
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Immunol., 10 May 2022
Sec. Viral Immunology
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.864838
This article is part of the Research TopicImmune Responses to HIV Infection: Basic, Clinical and Translational Research in East and Southeast AsiaView all 43 articles
Introduction: During the COVID-19 pandemic, people living with HIV (PLWH) were considered to be at risk of worse COVID-19 outcomes once infected. However, the existing evidence is inconsistent. This systematic review and meta-analysis aimed to compare the risk of SARS-CoV-2 infection, severe COVID-19 symptoms, and mortality among PLWH and patients without HIV.
Method: The articles included studies published in PubMed, Medline, Embase, and Cochrane between December 1, 2019, and December 1, 2021. We included the original studies published in English focusing on observational studies assessing the risk of SARS-CoV-2 infection, severe COVID-19 symptoms, and mortality among PLWH. Four independent reviewers extracted data. STrengthening the Reporting of OBservational studies in Epidemiology-Modified (STROBE-M) checklist was used for quality assessment. For the results with heterogeneity I2 >75%, a random-effects model was employed. Otherwise, a fixed-effects model was used. The risk of SARS-CoV-2 infection, severe COVID-19 symptoms, and mortality were compared with and without HIV.
Results: We included a total of 32 studies and 71,779,737 study samples, of whom 797,564 (1.11%) were PLWH. Compared with COVID-19 patients without HIV infection, PLWH had comparable risk of SARS-CoV-2 infection (adjusted Risk Ratio=1.07, 95% CI: 0.53-2.16, I2 = 96%, study n=6, n=20,199,805) and risk of developing severe COVID-19 symptoms (aRR=1.06, 95% CI: 0.97-1.16, I2 = 75%, n=10, n=2,243,370). PLWH, if infected with SARS-CoV-2, were found to have an increased risk of mortality compared with people without HIV (aRR=1.30, 95% CI: 1.09-1.56, I2 = 76%, study n=16, n=71,032,659). This finding was consistent across different subgroup analyses.
Conclusion: PLWH are at increased risk of COVID-19 related mortality once infected. The local health system should, on the one hand, strengthen COVID-19 prevention and clinical management among PLWH to avoid infection and, on the other hand, sustain the HIV care continuum for PLWH for HIV management.
Every minute, around 190 new Coronavirus Disease 2019 (COVID-19) cases are reported, and three individuals die from COVID-19 throughout the globe. The outbreak of COVID-19, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has resulted in one of the most devastating pandemics in human history (1). By January 20, 2021, it has caused over 3 billion illnesses and approximately 5.5 million deaths (2). As a result of the availability of the COVID-19 vaccine and a better understanding of the COVID-19 treatment, the overall COVID-19 mortality is decreasing. However, the immunocompromised populations are still at an increased risk of mortality. Due to abnormal humoral and cellular immunity, people living with HIV (PLWH) were considered to be at an increased risk of severe Covid-19 outcomes once infected (3, 4). On the other hand, PLWH of long-term use of ART also has a higher chance of suffering from different chronic conditions such as hypertension than HIV negative population, which as a result, worsen their outcome once infected by SARS-CoV-2 (4).
Considering that there are approximately 38 million PLWH globally and the potential increased risk of severe COVID-19 and mortality for this population, it is warranted to systematically assess the current vulnerability of PLWH SARS-CoV-2 infection and related outcomes (5). However, the recent epidemiological evidence on PLWH infected with SARS-CoV-2 has been inconsistent (6). Although there have been meta-analyses assessing the impact of HIV infection on COVID-19 outcomes among PLWH, the time frame of evidence inclusion was early, before January 2021, which did not cover cases with new variants (e.g., delta variant) (7, 8). After that, several variants have emerged and changed the global COVID-19 pandemic significantly. Meanwhile, more studies with larger sample sizes, more sophisticated study designs, and broader geographic coverage. It is high time we looked back for two years to summarize and elucidate how COVID-19 infection differs between PLWH and people without HIV infection. By filling up this knowledge gap, it will better characterize PLWH’s vulnerability during this COVID-19 pandemic and thus advocate for more attention and care for this population to achieve better health equity.
This systematic review and meta-analysis aimed to compare the risk of SARS-CoV-2 infection, severe COVID-19 symptoms, and death of PLWH with those without HIV.
This systematic review and meta-analysis were conducted and reported per Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (9). We searched PubMed, Medline, Embase, and Cochrane for publications from December 1st, 2019 to December 1st, 2021 using the combination of HIV/AIDS-related terms (“HIV”, “human immunodeficiency virus*”, “AIDS”, “acquired immunodeficiency syndrome”) and COVID-19 related terms (“COVID-19”, “SARS-CoV*”, “2019-nCoV”, “nCoV” and “novel coronavirus”). We also included terms related to observational studies such as “cohort”, “cross-sectional”, and “case-control” (See Supplementary Table 1 for detailed search terms).
For eligible studies, we included original observational studies published in English. The included studies should consist of both HIV positive and HIV negative COVID-19 cases in the study population. The outcomes of interests should at least contain one of the following three outcomes 1) the risk of SARS-CoV-2 infection, 2) the risk of developing severe COVID-19 symptoms, and 3) the risk of COVID-19 related mortality. We hereby defined severe COVID-19 symptoms according to the WHO COVID-19 clinical management guidelines (10). This study’s main objective is to pool and compare the outcomes between PLWH and people without HIV infection. On the other hand, due to age, sex, and comorbidities’ impact on the COVID-19 treatment outcomes, we required the included studies to have results adjusted for potential confounders. Studies without confounder adjustment were not included. Original studies focusing on molecular, genetics, and sociological perspectives without the above outcomes of interest were excluded. Studies without primary data, such as comments and reviews, were excluded. Case reports and case series were also excluded.
Two groups of reviewers (YW, YX, WT and SH, WA, YT) independently screened the literature titles, abstracts, and full-texts and identified eligible literature for data extraction. The two reviewers discussed any disagreement. A third reviewer (WT) was consulted for reconciliation if an agreement was not reached.
