- 1Department of Dermatology, Xiangya Hospital, Central South University, Changsha, China
- 2National Engineering Research Center of Personalized Diagnostic and Therapeutic Technology, Xiangya Hospital, Central South University, Changsha, China
- 3Hunan Key Laboratory of Skin Cancer and Psoriasis, Xiangya Hospital, Central South University, Changsha, China
- 4National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Central South University, Changsha, China
Chronic spontaneous urticaria (CSU) is defined as recurrent episodes of spontaneous wheal development and/or angioedema for more than six weeks and at least twice a week. The core link in the pathogenesis of CSU is the activation of mast cells, T cells, eosinophils, and other immune cells infiltrating around the small venules of the lesion. Increased vascular permeability, vasodilatation, and recruitment of inflammatory cells directly depend on mast cell mediators’ release. Complex regulatory systems tightly influence the critical roles of mast cells in the local microenvironment. The bias toward Th2 inflammation and autoantibodies derived from B cells, histamine expressed by basophils, and initiation of the extrinsic coagulation pathway by eosinophils or monocytes exerts powerful modulatory influences on mast cells. Cell-to-cell interactions between mast cells and eosinophils/T cells also are regulators of their function and may involve CSU’s pathomechanism. This review summarizes up-to-date knowledge regarding the crosstalk between mast cells and other immune cells, providing the impetus to develop new research concepts and treatment strategies for CSU.
Introduction
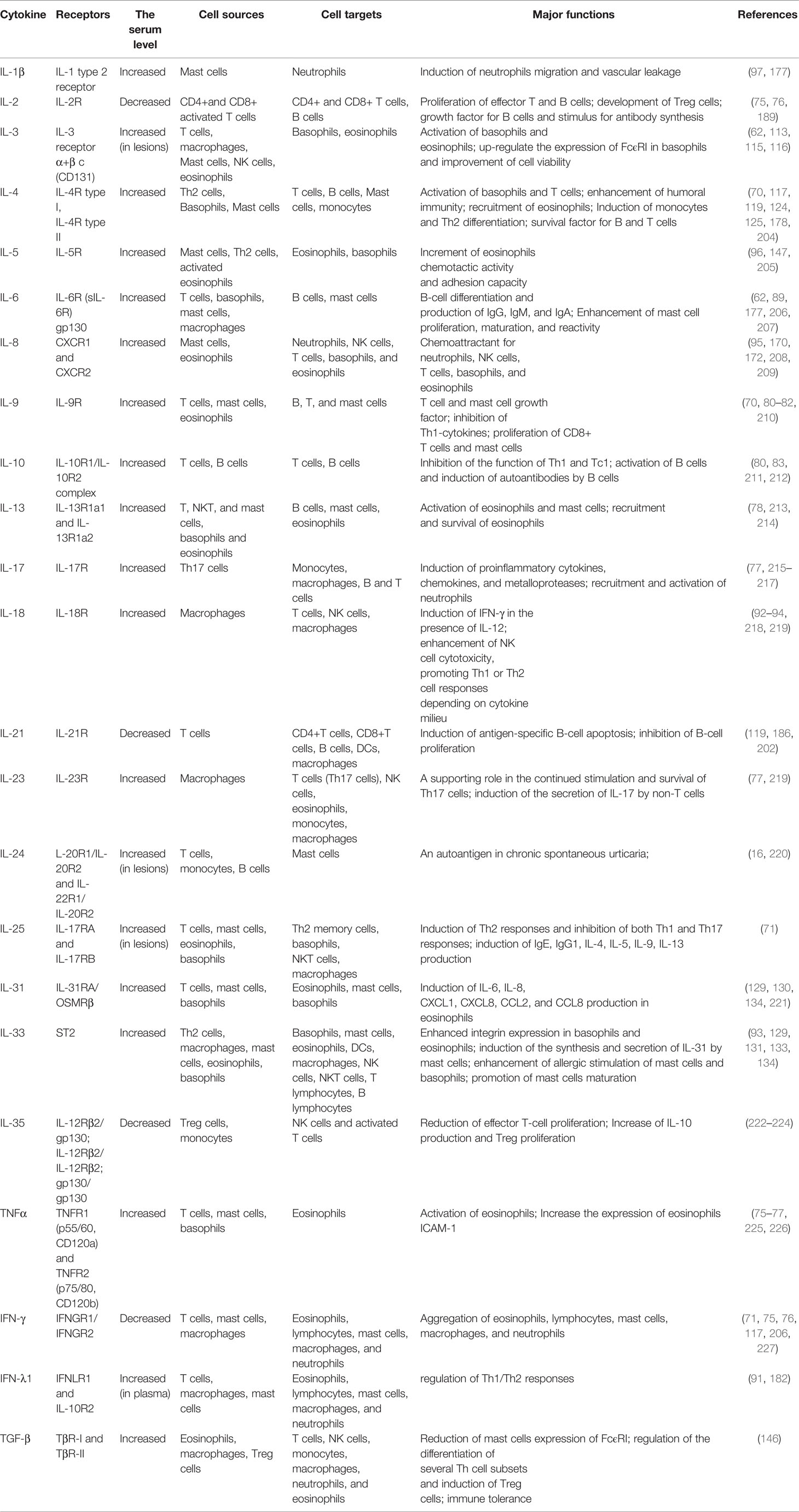
Over time, the prevalence of chronic urticaria has increased globally (1). The updated EAACI/GA2LEN/EDF/WAO guideline for chronic urticaria is now clearly divided into chronic inducible urticarias (CIndU) and chronic spontaneous urticaria (CSU), previously known as chronic idiopathic urticaria (CIU). Urticaria manifests as rapid wheals and/or angioedema, often accompanied by itching and/or burning (2). In addition, patients generally have milder systemic symptoms and may also have other autoimmune diseases, including autoimmune thyroid disease, vitiligo, rheumatoid arthritis, lupus, Type I diabetes, and psoriasis (3–5). The pathogenesis of CSU is multi-factorial, which is reported to be related to genetic factors, the environmental challenges like infections, food intolerance, the activation of coagulation cascade, dysregulation of intracellular signaling pathways within mast cells and basophils [i.e. imbalance of spleen tyrosine kinase (SYK) and Src homology 2-containing inositol 5’ phosphatase (SHIP)] and autoimmunity (Figure 1) (6–10). Autoimmunity response is considered an important mechanism in CSU pathogenesis.
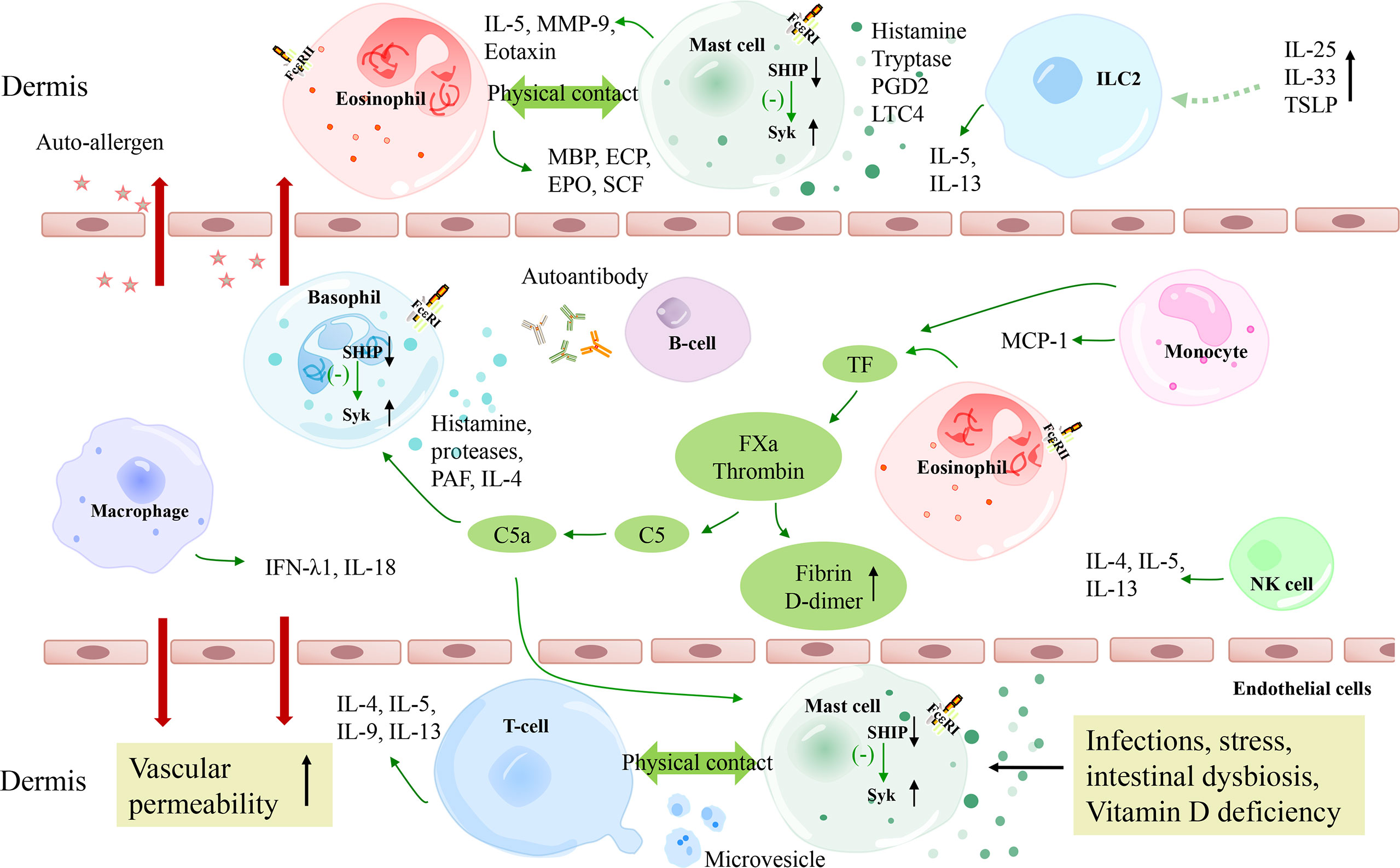
Figure 1 A schematic model of the pathogenesis of CSU. The extrinsic coagulation pathway is activated by tissue factors derived from eosinophils and monocytes, which contributes to the degranulation of mast cells and basophils. Autoimmunity, infections, stress, intestinal dysbiosis, and Vitamin D deficiency also lead to mast cell degranulation via different molecular pathways. TSLP combined with IL-25 and IL-33 are suggested to be activating factors of ILC2s, which release IL-5 and IL-13 to promote mast cell degranulation. T cells and eosinophils perform complex bidirectional crosstalk with mast cells. Besides, macrophages and NK cells may also play a role in CSU pathogenesis. C5a, complement 5a; FXa, activated factor X; TF, tissue factor; FceRI, high-affinity IgE receptor; Syk, spleen tyrosine kinase; SHIP, Src homology 2-containing inositol 5’ phosphatase; PGD2, prostaglandin D2; LTC4, Leukotriene C4; MMP-9, matrix metalloproteinase-9; TSLP, thymic stromal lymphopoietin; MCP-1, monocyte chemoattractant protein 1; ECP, eosinophil cationic protein; EPO, eosinophil peroxidase; MBP, major basic protein; SCF, stem cell factor.
There have been a couple of studies suggesting that subjects with CSU have an autoimmune basis; for example, autologous serum skin test (ASST), basophil activation test (BAT), and basophil histamine release assay (BHRA), etc. these diagnostic workups are helpful to diagnose autoimmune CSU (3, 11–13). IgG autoantibodies against IgE in patients or its high-affinity receptor (FcϵRI) are detected in nearly 45-50 percent of CSU patients (14, 15). Schmetzer et al (16) found that there are more than 200 kinds of IgE autoantigens in CSU patients and proposed that IL-24 is a common, specific and functional IgE autoantigens. IgE and IgG antibodies against thyroid peroxidase (TPO) were also found in a subgroup of CSU patients and were shown to activate mast cells (17–20). Subsequent studies have shown that IgG autoantibodies binding to FcϵRI/IgE result in mast cell degranulation and basophil activation to release a series of inflammatory mediators, finally leading to vasodilation in the lesional skin of CSU (14, 21) (Figure 2).
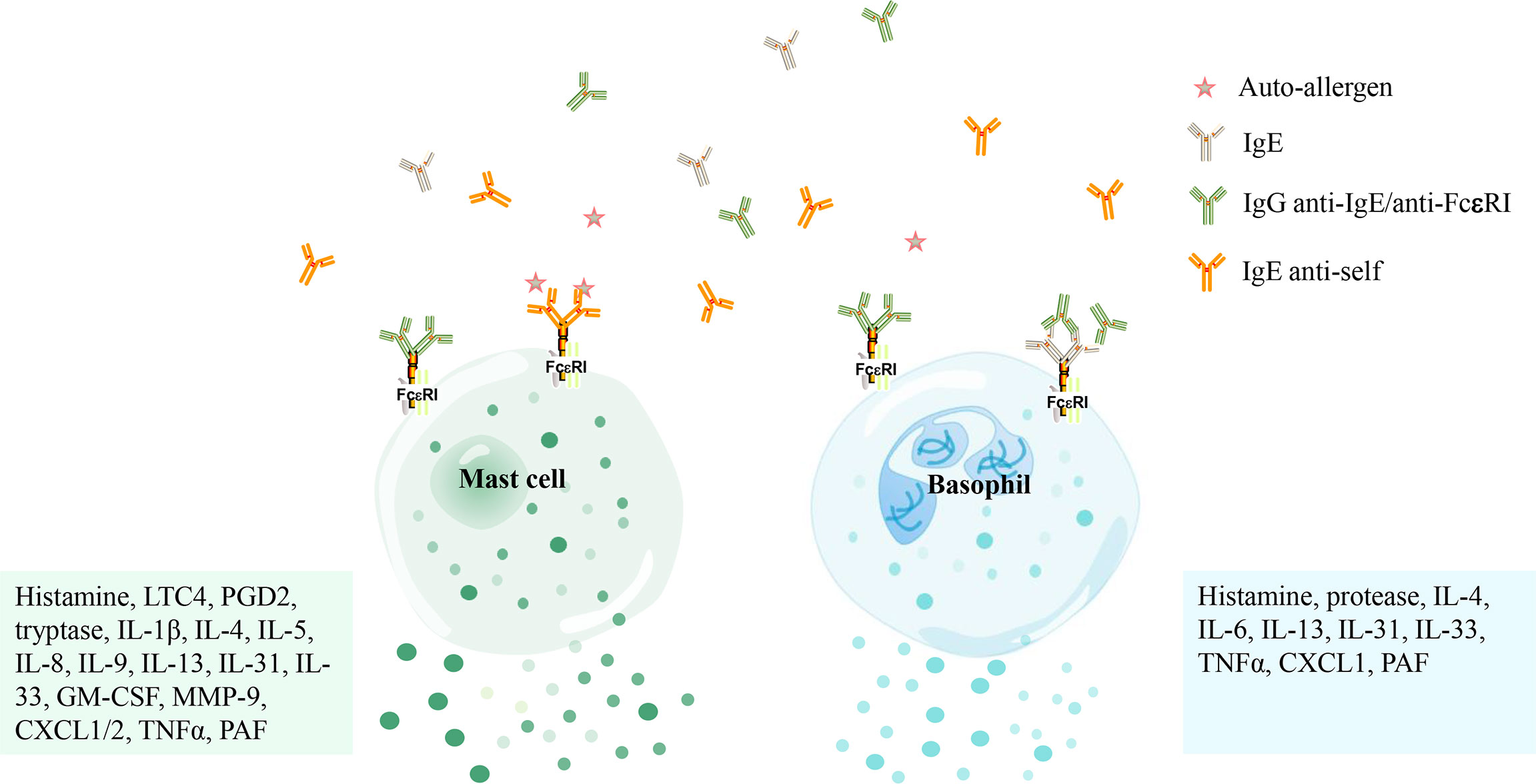
Figure 2 The activation of mast cells and basophils in patients with chronic spontaneous urticaria mediated by autoantibodies. Mast cells and basophils are activated by IgE antibodies against its high-affinity receptor (FcϵRI) or IgG antibodies against IgE/FcϵRI and release several mediators [i. e. histamine, tryptase, Leukotriene C4 (LTC4), prostaglandin D2 (PGD2), platelet-activating factor (PAF), granulocyte-macrophage colony-stimulating factor (GM-CSF), matrix metalloproteinase-9 (MMP-9), C-X-C motif chemokine ligand 1/2 (CXCL1/2), tumor necrosis factor α (TNFα), etc.] that concur to produce the marked vasodilation that stands at the basis of both wheal-and flare reaction and angioedema.
