- 1Department of Clinical Biochemistry, Sher-I-Kashmir Institute of Medical Sciences, Srinagar, JK, India
- 2Department of Microbiology, Government Medical College, Srinagar, JK, India
- 3Department of Ophthalmology, Government Medical College, Jammu, JK, India
- 4Department of Biochemistry, Maharishi Markandeshwar Institute of Medical Sciences and Research (MMIMSR), Ambala, HR, India
- 5Department of General Medicine, Sher-I-Kashmir Institute of Medical Sciences, Srinagar, JK, India
- 6Advanced Centre for Human Genetics, Sher-I-Kashmir Institute of Medical Sciences, Srinagar, JK, India
We systematically reviewed and summarized studies focusing on Bharat Biotech’s Whole Virion Inactivated Corona Virus Antigen BBV152 (Covaxin), which is India’s indigenous response to fighting the SARS-CoV-2 pandemic. Studies were searched for data on the efficacy, immunogenicity, and safety profile of BBV152. All relevant studies published up to March 22, 2022, were screened from major databases, and 25 studies were eventually inducted into the systematic review. The studies focused on the virus antigen (6 μg) adjuvanted with aluminium hydroxide gel and/or Imidazo quinolin gallamide (IMDG), aTLR7/8 agonist. Pre-clinical, phase I, and II clinical trials showed appreciable immunogenicity. Both neutralizing and binding antibody titers were significant and T cell responses were Th1-biased. Phase III trials on the 6 μg +Algel-IMDG formulation showed a 93.4% efficacy against severe COVID-19. Data from the trials revealed an acceptable safety profile with mostly mild-moderate local and systemic adverse events. No serious adverse events or fatalities were seen, and most studies reported milder and lesser adverse events with Covaxin when compared with other vaccines, especially Oxford-Astra Zeneca’s AZD1222 (Covishield). The immunogenicity performance of Covaxin, which provided significant protection only after the second dose, was mediocre and it was consistently surpassed by Covishield. One study reported adjusted effectiveness against symptomatic infection to be just 50% at 2 weeks after the second dose. Nonetheless, appreciable results were seen in previously infected individuals administered both doses. There was some evidence of coverage against the Alpha, Beta, and Delta variants. However, neither Covaxin nor Covishield showed sufficient protection against the Omicron variant. Two studies reported super-additive results on mixing Covaxin with Covishield. Further exploration of heterologous prime-boost vaccination with a combination of an inactivated vaccine and an adenoviral vector-based vaccine for tackling future variants may be beneficial.
Introduction
With SARS-CoV-2 infections affecting more than half a billion and resulting in around 6.3 million deaths at the time of writing, more than 220 countries faced a monumental task in combating the devastation inflicted by this virus (1). Extensive vaccination efforts were initiated and successfully concluded all over the world in record times. Unfortunately, the huge number of cases of SARS-CoV-2 worldwide provided fertile ground for genomic changes precipitating the emergence of newer variants, such as the Alpha variant (B.1.1.7), which emerged in the UK, Beta variant (B.1.351) in South Africa, Gamma (P.1) in Brazil, Epsilon (B.1.429) emerging from California, and Iota (B.1.526) in New York. The phenomenal increase in SARS-CoV-2 infections in the Indian subcontinent during the second wave and the flouting of social distancing norms at religious and political gatherings provided the opportunity for the emergence of new variants, the Delta and Kappa (1.617.1 and 1.617.2, respectively) emerging in the country around December 2020 (2).
Bharat Biotech’s Covaxin (BBV152) was the second-most administered vaccine in India at 327 million doses received at the time of writing (3). It is a β-propiolactone inactivated vaccine (4). The inactivated whole-virion structure is combined with an adjuvant, Imidazo -quinoline gallamide, which is a toll-like receptor 7/8 agonist molecule adsorbed to alum (Algel-IMDG). The formulation improves homing of vaccine antigen onto draining lymph nodes without systemic spillage. A genetically stable strain NIV-2020-770 containing the Asp614Gly mutation used for making Covaxin was isolated from an asymptomatic SARS-CoV-2 positive patient at the National Institute of Virology (NIV), Pune (5). Biosafety level 3 (BSL3) manufacturing facilities with a vero cell manufacturing platform were utilized in the manufacturing process (6). The strain used is 99.7% identical to Wuhan Hu-1 (7). Five to 10% newborn calf serum in Dulbecco’s Modified Eagle medium (DMEM) was used to grow Vero CCL-81 cells in tissue culture flasks and cell stacks. Further virus propagation was achieved in bioreactors which maintained a temperature of 36 ± 1°C. Harvesting was done at 36-72 h post-infection and supernatants were processed. Additional purification and concentration were done by column chromatography and a tangential flow filtration system, respectively (8). The purified final bulk obtained from the inactivation procedure has been found to contain spike and nucleocapsid protein. Transmission electron microscopy (TEM) shows intact, oval structures with the characteristic crown shape (7). Covaxin got early approval from Indian Drug regulatory agencies (9). Studies on vaccine safety have always presented challenges. Antibody-dependent enhancement (ADE) has been a worrying concern with inactivated vaccines and the changes in conformations of spike proteins on inactivation with β-propiolactone may be a cause for concern (10). Autoimmune glomerular disorders have been reported 2 weeks after vaccination with Covaxin (11). A case of Cutaneous small-vessel vasculitis was reported 5 days after inoculation (12). There was also a report of varicella zoster reactivation in a 72-year-old woman a week after receiving the vaccine (13). Coronary thromboembolic phenomena have also been seen, though on a much lesser scale compared to other vaccines (14). Nonetheless, the WHO accorded Emergency use listing (EUL) approval to Covaxin on 3 November 2021 after several delays, its Strategic Advisory Group of Experts on Immunization (SAGE) having previously recommended two doses spaced 4 weeks apart in all adults (15). Several South American and African nations have also been using it in their programs, though not without reservations (16, 17).
