- College of Korean Medicine, Dongguk University, Goyang, South Korea
Purpose: Although respiratory diseases (RD) are rapidly becoming a global health issue due to their high mortality and prevalence, there are limitations to the currently available treatments. Acupuncture has been recognized to mitigate many diseases by reducing inflammation and modulating cytokines. However, no systematic analysis has been performed to examine the effects of acupuncture on RD. We aimed to evaluate the effects of acupuncture on rodent animal models of RD.
Methods: PubMed, EMBASE, MEDLINE, and the Research Information Service System were searched to retrieve studies that met our inclusion/exclusion criteria. The quality of each included study was evaluated using a 10-item checklist modified from the Collaborative Approach to Meta-Analysis and Review of Animal Data from Experimental Studies. With adequate data extracted, meta-analysis was performed using RevMan software.
Results: A total of 18 studies were included, and the mean quality assessment was 5.7. The meta-analysis revealed that acupuncture had a significant effect on changing the cytokine levels, including pro-/anti-inflammatory, Th1-, Th2- and Th17- specific cytokines.
Conclusion: Although there were limitations in the number of included studies, the results suggest that acupuncture can be a possible treatment for RD through its modulation of various cytokines, leading to reduced inflammation.
Introduction
Respiratory diseases (RD) are a major global health issue that have been a serious financial burden owing to the difficulty in treatment. RD encompasses various disorders such as sinusitis, asthma, chronic obstructive pulmonary disease (COPD), and even acute lung injury caused by other diseases. Although the lesions and symptoms of the aforementioned diseases may differ, most of them are associated with acute/chronic inflammation (1). Inflammation is a physiological immune process that includes both inflammatory and restorative responses. Among the factors that participate in this course of action, cytokines function as key mediators with different roles. For example, pro-inflammatory cytokines induce inflammation, while anti-inflammatory cytokines reduce inflammation and participate in the healing process (2). Cytokines can also be categorized according to the type of lymphocytes they are related to. Interleukin (IL)-4, 5, 9, and 13, which are known to be the CD4+ T helper 2 (Th2) cell-responsive cytokines, contribute to allergic airway inflammation. Meanwhile, IL-17, which is released from T helper 17 (Th17) cells, is known to exacerbate chronic lung inflammation (3). Although corticosteroids are currently being used for RD treatment, there are limitations yet to be solved as some cytokines mediate steroid-resistant airway inflammation and obstruction (4).
Acupuncture, which originates from traditional Chinese medicine, has long been acknowledged to mitigate many diseases. Acupuncture at the specific acupoint of ST36 has been reported to promote anti-inflammatory, anti-oxidative, and immune-enhancing effects (5–7). Moreover, the latest studies using animal models of sepsis showed that electroacupuncture at ST36 modulates endotoxin-induced systemic inflammation through the vagal-adrenal anti-inflammatory axis (6, 8). Recently, clinical research in COPD (9), allergic rhinitis (10), asthma (11, 12) showed the possibility of acupuncture as a treatment for these pulmonary diseases. Moreover, there has been increasing evidence of potential therapeutic effects of acupuncture from research on the coronavirus disease 2019 (13). Although pulmonary dysfunction has various etiologies, inflammation mechanisms occur in the lung tissue and fluid. Therefore, it is important to determine which anti-inflammatory mechanisms are linked to the effect of acupuncture on pulmonary diseases.
In this review, we performed a meta-analysis on inflammatory cytokines in various animal models of RD to determine the effects of acupuncture on RD.
Material and Methods
Searching
Reports that examined the effect of acupuncture on inflammatory cytokines in rodent models of RD that were written in English were included in the present study. PubMed, EMBASE, MEDLINE, and Research Information Service System were searched from inception until December 2021 using the following search terms: “mouse (mice)” or “rat (rats)”, and “acupuncture (electroacupuncture)” and “respiratory disease.”
Inclusion/Exclusion Criteria
Studies were included based on the following criteria: subjects (rodent models of RD), interventions (acupuncture as the main intervention but limited to manual acupuncture and electroacupuncture), and outcomes (the levels of each inflammatory cytokine were the main outcomes to evaluate the efficacy of acupuncture). Lung function test data were included as the subsequent outcomes. Along with those that did not provide access to the full text, studies that were not written in English or focused on unrelated topics were excluded. After screening the full text, studies that did not meet our criteria in the methods and results were also excluded.
Data Extraction
Two authors (Kim and Lee) independently extracted the data on the publication year, name of the first author, type of rodent RD model, disease/condition, sample size, type of acupuncture, and type of specimen. Along with the mean value of inflammatory cytokines within each group (e.g., control group, disease group, intervention group), standard deviation or standard error of the mean were extracted to determine the effect measures and effect size of acupuncture on RD. Each value from the studies was extracted using the Engauge Digitizer software version 12. We estimated the number of animals in groups by calculating the mean number of animals per study. Lung function data, including the type of lung function test and the result, were collected as a secondary outcome.
