- 1Department of Oral and Maxillofacial Surgery, Hospital of Stomatology, Jilin University, Changchun, China
- 2Laboratory Animal Center, College of Animal Science, Jilin University, Changchun, China
- 3Jilin Provincial Key Laboratory of Tooth Development and Bone Remodeling, Hospital of Stomatology, Jilin University, Changchun, China
Coronavirus disease 2019 (COVID-19) is a respiratory infectious disease that seriously threatens human life. The clinical manifestations of severe COVID-19 include acute respiratory distress syndrome and multiple organ failure. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causal agent of COVID-19, spreads through contaminated droplets. SARS-CoV-2 particles have been detected in the saliva of COVID-19 patients, implying that the virus can infect and damage the oral cavity. The oral manifestations of COVID-19 include xerostomia and gustatory dysfunction. Numerous studies showed that the four structural proteins of SARS-CoV-2 are its potential pathogenic factors, especially the S protein, which binds to human ACE2 receptors facilitating the entry of the virus into the host cells. Usually, upon entry into the host cell, a pathogen triggers the host’s immune response. However, a mount of multi-omics and immunological analyses revealed that COVID-19 is caused by immune dysregulation. A decrease in the number and phenotypes of immune cells, IFN-1 production and excessive release of certain cytokines have also been reported. In conclusion, this review summarizes the oral manifestations of COVID-19 and multi-omics analysis of SARS-CoV-2 infection.
1 Introduction
Coronavirus disease 2019 (COVID-19) is an ongoing pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (1, 2). SARS-CoV-2 is a kind of zoonotic virus affecting both humans and animals (3). It mainly infects the respiratory tract (4), the nervous system (5, 6), and the gastrointestinal tract (7). COVID-19 can develop into acute respiratory distress syndrome (ARDS), causing multiple organ failure and death (8). Since the oral cavity is directly connected to the external environment, it is easy to come into contact with viruses and other microorganisms through the oral cavity, including herpesvirus, retrovirus, cytomegalovirus, influenza virus, etc. (9). A variety of viruses can infect oral mucosa and salivary glands, causing oral symptoms. SARS-CoV-2 can be transmitted through droplets, aerosols, and contact with contaminated surfaces. Therefore, growing evidence suggests that the SARS-CoV-2 infection occurs when a person touches surfaces contaminated with SARS-CoV-2 and then directly touches the mucous membranes of the oral cavity and nose (10, 11). In addition to affecting the respiratory and immune systems, COVID-19 is manifested through different oral pathological features, including gustatory dysfunction, xerostomia, and salivary gland diseases (9, 12).
SARS-CoV-2 is a member of β-coronavirus genus (13). It contains four major structural proteins, including the spike (S) protein (14), which is an important virulence factor of SARS-CoV-2, mediating the entry of the virus into the host cells (4). Increasing evidence suggests that the occurrence and development of COVID-19 are related to the immune dysregulation caused by SARS-CoV-2 (15, 16). SARS-CoV-2 inhibits the secretion of type I interferon (IFN-1) and causes the cytokine storm (17, 18). Since the binding of SARS-CoV-2 to the oral cavity host cells is mediated by the angiotensin-converting enzyme 2 (ACE2) receptors (19), the virus can infect the epithelial cells of the oral mucosa and salivary glands, especially the epithelial cells of the tongue (20–22). In this review, we summarize the oral manifestations of COVID-19 and clarify the etiology and immunological pathogenesis of COVID-19 using multi-omics analysis.
2 Oral Manifestations of COVID-19
COVID-19 is a respiratory disease that manifests with fever, cough, dyspnea, headache, chest discomfort, and general body pain (23). Loss of taste and smell in early COVID-19 infection has been reported in some patients (24). A systematic analysis of COVID-19 clinical symptoms revealed that some patients present with unique symptoms, including oral disorders, such as gustatory dysfunction, oral mucosal diseases, salivary gland diseases, gingivitis, and periodontitis (9, 25).
2.1 Gustatory Dysfunction
Gustatory dysfunction is one of the most common oral manifestations of COVID-19 (26). Some COVID-19 patients reported taste and smell dysfunctions (25, 27–29). Given the increase in the number of COVID-19 patients with taste and smell dysfunctions, the Centers for Disease Control and Prevention (CDC) has included “New loss of taste or smell” as a symptom of COVID-19 diagnosed as SARS-CoV-2 infection. In one research involving 69 patients with olfactory and taste dysfunctions, 75.4% were diagnosed with COVID-19 (30). In addition, gustatory dysfunction can be used as a criterion for diagnosing COVID-19 (31). Overall, these findings suggested that gustatory dysfunction is a critical symptom of COVID-19, which may be helpful for the diagnosis of COVID-19.
2.2 Salivary Gland Diseases
Xerostomia is a common oral symptom of the early stage of COVID-19 disease (22, 25, 32, 33). A report showed the appearance of xerostomia symptoms in COVID-19 (34). In one research, over 70% of patients with xerostomia and loss of taste and smell tested positive before the COVID-19 diagnosis (35). Therefore, xerostomia and taste and smell dysfunctions are prodromal or unique early symptoms of COVID-19 and can be relied on to control the spread of the virus.
Dysphagia and frequent swelling or pain in the salivary glands or face are other oral COVID-19-related symptoms (36, 37). Salivary gland ectasia is a common oral manifestation (32). Reports of COVID-19-related parotitis and sialadenitis of the submandibular gland suggest that acute parotitis may be an early manifestation of COVID-19 (38, 39). In an analysis of oral involvement, salivary gland ectasia was observed in 43% of COVID-19 patients, suggesting that excessive inflammatory response in the salivary glands may indicate SARS-CoV-2 (32). Interestingly, SARS-CoV-2 virions have been detected in the patients’ saliva prior to the apparent lung lesions, which may be caused by SARS-CoV-2 infection in the salivary glands, explaining the asymptomatic COVID-19 infection (40). These reports show that oral diseases may be directly related to SARS-CoV-2 infection. These findings suggest that certain oral symptoms are strong indicators of SARS-CoV-2 infection. SARS-CoV-2 enters the host cells via ACE2 receptors abundant in the epithelial cells of the oral cavity, which might explain the involvement of the oral cavity in SARS-CoV-2 infection.
3 Structure of SARS-CoV-2
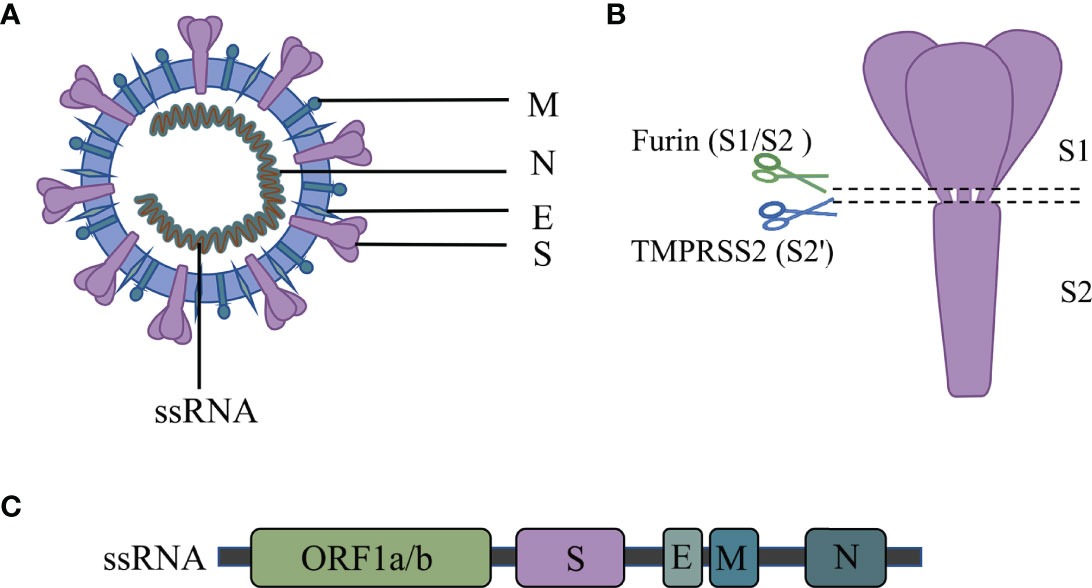
The SARS-CoV-2 is a single-stranded RNA virus. Its RNA encodes four major structural proteins, which include spike protein (S), envelope protein (E), membrane protein (M), and nucleocapsid protein (N) (41) [Figure 1]. Besides, 16 nonstructural proteins (NSPs) and 9 accessory proteins are included in the 29 proteins encoded (42).
The S protein mediates the virus’s entry into host cells and plays a key role in coronavirus infection (43). The S protein comprises the S1 receptor binding subunit and the S2 membrane fusion subunit (44). SARS-CoV-2 binds to the ACE2 receptor via the RBD region on the S1 subunit (45). The S2 subunit fuses with the host and viral membranes, facilitating the delivery of the viral genome into the host cells (43). The S protein is thus a vital component of the SARS-CoV-2 virus pathogenicity and might be used for COVID-19 diagnosis.
The E protein participates in the infection, replication, assembly, release, and virulence effect of the SARS-CoV-2 life cycle (46, 47). The E protein mediates the assembly and budding of the virus by interacting with the M protein (48). Moreover, E protein induces the host immune responses by promoting the activation of the NLRP3 inflammasome (49–51). Inhibiting or loss of expression of the E protein reduces titers of virions and induces incomplete viral maturation (52, 53).
Like the E protein, the M protein also inhibits the innate immune response. For instance, the M protein suppresses the signal transduction of RIG-I and MDA5 by targeting the mitochondrial antiviral signaling (MAVS) protein and then inhibits the virus-induced activation of the IFN-β promoter (54, 55).
The N protein has two main functions: it mediates the assembly of the helical capsid around the viral RNA and regulates the transcription of the viral genome (56). Also, the N protein promotes the expression of cytokines by activating the NLRP3 inflammasome signaling pathway (57). The nucleocapsid (N) proteins have dual regulatory effects on the innate immune response. At a low dose, the N protein inhibits the expression of IFN-1; however, at a high concentration, the N protein promotes the secretion of IFN-1 and cytokine release (58).
In addition to structural proteins, NSPs and accessory proteins of SARS-CoV-2 have a role in pathogenicity by influencing the host cell signaling (59). In general, the SARS-CoV-2 proteins play different critical roles in the immune invasion of the virus and modulation of the host immune response. Therefore, understating the role of SARS-CoV-2 proteins can lead to the identification of important diagnostic and therapeutic targets for vaccines against COVID-19.
For example, subunit vaccines, viral vector vaccines and inactivated viral vector vaccines induce antibodies targeting the S protein of SARS-CoV-2 (60–66).
4 Multi-omics Analysis of COVID-19
Multi-omics analysis reveals the pathogenic mechanism of organisms, including how they evade the immune system.
Transcriptomics, proteomics, metabolomics, immunomics, and single-cell transcriptomics are useful tools for analyzing biomolecules such as mRNAs, proteins, metabolites, and single cells (67) (Table 1). Therefore, they can clarify the pathogenesis and progression of COVID-19. Bronchoalveolar lavage fluid (BALF) and peripheral blood mononuclear cells (PBMC) of COVID-19 patients are common samples used for analyses (68, 74).