We aim to systematically review the overall efficacy, immunogenicity, and safety of BBV152 Covaxin vaccine, which could potentially guide public health policy in relation to combating the threat posed by SARS-CoV-2, especially in those countries that are actively considering adding it to their regimens.
Methods
Search strategy
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses Statement (PRISMA) recommendations were followed in this analysis. (Figure 1) A systematic literature search with no language restriction was performed in electronic databases, including PubMed, Google Scholar, Directory of Open Access Journals (DOAJ), as well as Lancet, to identify eligible studies published up to March 22, 2022. The search strategy was based on the following keywords and MeSH terms: “BBV152”, “Covaxin”, “vaccine”, “vaccination”, “safety” and “efficacy”. Reference lists of selected studies were also screened. In addition, internet engines were utilized to search for web pages that might have references of interest.
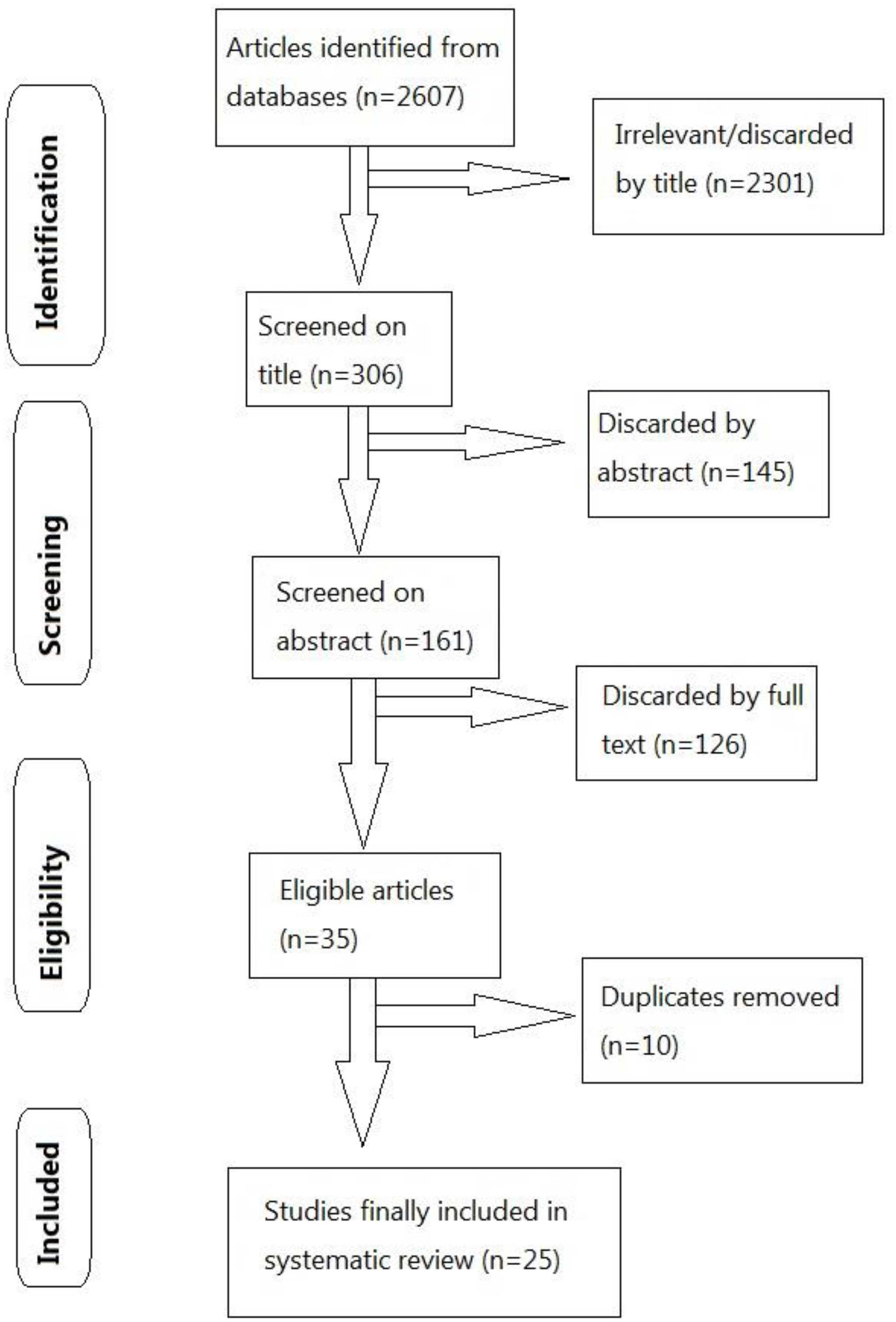
Figure 1 Flow diagram utilizing PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) for study selection.
Study selection
Two investigators (TIA and SR) independently performed the literature screening to identify eligible studies. Studies eligible for inclusion were studies of any design (case–control, case–cohort, prospective cohort, randomized control trials, cross-sectional, human, as well as non-human studies), which reported the effectiveness of the BBV152 vaccine to prevent reverse transcription–polymerase chain reaction (RT-PCR) confirmed COVID-19 (through comparison between vaccinated and unvaccinated individuals) and adjusted for covariates. For multiple studies based on the same data, or where preprints were succeeded by publication in indexed journals, the most recent ones were mentioned. Studies involving heterologous administration of BBV152 with other vaccines had interesting results and were included. One study focusing on chemokine and cytokine subsets elicited was not excluded. Studies comparing BBV152 with other vaccines or involving individuals previously infected with SARS-CoV-2 were included.
Exclusion criteria
We excluded studies that reported unadjusted effectiveness estimates, or which did not use RT-PCR to confirm the diagnosis of COVID-19. Uncorrected manuscripts and pre-prints were not included. One study focusing on breakthrough infections had to be excluded as data for both Covaxin and another vaccine codenamed AZD1222 (brand name Covishield) were grouped together and individual data for Covaxin could not be retrieved (18). Two other studies also had to be excluded due to a lack of Covaxin-specific subgroup analysis (19, 20).