Quality Assessment
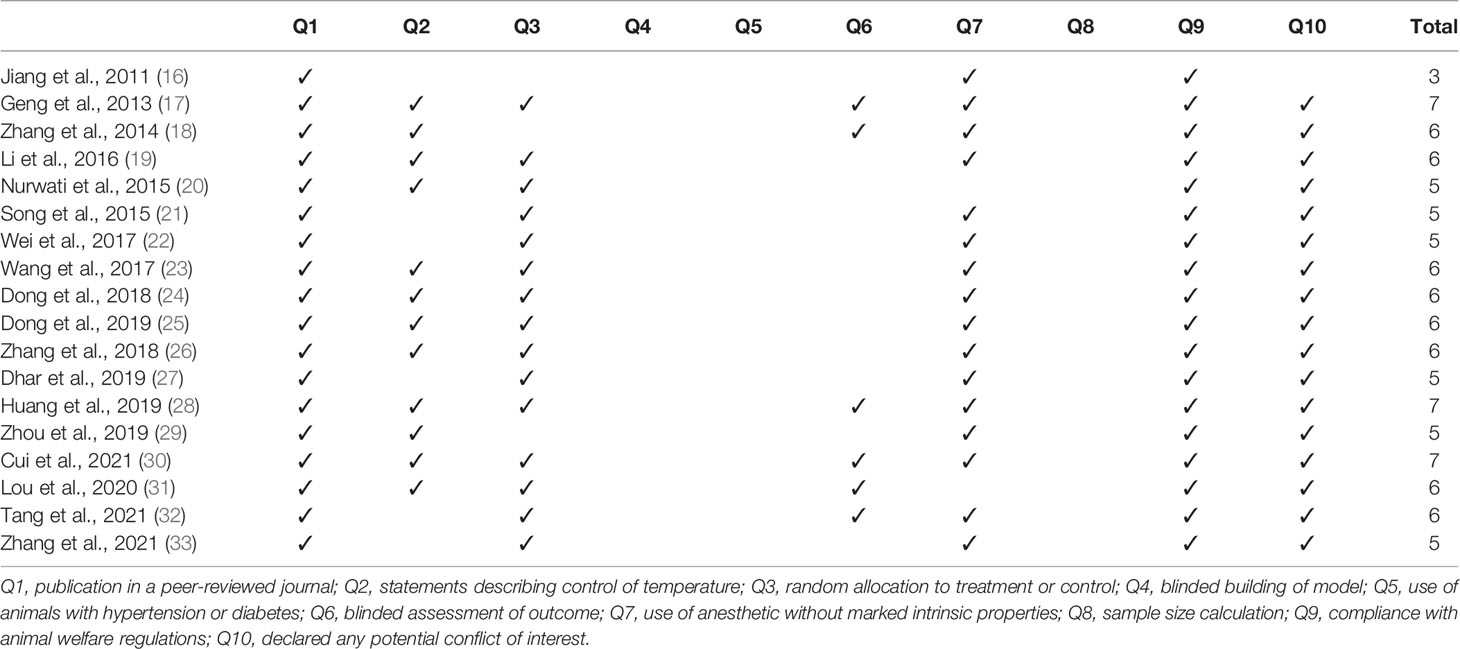
The methodological quality of each included study was assessed by two authors (Kim and Lee) using a 10-item checklist modified from the Collaborative Approach to Meta-Analysis and Review of Animal Data from Experimental Studies (CAMARADES) checklist (14, 15): publication in a peer-reviewed journal, statements describing control of temperature, random allocation to treatment or control, blinded building of model, use of animals with hypertension or diabetes, blinded assessment of outcomes, use of anesthetic without marked intrinsic properties, sample size calculation, compliance with animal welfare regulations, and declared any potential conflict of interest. The sum of the quality scores was recorded for each article, with a possible total score of 10 points.
Statistics
In the present study, the ratios of cytokine levels in the disease and intervention groups to the control group were considered as continuous data. To compare studies that used different units for cytokine levels, the difference between the disease/control ratio and acupuncture/control ratio was calculated. Since all 18 studies set RD as an independent variable and analyzed the correlation with the outcomes, the standardized mean difference (SMD) was estimated based on a fixed-effect model. The meta-analysis was performed on each cytokine subgroup using RevMan version 5.4 (Foundation for Statistical Computing, Vienna, Austria). The confidence interval (CI) was established at 95%, and a p-value < 0.05 was considered statistically significant. For the assessment of study heterogeneity, chi-squared and I2 statistics were used.
Results
Study Inclusion
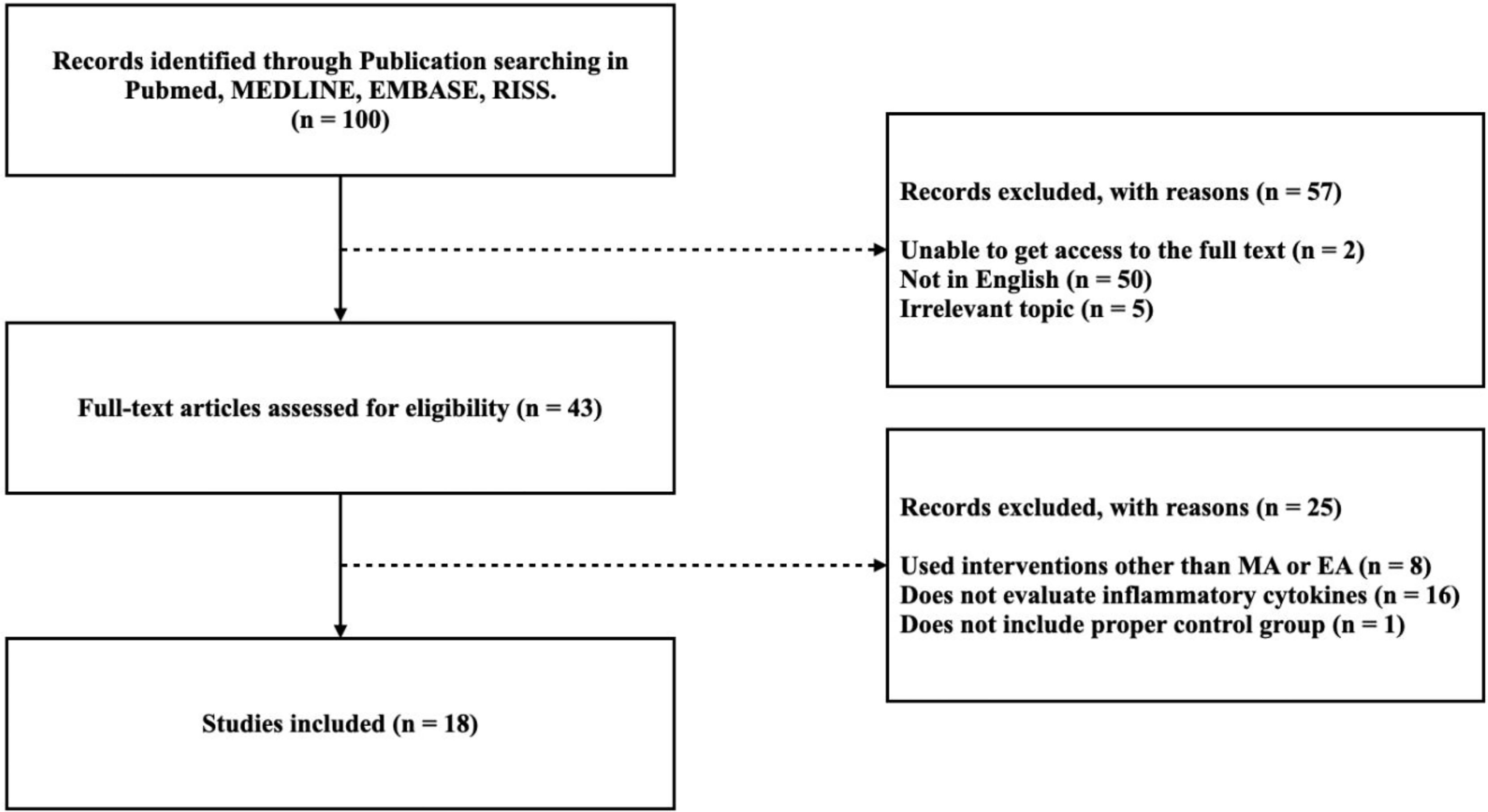
Among the 100 studies selected, 57 studies were excluded because 50 were not written in English, five did not cover relevant topics, and two did not allow access to the full text. The full-text screening was performed on the remaining 43 studies, of which 25 were excluded due to deficiencies in the methodology and results. Eight studies used intervention other than manual acupuncture or electroacupuncture, 16 did not evaluate inflammatory cytokines, and 1 study did not include proper control group. Therefore, a total of 18 studies were included in this review. A flow diagram of the study selection process is shown in Figure 1.
