- Department of Clinical Pharmacy & Toxicology, Leiden University Medical Center, Leiden, Netherlands
The use of monoclonal antibodies (mAbs) in the clinic has successfully expanded to treatment of cancer, viral infections, inflammations, and other indications. However, some of the classes of mAbs that are used in the clinic show the formation of anti-drug antibodies (ADAs) leading to loss of efficacy. This review describes ADA formation for the various mAbs, and its clinical effect. Lastly, this review considers the use of HLA-haplotypes as biomarkers to predict vulnerability of patients sensitive to formation of ADAs.
Introduction – What Exactly Is a Monoclonal Antibody
In order to recognize and neutralize alien organisms or antigens, B-cells secrete antibodies (Abs). These Abs are glycoproteins that belong to the superfamily of immunoglobulins. The Abs consist of two heavy chains and two light chains that characterize the isotype of the Ab (1). In the clinic, therapeutic monoclonal antibodies (mAbs) usually are of the γ-immunoglobulin (IgG) isotype. For this IgG isotype, the hypervariable regions of the heavy chains and light chains connect to form the antigen binding site (Fab-domain). The two constant domains together function as the fragment crystallizable (Fc-domain), which is responsible for the effector function of the immunoglobulin. Due to these Fab and Fc-domains, the IgG molecule is bivalent (2). In mice, the neonatal Fc receptor (FcRn) regulates the serum half-life of the IgG molecule through pH-dependent antibody recycling (2, 3).
Most therapeutic mAbs can target multiple disease targets (4). Of the mAbs currently on the market, 54% are fully human, meaning that they only feature human genetic sequences. 32% of the market is humanized, and 14% is chimeric (5).
Repeated Administration of mAbs Elicits Efficacy Reducing Immune Response
Even though mAbs are currently used in the treatment of a variety of diseases and have a promising future, their use has shown to be highly immunogenic and can cause an anti-drug antibody (ADA) response (2). These ADA responses intervene with the efficacy of a drug, or neutralizes a drug completely. Thereby, ADAs can alter the pharmacokinetic (PK) and pharmacodynamic (PD) properties of the mAb. ADAs can also eventually lead to severe adverse immune reactions in humans (5, 6).
The formation of these ADA responses is believed to depend on the interaction between the drug itself (e.g., its glycosylation, impurities, aggregation, or non-human sequences), the patient (e.g., the type of disease, genetic factors, concomitant immunomodulators) and to the route and regularity of administration of the mAb. Because the molecular mechanism of the development of these ADA responses are not fully understood, several researchers believed that the murine origin of the mAbs was the cause of the development of the immunogenic reaction. For that reason, humanized and chimeric mAbs were developed aimed at avoiding the human antimouse antibody response (HAMA) in the clinic (5, 7–9).
However, this did not eradicate the immunogenicity potential of mAbs and the related ADA response (2). Besides the questions of how and why ADA responses occur, the difficulty of the situation is further increased by the observation that some patients do develop ADA, but others don’t. It is also complicated more by the notion that the immunogenicity differences in patients receiving the same mAbs as treatment (10). These differences not only caused by regular interindividual factors, but also by geographical and racial differences (11).
The consequences of the immunogenicity elicited by mAbs range from absence of effect of the mAb to severe and life-threatening responses (12–14). Registered consequences include infusion reaction, anaphylaxis, immune complex-mediated diseases and loss of efficacy (15–21).
The anti-drug antibody responses that are elicited in mAbs-receiving patients can be classified into two categories, neutralizing ADA (ntADA) which influence the binding capacity of the drug directly by targeting the antigen-binding site, and the non-ntADA which recognizes other epitopes that are present on the drug while still facilitating mAb binding activity. However, the non-ntADA’s can in fact be malignant (22, 23).
ADA-Responses Elicited by mAbs and Their Clinical Effects
Over 90% of the therapeutic proteins cause immunogenicity, with the production of ADAs as a result (24). However, this immunogenic reaction is not the same in all patients and also not the same for all mAbs. The concentration of ADA can be measured (i.e., in blood), which is indicated as the titer. However, there is currently a lack of consitent and reliable immunogenicity assays to use for clinical desiscion making (25).
Some patients show a low ADA titer, in whom the concentration of free drug may still be high enough to be effective. Other patients may show a high ADA titer, causing the majority of the drug to be neutralized resulting in an absence of clinical response (23, 24, 26). As such, the formation of ADAs upon mAb use is of major clinical importance.
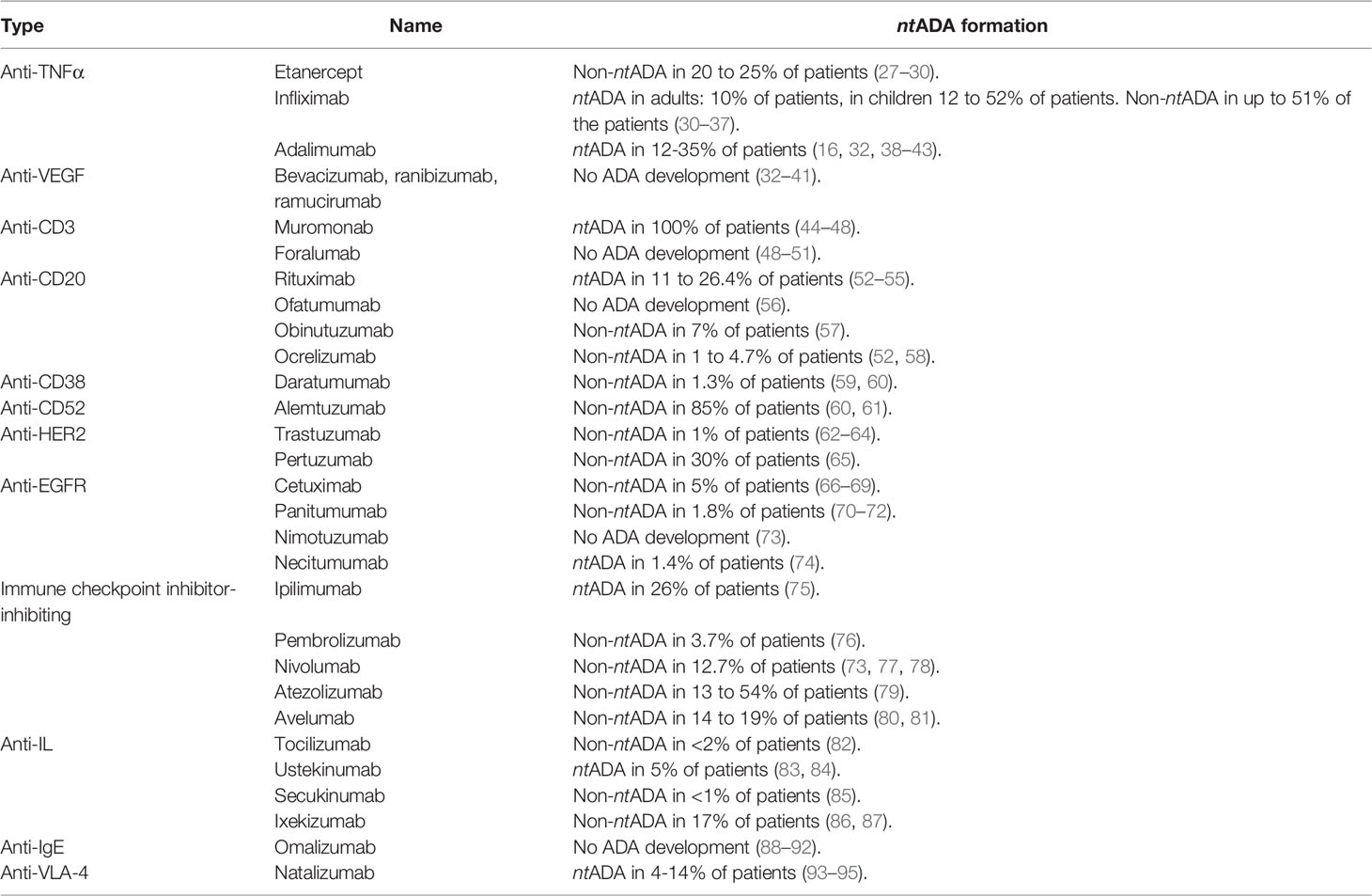
As the ADA responses are not only different between patients but also between mAb-classes, these were studied separately to describe which class of mAb shows an ADA response, when in the treatment that response is occurring and what the clinical effect of this response is (11). A summary of this literature study can be found in Table 1.
Method
For this literature study, a search of English publications listed in the electronic databases of the NCBI through PubMed was performed. Included in this search were the terms ‘Pharmacogenomic variants’, ‘Antidrug Antibody’, ‘Human Leukocyte Antigens’ and ‘Monoclonal Antibodies’ and MESH-terms related to them. After this PubMed search, an analytical review based on inclusion of humans in ADA response development tests was performed.
