
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Immunol. , 25 May 2022
Sec. Cancer Immunity and Immunotherapy
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.875488
This article is part of the Research Topic Immunotherapy in Specific Patients with Lung Cancer View all 38 articles
 Xianjing Chu1†
Xianjing Chu1† Lishui Niu1†
Lishui Niu1† Gang Xiao1
Gang Xiao1 Haiqin Peng1
Haiqin Peng1 Fuxing Deng1
Fuxing Deng1 Zhiyuan Liu1
Zhiyuan Liu1 Honghua Wu1
Honghua Wu1 Lei Yang1
Lei Yang1 Zhuguilong Tan1
Zhuguilong Tan1 Zhanzhan Li1*
Zhanzhan Li1* Rongrong Zhou1,2,3*
Rongrong Zhou1,2,3*Background: Although immunotherapy has been widely used, there is currently no research comparing immunotherapy for non-small cell lung cancer (NSCLC) patients with brain metastases (BMs). This meta-analysis addresses a gap in the comparison of immunotherapy efficacy, including immune checkpoint inhibitors (ICIs), chemotherapy (CT), radiotherapy (RT), and ICI combined CT or RT.
Methods: A search of Pubmed, Cochrane, EMBASE, and ClinicalTrial.gov was conducted to identify studies which enrolled NSCLC patients with BM treated with ICIs. The outcomes consisted of intracerebral overall response rate (iORR), intracerebral disease control rate (iDCR), extracranial overall response rate (EORR), distant brain failure (DBF), local control (LC), progression-free survival (PFS), and overall survival (OS).
Results: A total of 3160 participants from 46 trials were included in the final analysis. Patients treated with immunotherapy were associated with a longer PFS (0.48, 95%CI: 0.41-0.56), and a longer OS (0.64, 95%CI: 0.60-0.69) compared with immunotherapy-naive patients. In prospective studies, dual ICI combined CT and ICI combined CT achieved a better OS. The hazard ratio (HR) of dual ICI combined CT versus dual ICI was 0.61, and the HR of ICI combined CT versus ICI monotherapy was 0.58. Moreover, no statistical difference in PFS, OS, EORR, iORR, iDCR, and EDCR was found between patients with ICI monotherapy and ICI combined cranial radiotherapy. Concurrent ICI combined RT was shown to decrease the rate of DBF (OR = 0.15, 95% CI: 0.03-0.73) compared with RT after ICI. Patients treated with WBRT might have an inferior efficacy than those with SRS because the iORR of SRS was 0.75 (0.70, 0.80) and WBRT was 0. Furthermore, no obvious difference in PFS and OS was observed among the three different types of ICI, which targets PD-1, PD-L1, and CTLA-4, respectively.
Conclusions: Patients treated with ICI got superior efficacy to those without ICI. Furthermore, dual ICI combined CT and ICI combined CT seemed to be optimal for NSCLC patients with BM. In terms of response and survival, concurrent administration of SRS and ICI led to better outcomes for patients with BMs than non-concurrent or non-SRS.
Importance of the Study: In the new era of immunotherapy, our meta-analysis validated the importance of immunotherapy for non-small cell lung cancer (NSCLC) patients with brain metastases (BMs). By comparing the long-term and short-term impacts of various regimens, all immunotherapy treatments had superior efficacy to immunotherapy-naive. At the same time, through pairwise comparison in immunotherapy, our findings can help clinicians to make treatment decisions for NSCLC patients with BMs.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=269621, identifier CRD42021269621.
1. Immunotherapy improved OS and PFS compared to immunotherapy-naive regimens.
2. Dual ICI combined CT and ICI combined CT might be the two first-line recommendations for NSCLC patients with BM.
3. Efficacy of ICI combined RT in NSCLC patients with BM depends on the specific method of RT, and the sequence of RT and ICI.
Lung cancer has the characteristics of high incidence, high mortality, and low detection rate (1, 2). Metastasis is considered a leading reason for lung cancer patients’ death, especially brain metastases (BMs) (3). Based on the pathological type, lung cancer consists of small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC) (4). The mean survival time of untreated NSCLC patients with BM is as short as 1-2 months (5).
Although advances have been achieved in BM patients’ treatment recently, the survival rate is still unsatisfactory, possibly because the blood-brain barrier (BBB) hinders drug entry into the brain, such as chemotherapy (CT), of which the median overall survival was only 4-8 months (6–9). Surgery, whole-brain radiation therapy (WBRT), and stereotactic radiation surgery (SRS) are often referred to as conventional local treatments for BM (10). However, WBRT and SRS have certain limitations, such as radiation neurotoxicity, cognitive deterioration, etc. (11–13). In recent years, the emergence of tyrosine kinase inhibitors (TKIs) changed the treatments for BM, particularly in those patients with positive driver genes like epidermal growth factor receptor (EGFR), anaplastic lymphoma kinase (ALK), and c-ros oncogene 1 (ROS1) (14). Compared with first- and second-generation EGFR-TKIs, third-generation EGFR-TKIs demonstrated truly higher BBB permeability and better efficacy in patients with BM (15). Still, approximately 26% of patients with BM have no driver gene mutations (16).
Therefore, the emergence of immune checkpoint inhibitors (ICIs) that target PD-1, PD-L1, or CTLA-4 offers hope to advanced NSCLC patients with negative driver genes (17). Cohen JV et al. proposed that ICIs and active T cells can penetrate BBB (18), which is necessary for ICIs to work (19). Keynote-024 established immunotherapy as a first-line treatment for advanced NSCLC patients with positive PD-L1 (20). However, for those with unknown PD-L1 expression levels, the response rate was only 17%-19% (21–23). In addition, the results of previous clinical trials also showed that immunotherapy in combination with radiotherapy (RT) or CT might improve the survival of NSCLC patients (24–26).
However, the efficacy of immunotherapy in BM remains controversial, depending on various immunotherapy regimens (27, 28). Besides, most studies included in Alencar’s analysis (29) were retrospective with limited sample size and long-term efficacy. Although Yin et al. and Vivianedid et al. did some analyses about the efficacy of BMs immunotherapy, but the number of studies they included was limited and their analyses lacked efficacy comparison between diversified immunotherapy approaches (30, 31). Therefore, we designed and conducted this meta-analysis to evaluate the efficacy of immunotherapy more comprehensively in NSCLC patients with BM.
This meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) statement. And it was registered at the International Prospective Register of Systematic Reviews (PROSPERO) (number: CRD42021269621). Pubmed, Cochrane, EMBASE, Web of Science, and ClinicalTrial.gov were used to search literature by entering keywords and setting constraints. We collected all qualified clinical trials before September 25, 2021. The term words are “Carcinoma, Non-Small-Cell Lung,” “Immunotherapy,” “Immune Checkpoint Inhibitors,” “Pembrolizumab,” “Nivolumab,” “Atezolizumab,” “Durvalumab,” “Cemiplimab,” “Camrelizumab,” “Sintilimab,” “Tislelizumab,” or “Toripalimab.” And this literature retrieval process was performed independently by two authors (Xianjing Chu and Lishui Niu).
Literature titles and abstracts were screened by two authors independently, and then the results were combined to delete duplicate results. In case of disagreement, a third researcher was required. There were no language restrictions. Studies that meet the following standards are regarded as eligible studies. The inclusion standards are: 1) the participants are stage IV NSCLC patients; 2) the type of studies is randomized controlled trials or cohort studies; 3) intervention is immunotherapy; 4) outcomes included one or more of the following indexes: iORR, iDCR, EORR, OS, PFS, DBF, LC; 5) the studies are about NSCLC with BM. Studies of reviews, editorials, comments, case reports, animal trails, or letters are excluded.
Two investigators extracted data independently by browsing full text of studies. The following information was extracted: 1) authors and publication year; 2) study type; 3) median follow-up time; 4) interventions; 5) number of total participants; 6) number of participants with BM; 7) sex; 8) age; 9) smoking history; 10) the state of driving gene mutation; 11) PD-L1 expression; 12) the histological types; 13) radiotherapy history; 14) number of metastases lesions; 15) max diameter of metastases; 16) EORR; 17) iORR; 18) iDCR; 19) hazard ratio (HR) for PFS; 20) HR for OS; 21) DBF 22) LC. Two authors independently evaluated the methodological quality (risk of bias).
For non-RCT studies, Newcastle-Ottawa Scale (NOS) was used to calculate the risk of bias. In NOS, there are three assessing criteria for cohort studies including selection of cohorts (4 points), comparability of cohorts (2 points), and assessment of outcome (3 points). For case-control studies, selection (4 points), comparability (2 points), and exposure (3 points) are key criteria. A total score of 5 or above is considered high quality (32).
For RCT studies, the risk of bias and applicability concerns graph was created by using the Cochrane risk of bias tool. Version 2 of the Cochrane risk-of-bias tool divides the main bias types into five domains which are bias arising from the randomization process, bias due to deviations from intended interventions, bias due to missing outcome data, bias in measurement of the outcome, and bias in selection of the reported result. Risk-of-bias judgments within domains were then mapped to an overall judgment for the outcome. The outcomes include high risk of bias, some concerns, and low risk of bias (33).
