- 1Department of Gastroenterology, The First Affiliated Hospital of University of Science and Technology of China (USTC), Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei, China
- 2Department of Infectious Diseases, The First Affiliated Hospital of University of Science and Technology of China (USTC), Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei, China
Liver failure is characterized by serious liver decompensation and high mortality. The activation of systemic immune responses and systemic inflammation are widely accepted as the core pathogenesis of liver failure. Glucocorticoids (GCs) are most regularly utilized to suppress excessive inflammatory reactions and immunological responses. GCs have been used in the clinical treatment of liver failure for nearly 60 years. While there has been no unanimity on the feasibility and application of GC treatment in liver failure until recently. The most recent trials have produced conflicting results when it comes to the dose and time for GC therapy of different etiology of liver failure. Our review outlines the issues and options in managing GC treatment in liver failure based on an investigation of the molecular mechanism that GC may give in the treatment.
1 Introduction
Liver failure (LF) is a life-threatening syndrome defined as the acute decompensation of liver function with varied etiology and multiple organ dysfunctions (1, 2). In China, viral hepatitis is the leading cause of liver failure, followed by alcohol and toxic drugs. Hepatitis B virus (HBV) related acute-on-chronic liver failure (ACLF) is the most common type of end-stage liver disease in chronic HBV infection patients, characterized by rapid deterioration, with muti-organ failure and high short-term mortality (3). At present, there is still no specific treatment of LF. Currently treatment is mostly based on comprehensive medical care, artificial liver support systems (ALSSs), and liver transplantation (LT). LT is an effective therapy even in patients at advanced stages, nevertheless, LT is limited by the availability of donor organs and the high medical cost, the mortality rate of LF remains high (4, 5).
Activation of immune response and systemic inflammation are considered as the key role of LF, glucocorticoids (GCs) have been used in the clinical treatment of LF for many years with the function that can rapidly suppress excessive inflammatory reactions and immune response. However, their usage has been contentious. For over half a century, there are numerous studies have been published (6–9). Although some clinical and experimental research are currently being conducted to determine the efficacy of GC treatment of LF, countries and organizations have yet to reach a consensus (10–15).
This study covers advances on the mechanism, value, existing difficulties, and application tactics of GC application in different etiology LF to identify ideas for further research in related domains and to provide assistance for the clinical management of LF.
2 The Mechanism of GC Treatment in Liver Failure
2.1 The Core Pathogenesis of Liver Failure
Liver acts as an immune organ and plays a key role in innate immune defenses against pathogens (16–18). LF has the features of systemic inflammation, cellular immune depression, and progression to multiple organ dysfunction. Activation of systemic immune responses should be considered playing a significant role in the pathogenesis and prognosis of LF (19, 20). Cytokines also play a pivotal role in LF pathophysiology including hepatocellular death, extrahepatic complications, and hepatocyte regeneration. And cytokines mediated liver injuries are tightly associated with hepatocyte proliferation and regeneration (21, 22). Suppressor of cytokine signaling (SOCS) family, signal transducer and activator of transcription (STAT) and nuclear factor kB (NF-kB)-mediated pathways have been shown closely linked with liver injury (23, 24).
Patients with ALF and ACLF display evidence of a pro-inflammatory state with local liver inflammation, features of systemic inflammatory response syndrome (SIRS) and vascular endothelial dysfunction that drive progression to multi-organ failure (25). The sooner SIRS emerges, the worse the prognosis (26). “Endotoxin-macrophage-cytokine storm” is the core pathogenesis of liver failure (27). The immunological balance is disrupted in the latter stages of liver failure, resulting in “immune paralysis” and a reduction in the total number and activity of peripheral blood lymphocytes, both of which aided in the progression and exacerbation of LF (28–30).
The “first hit” in the “three hits hypothesis” is the initial immunological insult to the liver produced by viruses, medications, and other factors, which immediately leads to the degeneration and necrosis of hepatocytes. The loss of hepatic sinusoids, microvascular embolism, and microcirculation disturbances result in ischemia and hypoxia of liver tissue, as well as additional reperfusion damage, resulting in the “second hit”. The liver’s detoxifying and endotoxin-scavenging abilities were reduced by the first two strikes, resulting in intestinal endotoxin-induced endotoxemia, which released a significant number of inflammatory factors such as IL-6 and tumor necrosis factor α (TNF-α), culminating in the “third hit” (31–33). GCs can impede macrophage phagocytosis and antigen treatment, as well as reduce the generation of inflammatory cytokines, since they are the most often utilized anti-inflammatory and immunosuppressive medicines. As a result, there is a theoretical foundation for using GCs to treat liver failure.
2.2 Immune Response Inhibition and Anti-Inflammatory Mechanisms of GCs
GCs can swiftly suppress excessive immune response and inflammatory reaction. In addition to inhibiting cytotoxic liver damage, GC intervention in LF can also control humoral immunity. GCs can influence the fraction of CD4+ lymphocyte subsets that are distributed, raise the proportion of Treg cells, and boost the immunomodulatory activity of Treg cells, all of which contribute to increased negative inflammatory control (34). Furthermore, GCs can directly decrease CD8+ cell immunological activity and diminish cytotoxicity. ICAM-1 (intercellularcelladhesionmolecule-1, ICAM-1) is a part of the immunoglobulin superfamily which found on the cell membrane of hepatocytes and many other cells. ICAM-1-mediated cell adhesion is critical for cytotoxic T lymphocyte (CTL) attachment to hepatocytes and can improve CTL assault on target cells. At the receptor level, GCs can block the production of ICAM-1, effectively preventing CD8+ lymphocytes from attacking hepatocytes. Furthermore, GCs can minimize the liver tissue damage induced by T/NKT cell infiltration by inhibiting the killing impact of T/NKT cells (35, 36).
GCs can induce apoptosis of inflammatory cells and inhibit antigen presentation as well as the generation and release of proinflammatory cytokines including IL-1, IL-6, TNF-α, and IL-17 (37, 38). GCs can also boost the synthesis of the anti-inflammatory cytokine IL-10 and improve the negative control of inflammatory factor storms at the same time (37). Furthermore, through modulating the immunological signal transduction pathway, GCs can decrease the inflammatory response. Important negative cytokine regulatory factors include suppressor of cytokine signaling 1 (SOCS1), suppressor of cytokine signaling 2 (SOCS2), and interleukin-1 receptor-associated kinase M (IRAK-M) (39). GCs can improve the inhibitory impact of SOCS1 and SOCS2 on the JAK/STAT inflammatory signaling pathway, as well as the inhibitory effect of IRAK-M on the Toll-like receptor 4 (TLR4) inflammatory signaling pathway (40, 41). Nucleotide-binding oligomerisation domain-like receptors (NLRs) Family Pyrin Domain Containing 3(NLRP3) is related to innate immunity and can produce proinflammatory cytokines via caspase-1 (42). NLRP3 has been found increased in HBV-related ACLF patients and downregulated by GCs in surviving patients (43). GCs can also cause lymphocytes to migrate out of blood vessels, reducing the number of lymphocytes in blood vessels. It may also enhance the local microcirculation of the liver, as well as lessen the disturbances of ischemia, hypoxia, and reperfusion of hepatocytes.
2.3 GCs Can Enhance the Protective Effect of Hepatocytes
By inhibiting caspase-8 activation and the mitochondria-dependent apoptosis pathway, dexamethasone (DEX) pretreatment protected hepatocytes from TNF-α, plus actinomycin D (ActD)-induced apoptosis (44). Considering that GCs are thought to have a significant stabilizing impact on cell membranes, they can prevent hepatocyte disintegration and necrosis, slowing the course of liver damage.
3 GC Therapy in Different Etiology of Liver Failure
3.1 HBV Related ACLF
According to the evidence shows that HBV mainly causes liver damage through cytotoxic T-lymphocyte-mediated cytolytic pathways in HBV-infected hepatocytes (45, 46), using GCs to treat severe hepatitis B infections is appropriate because of the particular effects of GCs to inhibit immune responses and prevent cytolysis in infecting hepatocytes (47). Multiple studies suggest that using GCs in the early period of severe hepatitis can help prevent liver cells necrosis and afford a possibility of liver regeneration (48–50) but might enhance HBV replication (51), and lead to LF (12, 52–54). Thus, GCs have not been widely used for the treatment of severe hepatitis B in clinic. However, in recent years, due to the new generation of nucleoside analogs (NA), using GCs to treat HBV related LF has become much safer (55–57).
