- 1Department of Breast Surgery, The First Hospital of Lanzhou University, Lanzhou, China
- 2Department of General Surgery, The First Hospital of Lanzhou University, Lanzhou, China
- 3Department of Breast Surgery, Fudan University Shanghai Cancer Center, Shanghai, China
Background: Dual-targeted therapy is the standard treatment for human epidermal growth factor receptor 2 (HER2)-positive breast cancer, and effective biomarkers to predict the response to neoadjuvant trastuzumab and pertuzumab treatment need further investigation. Here, we developed a predictive model to evaluate the dual-targeted neoadjuvant treatment efficacy in HER2 gene-amplified breast cancer.
Method: This retrospective study included 159 HER2-amplified patients with locally advanced breast cancer who received neoadjuvant trastuzumab, pertuzumab, and chemotherapy. The correlation between clinicopathological factors and pathological complete response (pCR, in the breast and axilla) was evaluated. Patients were randomly assigned into the training set (n=110) and the testing set (n=49). We used an independent cohort (n=65) for external validation. We constructed our predictive nomogram model with the results of risk variables associated with pCR identified in the multivariate logistic analysis. The area under the curve (AUC) of the receiver operating characteristic (ROC) curve, decision curve analysis, and calibration curves were employed to assess the nomogram’s performance.
Results: We revealed that the HER2/CEP17 ratio (p=0.001), CD8 levels (p=0.005), and histological grade (p=0.007) were independent indicators for pCR in dual-targeted neoadjuvant treatment after multivariate adjustment. The combined prediction efficacy of the three indicators was significantly higher than that of each single indicator alone. The AUCs were 0.819, 0.773, and 0.744 in the training, testing, and external validation sets, respectively.
Conclusions: The HER2/CEP17 ratio, CD8 levels, and histological grade were significantly correlated with pCR in dual-targeted neoadjuvant treatment. The combined model using these three markers provided a better predictive value for pCR than the HER2/CEP17 ratio, CD8 levels, and the histological grade alone, which showed that an immunological effect partially mediates the predictive impact of neoadjuvant treatment.
Introduction
The human epidermal growth factor receptor 2 (HER2) gene, which plays a pivotal part in tumor formation and growth processes, is amplified to roughly 20%–30% of all breast cancers (1). HER2 amplification can be defined by protein expression, HER2 gene copy number alterations, or the HER2/CEP17 (centromere enumerator probe 17) ratio (2). Immunohistochemistry (IHC) and fluorescence in situ hybridization (FISH) are widely used to define HER2 status according to the American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) guidelines for HER2 testing (3). HER2 gene amplification is associated with poor clinical outcomes (4, 5). Pathological complete response (pCR) of the tumor has been extensively used as the major endpoint in neoadjuvant chemotherapy (NAC) trials. It is an essential independent indicator in the prognosis of prolonged disease-free and overall survival time (6). Anti-HER2 therapy is currently the standard treatment for HER2-amplified patients in adjuvant and neoadjuvant settings. More recently, neoadjuvant treatment for HER2 gene-amplified locally advanced breast cancer patients has significantly expanded with the advent of dual-targeted treatment by trastuzumab and pertuzumab. The combination of trastuzumab with pertuzumab and standard chemotherapy confirmed the improvement of the pCR rate in the TRYPHAENA and NeoSphere studies (7, 8). The patients included in this study were consecutive incident cases who had HER2-amplified locally advanced breast cancer and had received six cycles of docetaxel and carboplatin (TCb) NAC with trastuzumab and pertuzumab.
Previous research confirmed that a higher pCR rate was associated with HER2-amplified patients in neoadjuvant treatment. However, the relationship between the HER2 gene amplification level and pCR to concurrent neoadjuvant therapy is still unclear. A study from Yu found a better pCR rate with both a higher HER2/CEP17 ratio and a higher HER2 copy number and then demonstrated that HER2-amplified breast cancer was sensitive to NAC (9). This conclusion was also confirmed in trastuzumab-treated HER2-amplified patients from neoadjuvant trials by Wu (10). A recent study suggested that proteomic changes after a single cycle of HER2-targeted therapy could help identify tumors that would eventually undergo a pCR, validated a single marker CD45 as a classifier that robustly predicted pCR, and indicated that CD45-positive cell counts measured by conventional immunohistochemistry had similar results (11). These findings indicate that screening predictive markers in neoadjuvant treatment and the application of predictive models will have guiding significance for adjusting subsequent therapies. Therefore, there is an urgent need for pCR predictors in medicine. Some studies showed that pCR is predictive for long-term outcomes in HER2-positive and triple-negative breast cancer (12). Markers with predictive value can improve the probability of predicting pCR and also provide a reference for the follow-up treatment after neoadjuvant therapy. At present, studies performed in neoadjuvant treatment with trastuzumab and pertuzumab are still insufficient.
The immune system has become a promising new target for the diagnosis of breast cancer. Tumor-infiltrating lymphocytes (TILs) are included in the microenvironment of breast cancer, and the levels of TILs may also be considered prognostic factors (13). TILs consist of different cells such as CD3+ TILs, CD4+ TILs, CD8+ TILs, and FoxP3+ TILs (14). Treg cells induce the inactivation of CD8+ TILs, which plays a part in causing the death of tumor cells (15). A recent study reported that a high ratio of CD8+TILs to FOXP3+ regulatory T cells significantly improved patient survival in breast cancer (16). In our previous study, the original intention of measuring CD8 levels was to screen the immunomodulatory (IM) type in triple-negative breast cancer (TNBC). However, we found that CD8+ TILs in our patients could be used as a robust marker for predicting the rate of pCR to neoadjuvant treatment, considering the HER2/CEP17 ratio and histological grade. Although some studies have shown that TILs might have significant predictive values for NAC response (17) and indicate good survival in the adjuvant setting (18), no proven results of their roles in predicting the pCR rate in a neoadjuvant treatment setting have been found.
In summary, previous studies revealed that pCR was closely correlated with the HER2/CEP17 ratio in neoadjuvant anti-HER2 dual-targeted treatment (19). A few studies have shown the relationship between CD8 levels and response in dual-targeted neoadjuvant therapy with trastuzumab and pertuzumab. In this study, we investigated the relationship among HER2 amplification, CD8 levels, and pCR to find a better predictive value for HER2-amplified patients.
Patients and Methods
Patients
We retrospectively selected 159 consecutive patients with HER2-amplified breast cancer, histologically confirmed by core needle biopsy, who received neoadjuvant treatment with pertuzumab and trastuzumab plus chemotherapy in the Department of Breast Surgery, Fudan University Shanghai Cancer Center (FUSCC, Shanghai, China), from January 1, 2020 to June 30, 2021. Furthermore, we also collected an external validation set from the First Hospital of Lanzhou University (Lanzhou, Gansu, China), including 65 patients treated in the same way and during the same period. HER2-amplified patients were defined as those 2+ or 3+ positive for HER2 over-expression by IHC and amplified by fluorescence in situ hybridization (FISH) (Figures 1, 2). The detailed inclusion criteria were as follows: the absence of metastases, as assessed using chest computed tomography (CT) scan, bone scan, and positron emission tomography (PET)/CT; normal hematopoietic, hepatic, and renal functions; and a normal echocardiogram without severe cardiac arrhythmia or heart failure. The exclusion criteria were a previous malignancy history (except for inactive non-melanoma skin cancer and in situ carcinoma of the cervix), grade 2 or higher neurotoxicity according to the National Cancer Institute Common Toxicity Criteria (version 3.0) (http://ctep.cancer.gov), active infection, and other comorbid conditions that would affect drug tolerance or impair compliance. The studies involving participants were reviewed and approved by the independent ethics committee/institutional review board of The First Hospital of Lanzhou University Ethical Committee and Fudan University Shanghai Cancer Center Ethical Committee. Written informed consent was provided by all patients.
