
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 11 April 2022
Sec. Autoimmune and Autoinflammatory Disorders
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.887256
This article is part of the Research Topic Dissecting the Immunological, Pathological, and Clinical Aspects of Autoimmune Gastritis and its Neoplastic Complications View all 6 articles
 Chiara Della Bella1
Chiara Della Bella1 Antonio Antico2
Antonio Antico2 Maria Piera Panozzo2
Maria Piera Panozzo2 Nagaja Capitani3
Nagaja Capitani3 Marisa Benagiano1
Marisa Benagiano1 Luisa Petrone4
Luisa Petrone4 Annalisa Azzurri5
Annalisa Azzurri5 Sara Pratesi1
Sara Pratesi1 Sofia D’Elios6
Sofia D’Elios6 Fabio Cianchi1
Fabio Cianchi1 Diana Ortiz-Princz7
Diana Ortiz-Princz7 Nicola Bizzaro8,9
Nicola Bizzaro8,9 Mario Milco D’Elios1*
Mario Milco D’Elios1*Pernicious anemia (PA) is a megaloblastic anemia consisting of hematological, gastric and immunological alterations. The immunopathogenesis of PA is sustained by both autoantibodies (e.g. intrinsic factor (IFA) antibodies and anti parietal cell (PCA) antibodies and autoreactive T cells specific for IFA and the parietal cell proton pump ATPase. Iron deficient anemia (IDA) is a microcytic anemia and represents the most common cause of anemia worldwide. Our work aimed to investigate serum levels of several interleukins (IL) of the IL-20 cytokine subfamily in patients with PA, with IDA and in healthy subjects (HC). We compared serum levels of IL-19, IL-20, IL-26, IL-28A and IL-29 in 43 patients with PA and autoimmune gastritis, in 20 patients with IDA and no autoimmune gastritis, and in 47 HC. Furthermore, we analyzed the IL-19 cytokine production by gastric lamina propria mononuclear cells (LPMC) in eight patients with PA and four HC. We found that patients with PA have significantly higher serum levels of IL-19 (163.68 ± 75.96 pg/ml) than patients with IDA (35.49 ± 40.97 pg/ml; p<0.001) and healthy subjects (55.68 ± 36.75 pg/ml; p<0.001). Gastric LPMC from all PA patients were able to produce significantly higher levels of IL-19 (420.67 ± 68.14 pg/ml) than HC (53.69 ± 10.92 pg/ml) (p<0.01). Altogether, our results indicate that IL-19 serum levels are significantly increased in patients with PA but not with IDA and that IL-19 is produced in vivo in the stomach of PA patients. These data open a new perspective on PA pathogenesis and suggest that IL-19 may represent a novel important tool for the management of patients with PA.
Anemia is a condition in which the haemoglobin concentration is lower than normal and might be macrocytic or microcytic. Pernicious anemia (PA) is a megaloblastic anemia consisting of hematological, gastric and immunological alterations. It is an autoimmune disease with a multifactorial etiology involving genetic susceptibility, environmental and immunological factors. Estimate of PA prevalence is 0.1% in the general population which increases to 1.9% in patients aged older than 60 years (1). In older adults of European and African ancestry it is more common (4.0% and 4.3%, respectively) than in people of Asian descent (0.43%) (2, 3).
The immunopathogenesis of pernicious anemia is sustained by autoreactive T cells and by autoantibodies, e.g. intrinsic factor antibodies (IFA) and parietal cell antibodies (PCA). Autoimmunity begins with the activation of gastric dendritic cells, which in turn trigger CD4+ T cell lymphocytes in perigastric lymph nodes (4, 5). These autoreactive CD4+ T cells as well as the autoantibodies produced by autoreactive B cells are specific for intrinsic factor and the proton pump ATPase, thus attack gastric parietal cells and ultimately lead to gastric cell death and gastric atrophy (6–8). The gastric mucosa atrophy impairs absorption of dietary cobalamin resulting in vitamin B12 deficiency.
Patients develop unexplained fatigue, pallor, cardio-respiratory symptoms like shortness of breath and tachycardia, gastrointestinal manifestations and neurologic abnormalities such as memory loss, poor concentration and paresthesia. It often occurs in combination with other autoimmune disorders such as autoimmune thyroiditis, Addison’s disease, vitiligo, and type 1 diabetes (9, 10).
PA diagnosis includes the serum B12 assay to establish cobalamin deficiency (B12<200ng/L with normal or reduced folate levels) and the detection of serum IFA or PCA (11).
On the other hand, iron deficiency anemia (IDA) is the most common cause of anemia worldwide affecting >12% of the world’s population, especially women, children, and individuals living in under-resourced and middle-income countries. Diagnosis of IDA is based on medical history, microcytic anemia, and a serum ferritin level <15 mg/L (12).