In patients with CSU, infiltrating inflammatory cells are mainly located in the dermis and deep dermis, and there is almost no difference between patients with and without autoantibodies against FcϵRI/IgE (22). Evidence shows that eosinophils, neutrophils, basophils, and macrophages in lesions are significantly higher than in healthy subjects (23–25). It is controversial whether the number of mast cells in the lesions of CSU patients is increased. Some of the studies reported an increase (23, 26), while others reported that the level of mast cells in CSU patients decreased slightly compared with that in controls (27).
It is widely known that mast cells are key contributors to CSU (8, 26). Mast cells are innate immune component cells distributed around blood vessels and nerves (28). Activation of mast cells can be triggered by various extracellular stimuli like antigen-IgE complex, cytokines expressed by other inflammatory cells, viruses, bacteria, and microvesicles (29, 30). Many past and recent studies have emphasized that IgE-dependent mast cell degranulation plays a crucial role in CSU, which could be promoted by various outer factors (cold, heat, pressure) or allergens (31–33). Beyond IgE induced activation of mast cells, Mas-related G-protein coupled receptor-X2 (MRGPRX2) has been reported to have a vital role in CSU, and the MRGPRX2 receptor is expressed at high levels in mast cells of the skin. In contrast, MRGPRX2 activation resulted in a more uniform and rapid release of individual granules from mast cells (34, 35). In addition, we learned that mast cells’ local and systemic effects are due to soluble mediators, partly through cell-to-cell contacts and microvesicles. Microvesicles are nanoscale vesicular structures secreted by various cell types and commonly found in most body fluids. Additional evidence suggests that the physical contact between mast cells and eosinophils/T cells has been observed in inflammation, although it has not been widely studied in CSU (36, 37). Microvesicles serve as vehicles for intercellular communication, with both resting and degranulating mast cells capable of secreting microvesicles. Recent reports have also documented that microvesicles derived from T cells can activate mast cells (38, 39), which is mentioned below. These studies highlight new possibilities beyond cell-to-cell contacts or soluble mediators in functional interrelationships between mast cells and other cells. Activated mast cells release preformed and de novo mediators, including histamine, tryptase, chymase, carboxypeptidase, cathepsin G, platelet-activating factor, leukotrienes, prostaglandins, cytokines, chemokines, which will lead to the increase of vascular permeability, chemotaxis of other inflammatory cells and the formation of a wheal and flare-type skin reaction (40, 41).
Here, we reviewed and summarized the immunopathogenesis of CSU, focusing on the crosstalk between immune cells involved in this disease to complete our understanding of mast cell and non-mast cell contributors to CSU.
The Crosstalk of Immune Cells in CSU
Innate immunity [such as mast cells, basophils, eosinophils, neutrophils, monocytes, macrophages, Group 2 innate lymphocytes (ILC), natural killer (NK) cells, and the complement system] and adaptive immunity (such as Th1, Th2, Th9, Th17, regulatory T cells, B cells, and antibodies) play extremely complex interactions in CSU guided through soluble inflammatory factor, microvesicles or cell-to-cell contacts. Within the frames of innate arms of immunity, Mast cells produce tryptase and chymase to activate complement, which acts on complement receptors expressed on mast cells in an autocrine manner (42). Eosinophils and monocytes release tissue factor (TF) to activate the coagulation cascade and promote complement activation (43, 44). Mast cells and eosinophils modulate each other also by forming allergic effector units (36). Besides, mast cells are vital links between innate and adaptive immunity. Mast cells involve in the initiation and dynamics of adaptive immunity through (at least) four modes of action in CSU:(a) Inducing or modulating T-cell activation and polarization; (b) Promoting the production of IgE in B cells (through IL-4, IL-13);(c) Producing TNFα and other mediators that up-regulate the expression of adhesion molecules on vascular endothelial cells to promote the recruitment of T cells;(d) Allergen-specific Th2 cells stimulate B cells to produce IgE antibodies that activate mast cells and basophils (45–47).
The changes in the release of inflammatory mediators after activation of each type of immune cell also affect other cells, including histamine, prostaglandin D2 (PGD2), major basic protein (MBP), C5a, thrombin, TF, eosinophil cationic protein (ECP), cytokines (interleukin, chemokine, interferon, and tumor necrosis factor), etc., resulting in increased vascular permeability and edema formation. The above immune cells involved in CSU and the primary activation mechanism are summarized in Table 1.
Considering the importance of mast cells in the pathogenesis of urticaria, both the crosstalk among mast cells and other immune cells (Figure 3) and the interaction between B lymphocytes and other immune cells are discussed in detail.
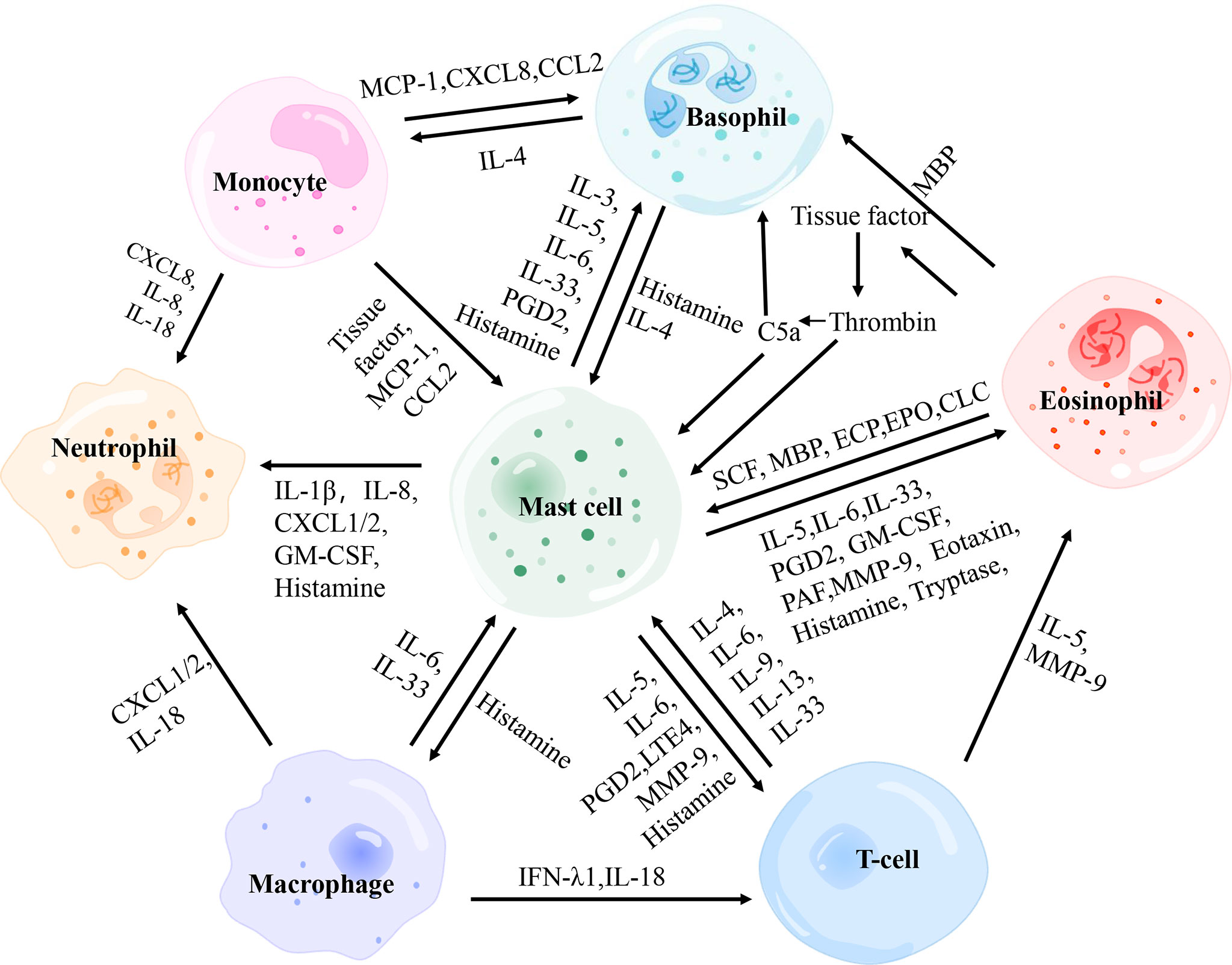
Figure 3 The interactions between main effector cells involved in chronic spontaneous urticaria. PGD2, prostaglandin D2; MBP, major basic protein; MCP-1, monocyte chemotactic and stimulating factor; CCL, C-C motif chemokine ligand; CXCL, C-X-C motif chemokine ligand; SCF, stem cell factor; ECP, eosinophil cationic protein; EPO, eosinophil peroxidase; GM-CSF, granulocyte-macrophage colony-stimulating factor; PAF, platelet-activating factor; MMP-9, matrix metalloproteinase-9; IFN-λ1, interferon-λ1.
Mast Cells and Basophils
Mast cells and basophils play a critical role in allergic inflammation originating from CD34+hemopoietic stem cells in the bone marrow, yet they differ in development, distribution, proliferation, and survival time (98). These two types of cells rapidly degranulate and release histamine after IgE stimulation. However, their ability to secrete diverse cytokines and chemokines in response to non-IgE stimulation is different (99, 100). The activation of basophils and skin mast cells with consequent release of histamine and other pro-inflammatory mediators [i.e. leukotriene C4 (LTC4), platelet-activating factor (PAF), IL-13, IL-25, CXCL8/IL-8] is responsible for vasodilation in the lesional skin of CSU. Moreover, mast cells selectively secrete several preformed mediators (I.e. heparin, tryptase, chymase, cathepsin G, carboxypeptidase A3, and renin) and PGD2 (26). Recent evidence suggests that baseline basophil count and basophil functional phenotype are linked to the efficacy of omalizumab in CSU (61, 101). In CSU, basopenia was associated with the more severe disease, while the basophil responder phenotype was associated with the more prolonged disease (102). This gives basophils a different significance in CSU than mast cells.
Histamine is released by activated mast cells and basophils; and acts on these two kinds of cells through H4R, one of the histamine receptors (103). The binding of histamine to H4R affects mast cell function mainly through the following three aspects. At first, histamine up-regulates the expression of FcϵRI on the surface of mast cells and induces mast cell activation (104). Secondly, histamine affects mast cell function by promoting intracellular calcium mobilization (105). Thirdly, H4R-mediated mast cell activation triggers the expression of several proinflammatory mediators, such as tumor necrosis factor α (TNFα), tumor growth factor-β1 (TGF-β1), macrophage inflammatory protein 1α (MIP-1α), regulated upon activation, normal T-cell expressed and secreted (RANTES), IL-4, IL-5, IL-6, IL-8, and monocyte chemoattractant protein 1 (MCP-1) (106). Histamine also induces the chemotaxis migration of basophils through H4R and regulates the IgE-dependent activation by participating in a negative feedback loop (107, 108).
Activated mast cells release PGD2 (109), an endogenous agonist of receptor chemoattractant receptor homologous molecule 2 (CRTH2) expressed on various cell types, including basophils and mast cells (110). PGD2/CRTH2 signaling pathway is believed to play a vital role in allergies. Interestingly, CRTH2 expression was inhibited in CSU patients (111). Upon binding with CRTH2, PGD2 induces intracellular calcium mobilization, up-regulates CD11b, and enhances antigen-mediated histamine release of basophils (112).
Patients with CSU have elevated levels of IL-3 in lesions; it may be released by activated mast cells (113). IL-3 is a cytokine essential for the growth and development of basophils (114). High IL-3 receptor expression on basophils was detected in ASST-positive CSU patients. In addition, IL-3 enhances the responsiveness of basophils to other stimuli and up-regulates the expression of FcϵRI in basophils of patients with CSU (115, 116).
Several research reported that the serum levels of IL-4 were reduced in CSU patients (76, 117, 118). However, other studies have suggested that the serum IL-4 level was increased (70) and plasma IL-4 levels in CSU patients are increased and positively correlated with the total IgE level (119). Since total IgE serum levels are often elevated (up to 50%) in CSU patients, normal or deficient total IgE levels were also observed (120). Therefore, the role of IL-4 in CSU is also worthy of attention. The significant expression of IL-4 in diseased skin has not been disputed (27, 48, 121). IL-4 is mainly derived from T cells, basophils, and mast cells. Robust IL-4 production of basophils occurs in response to IgE-dependent and IgE-independent stimuli (100). Additionally, IL-4 has been an effective regulator of human mast cell phenotype, growth, and differentiation (122). Some reports have shown that IL-4 synergized with IgE to upregulate the expression of FcϵRI on the mast cell surface (123). Thienemann et al. (124) elegantly described that in mature cutaneous mast cells, IL-4 treatment increased the survival rate of cutaneous mast cells. However, no effect of IL-4 on the expression of c-KIT or FcϵRI-α was observed, which means the impact of IL-4 depends on the differentiation state of mast cells. Further, IL-4 reduces the ability of mast cells to adhere to the extracellular matrix (125). Thus, basophils influence mast cells in the inflammatory site by producing IL-4.
MRGPRX2 is a receptor associated with IgE-independent activation on mast cells, basophils, and eosinophils. It has been reported that serum MRGPRX2 levels are higher in patients with severe CSU than controls (34). Previous studies have shown that MRGPRX2 and MRGPRX2 containing vesicles are found in the serum of atopic individuals to release exocytosis and plasma membrane budding (126). Mast cell-derived extracellular vesicles interact with other cells located nearby or far away, regulating inflammation; and allergic reactions (39). Whether mast cells communicate with other cells in this way remains to be verified in CSU. In addition, the involvement of MRGPRX2 is supposed to be associated with proinflammatory basophilic and eosinophilic effects, such as calcium mobilization, increased survival, and cytokine release. Mast cells produce IL-3 and IL-5, which enhance the expression of MRGPRX2, which may lead to a vicious pro-inflammatory cycle (127, 128).
IL-31 and IL-33 levels in the serum of CSU patients were higher than those in healthy controls (129). IL-31 is released from basophils after anti-IgE, IL-3, or N-formylmethionyl-leucyl-phenylalanine (fMLP) stimulation. IL-31 also induces the release of IL-4 and IL-13 from basophils (130). IL-33 in serum mainly originates from activated CD4+ T cells, and IL-33 is also released by skin mast cells, and macrophages in CSU (71). IL-33 acts through its receptor, tumorigenicity 2 receptor (ST2), which is highly expressed on the surface of mast cells, basophils, Th2 cells, eosinophils, and innate lymphocytes (131, 132). IL-33 pretreatment increases the number of activated mast cells and enhances the activation of individual mast cells (133). Although IL-33 itself does not induce mast cell degranulation, it enhances the allergic response in mast cells and basophils, promotes the maturation of mast cells, and can be released by mast cells after activation. Moreover, IL-33 induces the synthesis and secretion of IL-31 from LAD2 mast cells. The induction effect is enhanced in the presence of IgE or IgG antibodies, as is IL-4 (134). A study demonstrated a cellular crosstalk mechanism through which activated mast cells communicated with ST2-expressing basophils; stimulating these basophils produces a unique response signal including neutrophil-attracting chemokine CXCL1 (131).
