- 1Department of Radiation Oncology, Chongqing University Cancer Hospital & Chongqing Cancer Institute & Chongqing Cancer Hospital, Chongqing, China
- 2Chongqing Public Health Medical Center, Chongqing, China
- 3Department of Pharmacy, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China
- 4Department of Pharmacy, The First Affiliated Hospital of Chongqing Medical and Pharmaceutical College, Chongqing, China
Accumulating evidence indicates that patients with inflammatory bowel disease (IBD) have a significantly higher risk of developing different cancers, while the exact mechanism involved is not yet fully understood. Malassezia is a lipid-dependent opportunistic yeast, which colonizes on mammalian skin and internal organs. Also, dysbiosis in fungal communities accompanied by high level of Malassezia are fairly common in inflammatory diseases such as IBD and various cancers. In cancer patients, higher levels of Malassezia are associated with worse prognosis. Once it is ablated in tumor-bearing mice, their prognostic conditions will be improved. Moreover, Malassezia manifests multiple proinflammatory biological properties, such as destruction of epithelial barrier, enrichment of inflammatory factors, and degradation of extracellular matrix (ECM), all of which have been reported to contribute to tumor initiation and malignant progression. Based on these facts, we hypothesize that high levels of Malassezia together with mycobiome dysbiosis in patients with IBD, would aggravate the microecological imbalance, worsen the inflammatory response, and further promote tumorigenesis and deterioration. Herein, we will discuss the detrimental properties of Malassezia and explore the key role of this fungus in the correlation between IBD and cancer, in order to take early surveillance and intervention to minimize the cancer risk in individuals with IBD.
Introduction
Inflammatory bowel disease (IBD) belongs to chronic idiopathic gastrointestinal (GI) inflammatory diseases, characterized by imbalance of the intestinal microbiome (1). Typically, IBD contains Crohn’s disease (CD) and ulcerative colitis (UC), of which the common characteristic is the inflammation in the GI wall. Their main distinction is the site and depth of lesions. UC is generally limited to the colon, while CD may include the whole intestine ranging from the mouth to the rectum (2). The rapid pace of IBD expansion over only several decades is disturbing, which has led to serious socioeconomic burden (3).
Emerging evidence suggests that the damaged intestine barrier and gut microbiome dysbiosis are closely linked with the genesis of IBD (4–6). Generally, the batches of bacteria, viruses, archaea, fungi, and eukaryotic microbes inhabiting in the GI tract are referred as gut microbiota, which have already formed a mutually beneficial correlation with the host (7, 8). Among the collection of bacteria, persistent enteric pathogens including adhesion-invasive Escherichia coli (AIEC) and Clostridium difficile, may act as a trigger for IBD (6). Besides bacterial contribution, there is increasing awareness regarding the impact of mycobiome as immunologically reactive components. On the whole, IBD and ordinary population differ in mycobiome. Alterations in gut mycobiome as well as their communications with other intestine microbiota are essential to maintain the intestinal barrier, such as in IBD (9–14). Reports showed that higher abundance of Candida albicans was observed in the intestinal tract of UC patients, when compared to controls, conversely, the decreased amount of C. albicans indicated the improvement and recovery of UC (12). Studies also confirmed that when compared with healthy people, one specific commensal fungi Malassezia yeast has been identified particularly abundant in people with IBD, which may scale up inflammatory cytokine production and aggravate inflammation in IBD (10, 11, 13, 14). In addition, when the microflora balance is disturbed or the host immune defense is impaired, fungi may spread or transfer from the original symbiotic habitat to other important organs such as the gut, thus becoming a susceptible factor for life-threatening infection (15). The mouse model of psoriasis showed that pre-exposure of symbiotic fungus may significantly worsen tissue inflammation through enhancing T helper type 17 (Th17)-dependent immune responses and phagocytosis of neutrophils (16).
Recently, it has been noticed that malignancies are prevalent in people with IBD (17–20). Reports showed that standardized incidence ratios (SIRs) for pancreatic cancer (PC) in IBD cases were 7.6-fold higher than the ordinary persons (21). For colorectal cancer (CRC), when compared with the reference individuals, the cancer risk in patients with IBD increased considerably, up to around 10 times (22, 23). Studies indicated a strong association between IBD and bile duct cancer, and the SIR could rise remarkably in patients with UC for intrahepatic cholangiocarcinoma (24, 25). Extraintestinal analyses also revealed that the IBD group had a significantly elevated risk for skin cancer (e.g., melanoma), particularly among CD patients or elder population (26–28). Furthermore, evidence has demonstrated that inflammation acts a substantial role in oncogenesis (29, 30).
However, the underlying mechanism linking IBD to cancer remains to be further clarified. Malassezia, as representative fungal commensals, may be the key to push aside this dense fog. Based on this, this work is intended to discuss and elaborate the interplay between Malassezia, IBD, and cancer.
Hypothesis
Considering current evidence, we hypothesize that the particular enrichment of Malassezia genius in the gut microbiome could promote inflammatory responses in IBD patients through the following microbiological characteristics: disrupting the integrity of epithelial barrier; increasing the release of proinflammatory molecules; and degrading the extracellular matrix (ECM). In this manner, Malassezia can further induce neoplasia and raise cancer incidence in the IBD population under inflammatory conditions (Figure 1).
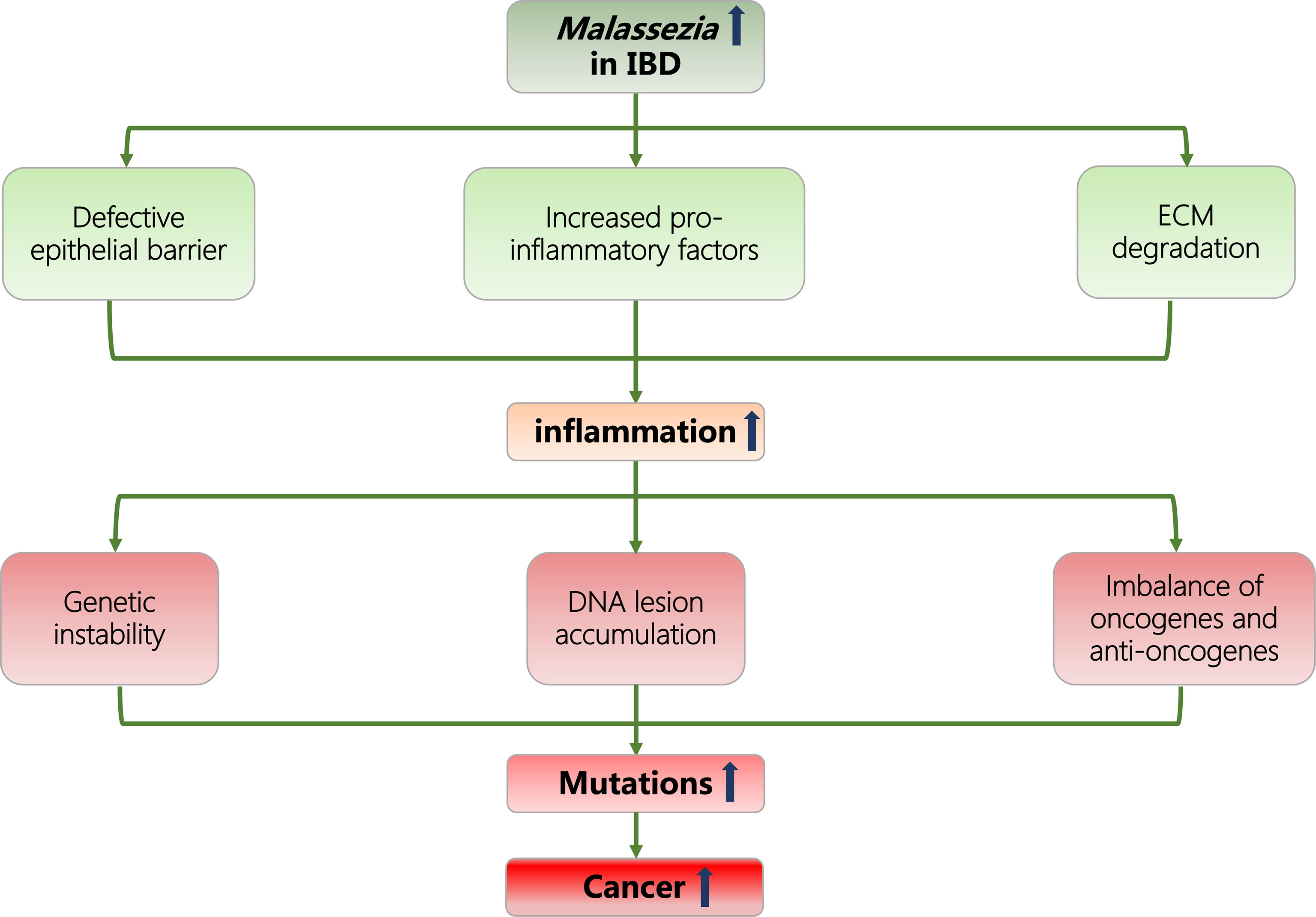
Figure 1 Flowcharts representing the association of Malassezia, IBD, inflammation, and cancer. IBD, inflammatory bowel disease; ECM, extracellular matrix.
