
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 09 May 2022
Sec. B Cell Biology
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.876306
This article is part of the Research Topic SARS-CoV-2 Variants, B Lymphocytes, and Autoreactivity View all 10 articles
 Lisa Paschold1
Lisa Paschold1 Bianca Klee2
Bianca Klee2 Cornelia Gottschick2
Cornelia Gottschick2 Edith Willscher1
Edith Willscher1 Sophie Diexer2
Sophie Diexer2 Christoph Schultheiß1
Christoph Schultheiß1 Donjete Simnica1
Donjete Simnica1 Daniel Sedding3
Daniel Sedding3 Matthias Girndt4
Matthias Girndt4 Michael Gekle5
Michael Gekle5 Rafael Mikolajczyk2
Rafael Mikolajczyk2 Mascha Binder1*
Mascha Binder1*The COVID-19 pandemic shows that vaccination strategies building on an ancestral viral strain need to be optimized for the control of potentially emerging viral variants. Therefore, aiming at strong B cell somatic hypermutation to increase antibody affinity to the ancestral strain - not only at high antibody titers - is a priority when utilizing vaccines that are not targeted at individual variants since high affinity may offer some flexibility to compensate for strain-individual mutations. Here, we developed a next-generation sequencing based SARS-CoV-2 B cell tracking protocol to rapidly determine the level of immunoglobulin somatic hypermutation at distinct points during the immunization period. The percentage of somatically hypermutated B cells in the SARS-CoV-2 specific repertoire was low after the primary vaccination series, evolved further over months and increased steeply after boosting. The third vaccination mobilized not only naïve, but also antigen-experienced B cell clones into further rapid somatic hypermutation trajectories indicating increased affinity. Together, the strongly mutated post-booster repertoires and antibodies deriving from this may explain why the third, but not the primary vaccination series, offers some protection against immune-escape variants such as Omicron B.1.1.529.
Until February 2022, the World Health Organization (WHO) counted 400 million severe acute coronavirus disease 2019 (COVID-19) infections caused by respiratory syndrome coronavirus 2 (SARS-CoV-2). By then, the number of deaths had totaled almost 6 million individuals globally. While mRNA-based and adenovirus-vectored vaccines have been developed at unprecedented speed, global vaccination strategies remain challenging and new SARS-CoV-2 variants with varying potential to evade adaptive immunity and/or to enhance transmissibility constantly emerge. Since memory B cell populations play a decisive role in severity reduction of COVID-19 and early antibody-mediated virus neutralization may even prevent infections, understanding infection- and vaccine-induced SARS-CoV-2 specific B cell immunity is critical (1–3).
As recently reviewed by Laidlaw et al. (4), COVID-19 generates both germinal center and extrafollicular B cell responses in unvaccinated individuals – depending on the severity of infection - that converge on B cells expressing antigen receptors with preferential immunoglobulin heavy chain variable-joining gene (IGHV-J) usage (1, 5–12). Interestingly, even B cells with low or absent IGHV affinity maturation can generate antibodies that specifically recognize and neutralize the ancestral strain of SARS-CoV-2 (6, 11, 13–15). Yet, a continued evolution of the humoral response appears to take place over at least six months after infection – even without re-infection – as demonstrated by sustained acquisition of IGHV somatic hypermutation despite waning antibody titers (16–21). There is emerging evidence that these rather prolonged B cell maturation dynamics may also be characteristic for vaccine-induced anti-SARS-CoV-2 immune responses (22, 23).
With the advent of SARS-CoV-2 variants of concern, affinity to the ancestral strain’s S protein (currently used in all licensed vaccines) does not necessarily predict antibody neutralization potency. Immune escape can affect clones with high affinity against the ancestral strain but, depending on the targeted epitope, some clones also retain their neutralizing potency against variants of concern (24). This is in clear contrast to clones that have been induced by the ancestral strain and show only low affinity to this strain. Such clones constantly fail to neutralize variants (24). This finding suggests that high affinity binding to the ancestral strain may provide some flexibility in compensating the effect of individual immune escape mutations (24). Therefore, in times of emerging viral variants an optimal vaccination strategy should aim at inducing the highest possible level of affinity maturation through somatic hypermutation, even if the available vaccines are targeted at the ancestral strain.
In the study presented here, we used two cohorts of not previously infected patients to compare antibody levels and somatic hypermutation trajectories across a primary series of two standard vaccinations with those induced by a third “booster” vaccination using immune repertoire sequencing. We show that B lineage evolution is low after the priming vaccinations. In contrast, the maturation trajectories induced by the third vaccination is compatible with selective mobilization and germinal center recruitment of naïve but also previously matured memory B cell lineages to undergo fast and extensive somatic hypermutation. This considerable affinity maturation and the resulting high antibody titers may explain the increased protection of the third “booster” vaccination against variants such as the Omicron variant B.1.1.529.
This study was registered as non-interventional study (NIS) at the Paul-Ehrlich-Institute (NIS635). It consisted of data and biological samples collected in the DigiHero and HACO cohorts.
DigiHero is a population-based cohort study for digital health research in Germany conducted in the city of Halle (Saale) which registered 8,077 participants until November 2021. The recruitment was conducted in different waves and included mailed invitation to all 129,733 households in Halle as well as promotion via media. The study was approved by the institutional review board (approval number 2020-076). Its digital design allowed targeted invitation of participants to modules that included different surveys and blood biobanking subprojects. The COVID-19 module of DigiHero recruited participants with prior positive SARS-CoV-2 testing in their households. Until December 2021, 514 individuals had completed the survey on their COVID-19 history as well as on their vaccination status and had donated blood for this module at this data cut. These samples were used for antibody analyzes. The SARS-CoV-2 booster vaccination module of DigiHero recruited participants willing to respond to a survey on the third SARS-CoV-2 booster vaccination. Until December 2021, 4,670 participants had completed the survey. Fifteen randomly chosen participants without prior COVID-19 infection donated blood prior to and on day 14 (d14) after booster vaccination at this data cut. These samples were used for antibody and B cell repertoire next-generation sequencing analyzes.
As a reference within NIS635, 40 samples from 20 uninfected control cases completing their primary vaccination series with the BioNTech/Pfizer vaccine were recovered from the biobank of the Halle COVID-19 cohort (HACO). These 20 control cases had donated blood prior to and at d28 of their primary vaccination series (d7 after the second vaccination). Informed written consent was obtained and the study was approved by the institutional review board (approval number 2020-039). These samples were used for antibody and B cell repertoire next-generation sequencing analyzes.
Table 1 summarizes all relevant participant numbers, their basic characteristics and biological samples used in NIS635. The study was conducted in accordance with the ethical principles stated by the Declaration of Helsinki. Informed written consent was obtained from all participants or legal representatives.
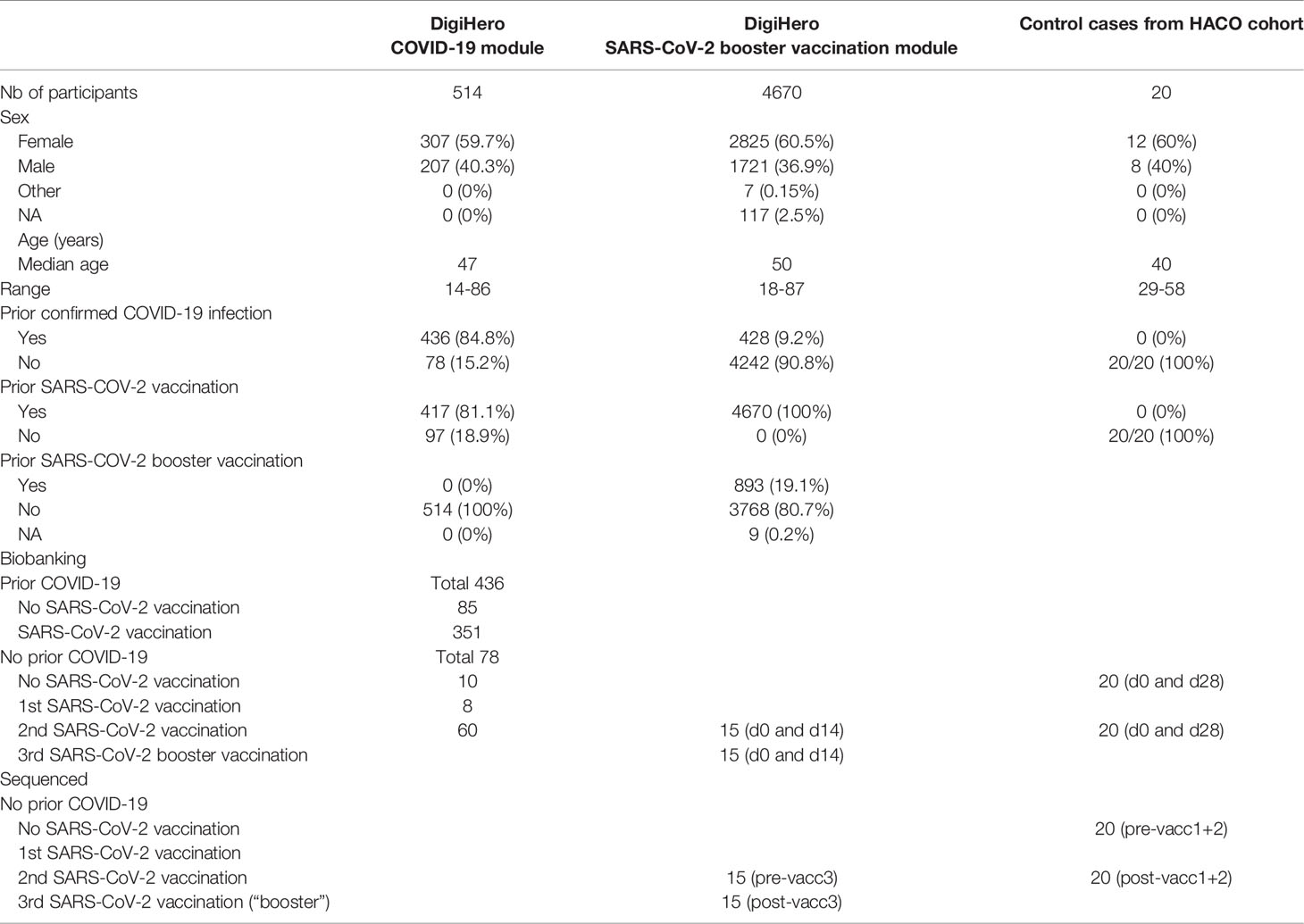
Table 1 Characteristics of participants in the DigiHero COVID-19 and SARS-CoV-2 booster vaccination modules and the HACO subcohort used for NIS635.
