- 1Howard University College of Medicine, Washington, DC, United States
- 2Cooper Medical School of Rowan University, Camden, NJ, United States
- 3Department of Psychiatry, Creighton University School of Medicine, Phoenix, AZ, United States
- 4Department of Dermatology, Howard University College of Medicine, Washington, DC, United States
Currently, there is a lack of racial/ethnic heterogeneity in research databases, exposing a systematic issue in studies exploring inflammation-mediated diseases, such as hidradenitis suppurativa (HS). HS is a chronic inflammatory skin condition that disrupts normal structure and functioning of terminal hair follicles, resulting in the formation of recurrent abscesses, nodules, and sinus tracts within intertriginous regions. Studies have described higher serum levels of inflammation-mediated C-reactive protein (CRP) in patients with HS, a disease that predominantly affects skin of color (SOC) populations. Herein, we explore the role of CRP levels in the context of HS disease presentation, management, and psychosocial implications in SOC patients to determine existing disparities in research studies.
Introduction
Skin of color (SOC) refers to individuals of African, Asian, Native American, Middle Eastern, and Hispanic backgrounds (1). According to the 2020 United States (US) Census, these persons collectively constitute nearly half of the population (2). Among the US and United Kingdom (UK) populations, the Black community accounts for 13.4% of 328 million and 3.3% of 56.1 million, respectively (3). Despite being one of the largest minority groups in both countries, this population is marginally included in research.
A recent article by Nagar et al. revealed higher serum levels of inflammation-mediated C-reactive protein (CRP) in UK populations composed largely of older, Black females. Notably, the sample included only 6,456 (0.1%) Black subjects and 426,842 (98.5%) White subjects (4). Compared to census demographics reported during the same time period (2006-2011), this sampled population underrepresents Black communities (3). Their findings suggest that socio-environmental factors play a consequential role in explaining these disparities. Yet, the lack of racial/ethnic heterogeneity in their dataset demonstrates a systematic issue in studies exploring inflammation-mediated diseases. This fundamental representation gap limits our understanding of disease biomarker influence on inflammatory skin conditions that disproportionately affect minority patients.
CRP is an acute phase reactant produced by the liver during inflammation. With pro-inflammatory and anti-inflammatory properties, CRP is essential in the clearance of foreign antigens and damaged cells (5). Traditionally, it has been used as a biomarker for infectious and cardiovascular events (6), but various studies also demonstrate a correlation between CRP levels and disease severity in inflammatory skin conditions (7–9) such as HS (10). Research shows that socioeconomic and psychosocial factors likely contribute to variations in CRP levels between Blacks and Whites. Low-income status and smoking activity contributed most to elevated CRP levels among older Black males, whereas obesity mainly contributed to elevated CRP levels in older Black females (11). Moreover, self-reported daily and lifetime discrimination by Blacks/African Americans (AA) correlated with increased CRP levels (12).
Here, we highlight the limited correlative research on CRP in SOC HS patients to address this population’s existing health disparities and poor psychosocial outcomes. The present study found that though differences in CRP levels have been related to HS severity, there is paucity of research exploring its correlation to race in the disease. Our aim is to encourage dialogue about the underrepresentation of Blacks/AA within CRP as well as HS investigations, underscoring the need for diversity in clinical and biomedical studies to produce generalizable data and results.
Hidradenitis Suppurativa
HS is a chronic inflammatory skin condition that disrupts normal structure and functioning of terminal hair follicles. It is characterized by the formation of recurrent abscesses, nodules, and sinus tracts in intertriginous regions, with severe complications including soft tissue infection, lymphedema, and sepsis (13–15). Although the pathogenesis is not entirely understood, studies show that HS is three times more prevalent in Blacks/AA compared to Whites (16, 17). Yet, there is a scarcity of diverse representation in research examining CRP's role in inflammatory diseases despite its strong association and increased levels in SOC patients.
