- 1The Jackson Laboratory, Bar Harbor, ME, United States
- 2Graduate School of Biomedical Sciences and Engineering, University of Maine, Orono, ME, United States
- 3Graduate School of Biomedical Sciences, Tufts University School of Medicine, Boston, MA, United States
T follicular helper (Tfh) cells provide support to B cells upon arrival in the germinal center, and thus are critical for the generation of a robust adaptive immune response. Tfh express specific transcription factors and cellular receptors including Bcl6, CXCR5, PD-1, and ICOS, which are critical for homing and overall function. Generally, the induction of an immune response is tightly regulated. However, deviation during this process can result in harmful autoimmunity or the inability to successfully clear pathogens. Recently, it has been shown that Tfh differentiation, activation, and proliferation may be linked with the cellular metabolic state. In this review we will highlight recent discoveries in Tfh differentiation and explore how these cells contribute to functional immunity in disease, including autoimmune-related disorders, cancer, and of particular emphasis, during infection.
Introduction
T follicular helper cells (Tfh) are a critical cellular subset in adaptive immunity, serving as a bridge between both cell-mediated and humoral immune responses. Both Tfh and follicular regulatory T (Tfr) cells are responsible for modulating a germinal center (GC) response, an antibody response essential for humoral immunity in response to antigen (1–4). Tfr cells express CD25 and transcription factor (TF) Foxp3, and can secrete anti-inflammatory cytokines, such as TGF-β (5). Tfh, on the other hand, express CXCR5, ICOS, TF Bcl6, and produce IL-21 and IL-4 (6–8). Induction of TF Bcl6 in these cells is largely due to IL-6 presence during differentiation (9, 10). In addition to Bcl6, these cells also express signal transducers and transcriptional activators (STAT) 1, 3, and 4, Batf, Maf, and Acl2 (11). The cytokines that are present during peripheral T cell differentiation (at the time of antigen encounter) can dictate which cell subtype the naïve CD4+ T cells will differentiate into, largely through regulation of TF expression (12). The cocktail of TFs present in a cell controls the surface expression of specific receptors. For Tfh, the surface markers expressed are CXCR5, ICOS, CD40L, PD-1, and BTLA (7, 13). It is the differential expression of these receptors, in addition to the intracellular expression of TFs, that are used widely to define the Tfh subset. Although TF Bcl6 is considered the master regulator of Tfh, these cells also express cytoplasmic signaling molecule SLAM-associated protein (SAP), which is involved in the secretion of IL-21 and terminal Tfh differentiation (7, 14).
The mechanisms underlying Tfh differentiation are complex; This review summarizes the key findings in the processes underlying Tfh differentiation with a particular emphasis on the metabolic pathways involved, and the mechanisms by which Tfh may influence the pathogenesis of infection, cancer, and autoimmune diseases. The insights provided from the work cited herein are key for the development of improved, targeted, therapeutics.
Differentiation of Tfh
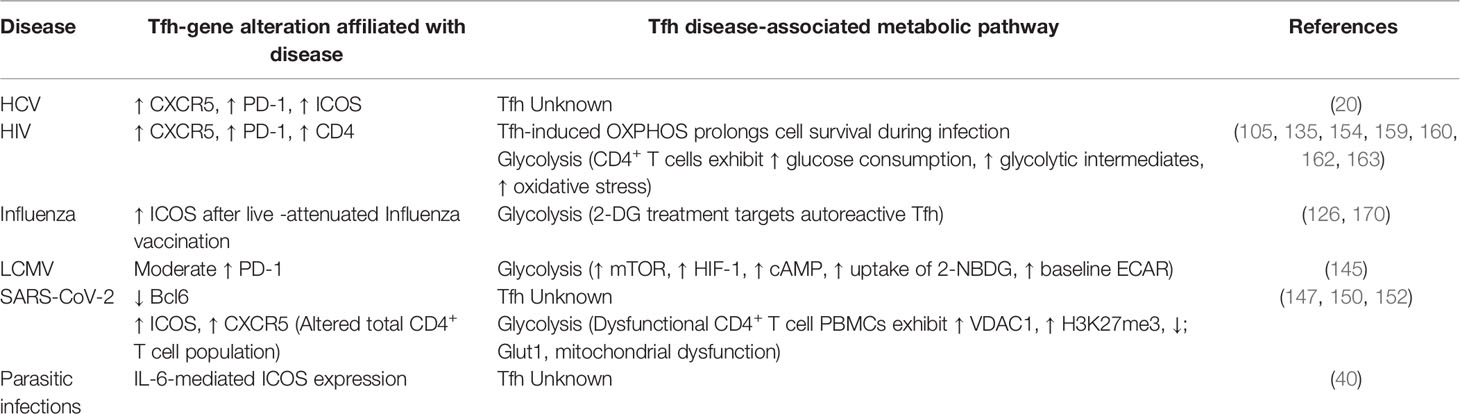
It has been proposed that Tfh differentiation progressively occurs in three different steps (7, 15–18) (Figure 1). The localization of Tfh may contribute to overall function, resulting in subdifferentiation of tissue-specific Tfh lineages. The initial phase in Tfh differentiation occurs during the localization of progenitor Tfh to B cell-containing follicles (10). The trigger for this migration event is through antigen signaling by dendritic cells resulting in T cell receptor (TCR) activation on naïve CD4+ T cells (19). During the interaction of antigen-presenting cells, such as dendritic cells (DC) and CD4+ T cell priming event, two interactions underly initial signaling necessary for pre-Tfh commitment, first, through binding of the TCR to the major histocompatibility complex (MHC), and secondly, binding of CD28 on T cells by DC-expressing CD80/86 in a co-stimulation mechanism. Interactions between naïve T cells and DCs are further regulated by the expression of cytokines and receptors including IL-6, ICOS, and IL-2 (19, 20). Secretion of IL-6, largely by DCs, stimulates the production of transcription factors central to Tfh differentiation, primarily Bcl6, but also includes TCF-1 and LEF-1, among others (21, 22). The nuances of DC modulation of Tfh differentiation have been extensively reviewed (23, 24). Induction of Bcl6 signaling results in expression CXCR5 on the cell surface, regulates Tfh intracellular calcium signaling, and modulates expression of CD40L (9, 10, 25). While the deletion of one copy of the Bcl6 gene did not significantly alter activation of Tfh or localization of Tfh to follicles, GC formation and maintenance was inhibited. Moreover, overexpression of CD40L could partially rescue GC formation and Tfh maintenance after deletion of the single copy of the Bcl6 gene (25). This highlights the importance of Bcl6 for Tfh maintenance and signifies that there may be overlapping mechanisms of Bcl6 and CD40L in mediating T:B cell interactions. Co-stimulatory receptor OX40 also regulates Tfh differentiation, though is attributed as both a positive and a negative regulator: LCMV-infected OX40-deficient mice exhibit impaired Tfh differentiation (26) while enhanced OX40 signaling, in combination with anti-PD-1 blockade, in Plasmodium infection diminished the Tfh response, through upregulation of inhibitory Blimp1 and IFN-γ signaling (27). ICOS expression also plays a major role in the regulation of Tfh migration and differentiation (28, 29). Likewise, its corresponding ligand ICOSL, expressed by B cells, is important for Tfh differentiation, through manipulation of the glycolytic environment of Th cells (24, 30). Thus, expression of cellular receptors can largely influence the cellular differentiation fate.
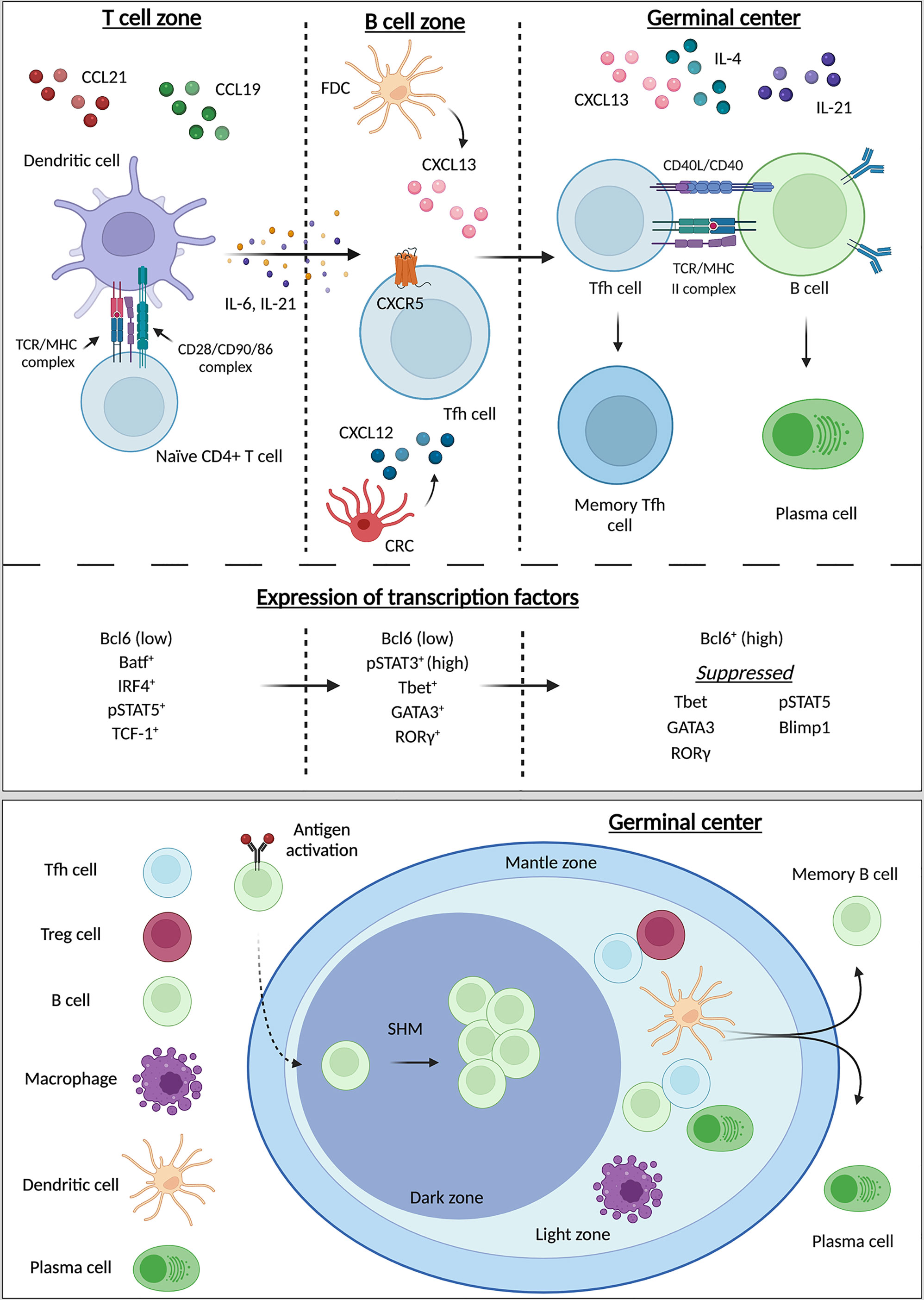
Figure 1 Differentiation, maturation of Tfh and germinal center organization. Tfh differentiation progressively occurs in three different zones: the T cell zone, the B cell zone, and the germinal center (GC). From the T cell zone to the GC, naïve CD4+ T cells differentiate into Tfh with final maturation occurring within the GC. In the T cell zone interactions between CD4+ naïve T cells and dendritic cells (DC) initiate Tfh differentiation and promote the release of IL-6, IL-21, and the upregulation of CXCR5 on the pre-Tfh. These pro-Tfh cytokines result in the release of CXCL13 by follicular dendric cells (FDCs) and CXCL12 by CXCL12-producing reticular cells (CRCs) in the B cell zone. Recognition of CXCR5 by CXCL13 results in recruitment of the immature Tfh into the B cell zone. Both CXCR5 and ICOS signaling promote homing of the now-mature Tfh into the GC. Interactions with B cells in the GC result in the maturation of B cells into plasma cells for the production of antibodies. During these three stages of Tfh differentiation the transcriptional environment changes as differentiation progresses. Initially expression Bcl6 is low, and the pre-Tfh cell enhances expression of Batf, IRF1, pSTAT5, and TCF-1. During the second stage of development Bcl6 expression remains low while pSTAT3, Tbet, and RORg increase in expression. In the final stage of Tfh maturation Bcl6 is now highly expressed, while suppression of Tbet, GATA3, RORg, pSTAT5, and Blimp1 are suppressed. The GC is comprised of three zones: the mantle zone, light zone, and dark zone. The light zone primarily houses T cells and the majority of T:B cell interactions occur here. Within the dark zone B cells undergo somatic hyper mutation (SHM) for differentiation into plasma cells and long-lived memory B cells. Created with BioRender.com.
Fibroblastic reticular cells produce chemoattractants CCL19, CCL21, and CXCL12; expression of CCL19/21 induces migration of CCR7-expressing naïve CD4+ T cells and DCs to the T cell zone, resulting in T cell priming (7, 31–34). Then, a series of changes occur. CXCL13, responsible for recognition of CXCR5, is produced by follicular dendritic cells (FDCs), one of two stromal cell types contained within B cell follicles, with the other being CXCL12-producing reticular cells (CRCs), responsible for recognition of CXCR4. The production of CXCL13/12 increases migration of B cells during the induction of a germinal center response (35, 36). Interactions between DCs and naïve CD4+ T cells in the T cell zone promote CD4+ Tfh differentiation resulting in the upregulation of CXCR5 and subsequent down regulation of CCR7, and the migration of the T cells to the CXCL13-rich B cell zone contained within follicles (23).