4.1 The Target Cells Infected by SARS-CoV-2 in Oral Cavity
A study on Rhesus Macaques demonstrated that ACE2 (+) epithelial cells in salivary glands duct were the early target cells of SARS-CoV infection (78). SARS-CoV-2 is also recognized by ACE2 receptors. These findings suggest that SARS-CoV-2 targets ACE2 (+) salivary glands duct epithelial cells. Single-cell RNA sequencing (scRNA-seq) was used to evaluate the specific expression of ACE2 in oral cells. The data showed that compared to buccal and gingiva tissues, the expression of ACE2 was higher in tongue tissues (20). Interestingly, analysis of 7 kinds of cell lines of oral cavity showed that the expression of ACE2 was enriched in epithelial cells (20). This finding indicates that SARS-CoV-2 has ability to influence oral epithelial cells which is a potential pathway of SARS-CoV-2 infection in oral cavity. Evidence suggested that Furin could promote the virus-cell fusion by acting on the cleavage site of S protein to make the virus enter the target cell (79). ScRNA-seq and immunohistochemical (IHC) analysis of oral cells showed that ACE2 receptors, Furin and TMPRSS2 were enriched in oral mucosal and salivary glands cells, especially in epithelial cells (80, 81). Therefore, these data indicate that ACE2 receptor, Furin and TMPRSS2 play an essential role in SARS-CoV-2 infection in oral epithelial cells. In addition, a report showed S protein of SARS-CoV-2 had been detected in epithelial cells of dorsum of the tongue (82). Moreover, a previous study showed that SARS-CoV-2 could infect epithelial cells in situ and then shed into saliva which confirmed by scRNA-seq, orthogonal RNA, and protein expression analysis (83). Furthermore, it was demonstrated the inhibited expression of ACE2 and Furin through Maackia amurensis seed lectin (MASL) which has a potential therapeutic effect on COVID-19 by decreasing the expression of inflammatory mediators by oral epithelial cells (84). Considering of host response in SARS-CoV-2 infection, scRNA-seq and transcriptomic analysis were performed. The data showed that upregulated pro-inflammatory signaling and immune dysregulation were observed in epithelial cells of the lung (85, 86). Moreover, the expression of proinflammatory cytokine genes was demonstrated in gingival epithelial cells, which also confirmed the antiviral defense mechanism in oral cavity (87). Besides, nCounter analysis of oral mucosa in severe patients showed signals of cell arresting which was correlated with systemic immune response abnormalities (88). Furthermore, the intense lymphocytic infiltration was detected in minor salivary glands (89). These studies indicate that SARS-CoV-2 could infect oral epithelial cells and be involved in abnormal immune regulation.
4.2 Omics Analysis of the Immune Response in COVID-19
Proteomic analysis of COVID-19 patients has shown that high levels of viremia are associated with sustained elevated levels of certain entry factors, such as ACE2 receptor, Furin and cathepsin B/L (CTSB/CTSL) (90). Previous report demonstrated that SARS-CoV-2 failed to enter cells which loss expressed ACE2 receptor (91). These results suggested that ACE2 receptor of host cell has a role in the infection of SARS-CoV-2. In addition, research shows that IFN-1 and IFN-III are under-expressed, whereas inflammatory cytokines such as IL-6, IL1RA, CCL2, CCL8, CXCL2, CXCL8, CXCL9, and CXCL16 are overexpressed in the serum of COVID-19 patients (17, 92). Furthermore, CCL4, CXCL10, IL-7, and IL-1α exacerbate the COVID-19 disease (93).
A positive correlation has been reported between the proliferation of monocytes and DCs that express MKI67 and TOP2A and the severity of COVID-19 disease (93). A decrease in the proportion of CD21+ and CD27+ B cells has been reported in the moderate and severe COVID-19 cases (94). Compared with moderate and mild COVID-19, the expansion of plasmablasts and plasma cells is lower than that in critical and severe cases (93). A similar trend is observed for B cell response to IFN-α (93).
Compared with healthy or patients with mild COVID-19, there is a decrease in the proportion of T lymphocytes, monocytes, dendritic cells, and natural killer cells, but a significant increase in neutrophils, hyperactivated T cells, and cytotoxic CD8+ T cells in patients with severe COVID-19 (94, 95). The proportion of lymphocytes also changes in COVID-19 patients, which shows that the proportion of CD4-CTLs increased, whereas the proportion of reactive Treg cells decreased (96). T-cell signaling is present in mild patients, but absent in severe patients (97). Moreover, both NLR (neutrophil count-to-lymphocyte count ratio) and NTR (neutrophil-to-T cell ratio) are elevated in severe COVID-19 patients (94). Neutrophilia and lymphocyte dysfunction may be related to tissue damage caused by the massive release of cytokines. High plasmablasts, circulating megakaryocytes, and erythropoiesis have been reported in severe COVID-19 cases (17, 69, 97–99).
In fact, the progression of the SARS-CoV-2 infection differs among patients. Multi-omics can reveal the changes in the increased secretion of cytokines, an increased proportion of neutrophils, and a decreased proportion of lymphocytes, which can open up new horizons in treating COVID-19 and the pathogenic mechanism of SARS-CoV-2. In the present study, the multi-omics analysis revealed increased secretion of cytokines and the decreased expression of IFN, respectively, in COVID-19 patients, further indicating that SARS-CoV-2 affects the function of the immune system.
4.3 Omics Analysis of Biomarkers of COVID-19
Notably, the potential therapeutic and diagnostic markers of COVID-19 were screened by omics (Table 2). Considering the invasion of SARS-CoV-2 in mammalian cells, omics analysis is a powerful tool for studying the roles of ACE2 receptor, cathepsin L1 (CTSL), and transmembrane serine proteinase 2 (TMPRSS2) (74). Furthermore, proteomic analysis of COVID-19 patients revealed a significant increase in cathepsin L1 in the lung (109). Thus, ACE2 receptors, CTSL 1, and TMPRSS2 can be targets for preventing and treating COVID-19. Moreover, studies have shown that soluble ACE2 and TMPRSS2 inhibitors have antiviral effects by blocking viral infection (19, 110). Proteomic analysis of SARS-CoV-2-infected host cells revealed that SARS-CoV-2 reshapes central cellular pathways of translation, splicing, carbon metabolism, protein homeostasis, and nucleic acid metabolism (100). In addition, the application of translation inhibitors significantly inhibits the replication of SARS-COV-2 (100). Multi-omics analysis of SARS-COV-2-infected cells showed that CIGB-300 interferes with RNA splicing by targeting casein kinase II (CK2) at the early stage of viral infection, suggesting that cigB-300 has antiviral effects (101). Transcriptome analysis showed increased HSP90AA1 mRNA levels in virus-infected cells, reducing viral replication and pro-inflammatory cytokine expression by inhibiting HSP90 activity (102).
In addition, multi-omics can be used to reveal the progression of COVID-19. For example, the mRNA level of S100s (107), pro-inflammatory signaling molecules of IL-6 are upregulated, and down-regulation of proteins in albumin (ALB), apolipoprotein A1 (APOA1), apolipoprotein C1 (APOC1), gelatins (GSN) and transferrin (TF) is seen in severe COVID-19 disease (108). These biomarkers have potential applications in the diagnosis of COVID-19.
5 Pathogenic Mechanisms in COVID-19
SARS-CoV-2 infection induces several immune responses. Firstly, upon entry into the body, the antigen-presenting cells (APCs) recognize the pathogen-associated molecular patterns (PAMPs) of SARS-CoV-2 through multiple pattern recognition receptors (PRRs) (111). Activated immune cells then produce numerous cytokines, such as IFNs, TNF-α, and interleukins, to destroy the virus- infected cells (112–114). The pathogenesis of SARS-CoV-2 is related to the inhibition of IFN production and the related cytokine storm (115).
5.1 SARS-CoV-2 Receptors
Studies have shown that the ACE2 receptor is the cellular receptor of SARS-CoV-2 (116, 117). ACE2 is expressed on the oral mucosa and salivary gland cells, suggesting that the oral cavity participates in the SARS-CoV-2 infection (20, 21). Once in the body, S protein is activated by TMPRSS2, which promotes the release of the SARS-CoV-2 genome into host cells (118) [Figure 2]. In general, ACE2 and TMPRSS2 are critical for SARS-CoV-2 infection. Reports show that ACE2 and TMPRSS2 are both expressed on the epithelial cells of the oral mucosa and salivary glands (83, 119, 120). ACE2 and TMPRSS2 are both expressed in taste buds cells; moreover, ACE2 is highly enriched in the epithelial cells of the tongue, which may be related to gustatory dysfunction (121). Interestingly, the expression of ACE2 on small salivary glands is higher than that in lungs, and the positive rate of SARS-CoV-2 in the saliva of asymptomatic infected patients is as high as 91.7% (40). The above findings underscore the critical role of ACE2 receptors in SARS-CoV-2.
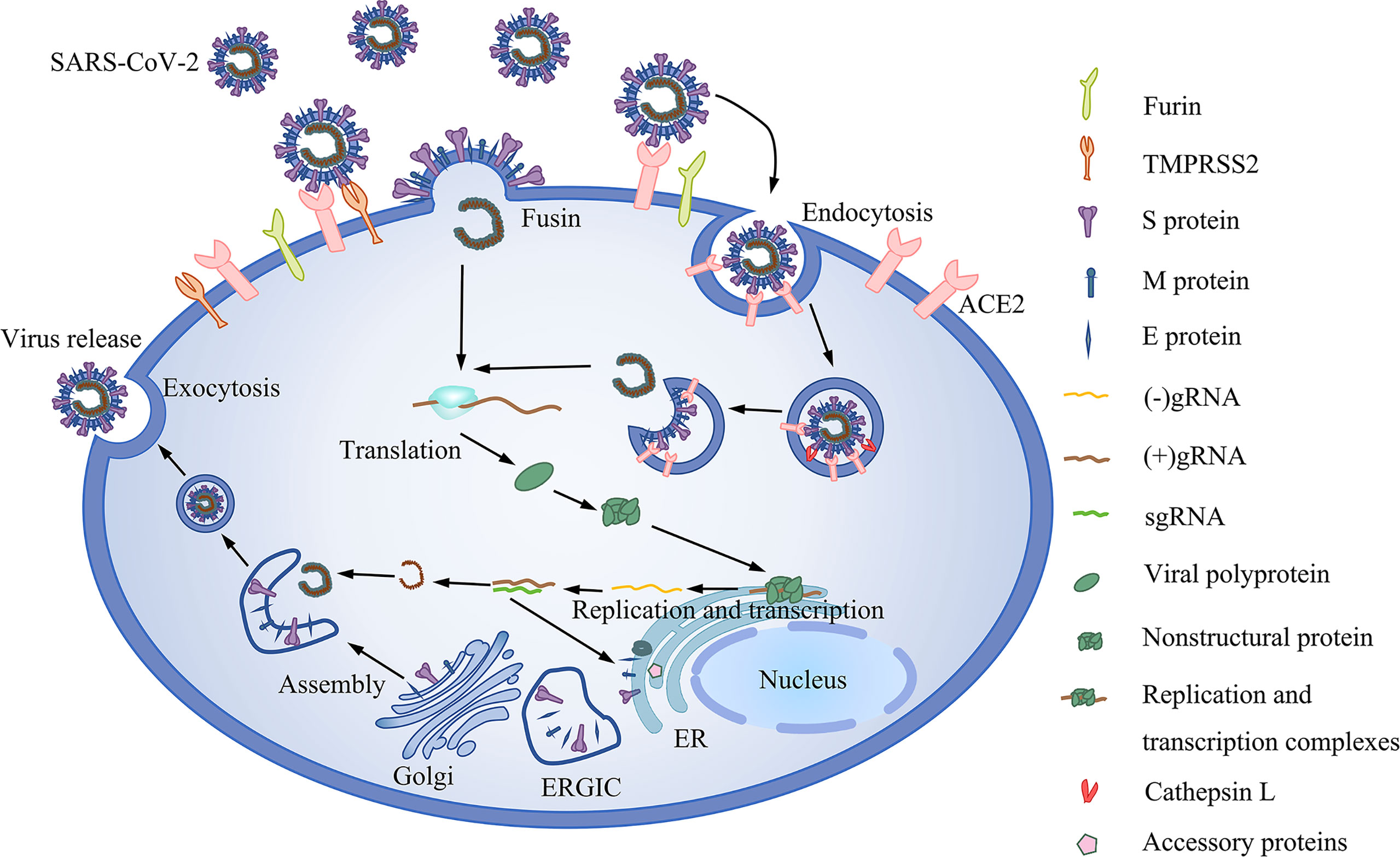
Figure 2 The life cycle of SARS-CoV-2. It includes viral entry, replication and transcription, assembly and release. Binding of SARS-CoV-2 to the ACE2 receptor and the subsequent activation of the virus by Furin and TMPRSS2. In the absence of TMPRSS2, the virus is activated by intracellular cathepsin. Upon entry into the cell, ORF1ab of the virus is translated to polyproteins, which are then cleaved into nonstructural proteins before assembly into replication and transcription complexes. Replication and transcription of the genome generate gRNA and subgenomic RNA (sgRNA). Shorter sgRNAs encode structural proteins and accessory proteins. The ERGIC is then assembled into mature SARS-CoV-2 virions.
