
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Immunol., 30 March 2022
Sec. Primary Immunodeficiencies
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.864449
This article is part of the Research TopicNovel Aspects in Treatment of Skin Disorders in Primary ImmunodeficienciesView all 5 articles
 Anouk E. M. Nouwen1
Anouk E. M. Nouwen1 Renske Schappin1†
Renske Schappin1† N. Tan Nguyen1†
N. Tan Nguyen1† Aviël Ragamin1
Aviël Ragamin1 Anette Bygum2,3
Anette Bygum2,3 Christine Bodemer4
Christine Bodemer4 Virgil A. S. H. Dalm5,6
Virgil A. S. H. Dalm5,6 Suzanne G. M. A. Pasmans1*
Suzanne G. M. A. Pasmans1*Background: Comèl-Netherton syndrome (NS) is a rare disease caused by pathogenic variants in the SPINK5 gene, leading to severe skin barrier impairment and proinflammatory upregulation. Given the severity of the disease, treatment of NS is challenging. Current treatment regimens are mainly topical and supportive. Although novel systemic treatment options for NS have been suggested in recent literature, little is known about their outcomes.
Objective: to provide an overview of systemic treatment options and their outcomes in adults and children with NS.
Methods: Embase, MEDLINE, Web of Science, Cochrane Central Register of Controlled Trials, and Google Scholar were searched up to July 22, 2021. Empirical studies published in English language mentioning systemic treatment in NS were enrolled. Studies that did not define a treatment period or report at least one outcome were excluded. Methodological quality was evaluated by the Joanna Briggs Institute critical appraisal checklist for case reports or case series. Overall quality of evidence of the primary outcome, skin, was assessed by the GRADE approach.
Results: 36 case series and case reports were included. The effects of 15 systemic therapies were described in 48 patients, of which 27 were children. Therapies included retinoids, prednisolone, cyclosporine, immunoglobulins, and biologicals. In retinoids both worsening (4/15 cases) and improvement (6/15 cases) of the skin was observed. Use of prednisolone and cyclosporine was only reported in one patient. Immunoglobulins (13/15 cases) and biologicals (18/21 cases) showed improvement of the skin. Certainty of evidence was rated as very low.
Conclusion: NS is a rare disease, which is reflected in the scarce literature on systemic treatment outcomes in children and adults with NS. Studies showed large heterogeneity in outcome measures. Adverse events were scarcely reported. Long-term outcomes were reported in a minority of cases. Nonetheless, a general beneficial effect of systemic treatment was found. Immunoglobulins and biologicals showed the most promising results and should be further explored. Future research should focus on determining a core outcome set and measurement instruments for NS to improve quality of research.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=217933, PROSPERO (ID: 217933).
Comèl-Netherton syndrome (NS; OMIM 256500) is a rare and severe, potential life-threatening disorder of epidermal maturation and keratinization (1). It has an incidence of 1 per 200.000 births and an estimated prevalence of 1-9/1.000.000 (2). It is caused by pathogenic autosomal recessive homozygous or compound heterozygous variants in the serine protease inhibitor of the Kazal type 5 (SPINK5) gene on chromosome 5q32 (1, 3, 4). To date, over 80 different variants have been reported (4). The SPINK5 gene normally encodes LEKTI (lympho-epithelial Kazal-type-related inhibitor), an epidermal serine protease inhibitor that is crucial for maintaining skin barrier function (5–7). The result of pathogenic variants in SPINK5 is a continuous break-down of the skin barrier with secondary inflammation. Although SPINK5 is also expressed in other tissues including esophagus, tongue, and thymus, the consequences of the pathogenic variants in SPINK5 in these tissues remain unclear (7, 8).
LEKTI deficiency in the granular layer of the epidermis and in the inner root sheets of hair follicles leads to a loss of inhibition of serine proteinases and unopposed activity of kallikrein-related peptidase 5 (KLK5), which activates KLK7, KLK14, and elastase 2 (ELA2) (5, 9–14). The deregulated activity of KLKs leads to an increased degradation of desmosomal cadherin component desmoglein 1 (DSG1) and other corneodesmosomal proteins resulting in loss of cell adhesion, desquamation, and early detachment of the stratum corneum (SC) (15–17). This causes severe skin barrier impairment. Through proteinase-activated receptor 2 (PAR2) signaling in keratinocytes, KLK5 triggers the secretion of proinflammatory and proallergic cytokines, including tumor necrosis factor-a (TNF-a), Intercellular Adhesion Molecule 1 (ICAM-1), Interleukin-8 (IL-8), and thymic stromal lymphopoietine (TSLP) (18–20). This further enhances allergic predisposition and secondary inflammation (21). Furthermore, a recent study in patients with NS showed striking upregulation of the T helper type 17 (Th17)/IL-23 pathway and IL-1β expression, suggesting a potential target for systemic treatment (22).
NS is clinically characterized by congenital ichthyosiform erythroderma, trichorrhexis invaginata (bamboo hair), and atopic manifestations (food allergy, asthma, rhinoconjunctivitis) with high IgE levels and eosinophilia (23–25). Especially in the first year, patients have a high risk of life-threatening complications, including hypernatriemic dehydration due to extensive transepidermal waterloss, hypothermia, failure to thrive, and sepsis (1, 26–29). In a majority of patients, the erythroderma present at birth gradually evolves into ichthyosis linearis circumflexa with typical ‘double-edged’ scales (30). Although symptoms can vary widely, most patients experience extensive pruritus and recurrent skin infections (24, 29, 31). Furthermore, NS is considered an immunodeficiency, based on previous studies that showed defects in immune cell function (29, 32, 33). However, a recent study suggests a local skin barrier defect in the absence of an underlying systemic immunodeficiency (24).
Given the severity of the disease, treatment of NS is important but often challenging. Little is known about therapeutic options and their outcomes, with treatment regimens hitherto being only supportive in nature. Furthermore, current treatment strategies mainly target skin symptoms. Although lifelong treatment is required, there are no registered treatments and treatment guidelines available for patients with NS. First-step treatment options include topical corticosteroids (34). Also topical calcineurin inhibitors and narrowband ultraviolet B phototherapy have been reported in NS patients (35, 36). For many patients systemic agents are required. Retinoids have been described in several studies (37–39). In recent years, novel systemic treatment options for NS have been suggested in the literature, such as treatment with immunoglobulins, and biologicals targeting specific inflammatory pathways (29, 40, 41).
A recent systematic review summarized the different therapeutic options for children with NS (42). Here, we further explore treatment regimens for NS, specifically focusing on systemic treatment options in both children and adults. This systematic review aims to give an overview of systemic treatment options and their outcomes in patients with NS as a basis for future guideline development.
A comprehensive systematic literature search was performed in Embase, MEDLINE, Web of Science, Cochrane Central Register of Controlled Trials, and Google Scholar up to July 22, 2021.We performed a broad search for all articles including NS patients. For the full search strategy see Supplementary 1.
A pragmatic approach was used to include as many studies as possible for this systematic review. We included all English language clinical empirical studies reporting systemic therapy for patients with NS. Systemic therapy included oral, subcutaneous, and intravenous administered medication. There were no restrictions on age of patients. Abstracts, summaries of meetings, and summaries of oral presentations were manually excluded. We excluded studies if: 1) patients were treated for diseases other than NS; 2) systemic treatment lacked specification of type of medication, treatment duration, or treatment related outcome, and 3) if no full text was available.
Duplicates were removed from the identified articles. Two reviewers (AN; SP or TN) independently assessed titles and abstracts for potential eligibility, using Rayyan software (43). Potentially eligible studies were reviewed in full text. All references from included articles were manually screened for additional eligible articles. Any disagreements between reviewers were resolved by discussion with a third reviewer (VD). If studies were considered eligible but lacked specific details, authors were contacted to provide these details. If necessary data could not be provided, studies were excluded.
Data were extracted independently by two reviewers (AN and TN) using a predefined data extraction template in Excel. Discrepancies were resolved by discussion with a third reviewer (SP, RS, or VD). The following data were extracted if available: author, year of publication, country of inclusion, study design, total number of patients, number of NS patients, number of eligible NS patients, age and sex of patients, given treatment and dosage, duration of treatment, overall treatment effect, primary treatment outcome (effect of treatment on the skin), secondary treatment outcomes (e.g. pruritus, quality of life, frequency of flare-ups), and side effects as provided by the authors. Furthermore, previous and concomitant treatment were noted. Some patients received different systemic treatments consecutively. In this case, each treatment period was described separately and denoted as a treatment case.