Excessive systemic inflammation and susceptibility to infection are two pathophysiological characteristics of ACLF. Immunotherapies, such as glucocorticoids are effective on ACLF. Some studies have reported that GC treatment improve the survival rate of the patients with HBV-ACLF. A prospective multi-center clinical trial totally included 171 HBV-ACLF patients, 83 patients treated with methylprednisolone [1.5 mg/kg/day (day 1-3), 1 mg/kg/day (day 4-5), and 0.5 mg/kg/day (day 6-7)] for 7 days, the results showed methylprednisolone treatment can increase the 6-month survival rate of HBV-ACLF patients (27). And there is a retrospective study included 349 patients with HBV-ACLF in 2021. 155 patients used methylprednisolone or prednisone. The results showed that GC treatment could not improve the liver function of ACLF patients but might reduce their 28 days mortality rate (58). Similar results were also demonstrated by Zhao et al (59). No matter the patients used antivirus or not. The explanation for this might be that infectious complications are both the primary cause of ACLF and the leading cause of mortality from ACLF, these patients are susceptible immune paresis (60). Thus even though GC therapy did not improve liver function or short-term health, it may be required in critical patients. Nevertheless, this effect has not been validated by others. A retrospective, controlled trial with 31 HBV-related ACLF patients under dexamethasone injection for three times and followed up for 12 weeks, the results showed that dexamethasone cannot improve liver functions and 12-week survival rates of patients with HBV-related ACLF (61). A Ten-year cohort study in a University Hospital in East China also showed that steroid treatment did not improve transplant free survival in ACLF patients precipitated by hepatitis B (15).
The timing of GCs treatment in HBV-related ACLF is very important. Zhang et al. found that dexamethasone (10 mg/day, i.v.) for 5 days based on lamivudine (LMV) treatment is effective in improving the liver function and survival rate of patients with pre-ACLF (62). Another study included 87 patients with early-stage HBV-related subacute liver failure, 43 patients in the control group received LMV and routine integrated treatment, and those in the treatment group were given additional short-term low-dose glucocorticoid treatment. The results showed that GC treatment can improve survival rate and shorten the mean hospital stay of patients with HBV-related early-stage subacute liver failure patients (63). Thus, GCs should theoretically be able to control excessive systemic inflammation and hepatic inflammation in the early stages of ACLF, whereas they aggravate immune paralysis in the late stages. And another noteworthy issue is that nucleoside analog should be used as a basic treatment in HBV-ACLF patients. Overall, low dose, short term GC treatment combined with NA in the early stage of HBV-ACLF patients is safe and effective.
3.2 HBV Related ALF
Nearly half a century ago, researchers used double-blind, randomized trials of methylprednisolone(38-48mg/day) vs. placebo in severe viral hepatitis and showed the conclusion that methylprednisolone does not enhance survival in patients with severe viral hepatitis (6, 7). In 2006, Kotoh et al. used a high-dose methylprednisolone (1000 mg/day for 3 continuous days) to treat patients with severe acute hepatic failure and found methylprednisolone might effectively prevent the progression of severe acute hepatic failure (64). Fujiwara et al. used 1000 mg of methylprednisolone daily for 3 days followed by the reduced doses according to the treatment response in the early stage of viral acute liver failure, which indicated an effective suppressing of hepatocytes destruction and a slightly higher survival rate (65). And high dose of GCs treated in ALF did not significantly increase the incidence of infection (66). Then Fujiwara reported combination therapy with GCs and NA for HBV-ALF induces the rapid resolution of inflammation leading to a rapid recovery of the liver function. When it is administered at a sufficiently early stage, it would have a survival benefit and prevent persistent infection (67). In a whole, when treated in ALF, high dose GCs might be more effective, the early stage of the ascending period would be the best timing, and NA is also very important in HBV related ALF.
3.3 AIH Induced Liver Failure
Autoimmune hepatitis (AIH) is an immune-mediated necroinflammatory disease of the liver parenchyma. Although AIH is linked with minor symptoms in most patients, it can also be associated with severe symptoms and develop to ALF, or ACLF. Acute severe autoimmune hepatitis (AS-AIH) is a relatively rare cause of ALF, which is often neglected and delayed in treatment. The standard paradigm of management in acute AIH involved corticosteroid therapy. This can achieve a remission in more than 80% (68). However, GCs use in AIH-induced LF remains controversial. A single-center French study in 2007 looked at the role of GSs in patients with a severe presentation of AIH. They found that GC therapy is of little benefit in severe and fulminant forms of AIH; It may increase the risk of septic complications and should not delay liver transplantation (LT) (69). A retrospective analysis of patients with autoimmune, indeterminate, and drug-induced ALF included 66 patients with AIH. The study compared 25 patients who were given prednis(ol)one (median dosage 60 mg/day) with 41 patients who were not. GCs did not increase overall or spontaneous (without-LT) survival in autoimmune ALF. Furthermore, GCs use was linked to an increased mortality in the group of patients with the highest MELD scores (70). While there are also researchers suggested GCs should be considered as soon as possible in AS-AIH patients (71). Recently, a retrospectively study enrolled 32 patients with AIH-induced ALF compared with 93 age- and sex-matched patients with chronic AIH (cAIH), the patients received prednis(ol)one with an average dose of 153.9 mg daily for the first group and from 61.8 mg daily for the second group. GCs therapy was not associated with high mortality or sepsis in AIH-induced ALF and suggested that GCs treatment of AIH-mediated ALF may improve the outcome (72). Another study included 128 AS-AIH patients, 115 (90%) were treated with GCs within a median of 6 (2–10) days of their admission to hospital. Seventy-eight patients (73%) received prednis(ol)one with a dosage of 1 mg/kg/d while 37 (27%) received 0.5 mg/kg/d. Thirteen patients (10%) did not receive GCs therapy, the results showed that non-treated patients were more seriously ill than treated patients (73). Zachou et al. present an open, real-world observational study included 34 AS-AIH patients were treated with either 1g methylprednisolone for 3 consecutive days followed by intravenous prednisolone (1mg/kg/day) or prednisolone (1.5mg/kg/day) from the beginning. And indicated that high-dose intravenous GCs in original AS-AIH seems safe and efficient as it prevents disease deterioration and the need of liver transplantation (74). A recent Asian-Pacific study included 82 patients with AIH induced ACLF. A survival benefit was demonstrated in those who received GCs. Moreover, patients with high MELD scores and encephalopathy had unfavorable responses to GCs (75). In general, GC treatment is effective in both ALF and ACLF induced by AIH. High doses are also relatively safe. GCs should be used as early as possible, with an increased risk if MELD score is very high or encephalopathy present.
3.4 Drug Induced Liver Failure
Drug-induced liver injury (DILI) is a liver toxicity induced by drugs or their metabolites. Patients with DILI may present with various clinical manifestations, ranging from abnormal liver function test results but without symptoms to ALF (76). For drug-induced ALF, there are two primary therapeutic options: a) fast depuration of the body from the toxic chemical to prevent additional aggressiveness before the agent reaches the liver; and b) administration of an antidote to prevent and/or stop the aggression once the toxin reaches the liver. The newest EASL clinical practice guidelines suggested that GCs are usually given when all else fails to produce results (77). In early trials, GC therapy for all kinds of ALF demonstrated limited benefits (70, 78). A single-centre retrospective study used two kinds of GCs administration methods (Methylprednisolone, range 60-120 mg/day or prednisone, range 40-60 mg/day for 3-5 days and then prednisone 20 mg/day and 5-10 mg weekly reduction) or (Methylprednisolone, range 60-120 mg/day for 3-5days) to treat severe drug-induced liver injury (DILI) patients. The results showed that short-term use of GCs can improve the liver injury and patient survival of severe DILI patients with hyperbilirubinemia (TBil >243 µmol/L) (79). GCs combined with ursodesoxycholic acid appears to be safe, and leads to a more rapid reduction in bilirubin and transaminases after severe DILI (80). However, opposite result was found by Wan et al. that prednisone was not beneficial for the treatment of severe DILI (81). Heretofore, among the several liver diseases, AIH is the most reliable clinical indication for GC treatment (82). In patients with suspected drug induced AIH who are receiving GCs therapy, withdrawal of treatment once the liver injury has resolved should be followed by careful monitoring (83). Antiepileptic drug-induced liver injury is commonly related with hypersensitivity symptoms and may respond to GCs treatment (84). GCs should be administered in patients with severe alcoholic hepatitis (AH) if there are no contraindications (85). Overall, drug-induced liver failure needs evidence of immunopathogenicity to restore the condition through GCs blocking immune responses.