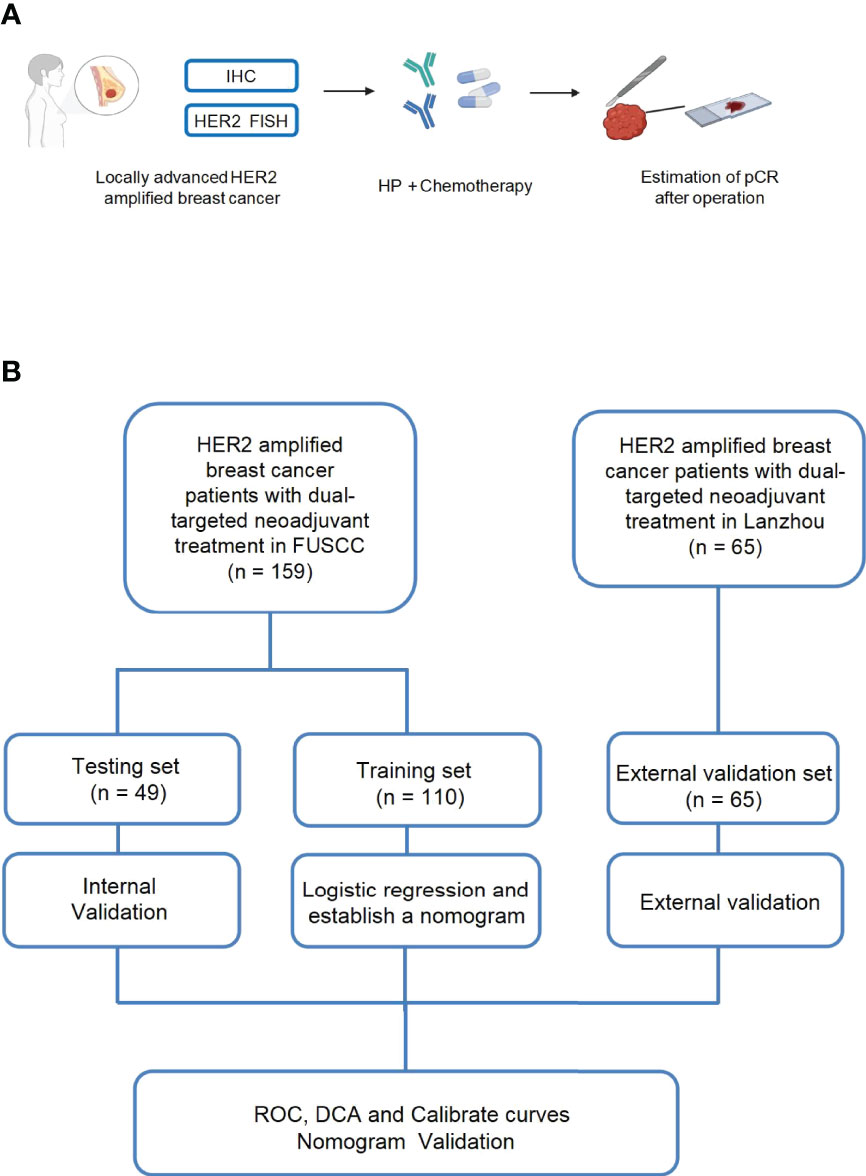
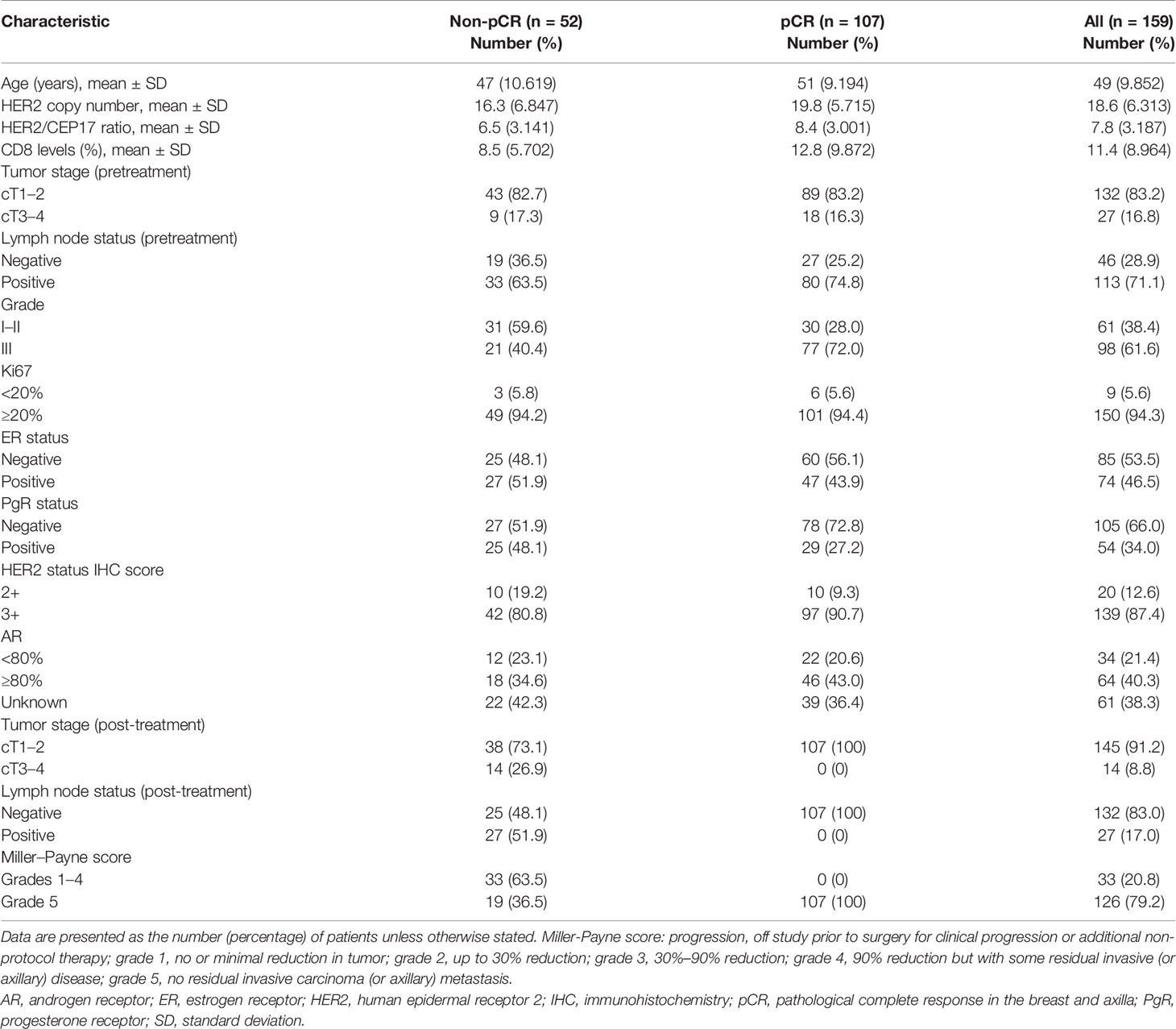
Figure 1 Schematic illustration of our experimental design (A, B). One hundred fifty-nine patients with locally advanced HER2-amplified breast cancer, histologically confirmed by core needle biopsy, were selected. HER2-amplified patients were defined as those who were 2+ or 3+ positive for HER2 overexpression by immunohistochemistry (IHC) or HER2 amplified by fluorescence in situ hybridization (FISH) and received neoadjuvant treatment with pertuzumab and trastuzumab plus chemotherapy. Then, pCR was estimated after the surgery.
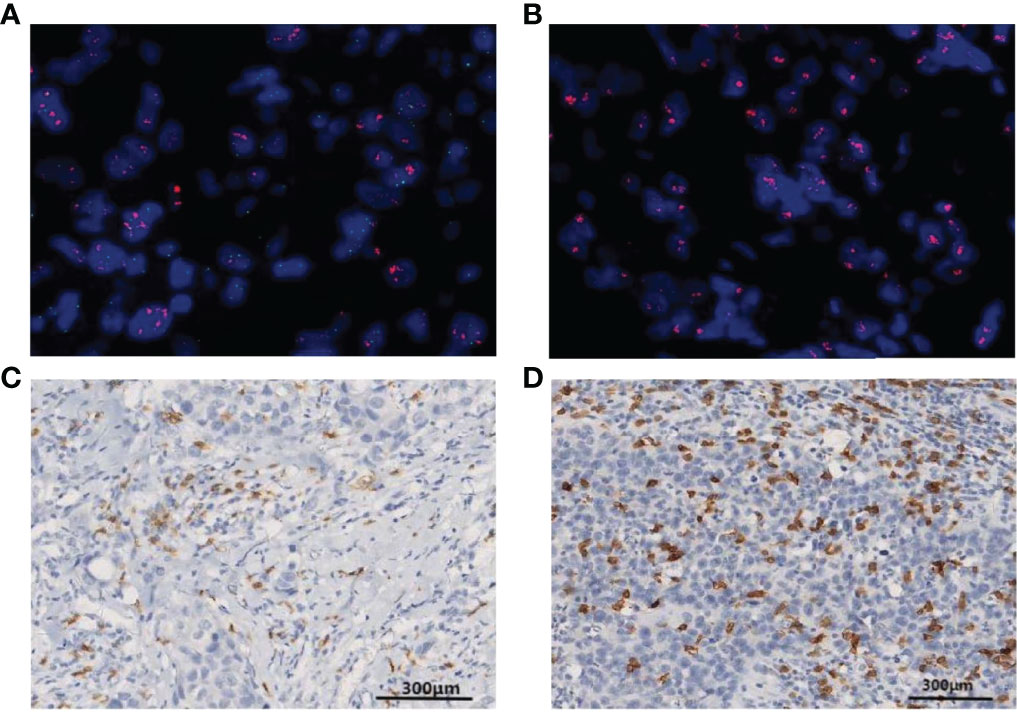
Figure 2 Fluorescence in situ hybridization (FISH) is shown for HER2 gene amplification in specimens and in corresponding histological sections (HER2, red signal; CEP17, green signal). (A) A patient with positive HER2 amplification status of a HER2/CEP17 ratio <8. (B) A patient with positive HER2 amplification status of a HER2/CEP17 ratio ≥8. (C) Patterns of CD8+ T-lymphocyte IHC staining >10% CD8 IHC staining (100× magnification). (D) More than 20% CD8 IHC staining (100× magnification).