Effect of treatment per received systemic treatment (treatment case) was converted into a 3-level scale reflecting overall treatment effect. Levels were: ‘-’ (worsening of the condition); ‘0’ (no change; or temporary improvement; or a combination of worsening and improvement; or temporary worsening); and ‘+’ (improvement).
For each type of systemic treatment the number of studies, number of treatment cases, number of children, number of females, and number of treatment cases with overall improvement were calculated. Age range and range of treatment duration were collected. Furthermore, reported side effects were summarized per type of treatment.
The Joanna Briggs Institute (JBI) critical appraisal checklist for case reports and JBI critical appraisal checklist for case series were used for quality assessment of the case reports and case series respectively (44). Two reviewers (SP and AN) performed the quality assessment. Disagreements were resolved by discussion with a third reviewer (RS). Case series that included only one NS patient were assessed as case reports. Quality assessment was focused on the reported systemic treatments, which was not always the main subject of the included studies. The overall quality of evidence of this review’s primary outcome, effect of treatment on the skin, was assessed using an adapted version of the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology (45).
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (46). The review protocol was registered in PROSPERO (ID: 217933) and can be accessed at https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=217933(ref PROSPERO).
A total of 1484 unique studies were identified through database searching and screening of reference lists, with 166 studies eligible for full-text review based on title and abstract screening. Following the full-text review and approach of authors for missing details, we included 36 studies in the final qualitative analysis (Figure 1). Of these articles, 21 studies were case reports and 15 studies were case series. 8 case series only reported one patient with NS. An overview of patient characteristics, study characteristics, and treatment outcomes is provided in Table 1. Previous treatments are listed in Table S1.
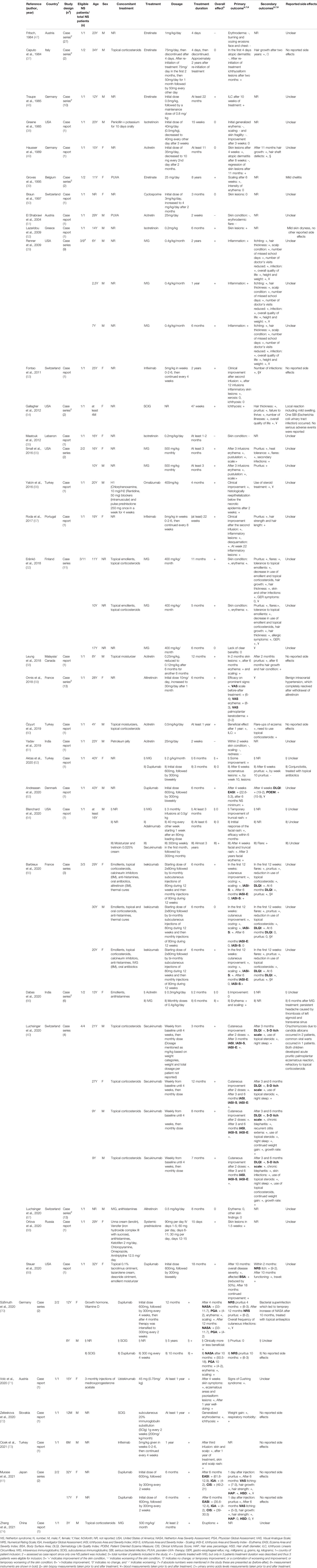
Table 1 Characteristics of included studies evaluating systemic therapy in patients with netherton syndrome.
Overall, 48 patients with NS treated with systemic agents were included. The study sample consisted of 19 adults and 27 children. The youngest patients were a few months old, the oldest patient was 43 years of age. In two patients, age was not reported (50, 67). 25 patients were male and 20 patients were female. In three patients, sex was not reported (32).
An overview of all reported therapies is presented in Tables 2, S2 and Figure 2. Overall, 15 different types of systemic therapies were investigated, belonging to 5 treatment groups. Included treatment groups were systemic retinoids (n= 15), systemic prednisolone (n=1), cyclosporine (n=1), immunoglobulins (n=15), and biologicals (n=21). Of all therapies, intravenous immunoglobulins (IVIG; n=12) and dupilumab (n=7) were most studied.
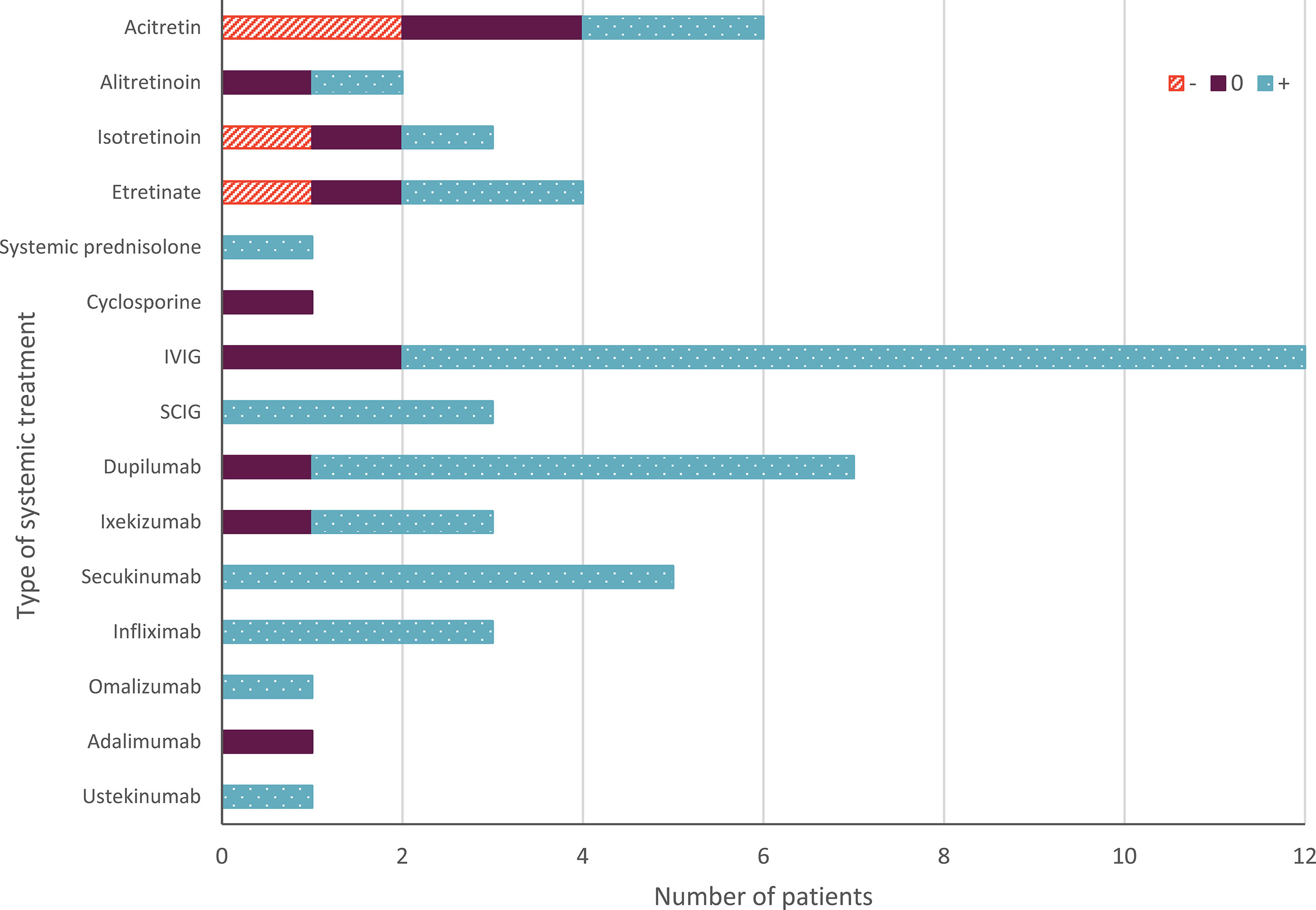
Figure 2 Overall effect of systemic treatment in Netherton syndrome. The orange upward diagonal lines indicate worsening (-) the skin condition; blue with white dots indicates improvement (+) of the skin condition. Purple indicates no change; or temporary improvement; or a combination of worsening and improvement; or temporary worsening (0). The number of patients on the X-axis indicates absolute numbers. IVIG, intravenous immunoglobulins; SCIG, subcutaneous immunoglobulins.
