
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 06 April 2022
Sec. Inflammation
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.886421
This article is part of the Research Topic Organ Cross Talk and its Impact on the Clinical Course in Multiple Trauma and Critical Illness View all 18 articles
 Nikita M. Patel1*
Nikita M. Patel1* Noriaki Yamada1,2
Noriaki Yamada1,2 Filipe R. M. B. Oliveira3
Filipe R. M. B. Oliveira3 Lara Stiehler1,4
Lara Stiehler1,4 Elisabeth Zechendorf4
Elisabeth Zechendorf4 Daniel Hinkelmann4
Daniel Hinkelmann4 Sandra Kraemer4
Sandra Kraemer4 Christian Stoppe5
Christian Stoppe5 Massimo Collino6
Massimo Collino6 Debora Collotta6
Debora Collotta6 Gustavo Ferreira Alves6
Gustavo Ferreira Alves6 Hanna Pillmann Ramos3
Hanna Pillmann Ramos3 Regina Sordi3
Regina Sordi3 Ingo Marzi7
Ingo Marzi7 Borna Relja7,8
Borna Relja7,8 Gernot Marx4
Gernot Marx4 Lukas Martin1,4*†
Lukas Martin1,4*† Christoph Thiemermann1†
Christoph Thiemermann1†Objective: The aim of this study was to investigate (a) macrophage migration inhibitory factor (MIF) levels in polytrauma patients and rats after haemorrhagic shock (HS), (b) the potential of the MIF inhibitor ISO-1 to reduce multiple organ dysfunction syndrome (MODS) in acute (short-term and long-term follow-up) HS rat models and (c) whether treatment with ISO-1 attenuates NF-κB and NLRP3 activation in HS.
Background: The MODS caused by an excessive systemic inflammatory response following trauma is associated with a high morbidity and mortality. MIF is a pleiotropic cytokine which can modulate the inflammatory response, however, its role in trauma is unknown.
Methods: The MIF levels in plasma of polytrauma patients and serum of rats with HS were measured by ELISA. Acute HS rat models were performed to determine the influence of ISO-1 on MODS. The activation of NF-κB and NLRP3 pathways were analysed by western blot in the kidney and liver.
Results: We demonstrated that (a) MIF levels are increased in polytrauma patients on arrival to the emergency room and in rats after HS, (b) HS caused organ injury and/or dysfunction and hypotension (post-resuscitation) in rats, while (c) treatment of HS-rats with ISO-1 attenuated the organ injury and dysfunction in acute HS models and (d) reduced the activation of NF-κB and NLRP3 pathways in the kidney and liver.
Conclusion: Our results point to a role of MIF in the pathophysiology of trauma-induced organ injury and dysfunction and indicate that MIF inhibitors may be used as a potential therapeutic approach for MODS after trauma and/or haemorrhage.
Trauma is one of the leading causes of death and disability in young people aged under 44 and exceeds the number of deaths caused by HIV, tuberculosis and malaria combined (1). Globally, injuries are responsible for over 9% of all mortalities and annually there are approximately 6 million trauma-related deaths (1, 2). Trauma-associated haemorrhage and haemorrhagic shock (HS) account for nearly 40% of all trauma deaths and is a key driver of multiple organ dysfunction (MODS) (3–7).
Whilst the number of early post-injury deaths have decreased in recent years secondary to improved care in the pre-hospital setting, there has been an accompanying increase in deaths attributed to MODS during the late post-injury phase (4, 8, 9). The mechanisms contributing to MODS include (a) an excessive systemic inflammatory response secondary to the release of damage-associated molecular patterns (DAMPs) from extensive tissue damage and (b) ischaemia-reperfusion (I/R) injury (8, 10).
DAMPs activate the immune system, leading to the release of cytokines which can cause organ injury and dysfunction (11). Moreover, raised cytokine levels are linked to worse prognosis in critically ill patients (12–14). One such cytokine is macrophage migration inhibitory factor (MIF) which is pro-inflammatory and possesses chemokine-like properties by promoting the expression or production of several pro-inflammatory mediators including IL-1β, IL-2, IL-6, IL-8, IL-12, IFN-γ, nitric oxide, TNF-α, cyclooxygenase 2 and matrix metalloproteinases (15–23). Consequently, further leukocytes are directed to the site of injury and/or infection (24–26). MIF also has a role in counter-regulating the immunosuppressive and anti-inflammatory effects of glucocorticoids (27, 28).
It has previously been shown that MIF concentration in the plasma/serum of trauma patients was higher than that of healthy controls (29, 30) and serum MIF levels in blunt trauma patients with MODS were significantly greater than patients without MODS (31, 32). Currently, there are no specific pharmacological treatments which prevent the onset of MODS associated with HS. Therefore, the aim of this study was to investigate the effects of blocking MIF activity with the inhibitor ISO-1 [(S,R)-3-(4-hydroxyphenyl)-4,5-dihydro-5-isoxazole acetic acid methyl ester] on the HS-induced MODS in rats.
Original data was obtained under Gene Expression Omnibus (GEO) accession GSE36809, published by Xiao and colleagues (33). RNA was extracted from whole blood leukocytes of severe blunt trauma patients (n = 167) over the course of 28 days and healthy controls (n = 37) and hybridised onto an HU133 Plus 2.0 GeneChip (Affymetrix) according to the manufacturer’s recommendations. The dataset was reanalysed for MIF gene expression.
Blood samples of 208 patients were collected after written informed consent was obtained from either the patient or a nominated legally authorised representative. Samples were collected between 2010-2014 from University Hospital Frankfurt of Goethe-University and approved by an institutional ethics committee (Number 312/10) in accordance with the declaration of Helsinki and following STROBE-guidelines (34).
For the short-term follow-up acute HS model, all animal procedures were approved by the Animal Welfare Ethics Review Board of Queen Mary University of London and by the Home Office (Licence number PC5F29685). For the long-term follow-up acute HS model, all animal procedures were approved by the Universidade Federal de Santa Catarina Institutional Committee for Animal Use in Research (Licence number 7396250219) in accordance with the Brazilian Government Guidelines for Animal Use in Research. All in vivo experiments are reported in accordance to ARRIVE guidelines.
Blood samples from patients (18-80 years) with blunt or penetrating trauma and ISS ≥ 16 were obtained on Day 0 (arrival to the emergency room); Day 2; Day 5 and Day 7. Exclusion criteria were patient death in the emergency room or within 24 h of hospital admission, known pre-existing immunological disorders, treatment with immunosuppressive or anti-coagulant medication, burns, concomitant acute myocardial infarction and thromboembolic events. Blood samples were collected in pre-chilled ethylenediaminetetraacetic acid tubes (BD vacutainer, Becton Dickinson Diagnostics, Aalst, Belgium) and kept on ice. Blood was centrifuged at 2,000 g for 15 min at 4°C to separate serum and stored at −80°C for further analysis.
Male Wistar rats (for short-term follow-up acute model: Charles River Laboratories Ltd., UK; for long-term follow-up acute model: Universidade Federal de Santa Catarina, Brazil) weighing 250-350 g were kept under standard laboratory conditions and received a chow diet and water ad libitum. ISO-1 (25 mg/kg; Tocris, UK) was diluted in 5% DMSO + 95% Ringer’s Lactate (vehicle) and rats were treated (i.v. in short-term follow-up and i.p. in long-term follow-up) upon resuscitation.
The pressure-controlled short-term follow-up acute HS model was performed as previously described (35–38). Briefly, forty rats were anaesthetised with sodium thiopentone (120 mg/kg i.p. initially and 10 mg/kg i.v. for maintenance as needed and randomised into four groups (n = 10 per group): Sham + vehicle; Sham + ISO-1 (25 mg/kg), HS + vehicle; HS + ISO-1 (25 mg/kg) using the GraphPad online random number generator. The investigator was blinded to the intervention (vehicle or ISO-1) and treatment group allocation was revealed following data analysis. Analgesia was not administered as the rats remain anaesthetised for the duration of the experiment (non-recovery procedure) and as such do not expect the animals to feel pain. Adequacy of anaesthesia was ascertained throughout the experiment by testing the pedal reflex. No animals died during the course of the study, thus all data have been included. Blood was withdrawn to achieve a fall in mean arterial pressure (MAP) to 35 ± 5 mmHg, which was maintained for 90 min. At 90 min after initiation of haemorrhage (or when 25% of the shed blood had to be reinjected to sustain MAP at 35 ± 5 mmHg), resuscitation was performed with the shed blood over a period of 5 min. At 4 h post-resuscitation, blood was collected for the measurement of biomarkers of organ injury/dysfunction (MRC Harwell Institute, Oxfordshire, UK) and organs for ex vivo analysis. Sham-operated rats were used as control and underwent identical surgical procedures, but without haemorrhage or resuscitation. Detailed description of the short-term follow-up model can be found in the supplemental (Supplemental Figure 1A).
The pressure-controlled long-term follow-up acute HS model was performed as previously described (36). Briefly, thirty rats were administered analgesia with tramadol (10 mg/kg i.p.) 15 min prior to anaesthesia induction with ketamine and xylazine (100 mg/kg and 10 mg/kg i.m. respectively) and randomised into three groups: Sham + vehicle (n = 6); HS + vehicle (n = 12); HS + ISO-1 (25 mg/kg; n = 12) using the GraphPad online random number generator. The investigator was blinded to the intervention (vehicle or ISO-1) and treatment group allocation was revealed following data analysis. Adequacy of anaesthesia throughout the experiment was ascertained by testing the pedal reflex. No animals died during the course of the study, thus all data have been included. Blood was withdrawn to achieve a fall in MAP to 40 ± 2 mmHg, which was maintained for 90 min. At 90 min after initiation of haemorrhage (or when 25% of the shed blood had to be reinjected to sustain MAP at 40 ± 2 mmHg), resuscitation was performed with the shed blood over a period of 5 min plus 1.5 mL/kg Ringer’s lactate. At 24 h post-resuscitation, blood was collected for the measurement of organ injury/dysfunction parameters (Hospital Universitário Professor Polydoro Ernani de São Thiago, Brazil) and organs for ex vivo analysis. Sham-operated rats were used as control and underwent identical surgical procedures, but without haemorrhage or resuscitation. Detailed description of the long-term follow-up model can be found in the supplemental (Supplemental Figure 1B).