Overview of Antidrug Antibodies Elicited Against Monoclonal Antibodies and Their Clinical Effects
Tumour Necrosis Factor Alpha Antagonistic mAbs
Tumour necrosis factor alpha (TNFα) antagonistic mAbs are used in the clinic to treat rheumatoid arthritis, spondylarthritis, psoriasis and inflammatory bowel disease. Three biologic therapies are currently approved: etanercept, infliximab and adalimumab. Etanercept is a dimeric fusion protein that blocks the effect of TNFα, infliximab is a chimeric monoclonal receptor antibody and adalimumab is a human monoclonal TNFα antibody. While all these mAbs elicit an ADA response, this response can be attenuated by immunosuppressors (especially methotrexate) as much as a reduction of 80% (16, 27). For this reduction, the mechanism is still unknown (27).
Immunogenicity of Etanercept
ADAs against etanercept are formed in 20 to 25% of the patients receiving the drug. However, the antibodies formed are all non-neutralizing. Patients that were monitored for the formation of these antidrug antibodies in a study that lasted 120 weeks, showed an increase of antibodies with an increase in the duration of the study. Despite the presence of these antibodies, these have no correlation to the clinical response or adverse effects (16, 96). This suggests the presence of drug binding antibodies rather than drug neutralizing antibodies (27–30).
Immunogenicity of Infliximab
In adults receiving treatment with infliximab, about 10% show development of ntADAs, and this rate goes up to 51% for non-ntADAs (30, 31). The incidence rates of ntADAs is even higher in patients with Crohn’s Disease that receive infliximab after drug-free intervals of maximum 16 weeks (32). The patients in which these antibodies are present, infusion reactions, low titers and loss of drug efficacy are seen more often than in antibody-negative patients receiving the same treatment (16, 33–37). The development of these ADA responses is seen less often in patients that simultaneously receive immunosuppressive therapies like methotrexate (32).
In children, the incidence of ADA responses is higher. In a study that include paediatric patients with Crohn’s Disease, 12% of the tested population was antibody-positive despite the simultaneous administration of immunosuppressive therapies. In the study covering paediatric patients with ulcerative colitis, 52% of the tested population was antibody-positive after a median 24-month period. Here, there was no simultaneous administration of immunosuppressive therapies (30, 32).
Immunogenicity of Adalimumab
In rheumatoid arthritis-patients receiving treatment with adalimumab, the patients treated with only adalimumab showed higher incidence of immunogenicity against the mAb after 6 months than patients treated with adalimumab and concomitant immunosuppressants (12% versus 1%) (32, 38–43). The levels of non-ntADAs in patients ranges up to 35% (42). No apparent correlation was shown between the development of the antibodies to the adverse reactions. The antibodies that were formed in the patients, were neutralizing. The formation of the antibodies was seen more often in patients that received their dosage every other week than it was in patients receiving their dosage weekly. The same effects were seen in patients with other diseases as is described in the Humira (adalimumab) label distributed by the FDA (16, 32).
For the anti-TNFα therapies discussed here, reports show that dose titration reduces the incidence of ADA responses and improves the drug response (18, 97). However, other studies show that this dose escalation boosts the immune response with consequences like infusion-related adverse events or severe thromboembolic phenomena (27, 98, 99). The mechanisms behind this remains unclear (27).
Vascular Endothelial Growth Factor Antagonistic mAbs
As vascular endothelial growth factor (VEGF) is a mediator of angiogenesis related to tumours and conditions like diabetic retinopathy and age-related macular degeneration, anti-VEGF therapy is used in the clinic predominantly for therapy of solid tumours (100, 101). Anti-VEGF therapies that have been developed and are currently used in the clinic are bevacizumab, ranibizumab and ramucirumab (102). Bevacizumab is a recombinant humanized IgG mAb targeting VEGF-A, thus preventing the complex of VEGF-A and VEGFR-2 to form (102, 103). Ranibizumab is an affinity-matured antibody Fab-domain, which is developed against all fragments of the VEGF (104, 105). Ramucirumab is a fully human mAbs that inhibits VEGFR2 via the blockage of the VEGFR2-VEGF ligand interaction (102, 106, 107). None of the mentioned VEGF-inhibiting mAbs show immunogenicity (102–110).
Cell Surface Antigen-Targeted mAbs
CD3-inhibiting mAbs
Immunogenicity of Muromonab
The first murine mAb available on the market was Ortho Kung T3 (OKT3, muromonab) (44). This mAb is from murine origin, and showed extreme immunogenicity with a high incidence of ADA responses, but also a wide variety of severe side effects including cytokine syndrome, seizures, encephalopathy and graft thrombosis (45–47). OKT3 is a mouse IgG2a-antibody that targets the CD3ε-chain in the CD3/TCR that complex characterizes T-lymphocytes (44).
Muromonab was later humanized by grafting the complementary-determining region into a structure with a human IgG-backbone, which in phase I studies did not show immunogenicity when administered orally (48).
Immunogenicity of Foralumab
Foralumab is a completely human anti-CD3 mAb. For this mAb, the Fc-fragment of human IgG1 was mutated so the mAb shows no FcR-binding in vitro and displays minor cytokine release in vivo while sustaining the modulation of the CD3/TCR-complex and T-cell depletion (49).
The present generation of anti-CD3 mAb presents a reduced affinity for Fc-receptors, resulting in decreased side effects when compared to the original FcR-biding antibodies that were derived from rodents (44). This generation shows very promising results in preclinical studies looking at the possibility of oral or nasal administration (48–51).
CD20-inhibiting mAbs
Anti-CD20 mAbs are used in the clinic to achieve B-cell depletion. Initially, this class of mAbs was developed to treat B-cell proliferative disorders such as non-Hodgkin’s lymphoma and chronic lymphocytic leukaemia, but the therapies are currently also applied in the therapy of rheumatoid arthritis and other autoimmune diseases with both T-cell and B-cell etiology (52).
Immunogenicity of Rituximab
Rituximab is a murine-human chimeric anti-CD20 mAb. The development of ntADA responses in the clinic is seen often, which is believed to be caused by the chimeric nature of the antibody (52). A study focused on the safety of rituximab in patients with rheumatoid arthritis that administered rituximab together with methotrexate showed that 11% of the patients enrolled in the study showed an ADA response within 8 to 12 weeks after administration (53). Another study, the HERMES trial, presented 26.4% of the patients with immunogenicity against the drug at week 48 (54). Similar studies show that anti-rituximab antibodies reduce the efficacy of the drug (55).
Immunogenicity of New-Generation Anti-CD20 mAbs
Ofatumumab is one of the new-generation anti-CD20 mAbs currently used in the clinic (52). In one of the phase II trials that was conducted for ofatumumab, the none of the patients developed an ADA response in week 48 (56).
Obinutuzumab is another new-generation anti-CD20 mAb that is FDA-approved for the treatment of chronic lymphocytic leukaemia (52). In the clinical trials, 7% of the patients have developed an ADA response against this drug during treatment; the ADA were found in follow-up tests performed 6-months or more after treatment and did not have any effect on the efficacy of the drug. Other studies did not show development of ADA responses at all (57).
Ocrelizumab has been FDA-approved in the treatment of relapsing-remitting MS (52). Several trials were conducted to assess the safety of ocrelizumab, which all showed comparable between the ocrelizumab/methotrexate and placebo/methotrexate groups, ranging from 1 to 4.7% (52, 58). Of the patients that developed an ADA response, there was no correlation between immunogenicity and reduced efficacy or adverse effects of the drug (52).
For other new-generation anti-CD20 mAb, studies showed development of ADA responses but no effect on the efficacy of the drugs (111). This shows that the new generation of anti-CD20 mAbs is as efficient as the ‘old’ generation, but with a better safety profile in terms of immunogenicity (52).
CD38-inhibiting mAbs
CD38 functions as cell surface ectoenzyme. It contributes to the modification of NAD+ released from damaged cells in inflammation, to immunosuppressive extracellular adenosine. CD38 might also play a role in the promotion of tumour growth via the suppression of effector T-cell responses in a tumour environment (112). An anti-CD38 specific mAb used in the clinic is daratumumab, which shows to be effective in multiple myeloma (112).
Immunogenicity of Daratumumab
Daratumumab is generated from CD38-immunized transgenic mice carrying the genomic loci encoding human IgH and IgI (112). When administered intravenously, the drug shows a low risk of immunogenicity (1.3% of the patients). The presence of antibodies increased with increased dosage. The antibodies that were formed, were neutralizing but did not affect pharmacokinetics (59, 60).
CD52-inhibiting mAbs
The membrane glycoprotein CD52 is expressed by lymphocytes, monocytes, subsets of dendritic cells and subsets of epithelial cells. Inhibiting this glycoprotein causes the depletion of lymphocytes and blood dendritic cells in vivo, making it a target for mAbs designed to treat graft versus host disease in bone marrow transplantation, relapsing multiple sclerosis and chronic lymphocytic leukaemia (113). The anti-CD52 mAb used in the clinic is alemtuzumab, which is a humanized mAb.