In 4 patients the consecutive use of two or more systemic therapies was reported, for which outcome and treatment duration were described per therapy and therefore count as multiple cases (62, 64, 66, 70). In total 53 treatment cases were reported in 48 patients. Many patients received previous topical and systemic therapy, but this was not consistently reported in all studies (see Table S1). In 18 patients simultaneous use of topical treatments including corticosteroids was reported during treatment with the systemic agents (see Table 1). 7 patients received multiple systemic treatments for NS simultaneously which sometimes prevented interpretation of treatment results (38, 56, 65, 67, 70).
Overall, treatment duration varied widely. In most treatment cases outcomes were only assessed short-term, with treatment durations of 6 months or shorter. Only 30% of the cases had treatment durations of one year or longer. Duration of treatment in patients with retinoids varied from 4 days to 2 years, with one outlier having a treatment duration of at least 8 years. In patients treated with immunoglobulins and biologicals the treatment duration ranged between 2 months to 5 years and 3 months to 3 years, respectively (see Tables 1, 2 and S2).
Outcomes were measured in numerous ways using both descriptive methods and standardised measurement instruments (see Tables 1, 2).
All studies reported the effect of treatment on the skin. Most studies used descriptive methods, and focused on different aspects of the skin (e.g. erythema, scaling), resulting in a large inter-study variability. A total of 10 different measurement instruments were reported in 7 studies, including the Eczema Area and Severity Index (EASI), Ichthyosis Area and Severity Index (IASI), Ichthyosis Area and Severity Index – Scaling (IASI-S), Ichthyosis Area and Severity Index – Erythema (IASI-E), Netherton Area Severity Assessment (NASA), Physician Global Assessment (PGA), Clinical Ichthyosis Score (CIS), Investigator Global Assessment (IGA), Visual Analogue Scale (VAS; e.g. for erythema), and Body Surface Area (BSA) (see Table 1).
22 studies reported the effect of systemic treatment on secondary outcomes (see Table 1). Pruritus or itch, effects on hair, use of topical corticosteroids, infections, and quality of life were mostly reported. Other notable outcomes were height and weight (especially in children), and frequency of flare-ups. Most studies used descriptive methods. A total of 7 different measurement instruments were reported for the secondary outcomes: 5-D Itch Scale, Patient Oriented Eczema Measure (POEM), Dermatology Life Quality Index (DLQI), Numerical Rating Scale (NRS) and the Visual Analogue Scale (VAS) for pruritus, Hair Area Percentage (HAP), and Hair Shaft Diameters (HSD) (see Table 1).
Most reported treatments showed improvement of the patient’s condition (38/53 treatment cases). Immunoglobulins showed improvement in 13/15 cases, and biologicals in 18/21 of the cases. In cases treated with retinoids, both worsening (4/15 cases) and improvement (6/15 cases) of the skin condition was reported (see Tables 2, S2 and Figure 2).
Occurrence of adverse events or side effects was reported in 8 of the 36 studies (see Tables 1 , 2). Overall, 11 adverse events or side effects were reported, such as cheilitis, local swelling, conjunctivitis, and onychomycosis. The highest number of side effects (3 types of events) was reported in patients treated with secukinumab, who experienced onychomycosis due to Candida albicans, common viral warts, and palmoplantar eczematous reactions (40) (see Table 2). In 9 patients (8 studies) it was reported that no side effects had occurred (see Table 1).
The results of the JBI critical appraisal checklist for case reports and case series are summarized in Tables 3, 4 respectively. Overall, study quality of the included studies was moderate-to-good. Most of the case reports scored 6 out of 8 quality criteria or higher (see Table 3). All case series scored 6 out of 10 quality criteria or higher (see Table 4). The quality of evidence of the effect of systemic treatment on the skin according to the GRADE methodology is reported in Table 5.
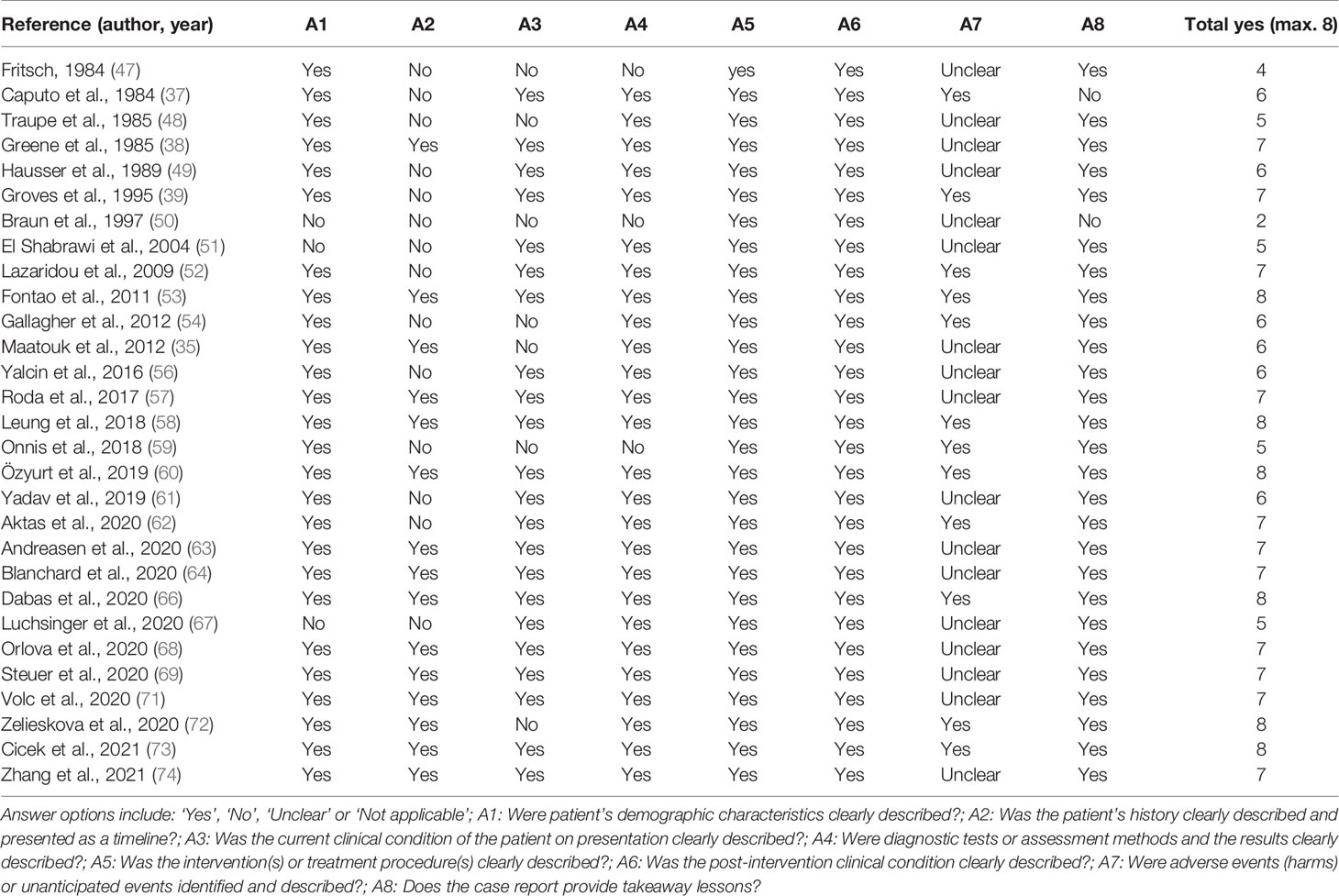
Table 3 Quality assessment of the included case reports using the Joanna Briggs Institute (JBI) Critical Appraisal Checklist for Case Reports.
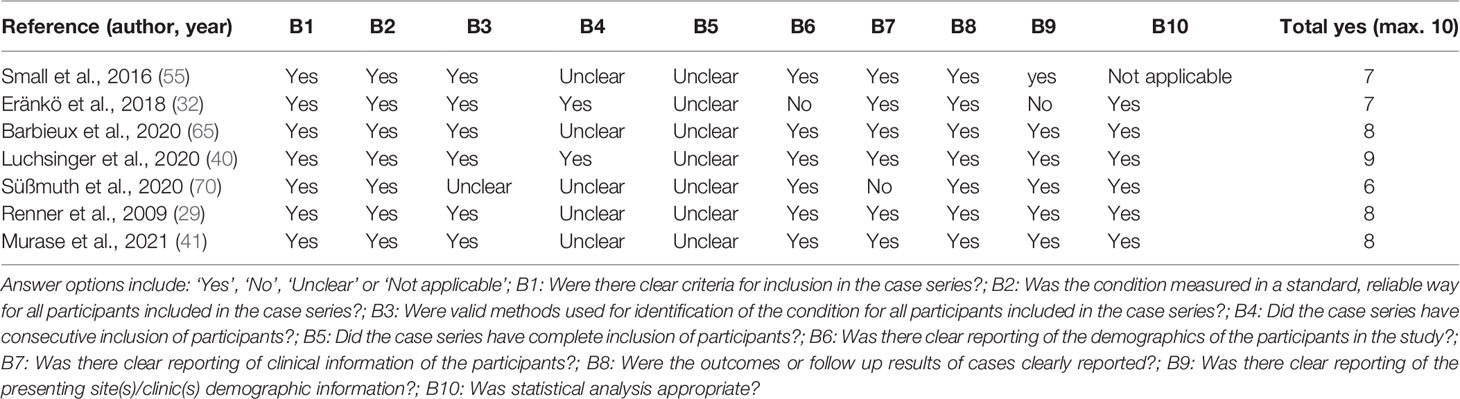
Table 4 Quality assessment of the included case series using the Joanna Briggs Institute (JBI) Critical Appraisal Checklist for Case Series.