The collected plasma samples were isolated by centrifugation of whole blood for 15 min at 2,000xg, followed by centrifugation at 12,000xg for 10 min and stored at - 80°C. Peripheral mononuclear cells (PBMC) were isolated by standard Ficoll gradient centrifugation. Genomic DNA was extracted from PBMCs using the GenElute Mammalian Genomic DNA Miniprep Kit (Sigma-Aldrich, St. Louis, USA).
Antibodies against the S1 domain of the spike (S) protein and the nucleocapsid protein (NCP) of SARS-CoV-2 were determined by Anti-SARS-CoV-2-ELISA IgA/IgG and Anti-SARS-CoV-2-NCP-ELISA kits from Euroimmun (Lübeck, Germany). Readouts were performed at 450 nm using a Tecan Spectrophotometer SpectraFluor Plus (Tecan Group Ltd., Männedorf, Switzerland).
Immunosequencing of B cell repertoires was performed as described in (25). In brief, V(D)J rearranged IGH loci were amplified from 500 ng of genomic DNA using a multiplex PCR, pooled at 4 nM and quality-assessed on a 2100 Bioanalyzer (Agilent Technologies). Sequencing was performed on an Illumina MiSeq (paired-end, 2 x 301-cycles, v3 chemistry). Rearranged IGH loci were annotated using MiXCR v3.0.13 (26) and the IMGT 202011-3.sv6 IGH library as reference. Non-productive reads and sequences with less than 2 counts were discarded. All repertoires were normalized to 30,000 reads. Each unique complementarity-determining region 3 (CDR3) nucleotide sequence was considered a clone. Broad repertoire metrics (clonality, diversity, richness) were analyzed as previously described (27). IGHV genes were regarded as somatically hypermutated if they showed < 98% identity to the germline sequence and B cell clones with hypermutated IGHV gene were considered antigen-experienced.
We searched our IGH repertoires for validated SARS-CoV-2 antibody rearrangements with identical or highly similar CDR3 amino acid sequence (Levenshtein distance of ≤ 2) and identical IGHV-J gene usage as described in (13). The validated SARS-CoV-2 antibody sequences were derived from CoV-AbDab accessed at 17th December 2021 (28) and classified into 3,195 total SARS-CoV-2 binding sequences and 1,147 SARS-CoV-2 neutralizing sequences. A list of the target sequences is provided in Supplementary Table 1.
To calculate network connectivity in BCR repertoires, we used the Levenshtein distance of all unique CDR3 amino acid (aa) sequences per repertoire using the imnet tool (https://github.com/rokroskar/imnet). Sequences with Levenshtein distance ≤ 3 were connected. For visualization as petri dish plots we used R package igraph and the fruchterman-reingold layout (29). Each dot represents a different unique CDR3aa sequence, which is termed a ‘clone’. The number of identical CDR3aa sequences (= frequency of the clone in the repertoire) is not reflected in this kind of graphical presentation. CDR3aa sequences with a Levenshtein distance of ≤ 3 are connected. Data analysis and plotting was performed using R version (v4.1.2).
We identified overlapping B cell lineages in the pre- and post-vaccination time point per patient. A B cell lineage was defined as a group of B cell clonotypes that share a common V and J gene and a CDR3 sequence differing only in up to 10% of its amino acid positions (30). Lineages and their evolution were visualised as stream plots with function plot.stacked from (https://www.r-bloggers.com/2013/12/data-mountains-and-streams-stacked-area-plots-in-r/). Data analysis and plotting was performed using R version (v4.1.2).
Differences between the four groups were analyzed by ordinary one-way ANOVA and post-ANOVA analyses between individual columns were performed using Tukey’s multiple comparisons test. Differences between two groups were studied by unpaired, two-tailed student’s t-test or in the case of paired samples by paired, two-tailed student’s t-test. All statistical analyses were performed using R version 4.1.2 and GraphPad Prism 8.3.1 (GraphPad Software, La Jolla, CA, USA).
This study was registered as non-interventional study (NIS) at the Paul-Ehrlich-Institute (NIS635). DigiHero and HACO were approved by the institutional review board (approval numbers 2020-076 and 2020-039). Written informed consent was received prior to participation.
For NIS635, we used a classical biobanking study (HACO) and a digital cohort study (DigiHero) with flexible recruitment of participants into different survey modules to obtain COVID-19 and vaccination data in a large cohort and to acquire relevant biological samples from subgroups of interest. In DigiHero, 514 participants donated blood and completed the survey of the COVID-19 module until December 2021. 4,670 participants completed the survey on the third SARS-CoV-2 “booster” vaccination until the same data cut. In HACO, data and sample collection had been completed in January 2021. Using this data, complete COVID-19 and vaccination histories could be deduced for all participants of NIS635. Details for all subcohorts are given in Table 1.
In the DigiHero SARS-CoV-2 „booster” vaccination survey, the majority of participants indicated to have already received their third vaccination or to be planning to receive it shortly (Figure 1A). The time between completion of the primary vaccination series and the third booster vaccination is shown in Figure 1B. The majority of participants received BioNTech/Pfizer as their third vaccine (Figure 1C). The tolerability of the “booster” was roughly comparable to that of first and second SARS-CoV-2 vaccinations (Figure 1D). Most frequent side effects were local reactions, fatigue, and headache with comparable tolerability of both mRNA vaccines (Figure 1E).