Although CXCR5 expression is sufficient for T cell arrival at the B cell zone, not all CXCR5-expressing T cells cross the T-B cell junction. Arrival within the B cell zone is highly dependent on PI3K signaling (37). Expression of CXCR5 and ICOS on the cell surface promotes PI3K signaling, while T cell PD-1 interaction with B cell PD-L1 results in PI3K inhibition; inhibition which is overcome by the ICOS-linked PI3K activation (38). In addition to increasing PI3K activation, ICOS also enhances the formation of T cell pseudopodia and migration (39). Lastly, T cells from the T cell zone, migrate across the T-B cell junction, marking phase two of Tfh differentiation, into the B cell zone (7). Signaling pathways necessary for early Tfh differentiation are controlled by CXCR5 expression and Bcl6, as well as pathways largely regulated by DCs, including IL-6, ICOS, TCR, and IL-2 signaling pathways (20). Some of these interleukins are also usurped during disease. For example, Plasmodium infection increases IL-6, resulting in enhanced B- and CD4+ T cell activation (40). This in turn promotes parasite-specific antibody production. Together, this demonstrates the critical roles that signaling networks may play early in the fate of Tfh.
Subsequently following T:B cell interactions, the third phase of Tfh differentiation occurs. This final phase in the maturation of Tfh happens in specialized structures within B cell zones known as germinal centers (GCs) (41, 42). Tfh express SAP resulting in secretion of IL-21, which is not only necessary for final maturation of Tfh within the GC but is also critical for normal B cell development (43, 44). Within GCs, B cells produce high-affinity antibodies and further differentiate into memory B cells or plasma cells after undergoing randomized somatic hypermutation (SHM) which generates an improved antigen affinity (45). Moreover, GCs are divided into two distinct compartments, the light zone (LZ) and dark zone (DZ), each compartment caters to specific GC-associated cell types. The light zone primarily houses Tfh and Tfr cells that have entered the GC. Here, Tfh assist in the production of GCs and aid B cells, providing signaling for B cell differentiation into plasmablasts and plasma cells for production and secretion of antibodies. Meanwhile, FoxP3+ Tfr are also critical for GC regulation, as these cells suppress Tfh/B cell signaling to prevent excessive antibody production (46). Tfr are a specialized subset of Treg cells that have adopted a Tfh phenotype, likely due to the cytokine milieu in which they were differentiated. This generates a Treg cell type that is capable of homing to GC, due to high expression of Tfh markers CXCR5 and Bcl6 (47). The numerical balance between Tfh and Tfr is a large factor in regulation of the GC microenvironment and is essential for avoiding the development of autoimmunity. Meanwhile, the DZ supports B cell SHM and B cell differentiation and maturation (45). Underlying B cell SHM is the expression of CXCR4. Expression of CXCR4 retains B cells within the DZ where these cells undergo rapid proliferation until CXCR4 expression decreases, at which time these cells migrate into the LZ where interactions with FDCs and Tfh occur (48) (Figure 1).
Although Tfh reach maturation within GCs, Tfh can migrate between GCs or even through blood or lymph (49). During this migration Bcl6 is downregulated, and these Tfh are now termed memory Tfh, and upon IL-7Rα upregulation, become resting memory Tfh. Resting memory Tfh express even lower levels of Bcl6. Memory Tfh are found in the blood, lymph, and secondary lymphoid organs. It is estimated that approximately 20% of the circulating CD4+ memory T cells are memory Tfh (50). These memory Tfh express comparable levels of CXCR5 to Tfh which reside within lymph nodes, though they express lower levels of PD-1 and ICOS (51). It is speculated that circulating Tfh may differentiate into memory Tfh. Memory Tfh may circulate scouring for antigen. Upon arrival within lymph nodes, these cells require priming by DCs to functionally produce and secrete cytokines, which are produced at higher levels than lymph node-resident Tfh. It is posited that circulating memory Tfh are on the frontline for defense against re-exposure to antigen (50).
Cytokine and Transcriptional Regulation of Tfh Differentiation
The transcriptional environment of cells can promote Tfh differentiation (52). During cell division and growth, differential expression of transcription factors (TFs) influences the maturation and differentiation of those cells. Multiple TFs have been positively and negatively associated with Tfh differentiation (52) (Figure 1).
Bcl6 is a transcriptional repressor critical for Tfh differentiation, as these cells cannot form in its absence (53, 54). Moreover, induction of Bcl6 induces expression of CXCR5, ICOS, and PD-1 and robust Tfh differentiation in Bcl6-/- mice (54–56). It has been proposed that Bcl6 dictates Tfh differentiation through both direct and indirect methods. Via direct means, Bcl6 can modulate T cell fate through DNA-binding capabilities; Bcl6 represses signaling pathways that would generate alterative CD4+ T cell subtypes (56). Through indirect means, acting as a ‘repressor of repressors’ Bcl6 can inhibit miRNAs that would otherwise serve to inhibit Tfh differentiation, typically through inhibition of a Tfh-specific marker like CXCR5 (56). Bcl6 can also indirectly enhance CXCR5 and PD-1 signaling through suppression of p-selectin glycoprotein 1 (PDG-1) (57) and through suppression of Blimp1 (58). Although Bcl6 suppression of alternative TFs has been reported, including T-bet, ROR-γ, and GATA-3 (59, 60), direct modulation of these TFs that are often expressed concurrently with Bcl6 require further experimentation. The differential TF expression may generate specific genetic patterns under varying conditions. Bcl6 can also influence the cytokine milieu during differentiation (8) and receptor expression and cellular migration (8, 61, 62). Thus, while the many facets of its involvement in direct and indirect manipulation of T cell lineages are still unraveling, Bcl6 is clearly essential for Tfh differentiation.
STATs promote the differentiation of Tfh, with particular emphasis on STATs 1, 3, and 4 (11, 63). Expression of CXCR5 and Bcl6 enhances activity of STAT1 in murine cells to interact with Maf and Batf, which in turn further enhance CXCR5 expression (52, 64). Transcription factor Maf is very important for induction of Tfh. Maf expression enhances CXCR5, IL-21, and IL-4, with Maf directly influencing the production of IL-17 and IL-21, all of which are critical for Tfh differentiation and function (28, 65). Maf is activated through ICOS signaling (65). Batf enhances Bcl6 expression (66, 67). Under conditions of low Batf expression in vivo, induction of both Bcl6 and Maf is necessary for the induction of CXCR5 (66). Although expression of Batf is not largely enhanced in Tfh, mice that fail to express Batf (Batf-/-) are incapable of producing Tfh, underscoring the influence of small changes in Batf expression on Tfh differentiation (66).
STAT3 has also been linked with the promotion of Tfh in mouse cells, while STAT3 and STAT4 are important for Tfh induction in humans (52). Although STATs largely enhance Tfh expression through transcriptional regulation, STAT5 negatively influences Tfh differentiation (67, 68). Multiple TFs can bind to the Bcl6 promotor, thereby influencing Bcl6 mRNA expression (69). The binding of TFs to the Bcl6 promotor is influenced by IL-2 expression; under conditions of low IL-2, STAT3 competitively binds to the promotor, enhancing Bcl6 mRNA expression. However, when IL-2 is high, STAT5 instead binds to the Bcl6 promotor, thereby inhibiting Bcl6 expression (69). However, mature GC-Tfh produce high levels of IL-2. It has since been determined that Tfh within GCs maintain IL-2 hyporesponsiveness through IL-6 signaling. IL-6 inhibited the upregulation of CD122 on the cell surface, which would typically result in increased STAT5 signaling and would be thus susceptible to the effects of IL-2 (70).
The negative influence of TFs in Tfh induction has also been described. Expression of Foxp1 and Foxo1 is necessary for maintaining immature CD4+ T cells (71), and inhibition of these TFs enhances the induction of Tfh. As such, the regulation of Foxp1 and Foxo1 by other factors directly influences the fate of the immature CD4+ T cells. Regulation of ICOS via ICOSL stimulated PI3K signaling, downregulates Foxo1, and upregulated Bcl6 signaling (72, 73). Blimp1 is also an important transcriptional regulator that negatively regulates Tfh differentiation (14). Bcl6 and Blimp1 are transcription factors that are antagonistic, known as the Bcl6/Blimp1 axis (74). CD4+ T cells that differentiate into non-Tfh lineages express high levels of Blimp1. Moreover, CD4+ T cells that fail to express Blimp1 (Prdm1-/-) preferentially differentiate into Tfh, at an accelerated rate, in vivo, generally resulting in autoimmunity (75).
Together, while this only presents a subset of the TFs involved, the transcriptional profile of cells greatly influences the fate of CD4+ T cells during differentiation. The intricacies of TF-Tfh regulation have been thoroughly summarized elsewhere (52, 76, 77). This suggests that CD4+ T cell Tfh lineage requires the appropriate balance of the aforementioned factors, which under healthy conditions, are tightly regulated.
Cytokines play a critical role in regulating Tfh differentiation. It is argued that IL-6 is the most important cytokine in the differentiation of Tfh. IL-6 expression initiates Bcl6 induction during pre-Tfh development, while also inducing IL-21 expression in murine CD4 T cells (20). IL-6 can be secreted by a number of immune-related cell types, including DCs, B cells, and macrophages, among others (24). These cells produce IL-6 in response to internal and external pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs). While IL-6 is critical for early induction of pre-Tfh phenotype, addition of IL-21 and IL-27 may compensate for the lack of IL-6 in early Tfh differentiation (20, 78–80).
It has long been reported that IL-2 negatively regulates differentiation of Tfh (81). This has been supported by evidence that IL-2/STAT5 signaling impairs expression of Bcl6 (82). This inhibition is through the promotion of Blimp1 signaling, tipping the Bcl6/Blimp1 axis. Moreover, high expression of PD-1 within GCs by Tfh results in the inhibition of IL-2; expression of IL-2 would typically serve to inhibit Tfh differentiation, suggesting that expression of IL-2 may directly affect PD-1 expression, thereby influencing Tfh differentiation fate (82). Interestingly, IL-2 is initially highly expressed during Tfh differentiation, and is necessary for their selective differentiation, and appears to wane as differentiation progresses (81). A complete picture of how differential and temporal IL-2 expression regulates Tfh differentiation requires further experimentation. Though not discussed here, numerous other cytokines can influence Tfh differentiation. Comprehensive reviews of cytokine regulation of Tfh differentiation are available (10, 83, 84).
Until the last decade it was believed that T cells that provided help to B cells were specifically derived from naïve CD4+ T cells and were classified as Tfh. However, it is now appreciated that CD8+ T cells may, under certain conditions, provide help to B cells in a similar manner, in what is described as Tfh-like activity. One group reported that CD8+ T cells could function in this capacity using a mouse model that was deficient in IL-2 (85). These mice generated spontaneous autoimmune disease in what, at the time, was believed to be due to unimpeded CD4+ Tfh differentiation. This was supported by the finding that depletion of CD4+ T cells in these mice prevented the onset of the autoimmune disease and thus improved survival. However, when the research group depleted CD8+ T cells using this model, they found that depletion of CD8+ T cells also delayed disease and prolonged survival. RNA sequencing of CD8+ T cells in these mice revealed cells that exhibited many of the major Tfh markers, including expression of CXCR5, Bcl6, ICOS, PD-1, and IL-21, and thus may exhibit similar metabolic profiles. These cells, defined as CD8+ Tfh-like cell population, are capable of localizing with and providing help to B cells within GCs and appear to contribute to the onset of autoimmune disease, through the dysregulation of Tfh populations (58, 85–89). CD8+ Tfh-like cells have been increasingly implicated in immune activity. In patients with rheumatoid synovitis, CD8+ Tfh-like cells that express CD40L, and therefore interact with B cells within the GC, have been heavily implicated in disease development (87). These cells also develop under conditions of other inflammatory diseases and in chronic infection and have been described to also express GC-homing Tfh marker CXCR5 (58, 88, 90–92). Moreover, these CD8+ Tfh-like cells were capable of supporting survival of B cells ex vivo (93). Thus, in certain conditions of disease, CD8+ T cells may adopt Tfh-like characteristics and functionality, and therefore these cells cannot be ignored in discussions pertaining to the roles of Tfh in modulation of diseases involving inflammation or infection. Moreover, the metabolic requirements of CD8+ Tfh-like cells is an entirely unexplored field of research. Given the implications of these cells that are now emerging for numerous disease states, and given their similarities with canonical Tfh, an understanding of how these cells contribute to the immunological environment is warranted.
Collectively, the local environment directly influences an immune response through regulation of the balance of Tfh induction, through expression of cellular receptors, cytokines, and induction or suppression of transcription factors. Moreover, the intricacies of CD4+- versus CD8+-derived T cells convolute this multifaceted process. Arguably, metabolic changes underly Tfh differentiation, and these alterations correlate with the expression of Tfh-specific markers.
Metabolic Regulation of Tfh Differentiation
The metabolic environment has been reported to greatly influence the differentiation of CD4+ T cells into functionally mature T cells. Some studies suggest that oxidative phosphorylation (OXPHOS) plays a critical role in Tfh differentiation. However, other sources suggest that glycolysis, rather than OXPHOS is necessary for Tfh induction, due to heightened mTOR and HIF1α signaling (Figure 2).