The following variables were extracted from the final included studies: the first author, published year, country, study design, study settings, study periods, sample size, diagnosis methods, patient-basic information (e.g., mean age and sex), HIV-related information [e.g., latest CD4 count, latest HIV viral load and whether current receiving antiviral therapy (ART)], comorbidities, and COVID-19 related outcomes (infection, severe COVID-19 symptoms, and morality). If reported, we extracted both the crude and adjusted estimation from the included studies. The adjusted results with the maximum number of adjustments were selected (11).
To synthesize the impact of the HIV/SARS-CoV-2 co-infection on the three outcomes, we conducted pooled analyses based on the gathered effect estimates and standard errors. Pooling was accomplished using the inverse variance method. Considering the variation in the types of effect estimation, including odds ratio (OR), risk ratio (RR), and hazard ratio (HR), and for ease of interpretation, we converted these estimations into a standard metric, risk ratio (12, 13). The pool results were expressed as RR, with 95% confidence intervals, I2, the heterogeneity metric, number of studies pooled, and number of sample sizes included. The heterogeneity between the studies in the comparison was assessed by calculating I2. A fix-effects model would be employed for the results with I2 ≤75%. A random-effects model would be employed for the results with I2 >75% (14). Statistical significance was based on a p-value of less than 0.05.
Considering the diversities in the healthcare system and variations in the study setting and their potential impact on the pooling, we conducted subgroup analyses based on World Bank income classification (high-income (HIC) vs. low-middle-income countries (LMIC)), studying inpatients cases only or both inpatient and outpatient cases, single-center or multi-center study. If an investigation could not be classified into either subgroup, it was excluded from the subgroup analysis. Adjusted RRs were used in the subgroup analysis.
Two reviewers (SH and WA) independently assessed the quality of the included studies. The STrengthening, the Reporting of OBservational studies in Epidemiology-Modified (STROBE-M) checklist, was used for the quality assessment (15, 16). We calculated the adherence score based on the checklist results. The studies scored ≥ 85 would be ranked as “Excellent” in quality. The studies scored between 70 to 85 would be ranked as “Good”. The studies scored between 50 to 70 would be ranked as “Fair”. Those studies scored below 50 would be ranked as “Poor” (16). We generated funnel plots that utilized Eggers’ test to examine small sample effects as a possible indicator of publication bias (p<0.05) in our meta-analysis models to assess the potential publication bias. Articles were downloaded and managed by Endnote X9. Data were extracted and entered into Microsoft Excel (2016). The meta-analyses were performed by the R v4.02 and R Studio (2020), using the R package meta. Quality assessment was performed by using the STROBE-M checklist.
A total of 2,225 articles were identified from the databases. Overall, 122 of them were detected to be duplicates by Endnote, leaving 2103 articles for the initial title & abstract screening. Based on the abstract and title screening, 1,914 articles were excluded. Among the 189 articles screened for full text, an additional 157 articles were excluded, leaving the rest 32 articles included for the analyses (Figure 1) (17–48).
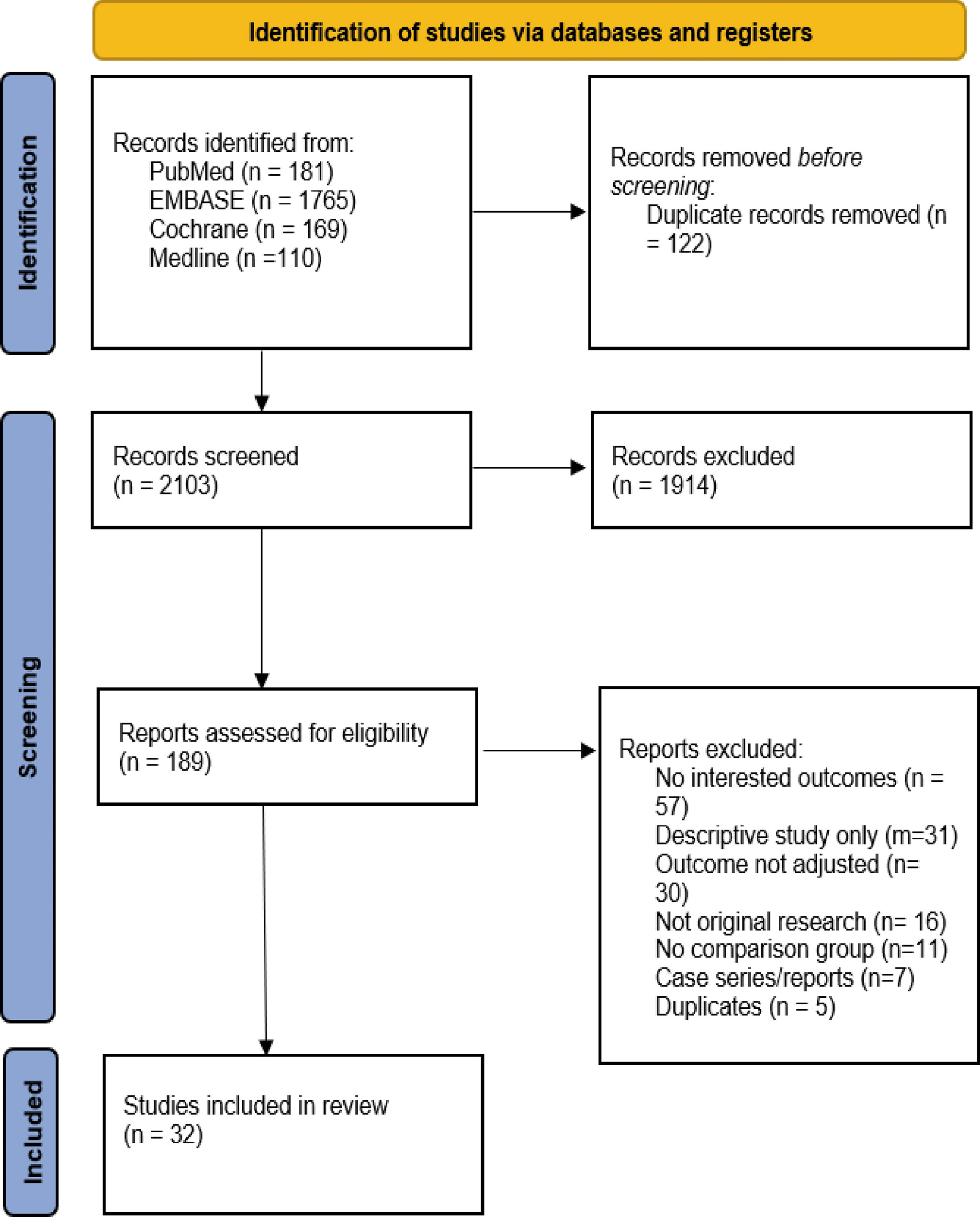
Figure 1 Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) chart for the literature search.