Mast Cells and Eosinophils
Mast cells and eosinophils are key effector cells of CSU. Some studies suggested that there was physical contact between mast cells and eosinophils in the late and chronic stages of allergic inflammation. Curiously, a study found co-localization of mast cells and eosinophils in the urticarial area of CU patients (135). Transmission electron microscopy (TEM) showed that mast cells and eosinophils adhere to each other during co-culture in vitro (136). In other words, mast cells and eosinophils show signs of physical contact and mutual activation during co-culture. These results indicate that mast cells and eosinophils may form an effector unit in allergic diseases (Figure 4). It has been reported that this MC-Eos interplay improved the survival rate of eosinophils in vitro. There is a complex network of paracrine and membrane interactions between mast cells and eosinophils (137, 138). It was found that CD48-2B4 mediates the physical contact between mast cells and eosinophils (36). Eosinophils enhance the release of basal mast cell mediators with CD48-2B4 and jointly stimulate IgE-activated mast cells. Eosinophils also lower the IgE response threshold of mast cells by delivering co-stimulatory signals integrated into IgE-mediated pathways. However, mast cell-induced eosinophil activation does not require CD48-2B4 exposure. Mast cells induce eosinophil migration and activation via paracrine signaling. Eosinophils show enhanced expression of intercellular adhesion molecule-1 (ICAM-1), dependent on direct contact with mast cells. An increase in TNFα release has also been observed in long-term co-culture, which increases ICAM-1 in eosinophils. ICAM-1 signaling is associated with prolonged survival of eosinophils and enhanced MC-Eos adhesion. The binding of mast cell DNAX accessory molecule 1 (DNAM-1/CD226) to eosinophil Nectin-2 (CD112) has also been implicated in eosinophil-augmented activation of mast cells because CD226 synergized with FcϵRI on mast cells to promote mast cell degranulation. This co-stimulatory response might be a critical component in allergic inflammation, manifesting in ailments such as rhinitis, asthma, and CSU, which are closely related to autoimmunity (139, 140). Thus, it is crucial to demonstrate the mast cell-eosinophil interplay in skin lesions of CSU patients, and blocking this interface may have critical value in CSU therapy in the future.
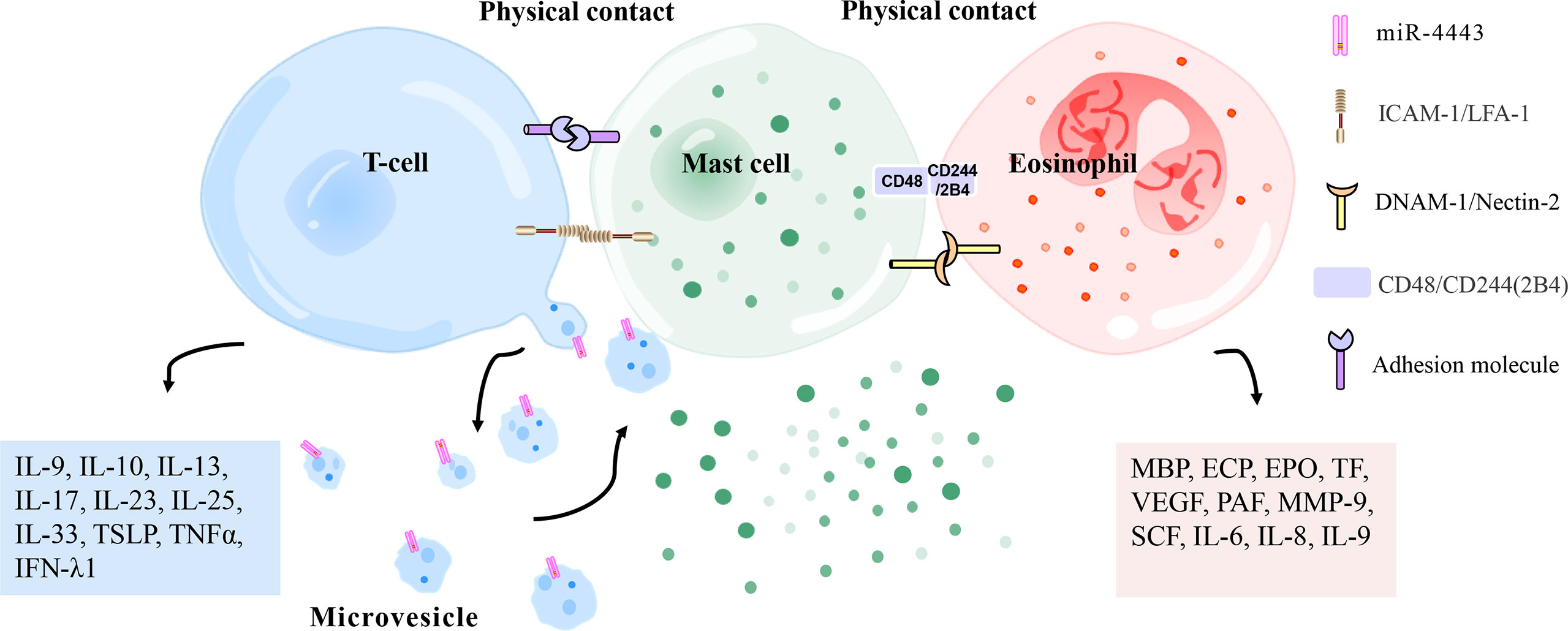
Figure 4 Physical contact between mast cells and T cells/eosinophils. Mast cells and activated T cells in the inflammation site can perform physical contact (heterotypic adhesion) mediated by adhesion molecules (i. e. ICAM-1 [on mast cells], LFA-1 [on T cells]), thereby being activated to release inflammation-related mediators (histamine, TNFα, MMP-9, interleukin, metallopeptidase inhibitor 1, etc.). Heterotypic adhesion also shows that mast cells have a broad ability to directly mediate T cell activation. In addition, mast cells can be activated by microvesicles released by T cells that carry activating factors, responding to the site of inflammation without contact with T cells. Mast cells and eosinophils have been observed in the late and chronic stages of allergic inflammation to regulate each other’s functions by forming an effect unit. CD48 (on mast cells) and CD244 (on eosinophils), DNAM-1 (on mast cells), and Nectin-2 (on eosinophils) have been reported to mediate this effect. ECP, eosinophil cationic protein; PAF, platelet-activating factor; MMP-9, matrix metalloproteinase-9; IFN-λ1, interferon-λ1; EPO, eosinophil peroxidase; MBP, major basic protein; VEGF, vascular endothelial growth factor; SCF, stem cell factor; TSLP, thymic stromal lymphopoietin; TNFα, tumor necrosis factor α; TF, tissue factor; ICAM-1, intercellular adhesion molecule-1; LFA-1, leukocyte function-related antigen-1; DNAM-1, DNAX accessory molecule 1.
In addition to physical contact, mast cells and eosinophils interact through inflammatory mediators and related receptors. Eosinophils express activated receptors of various chemokines (i.e., CCR3, CXCR3, CXCR4, CCR5, CCR6, etc.), interleukins (i.e., IL-3R, IL-4R, IL-5R, IL-13R, ST2, etc.), amines (i.e., histamine receptors), phosphoryl-associated molecular pattern molecules (i.e., Toll-like receptors), lipid mediators [CRTh2, cysteinyl leukotrienes receptor 1 (CysLT1R)] and complement systems (i.e., C3a, C5a, etc.) on their surface, as well as inhibitory receptors, such as CD300a and Sialic acid-binding immunoglobulin-type lectins (Siglecs) (109).
Mast cells recruit eosinophils to the diseased skin by releasing eotaxin, an effective agonist of CCR3 (141, 142). Mast cells release a large amount of histamine after activation, and one of the histamine receptors, H4R, is expressed on eosinophils (103). Histamine enhances the expression of eosinophil adhesion molecules through H4R, resulting in increased eosinophil migration (143). PGD2 released by mast cells also induces chemotaxis of eosinophils (110), promoting the activation of eosinophils and the release of ECP (144). Tryptase, produced by mast cells, stimulates the activation of eosinophils to produce IL-6 and IL-8 by cleavage of protease-activated receptor 2 (PAR-2) (145).
Mast cells are a significant source of IL-5 and IL-6 (49). Hong et al. (146) showed that the levels of histamine, LTC4, TNFα, TGF-β, IL-4, IL-5, and IL-6 in serum samples from patients with CSU are significantly higher than those in healthy controls. In humans, the effects of IL-5 are limited to basophils and eosinophils. The expression of IL-5Rα on basophils is three times lower than that on mature eosinophils. IL-5 plays an essential role in the initiation and survival of eosinophils as well as the proliferation and maturation of their progenitor cells. It is speculated that IL-5 is involved in the development and maintenance of the innate inflammatory process in spontaneous wheals (96, 147).
Selective expression of Siglec-8 in human eosinophils and mast cells has been demonstrated. Lirentelimab against Siglec-8 is effective in antihistamine refractory CSU (148). In eosinophils, the involvement of Siglec-8 leads to apoptosis (149), and IL-33 (produced by mast cells) triggers Siglec-8-mediated eosinophil apoptosis through β2 integrins (150). In mast cells, Siglec-8 crosslinking resulted in severe inhibition of IgE receptor-induced histamine and PGD2 release without apoptosis (151–153).
In the plasma of CSU patients, increased Plasma matrix metalloproteinase-9 (MMP-9) levels have been detected. Recent studies suggested that TNFα induced the up-regulation of these two genes in mast cells, and MMP-9 levels were correlated with disease severity in children with CSU (154–156). Mast cells, eosinophils, or activated T cells may be potential sources of MMP-9 that promotes the migration of eosinophils and lymphocytes (especially CD4+ T cells) to the skin (157).
Besides, eosinophils affect mast cells in the following ways. The extrinsic coagulation cascade in CSU is activated by eosinophil-derived TF (43, 158), triggering the production of thrombin and C5a. Thrombin acts on PARs (PAR1 and PAR4) to mediate mast cell degranulation (159). It also causes increased endothelial cell permeability, resulting in the formation of cutaneous wheals and angioedema (160). However, another study has shown that activated exogenous coagulation factors do not activate human skin mast cells and basophils by themselves but by producing C5a that acts on the C5a receptor (C5aR) (50).
Activated eosinophils release inflammatory mediators including MBP, ECP, eosinophil peroxidase (EPO) (66), which induce histamine release from mast cells and basophils through MRGPRX2 (127, 161, 162). In addition, MBP activates human mast cells through integrin-b1 (expressed on the surface of mast cells) (135). Furthermore, Research shows that eosinophil-derived stem cell factors may recruit and activate mast cells (163).
Translation control tumor protein (TCTP), also known as a histamine-releasing factor. The expression of dimer TCTP is increased in the sera of CSU patients. After stimulation with dimer TCTP, the activation of basophils and mast cells is increased dramatically. A study found that the level of TCTP dimer was positively correlated with the level of ECP, indicating that eosinophils may indirectly participate in the activation of basophils and mast cells through this mechanism (164).
Mast Cells and T Cells
Several reports have shown a complex interaction between mast cells and activated T lymphocytes at the site of inflammation (Figure 4). Mast cells and activated T lymphocytes make physical contact (heterotypic adhesion) through adhesion molecules. Mast cells express the co-stimulatory molecules CD80, CD86, and the adhesion molecule CD54 (ICAM-1), all of which are involved in T cell activation (72, 165). The interaction between mast cells and T cells is at least partially mediated by the adhesion molecule ICAM-1 and its ligand leukocyte function-related antigen-1 (LFA-1) because the addition of antibodies against these two molecules inhibits adherent-induced degranulation of mast cells (166). Activated-mast cells release inflammation-related mediators (histamine, TNFα, MMP-9, IL-4, TNFα, IL-6, etc.), which regulate extracellular matrix degradation during T cell-mediated inflammation and are also essential for leukocyte extravasation and recruitment to affected parts (167–169). This activation pathway stimulates the expression and release of IL-8, which is an effective chemokine to induce neutrophil migration (170). These studies suggest that activated T cells may play a role in the pathogenic activation of mast cells. Heterotypic adhesion suggests that mast cells have a general ability to directly mediate the activation of T cells, suggesting that human mast cells may be involved in inducing adaptive immune responses by recruiting and activating T cells in allergic reactions or autoimmune diseases. However, the limited evidence for this effect comes from the use of in vitro co-culture systems (166, 168, 171). Because of the heterogeneity of mast cells from different species and tissues, the development of models to evaluate these effects in vivo will be a significant advancement in mast cell and T cell biology. Especially in patients with CSU, mast cells and T cells are abundant in the lesion area, but whether there is heterotypic adhesion between mast cells and T cells needs to be determined by immunofluorescence or electron microscopy.
Microvesicles released by T cells are stimuli for activation of mast cells, allowing them to respond to the inflammatory site without contact with T cells (Figure 4). Activated T cells release microvesicles carrying similar mast cell activators. Thus, by releasing microvesicles, T cells deliver activated surface molecules in a way that does not require physical contact between cells and encourages mast cells to release inflammatory mediators (172). Further analysis showed that T-cell-derived microvesicles, rather than FcϵRI crosslinking, induced IL-24 gene transcription and protein production in mast cells (51). Shefler et al. (38) elegantly described that T-cell-derived microvesicles, as intercellular vectors of functional miR-4443, may regulate PTPRJ gene expression heteromorphically in mast cells, thereby regulating ERK phosphorylation and IL-8 release in mast cells. Mast cell-microvesicle interactions enable activated T cells to promote the remote contact-mediated activation of mast cells. Mast cells are activated at the inflammation sites by these pathways, which provides a new mechanism for chronic inflammatory skin disease, but their role in CSU needs to be confirmed.
Beyond physical contact and microvesicles from T cells, mast cells and T cells may also interact with each other through inflammatory mediators and related receptors. One of the histamine receptors, H4R, is also expressed on T cells (173). H4R is involved in the pathogenesis of allergies and inflammation as it activates Th2 and Th17 cells (174). Histamine mediates the enhancement of Th2 cytokine secretion (such as IL-5, IL-4, IL-10, and IL-13) and the inhibition of Th1 cytokine production (IFN- γ, IL-12, and IL-2). Thus, histamine regulates the efficient balance between Th1 and Th2 cells by aiding the transfer to Th2 cells (175). PGD2 and leukotriene E4 (LTE4) derived from mast cells promote the survival, migration, and activation of Th2 cells (176). In addition, T cells enhance mast cell proliferation, maturation, and reactivity by secreting IL-6 after FcϵRI aggregation (89, 177). T-cell-derived IL-4 also induces mast cell chemotaxis (178).
Mast Cells and Neutrophils
Mast cells influence neutrophils in the following ways. Mast cells initiate the early stage of neutrophilic recruitment by releasing the chemical inducer CXCL1/CXCL2. Upon reaching the stimulated tissue, neutrophils further penetrate the tissue in a macrophage-dependent manner (macrophages also synthesize CXCL1/CXCL2 neutrophil chemokines) (90). Serum levels of granulocyte-macrophage colony-stimulating factor (GM-CSF) were higher in ASST-positive CSU patients than in ASST-negative patients (73). GM-CSF derived from mast cells and activated by IgE cross-linking, appreciably prolongs the survival of neutrophils (179). IL-1β expression was elevated in both diseased and non-diseased skin of CSU patients, and mast cells were shown to secrete IL-1β and induced neutrophil migration and vascular leakage (177). The heterotypic adhesion of mast cells to T cells promotes the expression and release of IL-8, which is an effective chemokine that induces neutrophil migration, thereby promoting neutrophil aggregation in diseased skin (170).
Mast Cells and Monocytes
The expression of chemokines CCL2 and CXCL8 in monocytes of CSU patients was upregulated, reflecting the high responsiveness of monocytes. CXCL8/IL-8 is a chemokine and activator of various immune cells, which is related to chronic inflammatory diseases. CCL2 activates mast cells, mainly basophils (95). After being activated, monocytes release MCP-1, an influential histamine-releasing factor of mast cells and basophils, which activates mast cells and basophils, causing them to release histamine and other inflammatory mediators (180). In addition to chemokines, monocytes influence mast cells by releasing TF. Mononuclear TF expression was enhanced in CSU patients compared with healthy donors. Mononuclear TF expression may be induced by agonists of toll-like receptors 1, 2, 4, and 5, triggering exogenous coagulation pathways and increasing vascular permeability in a histamine-independent manner. This occurrence indirectly triggers the activation of mast cells and basophils, leading to the formation of wheals and angioedema (44).
