the Virus. Am J Transplant (2021) 21(8):2719–26. doi: 10.1111/ajt.16615
35. Azzi Y, Raees H, Wang T, Cleare L, Liriano-Ward L, Loarte-Campos P, et al. Risk Factors Associated With Poor Response to Covid-19 Vaccination in Kidney Transplant Recipients. Kidney Int (2021) 100(5):1127–8. doi: 10.1016/j.kint.2021.08.019
36. Ruether DF, Schaub GM, Duengelhoef PM, Haag F, Brehm TT, Fathi A, et al. Sars-Cov2-Specific Humoral and T-Cell Immune Response After Second Vaccination in Liver Cirrhosis and Transplant Patients. Clin Gastroenterol Hepatol (2022) 20(1):162–72.e9. doi: 10.1016/j.cgh.2021.09.003
37. Russo G, Lai Q, Poli L, Perrone MP, Gaeta A, Rossi M, et al. SARS-CoV-2 Vaccination With Bnt162b2 in Renal Transplant Patients: Risk Factors for Impaired Response and Immunological Implications. Clin Transplant (2022) 36(1):e14495. doi: 10.1111/ctr.14495
38. Tang W, Gartshteyn Y, Ricker E, Inzerillo S, Murray S, Khalili L, et al. The Use of Covid-19 Vaccines in Patients With Sle. Curr Rheumatol Rep (2021) 23(11):79. doi: 10.1007/s11926-021-01046-2
39. Colmenero J, Rodríguez-Perálvarez M, Salcedo M, Arias-Milla A, Muñoz-Serrano A, Graus J, et al. Epidemiological Pattern, Incidence, and Outcomes of Covid-19 in Liver Transplant Patients. J Hepatol (2021) 74(1):148–55. doi: 10.1016/j.jhep.2020.07.040
40. Terrec F, Jouve T, Malvezzi P, Janbon B, Naciri Bennani H, Rostaing L, et al. Belatacept Use After Kidney Transplantation and Its Effects on Risk of Infection and Covid-19 Vaccine Response. J Clin Med (2021) 10(21):5159. doi: 10.3390/jcm10215159
41. Collier DA, Ferreira I, Kotagiri P, Datir RP, Lim EY, Touizer E, et al. Age-Related Immune Response Heterogeneity to SARS-CoV-2 Vaccine Bnt162b2. Nature (2021) 596(7872):417–22. doi: 10.1038/s41586-021-03739-1
42. Chen WH, Kozlovsky BF, Effros RB, Grubeck-Loebenstein B, Edelman R, Sztein MB. Vaccination in the Elderly: An Immunological Perspective. Trends Immunol (2009) 30(7):351–9. doi: 10.1016/j.it.2009.05.002
43. Chen JJ, Lee TH, Tian YC, Lee CC, Fan PC, Chang CH. Immunogenicity Rates After SARS-CoV-2 Vaccination in People With End-Stage Kidney Disease: A Systematic Review and Meta-Analysis. JAMA Netw Open (2021) 4(10):e2131749. doi: 10.1001/jamanetworkopen.2021.31749
44. Benotmane I, Gautier G, Perrin P, Olagne J, Cognard N, Fafi-Kremer S, et al. Antibody Response After a Third Dose of the mRNA-1273 SARS-CoV-2 Vaccine in Kidney Transplant Recipients With Minimal Serologic Response to 2 Doses. JAMA (2021) 326(11):1063–5. doi: 10.1001/jama.2021.12339
45. Westhoff TH, Seibert FS, Anft M, Blazquez-Navarro A, Skrzypczyk S, Zgoura P, et al. A Third Vaccine Dose Substantially Improves Humoral and Cellular SARS-CoV-2 Immunity in Renal Transplant Recipients With Primary Humoral Nonresponse. Kidney Int (2021) 100(5):1135–6. doi: 10.1016/j.kint.2021.09.001
46. Dolff S, Zhou B, Korth J, Luo D, Dai Y, Jahn M, et al. 21evidence of Cell-Mediated Immune Response in Kidney Transplants With a Negative mRNA Vaccine Antibody Response. Kidney Int (2021) 100(2):479–80. doi: 10.1016/j.kint.2021.05.013
Keywords: organ transplantation, COVID19, SARS-CoV-2 vaccine, risk factors, antibody response, meta-analysis
Citation: Zong K, Peng D, Yang H, Huang Z, Luo Y, Wang Y, Xiang S, Li T, Mou T and Wu Z (2022) Risk Factors for Weak Antibody Response of SARS-CoV-2 Vaccine in Adult Solid Organ Transplant Recipients: A Systemic Review and Meta-Analysis. Front. Immunol. 13:888385. doi: 10.3389/fimmu.2022.888385
Received: 02 March 2022; Accepted: 05 May 2022;
Published: 14 June 2022.
Edited by:
Estela Paz Artal, University Hospital October 12, SpainReviewed by:
Javier Carbone, Gregorio Marañón Hospital, SpainAngel Manuel Sevillano Prieto, Hospital Universitario 12 de Octubre, Spain
Copyright © 2022 Zong, Peng, Yang, Huang, Luo, Wang, Xiang, Li, Mou and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhongjun Wu, d3pqdGN5QDEyNi5jb20=
†These authors have contributed equally to this work and share last authorship
 Kezhen Zong†
Kezhen Zong† Hang Yang
Hang Yang Yihua Wang
Yihua Wang Zhongjun Wu
Zhongjun Wu