- 1Institute of Rheumatology, Shenzhen Futian Hospital for Rheumatic Diseases, Shenzhen, China
- 2Institute of Biochemistry and Immunology, Chung Shan Medical University, Taichung, Taiwan
- 3School of Medicine, Chung Shan Medical University, Taichung, Taiwan
- 4Department of Applied Foreign Languages, Chung Shan Medical University, Taichung, Taiwan
- 5Department of Medical Research, Chung Shan Medical University Hospital, Taichung, Taiwan
- 6Division of Allergy, Immunology and Rheumatology, Chung Shan Medical University Hospital, Taichung, Taiwan
- 7Institute of Medicine, Chung Shan Medical University, Taichung, Taiwan
- 8Graduate Institute of Integrated Medicine, China Medical University, Taichung, Taiwan
Objectives: Previous research has shown a possible relationship between endometriosis and autoimmune diseases. However, the relationship between endometriosis and ankylosing spondylitis (AS) is lacking. Therefore, we intended to find possible associations between endometriosis and AS using ICD-9 coding data in a population-based retrospective cohort study in Taiwan.
Method: Data for this retrospective cohort study were collected from the Taiwan National Health Insurance Research Database (NHIRD) between 2000–2012. We collected 13,145 patients with endometriosis and a 78,870 non-endometriosis comparison cohort. Diagnoses of endometriosis and AS were defined by the International Classification of Diseases-9 (ICD-9-CM) code for at least 3 outpatients or 1 hospitalization. Propensity score matching by comorbidities, corticosteroids, and non-steroidal anti-inflammatory drugs (NSAIDs) usage were done for baseline comparability. Cox proportional hazard models were used to evaluate crude and adjusted hazard ratios.
Results: The cumulative incidence of AS was higher in patients with endometriosis compared to the non-endometriosis comparison cohort (log-rank test, p = 0.015). The adjusted hazard ratio (aHR) of incidental AS in patients with endometriosis was 1.61 (95% CI = 1.11 to 2.35) in comparison to the non-endometriosis comparison cohort. An increased risk of AS was also observed in subjects with major depressive disorder (aHR = 5.05, 95% CI = 1.85 to 13.78). Stratified analyses of age subgroups showed consistent results. NSAID users had a lower risk of AS than NSAID non-users (aHR 4.57 vs 1.35, p for interaction = 0.031).
Conclusions: In this retrospective population-based cohort study, we found a higher risk of AS in patients with endometriosis. We suggest that clinicians should pay attention to the occurrence of AS in patients with endometriosis.
Introduction
Endometriosis is a common benign gynecologic disease with active endometrial cells outside the uterine cavity. Epidemiological studies show that the prevalence rate is about 10–15% of women within the reproductive age bracket worldwide (1). The pathogenesis of endometriosis has not been completely elucidated. Studies have shown that the immune system plays an important role in the pathogenesis of endometriosis (2). The imbalance of the immune system is associated with invasion, proliferation, and angiogenesis of the endometrium. In terms of endometriosis patients, the elevated number of macrophages and increased level of tumor necrosis factor (TNF)-α could provide an inflammatory environment for endometriosis progress; the decreased cytotoxicity of natural killer (NK) cells and elevated concentration of regulatory T cells could decrease immune surveillance and then promote the establishment of endometriotic lesions. Furthermore, Th17 cell subset may also play a role in endometriosis.
A recent meta-analysis has summarized the association between endometriosis and autoimmune diseases from several previous studies (3). Some studies have demonstrated that patients with endometriosis have a higher incidence of autoimmune diseases, namely, rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), Sjögren’s syndrome (SS), and multiple sclerosis (MS). However, the association between endometriosis and ankylosing spondylitis (AS) has never been reported. Endometriosis and AS share the same immunologic characteristics such as TNF-α and Th17 pathway. Therefore, in this study, we designed a retrospective cohort study to investigate the risk of AS in endometriosis women.
methods
Data Source
The research was a retrospective cohort study based on the National Health Insurance Research Database (NHIRD), which enrolled almost 99% of the population of 23 million beneficiaries in Taiwan. The database included all insurance claims data, including outpatient visits, emergency room visits, and hospitalizations. One million subjects were sampled from the 23 million beneficiaries, and the data were collected from 1999 to 2013. The sampled database was deidentified, and the study was approved by the Institutional Review Board of Chung Shan Medical University Hospital.