The interleukin (IL)-20 family members, IL-19, IL-20, IL-22, and IL-26 are grouped into the same family based on shared common receptor subunits (the IL-20 receptor β chain, IL-20Rβ) and signaling pathways. All IL-20 subfamily members activate the Janus Kinase (JAK) and signal transducer and activator of transcription (STAT) pathway, particularly STAT3 (13, 14). These subfamily members are part of the larger IL-10 family of cytokines, which besides IL-10 itself, includes the more distantly related IL-28A and IL-29 (which are also known as IFNλ2 and IFNλ1, respectively) (15). The interleukin−20 (IL−20) cytokine subfamily constitutes a key link between the immune system and epithelial tissues, and exerts essential functions in innate mucosal immunity (16). IL-28A and IL-29 are able to induce antiviral responses similar to those of type I interferons and mainly act on epithelial cells (17).
IL-19, IL-20, IL-22, IL-26, IL-28A and IL-29 are produced by different inflammatory cells, primarily keratinocytes, osteocytes, epithelial cells, myeloid cells, B and T cells (14, 18, 19). IL-19 expression can be induced by different stimuli such as TNF-α and IL-17 (20).
IL-19 functions as an anti-inflammatory and pro-angiogenic factor (21). IL-19 was discovered in 2000 and has been implicated in different diseases, such as psoriasis, type I diabetes, endotoxic shock, periodontal disease, vascular diseases and rheumatoid arthritis (22, 23). Xiao et al. recently identified IL-19 as a potent cytokine able to promote the expansion and proliferation of neutrophils. The administration of IL-19 protected wild-type mice from neutropenia induced by both chemotherapy and radiation, demonstrating the therapeutic efficacy of this cytokine. It still remains unknown whether IL-19 has additional effects on other organs or tissues but its potential application in neutropenic state is undoubted, being more efficient than granulocyte colony-stimulating factor (G-CSF). Cancer patients who underwent chemotherapy and had reduced activity to promote bone marrow neutrophil formation, had striking downregulation of serum IL-19 (24).
We wondered whether levels of IL-19 and other related cytokines could be abnormal in patients with anemia, such as PA or IDA. We thus measured serum IL-19, IL-20, IL-26, IL-28A and IL-29 levels in patients with PA, in patients with IDA and in healthy subjects (HC). We then investigated the in vivo production of IL-19 by lamina propria mononuclear cells (LPMC) derived from the stomach of patients with PA and controls.
Upon approval of the local Ethical Committee (Ethic statement n.14936/CAM_BIO), 110 subjects were enrolled in this study. Briefly, we studied serum levels of IL-19 and other related cytokines in 43 untreated patients with PA and autoimmune gastritis (diagnosed by histology), 20 untreated patients with IDA and no autoimmune gastritis (as defined by histology), and 47 HC. Patients groups, age and sex are detailed in Table 1. Eight PA patients out of 43 suffered also from autoimmune thyroiditis. No other autoimmune comorbidities were present in PA, IDA and HC subjects. None of the patients suffered from peptic ulcer, gastric cancer, gastric lymphoma nor had proton pump inhibitors assumption in the previous six months. Active H. pylori infection, as ruled out by histopathology, was not present in any patient or control. All PA, IDA patients and controls were investigated by serology (Helori CTX, Eurospital, Trieste, Italy) and three PA patients were seropositive for H. pylori. Diagnosis of PA was based on medical history, macrocytic anemia in peripheral blood, erythroblastosis with megaloblastic changes in bone marrow, low serum levels of vitamin B12 (below 200ng/L). All participants were tested for intrinsic factor and anti-parietal cell autoantibodies. All PA patients had serum intrinsic factor autoantibodies, as assessed by immunofluorenzymatic assay (EliA, Thermo Fisher, Uppsala, Sweden) and/or anti-gastric parietal cells autoantibodies detectable by indirect immunofluorescence assay on rodent tissue (Table 2) (8, 11). 12 out of 43 PA patients had serum gastric parietal cells autoantibodies, 15 PA patients had serum autoantibodies against intrinsic factor, and 16 PA patients had both PCA and IFA autoantibodies. Diagnosis of IDA was based on medical history, microcytic anemia, and a serum ferritin level of 15 mg/L or less (12). IDA patients and HC had no intrinsic factor and anti-parietal cell autoantibodies. Normal white blood cell and reticulocyte counts were found in PA, IDA and HC. In eight patients (six females and two males, mean age 52; range 37-64 years) with PA and four HC (two females and two males, mean age 46; range: 42-48 years) following informed consent, biopsy specimens were obtained from the gastric mucosa in order to study LPMC.