Alemtuzumab
Alemtuzumab is the first humanized mAb. The humanization was performed by removing rodent constant regions and grafting the complementarity-determining regions onto human framework regions. This process, however, seemed not enough to prevent ADA responses to occur. Studies reveal the presence of antibodies in 85% of cases within 24 months, even though these have no reported clinical significance (60, 61). It was mentioned that the development of these ADAs can become more problematic with increasing number of treatment cycles and will therefore need monitoring in the clinic, as they can persist for years (61).
HER2-inhibiting mAbs
The human epidermal growth factor receptor-2 (HER2) is a proto-oncogene, encoding a transmembrane tyrosine kinase receptor (114). Amplification of the HER2-gene and overexpression of this tyrosine kinase receptor is observed in 20-30% of women with breast cancer, which is associated with a worse prognosis. Amplification or overexpression of HER2 is believed to have a role in the pathogenesis of breast cancer (115). Trastuzumab and pertuzumab are anti-HER2 mAbs currently used in the clinic. The mAbs are used separately, but also in combination (62, 63, 65).
Immunogenicity of Trastuzumab
Trastuzumab, a humanized anti-HER2 mAb, inhibits the growth of HER2-overexpressing tumour cells. It provides a significant survival benefit on its own and in combination with chemotherapeutic agents (115). Studies show the relation between the number of administered doses and the percentage of ADA-positive patients up to 3 administered doses. Higher administered doses present a dose-dependent reduction in percentage of positive samples which could be due to a developed tolerance after initial administered doses. Only a low percentage (1%) of the patients show ADA responses, but these do have high titers of anti-trastuzumab antibodies. These ADAs do not impact the efficacy of the drug (62–64).
Immunogenicity of Pertuzumab
Pertuzumab blocks the formation of the HER2-dimer and its receptor, by binding to HER2. It thereby inhibits the activation of the Pi3K-PKB/Akt signalling pathway Thereby, pertuzumab inhibits cancer cell activation, slows tumour growth and reduces the recurrence rate (65). However, a study shows that 30% of patients included in the trial develop an ADA response, 70 days after administration. The antibodies did not neutralize or affect pertuzumab (65).
EGFR-inhibiting mAbs
The epidermal growth factor receptor (EGFR) is a tyrosine kinase receptor that plays a role in the homeostatic regulation of normal cells and carcinogenesis of epithelial malignancies (116). There are currently four anti-EGFR mAbs approved for use in the clinic; cetuximab, panitumumab, nimotuzumab and necitumumab.
Immunogenicity of Cetuximab
Cetuximab is a human/mouse chimeric IgG1 mAb that blocks the interaction between EGFR and its ligands, by which the downstream RAS-signalling pathway and ERK-activation is inhibited (117). In studies assessing the immunogenicity, only non-neutralizing anti-cetuximab antibodies were found in 5% of the patients, indicating that there is no relation between these antibodies and the pharmacokinetics or efficacy and safety profile (66–69).
Immunogenicity of Panitumumab
Panitumumab is a fully human IgG2 antibody that, like cetuximab, binds the EGFR (117). Immunogenicity of panitumumab in combination therapy with cytostatics is similar to the immunogenicity observed in panitumumab monotherapy (1.8%). The antibodies formed do not have a clinical effect as these do not alter the pharmacokinetics or safety profile (70–72).
Immunogenicity of Nimotuzumab
Nimotuzumab is a humanized IgG1-mAb that binds the EGFR to inhibit the EGFR-pathway (73, 116). In studies testing the immunogenicity, no anti-nimotuzumab antibodies were detected (73).
Immunogenicity of Necitumumab
Necitumumab is a fully human IgG2 anti-EGFR antibody that stimulates natural killer (NK) cell-driven cytotoxicity against tumour cells via the interaction of its constant region and CD16 receptor on NK-cells (118). Clinical trials show neutralizing anti-necitumumab antibodies in 1.4% of the tested population (74).
Immune Checkpoint Inhibitor-Inhibiting mAbs
The cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) and programmed death 1 (PD-1) immune checkpoint pathways play key roles in the peripheral tolerance. CTLA-4 is often considered ‘leader’ of immune checkpoint inhibitors, due to its ability to prevent autoreactive T-cells at initial stage of naïve T-cell activation, primarily in lymph nodes. The previously activated T-cells are in a later stage of the immune response regulated by PD-1 (119–121). In cancer immunotherapy, tumour cells have developed ways to avoid recognition by T-cells, by which the cells can take advantage of peripheral tolerance (120).
Immunogenicity of Ipilimumab
Ipilimumab is a fully human IgG1 mAb. Neutralizing anti-ipilimumab antibodies have been found in 26% of the patients after administration of the drug. These neutralize drug target binding or enhance drug clearance, which both cause loss of treatment efficacy and suboptimal levels of active drugs. Also, several hypersensitivity reactions have been registered in relation to the ADA response. Studies even show shortened overall survival and shortened progression-free survival (75).
Immunogenicity of Pembrolizumab
Pembrolizumab is a humanized IgG4 mAb, targeting immune checkpoint PD-1. The incidence of ADA responses against pembrolizumab is low, as anti-pembrolizumab antibodies were found in 3.4% of the patients. The antibodies are non-neutralizing; this immunogenicity has no clinically relevant effects on exposure, safety or efficacy of the drug (76).
Immunogenicity of Nivolumab
Nivolumab is a fully human IgG4 mAb that targets the immune checkpoint PD-1. The incidence of ADA responses against nivolumab is low (12.7% of patients were ADA-positive), and the antibodies that are formed do not affect the pharmacokinetic profile or efficacy of the drug (73, 77, 78).
Immunogenicity of Atezolizumab
Atezolizumab is a humanized IgG1 mAb that binds programmed death-ligand 1 (PD-L1), thereby inhibiting interactions between PD-1 and CD80 receptors. The drug shows high variability of ADA response rates, ranging from 13 to 54%. The antibodies formed, however, did not show impact on the pharmacokinetic profile and efficacy of the drug (79).
Immunogenicity of Avelumab
Avelumab is a fully human IgG1 mAb which targets PD-L1. An ADA response was observed in 14 to 19% of the tested patients after 2.5 months. The presence of the antibodies, however, had no influence on pharmacokinetics or safety of the drug (80, 81).
Interleukin-targeted mAbs
Immunogenicity of Tocilizumab
Tocilizumab is a humanized IgG1 mAb that blocks the binding between IL-6 and IL-6R, by which it inhibits IL-6 activity. Studies show low immunogenicity of the drug (<2% of patients), with the antibodies present not affecting pharmacokinetics or safety profiles (82, 122).
Immunogenicity of Ustekinumab
Ustekinumab is a human IgG1 mAb that targets the shared p40 subunit of IL-12 and IL-23. ADA responses occur in 5% of patients, of which the majority consist of neutralizing antibodies which affect the effectivity of the drug (83, 84).
Immunogenicity of Secukinumab
Secukinumab is a fully human IgG1 mAb that targets IL-17. ADA responses were seen in <1% of patients, and was not linked to immunogenicity-related adverse effects and therefore there is no clinical effect on the efficacy and/or the pharmacokinetics of the drug (85).
Immunogenicity of Ixekizumab
Ixekizumab is a humanized mAb targeted against IL-17A. In clinical trials, 17% of patients receiving the drug developed non-ntADAs where the presence of the anti-ixekizumab antibodies did not interfere with serum concentrations and drug efficacy (86, 87).
IgE-inhibiting mAbs
IgE antibodies are known for their effector functions and their function in mediating allergic reactions. This effector function is an interesting target for mAb (123).
Immunogenicity of Omalizumab
Omalizumab is an anti-IgE mAb which inhibits the effector function of IgE, as well as the activation of mast cells and basophils (124). No antibodies against omalizumab are detected (88).
VLA4-inhibiting mAbs
Very late active antigen (VLA)-4 functions as receptor for the vascular cellular adhesion molecule 1 (VCAM-1) (93).
Immunogenicity of Natalizumab
Natalizumab is a IgG4 mAb targeting VLA-4. The drug is prescribed in multiple sclerosis-patients. Here, it effectively reduces the disease activity and decreases the risk of disability progression (94). In 4 to 14% of the patients, anti-natalizumab antibodies are found. The presence of these antibodies is associated with an increased risk for infusion-related adverse effects, but it also causes a reduced clinical effect (93–95).
HLA Haplotypes as biomarker to Predict the Occurrence of ADA Responses in Vulnerable Individuals
Our review indicates that there is no clear path to say if and which ADA responses will occur based on the structure of the mAb. The summarising Table 1 clearly shows an overview of the ADA responses against commonly used mAbs in the clinic. The range of frequencies of ADAs reported are due to various factors, few of them being the lack of standardized tests to detect ADAs, different patient populations used in the studies, different diseases present in the studied populations and so on. This is especially true for the older molecules like infliximab, adalimumab and rituximab, but is also an important note for the ‘newer’ molecules.
Recently, studies have focused on the possibility to predict the occurrence of ADA responses in individual patients. There is evidence that patients carrying specific HLA haplotypes are more vulnerable to form ADAs and this may be useful to personalize mAb treatment (125–129).