Treatment
Invasive carcinomas were diagnosed by core needle biopsy in all patients. Ultrasound, breast magnetic resonance imaging (MRI), and CT scans of the chest and abdomen were conducted for clinical staging. Fine needle aspiration cytology was performed when metastasis to axillary lymph nodes was suspected or found. All patients received carboplatin [area under curve (AUC)=6] and docetaxel [75 mg/m2, without dose escalation] plus an 8-mg/kg loading dose of trastuzumab, followed by a 6-mg/kg maintenance dose, and an 840-mg pertuzumab loading dose followed by a 420-mg maintenance dose every 3 weeks for 6 cycles (the TCbHP regimen). No patient required reducing the dosage owing to tolerance difficulties (8).
Immunohistochemistry
IHC assessment was carried out with paraffin-embedded tumor samples biopsied prior to the neoadjuvant treatment. Estrogen receptor (ER), progesterone receptor (PgR), the Ki67 labeling index, and HER2 expression were evaluated by IHC in all patients (20). For comparison, each HER2-amplified tumor was ultimately evaluated based on the updated 2018 American Society of Clinical Oncology (ASCO)/College of American Pathologists (CAP) Clinical Practice Guidelines as follows (1): IHC 3+ positive, circumferential complete, intense membrane staining in >10% of tumor cells (2); IHC 2+ equivocal, weak to moderate complete membrane staining in >10% of tumor cells, moderate to intense but incomplete (basolateral or lateral) staining, or circumferential intense staining in ≤10% of cells (3); IHC 1+ negative, incomplete membrane staining that is faint/barely perceptible in >10% of tumor cells; and (4) IHC 0 negative, no staining or incomplete, faint/barely perceptible staining in ≤10% of tumor cells (21). Tumors were positive for ER and PgR, with ≥1% of cells being positive (22). IHC for CD8 levels and androgen receptor (AR) staining was performed on the tumors of all patients. The proportion of CD8+ T cells was calculated as the percentage of CD8+ T cells among total cells. Any cell with CD8-positive staining was counted as CD8+ TILs. Results were recorded as percentage of IHC-stained cells and adjudicated by two pathologists (23). The following primary antibodies were used: anti-AR (Abcam, ab133273), anti-CD8 (SP57, Ventana), anti-ER (Abcam, ab108398), and anti-PgR (Abcam, ab16661).
HER2 FISH
The analysis of FISH was carried out on deparaffinized 5-μm tissue sections using the Path Vysion HER2 DNA probe kit and the HER2/CEP17 probe mixture (Abbott Molecular, Des Plaines, IL, USA) (24). Experienced pathologists in the Department of Pathology of FUSCC and the Department of Pathology of the First Hospital of Lanzhou University assessed the FISH. The pathologists reported the average copy numbers of HER2 and CEP17 and the HER2/CEP17 ratio for each patient. The standard College of American Pathologists and the American College of Medical Genetics guidelines were used to assess the quality control of the HER2 FISH test routinely (25). All samples in our study were HER2 amplified with a HER2/CEP17 ratio ≥ 2.0 (26).
Evaluation of Clinical Response and Pathological Response
The modified Response Evaluation Criteria in Solid Tumors (RECIST1.1) criteria defined the clinical response (27). The semi-quantitative Miller–Payne grading system was used to measure pathological response. This measures the reduction in percentage in invasive tumor volume and cellularity based on the pathological assessment of surgical samples after neoadjuvant treatment (28, 29). In our study, pCR was defined both in the breast (grade 5 of the Miller–Payne score) and axillary regions.
Construction and Validation of a Nomogram Model
All the included samples were assigned into a training set and a testing set randomly according to a ratio of 7:3 based on an approach of splitting the data by the hospitalization date (30). We used an independent cohort (n=65) from the First Hospital of Lanzhou University as an external validation set. We selected the HER2/CEP17 ratio, CD8 levels, and histological grade as variables, and used the logistic regression to construct a nomogram model in training set (31). The fit of the model was assessed by the Hosmer–Lemeshow goodness-of-fit chi-square test. Each variable was assigned a point value by drawing a vertical line between the appropriate variable value and the scale in the first row. We can get the total nomogram score by summing up the scores for each of the variables. Then, a probability of pCR can be determined by drawing a vertical line between the total score and the scale in the last row. The area under the curve (AUC) of the receiver operating characteristic (ROC) curve, calibration curves, and decision curve analysis (DCA) were employed to evaluate the nomogram’s performance.
Statistical Analysis
General characteristics of study subjects were presented as the mean ± standard deviation (SD) for continuous variables and the number (percentage) for categorical variables. Categorical data were compared using the chi-square or Fisher’s exact test. We used the logistic regression to obtain the odds ratio (OR) with 95% confidence interval (CI) for the association with the response. ROC curve analysis was used to assess the prediction power of the HER2/CEP17 ratio, CD8 levels, and histological grade. For all analyses, a p-value <0.05 was considered significant. All statistical analyses were carried out in R (version 3.6.3). In addition, “glm,” “rms,” “pROC,” “Calibration Curves,” and “Decision Curve” packages were used.
Results
Patient Characteristics
We recruited 159 eligible HER2-amplified patients with locally advanced breast cancer from January 2020 to June 2021, and 142 of them were premenopausal. All of the patients received TCb plus trastuzumab and pertuzumab as neoadjuvant treatment for six cycles. Clinicopathological characteristics are shown in Table 1. The female patients had a mean age of 49 years (SD=9.852). The number of pretreatment patients in tumor stage T1–2 was 132 (83.2%), and in tumor stage T3–4, it was 27 (16.8%). The histological grades were GI–II (n=61, 38.4%) and GIII (n=98, 61.6%). The Ki67 value was over 20% in most patients (n=150, 94.3%). A total of 113 patients were positive for lymph node metastasis (pretreatment) (71.1%), 46.5% were ER positive, 34.0% were PgR positive, and 40.3% were over 80% AR positive. In the pretreatment FISH HER2 assessment, the mean HER2/CEP17 ratio was 7.8 (SD=3.187), and the mean HER2 copy number was 18.6 (SD=6.313). The number of CD8 levels was 11.4% (SD=8.964). There were 145 (91.2%) posttreatment patients in tumor stage T1–2 and 14 (8.8%) in tumor stage T3–4. One hundred thirty-two patients were negative for lymph node metastasis after treatment (83.0%) (Table 1).
Treatment Response and Predictors of Response
In univariate logistic regression analyses in the training set, the HER2/CEP17 ratio (OR=1.307, p=0.001, 95% CI: 1.117–1.530), HER2 copy number (OR=1.097, p=0.012, 95% CI: 1.021–1.180), and CD8 level (OR=1.072, p=0.024, 95% CI: 1.009–1.139) (Table 2) were better associated with pCR. Regarding other predictors of pCR, PgR positivity and histological grade had a significant effect on pCR in the breast and axilla. Nevertheless, tumor size, Ki67, lymph node status, and ER status at baseline had no significant effect on tumor response. In the univariate analysis, compared with pCR, the HER2/CEP17 ratio had a higher OR value than the HER2 copy number. The HER2 copy number was excluded from the multivariate analysis to avoid confounding.