Serum samples from 110 enrolled subjects were tested for IL-19, IL-20, IL-26, IL-28A and IL-29 levels with the Bio-Plex Pro™ assay (BIO-RAD, Hercules, California, USA) based on Luminex xMAP technology. Briefly, the 96 wells plate was seeded with fluorescently dyed magnetic beads, each with a specific color code to permit discrimination of tested analytes within multiplex suspension. Sera were diluted 1:4 with sample diluent provided with the kit. Calibrator vial was reconstituted according to the manufacturer’s instructions and the standard dilution series 1:3 was performed. Sera and calibrators were added following the plate layout. After 30 minutes incubation at room temperature (RT) and three washes, detection antibody was added and the plate was incubated 30 minutes further at RT. After streptavidin-phycoerythrin addition, the plate was finally washed and, after resuspended in assay buffer, data were acquired on MAGPIX System (Luminex, Texas, USA). Bio-Plex Manager™ software was used for data analysis of cytokines concentration by fitting fluorescence intensity values into the calibration curve.
The gastric biopsies were obtained from eight PA patients and four healthy controls. LPMC were isolated by the DTT-EDTA-collagenase sequence as previously described (25). To induce cytokine production by gastric LPMC, 105 cells were stimulated for 36 hours with phorbol 12-myristate 13-acetate (PMA) (10 ng/ml) plus ionomycin (0.5 mM) in 100 μl complete medium, as detailed elsewhere (26). Culture supernatants were assayed for IL-19 content. The quantitative determination of IL-19 was performed by Bio-Plex IL-19 singleplex assay (BIO-RAD).
Descriptive statistic was applied for the calculation of qualitative data, as well as for mean, standard deviation, median and interquartile range. Distributions of obtained results were evaluated for their normality and then compared by Student T-test or by Mann-Whitney U test.
p<0.05 was considered statistically significant.
Serum cytokine concentrations values falling below the lower limit of quantification (LLOQ) were replaced with one-half the respective LLOQ for descriptive purposes (27); no subject had values above the upper limit of quantification (ULOQ).
Luminex assay accuracy in discriminating between two compared groups was computed by ROC (Receiving Operating Characteristic) analysis. Plotting a ROC curve, the obtained AUC (Area Under the Curve) defines the test accuracy (28).
Statistical analysis were carried out using IBM®SPSS Statistic version 27.0.
Results for the five cytokines in the groups of patients and controls are detailed in Table 3. Distribution of the levels of each cytokine is shown in Figure 1.
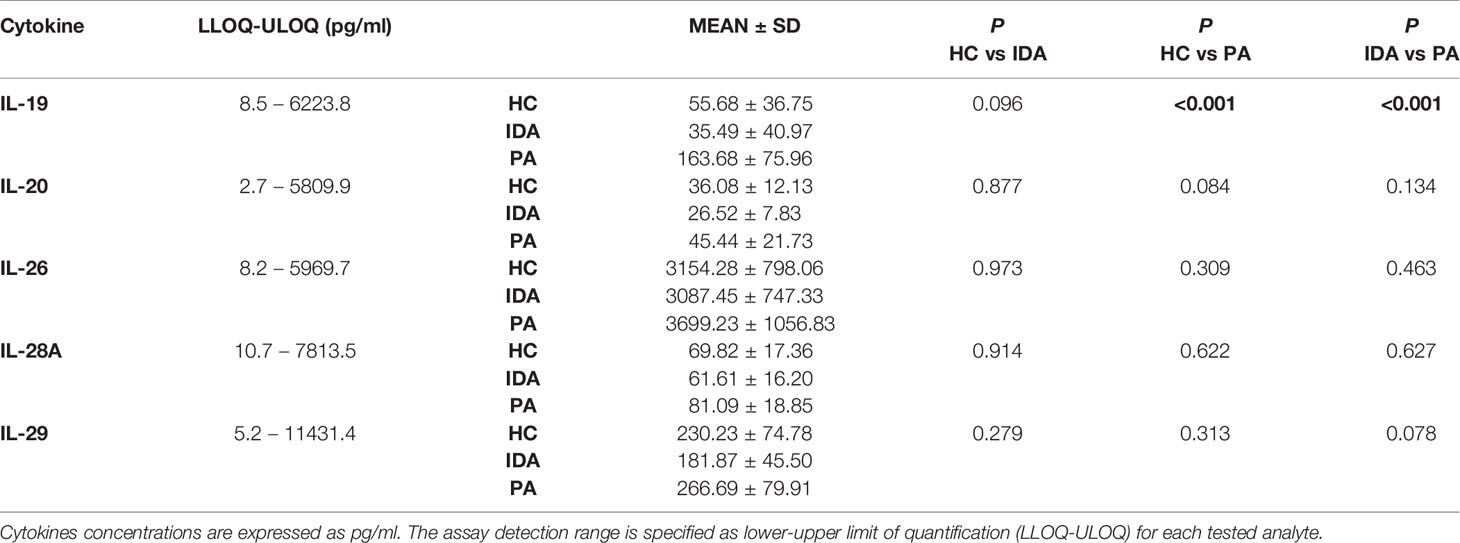
Table 3 Luminex assay results for IL-19, IL-20, IL-26, IL-28A and IL-29 levels in sera of HC, Healthy controls; IDA, Iron deficiency anemia patients; PA, Pernicious anemia patients.
