
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 04 July 2022
Sec. Autoimmune and Autoinflammatory Disorders
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.871705
This article is part of the Research Topic Intestinal and Liver Autoimmunity: Unique Pathogenesis, Diseases and Crosstalk View all 5 articles
Aim: Our objective was to investigate whether Bifidobacterium infantis inhibits PI3K-Akt-mTOR signaling and upregulates Foxp3 expression through PD-L1 and to explore the possible mechanism of action of B. infantis in cellular immunosuppression.
Method: The effects of B. infantis supernatant on PD-L1, PD-1, Foxp3, and the PI3K-Akt-mTOR signaling pathway were observed by culturing HCT-116 cells. Simultaneously, the effects of blocking PD-L1 on PD-1, on Foxp3 protein and mRNA, and on the PI3K-Akt-mTOR signaling pathway protein were observed.
Results: B. infantis supernatant was able to upregulate the protein and mRNA expression of PD-L1 and Foxp3 and downregulate the phosphorylated protein expression of PI3K, Akt, and mTOR (P < 0.05); however, for PI3K, Akt, and mTOR, there was no change in the total protein expression. After the blocking of PD-L1, the stimulatory effect of B. infantis supernatant on Foxp3 and the inhibitory effect on the phosphorylated protein expression of PI3K, Akt, and mTOR were weakened.
Conclusion: B. infantis may inhibit the PI3K-Akt-mTOR signaling pathway and promote the expression of Foxp3 through PD-L1, which may be a target via which B. infantis exerts its immunosuppressive effect.
Inflammatory bowel disease (IBD) is a disease of the digestive system that seriously endangers the health of the sufferer (1). IBD often damages organs outside the digestive tract, including the eyes, skin, mucosa, joints, liver, and pancreas. Patients suffering from IBD usually exhibit gastrointestinal bleeding, fistula, perforation, cancer, and other complications, which can affect their quality of life and ability to work (2). These conditions endanger the health of the patients and impose an economic burden on their families (3, 4). It is therefore important to find an effective treatment for this condition.
Forkhead box protein 3 (Foxp3) is a regulatory T cell (Treg) marker that exerts an immunosuppressive effect (5). Foxp3+Tregs, which stably express Foxp3, are primarily produced in the thymus and move to the periphery where they exert an immunosuppressive effect, while Foxp3−Tregs have no such function (6). Therefore, Foxp3 plays a key role in immunosuppression and cell stability. Our research group previously found that Bifidobacterium infantis (B. infantis) can promote the expression of Foxp3 in Tregs by upregulating PD-L1, thereby promoting cell proliferation and improving the anti-inflammatory function of these cells, so as to inhibit the intestinal immune response, and this provided new clues for the immunosuppressive treatment of IBD (7). However, the mechanism by which B. infantis promotes Foxp3 expression remains to be evaluated.
The PI3K-Akt-mTOR signaling pathway is involved in cell growth, differentiation, and apoptosis and shows crosstalk with other important signal transduction pathways (8). The differentiation and proliferation of initial T lymphocytes depends on the activation of the Akt-mTOR signaling pathway (9). Activation of this signaling pathway can inhibit the differentiation of Tregs (10), while its inhibition promotes an increase in Foxp3 expression and affects the Treg counts in animals. Francisco et al. (11) found that PD-L1-negative antigen-presenting cells induce the transformation of CD4+T cells into Foxp3+Tregs to a minimal extent, suggesting the importance of PD-L1 in Treg differentiation. Simultaneously, they found that PD-L1 can antagonize the Akt-mTOR signaling pathway, which provided a new idea for studies on the mechanism by which PD-L1 regulates Tregs. However, there are few studies on the identity of the signaling pathway modulated by B. infantis, which triggers the immune response.
Based on the above theory, we speculated that B. infantis could increase the expression of Foxp3 by upregulating PD-L1 and inhibiting PI3K-Akt-mTOR signaling. Several studies have shown that Foxp3, PD-L1, and PD-1 are expressed in HCT-116 cells (12–14). Therefore, we chose this cell line as a model to study whether B. infantis could inhibit PI3K-Akt-mTOR signaling and upregulate Foxp3 expression through PD-L1, so as to further explore the possible mechanism by which B. infantis exerts an immunosuppressive effect.
The HCT-116 cell line was purchased from ATCC, USA. B. infantis CGMCC No. 0313-2 (Batch No. 2017012) was provided Kexing Biological Products Co., Ltd, Shandong, China. Anti-PD-L1 was purchased from BIOX cell, New Hampshire, USA. Protein marker (m000624) was purchased from Kingsley Biotechnology Co., Ltd, Nanjing, Jiangsu, China. Polyvinylidene fluoride (PVDF) membranes were purchased from the GE company, Fairfield, Connecticut, USA. GAPDH antibody, phospho-PI3K antibody, and phospho-mTOR antibody were purchased from Abcam, USA. PD-L1, PD-1, Foxp3, AKT1, and mTOR antibodies were purchased from Proteintech, USA. PI3K, phospho-Akt, PTEN, and phospho-PTEN antibodies were purchased from CST, USA. Sensitive chemiluminescence solution was sourced from Millipore, USA. PrimeScript RT reagent kit with gDNA Eraser was purchased from TaKaRa Bio, Japan. The qRT-PCR kit SYBR Premix Ex Taq II (TLI RNaseH Plus) was from TaKaRa. TPY agar medium and TPY broth were purchased from Qingdao Haibo Biotechnology Co., Ltd, China. All primers were purchased from China Bio Engineering Co., Ltd. The turbidimetric tube was purchased from China Wenzhou Kangtai Biotechnology Co., Ltd.
HCT-116 cells were cultured in McCoy’s 5A medium containing 10% fetal bovine serum, 1% penicillin-streptomycin, and double antibody at 37°C and 5% carbon dioxide. The culture medium was changed every 24–48 hours. When the confluency reached ~80%, the cell line was passaged.
B. infantis lyophilized powder was diluted in a sterile solution. A loopful of liquid bacterial culture was inoculated with the bacterial liquid and then inoculated on the TPY plate medium according to the three-zone marking method under a flame. The culture dish was placed in an inverted position in a round-bottom three-dimensional anaerobic culture bag and incubated at 37°C for 48 h.