Human MIF plasma levels (R&D SYSTEMS Human MIF DuoSet) and rat MIF serum levels from the acute HS (short-term follow-up) model (Cusabio Biotech, Wuhan, China) were detected by commercially available ELISAs according to the manufacturer protocol. Detection occurred at 450 nm and 540 nm using iMark® microplate absorbance reader (BioRad). Further details can be found in the Supplemental Table 1.
Semi-quantitative immunoblot analysis was carried out in kidney and liver samples as previously described (36). Detailed description of the method can be found in the supplemental.
Lung tissue sections were deparaffinised and hydrated and stained for CD68. Detailed description of the method can be found in the supplemental.
Determination of myeloperoxidase activity in lung and liver tissue samples was performed as previously described (36). Detailed description of the method can be found in the supplemental.
All figures are expressed as median with range of n observations, where n represents the number of animals/experiments/subjects studied. Measurements obtained from the vehicle and ISO-1 treated animal groups were analysed by one-way ANOVA followed by a Bonferroni’s post-hoc test on GraphPad Prism 8.0 (GraphPad Software, Inc., La Jolla, CA, USA). The distribution of the data was verified by Shapiro-Wilk normality test, and the homogeneity of variances by Bartlett test. When necessary, values were transformed into logarithmic values to achieve normality and homogeneity of variances. To investigate the relationship between the variables, Pearson correlation r was performed. P<0.05 was considered statistically significant.
Xiao and colleagues (33) compared genome-wide expression in leukocytes from trauma patients against matched healthy controls. We reanalysed this dataset for MIF expression. When compared to healthy controls, MIF expression was significantly elevated at all time points except Day 1 (p<0.05; Supplemental Figure 2). An initial increase was noted at 12 h followed by a later peak at Day 7. MIF expression remained elevated at Day 28, the latest timepoint measured.
Xiao and colleagues (33) also stratified their trauma patient cohort into uncomplicated (recovery in <5 days) and complicated (recovery after 14 days, no recovery by Day 28 or death) to further identify genotypic differences. We reanalysed this dataset for MIF expression using this stratification. When comparing uncomplicated and complicated patients, there were no significant differences at any of the timepoints measured (p>0.05; Supplemental Figure 3).
To investigate the role of MIF in trauma, 208 patients were included in the study. Detailed patient characteristics can be found in Table 1. Polytrauma patients showed significantly increased MIF levels on Day 0 (on arrival to the emergency room, 12398 ± 1262 pg/mL) compared to Day 2 (2866.9 ± 377.8 pg/mL), Day 5 (2335.7 ± 203.4 pg/mL) and Day 7 (2114.6 ± 165.3 pg/mL) (all p<0.001; Figure 1A). Furthermore, we found a weak positive correlation between MIF levels on Day 0 and both hospital (r = 0.22, n = 199, p<0.01; Figure 1C) and ICU (r = 0.26, n = 198, p<0.01; Figure 1D) stays. MIF levels on Day 0 were not correlated with baseline characteristics such as age (r = -0.05, n = 200, p = 0.49) and sex (r = -0.07, n = 200, p = 0.3) and only weakly correlated with ISS score (r = 0.15, n = 177, p<0.05) (Figure 1B).
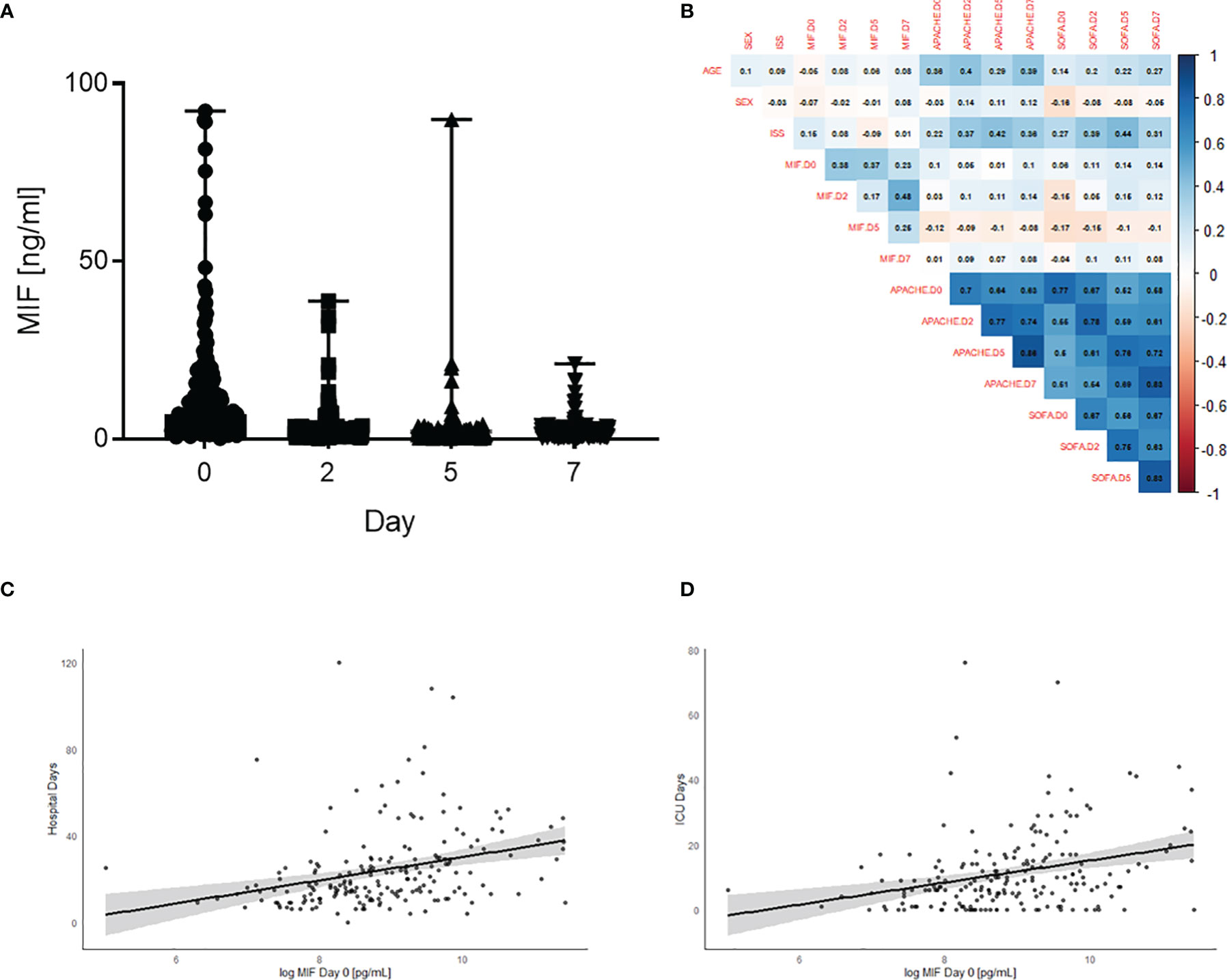
Figure 1 Plasma MIF levels are elevated in polytrauma patients and associated with longer stay in ICU and hospital. (A) Plasma MIF levels in trauma patients (n = 208) at different time points: Day 0 (Emergency room), Day 2, Day 5 and Day 7. Data are expressed as median with range. (B) Heatmap for correlations between MIF levels and baseline characteristics sex, age, SOFA, ISS and APACHE score. Scatter plot for Day 0 MIF levels against (C) hospital stay and (D) intensive care unit (ICU) stay.
Having found elevated plasma MIF levels in patients with trauma-haemorrhage, we investigated whether haemorrhage alone (in the absence of physical trauma) is sufficient to drive increases in MIF. To address this question, we used a model of severe haemorrhage followed by resuscitation in the rat. When compared to sham-operated rats, haemorrhage followed by resuscitation resulted in a significant increase in MIF levels (p<0.001; Figure 2A). Although ISO-1 has been reported to inhibit the effects, rather than the formation, of MIF in vivo, we report here that treatment of HS rats with ISO-1 resulted in a significantly lower MIF level when compared to HS rats treated with vehicle (p<0.05; Figure 2A). Administration of ISO-1 to sham-operated rats had no effect on MIF levels (p>0.05; Figure 2A).