4 The Application Strategy of GC Treatment in Liver Failure
4.1 The Dose and Timing of GC Treatment in Liver Failure
There is currently no consensus on the type and dosage of GCs used in LF. GC dose is generally controlled in methylprednisolone (1∼2mg/kg/d) according to current clinical studies. Kotoh et al. investigated the possibility of using high dose of GCs to treat LF. 17 ALF patients underwent three days treatment of 1000mg methylprednisolone daily, and 13 of them were cured without serious complications, two died, and two received LT (64). The relevance of high-dose GCs in the treatment of severe acute exacerbation of CHB and the early stage of ALF was explored by other researchers. They indicated a slim advantage in terms of survival and liver regeneration in the GC treated group, but there was no significant difference, whereas patients with a poor basic condition and advanced liver damage at the start of treatment had a poor prognosis (50, 65). It will not function if a high dose of GCs are given during LF due to a decrease in the number of GC receptors on the surface of cells in the liver tissue, and there may also be an increase in the likelihood of GC adverse effects because GCs have the potential to cause substantial liver damage (65, 86, 87). As a result, high-dose GCs are more usually used in ALF patients compared to ACLF patients. And not indicated for individuals particularly with poor basic condition. Low and medium doses are generally used. Currently, some researchers utilize 10 mg dexamethasone once a day for three days to treat patients with HBV-related ACLF. The results revealed that early in the course of a severe acute exacerbation of chronic hepatitis B, combined with standard treatment, low-dose, short-term glucocorticoid treatment dramatically decreased the probability of progression to liver failure and shortened hospitalization time, without raising the complication rate (88). Low doses of GCs primarily depress cellular immunity, but high doses of GCs lower humoral immunity by suppressing B cells and antibody generation (89). However, there are certain variances in the dosage of GC used for various reasons. Prednisone 40 mg/d, according to some research, can be taken early in alcohol induced LF (90). When AIH is induced to LF, an initial dose of 20-50 mg/d methylprednisolone is used to provide a stronger curative effect (91). Some studies employed 1.5 mg/kg/d as the beginning dose for CHB-related LF and eventually reached excellent outcomes after progressively lowering the dose according to the disease (92).
When the effectiveness of GC treatment cannot be established in a clinical setting, the concept of safety requires that any potential adverse effects of GCs be maintained within a manageable range. GCs can considerably lower the number of lymphocytes in circulation by inhibiting the presence of phagocytic cells to the antigen, promoting the destruction and disintegration of lymphocytes, and developing the removal of lymphocytes from blood vessels (93). Even though that GCs can raise the risk of infection and upper gastrointestinal bleeding, as well as other complications, their adverse effects are manageable. As a result, it is critical to screen for and monitor adverse effects in individuals with liver failure who are taking GCs.
GC intervention in the early stages of LF has been demonstrated in several studies to improve prognosis and minimize death (63). Zhao et al. believed that GCs should be utilized when the MELD score is less than 35, the HE score is less than 4, and the ALT level is ≥ 30 ULN (14). When the MELD score is less than 27 and hepatic encephalopathy is less than stage II in AIH-related LF, the benefit of GCs is greatest (94). However, until recently, there were no clear quantitative indicators for GC therapy in LF, we believe that the age, basic conditions, and complications of the patients should all be considered. As a result, more clinical experience should be very important for doctors.
4.2 Problems of GCs Application in Liver Failure
4.2.1 GC Resistance
Some ACLF patients have a low sensitivity to GCs treatment (95). GCs via binding to their intracellular receptor (GR) to have powerful anti-inflammatory activities. Decreased GR in many inflammatory diseases confers GC resistance (GCR) and undermines glucocorticoid therapy efficacy. GCR is clearly acquired through persistent inflammatory injury (96–98). Tjandra et al. reported a significant decrease in hepatic T lymphocyte GR mRNA and protein levels in experimental cholangitis rats, demonstrating that hepatic T cell resistance to increased cortisol levels is at least partially mediated by decreased GR expression (99). In AIH patients, GR expression in peripheral mononuclear cells was shown to be closely related with GCR and to impact the outcome of therapy and the degree of disease severity (100, 101). Moreover, there is a dynamic process in the immune state of LF. It is reported that inactivation of functional T cells is a key step in the progression of systemic immunological dysfunction in ALF (102). And proinflammatory cytokines are involved in the pathogenesis of ALF (103). Further studies reported that the serum cortisol level and the percentage of GR+T lymphocytes were significantly decreased in HBV-ACLF patients compared with CHB patients and healthy controls. The relative GR alpha mRNA expression was significant decreased in ACLF patients (95). Recently, Wang et al. noted that in ACLF patients, GR alpha expression was negatively regulated by miR-124a. MicroRNA-124a contributes to GCR in ACLF by negatively regulating GR alpha (104).
4.2.2 Side Effects of GC Therapy in Liver Failure
The immune system is depressed during GC therapy, which raises the risk of secondary infection and the spread and aggravation of the primary illness, as well as the chance of systemic infection and sepsis (14, 66, 105). According to the CANONIC research of the EASL chronic liver failure (EASL-CLIF) Alliance, almost a third of patients with ACLF will be complicated with bacterial infection. Similarly, the EASL-CLIF and the North American Federation of End-stage Liver Diseases (NACSELD) have discovered that some individuals with liver cirrhosis develop ACLF due to coinfection (106, 107). When LF strikes, the intestinal barrier weakens and microecological changes occur, allowing intestinal flora to migrate and endotoxin to enter the bloodstream, resulting in infection (108). At the same time, microorganisms increase the risk of infection after avoiding the immune system and entering the circulation owing to immune escape and immunological paralysis during LF (109). Furthermore, genetic variables have a role in raising the likelihood of coinfection in LF patients (110, 111).
Sepsis is a common complication of ACLF (112), GCs have been tested and widely used in sepsis patients (113). Although it is an acute systemic inflammatory disease, GCs are hardly useful in sepsis (114). One reason to explain the rather poor successes of GCs in sepsis is that a profound GCR has developed. GCR has already developed by the time sepsis are diagnosed and treated. Many researchers have described GCR in cohorts of sepsis patients. Levels of GR mRNA in peripheral mononuclear cells (PBMCs) were found reduced in sepsis children (115). And Dekelbab et al. reported reduced GR protein levels in some organs (such as liver, brain, muscle) during sepsis (116). An increased expression of miR124 was associated with reduced GR expression in T cells of sepsis patients was also reported (117). Furthermore, Guerrero et al. found a temporary increase of the dominant negative GR beta in PBMCs during sepsis (118). Sepsis is associated with GCR significantly, which might be due to a decrease in GR expression or response.
GCs can also suppress gastric mucus secretion while increasing stomach acid and pepsin secretion, resulting in ulcers, perforations, and gastrointestinal (GI) bleeding. As LF is associated with a high risk of severe GI bleeding, using GCs in LF will significantly increase the risk of causes. Although the specific mechanism by which GCs may cause GI bleeding is unknown, GCs may inhibit tissue repair, thus causing delayed wound healing (119). Furthermore, aberrant blood pressure may emerge because of the pharmacological properties of GCs, as well as electrolyte and blood glucose abnormalities, potentially increasing the risk of hepatic encephalopathy, hepatorenal syndrome, and other consequences in LF.
4.3 Strategies of GCs Application in Liver Failure
Secondary infection is one of the most serious concerns associated with the use of GCs in LF, posing a secondary threat to the prognosis. Antibiotic prophylaxis can avoid LF consequences including peritonitis and upper gastrointestinal bleeding (120). Currently, it is generally recommended to choose quinolones for prophylactic anti-infective treatment (121). Simultaneously, by increasing gut flora, stimulating gastrointestinal peristalsis, managing autoimmunity, and strengthening nursing care, we can lower the chance of infection (122–124). Opportunistic fungal infections have emerged as a major cause of morbidity and mortality in immunocompromised patients including those who have received GC treatment (125). Although some studies have found that the prognosis of ACLF patients with fungal infection does not improve after active antifungal therapy, since all survivors have received antifungal therapy, it is still recommended to begin antifungal therapy as soon as possible in the early detection of fungal infection (126).
GI bleeding is common in LF patients, especially in ACLF patients with esophageal varices. The current GI bleeding in ALF patients is 1.5% (127). Patients who were taking high-dose glucocorticoids alone had a slight increased relative risk for developing GI bleeding (128). Pharmacologic suppression of stomach acid secretion has been proven to prevent GI bleeding (129, 130). Proton pump inhibitors (PPIs) are effective method to prevent peptic ulcer disease and GI bleeding in ALF (131). It is reported that in drug-induced liver injury, a PPI might be useful to prevent GI bleeding when GCs used (132). A study of HBV-related LF showed that GC therapy accompanied by prophylactic medication with PPI can prevent the severe side effects of GI bleeding of GC therapy (92). As a result, while using GCs in LF patients, more attention must be taken, and the stomach mucosa should be actively preserved to avoid GI bleeding.
Because of the immunosuppression caused by using GCs, LF patients with basic viral hepatitis may activate the virus. HBsAg positive LF patients should start antiviral therapy as soon as possible to inhibit virus replication. GCs can cause feelings of euphoria, excitation, sleeplessness, and even severe mental problems including hallucinations and insanity. It should be given special attention to patients’ mental states. Medication should be discontinued as soon as significant mental problems are discovered. Patients taking GCs for a long time will develop osteoporosis, timely calcium supplement will be useful. Since the proposal of GCs in the treatment of LF has been controversial, its strong immunosuppressive effect and significant efficacy not only bring great temptation to us but also make us face the risk of serious adverse reactions.
