
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol. , 15 June 2022
Sec. Vaccines and Molecular Therapeutics
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.855078
This article is part of the Research Topic Insights in Vaccines and Molecular Therapeutics: 2021 View all 11 articles
The lncRNA MIR4435-2 host gene (MIR4435-2HG) is located on human chromosome 2q13, and its expression is up-regulated in 18 tumors. MIR4435-2HG participates in 6 signaling pathways to promote tumorigenesis, including the TGF-β signaling pathway, Wnt/β-catenin signaling pathway, MDM2/p53 signaling pathway, PI3K/AKT signaling pathway, Hippo signaling pathway, and MAPK/ERK signaling pathway. MIR4435-2HG competitively binds with 20 miRNAs to form a complex ceRNA network, thereby regulating the expression of downstream target genes. The high expression of MIR4435-2HG is also closely related to the clinicopathological characteristics and poor prognosis of a variety of tumors. Also, the high expression of MIR4435-2HG in peripheral blood or serum has the value of predicting the risk of 9 tumors. In addition, MIR4435-2HG participates in the mechanism of action of three cancer drugs, including resveratrol for the treatment of lung cancer, cisplatin for non-small cell lung cancer and colon cancer, and carboplatin for triple-negative breast cancer. This article systematically summarizes the diagnostic and prognostic value of MIR4435-2HG in a variety of tumors and outlines the ceRNA network and signaling pathways related to MIR4435-2HG, which will provide potential directions for future MIR4435-2HG research.
Long non-coding RNAs (lncRNAs) are transcripts longer than 200 nucleotides that can not be translated into proteins. With the rapid development of high-throughput sequencing technology, more and more lncRNAs have been reported to participate in tumor differentiation, stemness, migration, invasion, apoptosis, and proliferation (1).
The lncRNA MIR4435-2 host gene (MIR4435-2HG) is located on human chromosome 2q13, also known as lncRNA-AWPPH, LINC00978, and AK001796. In 2015, MIR4435-2HG was first discovered to be involved in the cell growth inhibition of resveratrol in lung cancer (2). At present, MIR4435-2HG has been proven to be an oncogenic lncRNA, and its abnormal up-regulation can promote the occurrence and development of 18 tumors. In addition, MIR4435-2HG is abnormally up-regulated in the blood of patients with at least 9 tumors, suggesting that MIR4435-2HG can be used as a non-invasive diagnostic marker for these 9 tumors.
Competitive endogenous RNA (ceRNA) can sponge miRNA to regulate downstream mRNA of miRNA (3). MIR4435-2HG is the ceRNA of 20 miRNAs, which can regulate many downstream genes. MIR4435-2HG participates in at least 6 signaling pathways, including TGF-β signaling pathway, Wnt/β-catenin signaling pathway, MDM2/p53 signaling pathway, PI3K/AKT signaling pathway, Hippo signaling pathway, and MAPK/ERK signaling pathway.
The abnormal up-regulation of MIR4435-2HG is closely related to the clinicopathological characteristics of 11 tumors, including tumor size, TNM stage, lymph node metastasis, etc. The high expression of MIR4435-2HG12 is associated with the poor prognosis of 12 tumors. In addition, MIR4435-2HG is related to the mechanism of action of cancer drugs, including resveratrol for the treatment of lung cancer (2), cisplatin for non-small cell lung cancer and colon cancer (4, 5), and carboplatin for triple-negative breast cancer (6).
There is no comprehensive overview related to MIR4435-2HG. Here, this article summarizes the diagnostic and prognostic value of MIR4435-2HG in tumors, clarifies its gene regulatory network, and discusses the future directions and challenges of MIR4435-2HG research.
We downloaded the expression data of MIR4435-2HG in TCGA, TARGET, and GTEx of 32 cancer types from the UCSC Xena (https://xenabrowser.net/) database, and further performed log2(x+1) transform for the expression data.
We compared the expression differences of MIR4435-2HG between normal and tumor samples in each cancer type using the unpaired Wilcoxon Test method of R software (version 4.1.1). As shown in Figure 1A, we observed significant upregulation of MIR4435-2HG in 27 tumors, significant downregulation of MIR4435-2HG in 3 tumors (ALL, PRAD, and KICH), and no significant difference in 2 tumors (PCPG and THYM). In addition, we evaluated the median expression of MIR4435-2HG among all ncRNAs in 32 tumors and corresponding non-tumor tissues. As shown in Figure 1B, expression of MIR4435-2HG exceeded at least 75% of lncRNAs in all tumors, suggesting the value of MIR4435-2HG in pan-cancer.
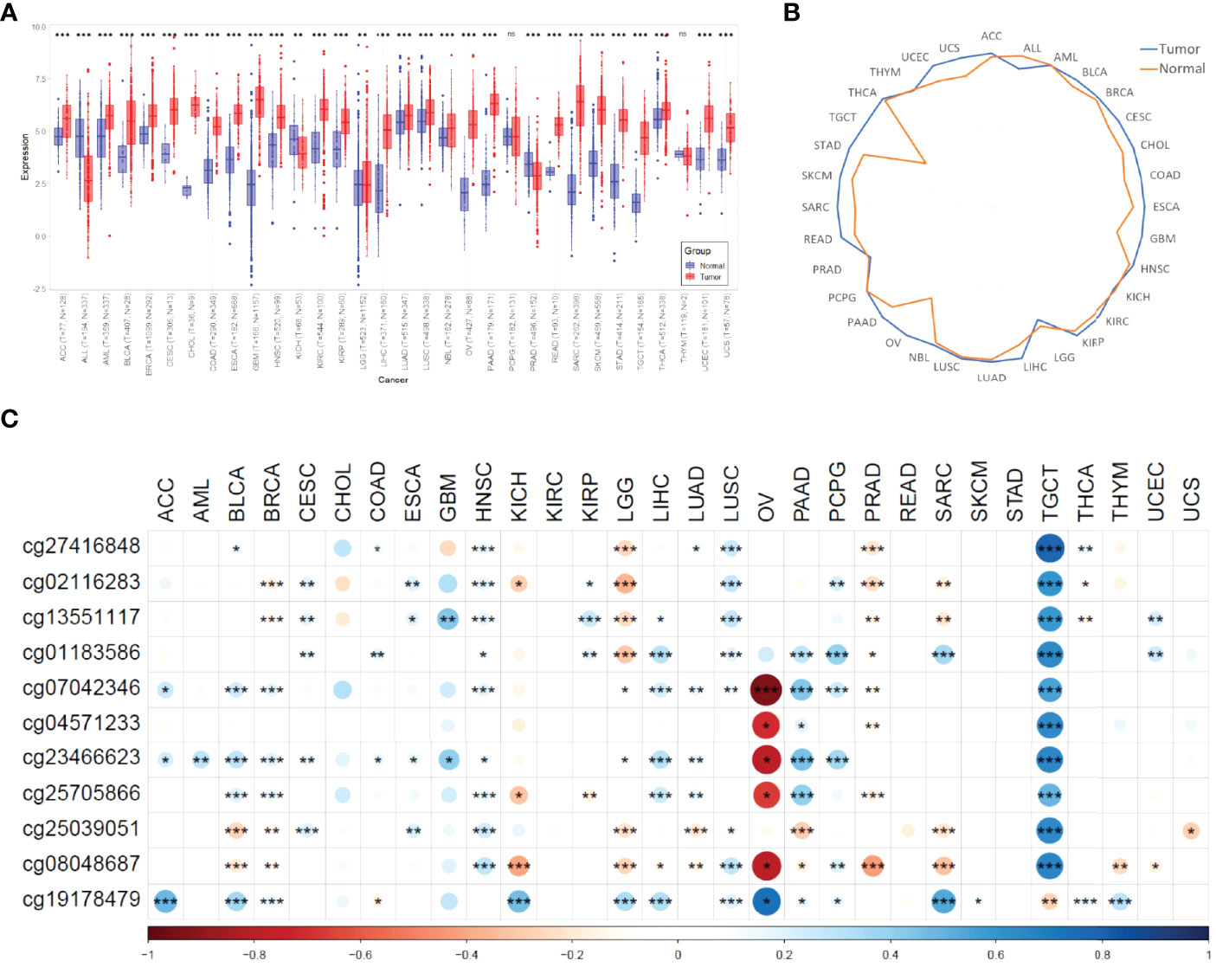
Figure 1 A pan-cancer analysis of MIR4435-2HG. (A) MIR4435-2HG is dysregulated in 32 cancer types. (*** means p < 0.001, ** means p<0.01, * means p<0.05, ns means no significant difference); (B) quantile expression of MIR4435-2HG in 32 cancer types; (C) The correlation tests between MIR4435-2HG expression and methylation of MIR4435-2HG CpG sites (*** means p<0.001, ** means p<0.01, * means p<0.05). ACC, Adrenocortical carcinoma; ALL, Acute lymphoblastic leukemia; AML, Acute myeloid leukemia; BLCA, Bladder urothelial carcinoma; BRCA, Breast invasive carcinoma; CESC, Cervical squamous cell carcinoma and endocervical adenocarcinoma; CHOL, Cholangiocarcinoma; COAD, Colon adenocarcinoma; ESCA, Esophageal carcinoma; GBM, Glioblastoma multiforme; HNSC, Head and Neck squamous cell carcinoma; KICH, Kidney chromophobe; KIRC, Kidney renal clear cell carcinoma; KIRP, Kidney renal papillary cell carcinoma; LGG, Brain lower grade glioma; LIHC, Liver hepatocellular carcinoma; LUAD, Lung adenocarcinoma; LUSC, Lung squamous cell carcinoma; NBL, Neuroblastoma; OV, Ovarian serous cystadenocarcinoma; PAAD, Pancreatic adenocarcinoma; PCPG, Pheochromocytoma and Paraganglioma; PRAD, Prostate adenocarcinoma; READ, Rectum adenocarcinoma; SARC, Sarcoma; STAD, Stomach adenocarcinoma; SKCM, Skin cutaneous melanoma; TGCT, Testicular germ cell tumors; THCA, Thyroid carcinoma; THYM, Thymoma; UCEC, Uterine corpus endometrial carcinoma; UCS, Uterine carcinosarcoma.
At present, MIR4435-2HG has been confirmed to be an oncogenic lncRNA of 18 cancers. These cancers involve the digestive, respiratory, reproductive, urinary, and nervous systems in humans (Table 1). Among them, the experimental results in most tumors were consistent with the bioinformatic analysis results, except for ALL and prostate cancer.
As shown in Figure 2, the expression of MIR4435-2HG is up-regulated in the five digestive system cancers. MIR4435-2HG is highly expressed in blood and tumor cell lines of oral squamous cell carcinoma (OSCC) (7), in tumor tissues and tumor cell lines of esophageal squamous cell carcinoma and hepatocellular carcinoma (8, 9, 18–23), and in serum, tumor tissues, and tumor cell lines of gastric cancer and colorectal cancer (1, 10–12, 14–17).