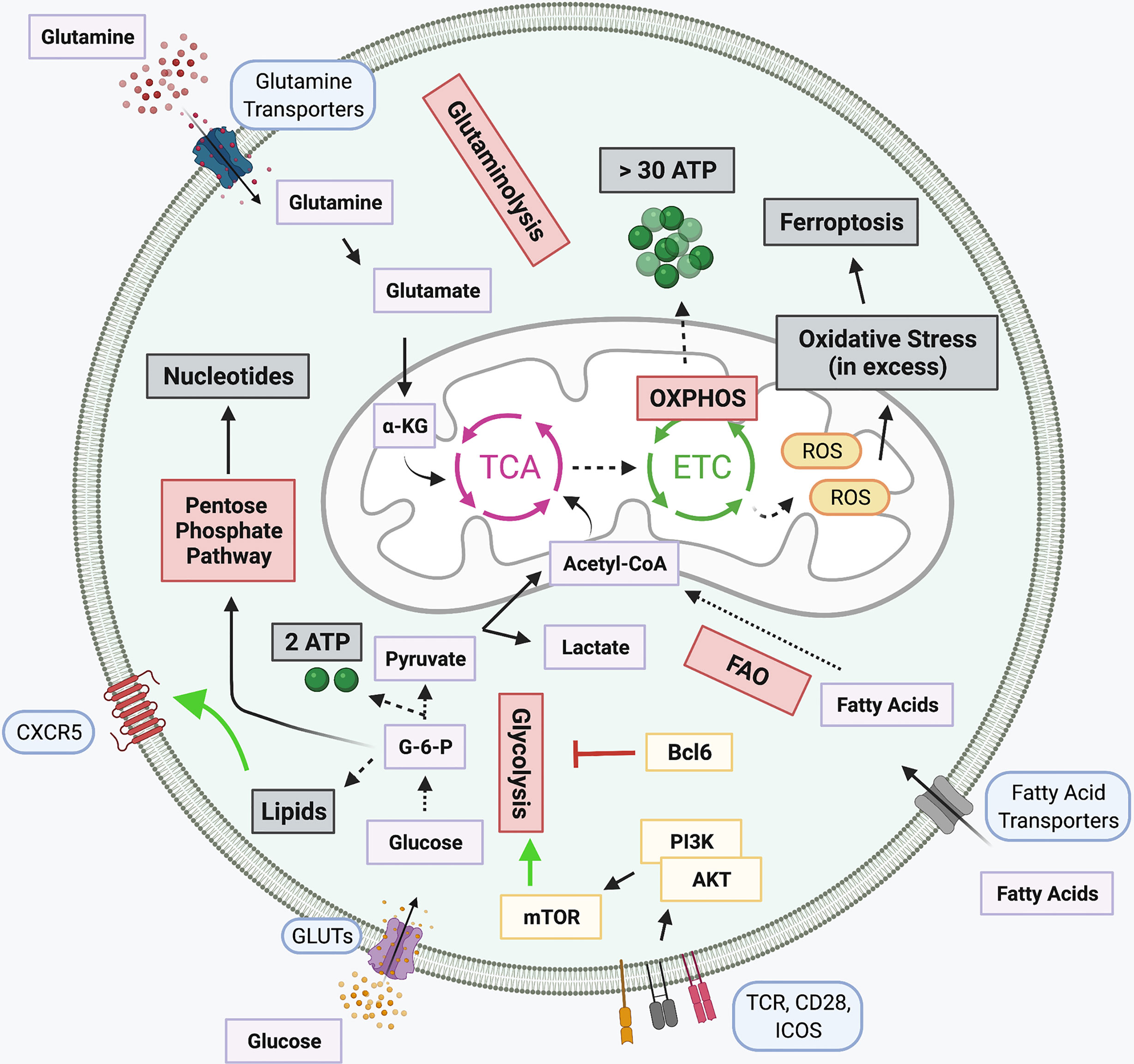
Figure 2 Metabolic pathways in T cells and Tfh-specific alterations. Several signaling networks underly cellular metabolism. Glucose is broken down to produce glycolysis and two ATP molecules in a process known as Glycolysis. Glycolytic intermediates support the pentose phosphate pathway for generation of nucleotides. The pyruvate produced during glycolysis is either converted into lactate or Acetyl-CoA, the latter is subsequently funneled into the tricarboxylic acid (TCA) cycle. Glutaminolysis also fuels the TCA cycle through the breakdown of glutamine into α-ketoglutarate (α-KG). Products of the TCA cycle, along with the oxidation of fatty acids (FAO), support the electron transport chain (ETC) which is coupled with OXPHOS for the generation of over 30 ATP molecules. When in excess, Reactive oxygen species (ROS) generated by the ETC, induce cellular oxidative stress, promoting apoptotic signaling. Several Tfh signaling pathways either promote or inhibit select metabolic pathways. Lipid metabolism, responsible for the generation of lipids, promotes expression of CXCR5 on the cell surface. Bcl6 can repress the transcription of several glycolytic intermediates, thought to prevent differentiation of alternative T cell subtypes, while TCR, CD28, and ICOS signaling in Tfh potently activate glycolysis through PI3K-linked activation of mTOR signaling. Created with BioRender.com.
Glycolysis is the process by which glucose is anabolized to produce pyruvate. Glycolysis also involves the production of several intermediates which can be utilized by alternative metabolic pathways, including the pentose phosphate pathway. In addition to the production of pyruvate, glycolysis also generates a small amount of cellular energy. From glycolysis, pyruvate is either transformed into lactate or is shuttled into the mitochondria and fed into the tricarboxylic acid (TCA) cycle. The TCA cycle supports the electron transport chain (ETC). The generated ETC gradient produces significantly more cellular energy than glycolysis in a process known as OXPHOS. However, pyruvate is not the only intermediate fed into the TCA cycle. Products like α-ketoglutarate from glutaminolysis, the breakdown of glutamine, and fatty acids from fatty acid oxidation (FAO) can also be oxidized in the TCA cycle. Acetyl-CoA generated from oxidizing these organic fuel molecules participates in the production of the intra-mitochondrial ETC proton gradient for OXPHOS. Shortcomings of the ETC results in the production of reactive oxygen species (ROS), which when in excess can be detrimental for cellular survival (94–97).
Mitochondrial respiration has been implicated with Tfh cell differentiation. Murine Tfh cells have been reported to exhibit features of ferroptosis, a type of programmed cell death, associated with iron-dependent excessive production of ROS, linked with TCR, CD28, and PD-1 stimulation (98). Moreover, it has been determined that prolonged Tfh-GCB interaction promotes the production of ROS in Tfh, ultimately resulting in cell death via ferroptosis (98). As ROS is resultant of ETC and lipid metabolism, this implicates these pathways, and in general, mitochondrial respiration, in the critical regulation of Tfh (98). Several reports highlight the importance of OXPHOS rather than glycolysis in metabolic regulation of Tfh differentiation. This is in part due to a report that Tfh express reduced levels of glycolysis, lower mTOR activity, and decreased mitochondrial respiration (99). At rest, T cells primarily utilize mitochondrial OXPHOS to generate fuel. Following antigen presentation, TCR, CD28, and PI3K/Akt signaling positively favor glycolysis as the primary metabolic pathway. Under these glycolytic conditions, CD4+ T cell differentiate into specialized T cell lineages, including Th1 and Th17 cells. However, a MAPK phosphatase, DUSP6, reduces Tfh differentiation through TCR-mediated glycolysis (100). Expression of the Tfh hallmark transcription factor Bcl6 can be modulated through regulation of metabolism. Under glucose restricted conditions or treatment with a potent glycolysis inhibitor 2-deoxyglucose (2-DG), Bcl6 expression is enhanced (101). However, a caveat to this increased Bcl6 expression is that under conditions of low glucose, cellular activation is dampened. When glucose levels are low AMPK is activated, and inhibition of AMPK reduces Bcl6 levels under glucose restriction. However, this Bcl6 induction is insensitive to IL-2 levels. Importantly, inhibition of cellular metabolism promotes Tfh differentiation, prior to cellular maturation, rather than being attributed as a secondary effect. It is thought that the two signaling mechanisms, Bcl6 induction through AMPK signaling under conditions of high cellular proliferation, low energy, and glycolytic demands occur simultaneously (101–103). While this pathway appears to be tightly regulated in this process, the nuances of the mechanisms involved require further clarification.
Other studies have demonstrated that Bcl6 expression results in the suppression of key glycolytic genes (104). Bcl6 can localize directly to foci for glycolytic genes, including Glut1, Glut3, hexokinase 2, and pyruvate kinase M, and functionally repress their transcription in both B and T cells (104). Moreover, this gene repression was sensitive to the expression of IL-2 and CD4+ T cells cultured in vitro in the absence of glucose exhibited enhanced Tfh differentiation (104). This may provide support for metabolic pathways alternative to glycolysis that drive Tfh differentiation. Primary human GC Tfh sorted from tonsillar tissue analyzed via RNA sequencing identified that genes associated with fatty acid oxidation (FAO) were enriched while there was reduced expression of glycolytic genes (105). Moreover, a Seahorse mito-stress test reported higher OXPHOS in GC Tfh cells when compared to other CD4+ cell types as the basal oxygen consumption rate, spare respiratory capacity, and ATP production were elevated (105). It is speculated that a precise Bcl6 expression gradient is needed to drive Tfh differentiation from other cell types. This is in conjuncture with a report that T-bet can suppress Bcl6 glycolytic repression to promote glycolytic pathways and Effector T cell function (104). PD-1, highly expressed upon mature Tfh commitment, can also alter metabolic pathways in T cells. PD-1 interaction with ligand PD-L1 suppresses glycolysis and activates genes affiliated with FAO (106). While there is support for OXPHOS for Tfh differentiation and function, reliance on glycolysis is also reported. In a murine study, deletion of an OXPHOS regulator, cytochrome c oxidase, impaired GC formation and antibody responses, though did not impact the overall number of Tfh cells (107), suggesting either their ability to adapt to metabolic changes with ease or the involvement of concurrent metabolic pathways in Tfh regulation.
Intraperitoneal injection of mice with 2-DG results in a hindered immune response through decreased differentiation and impaired function of Tfh (108), establishing a link between glycolysis and proper Tfh function. Glycolysis results in the activation of mTOR-related signaling, through activation of mTOR complex 1 (mTORC1) and complex 2 (mTORC2). Both mTORC1 and mTORC2 have been affiliated with the differentiation of various T cell lineages (109, 110). For Tfh, both mTORC1 and mTORC2 signaling has been reported. It has been shown that Rptor and Rictor knockout mice limit the humoral immune response through a decrease in the generation of Tfh (110). Raptor and Rictor are critical components of mTORC1 and mTORC2 signaling complexes, respectively. mTORC1 activity has also been linked with enhanced Bcl6 expression, through the downstream activation of 4E-BP1 and eIF4E, in vitro (111). Insufficient mTOR activity has been associated with a diminished Tfh response to vaccination (112). In this report, low leptin levels in serum correlated with a diminished Tfh response to vaccination. Leptin is a metabolic hormone secreted by adipose tissue that can modulate cell types of both the innate and adaptive immune systems (113–115). Importantly, the leptin-associated regulation was found to be linked with the stimulation of cellular glycolysis, through STAT3 and mTORC1 signaling (112), both of which are attributed to Tfh differentiation and function. Another group has linked mTOR signaling with the promotion of Tfh differentiation in vivo, through ICOS-mediated upregulation of glycolysis (18). However, others suggest that mTORC1 negatively regulates Tfh differentiation through the promotion of IL-2, as determined through in vitro methods (99). Although Bcl6 has been attributed to the inhibition of glycolysis, this may be a means of inhibiting differentiation of alternative T cell subtypes, like Th1 cells, thereby indirectly enhancing Tfh differentiation through repressor of repressor functions (104). Furthermore, while this metabolic dichotomy exists, it is important to note that the processes by which cells are differentiated and systemic effector fluctuations, can greatly alter the intra-cellular metabolic environment, thus contributing to the phenotypic and functional profiles of these cells.
There is further support for Tfh dependence on mTOR-mediated glycolysis for proper cellular differentiation and maturation. Cells that exhibit lower levels of mTOR inhibitor PTEN undergo heightened Tfh induction and result in enhanced production of GCs (110, 116). Roquin, an inhibitor of PI3K-mTOR signaling, impairs the Tfh response; knockout of Roquin results in an increase in Tfr (117). As PI3K is intimately linked with Bcl6 induction through ICOS/ICOSL signaling (73), enhanced Tfr differentiation is expected. Furthermore, in Peyer’s patches, mTORC1 and mTORC2 signaling enhances transcriptional promotion of Tfh through ICOS signaling (110). However, mTORC1 signaling has been affiliated with the differentiation of circulating Tfr, which, like Tfh, express CXCR5 and Bcl6. Because of this similarity, it is speculated that mTORC1 plays a direct role in pathways resulting in expression of CXCR5 and Bcl6 (118). Moreover, Tfh from K/BxN mice that develop spontaneous rheumatoid arthritis exhibit a heightened glycolytic demand and inhibition of glycolysis impairs their function reducing disease severity (102). Further support of the requirement of glycolysis for proper Tfh functioning is through reports of human rheumatoid arthritis patients expressing higher levels of hexokinase 2 in peripheral blood mononuclear cells in comparison to their healthy counterparts (119). Moreover, in vitro Tfh differentiation is diminished when cultured in low glucose (110). Also, glucose uptake and utilization are enhanced by ICOSL-ICOS stimulation via PI3K signaling, and transgenic overexpression of a glucose transporter Glut1 augments Tfh differentiation (110). Together, these data support the claim that Tfh engage a high glycolysis rate; however, the underlying reasons for enhanced glycolytic demand require further investigation. In addition, enhanced glycolytic Tfh dependance has been reported under hypoxic conditions. During malignant pleural effusion (MPE), Tfh cell exhibited differential expression of genes associated with glycolysis, cysteine, and methionine metabolism, while markers of OXPHOS and FAO were slightly elevated. Moreover, these cells show marketed inhibition of the pentose phosphate pathway, arachidonic acid, and α-linoleic acid metabolism (120). Together, this suggests that Tfh may rely on the interconnectedness of various metabolic pathways for fueling cellular demands.
Tfh may also be metabolically regulated by protein kinase C (PKC). Mice that fail to produce PKC-β (Prkcb-/-) were used to generate a chimera where only B cells were Prkcb-/-, while other cell populations were mostly wildtype. These mice exhibit B cells with impaired mitochondrial function and also produce lower numbers of splenic Tfh when compared to wildtype mice, suggesting that B cell expression of PKC-β affects Tfh development (121). Moreover, these mice fail to produce sufficient levels of GCs to elicit an effective immune response, thus they produce lower levels of plasma cells and IgG. These works support the idea that mTOR may regulate mitochondrial respiration through the actions of PKC (121).