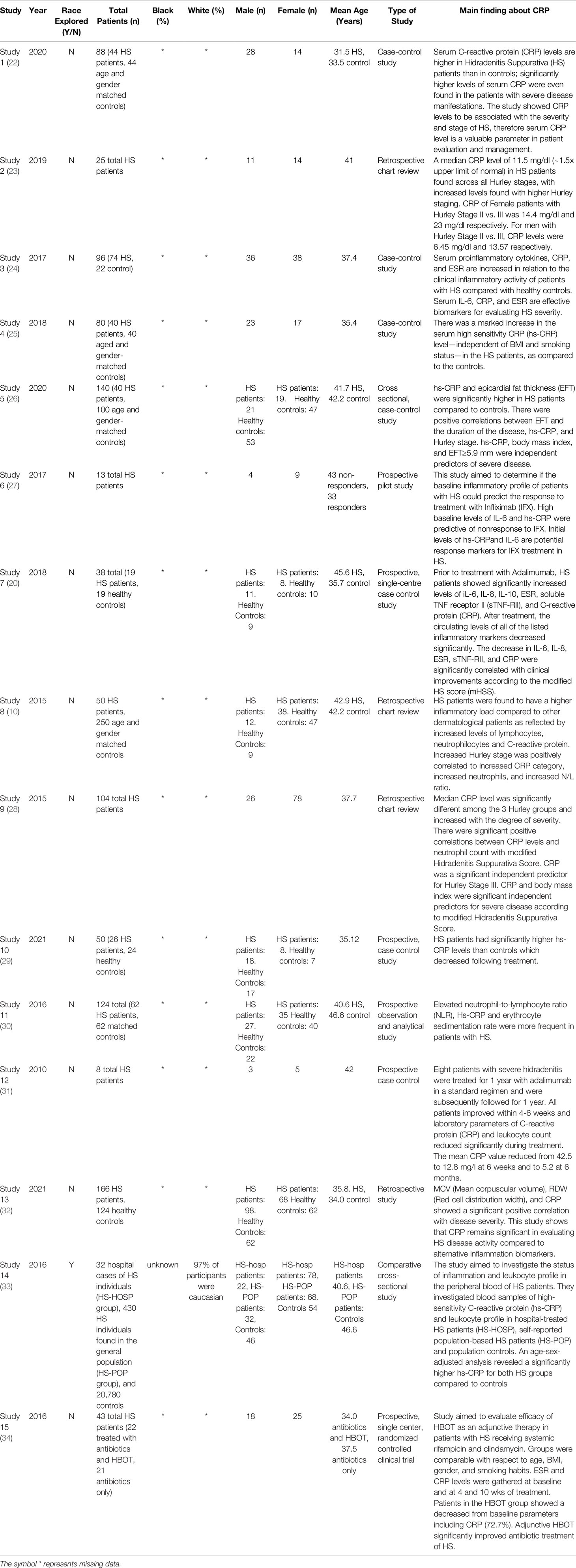
Quantitative evaluation has revealed higher CRP levels among HS patients than matched controls, independent of BMI and smoking status (10, 18). Moreover, CRP levels were found to be an independent predictor of Hurly Stage III, the most severe form of the disease (19). In patients with moderate-to-severe HS, Adalimumab treatment led to significant improvement in the clinical inflammatory load. These patients also showed reductions in serum CRP levels from baseline following treatment (20). All of these studies account for various predisposing factors, such as smoking status, obesity (BMI), gender, and age while neglecting to consider race (10, 19, 21). Table 1 shows a summary of the information extracted from these studies. Though several studies suggest CRP as a potential biomarker for HS, the scarcity of representation of SOC in such studies prevents the generalizability of this data. Monitoring race in studies such as these can further explain extenuating aspects that cause minority patients to be affected by common disease processes differently.