Short-term efficacy indicators and long-term indicators are extracted. The short-term indicators include progression-free survival (PFS: the time from randomization to objective tumor progression), intracerebral objective response rate (iORR), intracerebral disease control rate (iDCR), extracranial overall response rate (EORR), extracranial disease control rate (EDCR), distant brain failure (DBF), and local control (LC), while long-term indicators consist of overall survival (OS: the time from randomization to all-cause death) (34). ORR is the sum of proportion of patients getting complete intracranial response (CR: disappearance of every target lesion, and short axis of pathological lymph nodes to be within 10 mm) and partial intracranial response (PR: at least 30% decrease of target lesions’ diameters) (35, 36). DCR refers to the ratio between patients getting complete intracranial response, partial intracranial response, and stable disease (SD: either sufficient shrinkage <30% or sufficient increase <20%). Additionally, DBF is defined as the rate between the number of patients with the appearance of new BM or a stable or decreasing lesion size and the total number of BM people. Local control (LC) is defined as a stable or decreasing lesion size (37).
The primary outcomes in this study were iORR, iDCR, EORR, DBF, and HRs for PFS and OS. The heterogeneity within studies was assessed using the Chi-square test and I2 statistics. p<0.05, or I2>50% indicated significant heterogeneity. The random-effect model was used for later analysis in terms of significant heterogeneity, otherwise, the fixed-effect model was used. For ORR, DCR, EORR, and DBF, the odds ratios (OR) and their 95% confidence intervals (CIs) of these outcomes were estimated. The hazard ratios (HRs) and their 95%CIs were calculated for evaluating the efficacy of the following groups: ICI vs CT, ICI+CT vs CT, ICI+RT vs ICI, and ICI+RT vs RT in NSCLC patients with BM. Subgroup analysis was performed for the sequencing of ICI and radiotherapy (concurrent vs. sequential), different intracranial radiation methods (SRS vs. WBRT), and design of studies (retrospective vs. prospective). To make the results more intuitive, forest plots were created. The pair-wise network meta-analyses of different ICI regimens and ICI types in prospective studies were performed by R version 3.2.1 and the STATA 14.0, using the fixed-effects model. Publication bias was assessed using Begg and Egger tests. Sensitivity analyses were performed for evaluating the influence of each study by omitting one study each time. Other analyses were completed using Stata software version 14.0 (Corp, College Station TX, USA) and Rev Manager 5.3. p<0.05 was considered significant unless otherwise specified.
Our literature identified 4443 studies initially, of which 2189 studies were regarded as duplicate records, 226 studies were marked as ineligible by automation tools, and 334 studies were removed for other reasons, such as lack of abstract. After screening the remaining 1684 abstracts, 1080 abstracts were excluded due to irrelevance. Following a retrieving process of relevant 604 records, we could not find 182 of them in publication. Among the remaining 422 articles, 102 were excluded for the article types (reviews, case reports, et al.), 119 did not include outcomes of BM subgroup, 69 were non-immunotherapy, and 86 articles contained results of other cancers such as melanoma. Subsequently, 46 studies were incorporated into the final analysis (27–29, 37–80). We illustrated the detailed process of the literature searches in a flow chart (Figure 1).
These included trials were heterogeneous, of which 22 were prospective, and 24 were retrospective. The immunotherapy group comprised patients treated with ICI monotherapy, ICI combined RT and ICI combined CT. Patients who were treated without ICI were incorporated into the immunotherapy-naive group. ICI monotherapy was defined as non-irradiation or non-chemotherapy 4 weeks prior to the ICI therapy, and the remaining immunotherapy groups were ICI combined RT or ICI combined CT group. To assess the efficacy of the sequencing of immunotherapy and radiotherapy on BM, ICI combined RT was divided into concurrent, RT before ICI, and RT after ICI.
Among the immunotherapy group, 23 arms were ICI monotherapy, nine were ICI combined CT, and 22 were ICI combined RT (Figure S1A), which contained 14 cohorts with RT before ICI, 12 concurrent, and four with RT after ICI (Figure S1C). Furthermore, seven groups received ICI combined SRS, one cohort received ICI combined WBRT, and the rest called mixed regimens did not differentiate between the two alternatives. But ICI combined RT groups were all analyzed in retrospective studies. Moreover, four dual ICI combined therapy were enrolled. A total of 33 trials were treated with PD-1 inhibitors. PD-1 combined CLTA-4 inhibitors were used in two studies. Four of the studies used PD-L1 inhibitors, and one involved PD-L1 and CLTA-4 inhibitors. Mixed regimens were classified as those in which the type of ICI was unknown, containing six trials.
We summarized participants’ sex, age, smoking history, EGFR/KRAS/ALK mutation, the expression of PD-L1, number and diameter of BM lesions, and pathological type in both Table 1 and Figure S1B, and the efficacy in Table S3.
Strong evidence in Figure 2A showed that when compared with the ICI naive group, the immunotherapy group was associated with significantly longer PFS (0.48, 95% CI: 0.41-0.56) and OS (0.64, 95%CI: 0.60-0.69). 501 patients with BM were included to evaluate PFS of ICI monotherapy against CT, and 823 patients were enrolled to compare OS. The HRs of PFS and OS were 0.75 (0.58, 0.91) and 0.61 (0.47-0.75). The HR of ICI combined CT versus CT was 0.40 (95%CI: 0.30-0.50), and 0.43 (95%CI: 0.30-0.56). The HRs were 0.50 (0.26-0.74) and 0.76 (0.70-0.92) respectively in ICI combined RT versus RT. A significant difference was also observed between immunotherapy and immunotherapy-naive groups in the retrospective and prospective studies respectively. The HR of PFS was 0.46 and OS was 0.76 in retrospective studies, which was 0.50 and 0.49 in prospective studies (Figure 2B).
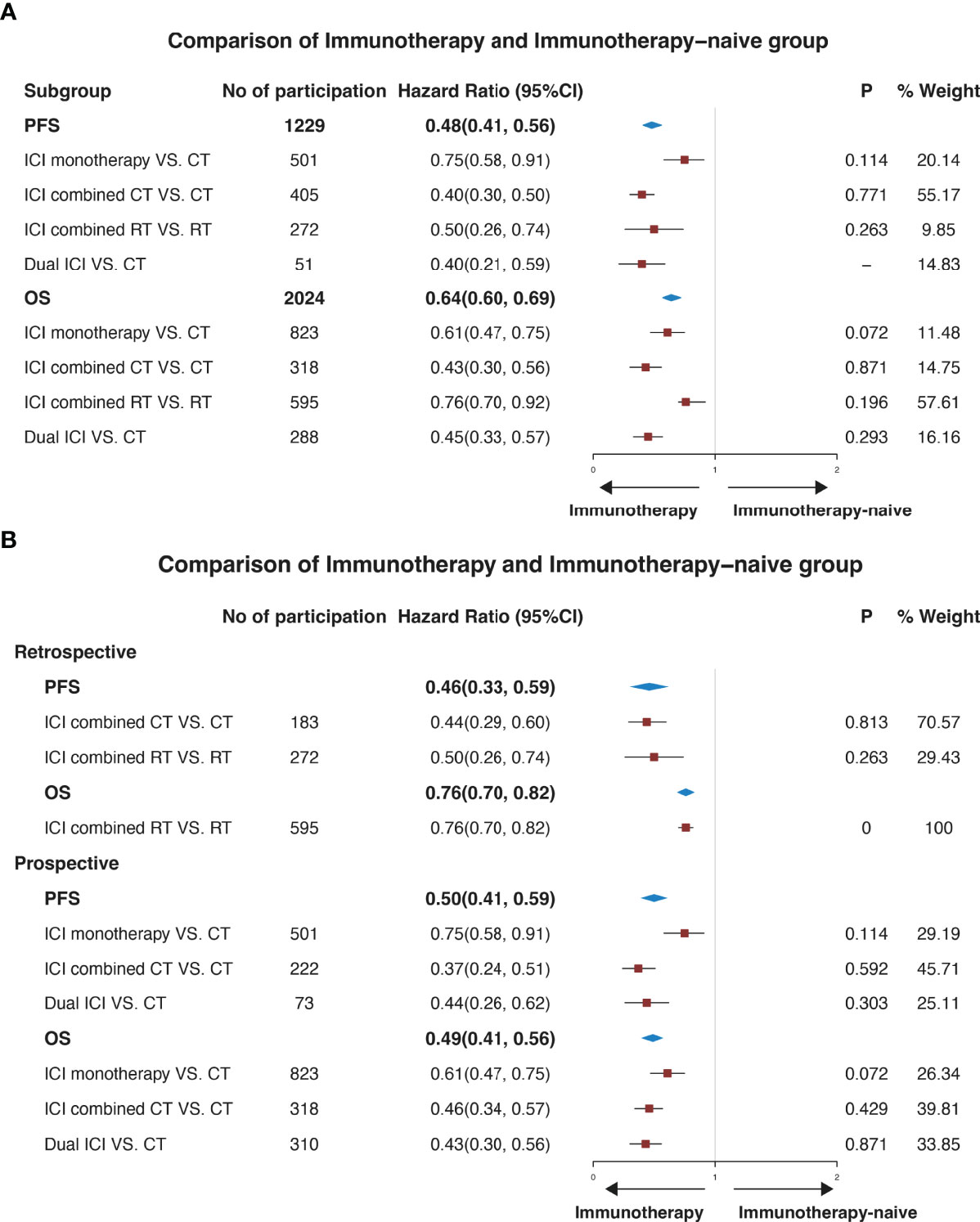
Figure 2 Forest plots illustrating pooled results of efficacy for the comparison of patients with or without immune checkpoint inhibitors. The immunotherapy group was defined as patients who received ICI. The immunotherapy-naive group was defined as patients who didn’t receive ICI. (A) illustrates a pooled result in all studies, and (B) illustrates a pooled result of subgroup analysis according to the type of study. No, number; CI, confidence interval; P, p-value of heterogeneity. PFS, progression-free survival. OS, overall survival; CT, chemotherapy; RT, radiotherapy.
