
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol., 01 April 2022
Sec. Cancer Immunity and Immunotherapy
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.858021
This article is part of the Research TopicPathogenesis, Immune Escape, Prognosis and Novel Management of Lymphoid Proliferative DisordersView all 18 articles
 Wei Sang1,2,3†
Wei Sang1,2,3† Xiangmin Wang1,2,3†
Xiangmin Wang1,2,3† Hongzhi Geng4†
Hongzhi Geng4† Tianci Li1,2,3
Tianci Li1,2,3 Dashan Li1,2,3
Dashan Li1,2,3 Bingpei Zhang1,2,3
Bingpei Zhang1,2,3 Yi Zhou1,2,3
Yi Zhou1,2,3 Xuguang Song1,2,3
Xuguang Song1,2,3 Cai Sun1,2,3
Cai Sun1,2,3 Dongmei Yan1,2,3
Dongmei Yan1,2,3 Depeng Li1,2,3
Depeng Li1,2,3 Zhenyu Li1,2,3
Zhenyu Li1,2,3 Caixia Li4*
Caixia Li4* Kailin Xu1,2,3*
Kailin Xu1,2,3*Anti-CD30 CAR-T is a potent candidate therapy for relapsed/refractory (r/r) CD30+ lymphomas with therapy limitations, and the efficacy needed to be further improved. Herein a multi-center phase II clinical trial (NCT03196830) of anti-CD30 CAR-T treatment combined with PD-1 inhibitor in r/r CD30+ lymphoma was conducted. After a lymphocyte-depleting chemotherapy with fludarabine and cyclophosphamide, 4 patients in cohort 1 and 3 patients in cohort 2 received 106/kg and 107/kg CAR-T cells, respectively, and 5 patients in cohort 3 received 107/kg CAR-T cells combined with anti-PD-1 antibody. The safety and the efficacy of CAR-T cell therapy were analyzed. Cytokine release syndrome (CRS) was observed in 4 of 12 patients, and only 1 patient (patient 9) experienced grade 3 CRS and was treated with glucocorticoid and tocilizumab. No CAR-T-related encephalopathy syndrome was observed. Only two patients in cohorts 2 and 3 experienced obviously high plasma levels of IL-6 and ferritin after CD30 CAR-T cell infusion. The overall response rate (ORR) was 91.7% (11/12), with 6 patients achieving complete remission (CR) (50%). In cohorts 1 and 2, 6 patients got a response (85.7%), with 2 patients achieving CR (28.6%). In cohort 3, 100% ORR and 80% CR were obtained in 5 patients without ≥3 grade CRS. With a median follow-up of 21.5 months (range: 3-50 months), the progression-free survival and the overall survival rates were 45 and 70%, respectively. Of the 11 patients who got a response after CAR-T therapy, 7 patients (63.6%) maintained their response until the end of follow-up. Three patients died last because of disease progression. Taken together, the combination of anti-PD-1 antibody showed an enhancement effect on CD30 CAR-T therapy in r/r CD30+ lymphoma patients with minimal toxicities.
Chimeric antigen receptor (CAR) T-cell therapy offers an effective therapeutic option for patients with lymphoid malignancies (1–3). CD19 antigen was commonly selected in CAR-T treatment of B cell hematologic malignancies and got a response of 80% overall response rate (ORR) in lymphomas (2, 4) and higher than 90% ORR in B acute lymphocytic leukemia (B-ALL) patients (5) with controllable toxicities. However, most patients do not have a durable response, and there remains a room for improvement (6). The use of combinatorial approaches, including immunomodulatory drugs, checkpoint inhibitors, BTK inhibitor, etc., have been tried in CAR-T cell therapy and showed a synergetic anti-tumor effect with endurable toxicities (7).
CD30 is a target universally expressed in virtually all classical Hodgkin lymphomas (cHL), anaplastic large cell lymphomas (ALCL), and in a proportion of other lymphoma types (8). Intensive chemotherapy followed by hematopoietic stem cell transplantation (HSCT) is commonly used in those r/r lymphoma patients, but a considerable proportion of patients eventually relapse after treatment (9). The CD30-specific antibody drug conjugate brentuximab vedotin treatment is an important immunotherapy option at present, with promising anti-tumor activity and manageable toxicity in cHL and CD30-positive peripheral T-cell lymphoma (10), but the long-term disease control capacity really needed to be improved. More than half of patients will eventually relapse (11), so it is imperative to develop novel effective therapeutics to improve the prognosis for patients of those lymphoma subtypes.
CD30-directed CAR-T cell therapy offers a remarkable opportunity to these r/r CD30+ lymphoma patients. However, although well tolerated, the anti-tumor activity of CD30 CAR-Ts in r/r cHL or ALCL needed to be further improved (12, 13). In a previous phase I study, the anti-CD30 CART cell infusion just yielded a 39% ORR (12). Strategies to enhance the curative effect of CD30 CAR-T cells, including lymphodepleting chemotherapy regimen optimization (14) and combination of PD-1 inhibitor (15), have emerged as new foci on research in recent years. PD-1 was shown to be upregulated by nearly 40% in activated CAR-T cells (16). Besides this, following activation, CAR-T cells could upregulate programmed death ligand-1 (PD-L1) expression on cancer cells, which leads to the lack of clinical efficacy of CAR-T cells (17). Furthermore, PD-1 blockade was shown to improve the expansion and persistence of CAR-T cells through interrupting the PD-1/PD-L1 pathway (18). Based on the critical role of the PD-1/PD-L1 axis in the anti-CD30 CAR-T cell therapies, the combination treatment with PD-1-blocking antibody has become a work worthy of exploration in the future.