Table 5 The certainty of evidence for the primary outcome, effect of treatment on the skin, rated using the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) approach by Murad et al. (45).
In this systematic review, we analyzed the use and outcomes of systemic treatment in patients with NS. Despite uncertainties, immunoglobulins and biologicals seem most effective in treatment of NS on both skin-related and secondary outcomes. Other treatments such as retinoids were described to be less effective. NS is a rare disease and although available literature is scarce, we included 36 studies describing 48 patients among which 27 were children. We identified 15 different types of systemic treatment for patients with NS. The primary outcome, effect of treatment on the skin, was reported very heterogeneously using descriptive methods and various measurement instruments, focusing on multiple aspects of the skin. This hinders accurate comparison between studies. Secondary outcomes were not mentioned in all studies, and if mentioned, mainly focused on skin related problems, including pruritus, use of topical corticosteroids, infections, and hair. Quality of life was only reported in 5 studies (29, 40, 54, 63, 65). The strong focus on skin related problems combined with sparse attention for patients’ functioning as a person does not reflect the full range of impact that NS has on patient’s lives (75). Furthermore, side effects were infrequently reported, which may flatter treatment outcomes. Little is known about long-term outcomes of systemic therapy in NS.
Four systemic retinoids (acitretin, alitretinoin, isotretinoin, and etretinate) have been used in NS, with varying results. Retinoids are synthetic analogues of vitamin A that act via an anti-keratinizing effect and have been used in the treatment of ichthyosis for decades (76, 77). Despite the fact that retinoids have been used in dermatology for a significant amount of time, the majority of studies had only several months of retinoid use. One single study reported the use of etretinate for at least 8 years, with positive effects on scaling (39). In general, long-term effects and potential side effects of retinoids in NS remain unclear.
In most children and adults treated with retinoids, only skin related outcomes were reported. If secondary outcomes were mentioned, they usually focused on aspects of hair. Most studies reporting outcomes of retinoids in NS were published before SPINK5 mutation analysis was available or commonly used (78). Therefore, NS diagnosis in these studies was usually based on investigation of the hair to characterize trichorrhexis invaginata (bamboo hair). This may explain the elaborate description of outcomes based on hair in these studies.
There are currently no factors that can predict treatment outcome of retinoids in patients with NS. Although four patients with NS worsened during retinoid therapy, six patients improved, with less scaling, erythema, and pruritus, accompanied by a reduction in use of topical corticosteroids. In one patient treated with isotretinoin, worsening of the skin condition stopped when the dosage was reduced (38). This could be due to the course of the disease, or may indicate that patients with NS benefit from a lower dose. In contrast, several other NS patients improved during therapy with high dose retinoids. However, the effect of treatment dosages on outcome is difficult to determine since weight has been infrequently reported and dosages were reported as either total milligrams per day or milligrams per kilogram.
Considering the anti-keratinizing effect of retinoids, which results in removing scales and thinning of hyperkeratosis, it could be hypothesized that retinoids are effective in NS patients in which scaling is more pronounced compared to erythroderma. However, scaling was not assessed consistently, nor has it been measured using standardized instruments across studies. Therefore, no definitive conclusion can be drawn on the effectiveness of retinoids on certain subgroups of the NS population. Based on the current data and compared to immunoglobulins and biologicals, retinoids could be a secondary choice for therapy.
Prednisolone is a synthetic corticosteroid closely related to prednisone (79). Corticosteroids are used in NS for their anti-inflammatory and immunosuppressive properties (79, 80). Short-term systemic prednisolone for 15 days resulted in an improvement of skin lesions in one adult patient (68). Additionally, one adult patient used prednisolone for 4 weeks as concomitant therapy during the 4-month treatment with omalizumab (56). Although clinical improvement was observed in this patient, it is unclear whether the improvement can be attributed to prednisolone alone. Furthermore, six studies reported previous use of systemic corticosteroids in both children and adults with NS (41, 63–66, 71). Based on the mechanism of action and known side effects, systemic corticosteroids might not be the first choice of treatment in NS, but could be useful in flare-ups.
Cyclosporine is a calcineurin inhibitor, which can be used as an immunosuppressant in auto-immune and cutaneous diseases, including atopic dermatitis (81). Cyclosporine showed no effect on skin lesions in a single patient of unknown age treated for 3 months (50). Three other patients had been previously treated with cyclosporine, but the effects of treatment were not described (62, 64, 69). Based on one reported patient, we cannot determine whether treatment with cyclosporine is beneficial in patients with NS.
The use of both IVIG and SCIG has been reported in NS and thirteen out of fifteen patients showed clinical improvement after treatment initiation (see Table 2).
Immunoglobulins have been used in the treatment of primary immunodeficiencies and chronic inflammatory diseases (82–84). Since NS has been described as a primary immunodeficiency disorder, it has been hypothesized that the disorder may respond to treatment with immunoglobulins (29, 32, 33). Interestingly, most patients showed normal serum IgG levels, suggesting that the potential beneficial effects of immunoglobulin replacement therapy (IGRT) in these patients is merely based on the immunomodulatory mode of actions of IGRT (29, 32, 54, 85). This hypothesis is supported by the increase in NK cell cytotoxicity, changes in proportion of CD cells (including CD4, CD8 and CD16), and normalization of certain lymphocyte subclasses (including memory T cells, transitional and activated B cells and plasmablasts) observed during IVIG therapy (29, 32).
However, the exact mechanism of action remains to be elucidated.
The beneficial effects described in patients on supplemental dosage of IGRT is remarkable, since for immunomodulatory purposes in other inflammatory diseases mostly higher doses are prescribed (83, 85). The only adult NS patient that was described, received IVIG at a dose of 2 grams/kilogram/month, with some clinical improvement (62). The pediatric NS patients receiving IVIG were mostly treated at a dosage of 400-500mg/kg/month which is at supplemental dose (86).
Most NS patients treated with immunoglobulins were children (n=14), and in a majority (n=12) a positive clinical effect was observed during a 2 months to 5 years treatment period. Primary effects were a decrease in inflammation, erythema, scaling, and pustulation of the skin. Furthermore, a decrease in itch and infections, decrease in use of topical steroids, improvement of the quality of hair, and improvement in overall quality of life and functioning was reported (see Tables 1, S2). Remarkably, in 5 children, aged between 4 months and 2 years, a significant increase in weight and height was noted (29, 54, 72). This effect might be attributed to a general improvement in NS symptoms, thereby enabling growth. Another explanation may be that this is a treatment-specific effect.
Clinical data of immunoglobulin treatment in adult NS patients are lacking. However, based on the mechanism of action, immunoglobulins could be equally effective in children and in adults. The efficacy of immunoglobulins has only been reported in one adult (62). During a 6-month treatment period this adult patient experienced some improvement of her skin condition. To assess the role of immunoglobulins in adult patients, more data is needed.
In all use of biologicals initial improvement of the skin condition in NS was observed, suggesting an important role for targeted therapy in the future. As a consequence of the SPINK5 gene mutation and LEKTI deficiency, allergic and inflammatory pathways are upregulated in patients with NS (18, 20, 22, 29, 87). Increased activity of both Th2 and Th17 pathway, and high levels of TNF-a and IgE have been observed (18, 20, 22–24, 29, 87–89). In previous studies the effect of seven biologicals has been investigated (see Table 2). These biologicals specifically target TNF-a (adalimumab, infliximab), IL-17 (secukinumab, ixekizumab), IL-12 and IL-23 (ustekinumab), IL-4 and IL-13 (dupilumab), and IgE (omalizumab). Dupilumab (n=7) and secukinumab (n=5) were most studied in children and adults with NS (see Table 2). In 18 cases treated with biologicals, of which 8 were children, improvement was observed, resulting in less inflammatory lesions, desquamation, and oozing. Also, pruritus, quality of life, hair quality, and sleep improved, and use of topical corticosteroids and occurrence of infections decreased. Similar to the observations in pediatric patients treated with immunoglobulins, one study reported an increased growth rate in two children treated with secukinumab (40). Although these patients were slightly older (both 9 years) than the patients treated with immunoglobulins, this may indicate that growth and weight improve if NS symptoms improve, regardless of the type of medication.