Quality Assessment
The quality assessments of the included studies are summarized in Table 1. The quality score of the included studies ranged from 3 to 7 out of 10 points: three studies scored 7, eight scored 6, six scored 5, and one scored 3 points. All 18 studies were peer-reviewed and met animal welfare regulations. Twelve studies included statements describing the control of temperature. Fifteen studies specified the random allocation of subjects to treatment or control groups, and six described blinded assessment of the outcomes. Sixteen studies used anesthetics with no intrinsic properties, and 17 declared no potential conflicts of interest with respect to the research. None of the 18 studies conducted blind building of the model or sample size calculation, and none used animals with hypertension or diabetes.
Study Characteristics
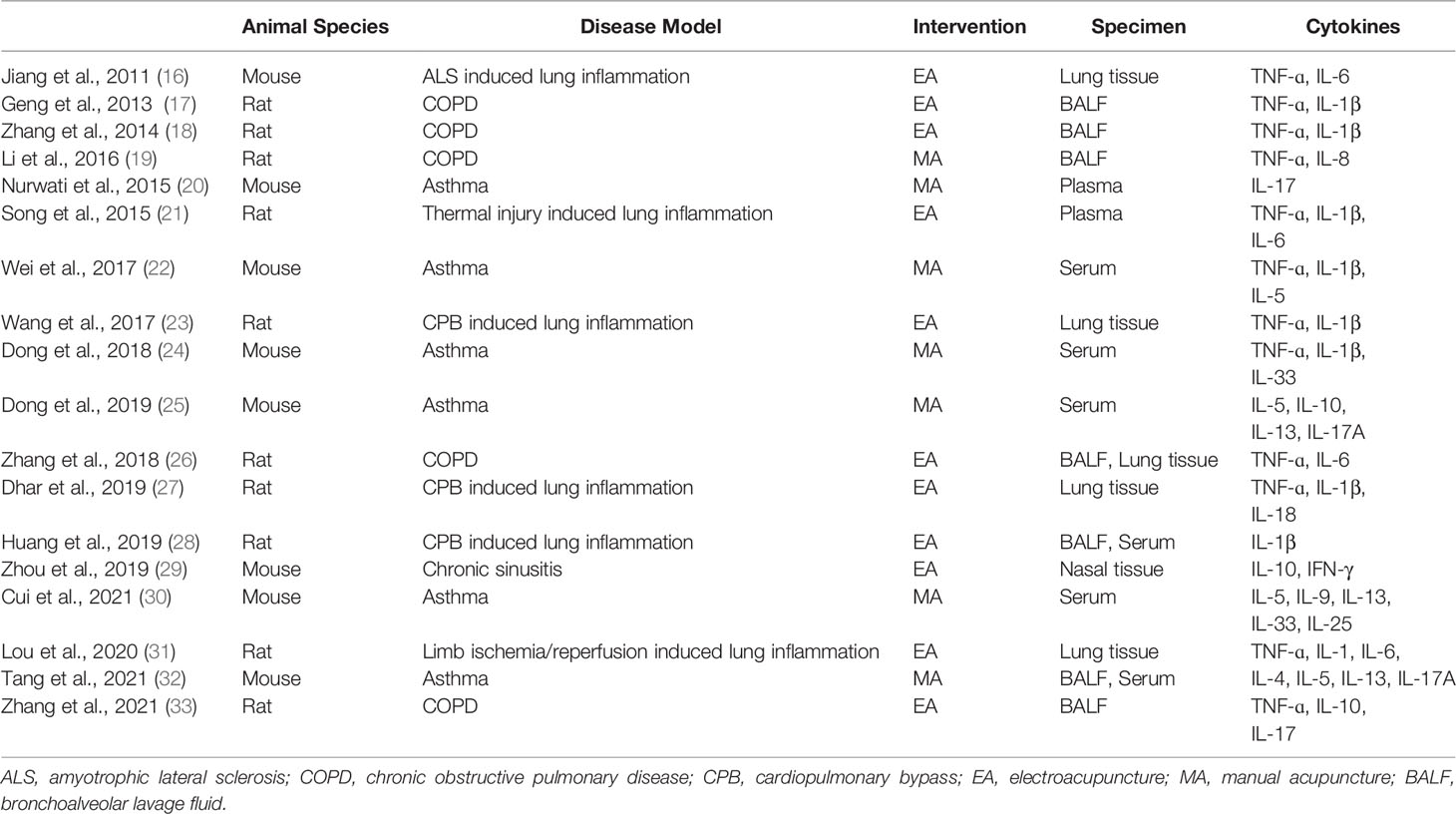
The characteristics of the included studies are summarized in Table 2. Among the 18 studies, 10 and eight studies were conducted using rats and mice, respectively. Six studies used an asthma model. Meanwhile, five used a COPD model, and only one used a chronic sinusitis model. Furthermore, six studies used an acute lung inflammation model induced by various preceding conditions, such as amyotrophic lateral sclerosis, thermal injury, cardiopulmonary bypass, or limb ischemia and reperfusion. Eleven studies used electroacupuncture, while seven used manual acupuncture. Specimens were collected to measure the levels of cytokines, including lung tissue, bronchoalveolar lavage fluid, plasma, serum, and nasal tissue.