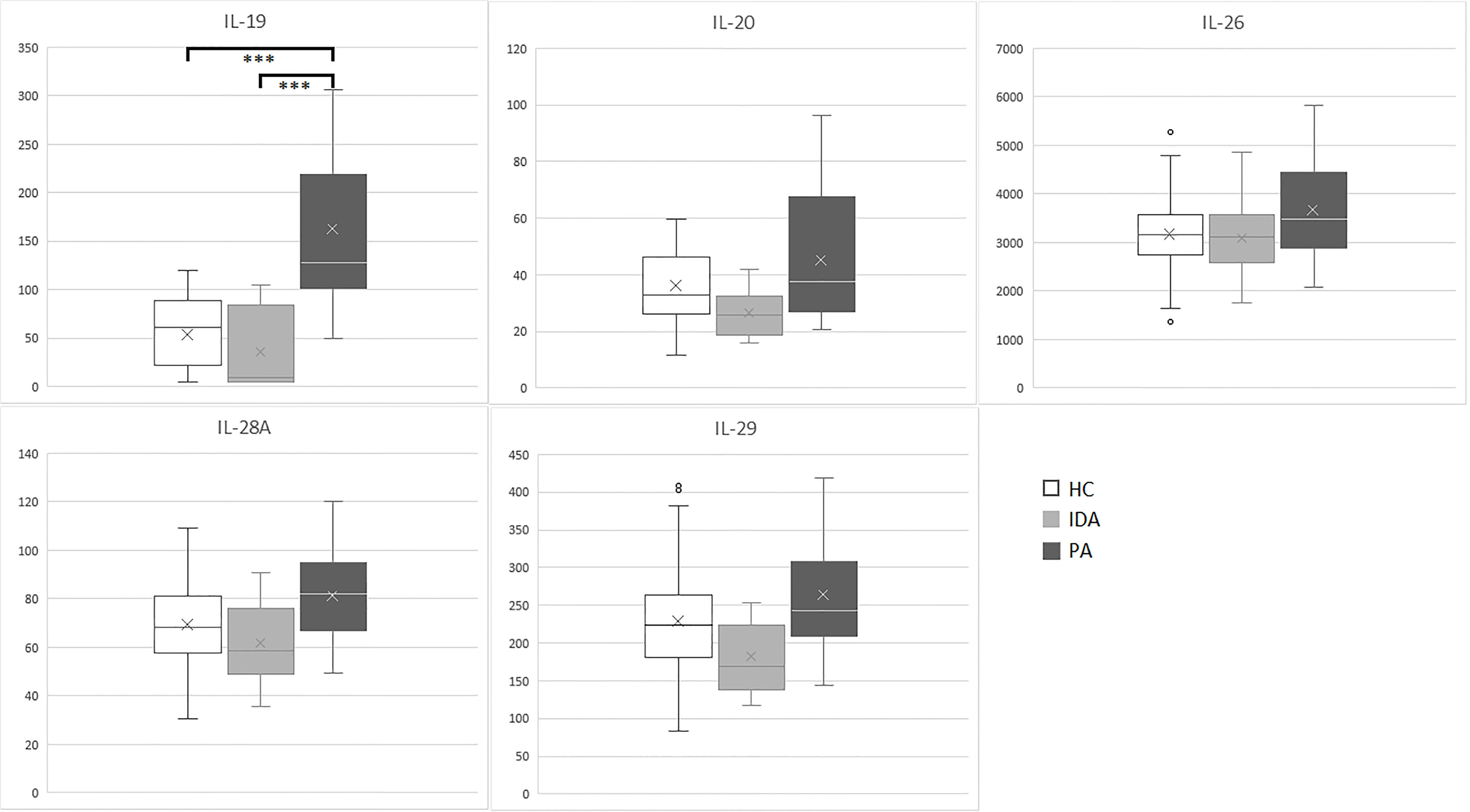
Figure 1 IL-19, IL-20, IL-26, IL-28A and IL-29 (pg/ml) levels in sera samples of enrolled subjects (PA, pernicious anemia; IDA, iron deficient anemia; HC, healthy control). ***p <0.001.
Significant differences were identified exclusively in IL-19 levels between patients with PA vs. HC (p<0.001) and between patients with PA vs. IDA patients (p<0.001). IL19 levels in PA, IDA and HC did not differ between males and females. No differences were found for the other cytokines.
With the purpose of using serum IL-19 quantification by Luminex assay as a useful tool to discriminate PA from IDA, we calculated the AUC of the ROC curve to assess the test performance. As graphed in Figure 2A, the ROC curve analyzing serum IL-19 amounts in HC compared to PA patients, at a cutoff of 91.05 pg/ml, provides an AUC = 0.937, with a sensitivity of 88.4% and a specificity of 84.8%. The comparison by ROC analysis of the IL-19 serum amounts in PA and IDA patients, found an AUC = 0.95, with a sensitivity of 95.3% and a specificity of 80.0%, choosing as best cutoff 88.08 pg/ml (Figure 2B).