5.2 Analysis of Pathological Process in SARS-COV-2 Infected Oral Cells
After SARS-CoV-2 infects oral cells by recognizing of ACE2 receptors, it causes damage to tissues or cells, thus leading to oral manifestations of COVID-19. ACE2 specific antibody test proved that the gustatory dysfunction of COVID-19 patients was related to the directly infected human taste cells in the dorsum of the tongue (122). Moreover, SARS-CoV-2 was also detected in submandibular gland of the COVID-19 patients (123, 124). Besides, IHC analysis of lip tissues with blister-like lesions showed that SARS-CoV-2 spike protein was positive in minor salivary acinus and duct cells (125). Interestingly, micronucleus test demonstrated that the death of oral mucosal cells was induced by SARS-CoV-2 (126). These indicate that SARS-CoV-2 induces cell death when it infects the salivary glands. Moreover, it was proposed that the infected salivary gland epithelial cells lysis stimulated the excessive secretion of inflammatory cytokines, causing salivary gland tissue damage (127). More importantly, in situ hybridization (ISH) and immunophenotyping showed that the most common histological feature of infected salivary glands was chronic salivary gland inflammation including lymphocytic inflammation and epithelial injury (83). These indicate that SARS-CoV-2 infection results in salivary gland dysfunction and xerostomia through excessive inflammatory response and the direct damage to ducts and acinar cells.
5.3 The Innate Immune Response Induced by SARS-CoV-2
IFN-1 is an important component of the innate immune response against viral infections. Recognition of PAMPs via the PRRs rapidly triggers the release of IFN-1 and many other pro-inflammatory cytokines, including interleukin (IL)-1β, IL-2, IL-6, IL-7, granulocyte colony-stimulating factor (GCSF), IFN-γ, and tumor necrosis factor-α (TNF-α) (128, 129). PRRs include Toll-like receptors (TLR), retinoic acid-inducible gene I (RIG-I)-like receptors (RLR), and C-type lectin receptors (CLR) (130, 131). IFN can regulate antiviral T cell responses and induce the expression of interferon-stimulated genes (ISG) via the JAK/STAT signaling pathway (132–134).
TLRs recruit specific adaptor molecules of downstream of the signaling cascade to initiate innate immune responses via the TLR/MyD88/NF-κB and TRIF/IFN-β pathway signaling pathways (135) [Figure 3]. Apart from TLRs, immune cells often recognize PAMPs via the RLRs, which induces the production of IFN. The RIG-I and MDA5 are TLRs that recognize and initiate an immune response against SARS-CoV-2 (136). Activated RIG-I and MDA5 interact with the downstream adapter MAVS to induce the expression of IFN-β and early ISGs (134) [Figure 3].
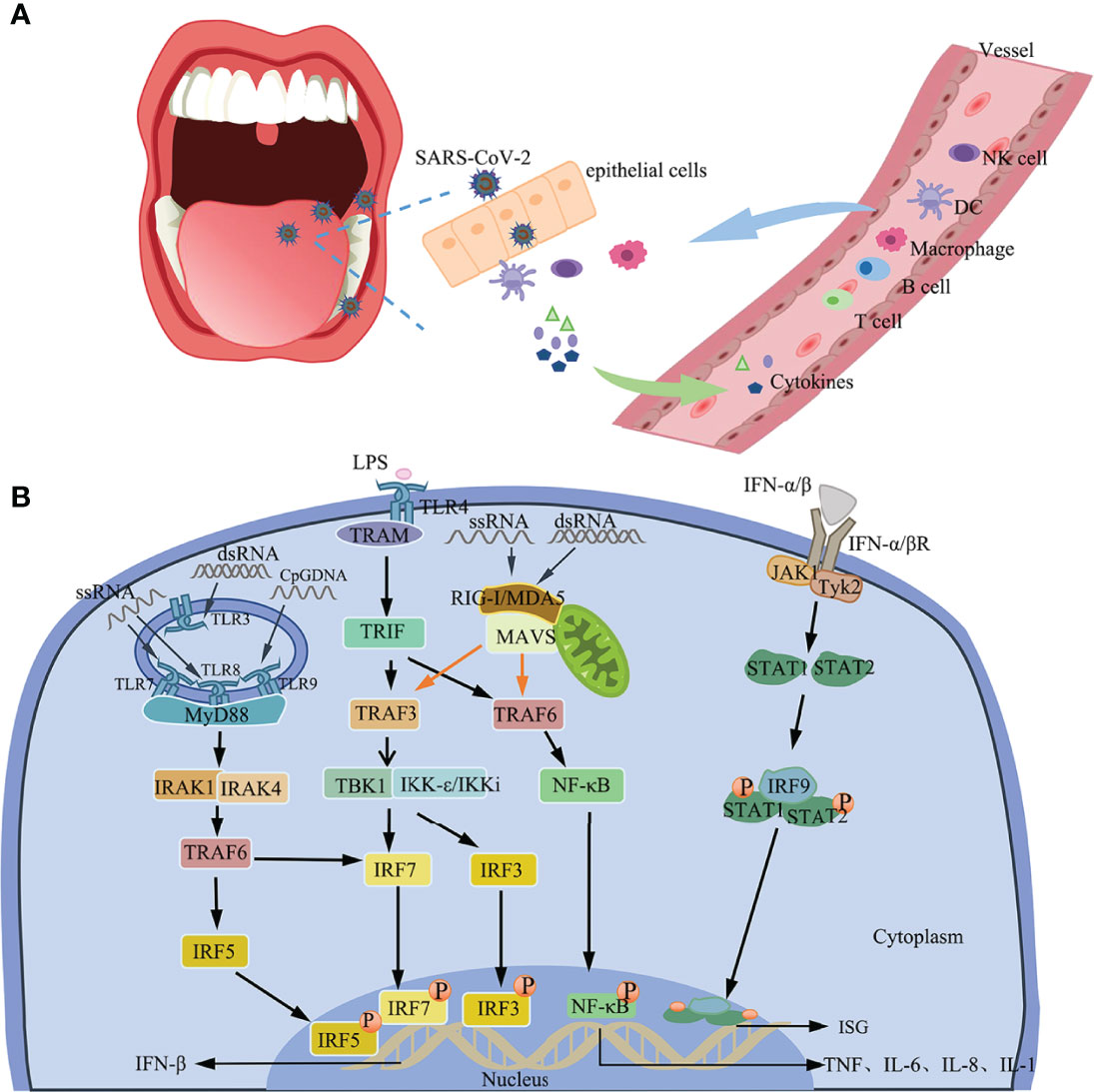
Figure 3 Immune response. (A) Immune response to SARS-CoV-2 in the oral cavity. (B) IFN induction and the positive feedback signaling pathway. The production of IFN-β by TLR4-TBK1/IKKi, TLR7/8/9-MyD88/IRAK1/IRAK4, and RIG-I/MDA5/MAVS signals. IFN-1 induces the expression of ISG via the Tyk2/JAK1/STAT signaling pathway by binding to IFNARs.
5.4 Adaptive Immunity Against SARS-CoV-2
Innate immunity performs two main functions: it directly kills pathogens and initiates adaptive immune responses (137). Adaptive immunity comprises humoral immunity and cellular immunity.
5.4.1 Cellular Immunity Against SARS-CoV-2
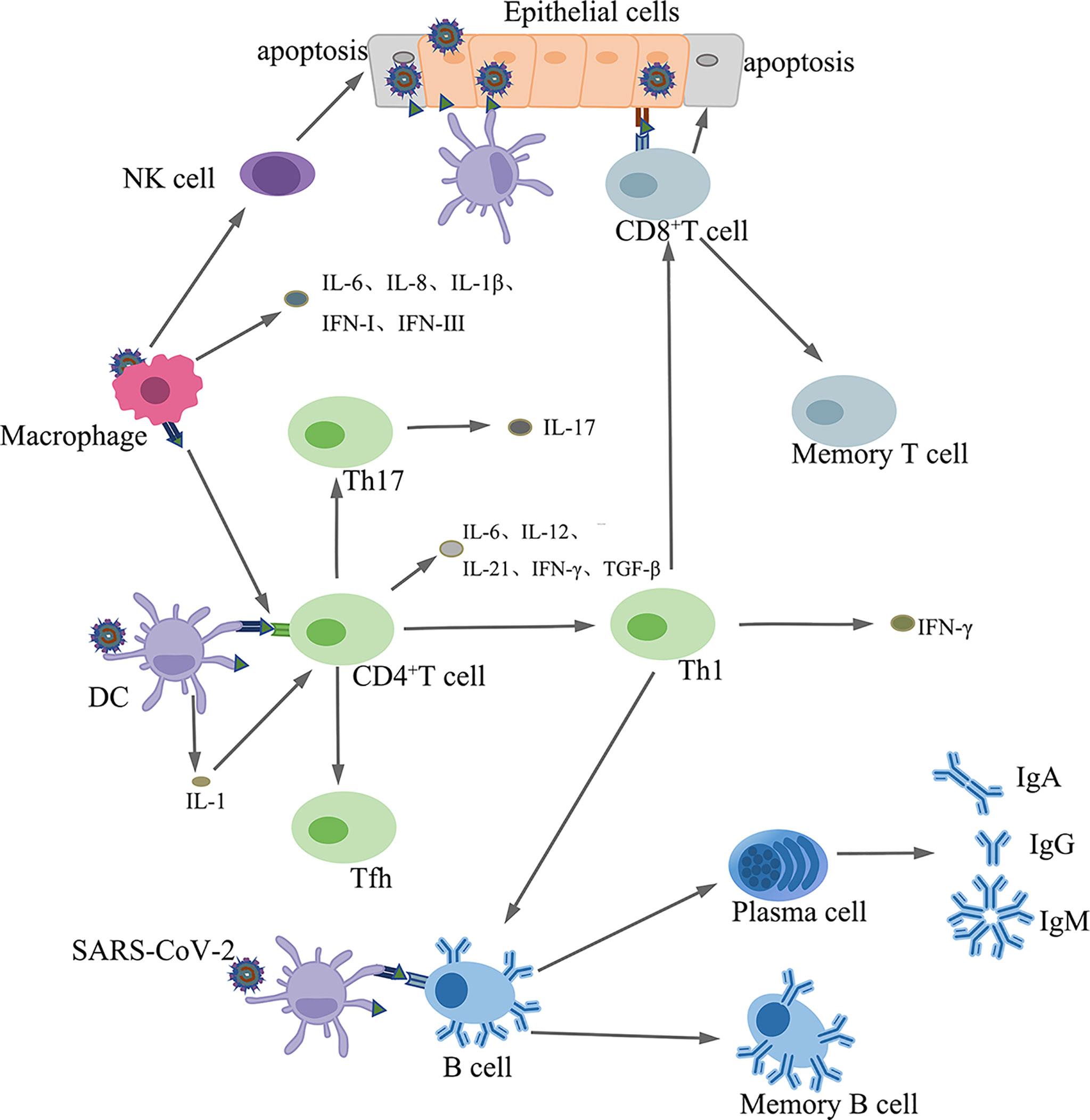
APCs present SARS-CoV-2 antigens to CD4+ T cells, which differentiate into Th1 sub-types that secret interleukin-12 (IL-12), which further stimulates Th1 cells. Th1 cells also stimulate CD8+ T killer cells (Tk) that kill virus infected cells (138). In addition, activated Th1 cells stimulate B cells to produce antigen-specific antibodies (139) [Figure 4]. Coronaviruses induce the production of proinflammatory cytokines, such as IL-17, by the helper T cell (Th) 17, which recruits monocytes and neutrophils to the sites of infection. Furthermore, IL-17 promotes the production of inflammatory cytokines, such as TNF-α, IL-1, IL-6, IL-8, and MCP-1 (140, 141).