Life and Properties of Malassezia
General Properties of Malassezia
Malassezia pertains to the category of lipid-dependent yeast and is an essential symbiotic organism resident on the mammalian skin, hair, and GI tract (31). Under certain scenario, Malassezia spp. may switch to opportunistic pathogens.
When referring to microbial variability in different body sites, the skin microbiome is known for its relatively high fungal presence (32). Malassezia is the dominated eukaryotic component of microbial communities identified in human and is most abundant in craniofacial sebaceous glands and relatively low in arms or trunk, due to the enrichment of the lipid nutritional sources (33, 34). So far, as many as 20 species of Malassezia strains have been identified (35, 36). Malassezia restricta and Malassezia globosa are the top 2 most abundant in human skin (37). However, the distribution of Malassezia is not confined to the skin, high-throughput sequencing revealed that they are also detected at a relatively high frequency in the GI and respiratory tract sample sets, with positive rates of 88% and 86%, respectively (38, 39).
Shaping on fungal community is changing with age. The skin microbiota of neonates delivered vaginally resembled their mother’s vaginal mycobiome, while that of neonates born through cesarean section was similar to the skin surface of mothers (40, 41). Malassezia colonized once after birth and was more resembling adult microbiome assemblage, as driven by maternal hormones (42, 43). The relative abundance of Malassezia in infant skin was only 2%, while cesarean-born infants owned lower (44). The sebaceous gland entered a dormant state within 6 months after birth, thus Malassezia returned to low enrichment. M. globosa predominately colonized on prepubertal skin. During adolescence, the increase of lipid levels in the sebaceous glands can lead to a simultaneously increased percentage with Malassezia (45). Skin fungal community analysis showed that Malassezia fungus was absolutely dominant in adults, with predominance up to over 90%. In contrast, Malassezia was relatively lower in children under 14, but the fungal community was more diverse (46).
Pathogenic Properties of Malassezia
Studies showed that Malassezia spp. were associated with numerous inflammatory diseases, such as dermal inflammation (16, 47), IBD (13, 14), CRC (48), pancreatic carcinoma (31), and severe infections (49). In general, Malassezia is mainly colonized in the skin (50). However, the availability of lipid nutrition within the GI tract may facilitate the localization and survival of Malassezia in the gut, and its nutrition can be accessed from host diet or intestinal fungal synthesis (51, 52). It is speculated that Malassezia might immigrate to the GI tract along with the diet; however, solid evidence is lacking. Manifold factors can influence the pathogenicity of Malassezia, which involves the virulence and quantity of Malassezia, environmental conditions including humidity, temperature, oxygen, and fatty acid nutrients, as well as the susceptibility of the host (53). So far, several viewpoints have been proposed to reveal the pathogenic behaviors of Malassezia, which may bring more dawn for understanding the inflammatory or infectious pathogenesis.
Epithelial Barrier Defects
The epithelial barrier is organized as a protective and complicated system that allows nutrient exchange while preventing the displacement of microorganisms and their metabolites. This tight barrier is obviously a main hurdle which must be broken through for microbial antigens to enter the human body, for example, in the lumen of the gut (54). Malassezia could produce increased irritating free fatty acids through metabolism, typified by oleic acid (OA), which may damage the permeability of the skin barrier and lead to skin itching or even exfoliation (55, 56). Because of this, when applying OA to the scalp, it would induce scalp flaking in patients with seborrheic dermatitis (55). Malassezia species were kind of lacking synthase genes for fatty acid, which may be supplemented by enhancing the expression of genes encoding secretory hydrolases in Malassezia genome to generate fatty acid (57). Among them, the extracellular lipases and phospholipases secreted by Malassezia could severally influence its virulence factors on the release of distinct metabolites and the cell wall characteristics itself, in order to facilitate epithelium targeting, lesion aggravation, and barrier disruption (57, 58). It is reported that the lipase activity of Malassezia is linked to the pathogenesis of inflammatory skin diseases in vitro (59). Malassezia phospholipase activity has also been reported to be related to its virulence in dogs (60). What is more, other environmental determinants such as increased epidermal water loss, loss of tight junction proteins, decrease in both cholesterol and free fatty acid, and high pH value, could exhibit catalytic effects in the pathogenesis of Malassezia, thus inducing and exacerbating cutaneous inflammation (61, 62). Defective cutaneous barriers failed to provide adequate protection against microbes or allergens, instead they may assist Malassezia to enter the blood circulation system, resulting in immune activation and inflammatory process (63).
We own innate immunity in our genomes which provides a defense against Malassezia infection. As a positive regulator, mast cells (MC) can detect and control the fungi Malassezia at the infected site, which may be activated by Toll-like receptor 2 (TLR2) (64, 65). Macrophages can effectively defend the host against the attack of opportunistic fungal pathogens through phagocytosis and collection of phagocytic contents, affected by TLR9 (66, 67). As innate immune receptors, Dectin-2 and Mincle were mainly involved in the immune recognition to Malassezia, arousing the production of pro- and anti-inflammatory factors (68). Moreover, Malassezia may induce a reciprocal activation between natural killer (NK) cells and dendritic cells (DCs), in which NK cells would promote the maturation and costimulatory capacity of DCs, as well as accelerate the release of interleukin-8 (IL-8) in DCs (69, 70). These innate immune responses will play an important role in the subsequent adaptive immunity including activation of T cells. Malassezia may also promote the progression of inflammation directly or indirectly by mediating host receptor recognition, promoting the release of proinflammatory cytokines and secreting chemicals or vesicles. Within the host, C-type lectin receptor (CLR) family could specifically recognize fungal microorganisms and initiate adaptive immune responses (68, 71). CLRs were specialized in sensing the carbohydrates in the cell wall of Malassezia, mediating the signaling adaptor caspase recruitment domain-containing protein (CARD)-9 to drive the polarization of CD4+ T lymphocytes into IL-17-producing immune cells, such as γδ T cells or innate lymphoid cells (ILCs) (71, 72). Malassezia could generate indoles, the ligands for the aryl hydrocarbon receptor (AhR), which may also promote Th17 differentiation and IL-17 secretion through activation of AhR signaling (73, 74). By eliciting the production of inflammatory cytokine IL-17 strongly, Malassezia spp. has been reported to stimulate tissue inflammation, destroy skin integrity, and further contribute to or augment epicutaneous infections such as atopic dermatitis (AD) in murine models (72). Other proinflammatory cytokines including IL-18, IL-8, and IL-6 and Th22 chemokines including C-C Motif Chemokine Ligand 17 (CCL17) were significantly increased after exposure to Malassezia as well, so as to worsen local inflammation (75, 76). It has been confirmed that, compared with the negative group, Malassezia colonization can induce CARD9-S12N polymorphism and strongly enhance the release of cytokines such as IL-10 or tumor necrosis factor alpha (TNF-α) in either wild-type (WT) or Card9−/− colitis mice (13). Moreover, Malassezia was able to activate NLRP3 inflammasome through Dectin2/CARD9 signal, accelerating the generation of IL-1β to aggravate inflammation (77). Zhang et al. also demonstrated that Malassezia can produce nanovesicles rich in allergens or proteins, which may initiate and maintain inflammation by activating the nuclear factor-κB (NF-κB) pathway and upregulate IL-6 production in the immune microenvironment (78).
ECM Degradation
ECM is a highly dynamic acellular network composed of collagen, fibronectin, and several other proteins (79). It is of great importance in the inflammatory process. Reports showed that ECM was involved in the signal transmission to recruit inflammatory cells, stimulate cell migration, and restore inner homeostasis for coping with external stimulus (80). Variation or degradation of ECM components has been confirmed to have a close relationship with the progression of various inflammatory diseases, such as AD (81, 82). Some virulence factors including acid sphingomyelinase and aspartate protease, which were secreted by Malassezia, could mediate ECM degradation and participate in the pathogenesis (83). MgSAP1, one unique secreted M. globosa protease, has been implicated in hydrolyzing host proteins to provide nutrition and destroy ECM elements, so as to facilitate pathogen adhesion in the inflamed areas (84). Adhesion is a decisive step in the pathogenesis of microbial infection (85). One close homolog of secretory MgSAP1 protease produced by Malassezia furfur is MfSAP1, which owned high catalytic efficiency in extracellular proteins of human skin, particularly when substrate collagen was thermally denatured (86, 87). It has been proved that MfSAP1 was likely to modify the epidermal and dermal environment through degrading key components of skin-correlated ECM, such as vitronectin, fibronectin, and thrombospondin, even at low proportions of enzyme to the substratum. Accordingly, high concentrations of MfSAP1 could rapidly and sensitively cleave these ECM proteins and inhibit cell migration and attachment to the fundamental ECM, thus attenuating re-epithelization process and retarding cutaneous wound healing in an acute traumatic cell model (86).