Mast Cells and Macrophages
One of the histamine receptors, H4R, is also expressed in macrophages (103). In the local microenvironment dominated by Th2 cells, histamine exists at high concentrations, IL-4, and histamine-induced down-regulation of C3aR expression in human M2 macrophages. Reduced C3aR expression may have an anti-inflammatory effect in which it reduces sensitivity to C3a-induced downstream signals, thereby helping to regulate local inflammatory responses in the skin. This mechanism may be related to the pathogenesis of CSU (181).
After FcϵRI aggregation, macrophages secrete IL-6, enhancing mast cell proliferation, maturation, and reactivity (89, 177). The significant increase in IFN-λ1 (IL-29) levels in peripheral blood of CSU patients suggests that IFN-λ1 may play an important role in the pathogenesis of CSU. In the blood of CSU patients, CD8+ T cells express more IFN-λ1, and in the skin, mast cells, eosinophils, B cells, neutrophils, and macrophages may be sources of IFN-λ1 (91). IFN-λ1 has a paramount role in modulating the development of Th1 and Th2 cells (182). However, it has been reported that IFN-λ1 failed to induce histamine release from human mast cells (183). The role of IFN-λ1 in the pathogenesis of allergic inflammation requires further study.
Mast Cells and Innate Lymphoid Cells
Innate lymphoid cells (ILCs), as a recently discovered family of innate immune cells, play an essential role in autoimmune-related and inflammatory skin diseases (184). NK cells are members of the ILC family and possess the ability of cell killing and cytokine production. However, the function of NK cells in the development and effector phase of allergy is still controversial (185). The increased percentage of NK cells in peripheral blood of patients with CU suggests that innate immune pathways might contribute to wheal formation, although it has not been verified in CSU (186). Accumulating evidence indicates that the bias toward Th2 cytokine production occurs in CSU is conducive to the differentiation of NK cells into NK2 subsets, which produce Th2 cytokines (52, 187). NK2 cells are capable of producing many important cytokines, including IL-4, IL-5, and IL-13, which further aggravates the pathology of CSU (188). Consistent with this, decreased levels of IFN-γ in serum of patients with CSU suggest that NK1 cells are not dominant compared with NK2 cells (189).
The function of NK cells is also affected by cytokines produced by mast cells. IL-4 derived from mast cells drives NK cells toward a type 2 phenotype (185). Many details about the interaction between NK cells and mast cells in CSU are still unclear; however, contributions from NK cells to allergies and various skin diseases have emerged.
In addition to NK cells, the ILC family also includes Group 2 ILCs (ILC2s), enriched at mucosal barriers in the skin and associated with allergic diseases. ILC2s are critical drivers of type 2 inflammation by releasing IL-5 and IL-13 in an antigen-independent manner (190). ILC2s, located near the mast cells in the skin, perform complex bidirectional crosstalk with mast cells in the local tissue environment (191). IL-13 derived from ILC2 has been shown to modulate mast cell function and is a key factor in driving allergic reactions, which may be an interesting target for future treatment of CSU (192).
Lipid mediators, including PGD2 and LTC4, are effective modulators of ILC2 function. Mast cells also produce a variety of cytokines and chemokines, including IL-4, IL-5, IL-13, and IL-33, as well as thymic stromal lymphopoietin (TSLP). IL-4, IL-9, and TSLP act as costimulatory cytokines of ILC2s. Besides, TSLP; and IL- 9 activate STAT5 and induce ILC2 survival. IL-4, IL-9, and IL-10 also work on ILC2s in an autocrine manner to maintain cytokine secretion, forming a positive feedback loop (193–195).
IL-25, TSLP, and IL-33 are potent activators of ILC2s, which induce intense proliferation and production of cytokines (i.e. IL-5, IL-6, IL-13, GM-CSF), chemokines (eotaxin), and peptides (196). Costimulatory cytokines (IL-2, IL-7) and IL-33 synergistically promote the effective activation of ILC2s (184, 194). Recently, IL-25, IL-33, and TSLP have been shown to increase in lesional skin of CSU patients suggesting that ILC2s are important contributors to immune dysregulation and pathology of CSU (71). The frequency of ILC2s in lesional skin and non-lesional skin of CSU compared to healthy subjects needed to be confirmed in future studies.
It is essential to mention that Vitamin D deficiency was reported commonly in CSU patients (197). Vitamin D suppresses the function of ILC2s (198). The deficiency of Vitamin D may be an important factor in the functioning of ILCs in CSU pathogenesis.
In brief, ILC2s interact with mast cells and participate in driving pathology in CSU through cell interactions. Understanding the changes in ILC and mast cell-derived cytokines in local tissues during CSU will help design strategies to restore skin immune homeostasis.
Crosstalk Between B Lymphocytes and Other Immune Cells
Autoantigens (such as TPO, IL-4) in patients with CSU induce B cell production of IgE/IgG antibodies. IgE/IgG binds to FcϵRI-α of mast cells/basophils and other target cells in the FC segment. When the same antigen is contacted again, the antigen binds to two or more IgE molecules that have been bound to the target cells. FcϵRI is cross-linked, leading to a series of activation reactions and the release of many inflammatory mediators (199). Autoantibodies against low-affinity IgE receptors (FcϵRII/CD23) were found in a subgroup of CSUs, which were expressed on leukomonocytes and eosinophils. After the anti-CD23 antibody binds to CD23, eosinophils infiltrating the skin of patients release MBP, which may be related to the release of histamine by basophils (67, 200).
In addition, T cells can influence B cells by secreting cytokines. A low level of IL-21 was observed in CSU patients, which was negatively correlated with total IgE, suggesting that IL-21 may be involved in the immunopathogenesis of CSU (119). One of the functions of IL-21 is to induce apoptosis of antigen-specific B cells (201). Therefore, the decrease in IL-21 alleviates the inhibition of B cell proliferation, which may lead to an increase in B cells with the progression of CSU. IL-21 seems to be a key cytokine for maintaining low IgE levels since the lack of IL-21 greatly enhances the IgE homologous switch and antigen-driven clonal expansion of IgE+ cells, which triggers an increase in IgE and leads to the occurrence of diseases (202). IL-21 is considered to be a critical negative regulator of IgE responses (203).
IL-4 and IL-6 derived from basophils also act on B cells to enhance their survival, proliferation, and promote humoral immunity (62).
The above cytokines described in CSU subjects are summarized in Table 2.
Conclusion and Future Directions
Since approximately 45-50% of CSU patients have autoantibodies, there is no doubt that further research is needed to target the activation of immune cells by autoantibody pathways. In addition to the intervention of autoantibodies, the mechanisms of crosstalk among various immune cells include physical contact activation and other pathways. These additional pathways include heterotypic adhesion between mast cells and T cells and an effector unit formed by mast cells and neutrophils. Physical contact between cells promotes mutual activation and the release of many inflammatory factors to a certain extent. Beyond that, activated T cells also stimulate the release of histamine, IL-8, and other inflammatory mediators by mast cells through the function of microvesicles, which provides a new mechanism for the pathogenesis of chronic inflammatory skin diseases; however, this occurrence remains to be verified in CSU patients. Inflammatory mediators such as histamine, PGD2, C5a, thrombin, TF, MBP-1, ECP, and cytokines (i.e., interleukins, chemokines, interferons, and tumor necrosis factor) play an important role in regulating the activation or inhibition of immune cells through the communication network among these cells and further affect the incidence and mitigation of CSU.
Because mast cells and basophils play a major role in the pathogenesis of CSU, the current research primarily focuses on the single functions of these cells, while the implication of T cells, neutrophils, and eosinophils in this disease are still not unified. Also, whether the various types of immune cells have physical interactions remains to be determined. Along with the increase in disease rates over the years, some patients may suffer more than one episode of CSU during their lifetime. Considering the possibility of recurrence, disabling symptoms, and significant impact on quality of life, further studies are imperative to advance the understanding of pathogenic factors that trigger skin symptoms and even systemic symptoms in CSU patients, especially the specific role played by immune cells, and to assist in the selection of proper and effective therapeutics.
Author Contributions
All authors participated in the conceptual design. BZ and JL drafted the manuscript. All authors participated in reviewing and editing the manuscript and in the approval of finalized manuscript.
Funding
This work was supported by the funding from Grant No. 82073458, 81773341, 81830096, 81974476, 81773329 by the National Natural Science Foundation of China; and was supported by 2020YFA0112904 from the National Key Research and Development Project. This study also was supported by the Program of Introducing Talents of Discipline to Universities (211 Project, No. B20017) and the science and technology innovation Program of Hunan Province (2021RC4013).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Maurer M, Eyerich K, Eyerich S, Ferrer M, Gutermuth J, Hartmann K, et al. Urticaria: Collegium Internationale Allergologicum (CIA) Update 2020. Int Arch Allergy Immunol (2020) 181(5):321–33. doi: 10.1159/000507218
2. Zuberbier T, Abdul Latiff AH, Abuzakouk M, Aquilina S, Asero R, Baker D, et al. The International EAACI/GA²LEN/EuroGuiDerm/APAAACI Guideline for the Definition, Classification, Diagnosis, and Management of Urticaria. Allergy (2022) 77(3):734–66. doi: 10.1111/all.15090
3. Kolkhir P, Altrichter S, Asero R, Daschner A, Ferrer M, Giménez-Arnau A, et al. Autoimmune Diseases Are Linked to Type IIb Autoimmune Chronic Spontaneous Urticaria. Allergy Asthma Immunol Res (2021) 13(4):545–59. doi: 10.4168/aair.2021.13.4.545
4. Le M, Zhang L, Gabrielli S, Prosty C, Miles LM, Netchiporouk E, et al. Increased Prevalence of Autoimmune Diseases in Children With Chronic Spontaneous Urticaria. Pediatr Allergy Immunol (2022) 33(2):e13736. doi: 10.1111/pai.13736
5. Kolkhir P, Borzova E, Grattan C, Asero R, Pogorelov D, Maurer M. Autoimmune Comorbidity in Chronic Spontaneous Urticaria: A Systematic Review. Autoimmun Rev (2017) 16(12):1196–208. doi: 10.1016/j.autrev.2017.10.003
6. Radonjic-Hoesli S, Hofmeier KS, Micaletto S, Schmid-Grendelmeier P, Bircher A, Simon D. Urticaria and Angioedema: An Update on Classification and Pathogenesis. Clin Rev Allergy Immunol (2018) 54(1):88–101. doi: 10.1007/s12016-017-8628-1
7. MacGlashan D. Autoantibodies to IgE and Fcϵri and the Natural Variability of Spleen Tyrosine Kinase Expression in Basophils. J Allergy Clin Immunol (2019) 143(3):1100–7.e11. doi: 10.1016/j.jaci.2018.05.019
8. Yanase Y, Takahagi S, Ozawa K, Hide M. The Role of Coagulation and Complement Factors for Mast Cell Activation in the Pathogenesis of Chronic Spontaneous Urticaria. Cells (2021) 10(7):1759. doi: 10.3390/cells10071759
9. Saini SS, Paterniti M, Vasagar K, Gibbons SP Jr., Sterba PM, Vonakis BM. Cultured Peripheral Blood Mast Cells From Chronic Idiopathic Urticaria Patients Spontaneously Degranulate Upon IgE Sensitization: Relationship to Expression of Syk and SHIP-2. Clin Immunol (2009) 132(3):342–8. doi: 10.1016/j.clim.2009.05.003
10. Bansal CJ, Bansal AS. Stress, Pseudoallergens, Autoimmunity, Infection and Inflammation in Chronic Spontaneous Urticaria. Allergy Asthma Clin Immunol (2019) 15:56. doi: 10.1186/s13223-019-0372-z
11. Baumann K, Marcelino J, Skov PS, Santos MCP, Wyroslak I, Scheffel J, et al. Autologous Serum Skin Test Reactions in Chronic Spontaneous Urticaria Differ From Heterologous Cell Reactions. J Eur Acad Dermatol Venereol (2021) 35(6):1338–45. doi: 10.1111/jdv.17131
12. EL NE, Gharib K. The Role of Antifcϵriα Autoantibodies Detection and Autologous Serum Skin Test in Comparison To Histamine Release Assay in Diagnosis of Chronic Autoimmune Urticaria. Egypt J Immunol (2020) 27(1):141–55.