Colony morphology was observed on the culture medium and attention was paid to no presence of any miscellaneous bacteria. The anaerobic indicator color change was checked to ensure the anaerobic state. A single colony was selected, coated on the slide, dried, and fixed. After staining and decolorization, the morphology of the bacteria was observed under an oil immersion objective and photographed.
B. infantis colonies were collected and inoculated in sterile normal saline. The concentration was adjusted to 3 × 109 CFU/ml in turbidimetric tubes and centrifuged at 5000 rpm. B. infantis was cultured in TPY broth at 37°C for 96 h. After centrifugation, the supernatant was filtered using a 0.22-μm pore filter to confirm that the supernatant was sterile. Aseptic conditions were observed throughout the procedure.
Cells in the logarithmic growth phase were seeded in 6-well plates and cultured for 12 h. After confirming adherence, the cells were cultured for 24 h with the medium. In total, we tested five groups: control group, TPY group, TPY+B. infantis group, TPY+B. infantis +PD-L1 Blockade group, and TPY+PD-L1 Blockade group.
After immobilization with 4% paraformaldehyde, samples were blocked using endogenous peroxidase and nonspecific antigens. The primary antibodies were used to probe the samples at the following dilutions: PD-1, 1:200 (Proteintech, 18106-1-AP); PD-L1, 1:50 (Proteintech, 17952-1-AP); and Foxp3, 1:300 (Proteintech, 22228-1-AP). After incubation with the secondary antibody, horseradish labeling, DAB color development, restaining, and dehydration, three low-power microscope fields were randomly selected to investigate protein localization and detection.
The total cell proteins were extracted, the protein concentration was determined, and protein samples of 40 μg were prepared. The proteins were separated via 60-V constant pressure electrophoresis, membrane transferred at 100 V, and blocked using 2.5% BSA. The PVDF membrane was developed in a dark room to detect the protein bands. Gelpro software was used to analyze gel image results and perform protein quantitative analysis. The specific antibody dilutions were as follows:

qRT-PCR was carried out after RNA extraction and purity evaluation, reverse transcription, and cDNA synthesis and purity evaluation. The primer sequences are shown in Table 1.
SPSS 23.0 and GraphPad 7.0 statistical software were used for analysis. The measurement data are expressed as mean ± standard deviation. Analysis of variance was used for comparison between groups, with P < 0.05 regarded as statistically significant.
In the negative control group, the nuclei showed dark blue staining, cell membranes showed no obvious staining, and the cytoplasm showed light blue staining, with a small amount of uneven light brown particles, as shown in Figure 1A. After PD-L1 positioning, the nucleus was stained dark blue, a few brown granules were found in several nuclei, the cell membrane was stained with dark brown granules, the cytoplasm was uniformly stained light blue, and a few yellow granules were scattered, as shown in Figure 1B. After PD-1 positioning, the nucleus was