A network meta-analysis was conducted in prospective studies to compare the efficacy of various ICI systemic regimens for the treatment of NSCLC patients with BM (Figure S2). In terms of OS (Figure 3A), ICI monotherapy, ICI combined CT, dual ICI, and dual ICI combined CT exhibited a relatively better efficacy compared to CT (HR = 0.76, 95%CI = 0.62-0.92; HR = 0.44, 95%CI = 0.33-0.58; HR = 0.65, 95%CI = 0.45-0.94; HR =0.4, 95%CI =0.3-0.54, respectively). Dual ICI combined CT and ICI combined CT achieved the highest OS. When compared to ICI monotherapy, the HR of ICI combined CT was 0.58, and the HR of dual ICI combined CT was 0.53. Furthermore, the HR between dual ICI combined CT and dual ICI was 0.61(0.4, 0.94).
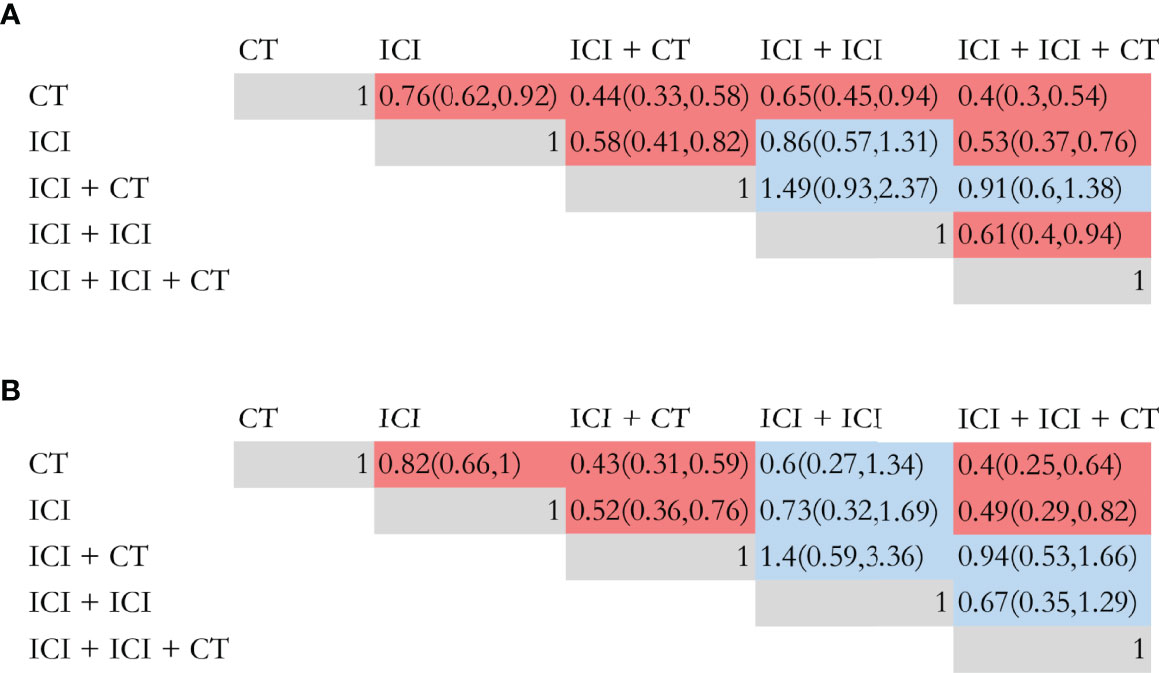
Figure 3 Efficacy of different immune checkpoint inhibitor regimens profiles based on overall survival (A) and progression-free survival (B). Each cell of the efficacy profiles contains the pooled hazard ratio and 95% confidence intervals; significant results are in red, otherwise, in blue. The pooled hazard ratio and 95% confidence intervals indicate the results of the column name compared with the row name. ICI, immune checkpoint inhibitor; CT, chemotherapy.
As shown in Figure 3B, ICI monotherapy, ICI combined CT, and dual ICI combined CT were all more effective than CT (HR = 0.83, 95%CI = 0.66-1; HR = 0.43, 95%CI = 0.31-0.59; HR = 0.4, 95%CI = 0.25-0.64). There was no difference between dual ICI and CT, and only Natasha (73) investigated the efficacy of dual ICI (durvalumab plus tremelimumab), which might cause bias in the comparison. The optimal for enhancing PFS were also dual ICI combined CT and ICI combined CT, of which the HR was 0.4 and 0.43, respectively.
The standard treatment for NSCLC patients with BM was cranial radiation previously, so the comparison was performed to analyze the effectiveness of ICI combined RT and ICI monotherapy (Figure 4). Surprisingly, there was no statistical difference in PFS (HR=0.97, 95%CI: 0.40-2.35), OS (HR=0.69, 95%CI: 0.23-1.15), EORR (OR=0.75, 95%CI: 0.28-2.01), iORR (OR=1.27, 95%CI: 0.65-2.47), iDCR (OR=1.52, 95%CI: 0.80-2.91), and EDCR (OR=0.99, 95%CI: 0.26-3.81) for patients with ICI combined intracranial radiation or ICI monotherapy (Figure 4A).
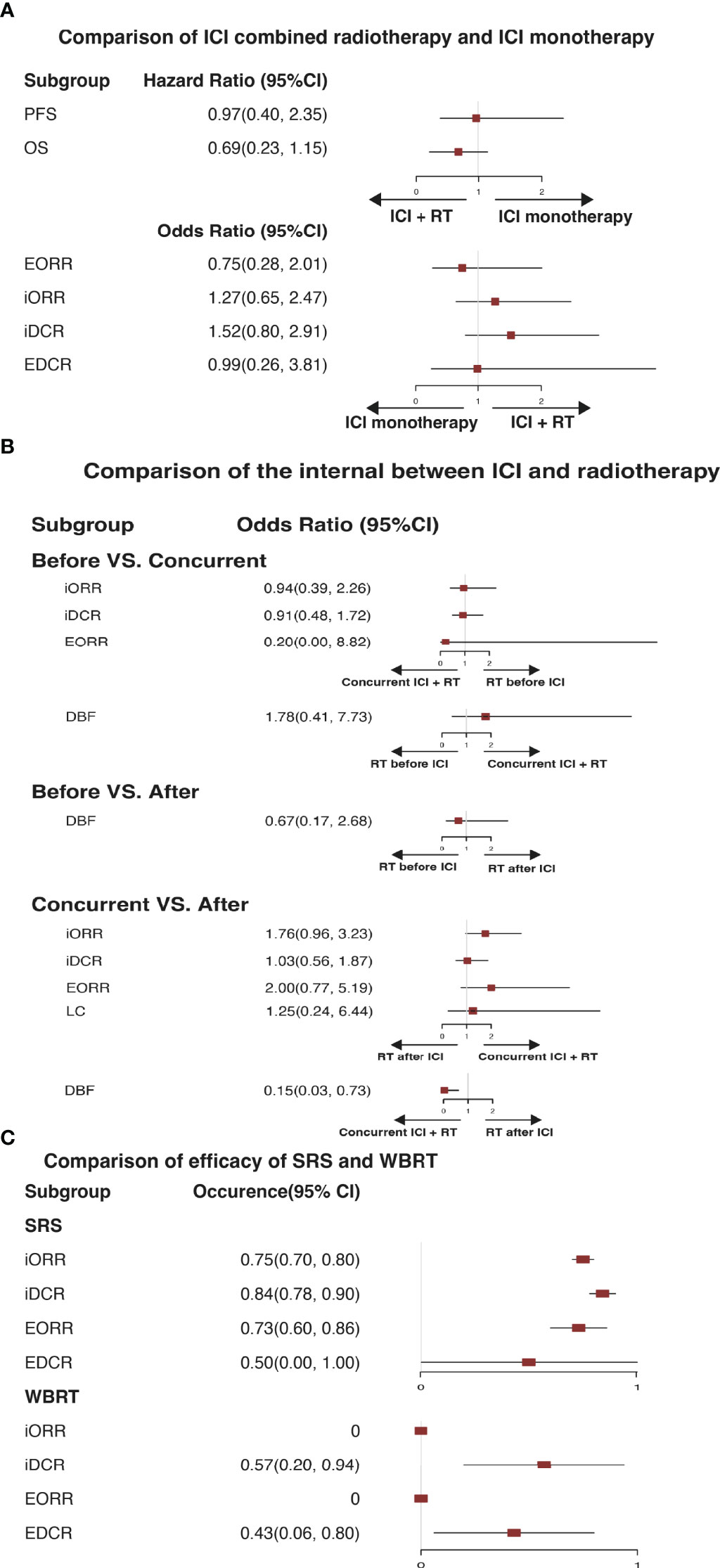
Figure 4 (A) Illustrates a pooled therapeutic result in comparison of immune checkpoint inhibitor monotherapy and immune checkpoint inhibitor combined radiotherapy. (B) Illustrates a pooled therapeutic result of comparison among radiotherapy before immune checkpoint inhibitor, concurrent immune checkpoint inhibitor combined radiotherapy, and radiotherapy after immune checkpoint inhibitor. (C) Illustrates a pooled result in comparison of stereotactic radiation surgery and whole-brain radiation therapy. ICI, immune checkpoint inhibitor; RT, radiotherapy; SRS, stereotactic radiation surgery; WBRT, whole-brain radiation therapy; PFS, progression-free survival. OS, overall survival; iORR, intracerebral objective response rate; iDCR, intracerebral disease control rate; EORR, extracranial overall response rate; EDCR, extracranial disease control rate; DBF, distant brain failure; LC, local control.
