- 1International School of Medicine, Istanbul Medipol University, Istanbul, Turkey
- 2Zabludowicz Center for autoimmune diseases, Sheba Medical Center, Ramat-Gan, Israel
- 3St. George School of Medicine, University of London, London, United Kingdom
- 4Laboratory for Industrial and Applied Mathematics (LIAM), Department of Mathematics and Statistics, York University, Toronto, ON, Canada
Despite their proven efficacy and huge contribution to the health of humankind, vaccines continue to be a source of concern for some individuals around the world. Vaccinations against COVID-19 increased the number of distressed people and intensified their distrust, particularly as the pandemic was still emerging and the populations were encouraged to be vaccinated under various slogans like “back to normal life” and “stop coronavirus”, goals which are still to be achieved. As fear of vaccination-related adverse events following immunization (AEFIs) is the main reason for vaccine hesitancy, we reviewed immune and autoimmune AEFIs in particular, though very rare, as the most worrisome aspect of the vaccines. Among others, autoimmune AEFIs of the most commonly administered COVID-19 vaccines include neurological ones such as Guillain-Barre syndrome, transverse myelitis, and Bell’s palsy, as well as myocarditis. In addition, the newly introduced notion related to COVID-19 vaccines, “vaccine-induced immune thrombotic thrombocytopenia/vaccine-induced prothrombotic immune thrombotic thrombocytopenia” (VITT/VIPITT)”, is of importance as well. Overviewing recent medical literature while focusing on the major immune and autoimmune AEFIs, demonstrating their rate of occurrence, presenting the cases reported, and their link to the specific type of COVID-19 vaccines represented the main aim of our work. In this narrative review, we illustrate the different vaccine types in current use, their associated immune and autoimmune AEFIs, with a focus on the 3 main COVID-19 vaccines (BNT162b2, mRNA-1273, and ChAdOx1). While the rate of AEFIs is extremely low, addressing the issue in this manner, in our opinion, is the best strategy for coping with vaccine hesitancy.
Introduction
Considered one of the fastest processes of manufacturing vaccines ever, “Coronavirus Disease 2019” (COVID-19) vaccines were discovered, studied, and produced in terms of months since the declaration of COVID-19 as a pandemic by the World Health Organization (WHO) (1). While the burden of the outbreak alongside the support of the governments were the main motivations for the accelerated vaccine production, the process itself aroused concerns regarding the safety of the COVID-19 vaccines and contributed to mistrust in health authorities as well as in the vaccines themselves (2, 3). Interestingly, distrust in COVID-19 vaccines was reported even before the vaccines were available (4). It is of great importance to mention that vaccines are highly effective in reducing the burden of infectious diseases as the history clearly shows (5, 6), and the COVID-19 pandemic is likely to follow a similar trend after the roll-out of the COVID-19 vaccination has been fully implemented, globally. However, the fear of the lay public for adverse events following immunization (AEFIs), especially those of severe and long-term features, may constitute the main obstacle in successfully fighting against COVID-19 vaccine hesitancy (7, 8). This is especially true for autoimmune and immune-mediated AEFIs such as idiopathic thrombocytopenia (ITP), or immune thrombocytopenia, Guillain-Barré syndrome (GBS), and transverse myelitis (TM), among others. Therefore, it is crucial to address the mounting concern by reviewing the past, present, and possible future AEFIs of vaccines in general and COVID-19 vaccines in particular, especially as millions were already vaccinated around the world, and more data has accumulated regarding the safety profile of the COVID-19 vaccines (9). Addressing AEFIs of autoimmune nature covers, to a great extent, the concerns generated by the public explicitly considering vaccine booster programs endorsed by the health authorities. The importance of facing and addressing public concerns in dealing with vaccine hesitancy cannot be overemphasized (10).
We hereby present a detailed, narrative literature review covering the various types of vaccines, their immune-mediated AEFIs, and we focus on the autoimmune AEFIs described in correlation with the main COVID-19 vaccines in use worldwide, that is to say, those most administered (approximately 95% of those delivered). The rarity of the AEFIs among vaccinated people, and the risk of severe complications that COVID-19 carries, are the key factor for fighting against vaccine hesitancy.
Types of Vaccines, Adverse Events Following Immunization, and COVID-19 Related Vaccines
Vaccines that were approved or in use against COVID-19 can be classified as follows:
Inactivated Vaccines
Inactivated or killed vaccines contain a pathogen that has been inactivated after being grown in a culture. The first utilized was the cholera vaccine introduced in 1896 (11). There are two subtypes of inactivated vaccines: the traditional whole-cell vaccine and the acellular vaccine. The latter is a more modern version and contains only 3-4 antigens rather than the hundreds of microbial antigens present in the whole-cell vaccine. This antigen reduction produces a more specific immune response, thereby reducing potential AEFIs (12), such as febrile responses (13). While traditional whole virion-inactivated influenza vaccines and whole cell pertussis vaccines commonly cause febrile responses within 24 hours of immunization, the split virion influenza vaccine and the acellular pertussis vaccine showed a decreased rate of febrile illness (14, 15). Concerning inactivated vaccines against COVID-19, the vaccine developed by the Beijing Institute of Biological Products, known as BBIBP-CorV, and referred to as Sinopharm, is an inactivated vaccine. In a randomized, double-blind, dose-escalation, controlled phases I and II trial of the BBIBP-CorV vaccine, an acceptable safety profile and a robust humoral response to the coronavirus were reported (16). The study was conducted among 18-80 years old, healthy individuals negative for serum-specific IgM or IgG antibodies against both N and S proteins of the virus. The inactivated BBIBP-CorV vaccine was immunogenic and elicited strong humoral responses, with a 100% seroconversion rate in all groups. However, lower seroconversion rates were found in the group of individuals aged 60 and older, probably due to the atrophy/ageing of the immune system (17). The degree of local and systemic AEFIs was generally mild and occurred most after the first dose of vaccination (18).
Viral Vector-Based Vaccines
Viral vectors were first introduced in relation to gene and cancer therapy but, since then, have been adapted to vaccine development (19). The utility of vector-based vaccines is determined by the capacity of viruses to infect cells (20). High efficiency gene transduction, extremely selective gene delivery to target cells, generation of powerful immunological responses, and improved cellular immunity are the advantages of viral vector-based vaccines (20). Multiple viruses ranging from very complex large DNA viruses such as poxviruses, to simple RNA viruses such as parainfluenza virus, have been deployed as viral vectors (21). Viral vector vaccines facilitate intracellular antigen expression and trigger a highly cytotoxic T-lymphocyte response (22). A significant emphasis was made on COVID-19 vaccines based on viral vectors like the AstraZeneca, Janssen/Johnson & Johnson, and CanSino vaccines (19), which are based on adenoviruses (23). In addition to the vaccines listed above, Sputnik V, also known as Gam-COVID-Vac, is a COVID-19 adenovirus viral vector vaccine (24). Injection site pain is considered the most common local AEFI of viral vector vaccines, together with headache, fatigue, muscle pain, malaise, chills, and joint pain (25).
mRNA Vaccines
Because of their high potency, ability to evolve quickly, and potential for low-cost manufacturing and safe delivery, mRNA vaccines were found to be a promising alternative to traditional vaccine techniques. In 1990, the first effective application of in vitro transcribed (IVT) mRNA in animals was described, when reporter gene mRNAs were injected into mice and protein synthesis was detected (26). However, this finding did not lead to an increase in the mRNA vaccine investment, due to concerns regarding mRNA instability, inefficient in vivo delivery, and high innate immunogenicity. Subsequently, over the past decade, the use of mRNA vaccines has been gradually implemented due to their low potential risk of infection and insertional mutagenesis (27). Actually, the concept behind the mRNA vaccine is to deliver the mRNA of the pathogen into the human body in order to trigger an immune response and produce antibodies against the pathogen. There are two types of mRNA vaccines available for prevention of infectious diseases: self-amplifying or replicon RNA vaccines (SAM) and non-replicating mRNA vaccines. SAM vaccines have the benefit of producing their own adjuvants in the form of dsRNA structures, replication intermediates, and other motifs, which may explain their high potency. Directly injectable, non-replicating mRNA vaccines are promising vaccination products due to their convenient way of administration and low cost, especially in resource-constrained environments (28). Two of the most widely distributed COVID-19 vaccines, namely the BNT162b2 (BioNTech/Pfizer) and the mRNA-1273 (Moderna), are mRNA-based vaccines. The BNT162b2 vaccine, for instance, proved to be around 95% efficient in preventing the disease, with a relatively high safety profile (29). However, mild and short-term AEFIs were reported such as pain at the injection site, fatigue, and headache. The incidence of serious AEFIs was low.
Roll-out of COVID-19 Vaccines
As herd immunity is achieved once a large portion of the population is immune against an infectious agent preventing it from widely spreading, it is a must in overcoming the COVID-19 pandemic (30). In addition, herd immunity is also necessary to protect individuals who are unable to get vaccinated such as infants, children under the recommended age, and immunocompromised individuals (31). In turn, people become immune against a specific infection in two ways, either through acquiring the infection naturally from a pathogen, or passively by vaccination. While COVID-19 can be severe and fatal, especially in people at risk such as the elderly, those with chronic cardiovascular and respiratory disease, among others (32), vaccinations is paramount in terms of preventing disease acquisition as well as its spread. The US Food and Drug Administration (FDA) authorized the first vaccine for COVID-19 on December 11th, 2020 (33). More specifically, the first vaccine authorized was BNT162b2 (BioNTech/Pfizer COVID-19 vaccine) under the Emergency Use Authorizations (EUA). The United Kingdom (UK) Medicines and Healthcare products Regulatory Agency (MHRA) had already authorized the use of the same vaccine, some days before, on December 2nd, 2020 (34). Subsequently, the European Union (EU) commission authorized the vaccine on December 21st, 2020 (35). Shortly afterward, the mRNA-1273 COVID-19 vaccine (Moderna) was authorized (36). Later on, the ChAdOx1 vaccine (AstraZeneca) was authorized as well by the UK MHRA (37). The three mentioned vaccines were the most commonly used vaccines across the globe. By the end of November, 2021, approximately 55% of the world population has received at least one dose of a COVID-19 vaccine, 7.9 billion doses have been given globally, and around 27 million new doses are administered daily (38). Asia had the most doses administered at 5.09 billion doses, followed by Europe with 942 million doses, North America with 739.54 million doses, South America with 581.31 million doses, Africa with 235.45 million doses, and finally Oceania with 49.67 million doses.
Autoimmune Adverse Events Following Immunization and Their Correlation With Vaccines in General and COVID-19 Vaccines in Particular
Autoimmune Neurological Adverse Events Following Immunization
Guillain-Barre’ Syndrome (GBS)
GBS is an autoimmune disorder characterized by an immune-mediated nerve damage generally triggered by an infectious agent leading to cross-reactive antibodies attacking axonal antigens and resulting in demyelinating polyneuropathy (39). Despite its rarity, GBS is considered as the most common cause of acute flaccid paralysis worldwide (40), with about 100,000 people developing the disorder every year (41). The disease is manifested by ascending and symmetrical flaccid paralysis leading to paresthesia, autonomic dysfunction, and respiratory muscle paralysis (42). In some instances, cranial nerve involvement is also seen, causing facial diplegia (43). The complications of GBS can be fatal, including respiratory and cardiac failure (44). The mortality rate of GBS varies widely from 1-18% (45, 46). Numerous reports have suggested a possible relationship between GBS and vaccines. However, solid evidence was not established (47, 48). During the COVID-19 pandemic, various reports addressed the correlation between COVID-19 and GBS; nevertheless, a concrete causal relationship is yet to be established (40).
BNT162b2 (BioNTech/Pfizer) and GBS
Several case reports were published regarding GBS following the BNT162b2 vaccine. A 82-years-old female presented two weeks following her first dose (49), and a 67-year-old male seven days after the first shot (50). In addition, a 71-year-old male with Miller-Fisher syndrome (MFS), a rare variant of GBS, presented 18 days following his first dose of BNT162b2 vaccine (22). GBS was also documented following the second dose of the vaccine. For instance, a 25-year-old female developed a clinical picture of GBS few days following her second dose of the vaccine, and a 73-year-old male twenty days following the second dose of the vaccine (51). Furthermore, Ben David and colleagues (52) conducted a retrospective cohort study aimed to assess the safety of mRNA-based COVID-19 vaccine in previously diagnosed cases of GBS between 2000-2020. Based on a database from a health organization serving more than 2.5 million members, the authors found that out of 702 members who had a diagnosis of GBS, 579 received at least one vaccine dose, and only one patient presented with a relapse of GBS several days following the second dose, which represents a minimal risk. In addition, Shasha et al. (53) investigated AEFIs profile following BNT162b2 vaccine in a sample size of over 400,000 individuals, and found that only one individual in the vaccinated group had GBS versus none in the control group. Similarly, seven cases of GBS were reported following the first dose of BNT162b2 mRNA vaccine among approximately 4 million recipients (incidence of 0.18/100,000), while no cases recorded following the second dose. The study was conducted over a period of 30 days following vaccination concluding that among recipients of the BNT162b2 mRNA vaccine, GBS may occur at the expected community-based rate (54).
mRNA-1273 (Moderna) and GBS
To the best of our knowledge, only two case reports have been published regarding GBS following Moderna, mRNA-1273 COVID-19 vaccine. Both of the cases developed after the second dose. In the first case (55), the symptoms appeared two days after vaccination presented by an axonal variant of GBS. In turn, in the second case (56), the symptoms appeared 6 weeks following the vaccination, while the electrophysiological test identified a mixed axonal and demyelinating type of GBS. Furthermore, among 16 cases of acute-onset polyradiculoneuropathy that presented within 4 weeks after first dose of SARS-CoV2 vaccines, only one person received the mRNA-1273 vaccine, whereas 14 received the ChAdOx1 vaccine (57).