Recently, CD4+ T cells have been shown to utilize the cytidine diphosphate (CDP)-ethanolamine pathway, the pathway used to produce phosphatidylethanolamine (PE) and plasmenyl PE, a class of phospholipids that form biological membranes (122). The importance of this pathway was related to several intermediates that assist post-transcriptionally in promoting the surface expression and function of CXCR5. The colocalization of CXCR5 and PE at the cell surface is speculated to prevent the internalization and degradation of the critical Tfh homing protein. In vivo CRISPR-Cas9 screening identified the CDP-ethanolamine pathway intermediates, as their deletion hindered Tfh differentiation (122). Although this pathway likely does not support Tfh cellular fuel demands for differentiation, it highlights the intricacies of metabolic regulation of Tfh for proper function and suggests that our understanding of these processes is just beginning to scratch the surface.
As there is support for both glycolysis and/or mitochondrial-associated respiration in the regulation of Tfh metabolism, if Tfh can utilize multiple metabolic pathways, it could be conceivably seen as advantageous as it may allow for these cells to adapt to varying conditions within GCs. This may be essential because Tfh cells typically reside within GCs and may frequently have to compete for metabolic resources with other highly proliferative cell types, like B cells. Thus, upon ICOS stimulation Tfh cells can engage glycolysis, but could also maintain function in glycolysis-restricted conditions through utilizing mitochondrial metabolism.
Tfh in Disease: Autoimmune Related Disorders
Many characteristics of autoimmunity have been closely correlated with an over production of Tfh, including SLE, rheumatoid arthritis (RA), psoriasis, autoimmune thyroid disease (AITD), and diabetes, among others.
Tfh are highly correlated to the disease state in individuals suffering from SLE. It is thought that the major impact of Tfh is due to the disproportionate T:B cell number ratio (123, 124). Recent pre-clinical findings show that long-term glycolytic inhibition via 2-DG treatment can normalize frequencies and numbers of autoreactive Tfh, and other hyperactive T and B cells in mice that are predisposed to develop lupus-like symptoms and improve their disease phenotypes (125, 126). This suggests that abnormal glycolysis in these activated cells is involved in lupus progression. However, the mechanisms behind this effect of glycolytic inhibition for the treatment of SLE are still under investigation. Persons suffering from AITD exhibit increased numbers of Tfh in addition to higher levels of Bcl6 and IL-21 mRNA (12, 127). This is in correlation with those afflicted by RA, who exhibit higher concentrations of IL-21 and numbers of circulating Tfh in the bloodstream, likely through enhanced B cell activity (128). Moreover, a link has been established with collagen-induced arthritis (CIA) and the expression of CXCR5. Mice deficient in CXCR5 are resistant to CIA, demonstrating the importance of CXCR5 expression for overall Tfh homing and function, and its potential correlation with CIA (129). Furthermore, it was determined that ICOS signaling in Tfh is linked with activation of PI3K signaling, resulting in expansion of inflammatory T cell populations, and that administration of a glycolysis inhibitor, 3-bromopyruvate, reduced joint inflammation. Together, this suggests a correlation between Tfh ICOS signaling and glycolysis for progression of SLE and rheumatoid arthritis (102, 130).
Tfh have also been implicated in disease severity of other metabolic-associated autoimmune diseases like diabetes and atherosclerosis. The implication of Tfh in these disorders has been attributed to Treg : Tfh imbalance and production of pro-Tfh cytokines, including IL-6 and IL-17A, resulting in increasing disease severity (131, 132). A type 1 diabetes (T1D) mouse model microarray analysis of islet-specific T cells identified an increased gene profile for Tfh, including upregulation of CXCR5 and IL-21 (133). In addition, children that are newly diagnosed with T1D exhibit increased levels of Tfh (134). Moreover, individuals with T1D harbor memory T cells with heightened levels of Tfh markers including CXCR5, Bcl6, and IL-21, and downregulation of IL-2 signaling pathways (133). It is tempting to speculate that the downregulation of IL-2 may be implicated in the elevated levels of Tfh, due to the IL2/Bcl6 axis (133).
CD8+ Tfh-like cells have been linked with autoimmune inflammatory diseases in both IL-2 deficient and scurfy-prone mice (85). Scurfy mice are deficient in Treg cells and develop severe autoimmunity due to a lack of Tfh regulation (135). These Tfh-like cells provide B cell help both in vitro and in vivo, and when in combination with CD4+ Tfh (85). This is supported by evidence that depletion of STAT5, specifically in CD8+ T cells, resulted in increased numbers of CD8+ Tfh-like cells and promotes the production of autoreactive antibodies (136). This is conjuncture with reports linking STAT5 signaling with the negative regulatory role of IL-2 on CD4 Tfh differentiation. CD8+ Tfh-like cells have also been shown to contribute to synovial RA. These cells were found within histological sections of GCs and the Tfh-like cells expressed high levels of CD40L, IFN-γ, and lacked expression of perforin in these microenvironments (86, 87). The CD8+ Tfh-like cells exhibited IL-17R, are found in circulation and may correlate with prolonged survival (93). Further investigation is warranted to understand the complexities of CD8+ Tfh-like and CD4+ Tfh mediated responses and how these similar cell populations may synergistically contribute to autoimmunity.
Tfh in Disease: Cancer
Much of the evidentiary reports of the role of Tfh in cancer are due to studies involving breast cancer and colorectal cancer. Tfh impact multiple aspects of the immune system during cancer. These cells play roles in recruiting natural killer, macrophages, and CD8+ T cells, as well as being critical for the formation of ectopic lymphoid structures, all of which are important for an effective anti-tumor response (137). A Tfh gene signature has been described in tumor tissue of breast cancer patients, and expression of this signature correlated with improved tumor clearance and enhanced survival rates and Tfh have been shown to promote B cell differentiation within tumors resulting in improved antitumor immunity (137, 138). For individuals afflicted with colorectal cancer, survival rates have been positively correlated with Tfh numbers. Moreover, tissue microarrays were used to determine that Tfh were more resilient during tumor progression than other T cell populations, the numbers of which experienced progressive reductions (139). Although many sources positively associate Tfh with survival, there are some instances where Tfh are reported to be detrimental to an anti-tumor response, correlated with Tfh production of IL-4. A study using mice lacking CNS2 reported a decrease in tumor growth when compared to wildtype mice. CNS2 is a major source of IL-4 production in Tfh (67). However, in addition to IL-4, Tfh also produce IL-21, which has been shown to promote tumor killing when used in combination with anti-PD-1 or anti-CLTA-4 immunotherapy (140). Although published work for Tfh in the tumor microenvironment is limited, this is an active area of research.
Largely, the metabolic profile of Tfh cells under conditions of cancer is unclear. However, a recent report demonstrates enhanced glycolytic Tfh dependance during malignant pleural effusion (MPE), MPE-derived Tfh cells exhibited differentially enhanced expression of genes associated with glycolysis, cysteine, and methionine metabolism, while markers of OXPHOS and FAO were slightly elevated, when compared to blood-resident Tfh counterparts (120). Moreover, these cells show marketed inhibition of the pentose phosphate pathway, arachidonic acid, and alpha-linoleic acid metabolism (120). Thus, a symphony of multiple metabolic networks is at play under conditions of MPE. The inherently hypoxic environment influences cellular metabolism and has been attributed to increased glycolytic rates and suppression of mitochondrial metabolic pathways (141, 142). From these results we can emphasize the importance of the local environment on cellular metabolism and function. Logically, much of the controversy pertaining to Tfh metabolic reliance can be attributed to these major differences in environmental conditions. Further insight into how Tfh support anti-tumor immunity is undoubtedly forthcoming and will only serve to enhance our understanding of the diverse roles these cells play in cancer, and metabolic cues underlying their differential functionality.
Tfh in Disease: Infection
The body’s main line of defense against pathogens is through the production of anti-pathogen antibodies. Antibodies are produced by plasma cells that have undergone affinity maturation and expansion within GCs. Tfh are critical for antibody production as they help GC resident B cells differentiate into plasma cells. It has been demonstrated that Tfh play an important role in mounting an immune response against various viruses, bacteria, and parasites, including lymphocytic choriomeningitis virus (LCMV), severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), human immunodeficiency virus (HIV), Plasmodium, Schistosomiasis, and influenza infection. Of these, only a few have reported direct evidence between metabolic regulation of Tfh and association with disease. Because of established metabolic links with specific intracellular signaling cascades, some metabolic inferences can be gleaned.
Mice that are infected with a persistent strain of LCMV results in the favorable generation of CD4+ Tfh over Th1 cells (143). Due to the relationship between Tfh and B cells to produce antibodies, Tfh are critical for the clearance of viral pathogens. Tfh-mediated clearance of LCMV relies heavily on IL-6 for the increase in Tfh numbers. This increase is linked with the promotion of Bcl6 in these cells (144). Tfh are also critical in mediating a defense against both chronic and acute HCV infection as Tfh numbers increase, identified by expression of CXCR5 and PD-1 (20). Moreover, in humans infected with HCV, Tfh produce more IL-21 and have enhanced expression of ICOS (20), which, given the increase in ICOS, may be associated with enhanced rates of cellular glycolysis (110). These conclusions could be supported by studies in models of murine LCMV infection, where Tfh are described to be long-lived, having persisted for over 400 days. Moreover, these cells were found to be highly glycolytic. These long-lived Tfh, speculated to be memory Tfh, could differentiate into effector and peripheral memory T cell populations (145).
An impaired Tfh and GC response has been found in individuals suffering from COVID-19 caused by SARS-CoV-2 infection. Lung tissue analysis was performed on deceased COVID-19 patients in comparison to healthy individuals who had undergone lung surgery. It was determined that numbers of T and B cells were decreased in patients who had recently died due to COVID-19 (146). Furthermore, reduced lymph node-resident GCs were observed in the deceased patients, correlating with reduced levels of IgG and IgM, when compared to COVID-19 patients that had recovered from disease. Together, the authors speculated that the decreasing GC response and aberrant levels of immune cell populations resulted in a hampered humoral immune response in persons who have died from COVID-19 (146). Increasing expression of ICOS and CXCR5 on CD4+ T cells has also been reported (147), and circulating Tfh numbers increase for the duration of infection (148), thus, the increase in ICOS and CXCR5 may be attributed to the enhanced differentiation of Tfh, and increased glycolysis (110). Tfh-ICOS signaling is critical for the generation of an anti-COVID-19 response; hospitalized COVID-19 patients exhibited B cells with a lower expression of ICOS-L, the ligand for ICOS on Tfh, when compared with ambulatory COVID-19 patients (149). Another study has reported that Bcl6 expression in Tfh and B cells decrease, and GCs are significantly reduced during COVID-19 infection (150). Through single cell sequencing another research group has reported that the differentiation of Tfh in COVID-19 patients is impaired and that dysregulated T cell differentiation results in heightened T cell activation and accelerated cell death, with a particular emphasis on CD25+ Th1 and Th2 cells (151). Moreover, the authors speculate that altered differentiation may be related to dysregulation of metabolic pathways in these cells, as determined through altered gene profiles, though which metabolic genes are altered, and whether they are glycolytic or OXPHOS genes, is not reported (151). Although Tfh cell numbers have been reported to both increase and decrease, this may be due to differences in disease severity. Meanwhile, another study reported that upon severe COVID-19 infection, a subset of circulating CD4+ T cells derived from peripheral blood mononuclear cells (PMBC) express high levels of Voltage-Dependent Anion Channel 1 (VDAC1) and H3K27me3, an epigenetic marker, which may contribute to mitochondrial dysfunction and associated metabolic irregularities (152). Importantly, this distinct T cell subpopulation was not found in individuals with mild/moderate disease, and thus metabolic dysregulation in T cells may contribute to advanced COVID-19 disease (152).
In individuals suffering from HIV, Tfh are the CD4+ T cell population with the highest rate of infection in vivo, and the single cell type most capable of supporting HIV infection in vitro (153, 154). Moreover, HIV-infected Tfh are more likely to be found in GCs (154–156). Upon infection, Tfh do not sufficiently support B cells, though Tfh differentiation is enhanced. The resultant impaired humoral immune response may be due to increased PD-L1 expression on B cells that persistently interacts with PD-1, suppressing the function of Tfh (153). Furthermore, some Tfh exhibit downregulated expression of CD4, PD-1, and CXCR5, and this phenotype is speculated to be a means for infected Tfh to leave the GC and disseminate (154). Although little is known of Tfh-specific metabolic alterations during HIV infection, infected CD4+ T cells undergo significant metabolic modulation. HIV manipulates the T cell metabolic environment resulting in a hyperactive and often exhausted T cell phenotype, thereby reducing T cell numbers and overall dysfunction (155, 157, 158). In HIV-infected adults, reduction in T cell function correlates with increases in glucose consumption, due to HIV-induced upregulation of Glut1 in CD4+ T cells (159). HIV-infected CD4+ T cells exhibit increased amounts of glycolytic intermediates (160) and inhibition of glycolysis reduces the production of viral progeny (161). Moreover, the decline in CD4+ T cell numbers could be due to oxidative stress induced by HIV, as HIV-associated proteins can hinder mitochondrial biogenesis (162, 163). Moreover, perhaps HIV is more likely to selectively infect Tfh cells over other CD4+ subtypes because of their altered metabolic environment. It appears that viruses can advantageously manipulate the metabolic state of the host cell, to better support infection and resultant production of viral progeny. Addressing these metabolic events underlying pathogen-host interactions will require in-depth single-cell experimentation and analysis.