Herein, in this study, we performed a combinatorial strategy with anti-PD-1 and anti-CD30 CAR-T cell treatment in patients with r/r CD30+ lymphomas and showed a synergetic anti-tumor activity of immune checkpoint inhibitor with minimal toxicities.
A multi-center phase II clinical trial (ClinicalTrials.gov identififiers: NCT03196830) was conducted in the Affiliated Hospital of Xuzhou Medical University and the First Affiliated Hospital of Soochow University. The inclusion criteria were as follows: (1), All the recruited relapsed or refractory lymphoma patients in this study must be confirmed CD30-positive through immunohistochemistry staining by at least two pathologists (2), Eastern Cooperative Oncology Group performance status of 2 or less (3), have ≥1 cm of measurable lesion, and (4) experienced disease progression after at least 1 line of systemic chemotherapy regimen concluded at least 1 month prior. The exclusion criteria include (1) severe organ dysfunction (2), a history of active systemic autoimmune or immunodeficiency disease, and (3) treatment history of immunosuppressive agents or glucocorticoids within the last month. All patients provided written informed consent in accordance with the Declaration of Helsinki before enrolling in the study. The study protocol and the consent forms were approved by the ethics committee of the Affiliated Hospital of Xuzhou Medical University and the First Affiliated Hospital of Soochow University.
CD30 CAR-Ts were generated via a lentiviral vector. After a lymphocyte-depleting chemotherapy with (FC regimen (three daily doses of fludarabine, 30 mg/m², -5–3 days before infusion; one dose of cyclophosphamide, 750 mg/m², -5 days), the patients received a single dose of autologous CD30 CAR-T cell infusion intravenously (Figure 1).
The patients enrolled in this study were divided into 3 cohorts. The patients of cohort 1 and cohort 2 received 106/kg and 107/kg of CAR-T cells, respectively. The patients of cohort 3 received additional anti-PD-1 antibody at 14 days after 107/kg CAR-T cell infusion, and this continued every 3 weeks (Figure 1). Sequential auto-HSCT was done in part of patients. A second infusion of CD30 CAR-Ts was allowed in patients who had progressive disease (PD) after the first treatment or after auto-HSCT.
The priority of this study was to assess the safety and feasibility of CD30 CAR-Ts. Cytokine release syndrome (CRS) was graded according to the ASBMT consensus (19). Grade 3 or higher CRS was considered to be severe. Neurotoxic effects were assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03. The anti-tumor activity of anti-CD30 CAR-T cells was assessed and shown as ORR, complete remission (CR), partial remission (PR), overall survival (OS), and progression-free survival (PFS). OS was defined as the time from infusion to the date of death from any cause. PFS was defined as days from CD30 CAR-T cell infusion to relapse, progression, or death.
The sample size was based on clinical considerations. This is an exploratory study, and all analyses are descriptive in nature. Frequencies or percentages for categorical variables were used to analyze the safety and efficacy of CAR-T therapy. Follow-up time was estimated using the Kaplan–Meier method, whereas OS and PFS were estimated using the Kaplan–Meier method. Data were analyzed using Graphpad Prism version 8.
From July 1, 2017 to July 31, 2021, 13 patients including 9 cHL, 2 angioimmunoblastic T-cell lymphoma (AITL), and 2 gray zone lymphoma patients were enrolled. Finally, a total of 12 patients were evaluated for response because 1 AITL patient was lost to follow-up. The patient characteristics are summarized in Table 1. The median age was 25 years old (range: 19–64 years), and 7 patients were male patients. Most of them have complex pretreatment histories, including chemotherapy, radiotherapy, anti-PD-1 antibody, or even HSCT. At the time of enrollment into the CD30 CAR-T cell protocol, 9 of the 12 patients have extranodal invasion, including lung, bone, liver, muscle, etc. The disease status of all patients was PD before CD30 CAR-T infusion. Three patients received two cycles of CD30 CAR-T treatment. All the patients received FC lympho-depleting chemotherapy prior to CD30 CAR-T infusion. Sequential auto-HSCT and allo-HSCT were conducted in 5 and 1 patient, respectively. In total, in cohort 1, 4 patients received 106/kg CAR-T cells; in cohort 2, 3 patients received 107/kg CAR-T cells; and in cohort 3, 5 patients received 107/kg CAR-T cells combined with anti-PD-1 antibody (Figure 2).