Exposure and Patient Selection
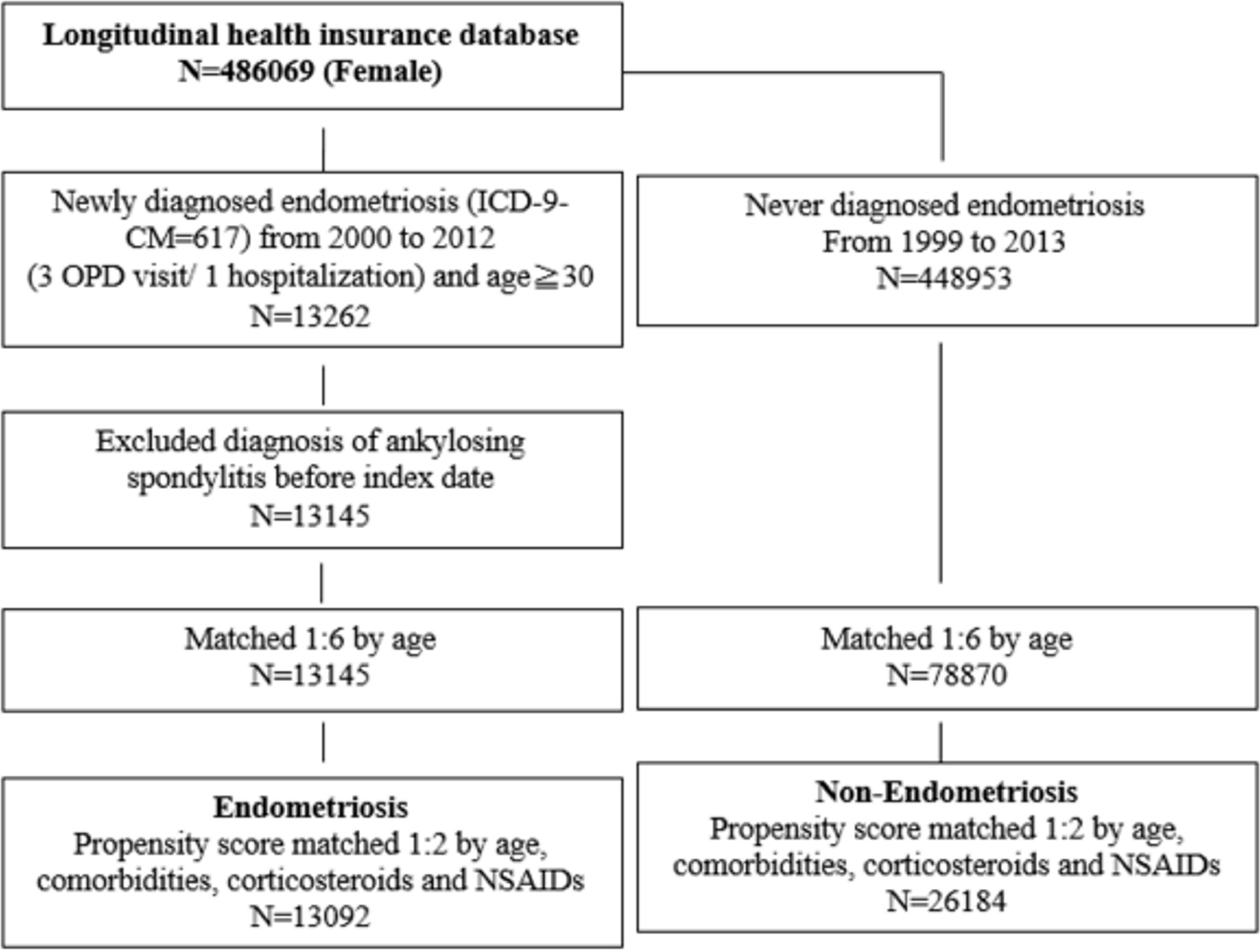
The study cohort included newly diagnosed female endometriosis from 2000 to 2012 with ICD-9-CM coding of 617 for outpatient visits ≥3 times or hospitalization ≥1 time in the NHIRD dataset. The index date was set as the date of the first endometriosis diagnosis for the patient. To ensure a new-onset subject, we excluded the diagnosis of ankylosing spondylitis (ICD-9-CM = 720.0) before 2000. The non-endometriosis comparison group was defined with participants who had never been diagnosed with endometriosis from 1999 to 2013.
Outcome
The outcome was the incidence of ankylosing spondylitis, defined by ICD-9-CM coding of 720.0 for at least three outpatient visits or one hospitalization after the index date of endometriosis. The study was followed until the occurrence of ankylosing spondylitis, or 31 December 2013, or withdrawal from the national insurance system, whichever occurred first.
Covariates
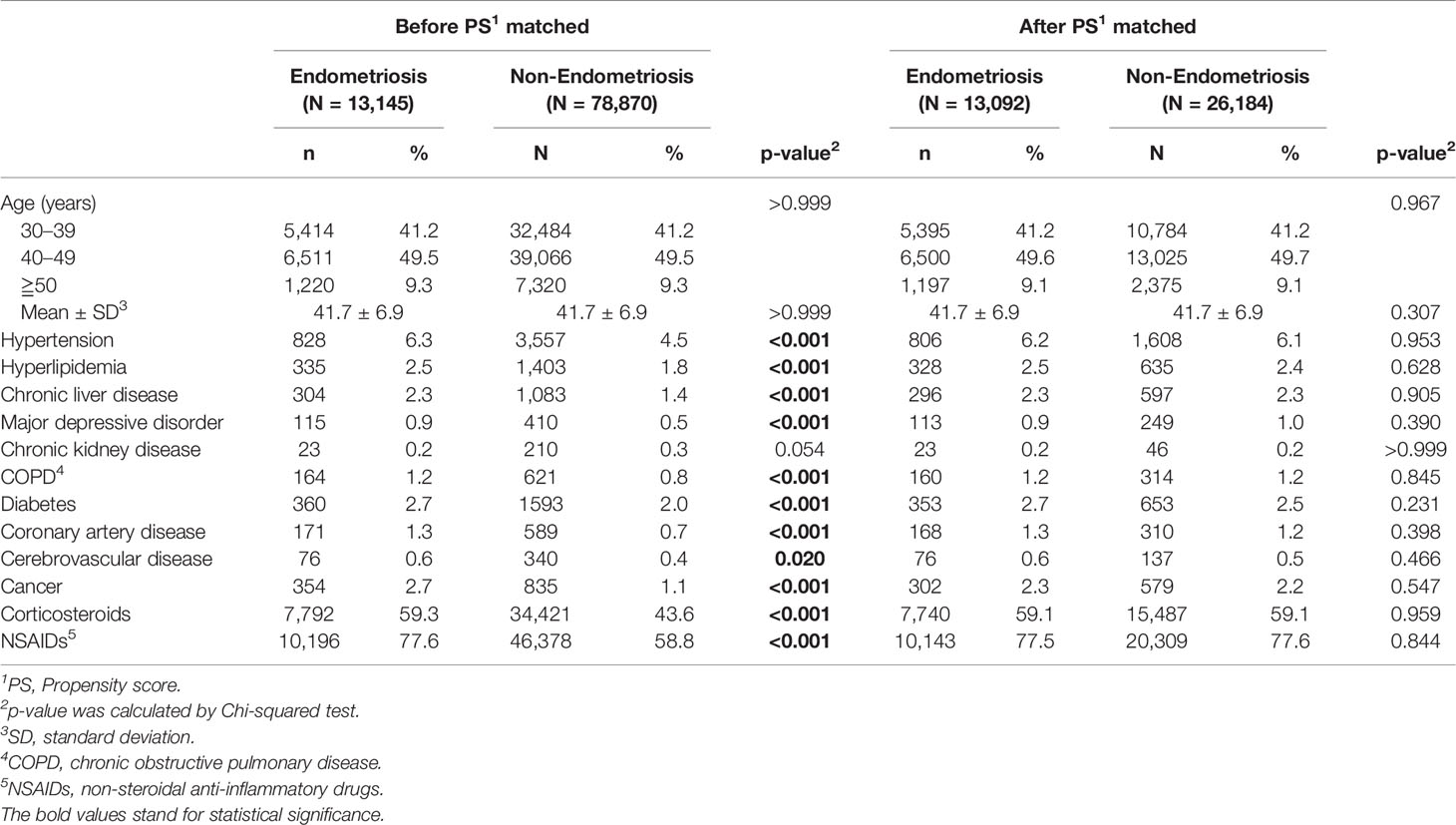
The baseline characteristics were matched by age, sex, and co-morbidities, namely, hypertension (ICD-9-CM = 401–405), hyperlipidemia (ICD-9-CM = 272.0–272.4), chronic liver disease (ICD-9-CM = 571), major depressive disorder (ICD-9-CM = 296.2, 296.3), chronic kidney disease (ICD-9-CM = 585), chronic obstructive pulmonary disease (ICD-9-CM = 490–492, 494, 496), diabetes (ICD-9-CM = 250), coronary artery disease (ICD-9-CM codes = 410–414), cerebrovascular disease (ICD-9-CM codes = 430–438), and cancer (ICD-9-CM = 140–208). Those comorbidities were defined before the index date within one year and at least three outpatient visits or once hospitalized. Additionally, corticosteroids and non-steroidal anti-inflammatory drugs (NSAIDs) usage, defined by a prescription for at least 30 days within the first year, was calculated.
Matching
As shown in Figure 1, 1:6 matching by age and index date was performed between both groups. Then, 1:2 propensity score matching was performed on comorbidities, corticosteroids, and NSAID usage between the two groups to ensure baseline comparability. The propensity score was a probability that was estimated through logistic regression. The binary variables were the endometriosis and non-endometriosis groups. By matching the propensity score, it could balance the heterogeneity between two groups.