Apart from proinflammatory motivation, Malassezia has manifested some other biological features, including unique cell-defense characteristics. Malassezia owns quite thick and unique multilayered cell walls, which could protect themselves from complex environmental stress and help to escape phagocytosis (88). In particular, Malassezia was able to form biofilms on their surface, which was correlated to the emergence of drug resistance and the maintenance of virulence (89). Beyond these described circumstances, Malassezia may promote disease progression by modulating the pathogenicity of other microorganisms, for instance Staphylococcus aureus through microbial communications (90).
Increased Malassezia and Its Relevance With Inflammation in IBD and Other Inflammatory Diseases
In recent years, the detection of Malassezia in the digestive tract has attracted public attention. Microbial diversity analysis showed that Basidiomycota phyla ranked as second major dominators, following Ascomycota phyla, within gut fungal microbiota in both healthy controls and IBD patients, although there were some variations among different disease phenotypes (14). As contrasted to healthy persons, Malassezia genera were observed with a high prevalence in people with IBD, which was one primary reason to the increase of Basidiomycetes in IBD (11, 14). Sokol and his team revealed that the higher abundance of Malassezia was noticed in the acute stage of IBD population (14). In one retrospective cohort, when compared with healthy volunteers, increased abundance of Malassezia was also detected in patients with IBD (50). Limon and his colleagues reported that Malassezia species, represented by M. restricta, were more enriched in the sigmoid colon mucosa of CD patients, where massive monocyte-derived DCs were involved in the following pathogenesis (13). Statistic data suggested that M. sympodialis and its extract were able to initiate MC to secret cysteinyl leukotrienes and intensify IgE-dependent immunoreactions in vitro, which might possibly lead to deteriorative inflammation in IBD as well (91, 92). The polymorphism of CARD-9 in CD would encourage the colonization of M. restricta yeast in the intestine, which may worsen or even exacerbate the intestinal inflammation by strongly evoking proinflammatory responses of macrophages and monocyte-derived DCs in murine models (13). Beyond this, Liguori et al. also demonstrated that the overall load of Malassezia in CD patients was significantly increased in contrast to ordinary ones (p < 0.05), suggesting that Malassezia may play a role in the pathogenesis of mucositis (11). Similar results have been obtained from the clinical analysis on UC. Regarding fecal fungal composition, there was a significant difference in only several fungal genera between UC patients and non-IBD controls, among which Malassezia owned a relatively higher abundance in patients with UC, particularly when at the active phase (93).
Aside from IBD, evidence suggests that the enrichment of Malassezia is also obviously increased in other chronic or inflammatory diseases, such as psoriasis, AD, and cystic fibrosis (CF). Scholars found out that the average quantity and species diversity of Malassezia in psoriatic patients were higher than those in healthy individuals, particularly in skin lesions (94). Malassezia-positive culture rate of scalp skin samples was 85% and 50% in patients with psoriasis and healthy subjects, respectively (95). The more serious the lesion, the higher is the positive rate. Studies also indicated that Malassezia allergens could induce immunoglobulin E (IgE)-mediated sensitization in AD subjects (96). There was a significant positive correlation between Malassezia-specific IgE levels and AD severity (97). Mittermann and colleagues reported that compared with healthy controls, Malassezia was more abundantly detected in sputum specimens of asthmatic patients, particularly in children (98). High detection rates of Malassezia were observed in respiratory tract of CF patients as well (99, 100). Beyond this, higher Malassezia colonization was detected in subjects with neurodegenerative diseases, such as Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis (101–103). In most cases, the severity of the mentioned diseases is positively correlated with the abundance of Malassezia detected.
It seems ubiquitous that the appearance frequency of Malassezia yeast differs in distinct human body site. As Malassezia strains are resident members of cutaneous mycobiome, it is not so esoteric to understand the relationship between the high prevalence of Malassezia and psoriasis or AD. Apart from this, Malassezia may be an overlooked fungal pathogen that is neglected to participate in inflammatory splanchnic diseases, particularly in IBD.
Roles of Malassezia and Inflammation in the Context of Cancer
Malassezia Is Associated With Cancer
Host–microflora interactions in the tumor microenvironment play an important driving role in tumorigenesis and progression. In various noninfectious diseases such as cancer, the emphasis of most research is concentrated in bacteriome. Recently, scholars have recognized the significance of mycobiome and microbial dysbiosis in genesis and development of neoplasms. Since its powerful potential in cancer development, the role of one cardinal fungus genus Malassezia has just begun to come under scrutiny.
Epidemiological data suggested that abundant yeast niches of Malassezia were overlapping with cancerous regions of basal cell carcinoma in humans and animals such as dogs or cats (96, 104). This yeast may promote dermal carcinogenesis through synthesis and activation of AhR ligands and further inhibition of cell caducity (104). Moreover, Aykut and his team reported that Malassezia was remarkably elevated in the pancreas of PC patients and mice models than that in healthy volunteers. Furthermore, they have demonstrated that repopulation with Malassezia could promote PC through mannose-binding lectin (MBL) signal pathway, thus accordingly its ablation with amphotericin B in murine models was found to slow oncogenic progression (31, 105). Malassezia also showed higher relative abundance in patients with oral squamous cell carcinoma (OSCC), compared with control volunteers (106). In addition, Gao and collaborators discovered that although there was no significant difference in stool mycobiota diversity between CRC patients and healthy controls, the fungal subgroup Malassezia genus was more enriched in people with CRC, which was positively correlated with tumor progression (48). Similar results were obtained in another analysis performed by Coker and coworkers. They found that when compared with control subjects, Malassezia strains were elevated significantly and the mycobiome diversity was specifically modified in CRC (107). This may provide a potentially predictive diagnostic marker for CRC. However, so far, there is no exact hypothesis about the underlying mechanism of Malassezia involved in cancer, and the relationship between Malassezia and CRC still awaits further study.
Inflammation and Cancer
There are many hypotheses about the pathogenesis of tumors, among which the theory of inflammatory mechanism is the widely accepted one. Usually, inflammation is fundamental to fight against harmful or pathogenic stimuli, hasten the wound to restore and maintain normal function of tissues, which involves in endothelial cells, immune cells, and inflammatory agents (108). Self-limited acute inflammation is beneficial in the healing process (109). However, when it is out of control, it may develop into chronic inflammation, induce tissue lesions and predispose to cancer (110), including tumorigenesis, progression, and metastasis (111). Only a small portion of cancers are ascribed to germ line mutations, while 90% of cancers are associated with somatic mutations and environmental hazards, and the latter is always linked to chronic inflammation or infections (112). Epidemiological investigations showed that inflammation was closely related to the occurrence of about 20% of all cancers (113). Triggers of chronic inflammation for enhanced risk or progression of cancer include microbial infections, such as Helicobacter pylori in gastric adenocarcinoma and chronic hepatitis B virus (HBV) in hepatocellular carcinoma (HCC), and inflammatory diseases (e.g., UC in CRC), which were closely bound up with genetic instability (114–116). Accordingly, with H. pylori being eradicated, the conditions of atrophic gastritis and intestinal metaplasia would be improved or eliminated, thus potentially suppressing the generation of gastric cancer (117). Moreover, existing evidence has shown that hypoxia-associated inflammatory cytokines or chemokines were significantly elevated in the tumor microenvironment, for example, IL-6, IL-1, and TNF (29, 118). Anti-inflammatory drugs may benefit cancer patients, such as TNF blockade and nonsteroidal anti-inflammatory drugs (NSAIDs) (119, 120).
Malassezia-Related Inflammation Contribute to Tumorigenesis
As mentioned above, Malassezia has exhibited profound proinflammatory effects and been linked to oncogenesis. On the other hand, inflammation can influence all the stages of tumorigenesis and drive tumor development and metastasis. Based on prior elaboration, Malassezia-associated inflammation may promote tumor initiation and malignant progression through multiple approaches:
(1) Barrier impairment—epithelial barrier deterioration, including aberrant production of mucin and defective expression or organization of tight junctional proteins, induced by oncogene activation, was an early malignant behavior in intestinal tumorigenesis (121). Correspondingly, loss of mucus would result in increased penetration of epithelial barriers and enhanced microbial translocation through the colon, leading to colorectal neoplasms in mouse models (122).