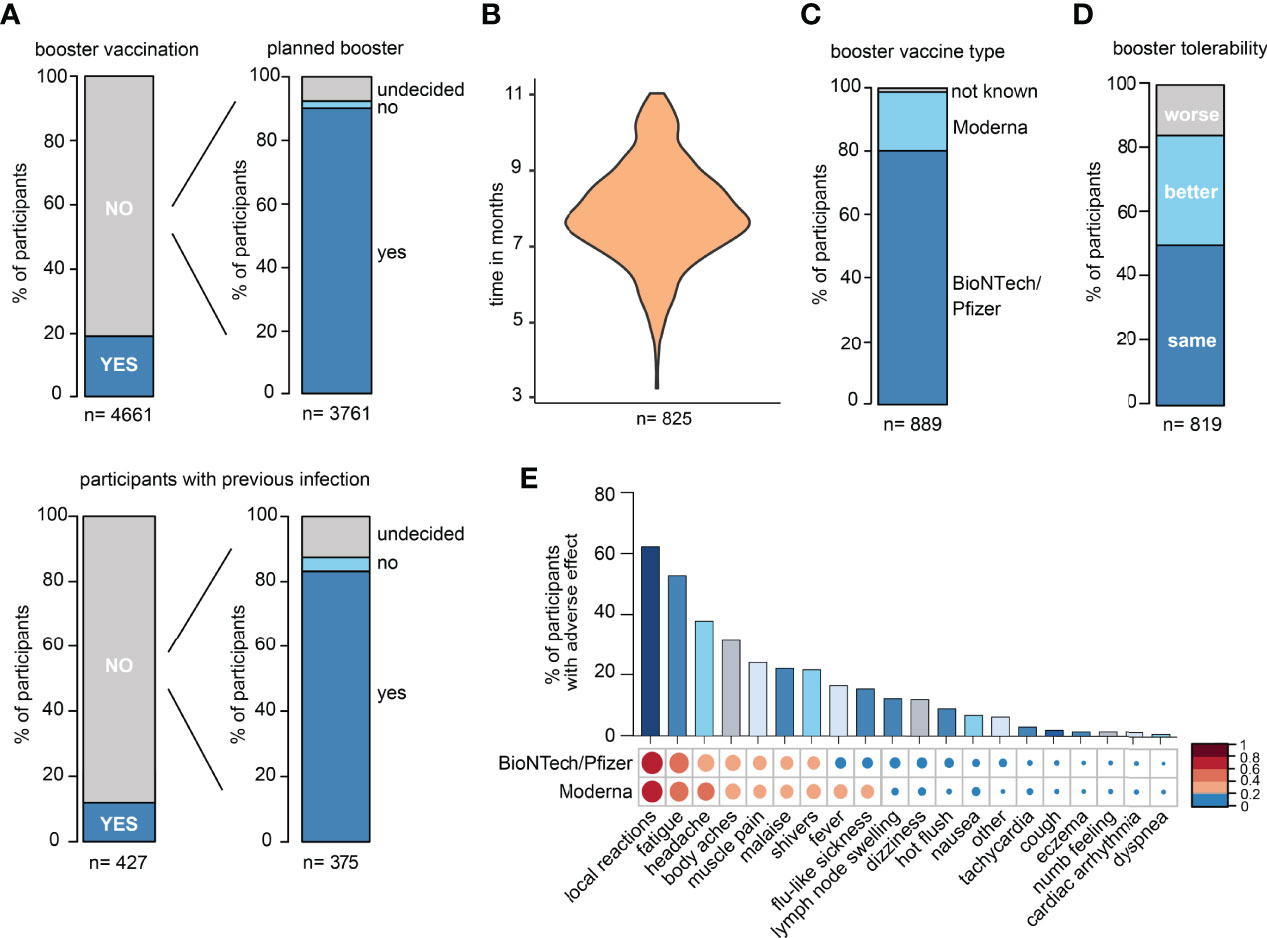
Figure 1 Survey data from the DigiHero SARS-CoV-2 booster vaccination module. (A) Statistics of participants with prior or planned third SARS-CoV-2 vaccination (booster). (B) Time between completion of the primary vaccination series and third vaccination in months. (C) SARS-CoV-2 third vaccination type. (D) Tolerability of the third vaccination compared to previous SARS-CoV-2 vaccinations. (E) Adverse events upon third vaccination.
Based on this survey, 15 participants without prior COVID-19 infection that planned a third vaccination within the next four weeks were randomly chosen and invited to donate blood before and after their third vaccination for antibody and B cell repertoire NGS analyzes.
All biobanked samples indicated in Table 1 were tested for S1 and NCP antibodies by ELISA. Figure 2A shows the distribution of antibody levels for the different subgroups. Participants vaccinated after infection (hybrid immunity) and participants after their third “booster” vaccination achieved the highest S1 antibody levels followed by previously uninfected participants that had only completed their primary vaccination series (Figure 2A). Only few individuals showed elevated NCP antibody levels despite having indicated no prior COVID-19 potentially pointing at unrecognized previous infection. All other participants showed antibody levels compatible with their infection/vaccination status. In all participants with matched pre- and post-vaccination samples, clear increases in S1 antibodies were noted with highest levels after the third vaccination, while NCP antibodies remained negative (Figure 2B).
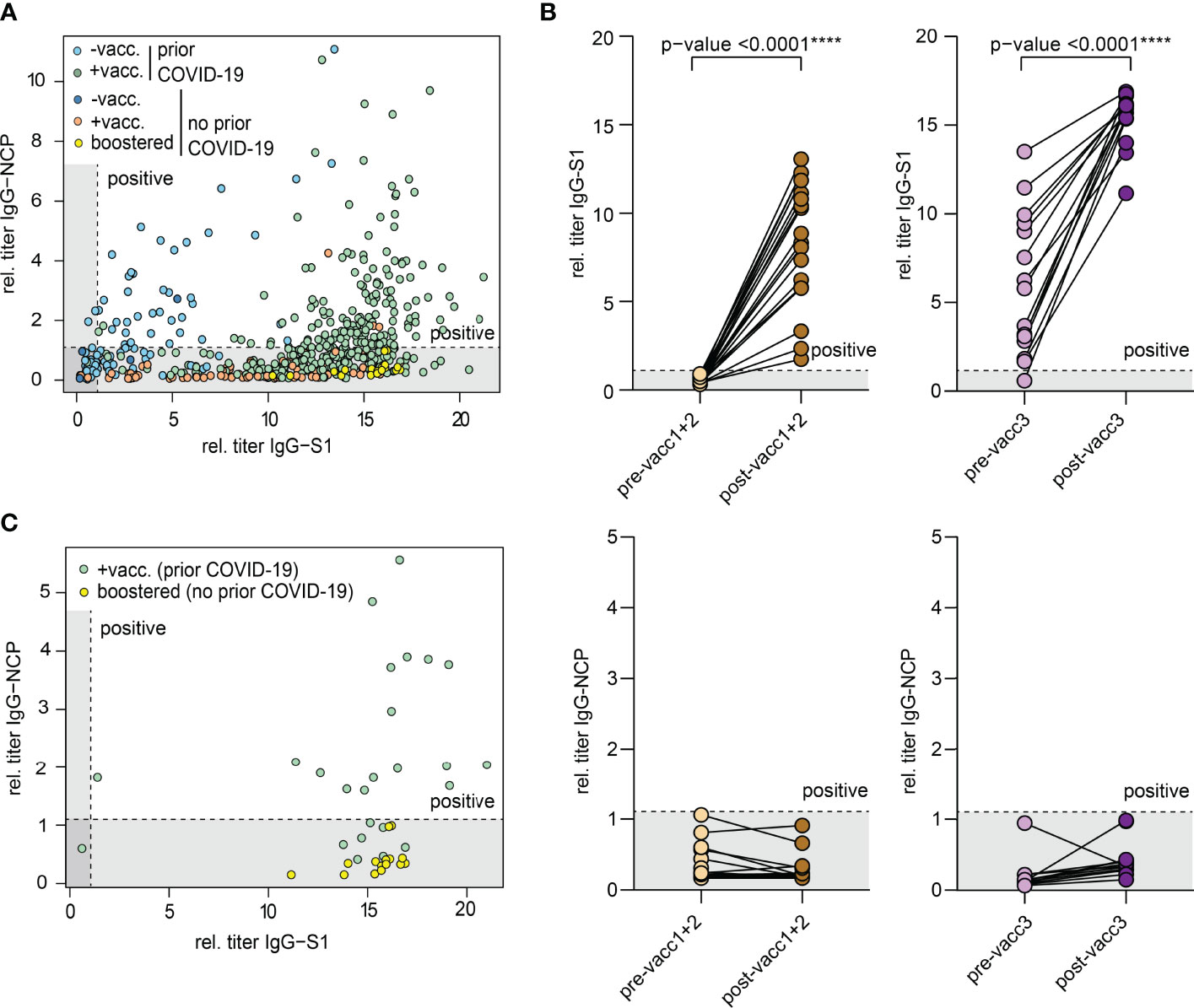
Figure 2 Antibodies against the S1 domain of the spike (S) protein and the nucleocapsid protein (NCP) of SARS-CoV-2. (A) Comparison of IgG-NCP and IgG-S1 antibodies in vaccinated individuals with or without prior COVID-19 infection. (B) Matched IgG-S1 and IgG-NCP antibody titers of previously uninfected individuals prior to and after the primary vaccination series (pre-/post-vacc1+2) and the third vaccination (pre-/post-vacc3). Statistical test: Two-tailed paired t-test. p-value cut-offs: <0.0001 extremely significant (****). (C) Comparison of IgG-NCP and IgG-S1 antibodies between previously infected participants that received a subsequent vaccination (green) and previously uninfected participants with three vaccinations (yellow). Both types of blood samples were collected at a maximum of 4 weeks from last vaccination. Cut-off values are presented as hatched lines.
Next, we compared antibody levels in participants with hybrid immunity to those after three vaccinations. Given the over-time decay in antibody titers both after infection and vaccination (16, 17, 20, 31–34), we included only participants in this analysis who donated blood in a standard interval of 2-4 weeks after the last vaccination dose. This analysis showed that antibody levels were similarly high in both subsets indicating that the third “booster” dose may mimic the hybrid-like response observed in individuals after infection and vaccination (Figure 2C).