Statistical Analyses
To compare the characteristics of the endometriosis and non-endometriosis groups, the Chi-square test for categorical variables and the Student’s t-test for continuous variables were used. The Kaplan–Meier analysis was used to calculate the cumulative incidence of AS and the log-rank test was used to test the significance. A Cox proportional hazard model was used to estimate the hazard ratio of AS between the endometriosis and non-endometriosis groups, and it was adjusted for age, comorbidities, corticosteroids, and NSAIDs. We made the proportional hazard assumption. The p-value of the time-dependent covariates was 0.771, which satisfied the proportional hazards assumption. The statistical software being used in this research was SPSS version 18.0 (SPSS Inc., Chicago, IL, USA).
Patient and Public Involvement
This research was conducted without participant involvement. The participants of the retrospective cohort study were not invited to comment on the study design and were not consulted to interpret the results. Participants were not invited to contribute to the writing or editing of this document for readability or accuracy.
Results
Analysis of Comorbidities and Medications in Endometriosis Patients
The characteristics of the enrolled 13,145 patients with endometriosis and 78,870 age-matched comparison cohorts are summarized in Table 1. Compared with the comparison cohort, patients with endometriosis were more likely to have the comorbidities, namely, hypertension (6.3% vs. 4.5%), hyperlipidemia (2.5% vs. 1.8%), chronic liver disease (2.3% vs. 1.4%), major depressive disorder (0.9% vs. 0.5%), COPD (1.2% vs. 0.8%), diabetes (2.7% vs. 2.0%), coronary artery disease (1.3% vs. 0.7%), cerebrovascular disease (0.6% vs. 0.4%), and cancer (2.7% vs. 1.1%), except for chronic kidney disease (0.2% vs. 0.3%). Consistent with the comorbidity analysis, most medications for these comorbidities, including costicosteroids (59.3% vs. 43.6%) and NSAIDs (77.6 vs. 58.8%), were significantly more frequently used in the endometriosis group than in the comparison cohort group.