ChAdOx1 (AstraZeneca) and GBS
During July 2021, the European Medicines agency (EMA) recommended that GBS should be added as a warning sign on the ChAdOx1 vaccine product information, despite being unable to decisively conclude about a causal association (58). This action was in part due to multiple reports of GBS cases occurring within a month following the first dose of the vaccine (59–67). The series of cases caused a major concern among leading experts. In fact, the GBS cases reported after the ChAdOx1 vaccine often presented as facial diplegia and paresthesia, a rather rare manifestation of the condition, with clinical improvement after corticosteroid and intravenous immune globulins (IVIG) therapy. The cases had no known exposure to the coronavirus and tested negative upon admission. However, as a recent study found that the COVID-19 infection was unlikely to cause GBS (68), the significance is debatable. Other baseline characteristics, such as age, gender and associated morbidity differed between cases, and scholars are therefore unable to make a clear-cut connection between them. The incidence of GBS was estimated to be approximately 0.89 to 1.89 cases per 100,000 person-years (69). A recent report issued by the UK Health Security Agency concluded that the risk of developing GBS after the first dose of ChAdOx1vaccine adds 5.6 extra cases of GBS per million doses, while having no association with respect to the second dose (70). As the condition has also been linked to the Ad26.COV2.S (Janssen/Johnson & Johnson) COVID-19 vaccine (71), another adenoviral vector vaccination, further investigations into the pathogenesis are required as large studies about GBS rate are lacking, and conclusions should not be drawn without further analysis.
Transverse Myelitis
TM is an immune-mediated acute or a subacute inflammatory disease of the spinal cord accompanied with motor, sensory, and autonomic symptoms (72). The clinical presentation of TM varies depending on the level of the spinal cord involved as the disease is manifested below the affected segment. Patients presenting with TM can be paraplegic, most having urinary bladder function disorders and paresthesia (73). The exact etiology of TM has not been established yet. Nevertheless, different types of vaccines were formerly linked to the appearance of TM including hepatitis B vaccine, MMRV and others (74). Most of the cases documented occurred between several days to several months from vaccination however, longer durations were also recorded.
BNT162b2 (BioNTech/Pfizer) and Transverse Myelitis
Actually, little evidence with no obvious association exists regarding the appearance of TM after the administration of BNT162b2 COVID-19 vaccine. Case reports include a 75-year-old Japanese patient who presented with TM 3 days following the first dose of the BNT162b2 vaccine (75). The authors could not elucidate a clear association between the vaccine and the clinical presentation and concluded that more epidemiological studies are needed. Furthermore, among more than 700,000 individuals who received the first dose of the BNT162b2 vaccine in Mexico, only 2 cases of TM were documented, equal to a rate of 0.28 per 100,000 cases (76).
mRNA-1273 (Moderna) and Transverse Myelitis
Concerning the mRNA-1273 COVID-19 vaccine, case reports of ADEM (77), neuromyelitis optica (78), and acute TM (79) were reported. Ismail et al. (80) reviewed central nervous system (CNS) demyelination disorders among recipients of various COVID-19 vaccinations. A total of 32 cases were registered. Among the cases, higher rates of women (68.8%) than men and a median age of 44 years were noticed. Moreover, most of the cases (71.8%) occurred following the first dose whereas more than a half of the cases had a previous history of immune-mediated diseases (53.1%). As for TM in particular, 6 cases occurred after receiving the mRNA-1273 vaccine. According to the same study, the other vaccines possibly correlating with TM were as follows: 11 cases following BNT162b2 vaccine, 8 after ChAdOx1 vaccine, 5 after Sinovac/Sinopharm vaccines, and one after each of the Sputnik and the Janssen/Johnson & Johnson vaccines.
ChAdOx1 (AstraZeneca) and Transverse Myelitis
During the phase 3 clinical trial of the ChAdOx1 vaccine, 3 participants were diagnosed with TM (81). As a result, the trial was temporarily paused enabling further investigations. Two of the cases were determined to be unrelated to the vaccine, the first one had pre-existing, undiagnosed multiple sclerosis; and the other was in fact in the control group with his symptoms appearing over 68 days post vaccination (82). As experts concluded the phenomenon was unlikely to be related to the vaccination the trial resumed however, the incidents raised global concerns regarding the safety profile of the ChAdOx1 vaccine. The longitudinally extensive TM (LETM) is a rare subtype of TM, in which the damage extends over 3 or more vertebrae (83). Reports regarding the LETM subtype started to emerge following the worldwide distribution of the COVID-19 vaccines (84–88). All cases occurred in patients under 60 years of age who presented with neurological symptoms starting within 3 weeks of receiving the first dose of the ChAdOx1 vaccine. As the COVID-19 infection was previously implicated in inducing acute TM (89), all reported cases tested negative on standard PCR testing. Furthermore, all cases completely recovered after treatment with either high dose corticosteroids or plasma exchange and were subsequently discharged.
Bell’s Palsy
Bell’s palsy, or facial nerve palsy, is the partial (paresis) or total (paralysis) loss of function of the facial nerve (90). The etiology of Bell’s palsy is most commonly idiopathic but may occur due to several factors such as viral infections (herpes viruses), ischemia, inflammatory and immune-mediated diseases (91). Bell’s palsy is divided into central facial palsy and peripheral facial palsy (92). Central palsy is characterized by contralateral sensory disturbances, as well as dry mouth. In turn, peripheral palsy is characterized by ipsilateral paralysis of the eyelid and forehead muscles.
In a case-control study from Switzerland which aimed to assess the correlation between the inactivated intranasal influenza vaccines and Bell’s palsy (93), a significantly increased risk of Bell’s palsy was found among vaccinated people. The risk of developing Bell’s palsy was 19 times higher than the control group. As a result, the vaccine was withdrawn from clinical use.
BNT162b2 (BioNTech/Pfizer) and Bell’s Palsy
The safety database of the BNT162b2 vaccine revealed a slight increase in the cases of Bell’s palsy in vaccinated individuals (94). There were 4 cases of Bell’s palsy in the vaccine group compared to none in the placebo group. As the rate was as expected in the general population, no causal relationship was established. Reviewing the reported safety data concluded that mRNA-based vaccines might be associated with Bell’s palsy (95). Accordingly, several case reports highlighted a similar association, including a healthy 37-year-old male who had Bell’s palsy several days following the first BNT162b2 vaccine dose (96). In addition, different population-based studies which investigated the adverse effects of the BNT162b2 vaccine reported an increase in the frequency of Bell’s palsy as well. For instance, in a nationwide study from Israel which analyzed more than 800 thousand people, the incidence of Bell’s palsy was higher in the vaccinated group compared to the control group, but without significant results (97). Similarly, Shibli and colleagues (98) researched a database of Bell’s palsy cases occurring within 21-days after the first dose and 30-days after the second dose of the vaccine in comparison to the expected cases based on a database from 2019. The authors found a slightly increased incidence of Bell’s palsy following the first dose, mainly among females aged 65 and older, with an estimated attributable risk of 4.46 per 100,000 vaccinated individuals. The study suggested an association between the vaccine and an increased risk of Bell’s palsy but with a small impact on public health. In contrast, Shasha et al. (53) reported no association between COVID-19 vaccines, including BNT162b2, and Bell’s palsy among more than 400 thousand vaccinated people compared to the control group.
mRNA-1273 (Moderna) and Bell’s Palsy
Three cases of Bell’s palsy were reported following vaccination with mRNA-1273 vaccine (99–101). The symptoms appeared 12 hours to 2 days after the vaccine was administered. Two cases occurred after the first dose, while one appeared following the second dose. In one patient, a prior episode of Bell’s palsy was recorded whereas the other 2 persons were healthy young people aged 35-36-year-old. Moreover, Sato et al. (102) showed that the rate of Bell’s palsy after both types of the mRNA COVID-19 vaccines (BNT162b2 and mRNA-1273) are lower or equivalent to the rate of Bell’s palsy after influenza vaccines. Additionally, a study from Singapore including 1.4 million subjects who received COVID-19 vaccination, 86.7% by BNT162b2 vaccine and 13.3% by mRNA-1273 vaccine, 11 patients were referred to hospital with Bell’s palsy and 27 patients had cranial mononeuropathy (103). According to data from the WHO pharmacovigilance database, no close association between BNT162b2 and mRNA-1273 COVID-19 vaccines and facial paralysis could be found (104).
ChAdOx1 (AstraZeneca) and Bell’s Palsy
While Bell’s palsy is a lower motor neuron disease manifesting as a unilateral facial paralysis (105), GBS may also present with facial paralysis, and most cases linked to the ChAdOx1 vaccine presented in this form (59–67). Therefore, it is difficult to distinguish between the two conditions. One study attempted to examine the risk of co-occurrence of Bell’s palsy and GBS following COVID-19 vaccinations and found an increased risk of co-occurrence following the ChAdOx1 vaccination (106). However, as previously mentioned, the study could not distinguish between the two conditions. Furthermore, a review of published literature failed to identify case reports of isolated Bell’s palsy linked to the ChAdOx1 vaccination. Therefore, it is impossible to draw conclusions of a possible link.
Encephalitis
Postvaccinal encephalitis was described in regard to COVID-19 vaccines. In a case series of three patients who presented with symptoms suspected of encephalitis in a range of 7-11 days of receiving the ChAdOx1 vaccine was previously documented (107). The symptoms included gait disturbance, aphasia, headaches, and seizures. As the criteria for autoimmune encephalitis was fulfilled in the three cases, treatment with systemic corticosteroids led to clinical improvement. Other causes of encephalitis including infectious agents were ruled out. All cases were mild and resolved without sequelae. Due to its rarity, the authors highlighted that the benefits of the vaccine outweigh the risks. It is noteworthy to mention hereby, that herpes simplex encephalitis was reported following ChAdOx1 vaccine (108) however, this can be regarded as an infection-related AEFI rather than autoimmune induced.
In addition, a case report of a Japanese lady admitted who developed diplopia the next day following the first dose of the BNT162b2 vaccine administration was described in the literature (109). The symptoms aggravated after the second dose of the vaccine and the patients was finally diagnosed with encephalitis based on brain MRI findings. The patient responded well and totally recovered after treatment with steroids was initiated. The authors could not prove any causal relationship in their case.
In regard to the mRNA-1273 COVID vaccine, a case report of a patient diagnosed with acute encephalitis, myoclonus and Sweet syndrome was described after receiving the first dose of the mRNA-1273 vaccine (110). The symptoms resolved following glucocorticoids treatment.
Myocarditis
Myocarditis is defined as the presence of inflammatory cellular infiltrate in the myocardium alongside tissue necrosis, which is not caused by coronary heart disease, and diagnosed by a combination of histological, immunological and immunohistochemical criteria (111). Based on etiologic factors, myocarditis can be divided into 2 subgroups: infectious, due to bacterial, viral, fungal, or parasitic infections; or non-infectious causes like autoimmunity, drugs, or vaccines (112). However, viral infections are seemingly the most common cause of myocarditis (113). Myocarditis can present with a range of symptoms from non-specific complaints such as fever and mild dyspnea, to fulminant hemodynamic imbalances and sudden death (114). In fact, myocarditis is considered as a common cause of sudden cardiac death (115). In a review article which included 1230 patients who initially had unexplained cardiomyopathy, 9% of the patients were eventually diagnosed with myocarditis (116). A link between vaccines and myocarditis was repeatedly reported in medical literature. For instance, in a review data of 35,188 individuals from the Vaccine Adverse Event Reporting System (VAERS), 8 cases of myocarditis were registered in individuals below 18 years of age, and 12 cases in elderly persons (117). However, it should be mentioned that VAERS presents some limitations, including the fact that it is a passive reporting system, and, therefore, information collected could be inaccurate, incomplete, coincidental, or unverifiable, warranting thorough epidemiological surveys to confirm such findings.
In another study designed to determine the incidence of cardiac symptoms and subclinical myocarditis/pericarditis after smallpox and trivalent influenza vaccine, out of 1081 individuals who received the smallpox vaccine, 4 Caucasian males were diagnosed with probable myocarditis and 1 female with suspected pericarditis. The study did not find any possible or probable cases of myocarditis/pericarditis following trivalent influenza vaccine however, some patients developed new cardiac symptoms like chest pain, dyspnea, and palpitations (118). One study that focused on myopericarditis following smallpox virus vaccination in the US military found that the incidence of myopericarditis was 7.5 times higher in soldiers who received the vaccine compared to the expected rate (119). Influenza vaccines as well were associated with cases of myocarditis (120–122). While myocarditis is well documented in regard to COVID-19 (123), the correlation with myocarditis and various types of COVID-19 vaccines is illustrated hereby.