Matched blood samples of two cohorts were subjected to next-generation sequencing of the B cell receptor repertoire. Cohort ‘vacc1+2’ consists of 20 individuals who donated blood before their first SARS-CoV-2 vaccination (pre-vacc1+2) and after their second vaccination (post-vacc1+2). Cohort ‘vacc3’ consists of 15 different individuals -not overlapping with individuals from cohort vacc1+2- who donated blood before (pre-vacc3) and after (post-vacc3) their third vaccination. The time points of blood collection are shown in Figure 3A. All of the sequenced samples included in this manuscript were derived from participants without a prior SARS-CoV-2 infection which was confirmed by negative levels of NCP antibodies (Figure 2B).
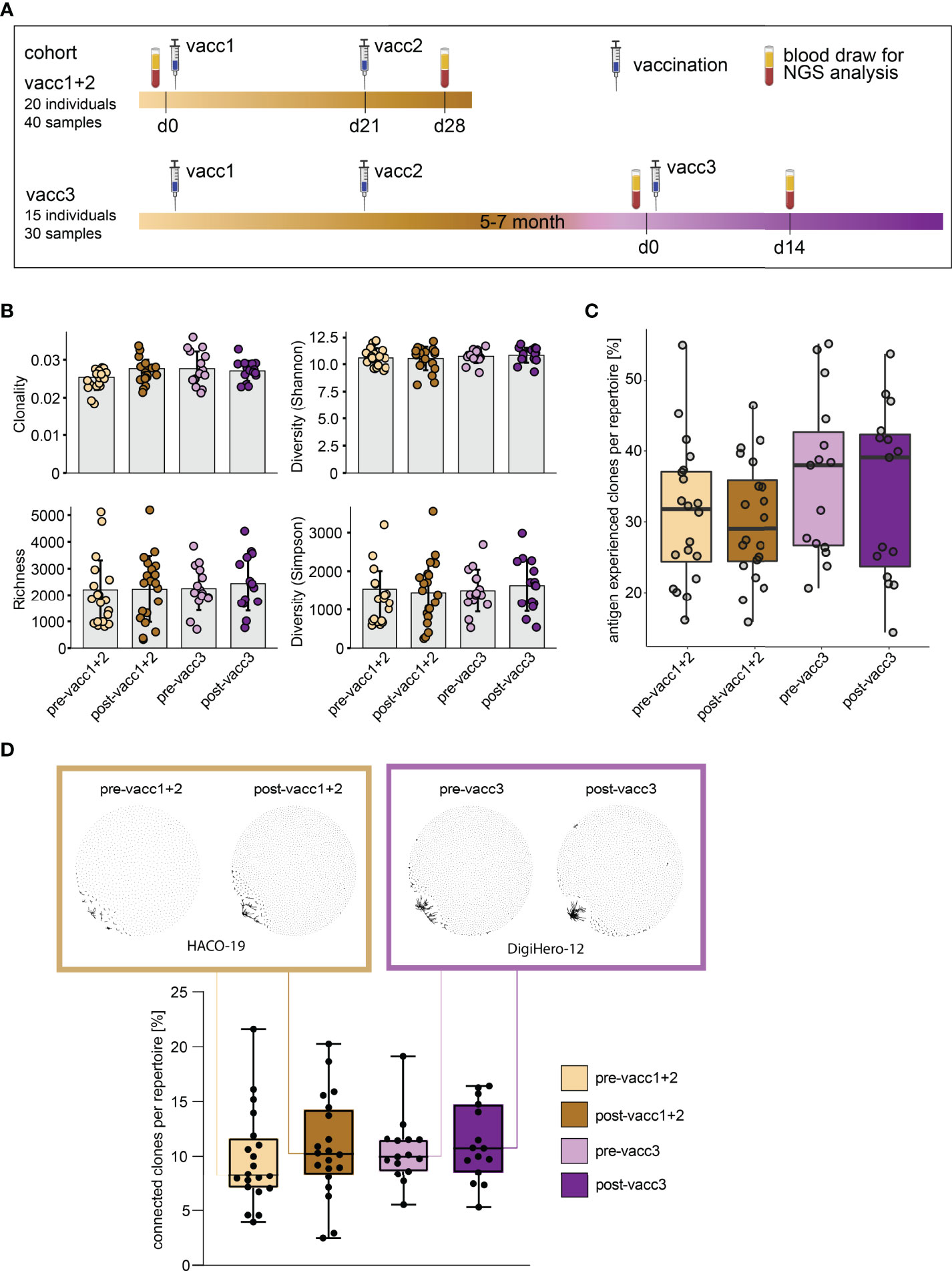
Figure 3 Matched pre- and post-SARS-CoV-2 vaccination blood sampling and global B cell repertoire analysis. (A) Vaccination and blood sampling scheme. (B) Broad B cell repertoire metrics. Bars indicate mean ± standard deviation. (C) Percentage of antigen experienced clones with somatic hypermutation (<98% identity to germline) per B cell repertoire. Box and whiskers plot are shown in the style of Tukey. (D) Quantitative connectivity analysis of B cell clones per repertoire. A clone is defined as a unique CDR3aa sequence. Clones are connected if they have a Levenshtein distance of ≤ 3 are connected. Boxes outline 25th to 75th percentile with a line at the median and whiskers from minimum to maximum. Petri dish plots of two representative pre-/post vaccination B cell repertoires are shown in brown and violet boxes on top (patients HACO-19 and DigiHero-12). Each dot in the petri dish plot represents one clone.
All patients included in this analysis had received mRNA vaccines; 8 of 15 participants received the BionTech/Pfizer vaccine as third vaccination. There were no global differences in immune repertoire metrics such as diversity, richness or clonality across groups (Figure 3B). In addition, B cell repertoire somatic hypermutation rates of IGHV genes that reflect antigen-mediated affinity maturation were roughly identical on the global immune repertoire level (Figure 3C). There were, however, trends in repertoire connectivity: Pre-vaccination B cell repertoires showed lowest connectivity between B cell clonotypes while samples taken after the third vaccination showed highest connectivity (Figure 3D, lower part). B cell connectivity plots of two patients with representative connectivity levels are shown in the upper part of Figure 3D.
While global B cell repertoire metrics were rather stable across the studied subgroups, we hypothesized that the subrepertoire of B cells with known SARS-CoV-2 specificity may provide more insight into affinity maturation in response to vaccination. We, therefore, searched our set of immune repertoires for 3,195 known SARS-CoV-2 antibody sequences (28) to determine blood circulation of B cells carrying SARS-CoV-2 reactive B cell receptors. 1,147 thereof derived from neutralizing SARS-CoV-2 antibodies. Interestingly, blood-circulation of such B cells appeared to be increased shortly after the first two vaccinations (Figures 4A, B). After the third vaccination, we also noted increases as compared to the matched time point before the third vaccination (Figures 4A, B). Yet, in absolute numbers, the increase in blood circulation of these clones was lower than after the primary vaccination series.