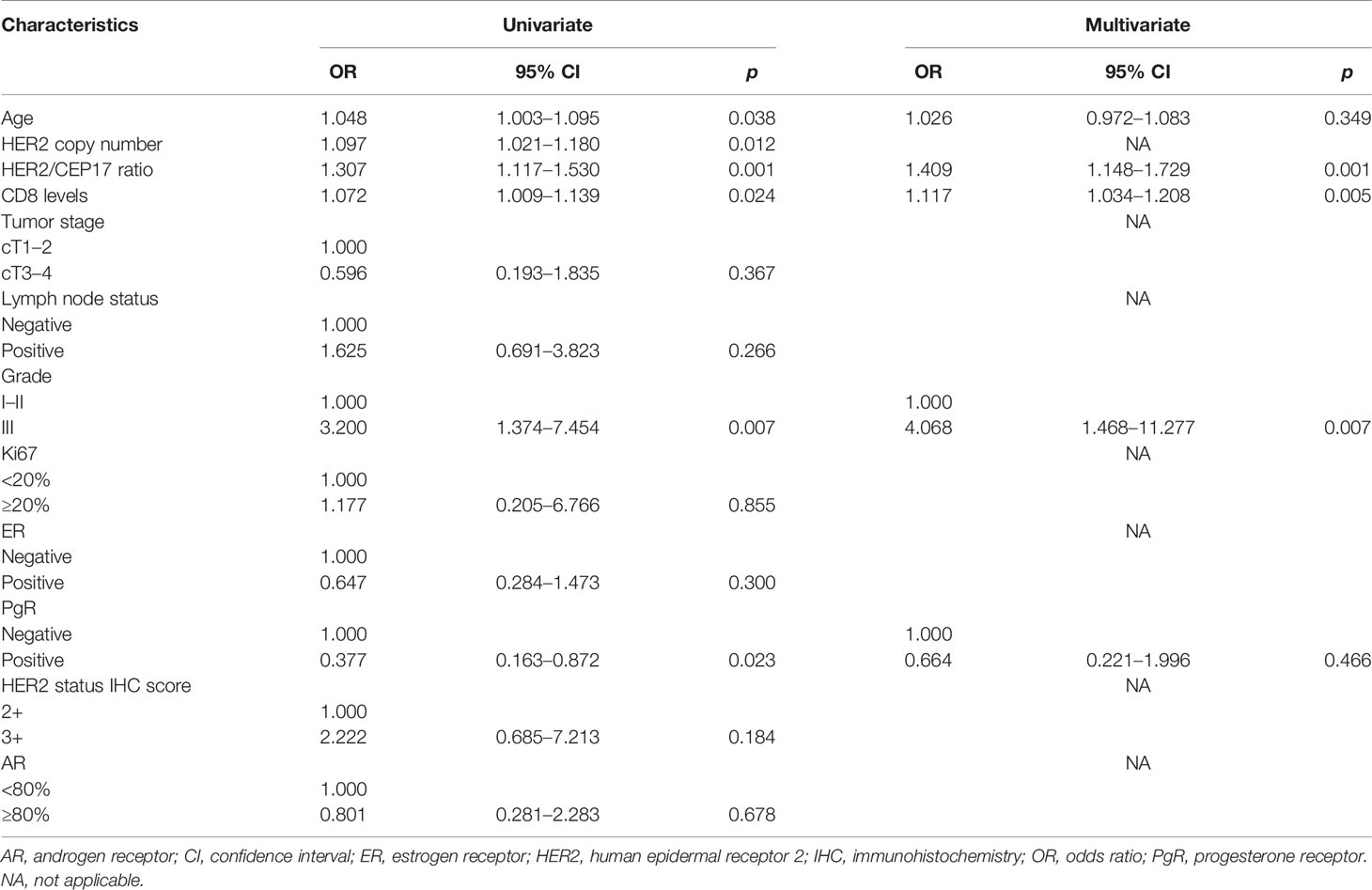
Table 2 Univariate and multivariate logistic regression models predicting pathological complete response after neoadjuvant treatment in training set.
Similarly, in the multivariate analysis, women were more likely to achieve pCR if tumors showed a higher HER2/CEP17 ratio (continuous) (OR=1.409, p=0.001, 95% CI: 1.148–1.729). CD8 levels (continuous) were predictive of pCR, with an OR of 1.117 (p=0.005, 95% CI: 1.034–1.208). The probability of achieving pCR was also depended on histological grades (OR=4.068, p=0.007, 95% CI: 1.468–11.277). Next, the multivariate analysis revealed that PgR positivity (OR=0.664, p=0.466, 95% CI: 0.221–1.996) was not associated with pCR (Table 2). We modeled the HER2/CEP17 ratio and CD8 levels as continuous variables and histological grade as a categorical variable.
Validation of a Predictive Nomogram Model
An ROC curve was constructed using the HER2/CEP17 ratio, CD8 levels, and histological grade to select the best responders to concurrent NAC with trastuzumab and pertuzumab. The AUC was 0.787 in the whole cohort. The AUC of the HER2/CEP17 ratio, CD8 levels, and histological grade was 0.685, 0.622, and 0.658, respectively, in the whole cohort (Figure 3A). We integrated pCR with the HER2/CEP17 ratio, CD8 levels, and histological grade to construct a prognostic nomogram model in training set (Figures 3B, C).
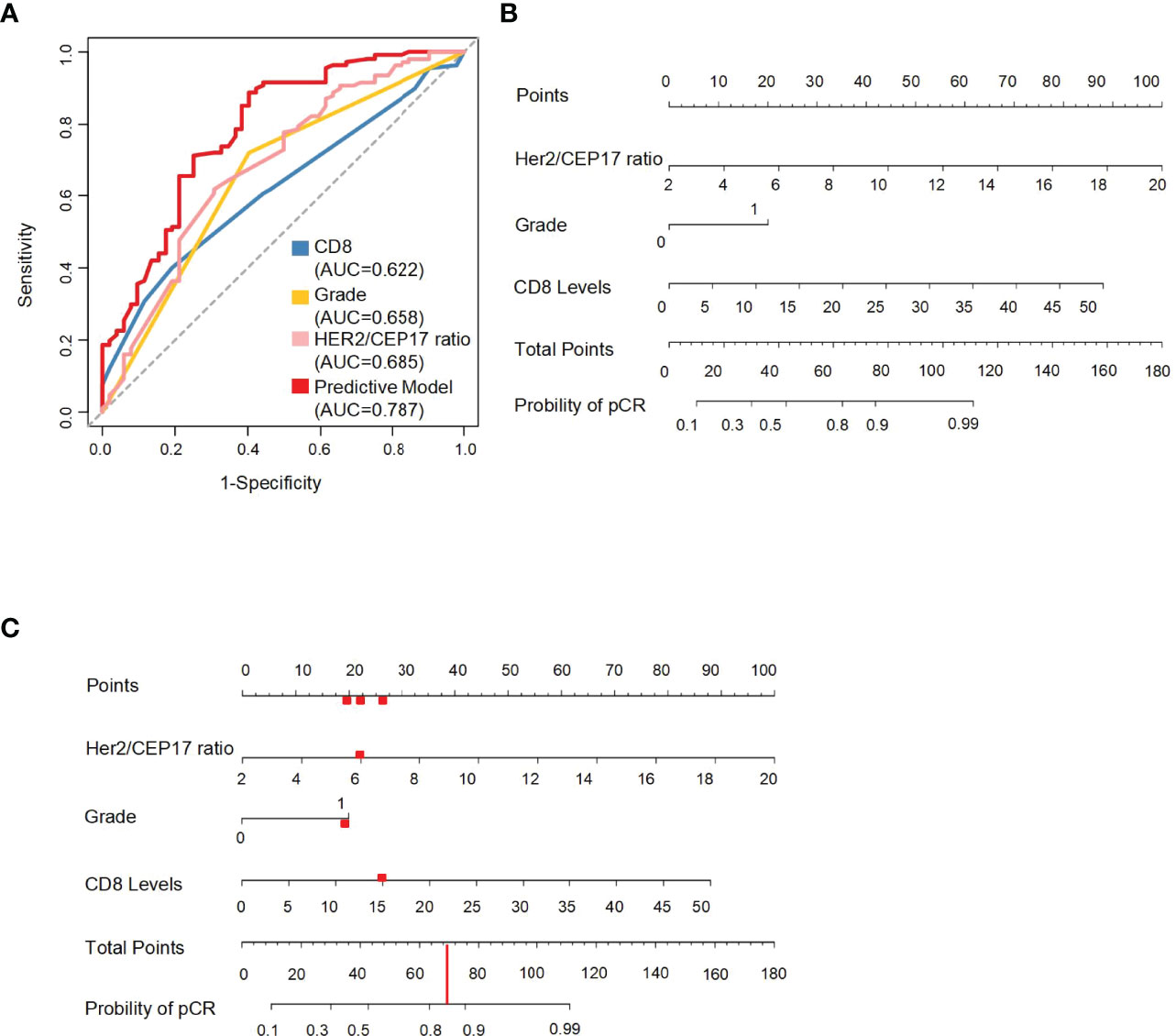
Figure 3 The ROC curves of the HER2/CEP17 ratio, CD8 levels, and histological grade and the predictive nomogram model. (A) ROC curves constructed using the HER2/CEP17 ratio, CD8 levels, and histological grade in the whole cohort. (B) Nomogram to predict the probability of pathological complete response (pCR). HER2/CEP17 ratio, CD8 levels, and histological grade (0 present grade I–II, 1 present grade III) in the training set. (C) The red line and dots demonstrate usage of the model: a patient of HER2-amplified breast cancer with histological grade 3 and 15% of CD8 levels, and HER2/CEP17 ratio is 6 would have a total of 70 points (20 for histological grade 3, 22 for HER2/CEP17 ratio, and 28 for CD8 levels). The predictive value of pCR after NAC for this patient is 85%.