stained dark blue, brown granules were deposited in individual nuclei, a large number of dark brown granules were observed in the cell membrane, the cytoplasm was uniformly stained light blue, and yellow granules were observed, as shown in Figure 1C. After Foxp3 positioning, the nucleus was stained dark blue, a large number of brown granules were found in the nucleolus and nuclear membrane, almost no dark brown granules were found in the cell membrane, the cytoplasm was uniformly stained light blue in the middle, and yellow granules were found in the cytoplasm of the individual cells, as shown in Figure 1D.
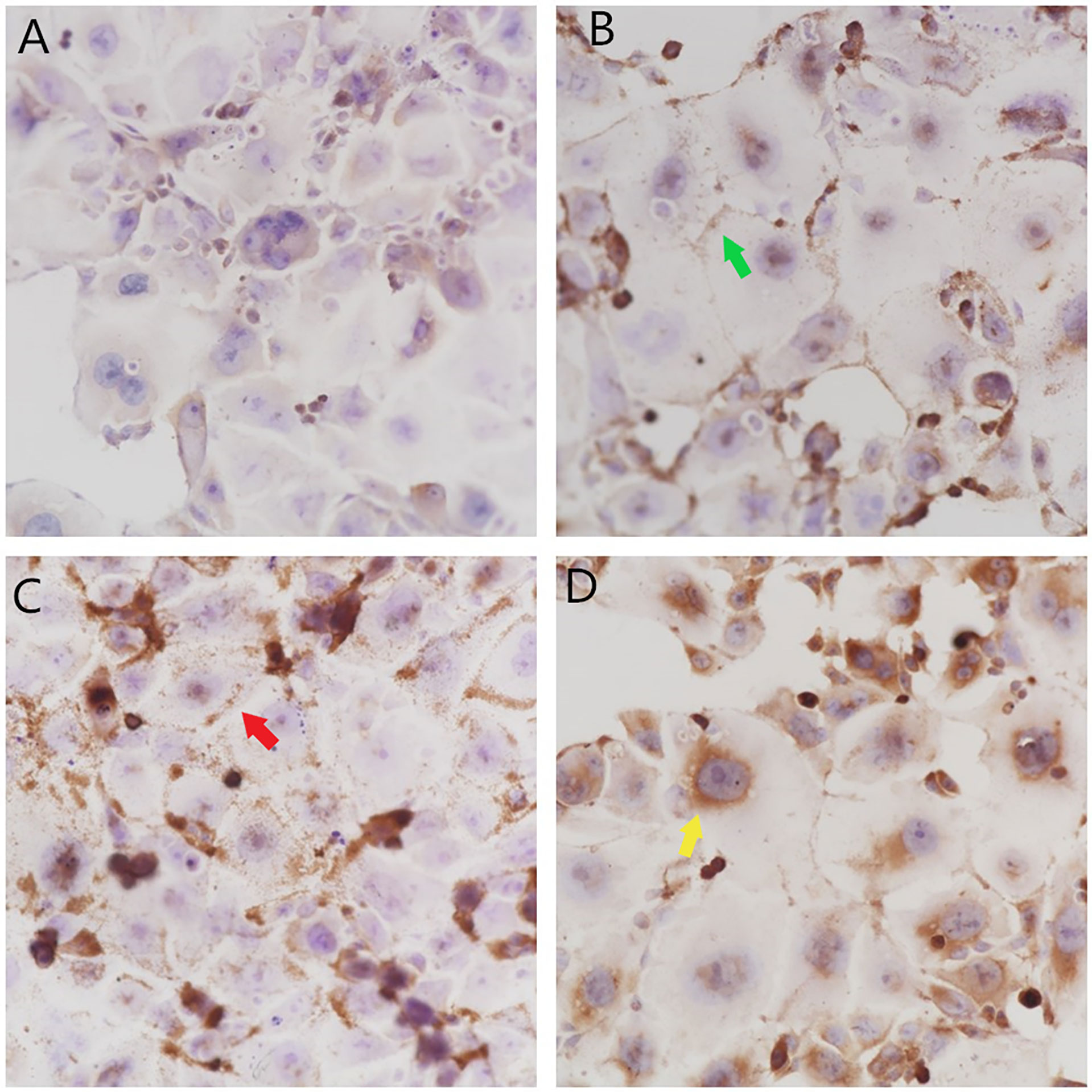
Figure 1 Localization and expression of PD-L1, PD-1, and Foxp3 in colon HCT-116 cells. (A) The negative control group (× 400) showed no obvious brown granule deposition. (B) The PD-L1 group (× 400) showed brown granule deposition on the cell membrane  and a small amount of brown granule deposition in the cytoplasm. (C) The PD-1 group (× 400) showed a large amount of brown granule deposition on the cell membrane
and a small amount of brown granule deposition in the cytoplasm. (C) The PD-1 group (× 400) showed a large amount of brown granule deposition on the cell membrane  and a small amount of brown granule deposition in the cytoplasm. (D) The Foxp3 group (× 400) showed a large amount of brown granule deposition in the nucleolus and on the nuclear membrane
and a small amount of brown granule deposition in the cytoplasm. (D) The Foxp3 group (× 400) showed a large amount of brown granule deposition in the nucleolus and on the nuclear membrane .
.
B.infantis impoved the expression of PD-L1.There was no significant difference in PD-L1 mRNA or protein expression between the TPY and control groups. Compared with that in the TPY group, the expression of the PD-L1 protein and mRNA in the TPY+B. infantis group was significantly higher (P < 0.05), as shown in Figure 2.
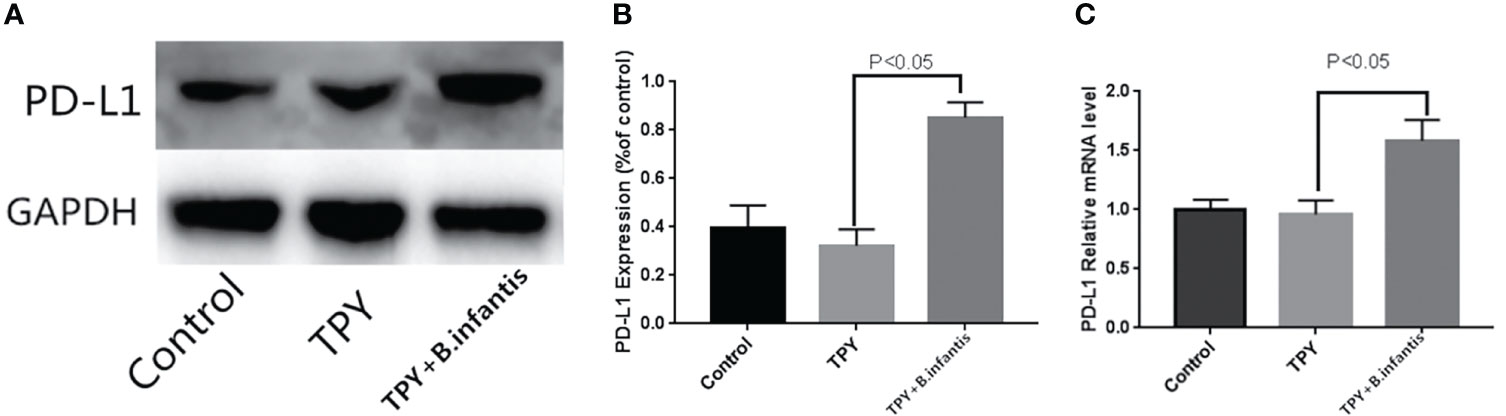
Figure 2 Effect of Bifidobacterium infantis supernatant on PD-L1 expression. (A) PD-L1 protein expression band. (B) Differences in PD-L1 protein expression. (C) Differences in PD-L1 mRNA expression. The data in the figure are expressed as mean ± standard deviation, and the sample comparison among the groups was conducted using one-way ANOVA. P < 0.05 was considered statistically significant.
B.infantis didn’t promote or lead to the protein expression of PD-1, but inhibited the RNA expression of PD-1 interestingly. TPY broth increased the RNA expression of PD1, and this promotion was weakened after blocking PD-L1. There was no significant difference in the expression of PD-1 protein between the TPY group and control group (P = 0.11). Similarly, there was no change in PD-1 protein expression between the TPY and TPY+B. infantis groups (P = 0.90). There was no significant difference in PD-1 protein expression between the TPY+B. infantis and TPY+B. infantis+PD-L1 Blockade groups (P = 0.13). Compared with that in the TPY group, there was no significant difference in the expression of the PD-1 protein in the TPY+PD-L1 Blockade group (P = 0.14), as shown in Figure 3.