Oversight of this inequality maintains and exacerbates existing psychosocial outcomes of Black HS patients. These communities report a lower health-related quality of life, which is attributed to debilitating chronic pain, poor mental health and diminished self-sufficiency (35–38). Phenotypic manifestations, including disfiguring nodules with malodorous discharge, perpetuate social stigma, low self-esteem and self-isolation. This is implicated in the disproportionate rates of depression, anxiety, substance use, and suicidal ideation among this population (37, 39–43). Interestingly, patients with major depressive disorder exhibit increased peripheral blood concentrations of CRP (44, 45), and elevated CRP levels predict resistance to standard antidepressant therapies (44, 46, 47). Black/AA patients are more likely to have severe HS with elevated CRP levels (48, 49). Thus, neglecting these communities hinders quality of life while deepening existing disparities (49, 50).
Discussion
Inflammatory skin conditions like HS have distinct features in SOC populations, and CRP is a commonly utilized biomarker that is linked to disease activity. Compared to individuals without disease, CRP levels are significantly elevated in individuals with HS. Despite the vast evidence of more severe disease manifestations among Black populations, there is a gross underrepresentation of racial minorities throughout clinical and biomedical research even in diseases that disproportionally affect SOC patients.
Addressing the racial disparity in clinical and biomedical research studies, databases, and biorepositories is therefore critical to reducing the disease burden experienced by SOC patients. Researchers should accurately report race/ethnicity data and establish race-matched healthy controls. Understanding mediators of inflammatory skin diseases among Black patients may reveal pertinent risk factors and optimal therapeutic strategies. This will inform tailored interventions to appropriately address the aforementioned psychological burdens, as this lack of diversity has culminated in poorer outcomes among vulnerable populations. While limitations associated with inadequate diversity in data collection have been established, little has been done to revolutionize change. Therefore, it is imperative that academic researchers prioritize racial/ethnic diversity during patient recruitment as well as biospecimen collection and analysis.
Data Availability Statement
The original contributions presented in the study are included in the article material. Further inquiries can be directed to the corresponding author.
Author Contributions
CO, JW, CI, PI, and AB identified the gap in the field and conceptualized the overarching idea. CO, JW, CI, and PI collected and summarized the data from literature searches and drafted the manuscript. RK, JP, GO, and AB provided critical review and revised this manuscript. All authors contributed to the article and approved the submitted version.
Funding
The publishing of this work was supported by the Skin of Color Society Career Development Award (ASB).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kundu RV, Patterson S. Dermatologic Conditions in Skin of Color: Part I. Special Considerations for Common Skin Disorders. Am Fam Physician (2013) 87:850–6.
2. US Census Bureau. Race and Ethnicity in the United States: 2010 Census and 2020 Census. (2021). Available at: https://www.census.gov/library/visualizations/interactive/race-and-ethnicity-in-the-united-state-2010-and-2020-census.html. [Accessed March 17, 2022].
3. Office for National Statistics. 2011 Census: Key Statistics and Quick Statistics for Local Authorities in the United Kingdom. (2013). Available at: https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/bulletins/keystatisticsandquickstatisticsforlocalauthoritiesintheunitedkingdom/2013-10-11#:~:text=The%20estimated%20population%20of%20England,million%20people%20in%20Northern%20Ireland. [Accessed March 17, 2022].
4. Nagar SD, Conley AB, Sharma S, Rishishwar L, Jordan IK, Marino-Ramirez L. Comparing Genetic and Socioenvironmental Contributions to Ethnic Differences in C-Reactive Protein. Front Genet (2021) 12:738485. doi: 10.3389/fgene.2021.738485
5. Nehring SM, Goyal A, Patel BC. C Reactive Protein. In: StatPearls. Treasure Island(FL): StatsPearls Publishing (2021).