The human leukocyte antigen (HLA) system is involved in rejecting foreign entities that enter the body. They encode highly polymorphic cell surface molecules (130). Due to their role in the immune response against foreign entities, HLA haplotypes are believed to play an important role in the formation of ADA responses. For example, the relation between anti-infliximab antibodies and the HLA-DRB1 alleles has been studied, as well as the relation between anti-adalimumab antibodies and HLA-DRB1*03 (128).
Another study mentions the link between genetic variations in HLA and a higher risk of anti-TNFα antagonist antibodies. A direct causal link has not been found, but the results suggest that polymorphisms in HLA-DQA1*05 not only cause immunogenicity against the drug, but also a lower efficacy and affected pharmacokinetic profile. A single nucleotide variation (rs2097432) that shows increased risk of immunogenicity against adalimumab and infliximab have been described (131–133). In a study covering this variation, the allele frequency of rs2097432 was 25.5%. The risk of developing ADA responses was higher in the variant carriers (adjusted HR = 7.29). The carriers of this variant also had an increased risk of loss of response (adjusted HR = 2.34) and discontinuation (adjusted HR = 2.27) (132).
The possibility for these HLA-haplotypes to predict the occurrence of ADA responses was also tested for rituximab and atezolizumab (134, 135). Here, the associated alleles were HLA-DRB1*01:01 for all ADA (p = 3.4*10−5, odds ratio = 1.96), and HLA-DQA1*01:01 when considering ntADA (p = 2.8 x 10−7, odds ratio = 2.31). These alleles both occur in common HLA haplotypes. The study indicates that the HLA class II genotype plays a role in the development of anti-atezolizumab antibodies. However, the study also suggests that these genetic factors are yet insufficient as clinically meaningful predictors of ADA responses (135).
The explorative studies on the relation of the HLA haplotypes and variants need validation before they can be used as reliable biomarker in the clinic. This is, however, by far not enough information to base all ADA responses against all mAbs upon, as it is unlikely that there is one general biomarker to predict ADA formation for all different mAbs. Therefore, future research should be focused on the possibility to use these variants in HLA haplotypes as biomarker in the clinic, to prevent the immunogenicity against the mAb and thereby increase the benefit patients can experience from the drugs.
Future Perspectives on the Use of HLA Haplotypes to Predict Vulnerable Patients
In conclusion, the occurrence of antidrug antibody responses against mAb is a serious clinical concern. However, despite many mAbs displaying these ADA responses, it is inappropriate to directly compare the incidence rates of these responses across molecules. The immunogenicity incidence rates are highly dependend on the type of assay that is used to identify the ADA response, the dosing regimen that is prescribed, the time points on which the samples for the ADA-assays are taken, the route of administration of the mAb, the state of the underlying disease, the use of concurrent immunosuppressive treatment and many other factors. Therefore, the direct comparison of immunogenicity rates across the biologicals described in this paper is difficult.
Additionally, it is currently not possible to predict the occurrence of ADA responses, neither based upon mAb related factors nor on patient related factors. While not being the only plausible risk factor for ADA formation, studies that explore the possibility to predict ADA formation suggest the involvement of genetic variants in HLA haplotypes. For some drugs and in some diseases, the possibility to use these genetic variants as biomarkers has been studied, but for the majority of mAbs this is yet unexplored.
In the future, HLA haplotype identification might play an important role in the prevention of ADA responses, thereby not only improving the experience of the patients receiving drugs, but also minimizing hospitalization due to ADA responses and improving the treatment of patients as the correct drugs that do not elicit an ADA response can be prescribed quicker. This technique can not only be applied to MoAbs, but can also be explored to be helpful in the prediction of reactions of the immune system against other classes of drugs.
Author Contributions
RM wrote the review under supervision of H-JG. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The author, RM, would like to thank fellow-author HJG for his supervision during the master’s research project that resulted in writing this review. RM would also like to thank the staff of the Walaeus Library in the Leiden University Medical Center for their help in conducting a search strategy based on MeSH-terms to use in the PubMed database.
References
1. Buss NA, Henderson SJ, Mcfarlane M, Shenton JM, De Haan L. Monoclonal Antibody Therapeutics: History and Future Introduction-A Brief History of Therapeutic Monoclonal Antibodies. Curr Opin Pharmacol (2012). doi: 10.1016/j.coph.2012.08.001
2. Vaisman-Mentesh A, Rosenstein S, Yavzori M, Dror Y, Fudim E, Ungar B, et al. Molecular Landscape of Anti-Drug Antibodies Reveals the Mechanism of the Immune Response Following Treatment With Tnfα Antagonists. Front Immunol (2019) 10:2921/BIBTEX. doi: 10.3389/FIMMU.2019.02921/BIBTEX
3. Ober RJ, Radu CG, Ghetie V, Ward ES. Differences in Promiscuity for Antibody-FcRn Interactions Across Species: Implications for Therapeutic Antibodies. Int Immunol (2001) 13:1551–9. doi: 10.1093/intimm/13.12.1551
4. Grilo AL, Mantalaris A. The Increasingly Human and Profitable Monoclonal Antibody Market. Trends Biotechnol (2019) 37:9–16. doi: 10.1016/J.TIBTECH.2018.05.014
5. Hansel TT, Kropshofer H, Singer T, Mitchell JA, George AJT. The Safety and Side Effects of Monoclonal Antibodies. Nat Rev Drug Discovery (2010) 9:325–38. doi: 10.1038/nrd3003
6. De Groot AS, Scott DW. Immunogenicity of Protein Therapeutics. Trends Immunol (2007) 28:482–90. doi: 10.1016/j.it.2007.07.011
7. Lynch DH, Yang XD. Therapeutic Potential of ABX-EGF: A Fully Human Anti-Epidermal Growth Factor Receptor Monoclonal Antibody for Cancer Treatment. Semin Oncol (2002) 29:47–50. doi: 10.1053/SONC.2002.31522
8. Bloem K. BH-B-T Drug, 2017 U. Immunogenicity of Therapeutic Antibodies: Monitoring Antidrug Antibodies in a Clinical Context. Ther Drug Monit (2017) 39:1.
9. Nelson A, Dhimolea E. Discovery JR-N Reviews Drug, 2010 U. Development Trends for Human Monoclonal Antibody Therapeutics. Nat Rev Drug Discovery (2010).
10. Ben-Horin S, Heap GA, Ahmad T, Kim H, Kwon T, Chowers Y. The Immunogenicity of Biosimilar Infliximab: Can We Extrapolate the Data Across Indications? Taylor Fr (2015) 9:27–34. doi: 10.1586/17474124.2015.1091307
11. Vaisman-Mentesh A, Gutierrez-Gonzalez M, DeKosky BJ, Wine Y. The Molecular Mechanisms That Underlie the Immune Biology of Anti-Drug Antibody Formation Following Treatment With Monoclonal Antibodies. Front Immunol (2020) 11:1951/BIBTEX. doi: 10.3389/FIMMU.2020.01951/BIBTEX
12. Kuus-Reichel K, Grauer LS, Karavodin LM, Knott C, Krusemeier M, Kay NE. Will Immunogenicity Limit the Use, Efficacy, and Future Development of Therapeutic Monoclonal Antibodies? Clin Diagn Lab Immunol (1994) 1:365–72. doi: 10.1128/CDLI.1.4.365-372.1994
13. Koren E, Zuckerman L, Mire-Sluis A. Immune Responses to Therapeutic Proteins in Humans–Clinical Significance, Assessment and Prediction. Curr Pharm Biotechnol (2002) 3:349–60. doi: 10.2174/1389201023378175
14. Schellekens H, Casadevall N. Immunogenicity of Recombinant Human Proteins: Causes and Consequences. J Neurol (2004) 251 Suppl 2. doi: 10.1007/S00415-004-1202-9
15. Mayer L, Young Y. Infusion Reactions and Their Management. Gastroenterol Clin North Am (2006) 35:857–66. doi: 10.1016/J.GTC.2006.09.006
16. Scheinfeld N. A Comprehensive Review and Evaluation of the Side Effects of the Tumor Necrosis Factor Alpha Blockers Etanercept, Infliximab and Adalimumab. J Dermatolog Treat (2004) 15:280–94. doi: 10.1080/09546630410017275
17. Korswagen LA, Bartelds GM, Krieckaert CLM, Turkstra F, Nurmohamed MT, Van Schaardenburg D, et al. Venous and Arterial Thromboembolic Events in Adalimumab-Treated Patients With Antiadalimumab Antibodies: A Case Series and Cohort Study. Arthritis Rheum (2011) 63:877–83. doi: 10.1002/ART.30209
18. Bartelds GM, Wijbrandts CA, Nurmohamed MT, Stapel S, Lems WF, Aarden L, et al. Clinical Response to Adalimumab: Relationship to Anti-Adalimumab Antibodies and Serum Adalimumab Concentrations in Rheumatoid Arthritis. Ann Rheum Dis (2007) 66:921–6. doi: 10.1136/ARD.2006.065615
19. Wolbink GJ, Aarden LA, Dijkmans BAC. Dealing With Immunogenicity of Biologicals: Assessment and Clinical Relevance. Curr Opin Rheumatol (2009) 21:211–5. doi: 10.1097/BOR.0B013E328329ED8B
20. Yanai H, Hanauer SB. Assessing Response and Loss of Response to Biological Therapies in IBD. Am J Gastroenterol (2011) 106:685–98. doi: 10.1038/ajg.2011.103
21. Schutgens REG. Rituximab-Induced Serum Sickness. Br J Haematol (2006) 135:147. doi: 10.1111/j.1365-2141.2006.06214.x
22. Bendtzen K. Immunogenicity of Anti-TNF-α Biotherapies: II. Clinical Relevance of Methods Used for Anti-Drug Antibody Detection. Front Immunol (2015) 6:109/FULL. doi: 10.3389/FIMMU.2015.00109/FULL
23. Garcês S, Demengeot J. The Immunogenicity of Biologic Therapies. Curr Probl Dermatol (2018) 53:37–48. doi: 10.1159/000478077
24. Shankar G, Arkin S, Cocea L, Devanarayan V, Kirshner S, Kromminga A, et al. Assessment and Reporting of the Clinical Immunogenicity of Therapeutic Proteins and Peptides—Harmonized Terminology and Tactical Recommendations. AAPS J (2014) 16:658. doi: 10.1208/S12248-014-9599-2
25. Gunn GR, Sealey DCF, Jamali F, Meibohm B, Ghosh S, Shankar G. From the Bench to Clinical Practice: Understanding the Challenges and Uncertainties in Immunogenicity Testing for Biopharmaceuticals. Clin Exp Immunol (2016) 184:137. doi: 10.1111/CEI.12742
26. Deehan M, Garcês S, Kramer D, Baker MP, Rat D, Roettger Y, et al. Managing Unwanted Immunogenicity of Biologicals. Autoimmun Rev (2015) 14:569–74. doi: 10.1016/J.AUTREV.2015.02.007
27. Garcês S, Demengeot J, Benito-Garcia E. The Immunogenicity of Anti-TNF Therapy in Immune-Mediated Inflammatory Diseases: A Systematic Review of the Literature With a Meta-Analysis. Ann Rheum Dis (2013) 72:1947–55. doi: 10.1136/annrheumdis-2012-202220