The analysis included a total of 71,779,737 sample size, of whom 797,564 (1.11%) were PLWH. These studies were conducted in 10 countries and represented North America (n=14), Africa (n=7), Europe (n=10), and Asia (n=1). 17 (53.12%) of the studies were conducted in hospital settings, and 15 of them were conducted in other settings such as registries, surveillance system databases, national cohorts. 25 (78.12%) of the included studies were multi-center studies, and 13 (40.62%) of these studies specifically focused on hospitalized COVID-19 cases. The study information was summarized in Supplementary Table 2.
Supplementary Table 3 summarized patient-level sociodemographic variables as well as clinical information of the patients. 58% of the study population were male. The mean age of PLWH was 52.12 (Standard deviation (SD): 6.82), while the mean age of patients without HIV was 55.34 (SD: 5.95). Among PLWH, on average, 91% of them were on ART (range: 70% - 100%). In terms of HIV viral suppression rate, on average, 77% of the studied population had achieved HIV viral suppression (range: 31%-97%). About 87% (92,714/106,568) of the PLWH had at least one comorbidity besides SARS-CoV-2 infection, which was higher than HIV- patients [79% (37,838/47,746)]. Hypertension, diabetes, and obesity were the top three most reported comorbidities among PLWH.
Figures 2, 3 showed the forest plots for the three meta-analyses related to the crude and adjusted risk of SARS-CoV-2 infection, severe COVID-19 symptoms, and COVID-19 mortality.
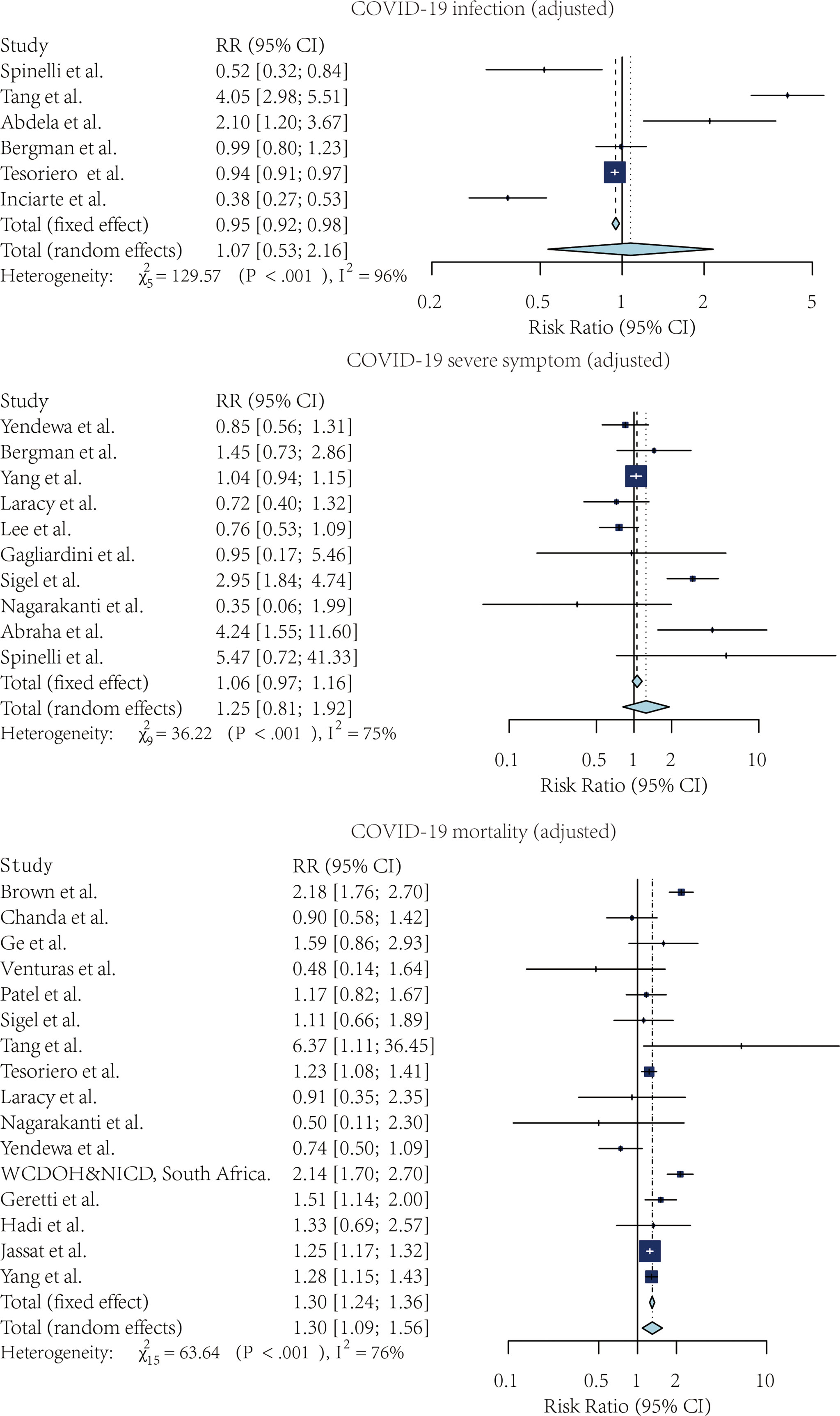
Figure 2 The forest plot of the crude risk ratio of SARS-CoV-2 infection, Severe COVID-19 symptoms, and COVID-19 mortality, comparing the patients with and without HIV.