5 Conclusions
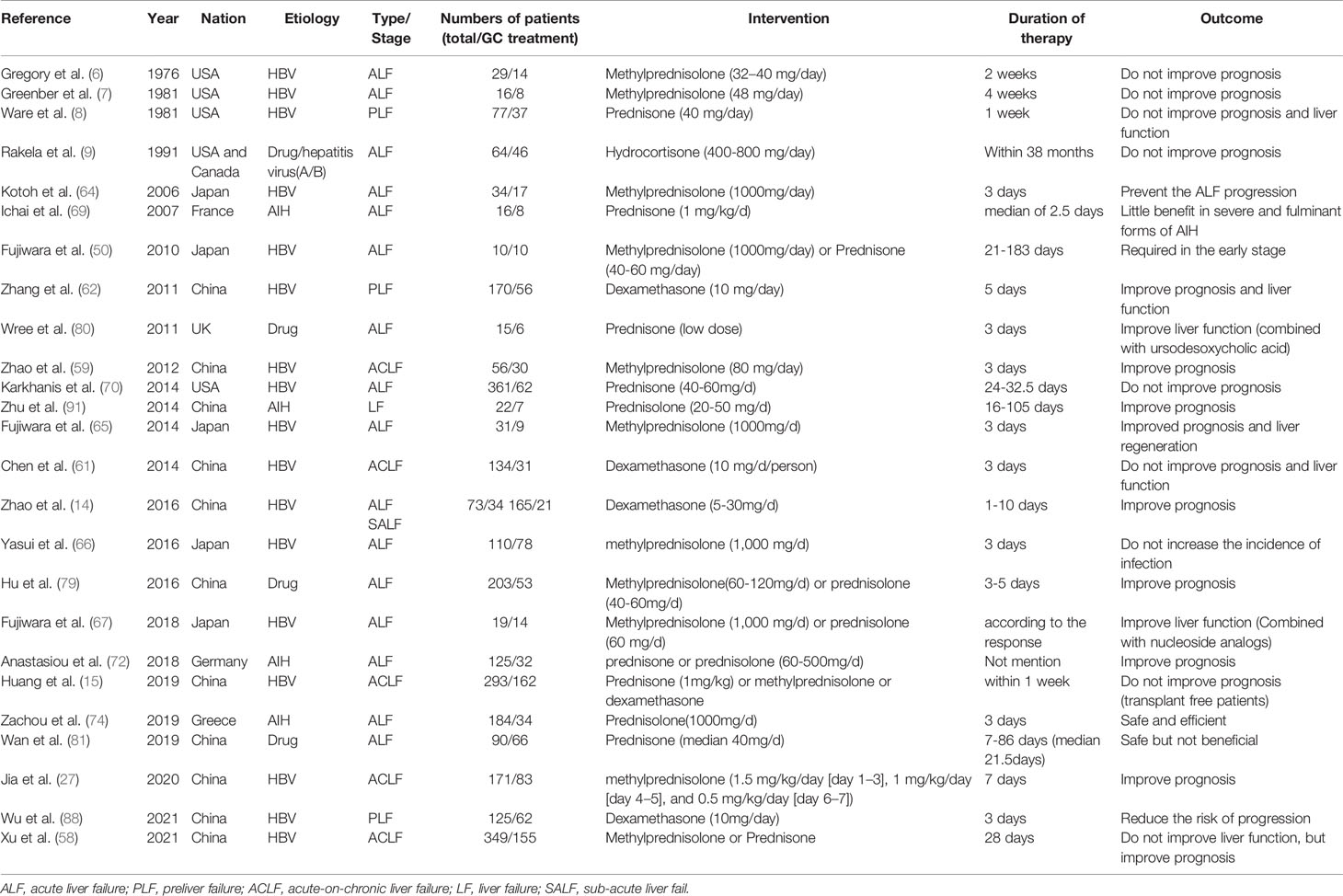
Although the notion of administering GCs to treat LF has circulated for a long time, no conclusive evidence has been provided of its therapeutic efficacy. Some data was from non-randomized studies or carried out in small groups (62, 64, 67). Here, we summarized several published articles referred GC treatment in different status of LF in Table 1.
Given all of that, due to the intricate pathophysiology of LF, it is critical to investigate immunological manifestations with various etiologies. In the treatment of LF, we should personalize each patient’s treatment plan, prioritize patient safety, monitor, and avoid any adverse responses to GCs in a timely manner, all of which will help patients obtain more benefit and improve their prognosis. To provide clinical professionals with a suitable treatment plan based on evidence-based medicine, further larger randomized clinical trials are required.
Author Contributions
CY wrote this manuscript, WL designed this manuscript, LL and KZ provided literatures review. All authors contributed to the article and approved the submitted version.
Funding
Provincial natural science foundation of Anhui (1908085QH331).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Xue R, Duan Z, Liu H, Chen L, Yu H, Ren M, et al. A Novel Dynamic Model for Predicting Outcome in Patients With Hepatitis B Virus Related Acute-on-Chronic Liver Failure. Oncotarget (2017) 8:108970–80. doi: 10.18632/oncotarget.22447
2. Singh T, Gupta N, Alkhouri N, Carey WD, Hanouneh IA. A Guide to Managing Acute Liver Failure. Cleve Clin J Med (2016) 83:453–62. doi: 10.3949/ccjm.83a.15101
3. Zhao RH, Shi Y, Zhao H, Wu W, Sheng JF. Acute-On-Chronic Liver Failure in Chronic Hepatitis B: An Update. Expert Rev Gastroenterol Hepatol (2018) 12:341–50. doi: 10.1080/17474124.2018.1426459
4. Asrani SK, Simonetto DA, Kamath PS. Acute-On-Chronic Liver Failure. Clin Gastroenterol Hepatol (2015) 13:2128–39. doi: 10.1016/j.cgh.2015.07.008
5. Singanayagam A, Bernal W. Update on Acute Liver Failure. Curr Opin Crit Care (2015) 21:134–41. doi: 10.1097/MCC.0000000000000187
6. Gregory PB, Knauer CM, Kempson RL, Miller R. Steroid Therapy in Severe Viral Hepatitis. A Double-Blind, Randomized Trial of Methyl-Prednisolone Versus Placebo. New Engl J Med (1976) 294:681–7. doi: 10.1056/NEJM197603252941301
7. Greenberg HB, Robinson WS, Knauer CM, Gregory PB. Hepatitis B Viral Markers in Severe Viral Hepatitis: Influence of Steroid Therapy. Hepatol (Baltimore Md) (1981) 1:54–7. doi: 10.1002/hep.1840010109
8. Ware AJ, Cuthbert JA, Shorey J, Gurian LE, Eigenbrodt EH, Combes B. A Prospective Trial of Steroid Therapy in Severe Viral Hepatitis. The Prognostic Significance of Bridging Necrosis. Gastroenterology (1981) 80:219–24. doi: 10.1016/0016-5085(81)90707-1
9. Rakela J, Mosley JW, Edwards VM, Govindarajan S, Alpert E. A Double-Blinded, Randomized Trial of Hydrocortisone in Acute Hepatic Failure. The Acute Hepatic Failure Study Group. Digestive Dis Sci (1991) 36:1223–8. doi: 10.1007/bf01307513
10. Wang F, Wang BY. Corticosteroids or non-Corticosteroids: A Fresh Perspective on Alcoholic Hepatitis Treatment. Hepatobiliary Pancreat Dis Int (2011) 10:458–64. doi: 10.1016/s1499-3872(11)60079-9
11. Yeoman AD, Westbrook RH, Zen Y, Bernal W, Al-Chalabi T, Wendon JA, et al. Prognosis of Acute Severe Autoimmune Hepatitis (AS-AIH): The Role of Corticosteroids in Modifying Outcome. J Hepatol (2014) 61:876–82. doi: 10.1016/j.jhep.2014.05.021
12. Yang CH, Wu TS, Chiu CT. Chronic Hepatitis B Reactivation: A Word of Caution Regarding the Use of Systemic Glucocorticosteroid Therapy. Br J Dermatol (2007) 157:587–90. doi: 10.1111/j.1365-2133.2007.08058.x
13. Ramachandran J, Sajith KG, Pal S, Rasak JV, Prakash JA, Ramakrishna B. Clinicopathological Profile and Management of Severe Autoimmune Hepatitis. Trop Gastroenterol (2014) 35:25–31. doi: 10.7869/tg.160
14. Zhao B, Zhang HY, Xie GJ, Liu HM, Chen Q, Li RF, et al. Evaluation of the Efficacy of Steroid Therapy on Acute Liver Failure. Exp Ther Med (2016) 12:3121–9. doi: 10.3892/etm.2016.3720
15. Huang C, Yu KK, Zheng JM, Li N. Steroid Treatment in Patients With Acute-on-Chronic Liver Failure Precipitated by Hepatitis B: A 10-Year Cohort Study in a University Hospital in East China. J Dig Dis (2019) 20:38–44. doi: 10.1111/1751-2980.12691
16. Gao B, Jeong WI, Tian Z. Liver: An Organ With Predominant Innate Immunity. Hepatol (Baltimore Md) (2008) 47:729–36. doi: 10.1002/hep.22034
17. Racanelli V, Rehermann B. The Liver as an Immunological Organ. Hepatol (Baltimore Md) (2006) 43:S54–62. doi: 10.1002/hep.21060
18. Crispe IN. The Liver as a Lymphoid Organ. Annu Rev Immunol (2009) 27:147–63. doi: 10.1146/annurev.immunol.021908.132629
19. Rolando N, Wade J, Davalos M, Wendon J, Philpott-Howard J, Williams R. The Systemic Inflammatory Response Syndrome in Acute Liver Failure. Hepatol (Baltimore Md) (2000) 32:734–9. doi: 10.1053/jhep.2000.17687
20. Wasmuth HE, Kunz D, Yagmur E, Timmer-Stranghoner A, Vidacek D, Siewert E, et al. Patients With Acute on Chronic Liver Failure Display “Sepsis-Like” Immune Paralysis. J Hepatol (2005) 42:195–201. doi: 10.1016/j.jhep.2004.10.019
21. Liu Q. Role of Cytokines in the Pathophysiology of Acute-on-Chronic Liver Failure. Blood Purif (2009) 28:331–41. doi: 10.1159/000232940
22. Li J, Zhu X, Liu F, Cai P, Sanders C, Lee WM, et al. Cytokine and Autoantibody Patterns in Acute Liver Failure. J Immunotoxicol (2010) 7:157–64. doi: 10.3109/15476910903501748
23. Yoshimura A, Naka T, Kubo M. SOCS Proteins, Cytokine Signalling and Immune Regulation. Nat Rev Immunol (2007) 7:454–65. doi: 10.1038/nri2093
24. Hong F, Jaruga B, Kim WH, Radaeva S, El-Assal ON, Tian Z, et al. Opposing Roles of STAT1 and STAT3 in T Cell-Mediated Hepatitis: Regulation by SOCS. J Clin Invest (2002) 110:1503–13. doi: 10.1172/JCI15841
25. Triantafyllou E, Woollard KJ, McPhail MJW, Antoniades CG, Possamai LA. The Role of Monocytes and Macrophages in Acute and Acute-On-Chronic Liver Failure. Front Immunol (2018) 9:2948. doi: 10.3389/fimmu.2018.02948
26. Jalan R, Gines P, Olson JC, Mookerjee RP, Moreau R, Garcia-Tsao G, et al. Acute-On Chronic Liver Failure. J Hepatol (2012) 57:1336–48. doi: 10.1016/j.jhep.2012.06.026