With this nomogram model, the AUC value was 0.819 in the training set and 0.773 and 0.744, respectively, in the testing and external validation sets. The AUC of the HER2/CEP17 ratio, CD8 levels, and histological grade was 0.718, 0.627, and 0.641 in the training set (Figure 4A). The AUC of the HER2/CEP17 ratio, CD8 levels and histological grade was 0.668, 0.640, and 0.706 in the testing set (Figure 4B). The AUC of the HER2/CEP17 ratio, CD8 levels, and histological grade was 0.664, 0.649, and 0.644, respectively, in the external validation set (Figure 4C). From the results above, it can be seen that the results of the testing set and external validation set were generally consistent with the training set, and the combined AUC value of the HER2/CEP17 ratio, CD8 levels, and histological grade was better than the individual indicators.
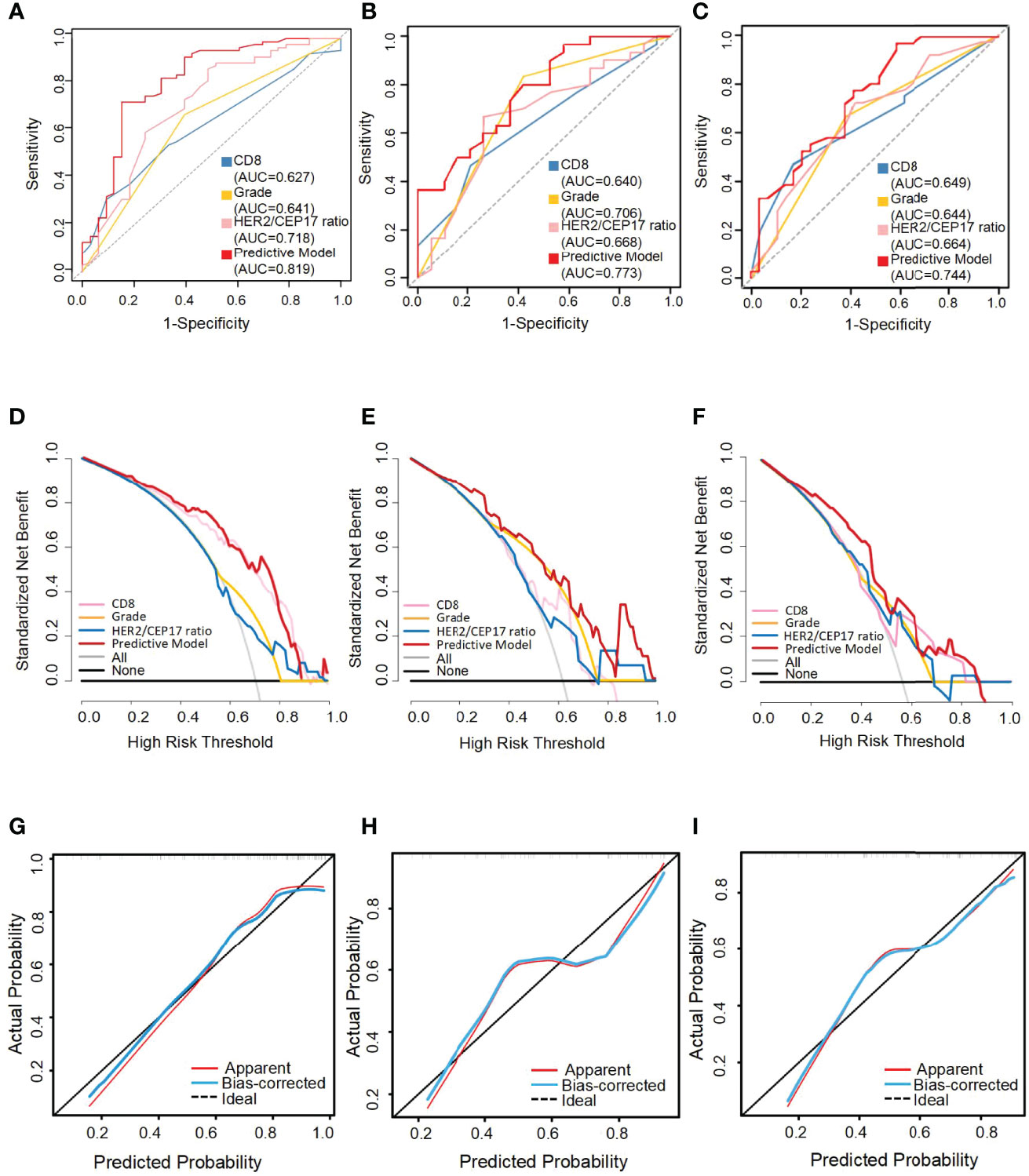
Figure 4 The receiver operating characteristic (ROC) curves, decision curve analysis (DCA), and calibration curve for the predictive nomogram model in the training set, testing set, and external validation set. AUC, area under the curve. The ROC curves of the training set (A), the testing set (B), and the external validation set (C). Decision curve analysis (DCA) of the training set (D), testing set (E), and external validation set (F). Calibration curve of the nomogram in the training set (G), the testing set (H), and the external validation set (I).
The performance of the nomograms was validated with ROC curve, calibration curve, and DCA for discriminative ability, accurate prediction, and clinical utility, respectively. The y-axis represents the net benefit in DCA curves. The red line indicates the predictive nomogram. The gray line represents the assumption that all patients obtain pCR. The horizontal black line represents the assumption that no patients get pCR. DCA was to show the clinical benefits of the nomogram in the training set, testing set, and external validation set. As was shown in Figure 4, DCA curves showed the clinical net benefits in the training set (Figure 4D), testing set (Figure 4E), and the external validation set (Figure 4F). The DCA curve of the predictive model (red) revealed better beneficial than the HER2/CEP17 ratio (pink), CD8 levels (blue), and histological grade (gold), respectively, in different sets. The calibration curve of the nomogram showed good agreement between the actual observations and the predicted outcomes in the training set (Figure 4G), testing set (Figure 4H), and external validation set (Figure 4I). As the prediction curves were close to the standard curve (Y=X), the final model had good performance and high applicability.
Discussion
In the neoadjuvant setting, dual-targeted treatment by trastuzumab and pertuzumab is standard for HER2 gene-amplified breast cancer. In the TRYPHAENA and NeoSphere study, the improvement of the pCR rate was confirmed by pertuzumab with trastuzumab and standard chemotherapy combined (7, 8). At present, studies evaluating the therapeutic effect are still insufficient. The existing indicators are used for single-targeted therapy but not dual-targeted therapy. Our study revealed that the HER2/CEP17 ratio, CD8 levels, and histological grade were associated with the response to the same neoadjuvant treatment. There are some potential reasons why HER2-amplified patients are more sensitive to dual-targeted therapy. First, trastuzumab induces internalization and degradation of the HER2 receptor by binding to the extracellular domain of the transmembrane HER2 receptor (32). Second, trastuzumab is related to immune and antibody-dependent cell-mediated cytotoxicity (ADCC). FCγR polymorphisms play a part in trastuzumab-mediated ADCC and also predict the clinical results in breast cancer patients (33). When trastuzumab binds to the FCγR of natural killer cells, it triggers ADCC and activates cell lysis (10). Lastly, trastuzumab leads to the inhibition of its downstream signaling via the RAS/MAPK and PI3K/AKT pathways, ultimately suppressing cellular growth and proliferation signaling. Pertuzumab prevents potent ligand-dependent HER2/HER3 heterodimerization, suppressing downstream PI3K and MAPK pathways (34, 35). The combination of the two drugs in chemotherapy should be taken into consideration for their contribution to the improved pCR outcome of NAC. Some studies in HER2-amplified breast cancer patients receiving neoadjuvant treatment focused on the level of HER2 amplification and its relationship with pCR. In a study conducted in South Korea, the HER2/CEP17 ratio was related with pCR in dual-targeted HER2 neoadjuvant treatment (19). Another study demonstrated that a higher HER2/CEP17 ratio was predictive of pCR to NAC in patients included in the National Cancer Database (36). Regarding the predictive value, we found that, compared with the HER2 copy number, the HER2/CEP17 ratio was more closely associated with pCR outcome, which agrees with previous findings. Kogawa showed a positive correlation between the HER2/CEP17 ratio and pCR (37). However, it is not reliable enough to depend on a single indicator as a predictor, so we built a combined model of the HER2/CEP17 ratio, CD8 level, and histological grade, which has a better predictive value for pCR.