5.4.2 Humoral Immunity Against SARS-CoV-2
Upon antigenic stimulation, B cells differentiate into plasma and memory B cells. Plasma cells synthesize and secrete antigen-specific antibodies (142) [Figure 4].
Neutralizing antibody titers to SARS-CoV-2 peak in the first few weeks after the onset of COVID-19 symptoms and decrease after that at a rate of up to 45% every month (143). In some individuals, SARS-CoV-2 neutralizing antibodies are undetectable within a few months of infection (143), suggesting that serum antibodies do not act as a protective factor for long-term immunity against SARS-CoV-2. A vaccine against the virus aims at increasing the antibody titers to higher levels compared to those induced by natural infection. A vaccine also induces the production of stable memory T and B cells that provide long-term immunity.
Inactivated and live attenuated virus vaccines are whole viruses that induce broader humoral and cellular immune responses (144, 145). However, the mutation of the virus may affect antibody production. The SARS-COV-2 Omicron variant is associated with more efficient cell entry, immune evasion, and increased infectivity (146). Research shows that the third dose of the BNT162b2 vaccine increases the neutralization efficiency of the Omicron variant compared to two doses, but even so, its efficacy is still lower than that against the Delta variant (147). BNT162b2 and mRNA-1273 are less effective in preventing Delta SARS-COV-2 infection but are highly efficacious in severe and critically ill patients (148).
5.5 Immune Evasion Induced by SARS-CoV-2
The IFN response is the first line of defense against viruses. However, SARS-CoV-2 strongly suppresses the production of IFN-1 and promotes the production of cytokines (17). SARS-CoV-2 inhibits the production of IFN mainly by (I) evading recognition by the host receptors (149–154) (II), interfering with IFN production (155) (III), blocking signal transmission (54, 156–158), and (IV) inhibiting the function of ISG effectors (58, 159).
Overall, the SARS-CoV-2 proteins mediate immune escape by disrupting the secretion of IFN.
5.6 Cytokine Storm
Immune response analysis showed that COVID-19 strongly inhibited the secretion of IFN-1, related to excessive inflammation (160). Clinical studies have shown that the severity of COVID-19 positively correlates with the serum levels of several cytokines, including TNF-α, IL-6, IL-7, IL-17, IL-18, granulocyte colony-stimulating factor (G-CSF), IP10, macrophage colony-stimulating factor (M-CSF), and chemokines. The secretion of cytokines is regulated through the (I) innate immune response signaling pathway (II), angiotensin II/angiotensin type I receptor signaling pathway, and (III) the ACE2 signaling pathway (115, 161).
6 Discussion
Some research findings on the oral manifestations of COVID-19 have been reported. The oral manifestations of COVID-19 primarily include gustatory dysfunction and xerostomia, but may also include ulceration, blisters, plaque-like lesions of the oral cavity, herpes simplex, swelling and/or pain in the salivary gland, halitosis, gingivitis, and periodontitis (162, 163). In some patients, xerostomia and gustatory dysfunction are the only manifestations or prodromal symptoms of COVID-19 (35).
The SARS-CoV-2 proteins, especially the S protein, play critical roles in the pathogenicity of the virus. Moreover, mutations might increase the pathogenicity of SARS-CoV-2. SARS-CoV-2 variants are more transmissible, pathogenic, and virulent (164). Indeed, a total of 93-mutations were detected in the SARS-CoV-2 genome. Among them, eight missense mutations occurred in the S surface glycoprotein. Three missense mutations (D354, Y364, and F367) occurred in the RBD of the S protein (165). Mutations may cause conformational changes in the related protein, which changes their antigenic properties (165). Mutations in the RBD domain of the S protein cause the virus to evade neutralizing Abs generated by vaccines (166). Other structural and nonstructural proteins that mediate the pathogenicity of the virus are also targets for COVID-19 treatment and SARS-CoV-2 vaccines’ development.
It has been reported that the healing of oral manifestations of COVID-19 and the regression of SARS-CoV-2 infection occurs simultaneously (162), indicating that the oral lesions might be associated with the infection of SARS-CoV-2. There is evidence that taste changes are caused by SARS-CoV-2 direct infection, which causes cell damage after virus infection, leading to taste dysfunction (122). However, some reports show that oral manifestations of COVID-19 are associated with inflammation, which is associated with immune cell-mediated cell death and tissue damage following SARS-CoV-2 infection (167). The application of omics may help solve this problem. Multi-omics can reveal how COVID-19 interacts with the immune response. The proportion of lymphocytes and neutrophils in the peripheral blood can be used to assess the severity of COVID-19 (168). Decreased lymphocyte counts in patients may lead to insufficient production of immune memory cells, making it difficult to deal with virus re-infection.
The entry of SARS-CoV-2 into host cells is mediated by ACE2 receptors and TMPRSS2. It has been proved that high expression of the ACE2 receptor was found in oral mucosa and salivary glands, and TMPRSS2 was co-expressed with the ACE2 receptor (83, 119, 120), indicating that the oral cavity is susceptible to SARS-CoV-2 infection. These receptors and enzymes facilitate the invasion and the subsequent oral manifestations of COVID-19. Upon entry into the oral host cells, SARS-CoV-2 first initiates a local immune response by inducing the production of IFN. However, SARS-CoV-2 causes a cytokine storm and induces excessive inflammatory responses through immune disorders, which might trigger damage to oral tissues. During the systemic response phase in patients with severe COVID-19, the virus dysregulates the immune response, increases the proportion of neutrophils, and decreases the proportion of lymphocytes. In the end, excessive inflammation damages the involved tissues. Multi-omics studies have confirmed that SARS-CoV-2 affects the immune system and causes immune disorders, suggesting that the pathogenesis of SARS-CoV-2 is related to the innate and adaptive immune responses (169).
7 Conclusion
SARS-CoV-2 infects cells of the oral cavity via the surface ACE2 receptors and TMPRSS2. The virus binds to its receptors via the S protein ligand. Multi-omics analyses further revealed that SARS-CoV-2 dysregulates the immune system mainly by decreasing the expression of IFN-1 and increasing cytokines levels.
Author Contributions
MH and DW wrote the manuscript. MH, DW, QX, SK, LC, HL, ZY, and WL searched PubMed and Web of Science for citations and prepared figures. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Fundamental Research Funds for the Central Universities (Grant Nos. 2019JCKT-70 and 2020JCXK-45), the Jilin Province Department of Finance (Grant No. JCSZ2019378-8 and jcsz2021893-13), the Jilin Scientific and Technological Development Program (Grant Nos. 20210101010JC and 20200801077GH), and the Changchun Scientific and Technological Development Program (Grant No. 21ZY26).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Comber L, OM E, Drummond L, Carty PG, Walsh KA, De Gascun CF, et al. Airborne Transmission of Sars-Cov-2 Via Aerosols. Rev Med Virol (2021) 31(3):e2184. doi: 10.1002/rmv.2184
2. Rana R, Tripathi A, Kumar N, Ganguly NK. A Comprehensive Overview on Covid-19: Future Perspectives. Front Cell Infect Microbiol (2021) 11:744903. doi: 10.3389/fcimb.2021.744903
3. Islam A, Ferdous J, Islam S, Sayeed MA, Rahman MK, Saha O, et al. Transmission Dynamics and Susceptibility Patterns of Sars-Cov-2 in Domestic, Farmed and Wild Animals: Sustainable One Health Surveillance for Conservation and Public Health to Prevent Future Epidemics and Pandemics. Transbound Emerg Dis (2021). doi: 10.1111/tbed.14356.