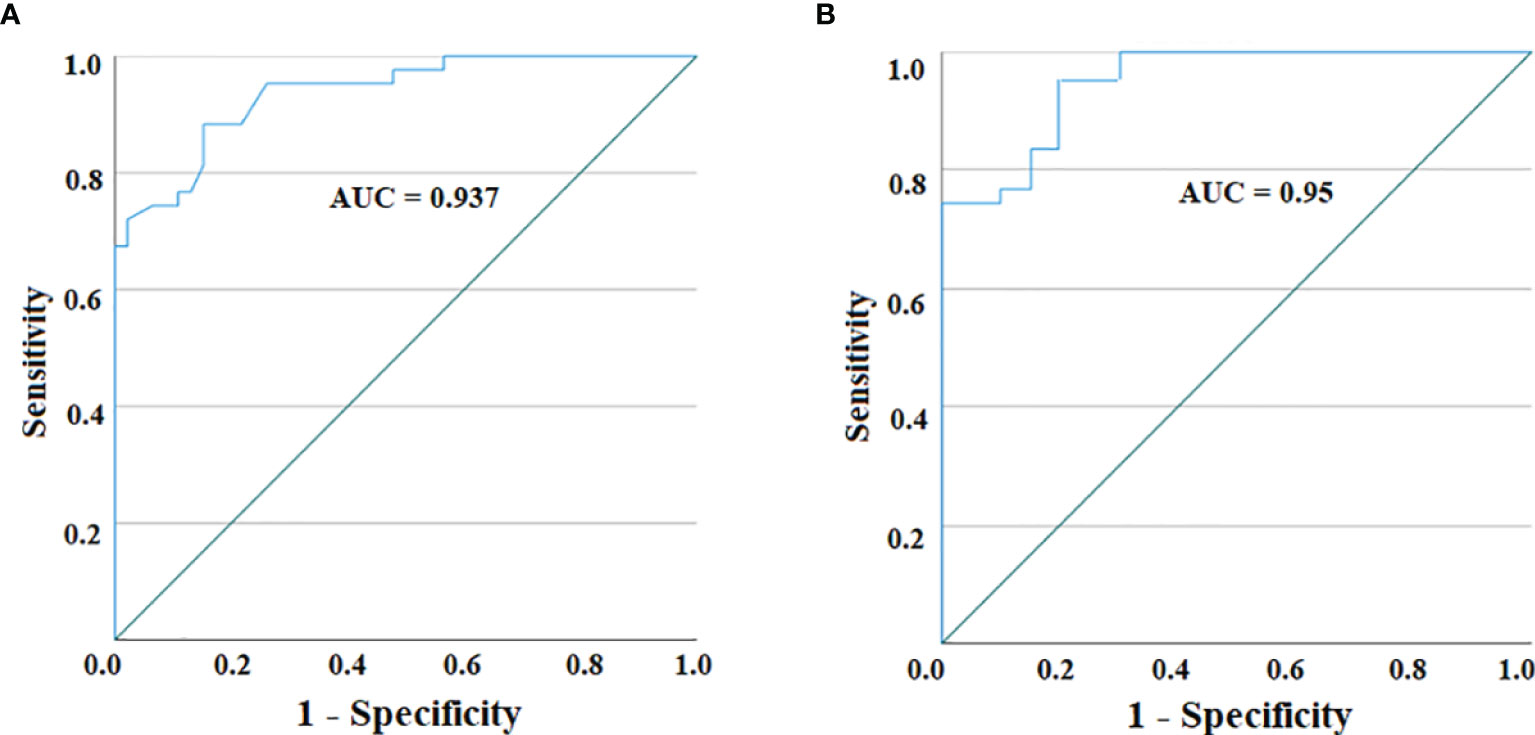
Figure 2 ROC curve for IL-19 serum levels in PA patients vs. HC (A) and between PA patients and IDA ones (B). The AUC for both curves reveal a highly accurate test performance (0.9 ≤ AUC < 1).
On the basis of results obtained at serum level, we then asked whether IL-19 is produced in vivo by patients with PA and autoimmune gastritis. Since gastric mucosal involvement was almost invariably present in patients with PA, gastric specimens were considered an appropriate source of in vivo LPMC. Eight patients with PA and four healthy controls studied at serum level were investigated for IL-19 cytokine production by LPMC derived from their gastric mucosa. Gastric LPMC were isolated by the DTT-EDTA-collagenase sequence. Following stimulation with PMA and ionomycin we measured IL-19 production by gastric LPMC in supernatants. A statistically significant difference of IL-19 production (p = 0.0045) was found between supernatant of gastric LPMC derived from all PA patients (mean 420.67 ± 68.14 pg/ml) and HC ones (mean 53.69 ± 10.92 pg/ml), as shown in Figure 3.