BNT162b2 (BioNTech/Pfizer) and Myocarditis
Myocarditis was documented by several case reports in individuals after receiving the COVID-19 vaccines (124–126). Montgomery et al. (127) investigated the association between myocarditis and the mRNA COVID-19 vaccines in healthy military members of the US army. After approximately 2.8 million mRNA vaccine doses, 23 persons were diagnosed with myocarditis within 4 days of vaccination. Seven out of the 23 cases (30%) received the BNT162b2 vaccine and most developed the symptoms following the second dose. The study concluded that further evaluation of this rare adverse effect is warranted. Furthermore, several large-scale studies were conducted to evaluate this association in Israel. For instance, Barda and colleagues (128) evaluated 884,828 people vaccinated with BNT162b2, based on data from the largest health care organization in Israel. While the BNT162b2 vaccine was not associated with most of the side effects searched, the vaccine was strongly associated with increased risk of myocarditis. The risk was calculated as 2.7 events per 100,000 people, with highest risk among young men with a median age of 25. Another study conducted by Witberg et al. (129) searched specifically regarding the diagnosis of myocarditis among individuals in the largest health care organization in Israel that received at least one dose of the BNT162b2 vaccine. Among more than 2.5 million vaccinated members who were 16 years of age or older, the estimated incidence of myocarditis was 2.13 cases per 100,000 people. The highest incidence was documented in young male patients aged 16-29, the majority with mild to moderate disease. In addition, Mevorach and colleagues (130) followed the diagnosis of myocarditis in Israel after approximately 5.1 million people were vaccinated with two doses of the mRNA COVID-19 vaccines. A total of 283 persons developed symptoms of myocarditis, 142 (50%) occurred after the BNT162b2 vaccine, whereas 136 were eventually diagnosed with probable or definitive myocarditis. Most of the cases were mild and the highest incidence was recorded following the second dose and in young male patients aged 16-19 years. In comparison with unvaccinated people, the rate ratio 30 days after the second dose was 2.35 (95% CI, 1.10 to 5.02). The authors concluded that despite the low incidence, it increased after the BNT162b2 vaccine, all were mild in severity. Following the extension of the FDA authorization of the emergency use of the BNT162b2 vaccine to include children aged 12-16 in May 2021, Dionne et al. (131) presented a case series of 15 children with myocarditis after receiving the BNT162b2 vaccine. Similarly, myocarditis developed mainly in boys and after the second dose, all with a mild course.
mRNA-1273 (Moderna) and Myocarditis
Myocarditis is a rare post-mRNA-vaccine sequela, and was seen mostly among young, vaccinated males. Up to June 2021, around 1226 reports of myocarditis have been reported in the US after more than 296 million doses of mRNA COVID-19 vaccines administered (132). Symptoms usually began 3 days after vaccination with more than 75% of the cases happened after receiving the second dose of mRNA vaccine. The median age was 26 years of age with at least 56% of the affected people being younger than 30 years old. About 76% of the cases were found in men. Experts estimated the rate of post-vaccination myocarditis among young males aged 12-29 as 40.6 cases per million second doses of mRNA COVID-19 vaccines and 2.4 per million second doses among males aged > 30. On the other hand, a million vaccinations among young males aged 12-29 could prevent 560 hospitalizations, 138 intensive care unit (ICU) admissions, and six deaths associated with the COVID-19 infection. In turn, according to the UK MHRA, toward the end of November 2021, a total of 103 reports of myocarditis after the use of mRNA-1273 were registered. A rate of 37 suspected myocarditis cases per million doses were calculated for the mRNA-1273 COVID-19 vaccine (133). No death cases were registered. Additionally, in a study conducted by Diaz et al. (134) the mean monthly number of the cases of myocarditis during the vaccine period was found significantly higher than that before the vaccine was available (27.3 vs.16.9). The study included about 2 million subjects who received at least one dose of COVID-19 vaccine. Despite the fact that more people received the BNT162b2 vaccine than the mRNA-1273 vaccine (52.6% vs 44.4%), 55% of the cases occurred after receiving mRNA-1273 (11/20). Cases were mild, few required hospital admission, and discharged after a median of 2 days. No deaths or readmissions were reported. As mentioned earlier regarding 2.8 million doses of mRNA COVID-19 vaccines in healthy members of the US army, 23 cases of myocarditis were reported, 16 cases among persons who received the mRNA-1273 COVID-19 vaccine (127). The majority occurred after the second dose. In terms of possible pathogenetic explanation of the appearance of myocarditis, Bozkurt and colleagues (135) suggested the involvement of various mechanisms such as molecular mimicry between the viral spike protein and self-antigens. In addition, triggering of pre-existing dysregulated immune pathways in certain individuals, activation of immunologic pathways and dysregulated cytokine release, were also proposed. Regarding the higher rates of myocarditis among males, the difference in the immune response of sex hormones and underdiagnosed cases of myocarditis among women were suggested.
ChAdOx1 (AstraZeneca) and Myocarditis
While myocarditis has been extensively reported following mRNA COVID-19 vaccines, very few reports exist regarding the development of myocarditis in relation to the ChAdOx1 vaccine (136). In the latter, the patient was given a diagnosis of myopericarditis with pleuritis. The European Medicines Agency (EMA) estimated that 38 cases of myocarditis and 47 cases of pericarditis were attributed to ChAdOx1 vaccine out of 40 million vaccine doses administered in Europe until May 2021 (137). The agency ensured a monthly review of new cases but refuted the need for an accelerated investigation. In turn, the UK Health Security Agency dismissed a connection between the vaccine and reported cases, claiming that considering the extensive distribution of the ChAdOx1 vaccine in the UK, the cases are more likely attributed to the background incidence rate (138).
Vaccine-Induced Immune Thrombotic Thrombocytopenia/Vaccine-Induced Prothrombotic Immune Thrombotic Thrombocytopenia
Since the appearance of the vaccine-induced immune thrombotic thrombocytopenia (VITT)/vaccine-induced prothrombotic immune thrombotic thrombocytopenia (VIPITT) was mainly attributable to the adenoviral vector vaccines, the ChAdOx1 (AstraZeneca) and the Ad26.COV2.S (Janssen/Johnson & Johnson) vaccines, the current section starts with the AstraZeneca vaccine, then the mRNA vaccines follow.
ChAdOx1 (AstraZeneca) and VITT/VIPITT
In early 2020, as the newly authorized COVID-19 vaccinations were being distributed worldwide, cases of thrombotic events as well as thrombocytopenia following vaccination with the ChAdOx1 vaccine began to emerge (139–141). While the EMA and the WHO issued statements declaring the reported incidence was not enough to deduce causation and cautioning against premature pausing of vaccination programs (142, 143), numerous European countries decided to halt the use of the vaccine pending further investigations. Subsequently, larger studies demonstrated slightly increased rates of venous thromboembolic events among the adenoviral vector vaccine recipients (144, 145). A thorough investigation by the Pharmacovigilance Risk Assessment Committee (PRAC) introduced for the first time the notion of VITT/VIPITT (146), as a rare adverse reaction to the vaccine. VITT/VIPITT is defined as presence of venous or arterial thrombosis, thrombocytopenia, and autoantibodies (anti-PF4–polyanion or anti-PF4–heparin antibodies) within 5–30 days of vaccination with either AstraZeneca or Janssen/Johnson & Johnson COVID-19 vaccines (147). In fact, VITT/VIPITT shares many similarities with heparin-induced thrombocytopenia (HIT) as both disorders are facilitated by platelet factor 4 (PF4) autoantibodies leading to platelet activation and consumption (147). The trigger for the autoantibody formation is poorly understood, as the cases reported had no previous exposure to heparin (148), and the autoantibodies were not found to cross-react with the viral spike proteins indicating a previous infection (139). Furthermore, epidemiological data illustrated that over 85% of VITT/VIPITT cases occurred in women under 60 years of age, despite higher rates of vaccination among the elderly (146). Therefore, the findings supported the assumption that VITT/VIPITT is most likely to be an autoimmune phenomenon. While its exact pathogenesis has not been established yet, a recent study demonstrated that ChAdOx1 COVID-19 vaccine induces higher rates of inflammation and platelet activation compared to other COVID-19 vaccines (149). The VITT/VIPITT autoantibodies consequently bind to PF4 (150), a chemokine secreted from activated platelets, in a site that corresponds to the heparin-binding site. In turn, immune complexes formation induces FcγRIIA receptor mediated platelet activation leading to widespread thrombosis with secondary thrombocytopenia due to platelet consumption (Figure 1) (148, 150). As the ChAdOx1 vaccination has proven to be effective in prevention of COVID-19 infection (151, 152), and due to the rarity of serious adverse events such as VITT/VIPITT, it is important to view this phenomenon in the proper clinical context.
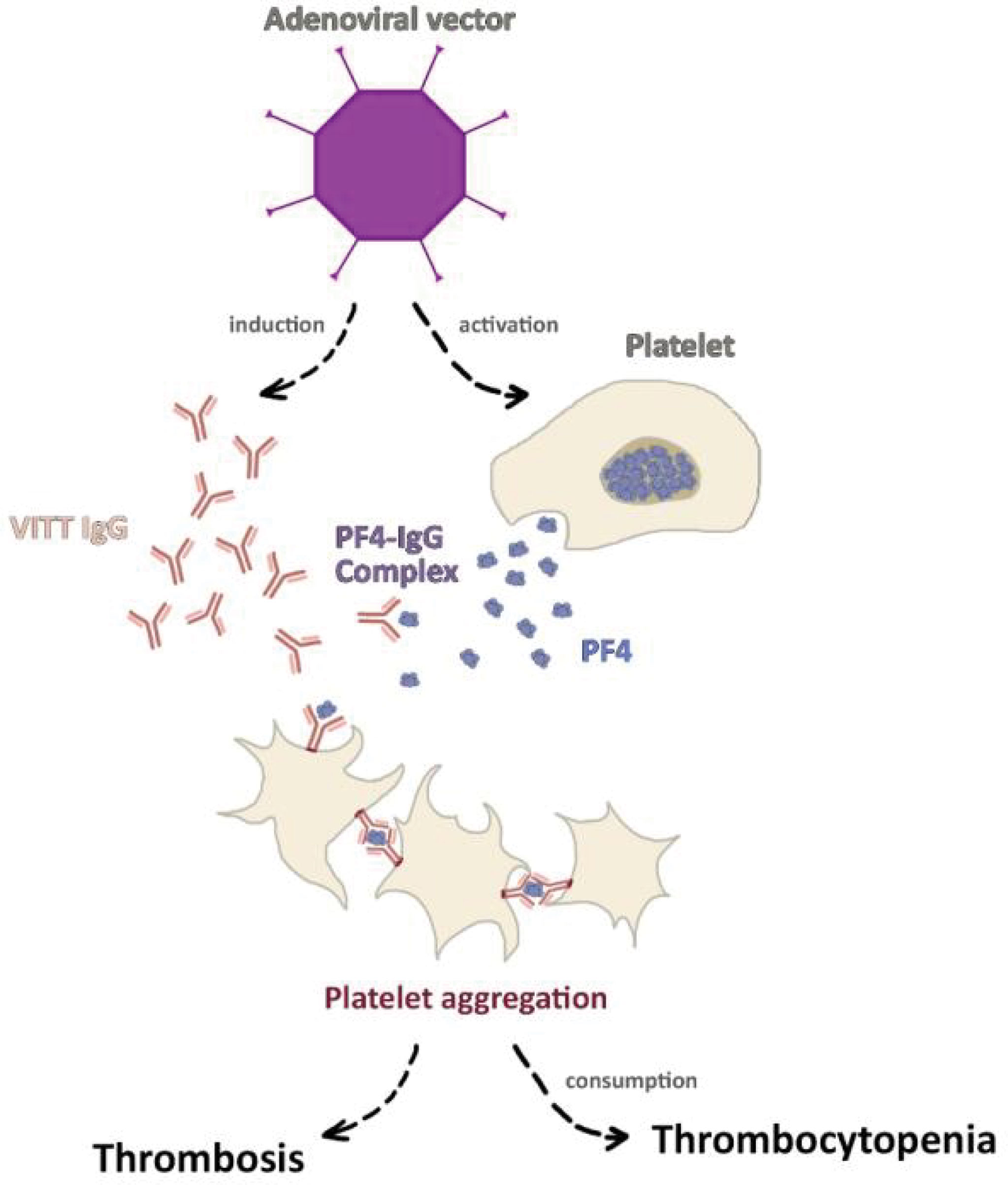
Figure 1 The pathogenesis of vaccine-induced immune thrombotic thrombocytopenia (VITT). Vector-based COVID-19 vaccines, particularly ChAdOx1 COVID-19 vaccine was shown to induce platelet activation alongside platelet factor 4 (PF4) autoantibodies formation. The activated platelets secret greater amount of PF4 which binds to autoantibodies forming the PF4-IgG complex. As a result, FcγRIIA receptor mediated platelet activation leads to widespread thrombosis and secondary thrombocytopenia.