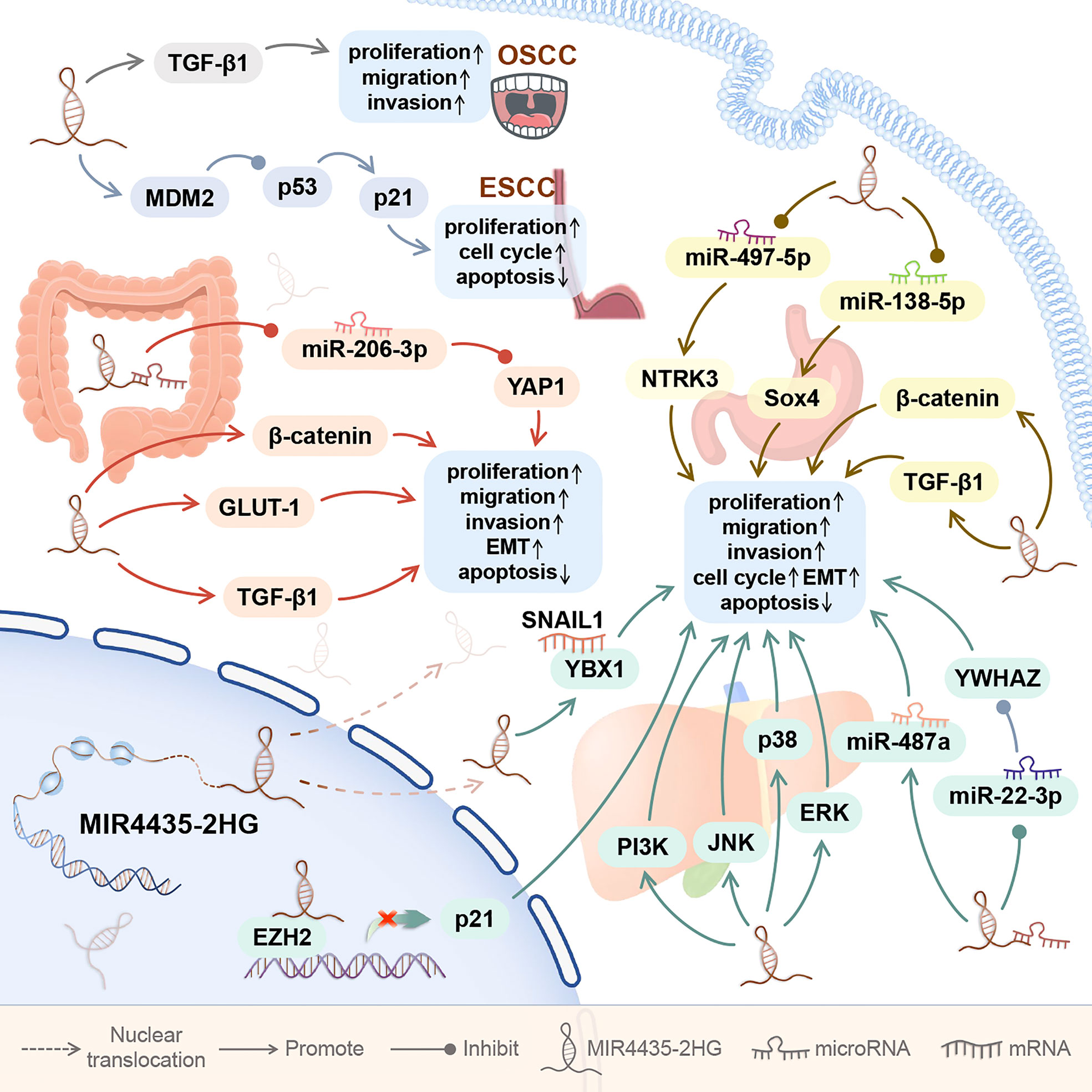
Figure 2 The role of MIR4435-2HG in digestive system cancer. In the digestive system, MIR4435-2HG can promote the growth of 5 types of tumors, including oral squamous cell carcinoma (OSCC), esophageal squamous cell carcinoma (ESCC), hepatocellular carcinoma (HCC), gastric cancer (GC), and colorectal cancer (CRC). By regulating downstream genes, MIR4435-2HG can affect tumor cell proliferation, migration, invasion, apoptosis, EMT, and cell cycle.
MIR4435-2HG is highly expressed in lung cancer tissues and tumor cell lines (2, 24–27, 29). Abnormal up-regulation of MIR4435-2HG was also detected in whole blood and serum of non-small cell lung cancer patients (27–30).
Among reproductive system cancers, MIR4435-2HG is highly expressed in tumor tissues and tumor cell lines of ovarian cancer (31–33), cervical cancer (34), and breast cancer (6, 35–37). In addition, MIR4435-2HG is highly expressed in prostate cancer cell lines and whole blood (38, 39), in serum and plasma of ovarian cancer patients (31, 32), and in plasma of triple negative breast cancer (TNBC) patients (6, 37).
In tumors of the urinary system, MIR4435-2HG is abnormally upregulated in cancer tissues and cancer cell lines of clear cell renal cell carcinoma and bladder cancer (3, 40, 41). In nervous system tumors, MIR4435-2HG is highly expressed in plasma, cancer tissues and cancer cell lines of glioma (42, 43), and in cancer tissues and cancer cell lines of glioblastoma (Figure 3) (44).
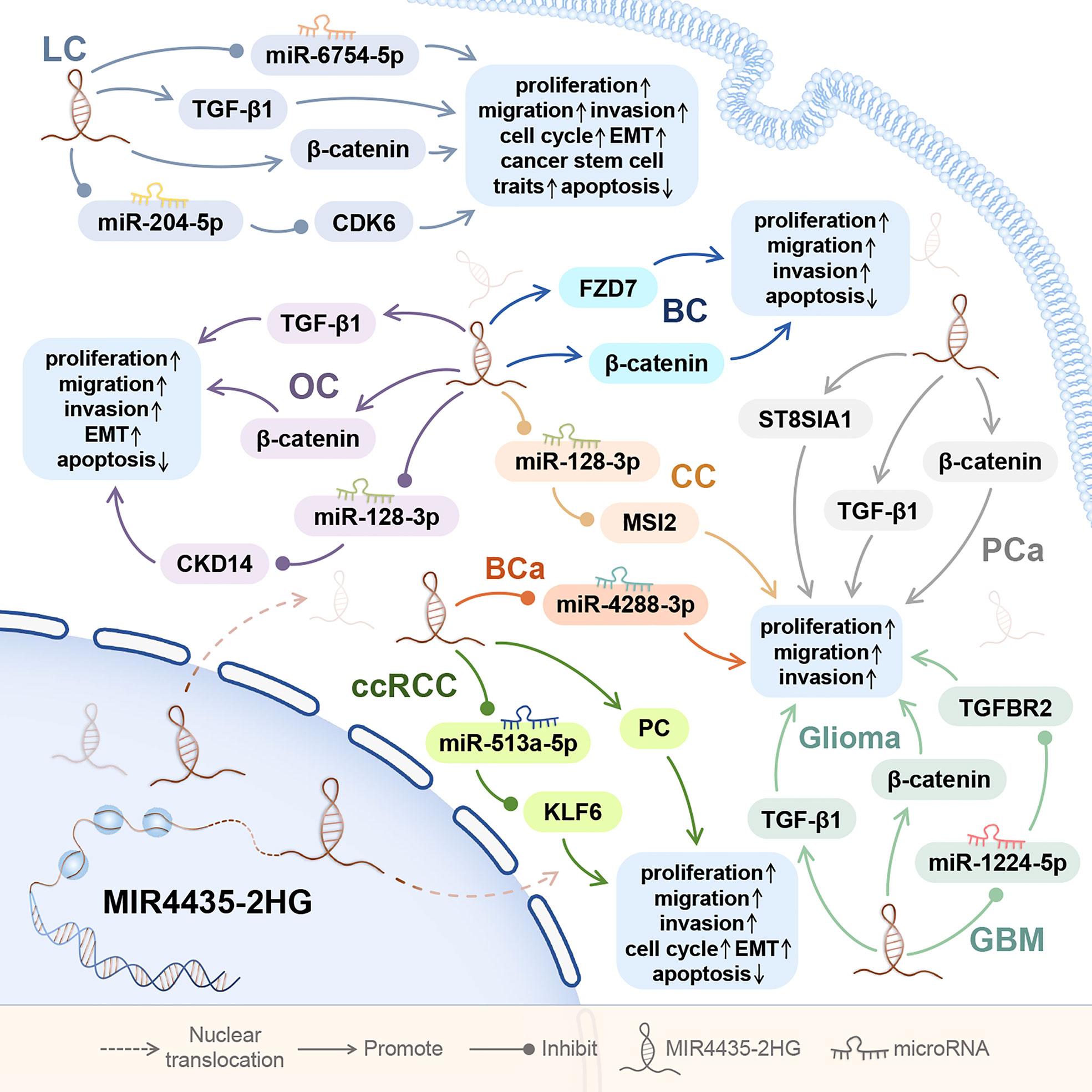
Figure 3 The role of MIR4435-2HG in tumors of the respiratory system, reproductive system, urinary system, and nervous system. MIR4435-2HG can also affect the proliferation, migration, invasion, apoptosis, EMT, and cell cycle of tumor cells by regulating downstream genes. Tumor of the respiratory system consists of lung cancer (LC); Tumors of the reproductive system consist of ovarian cancer (OC), cervical cancer (CC), breast (BC), and prostate cancer (PCa); Tumors of the urinary system include clear cell renal cell carcinoma (ccRCC) and bladder cancer (BCa); and tumor of the nervous system includes gliomas.
In addition, MIR4435-2HG was abnormally up-regulated in tissues and cell lines of osteosarcoma and head and neck squamous cell carcinoma (HNSC) and melanoma (45–48). MIR44435-2HG is highly expressed in childhood T-cell acute lymphoblastic leukemia (T-ALL) bone marrow and cell lines and nasopharyngeal carcinoma cell lines (Figure 4) (49, 50).