Studies were presented chronologically wherever possible.
We had three outcomes of interest, BBV-152
(a) vaccine efficacy, which is defined as ‘a proportionate reduction in disease attack rate (AR) between the unvaccinated (ARU) and vaccinated (ARV) study cohorts’ (21),
(b) immunogenicity of the vaccine, measured by estimating either binding antibodies by Enzyme-linked immunosorbent assays (ELISA) or Neutralizing Antibodies (NAb), by plaque reduction neutralization assays (PRNT90 or PRNT50), focus reduction neutralization titer (FRNT50), or microneutralization assay (MNT50), as well as cytokine and chemokine profiles, and
(c) vaccine safety.
Studies examining either efficacy, immunogenicity, or safety, or any combination of the three, were included. Each included study was studied independently by two investigators (TIA and SR), who also obtained details of the same under the headings of first author’s surname, study design, sample population and subgroups, number of participants, the incidence of COVID-19 (either asymptomatic, symptomatic with more than one grade of severity) in both vaccinated and unvaccinated individuals, and adjusted vaccine effectiveness estimates and covariates. Two investigators (SM and SI) utilized the Newcastle-Ottawa Scale to gauge the quality of included observational studies, and a score >7 was considered high quality and suitable for inclusion (22).
Results
A total of 25 articles met inclusion and quality criteria. Of these, three focused on efficacy, 19 examined immunogenicity, 10 included safety assessments. Three were animal-based studies. Full papers were assessed. Except for two articles based in Iran, all the studies were conducted in India. The articles were published in English between December 2020 and March 22, 2022. Several of the studies had the involvement of employees of Bharat Biotech, the company producing Covaxin.
Discussion
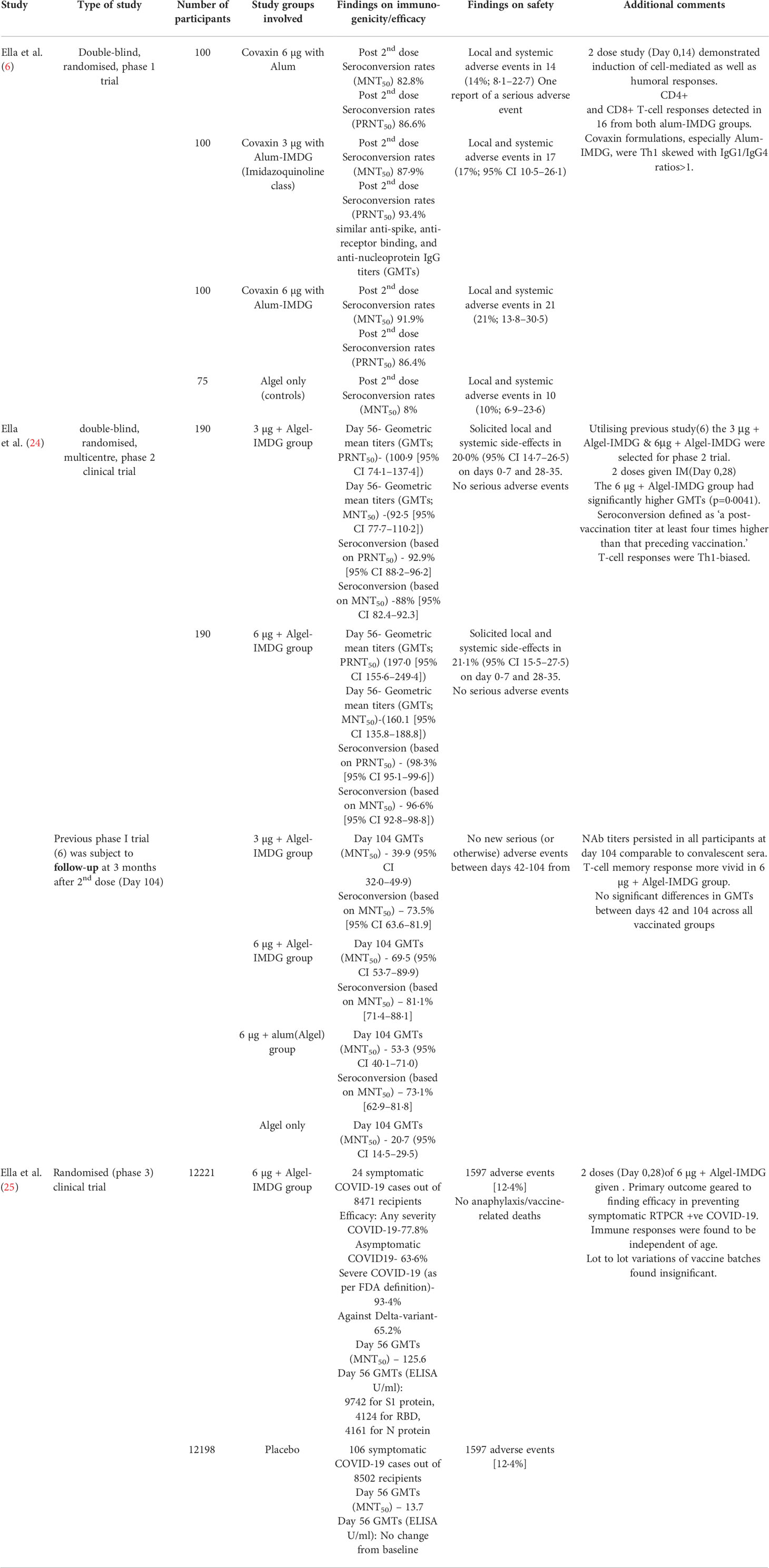
Inactivated vaccines have been traditionally used successfully for protection against historically notorious diseases like smallpox, polio, rabies, among others. Theoretically, the intact yet inactivated pathogen elicits a broader immune response compared to other platforms. Epitope coverage of inactivated vaccines is extensive and less prone to circumvention by newer variants (8). The pre-clinical animal studies on the Syrian Hamsters and Rhesus macaques suggested satisfactory efficacy and dose-sparing effect of the 3 µg BBV152 vaccine + Algel-IMDG (in comparison to the 6 μg + Algel-IMDG and other combinations). These animal studies showed high Nab titers, displayed prompt viral clearance from the lower respiratory tract, and displayed no evidence of radiographic abnormalities by the 7th day post-inoculation (8, 23). In the third animal study on three species, 100% seroconversion was observed in 21 days and peak titers were seen on day 28. This study showed sufficient levels of binding Ab and Nab lasting up to 98 days after the first dose. The reliability of the Algel-IMDG adjuvant was also established, as it was found to be non-mutagenic and induced Th1-biased antibody response (7).