28. Of H, Of H, Information P, Information P. Highlights of Prescribing Information. Metab Clin Exp (2008).
29. Gehin JE, Syversen SW, Warren DJ, Goll GL, Sexton J, Bolstad N. Serum Etanercept Concentrations in Relation to Disease Activity and Treatment Response Assessed by Ultrasound, Biomarkers and Clinical Disease Activity Scores: Results From a Prospective Observational Study of Patients With Rheumatoid Arthritis. Open (2021) 7:1985. doi: 10.1136/rmdopen-2021-001985
30. Yi H, Kim J, Jung H, Rim YA, Kim Y, Jung SM, et al. Induced Production of Anti-Etanercept Antibody in Collagen-Induced Arthritis. Mol Med Rep (2014) 9:2301–8. doi: 10.3892/mmr.2014.2127
31. Hoshino M, Yoshio T, Onishi S, Minota S. Influence of Antibodies Against Infliximab and Etanercept on the Treatment Effectiveness of These Agents in Japanese Patients With Rheumatoid Arthritis. Mod Rheumatol (2012) 22:532–40. doi: 10.1007/s10165-011-0567-8
32. Roblin X, Marotte H, Leclerc M, Del Tedesco E, Phelip JM, Peyrin-Biroulet L, et al. Combination of C-Reactive Protein, Infliximab Trough Levels, and Stable But Not Transient Antibodies to Infliximab Are Associated With Loss of Response to Infliximab in Inflammatory Bowel Disease. J Crohn’s Colitis. doi: 10.1093/ecco-jcc/jjv061
34. Barlow NL, Mohammed P, Berg JD. Serum Trough Infliximab and Anti-Infliximab Antibodies in a Cohort of Gastroenterology and Rheumatology Patients’ Infliximab Therapeutic Drug Monitoring. Ann Clin Biochem (2016) 53:477–84. doi: 10.1177/0004563215604866
35. Gomes LEM, Da Silva FAR, Pascoal LB, Ricci RL, Nogueira G, Camargo MG, et al. Serum Levels of Infliximab and Anti-Infliximab Antibodies in Brazilian Patients With Crohn’s Disease. Clinics (Sao Paulo) (2019) 74. doi: 10.6061/CLINICS/2019/E824
36. Raffáč Š, Gombošová L, Gabzdilová J, Novotná L, Šajtyová K, Pekárová T, et al. Detection of Anti-Infliximab Antibodies in Slovak IBD Patients and its Costs Saving Effect. Neuro Endocrinol Lett (2017) 38:5–9.
37. Subedi S, Gong Y, Chen Y, Shi Y. Infliximab and Biosimilar Infliximab in Psoriasis: Efficacy, Loss of Efficacy, and Adverse Events. (2019). doi: 10.2147/DDDT.S200147
38. Ogrič M, Poljšak KM, Lakota K, Žigon P, Praprotnik S, Semrl SSS, et al. Neutralizing Effects of Anti-Infliximab Antibodies on Synergistically-Stimulated Human Coronary Artery Endothelial Cells. Atherosclerosis (2019) 291:1–8. doi: 10.1016/J.ATHEROSCLEROSIS.2019.09.010
40. Hoxha A, Calligaro A, Tonello M, Ramonda R, Carletto A, Paolazzi G, et al. The Clinical Relevance of Early Anti-Adalimumab Antibodies Detection in Rheumatoid Arthritis, Ankylosing Spondylitis and Psoriatic Arthritis: A Prospective Multicentre Study. Jt Bone Spine (2016) 83:167–71. doi: 10.1016/J.JBSPIN.2015.04.020
41. Grossi V, Infantino M, Manfredi M, Basile U, Gulli F, Marino M, et al. Anti-Adalimumab and Anti-Certolizumab Antibodies Titers After Discontinuation of Adalimumab: Two Case Reports. Clin Chem Lab Med (2020) 58:E105–8. doi: 10.1515/CCLM-2019-0950
42. Ulijn E, Den Broeder N, Den Broeder N, Wientjes M, Van Herwaarden N, Van Herwaarden N, et al. Therapeutic Drug Monitoring of Adalimumab in RA: No Predictive Value of Adalimumab Serum Levels and Anti-Adalimumab Antibodies for Prediction of Response to the Next bDMARD. Ann Rheum Dis (2020) 79:867–73. doi: 10.1136/ANNRHEUMDIS-2020-216996
43. Van Schouwenburg PA, Kruithof S, Votsmeier C, Van Schie K, Hart MH, De Jong RN, et al. Functional Analysis of the Anti-Adalimumab Response Using Patient-Derived Monoclonal Antibodies. J Biol Chem (2014) 289:34482–8. doi: 10.1074/JBC.M114.615500
44. Brunelli JB, Silva CA, Pasoto SG, Saa CGS, Kozu KT, Goldenstein-Schainberg C, et al. Anti-Adalimumab Antibodies Kinetics: An Early Guide for Juvenile Idiopathic Arthritis (JIA) Switching. Clin Rheumatol (2020) 39:515–21. doi: 10.1007/S10067-019-04798-6
45. Ogrič M, Terčelj M, Praprotnik S, Tomšič M, Božič B, Sodin-Semrl S, et al. Detection of Adalimumab and Anti-Adalimumab Antibodies in Patients With Rheumatoid Arthritis: A Comprehensive Overview of Methodology Pitfalls and Benefits. Immunol Res (2017) 65:172–85. doi: 10.1007/S12026-016-8824-8
46. Wolbink GJ, Vis M, Lems W, Voskuyl AE, De Groot E, Nurmohamed MT, et al. Development of Antiinfliximab Antibodies and Relationship to Clinical Response in Patients With Rheumatoid Arthritis. Arthritis Rheum (2006) 54:711–5. doi: 10.1002/ART.21671
47. De Vries MK, Wolbink GJ, Stapel SO, De Vrieze H, Van Denderen JC, Dijkmans BAC, et al. Decreased Clinical Response to Infliximab in Ankylosing Spondylitis is Correlated With Anti-Infliximab Formation. Ann Rheum Dis (2007) 66:1252–4. doi: 10.1136/ARD.2007.072397
48. Pascual-Salcedo D, Plasencia C, Ramiro S, Nuño L, Bonilla G, Nagore D, et al. Influence of Immunogenicity on the Efficacy of Long-Term Treatment With Infliximab in Rheumatoid Arthritis. Rheumatol (Oxford) (2011) 50:1445–52. doi: 10.1093/RHEUMATOLOGY/KER124
49. Presta LG, Chen H, O’Connor SJ, Chisholm V, Meng YG, Krummen L, et al. Humanization of an Anti-Vascular Endothelial Growth Factor Monoclonal Antibody for the Therapy of Solid Tumors and Other Disorders. Cancer Res (1997) 57:4593–9.