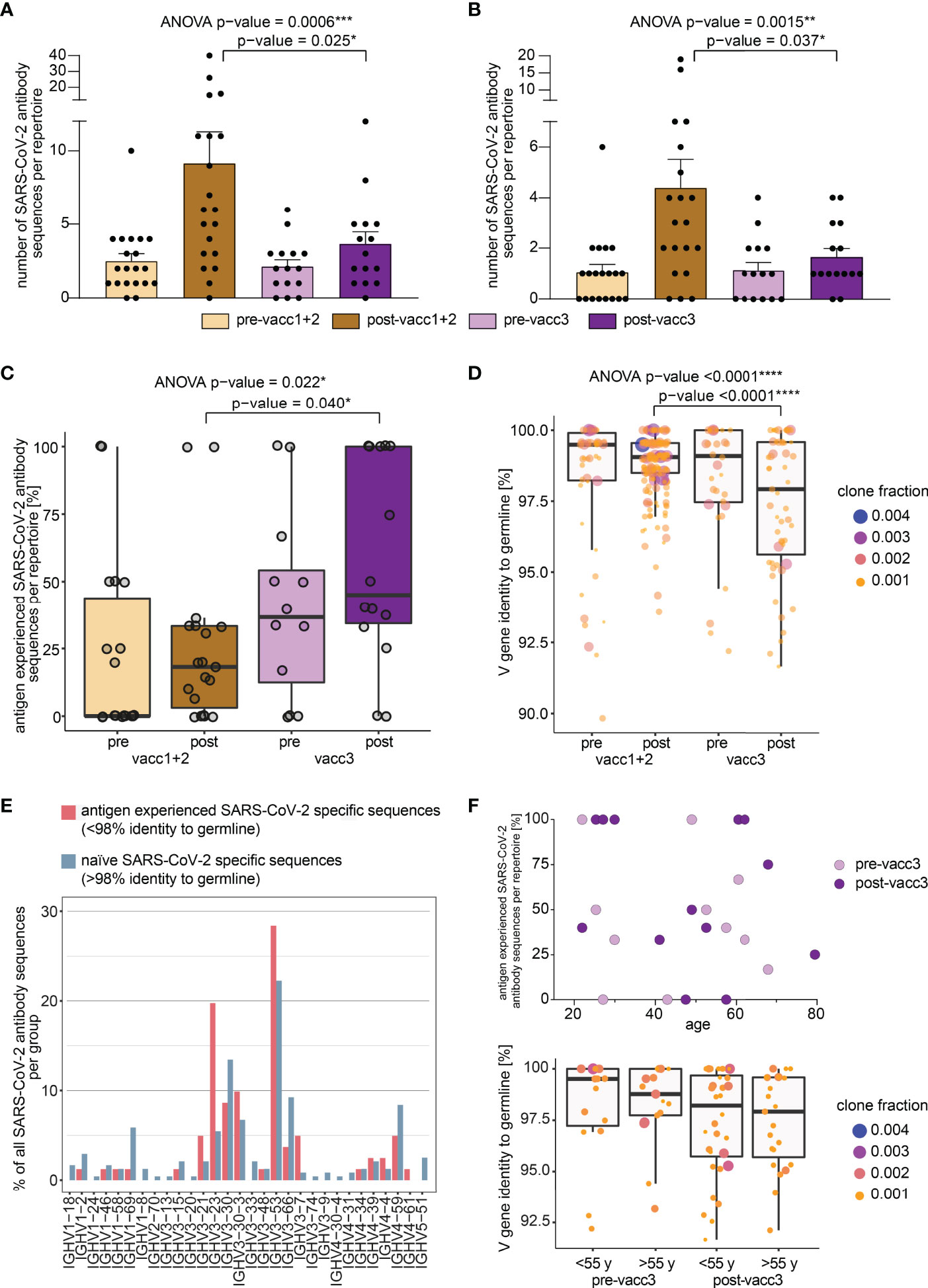
Figure 4 Search of SARS-CoV-2 directed B cell clonotypes in matched pre- and post-vaccination blood samples. Search of 3,195 total (A) and 1,147 neutralizing (B) antibody sequences in all immune repertoires. Bars indicate mean ± s.e.m. (C) Somatic hypermutation analysis of SARS-CoV-2 directed antibody sequences. The percentage of antigen experienced clones within the SARS-CoV-2 specific subrepertoires per patient is shown. A clone was considered antigen-experienced if the IGHV gene showed < 98% identity to the germline nucleotide sequence. (D) Somatic hypermutation load per SARS-CoV-2 directed antibody sequence is shown. Respective clone fractions are coded by color/size. Box and whiskers plot are shown in the style of Tukey. Ordinary one-way ANOVA was performed as statistical test and post-ANOVA analyses between individual columns were performed using Tukey’s multiple comparisons test. p-value cut-offs: <0.05 significant (*), <0.01 very significant (**), <0.001 extremely significant (***), <0.0001 extremely significant (****). (E) IGHV gene usage in naïve versus antigen-experienced SARS-CoV-2 directed antibody sequences. (F) Somatic hypermutation analysis of SARS-CoV-2 directed antibody sequences before and after the third vaccination (pre-/post-vacc3) in correlation to participant’s age. The percentage of antigen experienced clones within the SARS-CoV-2 specific subrepertoires per patient is shown in the upper panel. Somatic hypermutation load per SARS-CoV-2 directed antibody sequence is shown before and after the third vaccination (pre-/post-vacc3) subdivided into age groups of </> 55 years (y) in the lower panel. Respective clone fractions are coded by color/size. Box and whiskers plot are shown in the style of Tukey.
In a next step, we determined somatic hypermutation rates of SARS-CoV-2 specific clones from matched pre- and post-vaccination samples. We reasoned that the rate of somatically hypermutated clones should increase with the number of applied vaccinations. Indeed, we found a continuous increase in the fraction of somatically hypermutated B cell clones within the SARS-CoV-2 specific repertoire from pre-vaccination samples to samples acquired after the third vaccination (Figure 4C). Interestingly, the rate of somatically hypermutated SARS-CoV-2 specific clones was lower after completion of the primary vaccination series than prior to the third vaccination. This suggests that even „short-lived” mRNA vaccines trigger affinity maturation of B cells over months in line with recent data (22). Although the somatic hypermutation load per SARS-CoV-2 specific sequence numerically increased in primed participants, it was dramatically boosted by the third vaccination (Figure 4D). This was especially observed for BCR sequences encoded by the IGHV3-21, IGHV3-23, IGHV3-53 and IGHV3-30-3 genes (Figure 4E) which have been already linked to S-reactive antibodies with exceptional neutralizing potency (12, 35–39). However, IGHV3-23 is generally mutated more often in unselected B cells of vaccinated and unvaccinated controls (Supplementary Figure 1). The amount of hypermutation did not correlate with age (Figure 4F).
To be able to analyze individual maturation trajectories of B cells in the primary vaccination series versus upon third vaccination, we set out to identify developmental B lineages in all individual participants and to track them across the vaccination period. A B cell lineage is a group of clonotype-defined B cells that share a common V and J gene and a CDR3 sequence differing only in up to 10% of its amino acid positions (30). While we did not detect expanding B cell lineages in a substantial proportion of participants receiving their primary vaccination series, we found expanding lineages in the majority of patients receiving their third vaccination (Figure 5A). The repertoire space taken up by these vaccine-induced expanding B cell lineages was substantially higher after the third vaccination than after completion of the primary vaccination series (Figure 5B). The stream plots in Figure 5C and Supplementary Figure 2 show the development of B cell lineages from the pre- to the post-vaccination time point in all investigated cases. All overlapping, expanding lineages are shown as individual colored stream mapping its frequency within the pre- and post-vaccination repertoire. Detailed analysis of these maturation trajectories showed that the precursors of highly mutated post-booster clones were either naive or antigen-experienced cells that were mobilized into a secondary round of somatic hypermutation, most likely through a second recruitment to a germinal center. We found evidence for different scenarios: Further mutation of highly mutant clones and of clones with low numbers of mutations. Exemplary mutational trajectories induced by the third vaccination starting from naive or antigen-experienced clones are shown in Figure 5D. Finally, we looked at the IGHV gene usage of expanding lineages during the primary vaccination series and the third vaccination and found an overrepresentation of IGHV3-23 and IGHV3-53 in somatically hypermutated clones in both cohorts (Supplementary Figure 3).
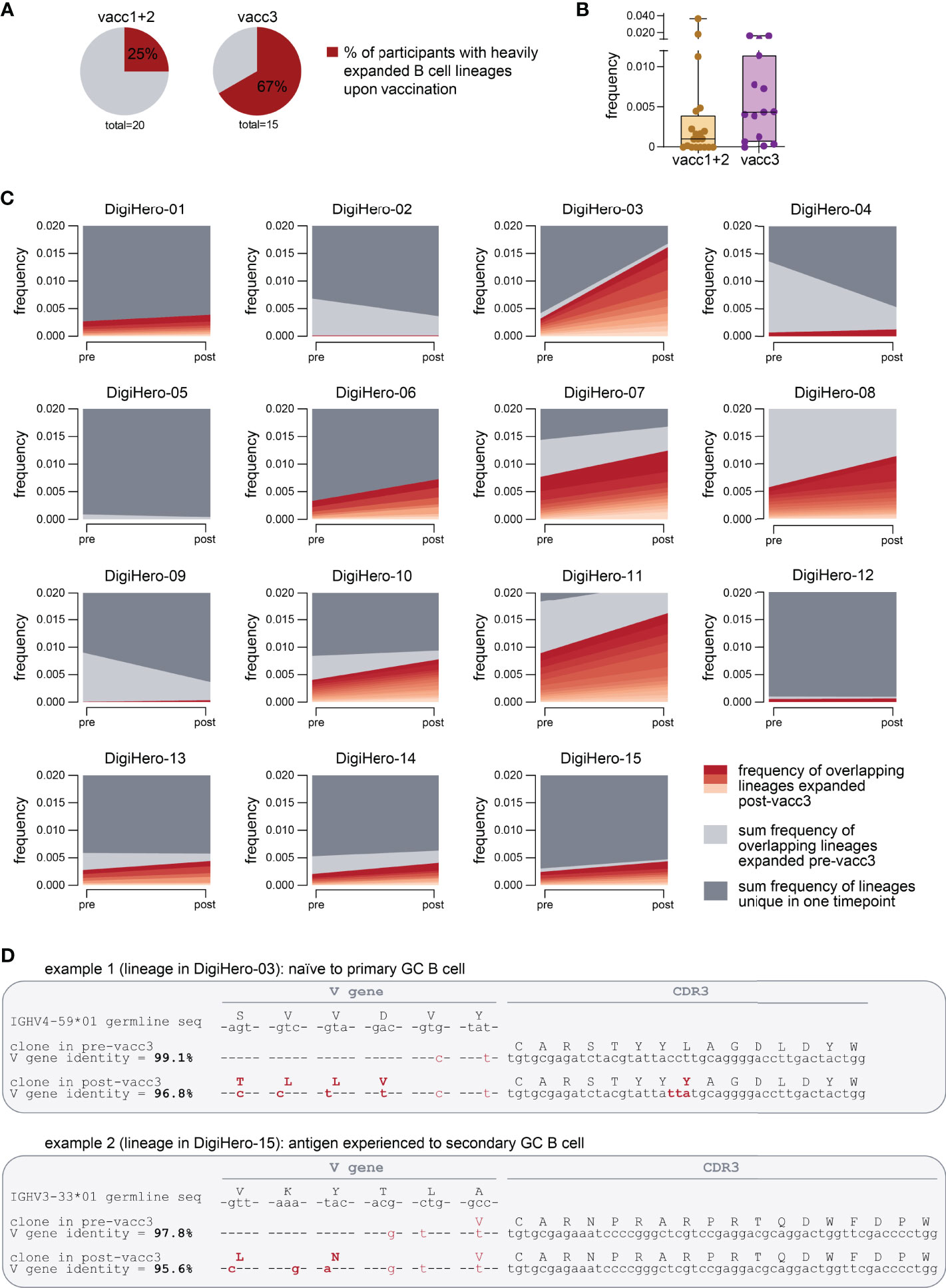
Figure 5 Expanding B cell lineages upon vaccination. B cell lineages in individual patients pre- and post-vaccination were constructed based on V and J gene identity as well as CDR3 sequence homology. (A) The percentage of participants with heavily expanded B cell lineages (more than 0.3% frequency within the post-vaccination repertoire taken up by overlapping expanded lineages) after the first/second (vacc1+2) or third vaccination (vacc3) are shown as pie charts. (B) Repertoire frequency of expanding B cell lineages at the post-vaccination time point. (C) Stream plots showing expanding B cell lineages in patients receiving their third SARS-CoV-2 vaccination (vacc3). (D) Exemplary detailed somatic hypermutation analysis of two antibody sequences. GC = germinal center. Seq = sequence.