The Effect of Acupuncture on Inflammatory Cytokines
Cytokines, the principal mediators of inflammation, are composed of various subfamilies that play different roles. First, they are known to have function as pro- or anti-inflammation. Second, cytokines also can be categorized with the immune cells where they are released from, including CD4+ Th1, Th2 or Th17 cells. Figures 2, 3 show that 14 cytokines were analyzed in this study. Due to the diverse properties of these cytokines, we performed the meta-analysis by categorizing cytokines into separate subgroups: (1) pro-/anti-inflammatory cytokines, and (2) Th1-, Th2-, and Th17-specific cytokines. The results revealed that rodent animal models with RD had higher levels of pro-inflammatory cytokines than the control group, and acupuncture lowered these levels (n = 207 for disease group, n = 205 for acupuncture group, SMD 2.18 [95% CI 1.90 ~ 2.46]; p < 0.00001; heterogeneity X2 = 89.74, I2 = 72%, Figure 2A). Anti-inflammatory cytokines showed the opposite result, showing a decrease in the disease model and an increase with acupuncture treatment (n = 31, SMD -3.09 [95% CI -3.98 ~ -2.21]; p < 0.00001; heterogeneity X2 = 20.14, I2 = 90%, Figure 2B). Most Th-1 specific cytokines seemed to have decreased by acupuncture treatment except for IFN-𝛾. (n=178, SMD 1.98 [95% CI 1.69 ~ 2.28]; p < 0.00001; heterogeneity X2 = 28.75, I2 = 73%, Figure 3A). Th2-specific cytokines had a similar tendency to that of pro-inflammatory cytokines (n = 124, SMD 2.24 [95% CI 1.84 – 2.64]; p < 0.00001; heterogeneity X2 = 15.77, I2 = 30%, Figure 3B). Lastly, Th-17 specific cytokines, which increased in the disease groups, were also shown to be regulated by acupuncture treatment (n = 39, SMD 1.73 [95% CI 1.18 – 2.27]; p < 0.00001; heterogeneity X2 = 1.92, I2 = 0%, Figure 3C).
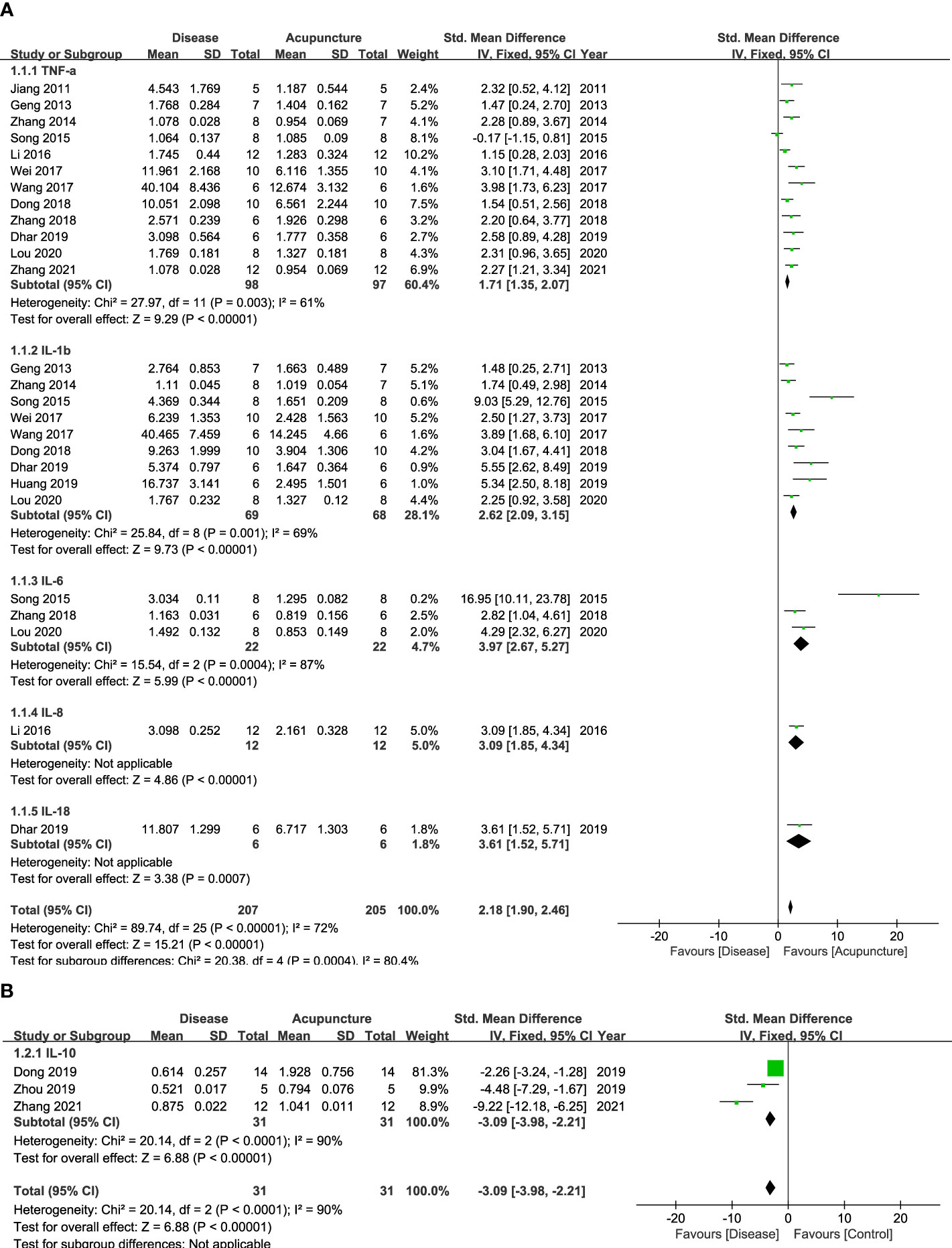
Figure 2 Forest Plot of Pro-/Anti-inflammatory Cytokines. (A) Pro-inflammatory Cytokines (B) Anti-inflammatory Cytokines.