13. Schoepke N, Asero R, Ellrich A, Ferrer M, Gimenez-Arnau A, EHG C, et al. Biomarkers and Clinical Characteristics of Autoimmune Chronic Spontaneous Urticaria: Results of the PURIST Study. Allergy (2019) 74(12):2427–36. doi: 10.1111/all.13949
14. Asero R, Marzano AV, Ferrucci S, Lorini M, Carbonelli V, Cugno M. Co-Occurrence of IgE and IgG Autoantibodies in Patients With Chronic Spontaneous Urticaria. Clin Exp Immunol (2020) 200(3):242–9. doi: 10.1111/cei.13428
15. De Swerdt A, Van Den Keybus C, Kasran A, Cadot P, Neyens K, Coorevits L, et al. Detection of Basophil-Activating IgG Autoantibodies in Chronic Idiopathic Urticaria by Induction of CD 63. J Allergy Clin Immunol (2005) 116(3):662–7. doi: 10.1016/j.jaci.2005.04.042
16. Schmetzer O, Lakin E, Topal FA, Preusse P, Freier D, Church MK, et al. IL-24 is a Common and Specific Autoantigen of IgE in Patients With Chronic Spontaneous Urticaria. J Allergy Clin Immunol (2018) 142(3):876–82. doi: 10.1016/j.jaci.2017.10.035
17. Sánchez J, Sánchez A, Cardona R. Clinical Characterization of Patients With Chronic Spontaneous Urticaria According to Anti-TPO IgE Levels. J Immunol Res (2019) 2019:4202145. doi: 10.1155/2019/4202145
18. Kolkhir P, Metz M, Altrichter S, Maurer M. Comorbidity of Chronic Spontaneous Urticaria and Autoimmune Thyroid Diseases: A Systematic Review. Allergy (2017) 72(10):1440–60. doi: 10.1111/all.13182
19. Zhang L, Qiu L, Wu J, Qi Y, Wang H, Qi R, et al. IgE and IgG Anti-Thyroid Autoantibodies in Chinese Patients With Chronic Spontaneous Urticaria and a Literature Review. Allergy Asthma Immunol Res (2022) 14(1):131–42. doi: 10.4168/aair.2022.14.1.131
20. Kolkhir P, Kovalkova E, Chernov A, Danilycheva I, Krause K, Sauer M, et al. Autoimmune Chronic Spontaneous Urticaria Detection With IgG Anti-TPO and Total IgE. J Allergy Clin Immunol Pract (2021) 9(11):4138–46.e8. doi: 10.1016/j.jaip.2021.07.043
21. Izaki S, Toyoshima S, Endo T, Kanegae K, Nunomura S, Kashiwakura JI, et al. Differentiation Between Control Subjects and Patients With Chronic Spontaneous Urticaria Based on the Ability of Anti-IgE Autoantibodies (AAbs) to Induce Fcϵri Crosslinking, as Compared to Anti-Fcϵriα AAbs. Allergol Int (2019) 68(3):342–51. doi: 10.1016/j.alit.2019.01.003
22. Sabroe RA, Poon E, Orchard GE, Lane D, Francis DM, Barr RM, et al. Cutaneous Inflammatory Cell Infiltrate in Chronic Idiopathic Urticaria: Comparison of Patients With and Without Anti-FcepsilonRI or Anti-IgE Autoantibodies. J Allergy Clin Immunol (1999) 103(3 Pt 1):484–93. doi: 10.1016/s0091-6749(99)70475-6
23. Kay AB, Ying S, Ardelean E, Mlynek A, Kita H, Clark P, et al. Elevations in Vascular Markers and Eosinophils in Chronic Spontaneous Urticarial Weals With Low-Level Persistence in Uninvolved Skin. Br J Dermatol (2014) 171(3):505–11. doi: 10.1111/bjd.12991
24. Batista M, Calado R, Gil F, Cardoso JC, Tellechea O, Gonçalo M. Histopathology of Chronic Spontaneous Urticaria With Occasional Bruising Lesions is Not Significantly Different From Urticaria With Typical Wheals. J Cutan Pathol (2021) 48(8):1020–6. doi: 10.1111/cup.13985
25. Martins CF, Morais KL, Figueroa P, Dias NF, Valente NS, Maruta CW, et al. Histopathological and Clinical Evaluation of Chronic Spontaneous Urticaria Patients With Neutrophilic and non-Neutrophilic Cutaneous Infiltrate. Allergol Int (2018) 67(1):114–8. doi: 10.1016/j.alit.2017.06.012
26. Church MK, Kolkhir P, Metz M, Maurer M. The Role and Relevance of Mast Cells in Urticaria. Immunol Rev (2018) 282(1):232–47. doi: 10.1111/imr.12632
27. Ying S, Kikuchi Y, Meng Q, Kay AB, Kaplan AP. TH1/TH2 Cytokines and Inflammatory Cells in Skin Biopsy Specimens From Patients With Chronic Idiopathic Urticaria: Comparison With the Allergen-Induced Late-Phase Cutaneous Reaction. J Allergy Clin Immunol (2002) 109(4):694–700. doi: 10.1067/mai.2002.123236
28. Varricchi G, Marone G. Mast Cells: Fascinating But Still Elusive After 140 Years From Their Discovery. Int J Mol Sci (2020) 21(2):464. doi: 10.3390/ijms21020464
29. Cildir G, Pant H, Lopez AF, Tergaonkar V. The Transcriptional Program, Functional Heterogeneity, and Clinical Targeting of Mast Cells. J Exp Med (2017) 214(9):2491–506. doi: 10.1084/jem.20170910
30. Falduto GH, Pfeiffer A, Luker A, Metcalfe DD, Olivera A. Emerging Mechanisms Contributing to Mast Cell-Mediated Pathophysiology With Therapeutic Implications. Pharmacol Ther (2021) 220:107718. doi: 10.1016/j.pharmthera.2020.107718
31. Panaszek B, Pawłowicz R, Grzegrzółka J, Obojski A. Autoreactive IgE in Chronic Spontaneous/Idiopathic Urticaria and Basophil/Mastocyte Priming Phenomenon, as a Feature of Autoimmune Nature of the Syndrome. Arch Immunol Ther Exp (Warsz) (2017) 65(2):137–43. doi: 10.1007/s00005-016-0417-7
32. Ertas R, Ozyurt K, Atasoy M, Hawro T, Maurer M. The Clinical Response to Omalizumab in Chronic Spontaneous Urticaria Patients is Linked to and Predicted by IgE Levels and Their Change. Allergy (2018) 73(3):705–12. doi: 10.1111/all.13345
33. Sánchez J, Amaya E, Acevedo A, Celis A, Caraballo D, Cardona R. Prevalence of Inducible Urticaria in Patients With Chronic Spontaneous Urticaria: Associated Risk Factors. J Allergy Clin Immunol Pract (2017) 5(2):464–70. doi: 10.1016/j.jaip.2016.09.029
34. Cao TBT, Cha HY, Yang EM, Ye YM. Elevated MRGPRX2 Levels Related to Disease Severity in Patients With Chronic Spontaneous Urticaria. Allergy Asthma Immunol Res (2021) 13(3):498–506. doi: 10.4168/aair.2021.13.3.498
35. Roy S, Chompunud Na Ayudhya C, Thapaliya M, Deepak V, Ali H. Multifaceted MRGPRX2: New Insight Into the Role of Mast Cells in Health and Disease. J Allergy Clin Immunol (2021) 148(2):293–308. doi: 10.1016/j.jaci.2021.03.049
36. Gangwar RS, Pahima H, Puzzovio PG, Levi-Schaffer F. Update on Eosinophil Interaction With Mast Cells: The Allergic Effector Unit. Methods Mol Biol (2021) 2241:221–42. doi: 10.1007/978-1-0716-1095-4_18
37. Mekori YA. Hershko AyT Cell-Mediated Modulation of Mast Cell Function: Heterotypic Adhesion-Induced Stimulatory or Inhibitory Effects. Front Immunol (2012) 3:6. doi: 10.3389/fimmu.2012.00006
38. Shefler I, Salamon P, Levi-Schaffer F, Mor A, Hershko AY, Mekori YA. MicroRNA-4443 Regulates Mast Cell Activation by T Cell-Derived Microvesicles. J Allergy Clin Immunol (2018) 141(6):2132–41.e4. doi: 10.1016/j.jaci.2017.06.045
39. Shefler I, Salamon P, Mekori YA. Extracellular Vesicles as Emerging Players in Intercellular Communication: Relevance in Mast Cell-Mediated Pathophysiology. Int J Mol Sci (2021) 22(17):9176. doi: 10.3390/ijms22179176
40. Wilcock A, Bahri R, Bulfone-Paus S, Arkwright PD. Mast Cell Disorders: From Infancy to Maturity. Allergy (2019) 74(1):53–63. doi: 10.1111/all.13657
41. Theoharides TC, Tsilioni I, Ren H. Recent Advances in Our Understanding of Mast Cell Activation - or Should it be Mast Cell Mediator Disorders? Expert Rev Clin Immunol (2019) 15(6):639–56. doi: 10.1080/1744666x.2019.1596800
42. Elieh Ali Komi D, Shafaghat F, Kovanen PT, Meri S. Mast Cells and Complement System: Ancient Interactions Between Components of Innate Immunity. Allergy (2020) 75(11):2818–28. doi: 10.1111/all.14413
43. Cugno M, Marzano AV, Tedeschi A, Fanoni D, Venegoni L, Asero R. Expression of Tissue Factor by Eosinophils in Patients With Chronic Urticaria. Int Arch Allergy Immunol (2009) 148(2):170–4. doi: 10.1159/000155748
44. Saito R, Yanase Y, Kamegashira A, Takahagi S, Tanaka A, Uchida K, et al. Increase of Tissue Factor Expression on the Surface of Peripheral Monocytes of Patients With Chronic Spontaneous Urticaria. Allergy (2020) 75(4):971–4. doi: 10.1111/all.14110
45. Katsoulis-Dimitriou K, Kotrba J, Voss M, Dudeck J, Dudeck A. Mast Cell Functions Linking Innate Sensing to Adaptive Immunity. Cells (2020) 9(12):2538. doi: 10.3390/cells9122538
46. Voss M, Kotrba J, Gaffal E, Katsoulis-Dimitriou K, Dudeck A. Mast Cells in the Skin: Defenders of Integrity or Offenders in Inflammation? Int J Mol Sci (2021) 22(9):4589. doi: 10.3390/ijms22094589
47. Cardamone C, Parente R, Feo GD, Triggiani M. Mast Cells as Effector Cells of Innate Immunity and Regulators of Adaptive Immunity. Immunol Lett (2016) 178:10–4. doi: 10.1016/j.imlet.2016.07.003
48. Caproni M, Volpi W, Macchia D, Giomi B, Manfredi M, Campi P, et al. Infiltrating Cells and Related Cytokines in Lesional Skin of Patients With Chronic Idiopathic Urticaria and Positive Autologous Serum Skin Test. Exp Dermatol (2003) 12(5):621–8. doi: 10.1034/j.1600-0625.2003.00010.x
49. Mukai K, Tsai M, Saito H, Galli SJ. Mast Cells as Sources of Cytokines, Chemokines, and Growth Factors. Immunol Rev (2018) 282(1):121–50. doi: 10.1111/imr.12634
50. Yanase Y, Matsuo Y, Takahagi S, Kawaguchi T, Uchida K, Ishii K, et al. Coagulation Factors Induce Human Skin Mast Cell and Basophil Degranulation via Activation of Complement 5 and the C5a Receptor. J Allergy Clin Immunol (2021) 147(3):1101–4.e7. doi: 10.1016/j.jaci.2020.08.018
51. Shefler I, Pasmanik-Chor M, Kidron D, Mekori YA, Hershko AY. T Cell-Derived Microvesicles Induce Mast Cell Production of IL-24: Relevance to Inflammatory Skin Diseases. J Allergy Clin Immunol (2014) 133(1):217–24.e1-3. doi: 10.1016/j.jaci.2013.04.035
52. Giménez-Arnau AM, DeMontojoye L, Asero R, Cugno M, Kulthanan K, Yanase Y, et al. The Pathogenesis of Chronic Spontaneous Urticaria: The Role of Infiltrating Cells. J Allergy Clin Immunol Pract (2021) 9(6):2195–208. doi: 10.1016/j.jaip.2021.03.033
53. Gimborn K, Lessmann E, Kuppig S, Krystal G, Huber M. SHIP Down-Regulates FcepsilonR1-Induced Degranulation at Supraoptimal IgE or Antigen Levels. J Immunol (2005) 174(1):507–16. doi: 10.4049/jimmunol.174.1.507
54. Wang D, Tang H, Shen Y, Wang F, Lin J, Xu J. Activation of the Blood Coagulation System in Patients With Chronic Spontaneous Urticaria. Clin Lab (2015) 61(9):1283–8. doi: 10.7754/clin.lab.2015.150226
55. Yanase Y, Takahagi S, Hide M. Chronic Spontaneous Urticaria and the Extrinsic Coagulation System. Allergol Int (2018) 67(2):191–4. doi: 10.1016/j.alit.2017.09.003
56. Peng S, Zhang T, Zhang S, Tang Q, Yan Y, Feng H. Integrated Bioinformatics and Validation Reveal IL1B and Its Related Molecules as Potential Biomarkers in Chronic Spontaneous Urticaria. Front Immunol (2022) 13:850993. doi: 10.3389/fimmu.2022.850993
57. He L, Yi W, Huang X, Long H, Lu Q. Chronic Urticaria: Advances in Understanding of the Disease and Clinical Management. Clin Rev Allergy Immunol (2021) 61(3):424–48. doi: 10.1007/s12016-021-08886-x
58. Asero R, Marzano AV, Ferrucci S, Genovese G, Cugno M. Baseline D-Dimer Plasma Levels Correlate With Disease Activity But Not With the Response to Omalizumab in Chronic Spontaneous Urticaria. Allergy (2019) 74(12):2538. doi: 10.1111/all.13936
59. Cugno M, Asero R, Ferrucci S, Lorini M, Carbonelli V, Tedeschi A, et al. Elevated IgE to Tissue Factor and Thyroglobulin are Abated by Omalizumab in Chronic Spontaneous Urticaria. Allergy (2018) 73(12):2408–11. doi: 10.1111/all.13587
60. Yanase Y, Morioke S, Iwamoto K, Takahagi S, Uchida K, Kawaguchi T, et al. Histamine and Toll-Like Receptor Ligands Synergistically Induce Endothelial Cell Gap Formation by the Extrinsic Coagulating Pathway. J Allergy Clin Immunol (2018) 141(3):1115–8.e7. doi: 10.1016/j.jaci.2017.07.026
61. Johal KJ, Chichester KL, Oliver ET, Devine KC, Bieneman AP, Schroeder JT, et al. The Efficacy of Omalizumab Treatment in Chronic Spontaneous Urticaria is Associated With Basophil Phenotypes. J Allergy Clin Immunol (2021) 147(6):2271–80.e8. doi: 10.1016/j.jaci.2021.02.038
62. Miyake K, Shibata S, Yoshikawa S, Karasuyama H. Basophils and Their Effector Molecules in Allergic Disorders. Allergy (2021) 76(6):1693–706. doi: 10.1111/all.14662
63. MacDonald SM, Vonakis BM. Association of the Src Homology 2 Domain-Containing Inositol 5' Phosphatase (SHIP) to Releasability in Human Basophils. Mol Immunol (2002) 38(16-18):1323–7. doi: 10.1016/s0161-5890(02)00082-2
64. Vonakis BM, Vasagar K, Gibbons SP Jr., Gober L, Sterba PM, Chang H, et al. Basophil FcepsilonRI Histamine Release Parallels Expression of Src-Homology 2-Containing Inositol Phosphatases in Chronic Idiopathic Urticaria. J Allergy Clin Immunol (2007) 119(2):441–8. doi: 10.1016/j.jaci.2006.09.035
65. MacGlashan D. Autoantibodies to IgE and FcepsilonRI and the Natural Variability of Spleen Tyrosine Kinase Expression in Basophils. J Allergy Clin Immunol (2019) 143(3):1100–7 e11. doi: 10.1016/j.jaci.2018.05.019
66. Altrichter S, Frischbutter S, Fok JS, Kolkhir P, Jiao Q, Skov PS, et al. The Role of Eosinophils in Chronic Spontaneous Urticaria. J Allergy Clin Immunol (2020) 145(6):1510–6. doi: 10.1016/j.jaci.2020.03.005
67. Puccetti A, Bason C, Simeoni S, Millo E, Tinazzi E, Beri R, et al. In Chronic Idiopathic Urticaria Autoantibodies Against Fc epsilonRII/CD23 Induce Histamine Release via Eosinophil Activation. Clin Exp Allergy (2005) 35(12):1599–607. doi: 10.1111/j.1365-2222.2005.02380.x
68. Sánchez J, Sánchez A, Munera M, Garcia E, Lopez JF, Velásquez-Lopera M, et al. Presence of IgE Autoantibodies Against Eosinophil Peroxidase and Eosinophil Cationic Protein in Severe Chronic Spontaneous Urticaria and Atopic Dermatitis. Allergy Asthma Immunol Res (2021) 13(5):746–61. doi: 10.4168/aair.2021.13.5.746
69. Kolkhir P, Church MK, Altrichter S, Skov PS, Hawro T, Frischbutter S, et al. Eosinopenia, in Chronic Spontaneous Urticaria, Is Associated With High Disease Activity, Autoimmunity, and Poor Response to Treatment. J Allergy Clin Immunol Pract (2020) 8(1):318–25.e5. doi: 10.1016/j.jaip.2019.08.025
70. Zheng R, Qian L, Yu J, Li M, Qian Q. Analysis of the Changes in Th9 Cells and Related Cytokines in the Peripheral Blood of Spontaneous Urticaria Patients. BioMed Rep (2017) 6(6):633–9. doi: 10.3892/br.2017.904
71. Kay AB, Clark P, Maurer M, Ying S. Elevations in T-Helper-2-Initiating Cytokines (Interleukin-33, Interleukin-25 and Thymic Stromal Lymphopoietin) in Lesional Skin From Chronic Spontaneous ('Idiopathic') Urticaria. Br J Dermatol (2015) 172(5):1294–302. doi: 10.1111/bjd.13621
72. Elieh Ali Komi D, Grauwet K. Role of Mast Cells in Regulation of T Cell Responses in Experimental and Clinical Settings. Clin Rev Allergy Immunol (2018) 54(3):432–45. doi: 10.1007/s12016-017-8646-z
73. Chen Q, Zhong H, Chen WC, Zhai Z, Zhou Z, Song Z, et al. Different Expression Patterns of Plasma Th1-, Th2-, Th17- and Th22-Related Cytokines Correlate With Serum Autoreactivity and Allergen Sensitivity in Chronic Spontaneous Urticaria. J Eur Acad Dermatol Venereol (2018) 32(3):441–8. doi: 10.1111/jdv.14541
74. Auyeung P, Mittag D, Hodgkin PD, Harrison LC. Autoreactive T Cells in Chronic Spontaneous Urticaria Target the IgE Fc Receptor Ialpha Subunit. J Allergy Clin Immunol (2016) 138(3):761–8 e4. doi: 10.1016/j.jaci.2016.04.036
75. Lopes A, Machado D, Pedreiro S, Henriques A, Silva I, Tavares B, et al. Different Frequencies of Tc17/Tc1 and Th17/Th1 Cells in Chronic Spontaneous Urticaria. Int Arch Allergy Immunol (2013) 161(2):155–62. doi: 10.1159/000345401
76. Dos Santos JC, Azor MH, Nojima VY, Lourenço FD, Prearo E, Maruta CW, et al. Increased Circulating Pro-Inflammatory Cytokines and Imbalanced Regulatory T-Cell Cytokines Production in Chronic Idiopathic Urticaria. Int Immunopharmacol (2008) 8(10):1433–40. doi: 10.1016/j.intimp.2008.05.016
77. Atwa MA, Emara AS, Youssef N, Bayoumy NM. Serum Concentration of IL-17, IL-23 and TNF-Alpha Among Patients With Chronic Spontaneous Urticaria: Association With Disease Activity and Autologous Serum Skin Test. J Eur Acad Dermatol Venereol (2014) 28(4):469–74. doi: 10.1111/jdv.12124
78. Caproni M, Cardinali C, Giomi B, Antiga E, D'Agata A, Walter S, et al. Serological Detection of Eotaxin, IL-4, IL-13, IFN-Gamma, MIP-1alpha, TARC and IP-10 in Chronic Autoimmune Urticaria and Chronic Idiopathic Urticaria. J Dermatol Sci (2004) 36(1):57–9. doi: 10.1016/j.jdermsci.2004.07.006
79. Sun RS, Sui JF, Chen XH, Ran XZ, Yang ZF, Guan WD, et al. Detection of CD4+ CD25+ FOXP3+ Regulatory T Cells in Peripheral Blood of Patients With Chronic Autoimmune Urticaria. Australas J Dermatol (2011) 52(3):e15–8. doi: 10.1111/j.1440-0960.2010.00658.x
80. Feng H, Feng J, Zhang Z, Xu Q, Hu M, Wu Y, et al. Role of IL-9 and IL-10 in the Pathogenesis of Chronic Spontaneous Urticaria Through the JAK/STAT Signalling Pathway. Cell Biochem Funct (2020) 38(4):480–9. doi: 10.1002/cbf.3481
81. Li J, Chen S, Xiao X, Zhao Y, Ding W, Li XC. IL-9 and Th9 Cells in Health and Diseases-From Tolerance to Immunopathology. Cytokine Growth Factor Rev (2017) 37:47–55. doi: 10.1016/j.cytogfr.2017.07.004
82. Sehra S, Yao W, Nguyen ET, Glosson-Byers NL, Akhtar N, Zhou B, et al. TH9 Cells are Required for Tissue Mast Cell Accumulation During Allergic Inflammation. J Allergy Clin Immunol (2015) 136(2):+. doi: 10.1016/j.jaci.2015.01.021
83. Bedke T, Muscate F, Soukou S, Gagliani N, Huber S. Title: IL-10-Producing T Cells and Their Dual Functions. Semin Immunol (2019) 44:101335. doi: 10.1016/j.smim.2019.101335
84. Mohamed RW, Fathy A, el-Sayed AE. Increased Circulating FcepsilonRII-Bearing B-Lymphocytes and Serum Levels of IL-4 in non-Autoreactive Chronic Idiopathic Urticaria. Egypt J Immunol (2003) 10(2):9–18.