Tumor histological grade refers to the degree of tumor tissue anaplasticity, including tumor differentiation, arrangement, number of mitoses, and local infiltration of cancer cells. The degree of tumor tissue anaplasticity can provide a reference basis for clinical treatment and prognosis estimation. The histological grade was associated with pCR in our research. Our findings are consistent with existing findings. A study from Rouzier found that the histological grade after the completion of neoadjuvant treatment was a good independent prognostic factor for pCR in patients with breast cancer (38). The correlation between pCR and long-term outcome was higher in patients with high-grade tumors than in those with low-grade tumors in neoadjuvant treatment (12). Furthermore, another article concludes the relationship between histological grade and neoadjuvant dual-target and chemotherapy response pCR in HER2-amplified tumors. Their analysis of the HER2-amplified tumors confirmed that a higher pCR rate was seen in histological grade III tumors treated with NAC and dual anti-HER2 therapy (39), which was consistent with our conclusion.
The immune microenvironment may affect dual-targeted neoadjuvant treatment efficacy. TILs can produce a positive effect on the frequency of pCR in TNBC and HER2-amplified breast cancer (13, 40). One study showed that high pre-NAC TIL levels were clearly predictive of pCR and can act as a surrogate marker to predict therapeutic effects of a dual-targeted neoadjuvant treatment regimen for HER2-amplified breast cancer (41). For multivariate analysis, our study found that CD8 levels could act as a robust marker to predict the pCR rate to neoadjuvant treatment, considering the HER2/CEP17 ratio and histological grade. Research has shown that an immunological signature consisting of the presence of a high number of CD8+TILs on final surgical biopsy samples of breast tumors treated with NAC is associated with pCR (42). Another possible reason is that the cell death induced by chemotherapy can lead to the release of tumor antigens, which can be processed by antigen-presenting cells (APCs) to CD8+ TILs, resulting in the death of cancer cells by activating CD8+ TILs (43).
Some data confirmed the hypothesis that breast cancer is immunogenic and may be targetable by immune-modulating therapies. CD8+ TILs play essential roles in the immune microenvironment, mainly by killing tumor cells through cytotoxic effects. TILs as an indicator of pre-existing immunogenicity might be useful for further stratification of breast cancer in clinical trials, including chemotherapy, anti-HER2 therapies, and future combinations with immune therapies (13). Trastuzumab also attracts cytotoxic innate immune cells to the tumor microenvironment, a concept commonly known as ADCC.
At the same time, we found that the HER2/CEP17 ratio, histological grade, and CD8 levels were positively associated with pCR, so we used an ROC curve to construct a predictive model for pCR with these three markers to select the best responder to concurrent NAC with trastuzumab and pertuzumab. It is noteworthy that in this model of neoadjuvant treatment, we confirm that the combined AUC value of the HER2/CEP17 ratio, CD8 levels, and histological grade was better than the individual indicators, which showed that an immunological effect partially mediates the predictive impact of neoadjuvant treatment. This study will provide more options for the clinical treatment of HER2-amplified breast cancer. The pCR prediction models were composed of different factors, which were currently widely studied, and there was no gold standard for models. The advantage of our prediction model was that we used HER2/CEP17 ratio and histological grade as two factors because they were recognized factors that were associated with pCR in HER2-positive breast cancer, and we also added CD8+TILs immune factor as one of the indicators. The AUC of the HER2/CEP17 ratio, CD8 levels, and histological grade was 0.718, 0.627 and 0.641, respectively, and the AUC was 0.819 of the combined model in the training set. Of course, this conclusion needs to be further verified by larger samples in the future.
We analyzed why hormone receptors (HRs) status is not predictive of pCR in our study and found that the predictive value of HR status remains unclear in HER2-amplified breast cancer. Previous studies demonstrated that HER2-amplified/hormone receptors-negative patients treated with the TCbHP regimen had higher pCR rates (71.1%–76.2%) (44, 45). However, in our study, ER and PgR status at baseline had no significant effect on tumor response (Table 2). Other studies also showed that the ER and PgR status was probably not a predictor for pCR (46), and the ER status was not predictive of pCR in subgroup analyses in HER2-amplified breast cancer patients (11). The small sample size perhaps is another explanation to our findings. Some potential factors were not found to be significant in our current study due to this reason. Prospective and randomized cohort study with larger sample size was needed to validate the predictive value of hormone status, and multi-omics profiling might facilitate the understanding of the effects of hormone status on dual-targeted therapy.
There are also some limitations in our study. First, studies conducted with larger sample sizes are required, and more clinical studies are needed to confirm our conclusions further. Second, a single IHC marker often provides inaccurate and incomplete prognostic indices, and more IHC markers should be included to predict pCR in dual-targeted neoadjuvant treatment. This study selected CD8 levels as the predictive marker because of its previous studies, which showed that CD8+TILs were associated with a positive effect of pCR in HER2-amplified breast cancer (47). Our advantage is that these model building indicators can be easily evaluated by pathologists, compared with multiplex techniques or proteomics. Thus, it is easy to apply this model in the clinical setting.
Consequently, it is crucial to find more effective molecular biological indicators to formulate individualized treatment plans and provide precision treatment for HER2-amplified breast cancer patients.
Conclusion
In conclusion, the HER2/CEP17 ratio, histological grade, and CD8 levels were positively associated with pCR. Furthermore, we constructed a nomogram model that could significantly predict the pCR rate of dual-targeted neoadjuvant treatment in HER2-amplified breast cancer patients.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving participants were reviewed and approved by the independent ethics committee/institutional review board of The First Hospital of Lanzhou University Ethical Committee and Fudan University Shanghai Cancer Center Ethical Committee. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
Conception/design: YX, KY, and XL. Provision of study material or patients: YX. Collection and/or assembly of data: YX and DM. Data analysis and interpretation: JD and SC. Manuscript writing: YX and JD. Final approval of manuscript: YX, JD, DM, SC, KY, and XL. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by the Science and Technology Planning Project of Gansu Province of China (21JR7RA371), the fund of The First Hospital of Lanzhou University of Gansu Province of China (ldyyyn2020-91), and The Wu Jieping Medical funding of China (320.6750.2021-10-100). The fund of Lanzhou Science and Technology Bureau of China (2022-ZD-96). The funders had no role in the design of the study and collection, analysis, interpretation of data, or writing of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Professor Lu Zhang from the Training Department, Xi’an International Studies University, for his help on the manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.877825/full#supplementary-material
References
1. Iqbal N. Human Epidermal Growth Factor Receptor 2 (HER2) in Cancers: Overexpression and Therapeutic Implications. Mol Biol Int (2014) 2014:852748. doi: 10.1155/2014/852748. Iqbal N.