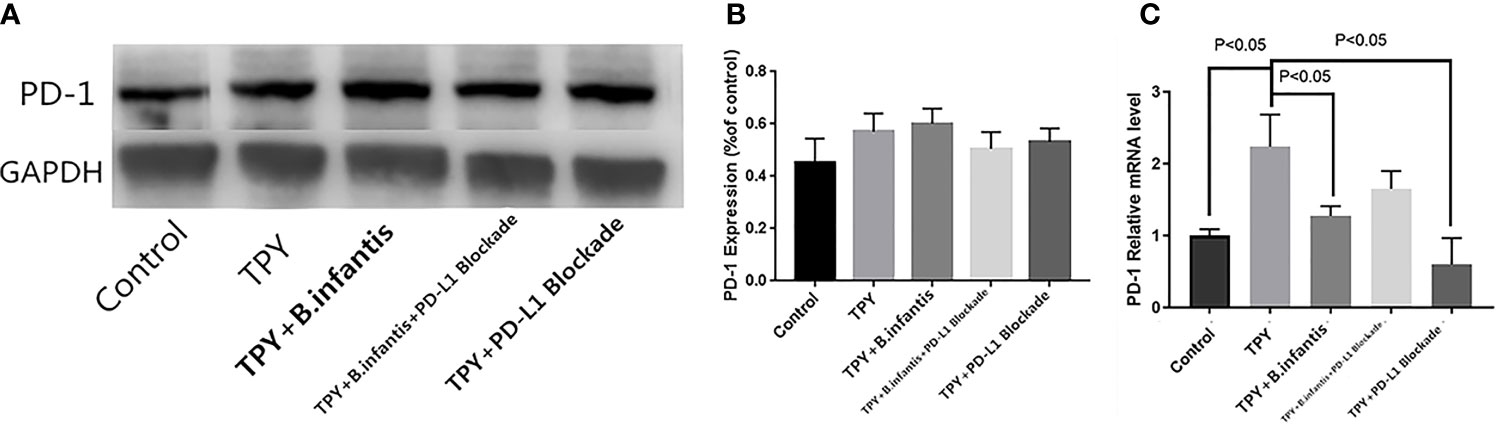
Figure 3 Effect of Bifidobacterium infantis supernatant on PD-1 expression. (A) PD-1 protein expression band. (B) Differences in PD-1 protein expression. (C) Differences in PD-1 mRNA expression. The data in the figure are expressed as mean ± standard deviation, and the sample comparison among the groups was conducted using one-way ANOVA. P < 0.05 was considered statistically significant.
The expression of PD-1 mRNA differed from the protein expression. Compared with that in the control group, the expression of PD-1 mRNA in the TPY group was significantly higher (P < 0.05), while compared with that in the TPY group, the expression of PD-1 mRNA in the TPY+B. infantis group was significantly lower (P < 0.05). After adding the PD-L1 blocker, the expression of PD-1 mRNA in the TPY group also decreased significantly (P < 0.05), as shown in Figure 3.
TPY broth dramatically decreased Foxp3 RNA, but had no significant effect on its protein. The addition of B.infantis significantly increased the expression of Foxp3. However, after blocking PD-L1, the promotion effect was also neutralized. Compared with that in the control group, Foxp3 protein expression in the TPY group showed no significant change (P = 0.13). Compared with that in the TPY group, Foxp3 protein expression in the TPY+B. infantis group was significantly higher (P < 0.05). Compared with that in the TPY+B. infantis+PD-L1 Blockade group, Foxp3 protein expression in the TPY+B. infantis group was significantly lower (P < 0.05). There was no significant difference in Foxp3 protein expression between the TPY group and the TPY+PD-L1 Blockade group (P = 0.14), as shown in Figure 4.
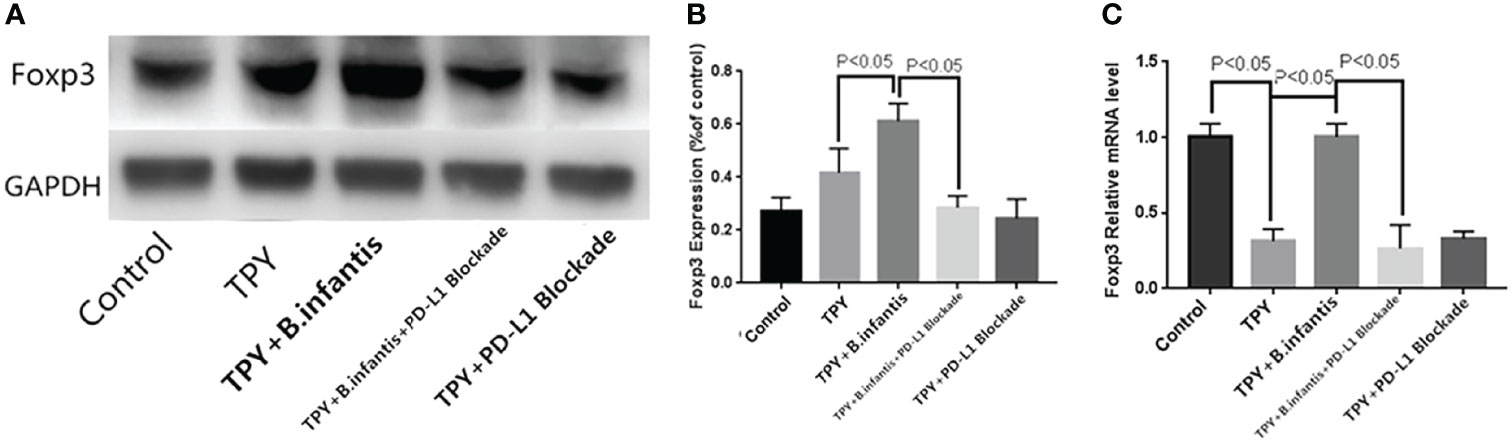
Figure 4 Effect of Bifidobacterium infantis supernatant on Foxp3 expression. (A) Foxp3 protein expression band. (B) Differences in Foxp3 protein expression. (C) Differences in Foxp3 mRNA expression. The data in the figure are expressed as mean ± standard deviation, and the sample comparison among the groups was conducted using one-way ANOVA. P < 0.05 was considered statistically significant.
Compared with that in the control group, Foxp3 mRNA expression in the TPY group was significantly higher (P < 0.05). Compared with that in the TPY group, the expression level of Foxp3 mRNA in the TPY+B. infantis group was significantly higher (P < 0.05). After adding the PD-L1 blocker, the expression of Foxp3 mRNA in the TPY+B. infantis group decreased significantly (P < 0.05). There was no significant difference in Foxp3 mRNA expression between the TPY group and TPY+PD-L1 Blockade group (P = 0.97), as shown in Figure 4.