85. Altrichter S, Zampeli V, Ellrich A, Zhang K, Church MK, Maurer M. IgM and IgA in Addition to IgG Autoantibodies Against FcvarepsilonRIalpha are Frequent and Associated With Disease Markers of Chronic Spontaneous Urticaria. Allergy (2020) 75(12):3208–15. doi: 10.1111/all.14412
86. Giménez-Arnau AM, Salman A. Targeted Therapy for Chronıc Spontaneous Urtıcarıa: Ratıonale and Recent Progress. Drugs (2020) 80(16):1617–34. doi: 10.1007/s40265-020-01387-9
87. Jang JH, Yang EM, Lee Y, Ye YM, Moon J, Ryu MS, et al. Increased Serum Free IgE Levels in Patients With Chronic Spontaneous Urticaria (CSU). World Allergy Organ J (2022) 15(2):100629. doi: 10.1016/j.waojou.2022.100629
88. Altrichter S, Zampeli V, Ellrich A, Zhang K, Church MK, Maurer M. IgM and IgA in Addition to IgG Autoantibodies Against Fcεriα are Frequent and Associated With Disease Markers of Chronic Spontaneous Urticaria. Allergy (2020) 75(12):3208–15. doi: 10.1111/all.14412
89. Desai A, Jung MY, Olivera A, Gilfillan AM, Prussin C, Kirshenbaum AS, et al. IL-6 Promotes an Increase in Human Mast Cell Numbers and Reactivity Through Suppression of Suppressor of Cytokine Signaling 3. J Allergy Clin Immunol (2016) 137(6):1863–71 e6. doi: 10.1016/j.jaci.2015.09.059
90. De Filippo K, Dudeck A, Hasenberg M, Nye E, van Rooijen N, Hartmann K, et al. Mast Cell and Macrophage Chemokines CXCL1/CXCL2 Control the Early Stage of Neutrophil Recruitment During Tissue Inflammation. Blood (2013) 121(24):4930–7. doi: 10.1182/blood-2013-02-486217
91. Wang SF, Gao XQ, Xu YN, Li DN, Wang HY, He SH. Elevated Plasma Level of Interferon-Lambda1 in Chronic Spontaneous Urticaria: Upregulated Expression in CD8(+) and Epithelial Cells and Induction of Inflammatory Cell Accumulation. Mediators Inflamm (2016) 2016:5032051. doi: 10.1155/2016/5032051
92. Kurt E, Aktas A, Aksu K, Keren M, Dokumacioglu A, Goss CH, et al. Autologous Serum Skin Test Response in Chronic Spontaneous Urticaria and Respiratory Diseases and its Relationship With Serum Interleukin-18 Level. Arch Dermatol Res (2011) 303(9):643–9. doi: 10.1007/s00403-011-1144-x
93. Puxeddu I, Italiani P, Giungato P, Pratesi F, Panza F, Bartaloni D, et al. Free IL-18 and IL-33 Cytokines in Chronic Spontaneous Urticaria. Cytokine (2013) 61(3):741–3. doi: 10.1016/j.cyto.2013.01.015
94. Rasool R, Ashiq I, Shera IA, Yousuf Q, Shah ZA. Study of Serum Interleukin (IL) 18 and IL-6 Levels in Relation With the Clinical Disease Severity in Chronic Idiopathic Urticaria Patients of Kashmir (North India). Asia Pac Allergy (2014) 4(4):206–11. doi: 10.5415/apallergy.2014.4.4.206
95. Santos JC, de Brito CA, Futata EA, Azor MH, Orii NM, Maruta CW, et al. Up-Regulation of Chemokine C-C Ligand 2 (CCL2) and C-X-C Chemokine 8 (CXCL8) Expression by Monocytes in Chronic Idiopathic Urticaria. Clin Exp Immunol (2012) 167(1):129–36. doi: 10.1111/j.1365-2249.2011.04485.x
96. Caproni M, Giomi B, Melani L, Volpi W, Antiga E, Torchia D, et al. Cellular Infiltrate and Related Cytokines, Chemokines, Chemokine Receptors and Adhesion Molecules in Chronic Autoimmune Urticaria: Comparison Between Spontaneous and Autologous Serum Skin Test Induced Wheal. Int J Immunopathol Pharmacol (2006) 19(3):507–15. doi: 10.1177/039463200601900306
97. Nakamura Y, Kambe N, Saito M, Nishikomori R, Kim YG, Murakami M, et al. Mast Cells Mediate Neutrophil Recruitment and Vascular Leakage Through the NLRP3 Inflammasome in Histamine-Independent Urticaria. J Exp Med (2009) 206(5):1037–46. doi: 10.1084/jem.20082179
98. Otsuka A, Kabashima K. Mast Cells and Basophils in Cutaneous Immune Responses. Allergy (2015) 70(2):131–40. doi: 10.1111/all.12526
99. Varricchi G, Raap U, Rivellese F, Marone G, Gibbs BF. Human Mast Cells and Basophils-How are They Similar How are They Different? Immunol Rev (2018) 282(1):8–34. doi: 10.1111/imr.12627
100. Miyake K, Shibata S, Yoshikawa S, Karasuyama H. Basophils and Their Effector Molecules in Allergic Disorders. Allergy (2021) 76(6):1693–706. doi: 10.1111/all.14662
101. Deza G, Bertolín-Colilla M, Sánchez S, Soto D, Pujol RM, Gimeno R, et al. Basophil FcεRI Expression is Linked to Time to Omalizumab Response in Chronic Spontaneous Urticaria. J Allergy Clin Immunol (2018) 141(6):2313–6.e1. doi: 10.1016/j.jaci.2018.02.021
102. Huang AH, Chichester KL, Saini SS. Association of Basophil Parameters With Disease Severity and Duration in Chronic Spontaneous Urticaria (CSU). J Allergy Clin Immunol Pract (2020) 8(2):793–5.e6. doi: 10.1016/j.jaip.2019.08.004
103. Thangam EB, Jemima EA, Singh H, Baig MS, Khan M, Mathias CB, et al. The Role of Histamine and Histamine Receptors in Mast Cell-Mediated Allergy and Inflammation: The Hunt for New Therapeutic Targets. Front Immunol (2018) 9:1873. doi: 10.3389/fimmu.2018.01873
104. Mirzahosseini A, Dalmadi B, Csutora P. Histamine Receptor H4 Regulates Mast Cell Degranulation and IgE Induced Fcϵri Upregulation in Murine Bone Marrow-Derived Mast Cells. Cell Immunol (2013) 283(1-2):38–44. doi: 10.1016/j.cellimm.2013.05.006
105. Hofstra CL, Desai PJ, Thurmond RL, Fung-Leung WP. Histamine H4 Receptor Mediates Chemotaxis and Calcium Mobilization of Mast Cells. J Pharmacol Exp Ther (2003) 305(3):1212–21. doi: 10.1124/jpet.102.046581
106. Jemima EA, Prema A, Thangam EB. Functional Characterization of Histamine H4 Receptor on Human Mast Cells. Mol Immunol (2014) 62(1):19–28. doi: 10.1016/j.molimm.2014.05.007
107. Kaplan AP. Basophil Histamine Release in Patients With Chronic Spontaneous Urticaria: Optimize or Minimize. J Allergy Clin Immunol (2019) 144(2):622–3. doi: 10.1016/j.jaci.2019.05.004
108. Mommert S, Kleiner S, Gehring M, Eiz-Vesper B, Stark H, Gutzmer R, et al. Human Basophil Chemotaxis and Activation are Regulated via the Histamine H4 Receptor. Allergy (2016) 71(9):1264–73. doi: 10.1111/all.12875
109. Gangwar RS, Landolina N, Arpinati L, Levi-Schaffer F. Mast Cell and Eosinophil Surface Receptors as Targets for Anti-Allergic Therapy. Pharmacol Ther (2017) 170:37–63. doi: 10.1016/j.pharmthera.2016.10.010
110. Nakashima C, Otsuka A, Kabashima K. Recent Advancement in the Mechanism of Basophil Activation. J Dermatol Sci (2018) 91(1):3–8. doi: 10.1016/j.jdermsci.2018.03.007
111. Oliver ET, Sterba PM, Devine K, Vonakis BM, Saini SS. Altered Expression of Chemoattractant Receptor-Homologous Molecule Expressed on T(H)2 Cells on Blood Basophils and Eosinophils in Patients With Chronic Spontaneous Urticaria. J Allergy Clin Immunol (2016) 137(1):304–6 e1. doi: 10.1016/j.jaci.2015.06.004
112. Yoshimura-Uchiyama C, Iikura M, Yamaguchi M, Nagase H, Ishii A, Matsushima K, et al. Differential Modulation of Human Basophil Functions Through Prostaglandin D2 Receptors DP and Chemoattractant Receptor-Homologous Molecule Expressed on Th2 Cells/DP2. Clin Exp Allergy (2004) 34(8):1283–90. doi: 10.1111/j.1365-2222.2004.02027.x
113. Hermes B, Prochazka AK, Haas N, Jurgovsky K, Sticherling M, Henz BM. Upregulation of TNF-Alpha and IL-3 Expression in Lesional and Uninvolved Skin in Different Types of Urticaria. J Allergy Clin Immunol (1999) 103(2 Pt 1):307–14. doi: 10.1016/s0091-6749(99)70506-3
114. Varricchi G, Poto R, Marone G, Schroeder JT. IL-3 in the Development and Function of Basophils. Semin Immunol (2021) 54:101510. doi: 10.1016/j.smim.2021.101510
115. Zellweger F, Buschor P, Hobi G, Brigger D, Dahinden CA, Villiger PM, et al. IL-3 But Not Monomeric IgE Regulates FcepsilonRI Levels and Cell Survival in Primary Human Basophils. Cell Death Dis (2018) 9(5):510. doi: 10.1038/s41419-018-0526-9
116. Lourenço FD, Azor MH, Santos JC, Prearo E, Maruta CW, Rivitti EA, et al. Activated Status of Basophils in Chronic Urticaria Leads to Interleukin-3 Hyper-Responsiveness and Enhancement of Histamine Release Induced by Anti-IgE Stimulus. Br J Dermatol (2008) 158(5):979–86. doi: 10.1111/j.1365-2133.2008.08499.x
117. Degirmenci PB, Kırmaz C, Vatansever S, Onur E, Nal E, Erdin S, et al. Analysis of the Association of Chronic Spontaneous Urticaria With Interlekin-4, -10, Transforming Growth Factor-β1, Interferon-γ, Interleukin-17A and -23 by Autologous Serum Skin Test. Postepy Dermatol Alergol (2017) 34(1):70–6. doi: 10.5114/pdia.2016.57679
118. Confino-Cohen R, Goldberg A, Aharoni D, Naiman L, Buchs A, Weiss M, et al. Low Stimulated IL-4 Secretion in PBMC From Patients With Chronic Idiopathic Urticaria. Cytokine (2004) 27(2-3):74–80. doi: 10.1016/j.cyto.2004.03.016
119. Gao C, Chen WC, Liu W, Chen Q, Chen S, Xu Y, et al. Pathogenic Role of Circulating CD4(+)CXCR5(+) Cell Subpopulations in Patients With Chronic Spontaneous Urticarial. Am J Transl Res (2020) 12(8):4434–44.
120. Altrichter S, Fok JS, Jiao Q, Kolkhir P, Pyatilova P, Romero SM, et al. Total IgE as a Marker for Chronic Spontaneous Urticaria. Allergy Asthma Immunol Res (2021) 13(2):206–18. doi: 10.4168/aair.2021.13.2.206
121. Caproni M, Giomi B, Volpi W, Melani L, Schincaglia E, Macchia D, et al. Chronic Idiopathic Urticaria: Infiltrating Cells and Related Cytokines in Autologous Serum-Induced Wheals. Clin Immunol (2005) 114(3):284–92. doi: 10.1016/j.clim.2004.10.007
122. Babina M, Guhl S, Artuc M, Zuberbier T. IL-4 and Human Skin Mast Cells Revisited: Reinforcement of a Pro-Allergic Phenotype Upon Prolonged Exposure. Arch Dermatol Res (2016) 308(9):665–70. doi: 10.1007/s00403-016-1688-x
123. Yamaguchi M, Sayama K, Yano K, Lantz CS, Noben-Trauth N, Ra C, et al. IgE Enhances Fc Epsilon Receptor I Expression and IgE-Dependent Release of Histamine and Lipid Mediators From Human Umbilical Cord Blood-Derived Mast Cells: Synergistic Effect of IL-4 and IgE on Human Mast Cell Fc Epsilon Receptor I Expression and Mediator Release. J Immunol (1999) 162(9):5455–65.