Dissection of infection-, but especially vaccine-induced B cell immunity to SARS-CoV-2 has become even more a priority in light of the advent of SARS-CoV-2 variants of concern that differ from the ancestral strain in transmissibility and immune evasion. In the second half of 2021, when the majority of blood samples for this analysis were collected, Delta (B.1.617.2) was the dominant SARS-CoV-2 variant worldwide. The Omicron variant B.1.1.529 has been first reported to WHO on the 24th of November 2021 and thereafter spread across the globe at unprecedented rate. In January 2022, Omicron replaced Delta as the dominant variant accompanied by a record of 15 million new COVID-19 cases worldwide in a single week. Both Delta and Omicron variants cause concerns also in fully vaccinated populations since the current vaccines are targeted at the ancestral SARS-CoV-2 strain (40–47). In addition, it was also reported that the type and/or sequence of different exposures triggers SARS-CoV-2-directed immune responses varying in specificity and neutralizing potency (48). It is therefore crucial to understand the biology of SARS-CoV-2 vaccination in detail to design better vaccination protocols and eventually vaccine updates providing sufficient protection against emerging variants.
In the study presented here, we show complex B cell maturation trajectories induced by the third “booster” vaccination that by far exceeded the level of somatic hypermutation measurable directly after completion of the primary vaccination series. Interestingly, the strong mutational activity was essentially restricted to few lineages that were present and mutated already before the third vaccination and therefore likely involved in previously induced memory B cells that were once again recruited to the lymph node’s germinal center for further refinement. Notably, we observed high mutational rates especially in IGHV3-21, IGHV3-23, IGHV3-53 and IGHV3-30-3 genes that were linked to antibodies isolated from elite neutralizers that have previously shown neutralizing potency against variants of concern after infection with the ancestral strain (12, 35–39). Interestingly, we did not observe any age-restriction for this maturation process indication that also older people benefit from a third vaccination although this needs further validation due to the limited size of the analyzed cohort. Since somatic hypermutation reflects affinity maturation, our data is not only well compatible with the strong increase in neutralization potential towards the ancestral strain induced by the third vaccination (49), it also may explain why individuals after a recent third vaccination are usually protected from infection with the Delta variant that shows only few immune-evasive S protein mutations (50–52). This postulated increase in affinity may also explain why the third vaccination but not the primary vaccination series produces some level of protection against infection with the Omicron strain that harbors a large number of immune-evasive mutations within the S protein (53, 54).
We found a gap between the fraction of somatically hypermutated SARS-CoV-2 directed B cell clones after completion of priming and directly before boosting. This suggested that SARS-CoV-2 vaccines might trigger long-term maturation and affinity selection in the B cell compartment over months even with mRNA vaccines that consist in short-lived injected molecules. This data is well compatible with recent findings from Sokal et al. (24) that show continuous maturation of B cells over months after vaccination by flow cytometry. From a translational perspective, this data may suggest that the shortening of the interval between priming and boosting may come at a price of lower booster efficacy if the time span for ongoing B cell maturation and affinity selection is too short. Increasing the time interval between the first and the second dose has been shown to promote effectiveness in the AstraZeneca vector vaccine trial for the first two vaccinations (55). At the same time, this needs to be balanced against the current risk associated with the inability of a primary vaccination series to protect against the current Omicron variant.
The third vaccination rapidly generated a considerable somatic hypermutation load in SARS-CoV-2 specific B cell receptor clonotypes given that the time interval between the pre- and post-booster vaccination sampling was only 14 days. To achieve a somatic hypermutation rate of the IGHV gene between 2 and 10% (typical for the antigen-experienced B cells seen in our study), between 6 and 30 nucleotide exchanges are required per clone. Given the increased somatic hypermutation rate of immunoglobulin genes of approximately 10(-3) mutations per base pair per cell division (56), more than three cell divisions are necessary to statistically exchange one nucleotide per IGHV gene. This very rough calculation may show the high proliferative stimulus of the third booster vaccination. The clinical correlate thereof may be the rather high rate of ipsilateral axillary lymph node swelling compared to other cohorts (57) observed by almost 10% of our DigiHero participants after the third vaccination.
The major limitation of our work is the purely computational approach. To derive firm conclusions regarding variant specificity of vaccination-induced B cell clones, functional validation of binding properties are clearly needed and part of many ongoing studies worldwide. Another limitation of the study design consists in a comparison of two vaccinations (the primary series) with just one third vaccination. Furthermore, it is noteworthy that our sequencing approach does not distinguish between memory B cells and plasmablasts, which may show different frequencies in both cohorts. This needs to be taken into account when comparing the primary vaccination series with the third vaccination.
Taken together, our data show that the primary vaccination series quickly generates antibodies from B cells that have only undergone low-level affinity maturation and may therefore not be protective for immune-escape viral variants such as Omicron B.1.1.529. Our analyzes confirm the role of the third SARS-CoV-2 “booster” to generate affinity-matured clones and mobilize them for antibody production.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below:
https://www.ebi.ac.uk/ena, PRJEB50803.
This study was registered as non-interventional study (NIS) at the Paul-Ehrlich-Institute (NIS635). DigiHero and HACO were approved by the institutional review board (approval numbers 2020-076 and 2020-039). Written informed consent was received prior to participation. The patients/participants provided their written informed consent to participate in this study.
Idea and design of research project: MB, LP, RM, CG. Supply of critical material (e.g. patient material, cohorts): RM, DSe, MB, MGe, MGi, SD, BK; Establishment of Methods: LP, CS, EW; Experimental work: LP, CS, DSi; Data analysis and interpretation: MB, LP, CS; Drafting of manuscript: MB, LP.