The phase I trials presented a Th1-skewed response. Post second dose Seroconversion rates of both the Algel-IMDG adjuvanted combinations of 3 μg and 6 μg were found to be comparable (6). Both cell-mediated and humoral immunity appeared to be sufficiently stimulated. An impressive safety profile with local and systemic adverse events in the range of 17%-21% (none severe) paved the way for phase II trials. In both the phase II trials as well as the follow-up to the phase I trials, the 6 µg + Algel-IMDG combination was found to have the highest immunogenicity and was eventually selected for phase III trials (Table 1) (24). These trials revealed a 93.4% efficacy against severe COVID-19, although efficacy against the Delta variant was lower at 65.2%. There were minimal lot-to-lot variations and the safety profile was similar to placebo (25). Notwithstanding the impressive safety profile (just 12.4% adverse events) established in the trials, an Iranian study reported 92.9% incidence of adverse events, with most of the complaints being of injection site pain. However, the Iranian study included only 42 Covaxin recipients compared to the much larger number in phase III trials. Also, similarly high adverse events were reported for the other vaccines, Oxford-Astra Zeneca’s AZD1222(Covishield) and Sputnik V, included in the study (10). The COVAT study showed an increased incidence of mild-moderate adverse events after the first dose in Covaxin (31.2%) compared to second dose (11.1%) and a 2.2% breakthrough infection rate. These rates were found to be better than that seen with AZD1222 recipients, who experienced 46.7% adverse events after the first dose, 18.1% after the second dose, and 5.5% breakthrough events (26). Basavaraja et al. also reported lower incidence rates for Covaxin compared to Covishield, mostly related to vaccination anxiety (27). On the other hand, on comparing two equally sized groups, Choudhary et al. reported higher breakthrough events with Covaxin compared to Covishield (28). Sharma et al. also reported a slightly higher breakthrough infection rate with Covaxin compared to Covishield (29). However, in the COVAT follow-up studies, breakthrough infection rates were similar for the two vaccines (30). Incidence rates varied from the high values reported by the Iranian study to the much lower values of 0.57% reported by Basavaraja et al. (27) The latter utilized spontaneous reporting of adverse events and supervised assessment was conducted for only 30 minutes, which might have resulted in under-reporting of any events occurring after that period. In another Iranian study, adenovirus-vector-based recipients, especially those getting Covishield, reported more numerous and intense side effects compared to vaccinees receiving inactivated formulations. This was attributed to the increased elicitation of cytokine/chemokine responses in the viral vector vaccines (31). Suffice to mention that no serious adverse events or deaths were reported with BBV152 in any study we included, and injection site pain was the decisively predominant adverse event.
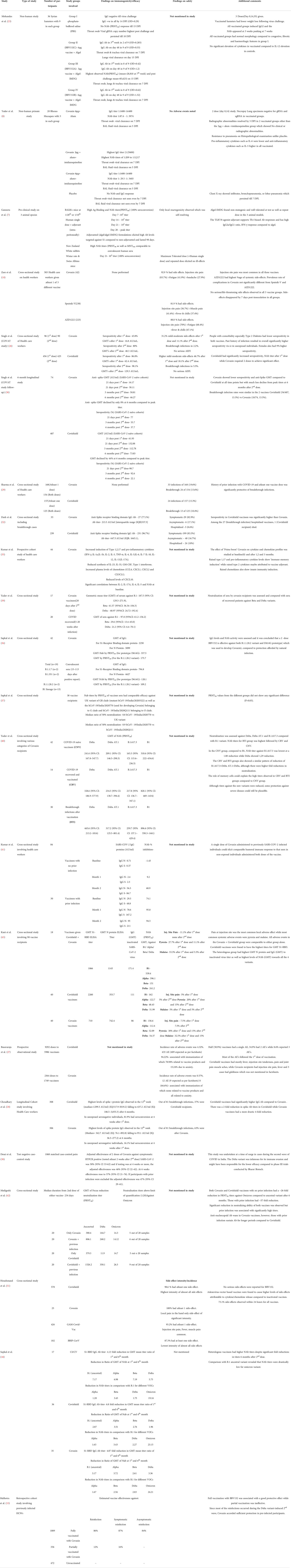
Immunogenicity assessments of Covaxin did not usually outperform that observed with other vaccines. In the COVAT study, Covishield surpassed BBV152 in the observed NAb titers and seropositivity rates after the first dose itself, which was barely equaled even after two doses of Covaxin had been administered (Table 2). Among fully vaccinated recipients, Covaxin could elicit an anti-spike Ab geometric mean titer (GMT) of only 48.3 AU/ml, which was less than half that observed with Covishield. The former also elicited a seropositivity of anti-Spike Ab of just 80% compared to 98.1% observed in the latter (26). This superiority in seroprevalence and peak GMT of Covishield was maintained at all time points from 1 to 6 months after the second dose, as seen in the follow-up to the COVAT study. Nonetheless, the declines in peak values were just as rapid, so that by the end of 6 months post the second dose, there was a narrowing of the Ab titer gap between the two vaccines. While Covishield showed a peak of almost 100% seropositivity 3 weeks after the second dose, Covaxin showed peak seropositivity of less than 80% (30). Dash et al. similarly reported higher seropositivity rates with Covishield, the IgG titers against Spike protein being three times that seen with Covaxin. However, they also reported a breakthrough infection related fatality with Covishield (32). The superiority of Covishield over BBV152 was again demonstrated by Choudhary et al., which showed several-fold higher elicitation of spike protein IgG by the former vaccine over the latter. The study noted a four-fold reduction in spike protein Ab titers at 6 months after the second dose for Covaxin, while Covishield showed only a two-fold reduction, which was at variance with the COVAT follow-up results. However, Choudhary et al. had a much higher number of vaccinees receiving Covaxin compared to the COVAT study, so could be considered more reliable. Both studies, however, agree