After initial improvement the effectiveness decreased in one out of seven patients receiving dupilumab, one out of three patients receiving ixekizumab and the single one patient receiving adalimumab (62, 64, 65). Barbieux et al. reported that the patient who showed decreased effectiveness of ixekizumab initially presented with erythroderma compared to the other two patients in the study who presented with ichthyosis linearis circumflexa (65). This may indicate that clinical phenotype could be an important factor when considering targeted therapy with biologicals. Furthermore, the varying clinical symptoms that are observed in NS might be caused by differences in pathway activity between patients. This could implicate that individual NS patients might benefit from different treatments, depending on phenotype.
Although current research is shifting towards therapies involving the Th17 pathway, this review shows that both biologicals targeting Th17 and Th2 pathway seem effective in NS. However, reports on the effects of biologicals in NS remain scarce and more importantly dispersed over seven types of biologicals. Furthermore, treatment duration varied between 3 months and 3 years, leaving long-term effects not yet well-established (see Table 2). More research is needed to assess the role of different types of biologicals in the treatment of NS. Nonetheless, biologicals seem promising agents for the future treatment of NS.
Large heterogeneity was observed in both primary and secondary outcomes. This hinders interpretation of outcomes and comparison of results between studies. In this review we observed that earlier studies used predominantly descriptive methods to evaluate treatment outcomes, whereas more recent publications employed more standardized measurement instruments, albeit with great heterogeneity within and across therapeutic groups. Therefore, it is difficult to determine the best treatment options for NS patients. Heterogeneity in reporting is probably due to NS being a rare disorder having small clusters or single cases scattered across the globe. International collaboration including consensus on a core outcome set and measurement instruments for NS will overcome this issue and improve the quality of NS research.
This systematic review included data on systemic treatment in both children and adults, on the short- and long term, focusing not only on the skin, but also on a variety of outcomes related to NS. With this broad focus we strived for equipoise, given all possible systemic treatments for NS. Furthermore, although NS is a rare disease, 53 treatment cases in 48 patients were included.
Although this review used the best available data, there are limitations that should be considered. Most studies were of moderate-good quality as assessed by the JBI critical appraisal checklist for case reports or case series respectively (see Tables 3 and 4). However, since all included studies were either case reports or case series with low numbers of patients, the quality of evidence of the effect of treatment on the skin was judged as having very low certainty (see Table 5). In a case (series) it is difficult to distinguish between the true treatment effect and the natural disease course of NS. As previously mentioned, large heterogeneity was observed in reported outcomes. This prevents thorough comparison and interpretation of outcomes between studies. Most outcomes were skin-related, which may not reflect the full range of patients’ symptoms or needs. In addition, side effects and adverse events of treatment were often not systematically reported. We cannot rule out that publication bias influenced results, leaving room for error in determining the true response to systemic agents. This publication bias was presumably enlarged as a consequence of our inclusion criterion to include only English language articles.
To date, the field of research in NS is evolving. Recently, a randomized controlled trial (RCT) investigating the efficacy and safety of secukinumab in ichthyoses completed its recruitment phase, including five patients with NS (90). Also, a pilot study investigating the efficacy and safety of dupilumab versus placebo in patients with NS is currently ongoing (91). It remains questionable whether classical RCTs, are a suitable, efficient, and ethical approach for evaluating treatment of rare (skin) diseases such as NS. Therefore, other methodologic strategies should become more accepted for these kind of diseases. Interesting strategies are study designs in which cases serve as their own control and repeated measurements are performed. Furthermore, adaptive designs that allow for statistical modification of elements of the RCT design can be used (92).
Another step to improve research in NS, is the adoption of standardized outcomes and instruments. The development of a core outcome set for NS by health care professionals and patients, together with a consensus on measurement instruments for future research, will improve data quality and comparability. Improvement of data quality will spur the development of new systemic treatments for NS.
Future research into pathogenesis and NS phenotypes may help predict effectiveness of treatment and identify targeted treatment for patients with NS. International embedded research is needed to evaluate the effect of targeted (systemic) therapy, including long-term use of immunoglobulins and biologicals. This progress in the field of rare diseases can only be obtained by large scale international collaboration between clinicians, researchers, patients, and industry. Ultimately, a treatment guideline is necessary to facilitate high-quality care for patients with NS throughout life.
Netherton syndrome (NS) is a rare disease, which is reflected in the scarce literature on systemic treatment outcomes in children and adults with NS. 36 case series and reports describing 27 children and 19 adults with NS were identified. Despite low quality of evidence and large heterogeneity in reported outcomes, a general beneficial effect of systemic treatment was found. Immunoglobulins and biologicals showed the most promising results, on skin-related symptoms, pruritus, hair quality, use of topical corticosteroids, infections, and quality of life. Both treatments should be further explored. Future research should first focus on determining a core outcome set and standardized measurement instruments for NS to improve quality of NS research. International cooperation between clinicians, researchers, patients, and industry is needed to develop better care for these patients with high unmet medical needs.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
SP, TN, VD, RS, AR, and AN designed the study. SP, AN, and TN were responsible for the formal screening of search results against eligibility criteria, data extraction and risk of bias assessment. Any disagreement was resolved by a discussion with VD or RS as third reviewer. AN, TN, and RS wrote the manuscript. RS supervised the study. All authors (AN, RS, TN, AR, AB, CB, VD, and SP) critically commented on the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
This study is part of the ERN-SKIN-subthematic group Ichthyosis (https://ern-skin.eu), The International Netherton Network (https://nethertonnetwork.com/thinc-2021), The Center of Rare Diseases Erasmus MC, and the Center of Rare Skin Diseases-Netherton Expert Center Erasmus MC, acknowledged by the Dutch Ministry of Health. We thank Kira Stuvel and Karin Veldman for their contributions to the Netherton projects. We thank all authors of the Netherton studies for their work and effort to improve knowledge and care in patients with Netherton syndrome. We thank Sabrina Gunput, Maarten Engel, and Wichor Bramer of the Erasmus MC Medical Library for their contribution to the search strategy.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.864449/full#supplementary-material
1. Hovnanian A. Netherton Syndrome: Skin Inflammation and Allergy by Loss of Protease Inhibition. Cell Tissue Res (2013) 351(2):289–300. doi: 10.1007/s00441-013-1558-1
2. Orphanet. Netherton Syndrome (2008). Available at: https://www.orpha.net/consor/cgi-bin/Disease_Search.php?lng=EN&data_id=938&Disease_Disease_Search_diseaseType=ORPHA&Disease_Disease_Search_diseaseGroup=634&Disease(s)/group%20of%20diseases=Comel-Netherton-syndrome&title=Comel-Netherton-syndrome&search=Disease_Search_Simple (Accessed January 24, 2022).