(2) Proinflammatory molecules—many inflammatory cytokines and growth factors have been reported to facilitate tumor development or antitumor immunity. In mice bearing breast cancer, IL-1 was upregulated and its related signaling was enhanced, which could promote angiogenesis, endothelial cell adhesion, lymphocyte polarization, and recruitment of myeloid cells, thus contributing to cancer progression and bone metastasis (123–125). Suppressed action of IL-1 dramatically inhibited tumor growth in ovarian cancer mouse models (126). IL-17 can promote GI tumorigenesis by binding to its receptor, and this signaling could induce the activation of the mitogen-activated protein kinase (MAPK) and NF-κB pathways and boost colonic epithelial cell proliferation and further support malignant transformation in mice (121, 127). TNF-α exhibited its protumorigenic features through activation of representative c-Jun N-terminal kinase (JNK) and NF-κB signaling pathways, resulting in enhanced epithelial to mesenchymal transition (EMT) and accelerated tumor cell invasion (128, 129).
(3) ECM remodeling—ECM remodeling such as degradation or stiffening was tumorigenic (130). It could contribute to tumor invasion and metastasis, in which integrin clustering could encourage focal adhesions, intensify ERK and PI3K pathways, and thus promote cell proliferation and invasion (131, 132).
Moreover, other mechanisms may also participate in the process of Malassezia-related inflammation-cancer transformation, such as DNA lesion accumulation and imbalance of oncogenes and antioncogenes. Inflammatory cells may release cytotoxic chemicals such as reactive oxygen species (RONS) to induce DNA damage (133). Continuous inflammatory conditions may result in aggravation and accumulation of DNA damage in cells, which may promote genetic mutations, generate genomic instability, and eventually cause carcinogenesis (134). Another powerful toxic polycyclic aromatic hydrocarbon, 7,12-dimethylbenz[a] anthracene (DMBA), has been reported to induce inflammation-dependent dermal tumorigenesis in mice through the cGAS-STING signaling pathway (135) and even distant metastasis in mouse models of breast cancer (136). Accumulated mutations in oncogenes such as c-Myc, which may be induced by inflammatory cytokines or DNA damage, could show synergism with inflammatory stimulus to enhance oncogenous process including enhanced cell proliferation, differentiation, and malignant transformation (137, 138). In addition, chronic inflammation may contribute to tumor protein 53 (TP53) mutations in the epithelium, and this accumulation could lead to deep loss of tumor suppressor functionality in cells. As a result, chromosomal instability increased and eventually cancer occurred (139, 140). In turn, the tumor microenvironment may exacerbate tumorigenic inflammation, leading to a persistent vicious circle between inflammation and cancer.
High-abundance Malassezia has already been detected in multiple cancers, implying that Malassezia may be a key in initiating and accelerating cancer development. Furthermore, the presence of Malassezia was inextricably linked with its induced inflammation. Based on this, during the process of Malassezia in cancer promotion, inflammation may be the biggest contributor.
Conclusion
In general, patients with IBD exhibit a high incidence in a series of cancers. For the IBD population, increased level of Malassezia is connected to the imbalance of microflora, fungal translocation, and inflammatory deterioration. In addition, the abundance of Malassezia is positively correlated with the pathogenesis and progression of various cancers, suggesting that Malassezia may be a key component to relate IBD with cancer. Accordingly, broad-spectrum antifungal drugs, aimed to reduce the production of Malassezia strains or their metabolites, can inhibit tumorigenesis and slow down tumor progression, by restoring internal fungal homeostasis and reducing inflammatory responses. This may provide new thoughts for cancer monitoring and novel therapeutic approaches. However, more studies are still required to verify the clinical benefits of Malassezia genus inhibition in IBD or cancer group. Although Malassezia is considered to be a pathogenic fungus which may participate in the pathogenesis of IBD and promote this disease to further develop into cancer, more informative pathogenesis still needs to be addressed. Multidisciplinary cooperation has become an inevitable trend, so the next arduous task is to deeply explore and fully utilize Malassezia-relevant mycology, and then combine it with metabolomics and immunology. This future research will reveal the potential of Malassezia strains as therapeutic targets, aiming at relieving inflammatory reaction, improving patient outcome in IBD, and further reducing the incidence of cancer.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Author Contributions
QY and JO wrote the first draft edition of the manuscript. DP provided critical revisions of this manuscript. LF and JY conceived and designed the manuscript. All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
Our work was funded by the Joint Medical Research Project (2020GDRC010) of Chongqing Science & Technology Bureau and Chongqing Health Commission and the Chinese Federation of Public Health foundation (GWLM202024).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Maaser C, Sturm A, Vavricka SR, Kucharzik T, Fiorino G, Annese V, et al. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: Initial Diagnosis, Monitoring of Known IBD, Detection of Complications. J Crohns Colitis (2019) 13:144–64. doi: 10.1093/ecco-jcc/jjy113
2. Lamas B, Richard ML, Leducq V, Pham HP, Michel ML, Da Costa G, et al. CARD9 Impacts Colitis by Altering Gut Microbiota Metabolism of Tryptophan Into Aryl Hydrocarbon Receptor Ligands. Nat Med (2016) 22:598–605. doi: 10.1038/nm.4102
3. Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, et al. Worldwide Incidence and Prevalence of Inflammatory Bowel Disease in the 21st Century: A Systematic Review of Population-Based Studies. Lancet (2018) 390:2769–78. doi: 10.1016/s0140-6736(17)32448-0
4. Alhagamhmad MH, Day AS, Lemberg DA, Leach ST. An Overview of the Bacterial Contribution to Crohn Disease Pathogenesis. J Med Microbiol (2016) 65:1049–59. doi: 10.1099/jmm.0.000331
5. Yang Q, Ouyang J, Sun F, Yang J. Short-Chain Fatty Acids: A Soldier Fighting Against Inflammation and Protecting From Tumorigenesis in People With Diabetes. Front Immunol (2020) 11:590685. doi: 10.3389/fimmu.2020.590685
6. Liu S, Zhao W, Lan P, Mou X. The Microbiome in Inflammatory Bowel Diseases: From Pathogenesis to Therapy. Protein Cell (2020) 12:331–45. doi: 10.1007/s13238-020-00745-3
7. Thursby E, Juge N. Introduction to the Human Gut Microbiota. Biochem J (2017) 474:1823–36. doi: 10.1042/bcj20160510