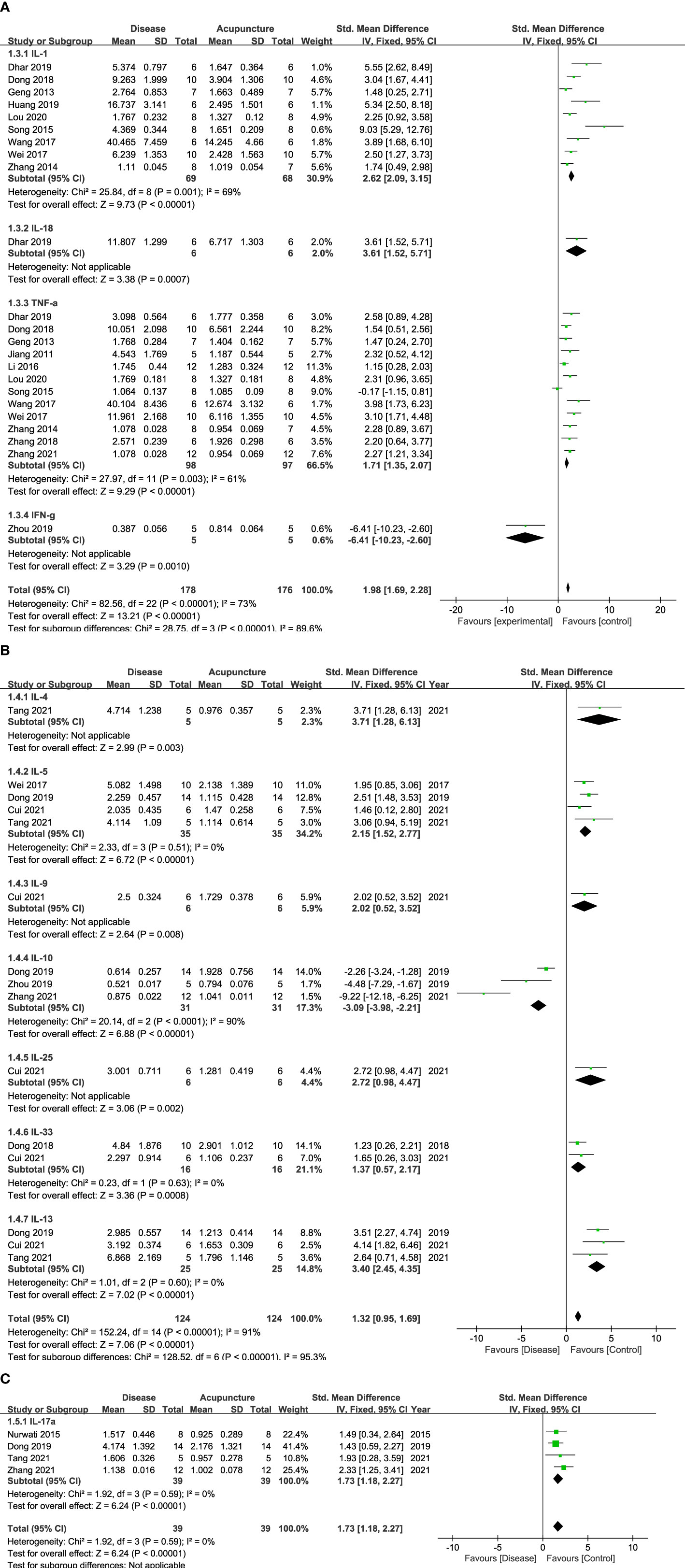
Figure 3 Forest Plot of Th cell-specific Cytokines. (A) Th1-specific Cytokines (B) Th2-specific Cytokines (C) Th17-specific Cytokines.
The Effect of Acupuncture on the Lung Function in Animal Models of RD
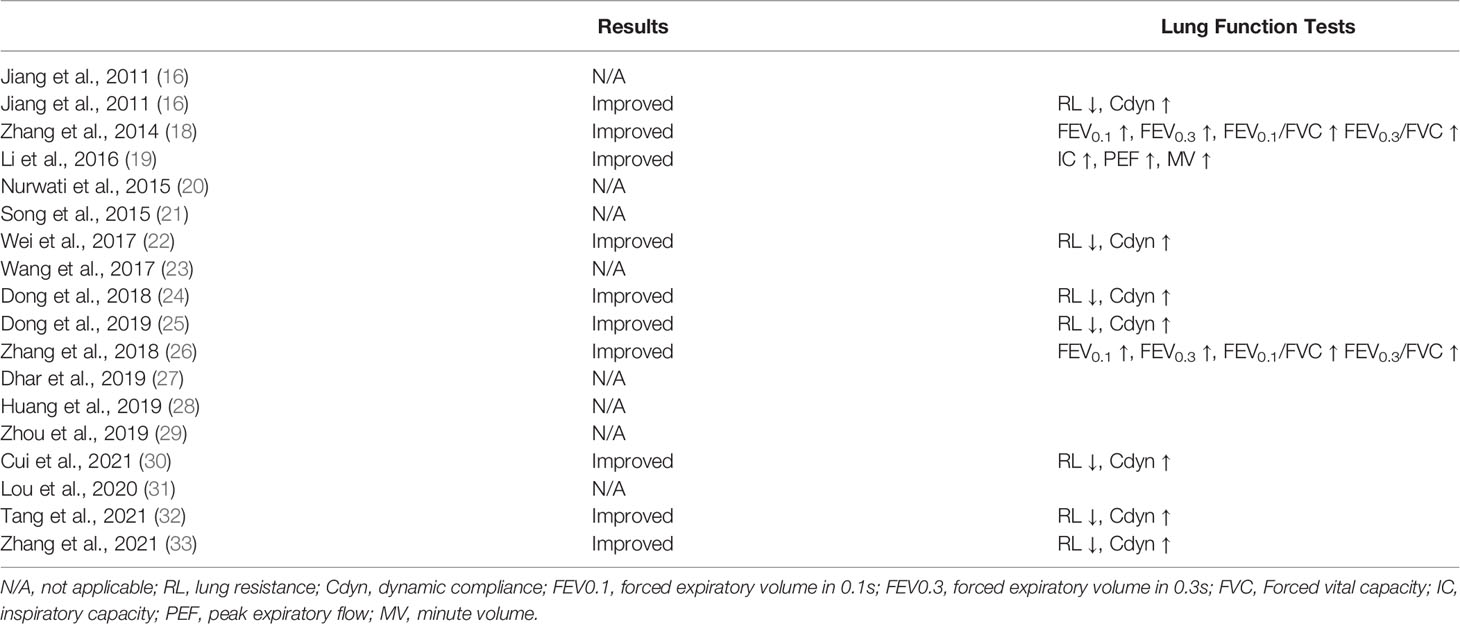
Table 3 shows how acupuncture affected lung function in RD models after examining the results of pulmonary function tests (PFTS), lung resistance (RL), and lung dynamic compliance (Cdyn). PFTS data included forced expiratory volume in 0.1s (FEV0.1), forced expiratory volume in 0.3s (FEV0.3), the ratio between the aforementioned two and forced vital capacity (FEV0.1/FVC, FEV0.3/FVC), inspiratory capacity, peak expiratory flow, and minute volume. Among the 18 studies, seven measured RL and Cdyn, three conducted PFTS, and eight did not mention any lung function tests. Acupuncture was found to increase Cdyn and alleviate RL. It was also shown that acupuncture improved lung function in general by increasing FEVs, FVC, inspiratory capacity, peak expiratory flow, and minute volume.