124. Thienemann F, Henz BM, Babina M. Regulation of Mast Cell Characteristics by Cytokines: Divergent Effects of Interleukin-4 on Immature Mast Cell Lines Versus Mature Human Skin Mast Cells. Arch Dermatol Res (2004) 296(3):134–8. doi: 10.1007/s00403-004-0486-z
125. Lorentz A, Schuppan D, Gebert A, Manns MP, Bischoff SC. Regulatory Effects of Stem Cell Factor and Interleukin-4 on Adhesion of Human Mast Cells to Extracellular Matrix Proteins. Blood (2002) 99(3):966–72. doi: 10.1182/blood.v99.3.966
126. An J, Lee JH, Won HK, Kang Y, Song WJ, Kwon HS, et al. Clinical Significance of Serum MRGPRX2 as a New Biomarker in Allergic Asthma. Allergy (2020) 75(4):959–62. doi: 10.1111/all.14084
127. Shtessel M, Limjunyawong N, Oliver ET, Chichester K, Gao L, Dong X, et al. MRGPRX2 Activation Causes Increased Skin Reactivity in Patients With Chronic Spontaneous Urticaria. J Invest Dermatol (2020) 141(3):678–81.e2. doi: 10.1016/j.jid.2020.06.030
128. Wedi B, Gehring M, Kapp A. The Pseudoallergen Receptor MRGPRX2 on Peripheral Blood Basophils and Eosinophils: Expression and Function. Allergy (2020) 75(9):2229–42. doi: 10.1111/all.14213
129. Lin W, Zhou Q, Liu C, Ying M, Xu S. Increased Plasma IL-17, IL-31, and IL-33 Levels in Chronic Spontaneous Urticaria. Sci Rep (2017) 7(1):17797. doi: 10.1038/s41598-017-18187-z
130. Raap U, Gehring M, Kleiner S, Rudrich U, Eiz-Vesper B, Haas H, et al. Human Basophils are a Source of - and are Differentially Activated by - IL-31. Clin Exp Allergy (2017) 47(4):499–508. doi: 10.1111/cea.12875
131. Hsu CL, Chhiba KD, Krier-Burris R, Hosakoppal S, Berdnikovs S, Miller ML, et al. Allergic Inflammation is Initiated by IL-33-Dependent Crosstalk Between Mast Cells and Basophils. PloS One (2020) 15(1):e0226701. doi: 10.1371/journal.pone.0226701
132. Cayrol C. IL-33, an Alarmin of the IL-1 Family Involved in Allergic and Non Allergic Inflammation: Focus on the Mechanisms of Regulation of Its Activity. Cells (2021) 11(1):107. doi: 10.3390/cells11010107
133. Joulia R, L'Faqihi FE, Valitutti S, Espinosa E. IL-33 Fine Tunes Mast Cell Degranulation and Chemokine Production at the Single-Cell Level. J Allergy Clin Immunol (2017) 140(2):497–509 e10. doi: 10.1016/j.jaci.2016.09.049
134. Petra AI, Tsilioni I, Taracanova A, Katsarou-Katsari A, Theoharides TC. Interleukin 33 and Interleukin 4 Regulate Interleukin 31 Gene Expression and Secretion From Human Laboratory of Allergic Diseases 2 Mast Cells Stimulated by Substance P and/or Immunoglobulin E. Allergy Asthma Proc (2018) 39(2):153–60. doi: 10.2500/aap.2018.38.4105
135. Fujisawa D, Kashiwakura J, Kita H, Kikukawa Y, Fujitani Y, Sasaki-Sakamoto T, et al. Expression of Mas-Related Gene X2 on Mast Cells is Upregulated in the Skin of Patients With Severe Chronic Urticaria. J Allergy Clin Immunol (2014) 134(3):622–33.e9. doi: 10.1016/j.jaci.2014.05.004
136. Minai-Fleminger Y, Elishmereni M, Vita F, Soranzo MR, Mankuta D, Zabucchi G, et al. Ultrastructural Evidence for Human Mast Cell-Eosinophil Interactions In Vitro. Cell Tissue Res (2010) 341(3):405–15. doi: 10.1007/s00441-010-1010-8
137. Elishmereni M, Alenius HT, Bradding P, Mizrahi S, Shikotra A, Minai-Fleminger Y, et al. Physical Interactions Between Mast Cells and Eosinophils: A Novel Mechanism Enhancing Eosinophil Survival In Vitro. Allergy (2011) 66(3):376–85. doi: 10.1111/j.1398-9995.2010.02494.x
138. Stassen M, Hartmann AK, Delgado SJ, Dehmel S, Braun A. Mast Cells Within Cellular Networks. J Allergy Clin Immunol (2019) 144(4s):S46–s54. doi: 10.1016/j.jaci.2019.01.031
139. Bachelet I, Munitz A, Mankutad D, Levi-Schaffer F. Mast Cell Costimulation by CD226/CD112 (DNAM-1/Nectin-2): A Novel Interface in the Allergic Process. J Biol Chem (2006) 281(37):27190–6. doi: 10.1074/jbc.M602359200
140. Elishmereni M, Bachelet I, Levi-Schaffer F. DNAM-1: An Amplifier of Immune Responses as a Therapeutic Target in Various Disorders. Curr Opin Investig Drugs (2008) 9(5):491–6.
141. Asero R, Cugno M, Tedeschi A. Eosinophils in Chronic Urticaria: Supporting or Leading Actors? World Allergy Organ J (2009) 2(9):213–7. doi: 10.1097/WOX.0b013e3181bb965f
142. Tedeschi A, Asero R, Lorini M, Marzano AV, Cugno M. Serum Eotaxin Levels in Patients With Chronic Spontaneous Urticaria. Eur Ann Allergy Clin Immunol (2012) 44(5):188–92.
143. Grosicki M, Wójcik T, Chlopicki S, Kieć-Kononowicz K. In Vitro Study of Histamine and Histamine Receptor Ligands Influence on the Adhesion of Purified Human Eosinophils to Endothelium. Eur J Pharmacol (2016) 777:49–59. doi: 10.1016/j.ejphar.2016.02.061
144. Oliver ET, Chichester K, Devine K, Sterba PM, Wegner C, Vonakis BM, et al. Effects of an Oral CRTh2 Antagonist (AZD1981) on Eosinophil Activity and Symptoms in Chronic Spontaneous Urticaria. Int Arch Allergy Immunol (2019) 179(1):21–30. doi: 10.1159/000496162
145. Temkin V, Kantor B, Weg V, Hartman ML, Levi-Schaffer F. Tryptase Activates the Mitogen-Activated Protein Kinase/Activator Protein-1 Pathway in Human Peripheral Blood Eosinophils, Causing Cytokine Production and Release. J Immunol (2002) 169(5):2662–9. doi: 10.4049/jimmunol.169.5.2662
146. Hong GU, Ro JY, Bae Y, Kwon IH, Park GH, Choi YH, et al. Association of TG2 From Mast Cells and Chronic Spontaneous Urticaria Pathogenesis. Ann Allergy Asthma Immunol (2016) 117(3):290–7. doi: 10.1016/j.anai.2016.06.026
147. Hassani M, Koenderman L. Immunological and Hematological Effects of IL-5(Rα)-Targeted Therapy: An Overview. Allergy (2018) 73(10):1979–88. doi: 10.1111/all.13451
148. Altrichter S, Staubach P, Pasha M, Singh B, Chang AT, Bernstein JA, et al. An Open-Label, Proof-of-Concept Study of Lirentelimab for Antihistamine-Resistant Chronic Spontaneous and Inducible Urticaria. J Allergy Clin Immunol (2021) 149,5(2022): 1683–90.e7. doi: 10.1016/j.jaci.2021.12.772
149. Legrand F, Cao Y, Wechsler JB, Zhu X, Zimmermann N, Rampertaap S, et al. Sialic Acid-Binding Immunoglobulin-Like Lectin (Siglec) 8 in Patients With Eosinophilic Disorders: Receptor Expression and Targeting Using Chimeric Antibodies. J Allergy Clin Immunol (2019) 143(6):2227–37 e10. doi: 10.1016/j.jaci.2018.10.066
150. Robida PA, Puzzovio PG, Pahima H, Levi-Schaffer F, Bochner BS. Human Eosinophils and Mast Cells: Birds of a Feather Flock Together. Immunol Rev (2018) 282(1):151–67. doi: 10.1111/imr.12638
151. Bochner BS. Siglec-8 on Human Eosinophils and Mast Cells, and Siglec-F on Murine Eosinophils, are Functionally Related Inhibitory Receptors. Clin Exp Allergy (2009) 39(3):317–24. doi: 10.1111/j.1365-2222.2008.03173.x
152. Kiwamoto T, Kawasaki N, Paulson JC, Bochner BS. Siglec-8 as a Drugable Target to Treat Eosinophil and Mast Cell-Associated Conditions. Pharmacol Ther (2012) 135(3):327–36. doi: 10.1016/j.pharmthera.2012.06.005
153. Youngblood BA, Leung J, Falahati R, Williams J, Schanin J, Brock EC, et al. Discovery, Function, and Therapeutic Targeting of Siglec-8. Cells (2020) 10(1):19. doi: 10.3390/cells10010019
154. Antiga E, Volpi W, Del Bianco E, Fabbri P, Caproni M. Plasma Levels of Metalloproteinase-9 are Elevated in Patients With Chronic Autoimmune Urticaria. Br J Dermatol (2009) 161(3):712–4. doi: 10.1111/j.1365-2133.2009.09368.x
155. Kolkhir P, André F, Church MK, Maurer M, Metz M. Potential Blood Biomarkers in Chronic Spontaneous Urticaria. Clin Exp Allergy (2017) 47(1):19–36. doi: 10.1111/cea.12870
156. Dilek F, Ozceker D, Ozkaya E, Tamay Z, Yazici M, Kesgin S, et al. Plasma Levels of Matrix Metalloproteinase-9 in Children With Chronic Spontaneous Urticaria. Allergy Asthma Immunol Res (2016) 8(6):522–6. doi: 10.4168/aair.2016.8.6.522
157. Kessel A, Bishara R, Amital A, Bamberger E, Sabo E, Grushko G, et al. Increased Plasma Levels of Matrix Metalloproteinase-9 are Associated With the Severity of Chronic Urticaria. Clin Exp Allergy (2005) 35(2):221–5. doi: 10.1111/j.1365-2222.2005.02168.x
158. Moosbauer C, Morgenstern E, Cuvelier SL, Manukyan D, Bidzhekov K, Albrecht S, et al. Eosinophils are a Major Intravascular Location for Tissue Factor Storage and Exposure. Blood (2007) 109(3):995–1002. doi: 10.1182/blood-2006-02-004945
159. Fang X, Li M, Zhang W, Li J, Zhu T. Thrombin Induces Pro-Inflammatory and Anti-Inflammatory Cytokines Secretion From Human Mast Cell Line (HMC-1) via Protease-Activated Receptors. Mol Immunol (2022) 141:60–9. doi: 10.1016/j.molimm.2021.11.012
160. Asero R, Tedeschi A, Riboldi P, Cugno M. Plasma of Patients With Chronic Urticaria Shows Signs of Thrombin Generation, and its Intradermal Injection Causes Wheal-and-Flare Reactions Much More Frequently Than Autologous Serum. J Allergy Clin Immunol (2006) 117(5):1113–7. doi: 10.1016/j.jaci.2005.12.1343
161. Subramanian H, Gupta K, Ali H. Roles of Mas-Related G Protein-Coupled Receptor X2 on Mast Cell-Mediated Host Defense, Pseudoallergic Drug Reactions, and Chronic Inflammatory Diseases. J Allergy Clin Immunol (2016) 138(3):700–10. doi: 10.1016/j.jaci.2016.04.051
162. O'Donnell MC, Ackerman SJ, Gleich GJ, Thomas LL. Activation of Basophil and Mast Cell Histamine Release by Eosinophil Granule Major Basic Protein. J Exp Med (1983) 157(6):1981–91. doi: 10.1084/jem.157.6.1981
163. Piliponsky AM, Gleich GJ, Nagler A, Bar I, Levi-Schaffer F. Non-IgE-Dependent Activation of Human Lung- and Cord Blood-Derived Mast Cells is Induced by Eosinophil Major Basic Protein and Modulated by the Membrane Form of Stem Cell Factor. Blood (2003) 101(5):1898–904. doi: 10.1182/blood-2002-05-1488
164. Ulambayar B, Lee H, Yang EM, Park HS, Lee K, Ye YM. Dimerized, Not Monomeric, Translationally Controlled Tumor Protein Induces Basophil Activation and Mast Cell Degranulation in Chronic Urticaria. Immune Netw (2019) 19(3):e20. doi: 10.4110/in.2019.19.e20
165. Hershko AY, Rivera J. Mast Cell and T Cell Communication; Amplification and Control of Adaptive Immunity. Immunol Lett (2010) 128(2):98–104. doi: 10.1016/j.imlet.2009.10.013
166. Inamura N, Mekori YA, Bhattacharyya SP, Bianchine PJ, Metcalfe DD. Induction and Enhancement of Fc(epsilon)RI-Dependent Mast Cell Degranulation Following Coculture With Activated T Cells: Dependency on ICAM-1- and Leukocyte Function-Associated Antigen (LFA)-1-Mediated Heterotypic Aggregation. J Immunol (1998) 160(8):4026–33.