BNT162b2 (BioNTech/Pfizer) and VITT/VIPITT
Since the mass vaccination campaign began with the BNT162b2 vaccine, several case reports have been published regarding the combination of thrombotic events together with thrombocytopenia, in a form of cerebral venous sinus thrombosis, VITT/VIPITT, and acquired thrombotic thrombocytopenic purpura (TTP) several days following vaccination with the BNT162b2 vaccine (153–162). In a large-scale study based on national data made up of 29,121,633 vaccinated individuals with the first dose of BNT162b2 vaccine (163), the association between VITT/VIPITT occurring as post-vaccine and post-infection was investigated. Among the enrollees, 9,513,625 were vaccinated with the BNT162b2 vaccine, and 1,758,095 were in the post-infection group as they were previously infected with COVID-19. An increased risk of arterial thromboembolism, cerebral venous sinus thrombosis, and ischemic stroke after the BNT162b2 vaccine was found however, an even higher risk of these associations was documented following COVID-19 infection. The study concluded an increased risk of hematological and vascular events for short-time intervals following the BNT162b2 vaccine. Still, most of these events were substantially higher and more prolonged after COVID-19 infection than after vaccination in the same population. In contrast, in another large-scale study using a national prospective cohort in Scotland with regard to hematologic and vascular events following vaccination with various vaccine types, no positive correlations were found between BNT162b2 and thrombocytopenic, thromboembolic, and hemorrhagic events (164).
mRNA-1273 (Moderna) and VITT7VIPITT
Cases of ITP and TTP after mRNA-1273 COVID-19 vaccine have been documented since the early vaccination campaign (165–168). A flare of familial thrombocytopenia has been reported as well (169). In addition, 13 cases of thrombocytopenia following 16 million doses of mRNA-1273 were found in the USA, suggesting a rate of 0.8 cases per million (170). Lee et al. (171) discussed 20 cases of thrombocytopenia among 20 million people who were vaccinated by at least one dose of the BNT162b2 or the mRNA1273 vaccines. A total of 17 cases considered new-onset ITP, and 11 out of the 20 cases received mRNA-1273. Furthermore, in a study based on the WHO Vigibase which includes 361 million COVID-19 vaccinated people, 2161 thrombotic events were noted, 325 of the cases appeared after the mRNA-1273 vaccine (172). Among the 325 thrombotic events, only 8 had associated thrombocytopenia. Regarding VITT/VIPITT, a German study assessed the rate of cerebral sinus and venous thrombosis (CVT) within 1 month of the first dose of the BNT162b2, ChAdOx1 and mRNA‐1273 COVID-19 vaccines and the frequency of VITT/VIPITT as the causing mechanism (173). The authors identified 45 cases of CVT, none of the cases occurred after the administration of mRNA-1273. Similar findings were shown by Krzywicka et al. (174) as out of 213 European patients with CVT, only one patient received the mRNA-1273 vaccine. According to the same study, out of 107 patients who had CVT alongside thrombocytopenia, no patient received the mRNA-1273 vaccine. In addition, only 5 possible cases of CVT were reported in Europe among 4 million subjects who received the mRNA-1273 vaccine (175). In summary, the risk of thrombocytopenia and thrombotic events in mRNA-1273 vaccinated people appears to be very low. In fact, the incidence of CVT among people vaccinated with the mRNA COVID-19 vaccines, both the BNT162b2 and mRNA-1273, was shown to be lower than that among people infected with COVID-19 (4.1 vs 39 per million) (176).
Other Autoimmune Side Effects
Immune Thrombocytopenia
ITP is a well-known autoimmune hematological condition characterized by a substantial reduction in peripheral platelet count to less than <100,000/microL due to platelet destruction by antiplatelet antibodies (177). Patients generally asymptomatic, may have minor mucocutaneous bleeding, and not uncommonly progress to life-threatening hemorrhages in severe cases (178). ITP was reported in correlation to COVID-19, COVID-19 vaccines, as well as other vaccines (179, 180).
Minimal Change Disease
Minimal change disease (MCD) is a histologically based renal pathology which is the leading cause of idiopathic nephrotic syndrome in adults and children (181). As cases of MCD were described after vaccination in the past (182–184), the reporting of cases following COVID-19 vaccines were not unforeseen.
BNT162b2 (BioNTech/Pfizer) and MCD
Several case reports have been published regarding new-onset or relapse of MCD following vaccination with the BNT162b2 vaccine. A 50-year-old healthy male presented with nephrotic syndrome and acute kidney injury four days following the first dose of the BNT162b2 vaccine (185). The diagnosis was confirmed by kidney biopsy. Renal function returned to normal within few days following treatment with corticosteroids. Following this report, similar cases in older people were presented (186, 187). The symptoms appeared seven days following the first dose of the vaccine. MCD was confirmed by kidney biopsy as well. In addition, a relapse of MCD was also described in a patient diagnosed with MCD 20 years prior to vaccination, developed proteinuria after vaccination, which resolved within 2 weeks following corticosteroids and cyclosporine treatment (188).
mRNA-1273 (Moderna) and MCD
Though autoimmune renal AEFIs of the mRNA-1273 COVID-19 vaccine were reported, MCD was not common among them. For instance, Thappy et al. (189) presented a case of 43-year-old man who developed symptoms of minimal change disease 7 days after receiving the first dose of the mRNA-1273 vaccine. The biopsy confirmed concomitant MCD and IgA nephropathy. The patient responded well to oral steroid treatment. In addition, biopsy proven IgA nephropathy, both as new onset and flare, was previously described following the mRNA-1273 COVID-19 vaccine, manifesting as hematuria (190, 191). Interestingly, the symptoms appeared 1-2 days after the second dose of the vaccine.
ChAdOx1 (AstraZeneca) and MCD
Several cases of MCD were described in the context of the ChAdOx1 vaccine (192–194). All these cases presented with a clinical picture of nephrotic syndrome that started up to 15 days after the first dose of the vaccine. The fairly short period from vaccination to presentation could be attributed to cytokine release by activated T-cells, as the ChAdOx1 vaccine has been shown to induce a robust T-cell response in most individuals (195). However, as the reported cases were limited, a definite conclusion could not be drawn. The number of cases of MCD following the vaccine was small, thus a strong association could not be inferred.
Vasculitis
As for vasculitis, two cases of antineutrophil cytoplasmic antibody (ANCA) associated vasculitis after the second dose of the mRNA-1273 COVID-19 vaccine were reported. As a result of the new onset ANCA vasculitis, one patient became dependent on dialysis (196), while in the other case renal improvement was achieved after plasma exchange, pulse steroid and cyclophosphamide therapy (197). Additional case of ANCA vasculitis which manifested as renal failure together with pulmonary hemorrhage 3 weeks after the first dose of mRNA-1273 was also described (198).
Miscellaneous
Autoimmune hepatitis was reported as well among individuals vaccinated with various types of COVID-19 vaccines (199–202). In a multicenter study conducted in several countries during the early implementation of the program of vaccination against the COVID-19, Watad and colleagues (203) investigated the association between new-onset or flares of immune-mediated diseases 28-days following mRNA COVID-19 vaccination. The authors detected 27 cases; 17 (63%) had flares of their illnesses whereas 10 (37%) cases had a new-onset disease. In total, 23 (85%) patients received the BNT162b2 vaccine, 2 (7.5%) received the mRNA-1273 vaccine, and 2 (7.5%) received the ChAdOx1 vaccine. Taking into consideration the great proportion of people vaccinated, the authors concluded that immune-mediated diseases flares or new-onset temporally associated with COVID-19 vaccination are rare. Furthermore, Ishay et al. (204) presented a case series of patients with new-onset or flares of autoimmune AEFIs after the BNT162b2 vaccine. Eight patients presented with either symmetric polyarthritis, panuveitis, pericarditis, temporal arteritis-like disease, fever of unknown origin (FUO), oligoarthritis, and myocarditis following either the first dose (62.5%) or the second dose (37.5%) of the vaccine. Based on their findings, the authors concluded that immune AEFIs might occur following vaccination however, they usually follow a mild course.
A brief summary of the studies addressing autoimmune AEFIs of the most utilized COVID-19 vaccines, including the study population and the conclusion of the works, is presented in Table 1.

Table 1 A brief summary of studies addressing immune and autoimmune side effects of COVID-19 vaccines.
Conclusion
Immune and autoimmune AEFIs after COVID-19 vaccines are rare and mostly non-life-threatening. Their rate follows 1 out of 3 possibilities, either it is very low, or similar to the occurrence rate in the general population, or even much lower in comparison to the COVID-19 infection itself. Of AEFIs mentioned, Bell’s palsy and myocarditis seemingly have the greatest risk when it comes to the mRNA-based COVID-19 vaccines however, in addition to their rarity, the disease course is mild and full recovery is the rule. In turn, GBS and VITT/VIPITT were found to be associated mainly with the adenovirus vector based COVID-19 vaccines, still with a low rate. Addressing this sort of AEFIs, their severity, and long-term effects of the COVID-19 vaccines while fighting against vaccine hesitancy is of great importance. Doubtlessly, such efforts will decrease public concern, increase vaccination coverage rate, and guide physicians towards the rapid identification of AEFIs, reassuring patients, and applying appropriate treatment in a timely manner.
Author Contributions
NM: Supervision, Writing- Review and Editing. NL: Writing - Original Draft, Software. AO: Writing - Original Draft. RS: Writing - Original Draft. AA: Writing - Original Draft. MA: Writing - Original Draft. MZ: Writing - Original Draft. NB: Conceptualization, Writing- Review and Editing. All authors contributed to the article and approved the subitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Organization WH. WHO Director-General's Opening Remarks at the Media Briefing on COVID-19 (2020). Available at: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19—11-march-2020.
2. Schernhammer E, Weitzer J, Laubichler MD, Birmann BM, Bertau M, Zenk L, et al. Correlates of COVID-19 Vaccine Hesitancy in Austria: Trust and the Government. J Public Health (Oxf) (2022) 44(1):e106–16. doi: 10.1093/pubmed/fdab122
3. Vaughan A. How to Stop Vaccine Hesitancy. New Sci (2020) 248(3309):12–3. doi: 10.1016/S0262-4079(20)32025-X
4. Group C. A Future Vaccination Campaign Against COVID-19 at Risk of Vaccine Hesitancy and Politicisation. Lancet Infect Dis (2020) 20(7):769–70.
5. Blume S, Geesink I. A Brief History of Polio Vaccines. Science (2000) 288(5471):1593–4. doi: 10.1126/science.288.5471.1593
6. Rottingen JA, Gouglas D, Feinberg M, Plotkin S, Raghavan KV, Witty A, et al. New Vaccines Against Epidemic Infectious Diseases. N Engl J Med (2017) 376(7):610–3. doi: 10.1056/NEJMp1613577
7. Lucia VC, Kelekar A, Afonso NM. COVID-19 Vaccine Hesitancy Among Medical Students. J Public Health (Oxf) (2021) 43(3):445–9. doi: 10.1093/pubmed/fdaa230
8. Schwarzinger M, Watson V, Arwidson P, Alla F, Luchini S. COVID-19 Vaccine Hesitancy in a Representative Working-Age Population in France: A Survey Experiment Based on Vaccine Characteristics. Lancet Public Health (2021) 6(4):e210–e21. doi: 10.1016/S2468-2667(21)00012-8
9. Lee GM. The Importance of Context in Covid-19 Vaccine Safety. N Engl J Med (2021) 385(12):1138–40. doi: 10.1056/NEJMe2112543
10. Liu PL, Zhao X, Wan B. COVID-19 Information Exposure and Vaccine Hesitancy: The Influence of Trust in Government and Vaccine Confidence. Psychol Health Med (2021) 7:1–10. doi: 10.1080/13548506.2021.2014910
11. Plotkin S. History of Vaccination. Proc Natl Acad Sci USA (2014) 111(34):12283–7. doi: 10.1073/pnas.1400472111
12. Verch T, Trausch JJ, Shank-Retzlaff M. Principles of Vaccine Potency Assays. Bioanalysis (2018) 10(3):163–80. doi: 10.4155/bio-2017-0176
13. Nakayama T. Causal Relationship Between Immunological Responses and Adverse Reactions Following Vaccination. Vaccine (2019) 37(2):366–71. doi: 10.1016/j.vaccine.2018.11.045
14. Kuno-Sakai H, Kimura M, Watanabe H. Verification of Components of Acellular Pertussis Vaccines That Have Been Distributed Solely, Been in Routine Use for the Last Two Decades and Contributed Greatly to Control of Pertussis in Japan. Biologicals (2004) 32(1):29–35. doi: 10.1016/j.biologicals.2003.11.001
15. Nakayama T. Vaccine Chronicle in Japan. J Infect Chemother (2013) 19(5):787–98. doi: 10.1007/s10156-013-0641-6
16. Xia S, Zhang Y, Wang Y, Wang H, Yang Y, Gao GF, et al. Safety and Immunogenicity of an Inactivated SARS-CoV-2 Vaccine, BBIBP-CorV: A Randomised, Double-Blind, Placebo-Controlled, Phase 1/2 Trial. Lancet Infect Dis (2021) 21(1):39–51. doi: 10.1016/S1473-3099(20)30831-8
17. Siegrist CA, Aspinall R. B-Cell Responses to Vaccination at the Extremes of Age. Nat Rev Immunol (2009) 9(3):185–94. doi: 10.1038/nri2508
18. Xia S, Duan K, Zhang Y, Zhao D, Zhang H, Xie Z, et al. Effect of an Inactivated Vaccine Against SARS-CoV-2 on Safety and Immunogenicity Outcomes: Interim Analysis of 2 Randomized Clinical Trials. JAMA (2020) 324(10):951–60. doi: 10.1001/jama.2020.15543
19. Lundstrom K. Application of Viral Vectors for Vaccine Development With a Special Emphasis on COVID-19. Viruses (2020) 12(11):1324. doi: 10.3390/v12111324
20. Ura T, Okuda K, Shimada M. Developments in Viral Vector-Based Vaccines. Vaccines (Basel) (2014) 2(3):624–41. doi: 10.3390/vaccines2030624
21. Lauer KB, Borrow R, Blanchard TJ. Multivalent and Multipathogen Viral Vector Vaccines. Clin Vaccine Immunol (2017) 24(1):e00298–16. doi: 10.1128/CVI.00298-16
22. Nishiguchi Y, Matsuyama H, Maeda K, Shindo A, Tomimoto H. Miller Fisher Syndrome Following BNT162b2 mRNA Coronavirus 2019 Vaccination. BMC Neurol (2021) 21(1):452. doi: 10.1186/s12883-021-02489-x
23. Tatsis N, Ertl HC. Adenoviruses as Vaccine Vectors. Mol Ther (2004) 10(4):616–29. doi: 10.1016/j.ymthe.2004.07.013
24. Jones I, Roy P. Sputnik V COVID-19 Vaccine Candidate Appears Safe and Effective. Lancet (2021) 397(10275):642–3. doi: 10.1016/S0140-6736(21)00191-4
25. Klugar M, Riad A, Mekhemar M, Conrad J, Buchbender M, Howaldt HP, et al. Side Effects of mRNA-Based and Viral Vector-Based COVID-19 Vaccines Among German Healthcare Workers. Biol (Basel) (2021) 10(8):752. doi: 10.3390/biology10080752
26. Wolff JA, Malone RW, Williams P, Chong W, Acsadi G, Jani A, et al. Direct Gene Transfer Into Mouse Muscle In Vivo. Science (1990) 247(4949 Pt 1):1465–8. doi: 10.1126/science.1690918
27. Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA Vaccines - a New Era in Vaccinology. Nat Rev Drug Discovery (2018) 17(4):261–79. doi: 10.1038/nrd.2017.243
28. Bhattacharya M, Sharma AR, Ghosh P, Patra P, Patra BC, Lee SS, et al. Bioengineering of Novel Non-Replicating mRNA (NRM) and Self-Amplifying mRNA (SAM) Vaccine Candidates Against SARS-CoV-2 Using Immunoinformatics Approach. Mol Biotechnol (2022) 64(5):510–25. doi: 10.1007/s12033-021-00432-6
29. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med (2020) 383(27):2603–15. doi: 10.1056/NEJMoa2034577
30. Randolph HE, Barreiro LB. Herd Immunity: Understanding COVID-19. Immunity (2020) 52(5):737–41. doi: 10.1016/j.immuni.2020.04.012
31. Fontanet A, Cauchemez S. COVID-19 Herd Immunity: Where Are We? Nat Rev Immunol (2020) 20(10):583–4. doi: 10.1038/s41577-020-00451-5
32. Jordan RE, Adab P, Cheng KK. Covid-19: Risk Factors for Severe Disease and Death. BMJ (2020) 368:m1198. doi: 10.1136/bmj.m1198
33. (FDA) USFDA. FDA Approves First COVID-19 Vaccine (2021). Available at: https://www.fda.gov/news-events/press-announcements/fda-approves-first-covid-19-vaccine.