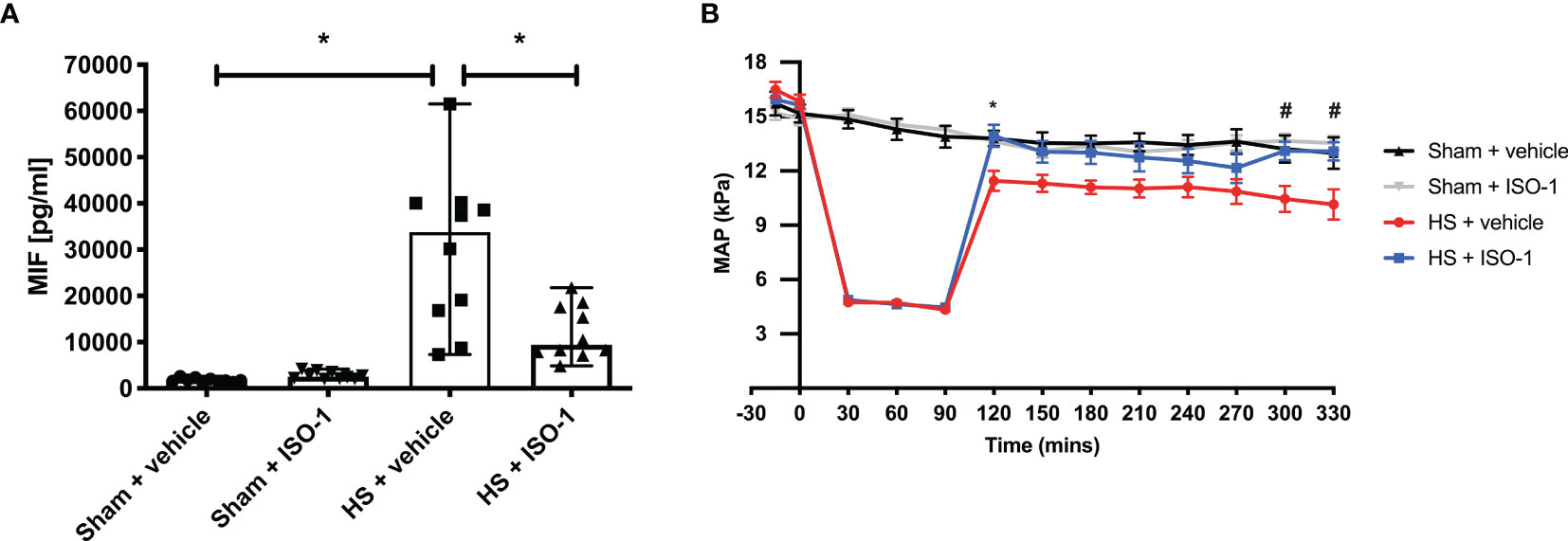
Figure 2 Serum MIF levels are elevated in HS-rats and ISO-1 improves HS-induced circulatory failure in a short-term follow-up acute HS model. (A) Serum MIF levels were detected by ELISA in vehicle or ISO-1 treated rats. Data are expressed as median with range of ten animals per group. (B) Mean arterial pressure (MAP) was measured from the completion of surgery to the termination of the experiment for all groups. Statistical analysis was performed using one-way ANOVA followed by a Bonferroni’s post-hoc test. *p < 0.05 Sham + vehicle vs. HS + vehicle; #p < 0.05 HS + vehicle vs. HS + ISO-1.
To investigate the effects of ISO-1 on circulatory failure, MAP was measured from the completion of surgery to the termination of the experiment. Baseline MAP values were similar amongst all four groups. Rats subjected to HS demonstrated a decline in MAP which was ameliorated by resuscitation, but still remained lower than that of sham-operated rats during resuscitation (at the equivalent time points, Figure 2B). When compared to sham-operated rats, HS-rats treated with vehicle exhibited a more pronounced decrease in MAP over time post-resuscitation. In contrast, MAP of HS-rats treated with ISO-1 was significantly higher than HS-rats treated with vehicle 4 h post-resuscitation (p<0.001; Figure 2B).
Having demonstrated that treatment with ISO-1 improves HS-induced circulatory failure, we next explored whether ISO-1 attenuates MODS associated with HS in rats. When compared to sham-operated rats, rats subjected to HS and treated with vehicle displayed increases in serum urea (p<0.001; Figure 3A) and creatinine (p<0.001; Figure 3B) and a decrease in creatinine clearance (p<0.001; Figure 3C) indicating the development of renal dysfunction. When compared to sham-operated rats, vehicle treated HS-rats exhibited significant increases in both ALT (p<0.001; Figure 3D) and AST (p<0.001; Figure 3E) indicating the development of hepatic injury, while the increases in amylase (p<0.001; Figure 3F) and CK (p<0.001; Figure 3G) denote pancreatic and neuromuscular injury, respectively. The significant increase in LDH (p<0.001; Figure 3H) in HS-rats treated with vehicle confirmed tissue injury whilst the increase in lactate (p<0.001; Figure 3I) indicated decreased transport of oxygen to the tissues developing from the state of hypoperfusion. Treatment of HS-rats with ISO-1 significantly attenuated the renal dysfunction, hepatic injury, pancreatic injury, neuromuscular injury and general tissue damage caused by HS (all p<0.05; Figures 3A–I). As HS causes macrophage infiltration into the lungs, we measured CD68+ positive cells as a marker for macrophage invasion. When compared to sham-operated rats (21.75 ± 2.29 per field), HS-rats treated with vehicle displayed a significant increase in macrophage count (47.33 ± 8.75 per field, p<0.05). Treatment with ISO-1 in HS-rats did not result in a significant decrease in macrophage count (35.90 ± 4.30 per field; Supplemental Figure 4).
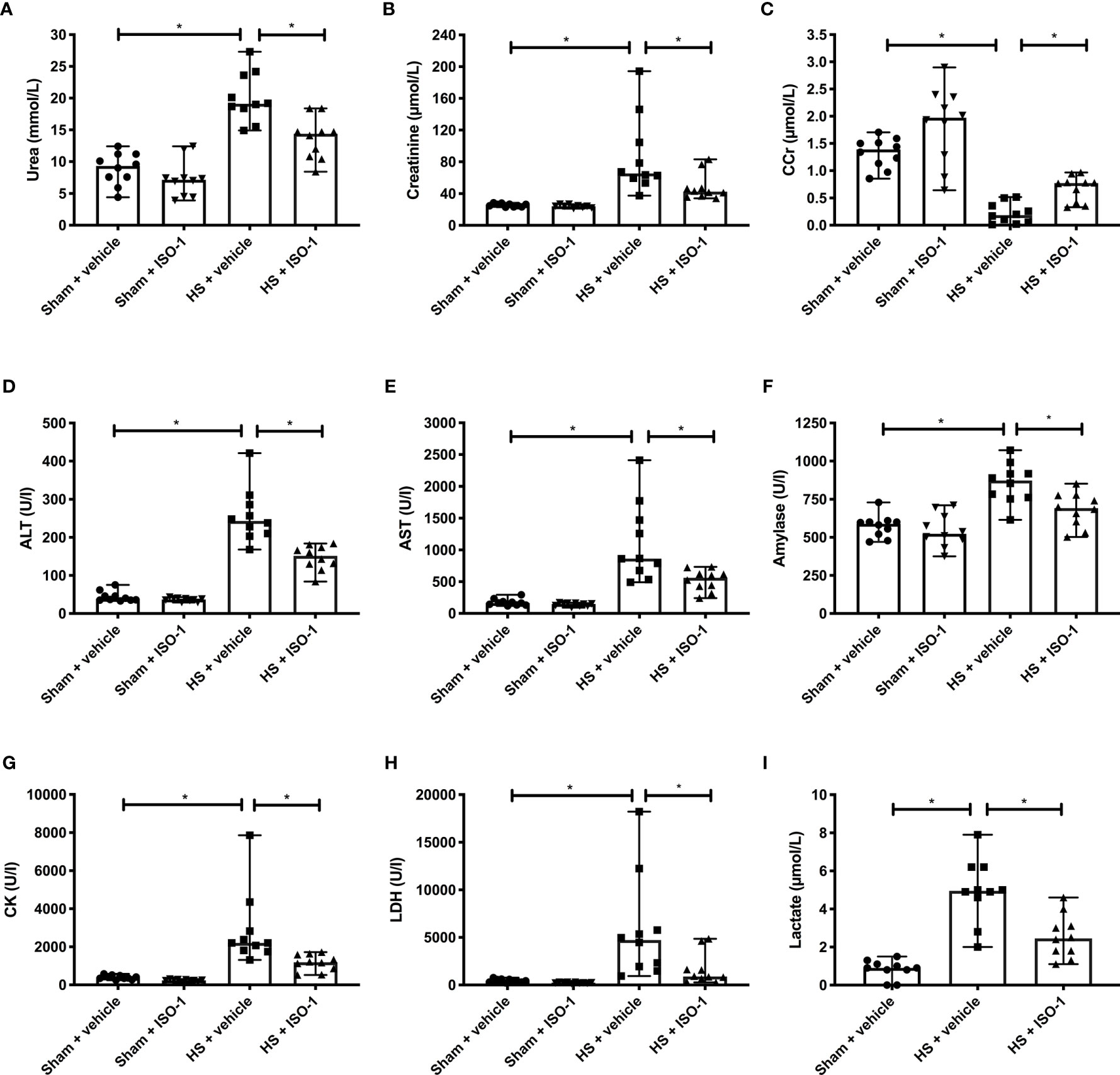
Figure 3 Treatment with ISO-1 attenuates HS-induced organ damage in a short-term follow-up acute HS model. Rats were subjected to haemorrhagic shock (HS) and 4 h after resuscitation, levels of serum (A) urea, (B) creatinine, (C) creatinine clearance (CCr), (D) alanine aminotransferase (ALT), (E) aspartate aminotransferase (AST), (F) amylase, (G) creatine kinase (CK), (H) lactate dehydrogenase (LDH) and (I) lactate were determined. Sham-operated rats were used as control. Data are expressed as median with range of ten animals per group. Statistical analysis was performed using one-way ANOVA followed by a Bonferroni’s post-hoc test. *p < 0.05 denoted statistical significance.
Having shown rats subjected to HS had elevated serum MIF levels, we wished to elucidate whether this observed increase correlates with clinical chemistry parameters (measured in serum collected 4 h post-resuscitation) and MAP (measured at 4 h post-resuscitation before sample collection). There was a positive correlation between MIF and all clinical chemistry parameters (p<0.05; Supplemental Figure 5 shown in blue, r values range from 0.64 – 0.77), with the strongest correlations between AST (r = 0.77) and LDH (r = 0.72). There was a negative correlation between MIF and MAP (p<0.05; Supplemental Figure 5 shown in red, r = -0.66).
The effect of MIF inhibition on the activation of the signalling events leading to the activation of NF-κB, was investigated in the kidney and liver. When compared to sham-operated rats, HS-rats treated with vehicle had significant increases in the phosphorylation of IKKα/β at Ser176/180 (p<0.001; Figure 4A and p<0.001; Figure 4B) and translocation of p65 to the nucleus (p<0.001; Figure 4C and p<0.05; Figure 4D). Treatment with ISO-1 significantly attenuated the increases in hepatic and renal phosphorylation of IKKα/β at Ser176/180 (p<0.001; Figure 4A and p<0.001; Figure 4B) and the nuclear translocation of p65 (p<0.001; Figure 4C and p<0.05; Figure 4D).