B.infantis had inhibitory effects on p-MTOR, p-Akt and p-PI3K, and this inhibitory effect disappeared after blocking PD-L1. The addition of B.infantis did not change mTOR, Akt and PI3K. The effect of B.infantis on p-PTEN and PTEN was opposite to that of PI3K-Akt-mTOR pathway. Compared with that in the TPY group, the whole phosphorylated protein expression of PI3K, Akt, and mTOR in the TPY+B. infantis group was significantly lower (P < 0.05), while the total protein content of PI3K, Akt, and mTOR showed no significant changes (P values of 0.186, 0.178, and 0.19, respectively). Compared with that in the TPY group, The phosphorylated protein and total protein PTEN contents were significantly higher (P < 0.05). The phosphorylated protein expression of mTOR, Akt, and PI3K in the TPY+B. infantis group was significantly higher than that in the TPY+B. infantis+PD-L1 Blockade group. After adding the PD-L1 blocker, the phosphorylated protein expression of mTOR and Akt and the total protein expression of Akt in the TPY group decreased significantly (P < 0.05), while the other proteins showed no significant change (Figure 5).
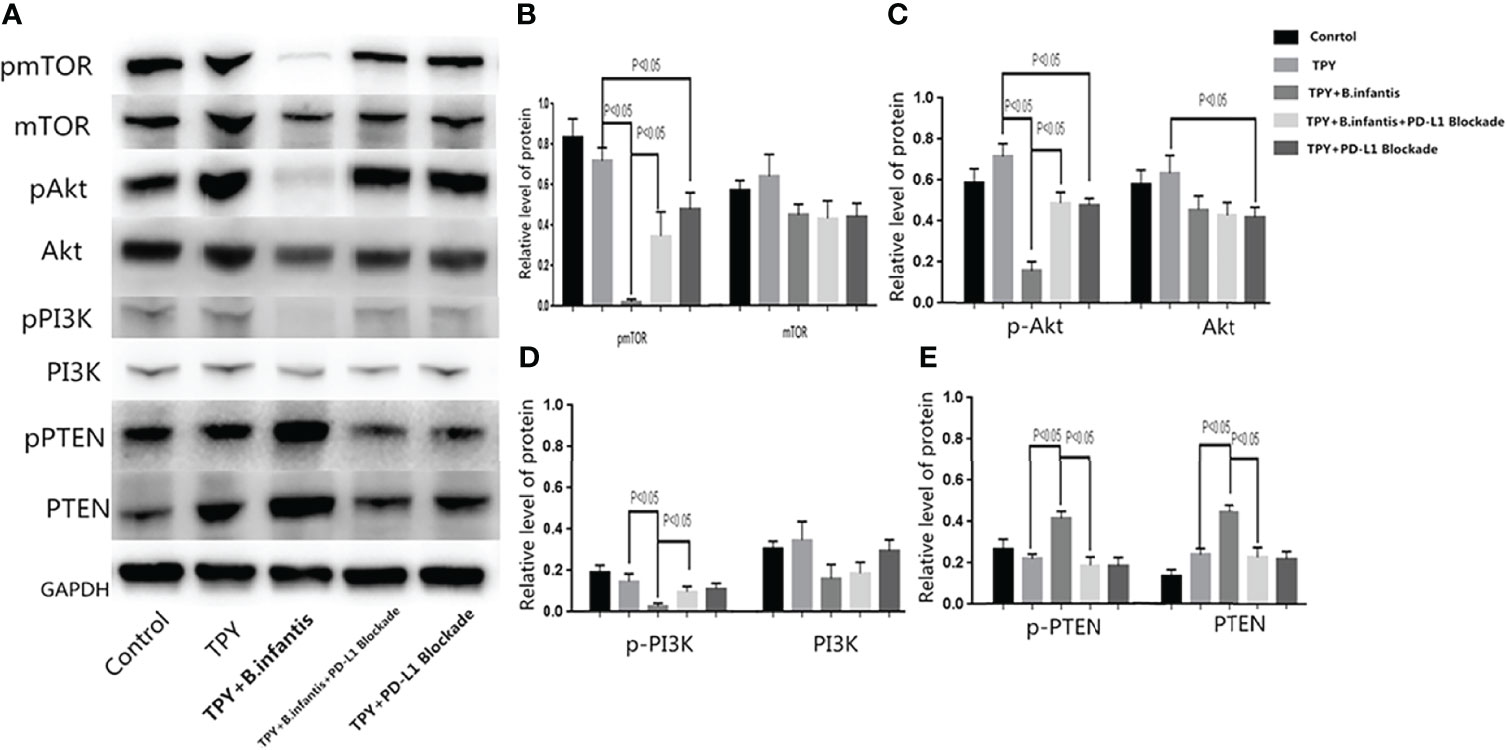
Figure 5 Effect of B. infantis supernatant on the PI3K-Akt-mTOR pathway after the blocking of PD-L1. (A) mTOR, Akt, PI3K, PTEN phosphorylated protein and total protein expression. (B) A statistical chart showing the p-mTOR and mTOR protein expression difference. (C) A statistical chart showing the p-Akt and Akt protein expression difference. (D) A statistical chart showing the p-PI3K and PI3K protein expression difference. (E) A statistical chart showing the p-PTEN and PTEN protein expression difference. The data in the figure are expressed as mean ± standard deviation, and the sample comparison among the groups was conducted using one-way ANOVA. P < 0.05 was considered statistically significant.
Probiotics are defined as living microorganisms that when given in sufficient quantities, can provide health benefits to the host (15). At present, the use of probiotics in the treatment of digestive system diseases is a promising alternative and adjuvant therapy (16) (17). Previous studies have shown that probiotics can play a therapeutic role in the regulation of immunity, gut flora composition, and protein and lipid metabolism (18, 19). We found that B. infantis mediated Foxp3 expression through PD-1/PD-L1 pathway, which promoted Tregs differentiation and improved IL-10 and TGF- β 1 expression, so as to reduce the immune and inflammatory response of mice with inflammatory bowel disease. In order to study how B. infantis transmits the signal to Foxp3, we designed the experiment in vitro. Recent studies have shown that supernatants of cultured probiotics, such as the supernatant of B. infantis, can exert the same beneficial effects as live bacteria, inhibiting the growth of pathogenic bacteria and improving epithelial barrier function and immune regulation (20, 21). However, the exact mechanism remains to be clarified.