8. Neish AS. Microbes in Gastrointestinal Health and Disease. Gastroenterology (2009) 136:65–80. doi: 10.1053/j.gastro.2008.10.080
9. Imai T, Inoue R, Kawada Y, Morita Y, Inatomi O, Nishida A, et al. Characterization of Fungal Dysbiosis in Japanese Patients With Inflammatory Bowel Disease. J Gastroenterol (2019) 54:149–59. doi: 10.1007/s00535-018-1530-7
10. Lam S, Zuo T, Ho M, Chan FKL, Chan PKS, Ng SC. Review Article: Fungal Alterations in Inflammatory Bowel Diseases. Aliment Pharmacol Ther (2019) 50:1159–71. doi: 10.1111/apt.15523
11. Liguori G, Lamas B, Richard ML, Brandi G, da Costa G, Hoffmann TW, et al. Fungal Dysbiosis in Mucosa-Associated Microbiota of Crohn's Disease Patients. J Crohns Colitis (2016) 10:296–305. doi: 10.1093/ecco-jcc/jjv209
12. Leonardi I, Paramsothy S, Doron I, Semon A, Kaakoush NO, Clemente JC, et al. Fungal Trans-Kingdom Dynamics Linked to Responsiveness to Fecal Microbiota Transplantation (FMT) Therapy in Ulcerative Colitis. Cell Host Microbe (2020) 27:823–9.e3. doi: 10.1016/j.chom.2020.03.006
13. Limon JJ, Tang J, Li D, Wolf AJ, Michelsen KS, Funari V, et al. Malassezia Is Associated With Crohn's Disease and Exacerbates Colitis in Mouse Models. Cell Host Microbe (2019) 25:377–88.e6. doi: 10.1016/j.chom.2019.01.007
14. Sokol H, Leducq V, Aschard H, Pham HP, Jegou S, Landman C, et al. Fungal Microbiota Dysbiosis in IBD. Gut (2017) 66:1039–48. doi: 10.1136/gutjnl-2015-310746
15. Alonso-Monge R, Gresnigt MS, Román E, Hube B, Pla J. Candida Albicans Colonization of the Gastrointestinal Tract: A Double-Edged Sword. PloS Pathog (2021) 17:e1009710. doi: 10.1371/journal.ppat.1009710
16. Hurabielle C, Link VM, Bouladoux N, Han SJ, Merrill ED, Lightfoot YL, et al. Immunity to Commensal Skin Fungi Promotes Psoriasiform Skin Inflammation. Proc Natl Acad Sci USA (2020) 117:16465–74. doi: 10.1073/pnas.2003022117
17. Olén O, Askling J, Sachs MC, Frumento P, Neovius M, Smedby KE, et al. Childhood Onset Inflammatory Bowel Disease and Risk of Cancer: A Swedish Nationwide Cohort Study 1964-2014. Bmj (2017) 358:j3951. doi: 10.1136/bmj.j3951
18. Olén O, Erichsen R, Sachs MC, Pedersen L, Halfvarson J, Askling J, et al. Colorectal Cancer in Ulcerative Colitis: A Scandinavian Population-Based Cohort Study. Lancet (2020) 395:123–31. doi: 10.1016/s0140-6736(19)32545-0
19. Hagen JW, Pugliano-Mauro MA. Nonmelanoma Skin Cancer Risk in Patients With Inflammatory Bowel Disease Undergoing Thiopurine Therapy: A Systematic Review of the Literature. Dermatol Surg (2018) 44:469–80. doi: 10.1097/dss.0000000000001455
20. Everhov ÅH, Erichsen R, Sachs MC, Pedersen L, Halfvarson J, Askling J, et al. Inflammatory Bowel Disease and Pancreatic Cancer: A Scandinavian Register-Based Cohort Study 1969-2017. Aliment Pharmacol Ther (2020) 52:143–54. doi: 10.1111/apt.15785
21. Jung YS, Han M, Park S, Kim WH, Cheon JH. Cancer Risk in the Early Stages of Inflammatory Bowel Disease in Korean Patients: A Nationwide Population-Based Study. J Crohns Colitis (2017) 11:954–62. doi: 10.1093/ecco-jcc/jjx040
22. Canavan C, Abrams KR, Mayberry J. Meta-Analysis: Colorectal and Small Bowel Cancer Risk in Patients With Crohn's Disease. Aliment Pharmacol Ther (2006) 23:1097–104. doi: 10.1111/j.1365-2036.2006.02854.x
23. Eaden JA, Abrams KR, Mayberry JF. The Risk of Colorectal Cancer in Ulcerative Colitis: A Meta-Analysis. Gut (2001) 48:526–35. doi: 10.1136/gut.48.4.526
24. Castro FA, Liu X, Försti A, Ji J, Sundquist J, Sundquist K, et al. Increased Risk of Hepatobiliary Cancers After Hospitalization for Autoimmune Disease. Clin Gastroenterol Hepatol (2014) 12:1038–45.e7. doi: 10.1016/j.cgh.2013.11.007
25. Tyson GL, El-Serag HB. Risk Factors for Cholangiocarcinoma. Hepatology (2011) 54:173–84. doi: 10.1002/hep.24351
26. Ha CY, Katz S. Clinical Outcomes and Management of Inflammatory Bowel Disease in the Older Patient. Curr Gastroenterol Rep (2013) 15:310. doi: 10.1007/s11894-012-0310-4
27. Hoffman A, Galle PR. Gastrointestinal Disorders and Dabigatran. Scand J Gastroenterol (2013) 48:9–16. doi: 10.3109/00365521.2012.706825
28. Long MD, Martin CF, Pipkin CA, Herfarth HH, Sandler RS, Kappelman MD. Risk of Melanoma and Nonmelanoma Skin Cancer Among Patients With Inflammatory Bowel Disease. Gastroenterology (2012) 143:390–9.e1. doi: 10.1053/j.gastro.2012.05.004
29. Singh N, Baby D, Rajguru JP, Patil PB, Thakkannavar SS, Pujari VB. Inflammation and Cancer. Ann Afr Med (2019) 18:121–6. doi: 10.4103/aam.aam_56_18
30. Khandia R, Munjal A. Interplay Between Inflammation and Cancer. Adv Protein Chem Struct Biol (2020) 119:199–245. doi: 10.1016/bs.apcsb.2019.09.004
31. Aykut B, Pushalkar S, Chen R, Li Q, Abengozar R, Kim JI, et al. The Fungal Mycobiome Promotes Pancreatic Oncogenesis via Activation of MBL. Nature (2019) 574:264–7. doi: 10.1038/s41586-019-1608-2
32. Oh J, Byrd AL, Park M, Kong HH, Segre JA. Temporal Stability of the Human Skin Microbiome. Cell (2016) 165:854–66. doi: 10.1016/j.cell.2016.04.008
33. Byrd AL, Belkaid Y, Segre JA. The Human Skin Microbiome. Nat Rev Microbiol (2018) 16:143–55. doi: 10.1038/nrmicro.2017.157
34. Jo JH, Kennedy EA, Kong HH. Topographical and Physiological Differences of the Skin Mycobiome in Health and Disease. Virulence (2017) 8:324–33. doi: 10.1080/21505594.2016.1249093
35. Theelen B, Cafarchia C, Gaitanis G, Bassukas ID, Boekhout T, Dawson TL Jr. Malassezia Ecology, Pathophysiology, and Treatment. Med Mycol (2018) 56:S10–s25. doi: 10.1093/mmy/myx134
36. Saheb Kashaf S, Proctor DM, Deming C, Saary P, Hölzer M, Taylor ME, et al. Integrating Cultivation and Metagenomics for a Multi-Kingdom View of Skin Microbiome Diversity and Functions. Nat Microbiol (2022) 7:169–79. doi: 10.1038/s41564-021-01011-w
37. Findley K, Oh J, Yang J, Conlan S, Deming C, Meyer JA, et al. Topographic Diversity of Fungal and Bacterial Communities in Human Skin. Nature (2013) 498:367–70. doi: 10.1038/nature12171
38. Nash AK, Auchtung TA, Wong MC, Smith DP, Gesell JR, Ross MC, et al. The Gut Mycobiome of the Human Microbiome Project Healthy Cohort. Microbiome (2017) 5:153. doi: 10.1186/s40168-017-0373-4
39. Hoggard M, Zoing M, Biswas K, Taylor MW, Douglas RG. The Sinonasal Mycobiota in Chronic Rhinosinusitis and Control Patients. Rhinology (2019) 57:190–9. doi: 10.4193/Rhin18.256
40. Dunn AB, Jordan S, Baker BJ, Carlson NS. The Maternal Infant Microbiome: Considerations for Labor and Birth. MCN Am J Matern Child Nurs (2017) 42:318–25. doi: 10.1097/nmc.0000000000000373
41. Georgountzou A, Papadopoulos NG. Postnatal Innate Immune Development: From Birth to Adulthood. Front Immunol (2017) 8:957. doi: 10.3389/fimmu.2017.00957
42. Ayhan M, Sancak B, Karaduman A, Arikan S, Sahin S. Colonization of Neonate Skin by Malassezia Species: Relationship With Neonatal Cephalic Pustulosis. J Am Acad Dermatol (2007) 57:1012–8. doi: 10.1016/j.jaad.2007.02.030