on the consistently higher titers of Covishield at all time points. Additionally, in previously unexposed seronegative individuals, an 81.9% seroconversion at 4 weeks after the first dose was observed with Covishield compared to just 16.1% with Covaxin (28). This was also proven in the study by Malhotra et al., which reported poor immunogenicity in individuals partially immunized with BBV152. Unlike Covishield, where antibody titers started peaking quickly after the first dose itself, it required at least two doses for Covaxin to be anywhere near as effective. In participants with prior viral exposure, two doses of BBV152 accorded sufficient protection, with an 87% efficiency against symptomatic reinfection, while a single dose was only 16% effective (43). A questionnaire-based study of health-care workers reported significantly reduced incidence and severity of COVID-19 infection in those receiving two doses of both Covaxin and Covishield compared to a single dose of either vaccine (44). Kumar et al. demonstrated the induction of innate, adaptive immune responses, as well as cytokine and chemokine induction. However, these responses were only observed after the second dose and lasted for 3 months, thus explaining the delayed peak of Covaxin action (33).
Response of Covaxin against SARS-CoV-2 variants
Sufficient action of vaccines against newer variants is essential to reduce mortality and control the spread of infection to manageable levels. The ameliorative action of Covaxin against several variants has been tested. A study by Sapkal et al. reported higher GMT of IgG for S1-receptor binding domain protein as well as higher NAb GMT for the B.1.1.28.2 and D614G strains in Covaxin vaccinee sera compared to convalescent sera (35). In another study by Sapkal et al., NAb titers of vaccinee sera had comparable efficacies against GR, G, and O clades of SARS-CoV-2 and could effectively neutralize the Alpha variant (36). Although BBV152 elicited comparatively reduced titers against the Delta and other newer variants, some rudimentary protection was still afforded. Desai et al. undertook their study at the peak of the second wave in India, probably triggered by the evasive Delta variant. They found an adjusted effectiveness against symptomatic infection to be 50% at 2 weeks after the second dose of BBV152, which rose to a somewhat reasonable figure of 57% at 6 weeks. A strong Th1 bias also allayed fears of serious adverse events (40). Ella et al. had reported efficacy of 65.2% against the Delta variant in the phase 3 trials (25). The study by Malhotra et al. conducted during the wave triggered by the Delta variant reported significant effectiveness (86%) of a two-dose Covaxin regimen (43) Yadav et al. observed neutralization of sera by Covaxin recipients in comparison with those of recovered patients and observed significantly higher levels of GMT against ancestral (B.1), Beta and Delta variants in vaccinees in comparison to the unvaccinated suggesting a somewhat ample coverage of these variants by the vaccine (34). In another study by the same author, action of Covaxin against B.1, Delta, Delta AY.1, and 1.617.3 was assessed, and it was inferred that a milder level of protection was nonetheless afforded by the vaccine against the newer variants (37). Higher titers were also observed in vaccinees who had been previously infected compared to vaccinees without any prior exposure. In fact, a significant humoral response was also observed by Kumar et al., who observed that a single dose of BBV152 administered to previously infected individuals had comparable effectiveness to non-exposed vaccinees administered both doses. IgG Ab against Spike proteins in individuals administered a single dose of Covaxin were markedly elevated (28 days after the first dose) at 167.2 AU/ml in recipients with prior viral exposure in comparison to just 2.3 AU/ml in those with no prior infections. However, the difference in titers between the two groups was less significant after two doses. Kumar et al. thus advocated saving on valuable vaccine doses by giving only a single dose of Covaxin to previously infected individuals; instead reserving the two-dose regimen for non-exposed individuals (38).
Response of Covaxin against the B.1.1.529 (Omicron) variant
Covaxin acted poorly against the B.1.1.529 variant and consequently, immune escape appeared widespread. Covishield fared no better. Despite extensive coverage of vaccination campaigns in the Indian subcontinent utilizing both the above vaccines, there were widespread incidences of breakthrough infections and reinfections (Table 2). Recipients of both vaccines with no prior virus exposure had a ~26-fold reduction in neutralization titers (FRNT50) against Omicron compared to the ancestral variant, 6 months after the second dose. However, those who had a history of prior exposure to infection had significantly higher titers, albeit these subsided twice as rapidly. Interestingly, Covaxin recipients sustained anti-nucleocapsid antibodies for longer periods as compared to Covishield (41).