Therefore, we hypothesized whether the sequencing of immunotherapy and RT affected the efficacy of ICI combined RT. Subsequently, a subgroup meta-analysis of three radiotherapy regimens, RT before ICI (BEFORE), concurrent RT and ICI (CONCURRENT), and RT after ICI (AFTER), was conducted (Figure 4B). In the comparison of Before and Concurrent groups, no obvious differences were observed in iORR, iDCR, EORR, and DBF (OR = 0.94, 95%CI: 0.39-2.26; OR = 0.91, 95%CI: 0.48-1.72; OR = 0.20, 95%CI: 0.00-8.82; OR = 1.78, 95%CI: 0.41-7.73, separately). The DBF of BEFORE versus AFTER group was 0.67, ranging from 0.17 to 2.68, indicating no difference. A discernible difference between CONCURRENT and AFTER was shown in DBF (OR = 0.15, 95%CI: 0.03-0.73), which demonstrated that concurrent treatment was associated with favorable locoregional disease control.
For NSCLC patients with BMs, WBRT and SRS are the preferred intracranial radiation treatments. In our study, seven trials assessed the short-term efficacy of ICI combined SRS or WBRT (Figure 4C). Referring to ICI combined SRS, the iORR, iDCR, EORR, and EDCR were 75%, 84%, 73%, and 50%, separately. In terms of ICI combined WBRT, these were 0%, 57%, 0%, and 43%, separately.
Moreover, a network meta-analysis was performed to compare the efficacy of PD-1, PD-L1, and PD-1 combined CLTA4 inhibitors (Figure 5). As shown in Figure 5A, PD-1, PD-L1, and PD-1 combined CLTA4 inhibitors all demonstrated a better OS (Figure 5A) and PFS (Figure 5B) in comparison with CT. However, no significant difference was observed among the three types of ICI for treating BM, which might indicate that they were all potential choices.
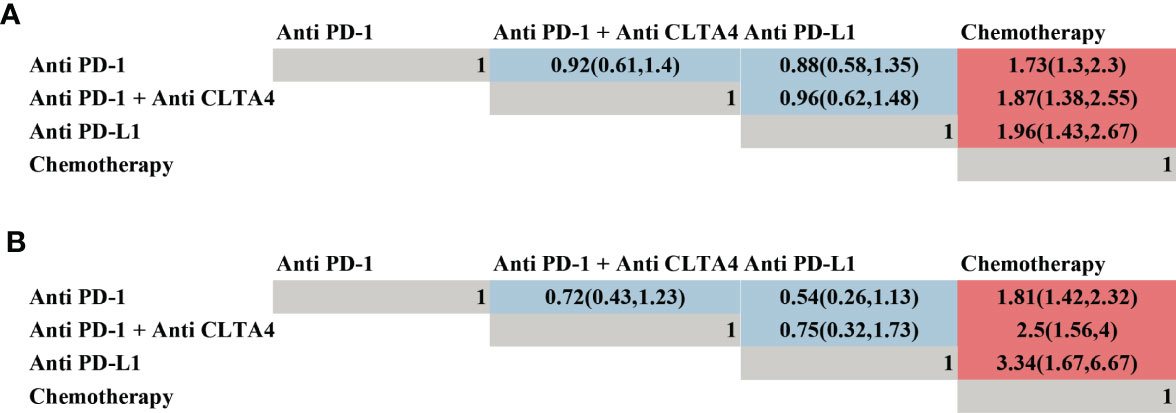
Figure 5 Efficacy of different types of immune checkpoint inhibitor profiles based on overall survival (A) and progression-free survival (B). Each cell of the efficacy profiles contains the pooled hazard ratio and 95% confidence intervals; significant results are in red, otherwise, in blue. The pooled hazard ratio and 95% confidence intervals indicate the results of the column name compared with the row name.
Sensitivity analysis was performed by excluding one individual study each time to assess the influence of each individual study on the pooled HRs for OS or PFS. The omission of any single study did not appreciably change the pooled HR, and the estimates in each case were well within the confidence limits of the overall estimate (Figure S4). The NOS results are listed in Table S2. Figure S5 provides the Cochrane risk of bias. Thus, our meta-analysis revealed a positive correlation between immunotherapy and the prognosis of NSCLC patients with BM.
NSCLC patients complicated with BMs, especially those whose driver genes mutations are negative or TKI resistant, remain a challenge to treat. However, the roles of ICI regimens for those patients are not completely established. We, therefore, summarized the ICI regimens in NSCLC patients with BMs and performed a meta-analysis to provide a theoretical basis for future treatment strategies. In total, we included 46 articles with 3160 NSCLC patients with BMs. Twenty-two articles were prospective studies, and 24 articles were retrospective studies. Three findings were yielded in our study.
Firstly, immunotherapy may be preferable over non-immunotherapy for NSCLC patients with BMs, with longer PFS (HR = 0.48) and OS (HR = 0.64). The advantage may be caused by the synergy between immunotherapy and chemotherapy/radiotherapy (81–86). For example, immunotherapy enhanced the radiotherapy-induced abscopal effect and reversed the immunosuppressive effect of radiation, by blocking the immune checkpoint between antigen-presenting cells and lymphocytes in regional lymph nodes and other organs. Meanwhile, radiation destroyed the endothelial junctions of the BBB, promoted tumor antigen release, and up-regulated T-cell mediated immune response and PD-L1 expression, which in turn prompted the efficacy of immunotherapy. Simultaneously, chemotherapy promoted tumor immunity by inducing immunogenic cell death as part of its intended therapeutic effect and disrupting strategies that tumors use to evade immune recognition. Moreover, CTLA-4 can impair the critical signal transmission of T-cell activation by competitively inhibiting the CD28 receptor’s binding to B-7 ligands, and PD-1 blocks T-cell activation directly. ICI could inhibit the two pathways so that active peripheral immune cells could penetrate BBB. Our findings were consistent with the conclusions of real-world clinical studies, which showed that immunotherapy had a therapeutic benefit in NSCLC patients with BM.
Secondly, no significant difference in PFS (HR = 0.97, 95%CI: 0.40-2.35), OS (HR = 0.69, 95%CI: 0.23-1.15), EORR (OR = 0.75, 95%CI: 0.28-2.01), iORR (OR = 1.27, 95%CI: 0.65-2.47), iDCR (OR = 1.52, 95%CI: 0.80-2.91), and EDCR (EORR (OR = 0.99, 95%CI: 0.26-3.81) was observed between ICI combined RT and ICI monotherapy, which is consistent with Alencar’s study (30). The difference in PD-L1 expression or lymphocyte tumor infiltration might be the biological mechanism of variable response rates on ICIs for BMs (87, 88). However, there were insufficient data to assess survival outcomes and stratify response based on other important factors like the time of BM diagnosis (newly diagnosed or recurrent), the number, size, and location of metastases, the presence of extracranial disease, the presence of actionable driver gene mutations, and PD-L1 expression.
Interestingly, the sequencing of immunotherapy and RT in the treatment of BM from NSCLC might influence the efficacy of ICI combined RT. Concurrent ICI combined RT might have a lower recurrence rate than sequential ICI combined RT, especially RT after ICI, with a DBF of 0.15. However, no significant improvement in efficacy was found in concurrent ICI combined RT, like iORR, iDCR, EORR, or EDCR, which is inconsistent with previous studies. The interval of ICI and cranial RT might be the core of inconsistency. The largest published retrospective study of patients receiving concurrent immuno-combined RT, the interval of which was shorter than 2 weeks, had a significantly longer OS, reduced incidence of new BM lesions, and an acceptable safety profile (89). The latest prospective study by Wang (unpublished, NCT02978404), a phase II study, in which the interval between nivolumab and SRS was also 2 weeks, achieved a median intracranial PFS of 6.5 months, and an OS of 21.4 months. Coincidentally, in the Emory trial of Khan (unpublished, NCT02858869), the interval of which between pembrolizumab and SRS was 2 to 3 days, also reached a median intracranial PFS of 7.5 months, and an OS of 32.8 months. In addition, a prospective study (unpublished, NCT02696993) that adopted concurrent SRS with dual immunotherapy (Nivolumab and Ipilimumab) with a 7-day interval, achieved a median intracranial PFS of 9.7 months, and a 4-month intracranial PFS rate of 75%. In combination with our analysis and the positive results of ongoing prospective trials, it provides strong support for the efficacy of concurrent ICI combined with RT in 4 weeks (90, 91), which is the drug wash-out period and the destruction of the BBB endothelial cell generated by radiotherapy, for NSCLC patients with BM.
Moreover, when evaluating the efficacy and tolerability of ICI combined RT for BM, it’s important to consider the variety of radiation treatment modalities and dose fractionation prescriptions used. Initial analysis suggested SRS achieved an obvious increase in iORR and EORR, compared to WBRT, which were 75% versus 0 and 73% versus 0, respectively. It was possibly connected to dosage distribution. WBRT delivers the same modest palliative radiation dosage to healthy brain tissue (non-ablative). While SRS delivers a strong ablative dosage solely to metastatic tissue (92). In our included research, the average number of BM was two, the diameter of which ranged from 0.5 to 2 cm, which was defined as small oligometastases. However, WBRT might be suitable for diffuse BM, and it had more acute toxicities than SRS, increases fatigue, lowers the quality of life, and impairs cognitive function (93). Our results correspond to multiple clinical studies, which indicated SRS might eventually replace WBRT for patients with localized (1-3) minor lesions (less than 4 cm in size).