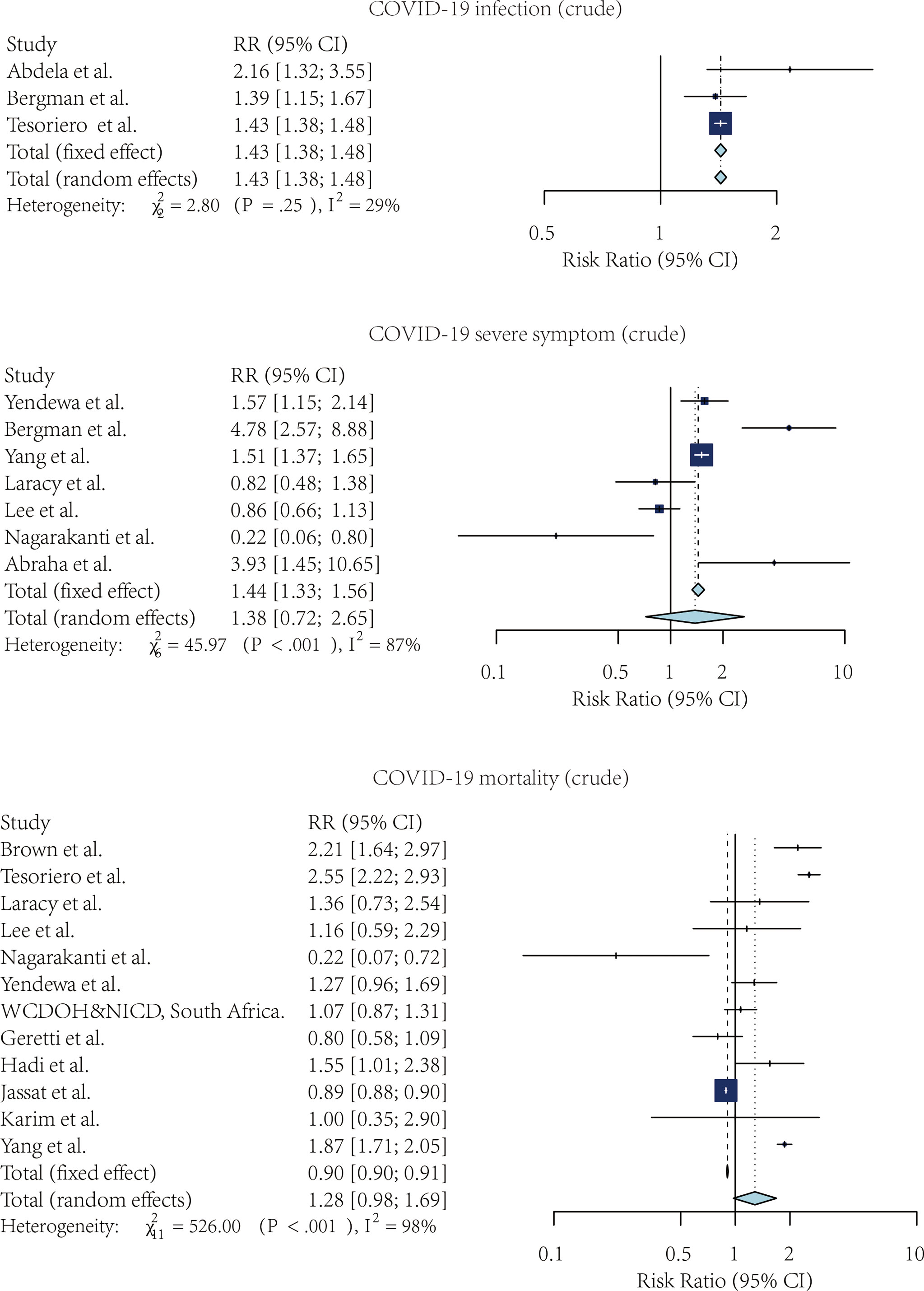
Figure 3 The forest plot of the adjusted risk ratio of SARS-CoV-2 infection, Severe COVID-19 symptoms, and COVID-19 mortality, comparing the patients with and without HIV.
Four studies compared the risk of SARS-CoV-2 infection without adjustment. According to the pooled crude risk ratio, there was an increased risk of SARS-CoV-2 infection among PLWH compared with people without HIV infection (RR=1.43, 95% CI: 1.38-1.48, I2 = 29%, study n=3, n=19,956,496). Eight studies provided an adjusted risk of SARS-CoV-2 infection estimation. After the adjustment, the susceptibility to SARS-CoV-2 infection was comparable between the two groups (aRR=1.07, 95% CI: 0.53-2.16, I2 = 96%, study n=6, n=20,199,805).
Nine studies compared the risk of developing severe COVID-19 symptoms between HIV-positive and negative groups. PLWH had a higher risk of developing severe SARS-CoV-2 symptoms once infected. This effect was marginally significant for both crude estimation (RR=1.38, 95% CI: 0.72-2.65, I2 = 87%, study n=7, n=2,239,955) and adjusted estimation (aRR=1.06, 95% CI: 0.97-1.16, I2 = 75%, n=10, n=2,243,370).
PLWH infected with COVID-19 also had a higher mortality risk than people without HIV infection. Overall, 13 studies provided crude estimations. The pooled crude risk ratio of mortality was 1.28 (RR=1.28, 95% CI: 0.98-1.69, I2 = 98%, study n=12, n=87,907,007), which was marginally significant. Nineteen studies had an adjusted mortality risk ratio. After adjustment, PLWH had a statistically significant higher risk of mortality than those not infected with HIV (aRR=1.30, 95% CI: 1.09-1.56, I2 = 76%, study n=16, n=71,032,659).
We compared the pooled crude estimates with the pooled adjusted estimates. The estimation was reduced as the estimates were adjusted for the potential confounders, including age, sex, and comorbidities, suggesting the importance of controlling for confounding minimizes the strength of association.
We hereby presented mortality, one of the most critical COVID-19 related outcomes, using adjusted RR (supplement 2: forest plots of subgroup analysis).
In high-income countries (HIC), the adjusted risk ratio was 1.33 (aRR=1.33, 95% CI:1.24-1.42, I2 = 71%, study n=12). In low- and middle-income countries (LMIC), the adjusted risk ratio was 1.22 (aRR=1.22, 95% CI: 0.74-2.00, I2 = 88%, study n=4).