50. Hsu JY, Wakelee HA. Monoclonal Antibodies Targeting Vascular Endothelial Growth Factor: Current Status and Future Challenges in Cancer Therapy. BioDrugs (2009) 23:289–304. doi: 10.2165/11317600-000000000-00000
51. Kong DH, Kim MR, Jang JH, Na HJ, Lee S. A Review of Anti-Angiogenic Targets for Monoclonal Antibody Cancer Therapy. Int J Mol Sci (2017) 18. doi: 10.3390/IJMS18081786
52. Braghiroli MI, Sabbaga J, Hoff PM. Bevacizumab: Overview of the Literature. Expert Rev Anticancer Ther (2012) 12:567–80. doi: 10.1586/era.12.13
53. Lien S, Lowman HB. Therapeutic Anti-VEGF Antibodies. Handb Exp Pharmacol (2008) 181:131–50. doi: 10.1007/978-3-540-73259-4_6
54. Barzelay A, Lowenstein A, George J, Barak A. Influence of non-Toxic Doses of Bevacizumab and Ranibizumab on Endothelial Functions and Inhibition of Angiogenesis. Curr Eye Res (2010) 35:835–41. doi: 10.3109/02713683.2010.489727
55. Spratlin J. Ramucirumab (IMC-1121b): Monoclonal Antibody Inhibition of Vascular Endothelial Growth Factor Receptor-2. Curr Oncol Rep (2011) 13:97–102. doi: 10.1007/S11912-010-0149-5
56. Spratlin JL, Mulder KE, MacKey JR. Ramucirumab (IMC-1121B): A Novel Attack on Angiogenesis. Future Oncol (2010) 6:1085–94. doi: 10.2217/FON.10.75
57. Ferrara N, Hillan KJ, Novotny W. Bevacizumab (Avastin), a Humanized Anti-VEGF Monoclonal Antibody for Cancer Therapy. (2005). doi: 10.1016/j.bbrc.2005.05.132
58. Gordon MS, Margolin K, Talpaz M, Sledge J, Holmgren E, Benjamin R, et al. Phase I Safety and Pharmacokinetic Study of Recombinant Human Anti-Vascular Endothelial Growth Factor in Patients With Advanced Cancer. J Clin Oncol (2001) 19:843–50. doi: 10.1200/JCO.2001.19.3.843
59. Nissim A, Chernajovsky Y. Historical Development of Monoclonal Antibody Therapeutics. Handb Exp Pharmacol (2008) 181:3–18. doi: 10.1007/978-3-540-73259-4_1
60. Kuhn C, Weiner HL. Therapeutic Anti-CD3 Monoclonal Antibodies: From Bench to Bedside. Immunotherapy (2016) 8:889–906. doi: 10.2217/IMT-2016-0049/ASSET/IMAGES/LARGE/FIGURE3.JPEG
61. Lobo PI, Patel HC. Murine Monoclonal IgG Antibodies: Differences in Their IgG Isotypes can Affect the Antibody Effector Activity When Using Human Cells. Immunol Cell Biol (1997) 75:267–74. doi: 10.1038/ICB.1997.41
62. Sgro C. Side-Effects of a Monoclonal Antibody, Muromonab CD3/orthoclone OKT3: Bibliographic Review. Toxicology (1995) 105:23–9. doi: 10.1016/0300-483X(95)03123-W
63. Chatenoud L, Baudrihaye MF, Chkoff N, Kreis H, Goldstein G, Bach JF. Restriction of the Human In Vivo Immune Response Against the Mouse Monoclonal Antibody OKT3. J Immunol (1986) 137.
64. Ilan Y, Zigmond E, Lalazar G, Dembinsky A, Ben Ya’Acov A, Hemed N, et al. Oral Administration of OKT3 Monoclonal Antibody to Human Subjects Induces a Dose-Dependent Immunologic Effect in T Cells and Dendritic Cells. J Clin Immunol 2009 301 (2009) 30:167–77. doi: 10.1007/S10875-009-9323-7
65. Dean Y, Dépis F, Kosco-Vilbois M. Combination Therapies in the Context of Anti-CD3 Antibodies for the Treatment of Autoimmune Diseases. Swiss Med Wkly 2012 47 (2012) 142. doi: 10.4414/SMW.2012.13711
66. Halota W, Ferenci P, Kozielewicz D, Dybowska D, Lisovoder N, Samira S, et al. Oral Anti-CD3 Immunotherapy for HCV-Nonresponders is Safe, Promotes Regulatory T Cells and Decreases Viral Load and Liver Enzyme Levels: Results of a Phase-2a Placebo-Controlled Trial. J Viral Hepat (2015) 22:651–7. doi: 10.1111/jvh.12369
67. Lalazar G, Mizrahi M, Turgeman I, Adar T, Ben Ya’acov A, Shabat Y, et al. Oral Administration of OKT3 MAb to Patients With NASH, Promotes Regulatory T-Cell Induction, and Alleviates Insulin Resistance: Results of a Phase IIa Blinded Placebo-Controlled Trial. J Clin Immunol 2015 354 (2015) 35:399–407. doi: 10.1007/S10875-015-0160-6
68. Du FH, Mills EA, Mao-Draayer Y. Next-Generation Anti-CD20 Monoclonal Antibodies in Autoimmune Disease Treatment. (2017) 8:12. doi: 10.1007/s13317-017-0100-y
69. Van Vollenhoven RF, Emery P, Bingham Iii CO, Keystone EC, Fleischmann R, Furst DE, et al. Personal non-Commercial Use Only. J Rheumatol (2010) 37:558–67. doi: 10.3899/jrheum.090856
70. Hauser SL, Waubant E, Arnold DL, Vollmer T, Antel J, Fox RJ, et al. B-Cell Depletion With Rituximab in Relapsing–Remitting Multiple Sclerosis. (2009) 358:676–88. doi: 10.1056/NEJMOA0706383
71. Tahir H, Rohrer J, Bhatia A, Wegener WA, Isenberg DA. Humanized Anti-CD20 Monoclonal Antibody in the Treatment of Severe Resistant Systemic Lupus Erythematosus in a Patient With Antibodies Against Rituximab [4]. Rheumatology (2005) 44:561–2. doi: 10.1093/rheumatology/keh533
72. Sorensen PS, Lisby S, Grove R, Derosier F, Shackelford S, Havrdova E, et al. Safety and Efficacy of Ofatumumab in Relapsing-Remitting Multiple Sclerosis. Neurology (2014) 82:573–81. doi: 10.1212/WNL.0000000000000125
73. Husar E, Solonets M, Kuhlmann O, Schick E, Piper-Lepoutre H, Singer T, et al. Hypersensitivity Reactions to Obinutuzumab in Cynomolgus Monkeys and Relevance to Humans. Toxicol Pathol (2017) 45:676–86. doi: 10.1177/0192623317723539
74. Emery P, Rigby W, Tak PP, Dö Rner T, Olech E, Martin C, et al. Safety With Ocrelizumab in Rheumatoid Arthritis: Results From the Ocrelizumab Phase III Program. doi: 10.1371/journal.pone.0087379
75. Ellebrecht CT, Choi EJ, Allman DM, Tsai DE, Wegener WA, Goldenberg DM, et al. Subcutaneous Veltuzumab, a Humanized Anti-CD20 Antibody, in the Treatment of Refractory Pemphigus Vulgaris. JAMA Dermatol (2014) 150:1331–5. doi: 10.1001/JAMADERMATOL.2014.1939
76. Bannas P, Koch-Nolte F, Malavasi F. Perspectives for the Development of CD38-Specific Heavy Chain Antibodies as Therapeutics for Multiple Myeloma. (2018). doi: 10.3389/fimmu.2018.02559
77. Usmani SZ, Nahi H, Mateos M-V, Van De Donk NWCJ, Chari A, Kaufman JL, et al. Subcutaneous Delivery of Daratumumab in Relapsed or Refractory Multiple Myeloma. (2019).