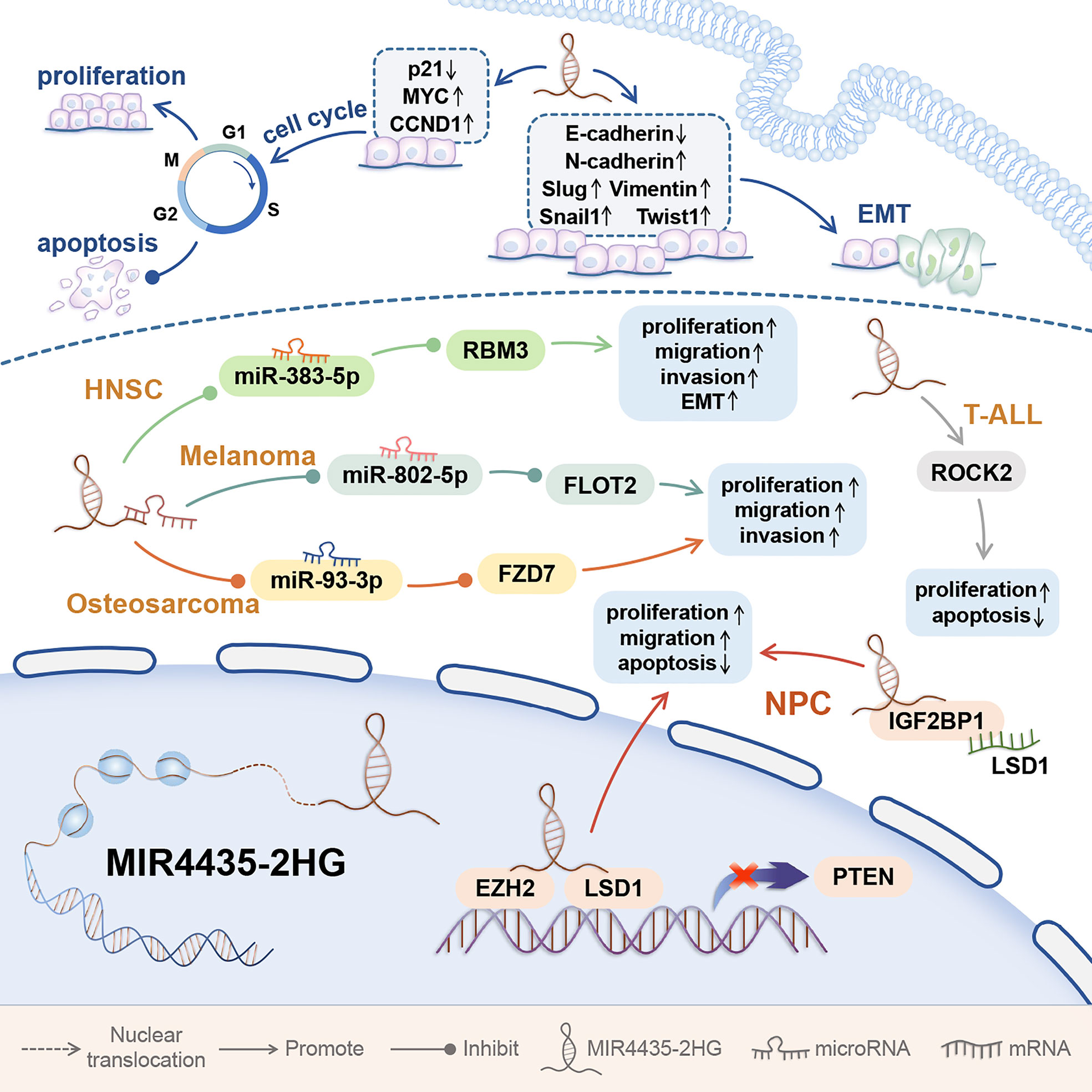
Figure 4 The mechanism of MIR4435-2HG affecting the behavior of tumor cells in other Systems. MIR4435-2HG can promote the progression of tumors in other systems, and affect the proliferation, invasion, migration, apoptosis, and EMT of tumor cells. Affecting EMT and cell cycle is an important mechanism for MIR4435-2HG to promote tumorigenesis. Head and neck squamous cell carcinoma (HNSC); T-cell acute lymphoblastic leukemia (T-ALL); nasopharyngeal carcinoma (NPC).
In the bioinformatics analysis of TCGA data, we analyzed tissue samples from prostate cancer and ALL. In experimental studies of prostate cancer, MIR4435-2HG expression was implicated in plasma and cell line samples (38, 39). In an experimental study of T-ALL, the expression of MIR4435-2HG was involved in the bone marrow and cell lines (49). Therefore, discrepancies in MIR4435-2HG results in prostate cancer and ALL may be due to sample differences. Further validation of the role of MIR4435-2HG in prostate cancer and ALL is required in the future.
The abnormal expression of MIR4435-2HG is closely related to cancer cell proliferation, apoptosis, invasion and migration. Cell cycle arrest can promote cell apoptosis and effectively inhibit cell proliferation (10). Inhibition of cell cycle progression is related to increased expression of genes that block the cell cycle and decreased expression of genes required for progression in G1, S, and M phases (2). p21 is a cyclin-dependent kinase (CDK) inhibitor, which is down-regulated in a variety of cancers. p21 can directly bind to kinases related to G1/S conversion and play a key role in cell cycle progression (21). In esophageal squamous cell carcinom (9), gastric cancer and hepatocellular carcinoma (12, 21), MIR4435-2HG knockdown can promote p21 expression. In addition, MIR4435-2HG knockdown can also reduce the expression of CCND1 and promote the cleavage of PARP and caspase-3 (12, 21). In gastric cancer and hepatocellular carcinoma (10–12, 20, 21), MIR4435-2HG knockdown can increase the proportion of G1 cells. In esophageal squamous cell carcinom (9), MIR4435-2HG knockdown can increase the ratio of G2/M-phase cells, decrease the ratio of S-phase cells, and promote cell apoptosis (Figure 4).
Epithelial-mesenchymal transition (EMT) usually induces the invasion and metastasis of cancer cells (24). EMT is essential in the early events of tumor cell metastasis. EMT can make cells more motile and aggressive, and it can confer cancer stem cell (CSC)-like traits on tumor cells (24). Transcription factors such as Snail1, SLUG, ZEB1, and TWIST1 can up-regulate the mesenchymal markers Vimentin and N-cadherin, and ultimately inhibit the expression of E-cadherin, a marker of epithelial status (24). In gastric cancer (10, 12), colorectal cancer (12), hepatocellular carcinoma (21), lung cancer (24), ovarian cancer (33), clear cell renal cell carcinoma (40), and HNSC (47), abnormal upregulation of MIR435-2HG can up-regulate the above-mentioned transcription factors, and ultimately promote the EMT process.
A study has shown that lncRNA expression can be activated by DNA hypomethylation in tumors (51). In glioma, Li et al. found that the up-regulation of MIR4435-2HG may be related to its abnormal methylation through HM450K methylation microarray data (52). Here, we systematically analyzed the correlation between the expression of MIR4435-2HG and the CpG methylation of MIR4435-2HG using the Pearson method. As shown in Figure 1C, the methylation of cg07042346 in OV was significantly reversely correlated with the expression of MIR4435-2HG (r<-0.5, p<0.01). However, in SARC and TGCT, the CpG sites of MIR4435-2HG were significantly positively correlated with the expression of MIR4435-2HG (r>0.5, p<0.01).
The oncogenic effect of MIR4435-2HG is related to the regulation of six signaling pathways, including the TGF-β signaling pathway, Wnt/β-catenin signaling pathway, MDM2/p53signalling pathway, PI3K/AKT signaling pathway, Hippo signaling pathway, and MAPK/ERK signaling pathway (Figure 5).
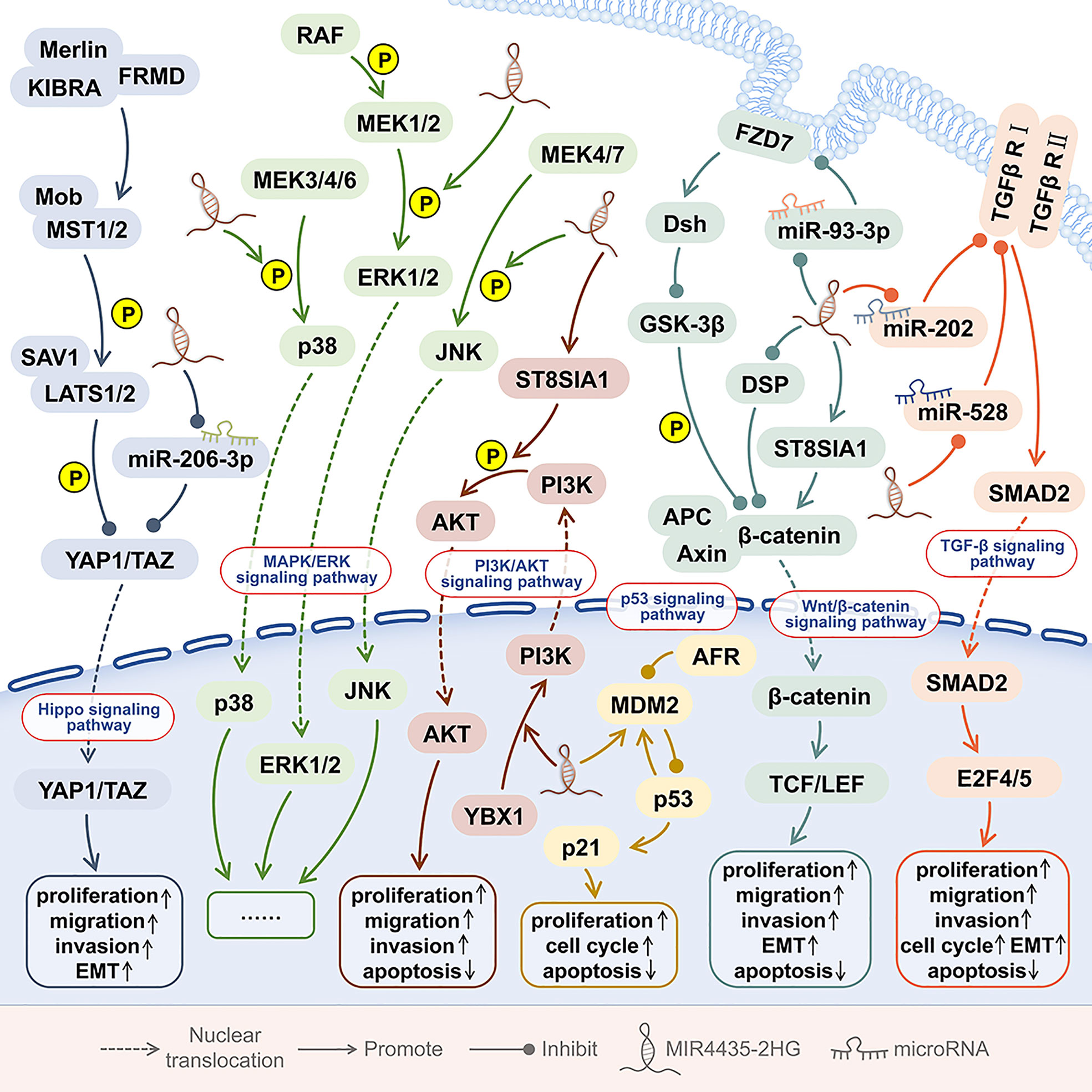
Figure 5 The signaling pathways involved in MIR4435-2HG. In human tumors, MIR4435 participates in at least 6 signaling pathways, including the TGF-β signaling pathway, Wnt/β-catenin signaling pathway, MDM2/p53 signaling pathway, PI3K/AKT signaling pathway, Hippo signaling pathway, and MAPK/ERK signaling pathway.