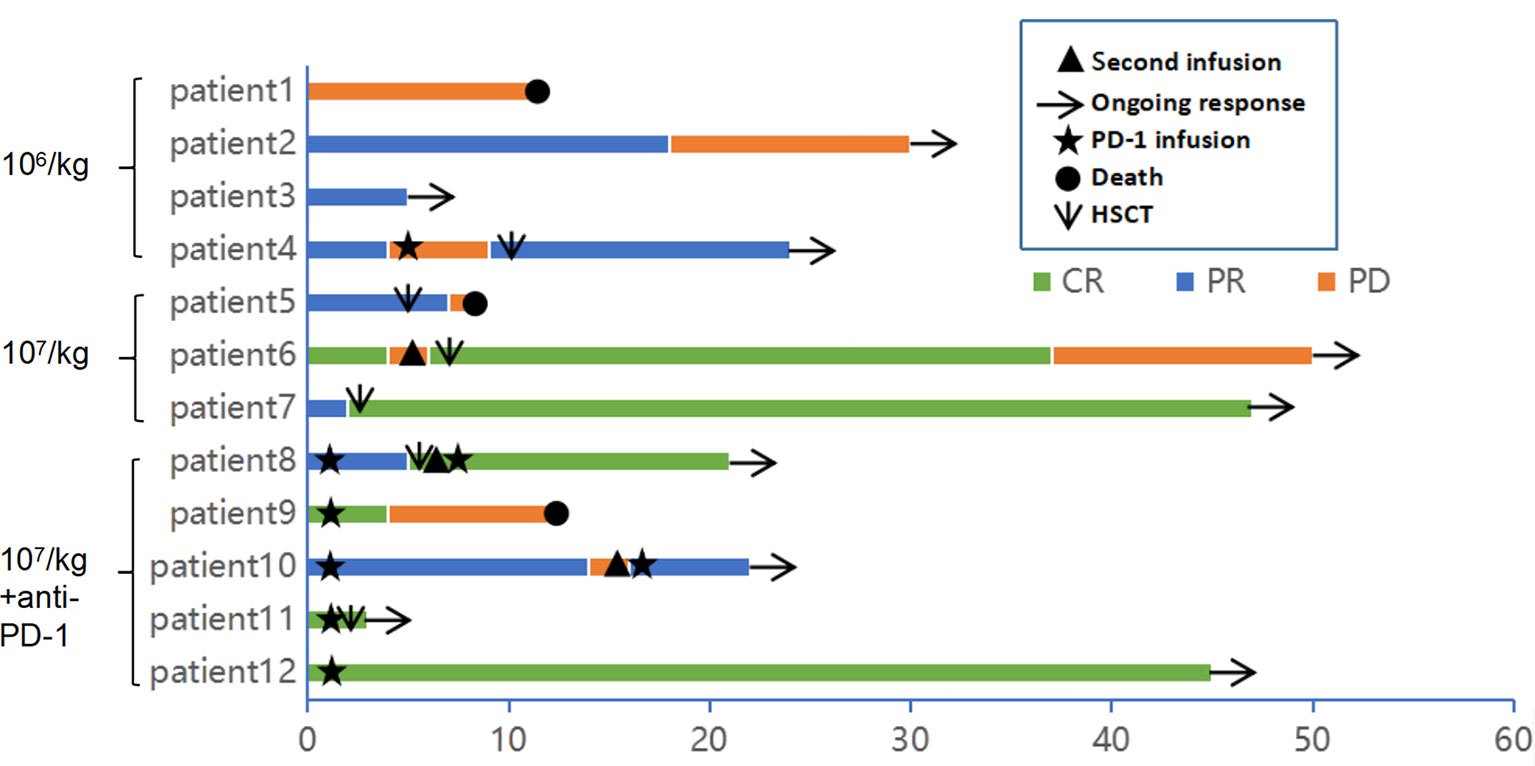
Figure 2 Clinical response and duration for patients after anti-CD30 CAR-T cell infusion. The color and the length of each bar indicate the response to the anti-CD30 CART treatment and the duration of response, respectively. CR, complete remission; PR, partial remission; PD, progressive disease. The black triangle indicates the start time of the second cycle of CAR-T cell infusion. The arrow indicates a sustained response. The star indicates the start time of anti-PD-1 antibody therapy. The original point and the downward pointing arrow represent the death and the start time of hematopoietic stem cell transplantation.
The CD30 CAR-T infusions were well tolerated, and no treatment-related deaths occurred during the study. CRS was the most common non-hematological adverse event after CD30 CAR-T infusion. In total, 4 patients (33.3%) experienced CRS, and severe CRS occurred in one patient (patient 5). Fever occurred in all the 4 CRS patients, hypoxia occurred in 1 patient (patient 9), and hypotension occurred in 1 patient (patient 5) (Table 2). Only patient 9 was treated with glucocorticoid and tocilizumab. Patient 5 experienced obviously elevated plasma levels of IL-6 and ferritin immediately after CD30 CAR-T cell infusion. Intriguingly, delayed IL-6 peak occurred at 1 month after cell infusion in patient 9 and was sustained for 1 week (Figure 3). Six patients (50%) experienced cytopenias, including neutropenia, thrombocytopenia, or anemia, among which grade 3 or higher hematological toxicities happened in 5 cases (41.7%). Transient elevation of ALT/AST was observed in 3 patients (25%). Fatigue, nausea/vomiting, diarrhea, and skin rash were observed in 4 patients (33.3%), 2 patients (16.7%), 1 patient (8.3%), and 1 patient (8.3%), respectively. No neurotoxicity was observed during this study (Table 2).