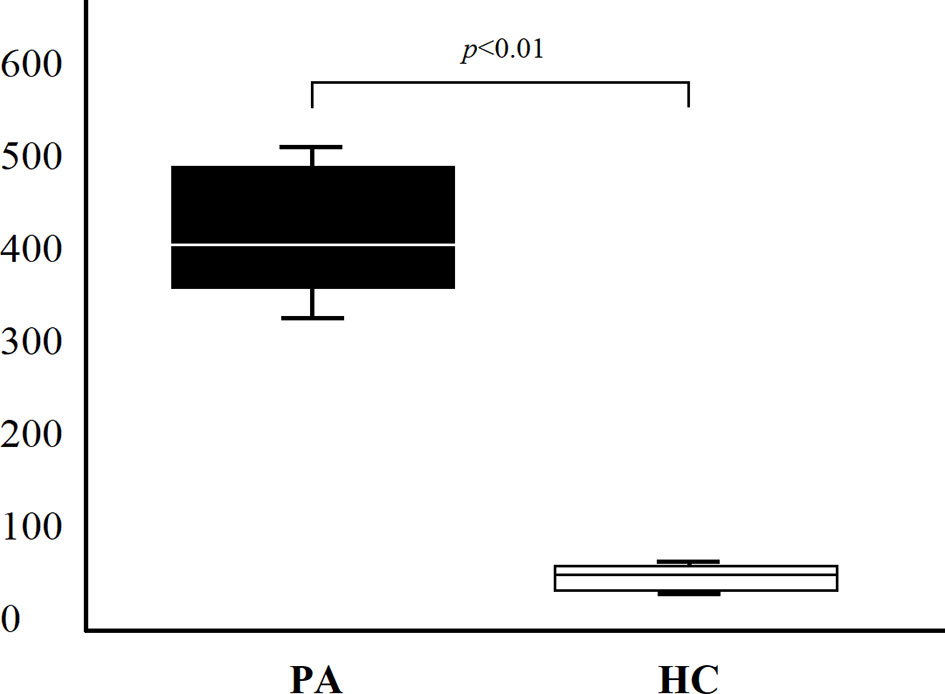
Figure 3 IL-19 production (pg/ml) of lamina propria mononuclear cells obtained from gastric specimens of 8 PA patients and 4 HC. Comparison of gastric IL-19 (mean ± SD) production between PA patients and HC.
The present study revealed several major findings that are relevant to the management and understanding the immunological basis of pernicious anemia and autoimmune gastritis.
We investigated the serum levels of different cytokines, such as IL-19, IL-20, IL-26, IL-28A and IL-29 in patients with pernicious anemia and autoimmune gastritis or iron deficient anemia without autoimmune gastritis. No differences in IL-20, IL-26, IL-28A and IL-29 serum levels were present among untreated PA patients, IDA patients or HC. On the other hand, significantly higher levels of IL-19 were found in the sera of PA patients compared to IDA or HC. It is of note that PA is a disease with a clear autoimmune pathogenesis, whose immunopathological features are gastric autoreactive T cells and autoreactive B cells specific for proton pump ATPase and IF, whereas the patients with IDA studied in this research had no autoimmune disease (29, 30). Our results support the notion that IL-19 is a relevant cytokine for the immunopathogenesis of PA but not of IDA.
We can hypothesize that the high serum level of IL-19 found in PA patients might be inversely related to the serum levels of vitamin B12 and that the substitution therapy with B12 will lead to both normal serum levels of B12 and IL-19. It can be further speculated that therapeutic regimen able to reduce IL-19 levels, such as monoclonal antibodies or siRNA, might be useful for the treatment of PA. Given that this investigation represents a pilot single-center study, future multi-center studies dealing with serum IL-19 levels would be very useful for a better definition of the test in PA and IDA.
To investigate whether at gastric level there was any IL-19 production in vivo, we analyzed the IL-19 cytokine production of LPMC derived from the gastric mucosa of patients with PA and HC. We found that LPMC obtained from all PA patients, but not HC, were able to produce high levels of IL-19. It was recently reported in patients with PA that gastric T cells specific for intrinsic factor produced very high level of IL-17 and TNF-α (8). It is of high interest that IL-19 expression can be induced by IL-17, TNF-α and lipopolysaccharide. Moreover, epithelial cells produce IL-19 following stimulation with IL-17, IL-22 and IL-1β (20). In PA patients a different IL-19 cytokine production might be related not only to the local inflammation but also to a different bone stimulation or to other yet unknown interfering factors in IL-19 cytokine production.