Transforming growth factor β (TGF-β) can bind to cell surface receptors and trigger the activation of multiple signal transduction pathways (54). In the early stage of the tumor, TGF-β can inhibit the proliferation of cancer cells, and TGF-β can promote tumor metastasis in the late stage of the tumor (55). In ovarian cancer, TGF-β targeted therapy needs to be carried out cautiously according to the cancer stage (32). The activation of the TGF-β signaling pathway is widely present in the development of tumors (17).
Overexpression of MIR4435-2HG can up-regulate TGF-β1 and promote the metastasis of 7 kinds of tumors, including oral squamous cell carcinoma (OSCC) (7), gastric cancer (12), colorectal adenocarcinoma (17), and non-small cell lung cancer (27, 28, 30), ovarian cancer (32), prostate cancer and glioma (38, 43). In addition, in OSCC and colorectal adenocarcinoma (7, 17), the up-regulation of MIR4435-2HG and TGF-β1 expression can promote tumor growth and metastasis. TGF-β is the main regulator of EMT and a key marker for the metastasis and progression of different malignant tumors (56). In gastric cancer and non-small cell lung cancer (12, 27), the upregulation of MIR4435-2HG and TGF-β1 can promote the EMT of tumor cells. In non-small cell lung cancer, the up-regulation of MIR4435-2HG and TGF-β1 is also closely related to postoperative tumor recurrence (28).
In OSCC (7), non-small cell lung cancer (27, 28, 30), ovarian cancer (32), prostate cancer (38), and glioma (43), the expression levels of MIR4435-2HG and TGF-β1was positively correlated with each other, while no correlation between MIR4435-2HG and TGF-β1 was found in plasma of healthy persons. Overexpression of MIR4435-2HG can up-regulate the expression of TGF-β1, while exogenous TGF-β1 treatment has no effect on the expression of MIR4435-2HG. In gastric cancer (10), colorectal cancer (15), lung cancer (24, 29), ovarian cancer (31), breast cancer (35), and osteosarcoma (45), the overexpression of MIR4435-2HG can increase β-catenin and promote tumorigenesis. β-catenin has been shown to interact with TGF-β (57). Therefore, β-catenin may mediate the interaction between MIR4435-2HG and TGF-β1 (32, 38, 43), but this still needs further research and verification.
β-catenin is located in the cell nucleus, and by controlling gene transcription, it can promote canceration and cancer cell metastasis, and induce cancer cell stemness and drug resistance (15, 24). β-catenin is a well-known oncogene and plays a key role in regulating the Wnt signaling pathway. The Wnt/β-catenin signaling pathway plays a key role in the growth of a variety of tumors (58). The increased cytoplasmic β-catenin content is a sign of the abnormal activation of Wnt/β-catenin pathway. β-catenin plays a key role in regulating the Wnt signaling pathway, and it controls the transcription of target genes in the nucleus (15).
In stomach cancer (10), colorectal cancer (15), lung cancer (24, 29), ovarian cancer (31), breast cancer and osteosarcoma (35, 37, 45), MIR4435-2HG can up-regulate the expression of β-catenin proportionally. In gastric cancer, desmoplakin (DSP) is the most abundant desmosomal protein. MIR4435-2HG can bind to DSP and inhibit DSP and its cascade reaction, thereby activating Wnt/β-catenin signal transduction, promoting tumor growth, metastasis, and EMT (10).
In lung cancer, MIR4435-2HG up-regulates β-catenin, promotes tumor growth, metastasis and EMT both in vivo and in vitro, and maintains the stemness of cancer cells (24). In non-small cell lung cancer, MIR4435-2HG can up-regulate β-catenin to promote cell proliferation and inhibit cell apoptosis (29). In ovarian cancer, overexpression of MIR4435-2HG significantly promotes the expression of β-catenin and promotes the growth, invasion, and migration of tumor cells (31). In addition, in breast cancer, MIR4435-2HG promotes tumor growth, invasion, metastasis, and EMT by activating Wnt/β-catenin signal transduction, and inhibits cell apoptosis (35); meanwhile, MIR44352HG knockdown can decreases the expression of total and nuclear β-catenin, reduces the expression of anti-apoptotic marker (Bcl2), proliferation marker (PCNA), and mesenchymal markers (N-cadherin, vimentin, and ZEB1), upregulates the cleaved PARP and the epithelial marker (E-cadherin), and activate caspase 3 and Bax of the apoptotic pathway (35).
Frizzled family receptor 7 (FZD7) is a Wnt signaling receptor, which is involved in the maintenance of cancer cell stemness and cancer progression. In triple-negative breast cancer (TNBC), overexpression of MIR4435-2HG promotes frizzled homolog 7 (FZD7) expression in cells, thereby activating the Wnt/β-catenin signaling pathway (37). In osteosarcoma, through the miR-93-3p/FZD7 axis, MIR4435-2HG can also up-regulate FZD7 and activate the Wnt/β-catenin signaling pathway, thereby promoting the proliferation, invasion, and migration of osteosarcoma cells (45). In addition, in prostate cancer tissues and cells, MIR4435-2GH increases the expression levels of β-catenin, p-FAK, p-AKT, c-MYC, and CCND1 by up-regulating ST8SIA1 (39).
In the above tumors, MIR4435-2HG can promote the expression of β-catenin, but the activation of Wnt/β-catenin signal transduction has no effect on the expression of MIR4435-2HG. Therefore, MIR4435-2HG is an upstream activator of the Wnt/β-catenin signaling pathway, which plays a role in the occurrence and development of cancer.
MDM2/p53 is one of the important signaling pathways, which can regulate cell growth and cell cycle (59). Mouse double minute 2 (MDM2) is an E3 ubiquitin ligase, which not only inhibits the transcriptional activity of p53, but also promotes the ubiquitination and degradation of p53 (9). p53 is an important tumor suppressor, which is activated in response to various stresses, thereby promoting cell apoptosis (9).
In esophageal squamous cell carcinoma tissues, both MIR4435-2HG and MDM2 were significantly up-regulated. Knockdown of MIR4435-2HG resulted in G2/M phase arrest in esophageal squamous cell carcinom cell lines (Eca-109 and TE-1), as well as decreased expression of MDM2, and increased expression of downstream p53 and p21 (9). The above shows that MIR4435-2HG promotes cell proliferation and cell cycle and inhibits cell apoptosis by regulating the MDM2/p53 signaling pathway.
When PI3K binds to growth factor receptors such as EGFR, RAS and PTEN, it can change the protein structure of AKT and activate AKT and its downstream effectors, thereby regulating cell proliferation, differentiation, apoptosis, and migration (60). The PI3K/AKT pathway is involved in the occurrence and development of a variety of cancers (60).
In the nucleus of hepatocellular carcinoma (HCC) cells, the interaction between MIR4435-2HG and the DNA binding protein Y-box binding protein 1 (YBX1) can enhance the binding of YBX1 to the PI3K promoter, thereby promoting the transcription of PI3K. MIR4435-2HG can activate the PI3K/AKT pathway, promote the proliferation and migration of HCC cells, and promote tumor growth and metastasis in mice. In the cytoplasm of HCC, MIR4435-2HG can interact with YBX1, up-regulate Snail1, and promote tumor progression (22). In prostate cancer tissues and cell lines, MIR4435-2HG promotes ST8SIA1 and up-regulates p-AKT levels (39).
In osteosarcoma cell lines (MG-63 and U2OS), MIR4435-2HG can up-regulate the protein levels of p-PI3K and p-AKT, suggesting that MIR4435-2HG may activate the PI3K/AKT pathway to promote the growth of osteosarcoma cells, Invasion, migration and apoptosis (46).
YAP1 is a Hippo signaling pathway gene, which is amplified in a variety of human cancers (61). YAP1 is a transcriptional regulator that is widely activated in human malignancies and can induce the proliferation, metastasis, stemness and chemotherapy resistance of cancer cells (61). In colorectal cancer, MIR4435-2HG binds miR-206-3p to up-regulate downstream YAP1 expression. MIR4435-2HG promotes the proliferation, invasion, migration and EMT of colorectal cancer cells by activating the Hippo signaling pathway, and promotes tumor growth in vivo (1).
The MAPK/extracellular signal-regulated kinase (ERK) signaling pathway is highly conserved. The MAPK/ERK signaling pathway involves a variety of biological events, including metabolic reprogramming, cell proliferation, survival, and differentiation (62). In the MAPK/ERK signaling pathway, mutations and dysfunctions of key genes are very common events in various human malignancies (62). In HCC cells, MIR4435-2HG can promote the phosphorylation of ERK, p38, and c-Jun N-terminal kinase (JNK), and activate the MAPK/ERK signaling pathway, thereby promoting HCC cell proliferation, cell cycle progression, and survival (20).
The competing endogenous RNA (ceRNA) hypothesis describes that lncRNA and mRNA may bind to the same miRNA through their miRNA response element (MRE) (52). lncRNAs and miRNAs are the two main subgroups of ncRNAs, and both have been shown to be key players in cancer biology (19).
Here, this article outlines the ceRNA network centered on MIR4435-2HG and its biological significance (Figure 6). MIR4435-2HG can be used as the ceRNA of 20 miRNAs, 19 of which are found in 14 cancers, including miR-22-3p (18), miR-206-3 (1), miR-296-5p (1), miR-497-5p (11), miR-138-5p (53), miR-203a-3p (13), miR-6754-5p (25), miR-204-5p (26), miR-528 (30), miR-202 (30), miR-125a-5p (52), miR125b-5p (52), miR-1224-5p (44), miR-4288-3p (41), miR-513a-5p (3), miR-128- 3p (33), miR-93-3p (45), miR-802-5p (48), and miR-383-5p (47). In addition, in osteoarthritis, MIR4435-2HG was found to sponge miR-510-3p (63).