2. Perez EA, Cortés J, Gonzalez-Angulo AM, Bartlett JM. HER2 Testing: Current Status and Future Directions. Cancer Treat Rev (2014) 40:2–276-84. doi: 10.1016/j.ctrv.2013.09.001
3. Wolff AC, Hammond M, Allison KH, Harry BE, Mangu PB, Bartlett JM, et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J Clin Oncol (2018) 36:20–2105-22. doi: 10.1200/JCO.2018
4. Michael F, Press LB, Thomas PA, Meisner LF, Zhou J-Y, Ma Y, et al. HER-2/Neu Gene Amplification Characterized by Fluorescence In Situ Hybridization: Poor Prognosis in Node-Negative Breast Carcinomas. J Clin Oncol (1997) 15:2894–904. doi: 10.1200/JCO.1997.15.8.2894
5. Gown AM, Goldstein LC, Barry TS, Kussick SJ, Kandalaft PL, Kim PM, et al. High Concordance Between Immunohistochemistry and Fluorescence In Situ Hybridization Testing for HER2 Status in Breast Cancer Requires a Normalized IHC Scoring System. Mod Pathol (2008) 21:10–1271-7. doi: 10.1038/modpathol.2008.83
6. Rastogi P, Anderson SJ, Bear HD, Geyer CE, Kahlenberg MS, Robidoux A, et al. Preoperative Chemotherapy: Updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol (2008) 26:5–778-85. doi: 10.1200/JCO.2007.15.0235
7. Gianni L, Pienkowski T, Im Y-H, Roman L, Tseng L-M, Liu M-C, et al. Efficacy and Safety of Neoadjuvant Pertuzumab and Trastuzumab in Women With Locally Advanced, Inflammatory, or Early HER2-Positive Breast Cancer (NeoSphere): A Randomised Multicentre, Open-Label, Phase 2 Trial. Lancet Oncol (2012) 13:1–25-32. doi: 10.1016/s1470-2045(11)70336-9
8. Schneeweiss A, Chia S, Hickish T, Harvey V, Eniu A, Hegg R, et al. Pertuzumab Plus Trastuzumab in Combination With Standard Neoadjuvant Anthracycline-Containing and Anthracycline-Free Chemotherapy Regimens in Patients With HER2-Positive Early Breast Cancer: A Randomized Phase II Cardiac Safety Study (TRYPHAENA). Ann Oncol (2013) 24:9–2278-84. doi: 10.1093/annonc/mdt182
9. Yu KD, Liu GY, Zhou XY, Zhou Y, Wu J, Chen CM, et al. Association of HER-2 Copy Number and HER-2/CEP-17 Ratio With Neoadjuvant Taxane-Containing Chemotherapy Sensitivity in Locally Advanced Breast Cancer. Oncologist (2012) 17:6–792-800. doi: 10.1634/theoncologist.2011-0381
10. Wu Z, Xu S, Zhou L, Yin W, Lin Y, Du Y, et al. Clinical Significance of Quantitative HER2 Gene Amplification as Related to Its Predictive Value in Breast Cancer Patients in Neoadjuvant Setting. Onco Targets Ther (2018) 11:801–8. doi: 10.2147/OTT.S157634
11. McNamara KL, Caswell-Jin JL, Joshi R, Ma Z, Kotler E, Bean GR, et al. Spatial Proteomic Characterization of HER2-Positive Breast Tumors Through Neoadjuvant Therapy Predicts Response. Nat Cancer (2021) 2:4–400-13. doi: 10.1038/s43018-021-00190-z
12. Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, et al. Pathological Complete Response and Long-Term Clinical Benefit in Breast Cancer: The CTNeoBC Pooled Analysis. Lancet (2014) 384:9938–164-72. doi: 10.1016/s0140-6736(13)62422-8
13. Denkert C, von Minckwitz G, Darb-Esfahani S, Lederer B, Heppner BI, Weber KE, et al. Tumour-Infiltrating Lymphocytes and Prognosis in Different Subtypes of Breast Cancer: A Pooled Analysis of 3771 Patients Treated With Neoadjuvant Therapy. Lancet Oncol (2018) 19:1–40-50. doi: 10.1016/s1470-2045(17)30904-x
14. Stanton SE, Disis ML. Clinical Significance of Tumor-Infiltrating Lymphocytes in Breast Cancer. J Immunother Cancer (2016) 4:59. doi: 10.1186/s40425-016-0165-6
15. Lee KH, Kim EY, Yun JS, Park YL, Do SI, Chae SW, et al. The Prognostic and Predictive Value of Tumor-Infiltrating Lymphocytes and Hematologic Parameters in Patients With Breast Cancer. BMC Cancer (2018) 18:1–938. doi: 10.1186/s12885-018-4832-5
16. Liu F, Lang R, Zhao J, Zhang X, Pringle GA, Fan Y, et al. CD8(+) Cytotoxic T Cell and FOXP3(+) Regulatory T Cell Infiltration in Relation to Breast Cancer Survival and Molecular Subtypes. Breast Cancer Res Treat (2011) 130:2–645-55. doi: 10.1007/s10549-011-1647-3
17. Denkert C, Loibl S, Noske A, Roller M, Muller BM, Komor M, et al. Tumor-Associated Lymphocytes as an Independent Predictor of Response to Neoadjuvant Chemotherapy in Breast Cancer. J Clin Oncol (2010) 28:1–105-13. doi: 10.1200/JCO.2009.23.7370
18. Mahmoud SM, Paish EC, Powe DG, Macmillan RD, Grainge MJ, Lee AH, et al. Tumor-Infiltrating CD8+ Lymphocytes Predict Clinical Outcome in Breast Cancer. J Clin Oncol (2011) 29:15–1949-55. doi: 10.1200/JCO.2010.30.5037
19. Choi JH, Jeon CW, Kim YO, Jung S. Pathological Complete Response to Neoadjuvant Trastuzumab and Pertuzumab Therapy Is Related to Human Epidermal Growth Factor Receptor 2 (HER2) Amplification Level in HER2-Amplified Breast Cancer. Med (Baltimore) (2020) 99:46–e23053. doi: 10.1097/MD.0000000000023053
20. Metzger-Filho O, Sun Z, Viale G, Price KN, Crivellari D, Snyder RD, et al. Patterns of Recurrence and Outcome According to Breast Cancer Subtypes in Lymph Node-Negative Disease: Results From International Breast Cancer Study Group Trials VIII and IX. J Clin Oncol (2013) 31:25–3083-90. doi: 10.1200/JCO.2012.46.1574
21. Wolff AC, Hammond MEH, Allison KH, Harvey BE, Mangu PB, Bartlett JMS, et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. Arch Pathol Lab Med/ (2018) 142:11–1364-82. doi: 10.5858/arpa.2018-0902-SA
22. Burstein HJ, Cirrincione CT, Barry WT, Chew HK, Tolaney SM, Lake DE, et al. Endocrine Therapy With or Without Inhibition of Epidermal Growth Factor Receptor and Human Epidermal Growth Factor Receptor 2: A Randomized, Double-Blind, Placebo-Controlled Phase III Trial of Fulvestrant With or Without Lapatinib for Postmenopausal Women With Hormone Receptor-Positive Advanced Breast Cancer-CALGB 40302 (Alliance). J Clin Oncol (2014) 32:35–3959-66. doi: 10.1200/JCO.2014.56.7941
23. Al-Saleh K, Abd El-Aziz N, Ali A, Abozeed W, Abd El-Warith A, Ibraheem A, et al. Predictive and Prognostic Significance of CD8(+) Tumor-Infiltrating Lymphocytes in Patients With Luminal B/HER 2 Negative Breast Cancer Treated With Neoadjuvant Chemotherapy. Oncol Lett (2017) 14:1–337-44. doi: 10.3892/ol.2017.6144
24. Press MF, Seoane JA, Curtis C, Quinaux E, Guzman R, Sauter G, et al. Assessment of ERBB2/HER2 Status in HER2-Equivocal Breast Cancers by FISH and 2013/2014 ASCO-CAP Guidelines. JAMA Oncol (2019) 5:3–366-75. doi: 10.1001/jamaoncol.2018.6012
25. Perez EA, Reinholz MM, Hillman DW, Tenner KS, Schroeder MJ, Davidson NE, et al. HER2 and Chromosome 17 Effect on Patient Outcome in the N9831 Adjuvant Trastuzumab Trial. J Clin Oncol (2010) 28:28–4307-15. doi: 10.1200/JCO.2009.26.2154
26. Finn RS, Press MF, Dering J, Arbushites M, Koehler M, Oliva C, et al. Estrogen Receptor, Progesterone Receptor, Human Epidermal Growth Factor Receptor 2 (HER2), and Epidermal Growth Factor Receptor Expression and Benefit From Lapatinib in a Randomized Trial of Paclitaxel With Lapatinib or Placebo as First-Line Treatment in HER2-Negative or Unknown Metastatic Breast Cancer. J Clin Oncol (2009) 27:24–3908-15. doi: 10.1200/JCO.2008.18.1925