4. Matheson NJ, Lehner PJ. How Does Sars-Cov-2 Cause Covid-19? Science (2020) 369(6503):510–1. doi: 10.1126/science.abc6156
5. Amruta N, Chastain WH, Paz M, Solch RJ, Murray-Brown IC, Befeler JB, et al. Sars-Cov-2 Mediated Neuroinflammation and the Impact of Covid-19 in Neurological Disorders. Cytokine Growth Factor Rev (2021) 58:1–15. doi: 10.1016/j.cytogfr.2021.02.002
6. Caporale N, Testa G. Covid-19 Lessons From the Dish: Dissecting Cns Manifestations Through Brain Organoids. EMBO J (2021) 40(2):e107213. doi: 10.15252/embj.2020107213
7. Guo M, Tao W, Flavell RA, Zhu S. Potential Intestinal Infection and Faecal-Oral Transmission of Sars-Cov-2. Nat Rev Gastroenterol Hepatol (2021) 18(4):269–83. doi: 10.1038/s41575-021-00416-6
8. Ballow M, Haga CL. Why Do Some People Develop Serious Covid-19 Disease After Infection, While Others Only Exhibit Mild Symptoms? J Allergy Clin Immunol Pract (2021) 9(4):1442–8. doi: 10.1016/j.jaip.2021.01.012
9. La Rosa GRM, Libra M, De Pasquale R, Ferlito S, Pedulla E. Association of Viral Infections With Oral Cavity Lesions: Role of Sars-Cov-2 Infection. Front Med (Lausanne) (2020) 7:571214. doi: 10.3389/fmed.2020.571214
10. Lotfi M, Hamblin MR, Rezaei N. Covid-19: Transmission, Prevention, and Potential Therapeutic Opportunities. Clin Chim Acta (2020) 508:254–66. doi: 10.1016/j.cca.2020.05.044
11. Ong SWX, Tan YK, Chia PY, Lee TH, Ng OT, Wong MSY, et al. Air, Surface Environmental, and Personal Protective Equipment Contamination by Severe Acute Respiratory Syndrome Coronavirus 2 (Sars-Cov-2) From a Symptomatic Patient. JAMA (2020) 323(16):1610–2. doi: 10.1001/jama.2020.3227
12. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and Clinical Characteristics of 99 Cases of 2019 Novel Coronavirus Pneumonia in Wuhan, China: A Descriptive Study. Lancet (2020) 395(10223):507–13. doi: 10.1016/S0140-6736(20)30211-7
13. Coronaviridae Study Group of the International Committee on Taxonomy of V. The Species Severe Acute Respiratory Syndrome-Related Coronavirus: Classifying 2019-Ncov and Naming It Sars-Cov-2. Nat Microbiol (2020) 5(4):536–44. doi: 10.1038/s41564-020-0695-z
14. Yan W, Zheng Y, Zeng X, He B, Cheng W. Structural Biology of Sars-Cov-2: Open the Door for Novel Therapies. Signal Transduct Target Ther (2022) 7(1):26. doi: 10.1038/s41392-022-00884-5
15. Li Y, Hou G, Zhou H, Wang Y, Tun HM, Zhu A, et al. Multi-Platform Omics Analysis Reveals Molecular Signature for Covid-19 Pathogenesis, Prognosis and Drug Target Discovery. Signal Transduct Target Ther (2021) 6(1):155. doi: 10.1038/s41392-021-00508-4
16. Kramer B, Knoll R, Bonaguro L, ToVinh M, Raabe J, Astaburuaga-Garcia R, et al. Early Ifn-Alpha Signatures and Persistent Dysfunction Are Distinguishing Features of Nk Cells in Severe Covid-19. Immunity (2021) 54(11):2650–69.e14. doi: 10.1016/j.immuni.2021.09.002
17. Blanco-Melo D, Nilsson-Payant BE, Liu WC, Uhl S, Hoagland D, Moller R, et al. Imbalanced Host Response to Sars-Cov-2 Drives Development of Covid-19. Cell (2020) 181(5):1036–45.e9. doi: 10.1016/j.cell.2020.04.026
18. Wang Y, Perlman S. Covid-19: Inflammatory Profile. Annu Rev Med (2022) 73:65–80. doi: 10.1146/annurev-med-042220-012417
19. Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, et al. Sars-Cov-2 Cell Entry Depends on Ace2 and Tmprss2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell (2020) 181(2):271–80.e8. doi: 10.1016/j.cell.2020.02.052
20. Xu H, Zhong L, Deng J, Peng J, Dan H, Zeng X, et al. High Expression of Ace2 Receptor of 2019-Ncov on the Epithelial Cells of Oral Mucosa. Int J Oral Sci (2020) 12(1):8. doi: 10.1038/s41368-020-0074-x
21. Sakaguchi W, Kubota N, Shimizu T, Saruta J, Fuchida S, Kawata A, et al. Existence of Sars-Cov-2 Entry Molecules in the Oral Cavity. Int J Mol Sci (2020) 21(17):6000. doi: 10.3390/ijms21176000
22. Chen L, Zhao J, Peng J, Li X, Deng X, Geng Z, et al. Detection of Sars-Cov-2 in Saliva and Characterization of Oral Symptoms in Covid-19 Patients. Cell Prolif (2020) 53(12):e12923. doi: 10.1111/cpr.12923
23. Kevadiya BD, Machhi J, Herskovitz J, Oleynikov MD, Blomberg WR, Bajwa N, et al. Diagnostics for Sars-Cov-2 Infections. Nat Mater (2021) 20(5):593–605. doi: 10.1038/s41563-020-00906-z
24. Borsetto D, Hopkins C, Philips V, Obholzer R, Tirelli G, Polesel J, et al. Self-Reported Alteration of Sense of Smell or Taste in Patients With Covid-19: A Systematic Review and Meta-Analysis on 3563 Patients. Rhinology (2020) 58(5):430–6. doi: 10.4193/Rhin20.185
25. Amorim Dos Santos J, Normando AGC, Carvalho da Silva RL, Acevedo AC, De Luca Canto G, Sugaya N, et al. Oral Manifestations in Patients With Covid-19: A 6-Month Update. J Dent Res (2021) 100(12):1321–9. doi: 10.1177/00220345211029637
26. Amorim Dos Santos J, Normando AGC, Carvalho da Silva RL, Acevedo AC, De Luca Canto G, Sugaya N, et al. Oral Manifestations in Patients With Covid-19: A Living Systematic Review. J Dent Res (2021) 100(2):141–54. doi: 10.1177/0022034520957289
27. Farid H, Khan M, Jamal S, Ghafoor R. Oral Manifestations of Covid-19-a Literature Review. Rev Med Virol (2022) 32(1):e2248. doi: 10.1002/rmv.2248
28. Lechien JR, Chiesa-Estomba CM, Place S, Van Laethem Y, Cabaraux P, Mat Q, et al. Clinical and Epidemiological Characteristics of 1420 European Patients With Mild-To-Moderate Coronavirus Disease 2019. J Intern Med (2020) 288(3):335–44. doi: 10.1111/joim.13089
29. Agyeman AA, Chin KL, Landersdorfer CB, Liew D, Ofori-Asenso R. Smell and Taste Dysfunction in Patients With Covid-19: A Systematic Review and Meta-Analysis. Mayo Clin Proc (2020) 95(8):1621–31. doi: 10.1016/j.mayocp.2020.05.030
30. Benazzo M, Cassaniti I, Maiorano E, Calastri A, Novazzi F, Bonetti A, et al. Sars-Cov-2 Virologic and Immunologic Correlates in Patients With Olfactory and Taste Disorders. Microorganisms (2020) 8(7):1052. doi: 10.3390/microorganisms8071052
31. Sudre CH, Keshet A, Graham MS, Joshi AD, Shilo S, Rossman H, et al. Anosmia, Ageusia, and Other Covid-19-Like Symptoms in Association With a Positive Sars-Cov-2 Test, Across Six National Digital Surveillance Platforms: An Observational Study. Lancet Digit Health (2021) 3(9):e577–e86. doi: 10.1016/S2589-7500(21)00115-1
32. Gherlone EF, Polizzi E, Tete G, De Lorenzo R, Magnaghi C, Rovere Querini P, et al. Frequent and Persistent Salivary Gland Ectasia and Oral Disease After Covid-19. J Dent Res (2021) 100(5):464–71. doi: 10.1177/0022034521997112
33. Biadsee A, Biadsee A, Kassem F, Dagan O, Masarwa S, Ormianer Z. Olfactory and Oral Manifestations of Covid-19: Sex-Related Symptoms-A Potential Pathway to Early Diagnosis. Otolaryngol Head Neck Surg (2020) 163(4):722–8. doi: 10.1177/0194599820934380
34. Freni F, Meduri A, Gazia F, Nicastro V, Galletti C, Aragona P, et al. Symptomatology in Head and Neck District in Coronavirus Disease (Covid-19): A Possible Neuroinvasive Action of Sars-Cov-2. Am J Otolaryngol (2020) 41(5):102612. doi: 10.1016/j.amjoto.2020.102612
35. Fantozzi PJ, Pampena E, Di Vanna D, Pellegrino E, Corbi D, Mammucari S, et al. Xerostomia, Gustatory and Olfactory Dysfunctions in Patients With Covid-19. Am J Otolaryngol (2020) 41(6):102721. doi: 10.1016/j.amjoto.2020.102721
36. El Kady DM, Gomaa EA, Abdella WS, Ashraf Hussien R, Abd ElAziz RH, Khater AGA. Oral Manifestations of Covid-19 Patients: An Online Survey of the Egyptian Population. Clin Exp Dent Res (2021) 7(5):852–60. doi: 10.1002/cre2.429
37. Riofrio G, Castillo S, Salcedo G, Alvitez-Temoche D, Watanabe R, Mayta-Tovalino F. Future Challenges of Covid-19 and Oral Manifestations in Daily Dental Practice: A Literature Review. J Int Soc Prev Community Dent (2021) 11(3):242–7. doi: 10.4103/jispcd.JISPCD_21_21
38. Chern A, Famuyide AO, Moonis G, Lalwani AK. Sialadenitis: A Possible Early Manifestation of Covid-19. Laryngoscope (2020) 130(11):2595–7. doi: 10.1002/lary.29083
39. Lechien JR, Chetrit A, Chekkoury-Idrissi Y, Distinguin L, Circiu M, Saussez S, et al. Parotitis-Like Symptoms Associated With Covid-19, France, March-April 2020. Emerg Infect Dis (2020) 26(9):2270–1. doi: 10.3201/eid2609.202059
40. Xu J, Li Y, Gan F, Du Y, Yao Y. Salivary Glands: Potential Reservoirs for Covid-19 Asymptomatic Infection. J Dent Res (2020) 99(8):989. doi: 10.1177/0022034520918518
41. Wang B, Zhong C, Tieleman DP. Supramolecular Organization of Sars-Cov and Sars-Cov-2 Virions Revealed by Coarse-Grained Models of Intact Virus Envelopes. J Chem Inf Model (2022) 62(1):176–86. doi: 10.1021/acs.jcim.1c01240
42. Zhang J, Li Q, Cruz Cosme RS, Gerzanich V, Tang Q, Simard JM, et al. Genome-Wide Characterization of Sars-Cov-2 Cytopathogenic Proteins in the Search of Antiviral Targets. mBio (2022) 13(1):e0016922. doi: 10.1128/mbio.00169-22
43. Li F. Structure, Function, and Evolution of Coronavirus Spike Proteins. Annu Rev Virol (2016) 3(1):237–61. doi: 10.1146/annurev-virology-110615-042301
44. Peng R, Wu LA, Wang Q, Qi J, Gao GF. Cell Entry by Sars-Cov-2. Trends Biochem Sci (2021) 46(10):848–60. doi: 10.1016/j.tibs.2021.06.001
45. Shereen MA, Khan S, Kazmi A, Bashir N, Siddique R. Covid-19 Infection: Origin, Transmission, and Characteristics of Human Coronaviruses. J Adv Res (2020) 24:91–8. doi: 10.1016/j.jare.2020.03.005
46. Schoeman D, Fielding BC. Is There a Link Between the Pathogenic Human Coronavirus Envelope Protein and Immunopathology? A Review of the Literature. Front Microbiol (2020) 11:2086. doi: 10.3389/fmicb.2020.02086