3. Chavanas S, Bodemer C, Rochat A, Hamel-Teillac D, Ali M, Irvine AD, et al. Mutations in SPINK5, Encoding a Serine Protease Inhibitor, Cause Netherton Syndrome. Nat Genet (2000) 25(2):141–2. doi: 10.1038/75977
4. Sarri CA, Roussaki-Schulze A, Vasilopoulos Y, Zafiriou E, Patsatsi A, Stamatis C, et al. Netherton Syndrome: A Genotype-Phenotype Review. Mol Diagn Ther (2017) 21(2):137–52. doi: 10.1007/s40291-016-0243-y
5. Deraison C, Bonnart C, Lopez F, Besson C, Robinson R, Jayakumar A, et al. LEKTI Fragments Specifically Inhibit KLK5, KLK7, and KLK14 and Control Desquamation Through a pH-Dependent Interaction. Mol Biol Cell (2007) 18(9):3607–19. doi: 10.1091/mbc.e07-02-0124
6. Magert HJ, Standker L, Kreutzmann P, Zucht HD, Reinecke M, Sommerhoff CP, et al. LEKTI, a Novel 15-Domain Type of Human Serine Proteinase Inhibitor. J Biol Chem (1999) 274(31):21499–502. doi: 10.1074/jbc.274.31.21499
7. Tartaglia-Polcini A, Bonnart C, Micheloni A, Cianfarani F, Andre A, Zambruno G, et al. SPINK5, the Defective Gene in Netherton Syndrome, Encodes Multiple LEKTI Isoforms Derived From Alternative pre-mRNA Processing. J Invest Dermatol (2006) 126(2):315–24. doi: 10.1038/sj.jid.5700015
8. Bitoun E, Micheloni A, Lamant L, Bonnart C, Tartaglia-Polcini A, Cobbold C, et al. LEKTI Proteolytic Processing in Human Primary Keratinocytes, Tissue Distribution and Defective Expression in Netherton Syndrome. Hum Mol Genet (2003) 12(19):2417–30. doi: 10.1093/hmg/ddg247
9. Bonnart C, Deraison C, Lacroix M, Uchida Y, Besson C, Robin A, et al. Elastase 2 Is Expressed in Human and Mouse Epidermis and Impairs Skin Barrier Function in Netherton Syndrome Through Filaggrin and Lipid Misprocessing. J Clin Invest (2010) 120(3):871–82. doi: 10.1172/JCI41440
10. Egelrud T, Brattsand M, Kreutzmann P, Walden M, Vitzithum K, Marx UC, et al. Hk5 and Hk7, Two Serine Proteinases Abundant in Human Skin, Are Inhibited by LEKTI Domain 6. Br J Dermatol (2005) 153(6):1200–3. doi: 10.1111/j.1365-2133.2005.06834.x
11. Fortugno P, Bresciani A, Paolini C, Pazzagli C, El Hachem M, D’Alessio M, et al. Proteolytic Activation Cascade of the Netherton Syndrome-Defective Protein, LEKTI, in the Epidermis: Implications for Skin Homeostasis. J Invest Dermatol (2011) 131(11):2223–32. doi: 10.1038/jid.2011.174
12. Hachem JP, Wagberg F, Schmuth M, Crumrine D, Lissens W, Jayakumar A, et al. Serine Protease Activity and Residual LEKTI Expression Determine Phenotype in Netherton Syndrome. J Invest Dermatol (2006) 126(7):1609–21. doi: 10.1038/sj.jid.5700288
13. Mitsudo K, Jayakumar A, Henderson Y, Frederick MJ, Kang Y, Wang M, et al. Inhibition of Serine Proteinases Plasmin, Trypsin, Subtilisin A, Cathepsin G, and Elastase by LEKTI: A Kinetic Analysis. Biochemistry (2003) 42(13):3874–81. doi: 10.1021/bi027029v
14. Schechter NM, Choi EJ, Wang ZM, Hanakawa Y, Stanley JR, Kang Y, et al. Inhibition of Human Kallikreins 5 and 7 by the Serine Protease Inhibitor Lympho-Epithelial Kazal-Type Inhibitor (LEKTI). Biol Chem (2005) 386(11):1173–84. doi: 10.1515/BC.2005.134
15. Borgono CA, Michael IP, Komatsu N, Jayakumar A, Kapadia R, Clayman GL, et al. A Potential Role for Multiple Tissue Kallikrein Serine Proteases in Epidermal Desquamation. J Biol Chem (2007) 282(6):3640–52. doi: 10.1074/jbc.M607567200
16. Descargues P, Deraison C, Bonnart C, Kreft M, Kishibe M, Ishida-Yamamoto A, et al. Spink5-Deficient Mice Mimic Netherton Syndrome Through Degradation of Desmoglein 1 by Epidermal Protease Hyperactivity. Nat Genet (2005) 37(1):56–65. doi: 10.1038/ng1493
17. Descargues P, Deraison C, Prost C, Fraitag S, Mazereeuw-Hautier J, D’Alessio M, et al. Corneodesmosomal Cadherins Are Preferential Targets of Stratum Corneum Trypsin- and Chymotrypsin-Like Hyperactivity in Netherton Syndrome. J Invest Dermatol (2006) 126(7):1622–32. doi: 10.1038/sj.jid.5700284
18. Briot A, Deraison C, Lacroix M, Bonnart C, Robin A, Besson C, et al. Kallikrein 5 Induces Atopic Dermatitis-Like Lesions Through PAR2-Mediated Thymic Stromal Lymphopoietin Expression in Netherton Syndrome. J Exp Med (2009) 206(5):1135–47. doi: 10.1084/jem.20082242
19. Briot A, Lacroix M, Robin A, Steinhoff M, Deraison C, Hovnanian A. Par2 Inactivation Inhibits Early Production of TSLP, But Not Cutaneous Inflammation, in Netherton Syndrome Adult Mouse Model. J Invest Dermatol (2010) 130(12):2736–42. doi: 10.1038/jid.2010.233
20. Hosomi N, Fukai K, Nakanishi T, Funaki S, Ishii M. Caspase-1 Activity of Stratum Corneum and Serum Interleukin-18 Level Are Increased in Patients With Netherton Syndrome. Br J Dermatol (2008) 159(3):744–6. doi: 10.1111/j.1365-2133.2008.08706.x
21. Meyer-Hoffert U. Reddish, Scaly, and Itchy: How Proteases and Their Inhibitors Contribute to Inflammatory Skin Diseases. Arch Immunol Ther Exp (Warsz) (2009) 57(5):345–54. doi: 10.1007/s00005-009-0045-6
22. Paller AS, Renert-Yuval Y, Suprun M, Esaki H, Oliva M, Huynh TN, et al. An IL-17-Dominant Immune Profile Is Shared Across the Major Orphan Forms of Ichthyosis. J Allergy Clin Immunol (2017) 139(1):152–65. doi: 10.1016/j.jaci.2016.07.019
23. Bitoun E, Chavanas S, Irvine AD, Lonie L, Bodemer C, Paradisi M, et al. Netherton Syndrome: Disease Expression and Spectrum of SPINK5 Mutations in 21 Families. J Invest Dermatol (2002) 118(2):352–61. doi: 10.1046/j.1523-1747.2002.01603.x
24. Stuvel K, Heeringa JJ, Dalm V, Meijers RWJ, van Hoffen E, Gerritsen SAM, et al. Comel-Netherton Syndrome: A Local Skin Barrier Defect in the Absence of an Underlying Systemic Immunodeficiency. Allergy (2020) 75(7):1710–20. doi: 10.1111/all.14197
25. Wilkinson RD, Curtis GH, Hawk WA. Netherton’s Disease; Trichorrhexis Invaginata (Bamboo Hair), Congenital Ichthyosiform Erythroderma and the Atopic Diathesis. A Histopathologic Study. Arch Dermatol (1964) 89:46–54. doi: 10.1001/archderm.1964.01590250052010
26. Dyer JA, Spraker M, Williams M. Care of the Newborn With Ichthyosis. Dermatol Ther (2013) 26(1):1–15. doi: 10.1111/j.1529-8019.2012.01555.x
27. Hausser I, Anton-Lamprecht I. Severe Congenital Generalized Exfoliative Erythroderma in Newborns and Infants: A Possible Sign of Netherton Syndrome. Pediatr Dermatol (1996) 13(3):183–99. doi: 10.1111/j.1525-1470.1996.tb01202.x
28. Pruszkowski A, Bodemer C, Fraitag S, Teillac-Hamel D, Amoric JC, de Prost Y. Neonatal and Infantile Erythrodermas: A Retrospective Study of 51 Patients. Arch Dermatol (2000) 136(7):875–80. doi: 10.1001/archderm.136.7.875
29. Renner ED, Hartl D, Rylaarsdam S, Young ML, Monaco-Shawver L, Kleiner G, et al. Comel-Netherton Syndrome Defined as Primary Immunodeficiency. J Allergy Clin Immunol (2009) 124(3):536–43. doi: 10.1016/j.jaci.2009.06.009
30. Judge MR, Morgan G, Harper JI. A Clinical and Immunological Study of Netherton’s Syndrome. Br J Dermatol (1994) 131(5):615–21. doi: 10.1111/j.1365-2133.1994.tb04971.x
31. De Palma AM, Mazereeuw-Hautier J, Giehl K, Hernandez-Martin A, Merlos M, Moons P, et al. Burden of Itch in Ichthyosis: A Multicentre Study in 94 Patients. J Eur Acad Dermatol Venereol (2019) 33(11):2095–100. doi: 10.1111/jdv.15613
32. Eranko E, Ilander M, Tuomiranta M, Makitie A, Lassila T, Kreutzman A, et al. Immune Cell Phenotype and Functional Defects in Netherton Syndrome. Orphanet J Rare Dis (2018) 13(1):213. doi: 10.1186/s13023-018-0956-6