Regarding the study population, we divided the studies into 1) only included inpatient cases and 2) included both inpatient and outpatient cases. For the studies with only hospitalized patients, the pool adjusted risk ratio of mortality was 1.25 (aRR=1.25, 95% CI:1.18-1.32, I2 = 14%, study n=6). For the studies with inpatient and outpatient cases, the pool adjusted risk ratio of mortality was 1.38 (aRR=1.38, 95% CI:1.06-1.79, I2 = 83%, study n=10). The findings were more consistent among inpatient studies.
We divided the studies into multi-center studies versus single-center studies regarding the study settings. We found a 44% increase in RR of mortality among PLWH compared with patients not infected with HIV (aRR=1.34, 95% CI:1.12-1.60, I2 = 78%, study n=14). On the other hand, the difference in mortality risk was not significant among single-center studies (aRR=0.49, 95% CI:0.19-1.27, I2 = 0%, study n=2).
The mean STROBE-M adherence score (%) was 69.1% (SD: 11.2, range: 40.5-88.1). Overall, the included studies’ quality is “Fair” (Supplementary Table 4). Two studies were of excellent quality, 15 were of good quality,13 were of fair quality, and two were of poor quality. Visual inspection of the funnel plot did not show obvious asymmetry. Egger’s test for asymmetry was insignificant (p=0.80). We could not conclude there was a significant publication bias among the included studies. The funnel plot was shown in (supplement 3: funnel plot).
Given COVID-19 is very likely to become a long-lasting infectious disease (49). Thus, understanding its interaction with other infectious diseases, such as HIV, will provide us with crucial messages for planning future measures. This was a two-year summary of the evidence on HIV and SARS-CoV-2 infection interactions. Compared with our last systematic review in early 2021 (8), there were more multi-center studies, more analyses with statistical adjustments, and more studies from different countries, both from HICs and LMICs.
We identified an increased risk of COVID-19 related mortality among PLWH compared with patients without HIV infection. Considering the role of age and comorbidities in impacting the COVID-19 infection outcome, after the adjustment, there was still an increased risk of mortality among PLWH, indicating HIV infection was possibly an independent risk factor of death caused by COVID-19. The subgroup analyses further confirmed the findings across LMICs and HICs, inpatient-only populations, and inpatient and outpatient mixed populations. This finding indicates the importance of COVID-19 prevention among PLWH and more careful management when PLWH presented with COVID-19.
We did not identify an increased risk of SARS-CoV-2 infection among PLWH. This was possibly due to public health prevention measures such as quarantine and shutdowns. Also, it was possible that PLWH, knowing their HIV infection, had a stronger awareness of COVID-19 prevention and avoided congregation. At the same time, the global-wide row out of the COVID-19 vaccine campaign also plays an indispensable role in stopping the spread.
Regarding the SARS-CoV-2 infection severity among PLWH, we did not identify a statistically significant increased risk of developing severe symptoms for PLWH. A few reasons may have led to this phenomenon. From an immunology perspective, since CD4 T cells play a pivotal role in innate and cellular immunity, the dysregulation of CD4 T cell activities and functions in PLWH could increase the risk of having worse outcomes once infected with infected SARS-CoV-2 compared with those not infected with HIV. One explanation could be due to the high viral suppression rate and ART uptake rate, which were important influencers of PLWH’s vulnerability to other infections. Since the majority of the PLWH had a controlled HIV status, this study was unable to assess the COVID-19 outcomes among patients with uncontrolled HIV due to limited study numbers and sample size. However, there has been evidence that patients with uncontrolled HIV would be more likely to develop severe Covid-19 symptoms, which aligned with the immunology mechanism (50, 51). On the other hand, the insignificant results could be due to the high heterogeneity of the included analyses, which led to a wider confidence interval estimation. Considering these facts, future studies and meta-analyses on how the outcome of SARS-CoV-2 infection are moderated by ART status, CD4 levels, COVID-19 vaccination status, and HIV viral load are warranted.
To our knowledge, this is the most recent review that covers the longest period since the detection of COVID-19 in December 2019. This review collected the relevant studies around the globe for a more comprehensive assessment of the SARS-CoV-2/HIV co-infection. In recognition of the impact of different potential confounders, we pooled the impact of crude interesting outcomes and the outcomes adjusted for potential confounders. We also conducted a series of subgroup analyses of mortality to assess the robustness of our findings.
Our studies have several limitations. Our studies could not assure if all SARS-CoV-2 infection outcomes were clinically confirmed since most of the studies confirmed the case based on RT-PCR only. There were possibilities of misclassification. Second, we were not able to analyze the outcomes of patients with poor HIV control compared with those without HIV infection due to the insufficiency of the evidence. However, a study has shown that patients with uncontrolled HIV are at risk of developing severe COVID-19 (50, 52). Third, the identified studies did not distinguish different SARS-CoV-2 variants with various contingencies and mortality rates (53).
Our study has several implications. From the policy perspective, the policymakers and healthcare system should reinforce the COVID-19 prevention among PLWH and maintain the HIV care continuum during the COVID-19 pandemic to ensure PLWH are at good HIV control (54–56). Also, it is important to advocate for the rolling out of the COVID-19 vaccine among PLWH to reduce the risk of developing severe COVID-19 and death among PLWH once infected (17), especially in countries with a high HIV burden. In terms of research perspective, first, future studies should focus more on evaluating the role of HIV treatment status, especially poor HIV control, its impact on SARS-CoV-2 infection outcomes. Second, considering the ever-mutating nature of SARS-CoV-2, it is necessary to assess different variants’ interactions with HIV. Third, as the availability of COVID-19 vaccine and therapies, how these preventive and treatment methods are going to impact the outcome of SARS-CoV-2/HIV co-infection needs to be further illustrated. Fourth, the studies must focus on the pathological and immunological perspective of the interaction between HIV and COVID-19.