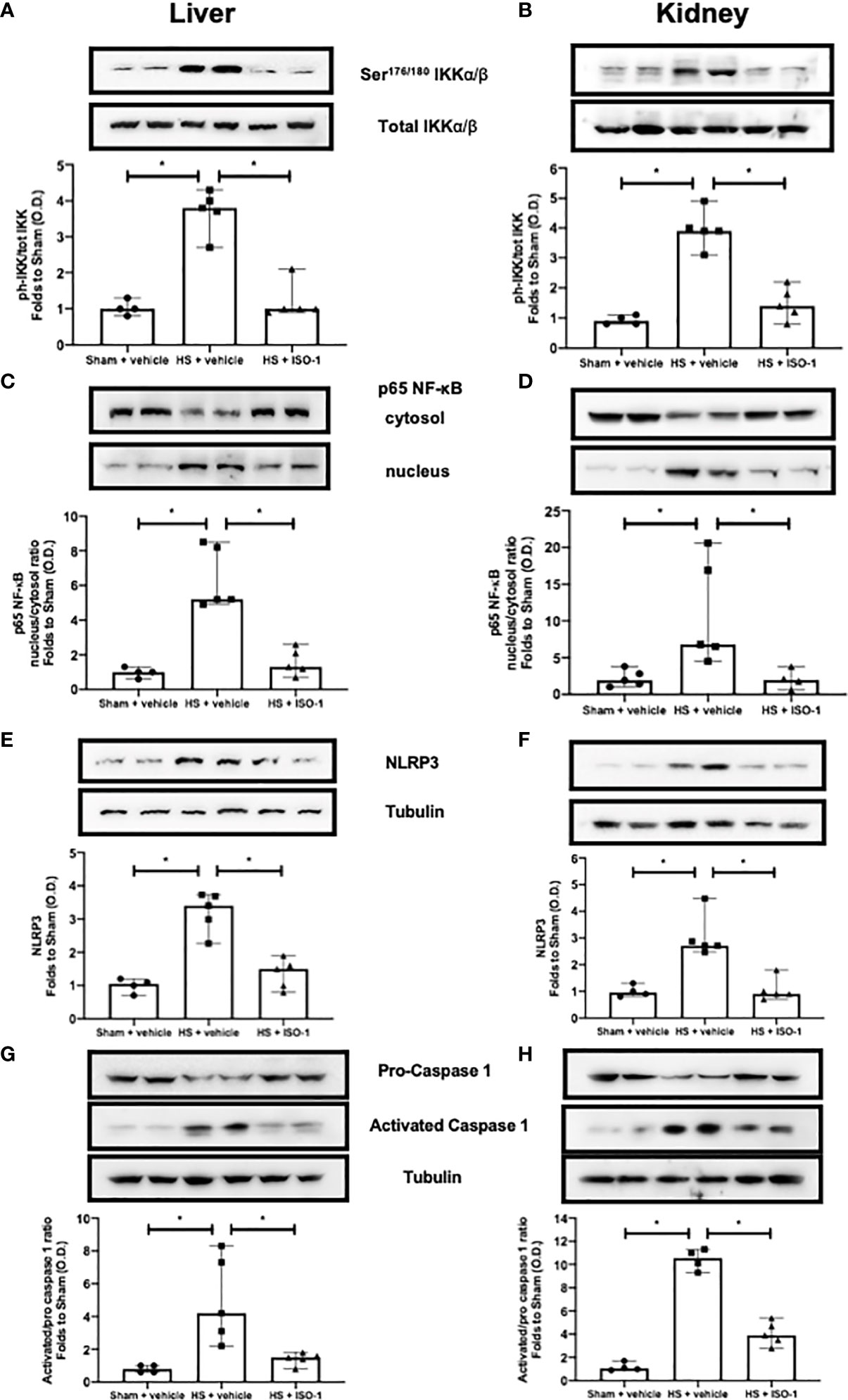
Figure 4 Treatment with ISO-1 attenuates NF-κB and NLRP3 activation in a short-term follow-up acute HS model. (A, B) The phosphorylation of IKKα/β at Ser176/180, (C, D) nuclear translocation of p65, (E, F) activation of NLRP3 and (G, H) cleavage of pro-caspase 1 of vehicle and ISO-1 treated rats were determined by western blot in the liver and kidney. Protein expression was measured as relative optical density (O.D.) and normalised to the sham band. Data are expressed as median with range of 4-5 animals per group. Statistical analysis was performed using one-way ANOVA followed by a Bonferroni’s post-hoc test. *p < 0.05 denoted statistical significance.
Having discovered treatment with ISO-1 significantly reduced the activation of NF-κB in the kidney and liver of rats subjected to HS, we next analysed the potential involvement of the NLRP3 inflammasome. When compared to sham-operated rats, HS-rats treated with vehicle exhibited a significantly increased expression of the NLRP3 inflammasome (p<0.001; Figure 4E and p<0.001; Figure 4F) and cleavage of pro-caspase 1 to caspase 1 (p=0.008; Figure 4G and p<0.001; Figure 4H). Treatment with ISO-1 significantly inhibited the hepatic and renal expression of NLRP3 (p<0.001; Figure 4E and p<0.001; Figure 4F) and cleavage of pro-caspase 1 to caspase 1 (p=0.015; Figure 4G and p<0.001; Figure 4H).
Having demonstrated treatment with ISO-1 improved blood pressure in a short-term follow-up model, we wished to determine whether ISO-1 would still be effective in a model in which the resuscitation period is prolonged to 24 h. When compared to sham-operated rats, HS-rats treated with vehicle had significantly lower MAP values at 24 h post-resuscitation (p<0.001; Figure 5A); highlighting that either cardiovascular dysfunction or excessive hypotension was still present. In contrast, MAP of HS-rats treated with ISO-1 was significantly higher at 24 h than vehicle treated rats (p<0.05; Figure 5A). There were no significant differences in HR between any of the three groups investigated (p>0.05; Figure 5B).
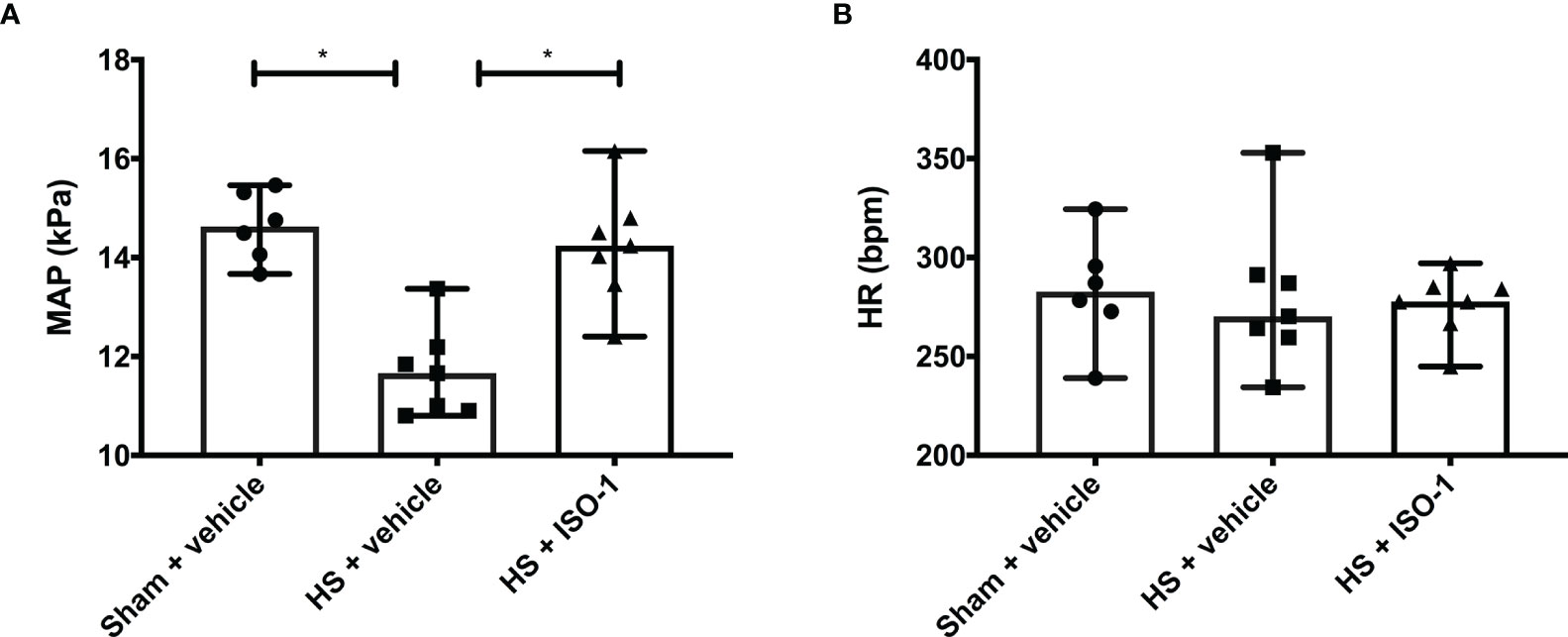
Figure 5 Treatment with ISO-1 improves HS-induced cardiac dysfunction in a long-term follow-up acute HS model. (A) Mean arterial pressure (MAP) and (B) heart rate (HR) were measured 24 h post resuscitation for vehicle and ISO-1 treated rats. Data are expressed as median with range. Sham + vehicle (n = 6), HS + vehicle (n = 7) and HS + ISO-1 (n = 7). Statistical analysis was performed using one-way ANOVA followed by a Bonferroni’s post-hoc test. *p < 0.05 denoted statistical significance.