Cumulative Risk for Ankylosing Spondylitis
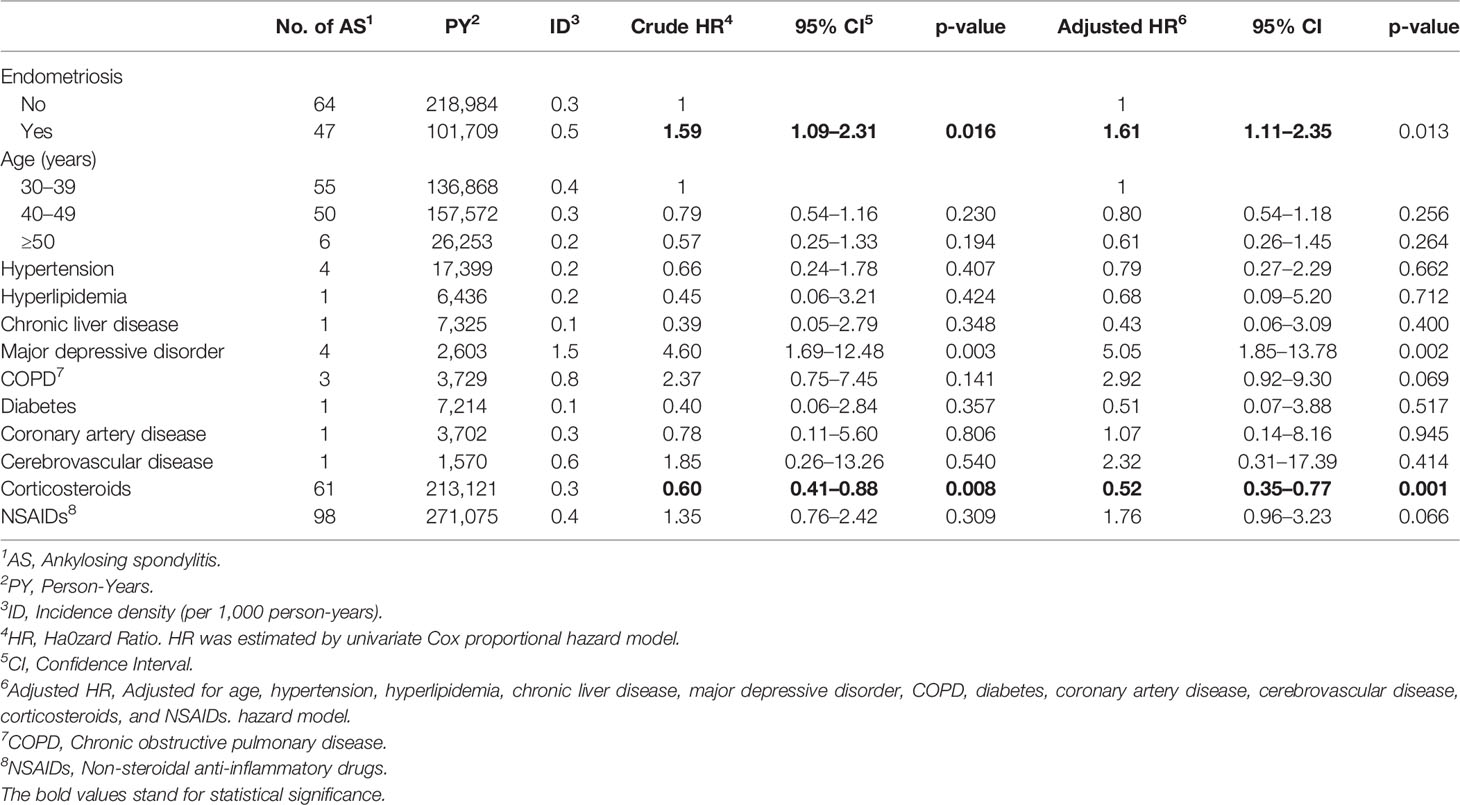
In a 14-year follow-up period, the cumulative incidence of AS in the endometriosis patients was significantly higher than that in those of non-endometriosis subjects (Figure 2, p = 0.015). The Cox proportional hazard regression analysis is shown in Table 2. Patients with endometriosis had an increased risk for subsequent AS compared with the non-endometriosis comparison cohort (HR = 1.61, 95% CI = 1.11 to 2.35, p = 0.013).
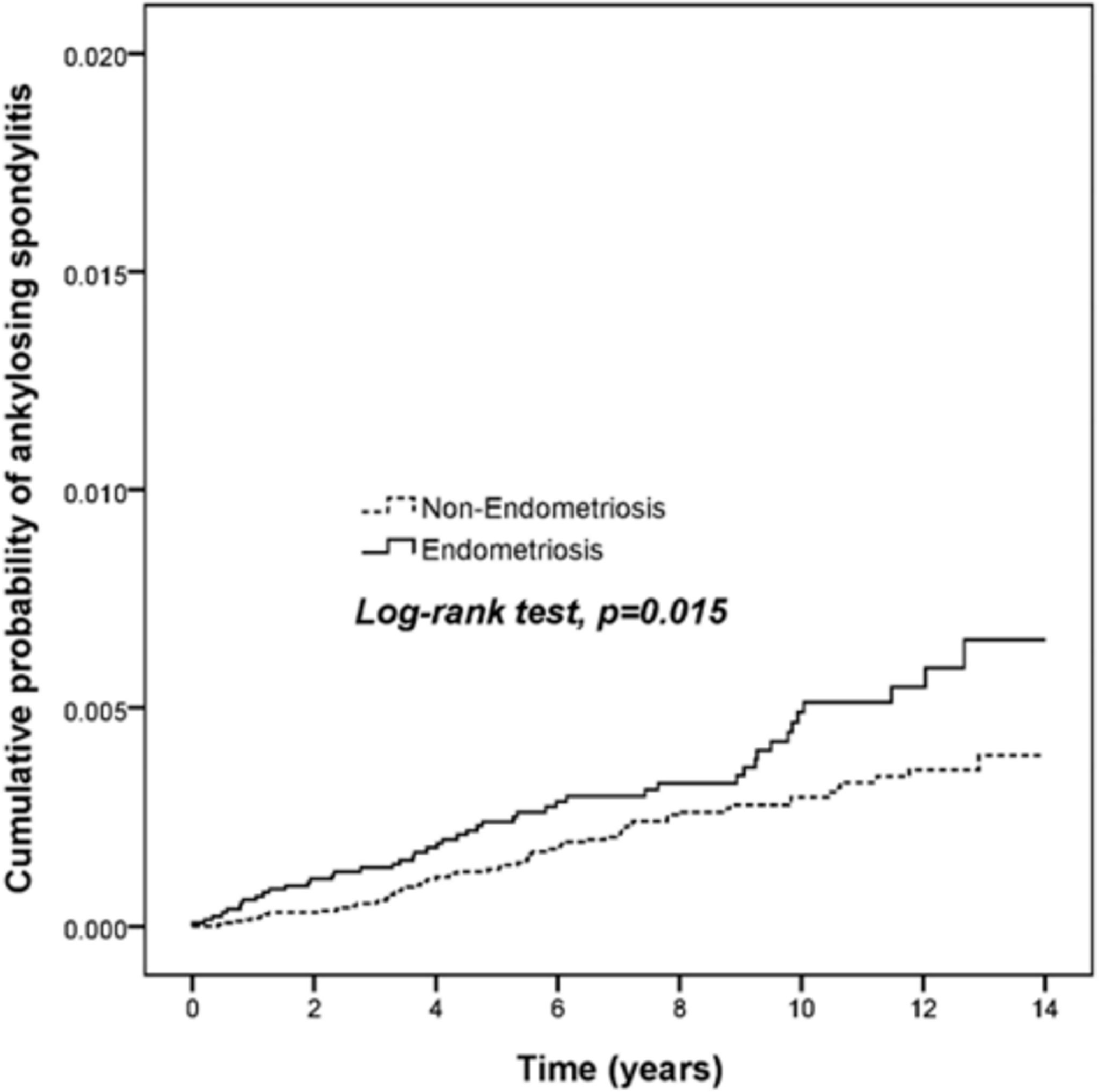
Figure 2 Endometriosis patients display a higher risk for ankylosing spondylitis. The cumulative probability among 13,145 endometriosis patients and 78,870 age and gender-matched comparison cohort was analyzed by log-rank test.