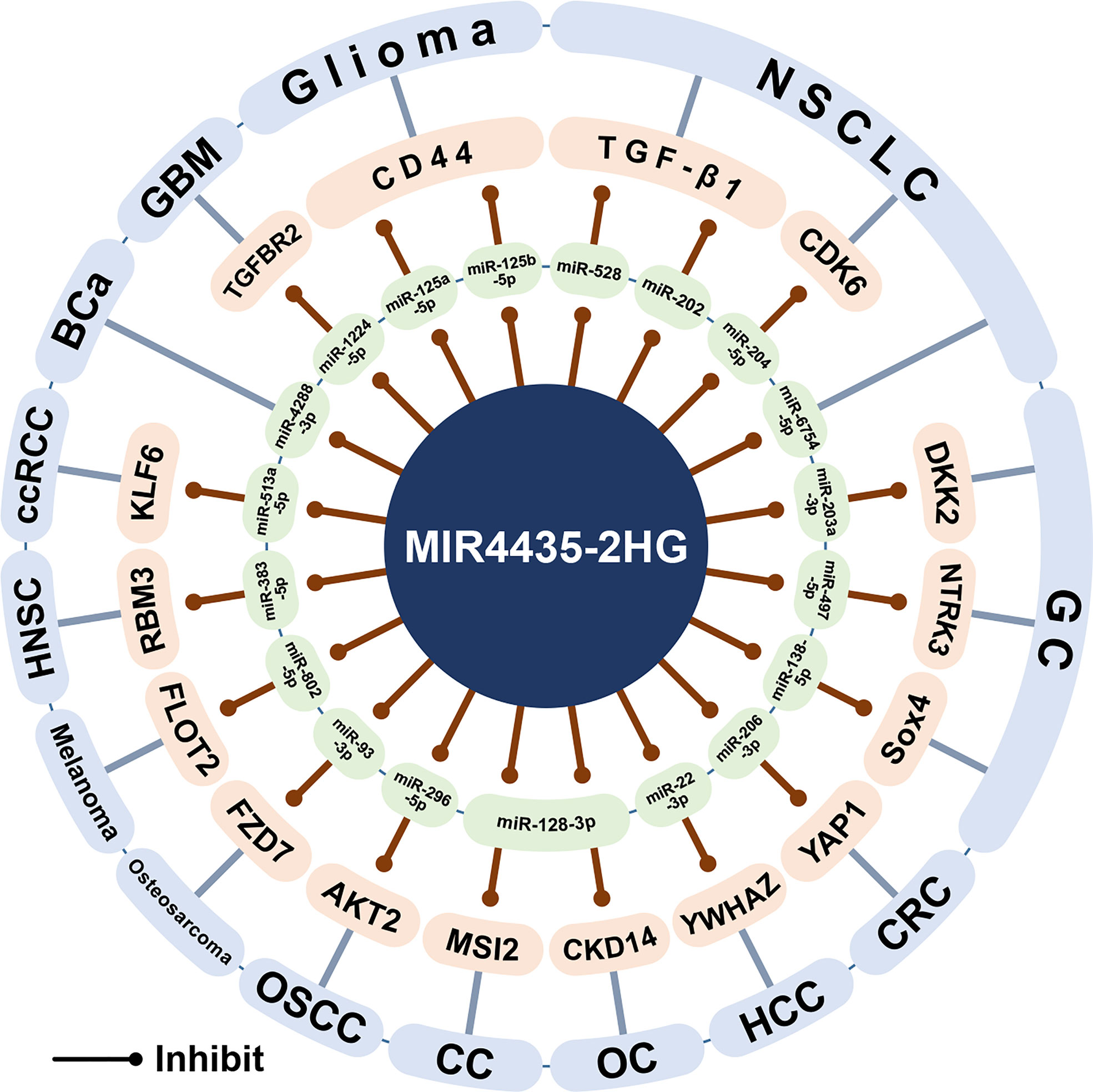
Figure 6 The ceRNA network of MIR4435-2HG. MIR4435-2HG can interact with 19 miRNAs in at least 14 cancers and osteoarthritis, and regulate the expression of its downstream target genes. HCC, Hepatocellular carcinoma; CRC, Colorectal cancer; OSCC, Oral squamous cell carcinoma; GC, Gastric cancer; NSCLC, Non-small cell lung cancer; GBM, Glioblastoma; BCa, Bladder cancer; ccRCC, clear cell renal cell carcinoma; OC, Ovarian cancer; CC, Cervical cancer; HNSC, Head and neck squamous cell carcinoma.
In the digestive system, MIR4435-2HG can up-regulate YWHAZ and YAP1 by competitively binding miR-22-3p and miR-206-3p to promote the progression of hepatocellular carcinoma and colorectal cancer, respectively (1, 18). In oral squamous cell carcinoma, the MIR4435-2HG/miR-296-5p axis inhibits AKT2, thereby promoting the expression of Snail1, an important transcription factor regulating EMT (64). In gastric cancer, MIR4435-2HG has been shown to promote gastric cancer progression through the miR-497-5p/NTRK3 axis (11) and the miR-138-5p/Sox4 axis (53). However, another study showed that the expression of MIR4435-2HG decreased in gastric cancer, and DKK2 was down-regulated through the MIR4435-2HG/miR-203a-3p axis to inhibit tumor progression (13). It is worth noting that the results of three gastric cancer studies have shown that the expression of MIR4435-2HG in gastric cancer cell lines (HGC-27, BGC-823, SGC-7901, SNU5, AGS, and MGC-803) is higher than that in gastric mucosal cell lines (GES-1) (10–12). However, one study showed that the expression of MIR4435-2HG in four gastric cancer cell lines (HGC-27, BGC-823, SGC-7901, and MKN-45) was lower than that of gastric mucosal cell line (GES-1) (13).
In non-small cell lung cancer, MIR4435-2HG can sponge miR-528 and miR-202, and subsequently up-regulate TGF-β1 to promote tumor growth (30). In addition, MIR4435-2HG/miR-204-5p/CDK6 axis and MIR4435-2HG/miR-6754-5p axis can promote the growth and invasion of non-small cell lung cancer (25, 26). In gliomas, MIR4435-2HG/miR-125a-5p axis and MIR4435-2HG/miR125b-5p axis can up-regulate CD44 and promote tumor progression (52). In glioblastoma, MIR4435-2HG competitively binds miR-1224-5p, thereby up-regulating TGFBR2 and promoting tumor growth (44). In bladder cancer, MIR4435-2HG can sponge miR-4288-3p and promote tumor growth and invasion (41). In clear cell renal cell carcinoma, MIR4435-2HG/miR-513a-5p promotes tumorigenesis and development by promoting the expression of KLF6 (3). In ovarian cancer and cervical cancer (33, 34), MIR4435-2HG can competitively bind miR-128-3p and up-regulate CKD14 and MSI2, thereby promoting tumor progression. In osteosarcoma, the MIR4435-2HG/miR-93-3p axis can up-regulate FZD7 and promote tumor progression (45). In melanoma, the MIR4435-2HG/miR-802-5p axis can promote FLOT2 expression and promote tumor growth and invasion (48). In HNSC, the MIR4435-2HG/miR-383-5p axis can up-regulate RBM3, thereby promoting tumor progression (47). In addition, in osteoarthritis, low expression of MIR4435-2HG can attenuate the MIR4435-2HG/miR-510-3p/IL-17A axis signal and activate the NF-κB signaling pathway, thereby mediating the process of osteoarthritis (63).
In summary, the carcinogenic effect of MIR4435-2HG is through sponging miRNAs to regulate the expression of the downstream target genes. In addition, in osteoarthritis, the low expression of MIR4435-2HG promotes the process of osteoarthritis through the ceRNA network.
As shown in Table 2, the abnormal up-regulation of MIR4435-2HG is closely related to the clinicopathological characteristics of 11 tumors. In tumors of the digestive system, high expression of MIR4435-2HG is associated with larger tumors, advanced TNM staging and lymph node metastasis in esophageal squamous cell carcinom (9), gastric cancer (10–12), colorectal cancer (1, 14, 16), and hepatocellular carcinoma (18–20, 23). In esophageal squamous cell carcinom, the upregulation of MIR4435-2HG is also related to tumor differentiation and advanced UICC (Union for International Cancer Control) staging (8, 9). In colorectal cancer, the up-regulated MIR4435-2HG is positively correlated with tumor grade and patient age (15). In hepatocellular carcinoma, high expression of MIR4435-2HG is significantly correlated with distant metastasis, advanced Edmondson grade, incomplete encapsulation, microvascular invasion, and advanced BCLC stage (18, 20, 22).
In breast cancer, MIR4435-2HG is negatively correlated with hormone receptor levels (36). In TNBC, the up-regulated MIR4435-2HG also points to larger tumors and higher TNM stages (37). In ovarian cancer and cervical cancer (33, 34), the high expression level of MIR4435-2HG is also related to advanced FIGO (Federation of Gynecology and Obstetrics) stage and lymph node metastasis. In ovarian cancer, MIR4435-2HG is also closely related to larger tumors and distant metastasis of tumors (31, 33). In lung cancer, high expression of MIR4435-2HG is significantly associated with larger tumors, higher TNM stages, stronger lymph node metastasis, and distant metastasis of the tumor (24, 25, 27, 29). The expression level of MIR4435-2HG in lung cancer tissues and serum of lung cancer patients is significantly positively correlated with tumor size and smoking habits (29). In addition, in clear cell renal cell carcinoma (40), HNSC (47), and osteosarcoma (45), MIR4435-2HG was found to be closely related to advanced TNM stages. In addition, MIR4435-2HG also points to large tumor size and advanced Fuhrman grade in clear cell renal cell carcinoma and distant metastasis of tumors in osteosarcoma (40, 45).
Abnormal up-regulation of MIR4435-2HG has potential value for cancer diagnosis and prognosis. The high expression of MIR4435-2HG is associated with a significant reduction in the overall survival (OS) of patients with 12 types of tumors (Table 2), including esophageal squamous cell carcinoma (8, 9), gastric cancer (11), colorectal cancer (1, 14, 17), hepatocellular carcinoma (18, 20, 22, 23), triple-negative breast cancer (TNBC) (37), ovarian cancer (31, 33), prostate cancer (38), lung cancer (24, 26, 29), glioblastoma (44), clear cell renal cell carcinoma (40), HNSC (47), and osteosarcoma (45). Among them, high MIR4435-2HG expression is significantly associated with shorter disease-free survival (DFS) in patients with esophageal squamous cell carcinoma (8), colorectal cancer (1, 14), breast cancer (36), or HNSC (47). In addition, high expression of MIR4435-2HG is also associated with shorter recurrence-free survival (RFS) in patients with hepatocellular carcinoma (22), clear cell renal cell carcinoma (40), or osteosarcoma (45). MIR4435-2HG is also positively correlated with postoperative distant recurrence in patients with non-small cell lung cancer (28).