27. Jia L, Xue R, Zhu Y, Zhao J, Li J, He WP, et al. The Efficacy and Safety of Methylprednisolone in Hepatitis B Virus-Related Acute-on-Chronic Liver Failure: A Prospective Multi-Center Clinical Trial. BMC Med (2020) 18:383. doi: 10.1186/s12916-020-01814-4
28. Chen P, Wang YY, Chen C, Guan J, Zhu HH, Chen Z. The Immunological Roles in Acute-on-Chronic Liver Failure: An Update. Hepatobiliary Pancreat Dis Int (2019) 18:403–11. doi: 10.1016/j.hbpd.2019.07.003
29. Takeuchi O, Akira S. Pattern Recognition Receptors and Inflammation. Cell (2010) 140:805–20. doi: 10.1016/j.cell.2010.01.022
30. Dong X, Gong Y, Zeng H, Hao Y, Wang X, Hou J, et al. Imbalance Between Circulating CD4+ Regulatory T and Conventional T Lymphocytes in Patients With HBV-Related Acute-on-Chronic Liver Failure. Liver International (2013) 33:1517–26. doi: 10.1111/liv.12248
31. Ambrosino G, Naso A, Cillo U, Basso S, Feltracco P, Carraro P, et al. CYTOCHINES AND LIVER FAILURE: MODIFICATION OF TNF-A AND IL-6 IN PATIENTS WITH ACUTE ON CHRONIC LIVER DECOMPENSATION TRETED WITH MOLECULAR ADSORBENT RECYCLING SYSTEM (MARS). Transplantation (2004) 78:739. doi: 10.1097/00007890-200407271-02040
32. Malhi H, Gores GJ. Cellular and Molecular Mechanisms of Liver Injury. Gastroenterology (2008) 134:1641–54. doi: 10.1053/j.gastro.2008.03.002
33. Zhang Z, Zou ZS, Fu JL, Cai L, Jin L, Liu YJ, et al. Severe Dendritic Cell Perturbation Is Actively Involved in the Pathogenesis of Acute-on-Chronic Hepatitis B Liver Failure. J Hepatol (2008) 49:396–406. doi: 10.1016/j.jhep.2008.05.017
34. Kim EJ, Lee JG, Kim JY, Song SH, Joo DJ, Huh KH, et al. Enhanced Immune-Modulatory Effects of Thalidomide and Dexamethasone Co-Treatment on T Cell Subsets. Immunology (2017) 152:628–37. doi: 10.1111/imm.12804
35. Dejager L, Vandevyver S, Petta I, Libert C. Dominance of the Strongest: Inflammatory Cytokines Versus Glucocorticoids. Cytokine Growth Factor Rev (2014) 25:21–33. doi: 10.1016/j.cytogfr.2013.12.006
36. Kwon HJ, Won YS, Park O, Feng D, Gao B. Opposing Effects of Prednisolone Treatment on T/NKT Cell- and Hepatotoxin-Mediated Hepatitis in Mice. Hepatol (Baltimore Md) (2014) 59:1094–106. doi: 10.1002/hep.26748
37. Oakley RH, Cidlowski JA. The Biology of the Glucocorticoid Receptor: New Signaling Mechanisms in Health and Disease. J Allergy Clin Immunol (2013) 132:1033–44. doi: 10.1016/j.jaci.2013.09.007
38. Nagy P, Kiss A, Schnur J, Thorgeirsson SS. Dexamethasone Inhibits the Proliferation of Hepatocytes and Oval Cells But Not Bile Duct Cells in Rat Liver. Hepatol (Baltimore Md) (1998) 28:423–9. doi: 10.1002/hep.510280220
39. Linossi EM, Babon JJ, Hilton DJ, Nicholson SE. Suppression of Cytokine Signaling: The SOCS Perspective. Cytokine Growth Factor Rev (2013) 24:241–8. doi: 10.1016/j.cytogfr.2013.03.005
40. Philip AM, Vijayan MM. Stress-Immune-Growth Interactions: Cortisol Modulates Suppressors of Cytokine Signaling and JAK/STAT Pathway in Rainbow Trout Liver. PLoS One (2015) 10:e0129299. doi: 10.1371/journal.pone.0129299
41. Miyata M, Lee JY, Susuki-Miyata S, Wang WY, Xu H, Kai H, et al. Glucocorticoids Suppress Inflammation via the Upregulation of Negative Regulator IRAK-M. Nat Commun (2015) 6:6062. doi: 10.1038/ncomms7062
42. Wei Q, Mu K, Li T, Zhang Y, Yang Z, Jia X, et al. Deregulation of the NLRP3 Inflammasome in Hepatic Parenchymal Cells During Liver Cancer Progression. Lab Invest (2014) 94:52–62. doi: 10.1038/labinvest.2013.126
43. Zhao Q, Wu CS, Fang Y, Qian Y, Wang H, Fan YC, et al. Glucocorticoid Regulates NLRP3 in Acute-On-Chronic Hepatitis B Liver Failure. Int J Med Sci (2019) 16:461–9. doi: 10.7150/ijms.30424
44. Oh HY, Namkoong S, Lee SJ, Por E, Kim CK, Billiar TR, et al. Dexamethasone Protects Primary Cultured Hepatocytes From Death Receptor-Mediated Apoptosis by Upregulation of cFLIP. Cell Death Diff (2006) 13:512–23. doi: 10.1038/sj.cdd.4401771
45. Chisari FV, Ferrari C. Hepatitis B Virus Immunopathogenesis. Annu Rev Immunol (1995) 13:29–60. doi: 10.1146/annurev.iy.13.040195.000333
46. Kondo Y, Kobayashi K, Asabe S, Shiina M, Niitsuma H, Ueno Y, et al. Vigorous Response of Cytotoxic T Lymphocytes Associated With Systemic Activation of CD8 T Lymphocytes in Fulminant Hepatitis B. Liver International (2004) 24:561–7. doi: 10.1111/j.1478-3231.2004.0982.x
47. Higuchi N, Kato M, Kotoh K, Kohjima M, Aishima S, Nakamuta M, et al. Methylprednisolone Injection via the Portal Vein Suppresses Inflammation in Acute Liver Failure Induced in Rats by Lipopolysaccharide and D-Galactosamine. Liver International (2007) 27:1342–8. doi: 10.1111/j.1478-3231.2007.01590.x
48. Czaja AJ, Davis GL, Ludwig J, Taswell HF. Complete Resolution of Inflammatory Activity Following Corticosteroid Treatment of HBsAg-Negative Chronic Active Hepatitis. Hepatol (Baltimore Md) (1984) 4:622–7. doi: 10.1002/hep.1840040409
49. Dumortier J, Durupt S, Chevallier M, Trepo C, Zoulim F. Favorable Course of Hepatitis B Virus Reactivation With Hepatocellular Insufficiency by a Treatment Combining Corticoids, Foscarnet and Ganciclovir. Gastroenterol Clin Biol (1997) 21:982–6. doi: GCB-12-1997-21-12-0399-8320-101019-ART78
50. Fujiwara K, Yasui S, Okitsu K, Yonemitsu Y, Oda S, Yokosuka O. The Requirement for a Sufficient Period of Corticosteroid Treatment in Combination With Nucleoside Analogue for Severe Acute Exacerbation of Chronic Hepatitis B. J Gastroenterol (2010) 45:1255–62. doi: 10.1007/s00535-010-0280-y
51. Schalm SW, Summerskill WH, Gitnick GL, Elveback LR. Contrasting Features and Responses to Treatment of Severe Chronic Active Liver Disease With and Without Hepatitis BS Antigen. Gut (1976) 17:781–6. doi: 10.1136/gut.17.10.781
52. Tygstrup N AP, Juhl E. Steroids in Chronic B-Hepatitis. A Randomized, Double-Blind, Multinational Trial on the Effect of Low-Dose, Long-Term Treatment on Survival. A Trial Group of the European Association for the Study of the Liver. Liver (1986) 6:227–32. doi: 10.1111/j.1600-0676.1986.tb01070.x
53. Inadomi T, Saito T, Kaneko M, Hashimoto T, Suzuki H. Bullous Pemphigoid in an HB Virus Carrier: Interaction Between Corticosteroids and HB Virus. J Dermatol (1997) 24:179–83. doi: 10.1111/j.1346-8138.1997.tb02768.x