167. Baram D, Vaday GG, Salamon P, Drucker I, Hershkoviz R, Mekori YA. Human Mast Cells Release Metalloproteinase-9 on Contact With Activated T Cells: Juxtacrine Regulation by TNF-Alpha. J Immunol (2001) 167(7):4008–16. doi: 10.4049/jimmunol.167.7.4008
168. Mekori YA, Baram D. Heterotypic Adhesion-Induced Mast Cell Activation: Biologic Relevance in the Inflammatory Context. Mol Immunol (2002) 38(16-18):1363–7. doi: 10.1016/s0161-5890(02)00089-5
169. Bhattacharyya SP, Drucker I, Reshef T, Kirshenbaum AS, Metcalfe DD, Mekori YA. Activated T Lymphocytes Induce Degranulation and Cytokine Production by Human Mast Cells Following Cell-to-Cell Contact. J Leukoc Biol (1998) 63(3):337–41. doi: 10.1002/jlb.63.3.337
170. Salamon P, Shoham NG, Gavrieli R, Wolach B, Mekori YA. Human Mast Cells Release Interleukin-8 and Induce Neutrophil Chemotaxis on Contact With Activated T Cells. Allergy (2005) 60(10):1316–9. doi: 10.1111/j.1398-9995.2005.00886.x
171. Kashiwakura J, Yokoi H, Saito H, Okayama Y. T Cell Proliferation by Direct Cross-Talk Between OX40 Ligand on Human Mast Cells and OX40 on Human T Cells: Comparison of Gene Expression Profiles Between Human Tonsillar and Lung-Cultured Mast Cells. J Immunol (2004) 173(8):5247–57. doi: 10.4049/jimmunol.173.8.5247
172. Shefler I, Salamon P, Reshef T, Mor A, Mekori YA. T Cell-Induced Mast Cell Activation: A Role for Microparticles Released From Activated T Cells. J Immunol (2010) 185(7):4206–12. doi: 10.4049/jimmunol.1000409
173. Dunford PJ, O'Donnell N, Riley JP, Williams KN, Karlsson L, Thurmond RL. The Histamine H4 Receptor Mediates Allergic Airway Inflammation by Regulating the Activation of CD4+ T Cells. J Immunol (2006) 176(11):7062–70. doi: 10.4049/jimmunol.176.11.7062
174. Mommert S, Gschwandtner M, Koether B, Gutzmer R, Werfel T. Human Memory Th17 Cells Express a Functional Histamine H4 Receptor. Am J Pathol (2012) 180(1):177–85. doi: 10.1016/j.ajpath.2011.09.028
175. Jutel M, Akdis CA. Histamine as an Immune Modulator in Chronic Inflammatory Responses. Clin Exp Allergy (2007) 37(3):308–10. doi: 10.1111/j.1365-2222.2007.02666.x
176. Xue L, Fergusson J, Salimi M, Panse I, Ussher JE, Hegazy AN, et al. Prostaglandin D2 and Leukotriene E4 Synergize to Stimulate Diverse TH2 Functions and TH2 Cell/Neutrophil Crosstalk. J Allergy Clin Immunol (2015) 135(5):1358–66.e1. doi: 10.1016/j.jaci.2014.09.006
177. de Montjoye L, Choteau M, Herman A, Hendrickx E, Cheou P, Baeck M, et al. IL-6 and IL-1beta Expression is Increased in Autologous Serum Skin Test of Patients With Chronic Spontaneous Urticaria. Allergy (2019) 74(12):2522–4. doi: 10.1111/all.13928
178. Olsson N, Taub DD, Nilsson G. Regulation of Mast Cell Migration by T and T Cytokines: Identification of Tumour Necrosis Factor-Alpha and Interleukin-4 as Mast Cell Chemotaxins. Scand J Immunol (2004) 59(3):267–72. doi: 10.1111/j.0300-9475.2004.01397.x
179. Lee KMC, Achuthan AA, Hamilton JA. GM-CSF: A Promising Target in Inflammation and Autoimmunity. Immunotargets Ther (2020) 9:225–40. doi: 10.2147/itt.S262566
180. Alam R, Lett-Brown MA, Forsythe PA, Anderson-Walters DJ, Kenamore C, Kormos C, et al. Monocyte Chemotactic and Activating Factor is a Potent Histamine-Releasing Factor for Basophils. J Clin Invest (1992) 89(3):723–8. doi: 10.1172/jci115648
181. Mommert S, Aslan D, Ratz L, Stark H, Gutzmer R, Werfel T. The Anaphylatoxin C3a Receptor Expression on Human M2 Macrophages Is Down-Regulated by Stimulating the Histamine H4 Receptor and the IL-4 Receptor. J Innate Immun (2018) 10(4):349–62. doi: 10.1159/000490426
182. Jordan WJ, Eskdale J, Srinivas S, Pekarek V, Kelner D, Rodia M, et al. Human Interferon Lambda-1 (IFN-Lambda1/IL-29) Modulates the Th1/Th2 Response. Genes Immun (2007) 8(3):254–61. doi: 10.1038/sj.gene.6364382
183. He S, Zhang H, Chen H, Yang H, Huang T, Chen Y, et al. Expression and Release of IL-29 by Mast Cells and Modulation of Mast Cell Behavior by IL-29. Allergy (2010) 65(10):1234–41. doi: 10.1111/j.1398-9995.2010.02349.x
184. Zhou S, Li Q, Wu H, Lu Q. The Pathogenic Role of Innate Lymphoid Cells in Autoimmune-Related and Inflammatory Skin Diseases. Cell Mol Immunol (2020) 17(4):335–46. doi: 10.1038/s41423-020-0399-6
185. Deniz G, van de Veen W, Akdis M. Natural Killer Cells in Patients With Allergic Diseases. J Allergy Clin Immunol (2013) 132(3):527–35. doi: 10.1016/j.jaci.2013.07.030
186. Huilan Z, Runxiang L, Bihua L, Qing G. Role of the Subgroups of T, B, Natural Killer Lymphocyte and Serum Levels of Interleukin-15, Interleukin-21 and Immunoglobulin E in the Pathogenesis of Urticaria. J Dermatol (2010) 37(5):441–7. doi: 10.1111/j.1346-8138.2010.00805.x
187. Zitti B, Bryceson YT. Natural Killer Cells in Inflammation and Autoimmunity. Cytokine Growth Factor Rev (2018) 42:37–46. doi: 10.1016/j.cytogfr.2018.08.001
188. Kucuksezer UC, Aktas Cetin E, Esen F, Tahrali I, Akdeniz N, Gelmez MY, et al. The Role of Natural Killer Cells in Autoimmune Diseases. Front Immunol (2021) 12:622306. doi: 10.3389/fimmu.2021.622306
189. Movahedi M, Tavakol M, Rahmani F, Amirzargar AA, Bidoki AZ, Heidari K, et al. Single Nucleotide Polymorphisms of IL-2, But Not IL-12 and IFN-γ, are Associated With Increased Susceptibility to Chronic Spontaneous Urticaria. Allergol Immunopathol (Madr) (2017) 45(4):333–8. doi: 10.1016/j.aller.2016.10.009
190. Akdis CA, Arkwright PD, Brüggen MC, Busse W, Gadina M, Guttman-Yassky E, et al. Type 2 Immunity in the Skin and Lungs. Allergy (2020) 75(7):1582–605. doi: 10.1111/all.14318
191. Orimo K, Tamari M, Saito H, Matsumoto K, Nakae S, Morita H. Characteristics of Tissue-Resident ILCs and Their Potential as Therapeutic Targets in Mucosal and Skin Inflammatory Diseases. Allergy (2021) 76(11):3332–48. doi: 10.1111/all.14863
192. Roediger B, Kyle R, Yip KH, Sumaria N, Guy TV, Kim BS, et al. Cutaneous Immunosurveillance and Regulation of Inflammation by Group 2 Innate Lymphoid Cells. Nat Immunol (2013) 14(6):564–73. doi: 10.1038/ni.2584
193. Sun H, Wu Y, Zhang Y, Ni B. IL-10-Producing ILCs: Molecular Mechanisms and Disease Relevance. Front Immunol (2021) 12:650200. doi: 10.3389/fimmu.2021.650200
194. Kabata H, Moro K, Koyasu S. The Group 2 Innate Lymphoid Cell (ILC2) Regulatory Network and its Underlying Mechanisms. Immunol Rev (2018) 286(1):37–52. doi: 10.1111/imr.12706
195. Lei A, He Y, Yang Q, Li X, Li R. Role of Myeloid Cells in the Regulation of Group 2 Innate Lymphoid Cell-Mediated Allergic Inflammation. Immunology (2020) 161(1):18–24. doi: 10.1111/imm.13232
196. Kim BS, Siracusa MC, Saenz SA, Noti M, Monticelli LA, Sonnenberg GF, et al. TSLP Elicits IL-33-Independent Innate Lymphoid Cell Responses to Promote Skin Inflammation. Sci Transl Med (2013) 5(170):170ra16. doi: 10.1126/scitranslmed.3005374
197. Tuchinda P, Kulthanan K, Chularojanamontri L, Arunkajohnsak S, Sriussadaporn S. Relationship Between Vitamin D and Chronic Spontaneous Urticaria: A Systematic Review. Clin Transl Allergy (2018) 8:51. doi: 10.1186/s13601-018-0234-7
198. Ruiter B, Patil SU, Shreffler WG. Vitamins A and D Have Antagonistic Effects on Expression of Effector Cytokines and Gut-Homing Integrin in Human Innate Lymphoid Cells. Clin Exp Allergy (2015) 45(7):1214–25. doi: 10.1111/cea.12568
199. Méndez-Enríquez E, Salomonsson M, Eriksson J, Janson C, Malinovschi A, Sellin ME, et al. IgE Cross-Linking Induces Activation of Human and Mouse Mast Cell Progenitors. J Allergy Clin Immunol (2022) 149(4):1458–63. doi: 10.1016/j.jaci.2021.08.019
200. Engeroff P, Vogel M. The Role of CD23 in the Regulation of Allergic Responses. Allergy (2021) 76(7):1981–9. doi: 10.1111/all.14724
201. Brandt K, Singh PB, Bulfone-Paus S, Rückert R. Interleukin-21: A New Modulator of Immunity, Infection, and Cancer. Cytokine Growth Factor Rev (2007) 18(3-4):223–32. doi: 10.1016/j.cytogfr.2007.04.003
202. Shang XZ, Ma KY, Radewonuk J, Li J, Song XY, Griswold DE, et al. IgE Isotype Switch and IgE Production are Enhanced in IL-21-Deficient But Not IFN-Gamma-Deficient Mice in a Th2-Biased Response. Cell Immunol (2006) 241(2):66–74. doi: 10.1016/j.cellimm.2006.07.011
203. Yang Z, Wu CM, Targ S, Allen CDC. IL-21 is a Broad Negative Regulator of IgE Class Switch Recombination in Mouse and Human B Cells. J Exp Med (2020) 217(5):e20190472. doi: 10.1084/jem.20190472
204. Yamanishi Y, Karasuyama H. Basophil-Derived IL-4 Plays Versatile Roles in Immunity. Semin Immunopathol (2016) 38(5):615–22. doi: 10.1007/s00281-016-0568-y
205. Shakoory B, Fitzgerald SM, Lee SA, Chi DS, Krishnaswamy G. The Role of Human Mast Cell-Derived Cytokines in Eosinophil Biology. J Interferon Cytokine Res (2004) 24(5):271–81. doi: 10.1089/107999004323065057
206. Grieco T, Porzia A, Paolino G, Chello C, Sernicola A, Faina V, et al. IFN-γ/IL-6 and Related Cytokines in Chronic Spontaneous Urticaria: Evaluation of Their Pathogenetic Role and Changes During Omalizumab Therapy. Int J Dermatol (2020) 59(5):590–4. doi: 10.1111/ijd.14812
207. Grzanka R, Damasiewicz-Bodzek A, Kasperska-Zajac A. Interplay Between Acute Phase Response and Coagulation/Fibrinolysis in Chronic Spontaneous Urticaria. Allergy Asthma Clin Immunol (2018) 14:27. doi: 10.1186/s13223-018-0255-8
208. Kasperska-Zając A, Damasiewicz-Bodzek A, Grzanka R, Skrzypulec-Frankel A, Bieniek K, Sikora-Żydek A, et al. Circulating Soluble LIGHT/TNFSF14 is Increased and Associated With IL-8 Concentration in Chronic Spontaneous Urticaria. Int J Immunopathol Pharmacol (2018) 32:2058738418784431. doi: 10.1177/2058738418784431
209. Kasperska-Zajac A, Grzanka A, Damasiewicz-Bodzek A, Bieniek K, Skrzypulec-Frankel A, Stencel-Gabriel K, et al. Elevated Plasma Il-8 Concentration is Related to Severity of Systemic Inflammation in Chronic Spontaneous Urticaria. J Biol Regul Homeost Agents (2017) 31(4):957–61.
210. Rojas-Zuleta WG, Sanchez E. IL-9: Function, Sources, and Detection. Methods Mol Biol (2017) 1585:21–35. doi: 10.1007/978-1-4939-6877-0_2
211. Irinyi B, Aleksza M, Antal-Szalmás P, Sipka S, Hunyadi J, Szegedi A. Cytokine Production of CD4+ and CD8+ Peripheral T Lymphocytes in Patients With Chronic Idiopathic Urticaria. Acta Derm Venereol (2002) 82(4):249–53. doi: 10.1080/000155502320323199
212. Moos Ł, Kapeluszna K, Okuniewicz R, Brzoza Z. The Role of Interleukin 10 and 18 in Chronic Spontaneous Urticaria Pathogenesis in the Context of Angioedema Coexistence. J Interferon Cytokine Res (2021) 41(5):172–6. doi: 10.1089/jir.2020.0256
213. Ryzhov S, Goldstein AE, Matafonov A, Zeng D, Biaggioni I, Feoktistov I. Adenosine-Activated Mast Cells Induce IgE Synthesis by B Lymphocytes: An A2B-Mediated Process Involving Th2 Cytokines IL-4 and IL-13 With Implications for Asthma. J Immunol (2004) 172(12):7726–33. doi: 10.4049/jimmunol.172.12.7726
214. Bae Y, Izuhara K, Ohta S, Ono J, Hong GU, Ro JY, et al. Periostin and Interleukin-13 Are Independently Related to Chronic Spontaneous Urticaria. Allergy Asthma Immunol Res (2016) 8(5):457–60. doi: 10.4168/aair.2016.8.5.457
215. Toubi E, Vadasz Z. The Emerging Role of IL-17 in the Immune-Pathogenesis of Chronic Spontaneous Urticaria. Immunotargets Ther (2020) 9:217–23. doi: 10.2147/itt.S266410
216. Sabag DA, Matanes L, Bejar J, Sheffer H, Barzilai A, Church MK, et al. Interleukin-17 is a Potential Player and Treatment Target in Severe Chronic Spontaneous Urticaria. Clin Exp Allergy (2020) 50(7):799–804. doi: 10.1111/cea.13616
217. Grzanka A, Damasiewicz-Bodzek A, Kasperska-Zajac A. The Relationship Between Circulating Concentrations of Interleukin 17 and C Reactive Protein in Chronic Spontaneous Urticaria. Allergy Asthma Clin Immunol (2017) 13:25. doi: 10.1186/s13223-017-0197-6
218. Boraschi D, Dinarello CA. IL-18 in Autoimmunity: Review. Eur Cytokine Netw (2006) 17(4):224–52.
219. Sharma P, Sharma PK, Chitkara A, Rani S. To Evaluate the Role and Relevance of Cytokines IL-17, IL-18, IL-23 and TNF-α and Their Correlation With Disease Severity in Chronic Urticaria. Indian Dermatol Online J (2020) 11(4):594–7. doi: 10.4103/idoj.IDOJ_396_19
220. de Montjoye L, Herman A, Hendrickx E, Chéou P, Blanchetot C, Hofman E, et al. Increased Expression of IL-24 in Chronic Spontaneous Urticaria. Allergy (2019) 74(9):1811–3. doi: 10.1111/all.13832
221. Chaowattanapanit S, Choonhakarn C, Salao K, Winaikosol K, Julanon N, Wongjirattikarn R, et al. Increased Serum IL-31 Levels in Chronic Spontaneous Urticaria and Psoriasis With Pruritic Symptoms. Heliyon (2020) 6(12):e05621. doi: 10.1016/j.heliyon.2020.e05621
222. Chen T, Fu LX, Sun QM, Zhou PM, Guo ZP. Decreased Interleukin-35 Serum Levels in Patients With Chronic Spontaneous Urticaria. Ann Allergy Asthma Immunol (2018) 121(4):503–4. doi: 10.1016/j.anai.2018.06.002
223. Zhang J, Zhang Y, Wang Q, Li C, Deng H, Si C, et al. Interleukin-35 in Immune-Related Diseases: Protection or Destruction. Immunology (2019) 157(1):13–20. doi: 10.1111/imm.13044
224. Su LC, Liu XY, Huang AF, Xu WD. Emerging Role of IL-35 in Inflammatory Autoimmune Diseases. Autoimmun Rev (2018) 17(7):665–73. doi: 10.1016/j.autrev.2018.01.017
225. Kamegashira A, Yanase Y, Takahagi S, Saito R, Uchida K, Kawaguchi T, et al. Histamine- or Vascular Endothelial Growth Factor-Induced Tissue Factor Expression and Gap Formation Between Vascular Endothelial Cells are Synergistically Enhanced by Lipopolysaccharide, Tumor Necrosis Factor-α, Interleukin (IL)-33 or IL-1β. J Dermatol (2020) 47(11):1293–300. doi: 10.1111/1346-8138.15516
226. Grzanka R, Damasiewicz-Bodzek A, Kasperska-Zajac A. Tumor Necrosis Factor-Alpha and Fas/Fas Ligand Signaling Pathways in Chronic Spontaneous Urticaria. Allergy Asthma Clin Immunol (2019) 15:15. doi: 10.1186/s13223-019-0332-7
Keywords: chronic spontaneous urticaria (CSU), mast cells, immune cells, crosstalk, pathogenesis
Citation: Zhou B, Li J, Liu R, Zhu L and Peng C (2022) The Role of Crosstalk of Immune Cells in Pathogenesis of Chronic Spontaneous Urticaria. Front. Immunol. 13:879754. doi: 10.3389/fimmu.2022.879754
Received: 20 February 2022; Accepted: 02 May 2022;
Published: 31 May 2022.
Edited by:
Francesco Liotta, Università degli Studi di Firenze, ItalyCopyright © 2022 Zhou, Li, Liu, Zhu and Peng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cong Peng, cGVuZ2Nvbmd4eUBjc3UuZWR1LmNu
†These authors share first authorship
 Bingjing Zhou
Bingjing Zhou Jie Li
Jie Li Runqiu Liu
Runqiu Liu Lei Zhu
Lei Zhu Cong Peng
Cong Peng