Heterologous prime boost vaccination
The study by Kant et al. observing the serendipitous ‘mix and match’ of Covaxin and Covishield reported the lowest Geometric mean titers (GMT) for both the S1-receptor binding domain antibodies as well as antibodies to the inactivated virus with Covaxin. However, neutralizing antibodies against B1, Alpha, Beta, and Delta were comparable to that observed with Covishield. Interestingly, mixing the two vaccines yielded better results than either vaccine taken alone. The heterologous group reported the highest titers for the N (nucleocapsid) protein and IgG to the inactivated virus. NAb’s against the four variants were also significantly higher than that seen in homologous groups. Nonetheless, neutralization of the sera of BBV152 vaccinees measured in geometric mean titer against the B1, Beta, and Delta variants was significantly higher than that seen with sera from recovered patients (39). Sapkal et al. assessed sera of vaccinees who had received heterologous vaccination (first dose Covishield, second dose Covaxin) and despite significant-fold reductions in GMT of NAb 6 months after the second dose, the heterologous group had consistently higher titers compared to the groups receiving homologous vaccination (either Covishield or Covaxin). NAb titers against the Omicron variant were remarkably reduced for both heterologous/homologous vaccination compared to ancestral, Alpha, Beta, and Delta variants. Nonetheless, heterologous vaccination was immunogenically superior to the homologous mode of vaccination (42).
Heterologous prime-boost vaccination was similarly encouraged by other studies which claimed higher inductions of immunogenicity with combinations of vector-based + inactivated vaccines, which suggests great scope for such regimens in tackling newer variants (45).
Conclusion
After a perusal of the studies included in the systematic review, the authors found the safety profile of Covaxin to be satisfactory and comparable with data from other vaccines, most of the complaints being of injection site pain. A study reported milder adverse effect profile of inactivated vaccines such as Covaxin compared to viral-vector-based ones. Although some studies reported slightly more breakthrough infections with the vaccine compared to other candidates, none of the studies reported any serious/severe adverse events or fatalities. Immunogenicity performance of BBV152, albeit higher than the natural immunity of recovered patients, with the added advantage of being Th1-cell biased, was not as competitive as Oxford–AstraZeneca’s AZD1222 (Covishield), as the latter consistently showed higher seroconversion rates and NAb titers. Covaxin displayed lower immunogenic parameters at almost all time points after the second dose, with titers usually lagging behind those seen with Covishield. While AZD1222 showed significant immunogenicity after the first dose itself, it required generally two doses of Covaxin to impart sufficient immunity. Previously infected individuals nonetheless showed good results with the administration of a single dose of Covaxin. Individuals with prior viral exposure administered at least two doses of Covaxin had the best results. In all, binding and neutralizing antibody titer values for Covaxin were not very impressive. Although some protection was afforded against strains such as Alpha, Beta, and Delta, it was not substantial. Neither Covaxin nor Covishield could provide sufficient immunity against the Omicron strain. However, a vaccination regimen including both vaccines displayed better immunogenicity, especially against multiple strains. Further experimentation with heterologous boost vaccination may be beneficial in tackling future variants.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
Conceptualization, TIA and SM; methodology, validation, TIA and SM; formal analysis, investigation, data curation, writing—original draft preparation, TIA and SM; writing—review and editing, supervision, TIA, SR, SI, JA, KH and SM. All authors have read and agreed to the published version of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Worldometers.info. COVID-19 CORONAVIRUS PANDEMIC STATISTICS (2022). Available at: https://www.worldometers.info/coronavirus/.
2. Kamath P. SC to hear plea for COVID-19 norms violation during kumbh mela, election rallies on may 10. Republic World (2021).
3. Ministry of Health and Family Welfare, COWIN. Vaccination statistics (2022). Available at: https://dashboard.cowin.gov.in/.
4. Kumar A, Dowling WE, Román RG, Chaudhari A, Gurry C, Le TT, et al. Status report on COVID-19 vaccines development. Curr Infect Dis Rep (2021) 23(6):1–12. doi: 10.1007/s11908-021-00752-3
5. Ganneru B, Jogdand H, Dharam VK, Molugu NR, Prasad S, Ella KM, et al. Evaluation of safety and immunogenicity of an adjuvanted, TH-1 skewed, whole virion InactivatedSARS-CoV-2 vaccine - BBV152. BioRxiv Preprint (2020). doi: 10.1101/2020.09.09.285445
6. Ella R, Vadrevu KM, Jogdand H, Prasad S, Reddy S, Sarangi V, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: a double-blind, randomised, phase 1 trial. Lancet Infect Dis (2021) 21:637–46. doi: 10.1016/S1473-3099(20)30942-7
7. Ganneru B, Jogdand H, Daram VK, Das D, Molugu NR, Prasad SD, et al. Th1 skewed immune response of whole virion inactivated SARS CoV 2 vaccine and its safety evaluation. ISCIENCE (2021) 24(4):102298. doi: 10.1016/j.isci.2021.102298
8. Yadav PD, Ella R, Kumar S, Patil DR, Mohandas S, Shete AM, et al. Immunogenicity and protective efficacy of inactivated SARS-CoV-2 vaccine candidate, BBV152 in rhesus macaques. Nat Commun (2021) 12(1386):1–11. doi: 10.1038/s41467-021-21639-w
9. Phelamei S. India’s first COVID-19 vaccine ‘COVAXIN’ by bharat biotech gets DCGI approval for human trials. Times Now. 30-Jun-2020
10. Zare H, Rezapour H, Mahmoodzadeh S, Fereidouni M. Prevalence of COVID-19 vaccines ( Sputnik V , AZD-1222 , and covaxin ) side effects among healthcare workers in birjand city , Iran. Int Immunopharmacol (2021) 101(Pt B):108351. doi: 10.1016/j.intimp.2021.108351
11. Ks JP, Muthukumaran A, Haridas N, Fernando E, Seshadri J, Kurien AA. Two cases of double-positive antineutrophil cytoplasmic autoantibody and antiglomerular basement membrane disease after BBV152 / covaxin vaccination. Kidney Int Rep (2021) 6(12):3090–1. doi: 10.1016/j.ekir.2021.10.004
12. Kar BR, Singh BS, Mohapatra L, Agrawal I. Cutaneous small-vessel vasculitis following COVID-19 vaccine. J Cosmetic Dermatol (2021) 20:3382–3. doi: 10.1111/jocd.14452
13. Muhie OA, Adera H, Tsige E, Afework A. Herpes zoster following covaxin receipt. Int Med Case Rep J (2021) 14:819–21. doi: 10.2147/IMCRJ.S345288
14. Showkathali R, Yalamanchi R, Narra L, Vinayagamoorthy N, Gunasekaran S, Nayak R, et al. Coronary thrombo-embolic events after covid-19 vaccination- a single centre study. Indian Heart J (2022) 74(2):131–34. doi: 10.1016/j.ihj.2022.01.002
15. WHO. WHO issues emergency use listing for eighth COVID-19 vaccine. www.who.int (2021). Available at: https://www.who.int/news/item/03-11-2021-who-issues-emergency-use-listing-for-eighth-covid-19-vaccine.