In conclusion, HIV infection remains an important risk factor for COVID-19 mortality. Therefore, it is highly recommended that PLWH should be protected from the COVID-19 pandemic through public health prevention and vaccines. On the other hand, overcoming current health system barriers (e.g., quarantine, social distancing, and community containment measurements) and securing continued ART supply as well as other HIV services is important for PLWH survival through the SARS-CoV-2 and HIV pandemics.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
WT and YW conceived the study idea; YW, YX, SH, WA, and YT conducted the searching, screening, and data extraction; YW, YX, FJ, and HT helped with the data analysis; WT, YW, and YX drafted the manuscript. All authors approved the manuscript for publication.
This work was supported by the National Natural Science Foundation of China (81903371) and NIH (R34MH119963).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor declared a shared affiliation with the authors YX, FJ, WT at the time of review.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.864838/full#supplementary-material
1. European Centre for Disease Prevention and Control. Timeline of ECDC's Response to COVID-19. Available at: https://www.ecdc.europa.eu/en/covid-19/timeline-ecdc-response (Accessed January 27, 2022).
2. WHO. Coronavirus Disease (COVID-19) Weekly Epidemiological Update and Weekly Operational Update. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports (Accessed January 27, 2022).
3. Zhang H, Wu T. CD4+T, Cd8+T Counts and Severe COVID-19: A Meta-Analysis. J Infect Dis (2020) 81(3):e82–4. doi: 10.1016/j.jinf.2020.06.036
4. Ho HE, Peluso MJ, Margus C, Matias Lopes JP, He C, Gaisa MM, et al. Clinical Outcomes and Immunologic Characteristics of Coronavirus Disease 2019 in People With Human Immunodeficiency Virus. J Infect Dis (2021) 223(3):403–8. doi: 10.1093/infdis/jiaa380
5. UNAIDS. Global HIV & AIDS Statistics — 2020 Fact Sheet. Available at: https://www.unaids.org/en/resources/fact-sheet (Accessed January 27, 2022).
6. Vishnevetsky A, Levy M. Rethinking High-Risk Groups in COVID-19. Mult Scler Relat Disord (2020) 42:102139. doi: 10.1016/j.msard.2020.102139
7. Ssentongo P, Heilbrunn ES, Ssentongo AE, Advani S, Chinchilli VM, Nunez JJ, et al. Epidemiology and Outcomes of COVID-19 in HIV-Infected Individuals: A Systematic Review and Meta-Analysis. Sci Rep (2021) 11(1):1–12. doi: 10.1038/s41598-021-85359-3
8. Wang Y, Xie Y, Nie J, Marly G, Tang W. The Interaction Between HIV Infection and COVID-19: A Systematic Review and Meta-Analysis (2020) Available at: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3716873.
9. Moher D, Liberati A, Tetzlaff J, Altman DG, medicine PGJP. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PloS Med (2009) 6(7):e1000097. doi: 10.1371/journal.pmed.1000097
10. WHO. Clinical Management of COVID-19: Interim Guidance. Available at: https://apps.who.int/iris/handle/10665/332196 (Accessed January 20, 2022).
11. Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. (editors). Cochrane Handbook for Systematic Reviews of Interventions. 2nd Edition. Chichester, UK: John Wiley & Sons (2019).
12. Grant RL. Converting an Odds Ratio to a Range of Plausible Relative Risks for Better Communication of Research Findings. Bmj (2014) 348. doi: 10.1136/bmj.f7450
13. Shor E, Roelfs D, Vang ZM. The "Hispanic Mortality Paradox" Revisited: Meta-Analysis and Meta-Regression of Life-Course Differentials in Latin American and Caribbean Immigrants' Mortality. Soc Sci Med (2017) 186:20–33. doi: 10.1016/j.socscimed.2017.05.049
14. Tang W, Mao J, Li KT, Walker JS, Chou R, Fu R, et al. Pregnancy and Fertility-Related Adverse Outcomes Associated With Chlamydia Trachomatis Infection: A Global Systematic Review and Meta-Analysis. Sex Transm Infect (2020) 96(5):322–9. doi: 10.1136/sextrans-2019-053999
15. Vandenbroucke JP, Von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, et al. Strobe Initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and Elaboration. PloS Med (2007) 4(10):e297. doi: 10.1371/journal.pmed.0040297
16. Limaye D, Limaye V, Pitani RS, Fortwengel G, Sydymanov A, Otzipka C, et al. Development of a Quantitative Scoring Method for Strobe Checklist. Acta Pol Pharm (2018) 75(5):1095–106. doi: 10.32383/appdr/84804
17. Menza TW, Capizzi J, Zlot AI, Barber M, Bush L. COVID-19 Vaccine Uptake Among People Living With HIV. AIDS Behav (2022), 1–5. doi: 10.1007/s10461-021-03570-9
18. Western Cape Department of Health in collaboration with the National Institute for Communicable Diseases SA. Risk Factors for Coronavirus Disease 2019 (COVID-19) Death in a Population Cohort Study From the Western Cape Province, South Africa. Clin Infect Dis (2021) 73(7):e2005–15. doi: 10.1093/cid/ciaa1198
19. Abdela SG, Abegaz SH, Demsiss W, Tamirat KS, van Henten S, van Griensven J. Clinical Profile and Treatment of COVID-19 Patients: Experiences From an Ethiopian Treatment Center. Am J Trop Med Hyg (2020) 104(2):532–6. doi: 10.4269/ajtmh.20-1356
20. Abraha HE, Gessesse Z, Gebrecherkos T, Kebede Y, Weldegiargis AW, Tequare MH, et al. Clinical Features and Risk Factors Associated With Morbidity and Mortality Among Patients With COVID-19 in Northern Ethiopia. Int J Infect Dis (2021) 105:776–83. doi: 10.1016/j.ijid.2021.03.037
21. Bennett CL, Ogele E, Pettit NR, Bischof JJ, Meng T, Govindarajan P, et al. Multi-Center Study of Outcomes Among Persons With HIV Who Presented to US Emergency Departments With Suspected SARS-CoV-2. J Acquir Immune Defic Syndr (2021) 88(4):406. doi: 10.1097/QAI.0000000000002795
22. Berenguer J, Díez C, Martín-Vicente M, Micán R, Pérez-Elías MJ, García-Fraile LJ, et al. Prevalence and Factors Associated With SARS-CoV-2 Seropositivity in the Spanish HIV Research Network Cohort. Clin Microbiol Infect (2021) 27(11):1678–84. doi: 10.1016/j.cmi.2021.06.023
23. Bergman J, Ballin M, Nordström A, Nordström P. Risk Factors for COVID-19 Diagnosis, Hospitalization, and Subsequent All-Cause Mortality in Sweden: A Nationwide Study. Eur J Epidemiol (2021) 36(3):287–98. doi: 10.1007/s10654-021-00732-w
24. Bhaskaran K, Rentsch CT, MacKenna B, Schultze A, Mehrkar A, Bates CJ, et al. HIV Infection and COVID-19 Death: A Population-Based Cohort Analysis of UK Primary Care Data and Linked National Death Registrations Within the OpenSAFELY Platform. Lancet HIV (2021) 8(1):e24–32. doi: 10.1016/s2352-3018(20)30305-2
25. Blanco JL, Casado JL, Blanco JR, Olalla J, De Lazzari EL, Berrocal LL, et al. A Prospective Case-Cohort Study of Covid-19 in Persons With Hiv: Covih-19 Study [Conference Abstract]. Top Antivir Med (2021) 29(1):248.