34. Agency UMHpR. Conditions of Authorisation for COVID-19 Vaccine Pfizer/BioNTech (Regulation 174) (2020). Available at: https://www.gov.uk/government/publications/regulatory-approval-of-pfizer-biontech-vaccine-for-covid-19/conditions-of-authorisation-for-pfizerbiontech-covid-19-vaccine.
35. Pfizer. Pfizer and BioNTech Receive Authorization in the European Union for COVID-19 Vaccine (2020). Available at: https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-receive-authorization-european-union.
36. (FDA) USFDA. (2020). Available at: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/moderna-covid-19-vaccine#additionalhttps://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/moderna-covid-19-vaccine#additional.
37. Agency UMHpR. Regulatory Approval of Vaxzevria (2020). Available at: https://www.gov.uk/government/publications/regulatory-approval-of-covid-19-vaccine-astrazeneca.
38. Data OWi. Coronavirus (COVID-19) Vaccinations (2021). Available at: https://ourworldindata.org/covid-vaccinations.
39. Esposito S, Longo MR. Guillain-Barre Syndrome. Autoimmun Rev (2017) 16(1):96–101. doi: 10.1016/j.autrev.2016.09.022
40. Shahrizaila N, Lehmann HC, Kuwabara S. Guillain-Barre Syndrome. Lancet (2021) 397(10280):1214–28. doi: 10.1016/S0140-6736(21)00517-1
41. Willison HJ, Jacobs BC, van Doorn PA. Guillain-Barre Syndrome. Lancet (2016) 388(10045):717–27. doi: 10.1016/S0140-6736(16)00339-1
42. van den Berg B, Walgaard C, Drenthen J, Fokke C, Jacobs BC, van Doorn PA. Guillain-Barre Syndrome: Pathogenesis, Diagnosis, Treatment and Prognosis. Nat Rev Neurol (2014) 10(8):469–82. doi: 10.1038/nrneurol.2014.121
43. Nanda SK, Jayalakshmi S, Ruikar D, Surath M. Twelfth Cranial Nerve Involvement in Guillian Barre Syndrome. J Neurosci Rural Pract (2013) 4(3):338–40. doi: 10.4103/0976-3147.118804
44. Wang Y, Zhang HL, Wu X, Zhu J. Complications of Guillain-Barre Syndrome. Expert Rev Clin Immunol (2016) 12(4):439–48. doi: 10.1586/1744666X.2016.1128829
45. Ancona P, Bailey M, Bellomo R. Characteristics, Incidence and Outcome of Patients Admitted to Intensive Care Unit With Guillain-Barre Syndrome in Australia and New Zealand. J Crit Care (2018) 45:58–64. doi: 10.1016/j.jcrc.2018.01.016
46. Malek E, Salameh J. Guillain-Barre Syndrome. Semin Neurol (2019) 39(5):589–95. doi: 10.1055/s-0039-1693005
47. Patja A, Paunio M, Kinnunen E, Junttila O, Hovi T, Peltola H. Risk of Guillain-Barre Syndrome After Measles-Mumps-Rubella Vaccination. J Pediatr (2001) 138(2):250–4. doi: 10.1067/mpd.2001.111165
48. Principi N, Esposito S. Vaccine-Preventable Diseases, Vaccines and Guillain-Barre' Syndrome. Vaccine (2019) 37(37):5544–50. doi: 10.1016/j.vaccine.2018.05.119
49. Waheed S, Bayas A, Hindi F, Rizvi Z, Espinosa PS. Neurological Complications of COVID-19: Guillain-Barre Syndrome Following Pfizer COVID-19 Vaccine. Cureus (2021) 13(2):e13426. doi: 10.7759/cureus.13426
50. Bouattour N, Hdiji O, Sakka S, Fakhfakh E, Moalla K, Daoud S, et al. Guillain-Barre Syndrome Following the First Dose of Pfizer-BioNTech COVID-19 Vaccine: Case Report and Review of Reported Cases. Neurol Sci (2021) 43(2):755–61. doi: 10.1007/s10072-021-05733-x
51. Razok A, Shams A, Almeer A, Zahid M. Post-COVID-19 Vaccine Guillain-Barre Syndrome; First Reported Case From Qatar. Ann Med Surg (Lond) (2021) 67:102540. doi: 10.1016/j.amsu.2021.102540
52. Shapiro Ben David S, Potasman I, Rahamim-Cohen D. Rate of Recurrent Guillain-Barre Syndrome After mRNA COVID-19 Vaccine Bnt162b2. JAMA Neurol (2021) 78(11):1409–11. doi: 10.1001/jamaneurol.2021.3287
53. Shasha D, Bareket R, Sikron FH, Gertel O, Tsamir J, Dvir D, et al. Real-World Safety Data for the Pfizer BNT162b2 SARS-CoV-2 Vaccine: Historical Cohort Study. Clin Microbiol Infect (2022) 28(1):130–4. doi: 10.1016/j.cmi.2021.09.018
54. Garcia-Grimshaw M, Michel-Chavez A, Vera-Zertuche JM, Galnares-Olalde JA, Hernandez-Vanegas LE, Figueroa-Cucurachi M, et al. Guillain-Barre Syndrome Is Infrequent Among Recipients of the BNT162b2 mRNA COVID-19 Vaccine. Clin Immunol (2021) 230:108818. doi: 10.1016/j.clim.2021.108818
55. Dalwadi V, Hancock D, Ballout AA, Geraci A. Axonal-Variant Guillian-Barre Syndrome Temporally Associated With mRNA-Based Moderna SARS-CoV-2 Vaccine. Cureus (2021) 13(9):e18291. doi: 10.7759/cureus.18291
56. Masuccio FG, Comi C, Solaro C. Guillain-Barre Syndrome Following COVID-19 Vaccine mRNA-1273: A Case Report. Acta Neurol Belg (2021) 12:1–3. doi: 10.1007/s13760-021-01838-4
57. Loo LK, Salim O, Liang D, Goel A, Sumangala S, Gowda AS, et al. Acute-Onset Polyradiculoneuropathy After SARS-CoV2 Vaccine in the West and North Midlands, United Kingdom. Muscle Nerve (2022) 65(2):233–7. doi: 10.1002/mus.27461
58. Agency EM. Meeting Highlights From the Pharmacovigilance Risk Assessment Committee (PRAC) (2021). Available at: https://www.ema.europa.eu/en/news/meeting-highlights-pharmacovigilance-risk-assessment-committee-prac-5-8-july-2021.
59. Allen CM, Ramsamy S, Tarr AW, Tighe PJ, Irving WL, Tanasescu R, et al. Guillain-Barre Syndrome Variant Occurring After SARS-CoV-2 Vaccination. Ann Neurol (2021) 90(2):315–8. doi: 10.1002/ana.26144
60. Bonifacio GB, Patel D, Cook S, Purcaru E, Couzins M, Domjan J, et al. Bilateral Facial Weakness With Paraesthesia Variant of Guillain-Barre Syndrome Following Vaxzevria COVID-19 Vaccine. J Neurol Neurosurg Psychiatry (2021) 93(3):341–2. doi: 10.1136/jnnp-2021-327027
61. Hasan T, Khan M, Khan F, Hamza G. Case of Guillain-Barre Syndrome Following COVID-19 Vaccine. BMJ Case Rep (2021) 14(6):e243629. doi: 10.1136/bcr-2021-243629
62. Introna A, Caputo F, Santoro C, Guerra T, Ucci M, Mezzapesa DM, et al. Guillain-Barre Syndrome After AstraZeneca COVID-19-Vaccination: A Causal or Casual Association? Clin Neurol Neurosurg (2021) 208:106887. doi: 10.1016/j.clineuro.2021.106887
63. Kanabar G, Wilkinson P. Guillain-Barre Syndrome Presenting With Facial Diplegia Following COVID-19 Vaccination in Two Patients. BMJ Case Rep (2021) 14(10):e244527. doi: 10.1136/bcr-2021-244527
64. Maramattom BV, Krishnan P, Paul R, Padmanabhan S, Cherukudal Vishnu Nampoothiri S, Syed AA, et al. Guillain-Barre Syndrome Following ChAdOx1-S/nCoV-19 Vaccine. Ann Neurol (2021) 90(2):312–4. doi: 10.1002/ana.26143
65. McKean N, Chircop C. Guillain-Barre Syndrome After COVID-19 Vaccination. BMJ Case Rep (2021) 14(7):e244125. doi: 10.1136/bcr-2021-244125
66. Min YG, Ju W, Ha YE, Ban JJ, Lee SA, Sung JJ, et al. Sensory Guillain-Barre Syndrome Following the ChAdOx1 Ncov-19 Vaccine: Report of Two Cases and Review of Literature. J Neuroimmunol (2021) 359:577691. doi: 10.1016/j.jneuroim.2021.577691
67. Oo WM, Giri P, de Souza A. AstraZeneca COVID-19 Vaccine and Guillain- Barre Syndrome in Tasmania: A Causal Link? J Neuroimmunol (2021) 360:577719. doi: 10.1016/j.jneuroim.2021.577719
68. Keddie S, Pakpoor J, Mousele C, Pipis M, Machado PM, Foster M, et al. Epidemiological and Cohort Study Finds No Association Between COVID-19 and Guillain-Barre Syndrome. Brain (2021) 144(2):682–93. doi: 10.1093/brain/awaa433
69. Yuki N, Hartung HP. Guillain-Barre Syndrome. N Engl J Med (2012) 366(24):2294–304. doi: 10.1056/NEJMra1114525
70. Agency UHS. Guidance: Information for Healthcare Professionals on Guillain-Barré Syndrome (GBS) Following COVID-19 Vaccination (2021). Available at: https://www.gov.uk/government/publications/covid-19-vaccination-guillain-barre-syndrome-information-for-healthcare-professionals/information-for-healthcare-professionals-on-guillain-barre-syndrome-gbs-following-covid-19-vaccination.
71. Woo EJ, Mba-Jonas A, Dimova RB, Alimchandani M, Zinderman CE, Nair N. Association of Receipt of the Ad26.COV2.S COVID-19 Vaccine With Presumptive Guillain-Barre Syndrome, February-July 2021. JAMA (2021) 326(16):1606–13.