Thirdly, dual ICI combined CT and ICI combined CT provided a better PFS and OS. Compared to dual ICI, the HRs of dual ICI combined CT were 0.61 and 0.67. The HRs of ICI combined CT versus ICI were 0.58 and 0.52, respectively. Surprisingly, there was no difference between dual ICI and ICI combined CT. The mechanisms and efficacy of ICI combined CT varied by different CT agents. Such as platinum-based CT, on the one hand, its favorable immunomodulation effects may boost tumor cells’ susceptibility to PD-1/PD-L1 inhibitors. On the other hand, down-regulation of intracellular PD-L1 expression by PD-1/PD-L1 inhibitors could make patients sensitive to platinum-based CT. However, the mechanisms and efficacy of ICI combined with CT varied by different chemotherapy agents. A dual ICI regimen was defined as a combined blockade of PD-1/L1 and CTLA-4, which offers a number of advantages over a single PD-1 inhibitor without the limitation of BBB. Firstly, PD-1/L1 inhibition is linked to CTLA-4 overexpression, thus anti-CTLA-4 inhibitors might prevent further immune escape directly. Secondly, myeloid-derived suppressor cells can severely limit T cell function inside the tumor microenvironment, but dual ICI can raise the fraction of CD8+ effector T cells relative to MDSCs synergistically. Thirdly, dual ICI could raise inflammatory cytokine production, such as TNF-α and IFN-γ, while lowering T cell anergy. Finally, dual ICI could lead to the growth of memory T cells, which facilitates longer-term anti-tumor immunity (94). But the brain was an immune-specialized environment, in which immune responses against tumors were restricted. Taken together, these confounding factors might weaken the difference of efficacy of ICI combined CT and dual ICI. After combining the results of dual ICI combined CT and ICI combined CT in our study, the ranking of efficacy and recommendation based on the five treatment groups were as follows: dual ICI combined CT, ICI combined CT, dual ICI, ICI monotherapy, and CT.
Another aspect that determines the effectiveness of ICIs is the choice of PD-1, PD-L1, or CTLA-4 inhibitors. Even though Duan et al. (95) found PD-1 inhibitors had better OS and PFS than PD-L1 inhibitors in various cancers, it’s uncertain if the three forms of ICIs, PD-1, PD-L1, and CLTA-4 inhibitors in NSCLC patients with BMs have different intracranial activity. Our analysis found no statistical difference in PD-1, PD-1 combined CLTA-4, and PD-L1 inhibitors for the first time. Still, further exploration was warranted to elucidate the specific mechanism.
Our strengths involved a comprehensive, systematic review of studies by a multidisciplinary team including specialists in NSCLC with BM and epidemiological methods. A broad search strategy was employed to catch all relevant studies. Therefore, our analysis was more comprehensive than other literature with similar topics. Furthermore, there was no proof of publication bias. Importantly, our study not only was the first meta-analysis concerning different ICI regimens, but also the first to compare the intracranial efficacy of WBRT and SRS, and three different ICI types for NSCLC patients with BM.
Our limitations deserve comments. In the analysis of ICI monotherapy versus ICI combined RT, most studies were retrospective, which makes pairwise analysis unavailable, and more prone to selection bias. Despite that, the results that ICI monotherapy might be effective as a single treatment for patients with BM were consistent with, and replenish, Alencar’s therapeutic perspective.
In conclusion, ICI should be considered for selected individuals lacking actionable driver gene mutations. Furthermore, concurrent ICI combined RT in 4 weeks demonstrated improved DBF, and SRS was superior to WBRT for localized and tiny BMs. Our findings revealed that dual ICI combined CT and ICI combined CT had better OS and PFS, giving possible efficacy speculations for clinical decisions. More prospective clinical studies evaluating the benefit of PD-1, PD-L1, and CTLA-4 inhibitors are required in the future, to elucidate why no significant difference in the efficacy of three distinct types of ICI was identified.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
All authors were involved in the design of the study, organization of the study, manuscript writing, and approval of final version of the manuscript. XC and LN generated the search strategy, performed the search, independently performed the article selection procedure, data extraction, and summarized the data. ZZL performed the data analyses, and RZ checked the data analysis and validity. XC and LN wrote the manuscript, had contributed equally to the manuscript. RZ and ZZL had contributed equally to the manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by Beijing Xisike Clinical Oncology and Research Foundation (Y-HR2019-0185) and National Multidisciplinary Cooperative Diagnosis and Treatment Capacity Building Project for Major Diseases of China (z027002).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank Dr. Mao Jiang and Dr. Taohua Liu for proofreading this article.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.875488/full#supplementary-material
1. Herbst RS, Morgensztern D, Boshoff C. The Biology and Management of Non-Small Cell Lung Cancer. Nature (2018) 553(7689):446. doi: 10.1038/nature25183
2. Nasim F, Sabath BF, Eapen GA. Lung Cancer. Med Clin North Am (2019) 103(3):463–73. doi: 10.1016/j.mcna.2018.12.006
3. Mujoomdar A, Austin JH, Malhotra R, Powell CA, Pearson GD, Shiau MC, et al. Clinical Predictors of Metastatic Disease to the Brain From non-Small Cell Lung Carcinoma: Primary Tumor Size, Cell Type, and Lymph Node Metastases. Radiology (2007) 242(3):882–8. doi: 10.1148/radiol.2423051707
4. Huo GW, Zuo R, Song Y, Chen WD, Chen WM, Chong DQ, et al. Effect of Antibiotic Use on the Efficacy of Nivolumab in the Treatment of Advanced/Metastatic Non-Small Cell Lung Cancer: A Meta-Analysis. Open Med (2021) 16(1):728–36. doi: 10.1515/med-2021-0272
5. Rangachari D, Yamaguchi N, VanderLaan PA, Folch E, Mahadevan A, Floyd SR, et al. Brain Metastases in Patients With EGFR-Mutated or ALK-Rearranged Non-Small-Cell Lung Cancers. Lung Cancer (2015) 88(1):108–11. doi: 10.1016/j.lungcan.2015.01.020
6. Yousefi, Bahrami T, Salmaninejad A, Nosrati R, Ghaffari P, Ghaffari SH. Lung Cancer-Associated Brain Metastasis: Molecular Mechanisms and Therapeutic Options. Cell Oncol (2017) 40(5):419–441. doi: 10.1007/s13402-017-0345-5
7. Robinet G, Thomas P, Breton JL, Lena H, Gouva S, Dabouis G, et al. Results of a Phase III Study of Early Versus Delayed Whole Brain Radiotherapy With Concurrent Cisplatin and Vinorelbine Combination in Inoperable Brain Metastasis of Non-Small-Cell Lung Cancer: Groupe Françcais De Pneumo-Cancéerologie (GFPC) Protocol 95. Ann Oncol (2001) 12(1):59–67. doi: 10.1023/A:1008338312647
8. Barlesi F, Gervais R, Lena H, Hureaux J, Berard H, Paillotin D, et al. Pemetrexed and Cisplatin as First-Line Chemotherapy for Advanced Non-Small-Cell Lung Cancer (NSCLC) With Asymptomatic Inoperable Brain Metastases: A Multicenter Phase II Trial (GFPC 07-01). Ann Oncol (2011) 22(11):2466–70. doi: 10.1093/annonc/mdr003
9. Dempke W, Edvardsen K, Lu S, Reinmuth N, Reck M, Inoue A. Brain Metastases in NSCLC - Are TKIs Changing the Treatment Strategy? Anticancer Res (2015) 35(11):5797.