Discussion
Recently, a number of clinical studies have reported the effect of acupuncture on RD. Acupuncture enhanced the strength of diaphragm while relieving the respiratory muscle fatigue of COPD patients (9), mitigated overall symptoms of allergic rhinitis/rhinoconjunctivitis (10), and showed therapheutic effects on bronchial asthma as well (12). However, no studies oversee the RD animal studies and systematically analyzes the effect of acupuncture on inflammatory cytokine as its underlying mechanism. In this review, we systematically analyzed 18 acupuncture studies that used rodent models of RD to determine whether acupuncture can improve RD symptoms and/or pathology. Rat/mouse models of asthma, COPD, cardiopulmonary bypass-, amyotrophic lateral sclerosis-, reperfusion-induced lung inflammation, and chronic sinusitis were administered with manual or electro-acupuncture, and the lung functions were then examined. To test lung function, the RL, Cdyn, FEV, FVC, peak expiratory flow, and minute volume were tested, and the results showed that acupuncture improved lung function in various inflammatory pulmonary disease animal models.
The following inflammatory cytokines were examined in lung tissue, fluids, or serum/plasma samples from animal models. Inflammatory cytokines can be specifically classified according to their functions: those that promote inflammation are known to be pro-inflammatory, while those that engage in restoring damages are considered anti-inflammatory cytokines. In pulmonary diseases, pro-inflammatory cytokines are known to trigger mucus secretion and airway fibrosis (34), while anti-inflammatory cytokines have been reported to be deficient, especially in asthma and COPD (35). In our results, pro-inflammatory cytokines, such as IL-1, 6, 8, 18, and TNF-α, were lowered by acupuncture. In contrast, anti-inflammatory cytokines, such as IL-10, showed an opposite tendency. Thus, our results suggest that acupuncture can possibly ameliorate inflammation by inducing anti-inflammatory cytokines while reducing pro-inflammatory ones depending on the set condition. Meanwhile, some cytokines are not simple enough to categorize by their pro-/anti-inflammatory roles. IFN-𝛾, for example, is a Th1 cytokine that counterbalances Th2 cytokines. However, its anti-inflammatory action has also been reported, suggesting that the functional roles of some cytokines are complex in immune diseases (36, 37).
Interestingly, acupuncture showed that it could possibility reduce Th2-related cytokine levels in asthma models, which might help maintain the balance between Th1 and Th2 cytokines. Asthma, an allergic inflammation driven by Th2 lymphocytes, shows representative pathology of higher levels of Th2 cytokines compared to Th1 cells (1). According to our study, Th2 cytokines such as IL-4, 5, 9, 13, 25, and 33 were downregulated by acupuncture treatment. This allows an additional interpretation of how acupuncture can normalize Th2 cytokine levels to alleviate inflammation in asthma. Furthermore, IL-17, which is released by Th17 cells, leads to the aggravation of allergic inflammation and airway hyper-responsiveness by causing neutrophilia and eosinophilia (38, 39). In our study, acupuncture significantly modulated abnormal levels of IL-17, again implying its effect on RD via Th17 cell control. As Th2 and Th17 cells play a crucial role in RD pathology (40), the T-cell-regulating ability of acupuncture deserves further investigation (41).
There are a few limitations to the present study. First, cytokines were not classified in a disease-specific manner, as this study encompassed overall effects of acupuncture on RD. Second, the fact that cytokines can play a different role depending on the situation could obscure the purpose of this review. This also caused some difficulties in categorizing them. In order to prevent aforementioned problem, we referred to how each study interpreted the role of cytokines. Third, the number of included studies was not conducive for a thorough systematic review, which implies the limited research investigating the effects of acupuncture. However, it is meaningful in that this study evaluated the efficacy of acupuncture on RD for the first time by analyzing inflammatory cytokines. In order to reveal the hidden mechanism of acupuncture, further research investigating each RD is needed.