6. Sproston NR, Ashworth JJ. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front Immunol (2018) 9:754. doi: 10.3389/fimmu.2018.00754
7. Arevalo-Bermudez MDP, Paradela S, Balboa-Barreiro V, Fonseca E. Cutaneous Lupus Erythematosus: Factors Related to Cutaneous Activity and Damage in a Cohort of 260 Patients From A Coruna, Spain. Lupus (2020) 29:1021–30. doi: 10.1177/0961203320930094
8. Ramos-Casals M, Retamozo S, Siso-Almirall A, Perez-Alvarez R, Pallares L, Brito-Zeron P. Clinically-Useful Serum Biomarkers for Diagnosis and Prognosis of Sarcoidosis. Expert Rev Clin Immunol (2019) 15:391–405. doi: 10.1080/1744666X.2019.1568240
9. Farshchian M, Ansar A, Sobhan M, Hoseinpoor V. C-Reactive Protein Serum Level in Patients With Psoriasis Before and After Treatment With Narrow-Band Ultraviolet B. Bras Dermatol (2016) 91:580–83. doi: 10.1590/abd1806-4841.20164655
10. Riis PT, Soeby K, Saunte DM, Jemec GB. Patients With Hidradenitis Suppurativa Carry a Higher Systemic Inflammatory Load Than Other Dermatological Patients. Arch Dermatol Res (2015) 307:885–9. doi: 10.1007/s00403-015-1596-5
11. Kraus VB, Stabler TV, Luta G, Renner JB, Dragomir AD, Jordan JM. Interpretation of Serum C-Reactive Protein (CRP) Levels for Cardiovascular Disease Risk Is Complicated by Race, Pulmonary Disease, Body Mass Index, Gender, and Osteoarthritis. Osteoarthritis Cartilage (2007) 15:966–71. doi: 10.1016/j.joca.2007.02.014
12. Sims KD, Sims M, Glover LM, Smit E, Odden MC. Perceived Discrimination and Trajectories of C-Reactive Protein: The Jackson Heart Study. Am J Prev Med (2020) 58:199–207. doi: 10.1016/j.amepre.2019.09.019
13. Zouboulis CC, Del Marmol V, Mrowietz U, Prens EP, Tzellos T, Jemec GB. Hidradenitis Suppurativa/Acne Inversa: Criteria for Diagnosis, Severity Assessment, Classification and Disease Evaluation. Dermatology (2015) 231:184–90. doi: 10.1159/000431175
14. Lee DE, Clark AK, Shi VY. Hidradenitis Suppurativa: Disease Burden and Etiology in Skin of Color. Dermatology (2017) 233:456–61. doi: 10.1159/000486741
15. Succaria F, Kerns M, Byrd A. A Comprehensive Guide to Hidradenitis Suppurativa. In: Chapter 5: Histopathology of the Pilosebaceous Unit and Interstitium of Hidradenitis Suppurativa. Philadelphia: ELSEVIER - HEALTH SCIENCE (2021).