Association of AS With Comorbidities and Medications
An increased risk of AS was observed in patients with major depressive disorder (HR = 5.05, 95% CI = 1.85 to 13.78, p = 0.002). A trend of increased risk was also found in patients with COPD, although the p-value was not significant (HR = 2.92, 95% CI = 0.92 to 9.30, p = 0.069). The application of corticosteroids was associated with a lower risk of AS (HR = 0.52, 95% CI = 0.35 to 0.77, p = 0.001), whereas the use of NSAIDs showed an increased risk of AS but not significantly (HR = 1.76, 95% CI = 0.96 to 3.23, p = 0.066).
Stratified Analyses
To determine which endometriosis patients were most susceptible to AS, stratified analyses were conducted in the endometriosis group (Table 3). Patients with endometriosis and aged between 40 and 50 years had a higher risk of AS compared with non-endometriosis patients (HR = 2.01, 95% CI = 1.15–3.49, p = 0.014). In patients without the use of NSAIDs, patients with endometriosis had a significantly higher incidence of AS compared with non-endometriosis patients (HR = 4.57, 95% CI = 1.41–14.84, p = 0.011). Moreover, the HR showed a statistical difference between the two groups with or without NSAID usage (p for interaction = 0.031).
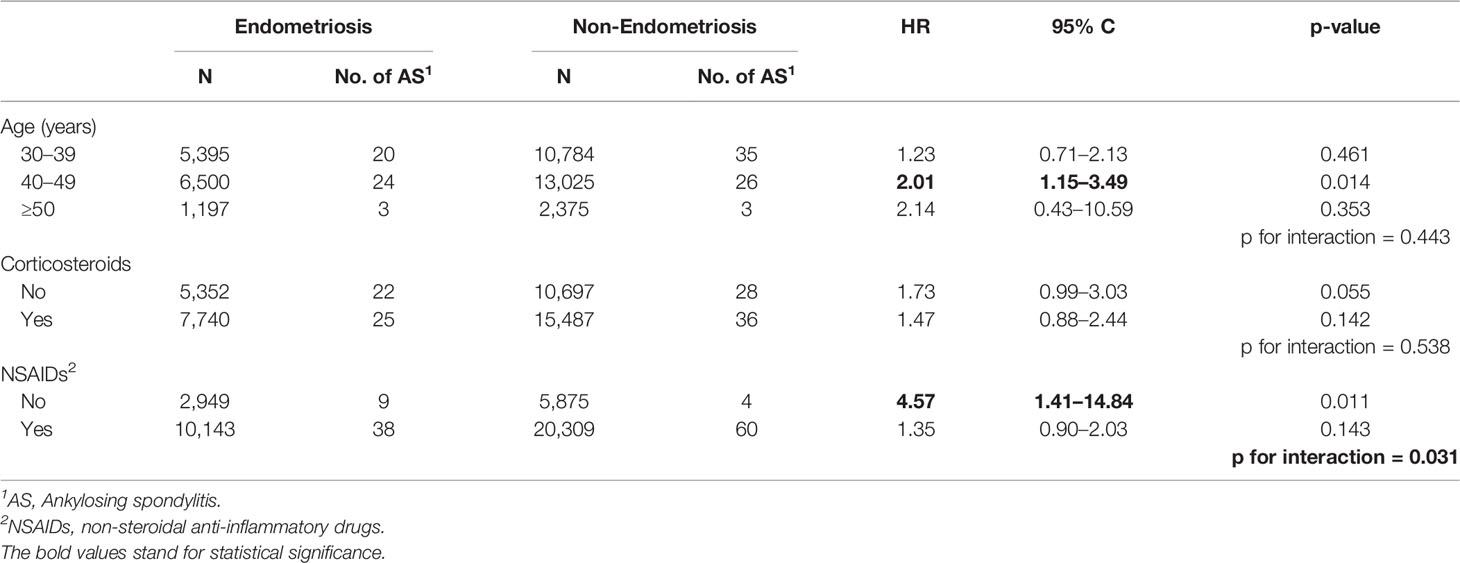
Table 3 Risk assessment of endometriosis or non-endometriosis subjects with age, corticosteroids, or NSAIDs.
The association of endometriosis and ankylosing spondylitis was more prominent at ages 40–49 compared with ages 30–39. The exact mechanism of this age-related association is unknown. One possibility is that at a younger stage, the pathogenesis of AS is contributed to by genetic and environmental factors. While in late-onset AS, hormones and chronic inflammation in the uterus may play more important roles.
Discussion
To our knowledge, this is the first study to use a nationwide population-based database to evaluate the relationship between endometriosis and AS. We found a statistically significant association between endometriosis and the risk of AS (HR = 1.59, 95% CI = 1.09–2.31, p = 0.016). Endometriosis patients without the use of NSAIDs were found to have a significantly higher incidence of AS compared with NSAID users (HR = 4.57, 95% CI = 1.41–14.84, p = 0.011). As such, we infer that endometriosis is probably an independent risk factor for AS.