Parasitic infections are reported to also impact Tfh populations. In mouse models of Plasmodium infection, infected IL-6 knockout mice (Il6-/-) resulted in a more severe infection when compared with infected wildtype mice. Moreover, the authors determined that the expression of IL-6 impacted not only B cell development but also Tfh populations, through increased expression of ICOS as well as enhanced homing of Tfh to splenic B cells (40). Furthermore, investigations in murine schistosomiasis, has shown that Tfh numbers are increased in splenic germinal centers and that the increase in Tfh correlates with progressing liver fibrosis (164). Higher numbers of circulating Tfh have also been demonstrated in human patients infected with Schistosoma species when compared with healthy individuals (165). Moreover, it is speculated that IL-4 secreting Tfh may play a role in protection from reinfection by Schistosoma species (166). Although direct evidence has not established metabolic alterations with Tfh function in Plasmodium infection, over the duration of malaria infection CD4+ T cells exhibit an exhausted phenotype (167). Moreover, in a recent report, cellular exhaustion correlated with reduced glycolysis, and was found to be associated with impaired mTORC1 activity (168). Implementation of immunotherapy targeted PD-1, CTLA-4, and IL-27 reduced cellular exhaustion and enhanced cellular rates of glycolysis (168). It is tempting to speculate that this population analysis of CD4+ T cells may reflect Tfh metabolic alterations, as mTORC1 has been demonstrated to be required for Tfh differentiation and function (110).
CD8+ Tfh-like cells have been described in simian-immunodeficiency virus (SIV) (169). The Tfh-like cells localize to follicles during SIV infection. Interestingly, Tfh-like cell homing to follicles may depend on IL-15, as shown following utilization of human IL-15 superagonist ALT-803. It is speculated that the increased homing of CD8+ Tfh-like cells under conditions of the superagonist may correlate with clearance of viral infection, as lower numbers of SIV-infected cells were contained within follicles (169). CD8+ Tfh-like cells have also been reported in conditions of chronic LCMV infection. The CD8+ Tfh-like cells exhibited many of the hallmarks of CD4+ Tfh including CXCR5, PD-1, ICOS, and TFs Batf and Maf, among others (136). Moreover, in Hodgkin’s lymphoma, genetic analysis determined that CXCR5+ ICOS+ CD8+ T cells are most closely related to CD4-derived Tfh than other cell populations (91), and thus these cells may undergo similar metabolic alterations under conditions of infection.
Collectively, Tfh are intimately associated with the severity of numerous diseases. Frequently this is due to dysregulation of these cells resulting in heightened production of pathogenic antibodies. A critical aspect of the manipulation of these cells is likely attributed to modulation of metabolic signaling; yet is a largely unexplored field. Current metabolic findings under conditions of infection are described in Table 1. An improved understanding of the metabolic demands of these cells will undoubtedly inform the identification and development of improved therapeutics to treat these diseases.
Conclusions
The importance of Tfh for mounting an adaptive immune response cannot be understated. Tfh are differentiated in response to antigen, and in turn they interact with GC B cells for production of pathogen-specific antibodies. In conditions of good health, Tfh are highly controlled; when this system is disrupted, Tfh may differentiate rapidly, resulting in autoimmunity. Likewise, Tfh differentiation may not occur at rates that allow for a sufficient immune response. In instances of cancer, Tfh may correlate with improved survival rates. However, they require continual access to nutrients to maintain functionality, which in a nutrient-restricted tumor environment often results in an insufficient T cell response for tumor clearance. Under conditions of infection, Tfh are typically associated with the progression of disease. The functionality of these cells is generally altered, resulting in an impaired humoral immune response. As the contributions of CD8+ Tfh-like cells to the immune response are now becoming appreciated, experimentation to understand the implications of these cells in generating an immune response under varying disease states will undoubtedly continue. Further investigation and comparison of differing Tfh populations would prove instrumental in unraveling the complexities of Tfh and Tfh-like cell differentiation, and how metabolism may contribute to Tfh development and function.
Arguably, Tfh functionality and differentiation can be largely attributed to metabolic regulation in activated CD4+ T cells. Currently, it is unclear whether Tfh ultimately require glycolysis or OXPHOS to fuel metabolic demands, or if these cells can use either, possibly with support of additional metabolic networks, depending on the local environment. It is speculated that many of the differences can be attributed to how Tfh are derived, whether in vitro or in vivo. Moreover, for in vivo implications, how the cells were generated (i.e., by vaccination, infection, or under conditions of autoimmunity, etc.), which may result in differences in metabolic demand according to the methods of activation. Moreover, the tissues-specific microenvironment is undoubtedly a clear metabolic influence, either through induced hypoxia, seen in many diseases, including autoimmunity, viral infections, and cancer. In addition, the competition for resources in these microenvironments are also key contributors to cellular function. These differing conditions could generate the dichotomy in metabolic states. However, currently there appears to be more substantial evidence for the role of glycolysis in fueling Tfh. More work is needed to provide clarity on the metabolic demands of Tfh under differing conditions. This insight will inform the generation of improved, metabolism-targeted, therapeutics for diseases in which Tfh are central, including autoimmunity, cancer, and infection.
Author Contributions
CM prepared the figures. CM, NL, JW, and C-HC wrote the manuscript. All authors have read and agreed to the published version of the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank all members of the Chang Laboratory for critical feedback and discussion. We are extremely grateful for the funding support from The Jackson Laboratory Director’s Innovation Fund (DIF-FY21-GREENE), The Mark Foundation for Cancer Research (19-036-ASP), The V Foundation (V2021-036), and the Pyewacket Fund.
Glossary
2-DG: 2-deoxyglucose
AITD: Autoimmune thyroid disease
AMPK: AMP-activated protein kinase
CIA: Collagen-induced arthritis
cDC: Conventional dendritic cells
COVID-19: Coronavirus disease 2019
CCL: C-C motif chemokine ligand
CDP: cytidine diphosphate
CNS2: conserved non-coding sequence 2
CRCs: CXCL12-producing reticular cells
CXCR: C-X-C motif chemokine receptor
DZ: Dark zone
DC: Dendritic cell
ID: DNA-binding inhibitor
DUSP6: Dual specificity phosphatase 6
ETC: Electron transport chain
LEF-1: Lymphoid enhancer-binding factor-1
Tfh: T follicular helper cell
Tfr: Follicular regulatory T cell
Th: Effector T helper cell
FDCs: Follicular dendritic cells
GC: Germinal center
Glut1: Glucose transport 1
HCV: Hepatitis C virus
HIV: Human immunodeficiency virus
ICOS: Inducible T cell co-stimulator
IL: interleukin
IFN: Interferon
LZ: Light zone
LCMV: Lymphocytic choriomeningitis virus
MHC: Major histocompatibility complex
mTOR: Mammalian target of rapamycin
OXPHOS: Oxidative phosphorylation
Ova: Ovalbumin
PBMC: Peripheral blood mononuclear cell
PD-1: Programmed cell death protein 1
PI3K: Phosphoinositide 3-kinase
PKC: Protein kinase C
SAP: SLAM-associated protein
TCA: Tricarboxylic acid cycle
TCF-1: T cell factor-1
Treg: Regulatory T cell
RA: Rheumatoid arthritis
SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2
STAT: Signal transducers and transcriptional activator
SLAM: Signaling lymphocyte activation molecule
SIM: Simian-immunodeficiency virus
SAP: SLAM-associated protein
SHM: Somatic hyper mutation
SLE: Systemic lupus erythematosus
TCR: T cell receptor
Tfh: T Follicular helper cell
TGF-β: Transforming growth factor-β
TF: Transcription factor
T1D: Type 1 diabetes
VDAC: Voltage Dependent Anion Channel 1
References
1. Lu Y, Craft J. T Follicular Regulatory Cells: Choreographers of Productive Germinal Center Responses. Front Immunol (2021) 12:679909. doi: 10.3389/fimmu.2021.679909
2. Wing JB, Tekguc M, Sakaguchi S. Control of Germinal Center Responses by T-Follicular Regulatory Cells. Front Immunol (2018) 9:1910. doi: 10.3389/fimmu.2018.01910
3. Krautler NJ, Suan D, Butt D, Bourne K, Hermes JR, Chan TD, et al. Differentiation of Germinal Center B Cells Into Plasma Cells Is Initiated by High-Affinity Antigen and Completed by Tfh Cells. J Exp Med (2017) 214:1259–67. doi: 10.1084/jem.20161533
4. Crotty S. Follicular Helper CD4 T Cells (TFH). Annu Rev Immunol (2011) 29:621–63. doi: 10.1146/annurev-immunol-031210-101400
5. Ono M. Control of Regulatory T-Cell Differentiation and Function by T-Cell Receptor Signalling and Foxp3 Transcription Factor Complexes. Immunology (2020) 160:24–37. doi: 10.1111/imm.13178
6. Ma CS, Suryani S, Avery DT, Chan A, Nanan R, Santner-Nanan B, et al. Early Commitment of Naive Human CD4(+) T Cells to the T Follicular Helper (T(FH)) Cell Lineage is Induced by IL-12. Immunol Cell Biol (2009) 87:590–600. doi: 10.1038/icb.2009.64
7. Crotty S. T Follicular Helper Cell Differentiation, Function, and Roles in Disease. Immunity (2014) 41:529–42. doi: 10.1016/j.immuni.2014.10.004
8. Hatzi K, Nance JP, Kroenke MA, Bothwell M, Haddad EK, Melnick A, et al. BCL6 Orchestrates Tfh Cell Differentiation via Multiple Distinct Mechanisms. J Exp Med (2015) 212:539–53. doi: 10.1084/jem.20141380
9. Rautajoki KJ, Kylaniemi MK, Raghav SK, Rao K, Lahesmaa R. An Insight Into Molecular Mechanisms of Human T Helper Cell Differentiation. Ann Med (2008) 40:322–35. doi: 10.1080/07853890802068582
10. Schmitt N, Ueno H. Regulation of Human Helper T Cell Subset Differentiation by Cytokines. Curr Opin Immunol (2015) 34:130–6. doi: 10.1016/j.coi.2015.03.007
11. Qiu H, Wu H, Chan V, Lau CS, Lu Q. Transcriptional and Epigenetic Regulation of Follicular T-Helper Cells and Their Role in Autoimmunity. Autoimmunity (2017) 50:71–81. doi: 10.1080/08916934.2017.1284821
12. Zhu J, Paul WE. Peripheral CD4+ T-Cell Differentiation Regulated by Networks of Cytokines and Transcription Factors. Immunol Rev (2010) 238:247–62. doi: 10.1111/j.1600-065X.2010.00951.x
13. Groom JR. Regulators of T-Cell Fate: Integration of Cell Migration, Differentiation and Function. Immunol Rev (2019) 289:101–14. doi: 10.1111/imr.12742
14. Choi YS, Yang JA, Yusuf I, Johnston RJ, Greenbaum J, Peters B, et al. Bcl6 Expressing Follicular Helper CD4 T Cells Are Fate Committed Early and Have the Capacity to Form Memory. J Immunol (2013) 190:4014–26. doi: 10.4049/jimmunol.1202963
15. Sallusto F, Lenig D, Mackay CR, Lanzavecchia A. Flexible Programs of Chemokine Receptor Expression on Human Polarized T Helper 1 and 2 Lymphocytes. J Exp Med (1998) 187:875–83. doi: 10.1084/jem.187.6.875
16. Nance JP, Belanger S, Johnston RJ, Takemori T, Crotty S. Cutting Edge: T Follicular Helper Cell Differentiation Is Defective in the Absence of Bcl6 BTB Repressor Domain Function. J Immunol (2015) 194:5599–603. doi: 10.4049/jimmunol.1500200
17. Linterman MA, Vinuesa CG. Signals That Influence T Follicular Helper Cell Differentiation and Function. Semin Immunopathol (2010) 32:183–96. doi: 10.1007/s00281-009-0194-z
18. Zhang Y, Garcia-Ibanez L, Toellner KM. Regulation of Germinal Center B-Cell Differentiation. Immunol Rev (2016) 270:8–19. doi: 10.1111/imr.12396
19. Goenka R, Barnett LG, Silver JS, O'Neill PJ, Hunter CA, Cancro MP, et al. Cutting Edge: Dendritic Cell-Restricted Antigen Presentation Initiates the Follicular Helper T Cell Program But Cannot Complete Ultimate Effector Differentiation. J Immunol (2011) 187:1091–5. doi: 10.4049/jimmunol.1100853
20. Eto D, Lao C, DiToro D, Barnett B, Escobar TC, Kageyama R, et al. IL-21 and IL-6 Are Critical for Different Aspects of B Cell Immunity and Redundantly Induce Optimal Follicular Helper CD4 T Cell (Tfh) Differentiation. PloS One (2011) 6:e17739. doi: 10.1371/journal.pone.0017739
21. Xu YD, Cheng M, Shang PP, Yang YQ. Role of IL-6 in Dendritic Cell Functions. J Leukoc Biol (2021). doi: 10.1002/JLB.3MR0621-616RR
22. Weber JP, Fuhrmann F, Feist RK, Lahmann A, Al Baz MS, Gentz LJ, et al. ICOS Maintains the T Follicular Helper Cell Phenotype by Down-Regulating Kruppel-Like Factor 2. J Exp Med (2015) 212:217–33. doi: 10.1084/jem.20141432
23. Ballesteros-Tato A, Randall TD. Priming of T Follicular Helper Cells by Dendritic Cells. Immunol Cell Biol (2014) 92:22–7. doi: 10.1038/icb.2013.62
24. Krishnaswamy JK, Alsen S, Yrlid U, Eisenbarth SC, Williams A. Determination of T Follicular Helper Cell Fate by Dendritic Cells. Front Immunol (2018) 9:2169. doi: 10.3389/fimmu.2018.02169
25. Liu D, Yan J, Sun J, Liu B, Ma W, Li Y, et al. BCL6 Controls Contact-Dependent Help Delivery During Follicular T-B Cell Interactions. Immunity (2021) 54:2245–2255.e4. doi: 10.1016/j.immuni.2021.08.003