The PI3K-Akt-mTOR signaling pathway is involved in cell proliferation, differentiation, apoptosis, and other types of cell regulation (22). When the ligand binds to the cell membrane receptor, the receptor activates phosphatidylinositol 3-kinase (PI3K), promoting the activation of a second messenger, further activating protein kinase B (Akt) and regulating cell proliferation and survival by phosphorylating mammalian target of rapamycin (mTOR) (23). PTEN is a tumor suppressor gene. As a key phosphatase, it can inhibit the formation of PI3K and reduce Akt activation and its downstream signal transduction (24). Our research showed that TPY exerted no statistically significant change in the PI3K-Akt-mTOR pathway proteins. We also found that the supernatant of B. infantis could reduce the protein expression of p-mTOR, p-Akt, and p-PI3K; however, it exerted no significant effect on the total protein content of mTOR, Akt, and PI3K, which may be due to the inhibition of PI3K-Akt-mTOR signaling/expression by fermentation products of B. infantis. B. infantis supernatant could significantly upregulate PD-L1 expression. Further, it was found that TPY broth could increase PD-1 mRNA expression but had no significant effect on the PD-1 protein. B. infantis supernatant could reduce PD-1 mRNA expression but had no effect on the PD-1 protein. We speculated that some components of the TPY broth could promote the gene transcription of PD-1, while B. infantis could counteract this effect. However, the specific mechanism remains to be studied further.
In recent years, studies have found that PD-L1 can inhibit PI3K-Akt-mTOR signaling (25, 26). PD-L1 promotes the high expression of PTEN, which can antagonize the PI3K-mediated signal, which promotes cell metabolism, growth, proliferation, and survival (27). The PI3K-Akt-mTOR signaling pathway is closely related to IBD (28). Studies have found that activation of this signal transduction pathway can activate NF-κB, which, in turn, activates TNF-α, IL-1, IL-6, and other inflammatory factors and mediators, thus amplifying and sustaining the inflammatory response. Lactobacillus can reduce the phosphorylated Akt levels in rat gastric sphincter and may further inhibit the pro-inflammatory factor IL-6, thus promoting TGF-β synthesis, so as to regulate the immune response (29). Studies have shown that B. infantis can inhibit the inflammatory response through the PI3K-Akt signaling pathway. B. infantis supernatant can regulate dendritic cell function by regulating mitogen activated protein kinase (MAPK), glycogen synthase kinase-3 (GSK3), and PI3K to different degrees through this cell pathway (30).
To confirm whether the supernatant of B. infantis affects PD-1 expression, the PI3K-Akt-mTOR signaling pathway, and Foxp3 by increasing PD-L1, we added the PD-L1 blocker to colon cells in vitro. The results showed that when PD-L1 was blocked, the B. infantis supernatant had no significant effect on the protein or mRNA expression of PD-1. The relationship between PD-L1 and PD-1 as ligands and receptors. We analyzed the blocking of ligands did not affect the changes of the receptors caused by B. infantis, while PD-L1 significantly inhibited the expression of PD-1 mRNA but did not change PD-1 protein levels. The protein expression of p-mTOR, p-Akt, and p-PI3K increased significantly in the PD-L1 antagonist group; however, the total protein expression of mTOR, Akt, and PI3K remained unchanged, while the phosphorylation and total PTEN expression decreased significantly. We believe that the action on PD-L1 is the main mechanism by which B. infantis exerts its immunosuppressive effect. After blocking PD-L1, the expression of PTEN decreased. Furthermore, the inhibition of PI3K-Akt-mTOR signaling was reduced. Compared with that in the TPY group, the expression of p-mTOR, p-Akt, and total Akt protein decreased after the PD-L1 blocker was added, suggesting that the PD-L1 protein itself may stimulate the activation of p-mTOR, p-Akt, and total Akt protein. We infer that B. infantis first promoted the massive expression of PD-L1, the combination of PD-L1 and PD-1, then inhibited the phosphorylation expression of PI3K, Akt and mTOR, finally promotes the translation of Foxp3, and then might induce Treg differentiation. However, after PI3K phosphorylation was inhibited, why did the expression of Akt and mTOR also change? We suspect that B. infantis might directly affect Akt and mTOR phosphorylation through PD-L1, rather than through the PI3K-Akt-mTOR signaling. However, the specific underlying mechanism requires further study.
B. infantis supernatant promoted the expression of Foxp3; however, the expression of Foxp3 mRNA and protein significantly decreased after blocking PD-L1, suggesting that PD-L1 is the main intermediate pathway of the B. infantis supernatant to promote the expression of Foxp3. TPY broth decreased the expression of Foxp3 mRNA; however, it had no significant effect on the Foxp3 protein. We speculated that some component of the TPY broth downregulated the transcription of Foxp3 but did not affect the translation process.
In conclusion, we found that B. infantis could inhibit PI3K-Akt-mTOR signaling and promote the expression of Foxp3 through PD-L1, so as to exert an immunosuppressive effect (see Figure 6). However, the specific mechanism needs to be explored further.