43. Nagata R, Nagano H, Ogishima D, Nakamura Y, Hiruma M, Sugita T. Transmission of the Major Skin Microbiota, Malassezia, From Mother to Neonate. Pediatr Int (2012) 54:350–5. doi: 10.1111/j.1442-200X.2012.03563.x
44. Vijaya Chandra SH, Srinivas R, Dawson TL Jr., Common JE. Cutaneous Malassezia: Commensal, Pathogen, or Protector? Front Cell Infect Microbiol (2020) 10:614446. doi: 10.3389/fcimb.2020.614446
45. Prohic A, Jovovic Sadikovic T, Krupalija-Fazlic M, Kuskunovic-Vlahovljak S. Malassezia Species in Healthy Skin and in Dermatological Conditions. Int J Dermatol (2016) 55:494–504. doi: 10.1111/ijd.13116
46. Jo JH, Deming C, Kennedy EA, Conlan S, Polley EC, Ng WI, et al. Diverse Human Skin Fungal Communities in Children Converge in Adulthood. J Invest Dermatol (2016) 136:2356–63. doi: 10.1016/j.jid.2016.05.130
47. Balaji H, Heratizadeh A, Wichmann K, Niebuhr M, Crameri R, Scheynius A, et al. Malassezia Sympodialis Thioredoxin-Specific T Cells Are Highly Cross-Reactive to Human Thioredoxin in Atopic Dermatitis. J Allergy Clin Immunol (2011) 128:92–9.e4. doi: 10.1016/j.jaci.2011.02.043
48. Gao R, Kong C, Li H, Huang L, Qu X, Qin N, et al. Dysbiosis Signature of Mycobiota in Colon Polyp and Colorectal Cancer. Eur J Clin Microbiol Infect Dis (2017) 36:2457–68. doi: 10.1007/s10096-017-3085-6
49. Roman J, Bagla P, Ren P, Blanton LS, Berman MA. Malassezia Pachydermatis Fungemia in an Adult With Multibacillary Leprosy. Med Mycol Case Rep (2016) 12:1–3. doi: 10.1016/j.mmcr.2016.05.002
50. Spatz M, Richard ML. Overview of the Potential Role of Malassezia in Gut Health and Disease. Front Cell Infect Microbiol (2020) 10:201. doi: 10.3389/fcimb.2020.00201
51. Nguyen NH, Suh SO, Marshall CJ, Blackwell M. Morphological and Ecological Similarities: Wood-Boring Beetles Associated With Novel Xylose-Fermenting Yeasts, Spathaspora Passalidarum Gen. Sp. Nov. And Candida Jeffriesii Sp. Nov. Mycol Res (2006) 110:1232–41. doi: 10.1016/j.mycres.2006.07.002
52. Suhr MJ, Hallen-Adams HE. The Human Gut Mycobiome: Pitfalls and Potentials–a Mycologist's Perspective. Mycologia (2015) 107:1057–73. doi: 10.3852/15-147
53. Torres M, de Cock H, Celis Ramírez AM. In Vitro or In Vivo Models, the Next Frontier for Unraveling Interactions Between Malassezia Spp. And Hosts. How Much Do We Know? J Fungi (Basel) (2020) 6:155. doi: 10.3390/jof6030155
54. Brenchley JM, Douek DC. Microbial Translocation Across the GI Tract. Annu Rev Immunol (2012) 30:149–73. doi: 10.1146/annurev-immunol-020711-075001
55. DeAngelis YM, Gemmer CM, Kaczvinsky JR, Kenneally DC, Schwartz JR, Dawson TL Jr. Three Etiologic Facets of Dandruff and Seborrheic Dermatitis: Malassezia Fungi, Sebaceous Lipids, and Individual Sensitivity. J Investig Dermatol Symp Proc (2005) 10:295–7. doi: 10.1111/j.1087-0024.2005.10119.x
56. Tanojo H, Boelsma E, Junginger HE, Ponec M, Boddé HE. In Vivo Human Skin Barrier Modulation by Topical Application of Fatty Acids. Skin Pharmacol Appl Skin Physiol (1998) 11:87–97. doi: 10.1159/000029813
57. Xu J, Saunders CW, Hu P, Grant RA, Boekhout T, Kuramae EE, et al. Dandruff-Associated Malassezia Genomes Reveal Convergent and Divergent Virulence Traits Shared With Plant and Human Fungal Pathogens. Proc Natl Acad Sci USA (2007) 104:18730–5. doi: 10.1073/pnas.0706756104
58. Hort W, Mayser P. Malassezia Virulence Determinants. Curr Opin Infect Dis (2011) 24:100–5. doi: 10.1097/QCO.0b013e328342f787
59. DeAngelis YM, Saunders CW, Johnstone KR, Reeder NL, Coleman CG, Kaczvinsky JR Jr., et al. Isolation and Expression of a Malassezia Globosa Lipase Gene, LIP1. J Invest Dermatol (2007) 127:2138–46. doi: 10.1038/sj.jid.5700844
60. Cafarchia C, Otranto D. Association Between Phospholipase Production by Malassezia Pachydermatis and Skin Lesions. J Clin Microbiol (2004) 42:4868–9. doi: 10.1128/jcm.42.10.4868-4869.2004
61. Galli E, Cinicola B, Carello R, Caimmi S, Brindisi G, De Castro G, et al. Atopic Dermatitis. Acta Biomed (2020) 91:e2020011. doi: 10.23750/abm.v91i11-S.10313
62. Danby SG, Cork MJ. pH in Atopic Dermatitis. Curr Probl Dermatol (2018) 54:95–107. doi: 10.1159/000489523
63. Saunders CW, Scheynius A, Heitman J. Malassezia Fungi Are Specialized to Live on Skin and Associated With Dandruff, Eczema, and Other Skin Diseases. PloS Pathog (2012) 8:e1002701. doi: 10.1371/journal.ppat.1002701
64. Blanco JL, Garcia ME. Immune Response to Fungal Infections. Vet Immunol Immunopathol (2008) 125:47–70. doi: 10.1016/j.vetimm.2008.04.020
65. Wheeler ML, Limon JJ, Underhill DM. Immunity to Commensal Fungi: Detente and Disease. Annu Rev Pathol (2017) 12:359–85. doi: 10.1146/annurev-pathol-052016-100342
66. Miceli MH, Díaz JA, Lee SA. Emerging Opportunistic Yeast Infections. Lancet Infect Dis (2011) 11:142–51. doi: 10.1016/s1473-3099(10)70218-8
67. Kasperkovitz PV, Khan NS, Tam JM, Mansour MK, Davids PJ, Vyas JM. Toll-Like Receptor 9 Modulates Macrophage Antifungal Effector Function During Innate Recognition of Candida Albicans and Saccharomyces Cerevisiae. Infect Immun (2011) 79:4858–67. doi: 10.1128/iai.05626-11
68. Ishikawa T, Itoh F, Yoshida S, Saijo S, Matsuzawa T, Gonoi T, et al. Identification of Distinct Ligands for the C-Type Lectin Receptors Mincle and Dectin-2 in the Pathogenic Fungus Malassezia. Cell Host Microbe (2013) 13:477–88. doi: 10.1016/j.chom.2013.03.008
69. Buentke E, D'Amato M, Scheynius A. Malassezia Enhances Natural Killer Cell-Induced Dendritic Cell Maturation. Scand J Immunol (2004) 59:511–6. doi: 10.1111/j.0300-9475.2004.01416.x
70. Gerosa F, Baldani-Guerra B, Nisii C, Marchesini V, Carra G, Trinchieri G. Reciprocal Activating Interaction Between Natural Killer Cells and Dendritic Cells. J Exp Med (2002) 195:327–33. doi: 10.1084/jem.20010938
71. LeibundGut-Landmann S, Gross O, Robinson MJ, Osorio F, Slack EC, Tsoni SV, et al. Syk- and CARD9-Dependent Coupling of Innate Immunity to the Induction of T Helper Cells That Produce Interleukin 17. Nat Immunol (2007) 8:630–8. doi: 10.1038/ni1460
72. Sparber F, De Gregorio C, Steckholzer S, Ferreira FM, Dolowschiak T, Ruchti F, et al. The Skin Commensal Yeast Malassezia Triggers a Type 17 Response That Coordinates Anti-Fungal Immunity and Exacerbates Skin Inflammation. Cell Host Microbe (2019) 25:389–403.e6. doi: 10.1016/j.chom.2019.02.002
73. Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, et al. Control of T(reg) and T(H)17 Cell Differentiation by the Aryl Hydrocarbon Receptor. Nature (2008) 453:65–71. doi: 10.1038/nature06880
74. Buommino E, Baroni A, Papulino C, Nocera FP, Coretti L, Donnarumma G, et al. Malassezia Pachydermatis Up-Regulates AhR Related CYP1A1 Gene and Epidermal Barrier Markers in Human Keratinocytes. Med Mycol (2018) 56:987–93. doi: 10.1093/mmy/myy004
75. Watanabe S, Kano R, Sato H, Nakamura Y, Hasegawa A. The Effects of Malassezia Yeasts on Cytokine Production by Human Keratinocytes. J Invest Dermatol (2001) 116:769–73. doi: 10.1046/j.1523-1747.2001.01321.x