As shown in Table 2, the high expression level of MIR4435-2HG in the tumor tissues and/or blood (whole blood, serum, and plasma) of cancer patients has proved to be of great diagnostic value in 9 cancers. In the tissues and serum of gastric cancer (12), colorectal cancer (15, 17), and non-small cell lung cancer (29), high expression of MIR4435-2HG can distinguish tumor patients from normal controls. In hepatocellular carcinoma serum (21), clear cell renal cell carcinoma tissue (40), and childhood T-ALL bone marrow (49), higher expression levels of MIR4435-2HG can distinguish tumor patients from normal controls. It is worth noting that the high expression of MIR4435-2HG in the serum of colon cancer and the plasma of TNBC and ovarian cancer can effectively distinguish patients with early-stage tumors (stage I-II) and healthy controls Group (6, 16, 32), suggesting that MIR4435-2HG may be used as an early diagnostic marker for these three tumors. In addition, in gliomas, the highly expressed MIR4435-2HG can distinguish metastatic tumors from healthy controls, but cannot effectively distinguish non-metastatic gliomas from normal healthy controls. This indicates that MIR4435-2HG may be involved in the process of glioma metastasis and can be used to diagnose glioma metastasis (42).
MIR4435-2HG has also been shown to be involved in the mechanism of action of a variety of tumor treatment drugs, including resveratrol for the treatment of lung cancer (2), cisplatin for non-small cell lung cancer and colon cancer (4, 5), and carboplatin for three-negative breast cancer (Figure 7) (6).
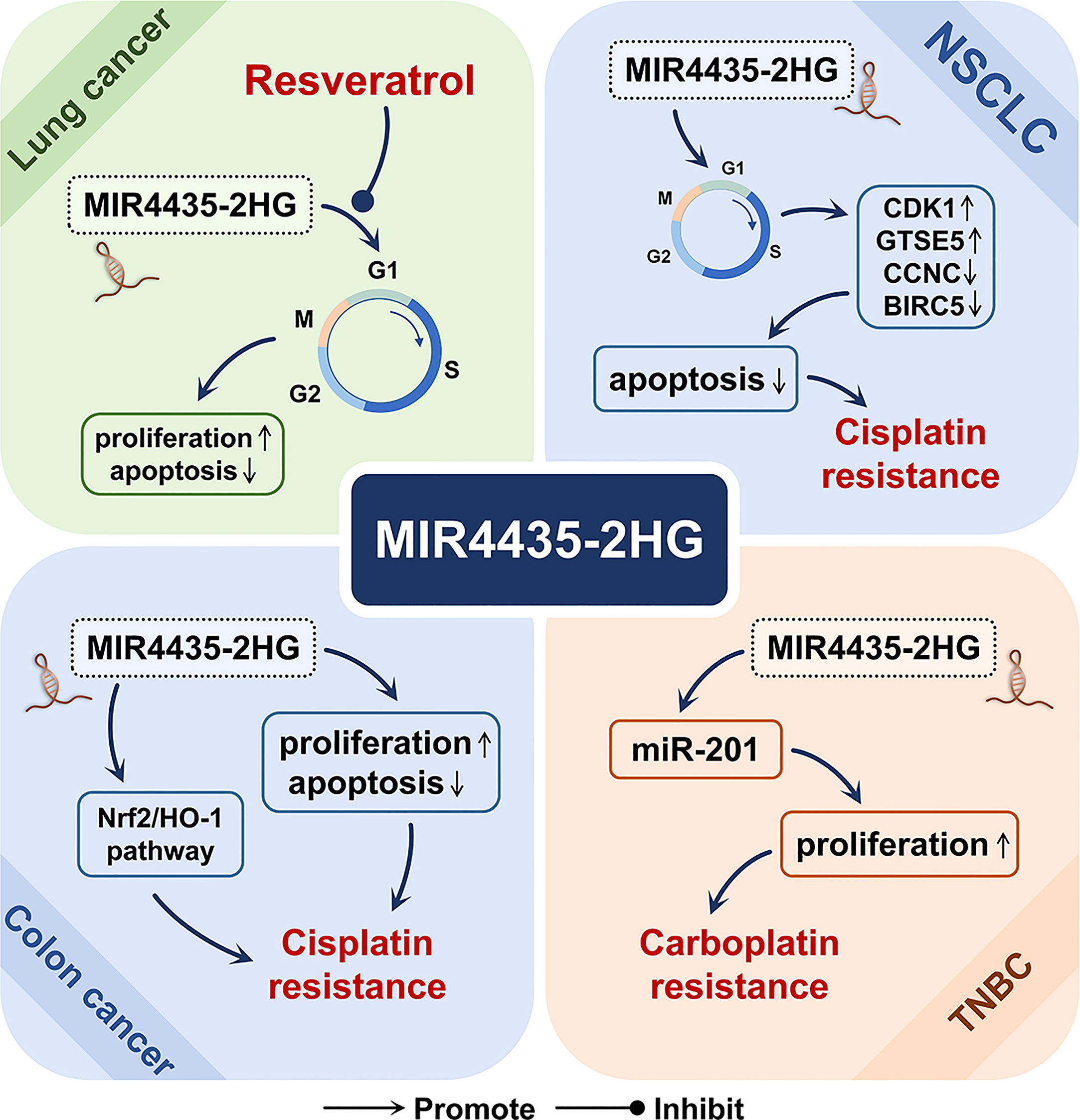
Figure 7 The role of MIR4435-2HG in cancer drugs. In lung cancer, MIR4435-2HG may be involved in the inhibitory effect of resveratrol on the growth of lung cancer cells. In non-small cell lung cancer (NSCLC) and colon cancer, MIR4435-2HG may be a driving factor for cisplatin resistance. In triple-negative breast cancer (TNBC), MIR4435-2HG may be involved in the development of carboplatin resistance.
Resveratrol is a natural polyphenol, found in various plants and Chinese herbal medicines. Due to its relatively low toxicity, it can promote cancer by targeting a variety of signaling molecules for cell survival and tumor growth. It is considered an ideal chemopreventive agent (2). As an oncogenic lncRNA, MIR4435-2HG is highly expressed in lung cancer cell lines, and its expression is down-regulated after resveratrol treatment, thereby inhibiting the proliferation and growth of lung cancer cells (2).
Cisplatin is widely used in the treatment of various cancers, but the emergence of cisplatin resistance is a serious clinical problem (5). In non-small cell lung cancer, MIR4435-2HG knockdown can reduce the cisplatin resistance and cell viability of the cisplatin-resistant cell line A549/DDP, and cause cell cycle arrest, which significantly increases the ratio in the G0/G1 phase. Meanwhile, MIR4435-2HG knockdown positively induced the expression of apoptosis-related factors (CCNC and BIRC5), and inhibited the expression of cell cycle-related factors (CDK1 and GTSE5), thereby promoting cell apoptosis (4). In colon cancer, MIR4435-2HG is highly expressed in the cisplatin-resistant cell line HCT116R, and MIR4435-2HG knockdown can significantly restore the sensitivity of cells to cisplatin, inhibit cell proliferation, and promote cell apoptosis (5). In addition, in colon cancer, MIR4435-2HG knockdown can reduce the transcription levels of key molecules (Nrf2 and HO-1) in the oxidative stress pathway (5).
Carboplatin is a cisplatin derivative with broad-spectrum anti-tumor activity. It can be used as a single drug or combined to treat multiple tumors (65). In triple-negative breast cancer (TNBC), overexpression of MIR4435-2HG and miR-21 can promote the proliferation of cancer cells treated with carboplatin, improve the viability of cancer cells, and induce chemotherapy resistance (6).
In summary, MIR4435-2HG may be involved in the inhibitory effect of resveratrol on the growth of lung cancer cells and may be an important driving factor for cisplatin and carboplatin resistance.
MIR4435-2HG is a lncRNA with great potential, which can be used as a diagnostic and prognostic biomarker for a variety of tumors, and a therapeutic target for a variety of tumors. MIR4435-2HG was abnormally up-regulated in tumor tissues and cell lines as an oncogene in 18 tumors, and its overexpression was also detected in the blood, plasma, or serum of 9 tumors. At the same time, MIR4435-2HG is closely related to the clinical characteristics and poor prognosis of 12 tumors. This may mean that MIR4435-2HG can be highly expressed and detected in human blood besides tumor tissues. In the future, the relationship between MIR4435-2HG and tumor development can be studied in more tumors. In addition, there is only one study on the methylation of MIR4435-2HG in gliomas (52), and the mechanism of MIR4435-2HG overexpression in these tumors has not been elucidated. Epigenetic research can provide hints for elucidating the molecular mechanism of MIR4435-2HG.
MIR4435-2HG can participate in at least 6 signal pathways and form a ceRNA network with miRNAs to promote the occurrence and development of tumors. In tumors, MIR4435-2HG can participate in the regulation of signaling pathways and affect different biological processes of tumors. For example, non-small cell lung cancer is involved in TGF-β signaling (27, 28, 30) and Wnt/β-catenin signaling (29). Colorectal cancer is involved in the Wnt/β-catenin signaling pathway (15) and the Hippo signaling pathway (1). This may provide ideas for exploring new tumor treatment strategies. However, the specific mechanism of MIR4435-2HG in the pathway has not been well explained. Meanwhile, the existing research on MIR4435-2HG mostly focuses on the “lncRNA-miRNA-mRNA” axis, however, the research on the relationship between other non-coding RNAs of MIR4435-2HG is still lacking. For example, MIR4435-2HG and BCL2L11 genes co-localize to chr2 q13, and the lncRNA Morrbid (a myeloid RNA regulator of BCL2L11-induced cell death) is involved in the regulation of N-ras splicing in mouse hepatocytes and is associated with tumorigenesis (66). In the future, it is necessary to further study the regulatory mechanism of MIR4435-2HG and improve its ceRNA network. In addition, the expression of MIR4435-2HG may also be closely associated with nearby genetic variants. For example, the rs17041869 site located in the enhancer of BCL2L11 can regulate the expression of the BCL2L11 gene near MIR4435-2HG (67), suggesting the need to explore genetic variants associated with MIR4435-2HG in the future.
In addition, it needs to be further explored for the application of MIR4435-2HG in the blood of tumor patients in the diagnosis and prognosis of tumors. The connection between MIR4435-2HG and tumor treatment drugs lays the foundation for the clinical treatment of tumors. In the future, it is necessary to test the role of MIR4435-2HG in the treatment of more cancer drugs.
SD, CY, and CZ contributed to the conception, design and final approval of the submitted version. CZ and ZX collected and analyzed literature. CZ, ZX, L-hZ, CY, and SD contributed to manuscript writing. All the authors conceived and gave the approval of the final manuscript.