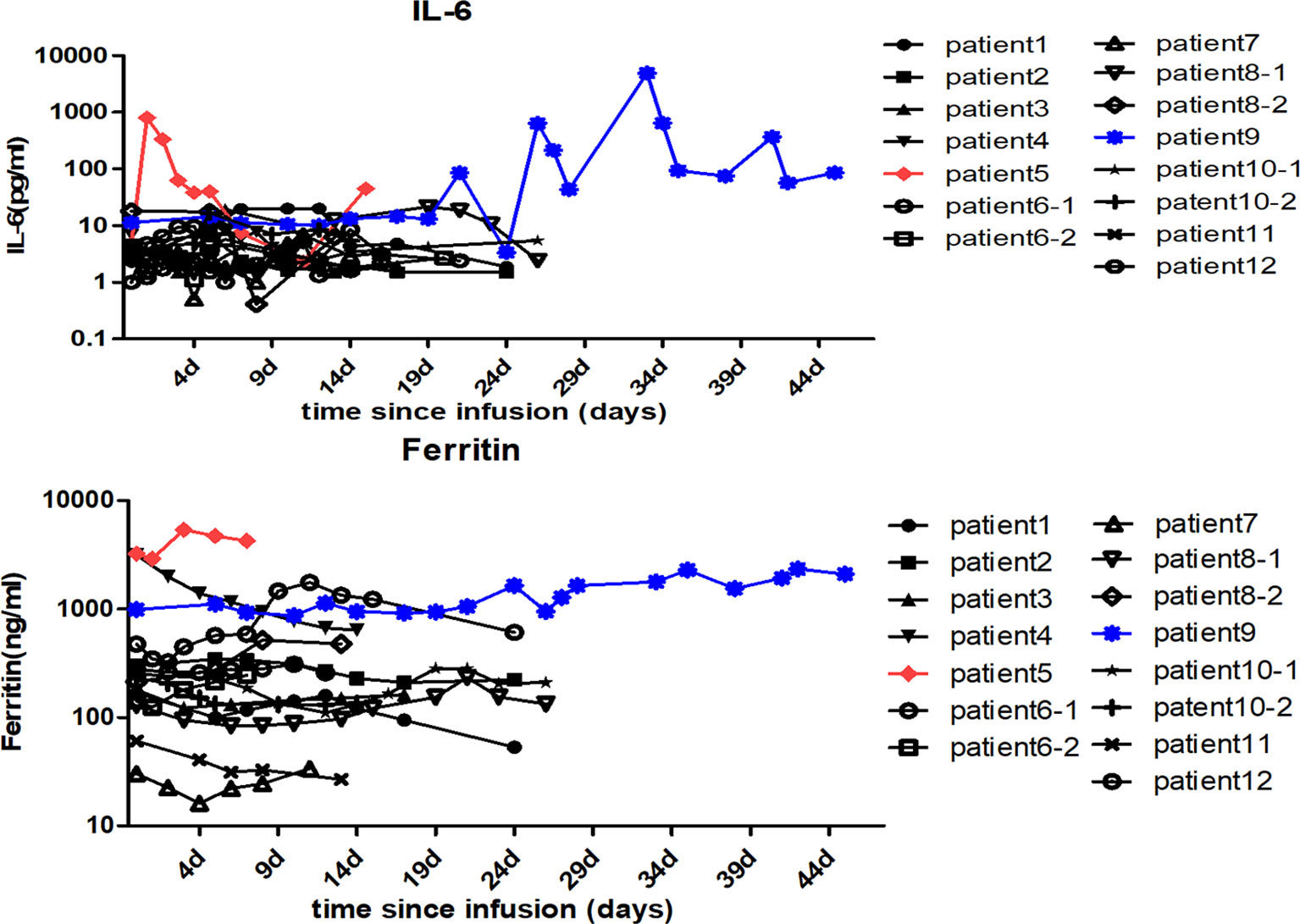
Figure 3 Changes in the patients’ serum cytokine levels after anti-CD30 CAR-T cell infusion. The serum IL-6 and ferritin levels of each patient were assessed before and at serial time points after anti-CD30 CAR-T cell infusion. The red and the blue lines, respectively, represent patient 5 and patient 9 with higher IL-6 and ferritin levels than the others.
Of the 12 evaluable patients, 6 patients had CR and 5 patients had PR after infusion, with 91.7% ORR and 50% CR rate (Figure 2, Table 1). In cohort 1, 3 of 4 patients achieved PR. In cohort 2, 2 of 3 patients achieved CR and 1 achieved PR, while in cohort 3, 100% ORR and 80% CR rate were achieved. Among 3 patients receiving a second CAR-T cell infusion, 2 patients achieved CR and 1 patient achieved PR. In the 11 patients responding to CAR-T treatment, 7 patients (7/11) continued to have responses until October 31, 2021. Of the 6 patients achieving CR, 4 patients continued to sustain a CR status until now. Among the 9 patients with cHL, 8 patients had response and 5 patients achieved CR (55.6%). Five of six (83.3%) cHL patients receiving 107/kg CAR-T cells ± PD-1-blocking antibody achieved CR, while no CR was obtained in patients receiving 106/kg CAR-T cells (cohort 1). Of 5 patients receiving HSCT after CAR-T cell infusion, 4 had CR and 1 had PR, with 100% ORR and 80% CR rate (Figure 2, Table 1). In particular, patient 8 in cohort 3, who received auto-HSCT and secondary CD30 CAR-T treatment immediately after achieving PR of the first CD30 CAR-Ts infusion, finally obtained CR and sustained remission until now (Figure 4). At a median follow-up of 21.5 months (range: 3–50 months), the PFS and OS were 45 and 70%, respectively (Figure 5). In total, 3 patients died lastly because of PD. Patient 1 (cohort 1) did not benefit from CAR-T and died 11 months after cell infusion. Patient 5 (cohort 2) and patient 9 (cohort 3) had PR and CR and died at 8 and 12 months post-CAR-T cell infusion, respectively.