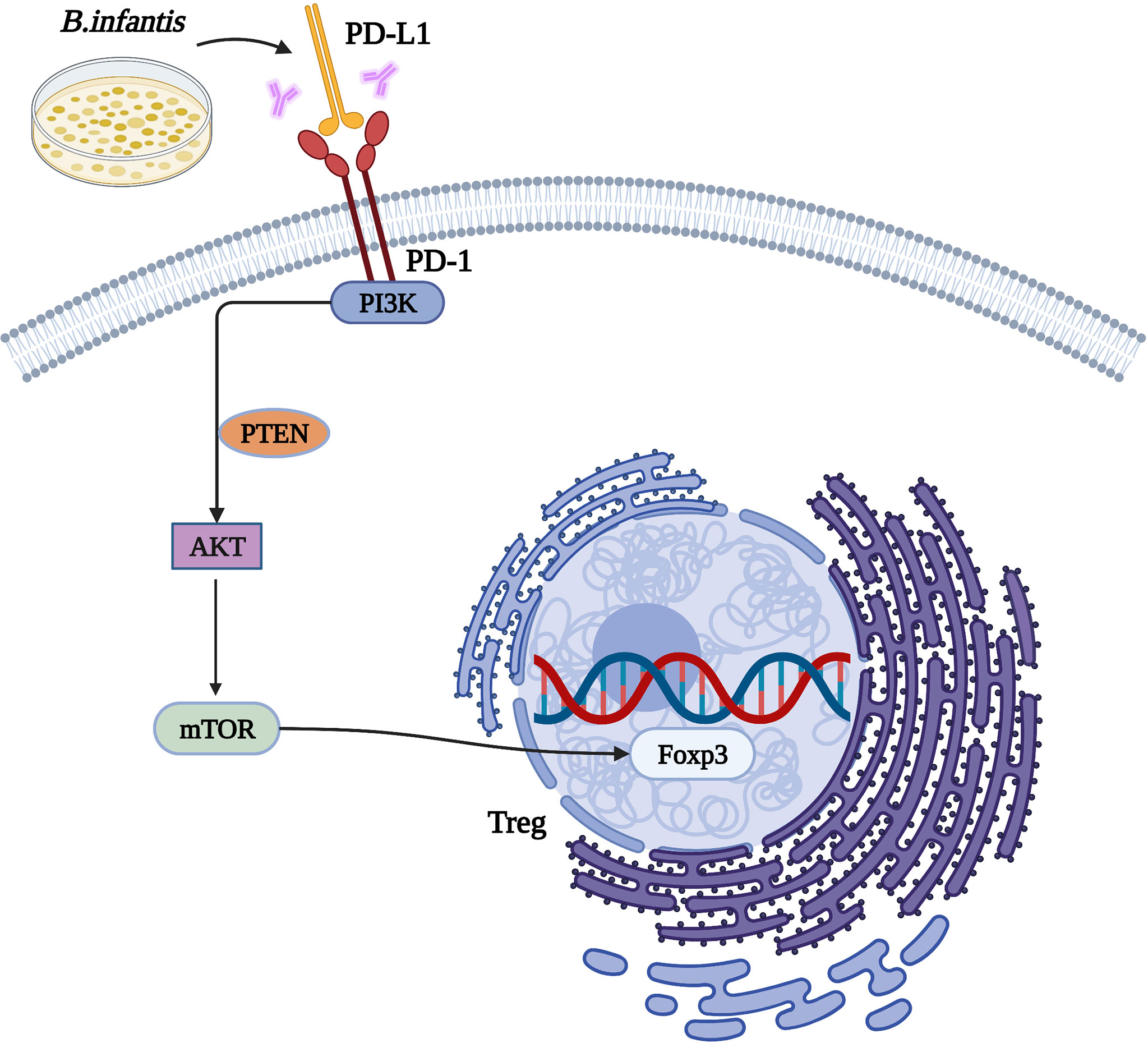
Figure 6 The mechanism by which Bifidobacterium infantis mediates the PD-1/PD-L1 pathway by inhibiting Foxp3 expression.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
LZ wrote manuscript, processed the samples, analyzed the raw data, obtained funding, and approved the final version of the manuscript. LZ and YX processed the samples, complete the experiment together. YL conceived the study protocol, critically revised the manuscript, and approved its final version. All authors contributed to the article and approved the submitted version. All authors contributed to the article and approved the submitted version.
This study was supported by the Doctoral Start-up Foundation of Liaoning Province (Contract No. 2021-BS-114).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Mahmoud R, Itzkowitz SH. Editorial: Missed Opportunities to Detect Colorectal Cancer in Inflammatory Bowel Disease—Getting to the Root. Alimentary Pharmacol Ther (2021) 53337–38. doi: 10.1093/jnci/djv303
2. Petra W, Vedel AD, Leth LEC, Johan B, Pia M. Occurrence of Colorectal Cancer and the Influence of Medical Treatment in Patients With Inflammatory Bowel Disease: A Danish Nationwide Cohort Study, 1997 to 2015. Inflammatory Bowel Dis (2021) 27:1795–803. doi: 10.1093/ibd/izaa340
3. Kaplan GG, Ng SC. Globalisation of Inflammatory Bowel Disease: Perspectives From the Evolution of Inflammatory Bowel Disease in the UK and China. Lancet Gastroenterol Hepatol (2016) 1:307–16. doi: 10.1016/S2468-1253(16)30077-2
4. Hashash JG, Binion DG. Exercise and Inflammatory Bowel Disease: Insights Into Etiopathogenesis and Modification of Clinical Course. Gastroenterol Clinics North America (2017) 46:895–905. doi: 10.1016/j.gtc.2017.08.010
5. Chen T, Hou X, Ni Y, Du W, Han H, Yu Y, et al. The Imbalance of FOXP3/GATA3 in Regulatory T Cells From the Peripheral Blood of Asthmatic Patients. J Immunol Res (2018) 2018:3096183. doi: 10.1155/2018/3096183
6. Xiong Y, Wang L, Giorgio ED, Akimova T, Hancock WW. Inhibiting the Coregulator CoREST Impairs Foxp3+ Treg Function and Promotes Antitumor Immunity. J Clin Invest 130 (2020) 130:1830–42. doi: 10.1172/JCI131375
7. Zhou L, Liu D, Xie Y, Yao X, Li Y. Bifidobacterium Infantis Induces Protective Colonic PD-L1 and Foxp3 Regulatory T Cells in an Acute Murine Experimental Model of Inflammatory Bowel Disease. Gut Liver (2019) 13:430–9. doi: 10.5009/gnl18316
8. Nunnery SE, Mayer IA. Targeting the PI3K/AKT/mTOR Pathway in Hormone-Positive Breast Cancer. Drugs (2020) 80:1685–97. doi: 10.1007/s40265-020-01394-w
9. Zhao Y, Huang Z, Qi M, Lazzarini P, Mazzone T. Immune Regulation of T Lymphocyte by a Newly Characterized Human Umbilical Cord Blood Stem Cell. Immunol Lett (2007) 108:78–87. doi: 10.1016/j.imlet.2006.10.007
10. Feuerer M, Hill JD. Foxp3+ Regulatory T Cells: Differentiation, Specification, Subphenotypes. Nat Immunol (2009) 10:689–95. doi: 10.1038/ni.1760
11. Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, et al. PD-L1 Regulates the Development, Maintenance, and Function of Induced Regulatory T Cells. J Exp Med (2009) 206:3015–29. doi: 10.1084/jem.20090847
12. Kim JE, Shin JS, Moon JH, Hong SW, Jung DJ, Kim JH, et al. Foxp3 is a Key Downstream Regulator of P53-Mediated Cellular Senescence. Oncogene 36 (2017) 36:219–30. doi: 10.1038/onc.2016.193
13. Maria Angelica C, Cristina I, David V, Xiaohong W, Peltier HJ, Yuping Y, et al. PDL1 Regulation by P53 via miR-34. J Natl Cancer Institute 108 (2015) 108. doi: 10.1093/jnci/djv303
14. Li X, Tian R, Liu L, Wang L, Huang C. Andrographolide Enhanced Radiosensitivity by Downregulating Glycolysis via the Inhibition of the PI3K-Akt-mTOR Signaling Pathway in HCT116 Colorectal Cancer Cells. J Int Med Res (2020) 48:030006052094616. doi: 10.1177/0300060520946169
15. Bigliardi B, Galati F. Innovation Trends in the Food Industry: The Case of Functional Foods. Trends Food Sci Technol (2013) 31:118–29. doi: 10.1016/j.tifs.2013.03.006
16. Zucker DM, Redulla R. Lactulose Management of Minimal Hepatic Encephalopathy: A Systematic Review. Gastroenterol Nurs (2019) 42:84–94. doi: 10.1097/SGA.0000000000000429
17. Wilkins T, Sequoia J. Probiotics for Gastrointestinal Conditions: A Summary of the Evidence. Am Family Physician (2017) 96:170–8.