33. Picard C, Al-Herz W, Bousfiha A, Casanova JL, Chatila T, Conley ME, et al. Primary Immunodeficiency Diseases: An Update on the Classification From the International Union of Immunological Societies Expert Committee for Primary Immunodeficiency 2015. J Clin Immunol (2015) 35(8):696–726. doi: 10.1007/s10875-015-0201-1
34. Petrova E, Hovnanian A. Advances in Understanding of Netherton Syndrome and Therapeutic Implications. Expert Opin Orphan Drugs (2020) 8(11):455–87. doi: 10.1080/21678707.2020.1857724
35. Maatouk I, Moutran R, Tomb R. Narrowband Ultraviolet B Phototherapy Associated With Improvement in Netherton Syndrome. Clin Exp Dermatol (2012) 37(4):364–6. doi: 10.1111/j.1365-2230.2011.04231.x
36. Yan AC, Honig PJ, Ming ME, Weber J, Shah KN. The Safety and Efficacy of Pimecrolimus, 1%, Cream for the Treatment of Netherton Syndrome: Results From an Exploratory Study. Arch Dermatol (2010) 146(1):57–62. doi: 10.1001/archdermatol.2009.326
37. Caputo R, Vanotti P, Bertani E. Netherton’s Syndrome in Two Adult Brothers. Arch Dermatol (1984) 120(2):220–2. doi: 10.1001/archderm.120.2.220
38. Greene SL, Muller SA. Netherton’s Syndrome. Report of a Case and Review of the Literature. J Am Acad Dermatol (1985) 13(2 Pt 2):329–37. doi: 10.1016/S0190-9622(85)70170-3
39. Groves S, Dezfoulian Bh, Bonardeaux C, de la Brassinne M. Netherton’s Syndrome in Two Sisters. A Ten Year Experience of Therapy With Retinoids. J Eur Acad Dermatol Venereol (1995) 5:173–6. doi: 10.1111/j.1468-3083.1995.tb00540.x
40. Luchsinger I, Knopfel N, Theiler M, Bonnet des Claustres M, Barbieux C, Schwieger-Briel A, et al. Secukinumab Therapy for Netherton Syndrome. JAMA Dermatol (2020) 156(8):907–11. doi: 10.1001/jamadermatol.2020.1019
41. Murase C, Takeichi T, Taki T, Yoshikawa T, Suzuki A, Ogi T, et al. Successful Dupilumab Treatment for Ichthyotic and Atopic Features of Netherton Syndrome. J Dermatol Sci (2021) 102(2):126–9. doi: 10.1016/j.jdermsci.2021.03.003
42. Barbati F, Giovannini M, Oranges T, Lodi L, Barni S, Novembre E, et al. Netherton Syndrome in Children: Management and Future Perspectives. Front Pediatr (2021) 9:645259. doi: 10.3389/fped.2021.645259
43. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-A Web and Mobile App for Systematic Reviews. Syst Rev (2016) 5(1):210. doi: 10.1186/s13643-016-0384-4
44. Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetcu R, et al. Chapter 7: Systematic Reviews of Etiology and Risk. In: Aromataris E, Munn Z, editors. JBI Manual for Evidence Synthesis. Adelaide: JBI (2020). Available at: https://synthesismanual.jbi.global. doi: 10.46658/JBIMES-20-08
45. Murad MH, Mustafa RA, Schunemann HJ, Sultan S, Santesso N. Rating the Certainty in Evidence in the Absence of a Single Estimate of Effect. Evid Based Med (2017) 22(3):85–7. doi: 10.1136/ebmed-2017-110668
46. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PloS Med (2009) 6(7):e1000097. doi: 10.1371/journal.pmed.1000097
47. Fritsch P. Austrian Experience With Etretinate. In: Cunliffe WJ, Milller AJ, editors. Retinoid Therapy: A Review of Clinical and Laboratory Research. The Proceedings of an International Conference, Held in London, 16-18 May 1983, Lancaster; Boston: MTP Press (1984). pp. 45–54.
48. Traupe H, Happle R. Etretinate Therapy in Children With Severe Keratinization Defects. Eur J Pediatr (1985) 143(3):166–9. doi: 10.1007/BF00442128
49. Hausser I, Anton-Lamprecht I, Hartschuh W, Petzoldt D. Netherton’s Syndrome: Ultrastructure of the Active Lesion Under Retinoid Therapy. Arch Dermatol Res (1989) 281(3):165–72. doi: 10.1007/BF00456387
50. Braun RP, Ramelet AA. Failure of Cyclosporine in Netherton’s Syndrome. Dermatology (1997) 195(1):75. doi: 10.1159/000245696
51. El Shabrawi-Caelen L, Smolle J, Metze D, Ginter-Hanselmayer G, Raghunath M, Traupe H, et al. Generalized Exfoliative Erythroderma Since Birth. Netherton Syndrome. Arch Dermatol (2004) 140(10):1275–80. doi: 10.1001/archderm.140.10.1275-a
52. Lazaridou E, Apalla Z, Patsatsi A, Trigoni A, Ioannides D. Netherton’s Syndrome: Successful Treatment With Isotretinoin. J Eur Acad Dermatol Venereol (2009) 23(2):210–2. doi: 10.1111/j.1468-3083.2008.02795.x
53. Fontao L, Laffitte E, Briot A, Kaya G, Roux-Lombard P, Fraitag S, et al. Infliximab Infusions for Netherton Syndrome: Sustained Clinical Improvement Correlates With a Reduction of Thymic Stromal Lymphopoietin Levels in the Skin. J Invest Dermatol (2011) 131(9):1947–50. doi: 10.1038/jid.2011.124
54. Gallagher JL, Patel NC. Subcutaneous Immunoglobulin Replacement Therapy With Hizentra(R) Is Safe and Effective in Two Infants. J Clin Immunol (2012) 32(3):474–6. doi: 10.1007/s10875-011-9645-0
55. Small AM, Cordoro KM. Netherton Syndrome Mimicking Pustular Psoriasis: Clinical Implications and Response to Intravenous Immunoglobulin. Pediatr Dermatol (2016) 33(3):e222–3. doi: 10.1111/pde.12856
56. Yalcin AD. A Case of Netherton Syndrome: Successful Treatment With Omalizumab and Pulse Prednisolone and Its Effects on Cytokines and Immunoglobulin Levels. Immunopharmacol Immunotoxicol (2016) 38(2):162–6. doi: 10.3109/08923973.2015.1115518
57. Roda A, Mendonca-Sanches M, Travassos AR, Soares-de-Almeida L, Metze D. Infliximab Therapy for Netherton Syndrome: A Case Report. JAAD Case Rep (2017) 3(6):550–2. doi: 10.1016/j.jdcr.2017.07.019
58. Leung AKC, Barankin B, Leong KF. An 8-Year-Old Child With Delayed Diagnosis of Netherton Syndrome. Case Rep Pediatr (2018) 2018:9434916. doi: 10.1155/2018/9434916
59. Onnis G, Chiaverini C, Hickman G, Dreyfus I, Fischer J, Bourrat E, et al. Alitretinoin Reduces Erythema in Inherited Ichthyosis. Orphanet J Rare Dis (2018) 13(1):46. doi: 10.1186/s13023-018-0783-9
60. Ozyurt K, Atasoy M, Ertas R, Ulas Y, Akkus MR, Kiraz A, et al. Netherton Syndrome Previously Misdiagnosed as Hyper IgE Syndrome Caused by a Probable Mutation in SPINK5 C. Turk J Pediatr (2019) 61(4):604–7. doi: 10.24953/turkjped.2019.04.020
61. Yadav N, Madke B, Kar S, Gangane N. Netherton Syndrome: An Atypical Presentation. Cutis (2019) 103(4):E27–9.
62. Aktas M, Salman A, Apti Sengun O, Comert Ozer E, Hosgoren Tekin S, Akin Cakici O, et al. Netherton Syndrome: Temporary Response to Dupilumab. Pediatr Dermatol (2020) 37(6):1210–1. doi: 10.1111/pde.14362
63. Andreasen TH, Karstensen HG, Duno M, Lei U, Zachariae C, Thyssen JP. Successful Treatment With Dupilumab of an Adult With Netherton Syndrome. Clin Exp Dermatol (2020) 45(7):915–7. doi: 10.1111/ced.14317
64. Blanchard SK, Prose NS. Successful Use of Secukinumab in Netherton Syndrome. JAAD Case Rep (2020) 6(6):577–8. doi: 10.1016/j.jdcr.2020.04.025
65. Barbieux C, Bonnet des Claustres M, de la Brassinne M, Bricteux G, Bagot M, Bourrat E, et al. Duality of Netherton Syndrome Manifestations and Response to Ixekizumab. J Am Acad Dermatol (2021) 84(5):1476–80. doi: 10.1016/j.jaad.2020.07.054
66. Dabas G, Mahajan R, De D, Handa S, Kumar R, Dayal D, et al. Managing Syndromic Congenital Ichthyosis at a Tertiary Care Institute-Genotype-Phenotype Correlations, and Novel Treatments. Dermatol Ther (2020) 33(6):e13816. doi: 10.1111/dth.13816
67. Luchsinger I, Vogler T, Schwieger-Briel A, Knopfel N, Walchli R, Weibel L, et al. Safe and Effective Use of Alitretinoin in Children With Recalcitrant Hand Eczema and Other Dermatoses - A Retrospective Analysis. J Eur Acad Dermatol Venereol (2020) 34(5):1037–42. doi: 10.1111/jdv.16088
68. Orlova E, Smirnova L, Grabovskaya O, Kayumova L. Netherton Syndrome in Combination With Iron-Deficiency Anemia. J Glob Pharma Technol (2020) 12(1):12–21.