Conclusion
In summary, this review showed that acupuncture treatment might ameliorate lung dysfunction in RD by regulating inflammatory cytokines. Results showed that when mitigating RD, acupuncture modulates pro/anti-inflammatory, Th1-, Th2-, and Th17-specific cytokines. We look forward to further research on the therapeutic effects of acupuncture and hope that the current review will be a good reference in this field of study.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
SL searched the database and extracted data. S-NK designed and supervised the study. SL and S-NK analyzed the data and wrote the paper. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Research Foundation of Korea funded by the Korean government (MSIT) (NRF-2020R1C1C1004107) and from the Ministry of Health & Welfare through the Korea Health Industry Development Institute (KHIDI) (grant no. HF21C0018).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Aghasafari P, George U, Pidaparti R. A Review of Inflammatory Mechanism in Airway Diseases. Inflammation Res (2019) 68:59–74. doi: 10.1007/s00011-018-1191-2
2. Opal SM, Depalo VA. Anti-Inflammatory Cytokines. Chest (2000) 117:1162–72. doi: 10.1378/chest.117.4.1162
3. Hirahara K, Nakayama T. CD4+ T-Cell Subsets in Inflammatory Diseases: Beyond the Th1/Th2 Paradigm. Int Immunol (2016) 28:163–71. doi: 10.1093/intimm/dxw006
4. Mckinley L, Alcorn JF, Peterson A, Dupont RB, Kapadia S, Logar A, et al. TH17 Cells Mediate Steroid-Resistant Airway Inflammation and Airway Hyperresponsiveness in Mice. J Immunol (2008) 181:4089–97. doi: 10.4049/jimmunol.181.6.4089
5. Torres-Rosas R, Yehia G, Pena G, Mishra P, Del Rocio Thompson-Bonilla M, Moreno-Eutimio MA, et al. Dopamine Mediates Vagal Modulation of the Immune System by Electroacupuncture. Nat Med (2014) 20:291–5. doi: 10.1038/nm.3479
6. Liu S, Wang Z, Su Y, Qi L, Yang W, Fu M, et al. A Neuroanatomical Basis for Electroacupuncture to Drive the Vagal-Adrenal Axis. Nature (2021) 598:641–5. doi: 10.1038/s41586-021-04001-4
7. Oh JE, Kim SN. Anti-Inflammatory Effects of Acupuncture at ST36 Point: A Literature Review in Animal Studies. Front Immunol (2021) 12:813748. doi: 10.3389/fimmu.2021.813748
8. Liu S, Wang ZF, Su YS, Ray RS, Jing XH, Wang YQ, et al. Somatotopic Organization and Intensity Dependence in Driving Distinct NPY-Expressing Sympathetic Pathways by Electroacupuncture. Neuron (2020) 108:436–50:e437. doi: 10.1016/j.neuron.2020.07.015
9. Liu Q, Duan H, Lian A, Zhuang M, Zhao X, Liu X. Rehabilitation Effects of Acupuncture on the Diaphragm Dysfunction in Chronic Obstructive Pulmonary Disease: A Systematic Review. Int J Chron Obstruct Pulmon Dis (2021) 16:2023–37. doi: 10.2147/COPD.S313439
10. Yin Z, Geng G, Xu G, Zhao L, Liang F. Acupuncture Methods for Allergic Rhinitis: A Systematic Review and Bayesian Meta-Analysis of Randomized Controlled Trials. Chin Med (2020) 15:109. doi: 10.1186/s13020-020-00389-9
11. Yin LM, Wang Y, Fan L, Xu YD, Wang WQ, Liu YY, et al. Efficacy of Acupuncture for Chronic Asthma: Study Protocol for a Randomized Controlled Trial. Trials (2015) 16:424. doi: 10.1186/s13063-015-0947-z
12. Hu J, Zhang C, Zhao S, Ge K, Di K, Wang H, et al. A Systematic Review and Meta-Analysis of Acupoint Application Combined With Western Medicine Therapy in the Treatment of Bronchial Asthma. Ann Palliat Med (2021) 10:11473–81. doi: 10.21037/apm-21-2507
13. Chen C, Zhan J, Wen H, Wei X, Ding L, Tao C, et al. Current State of Research About Acupuncture for the Treatment of COVID-19: A Scoping Review. Integr Med Res (2021) 10:100801. doi: 10.1016/j.imr.2021.100801
14. Sena E, van der Worp HB, Howells D, Macleod M. How can We Improve the Pre-Clinical Development of Drugs for Stroke? Trends Neurosci (2007) 30:433–9. doi: 10.1016/j.tins.2007.06.009
15. Auboire L, Sennoga CA, Hyvelin JM, Ossant F, Escoffre JM, Tranquart F, et al. Microbubbles Combined With Ultrasound Therapy in Ischemic Stroke: A Systematic Review of In-Vivo Preclinical Studies. PLoS One (2018) 13:e0191788. doi: 10.1371/journal.pone.0191788
16. Jiang JH, Yang EJ, Baek MG, Kim SH, Lee SM, Choi SM. Anti-Inflammatory Effects of Electroacupuncture in the Respiratory System of a Symptomatic Amyotrophic Lateral Sclerosis Animal Model. Neurodegener Dis (2011) 8:504–14. doi: 10.1159/000327911
17. Geng WY, Liu ZB, Song NN, Geng WY, Zhang GH, Jin WZ, et al. Effects of Electroacupuncture at Zusanli (ST36) on Inflammatory Cytokines in a Rat Model of Smoke-Induced Chronic Obstructive Pulmonary Disease. J Integr Med (2013) 11:213–9. doi: 10.3736/jintegrmed2013024
18. Zhang XF, Zhu J, Geng WY, Zhao SJ, Jiang CW, Cai SR, et al. Electroacupuncture at Feishu (BL13) and Zusanli (ST36) Down-Regulates the Expression of Orexins and Their Receptors in Rats With Chronic Obstructive Pulmonary Disease. J Integr Med (2014) 12:417–24. doi: 10.1016/S2095-4964(14)60040-6
19. Li J, Wu S, Tang H, Huang W, Wang L, Zhou H, et al. Long-Term Effects of Acupuncture Treatment on Airway Smooth Muscle in a Rat Model of Smoke-Induced Chronic Obstructive Pulmonary Disease. Acupunct Med (2016) 34:107–13. doi: 10.1136/acupmed-2014-010674
20. Nurwati I, Mudigdo A, Saputra K, Sutrisno T. Reduction of Interleukin-17 Level by Acupuncture at Feishu (BI 13) Is Strengthened by Acupuncture at Zusanli (ST 36) in a Mouse Model of Chronic Asthma: An Experimental Study. Med Acupunct (2015) 27:278–82. doi: 10.1089/acu.2015.1111