26. Boettler T, Moeckel F, Cheng Y, Heeg M, Salek-Ardakani S, Crotty S, et al. OX40 Facilitates Control of a Persistent Virus Infection. PloS Pathog (2012) 8:e1002913. doi: 10.1371/journal.ppat.1002913
27. Zander RA, Obeng-Adjei N, Guthmiller JJ, Kulu DI, Li J, Ongoiba A, et al. PD-1 Co-Inhibitory and OX40 Co-Stimulatory Crosstalk Regulates Helper T Cell Differentiation and Anti-Plasmodium Humoral Immunity. Cell Host Microbe (2015) 17:628–41. doi: 10.1016/j.chom.2015.03.007
28. Bauquet AT, Jin H, Paterson AM, Mitsdoerffer M, Ho IC, Sharpe AH, et al. The Costimulatory Molecule ICOS Regulates the Expression of C-Maf and IL-21 in the Development of Follicular T Helper Cells and TH-17 Cells. Nat Immunol (2009) 10:167–75. doi: 10.1038/ni.1690
29. Choi YS, Kageyama R, Eto D, Escobar TC, Johnston RJ, Monticelli L, et al. ICOS Receptor Instructs T Follicular Helper Cell Versus Effector Cell Differentiation via Induction of the Transcriptional Repressor Bcl6. Immunity (2011) 34:932–46. doi: 10.1016/j.immuni.2011.03.023
30. Zeng QH, Wei Y, Lao XM, Chen DP, Huang CX, Lin QY, et al. B Cells Polarize Pathogenic Inflammatory T Helper Subsets Through ICOSL-Dependent Glycolysis. Sci Adv (2020) 6(20). doi: 10.1126/sciadv.abb6296
31. Comerford I, Harata-Lee Y, Bunting MD, Gregor C, Kara EE, McColl SR. A Myriad of Functions and Complex Regulation of the CCR7/CCL19/CCL21 Chemokine Axis in the Adaptive Immune System. Cytokine Growth Factor Rev (2013) 24:269–83. doi: 10.1016/j.cytogfr.2013.03.001
32. Ma CS, Deenick EK, Batten M, Tangye SG. The Origins, Function, and Regulation of T Follicular Helper Cells. J Exp Med (2012) 209:1241–53. doi: 10.1084/jem.20120994
33. Weinstein JS, Herman EI, Lainez B, Licona-Limon P, Esplugues E, Flavell R, et al. TFH Cells Progressively Differentiate to Regulate the Germinal Center Response. Nat Immunol (2016) 17:1197–205. doi: 10.1038/ni.3554
34. Haynes NM, Allen CD, Lesley R, Ansel KM, Killeen N, Cyster JG. Role of CXCR5 and CCR7 in Follicular Th Cell Positioning and Appearance of a Programmed Cell Death Gene-1high Germinal Center-Associated Subpopulation. J Immunol (2007) 179:5099–108. doi: 10.4049/jimmunol.179.8.5099
35. Wang X, Cho B, Suzuki K, Xu Y, Green JA, An J, et al. Follicular Dendritic Cells Help Establish Follicle Identity and Promote B Cell Retention in Germinal Centers. J Exp Med (2011) 208:2497–510. doi: 10.1084/jem.20111449
36. Rodda LB, Bannard O, Ludewig B, Nagasawa T, Cyster JG. Phenotypic and Morphological Properties of Germinal Center Dark Zone Cxcl12-Expressing Reticular Cells. J Immunol (2015) 195:4781–91. doi: 10.4049/jimmunol.1501191
37. Preite S, Huang B, Cannons JL, McGavern DB, Schwartzberg PL. PI3K Orchestrates T Follicular Helper Cell Differentiation in a Context Dependent Manner: Implications for Autoimmunity. Front Immunol (2018) 9:3079. doi: 10.3389/fimmu.2018.03079
38. Shi J, Hou S, Fang Q, Liu X, Liu X, Qi H. PD-1 Controls Follicular T Helper Cell Positioning and Function. Immunity (2018) 49:264–274.e4. doi: 10.1016/j.immuni.2018.06.012
39. Xu H, Li X, Liu D, Li J, Zhang X, Chen X, et al. Follicular T-Helper Cell Recruitment Governed by Bystander B Cells and ICOS-Driven Motility. Nature (2013) 496:523–7. doi: 10.1038/nature12058
40. Sebina I, Fogg LG, James KR, Soon MSF, Akter J, Thomas BS, et al. IL-6 Promotes CD4(+) T-Cell and B-Cell Activation During Plasmodium Infection. Parasite Immunol (2017) 39. doi: 10.1111/pim.12455
41. Crotty S. T Follicular Helper Cell Biology: A Decade of Discovery and Diseases. Immunity (2019) 50:1132–48. doi: 10.1016/j.immuni.2019.04.011
42. Shulman Z, Gitlin AD, Targ S, Jankovic M, Pasqual G, Nussenzweig MC, et al. T Follicular Helper Cell Dynamics in Germinal Centers. Science (2013) 341:673–7. doi: 10.1126/science.1241680
43. Wu C, Nguyen KB, Pien GC, Wang N, Gullo C, Howie D, et al. SAP Controls T Cell Responses to Virus and Terminal Differentiation of TH2 Cells. Nat Immunol (2001) 2:410–4. doi: 10.1038/87713
44. Czar MJ, Kersh EN, Mijares LA, Lanier G, Lewis J, Yap G, et al. Altered Lymphocyte Responses and Cytokine Production in Mice Deficient in the X-Linked Lymphoproliferative Disease Gene SH2D1A/DSHP/SAP. Proc Natl Acad Sci USA (2001) 98:7449–54 doi: 10.1073/pnas.131193098
45. Pilzecker B, Jacobs H. Mutating for Good: DNA Damage Responses During Somatic Hypermutation. Front Immunol (2019) 10:438. doi: 10.3389/fimmu.2019.00438
46. Xie MM, Dent AL. Unexpected Help: Follicular Regulatory T Cells in the Germinal Center. Front Immunol (2018) 9:1536. doi: 10.3389/fimmu.2018.01536
47. Amiezer M, Phan TG. Disentangling Tfr Cells From Treg Cells and Tfh Cells: How to Untie the Gordian Knot. Eur J Immunol (2016) 46:1101–4. doi: 10.1002/eji.201646389
48. Wu BX, Zhao LD, Zhang X. CXCR4 and CXCR5 Orchestrate Dynamic Germinal Center Reactions and May Contribute to the Pathogenesis of Systemic Lupus Erythematosus. Cell Mol Immunol (2019) 16:724–6. doi: 10.1038/s41423-019-0244-y
49. Ma CS, Phan TG. Here, There and Everywhere: T Follicular Helper Cells on the Move. Immunology (2017) 152:382–7. doi: 10.1111/imm.12793
50. Sage PT, Alvarez D, Godec J, von Andrian UH, Sharpe AH. Circulating T Follicular Regulatory and Helper Cells Have Memory-Like Properties. J Clin Invest (2014) 124:5191–204. doi: 10.1172/JCI76861
51. Fazilleau N, Eisenbraun MD, Malherbe L, Ebright JN, Pogue-Caley RR, McHeyzer-Williams LJ, et al. Lymphoid Reservoirs of Antigen-Specific Memory T Helper Cells. Nat Immunol (2007) 8:753–61. doi: 10.1038/ni1472
52. Liu X, Nurieva RI, Dong C. Transcriptional Regulation of Follicular T-Helper (Tfh) Cells. Immunol Rev (2013) 252:139–45. doi: 10.1111/imr.12040
53. Wu S, Yang T, Pan F, Xia G, Hu Y, Liu L, et al. Increased Frequency of Circulating Follicular Helper T Cells in Patients With Ankylosing Spondylitis. Mod Rheumatol (2015) 25:110–5. doi: 10.3109/14397595.2014.902149
54. Johnston RJ, Poholek AC, DiToro D, Yusuf I, Eto D, Barnett B, et al. Bcl6 and Blimp-1 Are Reciprocal and Antagonistic Regulators of T Follicular Helper Cell Differentiation. Science (2009) 325:1006–10. doi: 10.1126/science.1175870
55. Nurieva RI, Chung Y, Martinez GJ, Yang XO, Tanaka S, Matskevitch TD, et al. Bcl6 Mediates the Development of T Follicular Helper Cells. Science (2009) 325:1001–5. doi: 10.1126/science.1176676
56. Yu D, Rao S, Tsai LM, Lee SK, He Y, Sutcliffe EL, et al. The Transcriptional Repressor Bcl-6 Directs T Follicular Helper Cell Lineage Commitment. Immunity (2009) 31:457–68. doi: 10.1016/j.immuni.2009.07.002
57. Poholek AC, Hansen K, Hernandez SG, Eto D, Chandele A, Weinstein JS, et al. In Vivo Regulation of Bcl6 and T Follicular Helper Cell Development. J Immunol (2010) 185:313–26. doi: 10.4049/jimmunol.0904023
58. Leong YA, Chen Y, Ong HS, Wu D, Man K, Deleage C, et al. CXCR5(+) Follicular Cytotoxic T Cells Control Viral Infection in B Cell Follicles. Nat Immunol (2016) 17:1187–96. doi: 10.1038/ni.3543
59. Gowthaman U, Chen JS, Zhang B, Flynn WF, Lu Y, Song W, et al. Identification of a T Follicular Helper Cell Subset That Drives Anaphylactic IgE. Science (2019) 365. doi: 10.1126/science.aaw6433
60. Kim CJ, Lee CG, Jung JY, Ghosh A, Hasan SN, Hwang SM, et al. The Transcription Factor Ets1 Suppresses T Follicular Helper Type 2 Cell Differentiation to Halt the Onset of Systemic Lupus Erythematosus. Immunity (2019) 50:272. doi: 10.1016/j.immuni.2018.12.023
61. Choi J, Diao H, Faliti CE, Truong J, Rossi M, Belanger S, et al. Bcl-6 is the Nexus Transcription Factor of T Follicular Helper Cells via Repressor-of-Repressor Circuits. Nat Immunol (2020) 21:777–89. doi: 10.1038/s41590-020-0706-5
62. Suan D, Nguyen A, Moran I, Bourne K, Hermes JR, Arshi M, et al. T Follicular Helper Cells Have Distinct Modes of Migration and Molecular Signatures in Naive and Memory Immune Responses. Immunity (2015) 42:704–18. doi: 10.1016/j.immuni.2015.03.002
63. Ray JP, Marshall HD, Laidlaw BJ, Staron MM, Kaech SM, Craft J. Transcription Factor STAT3 and Type I Interferons Are Corepressive Insulators for Differentiation of Follicular Helper and T Helper 1 Cells. Immunity (2014) 40:367–77. doi: 10.1016/j.immuni.2014.02.005
64. Schmitt N, Liu Y, Bentebibel SE, Munagala I, Bourdery L, Venuprasad K, et al. The Cytokine TGF-Beta Co-Opts Signaling via STAT3-STAT4 to Promote the Differentiation of Human TFH Cells. Nat Immunol (2014) 15:856–65. doi: 10.1038/ni.2947
65. Andris F, Denanglaire S, Anciaux M, Hercor M, Hussein H, Leo O. The Transcription Factor C-Maf Promotes the Differentiation of Follicular Helper T Cells. Front Immunol (2017) 8:480. doi: 10.3389/fimmu.2017.00480
66. Betz BC, Jordan-Williams KL, Wang C, Kang SG, Liao J, Logan MR, et al. Batf Coordinates Multiple Aspects of B and T Cell Function Required for Normal Antibody Responses. J Exp Med (2010) 207:933–42. doi: 10.1084/jem.20091548
67. Sahoo A, Wali S, Nurieva R. T Helper 2 and T Follicular Helper Cells: Regulation and Function of Interleukin-4. Cytokine Growth Factor Rev (2016) 30:29–37. doi: 10.1016/j.cytogfr.2016.03.011
68. Johnston RJ, Choi YS, Diamond JA, Yang JA, Crotty S. STAT5 Is a Potent Negative Regulator of TFH Cell Differentiation. J Exp Med (2012) 209:243–50. doi: 10.1084/jem.20111174
69. Qi H, Liu D, Ma W, Wang Y, Yan H. Bcl-6 Controlled TFH Polarization and Memory: The Known Unknowns. Curr Opin Immunol (2014) 28:34–41. doi: 10.1016/j.coi.2014.01.016
70. Papillion A, Powell MD, Chisolm DA, Bachus H, Fuller MJ, Weinmann AS, et al. Inhibition of IL-2 Responsiveness by IL-6 Is Required for the Generation of GC-TFH Cells. Sci Immunol (2019) 4. doi: 10.1126/sciimmunol.aaw7636
71. Miyazaki M, Miyazaki K, Chen S, Chandra V, Wagatsuma K, Agata Y, et al. The E-Id Protein Axis Modulates the Activities of the PI3K-AKT-Mtorc1-Hif1a and C-Myc/p19Arf Pathways to Suppress Innate Variant TFH Cell Development, Thymocyte Expansion, and Lymphomagenesis. Genes Dev (2015) 29:409–25. doi: 10.1101/gad.255331.114
72. Xiao N, Eto D, Elly C, Peng G, Crotty S, Liu YC. The E3 Ubiquitin Ligase Itch Is Required for the Differentiation of Follicular Helper T Cells. Nat Immunol (2014) 15:657–66. doi: 10.1038/ni.2912
73. Stone EL, Pepper M, Katayama CD, Kerdiles YM, Lai CY, Emslie E, et al. ICOS Coreceptor Signaling Inactivates the Transcription Factor FOXO1 to Promote Tfh Cell Differentiation. Immunity (2015) 42:239–51. doi: 10.1016/j.immuni.2015.01.017
74. Choi YS, Yang JA, Crotty S. Dynamic Regulation of Bcl6 in Follicular Helper CD4 T (Tfh) Cells. Curr Opin Immunol (2013) 25:366–72. doi: 10.1016/j.coi.2013.04.003
75. Kim SJ. Immunological Function of Blimp-1 in Dendritic Cells and Relevance to Autoimmune Diseases. Immunol Res (2015) 63:113–20. doi: 10.1007/s12026-015-8694-5
76. Ji LS, Sun XH, Zhang X, Zhou ZH, Yu Z, Zhu XJ, et al. Mechanism of Follicular Helper T Cell Differentiation Regulated by Transcription Factors. J Immunol Res (2020) 2020:1826587. doi: 10.1155/2020/1826587
77. Li P, Spolski R, Liao W, Leonard WJ. Complex Interactions of Transcription Factors in Mediating Cytokine Biology in T Cells. Immunol Rev (2014) 261:141–56. doi: 10.1111/imr.12199
78. Batten M, Ramamoorthi N, Kljavin NM, Ma CS, Cox JH, Dengler HS, et al. IL-27 Supports Germinal Center Function by Enhancing IL-21 Production and the Function of T Follicular Helper Cells. J Exp Med (2010) 207:2895–906. doi: 10.1084/jem.20100064
79. Coquet JM, Schuijs MJ, Smyth MJ, Deswarte K, Beyaert R, Braun H, et al. Interleukin-21-Producing CD4(+) T Cells Promote Type 2 Immunity to House Dust Mites. Immunity (2015) 43:318–30. doi: 10.1016/j.immuni.2015.07.015
80. Harker JA, Dolgoter A, Zuniga EI. Cell-Intrinsic IL-27 and Gp130 Cytokine Receptor Signaling Regulates Virus-Specific CD4(+) T Cell Responses and Viral Control During Chronic Infection. Immunity (2013) 39:548–59. doi: 10.1016/j.immuni.2013.08.010
81. DiToro D, Winstead CJ, Pham D, Witte S, Andargachew R, Singer JR, et al. Differential IL-2 Expression Defines Developmental Fates of Follicular Versus Nonfollicular Helper T Cells. Science (2018) 361. doi: 10.1126/science.aao2933
82. Ballesteros-Tato A, Leon B, Graf BA, Moquin A, Adams PS, Lund FE, et al. Interleukin-2 Inhibits Germinal Center Formation by Limiting T Follicular Helper Cell Differentiation. Immunity (2012) 36:847–56. doi: 10.1016/j.immuni.2012.02.012
83. Olatunde AC, Hale JS, Lamb TJ. Cytokine-Skewed Tfh Cells: Functional Consequences for B Cell Help. Trends Immunol (2021) 42:536–50. doi: 10.1016/j.it.2021.04.006
84. Luckheeram RV, Zhou R, Verma AD, Xia B. Cd4(+)T Cells: Differentiation and Functions. Clin Dev Immunol (2012) 2012:925135. doi: 10.1155/2012/925135
85. Valentine KM, Davini D, Lawrence TJ, Mullins GN, Manansala M, Al-Kuhlani M, et al. CD8 Follicular T Cells Promote B Cell Antibody Class Switch in Autoimmune Disease. J Immunol (2018) 201:31–40. doi: 10.4049/jimmunol.1701079
86. Kang YM, Zhang X, Wagner UG, Yang H, Beckenbaugh RD, Kurtin PJ, et al. CD8 T Cells Are Required for the Formation of Ectopic Germinal Centers in Rheumatoid Synovitis. J Exp Med (2002) 195:1325–36. doi: 10.1084/jem.20011565
87. Wagner UG, Kurtin PJ, Wahner A, Brackertz M, Berry DJ, Goronzy JJ, et al. The Role of CD8+ CD40L+ T Cells in the Formation of Germinal Centers in Rheumatoid Synovitis. J Immunol (1998) 161:6390–7.