76. Alhallaf R, Agha Z, Miller CM, Robertson AAB, Sotillo J, Croese J, et al. The NLRP3 Inflammasome Suppresses Protective Immunity to Gastrointestinal Helminth Infection. Cell Rep (2018) 23:1085–98. doi: 10.1016/j.celrep.2018.03.097
77. Wolf AJ, Limon JJ, Nguyen C, Prince A, Castro A, Underhill DM. Malassezia Spp. Induce Inflammatory Cytokines and Activate NLRP3 Inflammasomes in Phagocytes. J Leukoc Biol (2021) 109:161–72. doi: 10.1002/jlb.2ma0820-259r
78. Zhang YJ, Han Y, Sun YZ, Jiang HH, Liu M, Qi RQ, et al. Extracellular Vesicles Derived From Malassezia Furfur Stimulate IL-6 Production in Keratinocytes as Demonstrated in In Vitro and In Vivo Models. J Dermatol Sci (2019) 93:168–75. doi: 10.1016/j.jdermsci.2019.03.001
79. Golusda L, Kühl AA, Siegmund B, Paclik D. Extracellular Matrix Components as Diagnostic Tools in Inflammatory Bowel Disease. Biol (Basel) (2021) 10:1024. doi: 10.3390/biology10101024
80. Pfisterer K, Shaw LE, Symmank D, Weninger W. The Extracellular Matrix in Skin Inflammation and Infection. Front Cell Dev Biol (2021) 9:682414. doi: 10.3389/fcell.2021.682414
81. Bhattacharjee O, Ayyangar U, Kurbet AS, Ashok D, Raghavan S. Unraveling the ECM-Immune Cell Crosstalk in Skin Diseases. Front Cell Dev Biol (2019) 7:68. doi: 10.3389/fcell.2019.00068
82. Ringer P, Colo G, Fässler R, Grashoff C. Sensing the Mechano-Chemical Properties of the Extracellular Matrix. Matrix Biol (2017) 64:6–16. doi: 10.1016/j.matbio.2017.03.004
83. Angiolella L, Rojas F, Mussin J, Greco R, Sosa MLA, Zalazar L, et al. Biofilm Formation, Adherence, and Hydrophobicity of M. Sympodialis, M. Globosa and M. Slooffiae From Clinical Isolates and Normal Skinvirulence Factors of M. Sympodialis, M. Globosa and M. Slooffiae. Med Mycol (2020) 58:1162–8. doi: 10.1093/mmy/myaa017
84. Ianiri G, Heitman J, Scheynius A. The Skin Commensal Yeast Malassezia Globosa Thwarts Bacterial Biofilms to Benefit the Host. J Invest Dermatol (2018) 138:1026–9. doi: 10.1016/j.jid.2018.01.008
85. Tronchin G, Pihet M, Lopes-Bezerra LM, Bouchara JP. Adherence Mechanisms in Human Pathogenic Fungi. Med Mycol (2008) 46:749–72. doi: 10.1080/13693780802206435
86. Poh SE, Goh JPZ, Fan C, Chua W, Gan SQ, Lim PLK, et al. Identification of Malassezia Furfur Secreted Aspartyl Protease 1 (MfSAP1) and Its Role in Extracellular Matrix Degradation. Front Cell Infect Microbiol (2020) 10:148. doi: 10.3389/fcimb.2020.00148
87. Ashbee HR, Evans EG. Immunology of Diseases Associated With Malassezia Species. Clin Microbiol Rev (2002) 15:21–57. doi: 10.1128/cmr.15.1.21-57.2002
88. Celis A. M. WHAB, Triana S, Restrepo S, de Cock H. Malassezia Spp. Beyond the Mycobiota. SM Dermatol J (2017) 3:1019-1–1019-10. doi: 10.36876/smdj.1019
89. Angiolella L, Carradori S, Maccallini C, Giusiano G, Supuran CT. Targeting Malassezia Species for Novel Synthetic and Natural Antidandruff Agents. Curr Med Chem (2017) 24:2392–412. doi: 10.2174/0929867324666170404110631
90. Li H, Goh BN, Teh WK, Jiang Z, Goh JPZ, Goh A, et al. Skin Commensal Malassezia Globosa Secreted Protease Attenuates Staphylococcus Aureus Biofilm Formation. J Invest Dermatol (2018) 138:1137–45. doi: 10.1016/j.jid.2017.11.034
91. Wang S, Zhang YR, Yu YB. The Important Role of Fungi in Inflammatory Bowel Diseases. Scand J Gastroenterol (2021) 56:1312–22. doi: 10.1080/00365521.2021.1963838
92. Selander C, Engblom C, Nilsson G, Scheynius A, Andersson CL. TLR2/MyD88-Dependent and -Independent Activation of Mast Cell IgE Responses by the Skin Commensal Yeast Malassezia Sympodialis. J Immunol (2009) 182:4208–16. doi: 10.4049/jimmunol.0800885
93. He XX, Li YH, Yan PG, Meng XC, Chen CY, Li KM, et al. Relationship Between Clinical Features and Intestinal Microbiota in Chinese Patients With Ulcerative Colitis. World J Gastroenterol (2021) 27:4722–37. doi: 10.3748/wjg.v27.i28.4722
94. Rudramurthy SM, Honnavar P, Chakrabarti A, Dogra S, Singh P, Handa S. Association of Malassezia Species With Psoriatic Lesions. Mycoses (2014) 57:483–8. doi: 10.1111/myc.12186
95. Gomez-Moyano E, Crespo-Erchiga V, Martínez-Pilar L, Godoy Diaz D, Martínez-García S, Lova Navarro M, et al. Do Malassezia Species Play a Role in Exacerbation of Scalp Psoriasis? J Mycol Med (2014) 24:87–92. doi: 10.1016/j.mycmed.2013.10.007
96. Gaitanis G, Magiatis P, Hantschke M, Bassukas ID, Velegraki A. The Malassezia Genus in Skin and Systemic Diseases. Clin Microbiol Rev (2012) 25:106–41. doi: 10.1128/cmr.00021-11
97. Mittermann I, Wikberg G, Johansson C, Lupinek C, Lundeberg L, Crameri R, et al. IgE Sensitization Profiles Differ Between Adult Patients With Severe and Moderate Atopic Dermatitis. PloS One (2016) 11:e0156077. doi: 10.1371/journal.pone.0156077
98. Al Bataineh MT, Hamoudi RA, Dash NR, Ramakrishnan RK, Almasalmeh MA, Sharif HA, et al. Altered Respiratory Microbiota Composition and Functionality Associated With Asthma Early in Life. BMC Infect Dis (2020) 20:697. doi: 10.1186/s12879-020-05427-3
99. Abdillah A, Ranque S. Chronic Diseases Associated With Malassezia Yeast. J Fungi (Basel) (2021) 7:855. doi: 10.3390/jof7100855
100. Soret P, Vandenborght LE, Francis F, Coron N, Enaud R, Avalos M, et al. Respiratory Mycobiome and Suggestion of Inter-Kingdom Network During Acute Pulmonary Exacerbation in Cystic Fibrosis. Sci Rep (2020) 10:3589. doi: 10.1038/s41598-020-60015-4
101. Alonso R, Pisa D, Fernández-Fernández AM, Rábano A, Carrasco L. Fungal Infection in Neural Tissue of Patients With Amyotrophic Lateral Sclerosis. Neurobiol Dis (2017) 108:249–60. doi: 10.1016/j.nbd.2017.09.001
102. Alonso R, Pisa D, Fernández-Fernández AM, Carrasco L. Infection of Fungi and Bacteria in Brain Tissue From Elderly Persons and Patients With Alzheimer's Disease. Front Aging Neurosci (2018) 10:159. doi: 10.3389/fnagi.2018.00159
103. Pisa D, Alonso R, Carrasco L. Parkinson's Disease: A Comprehensive Analysis of Fungi and Bacteria in Brain Tissue. Int J Biol Sci (2020) 16:1135–52. doi: 10.7150/ijbs.42257
104. Gaitanis G, Velegraki A, Magiatis P, Pappas P, Bassukas ID. Could Malassezia Yeasts be Implicated in Skin Carcinogenesis Through the Production of Aryl-Hydrocarbon Receptor Ligands? Med Hypotheses (2011) 77:47–51. doi: 10.1016/j.mehy.2011.03.020
105. Lopez LR, Bleich RM, Arthur JC. Microbiota Effects on Carcinogenesis: Initiation, Promotion, and Progression. Annu Rev Med (2021) 72:243–61. doi: 10.1146/annurev-med-080719-091604
106. Mohamed N, Litlekalsøy J, Ahmed IA, Martinsen EMH, Furriol J, Javier-Lopez R, et al. Analysis of Salivary Mycobiome in a Cohort of Oral Squamous Cell Carcinoma Patients From Sudan Identifies Higher Salivary Carriage of Malassezia as an Independent and Favorable Predictor of Overall Survival. Front Cell Infect Microbiol (2021) 11:673465. doi: 10.3389/fcimb.2021.673465