10. Tayyeb B, Parvin M. Pathogenesis of Breast Cancer Metastasis to Brain: A Comprehensive Approach to the Signaling Network. Mol Neurobiol (2016) 53(1):446–54. doi: 10.1007/s12035-014-9023-z
11. Patchell RA, Tibbs PA, Walsh JW, Dempsey RJ, Maruyama Y, Kryscio RJ, et al. A Randomized Trial of Surgery in the Treatment of Single Metastases to the Brain. N Engl J Med (1998) 322(8):494–500. doi: 10.1056/NEJM199002223220802
12. Sheehan JP, Sun MH, Kondziolka D, Flickinger J, Lunsford LD. Radiosurgery for Non-Small Cell Lung Carcinoma Metastatic to the Brain: Long-Term Outcomes and Prognostic Factors Influencing Patient Survival Time and Local Tumor Control. J Neurosurg (2002) 97(6):1276. doi: 10.3171/jns.2002.97.6.1276
13. Castrucci WA, Knisely J. An Update on the Treatment of CNS Metastases in Small Cell Lung Cancer. Cancer J (2008) 14(3):138. doi: 10.1097/PPO.0b013e318172d6e1
14. Ernani V, Stinchcombe TE. Management of Brain Metastases in Non-Small-Cell Lung Cancer. J Oncol Pract (2019) 15(11):563–70. doi: 10.1200/JOP.19.00357
15. Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. New Engl J Med (2018) 378(2):113. doi: 10.1056/NEJMoa1713137
16. Kang Y, Jin Y, Li Q, Yuan X. Advances in Lung Cancer Driver Genes Associated With Brain Metastasis. Front Oncol (2021) 10:606300. doi: 10.3389/fonc.2020.606300
17. Soria J-C, Marabelle A, Brahmer JR, Gettinger S. Immune Checkpoint Modulation for Non-Small Cell Lung Cancer. Clin Cancer Res (2015) 21(10):2256–62. doi: 10.1158/1078-0432.CCR-14-2959
18. Cohen JV, Kluger HM. Systematic Immunotherapy for the Treatment of Brain Metastases. Front Oncol (2016) 6:49. doi: 10.3389/fonc.2016.00049
19. Prins RM, Vo DD, Khan-Farooqi H, Yang MY, Soto H, Economou JS, et al. NK and CD4 Cells Collaborate to Protect Against Melanoma Tumor Formation in the Brain. J Immunol (2006) 177(12):8448–55. doi: 10.4049/jimmunol.177.12.8448
20. Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Pembrolizumab Versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med (2016) 375(19):1823–33. doi: 10.1056/NEJMoa1606774
21. Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the Treatment of Non-Small-Cell Lung Cancer. N Engl J Med (2015) 372(21):2018–28. doi: 10.1056/NEJMoa1501824
22. Gettinger SN, Horn L, Gandhi L, Spigel DR, Antonia SJ, Rizvi NA, et al. Overall Survival and Long-Term Safety of Nivolumab (Anti-Programmed Death 1 Antibody, BMS-936558, ONO-4538) in Patients With Previously Treated Advanced Non-Small-Cell Lung Cancer. J Clin Oncol (2015) 33(18):2004–12. doi: 10.1200/JCO.2014.58.3708
23. Kong X, Lu P, Liu C, Guo Y, Yang Y, Peng Y, et al. A Combination of PD1/PDL1 Inhibitors: The Prospect of Overcoming the Weakness of Tumor Immunotherapy (Review). Mol Med Rep (2021) 23(5):362. doi: 10.3892/mmr.2021.12001
24. García-González J, Ruiz-Bañobre J, Afonso-Afonso FJ, Amenedo-Gancedo M, Areses-Manrique MDC, Campos-Balea B, et al. PD-(L)1 Inhibitors in Combination With Chemotherapy as First-Line Treatment for Non-Small-Cell Lung Cancer: A Pairwise Meta-Analysis. J Clin Med (2020) 9(7):2093. doi: 10.3390/jcm9072093
25. Sheng L, Gao J, Xu Q, Zhang X, Huang M, Dai X, et al. Selection of Optimal First-Line Immuno-Related Therapy Based on Specific Pathological Characteristics for Patients With Advanced Driver-Gene Wild-Type non-Small Cell Lung Cancer: A Systematic Review and Network Meta-Analysis. Ther Adv Med Oncol (2021) 13:17588359211018537. doi: 10.1177/17588359211018537
26. Jessurun CAC, Hulsbergen AFC, de Wit AE, Tewarie IA, Snijders TJ, Verhoeff JJC, et al. The Combined Use of Steroids and Immune Checkpoint Inhibitors in Brain Metastasis Patients: A Systematic Review and Meta-Analysis. Neuro Oncol (2021) 23(8):1261–72. doi: 10.1093/neuonc/noab046
27. Chou LQ, Barlesi F, Bertino EM, van denBent MJ, Wakelee H, Wen PY, et al. Results of the ASCEND-7 Phase II Study Evaluating ALK nhibitor (ALKi) Ceritinib in Patients (pts) with ALK+ Non-Small Cell Lung Cancer (NSCLC) Metastatic to the Brain. Ann Oncol (2019) 30(suppl_5):v602-60. doi: 10.1093/annonc/mdz260
28. Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, et al. Atezolizumab Versus Docetaxel in Patients With Previously Treated Non-Small-Cell Lung Cancer (OAK): A Phase 3, Open-Label, Multicentre Randomised Controlled Trial. Lancet (2017) 389(10066):255–65. doi: 10.1016/S0140-6736(16)32517-X
29. Shepard MJ, Xu Z, Donahue J, Eluvathingal Muttikkal TJ, Cordeiro D, Hansen L, et al. Stereotactic Radiosurgery With and Without Checkpoint Inhibition for Patients With Metastatic Non-Small Cell Lung Cancer to the Brain: A Matched Cohort Study. J Neurosurg (2019) 26:1–8. doi: 10.3171/2019.4.JNS19822
30. Teixeira Loiola de Alencar V, Guedes Camandaroba MP, Pirolli R, Fogassa CAZ, Cordeiro de Lima VC. Immunotherapy as Single Treatment for Patients With NSCLC With Brain Metastases: A Systematic Review and Meta-Analysis-the META-L-BRAIN Study. J Thorac Oncol (2021) 16(8):1379–91. doi: 10.1016/j.jtho.2021.04.014
31. Yang Y, Deng L, Yang Y, Zhang T, Wu Y, Wang L, et al. Efficacy and Safety of Combined Brain Radiotherapy and Immunotherapy in Non-Small-Cell Lung Cancer With Brain Metastases: A Systematic Review and Meta-Analysis. Clin Lung Cancer (2022) 23(2):95–107. doi: 10.1016/j.cllc.2021.06.009
32. Wells G, Shea B, O' Connell D, Peterson je, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analysis. Ottawa, Ontario: The Ottawa Health Research Institute (2011).
33. Santaguida PL, Ma CMR, Matchar DB. Chapter 8: Assessing Risk of Bias in a Randomized Trial. J Gen Internal Med (2012) 27(Suppl 1):S33–8. doi: 10.1007/s11606-012-2030-8
34. Kim R, Keam B, Hahn S, Ock CY, Kim M, Kim TM, et al. First-Line Pembrolizumab vs. Pembrolizumab Plus Chemotherapy vs. Chemotherapy Alone in Non-Small Cell Lung Cancer: A Systematic Review and Network Meta-Analysis. Clin Lung Cancer (2019) 20(5):331–8. doi: 10.1016/j.cllc.2019.05.009
35. Zhang B, Zhu W, Tao J, Li Y, Du CC, Chen YX, et al. Short-Term Efficacy of Different First-Line Chemotherapy Regimens for Advanced Non-Small Cell Lung Cancer: A Network Meta-Analysis. Clin Transl Sci (2020) 13(3):5897–98. doi: 10.1111/cts.12744
36. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New Response Evaluation Criteria in Solid Tumours: Revised RECIST Guideline (Version 1.1). Eur J Cancer (Oxford Engl 1990) (2009) 45(2):228–47. doi: 10.1016/j.ejca.2008.10.026
37. Schapira E, Hubbeling H, Yeap BY, Mehan Jr WA, Shaw AT, Oh K, et al. Improved Overall Survival and Locoregional Disease Control With Concurrent PD-1 Pathway Inhibitors and Stereotactic Radiosurgery for Lung Cancer Patients With Brain Metastases. Int J Radiat Oncol Biol Phys (2018) 101(3):624–9. doi: 10.1016/j.ijrobp.2018.02.175
38. Dudnik D, Yust-Katz S, Nechushtan H, Goldstein DA, Zer A, Flex D, et al. Intracranial Response to Nivolumab in NSCLC Patients With Untreated or Progressing CNS Metastases. Lung Cancer (2016) 98:114–7. doi: 10.1016/j.lungcan.2016.05.031
39. Watanabe H, Kubo T, Ninomiya T, Ohashi K, Ichihara E, Sato A, et al. The Effect of Nivolumab Treatment for Central Nervous System Metastases in Non-Small Cell Lung Cancer. J Clin Oncol (2017) 35(15_suppl):e20601–1. doi: 10.1200/JCO.2017.35.15_suppl.e20601
40. Geier M, Descourt R, Corre R, Léveiller G, Lamy R, Goarant E, et al. MA08.10 Real-Life Intracerebral Efficacy of Nivolumab in Non-Small Cell Lung Cancer Patients With Brain Metastases. J Thorac Oncol (2018) 13(10):S384–5. doi: 10.1016/j.jtho.2018.08.383
41. Kobayashi K, Nakachi I, Naoki K, Satomi R, Nakamura M, Inoue T, et al. Real-World Efficacy and Safety of Nivolumab for Advanced Non–Small-Cell Lung Cancer: A Retrospective Multicenter Analysis. Clin Lung Cancer (2018) 19(3):e349-58. doi: 10.1016/j.cllc.2018.01.001
42. Hendriks LEL, Henon C, Auclin E, Mezquita L, Ferrara R, Audigier-Valette C, et al. Outcome of Patients with Non-Small Cell Lung Cancer and Brain Metastases Treated with Checkpoint Inhibitors. J Thorac Oncol (2019) 14(7):1244–54. doi: 10.1016/j.jtho.2019.02.009
43. Zhang G, Cheng R, Wang H, Zhang Y, Yan X, Li P, et al. Comparable Outcomes of Nivolumab in Patients With Advanced NSCLC Presenting With or Without Brain Metastases: A Retrospective Cohort Study. Cancer Immunol Immunother (2020) 69(3):399–405. doi: 10.1007/s00262-019-02462-1
44. Bjørnhart B, Hansen KH, Jørgensen TL, Herrstedt J, Schytte T. Efficacy and Safety of Immune Checkpoint Inhibitors in a Danish Real Life Non-Small Cell Lung Cancer Population: A Retrospective Cohort Study. Acta Oncol (2019) 58(7):953–61. doi: 10.1080/0284186X.2019.1615636
45. Goldberg S, Schalper KA, Gettinger SN, Mahajan A, Herbst RS, Chiang AC, et al. Pembrolizumab for Management of Patients With NSCLC and Brain Metastases: Long-Term Results and Biomarker Analysis From a non-Randomised, Open-Label, Phase 2 Trial. Lancet Oncol (2020) 21(5):655–63. doi: 10.1016/S1470-2045(20)30111-X
46. ClinicalTrials.gov. Study of Nivolumab (BMS-936558) in Combination With Gemcitabine/Cisplatin, Pemetrexed/Cisplatin, Carboplatin/Paclitaxel, Bevacizumab Maintenance, Erlotinib, Ipilimumab or as Monotherapy in Subjects With Stage IIIB/IV Non-Small-Cell Lung Cancer (NSCLC) (Checkmate 012) (2020). Available at: https://clinicaltrials.gov/ct2/show/NCT01454102. (Accessed September 21, 2021).