88. He R, Hou S, Liu C, Zhang A, Bai Q, Han M, et al. Erratum: Follicular CXCR5-Expressing CD8+ T Cells Curtail Chronic Viral Infection. Nature (2016) 540:470. doi: 10.1038/nature20107
89. Yang R, Masters AR, Fortner KA, Champagne DP, Yanguas-Casas N, Silberger DJ, et al. IL-6 Promotes the Differentiation of a Subset of Naive CD8+ T Cells Into IL-21-Producing B Helper CD8+ T Cells. J Exp Med (2016) 213:2281–91. doi: 10.1084/jem.20160417
90. Im SJ, Hashimoto M, Gerner MY, Lee J, Kissick HT, Burger MC, et al. Defining CD8+ T Cells That Provide the Proliferative Burst After PD-1 Therapy. Nature (2016) 537:417–21. doi: 10.1038/nature19330
91. Xiao L, Jia L, Bai L, He L, Yang B, Wu C, et al. Phenotypic and Functional Characteristics of IL-21-Expressing CD8(+) T Cells in Human Nasal Polyps. Sci Rep (2016) 6:30362. doi: 10.1038/srep30362
92. Jiang J, Champion CI, Wei B, Liu G, Kelly KA. CD8(+)CXCR5(+) T Cells Regulate Pathology in the Genital Tract. Infect Dis Obstet Gynecol (2013) 2013:813238. doi: 10.1155/2013/813238
93. Quigley MF, Gonzalez VD, Granath A, Andersson J, Sandberg JK. CXCR5+ CCR7- CD8 T Cells Are Early Effector Memory Cells That Infiltrate Tonsil B Cell Follicles. Eur J Immunol (2007) 37:3352–62. doi: 10.1002/eji.200636746
94. Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science (2009) 324:1029–33. doi: 10.1126/science.1160809
95. Mills EL, Kelly B, O'Neill LAJ. Mitochondria Are the Powerhouses of Immunity. Nat Immunol (2017) 18:488–98. doi: 10.1038/ni.3704
96. Houten SM, Violante S, Ventura FV, Wanders RJ. The Biochemistry and Physiology of Mitochondrial Fatty Acid Beta-Oxidation and Its Genetic Disorders. Annu Rev Physiol (2016) 78:23–44. doi: 10.1146/annurev-physiol-021115-105045
97. Schieber M, Chandel NS. ROS Function in Redox Signaling and Oxidative Stress. Curr Biol (2014) 24:R453–62. doi: 10.1016/j.cub.2014.03.034
98. Yao Y, Chen Z, Zhang H, Chen C, Zeng M, Yunis J, et al. Selenium-GPX4 Axis Protects Follicular Helper T Cells From Ferroptosis. Nat Immunol (2021) 22:1127–39. doi: 10.1038/s41590-021-00996-0
99. Ray JP, Staron MM, Shyer JA, Ho PC, Marshall HD, Gray SM, et al. The Interleukin-2-Mtorc1 Kinase Axis Defines the Signaling, Differentiation, and Metabolism of T Helper 1 and Follicular B Helper T Cells. Immunity (2015) 43:690–702. doi: 10.1016/j.immuni.2015.08.017
100. Hsu WC, Chen MY, Hsu SC, Huang LR, Kao CY, Cheng WH, et al. DUSP6 Mediates T Cell Receptor-Engaged Glycolysis and Restrains TFH Cell Differentiation. Proc Natl Acad Sci USA (2018) 115:E8027–36 doi: 10.1073/pnas.1800076115
101. Xie MM, Amet T, Liu H, Yu Q, Dent AL. AMP Kinase Promotes Bcl6 Expression in Both Mouse and Human T Cells. Mol Immunol (2017) 81:67–75. doi: 10.1016/j.molimm.2016.11.020
102. Abboud G, Choi SC, Kanda N, Zeumer-Spataro L, Roopenian DC, Morel L. Inhibition of Glycolysis Reduces Disease Severity in an Autoimmune Model of Rheumatoid Arthritis. Front Immunol (2018) 9:1973. doi: 10.3389/fimmu.2018.01973
103. Liu RT, Zhang M, Yang CL, Zhang P, Zhang N, Du T, et al. Enhanced Glycolysis Contributes to the Pathogenesis of Experimental Autoimmune Neuritis. J Neuroinflamm (2018) 15:51. doi: 10.1186/s12974-018-1095-7
104. Oestreich KJ, Read KA, Gilbertson SE, Hough KP, McDonald PW, Krishnamoorthy V, et al. Bcl-6 Directly Represses the Gene Program of the Glycolysis Pathway. Nat Immunol (2014) 15:957–64. doi: 10.1038/ni.2985
105. Rane S. Inhibiting Glycolysis Enhances T Follicular Helper Cell Differentiation and Survival Upon Human Immunodeficienty Virus Infection. Indiana University (2020).
106. Patsoukis N, Bardhan K, Chatterjee P, Sari D, Liu B, Bell LN, et al. PD-1 Alters T-Cell Metabolic Reprogramming by Inhibiting Glycolysis and Promoting Lipolysis and Fatty Acid Oxidation. Nat Commun (2015) 6:6692. doi: 10.1038/ncomms7692
107. Tarasenko TN, Pacheco SE, Koenig MK, Gomez-Rodriguez J, Kapnick SM, Diaz F, et al. Cytochrome C Oxidase Activity Is a Metabolic Checkpoint That Regulates Cell Fate Decisions During T Cell Activation and Differentiation. Cell Metab (2017) 25:1254–1268.e7. doi: 10.1016/j.cmet.2017.05.007
108. Dong L, He Y, Zhou S, Cao Y, Li Y, Bi Y, et al. HIF1alpha-Dependent Metabolic Signals Control the Differentiation of Follicular Helper T Cells. Cells (2019) 8. doi: 10.3390/cells8111450
109. Yang J, Lin X, Pan Y, Wang J, Chen P, Huang H, et al. Critical Roles of mTOR Complex 1 and 2 for T Follicular Helper Cell Differentiation and Germinal Center Responses. Elife (2016) 5. doi: 10.7554/eLife.17936
110. Zeng H, Cohen S, Guy C, Shrestha S, Neale G, Brown SA, et al. Mtorc1 and Mtorc2 Kinase Signaling and Glucose Metabolism Drive Follicular Helper T Cell Differentiation. Immunity (2016) 45:540–54. doi: 10.1016/j.immuni.2016.08.017
111. Yi W, Gupta S, Ricker E, Manni M, Jessberger R, Chinenov Y, et al. The Mtorc1-4E-BP-Eif4e Axis Controls De Novo Bcl6 Protein Synthesis in T Cells and Systemic Autoimmunity. Nat Commun (2017) 8:254. doi: 10.1038/s41467-017-00348-3
112. Deng J, Chen Q, Chen Z, Liang K, Gao X, Wang X, et al. The Metabolic Hormone Leptin Promotes the Function of TFH Cells and Supports Vaccine Responses. Nat Commun (2021) 12:3073. doi: 10.1038/s41467-021-23220-x
113. Ganeshan K, Chawla A. Metabolic Regulation of Immune Responses. Annu Rev Immunol (2014) 32:609–34. doi: 10.1146/annurev-immunol-032713-120236
114. Abella V, Scotece M, Conde J, Pino J, Gonzalez-Gay MA, Gomez-Reino JJ, et al. Leptin in the Interplay of Inflammation, Metabolism and Immune System Disorders. Nat Rev Rheumatol (2017) 13:100–9. doi: 10.1038/nrrheum.2016.209
115. Naylor C, Petri WA Jr. Leptin Regulation of Immune Responses. Trends Mol Med (2016) 22:88–98. doi: 10.1016/j.molmed.2015.12.001
116. Shrestha S, Yang K, Guy C, Vogel P, Neale G, Chi H. Treg Cells Require the Phosphatase PTEN to Restrain TH1 and TFH Cell Responses. Nat Immunol (2015) 16:178–87. doi: 10.1038/ni.3076
117. Essig K, Hu D, Guimaraes JC, Alterauge D, Edelmann S, Raj T, et al. Roquin Suppresses the PI3K-mTOR Signaling Pathway to Inhibit T Helper Cell Differentiation and Conversion of Treg to Tfr Cells. Immunity (2017) 47:1067–1082.e12. doi: 10.1016/j.immuni.2017.11.008
118. Xu L, Huang Q, Wang H, Hao Y, Bai Q, Hu J, et al. The Kinase Mtorc1 Promotes the Generation and Suppressive Function of Follicular Regulatory T Cells. Immunity (2017) 47:538–551 e5. doi: 10.1016/j.immuni.2017.08.011
119. Zhou KL, Zhu ZH, Zhou JP, Zhao JJ, Zhang Y, Jiang B. Increased Hexokinase-2 as a Novel Biomarker for the Diagnosis and Correlating With Disease Severity in Rheumatoid Arthritis. Med (Baltimore) (2021) 100:e26504. doi: 10.1097/MD.0000000000026504
120. Huang ZY, Shao MM, Zhang JC, Yi FS, Du J, Zhou Q, et al. Single-Cell Analysis of Diverse Immune Phenotypes in Malignant Pleural Effusion. Nat Commun (2021) 12:6690. doi: 10.1038/s41467-021-27026-9
121. Tsui C, Martinez-Martin N, Gaya M, Maldonado P, Llorian M, Legrave NM, et al. Protein Kinase C-Beta Dictates B Cell Fate by Regulating Mitochondrial Remodeling, Metabolic Reprogramming, and Heme Biosynthesis. Immunity (2018) 48:1144–1159.e5. doi: 10.1016/j.immuni.2018.04.031
122. Fu G, Guy CS, Chapman NM, Palacios G, Wei J, Zhou P, et al. Metabolic Control of TFH Cells and Humoral Immunity by Phosphatidylethanolamine. Nature (2021) 595:724–9. doi: 10.1038/s41586-021-03692-z
123. Simpson N, Gatenby PA, Wilson A, Malik S, Fulcher DA, Tangye SG, et al. Expansion of Circulating T Cells Resembling Follicular Helper T Cells Is a Fixed Phenotype That Identifies a Subset of Severe Systemic Lupus Erythematosus. Arthritis Rheum (2010) 62:234–44. doi: 10.1002/art.25032
124. Choi JY, Ho JH, Pasoto SG, Bunin V, Kim ST, Carrasco S, et al. Circulating Follicular Helper-Like T Cells in Systemic Lupus Erythematosus: Association With Disease Activity. Arthritis Rheumatol (2015) 67:988–99. doi: 10.1002/art.39020
125. Yin Y, Choi SC, Xu Z, Perry DJ, Seay H, Croker BP, et al. Normalization of CD4+ T Cell Metabolism Reverses Lupus. Sci Transl Med (2015) 7:274ra18. doi: 10.1126/scitranslmed.aaa0835
126. Choi SC, Titov AA, Abboud G, Seay HR, Brusko TM, Roopenian DC, et al. Inhibition of Glucose Metabolism Selectively Targets Autoreactive Follicular Helper T Cells. Nat Commun (2018) 9:4369. doi: 10.1038/s41467-018-066860
127. Aust G, Sittig D, Becherer L, Anderegg U, Schutz A, Lamesch P, et al. The Role of CXCR5 and Its Ligand CXCL13 in the Compartmentalization of Lymphocytes in Thyroids Affected by Autoimmune Thyroid Diseases. Eur J Endocrinol (2004) 150:225–34. doi: 10.1530/eje.0.1500225
128. Liu R, Wu Q, Su D, Che N, Chen H, Geng L, et al. A Regulatory Effect of IL-21 on T Follicular Helper-Like Cell and B Cell in Rheumatoid Arthritis. Arthritis Res Ther (2012) 14:R255. doi: 10.1186/ar3707
129. Moschovakis GL, Bubke A, Friedrichsen M, Falk CS, Feederle R, Forster R. T Cell Specific Cxcr5 Deficiency Prevents Rheumatoid Arthritis. Sci Rep (2017) 7:8933. doi: 10.1038/s41598-017-08935-6
130. Panneton V, Bagherzadeh Yazdchi S, Witalis M, Chang J, Suh WK. ICOS Signaling Controls Induction and Maintenance of Collagen-Induced Arthritis. J Immunol (2018) 200:3067–76. doi: 10.4049/jimmunol.1701305
131. Zhang N, Tai J, Qu Z, Zhang Z, Zhao S, He J, et al. Increased Cd4(+)Cxcr5(+)T Follicular Helper Cells in Diabetic Nephropathy. Autoimmunity (2016) 49:405–13. doi: 10.1080/08916934.2016.1196677