Having shown that treatment with ISO-1 ameliorated the MODS associated with HS in a short-term follow-up model, we examined whether this effect was sustained when the resuscitation period was extended to 24 h. As with the short-term follow-up model, when compared to sham-operated rats, rats subjected to HS with long-term follow-up and treated with vehicle displayed significant increases in serum urea (p<0.05; Figure 6A) and creatinine (p<0.001; Figure 6B) indicating the development of renal dysfunction. When compared to sham-operated rats, vehicle treated HS-rats exhibited significant increases in ALT (p<0.001; Figure 6C), AST (p<0.001; Figure 6D), lipase (p<0.05; Figure 6E) and LDH (p<0.05; Figure 6F). Treatment of HS-rats with ISO-1 significantly attenuated the renal dysfunction, hepatic injury and tissue damage caused by HS (all p<0.05; Figures 6A–D, F). Having demonstrated that treatment with ISO-1 reduced the cell infiltration in the lung in a short-term follow-up acute HS model, we measured myeloperoxidase (MPO) activity in the lung and liver as an indicator of neutrophil infiltration. When compared to sham-operated rats, HS-rats treated with vehicle showed a significant increase in MPO activity in the lung (p<0.001; Figure 6G) and liver (p<0.05; Figure 6H). Treatment with ISO-1 in HS-rats significantly attenuated these rises in MPO activity (p<0.001; Figure 6G and p<0.05; Figure 6H).
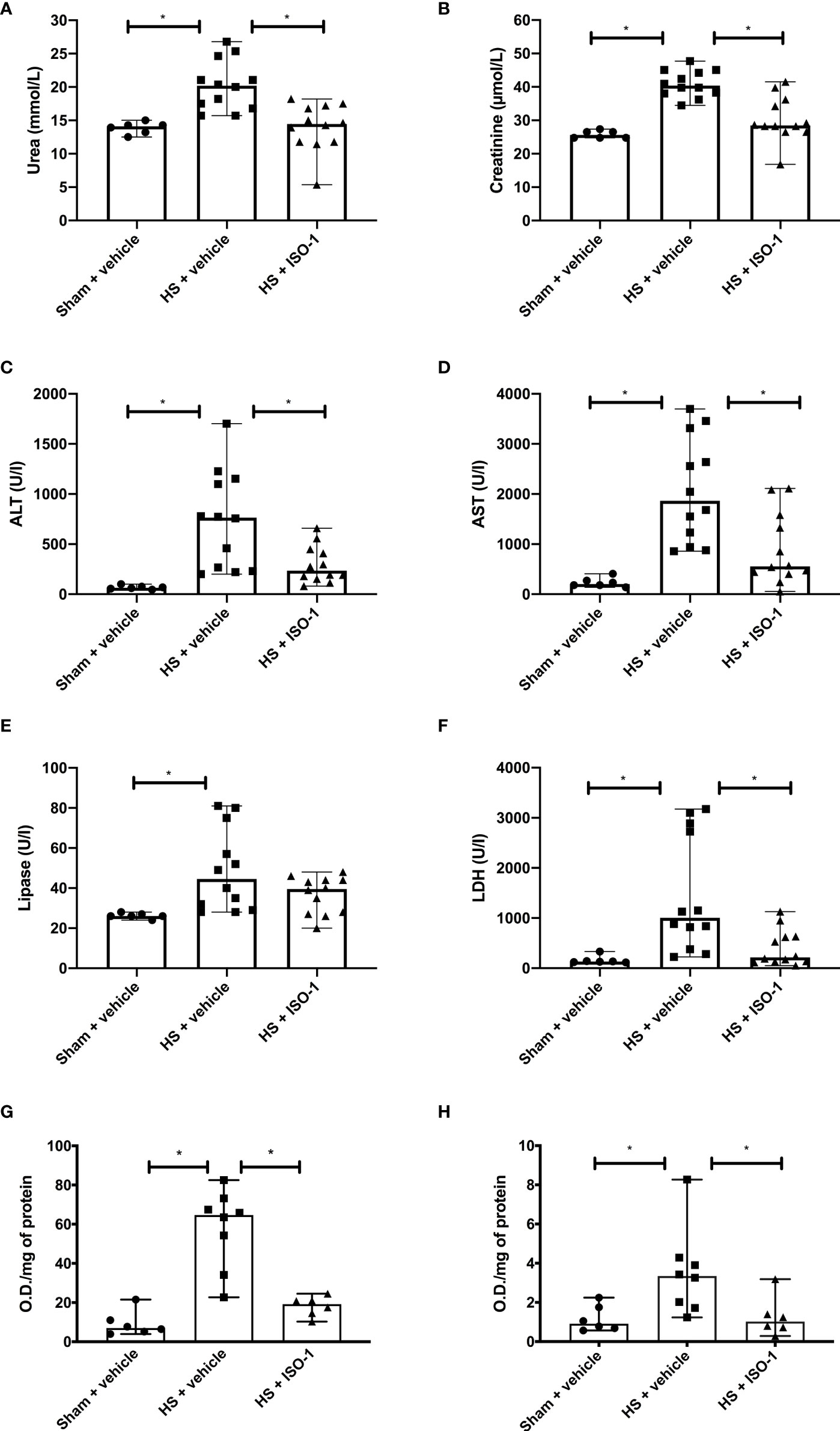
Figure 6 Treatment with ISO-1 attenuates HS-induced organ damage and myeloperoxidase activity in a long-term follow-up acute HS model. Rats were subjected to haemorrhagic shock (HS) and 24 h after resuscitation, levels of serum (A) urea, (B) creatinine, (C) alanine aminotransferase, (ALT), (D) aspartate aminotransferase (AST), (E) lipase and (F) LDH were determined were determined in vehicle and ISO-1 treated rats. Sham-operated rats were used as control. Sham + vehicle (n = 6), HS + vehicle (n = 12) and HS + ISO-1 (n = 12). Myeloperoxidase activity in (G) lung and (H) liver were determined for vehicle and ISO-1 treated rats. Sham + vehicle (n = 6), HS + vehicle (n = 8) and HS + ISO-1 (n = 6). Data are expressed as median with range. Statistical analysis was performed using one-way ANOVA followed by a Bonferroni’s post-hoc test. *p<0.05 denoted statistical significance.
This study reports that inhibition of MIF activity attenuates organ injury/dysfunction and circulatory failure in acute short-term follow-up (Figures 2, 3) and long-term follow-up (Figures 5, 6) rat models of HS. Having shown that MIF gene expression is significantly elevated in leukocytes of trauma patients (Supplemental Figure 2) and plasma MIF levels are raised in polytrauma patients (Figure 1), we used a reverse translational approach to investigate whether pharmacological intervention with ISO-1 ameliorates the MODS associated with HS in a well-established rat model. Inhibition of MIF activity significantly attenuated the fall in blood pressure (Figure 2 short-term follow-up and Figure 5 long-term follow-up) and, hence, the delayed vascular decompensation caused by HS (39). Moreover, ISO-1 significantly attenuated the renal dysfunction, hepatic injury and neuromuscular injury caused by HS (Figure 3 short-term follow-up and Figure 6 long-term follow-up); highlighting the drug efficacy at both timepoints. Similarly, ISO-1 also reduces disease severity in animal models of sepsis (40), acute pancreatitis (41–46), pneumonia (47), asthma (48), COPD (49), cystitis (50) and colitis (51).
We have illustrated that severe haemorrhage followed by resuscitation in the rat caused a significant increase in serum MIF levels, the magnitude of which was similar to the one seen in trauma patients. This finding implies that haemorrhage (rather than physical trauma) is the main driver for the observed increase in MIF in rats and possibly also in humans. Whilst the elevated plasma MIF levels in our cohort of polytrauma patients do not necessarily indicate an increase in synthesis, the increased gene expression measured by Xiao and colleagues (33) does suggest an associated rise in MIF production.
Post-traumatic complications can result in prolonged stays in ICU and in hospital. In the acute setting, there is a lack of diagnostic or risk stratification tools which allow the identification of the potential clinical outcome of trauma patients. We determined a significant positive correlation between elevated MIF levels in polytrauma patients and the overall length of ICU and hospital stays. These findings indicate that high plasma MIF levels at time of hospital admission are strongly predictive for a longer stay in ICU and hospital. Indeed, the human plasma MIF levels and gene expression data provide evidence supporting the role of MIF in trauma, as both gene expression and plasma levels increase following trauma. Most notably, MIF was a better predictor of hospital stay than either ISS or SOFA scores. For every further increase of MIF on admission by 5000 pg/mL, the stay of patients in hospital is prolonged by ~1.2 days. Similarly, Cho and colleagues showed that trauma patients with elevated MIF levels had longer ICU stays than patients with lower or normal MIF levels (52).
Nevertheless, we found a weak positive but significant correlation between MIF levels on admission and ISS which supports the findings of Chuang and colleagues’ study illustrating higher MIF levels were associated with worse clinical severity scores (APACHE II, RTS and TRISS) (30). Although clinical severity scores cannot be measured in rats, we found significant positive correlations between serum MIF levels and clinical chemistry parameters and a significant negative correlation between MIF and MAP both of which can be considered as indicators organ function (Supplementary Figure 5).
What, then, are the mechanisms by which ISO-1 attenuates HS-associated organ injury/dysfunction? It is recognised that key signalling pathways, such as those leading to NF-κB activation, initiate the production of pro-inflammatory mediators such as cytokines, chemokines and enzymes (53). As part of a positive feedback mechanism, these inflammatory mediators can induce activation of NF-κB and its upstream signalling machinery, further amplifying and propagating the NF-κB-mediated inflammatory responses. This can result in a more permeable endothelium, hypoxic/hypoperfused tissues, tissue injury and ultimately MODS (54). Trauma has been shown to increase the translocation of NF-κB to the nucleus (35–38). Inhibition of MIF activity with ISO-1 reduced NF-κB activation in the kidney and liver of HS-rats (Figure 4). This may suggest that inhibiting NF-κB activation contributes to the observed protective effects of ISO-1 in HS. It should be noted that MIF has been shown to upregulate TLR4 expression, leading to increased translocation of NF-κB into the nucleus (55) and interact with thioredoxin-interacting protein to induce NF-κB activity (56).