47. Schoeman D, Fielding BC. Coronavirus Envelope Protein: Current Knowledge. Virol J (2019) 16(1):69. doi: 10.1186/s12985-019-1182-0
48. Cao Y, Yang R, Lee I, Zhang W, Sun J, Wang W, et al. Characterization of the Sars-Cov-2 E Protein: Sequence, Structure, Viroporin, and Inhibitors. Protein Sci (2021) 30(6):1114–30. doi: 10.1002/pro.4075
49. Nieto-Torres JL, Verdia-Baguena C, Jimenez-Guardeno JM, Regla-Nava JA, Castano-Rodriguez C, Fernandez-Delgado R, et al. Severe Acute Respiratory Syndrome Coronavirus E Protein Transports Calcium Ions and Activates the Nlrp3 Inflammasome. Virology (2015) 485:330–9. doi: 10.1016/j.virol.2015.08.010
50. Jimenez-Guardeno JM, Nieto-Torres JL, DeDiego ML, Regla-Nava JA, Fernandez-Delgado R, Castano-Rodriguez C, et al. The Pdz-Binding Motif of Severe Acute Respiratory Syndrome Coronavirus Envelope Protein Is a Determinant of Viral Pathogenesis. PloS Pathog (2014) 10(8):e1004320. doi: 10.1371/journal.ppat.1004320
51. Chai J, Cai Y, Pang C, Wang L, McSweeney S, Shanklin J, et al. Structural Basis for Sars-Cov-2 Envelope Protein Recognition of Human Cell Junction Protein Pals1. Nat Commun (2021) 12(1):3433. doi: 10.1038/s41467-021-23533-x
52. Ortego J, Ceriani JE, Patino C, Plana J, Enjuanes L. Absence of E Protein Arrests Transmissible Gastroenteritis Coronavirus Maturation in the Secretory Pathway. Virology (2007) 368(2):296–308. doi: 10.1016/j.virol.2007.05.032
53. DeDiego ML, Alvarez E, Almazan F, Rejas MT, Lamirande E, Roberts A, et al. A Severe Acute Respiratory Syndrome Coronavirus That Lacks the E Gene Is Attenuated in Vitro and in Vivo. J Virol (2007) 81(4):1701–13. doi: 10.1128/JVI.01467-06
54. Fu YZ, Wang SY, Zheng ZQ, Yi H, Li WW, Xu ZS, et al. Sars-Cov-2 Membrane Glycoprotein M Antagonizes the Mavs-Mediated Innate Antiviral Response. Cell Mol Immunol (2021) 18(3):613–20. doi: 10.1038/s41423-020-00571-x
55. Lei X, Dong X, Ma R, Wang W, Xiao X, Tian Z, et al. Activation and Evasion of Type I Interferon Responses by Sars-Cov-2. Nat Commun (2020) 11(1):3810. doi: 10.1038/s41467-020-17665-9
56. Carlson CR, Asfaha JB, Ghent CM, Howard CJ, Hartooni N, Safari M, et al. Phosphoregulation of Phase Separation by the Sars-Cov-2 N Protein Suggests a Biophysical Basis for Its Dual Functions. Mol Cell (2020) 80(6):1092–103.e4. doi: 10.1016/j.molcel.2020.11.025
57. Pan P, Shen M, Yu Z, Ge W, Chen K, Tian M, et al. Sars-Cov-2 N Protein Promotes Nlrp3 Inflammasome Activation to Induce Hyperinflammation. Nat Commun (2021) 12(1):4664. doi: 10.1038/s41467-021-25015-6
58. Zhao Y, Sui L, Wu P, Wang W, Wang Z, Yu Y, et al. A Dual-Role of Sars-Cov-2 Nucleocapsid Protein in Regulating Innate Immune Response. Signal Transduct Target Ther (2021) 6(1):331. doi: 10.1038/s41392-021-00742-w
59. Suryawanshi RK, Koganti R, Agelidis A, Patil CD, Shukla D. Dysregulation of Cell Signaling by Sars-Cov-2. Trends Microbiol (2021) 29(3):224–37. doi: 10.1016/j.tim.2020.12.007
60. Ye T, Zhong Z, Garcia-Sastre A, Schotsaert M, De Geest BG. Current Status of Covid-19 (Pre)Clinical Vaccine Development. Angew Chem Int Ed Engl (2020) 59(43):18885–97. doi: 10.1002/anie.202008319
61. Du L, He Y, Zhou Y, Liu S, Zheng BJ, Jiang S. The Spike Protein of Sars-Cov–a Target for Vaccine and Therapeutic Development. Nat Rev Microbiol (2009) 7(3):226–36. doi: 10.1038/nrmicro2090
62. Richmond P, Hatchuel L, Dong M, Ma B, Hu B, Smolenov I, et al. Safety and Immunogenicity of S-Trimer (Scb-2019), a Protein Subunit Vaccine Candidate for Covid-19 in Healthy Adults: A Phase 1, Randomised, Double-Blind, Placebo-Controlled Trial. Lancet (2021) 397(10275):682–94. doi: 10.1016/S0140-6736(21)00241-5
63. Sanchez-Felipe L, Vercruysse T, Sharma S, Ma J, Lemmens V, Van Looveren D, et al. A Single-Dose Live-Attenuated Yf17d-Vectored Sars-Cov-2 Vaccine Candidate. Nature (2021) 590(7845):320–5. doi: 10.1038/s41586-020-3035-9
64. Liu X, Luongo C, Matsuoka Y, Park HS, Santos C, Yang L, et al. A Single Intranasal Dose of a Live-Attenuated Parainfluenza Virus-Vectored Sars-Cov-2 Vaccine Is Protective in Hamsters. Proc Natl Acad Sci U S A (2021) 118(50):e2109744118. doi: 10.1073/pnas.2109744118
65. Ewer KJ, Barrett JR, Belij-Rammerstorfer S, Sharpe H, Makinson R, Morter R, et al. T Cell and Antibody Responses Induced by a Single Dose of Chadox1 Ncov-19 (Azd1222) Vaccine in a Phase 1/2 Clinical Trial. Nat Med (2021) 27(2):270–8. doi: 10.1038/s41591-020-01194-5
66. Krammer F. Sars-Cov-2 Vaccines in Development. Nature (2020) 586(7830):516–27. doi: 10.1038/s41586-020-2798-3
67. Li CX, Gao J, Zhang Z, Chen L, Li X, Zhou M, et al. Multiomics Integration-Based Molecular Characterizations of Covid-19. Brief Bioinform (2022) 23(1):bbab485. doi: 10.1093/bib/bbab485
68. Su Y, Chen D, Yuan D, Lausted C, Choi J, Dai CL, et al. Multi-Omics Resolves a Sharp Disease-State Shift Between Mild and Moderate Covid-19. Cell (2020) 183(6):1479–95.e20. doi: 10.1016/j.cell.2020.10.037
69. Overmyer KA, Shishkova E, Miller IJ, Balnis J, Bernstein MN, Peters-Clarke TM, et al. Large-Scale Multi-Omic Analysis of Covid-19 Severity. Cell Syst (2021) 12(1):23–40.e7. doi: 10.1016/j.cels.2020.10.003
70. Thomas T, Stefanoni D, Dzieciatkowska M, Issaian A, Nemkov T, Hill RC, et al. Evidence of Structural Protein Damage and Membrane Lipid Remodeling in Red Blood Cells From Covid-19 Patients. J Proteome Res (2020) 19(11):4455–69. doi: 10.1021/acs.jproteome.0c00606
71. Bruzzone C, Bizkarguenaga M, Gil-Redondo R, Diercks T, Arana E, Garcia de Vicuna A, et al. Sars-Cov-2 Infection Dysregulates the Metabolomic and Lipidomic Profiles of Serum. iScience (2020) 23(10):101645. doi: 10.1016/j.isci.2020.101645
72. Bi X, Liu W, Ding X, Liang S, Zheng Y, Zhu X, et al. Proteomic and Metabolomic Profiling of Urine Uncovers Immune Responses in Patients With Covid-19. Cell Rep (2022) 38(3):110271. doi: 10.1016/j.celrep.2021.110271
73. Aschenbrenner AC, Mouktaroudi M, Kramer B, Oestreich M, Antonakos N, Nuesch-Germano M, et al. Disease Severity-Specific Neutrophil Signatures in Blood Transcriptomes Stratify Covid-19 Patients. Genome Med (2021) 13(1):7. doi: 10.1186/s13073-020-00823-5
74. Hou Y, Zhou Y, Gack MU, Lathia JD, Kallianpur A, Mehra R, et al. Multimodal Single-Cell Omics Analysis Identifies Epithelium-Immune Cell Interactions and Immune Vulnerability Associated With Sex Differences in Covid-19. Signal Transduct Target Ther (2021) 6(1):292. doi: 10.1038/s41392-021-00709-x
75. Downes DJ, Cross AR, Hua P, Roberts N, Schwessinger R, Cutler AJ, et al. Identification of Lztfl1 as a Candidate Effector Gene at a Covid-19 Risk Locus. Nat Genet (2021) 53(11):1606–15. doi: 10.1038/s41588-021-00955-3
76. Butler D, Mozsary C, Meydan C, Foox J, Rosiene J, Shaiber A, et al. Shotgun Transcriptome, Spatial Omics, and Isothermal Profiling of Sars-Cov-2 Infection Reveals Unique Host Responses, Viral Diversification, and Drug Interactions. Nat Commun (2021) 12(1):1660. doi: 10.1038/s41467-021-21361-7
77. Chiara M, Horner DS, Gissi C, Pesole G. Comparative Genomics Reveals Early Emergence and Biased Spatiotemporal Distribution of Sars-Cov-2. Mol Biol Evol (2021) 38(6):2547–65. doi: 10.1093/molbev/msab049
78. Liu L, Wei Q, Alvarez X, Wang H, Du Y, Zhu H, et al. Epithelial Cells Lining Salivary Gland Ducts Are Early Target Cells of Severe Acute Respiratory Syndrome Coronavirus Infection in the Upper Respiratory Tracts of Rhesus Macaques. J Virol (2011) 85(8):4025–30. doi: 10.1128/JVI.02292-10
79. Wu C, Zheng M, Yang Y, Gu X, Yang K, Li M, et al. Furin: A Potential Therapeutic Target for Covid-19. iScience (2020) 23(10):101642. doi: 10.1016/j.isci.2020.101642
80. Zhong M, Lin B, Pathak JL, Gao H, Young AJ, Wang X, et al. Ace2 and Furin Expressions in Oral Epithelial Cells Possibly Facilitate Covid-19 Infection Via Respiratory and Fecal-Oral Routes. Front Med (Lausanne) (2020) 7:580796. doi: 10.3389/fmed.2020.580796
81. Song J, Li Y, Huang X, Chen Z, Li Y, Liu C, et al. Systematic Analysis of Ace2 and Tmprss2 Expression in Salivary Glands Reveals Underlying Transmission Mechanism Caused by Sars-Cov-2. J Med Virol (2020) 92(11):2556–66. doi: 10.1002/jmv.26045
82. Marques BBF, Guimaraes TC, Fischer RG, Tinoco JMM, Pires FR, Lima Junior JDC, et al. Morphological Alterations in Tongue Epithelial Cells Infected by Sars-Cov-2: A Case-Control Study. Oral Dis (2021)00:1–6. doi: 10.1111/odi.13988
83. Huang N, Perez P, Kato T, Mikami Y, Okuda K, Gilmore RC, et al. Sars-Cov-2 Infection of the Oral Cavity and Saliva. Nat Med (2021) 27(5):892–903. doi: 10.1038/s41591-021-01296-8
84. Sheehan SA, Hamilton KL, Retzbach EP, Balachandran P, Krishnan H, Leone P, et al. Evidence That Maackia Amurensis Seed Lectin (Masl) Exerts Pleiotropic Actions on Oral Squamous Cells With Potential to Inhibit Sars-Cov-2 Infection and Covid-19 Disease Progression. Exp Cell Res (2021) 403(1):112594. doi: 10.1016/j.yexcr.2021.112594
85. Chen H, Liu W, Wang Y, Liu D, Zhao L, Yu J. Sars-Cov-2 Activates Lung Epithelial Cell Proinflammatory Signaling and Leads to Immune Dysregulation in Covid-19 Patients. EBioMedicine (2021) 70:103500. doi: 10.1016/j.ebiom.2021.103500