27. Di Maio M, Audisio M, Cardone C, De Luca E, Gargiulo P, Zichi C, et al. The Use of Not-Negative Conclusions to Describe Results of Formally Negative Trials Presented at Oncology Meetings. JAMA Oncol (2020) 6:6–926-7. doi: 10.1001/jamaoncol.2020.0475
28. Birkbak NJ, Wang ZC, Kim JY, Eklund AC, Li Q, Tian R, et al. Telomeric Allelic Imbalance Indicates Defective DNA Repair and Sensitivity to DNA-Damaging Agents. Cancer Discov (2012) 2:4–366-75. doi: 10.1158/2159-8290.CD-11-0206
29. Ogston KN, Miller ID, Payne S, Hutcheon AW, Sarkar TK, Smith I, et al. A New Histological Grading System to Assess Response of Breast Cancers to Primary Chemotherapy: Prognostic Significance and Survival. Breast (2003) 12:5–320-7. doi: 10.1016/s0960-9776(03)00106-1
30. Zhou ZR, Wang WW, Li Y, Jin KR, Wang XY, Wang ZW, et al. In-Depth Mining of Clinical Data: The Construction of Clinical Prediction Model With R. Ann Transl Med (2019) 7:23–796. doi: 10.21037/atm.2019.08.63
31. Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in Oncology: More Than Meets the Eye. Lancet Oncol (2015) 16:4–e173-e80. doi: 10.1016/s1470-2045(14)71116-7
32. Vu T, Claret FX. Trastuzumab: Updated Mechanisms of Action and Resistance in Breast Cancer. Front Oncol (2012) 2:62. doi: 10.3389/fonc.2012.00062
33. Musolino A, Naldi N, Bortesi B, Pezzuolo D, Capelletti M, Missale G, et al. Immunoglobulin G Fragment C Receptor Polymorphisms and Clinical Efficacy of Trastuzumab-Based Therapy in Patients With HER-2/Neu-Positive Metastatic Breast Cancer. J Clin Oncol (2008) 26:11–1789-96. doi: 10.1200/JCO.2007.14.8957
34. Nami B, Maadi H, Wang Z. Mechanisms Underlying the Action and Synergism of Trastuzumab and Pertuzumab in Targeting HER2-Positive Breast Cancer. Cancers (Basel) (2018) 10:10. doi: 10.3390/cancers10100342
35. Yamashita-Kashima Y, Shu S, Yorozu K, Moriya Y, Harada N. Mode of Action of Pertuzumab in Combination With Trastuzumab Plus Docetaxel Therapy in a HER2-Positive Breast Cancer Xenograft Model. Oncol Lett/ (2017) 14:4–4197-205. doi: 10.3892/ol.2017.6679
36. Greenwell K, Hussain L, Lee D, Bramlage M, Bills G, Mehta A, et al. Complete Pathologic Response Rate to Neoadjuvant Chemotherapy Increases With Increasing HER2/CEP17 Ratio in HER2 Overexpressing Breast Cancer: Analysis of the National Cancer Database (NCDB). Breast Cancer Res Treat (2020) 181:2–249-54. doi: 10.1007/s10549-020-05599-1
37. Kogawa T, Fouad TM, Liu DD, Wu J, Shen Y, Masuda H, et al. High HER2/Centromeric Probe for Chromosome 17 Fluorescence In Situ Hybridization Ratio Predicts Pathologic Complete Response and Survival Outcome in Patients Receiving Neoadjuvant Systemic Therapy With Trastuzumab for HER2-Overexpressing Locally Advanced Breast Cancer. Oncologist (2016) 21:1–21-7. doi: 10.1634/theoncologist.2015-0101
38. Rouzier R, Pusztai L, Delaloge S, Gonzalez-Angulo AM, Andre F, Hess KR, et al. Nomograms to Predict Pathologic Complete Response and Metastasis-Free Survival After Preoperative Chemotherapy for Breast Cancer. J Clin Oncol (2005) 23:33–8331-9. doi: 10.1200/JCO.2005.01.2898
39. Katayama A, Miligy IM, Shiino S, Toss MS, Eldib K, Kurozumi S, et al. Predictors of Pathological Complete Response to Neoadjuvant Treatment and Changes to Post-Neoadjuvant HER2 Status in HER2-Positive Invasive Breast Cancer. Mod Pathol (2021) 34:7–1271-81. doi: 10.1038/s41379-021-00738-5
40. Mao Y, Qu Q, Zhang Y, Liu J, Chen X, Shen K. The Value of Tumor Infiltrating Lymphocytes (TILs) for Predicting Response to Neoadjuvant Chemotherapy in Breast Cancer: A Systematic Review and Meta-Analysis. PloS One (2014) 9:12–e115103. doi: 10.1371/journal.pone.0115103
41. Hwang HW, Jung H, Hyeon J, Park YH, Ahn JS, Im YH, et al. A Nomogram to Predict Pathologic Complete Response (pCR) and the Value of Tumor-Infiltrating Lymphocytes (TILs) for Prediction of Response to Neoadjuvant Chemotherapy (NAC) in Breast Cancer Patients. Breast Cancer Res Treat (2019) 173:2–255-66. doi: 10.1007/s10549-018-4981-x
42. Ladoire S, Arnould L, Apetoh L, Coudert B, Martin F, Chauffert B, et al. Pathologic Complete Response to Neoadjuvant Chemotherapy of Breast Carcinoma Is Associated With the Disappearance of Tumor-Infiltrating Foxp3+ Regulatory T Cells. Clin Cancer Res (2008) 14:8–2413-20. doi: 10.1158/1078-0432.CCR-07-4491
43. Wang K, Xu J, Zhang T, Xue D. Tumor-Infiltrating Lymphocytes in Breast Cancer Predict the Response to Chemotherapy and Survival Outcome: A Meta-Analysis. Oncotarget (2016) 7:44288–98. doi: 10.18632/oncotarget.9988
44. Hurvitz SA, Martin M, Symmans WF, Jung KH, Huang C-S, Thompson AM, et al. Neoadjuvant Trastuzumab, Pertuzumab, and Chemotherapy Versus Trastuzumab Emtansine Plus Pertuzumab in Patients With HER2-Positive Breast Cancer (KRISTINE): A Randomised, Open-Label, Multicentre, Phase 3 Trial. Lancet Oncol (2018) 19:1–115-26. doi: 10.1016/s1470-2045(17)30716-7
45. Masuda N, Ohtani S, Takano T, Inoue K, Suzuki E, Nakamura R, et al. A Randomized, 3-Arm, Neoadjuvant, Phase 2 Study Comparing Docetaxel + Carboplatin + Trastuzumab + Pertuzumab (TCbHP), TCbHP Followed by Trastuzumab Emtansine and Pertuzumab (T-DM1+P), and T-Dm1+P in HER2-Positive Primary Breast Cancer. Breast Cancer Res Treat (2020) 180:1–135-46. doi: 10.1007/s10549-020-05524-6
46. Jia L, Ling Y, Li K, Zhang L, Wang Y, Kang H. A 10-Gene Signature for Predicting the Response to Neoadjuvant Trastuzumab Therapy in HER2-Positive Breast Cancer. Clin Breast Cancer (2021) 21:6–e654-e64. doi: 10.1016/j.clbc.2021.04.010
47. Harbeck N, von Schumann R, Kates RE, Braun M, Kuemmel S, Schumacher C, et al. Immune Markers and Tumor-Related Processes Predict Neoadjuvant Therapy Response in the WSG-ADAPT HER2-Positive/Hormone Receptor-Positive Trial in Early Breast Cancer. Cancers (Basel) (2021) 13:19. doi: 10.3390/cancers13194884
Keywords: trastuzumab, pertuzumab, predictive model, neoadjuvant treatment, HER2-amplified breast cancer
Citation: Xiao Y, Ding J, Ma D, Chen S, Li X and Yu K (2022) Predicting Pathological Complete Response in Neoadjuvant Dual Blockade With Trastuzumab and Pertuzumab in HER2 Gene Amplified Breast Cancer. Front. Immunol. 13:877825. doi: 10.3389/fimmu.2022.877825
Received: 17 February 2022; Accepted: 20 April 2022;
Published: 19 May 2022.
Edited by:
Shyamasree Ghosh, National Institute of Science Education and Research (NISER), IndiaReviewed by:
Nawale Hajjaji, Centre Oscar Lambret, FranceChao Cheng, Baylor College of Medicine, United States
Copyright © 2022 Xiao, Ding, Ma, Chen, Li and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xun Li, bGl4dW5kcjIxQG91dGxvb2suY29t; Keda Yu, eXVrZWRhQGZ1ZGFuLmVkdS5jbg==
†These authors have contributed equally to this work
 Yi Xiao1,2†
Yi Xiao1,2† Jiahan Ding
Jiahan Ding Xun Li
Xun Li Keda Yu
Keda Yu