47. Ashinuma H, Shingyoji M, Luchi T, Yoshida Y, Setoguchi T, Hasegawa Y, et al. P2.07-014 Immune Checkpoint Inhibitors for Brain Metastases of Non-Small-Cell Lung Cancer. J Thorac Oncol (2017) 12(11):S2420. doi: 10.1016/j.jtho.2017.11.073
48. Henon C, Mezquita L, Auclin E, Ammari S, Caramella C, Le Pechoux C, et al. P2.07-005 Impact of Baseline Leptomeningeal and Brain Metastases on Immunotherapy Outcomes in Advanced Non-Small Cell Lung Cancer (NSCLC) Patients. J Thorac Oncol (2017) 12(11):S2417. doi: 10.1016/j.jtho.2017.11.064
49. Molinier O, Audigier-Valette C, Cadranel J, Monnet I, Hureaux J, Hilgers W, et al. OA 17.05 IFCT-1502 CLINIVO: Real-Life Experience With Nivolumab in 600 Patients (Pts) With Advanced Non-Small Cell Lung Cancer (NSCLC). J Thorac Oncol (2017) 12(11):S1793. doi: 10.1016/j.jtho.2017.09.430
50. Dumenil C, Massiani MA, Dumoulin J, Giraud V, Labrune S, Chinet T, et al. Clinical Factors Associated With Early Progression and Grade 3–4 Toxicity in Patients With Advanced Non-Small-Cell Lung Cancers Treated With Nivolumab. PloS One (2018) 13(4):e0195945. doi: 10.1371/journal.pone.0195945
51. Wakuda K, Yabe M, Kodama H, Nishioka N, Miyawaki T, Miyawaki E, et al. Efficacy of Pembrolizumab in Patients With Brain Metastasis Caused by Previously Untreated Non-Small Cell Lung Cancer With High Tumor PD-L1 Expression. Lung Cancer (2020) 151(Suppl 4):60–8. doi: 10.1016/j.lungcan.2020.11.009
52. Crinò L, Bronte G, Bidoli P, Cravero P, Minenza E, Cortesi E, et al. Nivolumab and Brain Metastases in Patients With Advanced Non-Squamous Non-Small Cell Lung Cancer. Lung Cancer (2019) 129:35–40. doi: 10.1016/j.lungcan.2018.12.025
53. Cortinovis D, Chiari R, Catino A, Grossi F, Marinis DE F, Sperandi F, et al. Italian Cohort of the Nivolumab EAP in Squamous NSCLC: Efficacy and Safety in Patients With CNS Metastases. Anticancer Res (2019) 39(8):4265–71. doi: 10.21873/anticanres.13590
54. Powell SF, Abreu R, Langer CJ, Tafreshi A, Paz-Ares L, Kopp HG, et al. Pembrolizumab (Pembro) Plus Platinum-Based Chemotherapy (Chemo) in NSCLC With Brain Metastases: Pooled Analysis of KEYNOTE-021, 189, and 407. Ann Oncol (2019) 30(S5):606–7. doi: 10.1093/annonc/mdz260.005
55. Gadgeel SM, Lukas RV, Goldschmidt J, Conkling P, Park K, Cortinovis D, et al. Atezolizumab in Patients With Advanced non-Small Cell Lung Cancer and History of Asymptomatic, Treated Brain Metastases: Exploratory Analyses of the Phase III OAK Study. Lung Cancer (2018) 128:105–12. doi: 10.1016/j.lungcan.2018.12.017
56. Gauvain C, Vauléon E, Chouaid C, Le Rhun E, Jabot L, Scherpereel A, et al. Intracerebral Efficacy and Tolerance of Nivolumab in non–Small-Cell Lung Cancer Patients With Brain Metastases. Lung Cancer (2018) 116:62. doi: 10.1016/j.lungcan.2017.12.008
57. Sezer A, Kilickap S, Gümüş M, Bondarenko I, Özgüroǧlu M, Gogishvili M, et al. Cemiplimab Monotherapy for First-Line Treatment of Advanced non-Small-Cell Lung Cancer With PD-L1 of at Least 50%: A Multicentre, Open-Label, Global, Phase 3, Randomised, Controlled Trial. Lancet (2021) 397(10274):592–604. doi: 10.1016/S0140-6736(21)00228-2
58. Wu YL, Lu S, Cheng Y, Zhou C, Wang J, Mok T, et al. Nivolumab Versus Docetaxel in a Predominantly Chinese Patient Population With Previously Treated Advanced Non-Small Cell Lung Cancer: CheckMate 078 Randomized Phase III Clinical Trial. J Thorac Oncol (2019) 14(5):867–75. doi: 10.1016/j.jtho.2019.01.006
59. Goldman JW, Crino L, Vokes EE, Holgado E, Reckamp KL, Pluzanski A, et al. Nivolumab (Nivo) in Patients (Pts) With Advanced (Adv) NSCLC and Central Nervous System (CNS) Metastases (Mets). J Clin Oncol (2020) 34(S15):9038. doi: 10.1200/JCO.2016.34.15_suppl.9038
60. Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab Versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. New Engl J Med (2015) 373(17):16277–39. doi: 10.1056/NEJMoa1507643
61. Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Updated Analysis of KEYNOTE-024: Pembrolizumab Versus Platinum-Based Chemotherapy for Advanced Non–Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score of 50% or Greater. J Clin Oncol (2019) 37(7):537–46. doi: 10.1200/JCO.18.00149.
62. Mansfield AS, Herbst RS, Castro G, Hui R, Peled N, Kim DW, et al. Outcomes With Pembrolizumab (Pembro) Monotherapy in Patients (Pts) With PD-L1–Positive NSCLC With Brain Metastases: Pooled Analysis of KEYNOTE-001, -010, -024, and -042. Ann Oncol (2019) 30(Supplement_5):604–6. doi: 10.1093/annonc/mdz260.004
63. Afzal M, Dragnev K, Shirai K. A Tertiary Care Cancer Center Experience With Carboplatin and Pemetrexed in Combination With Pembrolizumab in Comparison With Carboplatin and Pemetrexed Alone in non-Squamous Non-Small Cell Lung Cancer. J Thorac Dis (2018) 10(6):3575–84. doi: 10.21037/jtd.2018.06.08
64. Zhou C, Wang Z, Sun Y, Cao L, Ma Z, Wu R, et al. GEMSTONE-302: A Phase III Study of Platinum-Based Chemotherapy (Chemo) With Placebo or CS1001, an Anti-PD-L1 Antibody, for First-Line (1L) Advanced Non-Small Cell Lung Cancer (NSCLC). Ann Oncol (2020) 31(suppl_6):S1386–406. doi: 10.1016/annonc/annonc367
65. Yy A, Wang Z, Fang J, Yu Q, Han B, Cang S, et al. Efficacy and Safety of Sintilimab Plus Pemetrexed and Platinum as First-Line Treatment for Locally Advanced or Metastatic Nonsquamous NSCLC: A Randomized, Double-Blind, Phase 3 Study (Oncology Program by InnovENT Anti-PD-1-11). J Thorac Oncol (2020) 15(10):1636–46. doi: 10.1016/j.jtho.2020.09.028
66. Singh C, Qian JM, Yu JB, Chiang VL. Local Tumor Response and Survival Outcomes After Combined Stereotactic Radiosurgery and Immunotherapy in Non-Small Cell Lung Cancer With Brain Metastases. J Neurosurg (2019) 132(2):512–7. doi: 10.3171/2018.10.JNS181371
67. Hellmann M, Paz-Ares L, Bernabe Caro R, Zurawski B, Kim SW, Carcereny Costa E. Nivolumab Plus Ipilimumab in Advanced Non–Small-Cell Lung Cancer. New Engl J Med (2019) 381(21):2020–31. doi: 10.1056/NEJMoa1910231
68. Hellmann MD, Paz-Ares L, Bernabe Caro R, Zurawski B, Kim SW, Carcereny Costa E, et al. Nivolumab plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer. N Engl J Med (2019) 381(21): 2020–31. doi: 10.1056/NEJMoa1910231
69. Enright T, Witt JS, Burr AR, Yadav P, Leal T, Baschnagel AM. Combined Immunotherapy and Stereotactic Radiotherapy Improves Neurologic Outcomes in Patients With Non–small-Cell Lung Cancer Brain Metastases. Clin Lung Cancer (2021) 22(2):110–19. doi: 10.1016/j.cllc.2020.10.014
70. Imber BS, Hellmann MD, Kris MG, Santomasso BD, Callahan MK, Osorio JC, et al. Lesion Response and Intracranial Control of Brain Metastases From Non–small Cell Lung Cancer After Stereotactic Radiosurgery or Hypofractionated Radiation Therapy Combined With Checkpoint Inhibitors. Int J Radiat Oncol Biol Physics (2017) 99(2):E465–6. doi: 10.1016/j.ijrobp.2017.06.1715