21. Song XM, Wu XJ, Li JG, Le LL, Liang H, Xu Y, et al. The Effect of Electroacupuncture at ST36 on Severe Thermal Injury-Induced Remote Acute Lung Injury in Rats. Burns (2015) 41:1449–58. doi: 10.1016/j.burns.2015.03.004
22. Wei Y, Dong M, Zhong L, Liu J, Luo Q, Lv Y, et al. Regulation of Hypothalamic-Pituitary-Adrenal Axis Activity and Immunologic Function Contributed to the Anti-Inflammatory Effect of Acupuncture in the OVA-Induced Murine Asthma Model. Neurosci Lett (2017) 636:177–83. doi: 10.1016/j.neulet.2016.11.001
23. Wang Z, Hou L, Yang H, Ge J, Wang S, Tian W, et al. Electroacupuncture Pretreatment Attenuates Acute Lung Injury Through Alpha7 Nicotinic Acetylcholine Receptor-Mediated Inhibition of HMGB1 Release in Rats After Cardiopulmonary Bypass. Shock (2018) 50:351–9. doi: 10.1097/SHK.0000000000001050
24. Dong M, Ma C, Wang WQ, Chen J, Wei Y. Regulation of the IL-33/ST2 Pathway Contributes to the Anti-Inflammatory Effect of Acupuncture in the Ovalbumin-Induced Murine Asthma Model. Acupunct Med (2018) 36:319–26. doi: 10.1136/acupmed-2017-011377
25. Dong M, Wang WQ, Chen J, Li MH, Xu F, Cui J, et al. Acupuncture Regulates the Balance of CD4(+) T Cell Subtypes in Experimental Asthma Mice. Chin J Integr Med (2019) 25:617–24. doi: 10.1007/s11655-018-3055-6
26. Zhang XF, Xiang SY, Geng WY, Cong WJ, Lu J, Jiang CW, et al. Electro-Acupuncture Regulates the Cholinergic Anti-Inflammatory Pathway in a Rat Model of Chronic Obstructive Pulmonary Disease. J Integr Med (2018) 16:418–26. doi: 10.1016/j.joim.2018.10.003
27. Dhar R, Zhang L, Li Y, Rana MN, Hu Z, Li Z, et al. Electroacupuncture Ameliorates Cardiopulmonary Bypass Induced Apoptosis in Lung via ROS/Nrf2/NLRP3 Inflammasome Pathway. Life Sci (2019) 238:116962. doi: 10.1016/j.lfs.2019.116962
28. Huang D, Chen M, Wang Z, Hou L, Yu W. Electroacupuncture Pretreatment Attenuates Inflammatory Lung Injury After Cardiopulmonary Bypass by Suppressing NLRP3 Inflammasome Activation in Rats. Inflammation (2019) 42:895–903. doi: 10.1007/s10753-018-0944-y
29. Zhou L, Hong J, Wan Z, Lu X, Shao Y. Effects of Electroacupuncture Combined With Interleukin10 on Chronic Sinusitis in Mice. Mol Med Rep (2019) 20:1952–8. doi: 10.3892/mmr.2019.10375
30. Cui J, Dong M, Yi L, Wei Y, Tang W, Zhu X, et al. Acupuncture Inhibited Airway Inflammation and Group 2 Innate Lymphoid Cells in the Lung in an Ovalbumin-Induced Murine Asthma Model. Acupunct Med (2021) 39:217–25. doi: 10.1177/0964528420924033
31. Lou Y, Yu Q, Xu K, Tu Y, Balelang MF, Lu G, et al. Electroacupuncture Preconditioning Protects From Lung Injury Induced by Limb Ischemia/Reperfusion Through TLR4 and NFkappaB in Rats. Mol Med Rep (2020) 22:3225–32. doi: 10.3892/mmr.2020.11429
32. Tang W, Dong M, Teng F, Cui J, Zhu X, Wang W, et al. TMT-Based Quantitative Proteomics Reveals Suppression of SLC3A2 and ATP1A3 Expression Contributes to the Inhibitory Role of Acupuncture on Airway Inflammation in an OVA-Induced Mouse Asthma Model. BioMed Pharmacother (2021) 134:111001. doi: 10.1016/j.biopha.2020.111001
33. Zhang XF, Xiang SY, Lu J, Li Y, Zhao SJ, Jiang CW, et al. Electroacupuncture Inhibits IL-17/IL-17R and Post-Receptor MAPK Signaling Pathways in a Rat Model of Chronic Obstructive Pulmonary Disease. Acupunct Med (2021) 39:663–72. doi: 10.1177/0964528421996720
34. Garth J, Barnes JW, Krick S. Targeting Cytokines as Evolving Treatment Strategies in Chronic Inflammatory Airway Diseases. Int J Mol Sci (2018) 19:3402. doi: 10.3390/ijms19113402
35. Barnes PJ. Cellular and Molecular Mechanisms of Asthma and COPD. Clin Sci (Lond) (2017) 131:1541–58. doi: 10.1042/CS20160487
36. Rosloniec EF, Latham K, Guedez YB. Paradoxical Roles of IFN-Gamma in Models of Th1-Mediated Autoimmunity. Arthritis Res (2002) 4:333–6. doi: 10.1186/ar432
37. Muhl H, Pfeilschifter J. Anti-Inflammatory Properties of Pro-Inflammatory Interferon-Gamma. Int Immunopharmacol (2003) 3:1247–55. doi: 10.1016/S1567-5769(03)00131-0
38. Finkelman FD, Hogan SP, Hershey GK, Rothenberg ME, Wills-Karp M. Importance of Cytokines in Murine Allergic Airway Disease and Human Asthma. J Immunol (2010) 184:1663–74. doi: 10.4049/jimmunol.0902185
39. Kudo M, Melton AC, Chen C, Engler MB, Huang KE, Ren X, et al. IL-17A Produced by Alphabeta T Cells Drives Airway Hyper-Responsiveness in Mice and Enhances Mouse and Human Airway Smooth Muscle Contraction. Nat Med (2012) 18:547–54. doi: 10.1038/nm.2684
40. Barnes PJ. Inflammatory Mechanisms in Patients With Chronic Obstructive Pulmonary Disease. J Allergy Clin Immunol (2016) 138:16–27. doi: 10.1016/j.jaci.2016.05.011
Keywords: respiratory diseases, animal models, inflammatory cytokines, acupuncture, meta-analysis
Citation: Lee S and Kim S-N (2022) The Effect of Acupuncture on Modulating Inflammatory Cytokines in Rodent Animal Models of Respiratory Disease: A Systematic Review and Meta-Analysis. Front. Immunol. 13:878463. doi: 10.3389/fimmu.2022.878463
Received: 18 February 2022; Accepted: 11 May 2022;
Published: 15 June 2022.
Edited by:
Masato Kubo, Tokyo University of Science, JapanReviewed by:
Priscilla Christina Olsen, Federal University of Rio de Janeiro, BrazilO. Sang Kwon, Wonkwang University, South Korea
Copyright © 2022 Lee and Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Seung-Nam Kim, c25raW1AZG9uZ2d1ay5lZHU=
 Serin Lee
Serin Lee Seung-Nam Kim
Seung-Nam Kim