Altogether, the results obtained so far indicate that IL-19 serum levels are significantly increased in patients with PA and that IL-19 is produced in vivo in the stomach of PA patients. Thus, we suggest that serum levels of IL-19 may represent a novel important tool to discriminate PA with autoimmune gastritis from IDA without autoimmune gastritis and that the measurement of serum IL-19 might be useful for monitoring the response to B12 vitamin therapy in PA patients.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Ethical Committee Area Vasta Centro Firenze. The patients/participants provided their written informed consent to participate in this study.
CDB, AAn, NB and MMD'E conceived and designed the study with MMD'E supervision. CDB, MPP, NC, MB, LP, AAz, SP, SD'E, FC, DO-P performed the experiments; analysis and interpretation of data were conducted by CDB, AAn, NB, MMD'E. CDB, AAn, NB, MMD'E wrote, reviewed and edited the manuscript. All the authors contributed to the article and approved the submitted version.
This work was supported by the Italian Ministry of University (grant 2010P3S8BR_005).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank Prof. Cosima T. Baldari for the critical reading of the manuscript and Ms. Mary Wilkins for the English editing of the manuscript.
1. Yousaf F, Spinowitz B, Charytan C, Galler M. Pernicious Anemia Associated Cobalamin Deficiency and Thrombotic Microangiopathy: Case Report and Review of the Literature. Case Rep Med (2017) 2017:9410727. doi: 10.1155/2017/9410727
2. Stabler SP. Vitamin B12 Deficiency. N Engl J Med (2013) 368(21):2041–2. doi: 10.1056/NEJMc1304350
3. Lenti MV, Rugge M, Lahner E, Miceli E, Toh BH, Genta RM, et al. Autoimmune Gastritis. Nat Rev Dis Primers (2020) 6(1):56. doi: 10.1038/s41572-020-0187-8
4. Massironi S, Zilli A, Elvevi A, Invernizzi P. The Changing Face of Chronic Autoimmune Atrophic Gastritis: An Updated Comprehensive Perspective. Autoimmun Rev (2019) 18(3):215–22. doi: 10.1016/j.autrev.2018.08.011
5. Toh BH. Diagnosis and Classification of Autoimmune Gastritis. Autoimmun Rev (2014) 13(4-5):459–62. doi: 10.1016/j.autrev.2014.01.048
6. Toh BH. Pathophysiology and Laboratory Diagnosis of Pernicious Anemia. Immunol Res (2017) 65(1):326–30. doi: 10.1007/s12026-016-8841-7
7. D’Elios MM, Bergman MP, Azzurri A, Amedei A, Benagiano M, De Pont JJ, et al. H(+),K(+)-Atpase (Proton Pump) Is the Target Autoantigen of Th1-Type Cytotoxic T Cells in Autoimmune Gastritis. Gastroenterology (2001) 120(2):377–86. doi: 10.1053/gast.2001.21187
8. Troilo A, Grassi A, Petrone L, Cianchi F, Benagiano M, Della Bella C, et al. Intrinsic Factor Recognition Promotes T Helper 17/ T Helper 1 Autoimmune Gastric Inflammation in Patients with Pernicious Anemia. Oncotarget (2019) 10(30):2921–9. doi: 10.18632/oncotarget.26874
9. Bizzaro N, Antico A. Diagnosis and Classification of Pernicious Anemia. Autoimmun Rev (2014) 13:565–8. doi: 10.1016/j.autrev.2014.01.042
10. Mohamed M, Thio J, Thomas RS, Phillips J. Pernicious Anemia. BMJ (2020) 369:m1319. doi: 10.1136/bmj.m1319
11. Andres E, Serraj K. Optimal Management of Pernicious Anemia. J Blood Med (2012) 3:97–103. doi: 10.2147/JBM.S25620
12. Miller JL. Iron Deficiency Anemia: A Common and Curable Disease. Cold Spring Harb Perspect Med (2013) 3(7):a011866. doi: 10.1101/cshperspect.a011866
13. Oral HB, Kotenko SV, Yilmaz M, Mani O, Zumkehr J, Blaser K, et al. Regulation of T Cells and Cytokines by the Interleukin-10 (IL-10)-Family Cytokines IL-19, IL-20, IL-22, IL-24 and IL-26. Eur J Immunol (2006) 36:380–8. doi: 10.1002/eji.200425523
14. Commins S, Steinke JW, Borish L. The Extended IL-10 Superfamily: IL-10, IL-19, IL-20, IL-22, IL-24, IL-26, IL-28, and IL-29. J Allergy Clin Immunol (2008) 121:1108–11. doi: 10.1016/j.jaci.2008.02.026
15. Ouyang W, Rutz S, Crellin NK, Valdez PA, Hymowitz SG. Regulation and Functions of the IL−10 Family of Cytokines in Inflammation and Disease. Annu Rev Immunol (2011) 29:71–109. doi: 10.1146/annurev-immunol-031210-101312