The underlying mechanism between endometriosis and AS with an increased comorbidity is unclear. We hypothesized several possibilities to explain the mechanism. First, endometriosis and AS are probably related to gene susceptibility. The Killer immunoglobulin-like two-domain short-tail receptor (KIR2DS5) is a key receptor of NK cells that promotes an early immune response to infection. Previous studies indicate that the KIR2DS5 gene is a protective factor against endometriosis and AS (4–6). In a special case, a woman with endometriosis was diagnosed with 13 co-morbidities, including SLE and AS (7). She was found homozygous for rs2476601 SNP of the PTPN22 gene, and heterozygous for both rs27434 and rs30187 SNPs of the ERAP1 gene, which have been proven to be related to AS (8–10). Secondly, both endometriosis and AS are involved in inflammatory responses induced by inflammatory mediators (11–13). For endometriosis, the vigorous inflammatory response will cause endometrial cells to release more chemokines, namely, IL-1β, IL-6, IL-8, TNF-α, and IL-17 (10, 11). Also, increased levels of IL-6, TNF-α, IL-1β, and IL-17 could be detected in AS patients (12, 14, 15). Moreover, both TNF-α and IL-17 inhibitors have therapeutic effects on AS (16, 17). Some studies also suggested that anti-TNF-α or TNF-inhibitors can significantly inhibit the progression of endometriosis lesions (18–20).
Patients with major depressive disorder were also associated with an increased risk of AS, with evidence suggesting that inflammatory cytokines are involved in the pathogenesis of major depression (21). Hypotheses such as ‘macrophage theory of depression’ and the ‘cytokine hypothesis of depression’ were proposed to describe a higher expression of IL-1, TNF‐α, IL-6, and IL-8 in patients suffering from depression (22). The balance between pro- and anti-inflammatory cytokines is supposed to be altered in subgroups of depressed patients, resulting in symptoms seen in comorbid depression associated with inflammation such as ankylosing spondylitis. Cytokine blockers may be used to improve clinical outcomes in major depressive disorders (23). Several studies also suggested that AS increases the risk of depressive disorders (24–26). Therefore, major depressive disorder and AS may interact with each other due to immune dysfunction.
Patients with COPD might show a higher risk of AS, with the p-value being around 0.05 (HR = 2.92, 95% CI = 0.92 to 9.30, p = 0.069). Two studies have demonstrated an increased incidence of COPD in patients with AS (27, 28). Our previous study showed that COPD was associated with a higher risk of SLE (HR = 1.75, 95% CI = 1.06 to 2.89, p = 0.028) (29). COPD patients could produce autoantibodies that were reactive to those antigens that are also found in many autoimmune diseases (30). All these findings indicate that autoimmunity may be linked to COPD and AS. The effect of smoking on both COPD and AS should be considered, but such information is excluded from the database.
Corticosteroids are associated with many complications, such as osteoporosis and subsequent bone fractures, hypertension, and neuropsychiatric disorders (31). However, our data showed that the cumulative daily dose of corticosteroids for more than 30 days has a lower risk of developing AS (HR = 0.52, 95% CI = 0.35 to 0.77, p = 0.001). This is the first study to display the protective effects of corticosteroids on endometriosis patients against the risks of AS. The exact mechanism remains unknown, perhaps because of its anti-inflammatory effects. In comparison, the use of NSAIDs seemed to increase the risk of AS, though without statistical significance (HR = 1.76, 95% CI = 0.96 to 3.23, p = 0.066). Therefore, clinicians are advised to assess the medication effect on AS risk during follow-up of endometriosis patients.
In our study, we also found the association of endometriosis and ankylosing spondylitis to be more prominent in the group aged 40–49 compared with individuals aged 30–39. The exact mechanism of this age-associated memory is still unknown. One possibility is that at a younger stage, the pathogenesis of AS is contributed to by genetic and environmental factors. While in late-onset AS, hormones and chronic inflammation in the uterus may play more important roles. Further studies are needed to elucidate the exact mechanistic causes and the correlation between age and disease onset.
There were some limitations to our study. Firstly, endometriosis might be associated with multifactorial factors such as BMI, alcohol use, smoking, family history, and menstrual status, which were not available in our database. Nevertheless, we matched alcohol and smoking-related diseases, including COPD, liver disease, and diabetes, to minimize these limitations. Secondly, laboratory data such as hormone levels and inflammatory markers were not available in this claim-based database. Moreover, the severity of endometriosis was not recorded in this database, but we had matched both groups on steroid and NSAID usage and did stratified analyses to reduce this bias.
However, the strength of our study is the population-based data based on the whole Taiwanese population, which provided enough sample size to minimize selection bias. Furthermore, the follow-up time of this work was 14 years, enabling us to observe the subsequent occurrence of AS, yet further studies are needed to support our findings.
Conclusion
In this retrospective population-based cohort study, we found a potentially higher risk of having AS in patients with endometriosis. NSAID use might reduce the risk of developing AS. Clinicians should pay attention to the risk of AS in patients with endometriosis and possible underlying endometriosis in managing AS patients. Further research may help clarify the possible mechanisms between endometriosis and AS.
Data Availability Statement
The data analyzed in this study is subject to the following licenses/restrictions: The data in this article is provided by and used under the permission of the Bureau of National Health Insurance. Data would be shared with permission of the Bureau of National Health Insurance. Requests to access these datasets should be directed to James Cheng-Chung Wei, amNjd2VpQGdtYWlsLmNvbQ==.
Ethics Statement
This study was approved by the Chung Shan Medical University Hospital (IRB, CS19009).
Author Contributions
ZHY, JCCW and ZZY accessed the data and supervised the project. H-YL, K-SH, YZ and YHW performed bioinformatics analysis and wrote the manuscript. BSC wrote the manuscript and revised the literature. All authors contributed greatly to the production and submission of the work.