This project was partially funded by the CRC 841 of the German Research Foundation (to MB) as well as by the Martin-Luther-University Halle (Saale).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank the participants of our DigiHero and HACO cohorts for their great support. Moreover, we thank Christoph Wosiek, Aline Patzschke, Jenny Wehde and Katrin Nerger for excellent technical assistance.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.876306/full#supplementary-material
1. Robbiani DF, Gaebler C, Muecksch F, Lorenzi JCC, Wang Z, Cho A, et al. Convergent Antibody Responses to Sars-Cov-2 in Convalescent Individuals. Nature (2020) 584:437–42. doi: 10.1038/s41586-020-2456-9
2. Schafer A, Muecksch F, Lorenzi JCC, Leist SR, Cipolla M, Bournazos S, et al. Antibody Potency, Effector Function, and Combinations in Protection and Therapy for Sars-Cov-2 Infection in Vivo. J Exp Med (2021) 218(3):e20201993. doi: 10.1084/jem.20201993
3. Israelow B, Mao T, Klein J, Song E, Menasche B, Omer SB, et al. Adaptive Immune Determinants of Viral Clearance and Protection in Mouse Models of Sars-Cov-2. Sci Immunol (2021) 6(64):eabl4509. doi: 10.1126/sciimmunol.abl4509
4. Laidlaw BJ, Ellebedy AH. The Germinal Centre B Cell Response to Sars-Cov-2. Nat Rev Immunol (2022) 22(1):7–18. doi: 10.1038/s41577-021-00657-1
5. Schultheiss C, Paschold L, Simnica D, Mohme M, Willscher E, von Wenserski L, et al. Next-Generation Sequencing of T and B Cell Receptor Repertoires From Covid-19 Patients Showed Signatures Associated With Severity of Disease. Immunity (2020) 53(2):442–55.e4. doi: 10.1016/j.immuni.2020.06.024
6. Seydoux E, Homad LJ, MacCamy AJ, Parks KR, Hurlburt NK, Jennewein MF, et al. Analysis of a Sars-Cov-2-Infected Individual Reveals Development of Potent Neutralizing Antibodies With Limited Somatic Mutation. Immunity (2020) 53(1):98–105.e5. doi: 10.1016/j.immuni.2020.06.001
7. Barnes CO, West AP Jr, Huey-Tubman KE, Hoffmann MAG, Sharaf NG, Hoffman PR, et al. Structures of Human Antibodies Bound to Sars-Cov-2 Spike Reveal Common Epitopes and Recurrent Features of Antibodies. Cell (2020) 182(4):828–42.e16. doi: 10.1016/j.cell.2020.06.025
8. Nielsen SCA, Yang F, Jackson KJL, Hoh RA, Roltgen K, Jean GH, et al. Human B Cell Clonal Expansion and Convergent Antibody Responses to Sars-Cov-2. Cell Host Microbe (2020) 28(4):516–25.e5. doi: 10.1016/j.chom.2020.09.002
9. Dejnirattisai W, Zhou D, Ginn HM, Duyvesteyn HME, Supasa P, Case JB, et al. The Antigenic Anatomy of Sars-Cov-2 Receptor Binding Domain. Cell (2021) 184(8):2183–200.e22. doi: 10.1016/j.cell.2021.02.032
10. Kreer C, Zehner M, Weber T, Ercanoglu MS, Gieselmann L, Rohde C, et al. Longitudinal Isolation of Potent Near-Germline Sars-Cov-2-Neutralizing Antibodies From Covid-19 Patients. Cell (2020) 182(4):843–54.e12. doi: 10.1016/j.cell.2020.06.044
11. Galson JD, Schaetzle S, Bashford-Rogers RJM, Raybould MIJ, Kovaltsuk A, Kilpatrick GJ, et al. Deep Sequencing of B Cell Receptor Repertoires From Covid-19 Patients Reveals Strong Convergent Immune Signatures. Front Immunol (2020) 11:605170. doi: 10.3389/fimmu.2020.605170
12. Yuan M, Liu H, Wu NC, Lee CD, Zhu X, Zhao F, et al. Structural Basis of a Shared Antibody Response to Sars-Cov-2. Science (2020) 369(6507):1119–23. doi: 10.1126/science.abd2321
13. Paschold L, Simnica D, Willscher E, Vehreschild MJ, Dutzmann J, Sedding DG, et al. Sars-Cov-2-Specific Antibody Rearrangements in Prepandemic Immune Repertoires of Risk Cohorts and Patients With Covid-19. J Clin Invest (2021) 131(1):e142966. doi: 10.1172/JCI142966
14. Zost SJ, Gilchuk P, Chen RE, Case JB, Reidy JX, Trivette A, et al. Rapid Isolation and Profiling of a Diverse Panel of Human Monoclonal Antibodies Targeting the Sars-Cov-2 Spike Protein. Nat Med (2020) 26(9):1422–7. doi: 10.1038/s41591-020-0998-x
15. Andreano E, Nicastri E, Paciello I, Pileri P, Manganaro N, Piccini G, et al. Extremely Potent Human Monoclonal Antibodies From Covid-19 Convalescent Patients. Cell (2021) 184(7):1821–35 e16. doi: 10.1016/j.cell.2021.02.035
16. Gaebler C, Wang Z, Lorenzi JCC, Muecksch F, Finkin S, Tokuyama M, et al. Evolution of Antibody Immunity to Sars-Cov-2. Nature (2021) 591(7851):639–44. doi: 10.1038/s41586-021-03207-w
17. Sokal A, Chappert P, Barba-Spaeth G, Roeser A, Fourati S, Azzaoui I, et al. Maturation and Persistence of the Anti-Sars-Cov-2 Memory B Cell Response. Cell (2021) 184(5):1201–13 e14. doi: 10.1016/j.cell.2021.01.050
18. Sakharkar M, Rappazzo CG, Wieland-Alter WF, Hsieh CL, Wrapp D, Esterman ES, et al. Prolonged Evolution of the Human B Cell Response to Sars-Cov-2 Infection. Sci Immunol (2021) 6(56):eabg6916. doi: 10.1126/sciimmunol.abg6916
19. Rodda LB, Netland J, Shehata L, Pruner KB, Morawski PA, Thouvenel CD, et al. Functional Sars-Cov-2-Specific Immune Memory Persists After Mild Covid-19. Cell (2021) 184(1):169–83.e17. doi: 10.1016/j.cell.2020.11.029
20. Dan JM, Mateus J, Kato Y, Hastie KM, Yu ED, Faliti CE, et al. Immunological Memory to Sars-Cov-2 Assessed for Up to 8 Months After Infection. Science (2021) 371(6529):eabf4063. doi: 10.1126/science.abf4063
21. Schultheiss C, Paschold L, Willscher E, Simnica D, Wostemeier A, Muscate F, et al. Maturation Trajectories and Transcriptional Landscape of Plasmablasts and Autoreactive B Cells in Covid-19. iScience (2021) 24(11):103325. doi: 10.1016/j.isci.2021.103325
22. Kim W, Zhou JQ, Sturtz AJ, Horvath SC, Schmitz AJ, Lei T, et al. Germinal Centre-Driven Maturation of B Cell Response to Sars-Cov-2 Vaccination. Nature (2022) 604:141–5. doi: 10.1038/s41586-022-04527-1
23. Pape KA, Dileepan T, Kabage AJ, Kozysa D, Batres R, Evert C, et al. High-Affinity Memory B Cells Induced by Sars-Cov-2 Infection Produce More Plasmablasts and Atypical Memory B Cells Than Those Primed by Mrna Vaccines. Cell Rep (2021) 37(2):109823. doi: 10.1016/j.celrep.2021.109823
24. Sokal A, Barba-Spaeth G, Fernandez I, Broketa M, Azzaoui I, de la Selle A, et al. Mrna Vaccination of Naive and Covid-19-Recovered Individuals Elicits Potent Memory B Cells That Recognize Sars-Cov-2 Variants. Immunity (2021) 54(12):2893–907.e5. doi: 10.1016/j.immuni.2021.09.011
25. Schultheiss C, Simnica D, Willscher E, Oberle A, Fanchi L, Bonzanni N, et al. Next-Generation Immunosequencing Reveals Pathological T-Cell Architecture in Autoimmune Hepatitis. Hepatology (2021) 73(4):1436–48. doi: 10.1002/hep.31473
26. Bolotin DA, Poslavsky S, Mitrophanov I, Shugay M, Mamedov IZ, Putintseva EV, et al. Mixcr: Software for Comprehensive Adaptive Immunity Profiling. Nat Methods (2015) 12(5):380–1. doi: 10.1038/nmeth.3364
27. Simnica D, Akyuz N, Schliffke S, Mohme M, VW L, Mahrle T, et al. T Cell Receptor Next-Generation Sequencing Reveals Cancer-Associated Repertoire Metrics and Reconstitution After Chemotherapy in Patients With Hematological and Solid Tumors. Oncoimmunology (2019) 8(11):e1644110. doi: 10.1080/2162402X.2019.1644110
28. Raybould MIJ, Kovaltsuk A, Marks C, Deane CM. Cov-Abdab: The Coronavirus Antibody Database. Bioinformatics (2021) 37(5):734–5. doi: 10.1093/bioinformatics/btaa739
29. Gabor C, Tamas N. The Igraph Software Package for Complex Network Research. InterJournal Complex Syst (2006) 1695(5):1–9.