78. Lundin J, Kimby E, Björkholm M, Broliden PA, Celsing F, Hjalmar V, et al. Phase II Trial of Subcutaneous Anti-CD52 Monoclonal Antibody Alemtuzumab (Campath-1H) as First-Line Treatment for Patients With B-Cell Chronic Lymphocytic Leukemia (B-CLL). Blood (2002) 100:768–73. doi: 10.1182/blood-2002-01-0159
79. Jordan MB, Mcclain KL, Yan X, Hicks J, Jaffe R. Anti-CD52 Antibody, Alemtuzumab, Binds to Langerhans Cells in Langerhans Cell Histiocytosis. Pediatr Blood Cancer (2005) 44:251–4. doi: 10.1002/pbc.20181
80. Chirmule N, Kang AS, Baker D, Ali L, Saxena G, Pryce G, et al. The Irony of Humanization: Alemtuzumab, the First, But One of the Most Immunogenic, Humanized Monoclonal Antibodies. Front Immunol | www.frontiersin.org (2020) 11:124. doi: 10.3389/fimmu.2020.00124
81. Yu S, Liu Q, Han X, Qin S, Zhao W, Li A. Development and Clinical Application of Anti-HER2 Monoclonal and Bispecific Antibodies for Cancer Treatment. Exp Hematol Oncol 2017 61 (2017) 6:1–15. doi: 10.1186/S40164-017-0091-4
82. Baselga J, Albanell J. Mechanism of Action of Anti-HER2 Monoclonal Antibodies. Ann Oncol Off J Eur Soc Med Oncol (2001) 12 Suppl 1. doi: 10.1093/ANNONC/12.SUPPL_1.S35
83. Kilany LAA, Gaber AAS, Aboulwafa MM, Zedan HH. Trastuzumab Immunogenicity Development in Patients’ Sera and in Laboratory Animals. BMC Immunol (2021) 22:1–15. doi: 10.1186/S12865-021-00405-Z/FIGURES/6
84. Cui Y, Cui D, Ren X, Chen X, Liu G, Liu Z. Pharmacokinetics, Immunogenicity and Safety Study for SHR-1309 Injection and Perjeta® in Healthy Chinese Male Volunteers. Front Pharmacol (2021) 12:660541/BIBTEX. doi: 10.3389/FPHAR.2021.660541/BIBTEX
85. Bordeau BM, Abuqayyas L, Nguyen TD, Chen P, Balthasar JP. Development and Evaluation of Competitive Inhibitors of Trastuzumab-HER2 Binding to Bypass the Binding-Site Barrier. Front Pharmacol (2022) 13:837744. doi: 10.3389/FPHAR.2022.837744
86. Padrón IM, García JG, Díaz RR, Lenza IC, Nicolás FG. Anti-Drug Antibodies Anti-Trastuzumab in the Treatment of Breast Cancer. J Oncol Pharm Pract (2021) 27:1354–6. doi: 10.1177/1078155220953873
87. Cai WQ, Zeng LS, Wang LF, Wang YY, Cheng JT, Zhang Y, et al. The Latest Battles Between EGFR Monoclonal Antibodies and Resistant Tumor Cells. Front Oncol (2020) 10:1249. doi: 10.3389/fonc.2020.01249
88. Pozzi C, Cuomo A, Spadoni I, Magni E, Silvola A, Conte A, et al. The EGFR-Specific Antibody Cetuximab Combined With Chemotherapy Triggers Immunogenic Cell Death. Nat Med (2016). doi: 10.1038/nm.4078
89. ERBITUX TM FDA. The EGFR-Specific Antibody Cetuximab Combined With Chemotherapy Triggers Immunogenic Cell Death. Nat Med (2016).
90. Dupont B, Mariotte D, Clarisse B, Galais MP, Bouhier-Leporrier K, Grellard JM, et al. Risk Factors Associated With Hypersensitivity Reactions to Cetuximab: Anti-Cetuximab IgE Detection as Screening Test. Futur Oncol (2014) 10:2133–40. doi: 10.2217/fon.14.153
91. Dupont B, Mariotte D, Dugué AE, Clarisse B, Grellard JM, Babin E, et al. Utility of Serum Anti-Cetuximab Immunoglobulin E Levels to Identify Patients at a High Risk of Severe Hypersensitivity Reaction to Cetuximab. Br J Clin Pharmacol (2017) 83:623–31. doi: 10.1111/bcp.13140
92. Lungulescu CV, Ungureanu BS, Turcu-Stiolica A, Ghimpau V, Artene SA, Cazacu IM, et al. The Role of IgE Specific for Galactose-α-1,3-Galactose in Predicting Cetuximab Induced Hypersensitivity Reaction: A Systematic Review and a Diagnostic Meta-Analysis. Sci Rep (2020) 10. doi: 10.1038/S41598-020-78497-7
93. Weeraratne D, Chen A, Pennucci JJ, Wu CY, Zhang K, Wright J, et al. Immunogenicity of Panitumumab in Combination Chemotherapy Clinical Trials. BMC Clin Pharmacol (2011) 11:17. doi: 10.1186/1472-6904-11-17
94. Kast J, Dutta S, Upreti VV. Panitumumab: A Review of Clinical Pharmacokinetic and Pharmacology Properties After Over a Decade of Experience in Patients With Solid Tumors. Adv Ther (2021) 38:3712–23. doi: 10.1007/S12325-021-01809-4
95. Peeters M, Balfourf J, Arnold D. Review Article: Panitumumab - A Fully Human Anti-EGFR Monoclonal Antibody for Treatment of Metastatic Colorectal Cancer. Aliment Pharmacol Ther (2008) 28:269–81. doi: 10.1111/j.1365-2036.2008.03717.x
96. Kato K, Ura T, Koizumi W, Iwasa S, Katada C, Azuma M, et al. Nimotuzumab Combined With Concurrent Chemoradiotherapy in Japanese Patients With Esophageal Cancer: A Phase I Study. Cancer Sci (2018) 109:785–93. doi: 10.1111/cas.13481
97. García-Foncillas J, Sunakawa Y, Aderka D, Wainberg Z, Ronga P, Witzler P, et al. Distinguishing Features of Cetuximab and Panitumumab in Colorectal Cancer and Other Solid Tumors. Front Oncol (2019) 9:849. doi: 10.3389/fonc.2019.00849
99. Fife BT, Bluestone JA. Control of Peripheral T-Cell Tolerance and Autoimmunity. via CTLA-4 PD-1 pathways. Immunol Rev (2008) 224:166–82. doi: 10.1111/J.1600-065X.2008.00662.X
100. Buchbinder EI, Desai A. CTLA-4 and PD-1 Pathways Similarities, Differences, and Implications of Their Inhibition. Am J Clin Oncol Cancer Clin Trials (2016) 39:98–106. doi: 10.1097/COC.0000000000000239
101. Krummel MF, Allison JP. CD28 and CTLA-4 Have Opposing Effects on the Response of T Cells to Stimulation. J Exp Med (1995) 182:459–65. doi: 10.1084/JEM.182.2.459
102. Kverneland AH, Enevold C, Donia M, Bastholt L, Svane IM, Nielsen CH. Development of Anti-Drug Antibodies is Associated With Shortened Survival in Patients With Metastatic Melanoma Treated With Ipilimumab. Oncoimmunology (2018) 7. doi: 10.1080/2162402X.2018.1424674
103. Kverneland AH, Enevold C, Donia M, Bastholt L, Svane IM, Nielsen CH. Development of Anti-Drug Antibodies is Associated With Shortened Survival in Patients With Metastatic Melanoma Treated With Ipilimumab. Oncoimmunology (2018) 7. doi: 10.1080/2162402X.2018.1424674
104. Van Vugt MJH, Stone JA, De Greef RHJMM, Snyder ES, Lipka L, Turner DC, et al. Immunogenicity of Pembrolizumab in Patients With Advanced Tumors. J Immunother Cancer (2019) 7. doi: 10.1186/s40425-019-0663-4
105. Agrawal S, Statkevich P, Bajaj G, Feng Y, Saeger S, Desai DD, et al. Evaluation of Immunogenicity of Nivolumab Monotherapy and Its Clinical Relevance in Patients With Metastatic Solid Tumors. Ther J Clin Pharmacol (2017) 57:394–400. doi: 10.1002/jcph.818
106. Kato R, Ikarashi D, Matsuura T, Maekawa S, Kato Y, Kanehira M, et al. Analyses of Nivolumab Exposure and Clinical Safety Between 3-Mg/Kg Dosing and 240-Mg Flat Dosing in Asian Patients With Advanced Renal Cell Carcinoma in the Real-World Clinical Setting. Transl Oncol (2020) 13. doi: 10.1016/J.TRANON.2020.100771
107. Wu B, Sternheim N, Agarwal P, Suchomel J, Vadhavkar S, Bruno R, et al. Evaluation of Atezolizumab Immunogenicity: Clinical Pharmacology (Part 1). Clin Transl Sci (2022) 130:130–40. doi: 10.1111/cts.13127
109. Marciscano AE, Gulley JL, Kaufman HL. Avelumab: Is it Time to Get Excited? Expert Rev Anticancer Ther (2018) 18:815–21. doi: 10.1080/14737140.2018.1493380
110. Burmester GR, Choy E, Kivitz A, Ogata A, Bao M, Nomura A, et al. Low Immunogenicity of Tocilizumab in Patients With Rheumatoid Arthritis. Ann Rheum Dis (2017) 76:1078–85. doi: 10.1136/annrheumdis-2016-210297
111. Uchiyama Y, Yorozu K, Hashizume M, Moriya Y, Mihara M. Tocilizumab, a Humanized Anti-Interleukin-6 Receptor Antibody, Ameliorates Joint Swelling in Established Monkey Collagen-Induced Arthritis. Biol Pharm Bull (2008) 31:1159–63. doi: 10.1248/BPB.31.1159
112. Chiu HY, Chu TW, Cheng YP, Tsai TF. The Association Between Clinical Response to Ustekinumab and Immunogenicity to Ustekinumab and Prior Adalimumab. PloS One (2015) 10. doi: 10.1371/journal.pone.0142930
113. De Keyser E, Busard CI, Lanssens S, Meuleman L, Hutten BA, Costanzo A, et al. Clinical Consequences of Antibody Formation, Serum Concentrations, and HLA-Cw6 Status in Psoriasis Patients on Ustekinumab. Ther Drug Monit (2019) 41:634–9. doi: 10.1097/FTD.0000000000000646
114. Deodhar A, Gladman DD, Mcinnes IB, Spindeldreher S, Martin R, Pricop L, et al. Secukinumab Immunogenicity Over 52 Weeks in Patients With Psoriatic Arthritis and Ankylosing Spondylitis. J Rheumatol J Rheumatol (2020) 47:539–86. doi: 10.3899/jrheum.190116
115. Reich K, Jackson K, Ball S, Garces S, Kerr L, Chua L, et al. Ixekizumab Pharmacokinetics, Anti-Drug Antibodies, and Efficacy Through 60 Weeks of Treatment of Moderate to Severe Plaque Psoriasis. J Invest Dermatol (2018) 138:2168–73. doi: 10.1016/J.JID.2018.04.019
116. Muram TM, Sloan JH, Chain JS, Komocsar WJ, Meiklejohn BI, Blauvelt A, et al. Konrad RJ. A Highly Sensitive and Drug-Tolerant Anti-Drug Antibody Screening Assay for Ixekizumab Using Affinity Capture Elution. J Invest Dermatol (2016) 136:1513–5. doi: 10.1016/J.JID.2016.01.040
117. Sutton B, Davies A, Bax H, Karagiannis S. IgE Antibodies: From Structure to Function and Clinical Translation. Antibodies (2019) 8:19. doi: 10.3390/antib8010019
118. D’Amato G, Stanziola A, Sanduzzi A, Liccardi G, Salzillo A, Vitale C, et al. Treating Severe Allergic Asthma With Anti-IgE Monoclonal Antibody (Omalizumab): A Review. Multidiscip Respir Med (2014) 9:1–6. doi: 10.1186/2049-6958-9-23/METRICS
119. D’Amato G, Salzillo A, Piccolo A, D’Amato M. Liccardi G. A Review of Anti-IgE Monoclonal Antibody (Omalizumab) as Add on Therapy for Severe Allergic (IgE-Mediated) Asthma. Ther Clin Risk Manag (2007) 3:613.