132. Niu Q, Huang ZC, Wu XJ, Jin YX, An YF, Li YM, et al. Enhanced IL-6/Phosphorylated STAT3 Signaling Is Related to the Imbalance of Circulating T Follicular Helper/T Follicular Regulatory Cells in Patients With Rheumatoid Arthritis. Arthritis Res Ther (2018) 20:200. doi: 10.1186/s13075-018-1690-0
133. Kenefeck R, Wang CJ, Kapadi T, Wardzinski L, Attridge K, Clough LE, et al. Follicular Helper T Cell Signature in Type 1 Diabetes. J Clin Invest (2015) 125:292–303. doi: 10.1172/JCI76238
134. Viisanen T, Ihantola EL, Nanto-Salonen K, Hyoty H, Nurminen N, Selvenius J, et al. Circulating CXCR5+PD-1+ICOS+ Follicular T Helper Cells Are Increased Close to the Diagnosis of Type 1 Diabetes in Children With Multiple Autoantibodies. Diabetes (2017) 66:437–47. doi: 10.2337/db16-0714
135. Hadaschik EN, Wei X, Leiss H, Heckmann B, Niederreiter B, Steiner G, et al. Regulatory T Cell-Deficient Scurfy Mice Develop Systemic Autoimmune Features Resembling Lupus-Like Disease. Arthritis Res Ther (2015) 17:35. doi: 10.1186/s13075-015-0538-0
136. Chen Y, Yu M, Zheng Y, Fu G, Xin G, Zhu W, et al. CXCR5(+)PD-1(+) Follicular Helper CD8 T Cells Control B Cell Tolerance. Nat Commun (2019) 10:4415. doi: 10.1038/s41467-019-12446-5
137. Gu-Trantien C, Migliori E, Buisseret L, de Wind A, Brohee S, Garaud S, et al. CXCL13-Producing TFH Cells Link Immune Suppression and Adaptive Memory in Human Breast Cancer. JCI Insight (2017) 2. doi: 10.1172/jci.insight.91487
138. Gu-Trantien C, Loi S, Garaud S, Equeter C, Libin M, de Wind A, et al. CD4(+) Follicular Helper T Cell Infiltration Predicts Breast Cancer Survival. J Clin Invest (2013) 123:2873–92. doi: 10.1172/JCI67428
139. Bindea G, Mlecnik B, Tosolini M, Kirilovsky A, Waldner M, Obenauf AC, et al. Spatiotemporal Dynamics of Intratumoral Immune Cells Reveal the Immune Landscape in Human Cancer. Immunity (2013) 39:782–95. doi: 10.1016/j.immuni.2013.10.003
140. Lewis KE, Selby MJ, Masters G, Valle J, Dito G, Curtis WR, et al. Interleukin-21 Combined With PD-1 or CTLA-4 Blockade Enhances Antitumor Immunity in Mouse Tumor Models. Oncoimmunology (2017) 7:e1377873 doi: 10.1080/2162402X.2017.1377873
141. Guzy RD, Hoyos B, Robin E, Chen H, Liu L, Mansfield KD, et al. Mitochondrial Complex III Is Required for Hypoxia-Induced ROS Production and Cellular Oxygen Sensing. Cell Metab (2005) 1:401–8. doi: 10.1016/j.cmet.2005.05.001
143. Fahey LM, Wilson EB, Elsaesser H, Fistonich CD, McGavern DB, Brooks DG. Viral Persistence Redirects CD4 T Cell Differentiation Toward T Follicular Helper Cells. J Exp Med (2011) 208:987–99. doi: 10.1084/jem.20101773
144. Harker JA, Lewis GM, Mack L, Zuniga EI. Late Interleukin-6 Escalates T Follicular Helper Cell Responses and Controls a Chronic Viral Infection. Science (2011) 334:825–9. doi: 10.1126/science.1208421
145. Kunzli M, Schreiner D, Pereboom TC, Swarnalekha N, Litzler LC, Lotscher J, et al. Long-Lived T Follicular Helper Cells Retain Plasticity and Help Sustain Humoral Immunity. Sci Immunol (2020) 5. doi: 10.1126/sciimmunol.aay5552
146. Duan YQ, Xia MH, Ren L, Zhang YF, Ao QL, Xu SP, et al. Deficiency of Tfh Cells and Germinal Center in Deceased COVID-19 Patients. Curr Med Sci (2020) 40:618–24. doi: 10.1007/s11596-020-2225-x
147. Neidleman J, Luo X, Frouard J, Xie G, Gill G, Stein ES, et al. SARS-CoV-2-Specific T Cells Exhibit Phenotypic Features of Helper Function, Lack of Terminal Differentiation, and High Proliferation Potential. Cell Rep Med (2020) 1:100081. doi: 10.1016/j.xcrm.2020.100081
148. Thevarajan I, Nguyen THO, Koutsakos M, Druce J, Caly L, van de Sandt CE, et al. Breadth of Concomitant Immune Responses Prior to Patient Recovery: A Case Report of Non-Severe COVID-19. Nat Med (2020) 26:453–5. doi: 10.1038/s41591-020-0819-2
149. Hanson A, Cohen H, Wang H, Shekhar N, Shah C, Dhaneshwar A, et al. Impaired ICOS Signaling Between Tfh and B Cells Distinguishes Hospitalized From Ambulatory CoViD-19 Patients MedRxiv (2020). doi: 10.1101/2020.12.16.20248343
150. Kaneko N, Kuo HH, Boucau J, Farmer JR, Allard-Chamard H, Mahajan VS, et al. Loss of Bcl-6-Expressing T Follicular Helper Cells and Germinal Centers in COVID-19. Cell (2020) 183:143–57.e13. doi: 10.1016/j.cell.2020.08.025
151. Kalfaoglu B, Almeida-Santos J, Tye CA, Satou Y, Ono M. T-Cell Hyperactivation and Paralysis in Severe COVID-19 Infection Revealed by Single-Cell Analysis. Front Immunol (2020) 11:589380. doi: 10.3389/fimmu.2020.589380
152. Thompson EA, Cascino K, Ordonez AA, Zhou W, Vaghasia A, Hamacher-Brady A, et al. Metabolic Programs Define Dysfunctional Immune Responses in Severe COVID-19 Patients. Cell Rep (2021) 34:108863. doi: 10.1016/j.celrep.2021.108863
153. Cubas RA, Mudd JC, Savoye AL, Perreau M, van Grevenynghe J, Metcalf T, et al. Inadequate T Follicular Cell Help Impairs B Cell Immunity During HIV Infection. Nat Med (2013) 19:494–9. doi: 10.1038/nm.3109
154. Kohler SL, Pham MN, Folkvord JM, Arends T, Miller SM, Miles B, et al. Germinal Center T Follicular Helper Cells Are Highly Permissive to HIV-1 and Alter Their Phenotype During Virus Replication. J Immunol (2016) 196:2711–22. doi: 10.4049/jimmunol.1502174
155. Perreau M, Savoye AL, De Crignis E, Corpataux JM, Cubas R, Haddad EK, et al. Follicular Helper T Cells Serve as the Major CD4 T Cell Compartment for HIV-1 Infection, Replication, and Production. J Exp Med (2013) 210:143–56. doi: 10.1084/jem.20121932
156. Pallikkuth S, Sharkey M, Babic DZ, Gupta S, Stone GW, Fischl MA, et al. Peripheral T Follicular Helper Cells Are the Major HIV Reservoir Within Central Memory CD4 T Cells in Peripheral Blood From Chronically HIV-Infected Individuals on Combination Antiretroviral Therapy. J Virol (2015) 90:2718–28. doi: 10.1128/JVI.02883-15
157. Petrovas C, Yamamoto T, Gerner MY, Boswell KL, Wloka K, Smith EC, et al. CD4 T Follicular Helper Cell Dynamics During SIV Infection. J Clin Invest (2012) 122:3281–94. doi: 10.1172/JCI63039
158. Lindqvist M, van Lunzen J, Soghoian DZ, Kuhl BD, Ranasinghe S, Kranias G, et al. Expansion of HIV-Specific T Follicular Helper Cells in Chronic HIV Infection. J Clin Invest (2012) 122:3271–80. doi: 10.1172/JCI64314
159. Palmer CS, Ostrowski M, Gouillou M, Tsai L, Yu D, Zhou J, et al. Increased Glucose Metabolic Activity Is Associated With CD4+ T-Cell Activation and Depletion During Chronic HIV Infection. AIDS (2014) 28:297–309. doi: 10.1097/QAD.0000000000000128
160. Hollenbaugh JA, Munger J, Kim B. Metabolite Profiles of Human Immunodeficiency Virus Infected CD4+ T Cells and Macrophages Using LC-MS/MS Analysis. Virology (2011) 415:153–9. doi: 10.1016/j.virol.2011.04.007
161. Hegedus A, Kavanagh Williamson M, Huthoff H. HIV-1 Pathogenicity and Virion Production are Dependent on the Metabolic Phenotype of Activated CD4+ T Cells. Retrovirology (2014) 11:98. doi: 10.1186/s12977-014-0098-4
162. Rodriguez-Mora S, Mateos E, Moran M, Martin MA, Lopez JA, Calvo E, et al. Intracellular Expression of Tat Alters Mitochondrial Functions in T Cells: A Potential Mechanism to Understand Mitochondrial Damage During HIV-1 Replication. Retrovirology (2015) 12:78. doi: 10.1186/s12977-015-0203-3
163. Perrin S, Cremer J, Roll P, Faucher O, Menard A, Reynes J, et al. HIV-1 Infection and First Line ART Induced Differential Responses in Mitochondria From Blood Lymphocytes and Monocytes: The ANRS EP45 "Aging" Study. PloS One (2012) 7:e41129. doi: 10.1371/journal.pone.0041129
164. Wang Y, Lin C, Cao Y, Duan Z, Guan Z, Xu J, et al. Up-Regulation of Interleukin-21 Contributes to Liver Pathology of Schistosomiasis by Driving GC Immune Responses and Activating HSCs in Mice. Sci Rep (2017) 7:16682. doi: 10.1038/s41598-017-16783-7
165. Zhang Y, Jiang Y, Wang Y, Liu H, Shen Y, Yuan Z, et al. Higher Frequency of Circulating PD-1(High) CXCR5(+)CD4(+) Tfh Cells in Patients With Chronic Schistosomiasis. Int J Biol Sci (2015) 11:1049–55. doi: 10.7150/ijbs.12023
166. Fairfax K, Nascimento M, Huang SC, Everts B, Pearce EJ. Th2 Responses in Schistosomiasis. Semin Immunopathol (2012) 34:863–71. doi: 10.1007/s00281-012-0354-4
167. Illingworth J, Butler NS, Roetynck S, Mwacharo J, Pierce SK, Bejon P, et al. Chronic Exposure to Plasmodium Falciparum Is Associated With Phenotypic Evidence of B and T Cell Exhaustion. J Immunol (2013) 190:1038–47. doi: 10.4049/jimmunol.1202438
168. Villegas-Mendez A, Khandelwal G, McGowan LM, Dookie RS, Haley MJ, George C, et al. Exhausted CD4(+) T Cells During Malaria Exhibit Reduced Mtorc1 Activity Correlated With Loss of T-Bet Expression. J Immunol (2020) 205:1608–19. doi: 10.4049/jimmunol.2000450
169. Webb GM, Li S, Mwakalundwa G, Folkvord JM, Greene JM, Reed JS, et al. The Human IL-15 Superagonist ALT-803 Directs SIV-Specific CD8(+) T Cells Into B-Cell Follicles. Blood Adv (2018) 2:76–84. doi: 10.1182/bloodadvances.2017012971
Keywords: T follicular helper cells, Tfh differentiation, cellular metabolism, germinal center, infection
Citation: Mayberry CL, Logan NA, Wilson JJ and Chang C-H (2022) Providing a Helping Hand: Metabolic Regulation of T Follicular Helper Cells and Their Association With Disease. Front. Immunol. 13:864949. doi: 10.3389/fimmu.2022.864949
Received: 29 January 2022; Accepted: 25 March 2022;
Published: 14 April 2022.
Edited by:
Qi-Jing Li, Duke University, United StatesCopyright © 2022 Mayberry, Logan, Wilson and Chang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chih-Hao Chang, THVjYXMuY2hhbmdAamF4Lm9yZw==
 Colleen L. Mayberry
Colleen L. Mayberry Natalie A. Logan1
Natalie A. Logan1 John J. Wilson
John J. Wilson Chih-Hao Chang
Chih-Hao Chang