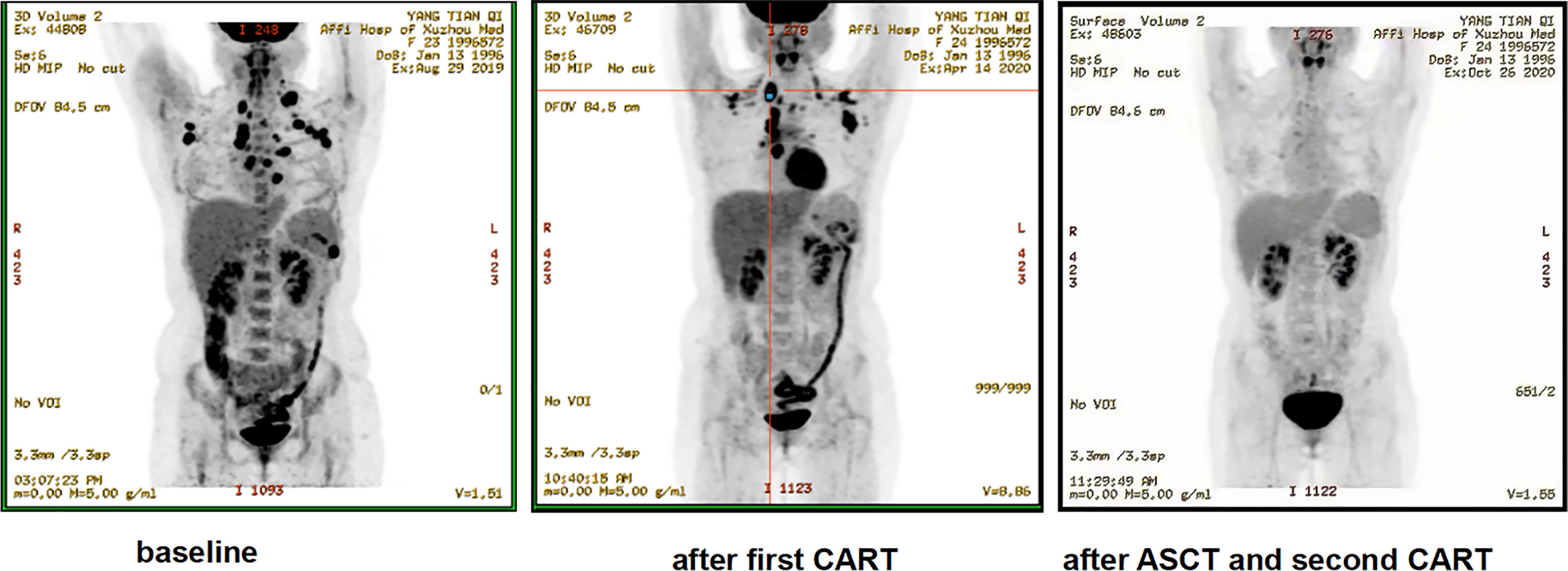
Figure 4 Representative clinical response images of the patient after anti-CD30 CAR-T cell infusion (patient 8). The second cycle of anti-CD30 CAR-T cells was infused 2 weeks after the autologous stem cell transplantation (ASCT). Positron emission tomography–computed tomography scans demonstrated a significant shrinkage of masses after the first cycle of anti-CD30 CAR-T cell infusion and complete disappearance of abnormal lymph nodes after ASCT and the second cycle of anti-CD30 CAR-T treatment.
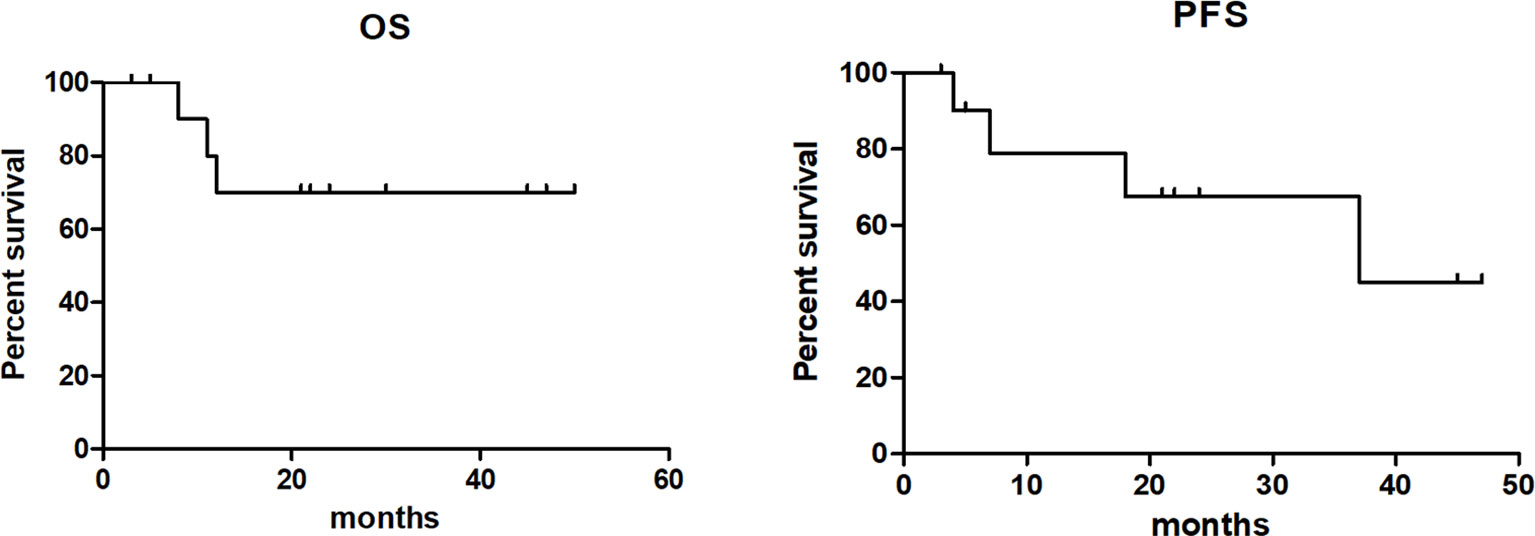
Figure 5 Overall survival and progression-free survival of patients after anti-CD30 CAR-T cell infusion.