120. Vennegoor A, Rispens T, Strijbis EMM, Seewann A, Uitdehaag BMJ, Balk LJ, et al. Clinical Relevance of Serum Natalizumab Concentration and Anti-Natalizumab Antibodies in Multiple Sclerosis. Mult Scler J (2013) 19:593–600. doi: 10.1177/1352458512460604
121. Lundkvist M, Engdahl E, Holmén C, Movérare R, Olsson T, Hillert J, et al. Characterization of Anti-Natalizumab Antibodies in Multiple Sclerosis Patients. Mult Scler J (2013) 19:757–64. doi: 10.1177/1352458512462920
122. Oliver B, Fernández Ó, Papais Alvarenga M, Jesú Pinto-Medel M, Guerrero M, Leó A, et al. Kinetics and Incidence of Anti-Natalizumab Antibodies in Multiple Sclerosis Patients on Treatment for 18 Months. . Short Rep Mult Scler J 17J:368–71. doi: 10.1177/1352458510385508
123. Nayak A, Casale T, David Miller S, Condemi J, McAlary M, Fowler-Taylor A, et al. Tolerability of Retreatment With Omalizumab, a Recombinant Humanized Monoclonal Anti-IgE Antibody, During a Second Ragweed Pollen Season in Patients With Seasonal Allergic Rhinitis. Allergy Asthma Proc (2003) 24:323–9.
124. Berger WE, Gupta N, McAlary M, Fowler-Taylor A. Evaluation of Long-Term Safety of the Anti-IgE Antibody, Omalizumab, in Children With Allergic Asthma. Ann Allergy Asthma Immunol (2003) 91:182–8. doi: 10.1016/S1081-1206(10)62175-8
125. Apsangikar P, Ghadge P, Naik M, Nair S. Randomized Comparative Clinical Study of First Global Omalizumab Biosimilar With Innovator Product in Moderate to Severe Persistent Asthma. J Assoc Physicians India (2020) 68:61–5.
126. Corren J, Casale TB, Lanier B, Buhl R, Holgate S, Jimenez P. Safety and Tolerability of Omalizumab. Clin Exp Allergy (2009) 39:788–97. doi: 10.1111/J.1365-2222.2009.03214.X
127. Thomas SS, Borazan N, Barroso N, Duan L, Taroumian S, Kretzmann B, et al. Comparative Immunogenicity of TNF Inhibitors: Impact on Clinical Efficacy and Tolerability in the Management of Autoimmune Diseases. A Systematic Review and Meta-Analysis. . BioDrugs (2015) 29:241–58. doi: 10.1007/s40259-015-0134-5
128. Schaeverbeke T, Truchetet ME, Kostine M, Barnetche T, Bannwarth B, Richez C. Immunogenicity of Biologic Agents in Rheumatoid Arthritis Patients: Lessons for Clinical Practice. Rheumatol (United Kingdom) (2015) 55:210–20. doi: 10.1093/rheumatology/kev277
129. Burmester GR, Durez P, Shestakova G, Genovese MC, Schulze-Koops H, Li Y, et al. Association of HLA-DRB1 Alleles With Clinical Responses to the Anti-Interleukin-17A Monoclonal Antibody Secukinumab in Active Rheumatoid Arthritis. Rheumatol (United Kingdom) (2016) 55:49–55. doi: 10.1093/rheumatology/kev258
130. Liu M, Degner J, Davis JW, Idler KB, Nader A, Mostafa NM, et al. Identification of HLA-DRB1 Association to Adalimumab Immunogenicity. PloS One (2018) 13. doi: 10.1371/journal.pone.0195325
131. Benucci M, Damiani A, Gobbi FL, Bandinelli F, Infantino M, Grossi V, et al. Correlation Between HLA Haplotypes and the Development of Antidrug Antibodies in a Cohort of Patients With Rheumatic Diseases. Biol Targets Ther (2018), 12–37. doi: 10.2147/BTT.S145941
132. Choo SY. The HLA System: Genetics, Immunology, Clinical Testing, and Clinical Implications. Yonsei Med J (2007) 48:11–23. doi: 10.3349/ymj.2007.48.1.11
133. Wilson A, Kim RB. Letter: Genetic Variation in the HLA-DQA1*05 Allele Predicts Tumour Necrosis Factor-α Antagonist Immunogenicity – Does Location Matter? Aliment Pharmacol Ther (2021) 53:1055–6. doi: 10.1111/apt.16338
134. Sazonovs A, Kennedy NA, Moutsianas L, Heap GA, Rice DL, Reppell M, et al. HLA-DQA1*05 Carriage Associated With Development of Anti-Drug Antibodies to Infliximab and Adalimumab in Patients With Crohn’s Disease. Gastroenterology (2020) 158:189–99. doi: 10.1053/J.GASTRO.2019.09.041
135. Wilson A, Peel C, Wang Q, Pananos AD, Kim RB. HLADQA1*05 Genotype Predicts Anti-Drug Antibody Formation and Loss of Response During Infliximab Therapy for Inflammatory Bowel Disease. Aliment Pharmacol Ther (2020) 51:356–63. doi: 10.1111/APT.15563
136. De Groot AS, Kazi ZB, Martin RF, Terry FE, Desai AK, Martin WD, et al. HLA- and Genotype-Based Risk Assessment Model to Identify Infantile Onset Pompe Disease Patients at High-Risk of Developing Significant Anti-Drug Antibodies (ADA). Clin Immunol (2019) 200:66–70. doi: 10.1016/J.CLIM.2019.01.009
Keywords: monoclonal antibodies, antidrug antibodies, human leukocyte antigen, HLA haplotypes, ntADA, biomarker
Citation: Mosch R and Guchelaar H-J (2022) Immunogenicity of Monoclonal Antibodies and the Potential Use of HLA Haplotypes to Predict Vulnerable Patients. Front. Immunol. 13:885672. doi: 10.3389/fimmu.2022.885672
Received: 01 March 2022; Accepted: 24 May 2022;
Published: 17 June 2022.
Edited by:
Cornelis Joseph Melief, Leiden University, NetherlandsReviewed by:
Anette Christine Karle, Novartis, SwitzerlandZuben E. Sauna, United States Food and Drug Administration, United States
Copyright © 2022 Mosch and Guchelaar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Henk-Jan Guchelaar, aC5qLmd1Y2hlbGFhckBsdW1jLm5s
 Romy Mosch
Romy Mosch Henk-Jan Guchelaar
Henk-Jan Guchelaar