16. Buenos Aires Province agrees to buy 10 million doses of covaxin vaccine. Buenos Aires Times (2021). Available at: https://www.batimes.com.ar/news/argentina/buenos-aires-province-agrees-to-buy-10-million-doses-of-covaxin-vaccine.phtml.
17. Anvisa allows for limited imports of Sputnik V and covaxin into Brazil. Mercopress (2021). Available at: https://en.mercopress.com/2021/06/05/anvisa-allows-for-limited-imports-of-sputnik-v-and-covaxin-into-brazil.
18. Tyagi K, Ghosh A, Nair D, Dutta K, Singh P, Ansari IA, et al. Breakthrough COVID19 infections after vaccinations in healthcare and other workers in a chronic care medical facility in new Delhi, India. Diabetes Metab Syndrome: Clin Res Rev (2021) 15(3):1007–8. doi: 10.1016/j.dsx.2021.05.001
19. Abhilash KP, Prabhakar, Mathiyalagan P, Krishnaraj VRK, Selvan S, Kanagarajan R, et al. Impact of prior vaccination with covishield TM and covaxin Ò on mortality among symptomatic COVID-19 patients during the second wave of the pandemic in south India during April and may 2021: a cohort study. Vaccine (2022) 40(13):2107–13. doi: 10.1016/j.vaccine.2022.02.023
20. Selvaraj P, Muthu S, Jeyaraman N, Prajwal GS, Jeyaraman M. Incidence and severity of SARS-CoV-2 virus post COVID-19 vaccination: A cross-sectional study in India. Clin Epidemiol Glob Health (2022) 14:100983. doi: 10.1016/j.cegh.2022.100983
21. Weinberg GA, Szilagyi PG. Vaccine Epidemiology: Efficacy , effectiveness , and the translational research roadmap. J Infect Dis (2010) 201(11):1607–10. doi: 10.1086/652404
22. Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. 2015.
23. Mohandas S, Yadav PD, Shete-Aich A, Abraham P, Vadrevu KM, Sapkal G, et al. Immunogenicity and protective efficacy of BBV152, whole virion inactivated SARS- CoV-2 vaccine candidates in the Syrian hamster model. ISCIENCE (2021) 24(2):102054. doi: 10.1016/j.isci.2021.102054
24. Ella R, Reddy S, Jogdand H, Sarangi V, Ganneru B, Prasad S, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine , BBV152: interim results from a double-blind , randomised , multicentre , phase 2 trial , and 3-month follow-up of a double-blind , randomised phase 1 trial. Lancet Infect Dis (2021) 21(7):950–61. doi: 10.1016/S1473-3099(21)00070-0
25. Ella R, Reddy S, Blackwelder W, Potdar V, Yadav P, Sarangi V, et al. Efficacy , safety , and lot-to-lot immunogenicity of an inactivated SARS-CoV-2 vaccine ( BBV152 ): interim results of a randomised , double-blind , controlled , phase 3 trial. Lancet (2021) 398(10317):2173–84. doi: 10.1016/S0140-6736(21)02000-6
26. Singh AK, Phatak SR, Singh R, Bhattacharjee K, Singh NK, Gupta A, et al. Antibody response after first and second-dose of ChAdOx1-nCOV ( covishield TM Ò ) and BBV-152 ( covaxin TM Ò ) among health care workers in India: The final results of cross-sectional coronavirus vaccine-induced antibody titre ( COVAT ) study. Vaccine (2021) 39(44):6492–509. doi: 10.1016/j.vaccine.2021.09.055
27. Basavaraja CK, Sebastian J, Ravi MD, John SB. Adverse events following COVID-19 vaccination: first 90 days of experience from a tertiary care teaching hospital in south India. Ther Adv Vaccines Immunother (2021) 9:1–12. doi: 10.1177/2515135
28. Choudhary HR, Parai D, Dash GC, Kshatri JS, Mishra N, Choudhary PK, et al. Persistence of antibodies against spike glycoprotein of SARS-CoV-2 in healthcare workers post double dose of BBV-152 and AZD1222 vaccines. Front Med (2021) 8:778129(778129). doi: 10.3389/fmed.2021.778129
29. Sharma P, Mishra S, Basu S, Kumar R, Tanwar N. Breakthrough infection with severe acute respiratory syndrome coronavirus 2 among healthcare workers in Delhi: A single-institution study. Cureus (2021) 13(10):e19070. doi: 10.7759/cureus.19070
30. Singh AK, Phatak SR, Singh R, Bhattacharjee K, Singh NK, Gupta A, et al. Humoral antibody kinetics with ChAdOx1-nCOV (CovishieldTM) and BBV-152 (CovaxinTM) vaccine among Indian healthcare workers: A 6- month longitudinal cross-sectional coronavirus vaccine-induced antibody titre (COVAT) study. Diabetes Metab Syndrome: Clin Res Rev (2022) 16(2):102424. doi: 10.1016/j.dsx.2022.102424
31. Houshmand B, Keyhan SO, Fallahi HR, Ramezanzade S, Sadeghi E. Vaccine-associated complications: a comparative multicenter evaluation among dental practitioners and dental students — which candidate vaccine is more safe in SARS COV II , gam-COVID-Vac ( Sputnik V ), ChAdOx1 nCoV-19 ( AstraZeneca ), BBV152 ( covaxin ). Maxillofac Plast Reconstructive Surg (2022) 44(3). doi: 10.1186/s40902-021-00330-6