Activation of the NLRP3 inflammasome stimulates IL-1β production which plays a crucial role in trauma-associated systemic inflammation and organ dysfunction/injury (36). Inhibition of MIF activity with ISO-1 reduced both the assembly and subsequent activation of the NLRP3 inflammasome in the kidney and liver of HS-rats (Figure 4). This may suggest that inhibiting NLRP3 inflammasome activation contributes to the observed beneficial effects of ISO-1 in HS by decreasing the pro-inflammatory effects related to increased IL-1β production and ensuing tissue inflammation (57). Of note, MIF has been proposed to play a role in the activation of the NLRP3 inflammasome (58, 59).
The sterile inflammation caused by HS is associated with increased recruitment of leukocytes to the tissues and is secondary to NF-κB and NLRP3 activation and their transcriptional regulation of pro-inflammatory cytokines (60–62). Furthermore, the leukocyte and endothelial cell surface expression of adhesion molecules is regulated by NF-κB and promotes leukocyte extravasation from the circulation to the injury site (63). We found a significant increase in CD68+ cells in the lung (Supplementary Figure 4) and in pulmonary and hepatic MPO activity (Figure 6), markers of macrophage and neutrophil recruitment respectively, after induction of HS in rats. Treatment of HS-rats with ISO-1 did not significantly reduce the number of pulmonary CD68+ cells and thus, macrophage infiltration into the lung. In contrast, administration of ISO-1 to HS-rats attenuated the rise in MPO activity related to increased neutrophil recruitment. Taken together, these results could imply that ISO-1 attenuates neutrophil, but not macrophage recruitment in HS. Indeed, an anti-MIF antibody was shown to attenuate the LPS-induced migration and accumulation of neutrophils in the lung (64). These observations can be explained by the chemokine-like properties of MIF, which facilitate the activation and recruitment of leukocytes during immune surveillance and inflammation (65, 66). Initially, MIF was eponymously described by its ability to inhibit random macrophage migration in vitro (67, 68). However, it is now known that MIF can mediate the recruitment of mononuclear cells in a number of disease states (69–72). A possible explanation as to why ISO-1 did not significantly reduce pulmonary macrophage invasion is that the presence of other inflammatory mediators, such as IL-1β, IL-6 and TNF-α, could have stimulated the migration of macrophages, but we would need to measure these mediators to confirm this theory.
Although ISO-1 displayed some striking, beneficial effects in the acute HS models, there are study limitations which should be taken into consideration. In total, 208 patients were included in the study, however, in some instances there were insufficient plasma volumes available for some of the measured timepoints. Therefore, some correlation analyses were performed with a reduced number of samples/patients. Following trauma, there is an increased inflammatory response, and we were able to measure elevated plasma MIF levels in polytrauma patients. However, we cannot exclude the possibility that the increased plasma levels are not exclusively associated with polytrauma. The animal models used in our study do not encompass all aspects of trauma/HS and further long-term survival experiments are needed to verify that the observed early reduction in MODS does, indeed, translate to improved outcome and ultimately reduced mortality. Hence, caution must be exercised when interpreting the pre-clinical results and extrapolating to the clinical scenario. Additionally, future studies in larger animals/higher species may be useful to confirm efficacy and to further examine the mechanism(s) of action (e.g. microcirculatory effects and blood gas analysis) of ISO-1 in HS. It should be noted that only healthy young male rats were used and, hence, age and gender differences and the presence of co-morbidities were not investigated (but may well impact outcome). Moreover, clinical studies with larger cohorts of trauma patients are required to robustly examine the relationship between MIF activity, inhibition and clinical outcomes in humans. Whilst we did not measure the long-term stability of MIF in the patient samples collected between 2010-2014, we were able to demonstrate significant and time-related elevations in MIF in the blood of patients with trauma and haemorrhage. Indeed, the peak levels of MIF observed in patients on admission to the hospital (measured in samples that were several years old) were similar to the peak levels measured in rats with severe haemorrhage (that we measured within weeks of completing the experiments). Nevertheless, we cannot, however, entirely rule out that the levels of MIF in patients with trauma-haemorrhage would have been even higher if the MIF determinations would have been carried out earlier after the trauma occurred.
In conclusion, we demonstrate here for the first time that MIF levels are elevated in polytrauma patients on arrival to the emergency room and higher MIF levels are associated with longer stays in ICU and hospital overall. The finding that HS alone (in the absence of physical trauma) in rats resulted in a rise in MIF levels similar to that seen in polytrauma patients supports the view that haemorrhage is the main driver for the elevations in MIF. Furthermore, treatment with ISO-1 reduces the organ injury/dysfunction and circulatory failure caused by severe haemorrhage in the rat, highlighting a role of MIF in disease pathogenesis. Administration of ISO-1 attenuates the degree of NF-κB and NLRP3 inflammasome activation (measured in the kidney and liver), both of which are key drivers of local and systemic inflammation. Thus, we propose that MIF inhibitors may be used in trauma patients to lower the organ injury and inflammation caused by severe haemorrhage and resuscitation.
Publicly available datasets were analysed in this study. This data can be found here: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=gse36809.
The studies involving human participants were reviewed and approved by University Hospital Frankfurt of Goethe-University - Institutional Ethics Committee (Number 312/10). The patients/participants provided their written informed consent to participate in this study. The animal study was reviewed and approved by Animal Welfare Ethics Review Board of Queen Mary University of London and by the Home Office (Licence number PC5F29685) and Universidade Federal de Santa Catarina Institutional Committee for Animal Use in Research (Licence number 7396250219).
Conception and design: NMP, LM, and CT. Animal experiments: NY, NMP, FRMBO, HPR, and RS. Human sample analysis: LM, EZ, GM, IM, BR, DH, LS, and CS. Animal sample analyses: NY, NMP, LM, CT, LS, EZ, FRMBO, HPR, SK, DC, MC, GFA, and RS. Clinical study and patient data analyses: LM, EZ, CT, IM, BR, DH, LS, NMP, and CS. Statistical analyses: NY, NMP, DH, LM, and CT. Drafting the manuscript for important intellectual content: LS, LM, EZ, NMP, and CT. All authors reviewed and approved the manuscript.
NMP was funded by the William Harvey Research Foundation. HPR and FRMBO were funded by National Council for Scientific and Technological Development (CNPq) fellowship. This study was supported by the German Research Foundation to LM (DFG, MA 7082/3-1), to CS (DFG, STO 1099/8-1) and by an intramural grant to EZ (START 131/19), National Council for Scientific and Technological Development to RS (CNPq, Brazil, Grant 409018/2018-0).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to thank Dr. Ronald Tompkins and Dr. Wenzhong Xiao for their support in the human MIF gene expression data analysis.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.886421/full#supplementary-material
1. World Health Organization. Injuries and Violence: The Facts 2014 (2014). Available at: www.who.int/healthinfo/global_burden_disease/projections/en/.
2. Haagsma JA, Graetz N, Bolliger I, Naghavi M, Higashi H, Mullany EC, et al. The Global Burden of Injury: Incidence, Mortality, Disability-Adjusted Life Years and Time Trends From the Global Burden of Disease Study 2013. Injury Prev (2016) 22(1):3–18. doi: 10.1136/injuryprev-2015-041616
3. Curry N, Hopewell S, Dorée C, Hyde C, Brohi K, Stanworth S. The Acute Management of Trauma Hemorrhage: A Systematic Review of Randomized Controlled Trials. Crit Care (2011) 15(2):1–10. doi: 10.1186/cc10096