16. Vlassova N, Kuhn D, Okoye GA. Hidradenitis Suppurativa Disproportionately Affects African Americans: A Single-Center Retrospective Analysis. Acta Derm Venereol (2015) 95:990–1. doi: 10.2340/00015555-2176
17. Garg A, Kirby JS, Lavian J, Lin G, Strunk A. Sex- and Age-Adjusted Population Analysis of Prevalence Estimates for Hidradenitis Suppurativa in the United States. JAMA Dermatol (2017) 153:760–64. doi: 10.1001/jamadermatol.2017.0201
18. Akdogan N, Alli N, Uysal PI, Topcuoglu C, Candar T, Turhan T. Visfatin and Insulin Levels and Cigarette Smoking Are Independent Risk Factors for Hidradenitis Suppurativa: A Case-Control Study. Arch Dermatol Res (2018) 310:785–93. doi: 10.1007/s00403-018-1867-z
19. Hessam S, Sand M, Gambichler T, Bechara FG. Correlation of Inflammatory Serum Markers With Disease Severity in Patients With Hidradenitis Suppurativa (Hs). J Am Acad Dermatol (2015) 73:998–1005. doi: 10.1016/j.jaad.2015.08.052
20. Jimenez-Gallo D, de la Varga-Martinez R, Ossorio-Garcia L, Collantes-Rodriguez C, Rodriguez C, Linares-Barrios M. Effects of Adalimumab on T-Helper-17 Lymphocyte- and Neutrophil-Related Inflammatory Serum Markers in Patients With Moderate-To-Severe Hidradenitis Suppurativa. Cytokine (2018) 103:20–4. doi: 10.1016/j.cyto.2017.12.020
21. Gonzalez-Manso A, Agut-Busquet E, Romani J, Vilarrasa E, Bittencourt F, Mensa A, et al. Hidradenitis Suppurativa: Proposal of Classification in Two Endotypes With Two-Step Cluster Analysis. Dermatology (2021) 237:365–71. doi: 10.1159/000511045
22. Akdogan N, Dogan S, Incel-Uysal P, Karabulut E, Topcuoglu, Yalcin B, et al. Serum Amyloid A and C−Reactive Protein Levels and Erythrocyte Sedimentation Rate Are Important Indicators in Hidradenitis Suppurativa. Arch Dermatol Res (2020) 312(1):255–62. doi: 10.2340/00015555-3647
23. Huang CM, Lowes MA, Cserti C, Alavi A. Hemoglobin Levels and Serum C-Reactive Protein in Patients With Moderate to Severe Hidradenitis Suppurativa. J Cutaneous Med Surg (2019) 23(5):501–6. doi: 10.3390/life11010034
24. Jiménez-Gallo D, de la Varga-Martínez R, Ossorio-García L, Albarrán-Planelles C, Rodríguez C, Linares-Barrios M. The Clinical Significance of Increased Serum Proinflammatory Cytokines, C-Reactive Protein, and Erythrocyte Sedimentation Rate in Patients With Hidradenitis Suppurativa. Mediators Inflammation (2017) 1):1–8. doi: 10.1111/bjd.16603
25. Akdogan N, Alli N, Incel-Uysal P, Topcuoglu C, Candar T, Turhan T. Visfatin and Insulin Levels and Cigarette Smoking are Independent Risk Factors for Hidradenitis Suppurativa: A Case–Control Study. Arch Dermatol Res (2018) 310(1):785–93. doi: 10.2147/PROM.S174299
26. Alatas ET, Biteker M, Alatas OD. Epicardial Fat Thickness Is Increased and Associated With Disease Severity in Hidradenitis Suppurativa. Arch Dermatol Res (2020) 312(1):467–72. doi: 10.21037/atm-20-1028
27. Montaudié H, Seitz-Polski B, Cornille A, Benzaken S, Lacour J, Passeron T. Interleukin 6 and High-Sensitivity C-Reactive Protein Are Potential Predictive Markers of Response to Infliximab in Hidradenitis Suppurativa. J Am Acad Dermatol (2017) 76(1):156–8. doi: 10.1001/jamadermatol.2019.2610