32. Dash GC, Subhadra S, Turuk J, Parai D, Rath S, Sabat J, et al. Breakthrough SARS-CoV-2 infections among BBV-152 (COVAXIN®) and AZD1222 (COVISHIELDTM) recipients: Report from the eastern state of India girish. J Med Virol (2021), 1–5. doi: 10.1002/jmv.27382
33. Kumar NP, Banurekha VV, GKC P, Nancy A, Padmapriyadarsini C, Mary AS, et al. Prime-boost vaccination with covaxin / BBV152 induces heightened systemic cytokine and chemokine responses. Front Immunol (2021) 12:752397(752397). doi: 10.3389/fimmu.2021.752397
34. Yadav PD, Sapkal GN, Ella R, Sahay RR, Nyayanit DA, Patil DY, et al. Neutralization of beta and delta variant with sera of COVID-19 recovered cases and vaccinees of inactivated COVID-19 vaccine BBV152 / covaxin. J Travel Med (2021) 28(7):1–3. doi: 10.1093/jtm/taab104
35. Sapkal G, Ph D, Yadav PD, Ph D, Ella R, Abraham P, et al. Neutralization of VUI b . 1 . 1 . 28 P2 variant with sera of COVID-19 recovered cases and recipients of covaxin an inactivated COVID-19 vaccine. J Travel Med (2021) 28(7):1–3. doi: 10.1093/jtm/taab077
36. Sapkal GN, Yadav PD, Ella R, Deshpande GR, Sahay RR, Gupta N, et al. Inactivated COVID-19 vaccine BBV152 / COVAXIN effectively neutralizes recently emerged b . 1 . 1 . 7 variant of SARS-CoV-2. J Travel Med (2021) 28(4):1–3. doi: 10.1093/jtm/taab051
37. Yadav PD, Sahay RR, Sapkal G, Nyayanit D, Shete AM, Deshpande G, et al. Comparable neutralization of SARS-CoV-2 delta AY . 1 and delta with individuals sera vaccinated with BBV152. Journal of Travel Medicine 28, no. 8 (2021): taab154. doi: 10.1093/jtm/taab154
38. Kumar NP, Padmapriyadarsini C, Devi KRU, Banurekha VV, Nancy A. Antibody responses to the BBV152 vaccine in individuals previously infected with SARS-CoV-2: A pilot study. Indian J Med Res (2021) 153(5–6):671–6. doi: 10.4103/ijmr.ijmr
39. Kant R, Dwivedi G, Zaman K, Sahay RR, Sapkal G, Kaushal H, et al. Immunogenicity and safety of a heterologous prime-boost COVID-19 vaccine schedule: ChAdOx1 vaccine covishield followed by BBV152 covaxin. J Travel Med (2021) 28(8):1–4. doi: 10.1093/jtm/taab166
40. Desai D, Khan AR, Soneja M, Mittal A, Naik S, Kodan P, et al. Effectiveness of an inactivated virus-based SARS-CoV-2 vaccine , BBV152 , in India: a test-negative , case-control study. Lancet Infect Dis (2020) S1473-3099(21):00674–5. doi: 10.1016/S1473-3099(21)00674-5
41. Medigeshi GR, Batra G, Murugesan DR, Thiruvengadam R, Chattopadhyay S, Das B, et al. Sub-Optimal neutralisation of omicron (B.1.1.529) variant by antibodies induced by vaccine alone or SARS-CoV-2 infection plus vaccine (hybrid immunity) post 6-months. eBioMedicine (2022) 78:103938. doi: 10.1016/j.ebiom.2022.103938
42. Sapkal G, Kant R, Dwivedi G, Sahay RR, Yadav PD, Deshpande GR, et al. Immune responses against different variants of SARS-CoV-2 including omicron following 6 months of administration of heterologous prime-boost COVID-19 vaccine. J Travel Med (2022), taac033. doi: 10.1093/jtm/taac033
43. Malhotra S, Mani K, Lodha R, Bakhshi S, Mathur VP, Gupta P, et al. SARS-CoV-2 reinfection rate and estimated effectiveness of the inactivated whole virion vaccine BBV152 against reinfection among health care workers in new Delhi, India. JAMA Netw Open (2022) 5(1):e2142210–e2142210. doi: 10.1001/jamanetworkopen.2021.42210
44. Parameswaran A, Apsingi S, Eachempati KK, Dannana CS, Jagathkar G, Iyer M, et al. Incidence and severity of COVID-19 infection post-vaccination: a survey among Indian doctors. Infection (2022). doi: 10.1007/s15010-022-01758-2
Keywords: BBV152, Covaxin, inactivated vaccine, Covishield, efficacy, immunogenicity, safety
Citation: Ahmed TI, Rishi S, Irshad S, Aggarwal J, Happa K and Mansoor S (2022) Inactivated vaccine Covaxin/BBV152: A systematic review. Front. Immunol. 13:863162. doi: 10.3389/fimmu.2022.863162
Received: 26 January 2022; Accepted: 27 June 2022;
Published: 09 August 2022.
Edited by:
Lee Mark Wetzler, Boston University, United StatesReviewed by:
Xionglin Fan, Huazhong University of Science and Technology, ChinaKamran Zaman, Indian Council of Medical Research - Regional Medical Research Centre (ICMR - RMRC) Gorakhpur, India
Copyright © 2022 Ahmed, Rishi, Irshad, Aggarwal, Happa and Mansoor. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sheikh Mansoor, bWFuc29vcnNoYWZpMjFAZ21haWwuY29t
 Tousief Irshad Ahmed
Tousief Irshad Ahmed Saqib Rishi2
Saqib Rishi2 Sheikh Mansoor
Sheikh Mansoor