4. Dewar D, Moore FA, Moore EE, Balogh Z. Postinjury Multiple Organ Failure. Injury (2009) 40:912–8. doi: 10.1016/j.injury.2009.05.024
5. Geeraedts LMG, Kaasjager HAH, van Vugt AB, Frölke JPM. Exsanguination in Trauma: A Review of Diagnostics and Treatment Options. Injury (2009) 40(1):11–20. doi: 10.1016/j.injury.2008.10.007
6. Dutton RP, Stansbury LG, Leone S, Kramer E, Hess JR, Scalea TM. Trauma Mortality in Mature Trauma Systems: Are We Doing Better? An Analysis of Trauma Mortality Patterns, 1997-2008. J Trauma - Injury Infect Crit Care (2010) 69(3):620–6. doi: 10.1097/TA.0b013e3181bbfe2a
7. Teixeira PGR, Inaba K, Hadjizacharia P, Brown C, Salim A, Rhee P, et al. Preventable or Potentially Preventable Mortality at a Mature Trauma Center. J Trauma (2007) 63(6):1338–47. doi: 10.1097/TA.0b013e31815078ae
8. Lord JM, Midwinter MJ, Chen YF, Belli A, Brohi K, Kovacs EJ, et al. The Systemic Immune Response to Trauma: An Overview of Pathophysiology and Treatment. Lancet (2014) 384:1455–65. Lancet Publishing Group. doi: 10.1016/S0140-6736(14)60687-5
9. Sauaia A, Moore EE, Johnson JL, Chin TL, Banerjee A, Sperry JL, et al. Temporal Trends of Postinjury Multiple-Organ Failure: Still Resource Intensive, Morbid, and Lethal. J Trauma Acute Care Surg (2014) 76(3):582. doi: 10.1097/TA.0000000000000147
10. Relja B, Mörs K, Marzi I. Danger Signals in Trauma. Eur J Trauma Emergency Surg (2018) 44(3):301. doi: 10.1007/s00068-018-0962-3
11. Chen GY, Nuñez G. Sterile Inflammation: Sensing and Reacting to Damage. Nat Rev Immunol (2010) 10:826–37. NIH Public Access. doi: 10.1038/nri2873
12. Halbgebauer R, Braun CK, Denk S, Mayer B, Cinelli P, Radermacher P, et al. Hemorrhagic Shock Drives Glycocalyx, Barrier and Organ Dysfunction Early After Polytrauma. J Crit Care (2018) 44:229–37. doi: 10.1016/j.jcrc.2017.11.025
13. Manson J, Thiemermann C, Brohi K. Trauma Alarmins as Activators of Damage-Induced Inflammation. Br J Surg (2012) 99:12–20. doi: 10.1002/bjs.7717
14. Jackman RP, Utter GH, Muench MO, Heitman JW, Munz MM, Jackman RW, et al. Distinct Roles of Trauma and Transfusion in Induction of Immune Modulation After Injury. Transfusion (2012) 52(12):2533–50. doi: 10.1111/j.1537-2995.2012.03618.x
15. Calandra T, Bernhagen J, Metz CN, Spiegel LA, Bacher M, Donnelly T, et al. MIF as a Glucocorticoid-Induced Modulator of Cytokine Production. Nature (1995) 377(6544):68–71. doi: 10.1038/377068a0
16. Bacher M, Metz CN, Calandra T, Mayer K, Chesney J, Lohoff M, et al. An Essential Regulatory Role for Macrophage Migration Inhibitory Factor in T-Cell Activation. Proc Natl Acad Sci USA (1996) 93(15):7849. doi: 10.1073/pnas.93.15.7849
17. Donnelly SC, Bucala R. Macrophage Migration Inhibitory Factor: A Regulator of Glucocorticoid Activity With a Critical Role in Inflammatory Disease. Mol Med Today (1997) 3:502–7. doi: 10.1016/S1357-4310(97)01133-7
18. Bozza M, Satoskar AR, Lin G, Lu B, Humbles AA, Gerard C, et al. Targeted Disruption of Migration Inhibitory Factor Gene Reveals its Critical Role in Sepsis. J Exp Med (1999) 189(2):341–6. doi: 10.1084/jem.189.2.341
19. Mitchell RA, Liao H, Chesney J, Fingerle-Rowson G, Baugh J, David J, et al. Macrophage Migration Inhibitory Factor (MIF) Sustains Macrophage Proinflammatory Function by Inhibiting P53: Regulatory Role in the Innate Immune Response. Proc Natl Acad Sci (2002) 99:345–50. doi: 10.1073/pnas.012511599
20. Calandra T, Roger T. Macrophage Migration Inhibitory Factor: A Regulator of Innate Immunity. Nat Rev Immunol (2003) 30:S27–35. doi: 10.1002/0471203076.emm0331
21. Rodríguez-Sosa M, Rosas LE, David JR, Bojalil R, Satoskar AR, Terrazas LI. Macrophage Migration Inhibitory Factor Plays a Critical Role in Mediating Protection Against the Helminth Parasite Taenia Crassiceps. Infection Immun (2003) 71(3):1247. doi: 10.1128/IAI.71.3.1247-1254.2003
22. Onodera S, Kaneda K, Mizue Y, Koyama Y, Fujinaga M, Nishihira J. Macrophage Migration Inhibitory Factor Up-Regulates Expression of Matrix Metalloproteinases in Synovial Fibroblasts of Rheumatoid Arthritis. J Biol Chem (2000) 275(1):444–50. doi: 10.1074/jbc.275.1.444
23. Onodera S, Nishihira J, Iwabuchi K, Koyama Y, Yoshida K, Tanaka S, et al. Macrophage Migration Inhibitory Factor Up-Regulates Matrix Metalloproteinase-9 and -13 in Rat Osteoblasts: Relevance To Intracellular Signaling Pathways. J Biol Chem (2002) 277(10):7865–74. doi: 10.1074/jbc.M106020200
24. Calandra T, Bernhagen J, Mitchell RA, Bucala R. The Macrophage is an Important and Previously Unrecognized Source of Macrophage Migration Inhibitory Factor. J Exp Med (1994) 179(6):1895–902. doi: 10.1084/jem.179.6.1895
25. Calandra T, Echtenacher B, le Roy D, Pugin J, Metz CN, Hültner L, et al. Protection From Septic Shock by Neutralization of Macrophage Migration Inhibitory Factor. Nat Med (2000) 6(2):164–70. doi: 10.1038/72262
26. Benigni F, Atsumi T, Calandra T, Metz C, Echtenacher B, Peng T, et al. The Proinflammatory Mediator Macrophage Migration Inhibitory Factor Induces Glucose Catabolism in Muscle. J Clin Invest (2000) 106(10):1291. doi: 10.1172/JCI9900
27. Baugh JA, Bucala R. Macrophage Migration Inhibitory Factor. Crit Care Med (2002) 30(1 Suppl):S27–35. doi: 10.1097/00003246-200201001-00004
28. Calandra T, Bucala R. Macrophage Migration Inhibitory Factor: A Counter-Regulator of Glucocorticoid Action and Critical Mediator of Septic Shock. J Inflammation (1995) 47(1–2):39–51.
29. Joshi PC, Poole GV, Sachdev V, Zhou X, Jones Q. Trauma Patients With Positive Cultures Have Higher Levels of Circulating Macrophage Migration Inhibitory Factor (MIF). Res Commun Mol Pathol Pharmacol (2000) 107(1–2):13–20.
30. Chuang C-C, Hung C-J, Tsai M-C, Yeh T-M, Chuang Y-C. High Concentrations of Circulating Macrophage Migration Inhibitory Factor in Patients With Severe Blunt Trauma: Is Serum Macrophage Migration Inhibitory Factor Concentration a Valuable Prognostic Factor? Crit Care Med (2004) 32(3):734–9. doi: 10.1097/01.CCM.0000117170.13320.F4
31. Hayakawa M, Katabami K, Wada T, Minami Y, Sugano M, Shimojima H, et al. Imbalance Between Macrophage Migration Inhibitory Factor and Cortisol Induces Multiple Organ Dysfunction in Patients With Blunt Trauma. Inflammation (2010) 34(3):193–7. doi:10.1007/s10753-010-9223-2
32. Shih HC, Huang MS, Lee CH. Polymorphonuclear Cell Priming Associated With NF-kB Activation in Patients With Severe Injury is Partially Dependent on Macrophage Migration Inhibitory Factor. J Am Coll Surgeons (2010) 211(6):791–7. doi: 10.1016/j.jamcollsurg.2010.07.028
33. Xiao W, Mindrinos MN, Seok J, Cuschieri J, Cuenca AG, Gao H, et al. A Genomic Storm in Critically Injured Humans. J Exp Med (2011) 208(13):2581. doi: 10.1084/jem.20111354
34. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. Lancet (London England) (2007) 370(9596):1453–7. doi: 10.1016/S0140-6736(07)61602-X
35. Sordi R, Chiazza F, Collotta D, Migliaretti G, Colas RA, Vulliamy P, et al. Resolvin D1 Attenuates the Organ Injury Associated With Experimental Hemorrhagic Shock. Ann Surg (2021) 273(5):1012–21. doi: 10.1097/SLA.0000000000003407
36. Patel NM, Oliveira FRMB, Ramos HP, Aimaretti E, Alves GF, Coldewey SM, et al. Inhibition of Bruton’s Tyrosine Kinase Activity Attenuates Hemorrhagic Shock-Induced Multiple Organ Dysfunction in Rats. Ann Surg (2021). doi: 10.1097/SLA.0000000000005357
37. Sordi R, Nandra KK, Chiazza F, Johnson FL, Cabrera CP, Torrance HD, et al. Artesunate Protects Against the Organ Injury and Dysfunction Induced by Severe Hemorrhage and Resuscitation. Ann Surg (2017) 265:408–17. doi: 10.1097/SLA.0000000000001664
38. Yamada N, Martin LB, Zechendorf E, Purvis GSD, Chiazza F, Varrone B, et al. Novel Synthetic, Host-Defense Peptide Protects Against Organ Injury/Dysfunction in a Rat Model of Severe Hemorrhagic Shock. Ann Surg (2018) 268:348–56. doi: 10.1097/SLA.0000000000002186
39. Thiemermann C, Szabo C, Mitchell JA, Vane JR. Vascular Hyporeactivity to Vasoconstrictor Agents and Hemodynamic Decompensation in Hemorrhagic Shock is Mediated by Nitric Oxide. Proc Natl Acad Sci USA (1993) 90(1):267–71. doi: 10.1073/pnas.90.1.267
40. Al-Abed Y, Dabideen D, Aljabari B, Valster A, Messmer D, Ochani M, et al. ISO-1 Binding to the Tautomerase Active Site of MIF Inhibits its Pro-Inflammatory Activity and Increases Survival in Severe Sepsis. J Biol Chem (2005) 280:36541–4. doi: 10.1074/jbc.C500243200
41. Wang B, Zhao K, Hu W, Ding Y, Wang W. Protective Mechanism of MIF Inhibitor ISO-1 on Intrahepatic Bile Duct Cells in Rats With Severe Acute Pancreatitis. Digestive Dis Sci (2021) 66(10):3415–26. doi: 10.1007/s10620-020-06674-9