71. Srivastava A, Abraham C, Filiput E, Huang J. OC-0495: Early PD-1 Blockade Improves Disease Control for NSCLC Brain Metastases Treated With Radiosurgery. Radiother Oncol (2018) 127:S254–5. doi: 10.1016/S0167-8140(18)30805-3
72. Ahmed KA, Kim S, Arrington J, Naghavi AO, Dilling TJ, Creelan BC, et al. Outcomes Targeting the PD-1/PD-L1 Axis in Conjunction With Stereotactic Radiation for Patients With Non-Small Cell Lung Cancer Brain Metastases. J Neuro-Oncol (2017) 133(2):331–8. doi: 10.1007/s11060-017-2437-5
73. Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab Plus Chemotherapy in Metastatic Non–Small-Cell Lung Cancer. New Engl J Med (2018) 378(22):2078–92. doi: 10.1056/NEJMoa1801005
74. Fehrenbacher L, von Pawel J, Park K, Rittmeyer A, Gandara DR, Ponce Aix S, et al. Updated Efficacy Analysis Including Secondary Population Results for OAK: A Randomized Phase III Study of Atezolizumab vs Docetaxel in Patients With Previously Treated Advanced Non-Small Cell Lung Cancer. J Thorac Oncol (2018) 13(8):1156. doi: 10.1016/j.jtho.2018.04.039
75. Nadal E, Rodriguez-Abreu D, Massuti B. Single Arm Phase II Study of Atezolizumab Plus Chemotherapy in Stage IV NSCLC With Untreated Brain Metastases. J Thorac Oncol (2021) 16(10):S863. doi: 10.1016/j.jtho.2021.08.062
76. Carbone D, Ciuleanu T, Cobo M, Schenker M, Zurawski B. David Paul Carbone, T.-E.C., Manuel Cobo, Michael Schenker, Bogdan Zurawski, First-line Nivolumab + Ipilimumab + Chemo in Patients With Advanced NSCLC and Brain Metastases: Results From CheckMate 9LA. Presented at: International Association for the Study of Lung Cancer 2021 World Conference on Lung Cancer. 2022. Virtual. Abstr OA09.01
77. Han X, Guo J, Tang X, Zhu H, Zhu D, Zhang X, et al. Efficacy and Safety of Sintilimab Pus Docetaxel in Patients With Previously Treated Advanced Non-Small Cell Lung Cancer: A Prospective, Single-Arm, Phase II Study in China. J Cancer Res Clin Oncol (2022) 28:1–9. doi: 10.1007/s00432-022-04023-z
78. Leighl NB, Laurie SA, Goss GD, Hughes BGM, Stockler MR, Tsao MS, et al. CCTG BR.34: A Randomized Trial of Durvalumab and Tremelimumab +/- Platinum-Based Chemotherapy in Patients With Metastatic (Stage IV) Squamous or Nonsquamous Non-Small Cell Lung Cancer (NSCLC). J Clin Oncol (2020) 38(S15):9502. doi: 10.1200/JCO.2020.38.15_suppl.9502
79. Garassino MC, Gadgeel S, Esteban E, Felip E, Speranza G, De Angelis F, et al. Abstract CT043: Outcomes Among Patients (Pts) With Metastatic Nonsquamous NSCLC With Liver Metastases or Brain Metastases Treated With Pembrolizumab (Pembro) Plus Pemetrexed-Platinum: Results From the KEYNOTE-189 Study. Cancer Res (2019) 79(13 Supplement):CT043–3. doi: 10.1158/1538-7445.AM2019-CT043
80. Amaral T, Kiecker F, Schaefer S, Stege H, Kaehler K, Terheyden P, et al. Combined Immunotherapy With Nivolumab and Ipilimumab With and Without Local Therapy in Patients With Melanoma Brain Metastasis: A DeCOG* Study in 380 Patients. J Immunother Cancer (2020) 8(1):e000333. doi: 10.1136/jitc-2019-000333
81. Xiao G, Liu Z, Gao X, Wang H, Peng H, Li J, et al. Immune Checkpoint Inhibitors for Brain Metastases in non-Small-Cell Lung Cancer: From Rationale to Clinical Application. Immunotherapy (2021) 13(12):1031–51. doi: 10.2217/imt-2020-0262
82. Herter-Sprie GS, Koyama S, Korideck H, Hai J, Deng J, Li YY, et al. Synergy of Radiotherapy and PD-1 Blockade in Kras-Mutant Lung Cancer. JCI Insight (2016) 1(9):e87415. doi: 10.1172/jci.insight.87415
83. Ngwa W, Irabor OC, Schoenfeld JD, Hesser J, Demaria S, Formenti SC. Using Immunotherapy to Boost the Abscopal Effect. Nat Rev Cancer (2018) 18(5):313–22. doi: 10.1038/nrc.2018.6
84. Sharabi AB, Lim M, DeWeese TL, Drake CG. Radiation and Checkpoint Blockade Immunotherapy: Radiosensitisation and Potential Mechanisms of Synergy. Lancet Oncol (2015) 16(13):e498–509. doi: 10.1016/S1470-2045(15)00007-8
85. Demaria S, Golden EB, Formenti SC. Role of Local Radiation Therapy in Cancer Immunotherapy. JAMA Oncol (2015) 1(9):1325–32. doi: 10.1001/jamaoncol.2015.2756
86. Li HB, Yang ZH, Guo QQ. Immune Checkpoint Inhibition for Pancreatic Ductal Adenocarcinoma: Limitations and Prospects: A Systematic Review. Cell Commun Signal: CCS (2021) 19(1):117. doi: 10.1186/s12964-021-00789-w
87. Mansfield AS, Aubry MC, Moser JC, Harrington SM, Dronca RS, Park SS, et al. Temporal and Spatial Discordance of Programmed Cell Death-Ligand 1 Expression and Lymphocyte Tumor Infiltration Between Paired Primary Lesions and Brain Metastases in Lung Cancer. Ann Oncol (2016) 27:1953–8. doi: 10.1093/annonc/mdw289
88. Kudo Y, Haymaker C, Zhang J, Reuben A, Duose DY, Fujimoto J, et al. Suppressed Immune Microenvironment and Repertoire in Brain Metastases From Patients With Resected non Small-Cell Lung Cancer. Ann Oncol (2019) 30:1521–30. doi: 10.1093/annonc/mdz207
89. Chen L, et al. Concurrent Immune Checkpoint Inhibitors and Stereotactic Radiosurgery for Brain Metastases in Non-Small Cell Lung Cancer, Melanoma, and Renal Cell Carcinoma. Int J Radiat Oncol Biol Phys (2018) 100(4):916–25. doi: 10.1016/j.ijrobp.2017.11.041
90. Yamada Y, Chang E, Fiveash JB, Knisely J. Radiotherapy in Managing Brain Metastases. Cham: Springer Cham (2020).
91. Cao Y, Tsien CI, Shen Z, Tatro DS, Ten Haken R, Kessler ML, et al. Use of Magnetic Resonance Imaging to Assess Blood-Brain/Blood-Glioma Barrier Opening During Conformal Radiotherapy. J Clin Oncol (2015) 23(18):4127–36. doi: 10.1200/JCO.2005.07.144
92. Zindler JD, Bruynzeel A, Eekers D, Hurkmans CW, Swinnen A, Lambin P. Whole Brain Radiotherapy Versus Stereotactic Radiosurgery for 4-10 Brain Metastases: A Phase III Randomised Multicentre Trial. BMC Cancer (2017) 17(1):500. doi: 10.1186/s12885-017-3494-z
93. Yue J, Yu J. SRS Versus WBRT for Resected Brain Metastases. Lancet Oncol (2017) 18(10):e559. doi: 10.1016/S1470-2045(17)30642-3
94. Dong Y, Wong J, Sugimura R, Lam KO, Li B, Kwok G, et al. Recent Advances and Future Prospects in Immune Checkpoint (ICI)-Based Combination Therapy for Advanced HCC. Cancers (2021) 13(8):1949. doi: 10.3390/cancers13081949
Keywords: non-small cell lung cancer, brain metastases, immunotherapy, combined immunotherapy, radiotherapy
Citation: Chu X, Niu L, Xiao G, Peng H, Deng F, Liu Z, Wu H, Yang L, Tan Z, Li Z and Zhou R (2022) The Long-Term and Short-Term Efficacy of Immunotherapy in Non-Small Cell Lung Cancer Patients With Brain Metastases: A Systematic Review and Meta-Analysis. Front. Immunol. 13:875488. doi: 10.3389/fimmu.2022.875488
Received: 14 February 2022; Accepted: 20 April 2022;
Published: 25 May 2022.
Edited by:
Dawei Chen, Shandong Cancer Hospital, ChinaReviewed by:
Zhenzhou Yang, Chongqing Medical University, ChinaCopyright © 2022 Chu, Niu, Xiao, Peng, Deng, Liu, Wu, Yang, Tan, Li and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rongrong Zhou, emhvdXJyQGNzdS5lZHUuY24=; Zhanzhan Li, bGl6aGFuemhhbkBjc3UuZWR1LmNu
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.