72. Beh SC, Greenberg BM, Frohman T, Frohman EM. Transverse Myelitis. Neurol Clin (2013) 31(1):79–138. doi: 10.1016/j.ncl.2012.09.008
73. Krishnan C, Kaplin AI, Pardo CA, Kerr DA, Keswani SC. Demyelinating Disorders: Update on Transverse Myelitis. Curr Neurol Neurosci Rep (2006) 6(3):236–43. doi: 10.1007/s11910-006-0011-1
74. Agmon-Levin N, Kivity S, Szyper-Kravitz M, Shoenfeld Y. Transverse Myelitis and Vaccines: A Multi-Analysis. Lupus (2009) 18(13):1198–204. doi: 10.1177/0961203309345730
75. Miyaue N, Yoshida A, Yamanishi Y, Tada S, Ando R, Hosokawa Y, et al. A Case of Refractory Longitudinally Extensive Transverse Myelitis After Severe Acute Respiratory Syndrome Coronavirus 2 Vaccination in a Japanese Man. Intern Med (2021) 61(5):739–42. doi: 10.2169/internalmedicine.8747-21
76. Garcia-Grimshaw M, Ceballos-Liceaga SE, Hernandez-Vanegas LE, Nunez I, Hernandez-Valdivia N, Carrillo-Garcia DA, et al. Neurologic Adverse Events Among 704,003 First-Dose Recipients of the BNT162b2 mRNA COVID-19 Vaccine in Mexico: A Nationwide Descriptive Study. Clin Immunol (2021) 229:108786. doi: 10.1016/j.clim.2021.108786
77. Kania K, Ambrosius W, Tokarz Kupczyk E, Kozubski W. Acute Disseminated Encephalomyelitis in a Patient Vaccinated Against SARS-CoV-2. Ann Clin Transl Neurol (2021) 8(10):2000–3. doi: 10.1002/acn3.51447
78. Fujikawa P, Shah FA, Braford M, Patel K, Madey J. Neuromyelitis Optica in a Healthy Female After Severe Acute Respiratory Syndrome Coronavirus 2 mRNA-1273 Vaccine. Cureus (2021) 13(9):e17961. doi: 10.7759/cureus.17961
79. Gao JJ, Tseng HP, Lin CL, Shiu JS, Lee MH, Liu CH. Acute Transverse Myelitis Following COVID-19 Vaccination. Vaccines (Basel) (2021) 9(9):1008. doi: 10.3390/vaccines9091008
80. Ismail II, Salama S. A Systematic Review of Cases of CNS Demyelination Following COVID-19 Vaccination. J Neuroimmunol (2022) 362:577765. doi: 10.1016/j.jneuroim.2021.577765
81. Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Safety and Efficacy of the ChAdOx1 Ncov-19 Vaccine (AZD1222) Against SARS-CoV-2: An Interim Analysis of Four Randomised Controlled Trials in Brazil, South Africa, and the UK. Lancet (2021) 397(10269):99–111. doi: 10.1016/S0140-6736(20)32661-1
82. Knoll MD, Wonodi C. Oxford-AstraZeneca COVID-19 Vaccine Efficacy. Lancet (2021) 397(10269):72–4. doi: 10.1016/S0140-6736(20)32623-4
83. Borchers AT, Gershwin ME. Transverse Myelitis. Autoimmun Rev (2012) 11(3):231–48. doi: 10.1016/j.autrev.2011.05.018
84. Hsiao YT, Tsai MJ, Chen YH, Hsu CF. Acute Transverse Myelitis After COVID-19 Vaccination. Med (Kaunas) (2021) 57(10):1010. doi: 10.3390/medicina57101010
85. Malhotra HS, Gupta P, Prabhu V, Kumar Garg R, Dandu H, Agarwal V. COVID-19 Vaccination-Associated Myelitis. QJM (2021) 114(8):591–3. doi: 10.1093/qjmed/hcab069
86. Notghi AA, Atley J, Silva M. Lessons of the Month 1: Longitudinal Extensive Transverse Myelitis Following AstraZeneca COVID-19 Vaccination. Clin Med (Lond) (2021) 21(5):e535–8. doi: 10.7861/clinmed.2021-0470
87. Pagenkopf C, Sudmeyer M. A Case of Longitudinally Extensive Transverse Myelitis Following Vaccination Against Covid-19. J Neuroimmunol (2021) 358:577606. doi: 10.1016/j.jneuroim.2021.577606
88. Tan WY, Yusof Khan AHK, Mohd Yaakob MN, Abdul Rashid AM, Loh WC, Baharin J, et al. Longitudinal Extensive Transverse Myelitis Following ChAdOx1 nCOV-19 Vaccine: A Case Report. BMC Neurol (2021) 21(1):395. doi: 10.1186/s12883-021-02427-x
89. Roman GC, Gracia F, Torres A, Palacios A, Gracia K, Harris D. Acute Transverse Myelitis (ATM):Clinical Review of 43 Patients With COVID-19-Associated ATM and 3 Post-Vaccination ATM Serious Adverse Events With the ChAdOx1 Ncov-19 Vaccine (Azd1222). Front Immunol (2021) 12:653786. doi: 10.3389/fimmu.2021.653786
91. Zhang W, Xu L, Luo T, Wu F, Zhao B, Li X. The Etiology of Bell's Palsy: A Review. J Neurol (2020) 267(7):1896–905. doi: 10.1007/s00415-019-09282-4
92. Reich SG. Bell's Palsy. Continuum (Minneap Minn) (2017) 23:447–66. doi: 10.1212/CON.0000000000000447
93. Mutsch M, Zhou W, Rhodes P, Bopp M, Chen RT, Linder T, et al. Use of the Inactivated Intranasal Influenza Vaccine and the Risk of Bell's Palsy in Switzerland. N Engl J Med (2004) 350(9):896–903. doi: 10.1056/NEJMoa030595
94. Administration USDaF. Vaccines and Related Biological Products Advisory Committee December 10, 2020 Meeting Briefing Document- FDA (2020). Available at: https://www.fda.gov/media/144245.
95. Ozonoff A, Nanishi E, Levy O. Bell's Palsy and SARS-CoV-2 Vaccines. Lancet Infect Dis (2021) 21(4):450–2. doi: 10.1016/S1473-3099(21)00076-1
96. Colella G, Orlandi M, Cirillo N. Bell's Palsy Following COVID-19 Vaccination. J Neurol (2021) 268(10):3589–91. doi: 10.1007/s00415-021-10462-4
97. Dagan N, Barda N, Kepten E, Miron O, Perchik S, Katz MA, et al. BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Mass Vaccination Setting. N Engl J Med (2021) 384(15):1412–23. doi: 10.1056/NEJMoa2101765
98. Shibli R, Barnett O, Abu-Full Z, Gronich N, Najjar-Debbiny R, Doweck I, et al. Association Between Vaccination With the BNT162b2 mRNA COVID-19 Vaccine and Bell's Palsy: A Population-Based Study. Lancet Reg Health Eur (2021) 11:100236. doi: 10.1016/j.lanepe.2021.100236
99. Cellina M, D'Arrigo A, Floridi C, Oliva G, Carrafiello G. Left Bell's Palsy Following the First Dose of mRNA-1273 SARS-CoV-2 Vaccine: A Case Report. Clin Imaging (2022) 82:1–4. doi: 10.1016/j.clinimag.2021.10.010
100. Iftikhar H, Noor SMU, Masood M, Bashir K. Bell's Palsy After 24 Hours of mRNA-1273 SARS-CoV-2 Vaccine. Cureus (2021) 13(6):e15935. doi: 10.7759/cureus.15935
101. Martin-Villares C, Vazquez-Feito A, Gonzalez-Gimeno MJ, de la Nogal-Fernandez B. Bell's Palsy Following a Single Dose of mRNA SARS-CoV-2 Vaccine: A Case Report. J Neurol (2022) 269(1):47–8. doi: 10.1007/s00415-021-10617-3
102. Sato K, Mano T, Niimi Y, Toda T, Iwata A, Iwatsubo T. Facial Nerve Palsy Following the Administration of COVID-19 mRNA Vaccines: Analysis of a Self-Reporting Database. Int J Infect Dis (2021) 111:310–2. doi: 10.1016/j.ijid.2021.08.071
103. Koh JS, Hoe RHM, Yong MH, Chiew HJ, Goh Y, Yong KP, et al. Hospital-Based Observational Study of Neurological Disorders in Patients Recently Vaccinated With COVID-19 mRNA Vaccines. J Neurol Sci (2021) 430:120030. doi: 10.1016/j.jns.2021.120030
104. Renoud L, Khouri C, Revol B, Lepelley M, Perez J, Roustit M, et al. Association of Facial Paralysis With mRNA COVID-19 Vaccines: A Disproportionality Analysis Using the World Health Organization Pharmacovigilance Database. JAMA Intern Med (2021) 181(9):1243–5. doi: 10.1001/jamainternmed.2021.2219
105. Eviston TJ, Croxson GR, Kennedy PG, Hadlock T, Krishnan AV. Bell's Palsy: Aetiology, Clinical Features and Multidisciplinary Care. J Neurol Neurosurg Psychiatry (2015) 86(12):1356–61. doi: 10.1136/jnnp-2014-309563
106. Patone M, Handunnetthi L, Saatci D, Pan J, Katikireddi SV, Razvi S, et al. Neurological Complications After First Dose of COVID-19 Vaccines and SARS-CoV-2 Infection. Nat Med (2021) 27(12):2144–53. doi: 10.1038/s41591-021-01556-7
107. Zuhorn F, Graf T, Klingebiel R, Schabitz WR, Rogalewski A. Postvaccinal Encephalitis After ChAdOx1 Ncov-19. Ann Neurol (2021) 90(3):506–11. doi: 10.1002/ana.26182
108. Moslemi M, Ardalan M, Haramshahi M, Mirzaei H, Sani SK, Dastgir R, et al. Herpes Simplex Encephalitis Following ChAdOx1 Ncov-19 Vaccination: A Case Report and Review of the Literature. BMC Infect Dis (2022) 22(1):217. doi: 10.1186/s12879-022-07186-9
109. Kobayashi Y, Karasawa S, Ohashi N, Yamamoto K. A Case of Encephalitis Following COVID-19 Vaccine. J Infect Chemother (2022) 28(7):975–7. doi: 10.1016/j.jiac.2022.02.009
110. Torrealba-Acosta G, Martin JC, Huttenbach Y, Garcia CR, Sohail MR, Agarwal SK, et al. Acute Encephalitis, Myoclonus and Sweet Syndrome After mRNA-1273 Vaccine. BMJ Case Rep (2021) 14(7):e243173. doi: 10.1136/bcr-2021-243173
111. Caforio AL, Pankuweit S, Arbustini E, Basso C, Gimeno-Blanes J, Felix SB, et al. Current State of Knowledge on Aetiology, Diagnosis, Management, and Therapy of Myocarditis: A Position Statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J (2013) 34(33):2636–48.48a-d. doi: 10.1093/eurheartj/eht210
113. Sagar S, Liu PP, Cooper LT Jr. Myocarditis. Lancet (2012) 379(9817):738–47. doi: 10.1016/S0140-6736(11)60648-X
114. Caforio AL, Marcolongo R, Basso C, Iliceto S. Clinical Presentation and Diagnosis of Myocarditis. Heart (2015) 101(16):1332–44. doi: 10.1136/heartjnl-2014-306363
115. Ammirati E, Frigerio M, Adler ED, Basso C, Birnie DH, Brambatti M, et al. Management of Acute Myocarditis and Chronic Inflammatory Cardiomyopathy: An Expert Consensus Document. Circ Heart Fail (2020) 13(11):e007405. doi: 10.1161/CIRCHEARTFAILURE.120.007405
116. Felker GM, Thompson RE, Hare JM, Hruban RH, Clemetson DE, Howard DL, et al. Underlying Causes and Long-Term Survival in Patients With Initially Unexplained Cardiomyopathy. N Engl J Med (2000) 342(15):1077–84. doi: 10.1056/NEJM200004133421502
117. Mei R, Raschi E, Forcesi E, Diemberger I, De Ponti F, Poluzzi E. Myocarditis and Pericarditis After Immunization: Gaining Insights Through the Vaccine Adverse Event Reporting System. Int J Cardiol (2018) 273:183–6. doi: 10.1016/j.ijcard.2018.09.054
118. Engler RJ, Nelson MR, Collins LC Jr., Spooner C, Hemann BA, Gibbs BT, et al. A Prospective Study of the Incidence of Myocarditis/Pericarditis and New Onset Cardiac Symptoms Following Smallpox and Influenza Vaccination. PLoS One (2015) 10(3):e0118283. doi: 10.1371/journal.pone.0118283
119. Arness MK, Eckart RE, Love SS, Atwood JE, Wells TS, Engler RJ, et al. Myopericarditis Following Smallpox Vaccination. Am J Epidemiol (2004) 160(7):642–51. doi: 10.1093/aje/kwh269
120. Kim YJ, Bae JI, Ryoo SM, Kim WY. Acute Fulminant Myocarditis Following Influenza Vaccination Requiring Extracorporeal Membrane Oxygenation. Acute Crit Care (2019) 34(2):165–9. doi: 10.4266/acc.2017.00045
121. Nagano N, Yano T, Fujita Y, Koyama M, Hasegawa R, Nakata J, et al. Hemodynamic Collapse After Influenza Vaccination: A Vaccine-Induced Fulminant Myocarditis? Can J Cardiol (2020) 36(9):1554.e5– 7. doi: 10.1016/j.cjca.2020.05.005
122. Rosenberg M, Sparks R, McMahon A, Iskander J, Campbell JD, Edwards KM. Serious Adverse Events Rarely Reported After Trivalent Inactivated Influenza Vaccine (TIV) in Children 6-23 Months of Age. Vaccine (2009) 27(32):4278–83. doi: 10.1016/j.vaccine.2009.05.023
123. Castiello T, Georgiopoulos G, Finocchiaro G, Claudia M, Gianatti A, Delialis D, et al. COVID-19 and Myocarditis: A Systematic Review and Overview of Current Challenges. Heart Fail Rev (2022) 27(1):251–61. doi: 10.1007/s10741-021-10087-9
124. Abu Mouch S, Roguin A, Hellou E, Ishai A, Shoshan U, Mahamid L, et al. Myocarditis Following COVID-19 mRNA Vaccination. Vaccine (2021) 39(29):3790–3. doi: 10.1016/j.vaccine.2021.05.087
125. Larson KF, Ammirati E, Adler ED, Cooper LT Jr., Hong KN, Saponara G, et al. Myocarditis After BNT162b2 and mRNA-1273 Vaccination. Circulation (2021) 144(6):506–8. doi: 10.1161/CIRCULATIONAHA.121.055913
126. Vidula MK, Ambrose M, Glassberg H, Chokshi N, Chen T, Ferrari VA, et al. Myocarditis and Other Cardiovascular Complications of the mRNA-Based COVID-19 Vaccines. Cureus (2021) 13(6):e15576. doi: 10.7759/cureus.15576
127. Montgomery J, Ryan M, Engler R, Hoffman D, McClenathan B, Collins L, et al. Myocarditis Following Immunization With mRNA COVID-19 Vaccines in Members of the US Military. JAMA Cardiol (2021) 6(10):1202–6. doi: 10.1001/jamacardio.2021.2833
128. Barda N, Dagan N, Ben-Shlomo Y, Kepten E, Waxman J, Ohana R, et al. Safety of the BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Setting. N Engl J Med (2021) 385(12):1078–90. doi: 10.1056/NEJMoa2110475
129. Witberg G, Barda N, Hoss S, Richter I, Wiessman M, Aviv Y, et al. Myocarditis After Covid-19 Vaccination in a Large Health Care Organization. N Engl J Med (2021) 385(23):2132–9. doi: 10.1056/NEJMoa2110737
130. Mevorach D, Anis E, Cedar N, Bromberg M, Haas EJ, Nadir E, et al. Myocarditis After BNT162b2 mRNA Vaccine Against Covid-19 in Israel. N Engl J Med (2021) 385(23):2140–9. doi: 10.1056/NEJMoa2109730
131. Dionne A, Sperotto F, Chamberlain S, Baker AL, Powell AJ, Prakash A, et al. Association of Myocarditis With BNT162b2 Messenger RNA COVID-19 Vaccine in a Case Series of Children. JAMA Cardiol (2021) 6(12):1446–50. doi: 10.1001/jamacardio.2021.3471
132. Gargano JW, Wallace M, Hadler SC, Langley G, Su JR, Oster ME, et al. Use of mRNA COVID-19 Vaccine After Reports of Myocarditis Among Vaccine Recipients: Update From the Advisory Committee on Immunization Practices - United States, June 2021. MMWR Morb Mortal Wkly Rep (2021) 70(27):977–82. doi: 10.15585/mmwr.mm7027e2
133. Agency UMHpR. Coronavirus Vaccine - Weekly Summary of Yellow Card Reporting (2021). Available at: https://www.gov.uk/government/publications/coronavirus-covid-19-vaccine-adverse-reactions/coronavirus-vaccine-summary-of-yellow-card-reporting#analysis-of-data.