69. Steuer AB, Cohen DE. Treatment of Netherton Syndrome With Dupilumab. JAMA Dermatol (2020) 156(3):350–1. doi: 10.1001/jamadermatol.2019.4608
70. Sussmuth K, Traupe H, Loser K, Stander S, Kessel C, Wittkowski H, et al. Response to Dupilumab in Two Children With Netherton Syndrome: Improvement of Pruritus and Scaling. J Eur Acad Dermatol Venereol (2021) 35(2):e152–e5. doi: 10.1111/jdv.16883
71. Volc S, Maier L, Gritsch A, Aichelburg MC, Volc-Platzer B. Successful Treatment of Netherton Syndrome With Ustekinumab in a 15-Year-Old Girl. Br J Dermatol (2020) 183(1):165–7. doi: 10.1111/bjd.18892
72. Zelieskova M, Banovcin P, Kozar M, Kozarova A, Nudzajova Z, Jesenak M. A Novel SPINK5 Mutation and Successful Subcutaneous Immunoglobulin Replacement Therapy in a Child With Netherton Syndrome. Pediatr Dermatol (2020) 37(6):1202–4. doi: 10.1111/pde.14318
73. Cicek F, Cekic S, Kilic SS. Infliximab Therapy in an Infant With Netherton Syndrome. Pediatr Dermatol (2021) 38(3):714–6. doi: 10.1111/pde.14590
74. Zhang Z, Pan C, Wei R, Li H, Yang Y, Chen J, et al. Netherton Syndrome Caused by Compound Heterozygous Mutation, C.80A>G Mutation in SPINK5 and Large-Sized Genomic Deletion Mutation, and Successful Treatment of Intravenous Immunoglobulin. Mol Genet Genomic Med (2021) 9(3):e1600. doi: 10.1002/mgg3.1600
75. Versteegh JJ, Dulfer K, Stuvel K, Pasmans SG, Utens EM. Netherton Syndrome; Neuropsychological and Psychosocial Functioning of Child and Adult Patients and Their Parents. J Health Psychol (2020) 25(13-14):2296–316. doi: 10.1177/1359105318790052
76. Torma H. Regulation of Keratin Expression by Retinoids. Dermatoendocrinol (2011) 3(3):136–40. doi: 10.4161/derm.3.3.15026
77. Vahlquist A, Duvic M eds. Retinoids and Carotenoids in Dermatology. 1st Ed. Boca Raton: CRC Press (2007). doi: 10.3109/9781420021189
78. Chavanas S, Garner C, Bodemer C, Ali M, Teillac DH, Wilkinson J, et al. Localization of the Netherton Syndrome Gene to Chromosome 5q32, by Linkage Analysis and Homozygosity Mapping. Am J Hum Genet (2000) 66(3):914–21. doi: 10.1086/302824
79. Czock D, Keller F, Rasche FM, Haussler U. Pharmacokinetics and Pharmacodynamics of Systemically Administered Glucocorticoids. Clin Pharmacokinet (2005) 44(1):61–98. doi: 10.2165/00003088-200544010-00003
80. Becker DE. Basic and Clinical Pharmacology of Glucocorticosteroids. Anesth Prog (2013) 60(1):25–31; quiz 2. doi: 10.2344/0003-3006-60.1.25
81. Kauvar AB, Stiller MJ. Cyclosporine in Dermatology: Pharmacology and Clinical Use. Int J Dermatol (1994) 33(2):86–96. doi: 10.1111/j.1365-4362.1994.tb01533.x
82. De Ranieri D, Fenny NS. Intravenous Immunoglobulin in the Treatment of Primary Immunodeficiency Diseases. Pediatr Ann (2017) 46(1):e8–12. doi: 10.3928/19382359-20161213-03
83. Gurcan HM, Ahmed AR. Efficacy of Various Intravenous Immunoglobulin Therapy Protocols in Autoimmune and Chronic Inflammatory Disorders. Ann Pharmacother (2007) 41(5):812–23. doi: 10.1345/aph.1K037
84. Perez EE, Orange JS, Bonilla F, Chinen J, Chinn IK, Dorsey M, et al. Update on the Use of Immunoglobulin in Human Disease: A Review of Evidence. J Allergy Clin Immunol (2017) 139(3S):S1–S46. doi: 10.1016/j.jaci.2016.09.023
85. Kaveri SV, Maddur MS, Hegde P, Lacroix-Desmazes S, Bayry J. Intravenous Immunoglobulins in Immunodeficiencies: More Than Mere Replacement Therapy. Clin Exp Immunol (2011) 164(Suppl 2):2–5. doi: 10.1111/j.1365-2249.2011.04387.x
86. Sriaroon P, Ballow M. Immunoglobulin Replacement Therapy for Primary Immunodeficiency. Immunol Allergy Clin North Am (2015) 35(4):713–30. doi: 10.1016/j.iac.2015.07.006
87. Malik K, He H, Huynh TN, Tran G, Mueller K, Doytcheva K, et al. Ichthyosis Molecular Fingerprinting Shows Profound TH17 Skewing and a Unique Barrier Genomic Signature. J Allergy Clin Immunol (2019) 143(2):604–18. doi: 10.1016/j.jaci.2018.03.021
88. Konishi T, Tsuda T, Sakaguchi Y, Imai Y, Ito T, Hirota S, et al. Upregulation of Interleukin-33 in the Epidermis of Two Japanese Patients With Netherton Syndrome. J Dermatol (2014) 41(3):258–61. doi: 10.1111/1346-8138.12410
89. Van Gysel D, Koning H, Baert MR, Savelkoul HF, Neijens HJ, Oranje AP. Clinico-Immunological Heterogeneity in Comel-Netherton Syndrome. Dermatology (2001) 202(2):99–107. doi: 10.1159/000051607
90. Paller A. The Efficacy and Safety of Secukinumab in Patients With Ichthyoses (2017). ClinicalTrials.gov. Available at: https://clinicaltrials.gov/ct2/show/study/NCT03041038 (Accessed January 24, 2022).
91. Mazereeuw-Hautier J. A Pilot Study of the Efficacy and Safety of Dupilumab Versus Placebo in Patients With Netherton Syndrome (NS-DUPI) (2020). ClinicalTrials.gov. Available at: https://clinicaltrials.gov/ct2/show/NCT04244006 (Accessed January 24, 2022).
Keywords: systematic review, Netherton syndrome, Ichthyosis linearis circumflexa, SPINK5, erythroderma, skin disease, ichthyosis, therapy
Citation: Nouwen AEM, Schappin R, Nguyen NT, Ragamin A, Bygum A, Bodemer C, Dalm VASH and Pasmans SGMA (2022) Outcomes of Systemic Treatment in Children and Adults With Netherton Syndrome: A Systematic Review. Front. Immunol. 13:864449. doi: 10.3389/fimmu.2022.864449
Received: 28 January 2022; Accepted: 07 March 2022;
Published: 30 March 2022.
Edited by:
Sara Sebnem Kilic, Uludag University, TurkeyReviewed by:
Hugo Chapdelaine, Montreal Clinical Research Institute (IRCM), CanadaCopyright © 2022 Nouwen, Schappin, Nguyen, Ragamin, Bygum, Bodemer, Dalm and Pasmans. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Suzanne G. M. A. Pasmans, cy5wYXNtYW5zQGVyYXNtdXNtYy5ubA==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.