54. Zhang B, Wang J, Xu W, Wang L, Ni W. Fatal Reactivation of Occult Hepatitis B Virus Infection After Rituximab and Chemotherapy in Lymphoma: Necessity of Antiviral Prophylaxis. Onkologie (2010) 33:537–9. doi: 10.1159/000319696
55. Shibolet O, Ilan Y, Gillis S, Hubert A, Shouval D, Safadi R. Lamivudine Therapy for Prevention of Immunosuppressive-Induced Hepatitis B Virus Reactivation in Hepatitis B Surface Antigen Carriers. Blood (2002) 100:391–6. doi: 10.1182/blood.v100.2.391
56. Lee WC, Wu MJ, Cheng CH, Chen CH, Shu KH, Lian JD. Lamivudine Is Effective for the Treatment of Reactivation of Hepatitis B Virus and Fulminant Hepatic Failure in Renal Transplant Recipients. Am J Kidney Dis (2001) 38:1074–81. doi: 10.1053/ajkd.2001.28607
57. Zhang XH, Feng R, Xu LP, Jiang Q, Jiang H, Fu HX, et al. Immunosuppressive Treatment Combined With Nucleoside Analog Is Superior to Nucleoside Analog Only in the Treatment of Severe Thrombocytopenia in Patients With Cirrhosis Associated With Hepatitis B in China: A Multicenter, Observational Study. Platelets (2015) 26:672–9. doi: 10.3109/09537104.2014.979339
58. Xu Y, Jiang Y, Li Y. Outcomes of Glucocorticoid Treatment in HBVAssociated Acute-On-Chronic Liver Failure Patients: A Retrospective Observational Study. Turk J Gastroenterol (2021) 32:473–80. doi: 10.5152/tjg.2021.20257
59. Zhao J, Zhang JY, Yu HW, He YL, Zhao JJ, Li J, et al. Improved Survival Ratios Correlate With Myeloid Dendritic Cell Restoration in Acute-on-Chronic Liver Failure Patients Receiving Methylprednisolone Therapy. Cell Mol Immunol (2012) 9:417–22. doi: 10.1038/cmi.2011.51
60. Bernsmeier C, Singanayagam A, Patel VC, Wendon J, Antoniades CG. Immunotherapy in the Treatment and Prevention of Infection in Acute-on-Chronic Liver Failure. Immunotherapy (2015) 7:641–54. doi: 10.2217/imt.15.27
61. Chen JF, Wang KW, Zhang SQ, Lei ZY, Xie JQ, Zhu JY, et al. Dexamethasone in Outcome of Patients With Hepatitis B Virus-Related Acute-on-Chronic Liver Failure. J Gastroenterol Hepatol (2014) 29:800–6. doi: 10.1111/jgh.12454
62. Zhang XQ, Jiang L, You JP, Liu YY, Peng J, Zhang HY, et al. Efficacy of Short-Term Dexamethasone Therapy in Acute-on-Chronic Pre-Liver Failure. Hepatol Res (2011) 41:46–53. doi: 10.1111/j.1872-034X.2010.00740.x
63. Wu JY, Li M, Zhang H. Effect of Glucocorticoid Treatment on the Clinical Outcome of Patients With Early-Stage Liver Failure. Nan Fang Yi Ke Da Xue Xue Bao (2011) 31:554–6.
64. Kotoh K, Enjoji M, Nakamuta M, Yoshimoto T, Kohjima M, Morizono S, et al. Arterial Steroid Injection Therapy Can Inhibit the Progression of Severe Acute Hepatic Failure Toward Fulminant Liver Failure. World J Gastroenterol (2006) 12:6678–82. doi: 10.3748/wjg.v12.i41.6678
65. Fujiwara K, Yasui S, Yonemitsu Y, Mikata R, Arai M, Kanda T, et al. Efficacy of High-Dose Corticosteroid in the Early Stage of Viral Acute Liver Failure. Hepatol Res (2014) 44:491–501. doi: 10.1111/hepr.12148
66. Yasui S, Fujiwara K, Haga Y, Nakamura M, Mikata R, Arai M, et al. Infectious Complications, Steroid Use and Timing for Emergency Liver Transplantation in Acute Liver Failure: Analysis in a Japanese Center. J Hepato-Biliary-Pancreatic Sci (2016) 23:756–62. doi: 10.1002/jhbp.399
67. Fujiwara K, Yasui S, Haga Y, Nakamura M, Yonemitsu Y, Arai M, et al. Early Combination Therapy With Corticosteroid and Nucleoside Analogue Induces Rapid Resolution of Inflammation in Acute Liver Failure Due to Transient Hepatitis B Virus Infection. Intern Med (2018) 57:1543–52. doi: 10.2169/internalmedicine.9670-17
68. Rahim MN, Liberal R, Miquel R, Heaton ND, Heneghan MA. Acute Severe Autoimmune Hepatitis: Corticosteroids or Liver Transplantation? Liver Transpl (2019) 25:946–59. doi: 10.1002/lt.25451
69. Ichai P, Duclos-Vallee JC, Guettier C, Hamida SB, Antonini T, Delvart V, et al. Usefulness of Corticosteroids for the Treatment of Severe and Fulminant Forms of Autoimmune Hepatitis. Liver Transpl (2007) 13:996–1003. doi: 10.1002/lt.21036
70. Karkhanis J, Verna EC, Chang MS, Stravitz RT, Schilsky M, Lee WM, et al. Steroid Use in Acute Liver Failure. Hepatol (Baltimore Md) (2014) 59:612–21. doi: 10.1002/hep.26678
71. Zheng L, Liu Y, Shang Y, Han Z, Han Y. Clinical Characteristics and Treatment Outcomes of Acute Severe Autoimmune Hepatitis. BMC Gastroenterol (2021) 21:93. doi: 10.1186/s12876-021-01653-4
72. Anastasiou OE, Dogan-Cavus B, Kucukoglu O, Baba H, Kahraman A, Gerken G, et al. Corticosteroid Therapy Improves the Outcome of Autoimmune Hepatitis-Induced Acute Liver Failure. Digestion (2018) 98:104–11. doi: 10.1159/000487940
73. De Martin E, Coilly A, Chazouilleres O, Roux O, Peron JM, Houssel-Debry P, et al. Early Liver Transplantation for Corticosteroid Non-Responders With Acute Severe Autoimmune Hepatitis: The SURFASA Score. J Hepatol (2021) 74:1325–34. doi: 10.1016/j.jhep.2020.12.033
74. Zachou K, Arvaniti P, Azariadis K, Lygoura V, Gatselis NK, Lyberopoulou A, et al. Prompt Initiation of High-Dose I.V. Corticosteroids Seems to Prevent Progression to Liver Failure in Patients With Original Acute Severe Autoimmune Hepatitis. Hepatol Res (2019) 49:96–104. doi: 10.1111/hepr.13252
75. Anand L, Choudhury A, Bihari C, Sharma BC, Kumar M, Maiwall R, et al. Flare of Autoimmune Hepatitis Causing Acute on Chronic Liver Failure: Diagnosis and Response to Corticosteroid Therapy. Hepatol (Baltimore Md) (2019) 70:587–96. doi: 10.1002/hep.30205
76. Chalasani NP, Maddur H, Russo MW, Wong RJ, Reddy KR, Practice Parameters Committee of the American College of G. ACG Clinical Guideline: Diagnosis and Management of Idiosyncratic Drug-Induced Liver Injury. Am J Gastroenterol (2021) 116:878–98. doi: 10.14309/ajg.0000000000001259
77. European Association for the Study of the Liver, Electronic Address eee, Clinical Practice Guideline Panel C, Panel M, Representative EGB. EASL Clinical Practice Guidelines: Drug-Induced Liver Injury. J Hepatol (2019) 70:1222–61. doi: 10.1016/j.jhep.2019.02.014
78. Tujios SR, Lee WM. Acute Liver Failure Induced by Idiosyncratic Reaction to Drugs: Challenges in Diagnosis and Therapy. Liver International (2018) 38:6–14. doi: 10.1111/liv.13535
79. Hu PF, Wang PQ, Chen H, Hu XF, Xie QP, Shi J, et al. Beneficial Effect of Corticosteroids for Patients With Severe Drug-Induced Liver Injury. J Dig Dis (2016) 17:618–27. doi: 10.1111/1751-2980.12383