42. Li M, Yu J, Zhao L, Mei F-C, Zhou Y, Hong Y-P, et al. Inhibition of Macrophage Migration Inhibitory Factor Attenuates Inflammation and Fetal Kidney Injury in a Rat Model of Acute Pancreatitis in Pregnancy. Int Immunopharmacol (2019) 68:106–14. doi: 10.1016/j.intimp.2018.12.068
43. Zhou Y, Zhao L, Mei F, Hong Y, Xia H, Zuo T, et al. Macrophage Migration Inhibitory Factor Antagonist (S,R)3-(4-hydroxyphenyl)-4,5-dihydro-5-isoxazole Acetic Acid Methyl Ester Attenuates Inflammation and Lung Injury in Rats With Acute Pancreatitis in Pregnancy. Mol Med Rep (2018) 17(5):6576–84. doi: 10.3892/mmr.2018.8672
44. Liu Y, Liu Y, Wang Q, Song Y, Chen S, Cheng B, et al. MIF Inhibitor ISO-1 Alleviates Severe Acute Pancreatitis-Associated Acute Kidney Injury by Suppressing the NLRP3 Inflammasome Signaling Pathway. Int Immunopharmacol (2021) 96:107555. doi: 10.1016/j.intimp.2021.107555
45. Guo ZD, Zhao L, Wang P, Deng WH, Shi Q, Zuo T, et al. Fetal Liver Injury Ameliorated by Migration Inhibitory Factor Inhibition in a Rat Model of Acute Pancreatitis in Pregnancy. J Obstetrics Gynaecol Res (2018) 44(3):374–83. doi: 10.1111/jog.13538
46. Zhu C, Liu Y, Song Y, Wang Q, Liu Y, Yang S, et al. Deletion of Macrophage Migration Inhibitory Factor Ameliorates Inflammation in Mice Model Severe Acute Pancreatitis. Biomedicine Pharmacotherapy = Biomedecine pharmacotherapie (2020) 125:109919. doi: 10.1016/j.biopha.2020.109919
47. Hou XQ, Gao YW, Yang ST, Wang CY, Ma ZY, Xia XZ. Role of Macrophage Migration Inhibitory Factor in Influenza H5N1 Virus Pneumonia. Acta Virologica (2009) 53(4):225–31. doi: 10.4149/av_2009_04_225
48. Chen PF, Luo YL, Wang W, Wang JX, Lai WY, Hu SM, et al. ISO-1, a Macrophage Migration Inhibitory Factor Antagonist, Inhibits Airway Remodeling in a Murine Model of Chronic Asthma. Mol Med (2010) 16(9–10):400–8. doi: 10.2119/molmed.2009.00128
49. Russell KE, Chung KF, Clarke CJ, Durham AL, Mallia P, Footitt J, et al. The MIF Antagonist ISO-1 Attenuates Corticosteroid-Insensitive Inflammation and Airways Hyperresponsiveness in an Ozone-Induced Model of COPD. PloS One (2016) 11(1):e0146102. doi: 10.1371/journal.pone.0146102
50. Vera PL, Iczkowski KA, Howard DJ, Jiang L, Meyer-Siegler KL. Antagonism of Macrophage Migration Inhibitory Factor Decreases Cyclophosphamide Cystitis in Mice. Neurourology Urodynamics (2010) 29(8):1451–7. doi: 10.1002/nau.20878
51. Shah YM, Ito S, Morimura K, Chen C, Yim SH, Haase VH, et al. Hypoxia-Inducible Factor Augments Experimental Colitis Through a MIF-Dependent Inflammatory Signaling Cascade. Gastroenterology (2008) 134(7):2036. doi: 10.1053/j.gastro.2008.03.009
52. Cho YD, Choi SH, Kim JY, Park SJ, Yoon YH, Cho HJ, et al. Macrophage Migration Inhibitory Factor Levels Correlate With an Infection in Trauma Patients. Ulusal travma ve acil cerrahi dergisi = Turkish J Trauma Emergency surgery: TJTES (2017) 201723(3):193–8. doi: 10.5505/tjtes.2016.04780
53. Senftleben U, Karin M. The IKK/NF-Kappa B Pathway. Crit Care Med (2002) 30:S18–26. doi: 10.1097/00003246-200201001-00003
54. Liu SF, Malik AB. NF-κb Activation as a Pathological Mechanism of Septic Shock and InflammationAmerican Journal of Physiology - Lung Cellular and Molecular Physiology. Am J Physiol Lung Cell Mol Physiol (2006) 290:L622–45. doi: 10.1152/ajplung.00477.2005.
55. Roger T, David J, Glauser MP, Calandra T. MIF Regulates Innate Immune Responses Through Modulation of Toll-Like Receptor 4. Nature (2001) 414(6866):920–4. doi: 10.1038/414920a
56. Kim MJ, Kim WS, Kim DO, Byun JE, Huy H, Lee SY, et al. Macrophage Migration Inhibitory Factor Interacts With Thioredoxin-Interacting Protein and Induces NF-κb Activity. Cell signalling (2017) 34:110–20. doi: 10.1016/j.cellsig.2017.03.007
57. Dinarello CA. Biologic Basis for Interleukin-1 in Disease. Blood (1996) 87:2095–147. American Society of Hematology. doi: 10.1182/blood.V87.6.2095.bloodjournal8762095
58. Lang T, Lee JPW, Elgass K, Pinar AA, Tate MD, Aitken EH, et al. Macrophage Migration Inhibitory Factor is Required for NLRP3 Inflammasome Activation. Nat Commun (2018) 9(1):1–15. doi: 10.1038/s41467-018-04581-2
59. Shin MS, Kang Y, Wahl ER, Park HJ, Lazova R, Leng L, et al. Macrophage Migration Inhibitory Factor Regulates U1 Small Nuclear RNP Immune Complex-Mediated Activation of the NLRP3 Inflammasome. Arthritis Rheumatol (Hoboken NJ) (2019) 71(1):109–20. doi: 10.1002/art.40672
60. Chen CC, Manning AM. Transcriptional Regulation of Endothelial Cell Adhesion Molecules: A Dominant Role for NF-κb. Agents Actions Suppl (1995) 47:135–41. doi: 10.1007/978-3-0348-7343-7_12
61. Collins T, Read MA, Neish AS, Whitley MZ, Thanos D, Maniatis T. Transcriptional Regulation of Endothelial Cell Adhesion Molecules: NF‐κb and Cytokine‐Inducible Enhancers. FASEB J (1995) 9(10):899–909. doi: 10.1096/fasebj.9.10.7542214
62. Campbell SJ, Anthony DC, Oakley F, Carlsen H, Elsharkawy AM, Blomhoff R, et al. Hepatic Nuclear Factor κb Regulates Neutrophil Recruitment to the Injured Brain. J Neuropathology Exp Neurol (2008) 67(3):223–30. doi: 10.1097/NEN.0b013e3181654957
63. Hayden MS, Ghosh S. NF-κb in Immunobiology. Cell Res (2011) 21(2):223–44. doi: 10.1038/cr.2011.13
64. Makita H, Nishimura M, Miyamoto K, Nakano T, Tanino Y, Hirokawa J, et al. Effect of Anti-Macrophage Migration Inhibitory Factor Antibody on Lipopolysaccharide-Induced Pulmonary Neutrophil Accumulation. Am J Respir Crit Care Med (1998) 158(2):573–9. doi: 10.1164/ajrccm.158.2.9707086
65. Bernhagen J, Krohn R, Lue H, Gregory JL, Zernecke A, Koenen RR, et al. MIF is a Noncognate Ligand of CXC Chemokine Receptors in Inflammatory and Atherogenic Cell Recruitment. Nat Med (2007) 13(5):587–96. doi: 10.1038/nm1567
66. Gregory JL, Leech MT, David JR, Yang YH, Dacumos A, Hickey MJ. Reduced Leukocyte–Endothelial Cell Interactions in the Inflamed Microcirculation of Macrophage Migration Inhibitory Factor–Deficient Mice. Arthritis Rheumatism (2004) 50(9):3023–34. doi: 10.1002/art.20470
67. David JR. Delayed Hypersensitivity In Vitro: Its Mediation by Cell-Free Substances Formed by Lymphoid Cell-Antigen Interaction. Proc Natl Acad Sci (1966) 56(1):72–7. doi: 10.1073/pnas.56.1.72
68. Bloom BR, Bennett B. Mechanism of a Reaction in Vitro Associated With Delayed-Type Hypersensitivity. Science (1966) 153(3731):80–2. doi: 10.1126/science.153.3731.80
69. Leech M, Metz C, Santos L, Peng T, Holdsworti-I SR, Bucala R, et al. Involvement of Macrophage Migration Inhibitory Factor in the Evolution of Rat Adjuvant Arthritis. Arthritis Rheumatism (1998) 41(5):910–7. doi: 10.1002/1529-0131(199805)41:5<910::AID-ART19>3.0.CO;2-E
70. Burger-Kentischer A, Göbel H, Kleemann R, Zernecke A, Bucala R, Leng L, et al. Reduction of the Aortic Inflammatory Response in Spontaneous Atherosclerosis by Blockade of Macrophage Migration Inhibitory Factor (MIF). Atherosclerosis (2006) 184(1):28–38. doi: 10.1016/j.atherosclerosis.2005.03.028
71. Schober A, Bernhagen J, Thiele M, Zeiffer U, Knarren S, Roller M, et al. Stabilization of Atherosclerotic Plaques by Blockade of Macrophage Migration Inhibitory Factor After Vascular Injury in Apolipoprotein E-Deficient Mice. Circulation (2004) 109(3):380–5. doi: 10.1161/01.CIR.0000109201.72441.09
Keywords: haemorrhagic shock, ischaemia-reperfusion, ISO-1, macrophage migration inhibitory factor, multiple organ dysfunction syndrome, trauma
Citation: Patel NM, Yamada N, Oliveira FRMB, Stiehler L, Zechendorf E, Hinkelmann D, Kraemer S, Stoppe C, Collino M, Collotta D, Alves GF, Ramos HP, Sordi R, Marzi I, Relja B, Marx G, Martin L and Thiemermann C (2022) Inhibition of Macrophage Migration Inhibitory Factor Activity Attenuates Haemorrhagic Shock-Induced Multiple Organ Dysfunction in Rats. Front. Immunol. 13:886421. doi: 10.3389/fimmu.2022.886421
Received: 28 February 2022; Accepted: 14 March 2022;
Published: 06 April 2022.
Edited by:
Tom E. Mollnes, University of Oslo, NorwayReviewed by:
Roman Pfeifer, University Hospital Zürich, SwitzerlandCopyright © 2022 Patel, Yamada, Oliveira, Stiehler, Zechendorf, Hinkelmann, Kraemer, Stoppe, Collino, Collotta, Alves, Ramos, Sordi, Marzi, Relja, Marx, Martin and Thiemermann. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lukas Martin, bG1hcnRpbkB1a2FhY2hlbi5kZQ==; Nikita M. Patel, bi5tLnBhdGVsQHFtdWwuYWMudWs=
†These authors have contributed equally to this work and share senior authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.