134. Diaz GA, Parsons GT, Gering SK, Meier AR, Hutchinson IV, Robicsek A. Myocarditis and Pericarditis After Vaccination for COVID-19. JAMA (2021) 326(12):1210–2. doi: 10.1001/jama.2021.13443
135. Bozkurt B, Kamat I, Hotez PJ. Myocarditis With COVID-19 mRNA Vaccines. Circulation (2021) 144(6):471–84. doi: 10.1161/CIRCULATIONAHA.121.056135
136. Hung YP, Sun KS. A Case of Myopericarditis With Pleuritis Following AstraZeneca Covid-19 Vaccination. QJM (2022) 114(12):879–81. doi: 10.1093/qjmed/hcab278
137. Agency EM. COVID-19 Vaccines: Update on Ongoing Evaluation of Myocarditis and Pericarditis (2021). Available at: https://www.ema.europa.eu/en/news/covid-19-vaccines-update-ongoing-evaluation-myocarditis-pericarditis.
138. Agenct UHS. Guidance: Myocarditis and Pericarditis After COVID-19 Vaccination: Clinical Management Guidance for Healthcare Professionals (2021). Available at: https://www.gov.uk/government/publications/myocarditis-and-pericarditis-after-covid-19-vaccination/myocarditis-and-pericarditis-after-covid-19-vaccination-guidance-for-healthcare-professionals.
139. Greinacher A, Selleng K, Mayerle J, Palankar R, Wesche J, Reiche S, et al. Anti-Platelet Factor 4 Antibodies Causing VITT do Not Cross-React With SARS-CoV-2 Spike Protein. Blood (2021) 138(14):1269–77. doi: 10.1182/blood.2021012938
140. Schultz NH, Sorvoll IH, Michelsen AE, Munthe LA, Lund-Johansen F, Ahlen MT, et al. Thrombosis and Thrombocytopenia After ChAdOx1 Ncov-19 Vaccination. N Engl J Med (2021) 384(22):2124–30. doi: 10.1056/NEJMoa2104882
141. Scully M, Singh D, Lown R, Poles A, Solomon T, Levi M, et al. Pathologic Antibodies to Platelet Factor 4 After ChAdOx1 Ncov-19 Vaccination. N Engl J Med (2021) 384(23):2202–11. doi: 10.1056/NEJMoa2105385
142. Agency EM. COVID-19 Vaccine AstraZeneca: PRAC Investigating Cases of Thromboembolic Events - Vaccine’s Benefits Currently Still Outweigh Risks (2021). Available at: https://www.ema.europa.eu/en/news/covid-19-vaccine-astrazeneca-prac-investigating-cases-thromboembolic-events-vaccines-benefits.
143. Nations U. WHO Backs AstraZeneca COVID Vaccine Amid Clotting Concerns; Green Lights Johnson & Johnson Shots (2021). Available at: https://news.un.org/en/story/2021/03/1087222.
144. Perry RJ, Tamborska A, Singh B, Craven B, Marigold R, Arthur-Farraj P, et al. Cerebral Venous Thrombosis After Vaccination Against COVID-19 in the UK: A Multicentre Cohort Study. Lancet (2021) 398(10306):1147–56. doi: 10.1016/S0140-6736(21)01608-1
145. Pottegard A, Lund LC, Karlstad O, Dahl J, Andersen M, Hallas J, et al. Arterial Events, Venous Thromboembolism, Thrombocytopenia, and Bleeding After Vaccination With Oxford-AstraZeneca ChAdOx1-S in Denmark and Norway: Population Based Cohort Study. BMJ (2021) 373:n1114. doi: 10.1136/bmj.n1114
146. Agency EM. Astrazeneca’s COVID-19 Vaccine: EMA Finds Possible Link to Very Rare Cases of Unusual Blood Clots With Low Blood Platelets (2021). Available at: https://www.ema.europa.eu/en/news/astrazenecas-covid-19-vaccine-ema-finds-possible-link-very-rare-cases-unusual-blood-clots-low-blood.
147. Marchandot B CA, Trimaille A, Sattler L, Grunebaum L, Morel O. Vaccine-Induced Immune Thrombotic Thrombocytopenia: Current Evidence, Potential Mechanisms, Clinical Implications, and Future Directions. Eur Heart J Open (2021) 1(2):oeab014. doi: 10.1093/ehjopen/oeab014
148. Dotan A, Shoenfeld Y. Perspectives on Vaccine Induced Thrombotic Thrombocytopenia. J Autoimmun (2021) 121:102663. doi: 10.1016/j.jaut.2021.102663
149. Ostrowski SR, Sogaard OS, Tolstrup M, Staerke NB, Lundgren J, Ostergaard L, et al. Inflammation and Platelet Activation After COVID-19 Vaccines - Possible Mechanisms Behind Vaccine-Induced Immune Thrombocytopenia and Thrombosis. Front Immunol (2021) 12:779453. doi: 10.3389/fimmu.2021.779453
150. Huynh A, Kelton JG, Arnold DM, Daka M, Nazy I. Antibody Epitopes in Vaccine-Induced Immune Thrombotic Thrombocytopaenia. Nature (2021) 596(7873):565–9. doi: 10.1038/s41586-021-03744-4
151. Falsey AR, Sobieszczyk ME, Hirsch I, Sproule S, Robb ML, Corey L, et al. Phase 3 Safety and Efficacy of AZD1222 (ChAdOx1 Ncov-19) Covid-19 Vaccine. N Engl J Med (2021) 385(25):2348–60. doi: 10.1056/NEJMoa2105290
152. Voysey M, Costa Clemens SA, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Single-Dose Administration and the Influence of the Timing of the Booster Dose on Immunogenicity and Efficacy of ChAdOx1 Ncov-19 (AZD1222) Vaccine: A Pooled Analysis of Four Randomised Trials. Lancet (2021) 397(10277):881–91. doi: 10.1016/S0140-6736(21)00432-3
153. Akiyama H, Kakiuchi S, Rikitake J, Matsuba H, Sekinada D, Kozuki Y, et al. Immune Thrombocytopenia Associated With Pfizer-BioNTech's BNT162b2 mRNA COVID-19 Vaccine. IDCases (2021) 25:e01245. doi: 10.1016/j.idcr.2021.e01245
154. de Bruijn S, Maes MB, De Waele L, Vanhoorelbeke K, Gadisseur A. First Report of a De Novo iTTP Episode Associated With an mRNA-Based Anti-COVID-19 Vaccination. J Thromb Haemost (2021) 19(8):2014–8. doi: 10.1111/jth.15418
155. Dias L, Soares-Dos-Reis R, Meira J, Ferrao D, Soares PR, Pastor A, et al. Cerebral Venous Thrombosis After BNT162b2 mRNA SARS-CoV-2 Vaccine. J Stroke Cerebrovasc Dis (2021) 30(8):105906. doi: 10.1016/j.jstrokecerebrovasdis.2021.105906
156. Ganzel C, Ben-Chetrit E. Immune Thrombocytopenia Following the Pfizer-BioNTech BNT162b2 mRNA COVID-19 Vaccine. Isr Med Assoc J (2021) 23(6):341.
157. King ER, Towner E. A Case of Immune Thrombocytopenia After BNT162b2 mRNA COVID-19 Vaccination. Am J Case Rep (2021) 22:e931478. doi: 10.12659/AJCR.931478
158. Maayan H, Kirgner I, Gutwein O, Herzog-Tzarfati K, Rahimi-Levene N, Koren-Michowitz M, et al. Acquired Thrombotic Thrombocytopenic Purpura: A Rare Disease Associated With BNT162b2 Vaccine. J Thromb Haemost (2021) 19(9):2314–7. doi: 10.1111/jth.15420
159. Matsumura A, Katsuki K, Akimoto M, Sakuma T, Nakajima Y, Miyazaki T, et al. [Immune Thrombocytopenia After BNT162b2 mRNA COVID-19 Vaccination]. Rinsho Ketsueki (2021) 62(11):1639–42.