CD30 CAR-T is an important treatment option for r/r CD30+ lymphoma, but the efficacy requires further improvement. In our multi-center study, based on the strategy of combinatorial treatment, anti-PD-1 antibody combined with CD30 CAR-T achieved encouraging results of 100% ORR and 80% CR rate with minimal toxicities.
For CD19 CAR-T, nearly 1/3 B-ALL patients and up to 13% of B-cell lymphoma patients were shown to undergo grade 3 or higher CRS (20, 21). While CAR-T targeting CD30 was shown to be well tolerated clinically, with rare cases of CRS and neurological toxicity (12, 13). In this study, based on the dosage explorations of CAR-T cells and the combinatorial strategy of PD-1 blockade, 3 cohorts of patients were conducted. In total, 8.3% severe CRS reaction was observed, and no neurotoxicity was found, and only 2 patients had significantly high plasma levels of IL-6 and ferritin after CAR-T treatment, which was consistent with the previous CD30 CAR-T studies of low toxicity characteristics (12, 13). Notably, our study showed that both 107/kg dose level of CAR-Ts and the combinatorial treatment of anti-PD-1 antibody were safe with acceptable toxicities. The most probable related adverse event was hematologic events, which happened in about 50% of patients and may be partly attributed to lymphodepletion. Severe cytopenia was common (41.7%) but recoverable with proper management. Other toxicities, including nausea, rash, and diarrhea, were transient and endurable in our study.
It was hard for CD30 CAR-Ts alone to get a satisfactory clinical response in the previous studies (12, 13). The strategy of combining with PD-1 inhibitor to refuel the CAR-T cells was being tried, and this obtained a gratifying initial result (18). In our study of evaluable patients, CD30 CAR-Ts have a significant clinical activity in heavily pretreated r/r CD30+ lymphoma patients with ORR of 91.7% and CR rate of 50%. However, the clinical efficacy of 106/kg CAR-T is not exciting because no patient obtained CR in cohort 1. In cohorts 2 and 3, a high dose of CAR-T and a combinatorial strategy of anti-PD-1 antibody significantly improved the therapeutic response of CAR-T, especially in cohort 3, of which 80% CR and 100% ORR was achieved. Furthermore, bridging HSCT after CAR-T treatment was done in 6 patients and got responses of 100% ORR and 66.7% CR rate, indicating a potential beneficial role of HSCT in improving the efficacy of CAR-T therapy. Notably, at a median follow-up of 21.5 months, nearly two-thirds of patients maintained their response until the end of follow-up.
After encountering a tumor-specific antigen in vivo, CARs could transmit an activation signal to T cells through the intracellular domain and cause T cell activation and expansion, which is dependent on the antigen density of tumor cells (22). For B-ALL, a dose of 105/kg CAR-T cells is enough to obtain a satisfactory curative effect (23), while at least 106/kg of CAR-T cells is needed in multiple myeloma (MM) and lymphoma (21, 24). Moreover, under the condition of 106/kg CAR-T cell treatment, B-ALL showed significantly more severe adverse events than MM and B-cell lymphoma (20, 21, 24). In our study, 106/kg of CD30 CAR-T cells failed to obtain a satisfactory response, which may be related to the special pathological characteristics of cHL. cHL is characterized by small numbers of large CD30+ Reed–Sternberg cells surrounded by a mixed infiltration of inflammatory and immune cells, which will show a low antigen density of CD30+ tumor cells. Therefore, CAR-T cell of a larger order of magnitude was tried in this study, and an exciting effect was obtained. The above-mentioned studies show that tumor load may be the key factor determining the dose level of CAR-T cell infusion, which will be further explored in the follow-up study.
PD-1 is an important negative costimulatory regulatory molecule to maintain T cell immune tolerance, and its ligand is commonly detected on cHL and NK/T lymphoma, etc. (8). Furthermore, upregulation of PD-1 could be seen in activated CAR-T cells (16). PD-1 blockade seems to be an ideal partner for CAR-T cell therapies. Therefore, immune checkpoint blockade in overcoming T cell exhaustion, including PD-1 gene knockout in CAR-T cells, engineering CAR-T cells to secrete anti-PD-1 and combinatorial treatment of anti-PD-1, etc., is continuously tried to be applied to CD30 CAR- T cells (15, 25, 26). Studies of PD-1 blockade enhancing the eradication of tumor cells of CAR-Ts revealed the potentially critical role of the PD-1/PD-L1 pathway in CAR-T cell immunotherapy (18). Similarly, our combinatorial treatment of PD-1 inhibitor also showed an encouraging result of 100% ORR. Moreover, in four CR patients in cohort 3, all of whom were previously resistant to the checkpoint inhibitor, the combinatorial treatment of anti-PD-1 antibody was still shown to be effective. CAR-T cell expansion improvement and activity enhancement by PD-1 blockade may be the potential reasons (17, 18). The above-mentioned study suggests that the synergistic effect of PD-1 blockade seems to be unaffected by the previous PD-1 resistance status. The anti-PD-1 antibody was added with the aim of improving the expansion and reducing the exhaustion of CAR-T cells and also have the potential risk of triggering or exacerbating a CRS reaction. Acute and severe CRS mostly occurred within 2 weeks after CAR-T cell infusion. Therefore, we add anti-PD-1 antibody at 14 days after CAR-T cell infusion and continued such every 3 weeks in our study. Further optimization of the administration time of the anti-PD-1 antibody needs to be further explored.