The research was supported by National Natural Science Foundation of China (32100521) and Qiantang Scholar Fund in Zhejiang University City College.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Dong X, Yang Z, Yang H, Li D, Qiu X. Long Non-Coding RNA MIR4435-2hg Promotes Colorectal Cancer Proliferation and Metastasis Through miR-206/YAP1 Axis. Front Oncol (2020) 10:160. doi: 10.3389/fonc.2020.00160
2. Yang Q, Xu E, Dai J, Liu B, Han Z, Wu J, et al. A Novel Long Noncoding RNA AK001796 Acts as an Oncogene and is Involved in Cell Growth Inhibition by Resveratrol in Lung Cancer. Toxicol Appl Pharmacol (2015) 285(2):79–88. doi: 10.1016/j.taap.2015.04.003
3. Zhu K, Miao C, Tian Y, Qin Z, Xue J, Xia J, et al. lncRNA MIR4435-2HG Promoted Clear Cell Renal Cell Carcinoma Malignant Progression via miR-513a-5p/KLF6 Axis. J Cell Mol Med (2020) 24(17):10013–26. doi: 10.1111/jcmm.15609
4. Liu B, Pan CF, Ma T, Wang J, Yao GL, Wei K, et al. Long Noncoding RNA AK001796 Contributes to Cisplatin Resistance of Nonsmall Cell Lung Cancer. Mol Med Rep (2017) 16(4):4107–12. doi: 10.3892/mmr.2017.7081
5. Luo P, Wu SG, Ji KB, Yuan X, Li HM, Chen JP, et al. LncRNA MIR4435-2HG Mediates Cisplatin Resistance in HCT116 Cells by Regulating Nrf2 and HO-1. PLoS One (2020) 15(11):e0223035. doi: 10.1371/journal.pone.0223035
6. Liu AN, Qu HJ, Gong WJ, Xiang JY, Yang MM, Zhang W. LncRNA AWPPH and miRNA-21 Regulates Cancer Cell Proliferation and Chemosensitivity in Triple-Negative Breast Cancer by Interacting With Each Other. J Cell Biochem (2019) 120(9):14860–6. doi: 10.1002/jcb.28747
7. Shen H, Sun B, Yang Y, Cai X, Bi L, Deng L, et al. MIR4435-2HG Regulates Cancer Cell Behaviors in Oral Squamous Cell Carcinoma Cell Growth by Upregulating TGF-Beta1. Odontology (2020) 108(4):553–9. doi: 10.1007/s10266-020-00488-x
8. Zong MZ, Shao Q, An XS. Expression and Prognostic Significance of Long Noncoding RNA AK001796 in Esophageal Squamous Cell Carcinoma. Eur Rev Med Pharmacol Sci (2019) 23(1):181–6.
9. Liu B, Pan CF, Yao GL, Wei K, Xia Y, Chen YJ. The Long non-Coding RNA AK001796 Contributes to Tumor Growth via Regulating Expression of P53 in Esophageal Squamous Cell Carcinoma. Cancer Cell Int (2018) 18:38. doi: 10.1186/s12935-018-0537-8
10. Wang HY, Wu MJ, Lu YM, He KF, Cai XL, Yu XF, et al. LncRNA MIR4435-2HG Targets Desmoplakin and Promotes Growth and Metastasis of Gastric Cancer by Activating Wnt/β-Catenin Signaling. Aging (2019) 11(17):6657–73. doi: 10.18632/aging.102164
11. Bu JY, Lv WZ, Liao YF, Xiao XY, Lv BJ. Long non-Coding RNA LINC00978 Promotes Cell Proliferation and Tumorigenesis via Regulating microRNA-497/NTRK3 Axis in Gastric Cancer. Int J Biol Macromol (2019) 123:1106–14. doi: 10.1016/j.ijbiomac.2018.11.162
12. Fu M, Huang Z, Zang X, Pan L, Liang W, Chen J, et al. Long Noncoding RNA LINC00978 Promotes Cancer Growth and Acts as a Diagnostic Biomarker in Gastric Cancer. Cell Prolif (2018) 51(1). doi: 10.1111/cpr.12425
13. Li L, Kou J, Zhong B. Up-Regulation of Long non-Coding RNA AWPPH Inhibits Proliferation and Invasion of Gastric Cancer Cells via miR-203a/DKK2 Axis. Hum Cell (2019) 32(4):495–503. doi: 10.1007/s13577-019-00277-x
14. Shen MY, Zhou GR, Zhang ZY. LncRNA MIR4435-2HG Contributes Into Colorectal Cancer Development and Predicts Poor Prognosis. Eur Rev Med Pharmacol Sci (2020) 24(4):1771–7.
15. Ghasemian M, Rajabibazl M, Mirfakhraie R, Razavi AE, Sadeghi H. Long Noncoding RNA LINC00978 Acts as a Potential Diagnostic Biomarker in Patients With Colorectal Cancer. Exp Mol Pathol (2021) 122:104666. doi: 10.1016/j.yexmp.2021.104666
16. Bai J, Xu J, Zhao J, Zhang R. Downregulation of lncRNA AWPPH Inhibits Colon Cancer Cell Proliferation by Downregulating GLUT-1. Oncol Lett (2019) 18(2):2007–12. doi: 10.3892/ol.2019.10515
17. Liu C, Han B, Xin J, Yang C. LncRNA-AWPPH Activates TGF-Beta1 in Colorectal Adenocarcinoma. Oncol Lett (2019) 18(5):4719–25. doi: 10.3892/ol.2019.10794
18. Shen XL, Ding YT, Lu F, Yuan HT, Luan WK. Long Noncoding RNA MIR4435-2HG Promotes Hepatocellular Carcinoma Proliferation and Metastasis Through the miR-22-3p/YWHAZ Axis. Am J Transl Res (2020) 12(10):6381–94. doi: 10.3389/fonc.2020.00160
19. Kong Q, Liang C, Jin Y, Pan Y, Tong D, Kong Q, et al. The lncRNA MIR4435-2HG is Upregulated in Hepatocellular Carcinoma and Promotes Cancer Cell Proliferation by Upregulating miRNA-487a. Cell Mol Biol Lett (2019) 24:26. doi: 10.1186/s11658-019-0148-y
20. Zhang Q, Cheng S, Cao L, Yang J, Wang Y, Chen Y. LINC00978 Promotes Hepatocellular Carcinoma Carcinogenesis Partly via Activating the MAPK/ERK Pathway. Biosci Rep (2020) 40(3). doi: 10.1042/BSR20192790
21. Xu X, Gu J, Ding X, Ge G, Zang X, Ji R, et al. LINC00978 Promotes the Progression of Hepatocellular Carcinoma by Regulating EZH2-Mediated Silencing of P21 and E-Cadherin Expression. Cell Death Dis (2019) 10(10):752. doi: 10.1038/s41419-019-1990-6
22. Zhao X, Liu Y, Yu S. Long Noncoding RNA AWPPH Promotes Hepatocellular Carcinoma Progression Through YBX1 and Serves as a Prognostic Biomarker. Biochim Biophys Acta Mol Basis Dis (2017) 1863(7):1805–16. doi: 10.1016/j.bbadis.2017.04.014
23. Han QL, Chen BT, Zhang KJ, Xia ST, Zhong WW, Zhao ZM. The Long non-Coding RNA AK001796 Contributes to Poor Prognosis and Tumor Progression in Hepatocellular Carcinoma. Eur Rev Med Pharmacol Sci (2019) 23(5):2013–9.
24. Qian H, Chen L, Huang J, Wang X, Ma S, Cui F, et al. The lncRNA MIR4435-2HG Promotes Lung Cancer Progression by Activating Beta-Catenin Signalling. J Mol Med (Berl) (2018) 96(8):753–64. doi: 10.1007/s00109-018-1654-5
25. Li X, Ren Y, Zuo T. Long Noncoding RNA LINC00978 Promotes Cell Proliferation and Invasion in Nonsmall Cell Lung Cancer by Inhibiting Mir67545p. Mol Med Rep (2018) 18(5):4725–32. doi: 10.3892/mmr.2018.9463
26. Wu D, Qin BY, Qi XG, Hong LL, Zhong HB, Huang JY. LncRNA AWPPH Accelerates the Progression of non-Small Cell Lung Cancer by Sponging miRNA-204 to Upregulate CDK6. Eur Rev Med Pharmacol Sci (2020) 24(8):4281–7.