107. Coker OO, Nakatsu G, Dai RZ, Wu WKK, Wong SH, Ng SC, et al. Enteric Fungal Microbiota Dysbiosis and Ecological Alterations in Colorectal Cancer. Gut (2019) 68:654–62. doi: 10.1136/gutjnl-2018-317178
108. Medzhitov R. Origin and Physiological Roles of Inflammation. Nature (2008) 454:428–35. doi: 10.1038/nature07201
109. Bannenberg GL, Chiang N, Ariel A, Arita M, Tjonahen E, Gotlinger KH, et al. Molecular Circuits of Resolution: Formation and Actions of Resolvins and Protectins. J Immunol (2005) 174:4345–55. doi: 10.4049/jimmunol.174.7.4345
110. Gordon S. Phagocytosis: An Immunobiologic Process. Immunity (2016) 44:463–75. doi: 10.1016/j.immuni.2016.02.026
111. Bishehsari F, Engen PA, Preite NZ, Tuncil YE, Naqib A, Shaikh M, et al. Dietary Fiber Treatment Corrects the Composition of Gut Microbiota, Promotes SCFA Production, and Suppresses Colon Carcinogenesis. Genes (2018) 9:102. doi: 10.3390/genes9020102
112. Coussens LM, Werb Z. Inflammation and Cancer. Nature (2002) 420:860–7. doi: 10.1038/nature01322
113. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2020. CA: Cancer J Clin (2020) 70:7–30. doi: 10.3322/caac.21590
114. Mittal S, El-Serag HB. Epidemiology of Hepatocellular Carcinoma: Consider the Population. J Clin Gastroenterol (2013) 47 Suppl:S2–6. doi: 10.1097/MCG.0b013e3182872f29
115. Plummer M, Franceschi S, Vignat J, Forman D, de Martel C. Global Burden of Gastric Cancer Attributable to Helicobacter Pylori. Int J Cancer (2015) 136:487–90. doi: 10.1002/ijc.28999
116. Bopanna S, Ananthakrishnan AN, Kedia S, Yajnik V, Ahuja V. Risk of Colorectal Cancer in Asian Patients With Ulcerative Colitis: A Systematic Review and Meta-Analysis. Lancet Gastroenterol Hepatology (2017) 2:269–76. doi: 10.1016/S2468-1253(17)30004-3
117. Lu B, Li M. Helicobacter Pylori Eradication for Preventing Gastric Cancer. World J Gastroenterol (2014) 20:5660–5. doi: 10.3748/wjg.v20.i19.5660
118. Naylor MS, Stamp GW, Foulkes WD, Eccles D, Balkwill FR. Tumor Necrosis Factor and Its Receptors in Human Ovarian Cancer. Potential Role in Disease Progression. J Clin Invest (1993) 91:2194–206. doi: 10.1172/jci116446
119. Haghnegahdar H, Du J, Wang D, Strieter RM, Burdick MD, Nanney LB, et al. The Tumorigenic and Angiogenic Effects of MGSA/GRO Proteins in Melanoma. J Leukoc Biol (2000) 67:53–62. doi: 10.1002/jlb.67.1.53
120. Thun MJ, Namboodiri MM, Calle EE, Flanders WD, Heath CW Jr. Aspirin Use and Risk of Fatal Cancer. Cancer Res (1993) 53:1322–7.
121. Grivennikov SI, Wang K, Mucida D, Stewart CA, Schnabl B, Jauch D, et al. Adenoma-Linked Barrier Defects and Microbial Products Drive IL-23/IL-17-Mediated Tumour Growth. Nature (2012) 491:254–8. doi: 10.1038/nature11465
122. Van der Sluis M, De Koning BA, De Bruijn AC, Velcich A, Meijerink JP, Van Goudoever JB, et al. Muc2-Deficient Mice Spontaneously Develop Colitis, Indicating That MUC2 Is Critical for Colonic Protection. Gastroenterology (2006) 131:117–29. doi: 10.1053/j.gastro.2006.04.020
123. Mantovani A, Barajon I, Garlanda C. IL-1 and IL-1 Regulatory Pathways in Cancer Progression and Therapy. Immunol Rev (2018) 281:57–61. doi: 10.1111/imr.12614
124. Garlanda C, Dinarello CA, Mantovani A. The Interleukin-1 Family: Back to the Future. Immunity (2013) 39:1003–18. doi: 10.1016/j.immuni.2013.11.010
125. Voronov E, Carmi Y, Apte RN. The Role IL-1 in Tumor-Mediated Angiogenesis. Front Physiol (2014) 5:114. doi: 10.3389/fphys.2014.00114
126. Negus RP, Stamp GW, Relf MG, Burke F, Malik ST, Bernasconi S, et al. The Detection and Localization of Monocyte Chemoattractant Protein-1 (MCP-1) in Human Ovarian Cancer. J Clin Invest (1995) 95:2391–6. doi: 10.1172/jci117933
127. Wang K, Kim MK, Di Caro G, Wong J, Shalapour S, Wan J, et al. Interleukin-17 Receptor a Signaling in Transformed Enterocytes Promotes Early Colorectal Tumorigenesis. Immunity (2014) 41:1052–63. doi: 10.1016/j.immuni.2014.11.009
128. Dash S. Analyzing the Role of TNF-α and Autophagy in Regulation of TGF-β Induced Epithelial to Mesenchymal Transition in Cancer Cells: BITS Pilani. (2018).
129. Wang X, Lin Y. Tumor Necrosis Factor and Cancer, Buddies or Foes? Acta Pharmacol Sin (2008) 29:1275–88. doi: 10.1111/j.1745-7254.2008.00889.x
130. Pickup MW, Mouw JK, Weaver VM. The Extracellular Matrix Modulates the Hallmarks of Cancer. EMBO Rep (2014) 15:1243–53. doi: 10.15252/embr.201439246
131. Gilkes DM, Semenza GL, Wirtz D. Hypoxia and the Extracellular Matrix: Drivers of Tumour Metastasis. Nat Rev Cancer (2014) 14:430–9. doi: 10.1038/nrc3726
132. Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, et al. Matrix Crosslinking Forces Tumor Progression by Enhancing Integrin Signaling. Cell (2009) 139:891–906. doi: 10.1016/j.cell.2009.10.027
133. Berti M, Vindigni A. Replication Stress: Getting Back on Track. Nat Struct Mol Biol (2016) 23:103–9. doi: 10.1038/nsmb.3163
134. Kawanishi S, Ohnishi S, Ma N, Hiraku Y, Murata M. Crosstalk Between DNA Damage and Inflammation in the Multiple Steps of Carcinogenesis. Int J Mol Sci (2017) 18:1808. doi: 10.3390/ijms18081808
135. Ahn J, Xia T, Konno H, Konno K, Ruiz P, Barber GN. Inflammation-Driven Carcinogenesis Is Mediated Through STING. Nat Commun (2014) 5:1–9. doi: 10.1038/ncomms6166
136. Singhal SS, Horne D, Singhal J, Vonderfecht S, Salgia R, Awasthi S. Synergistic Efficacy of RLIP Inhibition and 2′-Hydroxyflavanone Against DMBA-Induced Mammary Carcinogenesis in SENCAR Mice. Mol Carcinogenesis (2019) 58:1438–49. doi: 10.1002/mc.23026
137. Liao W, Overman MJ, Boutin AT, Shang X, Zhao D, Dey P, et al. KRAS-IRF2 Axis Drives Immune Suppression and Immune Therapy Resistance in Colorectal Cancer. Cancer Cell (2019) 35:559–72.e7. doi: 10.1016/j.ccell.2019.02.008
138. Kortlever RM, Sodir NM, Wilson CH, Burkhart DL, Pellegrinet L, Swigart LB, et al. Myc Cooperates With Ras by Programming Inflammation and Immune Suppression. Cell (2017) 171:1301–15. e14. doi: 10.1016/j.cell.2017.11.013
139. Donehower LA, Soussi T, Korkut A, Liu Y, Schultz A, Cardenas M, et al. Integrated Analysis of TP53 Gene and Pathway Alterations in The Cancer Genome Atlas. Cell Rep (2019) 28:1370–84.e5. doi: 10.1016/j.celrep.2019.07.001
Keywords: Malassezia, fungus, inflammation, inflammatory bowel disease, cancer
Citation: Yang Q, Ouyang J, Pi D, Feng L and Yang J (2022) Malassezia in Inflammatory Bowel Disease: Accomplice of Evoking Tumorigenesis. Front. Immunol. 13:846469. doi: 10.3389/fimmu.2022.846469
Received: 31 December 2021; Accepted: 10 February 2022;
Published: 04 March 2022.
Edited by:
Ian Marriott, University of North Carolina at Charlotte, United StatesReviewed by:
Giuseppe Ianiri, University of Molise, ItalyGuillaume Sarrabayrouse, Université de Paris, France
Copyright © 2022 Yang, Ouyang, Pi, Feng and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Feng, NDA1MDQ1ODVAcXEuY29t; Jiadan Yang, eWFuZ2ppYWRhbjEwMjZAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Qiyu Yang1†
Qiyu Yang1† Jing Ouyang
Jing Ouyang Jiadan Yang
Jiadan Yang