86. Huang J, Hume AJ, Abo KM, Werder RB, Villacorta-Martin C, Alysandratos KD, et al. Sars-Cov-2 Infection of Pluripotent Stem Cell-Derived Human Lung Alveolar Type 2 Cells Elicits a Rapid Epithelial-Intrinsic Inflammatory Response. Cell Stem Cell (2020) 27(6):962–73.e7. doi: 10.1016/j.stem.2020.09.013
87. Diamond G, Figgins EL, Robinson T, Senitko M, Abraham GE, Williams HB, et al. Examination of Gene Expression in Saliva Samples From Covid-19 Patients to Study the Host Defense Response Against Sars-Cov-2 in the Oral Cavity. Mol Oral Microbiol (2021) 36(2):157–8. doi: 10.1111/omi.12327
88. Gomez-Carballa A, Rivero-Calle I, Pardo-Seco J, Gomez-Rial J, Rivero-Velasco C, Rodriguez-Nunez N, et al. A Multi-Tissue Study of Immune Gene Expression Profiling Highlights the Key Role of the Nasal Epithelium in Covid-19 Severity. Environ Res (2022) 210:112890. doi: 10.1016/j.envres.2022.112890
89. Soares CD, Carvalho RA, Carvalho KA, Carvalho MG, Almeida OP. Letter to Editor: Oral Lesions in a Patient With Covid-19. Med Oral Patol Oral Cir Bucal (2020) 25(4):e563–e4. doi: 10.4317/medoral.24044
90. Li Y, Schneider AM, Mehta A, Sade-Feldman M, Kays KR, Gentili M, et al. Sars-Cov-2 Viremia Is Associated With Distinct Proteomic Pathways and Predicts Covid-19 Outcomes. J Clin Invest (2021) 131(13):e148635. doi: 10.1172/JCI148635
91. Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, et al. A Pneumonia Outbreak Associated With a New Coronavirus of Probable Bat Origin. Nature (2020) 579(7798):270–3. doi: 10.1038/s41586-020-2012-7
92. Arunachalam PS, Wimmers F, Mok CKP, Perera R, Scott M, Hagan T, et al. Systems Biological Assessment of Immunity to Mild Versus Severe Covid-19 Infection in Humans. Science (2020) 369(6508):1210–20. doi: 10.1126/science.abc6261
93. Stephenson E, Reynolds G, Botting RA, Calero-Nieto FJ, Morgan MD, Tuong ZK, et al. Single-Cell Multi-Omics Analysis of the Immune Response in Covid-19. Nat Med (2021) 27(5):904–16. doi: 10.1038/s41591-021-01329-2
94. Kuri-Cervantes L, Pampena MB, Meng W, Rosenfeld AM, Ittner CAG, Weisman AR, et al. Comprehensive Mapping of Immune Perturbations Associated With Severe Covid-19. Sci Immunol (2020) 5(49):eabd7114. doi: 10.1126/sciimmunol.abd7114
95. Tian Y, Carpp LN, Miller HER, Zager M, Newell EW, Gottardo R. Single-Cell Immunology of Sars-Cov-2 Infection. Nat Biotechnol (2022) 40(1):30–41. doi: 10.1038/s41587-021-01131-y
96. Meckiff BJ, Ramirez-Suastegui C, Fajardo V, Chee SJ, Kusnadi A, Simon H, et al. Imbalance of Regulatory and Cytotoxic Sars-Cov-2-Reactive Cd4(+) T Cells in Covid-19. Cell (2020) 183(5):1340–53.e16. doi: 10.1016/j.cell.2020.10.001
97. Chen YM, Zheng Y, Yu Y, Wang Y, Huang Q, Qian F, et al. Blood Molecular Markers Associated With Covid-19 Immunopathology and Multi-Organ Damage. EMBO J (2020) 39(24):e105896. doi: 10.15252/embj.2020105896
98. Xiong Y, Liu Y, Cao L, Wang D, Guo M, Jiang A, et al. Transcriptomic Characteristics of Bronchoalveolar Lavage Fluid and Peripheral Blood Mononuclear Cells in Covid-19 Patients. Emerg Microbes Infect (2020) 9(1):761–70. doi: 10.1080/22221751.2020.1747363
99. Bernardes JP, Mishra N, Tran F, Bahmer T, Best L, Blase JI, et al. Longitudinal Multi-Omics Analyses Identify Responses of Megakaryocytes, Erythroid Cells, and Plasmablasts as Hallmarks of Severe Covid-19. Immunity (2020) 53(6):1296–314.e9. doi: 10.1016/j.immuni.2020.11.017
100. Bojkova D, Klann K, Koch B, Widera M, Krause D, Ciesek S, et al. Proteomics of Sars-Cov-2-Infected Host Cells Reveals Therapy Targets. Nature (2020) 583(7816):469–72. doi: 10.1038/s41586-020-2332-7
101. Miranda J, Bringas R, Fernandez-de-Cossio J, Perera-Negrin Y. Targeting Ck2 Mediated Signaling to Impair/Tackle Sars-Cov-2 Infection: A Computational Biology Approach. Mol Med (2021) 27(1):161. doi: 10.1186/s10020-021-00424-x
102. Wyler E, Mosbauer K, Franke V, Diag A, Gottula LT, Arsie R, et al. Transcriptomic Profiling of Sars-Cov-2 Infected Human Cell Lines Identifies Hsp90 as Target for Covid-19 Therapy. iScience (2021) 24(3):102151. doi: 10.1016/j.isci.2021.102151
103. Shi R, Feng Z, Zhang X. Integrative Multi-Omics Landscape of Non-Structural Protein 3 of Severe Acute Respiratory Syndrome Coronaviruses. Genomics Proteomics Bioinf (2021) 19(5):707–726. doi: 10.1016/j.gpb.2021.09.007
104. Srinivasan S, Cui H, Gao Z, Liu M, Lu S, Mkandawire W, et al. Structural Genomics of Sars-Cov-2 Indicates Evolutionary Conserved Functional Regions of Viral Proteins. Viruses (2020) 12(4):360. doi: 10.3390/v12040360
105. Zeng HL, Chen D, Yan J, Yang Q, Han QQ, Li SS, et al. Proteomic Characteristics of Bronchoalveolar Lavage Fluid in Critical Covid-19 Patients. FEBS J (2021) 288(17):5190–200. doi: 10.1111/febs.15609
106. Ihling C, Tanzler D, Hagemann S, Kehlen A, Huttelmaier S, Arlt C, et al. Mass Spectrometric Identification of Sars-Cov-2 Proteins From Gargle Solution Samples of Covid-19 Patients. J Proteome Res (2020) 19(11):4389–92. doi: 10.1021/acs.jproteome.0c00280
107. Biji A, Khatun O, Swaraj S, Narayan R, Rajmani RS, Sardar R, et al. Identification of Covid-19 Prognostic Markers and Therapeutic Targets Through Meta-Analysis and Validation of Omics Data From Nasopharyngeal Samples. EBioMedicine (2021) 70:103525. doi: 10.1016/j.ebiom.2021.103525
108. Messner CB, Demichev V, Wendisch D, Michalick L, White M, Freiwald A, et al. Ultra-High-Throughput Clinical Proteomics Reveals Classifiers of Covid-19 Infection. Cell Syst (2020) 11(1):11–24.e4. doi: 10.1016/j.cels.2020.05.012
109. Nie X, Qian L, Sun R, Huang B, Dong X, Xiao Q, et al. Multi-Organ Proteomic Landscape of Covid-19 Autopsies. Cell (2021) 184(3):775–91.e14. doi: 10.1016/j.cell.2021.01.004
110. Monteil V, Kwon H, Prado P, Hagelkruys A, Wimmer RA, Stahl M, et al. Inhibition of Sars-Cov-2 Infections in Engineered Human Tissues Using Clinical-Grade Soluble Human Ace2. Cell (2020) 181(4):905–13.e7. doi: 10.1016/j.cell.2020.04.004
111. Carty M, Guy C, Bowie AG. Detection of Viral Infections by Innate Immunity. Biochem Pharmacol (2021) 183:114316. doi: 10.1016/j.bcp.2020.114316
112. Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, Matsui K, et al. Differential Roles of Mda5 and Rig-I Helicases in the Recognition of Rna Viruses. Nature (2006) 441(7089):101–5. doi: 10.1038/nature04734
113. Kadowaki N, Antonenko S, Lau JY, Liu YJ. Natural Interferon Alpha/Beta-Producing Cells Link Innate and Adaptive Immunity. J Exp Med (2000) 192(2):219–26. doi: 10.1084/jem.192.2.219
114. Vabret N, Britton GJ, Gruber C, Hegde S, Kim J, Kuksin M, et al. Immunology of Covid-19: Current State of the Science. Immunity (2020) 52(6):910–41. doi: 10.1016/j.immuni.2020.05.002
115. Ramasamy S, Subbian S. Critical Determinants of Cytokine Storm and Type I Interferon Response in Covid-19 Pathogenesis. Clin Microbiol Rev (2021) 34(3):e00299-20. doi: 10.1128/CMR.00299-20
116. Shang J, Ye G, Shi K, Wan Y, Luo C, Aihara H, et al. Structural Basis of Receptor Recognition by Sars-Cov-2. Nature (2020) 581(7807):221–4. doi: 10.1038/s41586-020-2179-y
117. Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh CL, Abiona O, et al. Cryo-Em Structure of the 2019-Ncov Spike in the Prefusion Conformation. Science (2020) 367(6483):1260–3. doi: 10.1126/science.abb2507
118. Glowacka I, Bertram S, Muller MA, Allen P, Soilleux E, Pfefferle S, et al. Evidence That Tmprss2 Activates the Severe Acute Respiratory Syndrome Coronavirus Spike Protein for Membrane Fusion and Reduces Viral Control by the Humoral Immune Response. J Virol (2011) 85(9):4122–34. doi: 10.1128/JVI.02232-10
119. Peng J, Sun J, Zhao J, Deng X, Guo F, Chen L. Age and Gender Differences in Ace2 and Tmprss2 Expressions in Oral Epithelial Cells. J Transl Med (2021) 19(1):358. doi: 10.1186/s12967-021-03037-4
120. Matuck BF, Dolhnikoff M, Duarte-Neto AN, Maia G, Gomes SC, Sendyk DI, et al. Salivary Glands Are a Target for Sars-Cov-2: A Source for Saliva Contamination. J Pathol (2021) 254(3):239–43. doi: 10.1002/path.5679
121. Brechbuhl J, Lopes AC, Wood D, Bouteiller S, de Valliere A, Verdumo C, et al. Age-Dependent Appearance of Sars-Cov-2 Entry Sites in Mouse Chemosensory Systems Reflects Covid-19 Anosmia-Ageusia Symptoms. Commun Biol (2021) 4(1):880. doi: 10.1038/s42003-021-02410-9
122. Doyle ME, Appleton A, Liu QR, Yao Q, Mazucanti CH, Egan JM. Human Type Ii Taste Cells Express Angiotensin-Converting Enzyme 2 and Are Infected by Severe Acute Respiratory Syndrome Coronavirus 2 (Sars-Cov-2). Am J Pathol (2021) 191(9):1511–9. doi: 10.1016/j.ajpath.2021.05.010
123. Guerini-Rocco E, Taormina SV, Vacirca D, Ranghiero A, Rappa A, Fumagalli C, et al. Sars-Cov-2 Detection in Formalin-Fixed Paraffin-Embedded Tissue Specimens From Surgical Resection of Tongue Squamous Cell Carcinoma. J Clin Pathol (2020) 73(11):754–7. doi: 10.1136/jclinpath-2020-206635
124. To KK, Tsang OT, Yip CC, Chan KH, Wu TC, Chan JM, et al. Consistent Detection of 2019 Novel Coronavirus in Saliva. Clin Infect Dis (2020) 71(15):841–3. doi: 10.1093/cid/ciaa149
125. Soares CD, Mosqueda-Taylor A, de Carvalho MGF, de Almeida OP. Oral Vesiculobullous Lesions as an Early Sign of Covid-19: Immunohistochemical Detection of Sars-Cov-2 Spike Protein. Br J Dermatol (2021) 184(1):e6. doi: 10.1111/bjd.19569
126. Pinto TG, Alpire MES, Ribeiro DA. Cytogenetic Biomonitoring in Buccal Mucosa Cells of Covid-19 Patients: Preliminary Findings. In Vivo (2021) 35(6):3495–9. doi: 10.21873/invivo.12651