28. Hessam S, Sand M, Gambichler T, Bechara FG. Correlation of Inflammatory Serum Markers With Disease Severity in Patients With Hidradenitis Suppurativa. J Am Acad Dermatol (2015) 73(6):998–1005. doi: 10.1007/s10880-019-09640-4
29. Saraç Öztürk G, Ergun T, Eyüboğlu IP, Akkiprik M. Serum High-Sensitivity C-Reactive Protein, Tumor Necrosis Factor-α, Interleukin (IL)-1β, IL-17A and IL-23 Levels in Patients With Hidradenitis Suppurativa. Cytokine (2021) 144(1):155585. doi: 10.1016/j.jid.2017.09.008
30. Pascual JC, González I, Corona D, Hispán P, Ramos JM, Sánchez-Paya J, et al. Assessment of Subclinical Atherosclerosis in Hidradenitis Suppurativa. J Eur Acad Dermatol Venereology (2016) 31(7):1229–38. doi: 10.1016/j.jaad.2018.02.053
31. Arenbergerova M, Gkalpakiotis S, Arenberger P. Effective Long-Term Control of Refractory Hidradenitis Suppurativa With Adalimumab After Failure of Conventional Therapy. Int J Dermatol (2010) 49(1):1445–9. doi: 10.1192/bjp.2018.66
32. Çetinarslan T, Ermertcan AT, Özyurt B, Gündüz K, et al. Evaluation of the Laboratory Parameters in Hidradenitis Suppurativa: Can We Use New Inflammatory Biomarkers? Dermatologic Ther (2021) 34(2):e14835. doi: 10.1016/j.bbi.2020.02.010
33. Miller IM, Ring HC, Prens EP, Rytgaard H, Mogensen UB, Ellervik C, et al. Leukocyte Profile in Peripheral Blood and Neutrophil-Lymphocyte Ratio in Hidradenitis Suppurativa: A Comparative Cross-Sectional Study of 462 Cases. Dermatology (2016) 232(1):511–9. doi: 10.1016/j.psyneuen.2017.01.023
34. Yildiz H, Senol L, Ercan E, Bilgili ME, Abuaf OK. A Prospective Randomized Controlled Trial Assessing the Efficacy of Adjunctive Hyperbaric Oxygen Therapy in the Treatment of Hidradenitis Suppurativa. Int J Dermatol (2016) 55(2):232–7. doi: 10.1016/j.psyneuen.2018.05.026
35. Sampogna F, Fania L, Mastroeni S, Fusari R, Napolitano M, Ciccone D, et al. Correlation Between Depression, Quality of Life and Clinical Severity in Patients With Hidradenitis Suppurativa. Acta Derm Venereol (2020) 100:adv00319. doi: 10.1016/j.jdin.2021.01.007
36. Krajewski PK, Matusiak L, von Stebut E, Schultheis M, Kirschner U, Nikolakis G, et al. Quality-of-Life Impairment Among Patients With Hidradenitis Suppurativa: A Cross-Sectional Study of 1795 Patients. Life (Basel, Switzerland) (2021) 11(1):34. doi: 10.3390/life11010034
37. Matusiak L. Profound Consequences of Hidradenitis Suppurativa: A Review. Br J Dermatol (2020) 183:e171–e77. doi: 10.1016/j.jnma.2016.09.002
38. Mac Mahon J, Kirthi S, Byrne N, O'Grady C, Tobin AM. An Update on Health-Related Quality of Life and Patient-Reported Outcomes in Hidradenitis Suppurativa. Patient Relat Outcome Meas (2020) 11:21–6. doi: 10.1007/s00403-019-02014-8
39. Phan K, Huo YR, Smith SD. Hidradenitis Suppurativa and Psychiatric Comorbidities, Suicides and Substance Abuse: Systematic Review and Meta-Analysis. Ann Transl Med (2020) 8:821. doi: 10.1177/1203475419858963
40. Reddy S, Orenstein LAV, Strunk A, Garg A. Incidence of Long-Term Opioid Use Among Opioid-Naive Patients With Hidradenitis Suppurativa in the United States. JAMA Dermatol (2019) 155:1284–90. doi: 10.1001/jamadermatol.2019.2610
41. Tugnoli S, Agnoli C, Silvestri A, Giari S, Bettoli V, Caracciolo S. Anger, Emotional Fragility, Self-Esteem, and Psychiatric Comorbidity in Patients With Hidradenitis Suppurativa/Acne Inversa. J Clin Psychol Med Settings (2020) 27:527–40. doi: 10.1007/s00403-018-1867-z