27. Huo Y, Li A, Wang Z. LncRNA AWPPH Participates in the Metastasis of non-Small Cell Lung Cancer by Upregulating TGF-Beta1 Expression. Oncol Lett (2019) 18(4):4246–52. doi: 10.3892/ol.2019.10754
28. Tang L, Wang T, Zhang Y, Zhang J, Zhao H, Wang H, et al. Long Non-Coding RNA AWPPH Promotes Postoperative Distant Recurrence in Resected Non-Small Cell Lung Cancer by Upregulating Transforming Growth Factor Beta 1 (TGF-Beta1). Med Sci Monit (2019) 25:2535–41. doi: 10.12659/MSM.912876
29. Song Z, Du J, Zhou L, Sun B. lncRNA AWPPH Promotes Proliferation and Inhibits Apoptosis of Nonsmall Cell Lung Cancer Cells by Activating the Wnt/betacatenin Signaling Pathway. Mol Med Rep (2019) 19(5):4425–32. doi: 10.3892/mmr.2019.10089
30. Yang M, He X, Huang X, Wang J, He Y, Wei L. LncRNA MIR4435-2HG-Mediated Upregulation of TGF-Beta1 Promotes Migration and Proliferation of Nonsmall Cell Lung Cancer Cells. Environ Toxicol (2020) 35(5):582–90. doi: 10.1002/tox.22893
31. Yu G, Wang W, Deng J, Dong S. LncRNA AWPPH Promotes the Proliferation, Migration and Invasion of Ovarian Carcinoma Cells via Activation of the Wnt/betacatenin Signaling Pathway. Mol Med Rep (2019) 19(5):3615–21. doi: 10.3892/mmr.2019.10029
32. Gong J, Xu X, Zhang X, Zhou Y. LncRNA MIR4435-2HG is a Potential Early Diagnostic Marker for Ovarian Carcinoma. Acta Biochim Biophys Sin (Shanghai) (2019) 51(9):953–9. doi: 10.1093/abbs/gmz085
33. Zhu L, Wang A, Gao M, Duan X, Li Z. LncRNA MIR4435-2HG Triggers Ovarian Cancer Progression by Regulating miR-128-3p/CKD14 Axis. Cancer Cell Int (2020) 20:145. doi: 10.1186/s12935-020-01227-6
34. Wang R, Liu L, Jiao J, Gao D. Knockdown of MIR4435-2hg Suppresses the Proliferation, Migration and Invasion of Cervical Cancer Cells via Regulating the miR-128-3p/MSI2 Axis In Vitro. Cancer Manag Res (2020) 12:8745–56. doi: 10.2147/CMAR.S265545
35. Chen D, Tang P, Wang Y, Wan F, Long J, Zhou J, et al. Downregulation of Long non-Coding RNA MR4435-2HG Suppresses Breast Cancer Progression via the Wnt/beta-Catenin Signaling Pathway. Oncol Lett (2021) 21(5):373. doi: 10.3892/ol.2021.12634
36. Deng LL, Chi YY, Liu L, Huang NS, Wang L, Wu J. LINC00978 Predicts Poor Prognosis in Breast Cancer Patients. Sci Rep (2016) 6:37936. doi: 10.1038/srep37936
37. Wang K, Li X, Song C, Li M. LncRNA AWPPH Promotes the Growth of Triple-Negative Breast Cancer by Up-Regulating Frizzled Homolog 7 (FZD7). Biosci Rep (2018) 38(6). doi: 10.1042/BSR20181223
38. Zhang H, Meng H, Huang X, Tong W, Liang X, Li J, et al. lncRNA MIR4435-2HG Promotes Cancer Cell Migration and Invasion in Prostate Carcinoma by Upregulating TGF-Beta1. Oncol Lett (2019) 18(4):4016–21. doi: 10.3892/ol.2019.10757
39. Xing P, Wang Y, Zhang L, Ma C, Lu J. Knockdown of lncRNA MIR44352HG and ST8SIA1 Expression Inhibits the Proliferation, Invasion and Migration of Prostate Cancer Cells In Vitro and In Vivo by Blocking the Activation of the FAK/AKT/betacatenin Signaling Pathway. Int J Mol Med (2021) 47(6). doi: 10.3892/ijmm.2021.4926
40. Wu K, Hu L, Lv X, Chen J, Yan Z, Jiang J, et al. Long non-Coding RNA MIR4435-1HG Promotes Cancer Growth in Clear Cell Renal Cell Carcinoma. Cancer biomark (2020) 29(1):39–50. doi: 10.3233/CBM-201451
41. Wang W, Xu Z, Wang J, Chen R. LINC00978 Promotes Bladder Cancer Cell Proliferation, Migration and Invasion by Sponging Mir4288. Mol Med Rep (2019) 20(2):1866–72. doi: 10.3892/mmr.2019.10395
42. Zhang T, Wang F, Liao Y, Yuan L, Zhang B. LncRNA AWPPH Promotes the Invasion and Migration of Glioma Cells Through the Upregulation of HIF1alpha. Oncol Lett (2019) 18(6):6781–6. doi: 10.3892/ol.2019.11018
43. Dai B, Xiao Z, Mao B, Zhu G, Huang H, Guan F, et al. lncRNA AWPPH Promotes the Migration and Invasion of Glioma Cells by Activating the TGF-Beta Pathway. Oncol Lett (2019) 18(6):5923–9. doi: 10.3892/ol.2019.10918
44. Xu H, Zhang B, Yang Y, Li Z, Zhao P, Wu W, et al. LncRNA MIR4435-2HG Potentiates the Proliferation and Invasion of Glioblastoma Cells via Modulating miR-1224-5p/TGFBR2 Axis. J Cell Mol Med (2020) 24(11):6362–72. doi: 10.1111/jcmm.15280
45. Li C, Wang F, Wei B, Wang L, Kong D. LncRNA AWPPH Promotes Osteosarcoma Progression via Activation of Wnt/beta-Catenin Pathway Through Modulating miR-93-3p/FZD7 Axis. Biochem Biophys Res Commun (2019) 514(3):1017–22. doi: 10.1016/j.bbrc.2019.04.203
46. Ding W, Wu D, Ji F, Zhang H. Inhibition of Long non-Coding RNA-AWPPH Decreases Osteosarcoma Cell Proliferation, Migration and Invasion. Oncol Lett (2019) 18(5):5055–62. doi: 10.3892/ol.2019.10898
47. Wang S, Chen X, Qiao T. Long Noncoding RNA MIR44352HG Promotes the Progression of Head and Neck Squamous Cell Carcinoma by Regulating the Mir3835p/RBM3 Axis. Oncol Rep (2021) 45(6). doi: 10.3892/or.2021.8050
48. Ma DM, Sun D, Wang J, Jin DH, Li Y, Han YE. Long non-Coding RNA MIR4435-2HG Recruits miR-802 From FLOT2 to Promote Melanoma Progression. Eur Rev Med Pharmacol Sci (2020) 24(5):2616–24. doi: 10.26355/eurrev_202003_20530
49. Li X, Song F, Sun H. Long non-Coding RNA AWPPH Interacts With ROCK2 and Regulates the Proliferation and Apoptosis of Cancer Cells in Pediatric T-Cell Acute Lymphoblastic Leukemia. Oncol Lett (2020) 20(5):239. doi: 10.3892/ol.2020.12102
50. Guo DQ, Liu F, Zhang L, Bian NN, Liu LY, Kong LX, et al. Long non-Coding RNA AWPPH Enhances Malignant Phenotypes in Nasopharyngeal Carcinoma via Silencing PTEN Through Interacting With LSD1 and EZH2. Biochem Cell Biol (2021) 99(2):195–202. doi: 10.1139/bcb-2019-0497
51. Wang ZH, Yang B, Zhang M, Guo WW, Wu ZY, Wang Y, et al. lncRNA Epigenetic Landscape Analysis Identifies EPIC1 as an Oncogenic lncRNA That Interacts With MYC and Promotes Cell-Cycle Progression in Cancer. Cancer Cell (2018) 33(4):706–20. doi: 10.1016/j.ccell.2018.03.006
52. Li ZJ, Tan H, Zhao WL, Xu YG, Zhang ZG, Wang MD, et al. Integrative Analysis of DNA Methylation and Gene Expression Profiles Identifies MIR4435-2HG as an Oncogenic lncRNA for Glioma Progression. Gene (2019) 5:705. doi: 10.1016/j.gene.2019.144012
53. Gao LF, Li W, Liu YG, Zhang C, Gao WN, Wang L. Inhibition of MIR4435-2HG on Invasion, Migration, and EMT of Gastric Carcinoma Cells by Mediating MiR-138-5p/Sox4 Axis. Front Oncol (2021) 11:661288. doi: 10.3389/fonc.2021.661288
54. Wakefield LM, Roberts AB. TGF-β Signaling: Positive and Negative Effects on Tumorigenesis. Curr Opin Genet Dev (2002) 12(2):22–9. doi: 10.1016/S0959-437X(01)00259-3
55. Mirzaei H, Faghihloo E. Viruses as Key Modulators of the TGF-Beta Pathway; a Double-Edged Sword Involved in Cancer. Rev Med Virol (2018) 28(2). doi: 10.1002/rmv.1967
56. Katsuno Y, Lamouille S, Derynck R. TGF-β Signaling and Epithelial-Mesenchymal Transition in Cancer Progression. Curr Opin Oncol (2013) 25(1):76–84. doi: 10.1097/CCO.0b013e32835b6371
57. Nishita M, Hashimoto MK, Ogata S, Laurent MN, Ueno N, Shibuya H, et al. Interaction Between Wnt and TGF-Beta Signalling Pathways During Formation of Spemann's Organizer. Nature (2000) 403(6771):781–5. doi: 10.1038/35001602
58. Li ZW, Zhao L, Wang QG. Overexpression of Long non-Coding RNA HOTTIP Increases Chemoresistance of Osteosarcoma Cell by Activating the Wnt/β-Catenin Pathway. Am J Transl Res (2016) 8(5):2385–93.
59. Giannikaki E, Kouvidou C, Tzardi M, Stefanaki K, Koutsoubi K, Gregoriou M, et al. P53 Protein Expression in Breast Carcinomas. Comparative Study With the Wild Type P53 Induced Proteins Mdm2 and P21/Waf1. Anticancer Res (1997) 17(3C):2123–7.
60. Porta C, Paglino C, Mosca A. Targeting PI3K/Akt/mTOR Signaling in Cancer. Front Oncol (2014) 4:64. doi: 10.3389/fonc.2014.00064
61. Zanconato F, Cordenonsi M, Piccolo S. YAP/TAZ at the Roots of Cancer. Cancer Cell (2016) 29(6):783–803. doi: 10.1016/j.ccell.2016.05.005
62. Asl ER, Amini M, Najafi S, Mansoori B, Mokhtarzadeh A, Mohammadi A, et al. Interplay Between MAPK/ERK Signaling Pathway and MicroRNAs: A Crucial Mechanism Regulating Cancer Cell Metabolism and Tumor Progression. Life Sci (2021) 278:119499. doi: 10.1016/j.lfs.2021.119499
63. Liu Y, Yang Y, Ding L, Jia Y, Ji Y. LncRNA MIR4435-2HG Inhibits the Progression of Osteoarthritis Through miR-510-3p Sponging. Exp Ther Med (2020) 20(2):1693–701. doi: 10.3892/etm.2020.8841
64. Zhang S, Li C, Liu J, Geng F, Shi X, Li Q, et al. Fusobacterium Nucleatum Promotes Epithelial-Mesenchymal Transiton Through Regulation of the lncRNA MIR4435-2hg/miR-296-5p/Akt2/SNAI1 Signaling Pathway. FEBS J (2020) 287(18):4032–47. doi: 10.1111/febs.15233
65. Lynce F, Nunes R. Role of Platinums in Triple-Negative Breast Cancer. Curr Oncol Rep (2021) 23(5):50. doi: 10.1007/s11912-021-01041-x
66. Fefilova A, Melnikov P, Prikazchikova T, Abakumova T, Kurochkin I, Mazin PV, et al. Murine Long Noncoding RNA Morrbid Contributes in the Regulation of NRAS Splicing in Hepatocytes In Vitro. Int J Mol Sci (2020) 21(16):5605. doi: 10.3390/ijms21165605
67. Kar SP, Beesley J, Olama AAA, Michailidou K, Tyrer J, Kote-Jarai Z, et al. Genome-Wide Meta-Analyses of Breast, Ovarian, and Prostate Cancer Association Studies Identify Multiple New Susceptibility Loci Shared by at Least Two Cancer Types. Cancer Discov (2016) 6(8):1052–67. doi: 10.1158/2159-8290.CD-15-1227
Keywords: MIR4435-2HG, cancer, competing endogenous RNA, diagnosis, prognosis
Citation: Zhong C, Xie Z, Zeng L-h, Yuan C and Duan S (2022) MIR4435-2HG Is a Potential Pan-Cancer Biomarker for Diagnosis and Prognosis. Front. Immunol. 13:855078. doi: 10.3389/fimmu.2022.855078
Received: 14 January 2022; Accepted: 23 May 2022;
Published: 15 June 2022.
Edited by:
Lee Mark Wetzler, Boston University, United StatesReviewed by:
Jun Ma, University of Minnesota Twin Cities, United StatesCopyright © 2022 Zhong, Xie, Zeng, Yuan and Duan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shiwei Duan, ZHVhbnN3QHp1Y2MuZWR1LmNu; Chunhui Yuan, Y2hfeXVhbkB6anUuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.