127. Wang C, Wu H, Ding X, Ji H, Jiao P, Song H, et al. Does Infection of 2019 Novel Coronavirus Cause Acute and/or Chronic Sialadenitis? Med Hypotheses (2020) 140:109789. doi: 10.1016/j.mehy.2020.109789
128. Schoggins JW. Interferon-Stimulated Genes: What Do They All do? Annu Rev Virol (2019) 6(1):567–84. doi: 10.1146/annurev-virology-092818-015756
129. Diamond MS, Kanneganti TD. Innate Immunity: The First Line of Defense Against Sars-Cov-2. Nat Immunol (2022) 23(2):165–76. doi: 10.1038/s41590-021-01091-0
130. Nan Y, Nan G, Zhang YJ. Interferon Induction by Rna Viruses and Antagonism by Viral Pathogens. Viruses (2014) 6(12):4999–5027. doi: 10.3390/v6124999
131. Kasuga Y, Zhu B, Jang KJ, Yoo JS. Innate Immune Sensing of Coronavirus and Viral Evasion Strategies. Exp Mol Med (2021) 53(5):723–36. doi: 10.1038/s12276-021-00602-1
132. Makris S, Paulsen M, Johansson C. Type I Interferons as Regulators of Lung Inflammation. Front Immunol (2017) 8:259. doi: 10.3389/fimmu.2017.00259
133. Crouse J, Kalinke U, Oxenius A. Regulation of Antiviral T Cell Responses by Type I Interferons. Nat Rev Immunol (2015) 15(4):231–42. doi: 10.1038/nri3806
134. Sa Ribero M, Jouvenet N, Dreux M, Nisole S. Interplay Between Sars-Cov-2 and the Type I Interferon Response. PLoS Pathog (2020) 16(7):e1008737. doi: 10.1371/journal.ppat.1008737
135. Bagchi A, Herrup EA, Warren HS, Trigilio J, Shin HS, Valentine C, et al. Myd88-Dependent and Myd88-Independent Pathways in Synergy, Priming, and Tolerance Between Tlr Agonists. J Immunol (2007) 178(2):1164–71. doi: 10.4049/jimmunol.178.2.1164
136. Loo YM, Gale M Jr. Immune Signaling by Rig-I-Like Receptors. Immunity (2011) 34(5):680–92. doi: 10.1016/j.immuni.2011.05.003
137. Fearon DT, Locksley RM. The Instructive Role of Innate Immunity in the Acquired Immune Response. Science (1996) 272(5258):50–3. doi: 10.1126/science.272.5258.50
138. Kervevan J, Chakrabarti LA. Role of Cd4+ T Cells in the Control of Viral Infections: Recent Advances and Open Questions. Int J Mol Sci (2021) 22(2):523. doi: 10.3390/ijms22020523
139. Crotty S. T Follicular Helper Cell Differentiation, Function, and Roles in Disease. Immunity (2014) 41(4):529–42. doi: 10.1016/j.immuni.2014.10.004
140. Mubarak A, Alturaiki W, Hemida MG. Middle East Respiratory Syndrome Coronavirus (Mers-Cov): Infection, Immunological Response, and Vaccine Development. J Immunol Res (2019) 2019:6491738. doi: 10.1155/2019/6491738
141. Martonik D, Parfieniuk-Kowerda A, Rogalska M, Flisiak R. The Role of Th17 Response in Covid-19. Cells (2021) 10(6):1550. doi: 10.3390/cells10061550
142. Long QX, Liu BZ, Deng HJ, Wu GC, Deng K, Chen YK, et al. Antibody Responses to Sars-Cov-2 in Patients With Covid-19. Nat Med (2020) 26(6):845–8. doi: 10.1038/s41591-020-0897-1
143. Muecksch F, Wise H, Batchelor B, Squires M, Semple E, Richardson C, et al. Longitudinal Serological Analysis and Neutralizing Antibody Levels in Coronavirus Disease 2019 Convalescent Patients. J Infect Dis (2021) 223(3):389–98. doi: 10.1093/infdis/jiaa659
144. Wang H, Zhang Y, Huang B, Deng W, Quan Y, Wang W, et al. Development of an Inactivated Vaccine Candidate, Bbibp-Corv, With Potent Protection Against Sars-Cov-2. Cell (2020) 182(3):713–21.e9. doi: 10.1016/j.cell.2020.06.008
145. Gao Q, Bao L, Mao H, Wang L, Xu K, Yang M, et al. Development of an Inactivated Vaccine Candidate for Sars-Cov-2. Science (2020) 369(6499):77–81. doi: 10.1126/science.abc1932
146. Del Rio C, Omer SB, Malani PN. Winter of Omicron-The Evolving Covid-19 Pandemic. JAMA (2022) 327(4):319–20. doi: 10.1001/jama.2021.24315
147. Nemet I, Kliker L, Lustig Y, Zuckerman N, Erster O, Cohen C, et al. Third Bnt162b2 Vaccination Neutralization of Sars-Cov-2 Omicron Infection. N Engl J Med (2022) 386(5):492–4. doi: 10.1056/NEJMc2119358
148. Tang P, Hasan MR, Chemaitelly H, Yassine HM, Benslimane FM, Al Khatib HA, et al. Bnt162b2 and Mrna-1273 Covid-19 Vaccine Effectiveness Against the Sars-Cov-2 Delta Variant in Qatar. Nat Med (2021) 27(12):2136–43. doi: 10.1038/s41591-021-01583-4
149. Hackbart M, Deng X, Baker SC. Coronavirus Endoribonuclease Targets Viral Polyuridine Sequences to Evade Activating Host Sensors. Proc Natl Acad Sci U S A (2020) 117(14):8094–103. doi: 10.1073/pnas.1921485117
150. Deng X, Hackbart M, Mettelman RC, O’Brien A, Mielech AM, Yi G, et al. Coronavirus Nonstructural Protein 15 Mediates Evasion of Dsrna Sensors and Limits Apoptosis in Macrophages. Proc Natl Acad Sci U S A (2017) 114(21):E4251–E60. doi: 10.1073/pnas.1618310114
151. VanBlargan LA, Goo L, Pierson TC. Deconstructing the Antiviral Neutralizing-Antibody Response: Implications for Vaccine Development and Immunity. Microbiol Mol Biol Rev (2016) 80(4):989–1010. doi: 10.1128/MMBR.00024-15
152. Kwong PD, Doyle ML, Casper DJ, Cicala C, Leavitt SA, Majeed S, et al. Hiv-1 Evades Antibody-Mediated Neutralization Through Conformational Masking of Receptor-Binding Sites. Nature (2002) 420(6916):678–82. doi: 10.1038/nature01188
153. Vigerust DJ, Shepherd VL. Virus Glycosylation: Role in Virulence and Immune Interactions. Trends Microbiol (2007) 15(5):211–8. doi: 10.1016/j.tim.2007.03.003
154. Shang J, Wan Y, Luo C, Ye G, Geng Q, Auerbach A, et al. Cell Entry Mechanisms of Sars-Cov-2. Proc Natl Acad Sci U S A (2020) 117(21):11727–34. doi: 10.1073/pnas.2003138117
155. Banerjee AK, Blanco MR, Bruce EA, Honson DD, Chen LM, Chow A, et al. Sars-Cov-2 Disrupts Splicing, Translation, and Protein Trafficking to Suppress Host Defenses. Cell (2020) 183(5):1325–39.e21. doi: 10.1016/j.cell.2020.10.004
156. Wang S, Dai T, Qin Z, Pan T, Chu F, Lou L, et al. Targeting Liquid-Liquid Phase Separation of Sars-Cov-2 Nucleocapsid Protein Promotes Innate Antiviral Immunity by Elevating Mavs Activity. Nat Cell Biol (2021) 23(7):718–32. doi: 10.1038/s41556-021-00710-0
157. Sui L, Zhao Y, Wang W, Wu P, Wang Z, Yu Y, et al. Sars-Cov-2 Membrane Protein Inhibits Type I Interferon Production Through Ubiquitin-Mediated Degradation of Tbk1. Front Immunol (2021) 12:662989. doi: 10.3389/fimmu.2021.662989
158. Shin D, Mukherjee R, Grewe D, Bojkova D, Baek K, Bhattacharya A, et al. Papain-Like Protease Regulates Sars-Cov-2 Viral Spread and Innate Immunity. Nature (2020) 587(7835):657–62. doi: 10.1038/s41586-020-2601-5
159. Hsu JC, Laurent-Rolle M, Pawlak JB, Wilen CB, Cresswell P. Translational Shutdown and Evasion of the Innate Immune Response by Sars-Cov-2 Nsp14 Protein. Proc Natl Acad Sci U S A (2021) 118(24):e2101161118. doi: 10.1073/pnas.2101161118
160. Hadjadj J, Yatim N, Barnabei L, Corneau A, Boussier J, Smith N, et al. Impaired Type I Interferon Activity and Inflammatory Responses in Severe Covid-19 Patients. Science (2020) 369(6504):718–24. doi: 10.1126/science.abc6027
161. Ni W, Yang X, Yang D, Bao J, Li R, Xiao Y, et al. Role of Angiotensin-Converting Enzyme 2 (Ace2) in Covid-19. Crit Care (2020) 24(1):422. doi: 10.1186/s13054-020-03120-0
162. Brandao TB, Gueiros LA, Melo TS, Prado-Ribeiro AC, Nesrallah A, Prado GVB, et al. Oral Lesions in Patients With Sars-Cov-2 Infection: Could the Oral Cavity Be a Target Organ? Oral Surg Oral Med Oral Pathol Oral Radiol (2021) 131(2):e45–51. doi: 10.1016/j.oooo.2020.07.014
163. Orilisi G, Mascitti M, Togni L, Monterubbianesi R, Tosco V, Vitiello F, et al. Oral Manifestations of Covid-19 in Hospitalized Patients: A Systematic Review. Int J Environ Res Public Health (2021) 18(23):12511. doi: 10.3390/ijerph182312511
164. Tao K, Tzou PL, Nouhin J, Gupta RK, de Oliveira T, Kosakovsky Pond SL, et al. The Biological and Clinical Significance of Emerging Sars-Cov-2 Variants. Nat Rev Genet (2021) 22(12):757–73. doi: 10.1038/s41576-021-00408-x
165. Phan T. Genetic Diversity and Evolution of Sars-Cov-2. Infect Genet Evol (2020) 81:104260. doi: 10.1016/j.meegid.2020.104260
166. Gupta RK. Will Sars-Cov-2 Variants of Concern Affect the Promise of Vaccines? Nat Rev Immunol (2021) 21(6):340–1. doi: 10.1038/s41577-021-00556-5
167. Marchesan JT, Warner BM, Byrd KM. The “Oral” History of Covid-19: Primary Infection, Salivary Transmission, and Post-Acute Implications. J Periodontol (2021) 92(10):1357–67. doi: 10.1002/JPER.21-0277
168. Canedo-Marroquin G, Saavedra F, Andrade CA, Berrios RV, Rodriguez-Guilarte L, Opazo MC, et al. Sars-Cov-2: Immune Response Elicited by Infection and Development of Vaccines and Treatments. Front Immunol (2020) 11:569760. doi: 10.3389/fimmu.2020.569760
Keywords: COVID-19, SARS-CoV-2, immune response, multi-omics, inflammation
Citation: Hao M, Wang D, Xia Q, Kan S, Chang L, Liu H, Yang Z and Liu W (2022) Pathogenic Mechanism and Multi-omics Analysis of Oral Manifestations in COVID-19. Front. Immunol. 13:879792. doi: 10.3389/fimmu.2022.879792
Received: 21 February 2022; Accepted: 10 June 2022;
Published: 04 July 2022.
Edited by:
Andrew Davidson, University of Bristol, United KingdomReviewed by:
Yanbao Yu, University of Delaware, United StatesSantosh Kumar Swain, Siksha O Anusandhan University, India
Copyright © 2022 Hao, Wang, Xia, Kan, Chang, Liu, Yang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weiwei Liu, bGl1d2Vpd0BqbHUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
 Ming Hao
Ming Hao Dongxu Wang
Dongxu Wang Qianyun Xia2
Qianyun Xia2 Huimin Liu
Huimin Liu Zhijing Yang
Zhijing Yang Weiwei Liu
Weiwei Liu