42. Thorlacius L, Cohen AD, Gislason GH, Jemec GBE, Egeberg A. Increased Suicide Risk in Patients With Hidradenitis Suppurativa. J Invest Dermatol (2018) 138:52–7. doi: 10.1007/s00403-019-02032-6
43. Garg A, Papagermanos V, Midura M, Strunk A, Merson J. Opioid, Alcohol, and Cannabis Misuse Among Patients With Hidradenitis Suppurativa: A Population-Based Analysis in the United States. J Am Acad Dermatol (2018) 79:495–500.e1. doi: 10.1016/j.jaad.2016.08.036
44. Chamberlain SR, Cavanagh J, de Boer P, Mondelli V, Jones DNC, Drevets WC, et al. Treatment-Resistant Depression and Peripheral C-Reactive Protein. Br J Psychiatry (2019) 214:11–9. doi: 10.1016/j.jaad.2015.08.052
45. Osimo EF, Pillinger T, Rodriguez IM, Khandaker GM, Pariante CM, Howes OD. Inflammatory Markers in Depression: A Meta-Analysis of Mean Differences and Variability in 5,166 Patients and 5,083 Controls. Brain Behav Immun (2020) 87:901–09. doi: 10.1016/j.bbi.2020.02.010
46. Jha MK, Minhajuddin A, Gadad BS, Greer T, Grannemann B, Soyombo A, et al. Can C-Reactive Protein Inform Antidepressant Medication Selection in Depressed Outpatients? Findings From the CO-MED Trial. Psychoneuroendocrinology (2017) 78:105–13. doi: 10.1016/j.psyneuen.2017.01.023
47. Haroon E, Daguanno AW, Woolwine BJ, Goldsmith DR, Baer WM, Wommack EC, et al. ASntidepressant Treatment Resistance Is Associated With Increased Inflammatory Markers in Patients With Major Depressive Disorder. Psychoneuroendocrinology (2018) 95:43–9. doi: 10.1111/j.1365-4632.2010.04638.x
48. Kilgour JM, Li S, Sarin KY. Hidradenitis Suppurativa in Patients of Color Is Associated With Increased Disease Severity and Healthcare Utilization: A Retrospective Analysis of 2 U.S. Cohorts. JAAD Int (2021) 3:42–52. doi: 10.1111/dth.14835
49. Soliman YS, Hoffman LK, Guzman AK, Patel ZS, Lowes MA, Cohen SR. African American Patients With Hidradenitis Suppurativa Have Significant Health Care Disparities: A Retrospective Study. J Cutan Med Surg (2019) 23:334–36. doi: 10.1159/000446021
Keywords: hidradenitis suppurativa, psychosocial impact, skin of color, inflammatory skin disease, immune mediated skin disease, dermatology, diversity, health care disparities
Citation: Okeke CAV, Williams JP, Iwuala CU, Igwe PK, Khanna R, Perry JD, Okoye GA and Byrd AS (2022) What's Race Got to Do With It? CRP Levels in Immune Mediated Skin Diseases: Considerations for Hidradenitis Suppurativa. Front. Immunol. 13:847050. doi: 10.3389/fimmu.2022.847050
Received: 04 January 2022; Accepted: 28 February 2022;
Published: 29 March 2022.
Edited by:
Richard Ahn, University of California, Los Angeles, United StatesReviewed by:
Wilson Liao, University of California, San Francisco, United StatesCopyright © 2022 Okeke, Williams, Iwuala, Igwe, Khanna, Perry, Okoye and Byrd. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Angel S. Byrd, YW5nZWxfYnlyZEBhbHVtbmkuYnJvd24uZWR1
 Chidubem A. V. Okeke
Chidubem A. V. Okeke Jonathan P. Williams
Jonathan P. Williams Callyn U. Iwuala
Callyn U. Iwuala Pearl K. Igwe
Pearl K. Igwe Raveena Khanna
Raveena Khanna Jessica D. Perry
Jessica D. Perry Ginette A. Okoye
Ginette A. Okoye Angel S. Byrd
Angel S. Byrd