30. Xiang H, Zhao Y, Li X, Liu P, Wang L, Wang M, et al. Landscapes and Dynamic Diversifications of B-Cell Receptor Repertoires in Covid-19 Patients. Hum Immunol (2022) 83(2):119–29. doi: 10.1016/j.humimm.2021.10.007
31. Kim N, Shin S, Minn D, Park S, An D, Park JH, et al. Sars-Cov-2 Infectivity and Antibody Titer Reduction for 6 Months After Second Dose of Bnt162b2 Mrna Vaccine in Healthcare Workers: A Prospective Cohort Study. J Infect Dis (2022) jiac035. doi: 10.1093/infdis/jiac035
32. Koerber N, Priller A, Yazici S, Bauer T, Cheng CC, Mijocevic H, et al. Dynamics of Spike-And Nucleocapsid Specific Immunity During Long-Term Follow-Up and Vaccination of Sars-Cov-2 Convalescents. Nat Commun (2022) 13(1):153. doi: 10.1038/s41467-021-27649-y
33. Seow J, Graham C, Merrick B, Acors S, Pickering S, Steel KJA, et al. Longitudinal Observation and Decline of Neutralizing Antibody Responses in the Three Months Following Sars-Cov-2 Infection in Humans. Nat Microbiol (2020) 5(12):1598–607. doi: 10.1038/s41564-020-00813-8
34. Iyer AS, Jones FK, Nodoushani A, Kelly M, Becker M, Slater D, et al. Persistence and Decay of Human Antibody Responses to the Receptor Binding Domain of Sars-Cov-2 Spike Protein in Covid-19 Patients. Sci Immunol (2020) 5(52):eabe0367. doi: 10.1126/sciimmunol.abe0367
35. Vanshylla K, Fan C, Wunsch M, Poopalasingam N, Meijers M, Kreer C, et al. Discovery of Ultrapotent Broadly Neutralizing Antibodies From Sars-Cov-2 Elite Neutralizers. Cell Host Microbe (2022) 30(1):69–82.e10. doi: 10.1016/j.chom.2021.12.010
36. Tong P, Gautam A, Windsor IW, Travers M, Chen Y, Garcia N, et al. Memory B Cell Repertoire for Recognition of Evolving Sars-Cov-2 Spike. Cell (2021) 184(19):4969–80 e15. doi: 10.1016/j.cell.2021.07.025
37. He B, Liu S, Wang Y, Xu M, Cai W, Liu J, et al. Rapid Isolation and Immune Profiling of Sars-Cov-2 Specific Memory B Cell in Convalescent Covid-19 Patients Via Libra-Seq. Signal Transduct Target Ther (2021) 6(1):195. doi: 10.1038/s41392-021-00610-7
38. Zhang Q, Ju B, Ge J, Chan JF, Cheng L, Wang R, et al. Potent and Protective Ighv3-53/3-66 Public Antibodies and Their Shared Escape Mutant on the Spike of Sars-Cov-2. Nat Commun (2021) 12(1):4210. doi: 10.1038/s41467-021-24514-w
39. Zou J, Li L, Zheng P, Liang W, Hu S, Zhou S, et al. Ultrapotent Neutralizing Antibodies Against Sars-Cov-2 With a High Degree of Mutation Resistance. J Clin Invest (2022) 132(4):e154987. doi: 10.1172/JCI154987
40. Hirabara SM, Serdan TDA, Gorjao R, Masi LN, Pithon-Curi TC, Covas DT, et al. Sars-Cov-2 Variants: Differences and Potential of Immune Evasion. Front Cell Infect Microbiol (2021) 11:781429. doi: 10.3389/fcimb.2021.781429
41. Hoffmann M, Kruger N, Schulz S, Cossmann A, Rocha C, Kempf A, et al. The Omicron Variant Is Highly Resistant Against Antibody-Mediated Neutralization: Implications for Control of the Covid-19 Pandemic. Cell (2022) 185(3):447–56.e11. doi: 10.1016/j.cell.2021.12.032
42. Dejnirattisai W, Huo J, Zhou D, Zahradnik J, Supasa P, Liu C, et al. Sars-Cov-2 Omicron-B.1.1.529 Leads to Widespread Escape From Neutralizing Antibody Responses. Cell (2022) 185(3):467–84.e15. doi: 10.1016/j.cell.2021.12.046
43. Thorne LG, Bouhaddou M, Reuschl AK, Zuliani-Alvarez L, Polacco B, Pelin A, et al. Evolution of Enhanced Innate Immune Evasion by Sars-Cov-2. Nature (2021) 602:487–95. doi: 10.1038/s41586-021-04352-y
44. Zhang J, Xiao T, Cai Y, Lavine CL, Peng H, Zhu H, et al. Membrane Fusion and Immune Evasion by the Spike Protein of Sars-Cov-2 Delta Variant. Science (2021) 374(6573):1353–60. doi: 10.1126/science.abl9463
45. Planas D, Veyer D, Baidaliuk A, Staropoli I, Guivel-Benhassine F, Rajah MM, et al. Reduced Sensitivity of Sars-Cov-2 Variant Delta to Antibody Neutralization. Nature (2021) 596(7871):276–80. doi: 10.1038/s41586-021-03777-9
46. McCallum M, Walls AC, Sprouse KR, Bowen JE, Rosen LE, Dang HV, et al. Molecular Basis of Immune Evasion by the Delta and Kappa Sars-Cov-2 Variants. Science (2021) 374(6575):1621–6. doi: 10.1126/science.abl8506
47. Mlcochova P, Kemp SA, Dhar MS, Papa G, Meng B, Ferreira I, et al. Sars-Cov-2 B.1.617.2 Delta Variant Replication and Immune Evasion. Nature (2021) 599(7883):114–9. doi: 10.1038/s41586-021-03944-y
48. Greaney AJ, Loes AN, Gentles LE, Crawford KHD, Starr TN, Malone KD, et al. Antibodies Elicited by Mrna-1273 Vaccination Bind More Broadly to the Receptor Binding Domain Than Do Those From Sars-Cov-2 Infection. Sci Transl Med (2021) 13(600):eabi9915. doi: 10.1126/scitranslmed.abi9915
49. Garcia-Beltran WF, St Denis KJ, Hoelzemer A, Lam EC, Nitido AD, Sheehan ML, et al. Mrna-Based Covid-19 Vaccine Boosters Induce Neutralizing Immunity Against Sars-Cov-2 Omicron Variant. Cell (2022) 185(3):457–66.e4. doi: 10.1016/j.cell.2021.12.033
50. Perez-Then E, Lucas C, Monteiro VS, Miric M, Brache V, Cochon L, et al. Neutralizing Antibodies Against the Sars-Cov-2 Delta and Omicron Variants Following Heterologous Coronavac Plus Bnt162b2 Booster Vaccination. Nat Med (2022) 28:481–5. doi: 10.1038/s41591-022-01705-6
51. Gross R, Zanoni M, Seidel A, Conzelmann C, Gilg A, Krnavek D, et al. Heterologous Chadox1 Ncov-19 and Bnt162b2 Prime-Boost Vaccination Elicits Potent Neutralizing Antibody Responses and T Cell Reactivity Against Prevalent Sars-Cov-2 Variants. EBioMedicine (2022) 75:103761. doi: 10.1016/j.ebiom.2021.103761
52. Levine-Tiefenbrun M, Yelin I, Alapi H, Katz R, Herzel E, Kuint J, et al. Viral Loads of Delta-Variant Sars-Cov-2 Breakthrough Infections After Vaccination and Booster With Bnt162b2. Nat Med (2021) 27(12):2108–10. doi: 10.1038/s41591-021-01575-4
53. van Gils MJ, van Willigen HDG, Wynberg E, Han AX, van der Straten K, Burger JA, et al. A Single Mrna Vaccine Dose in Covid-19 Patients Boosts Neutralizing Antibodies Against Sars-Cov-2 and Variants of Concern. Cell Rep Med (2022) 3(1):100486. doi: 10.1016/j.xcrm.2021.100486
54. Wang K, Jia Z, Bao L, Wang L, Cao L, Chi H, et al. Memory B Cell Repertoire From Triple Vaccinees Against Diverse Sars-Cov-2 Variants. Nature (2022) 603:919–25. doi: 10.1038/s41586-022-04466-x
55. Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Safety and Efficacy of the Chadox1 Ncov-19 Vaccine (Azd1222) Against Sars-Cov-2: An Interim Analysis of Four Randomised Controlled Trials in Brazil, South Africa, and the UK. Lancet (2021) 397(10269):99–111. doi: 10.1016/S0140-6736(20)32661-1
56. Odegard VH, Schatz DG. Targeting of Somatic Hypermutation. Nat Rev Immunol (2006) 6(8):573–83. doi: 10.1038/nri1896
Keywords: SARS-CoV-2, COVID-19, delta, B cell maturation, omicron variant, booster vaccination
Citation: Paschold L, Klee B, Gottschick C, Willscher E, Diexer S, Schultheiß C, Simnica D, Sedding D, Girndt M, Gekle M, Mikolajczyk R and Binder M (2022) Rapid Hypermutation B Cell Trajectory Recruits Previously Primed B Cells Upon Third SARS-Cov-2 mRNA Vaccination. Front. Immunol. 13:876306. doi: 10.3389/fimmu.2022.876306
Received: 15 February 2022; Accepted: 14 April 2022;
Published: 09 May 2022.
Edited by:
Marko Radic, University of Tennessee College of Medicine, United StatesReviewed by:
Mats Bemark, University of Gothenburg, SwedenCopyright © 2022 Paschold, Klee, Gottschick, Willscher, Diexer, Schultheiß, Simnica, Sedding, Girndt, Gekle, Mikolajczyk and Binder. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mascha Binder, TWFzY2hhLkJpbmRlckB1ay1oYWxsZS5kZQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.