26. Brown AE, Croxford SE, Nash S, Khawam J, Kirwan P, Kall M, et al. COVID-19 Mortality Among People With Diagnosed HIV Compared to Those Without During the First Wave of the COVID-19 Pandemic in England. HIV Med (2022) 23(1):90–102. doi: 10.1111/hiv.13167
27. Chanda D, Minchella PA, Kampamba D, Itoh M, Hines JZ, Fwoloshi S, et al. COVID-19 Severity and COVID-19-Associated Deaths Among Hospitalized Patients With HIV Infection - Zambia, March-December 2020. MMWR Morb Mortal Wkly Rep (2021) 70(22):807–10. doi: 10.15585/mmwr.mm7022a2
28. Eybpoosh S, Afshari M, Haghdoost AA, Afsar Kazerooni P, Gouya MM, Tayeri K. Severity and Mortality of COVID-19 Infection in HIV-Infected Individuals: Preliminary Findings From Iran. Med J Islam Repub Iran (2021) 35:33. doi: 10.47176/mjiri.35.33
29. Friedman EE, Devlin SA, McNulty MC, Ridgway JP. SARS-CoV-2 Percent Positivity and Risk Factors Among People With HIV at an Urban Academic Medical Center. PloS One (2021) 16(7):e0254994. doi: 10.1371/journal.pone.0254994
30. Gagliardini R, Lorenzini P, Vergori A, Cicalini S, Pinnetti C, Mazzotta V, et al. Characteristics and Outcomes of COVID-19-Related Hospitalization Among Plwh [Conference Abstract]. Top Antivir Med (2021) 29(1):206. doi: 10.3390/jcm11061546
31. Ge E, Li Y, Wu S, Candido E, Wei X. Association of Pre-Existing Comorbidities With Mortality and Disease Severity Among 167,500 Individuals With COVID-19 in Canada: A Population-Based Cohort Study. PloS One (2021) 16(10):e0258154. doi: 10.1371/journal.pone.0258154
32. Laracy J, Zucker J, Castor D, McMahon DJ, Guo TW, Yan M, et al. HIV-1 Infection Does Not Change Disease Course or Inflammatory Pattern of SARS-CoV-2-Infected Patients Presenting at a Large Urban Medical Center in New York City. Open Forum Infect Dis (2021) 8(2):ofab029. doi: 10.1093/ofid/ofab029
33. Lee MJ, Snell LB, Douthwaite ST, Fidler S, Fitzgerald N, Goodwin L, et al. Clinical Outcomes of Patients With and Without HIV Hospitalized With COVID-19 in England During the Early Stages of the Pandemic: A Matched Retrospective Multi-Centre Analysis (RECEDE-C19 Study). HIV Med (2022) 23(2):121–33. doi: 10.1111/hiv.13174
34. Nagarakanti SR, Okoh AK, Grinberg S, Bishburg E. Clinical Outcomes of Patients With COVID-19 and HIV Co-Infection. J Med Virol (2020) 93(3):1687–93. doi: 10.1002/jmv.26533
35. Patel VV, Felsen UR, Fisher M, Fazzari MJ, Ginsberg MS, Beil R, et al. Clinical Outcomes and Inflammatory Markers by HIV Serostatus and Viral Suppression in a Large Cohort of Patients Hospitalized With COVID-19. J Acquir Immune Defic Syndr (2021) 86(2):224–30. doi: 10.1097/qai.0000000000002578
36. Sigel K, Swartz T, Golden E, Paranjpe I, Somani S, Richter F, et al. Coronavirus 2019 and People Living With Human Immunodeficiency Virus: Outcomes for Hospitalized Patients in New York City. Clin Infect Dis (2020) 71(11):2933–8. doi: 10.1093/cid/ciaa880
37. Spinelli MA, Lynch KL, Yun C, Glidden DV, Peluso MJ, Henrich TJ, et al. SARS-CoV-2 Seroprevalence, and IgG Concentration and Pseudovirus Neutralising Antibody Titres After Infection, Compared by HIV Status: A Matched Case-Control Observational Study. Lancet HIV (2021) 8(6):e334–41. doi: 10.1093/cid/ciaa1198
38. Stoeckle K, Johnston CD, Jannat-Khah DP, Williams SC, Ellman TM, Vogler MA, et al. COVID-19 in Hospitalized Adults With HIV. Open Forum Infect Dis (2020) 7(8):ofaa327. doi: 10.1093/ofid/ofaa327
39. Tang ME, Gaufin T, Anson R, Cachay ER. HIV is Associated With a Higher Rate of COVID-19 Diagnosis But No Adverse Outcomes [Conference Abstract]. Top Antivir Med (2021) 29(1):204–5.