80. Wree A, Dechene A, Herzer K, Hilgard P, Syn WK, Gerken G, et al. Steroid and Ursodesoxycholic Acid Combination Therapy in Severe Drug-Induced Liver Injury. Digestion (2011) 84:54–9. doi: 10.1159/000322298
81. Wan YM, Wu JF, Li YH, Wu HM, Wu XN, Xu Y. Prednisone is Not Beneficial for the Treatment of Severe Drug-Induced Liver Injury: An Observational Study (STROBE Compliant). Med (Baltimore) (2019) 98:e15886. doi: 10.1097/MD.0000000000015886
82. European Association for the Study of the L. EASL Clinical Practice Guidelines: Autoimmune Hepatitis. J Hepatol (2015) 63:971–1004. doi: 10.1016/j.jhep.2015.06.030
83. Bjornsson ES, Bergmann O, Jonasson JG, Grondal G, Gudbjornsson B, Olafsson S. Drug-Induced Autoimmune Hepatitis: Response to Corticosteroids and Lack of Relapse After Cessation of Steroids. Clin Gastroenterol Hepatol (2017) 15:1635–6. doi: 10.1016/j.cgh.2017.05.027
84. Bjornsson E. Hepatotoxicity Associated With Antiepileptic Drugs. Acta Neurol Scand (2008) 118:281–90. doi: 10.1111/j.1600-0404.2008.01009.x
85. Singal AK, Bataller R, Ahn J, Kamath PS, Shah VH. ACG Clinical Guideline: Alcoholic Liver Disease. Am J Gastroenterol (2018) 113:175–94. doi: 10.1038/ajg.2017.469
86. D’Agnolo HM, Drenth JP. High-Dose Methylprednisolone-Induced Hepatitis in a Patient With Multiple Sclerosis: A Case Report and Brief Review of Literature. Netherlands J Med (2013) 71:199–202.
87. Oliveira AT, Lopes S, Cipriano MA, Sofia C. Induced Liver Injury After High-Dose Methylprednisolone in a Patient With Multiple Sclerosis. BMJ Case Rep (2015) 2015:bcr2015210722. doi: 10.1136/bcr-2015-210722
88. Zhe-Bin W, Ke W, Mo ZS, Zhen X, Yu-Bao Z, Ying Y, et al. Early, Short-Term, Low-Dose Glucocorticoid Therapy Effectively Blocks Progression of Severe Acute Exacerbation of Chronic Hepatitis B to Liver Failure. Clin Res Hepatol Gastroenterol (2021) 45:101505. doi: 10.1016/j.clinre.2020.07.010
89. Lim HY, Muller N, Herold MJ, van den Brandt J, Reichardt HM. Glucocorticoids Exert Opposing Effects on Macrophage Function Dependent on Their Concentration. Immunology (2007) 122:47–53. doi: 10.1111/j.1365-2567.2007.02611.x
90. Shasthry SM, Sarin SK. New Treatment Options for Alcoholic Hepatitis. World J Gastroenterol (2016) 22:3892–906. doi: 10.3748/wjg.v22.i15.3892
91. Zhu B, You SL, Wan ZH, Liu HL, Rong YH, Zang H, et al. Clinical Characteristics and Corticosteroid Therapy in Patients With Autoimmune-Hepatitis-Induced Liver Failure. World J Gastroenterol (2014) 20:7473–9. doi: 10.3748/wjg.v20.i23.7473
92. Bockmann JH, Dandri M, Lüth S, Pannicke N, Lohse AW. Combined Glucocorticoid and Antiviral Therapy of Hepatitis B Virus-Related Liver Failure. World J Gastroenterol (2015) 21:2214–9. doi: 10.3748/wjg.v21.i7.2214
93. Hamalainen M, Lilja R, Kankaanranta H, Moilanen E. Inhibition of iNOS Expression and NO Production by Anti-Inflammatory Steroids. Reversal Histone Deacetylase Inhibitors Pulm Pharmacol Ther (2008) 21:331–9. doi: 10.1016/j.pupt.2007.08.003
94. Mendizabal M, Marciano S, Videla MG, Anders M, Zerega A, Balderramo DC, et al. Fulminant Presentation of Autoimmune Hepatitis: Clinical Features and Early Predictors of Corticosteroid Treatment Failure. Eur J Gastroenterol Hepatol (2015) 27:644–8. doi: 10.1097/meg.0000000000000353
95. Gao L, Wang JF, Xiang M, Fan YC, Zhang ZG, Wang K. Expression of Human Glucocorticoid Receptor in T Lymphocytes in Acute-on-Chronic Hepatitis B Liver Failure. Digestive Dis Sci (2011) 56:2605–12. doi: 10.1007/s10620-011-1656-4
96. Dendoncker K, Libert C. Glucocorticoid Resistance as a Major Drive in Sepsis Pathology. Cytokine Growth Factor Rev (2017) 35:85–96. doi: 10.1016/j.cytogfr.2017.04.002
97. Rodriguez JM, Monsalves-Alvarez M, Henriquez S, Llanos MN, Troncoso R. Glucocorticoid Resistance in Chronic Diseases. Steroids (2016) 115:182–92. doi: 10.1016/j.steroids.2016.09.010
98. Straub RH, Cutolo M. Glucocorticoids and Chronic Inflammation. Rheumatol (Oxford) (2016) 55:ii6–14. doi: 10.1093/rheumatology/kew348
99. Tjandra K, Le T, Swain MG. Glucocorticoid Receptors are Downregulated in Hepatic T Lymphocytes in Rats With Experimental Cholangitis. Gut (2003) 52:1363–70. doi: 10.1136/gut.52.9.1363
100. Rai T, Ohira H, Tojo J, Abe K, Yokokawa J, Takiguchi J, et al. Expression of Human Glucocorticoid Receptor in Lymphocytes of Patients With Autoimmune Hepatitis. Hepatol Res (2004) 29:148–52. doi: 10.1016/j.hepres.2004.03.004
101. Rai T, Monoe K, Kanno Y, Saito H, Takahashi A, Irisawa A, et al. Expression of Human Glucocorticoid Receptor Beta of Peripheral Blood Mononuclear Cells in Patients With Severe Autoimmune Hepatitis. Fukushima J Med Sci (2006) 52:65–70. doi: 10.5387/fms.52.65
102. Xing T, Li L, Cao H, Huang J. Altered Immune Function of Monocytes in Different Stages of Patients With Acute on Chronic Liver Failure. Clin Exp Immunol (2007) 147:184–8. doi: 10.1111/j.1365-2249.2006.03259.x
103. Bemeur C, Qu H, Desjardins P, Butterworth RF. IL-1 or TNF Receptor Gene Deletion Delays Onset of Encephalopathy and Attenuates Brain Edema in Experimental Acute Liver Failure. Neurochem Int (2010) 56:213–5. doi: 10.1016/j.neuint.2009.11.010
104. Wang X, Xu H, Wang Y, Shen C, Ma L, Zhao C. MicroRNA-124a Contributes to Glucocorticoid Resistance in Acute-on-Chronic Liver Failure by Negatively Regulating Glucocorticoid Receptor Alpha. Ann Hepatol (2020) 19:214–21. doi: 10.1016/j.aohep.2019.08.007
105. Thursz MR, Richardson P, Allison M, Austin A, Bowers M, Day CP, et al. Prednisolone or Pentoxifylline for Alcoholic Hepatitis. N Engl J Med (2015) 372:1619–28. doi: 10.1056/NEJMoa1412278
106. Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, et al. Acute-On-Chronic Liver Failure Is a Distinct Syndrome That Develops in Patients With Acute Decompensation of Cirrhosis. Gastroenterology (2013) 144:1426–37.e9. doi: 10.1053/j.gastro.2013.02.042
107. Bajaj JS, O’Leary JG, Reddy KR, Wong F, Olson JC, Subramanian RM, et al. Second Infections Independently Increase Mortality in Hospitalized Patients With Cirrhosis: The North American Consortium for the Study of End-Stage Liver Disease (NACSELD) Experience. Hepatol (Baltimore Md) (2012) 56:2328–35. doi: 10.1002/hep.25947