In summary, our study suggested that PD-1 blockade yields a high clinical benefit to some extent for r/r CD30 lymphoma patients receiving anti-CD30 CAR-T therapy, and minimal toxicities were observed. These results are exciting and further support the combination of PD-1 inhibitor with anti-CD30 CAR-T therapy. In view of the small cohort in this study, larger clinical studies with more participants are required in the future to further confirm the critical role of the PD-1/PD-L1 axis in anti-CD30 CAR-T cell therapies.
The original contributions presented in the study are included in the article material. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by the ethics committee of the Affiliated Hospital of Xuzhou Medical University and the First Affiliated Hospital of Soochow University. The patients/participants provided their written informed consent to participate in this study.
KX, CL, and WS designed the research. XW, HG, TL, DL, BZ, YZ, XS, CS, DY, DL, and ZL collected the data. XW, WS, HG, and TL analyzed and interpreted the results. XW and WS wrote the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the Natural Science Foundation of Jiangsu Province (BK20190985), the National Natural Science Foundation of China (81900177), and the Key Young Medical Talents of Jiangsu Province (QNRC2016791).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank the numerous individuals who participated in this study.
1. Barrett DM, Singh N, Porter DL, Grupp SA, June CH. Chimeric Antigen Receptor Therapy for Cancer. Annu Rev Med (2014) 65:333–47. doi: 10.1146/annurev-med-060512-150254
2. Abramson JS. Anti-CD19 CAR T-Cell Therapy for B-Cell Non-Hodgkin Lymphoma. Transfus Med Rev (2020) 34:29–33. doi: 10.1016/j.tmrv.2019.08.003
3. Wang M, Munoz J, Goy A, Locke FL, Jacobson CA, Hill BT, et al. KTE-X19 CAR T-Cell Therapy in Relapsed or Refractory Mantle-Cell Lymphoma. N Engl J Med (2020) 382:1331–42. doi: 10.1056/NEJMoa1914347
4. Locke FL, Ghobadi A, Jacobson CA, Miklos DB, Lekakis LJ, Oluwole OO, et al. Long-Term Safety and Activity of Axicabtagene Ciloleucel in Refractory Large B-Cell Lymphoma (ZUMA-1): A Single-Arm, Multicentre, Phase 1-2 Trial. Lancet Oncol (2019) 20:31–42. doi: 10.1016/S1470-2045(18)30864-7
5. Chen W, Ma Y, Shen Z, Chen H, Ma R, Yan D, et al. Humanized Anti-CD19 CAR-T Cell Therapy and Sequential Allogeneic Hematopoietic Stem Cell Transplantation Achieved Long-Term Survival in Refractory and Relapsed B Lymphocytic Leukemia: A Retrospective Study of CAR-T Cell Therapy. Front Immunol (2021) 12:755549. doi: 10.3389/fimmu.2021.755549
6. Anagnostou T, Riaz IB, Hashmi SK, Murad MH, Kenderian SS. Anti-CD19 Chimeric Antigen Receptor T-Cell Therapy in Acute Lymphocytic Leukaemia: A Systematic Review and Meta-Analysis. Lancet Haematol (2020) 7:e816–e26. doi: 10.1016/S2352-3026(20)30277-5
7. Bansal R, Reshef R. Revving the CAR - Combination Strategies to Enhance CAR T Cell Effectiveness. Blood Rev (2021) 45:100695. doi: 10.1016/j.blre.2020.100695
8. Grover NS, Savoldo B. Challenges of Driving CD30-Directed CAR-T Cells to the Clinic. BMC Cancer (2019) 19:203. doi: 10.1186/s12885-019-5415-9
9. Schmitz N, Pfistner B, Sextro M, Sieber M, Carella AM, Haenel M, et al. Aggressive Conventional Chemotherapy Compared With High-Dose Chemotherapy With Autologous Haemopoietic Stem-Cell Transplantation for Relapsed Chemosensitive Hodgkin’s Disease: A Randomised Trial. Lancet (2002) 359:2065–71. doi: 10.1016/S0140-6736(02)08938-9
10. Younes A, Gopal AK, Smith SE, Ansell SM, Rosenblatt JD, Savage KJ, et al. Results of a Pivotal Phase II Study of Brentuximab Vedotin for Patients With Relapsed or Refractory Hodgkin’s Lymphoma. J Clin Oncol (2012) 30:2183–9. doi: 10.1200/JCO.2011.38.0410
11. Chen R, Gopal AK, Smith SE, Ansell SM, Rosenblatt JD, Savage KJ, et al. Five-Year Survival and Durability Results of Brentuximab Vedotin in Patients With Relapsed or Refractory Hodgkin Lymphoma. Blood (2016) 128:1562–6. doi: 10.1182/blood-2016-02-699850