18. Fujii T, Ohtsuka Y, Lee T, Kudo T, Shoji H, Sato H, et al. Bifidobacterium Breve Enhances Transforming Growth Factor Beta1 Signaling by Regulating Smad7 Expression in Preterm Infants. J Pediatr Gastroenterol Nutr (2006) 43:83–8. doi: 10.1097/01.mpg.0000228100.04702.f8
19. Maldonado Galdeano C, Cazorla SI, Lemme Dumit JM, Velez E, Perdigon G. Beneficial Effects of Probiotic Consumption on the Immune System. Ann Nutr Metab (2019) 74:115–24. doi: 10.1159/000496426
20. Kumar A, Hecht C, Priyamvada S, Anbazhagan AN, Alakkam A, Borthakur A, et al. Probiotic Bifidobacterium Species Stimulate Human SLC26A3 Gene Function and Expression in Intestinal Epithelial Cells. Am J Physiol Cell Physiol (2014) 307:C1084–92. doi: 10.1152/ajpcell.00194.2014
21. Ng SC, Hart AL, Kamm MA, Stagg AJ, Knight SC. Mechanisms of Action of Probiotics: Recent Advances. Inflammation Bowel Dis (2009) 15:300–10. doi: 10.1002/ibd.20602
22. Chen L, Liu P, Feng X, Ma C. Salidroside Suppressing LPS-Induced Myocardial Injury by Inhibiting ROS-Mediated PI3K/Akt/mTOR Pathway In Vitro and In Vivo. J Cell Mol Med (2017) 21:3178–89. doi: 10.1111/jcmm.12871
23. Yu YN, Han Y, Zhang F, Gao Z, Ma ML. Design, Synthesis, and Biological Evaluation of Imidazo[1,2-A]Pyridine Derivatives as Novel PI3K/mTOR Dual Inhibitors. J Med Chem X (2020) 63:3028–46. doi: 10.1021/acs.jmedchem.9b01736
24. Malaney P, Uversky VN, Dave V. PTEN Proteoforms in Biology and Disease. Cell Mol Life Sci (2017) 74:2783–94. doi: 10.1007/s00018-017-2500-6
25. Ding Y, Han R, Jiang W, Xiao J, Liu H, Chen X, et al. Programmed Death Ligand 1 Plays a Neuroprotective Role in Experimental Autoimmune Neuritis by Controlling Peripheral Nervous System Inflammation of Rats. J Immunol (Baltimore Md.:1950) (2016) 197:3831–40. doi: 10.4049/jimmunol.1601083
26. O'Donnell JS, Massi D, Teng MWL, Mandala M. PI3K-AKT-mTOR Inhibition in Cancer Immunotherapy, Redux. Semin Cancer Biol (2018) 48:91–103. doi: 10.1016/j.semcancer.2017.04.015
27. Dastmalchi N, Hosseinpourfeizi MA, Khojasteh SMB, Baradaran B, Safaralizadeh R. Tumor Suppressive Activity of miR-424-5p in Breast Cancer Cells Through Targeting PD-L1 and Modulating PTEN/PI3K/AKT/mTOR Signaling Pathway. Life Sci (2020) 259:118239. doi: 10.1016/j.lfs.2020.118239
28. Wang K, Zhang Z, Liu K, Yang X, Zou H. Neat1-Mirna204-5p-PI3K-AKT Axis as a Potential Mechanism for Photodynamic Therapy Treated Colitis in Mice. Photodiagnosis Photodyn Ther (2018) 24:349–57. doi: 10.1016/j.pdpdt.2018.10.020
29. Salazar N, Lopez P, Garrido P, Moran J, Cabello E, Gueimonde M, et al. Immune Modulating Capability of Two Exopolysaccharide-Producing Bifidobacterium Strains in a Wistar Rat Model. BioMed Res Int (2014) 2014:106290. doi: 10.1155/2014/106290
Keywords: IBD, Bifidobacterium Infantis, colon cell, signaling pathway, Foxp3
Citation: Zhou L, Xie Y and Li Y (2022) Bifidobacterium infantis Promotes Foxp3 Expression in Colon Cells via PD-L1-Mediated Inhibition of the PI3K-Akt-mTOR Signaling Pathway. Front. Immunol. 13:871705. doi: 10.3389/fimmu.2022.871705
Received: 08 February 2022; Accepted: 31 May 2022;
Published: 04 July 2022.
Edited by:
Narendra Prasad Singh, University of South Carolina, United StatesReviewed by:
Zaridatul Aini Ibrahim, University of Malaya, MalaysiaCopyright © 2022 Zhou, Xie and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Li, eWFubGkwMjI3QDEyNi5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.