160. Rodriguez C, Perez-Nieva A, Maiz L, Meijon MDM, Llamas P, Monreal M, et al. Vaccine-Induced Immune Thrombotic Thrombocytopenia After the BNT162b2 mRNA Covid-19 Vaccine: A Case Study. Thromb Res (2021) 208:1–3. doi: 10.1016/j.thromres.2021.10.002
161. Waqar SHB, Khan AA, Memon S. Thrombotic Thrombocytopenic Purpura: A New Menace After COVID Bnt162b2 Vaccine. Int J Hematol (2021) 114(5):626–9. doi: 10.1007/s12185-021-03190-y
162. Yoshida K, Sakaki A, Matsuyama Y, Mushino T, Matsumoto M, Sonoki T, et al. Acquired Thrombotic Thrombocytopenic Purpura Following BNT162b2 mRNA Coronavirus Disease Vaccination in a Japanese Patient. Intern Med (2021) 61(3):407–412. doi: 10.2169/internalmedicine.8568-21
163. Hippisley-Cox J, Patone M, Mei XW, Saatci D, Dixon S, Khunti K, et al. Risk of Thrombocytopenia and Thromboembolism After Covid-19 Vaccination and SARS-CoV-2 Positive Testing: Self-Controlled Case Series Study. BMJ (2021) 374:n1931. doi: 10.1136/bmj.n1931
164. Simpson CR, Shi T, Vasileiou E, Katikireddi SV, Kerr S, Moore E, et al. First-Dose ChAdOx1 and BNT162b2 COVID-19 Vaccines and Thrombocytopenic, Thromboembolic and Hemorrhagic Events in Scotland. Nat Med (2021) 27(7):1290–7. doi: 10.1038/s41591-021-01408-4
165. Hines A, Shen JG, Olazagasti C, Shams S. Immune Thrombocytopenic Purpura and Acute Liver Injury After COVID-19 Vaccine. BMJ Case Rep (2021) 14(7):e242678. doi: 10.1136/bcr-2021-242678
166. Karabulut K, Andronikashvili A, Kapici AH. Recurrence of Thrombotic Thrombocytopenic Purpura After mRNA-1273 COVID-19 Vaccine Administered Shortly After COVID-19. Case Rep Hematol (2021) 2021:4130138. doi: 10.1155/2021/4130138
167. Malayala SV, Mohan G, Vasireddy D, Atluri P. Purpuric Rash and Thrombocytopenia After the mRNA-1273 (Moderna) COVID-19 Vaccine. Cureus (2021) 13(3):e14099. doi: 10.7759/cureus.14099
168. Su PH, Yu YC, Chen WH, Lin HC, Chen YT, Cheng MH, et al. Case Report: Vaccine-Induced Immune Thrombotic Thrombocytopenia in a Pancreatic Cancer Patient After Vaccination With Messenger RNA-1273. Front Med (Lausanne) (2021) 8:772424. doi: 10.3389/fmed.2021.772424
169. Toom S, Wolf B, Avula A, Peeke S, Becker K. Familial Thrombocytopenia Flare-Up Following the First Dose of mRNA-1273 Covid-19 Vaccine. Am J Hematol (2021) 96(5):E134–5. doi: 10.1002/ajh.26128
170. Welsh KJ, Baumblatt J, Chege W, Goud R, Nair N. Thrombocytopenia Including Immune Thrombocytopenia After Receipt of mRNA COVID-19 Vaccines Reported to the Vaccine Adverse Event Reporting System (VAERS). Vaccine (2021) 39(25):3329–32. doi: 10.1016/j.vaccine.2021.04.054
171. Lee EJ, Cines DB, Gernsheimer T, Kessler C, Michel M, Tarantino MD, et al. Thrombocytopenia Following Pfizer and Moderna SARS-CoV-2 Vaccination. Am J Hematol (2021) 96(5):534–7. doi: 10.1002/ajh.26132
172. Smadja DM, Yue QY, Chocron R, Sanchez O, Lillo-Le Louet A. Vaccination Against COVID-19: Insight From Arterial and Venous Thrombosis Occurrence Using Data From VigiBase. Eur Respir J (2021) 58(1):2100956. doi: 10.1183/13993003.00956-2021
173. Schulz JB, Berlit P, Diener HC, Gerloff C, Greinacher A, Klein C, et al. COVID-19 Vaccine-Associated Cerebral Venous Thrombosis in Germany. Ann Neurol (2021) 90(4):627–39. doi: 10.1002/ana.26172
174. Krzywicka K, Heldner MR, Sanchez van Kammen M, van Haaps T, Hiltunen S, Silvis SM, et al. Post-SARS-CoV-2-Vaccination Cerebral Venous Sinus Thrombosis: An Analysis of Cases Notified to the European Medicines Agency. Eur J Neurol (2021) 28(11):3656–62. doi: 10.1111/ene.15029
175. Cines DB, Bussel JB. SARS-CoV-2 Vaccine-Induced Immune Thrombotic Thrombocytopenia. N Engl J Med (2021) 384(23):2254–6. doi: 10.1056/NEJMe2106315
176. Torjesen I. Covid-19: Risk of Cerebral Blood Clots From Disease is 10 Times That From Vaccination, Study Finds. BMJ (2021) 373:n1005. doi: 10.1136/bmj.n1005
177. Cooper N, Ghanima W. Immune Thrombocytopenia. N Engl J Med (2019) 381(10):945–55. doi: 10.1056/NEJMcp1810479
178. Neunert C, Noroozi N, Norman G, Buchanan GR, Goy J, Nazi I, et al. Severe Bleeding Events in Adults and Children With Primary Immune Thrombocytopenia: A Systematic Review. J Thromb Haemost (2015) 13(3):457–64. doi: 10.1111/jth.12813
179. Bizjak M, Bruck O, Kanduc D, Praprotnik S, Shoenfeld Y. Vaccinations and Secondary Immune Thrombocytopenia With Antiphospholipid Antibodies by Human Papillomavirus Vaccine. Semin Hematol (2016) 53 Suppl 1:S48–50. doi: 10.1053/j.seminhematol.2016.04.014
180. David P, Dotan A, Mahroum N, Shoenfeld Y. Immune Thrombocytopenic Purpura (ITP) Triggered by COVID-19 Infection and Vaccination. Isr Med Assoc J (2021) 23(6):378–80.
181. Vivarelli M, Massella L, Ruggiero B, Emma F. Minimal Change Disease. Clin J Am Soc Nephrol (2017) 12(2):332–45. doi: 10.2215/CJN.05000516
182. Clajus C, Spiegel J, Brocker V, Chatzikyrkou C, Kielstein JT. Minimal Change Nephrotic Syndrome in an 82 Year Old Patient Following a Tetanus-Diphteria-Poliomyelitis-Vaccination. BMC Nephrol (2009) 10:21. doi: 10.1186/1471-2369-10-21
183. Kielstein JT, Termuhlen L, Sohn J, Kliem V. Minimal Change Nephrotic Syndrome in a 65-Year-Old Patient Following Influenza Vaccination. Clin Nephrol (2000) 54(3):246–8.
184. Kikuchi Y, Imakiire T, Hyodo T, Higashi K, Henmi N, Suzuki S, et al. Minimal Change Nephrotic Syndrome, Lymphadenopathy and Hyperimmunoglobulinemia After Immunization With a Pneumococcal Vaccine. Clin Nephrol (2002) 58(1):68–72. doi: 10.5414/CNP58068
185. Lebedev L, Sapojnikov M, Wechsler A, Varadi-Levi R, Zamir D, Tobar A, et al. Minimal Change Disease Following the Pfizer-BioNTech COVID-19 Vaccine. Am J Kidney Dis (2021) 78(1):142–5. doi: 10.1053/j.ajkd.2021.03.010
186. D'Agati VD, Kudose S, Bomback AS, Adamidis A, Tartini A. Minimal Change Disease and Acute Kidney Injury Following the Pfizer-BioNTech COVID-19 Vaccine. Kidney Int (2021) 100(2):461–3. doi: 10.1016/j.kint.2021.04.035
187. Maas RJ, Gianotten S, van der Meijden WAG. An Additional Case of Minimal Change Disease Following the Pfizer-BioNTech COVID-19 Vaccine. Am J Kidney Dis (2021) 78(2):312. doi: 10.1053/j.ajkd.2021.05.003
188. Komaba H, Wada T, Fukagawa M. Relapse of Minimal Change Disease Following the Pfizer-BioNTech COVID-19 Vaccine. Am J Kidney Dis (2021) 78(3):469–70. doi: 10.1053/j.ajkd.2021.05.006
189. Thappy S, Thalappil SR, Abbarh S, Al-Mashdali A, Akhtar M, Alkadi MM. Minimal Change Disease Following the Moderna COVID-19 Vaccine: First Case Report. BMC Nephrol (2021) 22(1):376. doi: 10.1186/s12882-021-02583-9
190. Kudose S, Friedmann P, Albajrami O, D'Agati VD. Histologic Correlates of Gross Hematuria Following Moderna COVID-19 Vaccine in Patients With IgA Nephropathy. Kidney Int (2021) 100(2):468–9. doi: 10.1016/j.kint.2021.06.011
191. Negrea L, Rovin BH. Gross Hematuria Following Vaccination for Severe Acute Respiratory Syndrome Coronavirus 2 in 2 Patients With IgA Nephropathy. Kidney Int (2021) 99(6):1487. doi: 10.1016/j.kint.2021.03.002
192. Anupama YJ, Patel RGN, Vankalakunti M. Nephrotic Syndrome Following ChAdOx1 Ncov-19 Vaccine Against SARScoV-2. Kidney Int Rep (2021) 6(8):2248. doi: 10.1016/j.ekir.2021.06.024
193. Biradar V, Konnur A, Gang S, Hegde U, Rajapurkar M, Patel H, et al. Adult-Onset Nephrotic Syndrome Following Coronavirus Disease Vaccination. Clin Kidney J (2022) 15(1):168–70. doi: 10.1093/ckj/sfab153
194. Leclerc S, Royal V, Lamarche C, Laurin LP. Minimal Change Disease With Severe Acute Kidney Injury Following the Oxford-AstraZeneca COVID-19 Vaccine: A Case Report. Am J Kidney Dis (2021) 78(4):607–10. doi: 10.1053/j.ajkd.2021.06.008
195. Lineburg KE, Neller MA, Ambalathingal GR, Le Texier L, Raju J, Swaminathan S, et al. Rapid Whole-Blood Assay to Detect SARS-CoV-2-Specific Memory T-Cell Immunity Following a Single Dose of AstraZeneca ChAdOx1-S COVID-19 Vaccine. Clin Transl Immunol (2021) 10(8):e1326. doi: 10.1002/cti2.1326
196. Sekar A, Campbell R, Tabbara J, Rastogi P. ANCA Glomerulonephritis After the Moderna COVID-19 Vaccination. Kidney Int (2021) 100(2):473–4. doi: 10.1016/j.kint.2021.05.017
197. Anderegg MA, Liu M, Saganas C, Montani M, Vogt B, Huynh-Do U, et al. De Novo Vasculitis After mRNA-1273 (Moderna) Vaccination. Kidney Int (2021) 100(2):474–6. doi: 10.1016/j.kint.2021.05.016
198. Chen CC, Chen HY, Lu CC, Lin SH. Case Report: Anti-Neutrophil Cytoplasmic Antibody-Associated Vasculitis With Acute Renal Failure and Pulmonary Hemorrhage May Occur After COVID-19 Vaccination. Front Med (Lausanne) (2021) 8:765447. doi: 10.3389/fmed.2021.765447
199. Avci E, Abasiyanik F. Autoimmune Hepatitis After SARS-CoV-2 Vaccine: New-Onset or Flare-Up? J Autoimmun (2021) 125:102745. doi: 10.1016/j.jaut.2021.102745
200. Clayton-Chubb D, Schneider D, Freeman E, Kemp W, Roberts SK. Autoimmune Hepatitis Developing After the ChAdOx1 Ncov-19 (Oxford-AstraZeneca) Vaccine. J Hepatol (2021) 75(5):1249–50. doi: 10.1016/j.jhep.2021.06.014
201. Rela M, Jothimani D, Vij M, Rajakumar A, Rammohan A. Auto-Immune Hepatitis Following COVID Vaccination. J Autoimmun (2021) 123:102688. doi: 10.1016/j.jaut.2021.102688
202. Zin Tun GS, Gleeson D, Al-Joudeh A, Dube A. Immune-Mediated Hepatitis With the Moderna Vaccine, No Longer a Coincidence But Confirmed. J Hepatol (2021) 76(3):747–49. doi: 10.1016/j.jhep.2021.09.031
203. Watad A, De Marco G, Mahajna H, Druyan A, Eltity M, Hijazi N, et al. Immune-Mediated Disease Flares or New-Onset Disease in 27 Subjects Following mRNA/DNA SARS-CoV-2 Vaccination. Vaccines (Basel) (2021) 9(5):435. doi: 10.3390/vaccines9050435
204. Ishay Y, Kenig A, Tsemach-Toren T, Amer R, Rubin L, Hershkovitz Y, et al. Autoimmune Phenomena Following SARS-CoV-2 Vaccination. Int Immunopharmacol (2021) 99:107970. doi: 10.1016/j.intimp.2021.107970
205. Trimboli M, Zoleo P, Arabia G, Gambardella A. Guillain-Barre Syndrome Following BNT162b2 COVID-19 Vaccine. Neurol Sci (2021) 42(11):4401–2. doi: 10.1007/s10072-021-05523-5
206. Helmchen C, Buttler GM, Markewitz R, Hummel K, Wiendl H, Boppel T. Acute Bilateral Optic/Chiasm Neuritis With Longitudinal Extensive Transverse Myelitis in Longstanding Stable Multiple Sclerosis Following Vector-Based Vaccination Against the SARS-CoV-2. J Neurol (2022) 269(1):49–54. doi: 10.1007/s00415-021-10647-x
207. Kaulen LD, Doubrovinskaia S, Mooshage C, Jordan B, Purrucker J, Haubner C, et al. Neurological Autoimmune Diseases Following Vaccinations Against SARS-CoV-2: A Case Series. Eur J Neurol (2022) 29(2):555–63. doi: 10.1111/ene.15147
Keywords: COVID-19 vaccine, vaccine hesitancy, autoimmune side effects, Guillain-Barre syndrome, myocarditis, vaccine-induced immune thrombotic thrombocytopenia
Citation: Mahroum N, Lavine N, Ohayon A, Seida R, Alwani A, Alrais M, Zoubi M and Bragazzi NL (2022) COVID-19 Vaccination and the Rate of Immune and Autoimmune Adverse Events Following Immunization: Insights From a Narrative Literature Review. Front. Immunol. 13:872683. doi: 10.3389/fimmu.2022.872683
Received: 09 February 2022; Accepted: 30 May 2022;
Published: 05 July 2022.
Edited by:
Alain Le Moine, Université libre de Bruxelles, BelgiumReviewed by:
Harry Alexopoulos, National and Kapodistrian University of Athens, GreeceChris von Csefalvay, Starschema Inc., United States
Copyright © 2022 Mahroum, Lavine, Ohayon, Seida, Alwani, Alrais, Zoubi and Bragazzi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nicola Luigi Bragazzi, YnJhZ2F6emlAeW9ya3UuY2E=
 Naim Mahroum
Naim Mahroum Noy Lavine2,3
Noy Lavine2,3 Aviran Ohayon
Aviran Ohayon Mahmoud Alrais
Mahmoud Alrais Magdi Zoubi
Magdi Zoubi Nicola Luigi Bragazzi
Nicola Luigi Bragazzi