12. Wang CM, Wu ZQ, Wang Y, Guo YL, Dai HR, Wang XH, et al. Autologous T Cells Expressing CD30 Chimeric Antigen Receptors for Relapsed or Refractory Hodgkin Lymphoma: An Open-Label Phase I Trial. Clin Cancer Res (2017) 23:1156–66. doi: 10.1158/1078-0432.CCR-16-1365
13. Ramos CA, Ballard B, Zhang H, Dakhova O, Gee AP, Mei Z, et al. Clinical and Immunological Responses After CD30-Specific Chimeric Antigen Receptor-Redirected Lymphocytes. J Clin Invest (2017) 127:3462–71. doi: 10.1172/JCI94306
14. Ramos CA, Grover NS, Beaven AW, Lulla PD, Wu MF, Ivanova A, et al. Anti-CD30 CAR-T Cell Therapy in Relapsed and Refractory Hodgkin Lymphoma. J Clin Oncol (2020) 38:3794–804. doi: 10.1200/JCO.20.01342
15. Wang D, Zeng C, Xu B, Xu JH, Wang J, Jiang LJ, et al. Anti-CD30 Chimeric Antigen Receptor T Cell Therapy for Relapsed/Refractory CD30(+) Lymphoma Patients. Blood Cancer J (2020) 10:8. doi: 10.1038/s41408-020-0274-9
16. Li F, Zhang Z, Xuan Y, Zhang D, Liu J, Li A, et al. PD-1 Abrogates the Prolonged Persistence of CD8(+) CAR-T Cells With 4-1BB Co-Stimulation. Signal Transduct Target Ther (2020) 5:164. doi: 10.1038/s41392-020-00277-6
17. Rafiq S, Yeku OO, Jackson HJ, Purdon TJ, van Leeuwen DG, Drakes DJ, et al. Targeted Delivery of a PD-1-Blocking scFv by CAR-T Cells Enhances Anti-Tumor Efficacy. vivo Nat Biotechnol (2018) 36:847–56. doi: 10.1038/nbt.4195
18. Chong EA, Melenhorst JJ, Lacey SF, Ambrose DE, Gonzalez V, Levine BL, et al. PD-1 Blockade Modulates Chimeric Antigen Receptor (CAR)-Modified T Cells: Refueling the CAR. Blood (2017) 129:1039–41. doi: 10.1182/blood-2016-09-738245
19. Lee DW, Santomasso BD, Locke FL, Ghobadi A, Turtle CJ, Brudno JN, et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated With Immune Effector Cells. Biol Blood Marrow Transplant (2019) 25:625–38. doi: 10.1016/j.bbmt.2018.12.758
20. Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, et al. T Cells Expressing CD19 Chimeric Antigen Receptors for Acute Lymphoblastic Leukaemia in Children and Young Adults: A Phase 1 Dose-Escalation Trial. Lancet (2015) 385:517–28. doi: 10.1016/S0140-6736(14)61403-3
21. Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N Engl J Med (2017) 377:2531–44. doi: 10.1056/NEJMoa1707447
22. Gudipati V, Rydzek J, Doel-Perez I, Goncalves VDR, Scharf L, Konigsberger S, et al. Inefficient CAR-Proximal Signaling Blunts Antigen Sensitivity. Nat Immunol (2020) 21:848–56. doi: 10.1038/s41590-020-0719-0
23. Liu S, Deng B, Yin Z, Lin Y, An L, Liu D, et al. Combination of CD19 and CD22 CAR-T Cell Therapy in Relapsed B-Cell Acute Lymphoblastic Leukemia After Allogeneic Transplantation. Am J Hematol (2021) 96:671–9. doi: 10.1002/ajh.26160
24. Yan Z, Cao J, Cheng H, Qiao J, Zhang H, Wang Y, et al. A Combination of Humanised Anti-CD19 and Anti-BCMA CAR T Cells in Patients With Relapsed or Refractory Multiple Myeloma: A Single-Arm, Phase 2 Trial. Lancet Haematol (2019) 6:e521–e9. doi: 10.1016/S2352-3026(19)30115-2
25. Rupp LJ, Schumann K, Roybal KT, Gate RE, Ye CJ, Lim WA, et al. CRISPR/Cas9-Mediated PD-1 Disruption Enhances Anti-Tumor Efficacy of Human Chimeric Antigen Receptor T Cells. Sci Rep (2017) 7:737. doi: 10.1038/s41598-017-00462-8
Keywords: PD-1, chimeric antigen receptor, CD30, efficacy, relapsed/refractory CD30+ lymphoma
Citation: Sang W, Wang X, Geng H, Li T, Li D, Zhang B, Zhou Y, Song X, Sun C, Yan D, Li D, Li Z, Li C and Xu K (2022) Anti-PD-1 Therapy Enhances the Efficacy of CD30-Directed Chimeric Antigen Receptor T Cell Therapy in Patients With Relapsed/Refractory CD30+ Lymphoma. Front. Immunol. 13:858021. doi: 10.3389/fimmu.2022.858021
Received: 19 January 2022; Accepted: 10 March 2022;
Published: 01 April 2022.
Edited by:
Catherine Sautes-Fridman, INSERM U1138 Centre de Recherche des Cordeliers (CRC), FranceReviewed by:
Zheng Wei, Fudan University, ChinaCopyright © 2022 Sang, Wang, Geng, Li, Li, Zhang, Zhou, Song, Sun, Yan, Li, Li, Li and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kailin Xu, bGlobWRAMTYzLmNvbQ==; Caixia Li, bGljYWl4aWFAc3VkYS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.