Funding
This work was supported by the Sanming Project of Medicine in Shenzhen (grant number SZSM201602087), the National Natural Science Foundation of China (grant number 81102266), Medical Scientific Research Foundation of Guangdong Province (grant number A2018089), the Shenzhen Science and Technology Project (grant number JCYJ20180504170414637), and the Shenzhen Futian Public Welfare Scientific Research Project (grant numbers FTWS2021006).
Author Disclaimer
Researchers were independent of the funders. All authors had full access to all of the data (including statistical reports and tables) in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Giudice LC, Kao LC. Endometriosis. Lancet (2004) 364(9447):1789–99. doi: 10.1016/S0140-6736(04)17403-5
2. García-Gómez E, Vázquez-Martínez ER, Reyes-Mayoral C, Cruz-Orozco OP, Camacho-Arroyo I, Cerbón M. Regulation of Inflammation Pathways and Inflammasome by Sex Steroid Hormones in Endometriosis. Front Endocrinol (Lausanne) (2019) 10:935. doi: 10.3389/fendo.2019.00935
3. Shigesi N, Kvaskoff M, Kirtley S, Feng Q, Fang H, Knight JC, et al. The Association Between Endometriosis and Autoimmune Diseases: A Systematic Review and Meta-Analysis. Hum Reprod Update (2019) 25(4):486–503. doi: 10.1093/humupd/dmz014
4. Nowak I, Płoski R, Barcz E, Dziunycz P, Kamiński P, Kostrzewa G, et al. KIR2DS5 in the Presence of HLA-C C2 Protects Against Endometriosis. Immunogenetics (2015) 67(4):203–9. doi: 10.1007/s00251-015-0828-3
5. Nowak I, Majorczyk E, Wiśniewski A, Pawlik A, Magott-Procelewska M, Passowicz-Muszyńska E, et al. Does the KIR2DS5 Gene Protect From Some Human Diseases? PLoS One (2010) 5(8):e12381. doi: 10.1371/journal.pone.0012381
6. Rezaei R, Mostafaei S, Aslani S, Jamshidi A, Mahmoudi M. Association Study Between Killer Immunoglobulin-Like Receptor Polymorphisms and Ankylosing Spondylitis Disease: An Updated Meta-Analysis. Int J Rheum Dis (2018) 21(10):1746–55. doi: 10.1111/1756-185X.13408
7. Matalliotaki C, Matalliotakis M, Zervou MI, Trivli A, Matalliotakis I, Mavromatidis G, et al. Co-Existence of Endometriosis With 13 non-Gynecological Co-Morbidities: Mutation Analysis by Whole Exome Sequencing. Mol Med Rep (2018) 18(6):5053–7. doi: 10.3892/mmr.2018.9521
8. Tizaoui K, Kim SH, Jeong GH, Kronbichler A, Lee KS, Lee KH, et al. Association of PTPN22 1858c/T Polymorphism With Autoimmune Diseases: A Systematic Review and Bayesian Approach. J Clin Med (2019) 8(3):347. doi: 10.3390/jcm8030347
9. Evans DM, Spencer CC, Pointon JJ, Su Z, Harvey D, Kochan G, et al. Interaction Between ERAP1 and HLA-B27 in Ankylosing Spondylitis Implicates Peptide Handling in the Mechanism for HLA-B27 in Disease Susceptibility. Nat Genet (2011) 43(8):761–7. doi: 10.1038/ng.873
10. Cortes A, Pulit SL, Leo PJ, Pointon JJ, Robinson PC, Weisman MH, et al. Major Histocompatibility Complex Associations of Ankylosing Spondylitis are Complex and Involve Further Epistasis With ERAP1. Nat Commun (2015) 6:7146. doi: 10.1038/ncomms8146
11. Yang H, Chen Y, Xu W, Shao M, Deng J, Xu S, et al. Epigenetics of Ankylosing Spondylitis: Recent Developments. Int J Rheum Dis (2021) 24(4):487–93. doi: 10.1111/1756-185X.14080
12. Kong W, Tang Y, Tang K, Yan Z, Liu T, Tao Q, et al. Leukemia Inhibitory Factor is Dysregulated in Ankylosing Spondylitis and Contributes to Bone Formation. Int J Rheum Dis (2022) 25(5):592–600. doi: 10.1111/1756-185X.14312
13. Feng HY, Chan CH, Chu YC, Qu XM, Wang YH, Wei JC. Patients With Ankylosing Spondylitis Have High Risk of Irritable Bowel Syndrome: A Long-Term Nationwide Population-Based Cohort Study. Postgrad Med (2022) 134(3):290–6. doi: 10.1080/00325481.2022.2041338
14. Londono J, Romero-Sanchez MC, Torres VG, Bautista WA, Fernandez DJ, Quiroga Jde A, et al. The Association Between Serum Levels of Potential Biomarkers With the Presence of Factors Related to the Clinical Activity and Poor Prognosis in Spondyloarthritis. Rev Bras Reumatol (2012) 52(4):536–44. doi: 10.1590/S0482-50042012000400006
15. Raychaudhuri SP, Raychaudhuri SK. Mechanistic Rationales for Targeting Interleukin-17A in Spondyloarthritis. Arthritis Res Ther (2017) 19(1):51. doi: 10.1186/s13075-017-1249-5
16. Maxwell LJ, Zochling J, Boonen A, Singh JA, Veras MM, Tanjong Ghogomu E, et al. TNF-Alpha Inhibitors for Ankylosing Spondylitis. Cochrane Database Syst Rev (2015) 4(4):Cd005468. doi: 10.1002/14651858.CD005468.pub2