16. Rutz S, Wang X, Ouyang W. The IL−20 Subfamily of Cytokines - From Host Defence to Tissue Homeostasis. Nat Rev Immunol (2014) 14(12):783–95. doi: 10.1038/nri3766
17. Uze G, Monneron D. IL-28 and IL-29: Newcomers to the Interferon Family. Biochimie (2007) 89:729–34. doi: 10.1016/j.biochi.2007.01.008
18. Gallagher G, Dickensheets H, Eskdale J, Izotova LS, Mirochnitchenko OV, Peat JD, et al. Cloning, Expression and Initial Characterization of Interleukin-19 (IL-19), a Novel Homologue of Human Interleukin-10 (IL-10). Genes Immun (2000) 1:442–50. doi: 10.1038/sj.gene.6363714
19. Fonseca-Camarillo G, Furuzawa-Carballeda J, Granados J, Yamamoto-Furusho JK. Expression of Interleukin (IL)-19 and IL-24 in Inflammatory Bowel Disease Patients: A Cross-Sectional Study. Clin Exp Immunol (2014) 177(1):64–75. doi: 10.1111/cei.12285
20. Liao YC, Liang WG, Chen FW, Hsu JH, Yang JJ, Chang MS. IL-19 Induces Production of IL-6 and TNF-Alpha and Results in Cell Apoptosis Through TNF-Alpha. J Immunol (2002) 15169(8):4288–97. doi: 10.4049/jimmunol.169.8.4288
21. Leigh T, Scalia RG, Autieri MV. Resolution of Inflammation in Immune and Nonimmune Cells by Interleukin-19. Am J Physiol Cell Physiol (2020) 319(3):C457–64. doi: 10.1152/ajpcell.00247.2020
22. Gallagher G. Interleukin-19: Multiple Roles in Immune Regulation and Disease. Cytokine Growth Factor Rev (2010) 21:345–52. doi: 10.1016/j.cytogfr.2010.08.005
23. Leng RX, Pan HF, Tao JH, Ye DQ. IL-19, IL-20 and IL-24: Potential Therapeutic Targets for Autoimmune Diseases. Expert Opin Ther Targets (2011) 15:119–26. doi: 10.1517/14728222.2011.534461
24. Xiao M, Zhang W, Liu W, Mao L, Yang J, Hu L, et al. Osteocytes Regulate Neutrophil Development Through IL-19: A Potent Cytokine for Neutropenia Treatment. Blood (2021) 24:137(25):3533–47. doi: 10.1182/blood.2020007731
25. Luzza F, Parrello T, Monteleone G, Sebkova L, Romano M, Zarrilli R, et al. Up-Regulation of IL-17 Is Associated With Bioactive IL-8 Expression in Helicobacter Pylori-Infected Human Gastric Mucosa. J Immunol (2000) 165(9):5332–7. doi: 10.4049/jimmunol.165.9.5332
26. Capitani N, Codolo G, Vallese F, Minervini G, Grassi A, Cianchi F, et al. The Lipoprotein HP1454 of Helicobacter Pylori Regulates T-Cell Response by Shaping T-Cell Receptor Signalling. Cell Microbiol (2019) 21(5):e13006. doi: 10.1111/cmi.13006
27. Hornung RW, Reed LD. Estimation of Average Concentration in the Presence of Non Detectable Values. Appl Occup Environ Hyg (1990) 5:46–51. doi: 10.1080/1047322X.1990.10389587
28. Swet JA. Measuring the Accuracy of Diagnostic Systems. Science (1988) 240(4857):1285–93. doi: 10.1126/science.3287615
29. D’Elios MM, Amedei A, Azzurri A, Benagiano M, Del Prete G, Bergman MP, et al. Molecular Specificity and Functional Properties of Autoreactive T-Cell Response in Human Gastric Autoimmunity. Int Rev Immunol (2005) 24(1-2):111–22. doi: 10.1080/08830180590884611
Keywords: pernicious anemia, autoimmune gastritis, interleukin 19, serum, gastric mucosa
Citation: Della Bella C, Antico A, Panozzo MP, Capitani N, Benagiano M, Petrone L, Azzurri A, Pratesi S, D’Elios S, Cianchi F, Ortiz-Princz D, Bizzaro N and D’Elios MM (2022) Elevated IL-19 Serum Levels in Patients With Pernicious Anemia and Autoimmune Gastritis. Front. Immunol. 13:887256. doi: 10.3389/fimmu.2022.887256
Received: 01 March 2022; Accepted: 21 March 2022;
Published: 11 April 2022.
Edited by:
Marco Vincenzo Lenti, University of Pavia, ItalyReviewed by:
Laura Conti, Sapienza University of Rome, ItalyCopyright © 2022 Della Bella, Antico, Panozzo, Capitani, Benagiano, Petrone, Azzurri, Pratesi, D’Elios, Cianchi, Ortiz-Princz, Bizzaro and D’Elios. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mario Milco D’Elios, bWFyaW9taWxjby5kZWxpb3NAdW5pZmkuaXQ=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.