40. Tesoriero JM, Swain CAE, Pierce JL, Zamboni L, Wu M, Holtgrave DR, et al. COVID-19 Outcomes Among Persons Living With or Without Diagnosed HIV Infection in New York State. JAMA Netw (2021) 4(2):e2037069. doi: 10.1001/jamanetworkopen.2020.37069
41. Venturas J, Zamparini J, Shaddock E, Stacey S, Murray L, Richards GA, et al. Comparison of Outcomes in HIV-Positive and HIV-Negative Patients With COVID-19: HIV-Positive and -Negative Patients With COVID-19. J Infect (2021) 83(2):217–27. doi: 10.1016/j.jinf.2021.05.020
42. Yendewa GA, Perez JA, Schlick K, Tribout H, McComsey GA. Clinical Features and Outcomes of Coronavirus Disease 2019 Among People With Human Immunodeficiency Virus in the United States: A Multi-Center Study From a Large Global Health Research Network (TriNetX). Open Forum Infect Dis (2021) 8(7):ofab272. doi: 10.1093/ofid/ofab272
43. Geretti AM, Stockdale AJ, Kelly SH, Cevik M, Collins S, Waters L, et al. Outcomes of Coronavirus Disease 2019 (COVID-19) Related Hospitalization Among People With Human Immunodeficiency Virus (HIV) in the ISARIC World Health Organization (WHO) Clinical Characterization Protocol (UK): A Prospective Observational Study. Clin Infect Dis (2021) 73(7):e2095–106. doi: 10.1093/cid/ciaa1605
44. Hadi YB, Naqvi SFZ, Kupec JT, Sarwari AR. Characteristics and Outcomes of COVID-19 in Patients With HIV: A Multicentre Research Network Study. AIDS (Lond Engl) (2020) 34(13):F3–8. doi: 10.1097/QAD.0000000000002666
45. Inciarte A, Gonzalez-Cordon A, Rojas J, Torres B, de Lazzari E, de la Mora L, et al. Clinical Characteristics, Risk Factors, and Incidence of Symptomatic Coronavirus Disease 2019 in a Large Cohort of Adults Living With HIV: A Single-Center, Prospective Observational Study. AIDS (Lond Engl) (2020) 34(12):1775–80. doi: 10.1097/QAD.0000000000002643
46. Jassat W, Cohen C, Tempia S, Masha M, Goldstein S, Kufa T, et al. Risk Factors for COVID-19-Related in-Hospital Mortality in a High HIV and Tuberculosis Prevalence Setting in South Africa: A Cohort Study. Lancet HIV (2021) 8(9):e554–67. doi: 10.1016/S2352-3018(21)00151-X
47. Karim F, Gazy I, Cele S, Zungu Y, Krause R, Bernstein M, et al. HIV Status Alters Disease Severity and Immune Cell Responses in Beta Variant SARS-CoV-2 Infection Wave. Elife (2021) 10:e67397. doi: 10.7554/eLife.67397
48. Yang X, Sun J, Patel RC, Zhang J, Guo S, Zheng Q, et al. Associations Between HIV Infection and Clinical Spectrum of COVID-19: A Population Level Analysis Based on US National COVID Cohort Collaborative (N3C) Data. Lancet HIV (2021) 8(11):e690–700. doi: 10.1016/S2352-3018(21)00239-3
49. Murray CJ. COVID-19 Will Continue But the End of the Pandemic is Near. Lancet (2022). doi: 10.1016/S0140-6736(22)00100-3
50. Nomah DK, Reyes-Urueña J, Díaz Y, Moreno S, Aceiton J, Bruguera A, et al. Sociodemographic, Clinical, and Immunological Factors Associated With SARS-CoV-2 Diagnosis and Severe COVID-19 Outcomes in People Living With HIV: A Retrospective Cohort Study. Lancet HIV (2021) 8(11):e701–10. doi: 10.1016/s2352-3018(21)00240-x
51. Hoffmann C, Casado JL, Härter G, Vizcarra P, Moreno A, Cattaneo D, et al. Immune Deficiency Is a Risk Factor for Severe COVID-19 in People Living With HIV. HIV Med (2021) 22(5):372–8. doi: 10.1111/hiv.13037
52. Calza L, Bon I, Borderi M, Colangeli V, Borioni A, Re MC, et al. COVID-19 Outcomes in Patients With Uncontrolled HIV-1 Infection. J Acquired Immune Deficiency Syndromes (1999) 86(1):e15. doi: 10.1097/QAI.0000000000002537
53. Alexandar S, Ravisankar M, Kumar RS, Jakkan K. A Comprehensive Review on Covid-19 Delta Variant. Int J Pharmacol Clin Res (IJPCR) (2021) 5(83-85):7. doi: 10.1080/21645515.2021.2002083
54. Ridgway JP, Schmitt J, Friedman E, Taylor M, Devlin S, McNulty M, et al. HIV Care Continuum and COVID-19 Outcomes Among People Living With HIV During the COVID-19 Pandemic, Chicago, IL. AIDS Behav (2020) 24(10):2770–2. doi: 10.1007/s10461-020-02905-2
55. Shi L, Tang W, Hu H, Qiu T, Marley G, Liu X, et al. The Impact of COVID-19 Pandemic on HIV Care Continuum in Jiangsu, China. BMC Infect Dis (2021) 21(1):1–9. doi: 10.1186/s12879-021-06490-0
Keywords: human immunodeficiency virus (HIV), Coronavirus-19 (COVID-19), SARS-CoV-2, systematic review, meta-analysis
Citation: Wang Y, Xie Y, Hu S, Ai W, Tao Y, Tang H, Jing F and Tang W (2022) Systematic Review and Meta-Analyses of The Interaction Between HIV Infection And COVID-19: Two Years’ Evidence Summary. Front. Immunol. 13:864838. doi: 10.3389/fimmu.2022.864838
Received: 28 January 2022; Accepted: 13 April 2022;
Published: 10 May 2022.
Edited by:
Weibin Cheng, Guangdong Second Provincial General Hospital, ChinaReviewed by:
Zixin Wang, The Chinese University of Hong Kong, ChinaCopyright © 2022 Wang, Xie, Hu, Ai, Tao, Tang, Jing and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weiming Tang, d2VpbWluZ190YW5nQG1lZC51bmMuZWR1
†These authors have contributed equally to this work and share the first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.