17. Pavelka K, Kivitz A, Dokoupilova E, Blanco R, Maradiaga M, Tahir H, et al. Efficacy, Safety, and Tolerability of Secukinumab in Patients With Active Ankylosing Spondylitis: A Randomized, Double-Blind Phase 3 Study, MEASURE 3. Arthritis Res Ther (2017) 19(1):285. doi: 10.1186/s13075-017-1490-y
18. Kondo W, dal Lago EA, Noronha L, Olandoski M, Kotze PG, Amaral VF. Effect of Anti-TNF-α on Peritoneal Endometrial Implants of Rats. Rev Col Bras Cir (2011) 38(4):266–73. doi: 10.1590/S0100-69912011000400011
19. Liu Y, Sun L, Hou Z, Mao Y, Cui Y, Liu J. rhTNFR: Fc Suppresses the Development of Endometriosis in a Mouse Model by Downregulating Cell Proliferation and Invasiveness. Reprod Sci (2016) 23(7):847–57. doi: 10.1177/1933719115620495
20. Ajrawat P, Touma Z, Sari I, Taheri C, Diaz Martinez JP, Haroon N. Effect of TNF-Inhibitor Therapy on Spinal Structural Progression in Ankylosing Spondylitis Patients: A Systematic Review and Meta-Analysis. Int J Rheum Dis (2020) 23(6):728–43. doi: 10.1111/1756-185X.13829
21. Varan Ö, Babaoğlu H, Göker B. Associations Between Depressive Disorders and Inflammatory Rheumatic Diseases. Curr Top Med Chem (2018) 18(16):1395–401. doi: 10.2174/1568026618666180516100805
22. Buras A, Waszkiewicz N, Szulc A, Sierakowski S. [Monocytic Parameters in Patients With Rheumatologic Diseases Reflect Intensity of Depressive Disorder]. Pol Merkur Lekarski (2012) 33(198):325–9. doi: 10.5604/17322693.1196386
23. Shariq AS, Brietzke E, Rosenblat JD, Barendra V, Pan Z, McIntyre RS. Targeting Cytokines in Reduction of Depressive Symptoms: A Comprehensive Review. Prog Neuropsychopharmacol Biol Psychiatry (2018) 83:86–91. doi: 10.1016/j.pnpbp.2018.01.003
24. Shen CC, Hu LY, Yang AC, Kuo BI, Chiang YY, Tsai SJ. Risk of Psychiatric Disorders Following Ankylosing Spondylitis: A Nationwide Population-Based Retrospective Cohort Study. J Rheumatol (2016) 43(3):625–31. doi: 10.3899/jrheum.150388
25. Hakkou J, Rostom S, Mengat M, Aissaoui N, Bahiri R, Hajjaj-Hassouni N. Sleep Disturbance in Moroccan Patients With Ankylosing Spondylitis: Prevalence and Relationships With Disease-Specific Variables, Psychological Status and Quality of Life. Rheumatol Int (2013) 33(2):285–90. doi: 10.1007/s00296-012-2376-6
26. Ben Tekaya A, Mahmoud I, Hamdı I, Hechmı S, Saıdane O, Tekaya R, et al. [Depression and Anxiety in Spondyloarthritis: Prevalence and Relationship With Clinical Parameters and Self-Reported Outcome Measures]. Turk Psikiyatri Derg (2019) 30(2):90–8.
27. Hemminki K, Liu X, Ji J, Sundquist K, Sundquist J. Subsequent COPD and Lung Cancer in Patients With Autoimmune Disease. Eur Respir J (2011) 37(2):463–5. doi: 10.1183/09031936.00070410
28. Lambert J, Chekroun M, Gilet H, Acquadro C, Arnould B. Assessing Patients' Acceptance of Their Medication to Reveal Unmet Needs: Results From a Large Multi-Diseases Study Using a Patient Online Community. Health Qual Life Outcomes (2018) 16(1):134. doi: 10.1186/s12955-018-0962-3
29. Shi LH, Huang JY, Liu YZ, Chiou JY, Wu R, Wei JC. Risk of Systemic Lupus Erythematosus in Patients With Human Papillomavirus Infection: A Population-Based Retrospective Cohort Study. Lupus (2018) 27(14):2279–83. doi: 10.1177/0961203318809179
30. Packard TA, Li QZ, Cosgrove GP, Bowler RP, Cambier JC. COPD is Associated With Production of Autoantibodies to a Broad Spectrum of Self-Antigens, Correlative With Disease Phenotype. Immunol Res (2013) 55(1-3):48–57. doi: 10.1007/s12026-012-8347-x
Keywords: ankylosing spondylitis, autoimmune diseases, endometriosis, immunology, spine
Citation: Yin Z, Low H-Y, Chen BS, Huang K-S, Zhang Y, Wang Y-H, Ye Z and Wei JC-C (2022) Risk of Ankylosing Spondylitis in Patients With Endometriosis: A Population-Based Retrospective Cohort Study. Front. Immunol. 13:877942. doi: 10.3389/fimmu.2022.877942
Received: 17 February 2022; Accepted: 16 May 2022;
Published: 15 June 2022.
Edited by:
Ho-Chang Kuo, Kaohsiung Chang Gung Memorial Hospital, TaiwanReviewed by:
Hazan Karadeniz, Gazi University, TurkeyYutong Jiang, Third Affiliated Hospital of Sun Yat-sen University, China
Kuo-Chung Lan, Chang Gung Memorial Hospital, Taiwan
Malcolm Koo, Tzu Chi University of Science and Technology, Taiwan
Copyright © 2022 Yin, Low, Chen, Huang, Zhang, Wang, Ye and Wei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: James Cheng-Chung Wei, amNjd2VpQGdtYWlsLmNvbQ==; Zhizhong Ye, eWV6aGl6aG9uZzIwMDBAMTYzLmNvbQ==
†ORCID: James Cheng-Chung Wei, orcid. org/0000-0002-1235-0679
 Zhihua Yin1
Zhihua Yin1 Brian Shiian Chen
Brian Shiian Chen Yue Zhang
Yue Zhang Yu-Hsun Wang
Yu-Hsun Wang Zhizhong Ye
Zhizhong Ye James Cheng-Chung Wei
James Cheng-Chung Wei