108. Bellot P, Francés R, Such J. Pathological Bacterial Translocation in Cirrhosis: Pathophysiology, Diagnosis and Clinical Implications. Liver International (2013) 33:31–9. doi: 10.1111/liv.12021
109. Wasmuth HE, Kunz D, Yagmur E, Timmer-Stranghöner A, Vidacek D, Siewert E, et al. Patients With Acute on Chronic Liver Failure Display “Sepsis-Like” Immune Paralysis. J Hepatol (2005) 42:195–201. doi: 10.1016/j.jhep.2004.10.019
110. Appenrodt B, Grünhage F, Gentemann MG, Thyssen L, Sauerbruch T, Lammert F. Nucleotide-Binding Oligomerization Domain Containing 2 (NOD2) Variants are Genetic Risk Factors for Death and Spontaneous Bacterial Peritonitis in Liver Cirrhosis. Hepatol (Baltimore Md) (2010) 51:1327–33. doi: 10.1002/hep.23440
111. Senkerikova R, de Mare-Bredemeijer E, Frankova S, Roelen D, Visseren T, Trunecka P, et al. Genetic Variation in TNFA Predicts Protection From Severe Bacterial Infections in Patients With End-Stage Liver Disease Awaiting Liver Transplantation. J Hepatol (2014) 60:773–81. doi: 10.1016/j.jhep.2013.12.011
112. Hernaez R, Sola E, Moreau R, Gines P. Acute-On-Chronic Liver Failure: An Update. Gut (2017) 66:541–53. doi: 10.1136/gutjnl-2016-312670
113. Prigent H, Maxime V, Annane D. Clinical Review: Corticotherapy in Sepsis. Crit Care (2004) 8:122–9. doi: 10.1186/cc2374
114. Salluh JI, Povoa P. Corticosteroids in Severe Sepsis and Septic Shock: A Concise Review. Shock (2017) 47:47–51. doi: 10.1097/SHK.0000000000000704
115. van den Akker EL, Koper JW, Joosten K, de Jong FH, Hazelzet JA, Lamberts SW, et al. Glucocorticoid Receptor mRNA Levels Are Selectively Decreased in Neutrophils of Children With Sepsis. Intensive Care Med (2009) 35:1247–54. doi: 10.1007/s00134-009-1468-6
116. Dekelbab BH, Witchel SF, DeFranco DB. TNF-Alpha and Glucocorticoid Receptor Interaction in L6 Muscle Cells: A Cooperative Downregulation of Myosin Heavy Chain. Steroids (2007) 72:705–12. doi: 10.1016/j.steroids.2007.05.007
117. Ledderose C, Mohnle P, Limbeck E, Schutz S, Weis F, Rink J, et al. Corticosteroid Resistance in Sepsis is Influenced by microRNA-124–Induced Downregulation of Glucocorticoid Receptor-Alpha. Crit Care Med (2012) 40:2745–53. doi: 10.1097/CCM.0b013e31825b8ebc
118. Guerrero J, Gatica HA, Rodriguez M, Estay R, Goecke IA. Septic Serum Induces Glucocorticoid Resistance and Modifies the Expression of Glucocorticoid Isoforms Receptors: A Prospective Cohort Study and In Vitro Experimental Assay. Crit Care (2013) 17:R107. doi: 10.1186/cc12774
119. Narum S, Westergren T, Klemp M. Corticosteroids and Risk of Gastrointestinal Bleeding: A Systematic Review and Meta-Analysis. BMJ Open (2014) 4:e004587. doi: 10.1136/bmjopen-2013-004587
120. Fernández J, Acevedo J, Wiest R, Gustot T, Amoros A, Deulofeu C, et al. Bacterial and Fungal Infections in Acute-on-Chronic Liver Failure: Prevalence, Characteristics and Impact on Prognosis. Gut (2018) 67:1870–80. doi: 10.1136/gutjnl-2017-314240
121. Strnad P, Tacke F, Koch A, Trautwein C. Liver - Guardian, Modifier and Target of Sepsis. Nat Rev Gastroenterol Hepatol (2017) 14:55–66. doi: 10.1038/nrgastro.2016.168
122. Victor DW 3rd, Quigley EM. Microbial Therapy in Liver Disease: Probiotics Probe the Microbiome-Gut-Liver-Brain Axis. Gastroenterology (2014) 147:1216–8. doi: 10.1053/j.gastro.2014.10.023
123. Mookerjee RP, Pavesi M, Thomsen KL, Mehta G, Macnaughtan J, Bendtsen F, et al. Treatment With Non-Selective Beta Blockers Is Associated With Reduced Severity of Systemic Inflammation and Improved Survival of Patients With Acute-on-Chronic Liver Failure. J Hepatol (2016) 64:574–82. doi: 10.1016/j.jhep.2015.10.018
124. Madsen BS, Havelund T, Krag A. Targeting the Gut-Liver Axis in Cirrhosis: Antibiotics and non-Selective β-Blockers. Adv Ther (2013) 30:659–70. doi: 10.1007/s12325-013-0044-1
125. Lionakis MS, Kontoyiannis DP. Glucocorticoids and Invasive Fungal Infections. Lancet (2003) 362:1828–38. doi: 10.1016/S0140-6736(03)14904-5
126. Verma N, Singh S, Taneja S, Duseja A, Singh V, Dhiman RK, et al. Invasive Fungal Infections Amongst Patients With Acute-on-Chronic Liver Failure at High Risk for Fungal Infections. Liver International (2019) 39:503–13. doi: 10.1111/liv.13981
127. Acharya SK, Dasarathy S, Kumer TL, Sushma S, Prasanna KS, Tandon A, et al. Fulminant Hepatitis in a Tropical Population: Clinical Course, Cause, and Early Predictors of Outcome. Hepatol (Baltimore Md) (1996) 23:1448–55. doi: 10.1002/hep.510230622
128. Garcia Rodriguez LA, Lin KJ, Hernandez-Diaz S, Johansson S. Risk of Upper Gastrointestinal Bleeding With Low-Dose Acetylsalicylic Acid Alone and in Combination With Clopidogrel and Other Medications. Circulation (2011) 123:1108–15. doi: 10.1161/CIRCULATIONAHA.110.973008
129. Green FW Jr., Kaplan MM, Curtis LE, Levine PH. Effect of Acid and Pepsin on Blood Coagulation and Platelet Aggregation. A Possible Contributor Prolonged Gastroduodenal Mucosal Hemorrhage. Gastroenterology (1978) 74:38–43. doi: 10.1016/0016-5085(78)90352-9
130. Yacyshyn BR, Thomson AB. Critical Review of Acid Suppression in Nonvariceal, Acute, Uppergastrointestinal Bleeding. Dig Dis (2000) 18:117–28. doi: 10.1159/000051385
131. Polson J, Lee WM, American Association for the Study of Liver D. AASLD Position Paper: The Management of Acute Liver Failure. Hepatol (Baltimore Md) (2005) 41:1179–97. doi: 10.1002/hep.20703
Keywords: liver failure, glucocorticoids (GCs), inflammation suppression, immunosuppression, strategies
Citation: Ye C, Li W, Li L and Zhang K (2022) Glucocorticoid Treatment Strategies in Liver Failure. Front. Immunol. 13:846091. doi: 10.3389/fimmu.2022.846091
Received: 30 December 2021; Accepted: 23 February 2022;
Published: 16 March 2022.
Edited by:
Yu Shi, Zhejiang University, ChinaReviewed by:
Su Lin, First Affiliated Hospital of Fujian Medical University, ChinaSukriti Baweja, The Institution of Liver and Biliary Sciences (ILBS), India
Francesca M. Trovato, King’s College Hospital NHS Foundation Trust, United Kingdom
Copyright © 2022 Ye, Li, Li and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenyuan Li, bGl3ZW55dWFuQHVzdGMuZWR1LmNu
 Chao Ye
Chao Ye Wenyuan Li
Wenyuan Li Lei Li
Lei Li Kaiguang Zhang1
Kaiguang Zhang1